Abstract
Background
A fundamental feature underlying many movement disorders is increased variability in the motor response. Despite abnormalities of grip force control in people with dystonia, it is not clear whether dystonia is also associated with increased variability in force output and whether force variability in dystonia is affected by the presence or absence of visual feedback.
Objective
To examine force variability in 16 patients with writer’s cramp and 16 matched controls.
Methods
The variability of force output at the wrist under conditions of both vision and no vision was examined. The underlying frequency structure of the force signal was also compared across groups. Participants produced isometric wrist flexion to targets at 25% and 50% of their maximum voluntary contraction strength under conditions of both vision and no vision.
Results
Similar levels of force variability were observed in patients with dystonia and controls at the lower force levels, but patients with dystonia were less variable in their force output than controls at the higher force level. This reduction in variability in people with dystonia at 50% maximum voluntary contraction was not affected by vision. Although a similar dominant frequency in force output was observed in people with dystonia and controls, a reduced variability in the group with dystonia at the higher force level was due to reduced power in the 0-4-Hz frequency bin.
Conclusions
The first evidence of a movement disorder with reduced variability is provided. The findings are compatible with a model of dystonia, which includes reduced cortical activation in response to sensory input from the periphery and reduced flexibility in motor output.
Patients with focal hand dystonia (FHD) typically present with muscular spasms and apparent incoordination when attempting to write, often adopting abnormal postures of the hand and fingers when gripping a pen.1. They have a variety of general motor control deficits including slowness,2-7 weakness8 and grip force dysfunction9-11 when compared with healthy controls. The underlying cause of these motor deficits has not been established clearly.
A fundamental feature underlying many movement disorders is increased variability in the motor response. In laboratory studies investigating force control, the forces exerted fluctuate over time,12-14 and these dynamic changes in force control can be examined using the standard deviation (SD) of force as an index of variability.13 Higher force variability has previously been shown in patients with Parkinson’s disease,15 16 Huntington’s disease,17 18 cerebellar dysfunction19 and deafferentation20 than in healthy controls. To our knowledge, however, variability in force output has not been directly examined in a group of patients with dystonia. The purpose of this study was to examine force fluctuations over time in patients with FHD, both with and without the benefit of visual feedback. We used a task designed not to induce dystonic posturing to explore whether there was an underlying deficit in force control in these patients that was not due to the presence of dystonic contractions. On the basis of findings of increased force variability in patients with other movement disorders, we hypothesised that variability would be higher in the group with dystonia than in controls despite the non-dystonia-producing nature of the task. We also compared the underlying frequency structure of the force signal across groups to determine whether any differences in variability could be attributed to pathological tremor or whether differences were consistent with deficits in sensorimotor integration in patients with dystonia.21
Our second hypothesis was that force variability would increase more in the group with dystonia than in the control group when visual feedback was removed. Partial support for this hypothesis comes from the study by Inzelberg et al,22 who showed that patients with idiopathic torsion dystonia had increased movement times and decreased movement accuracy when carrying out non-ballistic reaching movements without visual feedback. Grip force abnormalities in dystonia have been attributed to disturbances in sensorimotor integration.9 10 If abnormal sensorimotor integration underlies the force control deficits in dystonia, we would expect that patients with dystonia would be more dependent on visual feedback to maintain force steadiness than would be healthy people. The findings from this study will provide insight into whether the force deficits previously found in patients with FHD are task-dependent manifestations of FHD or whether they show a global force control dysfunction in dystonia.
PARTICIPANTS AND METHODS
Participants
In all, 16 patients with primary FHD and 16 neurologically healthy controls matched for age, height, weight and sex participated in this study. Two-tailed t tests showed no significant differences between patients and controls in height, weight or age (all p>0.05). Patients were recruited from two surrounding medical centres, Rush University Medical Center, Chicago, Illinois and Northwestern Memorial Hospital, Chicago, Illinois. Inclusion criteria for participation were a diagnosis of writer’s cramp, an age range between 20 and 65 years, no history of other neurological problems or injury to the arms, and at least a 4-month period since receiving any botulinum toxin injection for treatment of dystonia. Table 1 shows a summary of the characteristics of patients with dystonia. The clinically most affected hand was tested, which for all patients except D16 was the dominant side. Dominance and the tested hand were matched across controls. Informed consent was obtained from each participant before testing, according to the Declaration of Helsinki. The Institutional Review Board of the University of Illinois at Chicago approved the protocol for use.
Table 1.
Patient characteristics
| Patient | Age (years) | Sex | Hand* | Symptom duration (years) | Dx† | Duration since treatment with botulinum toxin‡ | Movement scale severity (arm)§ | Pattern of dystonic posturing during writing¶ |
|---|---|---|---|---|---|---|---|---|
| D1 | 57 | F | L | 7 | DC | NA | 6 | Wrist, thumb, and F1-2 flex, F2 abd |
| D2 | 61 | F | R | 17 | WC | 13 months | 9 | F2-3 flex, wrist UD |
| D3 | 63 | F | R | 17 | DC | NA | 9 | Wrist flex and UD, F5 finger abd |
| D4 | 24 | F | R | 16 | WC | NA | 4 | Wrist flex and UD, F2 flex |
| D5 | 53 | F | R | 41 | DC | NA | 9 | Wrist flex and UD, F2-5 flex |
| D6 | 57 | F | L | 6 | WC | NA | 2 | Wrist, thumb and F2 flex |
| D7 | 45 | F | R | 2.5 | WC | NA | 2 | Wrist UD, thumb and F2 flex |
| D8 | 63 | F | R | 24 | DC | 6 months | 6 | F2 ext |
| D9 | 47 | M | R | 10 | WC | NA | 2 | Wrist ext, F2 abd and flex |
| D10 | 48 | M | L | 2.5 | DC | 6 months | 4 | Wrist UD, F5 flex |
| D11 | 56 | M | L | 5 | WC | NA | 2 | Wrist ext, F2 flex |
| D12 | 42 | M | R | 7 | WC | 14 months | 2 | Wrist ext, F2 flex |
| D13 | 45 | M | R | 23 | DC | 13 months | 12 | Wrist UD and flex, thumb and F2-3 ext |
| D14 | 33 | M | R | 5 | WC | 37 months | 2 | Wrist ext, F1-2 ext, thumb abd |
| D15 | 44 | M | R | 5 | WC | NA | 2 | Thumb ext |
| D16 | 54 | M | R | 11 | DC | NA | 6 | F2 flex, thumb add |
Hand dominance: L, left; R, right.
Diagnosis: DC, dystonic cramp (more than one task involved); WC, writer’s cramp (only writing affected).23
NA, not applicable, patient never treated with botulinum toxin.
Arm subscale from the Burke-Fahn-Marsden rating scale,24 with higher scores indicating more severe involvement of the arm (maximum score 16).
abd, abduction; add, adduction; ext, extension; flex, flexion; F2-5, fingers 2-5; UD, ulnar deviation.
Apparatus and procedures
A wrist manipulandum device held the wrist in neutral with regard to a position of flexion or extension (fig 1A). Joint torque was measured by a torque transducer mounted on a shaft at the axis of rotation. The torque signal was digitised at 1000 samples/s and stored on a computer for offline analysis. The mechanical signal was digitally low-pass-filtered at 20 Hz with a second-order Butterworth filter, dual passed.
Figure 1.
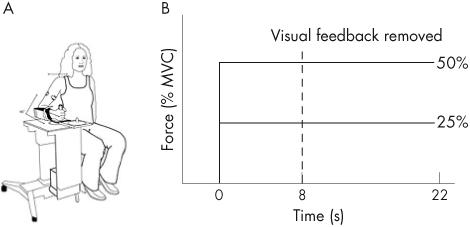
(A) Wrist manipulandum used for data collection. (B) Schematic of the experimental paradigm. The dashed vertical line indicates the point of visual feedback removal on alternate trials. MVC, maximum voluntary contraction.
Maximum voluntary contraction (MVC) values were established for each participant as the average peak torque in three 6-s trials of maximum effort of isometric wrist flexion. After this, 25% and 50% of MVC values were determined for each participant and these were the target torques set for each participant. We examined torque output to each target at the wrist under a condition of either vision or no vision.
For the vision trials, torque was displayed on a computer screen in front of the participant as a vertical marker. A second stationary marker bar on the screen represented target location. The width of the target always corresponded to 0.01 Nm of torque. The visual display was set to a high gain such that small changes in torque produced large deviations in the torque cursor with regard to the target. A computer-generated preparatory tone alerted the participant to prepare for a trial. A second “go” tone was generated 1 s after the preparatory tone as a signal for the participant to begin the trial. At the go tone, participants were instructed to isometrically move the torque marker to the target and to hold it there as steadily as possible until they heard the third tone. The third tone signalled the participant to end the trial. Figure 1B shows a schematic of the timing of the experimental paradigm. The no-vision trials began in the same way as the vision trials, but the screen was blanked after 8 s, thus eliminating visual feedback. Participants were instructed to continue to try maintaining the force steady at the memorised target after the visual display was blanked. Four trials of each condition were collected, and trials alternated between conditions of vision and withdrawal of vision.
Data analysis
The following measures were calculated:
Force variability: The SD of force was averaged across the last 10 s of each trial, and then the SD for each participant was averaged across the four trials in each vision condition. As any decay in force output after removal of visual feedback will affect the computed SD, a linear trend was fitted to the force output data over time and any linear trend was subtracted from the force data before the SD was calculated.
Modal force frequency and spectral power of the force signal: Fourier analysis was applied to the last 10 s of each force trial. Autospectral analysis was carried out with Welch’s averaged periodogram method with a Hanning window size of about 1024 to give a 0.9766-Hz frequency bin for each power spectral estimate. Within the power spectrum, the modal frequency at the peak power of the torque signal was determined. The modal frequency of force output represents the dominant frequency with which force is controlled. Additionally, the absolute power for the 0-4-Hz, 4-8-Hz, and 8-12-Hz frequency bins was determined. Previous research indicates that essentially all the power in the force signal is located in the 0-12-Hz frequency range.13 21
The dependent variables described here were analysed with t tests and repeated-measures analysis of variance with Statistica (StatSoft, Oklahama, USA). For the analysis of force variability, there was one between-subjects factor for group (control, dystonic) and two within-subjects factors for vision (vision, no vision) and force level (25% MVC, 50% MVC). For the analysis of spectral power, there was one between-subjects factor for group (control, dystonic) and three within-subjects factors for vision (vision, no vision), force level (25% MVC, 50% MVC) and frequency bin (0-4, 4-8 and 8-12 Hz). Each analysis of variance was interpreted as significant when there was less than a 5% chance of making a type-I error (ie, p<0.05). Interactions related to differences between controls and patients with dystonia were followed up with retrospective Tukey’s Honestly Significant Different Test.
RESULTS
Force variability with and without visual feedback
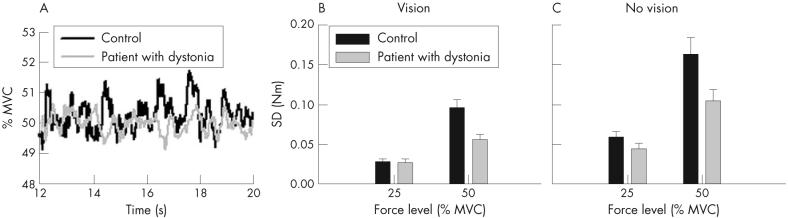
Figure 2A is a single-trial record for one patient with dystonia and a matched control at the 50%-MVC force level. The patient with dystonia shows a reduced amplitude of changes in the force signal across time compared with the control. Group data on force variability are shown in fig 2B and C. Across groups, we found a significant main-group effect on force variability that was qualified by a significant group by force interaction (table 2). Tukey’s Honestly Significant Different Test showed that variability was considerably lower in the group with dystonia than in the control group at the 50%-MVC force level but not at the 25%-MVC force level. Inspection of individual data showed that 13 of 16 patients had lower force variability than their controls at the 50%-MVC force level, indicating that this was a robust finding (data not shown). As expected, we found a significant main effect for vision with reduced force variability when visual feedback was available (table 2). The group by vision interaction was, however, not significant (table 2). Patients with dystonia were therefore no more variable than controls when vision was removed. To ensure that reduced variability was not simply related to a difference in target force between groups, we carried out an analysis of covariance on the 50%-MVC data with MVC as a covariate. The result did not change. Despite having a lower MVC than controls (F1,29 = 26.63, p<0.001, mean wrist MVC controls 10.5 Nm (SD 3.9); group with dystonia 7.88 Nm (SD 3.2)), patients with dystonia were still less variable at the 50%-MVC force level than controls averaged across vision conditions (F1,29 = 5.89, p = 0.022). Thus, adding MVC as a covariate did not significantly change the F value for the group effect (table 2).
Figure 2.
(A) Single-trial records from one patient with dystonia (grey line) and a matched control (black line) at 50% maximum voluntary contraction (MVC) with vision allowed throughout the trial. (B) Force variability across the group of controls (black bars) and patients with dystonia (grey bars) at 25%-MVC and 50%-MVC force levels with vision. (C) Force variability across the group of controls (black bars) and patients (grey bars) at both force levels without vision. Data are mean (SE).
Table 2.
Statistical results
| Force variability | Spectral power | ||||
|---|---|---|---|---|---|
| F | p Value | F | p Value | ||
| Group | 5.86 | 0.022 | Group | 8.41 | 0.007 |
| Vision | 61.86 | <0.001 | Vision | 1.69 | 0.203 |
| Force | 92.56 | <0.001 | Force | 39.18 | <0.001 |
| Group×Vision | 2.36 | 0.135 | Bin | 49.88 | <0.001 |
| Group×Force | 9.40 | 0.005 | Group×Vision | 1.33 | 0.258 |
| Vision×Force | 22.08 | <0.001 | Group×Force | 11.84 | 0.002 |
| Group×Vision×Force | 0.14 | 0.708 | Group×Bin | 8.78 | <0.001 |
| Vision×Force | 2.12 | 0.156 | |||
| Vision×Bin | 1.03 | 0.363 | |||
| Force×Bin | 39.78 | <0.001 | |||
| Group×Vision×Force | 1.29 | 0.265 | |||
| Group×Vision×Bin | 0.89 | 0.417 | |||
| Group×Force×Bin | 12.50 | <0.001 | |||
| Vision×Force×Bin | 1.50 | 0.232 | |||
| Group×Vision×Force×Bin | 0.85 | 0.432 | |||
p Values in bold highlight statistically significant results at p<0.05.
Frequency structure of force output
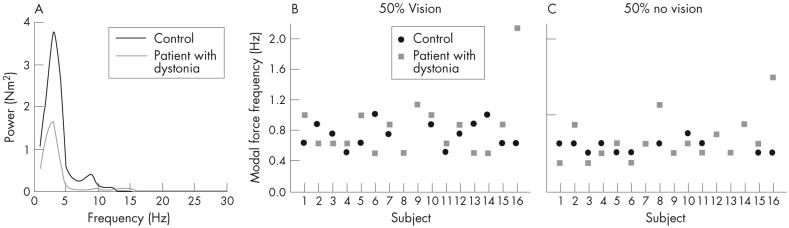
Figure 3A shows a power spectrum of the force signal from a patient with dystonia and a control at the 50%-MVC force level. The modal frequency was similar between them, but the patient showed reduced spectral power. Figure 3B and C gives individual data for modal force frequency for all participants and shows that the modal frequency was similar for patients and controls under both conditions of vision. Averaged across force level and vision conditions, an unpaired t test showed no significant difference in modal force frequency between groups (t30 = - 1.51, p = 0.14; control mean 0.69 (SD 0.18) Hz, group with dystonia mean 0.82 (SD 0.45) Hz).
Figure 3.
Power spectrum from the total force for a control (black line) and a patient with dystonia (grey line) at the 50%-maximum voluntary contraction (MVC) force level. (B) Modal force frequency for each control (black circles) and patient (grey squares) at the 50%-MVC force level with vision. (C) Modal force frequency for each control (black circles) and patient (grey squares) at the 50%-MVC force level without vision.
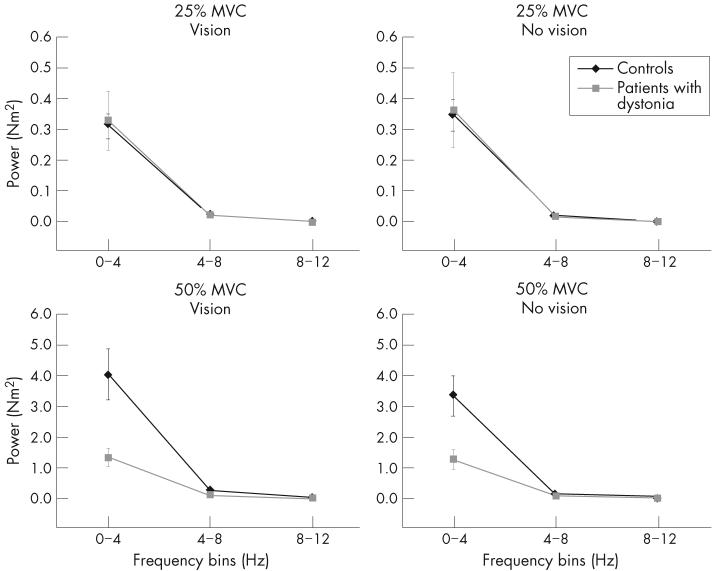
Figure 4 shows average spectral power in the 0-4-Hz, 4-8-Hz and 8-12-Hz frequency bins in patients and controls. At 25% MVC (fig 4, top panels) and 50% MVC (fig 4, bottom panels), most of the spectral power in the force signal was located in the 0-4-Hz frequency bin for both groups. Statistical analysis showed significant main effects for group, force and frequency bin (table 2). These, however, were qualified by a significant three-way interaction between group, force and bin (table 2). Retrospective analyses showed that the interaction was mainly caused by differences between groups at the 50%-MVC force level in the 0-4-Hz frequency bin—that is, although patients with dystonia and controls showed no difference in spectral power in the 0-4-Hz frequency bin at 25% MVC, patients had considerably less spectral power in the 0-4-Hz frequency bin compared with controls at 50% MVC. We found no differences between groups in the 4-8-Hz or 8-12-Hz frequency bins at either 25% MVC or 50% MVC.
Figure 4.
Average spectral power of the force signal for the 25%-maximum voluntary contraction (MVC; top panels) and 50%-MVC force target (bottom panels) under conditions of vision (left panels) and no vision (right panels) in the 0-4-Hz, 4-8-Hz and 8-12-Hz frequency bins. Controls are shown as black lines and diamonds and patients with dystonia as grey lines and squares. Values are mean (SE). The scale of the y axis is different at the different force targets.
DISCUSSION
In this study, we examined force variability in patients with FHD compared with healthy people, by using an isometric force control task designed not to induce dystonic posturing under conditions of visual feedback and no visual feedback. Three principal findings are as follows.
Patients with dystonia had similar levels of force variability to that of controls at the lower force level studied, but were less variable in their force output than controls at the higher (50% MVC) force level.
This reduction in variability was not affected by vision.
Although patients and controls showed a similar dominant frequency in their force output, reduced variability in the group with dystonia at 50% MVC was attributable to reduced power in the 0-4-Hz frequency bin.
Our first hypothesis was that patients with FHD would show increased variability in force output compared with controls. This hypothesis was not supported. Variability was similar between groups at the lower force level studied, but at the higher force level, patients with FHD were actually less variable than controls. To our knowledge, ours is the first study that has shown reduced variability in a movement disorder. It shows that there are clear behavioural differences between patients with dystonia and patients with other movement disorders. By using this same paradigm, higher force variability has been found in patients with Parkinson’s disease15 16 and in Huntington’s disease17 than in controls. We are not aware of any previous examination on variability in force control in patients with dystonia. Figure 2 in the paper by Odergren et al9 and fig 4 in that by Schenk and Mai,25 however, suggest increased variability in performing gripping tasks by patients with dystonia.
Variability in movement by patients with dystonia has previously been examined, although on a limited basis. Inzelberg et al22 showed that patients with idiopathic torsion dystonia made reaching movements with movement extent errors of a magnitude similar to that by healthy controls when vision of the moving limb and target were permitted during movement. They, however, made substantially more final movement errors than controls when vision of the moving limb was not permitted during movement. van der Kamp et al2 examined rapid elbow movements in patients with torsion dystonia and found that the amplitude of short 15° movements was more variable in patients with dystonia than in controls, whereas amplitude variability was not different between groups at the longer 30° movements. All movement tasks were performed with full vision. Agostino et al3 examined sequential arm movements in patients with dystonia whose upper limb was affected and found that variability in movement time was normal in patients with dystonia. Again, visual feedback was permitted during the task. Variability may be influenced by the task studied, the presence or absence of dystonic posturing, the joint studied and the task instructions. As such, our finding of reduced variability in a group of patients with dystonia is important in understanding this complex and interesting movement disorder further, as it shows clear behavioural differences between patients with dystonia and patients with other movement disorders.
Our second hypothesis was that force variability would increase more in the group with dystonia than in controls when visual feedback was removed, but this hypothesis was also not supported. The absence of visual feedback did not affect group differences in force variability, suggesting that patients with dystonia are not more dependent on visual feedback than controls. This finding also contrasts that observed in people with Parkinson’s disease who have been shown to be more dependent on visual information, using the same experimental paradigm26 and other paradigms.27-30 Previous studies have questioned whether force control abnormalities in people with FHD can be attributed to a generalised impairment of sensorimotor integration.25 11 As our study found that visual feedback did not differentially affect force variability in patients with dystonia compared with controls, we suggest that if a generalised sensorimotor impairment does exist in patients with dystonia, the visuomotor system does not have a role.
Two mechanisms that could underlie reduced force variability at the higher force level in the group with dystonia, although they are not mutually exclusive. Firstly, patients with dystonia may have reduced cortical activation in response to sensory input from the periphery. The modal force frequency was around 1 Hz in both patients and in controls, with little power above 4 Hz, which is consistent with previous findings on healthy people aged 20-90 years.31. This indicates that tremor cannot explain differences in force variability between groups. Instead, patients with dystonia had considerably less spectral power in the 0-4-Hz frequency range. This portion of the power spectrum has been associated with actions that require a continuous and fine sensory guidance for their motor output.32 It seems therefore that patients with FHD make corrections of lower amplitude in response to perceived or observed deviations from the force target. Reduced variability at higher force levels may be due to reduced cortical activation in response to sensory input from the periphery in patients with dystonia with increased task demands. This is partially consistent with findings from positron emission tomography, which showed reduced cerebral blood flow in the sensorimotor cortex of patients with dystonia during non-dystonia-producing tasks.33 34
A second mechanism that could underlie reduced force variability at the higher force level in the group with dystonia is related to down regulation of sensory input during a motor task. It has previously been suggested that sensory input in patients with writer’s cramp has little or no effect on motor output.35 Although healthy people constantly updated their motor output for an ongoing motor output in response to sensory feedback from the periphery, those with dystonia may down regulate sensory input during a non-dystonia-producing task and instead rely on a fixed motor output. This may become more apparent at higher force levels, when sensory input from the periphery would naturally be increased. Although maintaining a similar level of output may seem to be a positive feature of motor control in patients with dystonia, its consequence is a less flexible motor response. This is quite consistent with the clinical picture of FHD, whereby a particular task elicits a fixed and predictable hand posture that is difficult to change voluntarily. Changes in variability occurring only at the higher force level studied are consistent with the clinical observation that patients with other forms of dystonia show changes in the pattern and magnitude of involuntary dystonic movements with increasing effort.36 37
It was important to ensure that reduced variability in the group with dystonia was not simply because of differences in force target between the groups, as variability has previously been shown to scale with target force in healthy people.38 13. We do not believe that different target forces can explain our findings for several reasons. Firstly, a lower target level is not always associated with reduced force variability—for example, elderly people have a lower strength39 but a higher force variability than young healthy people.31 40 Secondly, reduced variability was not found in the group with dystonia at the lower force level in this study, suggesting that different responses can be elicited in patients with dystonia depending on the force levels studied. Thirdly, to reduce the likelihood of strength being a factor, we used the same percentage of MVC as the target force level for the group with dystonia and the controls. Between groups, effort was therefore similar. Finally, analysis of covariance using strength as a covariate did not change our findings of reduced variability in the group with dystonia compared with controls at the higher force level.
In conclusion, we have shown that patients with FHD are no more variable than controls at a lower force level, but have reduced force variability compared with controls at a higher force level regardless of whether visual feedback is present. We have suggested that these findings are compatible with a model of dystonia that is based on reduced flexibility in motor output, perhaps related to down regulation of sensory input during the performance of a motor task.
ACKNOWLEDGEMENTS
We thank the staff at the Section for Movement Disorders in the Department of Neurological Sciences at Rush University Medical Center, Chicago, Illinois, and Dr Tanya Simuni of the Department of Neurology at Northwestern Memorial Hospital, Chicago, Illinois, for their assistance in patient recruitment.
Funding: This study was supported in part by the National Institutes of Health Grants NS21827, NS40902 and NS52318.
Abbreviations
- FHD
focal hand dystonia
- MVC
maximum voluntary contraction
Footnotes
Competing interests: None.
REFERENCES
- 1.Sheehy MP, Rothwell JC, Marsden CD. Writer’s cramp. Adv Neurol. 1988;50:457–72. [PubMed] [Google Scholar]
- 2.van der Kamp W, Berardelli A, Rothwell JC, et al. Rapid elbow movements in patients with torsion dystonia. J Neurol Neurosurg Psychiatry. 1989;52:1043–9. doi: 10.1136/jnnp.52.9.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agostino R, Berardelli A, Formica A, et al. Sequential arm movements in patients with Parkinson’s disease, Huntington’s disease and dystonia. Brain. 1992;115:1481–95. doi: 10.1093/brain/115.5.1481. [DOI] [PubMed] [Google Scholar]
- 4.Inzelberg R, Flash T, Korczyn AD. Kinematic properties of upper-limb trajectories in Parkinson’s disease and idiopathic torsion dystonia. Adv Neurol. 1990;53:183–9. [PubMed] [Google Scholar]
- 5.Curra A, Berardelli A, Agostino R, et al. Movement cueing and motor execution in patients with dystonia: a kinematic study. Mov Disord. 2000;15:103–12. doi: 10.1002/1531-8257(200001)15:1<103::aid-mds1016>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.MacKinnon CD, Velickovic M, Drafta C, et al. Corticospinal excitability accompanying ballistic wrist movements in primary dystonia. Mov Disord. 2004;19:273–84. doi: 10.1002/mds.20017. [DOI] [PubMed] [Google Scholar]
- 7.Prodoehl J, MacKinnon CD, Comella C, et al. Rate of force production and relaxation is impaired in patients with focal hand dystonia. Parkinsonism Relat Disord. 2006 doi: 10.1016/j.parkreldis.2006.01.008. In press.
- 8.Prodoehl J, Mackinnon CD, Comella CL, et al. Strength deficits in primary focal hand dystonia. Mov Disord. 2006;21:18–27. doi: 10.1002/mds.20623. [DOI] [PubMed] [Google Scholar]
- 9.Odergren T, Iwasaki N, Borg J, et al. Impaired sensory-motor integration during grasping in writer’s cramp. Brain. 1996;119(Pt 2):569–83. doi: 10.1093/brain/119.2.569. [DOI] [PubMed] [Google Scholar]
- 10.Serrien DJ, Burgunder JM, Wiesendanger M. Disturbed sensorimotor processing during control of precision grip in patients with writer’s cramp. Mov Disord. 2000;15:965–72. doi: 10.1002/1531-8257(200009)15:5<965::aid-mds1030>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Nowak DA, Rosenkranz K, Topka H, et al. Disturbances of grip force behaviour in focal hand dystonia: evidence for a generalised impairment of sensory-motor integration? J Neurol Neurosurg Psychiatry. 2005;76:953–9. doi: 10.1136/jnnp.2004.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens JA, Taylor A. The effect of visual feedback on physiological muscle tremor. Electroencephalogr Clin Neurophysiol. 1974;36:457–64. doi: 10.1016/0013-4694(74)90202-8. [DOI] [PubMed] [Google Scholar]
- 13.Slifkin AB, Newell KM. Noise, information transmission, and force variability. J Exp Psychol Hum Percept Perform. 1999;25:837–51. doi: 10.1037//0096-1523.25.3.837. [DOI] [PubMed] [Google Scholar]
- 14.Laidlaw DH, Bilodeau M, Enoka RM. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve. 2000;23:600–12. doi: 10.1002/(sici)1097-4598(200004)23:4<600::aid-mus20>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Vaillancourt DE, Slifkin AB, Newell KM. Inter-digit individuation and force variability in the precision grip of young, elderly, and Parkinson’s disease participants. Motor Control. 2002;6:113–28. doi: 10.1123/mcj.6.2.113. [DOI] [PubMed] [Google Scholar]
- 16.Vaillancourt DE, Slifkin AB, Newell KM. Intermittency in the visual control of force in Parkinson’s disease. Exp Brain Res. 2001;138:118–27. doi: 10.1007/s002210100699. [DOI] [PubMed] [Google Scholar]
- 17.Reilmann R, Kirsten F, Quinn L, et al. Objective assessment of progression in Huntington’s disease: a 3-year follow-up study. Neurology. 2001;57:920–4. doi: 10.1212/wnl.57.5.920. [DOI] [PubMed] [Google Scholar]
- 18.Gordon AM, Quinn L, Reilmann R, et al. Coordination of prehensile forces during precision grip in Huntington’s disease. Exp Neurol. 2000;163:136–48. doi: 10.1006/exnr.2000.7348. [DOI] [PubMed] [Google Scholar]
- 19.Rost K, Nowak DA, Timmann D, et al. Preserved and impaired aspects of predictive grip force control in cerebellar patients. Clin Neurophysiol. 2005;116:1405–14. doi: 10.1016/j.clinph.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Lafargue G, Paillard J, Lamarre Y, et al. Production and perception of grip force without proprioception: is there a sense of effort in deafferented subjects? Eur J Neurosci. 2003;17:2741–9. doi: 10.1046/j.1460-9568.2003.02700.x. [DOI] [PubMed] [Google Scholar]
- 21.Allum JHJ, Dietz V, Freund HJ. Neuronal mechanism underlying physiological tremor. J Neurophysiol. 1978;41:557–71. doi: 10.1152/jn.1978.41.3.557. [DOI] [PubMed] [Google Scholar]
- 22.Inzelberg R, Flash T, Schechtman E, et al. Kinematic properties of upper limb trajectories in idiopathic torsion dystonia. J Neurol Neurosurg Psychiatry. 1995;58:312–9. doi: 10.1136/jnnp.58.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheehy MP, Marsden CD. Writers’ cramp—a focal dystonia. Brain. 1982;105(Pt 3):461–80. doi: 10.1093/brain/105.3.461. [DOI] [PubMed] [Google Scholar]
- 24.Burke RE, Fahn S, Marsden CD, et al. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35:73–7. doi: 10.1212/wnl.35.1.73. [DOI] [PubMed] [Google Scholar]
- 25.Schenk T, Mai N. Is writer’s cramp caused by a deficit of sensorimotor integration? Exp Brain Res. 2001;136:321–30. doi: 10.1007/s002210000599. [DOI] [PubMed] [Google Scholar]
- 26.Vaillancourt DE, Slifkin AB, Newell KM. Visual control of isometric force in Parkinson’s disease. Neuropsychologia. 2001;39:1410–8. doi: 10.1016/s0028-3932(01)00061-6. [DOI] [PubMed] [Google Scholar]
- 27.Cooke JD, Brown JD, Brooks V. Increased dependence on visual information for movement control in patients with Parkinson’s disease. Can J Neurol Sci. 1978;5:413–5. doi: 10.1017/s0317167100024197. [DOI] [PubMed] [Google Scholar]
- 28.Flowers KA. Visual “closed loop” and “open loop” characteristics of voluntary movement in patients with parkinsonism and intention tremor. Brain. 1976;99:269–310. doi: 10.1093/brain/99.2.269. [DOI] [PubMed] [Google Scholar]
- 29.Beuter A, Milton JG, Labrie C, et al. Delayed visual feedback and movement control in Parkinson’s disease. Exp Neurol. 1990;110:228–35. doi: 10.1016/0014-4886(90)90034-p. [DOI] [PubMed] [Google Scholar]
- 30.Romero DH, Van Gemmert AW, Adler CH, et al. Altered aiming movements in Parkinson’s disease patients and elderly adults as a function of delays in movement onset. Exp Brain Res. 2003;151:249–61. doi: 10.1007/s00221-003-1452-2. [DOI] [PubMed] [Google Scholar]
- 31.Vaillancourt DE, Newell KM. Aging and the time and frequency structure of force output variability. J Appl Physiol. 2003;94:903–12. doi: 10.1152/japplphysiol.00166.2002. [DOI] [PubMed] [Google Scholar]
- 32.Slifkin AB, Vaillancourt DE, Newell KM. Intermittency in the control of continuous force production. J Neurophysiol. 2000;84:1708–18. doi: 10.1152/jn.2000.84.4.1708. [DOI] [PubMed] [Google Scholar]
- 33.Ceballos-Baumann AO, Passingham RE, Warner T, et al. Overactive prefrontal and underactive motor cortical areas in idiopathic dystonia. Ann Neurol. 1995;37:363–72. doi: 10.1002/ana.410370313. [DOI] [PubMed] [Google Scholar]
- 34.Ceballos-Baumann AO, Sheean G, Passingham RE, et al. Botulinum toxin does not reverse the cortical dysfunction associated with writer’s cramp. A PET study. Brain. 1997;120(Pt 4):571–82. doi: 10.1093/brain/120.4.571. [DOI] [PubMed] [Google Scholar]
- 35.Rosenkranz K, Williamon A, Butler K, et al. Pathophysiological differences between musician’s dystonia and writer’s cramp. Brain. 2005;128:918–31. doi: 10.1093/brain/awh402. [DOI] [PubMed] [Google Scholar]
- 36.Sanger TD, Delgado MR, Gaebler-Spira D, et al. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111:e89–97. doi: 10.1542/peds.111.1.e89. [DOI] [PubMed] [Google Scholar]
- 37.Klap P, Cohen M, van Prooyen Keyzes S, et al. Laryngeal dystonia. Rev Neurol (Paris) 2003;159:916–22. [PubMed] [Google Scholar]
- 38.Enoka RM, Christou EA, Hunter SK, et al. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol. 2003;13:1–12. doi: 10.1016/s1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- 39.Roos MR, Rice CL, Connelly DM, et al. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve. 1999;22:1094–103. doi: 10.1002/(sici)1097-4598(199908)22:8<1094::aid-mus14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 40.Galganski ME, Fuglevand AJ, Enoka RM. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J Neurophysiol. 1993;69:2108–15. doi: 10.1152/jn.1993.69.6.2108. [DOI] [PubMed] [Google Scholar]