Abstract
Background/Purpose
There is a well-known relationship between multiple sclerosis (MS) and damage to the optic nerve, but advanced, quantitative magnetic resonance imaging (MRI) methods have not been applied to large cohorts. Our objective was to determine if a short imaging protocol (<10 minutes), implemented with standard hardware, could detect abnormal water diffusion in the optic nerves of MS patients.
Methods
We examined water diffusion in human optic nerves via diffusion tensor MRI (DTI) in the largest multiple sclerosis cohort reported to date (104 individuals, including 38 optic nerves previously affected by optic neuritis). We also assessed if such abnormalities are associated with loss of visual acuity (both high and low contrast) and damage to the retinal nerve fiber layer (assessed via optical coherence tomography).
Results
The most abnormal diffusion was found in the optic nerves of patients with secondary progressive MS, especially in optic nerves previously affected by optic neuritis (19% drop in fractional anisotropy). DTI abnormalities correlated with both retinal nerve fiber layer thinning (correlation coefficient 0.41) and loss of visual acuity, particularly at high contrast and in nerves previously affected by optic neuritis (correlation coefficient 0.54). However, diffusion abnormalities were overall less pronounced than retinal nerve fiber layer thinning.
Conclusion
Diffusion tensor imaging is sensitive to optic nerve damage in patients with MS, but a short imaging sequence added to standard clinical protocols may not be the most reliable indicator of optic nerve damage.
Introduction
Impaired vision is extremely common in multiple sclerosis (MS) and may arise from damage (demyelination and axonal loss) to different components (e.g., optic nerve, tract, and radiation) of the visual system. Of these, the optic nerve is the best-characterized, as optic neuritis (ON) is the presenting symptom in many cases of MS (1; 2). Nevertheless, recent research suggests that visual dysfunction accumulates even without prior ON. This dysfunction can be detected clinically using low-contrast letters to assess visual sensory function (3); optical coherence tomography (OCT) to assess damaged or destroyed visual axons by measuring peripapillary retinal nerve fiber layer (PRNFL) thickness and total macular volume (TMV) (4); and visual evoked potentials (VEPs) to assess speed and amplitude of impulse conduction (5).
Magnetic resonance imaging (MRI) can detect visual pathway damage and has been extensively investigated. MRI shows T2-weighted hyperintensities in optic nerves with prior ON, whereas in acute cases, post-contrast T1-weighted hyperintensities are appreciated (6). Recently, quantitative MRI techniques such as magnetization-transfer (7–9) and diffusion-weighted (10–16) imaging have revealed time-evolving abnormalities following an attack of ON. Unfortunately, both of these techniques are time consuming, prohibiting clinical application.
Diffusion tensor imaging (DTI) uses information contained in the diffusion properties of water to probe tissue microstructural abnormalities such as demyelination and axonal damage, both of which occur in ON. The most commonly reported DTI-derived indices are fractional anisotropy (FA), mean diffusivity (MD), and diffusion parallel (λ∥) and perpendicular (λ⊥) to the long axis of specific white-matter tracts (17). DTI studies of affected optic nerves in patients with a remote history of ON reveal reduced FA and elevated diffusivities as well as correlations with concurrent measurements of acuity, VEPs, and PRNFL thickness (10; 14; 16). However, sample sizes in these studies have been low due to technical hindrances related to the small diameter (≤3mm) and mobility of the optic nerves and their proximity to the paranasal sinuses, which induce susceptibility-related artifacts and interfere with conventional DTI. Additionally, lengthy acquisition times actually may accentuate the effects of optic-nerve motion.
The goals of this study were: (1) To develop and evaluate a rapid optic-nerve DTI sequence performed with standard coils in a clinically acceptable time frame (<10 minutes); and (2) To relate abnormal water diffusion in the optic nerve to measures of visual sensory function and retinal structure in the largest MS cohort reported to date. This cohort includes patients who reported an attack of acute ON more than 6 weeks prior to scanning; patients without a history of clinically recognized ON; and healthy volunteers. Thus, we examined optic-nerve abnormalities in a representative MS cohort rather than a cohort dominated by patients with ON. We hypothesized that DTI indices would distinguish MS optic nerves affected by prior ON from MS optic nerves without a history of ON, and healthy optic nerves. Furthermore, we hypothesized that, at least in MS, DTI-derived measurements would correlate with visual sensory dysfunction, PRNFL thinning, and loss of TMV.
Methods
Participants
Individuals with MS were recruited from the Johns Hopkins MS Center after the examining neurologist confirmed the diagnosis and the absence of confounding ophthalmologic or neurologic diseases. Healthy volunteers were recruited from the community. Participants were not selected based on history of ON; however, scans performed less than 6 weeks following the onset of acute ON were excluded. OCT and visual acuity testing using both high- and low-contrast (Sloan) letters were performed on all participants within 30 days of MRI. The median time between MRI and OCT/visual acuity testing was 0 days (range 0–23). Prior to testing, all participants gave signed, informed consent, and all studies were approved by the institutional review board.
OCT and Visual Acuity Testing
PRNFL thickness, TMV, and both high- and low-contrast visual acuity scores were measured as previously described (4). PRNFL and TMV measurements were obtained using an OCT-3 scanner (Stratus; Carl Zeiss Meditec, Dublin, CA) with the “Fast RNFL Thickness” and “Fast Macular Thickness” protocols, respectively. Participants were encouraged to use their corrective lenses when performing contrast-acuity testing with Sloan letter charts (Precision Vision, La Salle, IL) at 100%, 2.5%, and 1.25% contrast. Results were recorded as the percentage of letters, out of 70, correctly identified.
MRI Acquisition
MRI data were obtained on a Philips 3T Achieva (Philips Health Care, Best, The Netherlands) using the quadrature body coil for transmission and a 16-channel neurovascular coil for reception. Data were acquired during a 442-day period between March 26, 2008, and June 11, 2009. Multi-slice, fat-saturated, double-echo proton-density and T2-weighted (TR/TE1/TE2 = 3000/10/80ms, echo-train length 100, nominal acquired resolution 0.67mm × 0.88mm in plane, matrix 224 × 170, slice thickness 3.35mm, SENSE factor 2.5, 2 averages) and T1-weighted (TR/TE = 774/13ms, echo-train length 2, nominal acquired resolution 0.58mm × 0.74mm in plane, matrix 260 × 204, slice thickness 3mm, SENSE factor 2, 2 averages) sequences were used to provide anatomical information. In the MS cohort, the T1-weighted scans were performed after the intravenous administration of 0.1 mmol/kg gadopentetate dimeglumine (Magnevist; Bayer HealthCare, Leverkusen, Germany). The slices were oriented coronal to the head, which was oblique to the orbital portion of the optic nerves in some cases.
DTI was performed in the same plane as conventional MRI, with a pulsed-gradient, spin-echo with single-shot, echo-planar-imaging readout. The nominal acquired voxel size was 1.18mm × 1.18mm × 2.5mm, and the data were zero-padded in k-space to achieve a reconstructed in-plane resolution of 0.28mm × 0.28mm. The field of view was 80mm × 80mm, and 25 slices were obtained covering the anterior visual pathway from the globe to the optic chiasm. A high parallel-imaging factor (SENSE factor 3) enabled reduced TE, and high-order shims were combined with outer-volume-suppression to minimize susceptibility-related artifacts arising from the paranasal sinuses and tissues lateral to the optic nerves, respectively. Other parameters were: TR/TE = 5300/55ms, b-value = 500s/mm2, 15 gradient directions uniformly distributed about a sphere, and 5 minimally diffusion-weighted acquisitions (“b=0”; actual b-value~33s/mm2) that were averaged on the scanner. Two separate DTI acquisitions were obtained and entered into the tensor calculation as independent measurements without pre-averaging. No ocular fixation was used. The total scan time for the DTI acquisitions was 9min 22sec.
MRI Data Analysis
All diffusion data were processed using CATNAP (18). Diffusion-weighted images were coregistered to the first b=0 scan using a 6 degree-of-freedom registration algorithm supplied by AIR (19). Diffusion weighting was corrected for the rotational component of the registration, and the tensor was calculated in the standard fashion. Maps of FA, MD, λ⊥ and λ∥ were calculated (17).
The investigative team, which includes a neuroradiologist, two neuroophthalmologists, and four neurologists, determined that the MD maps displayed the best contrast between the optic nerves and the surrounding cerebrospinal fluid (CSF). Therefore, in each slice where the optic nerve was visible, a trained neuroophthalmologist (ZRW) placed regions of interest (ROIs) circumscribing the optic nerve on the MD images, as the MD images showed the greatest contrast between nerve and surrounding CSF. To minimize partial-volume effects, the outermost voxels of the ROIs were automatically removed using the erode function in Matlab (The Mathworks, Natick, MA). Diffusivity values that were spuriously (i.e., due to noise) negative were set to 0, and FA values that were spuriously greater than 1 were set to 1. This truncation prevents non-physiologic results while reducing bias by removing those slices entirely. DTI indices were then averaged across all slices, weighting the slice by the number of included voxels. These averaged values entered into the statistical calculations.
Statistics
Statistical calculations were performed in Stata 9.0 (Stata LP, College Station, TX). Stability of the quantitative measurements was assessed over the study's 442-day period using linear regression. There was no significant change in PRNFL thickness, TMV, visual acuity scores, expanded-disability-status-scale (EDSS) score (20), mean MS-severity score (MSSS) (21), or age (p>0.05 in all cases). However, diffusivity values gradually trended upward over time (p=0.05 for MD, p=0.01 for λ∥, and p=0.07 for λ⊥), or approximately 16% per year for MD, 14% for λ∥, and 22% for λ⊥. Scatter plots (not shown) revealed the relationship to be linear. Therefore, data were preprocessed by adjusting all DTI-indices to their expected value at the center date of the study (November 7, 2008), based on the slope of a mixed-effects regression using data from both optic nerves. There was no significant change in FA over time (p=0.36), but the FA values were adjusted similarly for consistency.
Intra-rater and Inter-rater Reliability
A subset of 10 consecutive data sets was analyzed. The same neuroophthalmologist analyzed 10 cases at two time points separated by >12 weeks; a second rater (JNR, MS neurologist) separately analyzed the same 10 cases. DTI indices were averaged across the right and left optic nerves, yielding a single measurement of each DTI index for each case. Bland-Altman analysis compared the results within the same rater as well as between the raters. Intraclass and interclass correlation coefficients were also calculated.
To assess differences between MS cases and healthy volunteers and to quantify the correlations between DTI indices and PRNFL thickness, TMV and visual acuity, mixed-effects regression models were used to account for multiple observations (i.e., from two optic nerves per person), with a p-value of 0.01 denoting statistical significance.
Results
Cohort characteristics are presented in Table 1. There was no difference in the age distribution between MS and healthy-volunteer groups (p=0.11, chi-squared test), although there was a higher proportion of women among the healthy volunteers. The overall disability level in our cohort was moderate: median EDSS score = 3.5, mean MSSS score = 4.8. In a linear mixed-effects model accounting for age and gender, individuals with MS had decreased PRNFL thickness (p=0.01), decreased TMV (p=0.004), and worse monocular visual acuity at 1.25% (p=0.03) compared with healthy volunteers. Monocular visual acuity was normal at 100% (p=0.41) and 2.5% (p=0.10) contrast in both groups. None of the optic nerves in the MS cases demonstrated enhancement on post-contrast T1-weighted images.
Table 1.
Cohort characteristics.
| all MS | RRMS | SPMS | PPMS | healthy | |
|---|---|---|---|---|---|
| # participants (# nerves) | 104 (197) | 63 (120) | 23 (42) | 18 (35) | 15 (29) |
| # women (%) | 67 (64%) | 44 (71%) | 14 (64%) | 9 (50%) | 13 (87%) |
| mean age, years (range) | 46 (20–67) | 40 (20–62) | 54 (45–67) | 53 (40–66) | 38 (23–56) |
| mean disease duration, years (range) | 11 (0–44) | 8 (0–25) | 21 (2–39) | 10 (1–44) | N/A |
| median EDSS (range) | 3.5 (0–7.5) | 2 (0–6.5) | 6 (2.5–7) | 5.5 (2.5–7.5) | N/A |
| mean MSSS (sd) | 4.8 (2.6) | 3.9 (2.5) | 5.8 (2.1) | 7.0 (1.6) | N/A |
| prior optic neuritis, # individuals (# eyes) | 35 (38) | 27 (29) | 7 (8) | 1 (1) | 0 |
| Mean PRNFL thickness, μm (sd) | 90 (14) | 91 (15) | 86 (13) | 92 (13) | 102 (14) |
| mean TMV, mm3 (sd) | 6.5 (0.5) | 6.5 (0.4) | 6.3 (0.5) | 6.6 (0.4) | 6.9 (0.4) |
| mean monocular 100% contrast visual acuity, % correct (sd) | 84 (14) | 87 (13) | 82 (9) | 77 (19) | 88 (11) |
| mean monocular 2.5% contrast visual acuity, % correct (sd) | 40 (17) | 42 (17) | 35 (15) | 37 (18) | 49 (14) |
| mean monocular 1.25% contrast visual acuity, % correct (sd) | 16 (15) | 18 (16) | 12 (12) | 14 (14) | 27 (16) |
Summary statistics (except for medians) are derived from all data and account for multiple observations per participant (i.e., right vs. left eye and across scans). For the MS cohort, optical-coherence-tomography and visual-acuity data were obtained within 30 days of each MRI scan. For the healthy-volunteer cohort, we did not require temporal proximity.
Abbreviations: MS, multiple sclerosis; RRMS, relapsing-remitting MS; SPMS, secondary progressive MS; PPMS, primary progressive MS. EDSS, expanded disability status scale. MSSS, MS severity score. PRNFL, peripapillary retinal nerve fiber layer; TMV, total macular volume. N/A, not applicable.
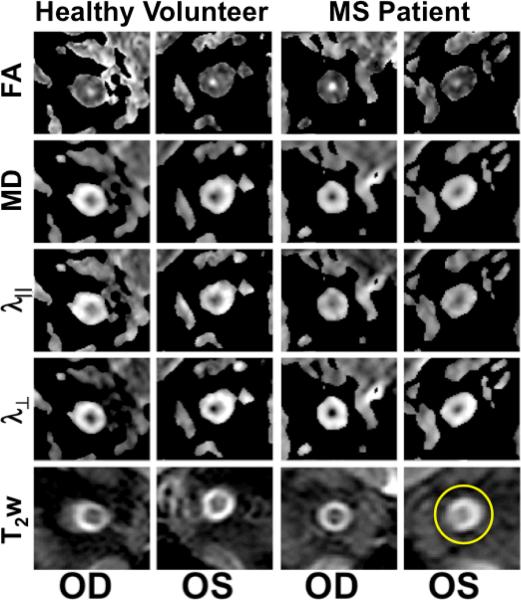
Figure 1 shows T2-weighted and DTI-index maps for the right (OD, left panel) and left (OS, right panel) optic nerves in a healthy volunteer. The optic nerve is clearly visible on all images. It is brighter than the surrounding CSF in the FA and darker in all other maps and on the T2-weighted images. Images from an MS patient with remote left (OS) ON are shown in the right panels. As is typical in ON, the T2-weighted signal is increased within the affected optic nerve (circle). In the same nerve, FA is lower and all directional diffusivities are higher. These results are consistent with data from prior studies of remote ON (10–16).
Figure 1.
Optic nerve DTI indices and T2-weighted images (T2w) for a healthy volunteer and an individual with MS with previous left (OS) optic neuritis. Note the high signal on the T2w in the left optic nerve (circle). FA, fractional anisotropy; MD, mean diffusivity; λ∥, parallel diffusivity; λ⊥, perpendicular diffusivity; OD, right eye.
Statistical Reliability
Reliability was assessed using Bland-Altman analysis (22). Differences among values derived by the same rater across both sessions are presented in Supplementary Table 1 (top panel). For all DTI indices, the difference between each session was less than 3%, and the 95% confidence interval for the difference overlapped 0. The 95% limits of agreement indicate a threshold for detecting real differences between pairs of values for each index and were <10% for FA and approximately 15–20% for diffusivities. Intraclass correlation coefficients were close to 1 for all indices, indicating excellent agreement.
Bland-Altman analysis of the difference between raters is shown in the bottom panel of Supplementary Table 1. As with the intra-rater analysis, all of the Bland-Altman 95% confidence intervals for the difference overlapped 0, indicating a non-significant deviation. The percent difference between raters was larger, however, up to 9.4% for λ⊥ but less than 5% otherwise. Inter-class correlation coefficients were again close to 1. The 95% limits of agreement were slightly larger for the inter-rater analysis and indicate that differences on the order of 10% for FA and 20% for the diffusivities can be considered significant.
Cohort Analyses
We obtained usable data from 226 of the 238 (95%) optic nerves; analysis of the other 12 nerves was marred by noise. We found no associations between any of the DTI indices and age, gender, or disease duration (p>0.1), so we made no adjustments for these variables.
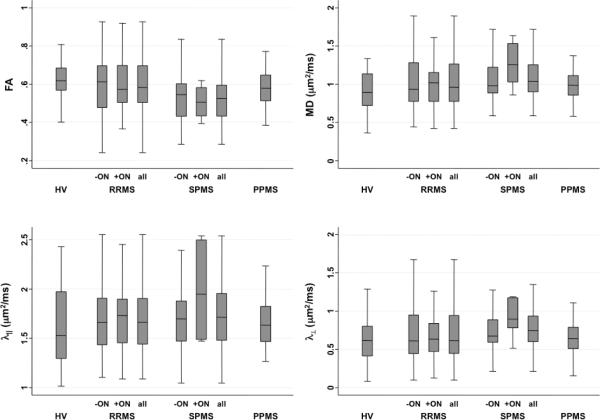
Figure 2 and Supplementary Table 2 present DTI indices for optic nerves with and without previous ON. The lowest FA and highest diffusivities were found in optic nerves with previous ON in patients with secondary progressive MS (SPMS). However, in mixed-effect models accounting for age and gender, only lower FA in SPMS compared with healthy volunteers (p=0.001) and participants with relapsing-remitting MS (RRMS) (p=0.01) was significant. These differences were persistent (p=0.005 and p=0.03, respectively) even when the analysis was restricted to optic nerves without prior ON, suggesting that damage to the optic nerves in SPMS may reflect diffuse neurodegeneration in addition to prior inflammation.
Figure 2.
Box plots showing DTI indices in optic nerves from healthy volunteers (HV) and relapsing-remitting (RRMS), secondary progressive (SPMS), and primary progressive (PPMS) MS, in optic nerves without (−ON) and with (+ON) previous optic neuritis. FA, fractional anisotropy; MD, mean diffusivity; λ∥, parallel diffusivity; λ⊥, perpendicular diffusivity.
Neither regression analysis in the full MS cohort nor paired t-tests comparing optic nerves in individuals with prior unilateral ON revealed differences in DTI indices between optic nerves with and without prior ON. This contrasts with findings for measures of retinal structure and low-contrast visual acuity, in which prior ON plays a major role. Specifically, mixed-effects regression analysis in eyes with prior ON revealed PRNFL thinning (p<0.001), loss of TMV (p<0.001), and decreased visual acuity at 2.5% (p<0.001) and 1.25% (p=0.001) contrast (but not at 100% contrast; p=0.16).
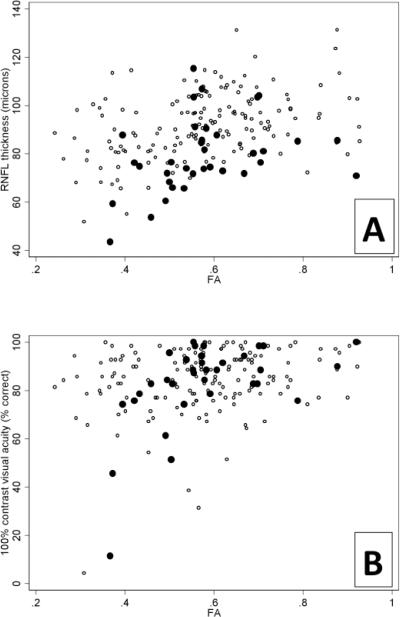
Mixed-effects regression analysis assessed the correlation between DTI-indices and OCT and visual acuity. The results are shown in Table 2 and reveal moderately strong correlations, particularly for PRNFL thickness vs. FA (Figure 3A) and λ⊥. We found correlation coefficients of similar magnitudes in the individual MS subgroups, but due to lower sample size, the corresponding p-values were higher.
Table 2.
Correlation of MRI indices with optical-coherence-tomography and visual-acuity measurements.
| FA | MD | λ ∥ | λ ⊥ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ± | − | + | ± | − | + | ± | − | + | ± | − | + | |
| PRNFL thickness | 0.41 § | 0.45 § | 0.35 | −0.35 ‡ | −0.39 ‡ | −0.40 | −0.29 * | −0.30 * | −0.40 | −0.39 § | −0.42 § | −0.43 |
| TMV | 0.25 ‡ | 0.27 * | 0.35 | −0.26 * | −0.28 * | −0.44 | −0.24 * | −0.20 * | −0.47 | −0.25 * | −0.27 * | −0.45 * |
| 100% acuity | 0.29 ‡ | 0.17 | 0.54 ‡ | −0.15 | −0.09 | −0.29 | −0.07 | −0.07 | 0.17 | −0.20 * | −0.10 | −0.39 |
| 2.5% acuity | 0.19 * | 0.13 | 0.36 | −0.18 | −0.10 | −0.27 | −0.13 | −0.11 | −0.15 | −0.18 | −0.08 | −0.35 |
| 1.25% acuity | 0.13 | 0.13 | 0.10 | −0.21 | −0.14 | 0 | −0.14 | −0.21 | 0.10 | −0.16 | −0.20 | 0.05 |
Correlation coefficients derived from mixed-effects regression analysis, accounting for correlations between optic nerves in the same individuals. Statistical significance is as follows:
(p<0.01)
(p<0.001)
(p<0.0001)
Prior optic neuritis status is denoted:
all optic nerves (197 nerves, 104 individuals)
no prior optic neuritis (158 nerves, 96 individuals)
prior optic neuritis (35 nerves, 32 individuals).
Abbreviations: FA, fractional anisotropy. MD, mean diffusivity. λ∥, parallel diffusivity. λ⊥,perpendicular diffusivity. PRNFL, peripapillary retinal nerve fiber layer. TMV, total macular volume.
Figure 3.
Scatter plots showing the association between fractional anisotropy (FA) and peripapillary retinal nerve fiber layer (PRNFL) thickness (A) and high contrast visual acuity (B) in optic nerves without (4) and with (3) previous optic neuritis.
Table 2 reveals that lower FA and higher diffusivities were associated with PRNFL thinning, TMV loss, and impaired visual acuity. Of the DTI indices, FA generated the strongest correlations whereas λ∥ generated the weakest. Correlations with visual acuity scores were generally weaker than with PRNFL thickness and TMV and were strongest at high contrast. These correlations weakened substantially when PRNFL was included as a covariate (Supplementary Table 3).
Finally, correlations of DTI indices with PRNFL thickness did not strongly depend on prior history of ON. For visual acuity scores, however, stronger correlations were the trend with a known history of ON (e.g. FA vs. 100% contrast visual acuity; see Figure 3B); this trend was also present for TMV. However, even the strongest of these correlations - between 100% contrast visual acuity and FA in optic nerves previously affected by ON - fell after adjusting for PRNFL thickness (Supplementary Table 3)
Discussion
This study describes the largest reported cohort of healthy and MS optic nerves investigated with DTI. We developed a rapid DTI protocol that was implemented using standard MRI hardware. We show DTI-derived indices that are consistent with previous reports (10; 13–16; 23). Contrary to many previous reports, but not all (12), we did not find many significant differences between MS (with or without history of ON) and healthy volunteer optic nerves, although trends were apparent. Consistent with these trends, DTI indices in MS optic nerves correlated with measures of retinal structural damage and visual acuity scores. In this section, we interpret these results in the context of literature reports and make some concluding remarks about the potential future uses of optic nerve DTI.
Technique and technical challenges
Technical challenges abound with DTI of the optic nerve in vivo, and many investigators have attempted to address these by using highly customized sequences with purpose-designed surface coils that require long acquisition times and extensive post-processing (11; 13; 16; 23–25). Those sequences were designed to minimize image distortions and maximize the signal-to-noise ratio, thus providing optimal results.Our goal was to devise a DTI acquisition that could be applied immediately on high-field clinical scanners with standard multi-channel, phased-array head coils within a clinically acceptable time frame. We therefore recognized that some image and data compromises would be necessary.
Our analysis procedure was straightforward, and initial processing of the images, including tensor and DTI-index calculation, followed a standard pipeline previously implemented in the brain (26; 27). As shown in Figure 1, we obtained high-quality images even without explicit B0 correction (15). Rather than using tractography, we drew ROIs on individual images and then eroded those ROIs to limit the effects of partial-volume averaging. We recognize, however, that given the oblique orientation of the optic nerves relative to the slice as well as the small cross-sectional diameter of the nerves, we were unable to eliminate fully partial-volume effects.
Compared with previous studies, our optic nerve DTI index values were generally in agreement. Standard deviations were slightly lower than those obtained in major cerebral white-matter tracts, including the corpus callosum and optic radiations (28; 29), but higher than those measured in the optic tract (30); all of these values were recorded in overlapping, but not identical, cohorts. Note, however, that DTI indices can vary across structures and are susceptible to imaging sequence parameters (26). Thus, we believe that our results represent reasonable estimates of the true DTI indices within the optic nerves and can be used to compare cohorts and assess relationships with non-DTI measurements.
Interpretation
Reduced anisotropy and increased diffusivity are expected in extralesional MS white matter (31–33), and these findings do not indicate specific types of tissue damage (34; 35). Such abnormalities also have been found in optic nerves following ON (36). Thus, our observation of a trend in the DTI indices in this direction is not surprising. Because we only studied optic nerves that either were not previously affected by ON or in which ON had occurred at least 6 weeks earlier, we found no evidence of decreased parallel diffusivity, as has been observed in the earliest stages of acute ON (16).
Contrary to our expectations and those in the literature, we did not observe extensive DTI abnormalities in optic nerves previously affected by ON (10; 14). It is well established that following an episode of ON, affected optic nerves show a persistent increase in T2-weighted signal and are atrophic (6; 8; 37; 38; 39); thus, T2-weighted imaging remains the clinical standard for detecting previous ON. Nevertheless, it is clear from our correlations that there is a connection with underlying optic nerve pathology. Abnormal DTI indices are associated with PRNFL thinning, macular volume loss, and, to a lesser extent, impaired visual acuity at high contrast. This last finding was contrary to our expectations, as low-contrast acuity is known to be particularly affected in patients with MS (3).
Portions of the two optic nerves merge following their partial decussation at the optic chiasm, so a comparison of the results obtained here with our previous measurements in the optic tracts (30) must be interpreted with caution. In the optic tract study, using a whole brain DTI protocol, we found that optic tract MD and λ⊥ were abnormally elevated and that FA was associated with both PRNFL thinning and TMV loss (but not visual acuity). Because the optic nerves and tracts are anatomically linked, it is not surprising that we found similar results with similar correlation strengths in this study.
Shortcomings
Because of the limited spatial resolution and SNR of our acquisition, coupled with slices that were oblique rather than perpendicular to the serpentine optic nerves, we reported summary rather than slice-wise measures. Thus, data from nerves previously affected by ON show an average of potentially severely damaged segments with data from other, undamaged, or less-affected segments. Moreover, the most damaged segments may have been systematically de-emphasized because we included only data from slices where we could confidently identify and demarcate the optic nerve. We suspect these factors contributed to our lack of sensitivity for detecting differences between unaffected and affected optic nerves.
Two additional limitations of our study were the lack of high-resolution anatomical data for assessing cross-sectional area and volume as well as the lack of VEP measurements. Both have been shown to correlate with each other and with DTI indices (10; 16) and are useful for a comprehensive analysis of optic nerve damage in MS.
A future study would rectify these shortcomings by: (1) unilateral rather than bilateral nerve imaging to minimize obliqueness of the imaging slice relative to the nerve axis; and (2) transitioning to non- EPI acquisitions or to a multi-shot EPI acquisition with phase correction.
Final Remarks
We suggest that optic nerve DTI, as a rapid, clinical adjunct to conventional T1-and T2-weighted imaging, is less sensitive to MS-induced tissue damage than OCT and low-contrast visual acuity measurements. Although DTI indices correlated with impaired visual acuity, much of that correlation could be accounted for by PRNFL thinning. Our results thus cast some doubt on the ultimate utility of DTI techniques derived from brain acquisitions as a tool in clinical care, at least in the chronic setting.
Supplementary Material
Acknowledgments
The study was supported by the National Multiple Sclerosis Society (TR3760A3); the National Institutes of Health (K01EB009120, K99NS064098, and P41RR015241, and the Intramural Research Program of the National Institute of Neurological Disorders and Stroke); and an unrestricted grant from EMD Serono to support data collection. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Terri Brawner, Kathleen Kahl, Ivana Kusevic, Stephanie Syc, and Christina Warner provided invaluable assistance with data collection. The CATNAP software was kindly provided by Dr. Bennett Landman. Dr. Peter van Zijl is a paid lecturer for Philips Healthcare and has technology licensed to Philips Healthcare; this arrangement has been approved by Johns Hopkins University in accordance with its Conflict of Interest policies.
Grant Support The study was supported by the National Multiple Sclerosis Society (grant TR3760A3); the National Institutes of Health (grants K01EB009120, K99NS064098, and P41RR015241, and the Intramural Research Program of the National Institute of Neurological Disorders and Stroke); and an unrestricted grant from EMD Serono to support data collection.
References
- 1.Balcer LJ. Optic neuritis. N Engl J Med. 2006;354(12):1273–1280. doi: 10.1056/NEJMcp053247. [DOI] [PubMed] [Google Scholar]
- 2.Shams PN, Plant GT. Optic neuritis: a review. Int MS J. 2009 Sep;16(3):82–89. [PubMed] [Google Scholar]
- 3.Baier ML, Cutter GR, Rudick RA, Miller D, Cohen JA, Weinstock-Guttman B, Mass M, Balcer LJ. Low-contrast letter acuity testing captures visual dysfunction in patients with multiple sclerosis. Neurology. 2005;64(6):992–5. doi: 10.1212/01.WNL.0000154521.40686.63. [DOI] [PubMed] [Google Scholar]
- 4.Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology. 2007;69(22):2085–92. doi: 10.1212/01.wnl.0000294876.49861.dc. [DOI] [PubMed] [Google Scholar]
- 5.Sisto D, Trojano M, Vetrugno M, Trabucco T, Iliceto G, Sborgia C. Subclinical visual involvement in multiple sclerosis: a study by MRI, VEPs, frequency-doubling perimetry, standard perimetry, and contrast sensitivity. Invest Ophthalmol Vis Sci. 2005;46(4):1264–8. doi: 10.1167/iovs.03-1213. [DOI] [PubMed] [Google Scholar]
- 6.Miller DH, Johnson G, McDonald WI, MacManus D, duBoulay EP, Kendall BE, Moseley IF. Detection of optic nerve lesions in optic neuritis with magnetic resonance imaging. Lancet. 1986 Jun;1(8496):1490–1491. doi: 10.1016/s0140-6736(86)91517-5. [DOI] [PubMed] [Google Scholar]
- 7.Boorstein JM, Moonis G, Boorstein SM, Patel YP, Culler AS. Optic neuritis: imaging with magnetization transfer. AJR Am J Roentgenol. 1997 Dec;169(6):1709–1712. doi: 10.2214/ajr.169.6.9393194. [DOI] [PubMed] [Google Scholar]
- 8.Inglese M, Ghezzi A, Bianchi S, Gerevini S, Sormani MP, Martinelli V, Comi G, Filippi M. Irreversible disability and tissue loss in multiple sclerosis: a conventional and magnetization transfer magnetic resonance imaging study of the optic nerves. Arch. Neurol. 2002 Feb;59(2):250–255. doi: 10.1001/archneur.59.2.250. [DOI] [PubMed] [Google Scholar]
- 9.Hickman SJ, Toosy AT, Jones SJ, Altmann DR, Miszkiel KA, MacManus DG, Barker GJ, Plant GT, Thompson AJ, Miller DH. Serial magnetization transfer imaging in acute optic neuritis. Brain. 2004;127(3):692–700. doi: 10.1093/brain/awh076. [DOI] [PubMed] [Google Scholar]
- 10.Kolbe S, Chapman C, Nguyen T, Bajraszewski C, Johnston L, Kean M, Mitchell P, Paine M, Butzkueven H, Kilpatrick T, Egan G. Optic nerve diffusion changes and atrophy jointly predict visual dysfunction after optic neuritis. Neuroimage. 2009;45(3):679–86. doi: 10.1016/j.neuroimage.2008.12.047. [DOI] [PubMed] [Google Scholar]
- 11.Hickman SJ, Wheeler-Kingshott CA, Jones SJ, Miszkiel KA, Barker GJ, Plant GT, Miller DH. Optic nerve diffusion measurement from diffusion-weighted imaging in optic neuritis. AJNR Am J Neuroradiol. 2005;26(4):951–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Frohman EM, Dwyer MG, Frohman T, Cox JL, Salter A, Greenberg BM, Hussein S, Conger A, Calabresi P, Balcer LJ, Zivadinov R. Relationship of optic nerve and brain conventional and non-conventional MRI measures and retinal nerve fiber layer thickness, as assessed by OCT and GDx: A pilot study. Journal of the Neurological Sciences. 2009 Jul;282(1–2):96–105. doi: 10.1016/j.jns.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Wheeler-Kingshott CA, Trip SA, Symms MR, Parker GJ, Barker GJ, Miller DH. In vivo diffusion tensor imaging of the human optic nerve: pilot study in normal controls. Magn Reson Med. 2006;56(2):446–51. doi: 10.1002/mrm.20964. [DOI] [PubMed] [Google Scholar]
- 14.Trip SA, Wheeler-Kingshott C, Jones SJ, Li WY, Barker GJ, Thompson AJ, Plant GT, Miller DH. Optic nerve diffusion tensor imaging in optic neuritis. Neuroimage. 2006;30(2):498–505. doi: 10.1016/j.neuroimage.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Techavipoo U, Okai AF, Lackey J, Shi J, Dresner MA, Leist TP, Lai S. Toward a practical protocol for human optic nerve DTI with EPI geometric distortion correction. Journal of Magnetic Resonance Imaging. 2009;30(4):699–707. doi: 10.1002/jmri.21836. [DOI] [PubMed] [Google Scholar]
- 16.Naismith RT, Xu J, Tutlam NT, Snyder A, Benzinger T, Shimony J, Shepherd J, Trinkaus K, Cross AH, Song SK. Disability in optic neuritis correlates with diffusion tensor-derived directional diffusivities. Neurology. 2009;72(7):589–594. doi: 10.1212/01.wnl.0000335766.22758.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 18.Landman BA, Farrell JA, Jones CK, Smith SA, Prince JL, Mori S. Effects of diffusion weighting schemes on the reproducibility of DTI-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. Neuroimage. 2007;36(4):1123–38. doi: 10.1016/j.neuroimage.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 20.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 21.Roxburgh RH, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S, Achiti I, Confavreux C, Coustans M, le Page E, Edan G, McDonnell GV, Hawkins S, Trojano M, Liguori M, Cocco E, Marrosu MG, Tesser F, Leone MA, Weber A, Zipp F, Miterski B, Epplen JT, Oturai A, Sorensen PS, Celius EG, Lara NT, Montalban X, Villoslada P, Silva AM, Marta M, Leite I, Dubois B, Rubio J, Butzkueven H, Kilpatrick T, Mycko MP, Selmaj KW, Rio ME, Sa M, Salemi G, Savettieri G, Hillert J, Compston DA. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology. 2005;64(7):1144–51. doi: 10.1212/01.WNL.0000156155.19270.F8. [DOI] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Measuring agreement in method comparison studies. Statistical Methods in Medical Research. 1999 Apr;8(2):135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 23.Chabert S, Molko N, Cointepas Y, Le Roux P, Le Bihan D. Diffusion tensor imaging of the human optic nerve using a non-CPMG fast spin echo sequence. J Magn Reson Imaging. 2005;22(2):307–10. doi: 10.1002/jmri.20383. [DOI] [PubMed] [Google Scholar]
- 24.Sarlls JE, Pierpaoli C. In vivo diffusion tensor imaging of the human optic chiasm at sub-millimeter resolution. Neuroimage. 2009 Oct;47(4):1244–1251. doi: 10.1016/j.neuroimage.2009.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinogradov E, Degenhardt A, Smith D, Marquis R, Vartanian TK, Kinkel P, Maier SE, Hackney DB, Lenkinski RE. High-resolution anatomic, diffusion tensor, and magnetization transfer magnetic resonance imaging of the optic chiasm at 3T. J Magn Reson Imaging. 2005;22(2):302–6. doi: 10.1002/jmri.20370. [DOI] [PubMed] [Google Scholar]
- 26.Farrell JA, Landman BA, Jones CK, Smith SA, Prince JL, van Zijl PC, Mori S. Effects of signal-to-noise ratio on the accuracy and reproducibility of diffusion tensor imaging-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5 T. J Magn Reson Imaging. 2007;26(3):756–67. doi: 10.1002/jmri.21053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reich DS, Smith SA, Zackowski KM, Gordon-Lipkin EM, Jones CK, Farrell JA, Mori S, van Zijl PC, Calabresi PA. Multiparametric magnetic resonance imaging analysis of the corticospinal tract in multiple sclerosis. Neuroimage. 2007;38(2):271–9. doi: 10.1016/j.neuroimage.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozturk A, Smith S, Gordon-Lipkin E, Harrison D, Shiee N, Pham D, Caffo B, Calabresi P, Reich D. MRI of the corpus callosum in multiple sclerosis: association with disability. Multiple Sclerosis. 2010 Feb;16(2):166–177. doi: 10.1177/1352458509353649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reich DS, Smith SA, Gordon-Lipkin EM, Ozturk A, Caffo BS, Balcer LJ, Calabresi PA. Damage to the optic radiation in multiple sclerosis is associated with retinal injury and visual disability. Arch. Neurol. 2009 Aug;66(8):998–1006. doi: 10.1001/archneurol.2009.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dasenbrock HH, Smith SA, Ozturk A, Farrell SK, Calabresi PA, Reich DS. Diffusion Tensor Imaging of the Optic Tracts in Multiple Sclerosis: Association with Retinal Thinning and Visual Disability. J Neuroimaging. 2010 Mar; doi: 10.1111/j.1552-6569.2010.00468.x. Internet. [cited 2010 Apr 17 ] Available from: http://www.ncbi.nlm.nih.gov/pubmed/20331501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciccarelli O, Werring DJ, Wheeler-Kingshott CA, Barker GJ, Parker GJ, Thompson AJ, Miller DH. Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology. 2001;56(7):926–33. doi: 10.1212/wnl.56.7.926. [DOI] [PubMed] [Google Scholar]
- 32.Roosendaal SD, Geurts JJ, Vrenken H, Hulst HE, Cover KS, Castelijns JA, Pouwels PJ, Barkhof F. Regional DTI differences in multiple sclerosis patients. Neuroimage. 2009;44(4):1397–1403. doi: 10.1016/j.neuroimage.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Ceccarelli A, Rocca M, Falini A, Tortorella P, Pagani E, Rodegher M, Comi G, Scotti G, Filippi M. Normal-appearing white and grey matter damage in MS. Journal of Neurology. 2007;254(4):513. doi: 10.1007/s00415-006-0408-4. [DOI] [PubMed] [Google Scholar]
- 34.Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57(4):688–95. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Jones M, DeBoy CA, Reich DS, Farrell JAD, Hoffman PN, Griffin JW, Sheikh KA, Miller MI, Mori S, Calabresi PA. Diffusion tensor magnetic resonance imaging of Wallerian degeneration in rat spinal cord after dorsal root axotomy. J. Neurosci. 2009;29(10):3160–3171. doi: 10.1523/JNEUROSCI.3941-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Sun SW, Naismith RT, Snyder AZ, Cross AH, Song SK. Assessing optic nerve pathology with diffusion MRI: from mouse to human. NMR Biomed. 2008;21(9):928–40. doi: 10.1002/nbm.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Size-selective neuronal changes in the anterior optic pathways suggest a differential susceptibility to injury in multiple sclerosis. Brain. 2001;124(Pt 9):1813–20. doi: 10.1093/brain/124.9.1813. [DOI] [PubMed] [Google Scholar]
- 38.Hickman SJ, Toosy AT, Jones SJ, Altmann DR, Miszkiel KA, MacManus DG, Barker GJ, Plant GT, Thompson AJ, Miller DH. A serial MRI study following optic nerve mean area in acute optic neuritis. Brain. 2004;127(11):2498–2505. doi: 10.1093/brain/awh284. [DOI] [PubMed] [Google Scholar]
- 39.Harnsberger HR, Wiggins RH, III, et al. Diagnostic Imaging: Head and Neck. Amirsys; Salt Lake City: 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.