Abstract
This report examines the relation of upper and lower extremity motor performance to functional impairment among 371 persons with probable AD. Cognitive and motor performance tests were administered at 6-month intervals for up to four years. Motor performance was assessed using three lower extremity tests and two upper extremity tests. Functional impairment was measured at 3-month intervals using caregiver ratings of impairments in activities of daily living, mobility and range of motion. Both lower and upper extremity performance were inversely related to functional impairments on all three scales (all ps < .001), after controlling for age, sex, and level of cognitive impairment. This suggests that motor performance contributes to functional impairments in AD, independent of cognitive impairment. It is important to preserve motor performance in individuals with AD because it influences physical function throughout the course of the disease.
Keywords: Alzheimer’s Disease, physical function, physical performance, longitudinal study
INTRODUCTION
Alzheimer’s disease (AD) is a major source of functional impairment in older people (1–3). Research on the development of functional impairment in AD has primarily focused on the role of cognitive impairment. These studies have found a substantial correlation between impaired functional activities of daily living and the severity of cognitive impairments (4–9), but rarely evaluated other potential contributing factors to functional impairment.
Motor performance warrants further study as a risk factor for functional impairments in AD, as most activities of daily living require basic motor skills. Motor symptoms, such as gait disturbances and extrapyramidal signs, are associated with increased risk of functional impairment in older people(10–11)and often develop over the course of AD(12–15). Post-mortem studies report substantial person-to-person variability in neuropathological changes in neural systems subserving motor performance (16–19). The few available studies (20–22) have found that motor symptoms are associated with increased risk of functional impairment in AD, but most did not longitudinally measure motor performance, limiting precision. One study of community-dwelling older adults with mild to moderate cognitive impairment found that lower extremity motor performance predicted the onset of dependence in basic activities of daily living (ADLs) at the 1-year follow-up (23). Two simple tests of motor performance (rapid gait and repeated chair stands) distinguished persons at low and high risk for ADL dependence.
The aim of this report is to examine cognitive and motor performance as predictors of functional impairments using data from a longitudinal study of persons with AD to test whether impairments in motor performance are related to functional impairment even in persons with AD. Our hypothesis is that impairments in motor performance will significantly predict functional performance independent of cognitive impairment. This report extends previous work because we had a longer follow-up period, more observations, and participants with greater cognitive impairment, functional impairment, and racial variation. We also tested both upper and lower motor performance and examined a broader range of functional impairment than previous studies. Cognitive and motor performance tests were administered every 6 months for up to four years. Impairments in daily functioning were evaluated at three-month intervals using structured interviews with a family member. The large number of observations per person enhances precision in estimating linear and non-linear trends in the development of functional impairments in persons with AD.
METHODS
Participants
Study participants were recruited from among all patients evaluated at the Rush Alzheimer’s Disease Center between June 1999 and April 2002. All participants were 65 years of age or older, met standard diagnostic criteria for possible or probable AD (24), and resided in a community setting. Of 559 persons eligible for this study, 396 (70.8%) consented to participate and 371 had a diagnosis of probable or highly probable AD and no missing baseline data and thus constituted the analytic sample. Participants were 70.1% female, 72.5% white, 26.4% black, 0.5% Asian-Pacific Islander, and 0.5% other. Mean age at baseline was 77.6 years (SD 9.0). Consent procedures were approved by the Institutional Review Board of Rush University Medical Center. All procedures were presented verbally by study representatives and specified in written consent documents. As an added precaution, participants with AD and a responsible family member jointly signed consent documents.
Measures
Cognitive Performance
The Mini-Mental State Examination (MMSE) (25) was selected as the primary index of cognitive impairment because of its widespread use in scaling dementia severity (26). Scores range from 0 to 30 indicating the number of correct responses. In addition, we used a global measure of cognitive performance that summarized nine cognitive function tests: two measures of episodic memory, three of semantic memory, one of working memory, two of visuaospatial memory and the MMSE that assessed global cognition. As previously described (27;28), each test was converted to a z-score using the mean and standard deviation of the entire study population at baseline. Then the nine z-scores were averaged. We tested our hypothesis using the MMSE and the global cognitive measure in separate analyses.
Motor Performance
The lower-extremity performance (LEP) battery consisted of three tests: 360-degree turn, measured walk, and repeated chair stands. These are commonly used tests of motor performance in elderly populations, and they have reasonable reliability (29;30) and well-established predictive validity (31;32). The 360-degree turn requires the subject to stand at a fixed point and make a complete rotation around that point. The score is based on the time it takes to complete the turn. The measured walk measures the time it takes a participant to complete an 8 ft. walk. The turn and walk tests were repeated twice and the average of the two measures was recorded. The chair stand requires the subjects to fold their arms across their chest and rise from a sitting position. The time it takes to get up from the chair 5 times is the score. In keeping with procedures established previously (31;33;34), recorded times were converted into quintiles with the shortest times scoring highest (5) and an additional category, coded 0, for those who were unable to complete the task. The scores from 0 to 5 on each of the three tests were summed to determine an overall LEP score (range, 0–15).
The upper extremity performance (UEP) battery consisted of finger tapping and Purdue pegboard tests. The finger tapping test(35) requires the subject to tap a key with their index finger as many times as possible for ten seconds. An electronic tapper (Western Psychological Services, Los Angeles, CA) was used to count the number of taps. Two trials were performed with each hand and the average of the four trials yielded the tapping score. The Purdue pegboard test(36) records the number of pegs that can be placed in the board in thirty seconds. Two trials were performed with each hand and the average of the four trials yielded the pegboard score. Each score was transformed into a standardized “z” score and the overall score for the UEP was the average of the z-scores with higher scores indicating better performance.
Functional Impairment
Functional impairments were measured using three standard self-report scales (37), modified slightly for use as informant ratings in the present study. Six items from the 7-item Katz scale were used to measure the ability to independently perform basic self-care activities (bathing, dressing, walking across a small room, transferring from bed to a chair, using the toilet, and eating; we excluded the grooming item to avoid possible gender bias. The 3-item Rosow-Breslau scale was used to measure mobility (walking a half-mile, walking up a flight of stairs, and strenuous work around the house). The 5-item Nagi scale was used to measure range of motion (reaching or extending arms above shoulder level; writing or handling small objects; pushing or pulling large objects; lifting objects weighing over 10 pounds; and stooping, crouching, or kneeling). For each measure, a summary score was calculated based on the number of items performed without assistance (Katz, Rosow-Breslau) or with little or no difficulty (Nagi) during the previous month. Inability to independently perform these basic physical activities (self-care, mobility, range of motion) is consistent with the concept of disability as outlined by the World Health Organization (38).
Other Variables
All analyses included age at baseline, gender, race (black or white) obtained using the 1980 census question. In sensitivity analyses, we considered nine chronic conditions obtained from informant report of history of cancer, heart attack, stroke, hypertension, diabetes, thyroid disease, head injury, Parkinson’s disease, or hip fracture. We also considered a tenth variable which was the count of the number of nine conditions reported.
Design and Procedures
Cognitive (MMSE and global cognitive function) and motor (LEP, UEP) performance tests were administered at baseline and every 6 months for up to 4 years. Baseline testing was conducted in a clinic setting; follow-up testing was conducted in the participant’s residence. Functional impairment scales (Katz, Nagi, Rosow-Breslau) were administered at baseline and every 3 months by telephone. All measures were administered by research technicians who completed four weeks of training, supervised administration, and standardized certification examinations. Errors in data collection were further minimized by the use of computer assisted testing procedures that specified item order, task instructions, and allowable response codes. Interrater reliability was monitored for each data collection measure every six months, with retraining provided as needed to maintain agreement at 90% or better. Average interrater reliability on all measures was 98.6% (range = 97.65% – 99.78%).
Analytic Methods
We used generalized linear models fitted by the method of Generalized Estimating Equations (GEE) (39;40) to examine associations with level over time in three measures of reported functional impairment. Specifically, we considered each of the three measures as a “proportion unimpaired” and modeled the score on the measure with a logistic link function and binomial error structure distribution. GEE allows for the inclusion of all observed data, that is, persons with some missing observations. We included time-in-study (since baseline), age at baseline, male sex, black race, and the interactions of age, sex, and race with time. These terms control for the potential confounding effects of age, sex, and race on both level and change over time in functional impairment. In addition, we included time-varying MMSE score in all models, to examine and also control for the degree of cognitive impairment. Time-varying LEP and UEP were added to individual models as the primary test of our hypotheses. We then fit an additional set of models, adding the interactions between LEP and UEP and the demographic terms to test for effect modification by age, sex, or race. All models were fit using SAS® (41).
Sensitivity analyses
Possible non-linear associations were tested by adding squared terms for study time, age, MMSE score, LEP and UEP to individual models. To see if analyses were sensitive to reasons for early exit from follow-up, we created three separate indicators: 1) death during follow-up, 2) nursing home placement during follow-up, 3) reaching a test floor during follow-up. We added these individually to analyses to see if they changed the estimated association of extremity performance with physical function. To see if a more comprehensive measure of cognitive function changed the association of extremity performance with physical function, we repeated analyses using a composite of nine cognitive tests (the global cognitive measure described earlier) instead of MMSE. Finally, we tested the effect of medical comorbidity by adding each of 9 conditions individually and a count of the number of nine conditions reported to separate models.
RESULTS
Follow-up participation
Participation rates ranged from 76.1 to 90.8% across the follow-up observations, with a median of 80.8%. Follow-up ranged from 0.2 to 4.3 years, with a mean of 2.7 years (SD= 1.1). The number of completed follow-up clinical evaluations per person ranged from 1 to 8, with mean of 4.8 (SD =1.9). Observation ended at death (n=124) or at the time of nursing home admission (n=109).
Changes in cognitive and motor performance, and functional impairments over time
Table 1 summarizes longitudinal observations on measures of cognitive performance, motor performance, and functional impairments. The mean baseline MMSE score (13.5) is near the mid-point of values considered to be in the impaired range. As expected for persons with AD, there was evidence of systematic decline in cognitive performance on the MMSE. Measures of LEP and UEP also declined over the study. There was marked functional impairment at baseline and significant decline on all three informant-rated disability scales.
Table 1.
Longitudinal observations on measures of cognitive performance, motor performance, and functional impairment in the Alzheimer’s disease study cohort (n =371).
| Measure | Baseline score mean (SD) | Number of participants reaching Score = 0 (floor) |
Annual Rate of change* mean (SE) | |
|---|---|---|---|---|
| Baseline | Follow-up | |||
| N | N | |||
| Cognitive performance | ||||
| MMSE | 13.5 (8.11) | 35 | 81 | −3.62 (4.29) |
| Motor performance | ||||
| LEP | 8.68 (4.67) | 26 | 101 | −2.08 (2.94) |
| UEP | 0.05 (0.96) | 22 | 90 | −0.45 (0.60) |
| Functional Impairment | ||||
| Katz (self-care) | 4.20 (2.12) | 42 | 56 | −0.91 (1.64) |
| Rosow-Breslau (mobility) | 1.60 (1.10) | 86 | 83 | −0.60 (0.99) |
| Nagi (range of motion) | 3.17 (1.78) | 37 | 59 | −0.71 (1.40) |
Notes: MMSE = Mini-Mental State Examination total score; LEP = Lower Extremity Performance battery summary score, UEP = Upper Extremity Performance battery summary score. Functional impairment scale scores are number of items the person is able to do “without assistance” (Katz; Rosow-Breslau) or with “little or no difficulty” (Nagi).
Estimate from least squares regression including only study time and excluding observations after the first “floor” observation.
Motor Performance and Functional Impairment
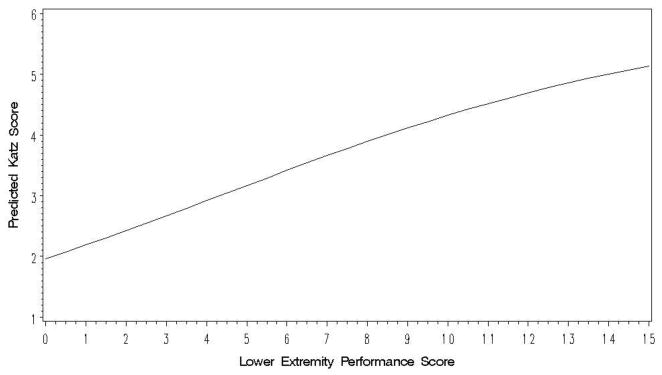
In the analyses of change in functional impairment, for each model we excluded observations after the first score of zero (the floor) on the outcome measure and included only people with at least 2 observations remaining. Adjusting for the effects of age, sex, and race, higher MMSE scores were significantly related to higher levels of functional ability on the Katz (β̂ = .107, SE= .007, p<.0001), Rosow-Breslau (β̂ = .038, SE= .007, p<.0001) and Nagi (β̂ = .044, SE= .008, p<.0001) functional impairment scales in models that included no extremity performance measure. The pattern indicates that functional impairments are greater with lower cognitive performance on the MMSE, as expected. We then added the primary predictors of interest, time-varying LEP and UEP scores, separately into these models. Additional models testing for effect modification by age, sex, or race showed no such effects, so we present as our final models the base models. As an example, Table 2 presents the results from the analysis of the association of the Katz score with LEP. As the model estimates refer to the outcome on the logit scale, we back-transformed the predicted values to the original scale to illustrate the model. For a person with average values of the other covariates, Figure 1 shows the modeled association between LEP and the number of Katz self-care items a person can do; the strong association between the two can be clearly seen. Consistent with the primary study hypothesis, lower LEP scores were associated with lower levels of functional ability on each of the other two scales, as well (see Table 3). Adding the LEP term also attenuated the effects of cognitive impairment on functional impairment. MMSE scores remained significant for the more complex self-care functional abilities measured by the Katz, but not for the Rosow-Breslau or Nagi scales. The same pattern of effects was also found using UEP battery as the index of motor performance (see Table 3).
Table 2.
Fitted GEE model predicting Katz self-care functional impairment scale score* as a function of covariates and lower extremity performance score
| Participants included | 254 | ||
| Total observations | 1,108 | ||
| Predictor | Estimate | (SE) | p |
| Intercept | −0.604 | (0.767) | 0.43 |
| Age at Baseline (Years) | −0.006 | (0.010) | 0.53 |
| Male Sex | 0.414 | (0.221) | 0.06 |
| Black Race | 0.155 | (0.197) | 0.43 |
| MMSE ** | 0.070 | (0.007) | < 0.001 |
| Time since Baseline (Years) | −0.619 | (0.396) | 0.12 |
| Age × Time | 0.006 | (0.005) | 0.27 |
| Sex × Time | −0.051 | (0.103) | 0.62 |
| Race × Time | 0.159 | (0.094) | 0.09 |
| LEP ** | 0.167 | (0.015) | < 0.001 |
Notes: GEE = Generalized Estimating Equations; MMSE = Mini-Mental State Examination total score; LEP = Lower Extremity Performance summary score
Modeled as the logit of the expected proportion of items “able to do” out of 6 total items.
These were modeled as time-varying covariates.
Figure 1.
Predicted Values of the Katz Functional Impairment Score as a Function of Lower Extremity Motor Performance Score Based on GEE Model Adjusted for Age, Sex, Race, and Cognitive Performance.
Table 3.
Summary of regression models predicting functional impairment on three scales (Katz, Rosow-Breslau, Nagi), adjusted for age, sex, and race.*
| Functional Impairment Scale** | ||||||
|---|---|---|---|---|---|---|
| Katz (Self-Care) | Rosow-Breslau (Mobility) | Nagi (Range of Motion) | ||||
| Lower Extremity Performance | ||||||
| N Participants | 254 | 228 | 254 | |||
| Total observations | 1,108 | 977 | 1,080 | |||
| Predictor | Estimate (SE) | P | Estimate (SE) | p | Estimate (SE) | p |
| MMSE | 0.070 (0.007) | <0.001 | 0.000 (0.007) | 0.95 | 0.006 (0.008) | 0.46 |
| LEP | 0.167 (0.015) | <0.001 | 0.125 (0.014) | <0.001 | 0.138 (0.013) | <0.001 |
| Upper Extremity Performance | ||||||
| N Participants | 261 | 231 | 263 | |||
| Total observations | 1,161 | 1,008 | 1,135 | |||
| Predictor | Estimate (SE) | P | Estimate (SE) | p | Estimate (SE) | p |
| MMSE | 0.063 (0.008) | <0.001 | 0.002 (0.008) | 0.76 | 0.009 (0.009) | 0.30 |
| UEP | 0.682 (0.069) | <0.001 | 0.484 (0.069) | <0.001 | 0.474 (0.067) | <0.001 |
Notes: MMSE = Mini-Mental State Examination total score; LEP = Lower Extremity Performance summary score; UEP = Upper Extremity Performance summary score
All models also included terms for age at baseline, sex, race, time since baseline, and the interactions of age, sex, and race with time; MMSE, LEP, and UEP were modeled as time-varying covariates.
Items able to do without assistance (Katz, Rosow-Breslau) or with “little or no difficulty” (Nagi)
Sensitivity Analyses
In tests for non-linear associations of time, age, MMSE score, LEP, UEP and study time with each of the three functional impairment scores, we found three significant quadratic effects, none of which changed the main conclusions presented (data not shown). In analyses including three indicators for death, nursing home admission, or reaching test floors during follow-up, the indicators had no substantive effect on the associations between MMSE score, motor performance (LEP, UEP) and functional impairment, demonstrating that results were not sensitive to reasons for early exit from the study. In models substituting the global measure of cognitive function for MMSE, the associations of LEP and UEP remained highly significant. Finally, in analyses including each of the nine medical conditions and the count of conditions, the associations between LEP and UEP and physical function were as strong or stronger than in analyses without them.
DISCUSSION
The findings of this longitudinal study indicate that impairments in both cognitive and motor performance are related to functional impairment in persons with AD. As expected from previous research, the need for assistance with basic self-care increased with lower cognitive performance. The novel findings of the study concern the contribution of motor performance to a broad range of functional impairments. Measures of LEP and UEP were associated with increased functional impairment in basic self-care activities, mobility, and range of motion, even after accounting for the effects of cognitive performance.
There is a paucity of longitudinal studies examining LEP and UEP in relation to functional impairments in persons with AD. Previous studies measured motor performance using clinician ratings of motor symptoms at one (20–22) or two(23) points in time. By contrast, the present study used performance tests at multiple points in time. Our data strongly support the hypothesis that motor performance contributes to functional impairments in AD, independent of cognitive performance. This pattern may reflect individual differences in neuropathology in nigrostriatal dopaminergic neural tracts (16–19). For example, gait disturbances have been linked to neurofibrillary tangles in the substantia nigra in persons with and without AD (18). We were not able to shed light on possible pathophysiological bases for this relationship because brain autopsy was not systematically obtained for this cohort.
Our findings are consistent with previous research (23) but also provide new insight into the association between motor performance and functional impairments. Our data show that motor performance is an independent predictor of a broad range of functional abilities, not just basic self-care activities, and that this association exists across a range of cognitive performance. Our data also show that both upper and lower motor performance predict functional impairments, independent of cognitive performance, suggesting that either UEP or LEP testing could be used to identify individuals with AD who are at high risk of becoming functionally dependent. The relevant clinical implication of our findings is that regardless of cognitive decline, it is important to preserve motor performance in individuals with AD because it influences physical function throughout the course of the disease.
Strengths of this study include a large sample size, repeated measurement of key variables over a period of up to four years, high rates of follow-up participation, and coverage of a broad spectrum of motor performance, functional impairments, and AD severity. There was a mean of 4.5 observations per person, facilitating the evaluation of linear and nonlinear trends in the data. Nursing home placement and death were monitored for all participations throughout the follow-up period. As a result, it was possible to evaluate the impact of these study endpoints on findings.
There are limitations in sampling that should be considered when evaluating our data. The study cohort was recruited from a memory disorders clinic in a large metropolitan area, and was limited to persons with the clinical diagnosis of AD. There are also limitations in measurement. First, cognitive performance was assessed using the MMSE, a brief scale consisting of basic attention, memory, language and praxis tasks, and with a more extensive composite measure of cognitive performance. It is possible but not likely that some specific measures not covered in the composite would have accounted for more variance in functional impairment. Executive function and other frontal system measures have shown promise in predicting functional impairments in AD (42). Second, measures of motor performance were limited to simple, timed performance tests of upper and lower extremity function. These measures capture speed of movement, but do not provide direct information on other dimensions of motor performance including strength, coordination, and movement disorders. Third, cognitive and motor performance tests are likely to share some task variance. For example, praxis items on the cognitive test require a motor response; the motor performance tests require rudimentary cognitive skills to comprehend and follow instructions. Finally, functional impairments were based on ratings by family members. This approach is widely used in research on disability in AD. However, these semi-quantitative ratings rely on subjective judgments by people responsible for providing informal care and assistance.
In summary, these data suggest that motor performance contributes to the development of functional impairment in persons with AD. We add to previous reports by showing that fluctuations in motor performance over the disease course were predictive of a broad range of functional impairments, including self-care deficits that require physical assistance from family and professional caregivers. UEP and LEP testing appears to provide a standardized and cost-efficient method to quantify motor performance over the disease course. Motor performance is also potentially modifiable, as physical activity interventions have shown promise in reducing functional impairment in AD (43).
Acknowledgments
This study was supported by Grants R01 AG10315 and R01 AG09966 from the National Institute on Aging, National Institutes of Health.
We thank the study participants and their family members for their time and commitment to this research project. We also thank Melinda Scheuer and her staff of research assistants for data collection activities, Woojeong Bang and her staff and Kenneth Tonnissen for assistance with analytic programming and analysis, and George Dombrowski and his staff for data management. The authors thank Dr. David Gilley for his contributions to conceptualization and interpretation of the issues. SAS® is a trademark or registered trademark of SAS Institute Inc. in the U.S. and other countries.
Footnotes
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Liesi E. Hebert, Email: Liesi_Hebert@rush.edu.
Julia L. Bienias, Email: jbienias@alum.wustl.edu.
Judith J. McCann, Email: jmccann@rush.edu.
Paul A. Scherr, Email: pas0@cdc.gov.
Robert S. Wilson, Email: Robert_S_Wilson@rush.edu.
Denis A. Evans, Email: Denis_Evans@rush.edu.
Reference List
- 1.Aguero-Torres H, Fratiglioni L, Guo Z, Viitanen M, von Strauss E, Winblad B. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health. 1998;88(10):1452–1456. doi: 10.2105/ajph.88.10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas VS. Excess functional disability among demented subjects? Findings from the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2001;12(3):206–210. doi: 10.1159/000051259. [DOI] [PubMed] [Google Scholar]
- 3.Sauvaget C, Yamada M, Fujiwara S, Sasaki H, Mimori Y. Dementia as a predictor of functional disability: a four-year follow-up study. Gerontology. 2002;48(4):226–233. doi: 10.1159/000058355. [DOI] [PubMed] [Google Scholar]
- 4.Zanetti O, Bianchetti A, Frisoni GB. Determinates of disability in Alzheimer’s disease. Int J Geriatr Psychiatry. 1993;8(7):581–586. [Google Scholar]
- 5.Hill RD, Backman L, Fratiglioni L. Determinants of functional abilities in dementia. J Am Geriatr Soc. 1995;43(10):1092–1097. doi: 10.1111/j.1532-5415.1995.tb07006.x. [DOI] [PubMed] [Google Scholar]
- 6.Suh GH, Ju YS, Yeon BK, Shah A. A longitudinal study of Alzheimer’s disease: rates of cognitive and functional decline. Int J Geriatr Psychiatry. 2004;19(9):817–824. doi: 10.1002/gps.1168. [DOI] [PubMed] [Google Scholar]
- 7.Feldman HH, Van Baelen B, Kavanagh SM, Torfs KE. Cognition, function, and caregiving time patterns in patients with mild-to-moderate Alzheimer disease: a 12-month analysis. Alzheimer Dis Assoc Disord. 2005;19(1):29–36. doi: 10.1097/01.wad.0000157065.43282.bc. [DOI] [PubMed] [Google Scholar]
- 8.Sarazin M, Stern Y, Berr C, Riba A, Albert M, Brandt J, et al. Neuropsychological predictors of dependency in patients with Alzheimer disease. Neurology. 2005;64(6):1027–1031. doi: 10.1212/01.WNL.0000154529.53488.30. [DOI] [PubMed] [Google Scholar]
- 9.Liu KP, Chan CC, Chu MM, Ng TY, Chu LW, Hui FS, et al. Activities of daily living performance in dementia. Acta Neurol Scand. 2007;116(2):91–95. doi: 10.1111/j.1600-0404.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- 10.Louis ED, Tang MX, Schupf N, Mayeux R. Functional correlates and prevalence of mild parkinsonian signs in a community population of older people. Arch Neurol. 2005;62(2):297–302. doi: 10.1001/archneur.62.2.297. [DOI] [PubMed] [Google Scholar]
- 11.Fleischman DA, Wilson RS, Schneider JA, Bienias JL, Bennett DA. Parkinsonian signs and functional disability in old age. Exp Aging Res. 2007;33(1):59–76. doi: 10.1080/03610730601006370. [DOI] [PubMed] [Google Scholar]
- 12.Lopez OL, Wisnieski SR, Becker JT, Boller F, DeKosky ST. Extrapyramidal signs in patients with probable Alzheimer disease. Arch Neurol. 1997;54(8):969–975. doi: 10.1001/archneur.1997.00550200033007. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, Bennett DA, Gilley DW, Beckett LA, Schneider JA, Evans DA. Progression of parkinsonian signs in Alzheimer’s disease. Neurology. 2000;54(6):1284–1289. doi: 10.1212/wnl.54.6.1284. [DOI] [PubMed] [Google Scholar]
- 14.Bennett DA, Shannon KM, Beckett LA, Wilson RS. Dimensionality of parkinsonian signs in aging and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 1999;54(4):M191–M196. doi: 10.1093/gerona/54.4.m191. [DOI] [PubMed] [Google Scholar]
- 15.Tsolaki M, Kokarida K, Iakovidou V, Stilopoulos E, Meimaris J, Kazis A. Extrapyramidal symptoms and signs in Alzheimer’s disease: prevalence and correlation with the first symptom. Am J Alzheimers Dis Other Demen. 2001;16(5):268–278. doi: 10.1177/153331750101600512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns JM, Galvin JE, Roe CM, Morris JC, McKeel DW. The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology. 2005;64(8):1397–1403. doi: 10.1212/01.WNL.0000158423.05224.7F. [DOI] [PubMed] [Google Scholar]
- 17.Holtzer R, Irizarry MC, Sanders J, Hyman BT, Wegesin DJ, Riba A, et al. Relation of quantitative indexes of concurrent alpha-synuclein abnormalities to clinical outcome in autopsy-proven Alzheimer disease. Arch Neurol. 2006;63(2):226–230. doi: 10.1001/archneur.63.2.226. [DOI] [PubMed] [Google Scholar]
- 18.Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol. 2006;59(1):166–173. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- 19.Attems J, Quass M, Jellinger KA. Tau and alpha-synuclein brainstem pathology in Alzheimer disease: relation with extrapyramidal signs. Acta Neuropathol. 2007;113(1):53–62. doi: 10.1007/s00401-006-0146-9. [DOI] [PubMed] [Google Scholar]
- 20.Mortimer JA, Ebbitt B, Jun SP, Finch MD. Predictors of cognitive and functional progression in patients with probable Alzheimer’s disease. Neurology. 1992;42(9):1689–1696. doi: 10.1212/wnl.42.9.1689. [DOI] [PubMed] [Google Scholar]
- 21.Stern Y, Albert M, Brandt J, Jacobs DM, Tang MX, Marder K, et al. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in Alzheimer’s disease: prospective analyses from the Predictors Study. Neurology. 1994;44(12):2300–2307. doi: 10.1212/wnl.44.12.2300. [DOI] [PubMed] [Google Scholar]
- 22.Scarmeas N, Albert M, Brandt J, Blacker D, Hadjigeorgiou G, Papadimitriou A, et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005;64(10):1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill TM, Richardson ED, Tinetti ME. Evaluating the risk of dependence in activities of daily living among community-living older adults with mild to moderate cognitive impairment. J Gerontol A Biol Sci Med Sci. 1995;50(5):M235–M241. doi: 10.1093/gerona/50a.5.m235. [DOI] [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 27.Wilson RS, McCann JJ, Li Y, Aggarwal NT, Gilley DW, Evans DA. Nursing home placement, day care use, and cognitive decline in Alzheimer’s disease. Am J Psychiatry. 2007;164(6):910–915. doi: 10.1176/ajp.2007.164.6.910. [DOI] [PubMed] [Google Scholar]
- 28.Wilson RS, Li Y, Aggarwal NT, Barnes LL, McCann JJ, Gilley DW, et al. Education and the course of cognitive decline in Alzheimer disease. Neurology. 2004;63(7):1198–1202. doi: 10.1212/01.wnl.0000140488.65299.53. [DOI] [PubMed] [Google Scholar]
- 29.Tager IB, Swanson A, Satariano WA. Reliability of Physical Performance and Self-Reported Functional Measures in an Older Population. J Gerontol Med Sci. 1998;53A(4):M295–M300. doi: 10.1093/gerona/53a.4.m295. [DOI] [PubMed] [Google Scholar]
- 30.Hoeymans N, Wouters ER, Feskens EJ, Van Den Bos GA, Kromhout D. Reproducibility of performance-based and self-reported measures of functional status. J Gerontol A Biol Sci Med Sci. 1997;52(6):M363–M368. doi: 10.1093/gerona/52a.6.m363. [DOI] [PubMed] [Google Scholar]
- 31.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 32.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 33.Guralnik JM, Seeman TE, Tinetti ME, Nevitt MC, Berkman LF. Validation and use of performance measures of functioning in a non-disabled older population: MacArthur studies of successful aging. Aging (Milano ) 1994;6(6):410–419. doi: 10.1007/BF03324272. [DOI] [PubMed] [Google Scholar]
- 34.Mendes De Leon CF, Barnes LL, Bienias JL, Skarupski KA, Evans DA. Racial disparities in disability: recent evidence from self-reported and performance-based disability measures in a population-based study of older adults. J Gerontol B Psychol Sci Soc Sci. 2005;60(5):S263–S271. doi: 10.1093/geronb/60.5.s263. [DOI] [PubMed] [Google Scholar]
- 35.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsyychological Test Battery: Theory and Interpretation. Tuscon, Ariz: Neuropsychology Press; 1985. [Google Scholar]
- 36.Tiffin J, Asher EJ. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32(3):234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- 37.Branch LG, Katz S, Kniepmann K, Papsidero JA. A prospective study of functional status among community elders. Am J Public Health. 1984;74(3):266–268. doi: 10.2105/ajph.74.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. International classification of impairments, disabilities and handicaps: A manual of classification relationg to the consequences of disease. Geneva: World Health Organization; 1980. [Google Scholar]
- 39.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 40.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 41.SAS Institute Inc. SAS/STAT(r) User’s Guide, Version 8. Cary, NC: SAS Institute Inc; 2000. [Google Scholar]
- 42.Boyle PA. Assessing and predicting functional impairment in Alzheimer’s disease: the emerging role of frontal system dysfunction. Curr Psychiatry Rep. 2004;6(1):20–24. doi: 10.1007/s11920-004-0033-9. [DOI] [PubMed] [Google Scholar]
- 43.Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003;290(15):2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]