Abstract
Combinations of the direct renin inhibitor aliskiren with angiotensin receptor blockers (ARBs) or diuretics are effective therapeutic regimens for the treatment of hypertension. A large database of safety information has become available during the past several years with aliskiren in combination trials. Data were pooled from nine short-term (8-week) and four longer-term (26–52-week) randomized, controlled trials of aliskiren in patients with hypertension. Adverse event (AE) rates were assessed for aliskiren combination therapy compared to component monotherapies. In short-term studies, overall AE rates were similar for those receiving aliskiren/valsartan or aliskiren/diuretic combinations (32.2–39.8%) and those receiving the component monotherapies (30.0–39.6%). In longer-term studies, AE rates with aliskiren/losartan (55.5%) and aliskiren/diuretic (45.0%) combination therapy were similar to those with losartan (53.9%) and diuretic (48.9%) alone. Angioedema and hyperkalemia occurred in similar proportions of patients on combination therapies versus monotherapy. In conclusion, the safety and tolerability profile of aliskiren in combination with the ARBs valsartan or losartan, or diuretic is similar to aliskiren, ARBs or diuretic alone.
Keywords: aliskiren, combination therapy, direct renin inhibitor, angiotensin receptor blocker, thiazide diuretic, hypertension, safety
INTRODUCTION
Many patients with hypertension require two or more antihypertensive agents to achieve recommended blood pressure (BP) goals (< 140/90 mmHg). Accordingly, current US and European treatment guidelines advocate that combination treatment should be considered as a first-line option for patients who have high initial BP (e.g. systolic BP > 20 mmHg above goal [< 140 mmHg] or diastolic BP > 10 mmHg above goal [< 90 mmHg]).1, 2 European guidelines also recommend combination therapy for patients with lesser degrees of BP elevation when other cardiovascular risk factors, and target organ damage, renal disease or a history of cardiovascular disease, are present.2 These recommendations reflect recent clinical trial evidence that the larger and more rapid reductions in BP conferred by early use of combination therapy and consequent prompt achievement of BP goals is associated with increased protection from cardiovascular events.3
It is clinically relevant to evaluate the safety profiles of fixed combinations of antihypertensive medications that promote effective BP reduction. The combination of drugs that target the renin–angiotensin system (RAS) with thiazide diuretics, is a widely used therapeutic approach. In 2007, the first oral direct renin inhibitor, aliskiren, became available for the treatment of hypertension. As aliskiren has a different mechanism of action compared to angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), it is possible that it may have a different safety profile to these agents. Clinical trials have shown that aliskiren is effective for reducing BP in patients with hypertension, when given alone or in combination with other antihypertensive therapies.4–13
In 2006, an independently chaired, multi-disciplinary aliskiren global safety committee was formed and funded by Novartis to analyze and review semi-annually all safety data related to aliskiren clinical trials of patients with hypertension and related co-morbid populations. The committee evaluated safety data pooled from short-term, placebo-controlled and longer-term, active-controlled aliskiren clinical trials, and have previously reported its findings for aliskiren monotherapy.14 In the present study, we report on clinical trial safety data for aliskiren in combination with ARBs and thiazide diuretic.
METHODS
Description of the Pooled Trials
Safety data from aliskiren studies were pooled every 6 months on an ongoing basis; all studies with a database lock before 31 August 2009 were included. Thirty-two studies were included in the pooled data set. Of these, 13 studies were excluded from the present analysis because they were not placebo-controlled, four studies were excluded because they had an open-label study design, and two studies were excluded because they were not studies of hypertension. Therefore, safety data from 13 randomized, double-blind clinical trials in patients with hypertension were evaluated: nine short-term (8-week) placebo-controlled studies9, 12, 15–21 and four longer-term (26-to 52-week) active-controlled studies.6, 10, 22, 23 The current analysis assessed the safety of aliskiren in combination with ARBs or thiazide diuretic. Studies enrolled patients with stage 1–2 hypertension (seated diastolic BP ≥ 90 or ≥ 95 mmHg and < 110 mmHg); three trials enrolled special populations (one included only overweight patients with left ventricular hypertrophy, and two included only patients aged ≥ 65 years with systolic hypertension [seated systolic BP of 140 or 150 mmHg to < 180 mmHg and diastolic BP < 110 mmHg]). Exclusion criteria were generally similar across the studies and included secondary hypertension, history of severe cardiovascular disease, type 1 or uncontrolled type 2 diabetes, and clinically significant hepatic, renal, or endocrine disorders.
Adverse Events – Search Terms
All adverse events that occurred during the treatment period of the studies were tabulated; the most common adverse events are presented in this paper. In addition, the pooled databases were searched for events of special interest that are related to agents that target the RAS, such as aliskiren: angioedema/urticaria, cough, rash, hypotension, hyperkalemia, peripheral edema, renal dysfunction, diarrhea, and colorectal events.24–31 The searches for events of special interest used broad terms that captured a wide variety of events. For example, the search for angioedema/urticaria included urticaria, edema of the head, neck, and mucous membranes; the search for hypotension included dizziness, syncope, vertigo, and positional vertigo; and the search for colorectal events included gastrointestinal neoplasms (benign and malignant), gastrointestinal hemorrhages, ulceration, and perforation.
This analysis also assessed all serious adverse events, regardless of whether they were classed as being of special interest. Serious adverse events were defined by guidelines from the International Conference on Harmonization-Good Clinical Practice (ICH-GCP). In brief, a serious adverse event is defined as one that is fatal or life-threatening; results in persistent or significant disability or incapacity; constitutes a congenital anomaly or birth defect; requires inpatient hospitalization or prolongation of existing hospitalization; or is otherwise medically significant.
Statistical Methods
Demographics, baseline characteristics, adverse events of special interest, and laboratory abnormalities were summarized by pooled treatment group, according to the treatment to which patients were randomized, regardless of doses used during any titration period. Pooled treatment groups for the short-term, placebo-controlled and longer-term, active-controlled studies included aliskiren 150 mg, aliskiren 300 mg, aliskiren monotherapy (150 or 300 mg), aliskiren/ARB combination therapy, aliskiren/thiazide diuretic combination therapy, ARB monotherapy, and thiazide diuretic monotherapy.
Pair-wise comparisons were performed for the adverse events of special interest (excluding regimens using the unlicensed 75 mg and 600 mg doses of aliskiren); relative risks with associated 95% confidence intervals (CIs) were determined for: aliskiren/ARB combination therapy versus ARB monotherapy (aliskiren/valsartan vs pooled valsartan, irbesartan and losartan for short-term studies; aliskiren/losartan for losartan for longer-term studies); aliskiren/ARB combination therapy versus aliskiren monotherapy (short- and longer-term studies); aliskiren/thiazide diuretic combination therapy versus thiazide diuretic monotherapy (short-term studies); and aliskiren and thiazide diuretic combination therapy versus aliskiren monotherapy (short-term studies). For the longer-term trials, relative risks were calculated for individual studies rather than for treatment groups pooled across studies, due to differences in study design and thus length of exposure.
RESULTS
Patients and Exposure
Safety data were available for 12,942 patients: 9,616 patients from the short-term, placebo-controlled studies and 3,326 patients from the longer-term, active-controlled studies. The cumulative exposure to study medication ranged from 82.6 to 245.6 patient-years in short-term placebo-controlled studies, and from 99.6 to 548.0 patient-years in longer-term, active controlled studies.
Baseline Patient Characteristics
Baseline characteristics of patients were generally similar between the monotherapy and combination therapy treatment groups (Table 1). In the short-term studies, the aliskiren/valsartan group contained a greater proportion of obese patients (44.7%) than the aliskiren or ARB (pooled valsartan, irbesartan and losartan) monotherapy groups (29.2–38.8%). Similarly, the aliskiren/losartan combination therapy and losartan monotherapy groups in the longer-term studies contained a greater proportion of patients who were obese (56.1% and 50.0%) than did the aliskiren monotherapy groups (40.7–41.9%), and a greater proportion of patients who had diabetes (27.1% and 22.1% vs 13.4–16.1%). In the short- and longer-term studies, baseline characteristics were similar between the aliskiren/HCT combination therapy, and aliskiren and HCT monotherapy groups.
Table 1.
Baseline patient characteristics
| A. Short-term, placebo-controlled studies | ||||||
|---|---|---|---|---|---|---|
| Baseline characteristic | Aliskiren 150 mg n = 1435 | Aliskiren 300 mg n = 1551 | Aliskiren/HCTa n = 1464 | HCTb n = 555 | Aliskiren/valsartanc n = 624 | ARBd n = 1069 |
| Age (years), mean ± SD | 55.8 ± 12.4 | 55.8 ± 12.1 | 54.6 ± 11.2 | 55.2 ± 12.2 | 53.4 ± 10.9 | 53.4 ± 10.9 |
| Age group, n (%) | ||||||
| < 65 years | 1034 (72.1) | 1146 (73.9) | 1177 (80.4) | 415 (74.8) | 525 (84.1) | 901 (84.3) |
| ≥ 65 years | 401 (27.9) | 405 (26.1) | 287 (19.6) | 140 (25.2) | 99 (15.9) | 168 (15.7) |
| ≥ 75 years | 94 (6.6) | 97 (6.3) | 44 (3.0) | 21 (3.8) | 14 (2.2) | 26 (2.4) |
| Male, n (%) | 881 (61.4) | 870 (56.1) | 792 (54.1) | 302 (54.4) | 372 (59.6) | 666 (62.3) |
| Race, n (%) | ||||||
| Caucasian | 847 (59.0) | 1172 (75.6) | 1261 (86.1) | 474 (85.4) | 500 (80.1) | 587 (54.9) |
| Black | 88 (6.1) | 142 (9.2) | 62 (4.2) | 31 (5.6) | 74 (11.9) | 129 (12.1) |
| Asian | 452 (31.5) | 157 (10.1) | 39 (2.7) | 12 (2.2) | 8 (1.3) | 311 (29.1) |
| Other | 48 (3.3) | 80 (5.2) | 102 (7.0) | 38 (6.8) | 42 (6.7) | 42 (3.9) |
| BMI (kg/m2), mean ± SD | 28.2 ± 5.2 | 29.5 ± 5.6 | 29.4 ± 5.4 | 29.4 ± 5.5 | 30.2 ± 5.6 | 29.2 ± 5.6 |
| Obese (BMI ≥ 30 kg/m2), n (%) | 419 (29.2) | 602 (38.8) | 557 (38.0) | 204 (36.8) | 279 (44.7) | 390 (36.5) |
| Diabetes, n (%) | 133 (9.3) | 165 (10.6) | 112 (7.7) | 42 (7.6) | 64 (10.3) | 95 (8.9) |
| Duration of hypertension (years), mean ± SD | 7.1 ± 7.1 | 8.3 ± 7.6 | 7.6 ± 7.3 | 7.8 ± 8.0 | 8.0 ± 7.7 | 7.4 ± 7.4 |
| Sitting SBP (mm Hg), mean ± SD | 153.9 ± 11.8 | 154.2 ± 11.6 | 153.7 ± 12.3 | 153.8 ± 12.1 | 153.0 ±12.4 | 153.5 ± 11.8 |
| Sitting DBP (mm Hg), mean ± SD | 98.0 ± 5.6 | 98.6 ± 5.6 | 99.2 ± 3.6 | 99.2 ± 3.4 | 99.7 ± 4.0 | 99.7 ± 4.0 |
| Baseline renal function (eGFR), n (%) | ||||||
| < 60 mL/min/1.73 m2 | 61 (4.3) | 69 (4.4) | 61 (4.2) | 20 (3.6) | 18 (2.9) | 30 (2.8) |
| ≥ 60 to < 90 mL/min/1.73 m2 | 685 (47.7) | 806 (52.0) | 807 (55.1) | 304 (54.8) | 324 (51.9) | 469 (43.9) |
| ≥ 90 mL/min/1.73 m2 | 689 (48.0) | 663 (42.7) | 596 (40.7) | 231 (41.6) | 270 (43.3) | 565 (52.9) |
| No data available | 0 | 13 (0.8) | 0 | 0 | 12 (1.9) | 5 (0.5) |
| B. Longer-term, active-controlled studies | ||||||
|---|---|---|---|---|---|---|
| Baseline characteristic | Aliskiren monotherapy (150 or 300 mg) n = 1593 | Aliskiren 150 mg n = 871 | Aliskiren 300 mg n = 722 | HCTa n = 558 | Aliskiren/losartanb n = 155 | Losartanc n = 154 |
| Age (years), mean ± SD | 60.1 ± 12.2 | 63.1 ± 12.6 | 56.6 ± 10.7 | 55.8 ± 10.9 | 58.8 ± 10.6 | 59.1 ± 11.0 |
| Age group, n (%) | ||||||
| < 65 years | 903 (56.7) | 356 (40.9) | 547 (75.8) | 430 (77.1) | 108 (69.7) | 101 (65.6) |
| ≥ 65 years | 690 (43.3) | 515 (59.1) | 175 (24.2) | 128 (22.9) | 47 (30.3) | 53 (34.4) |
| ≥ 75 years | 179 (11.2) | 156 (17.9) | 23 (3.2) | 19 (3.4) | 5 (3.2) | 8 (5.2) |
| Male, n (%) | 863 (54.2) | 443 (50.9) | 420 (58.2) | 312 (55.9) | 120 (77.4) | 118 (76.6) |
| Race, n (%) | ||||||
| Caucasian | 1402 (88.0) | 696 (79.9) | 706 (97.8) | 553 (99.1) | 147 (94.8) | 145 (94.2) |
| Black | 118 (7.4) | 117 (13.4) | 1 (0.1) | 0 | 1 (0.6) | 2 (1.3) |
| Asian | 37 (2.3) | 32 (3.7) | 5 (0.7) | 4 (0.7) | 1 (0.6) | 0 |
| Other | 36 (2.3) | 26 (3.0) | 10 (1.4) | 1 (0.2) | 6 (3.9) | 7 (4.5) |
| BMI (kg/m2), mean ± SD | 29.7 ± 5.3 | 29.9 ± 5.8 | 29.4 ± 4.6 | 29.1 ± 4.8 | 31.3 ± 4.0 | 30.8 ± 4.1 |
| Obese (BMI ≥ 30 kg/m2), n (%) | 659 (41.4) | 365 (41.9) | 294 (40.7) | 188 (33.7) | 87 (56.1) | 77 (50.0) |
| Diabetes, n (%) | 237 (14.9) | 140 (16.1) | 97 (13.4) | 60 (10.8) | 42 (27.1) | 34 (22.1) |
| Duration of hypertension (years), mean ± SD | 9.0 ± 8.7 | 9.8 ± 9.4 | 8.1 ± 7.6 | 6.9 ± 6.8 | 12.7 ± 10.1 | 12.3 ± 9.1 |
| Sitting SBP (mm Hg), mean ± SD | 153.3 ± 12.0 | 154.1 ± 11.6 | 152.4 ± 12.4 | 154.3 ± 11.0 | 144.4 ± 13.9 | 146.3 ± 13.4 |
| Sitting DBP (mm Hg), mean ± SD | 94.2 ± 8.8 | 91.9 ± 9.8 | 96.8 ± 6.7 | 99.0 ± 3.4 | 88.4 ± 8.5 | 89.1 ± 10.1 |
| Baseline renal function (eGFR), n (%) | ||||||
| < 60 mL/min/1.73 m2 | 146 (9.2) | 113 (13.0) | 33 (4.6) | 38 (6.8) | 12 (7.7) | 13 (8.4) |
| ≥ 60 to < 90 mL/min/1.73 m2 | 896 (56.2) | 474 (54.4) | 422 (58.4) | 336 (60.2) | 88 (56.8) | 91 (59.1) |
| ≥ 90 mL/min/1.73 m2 | 549 (34.5) | 282 (32.4) | 267 (37.0) | 184 (33.0) | 55 (35.5) | 50 (32.5) |
| No data available | 2 (0.1) | 2 (0.2) | 0 | 0 | 0 | 0 |
Data are summarized according to the treatment to which patients were randomized.
Aliskiren/HCT 150/6.25 mg 300/25 mg;
6.25–25 mg;
aliskiren/valsartan 75/80 mg 300/320 mg;
losartan 50 mg, irbesartan 150 mg, or valsartan 80–320 mg.
ARB, angiotensin receptor blocker; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HCT, hydrochlorothiazide; SBP, systolic blood pressure; SD, standard deviation.
Data are summarized according to the treatment to which patients were randomized.
HCT 12.5–25 mg; HCT/amlodipine 25/5–10 mg (in one study, amlodipine was added to HCT if blood pressure was not controlled to <140/90 mmHg);22
aliskiren/losartan 150/50 mg 300/100 mg;
losartan 50–100 mg.
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HCT, hydrochlorothiazide; SBP, systolic blood pressure; SD, standard deviation.
Serious Adverse Events
Overall 0.7% of patients in the short-term studies and 4.5% of patients in the longer-term studies had a serious adverse event. In the short-term studies, serious adverse events were experienced by a similar proportion of patients who received aliskiren in combination with valsartan (0.6%) to those who received aliskiren monotherapy (150 mg, 0.5%; 300 mg, 0.4%), ARB (valsartan 80–320 mg, irbesartan 150 mg or losartan 50 mg) monotherapy (0.8%) or placebo (0.7%). The incidence of serious adverse events with aliskiren/thiazide diuretic combination therapy (1.1%) was similar to that with thiazide diuretic monotherapy (0.9%) and slightly greater than that with aliskiren monotherapy (150 mg, 0.5%; 300 mg, 0.4%) or placebo (0.7%). In the longer-term studies, the proportion of patients who experienced serious adverse events while receiving combination therapy with aliskiren/losartan (5.2%) was lower than that in patients receiving losartan monotherapy (8.4%) and similar to that in patients receiving aliskiren monotherapy (3.4%); for the aliskiren/thiazide diuretic combination, the proportion of patients who experienced serious adverse events (2.5%) was similar to that for each of the individual monotherapies (3.4% and 1.7% for aliskiren and thiazide diuretic monotherapy, respectively). Most of the serious adverse events in each treatment group were experienced by only one patient, and there was no apparent clustering of events.
Any Adverse Event
In the short-term studies, adverse events were experienced by a similar proportion of patients receiving combination therapy with aliskiren/valsartan or with aliskiren/thiazide diuretic (32.2–39.8%) to those receiving the individual agents as monotherapy (30.0–39.6%) or placebo (35%). In the longer-term studies, the proportion of patients who experienced adverse events with aliskiren/losartan and aliskiren/thiazide diuretic combination therapy (55.5% and 45.0%, respectively) was similar to those receiving losartan monotherapy and thiazide diuretic monotherapy (53.9% and 48.9%, respectively). Discontinuations due to adverse events were uncommon (≤ 1.9% in the short-term studies and ≤ 5.9% in the longer-term studies). A smaller proportion of patients discontinued with combination treatment than with the component monotherapies in the longer-term studies (aliskiren/losartan, 1.9%; aliskiren/thiazide diuretic, 1.8%; losartan monotherapy, 6.5%; thiazide diuretic monotherapy, 3.3%; and aliskiren monotherapy, 4.3%).
In the short-term studies, the most common adverse events were headache, nasopharyngitis and diarrhea (Table 2A). The incidences of individual adverse events were generally similar between treatments, although there was a higher rate of headache in the aliskiren/HCT combination therapy and HCT monotherapy groups (6.5% and 7.0%, respectively) than in the other treatment groups (3.6–4.5%). The most common adverse events in the long-term studies were headache, dizziness, nasopharyngitis and diarrhea (Table 2B). The rates of individual adverse events were generally low; the incidence of nasopharyngitis was higher with aliskiren/losartan combination therapy (7.1%) and losartan monotherapy (8.4%) than with the other treatments (1.7–3.0%).
Table 2.
Any adverse event
| A. Short-term, placebo-controlled studies | |||||||
|---|---|---|---|---|---|---|---|
| Adverse event | Aliskiren monotherapy (150 or 300 mg) n = 3380 | Aliskiren 150 mg n = 1872 | Aliskiren 300 mg n = 1508 | Aliskiren/HCTa n = 1464 | HCTb n = 555 | Aliskiren/valsartanc n = 624 | ARBd n = 1069 |
| Most frequent adverse events occurring in ≥ 1% of patients in any group | |||||||
| Headache | 126 (3.7) | 67 (3.6) | 59 (3.9) | 95 (6.5) | 39 (7.0) | 28 (4.5) | 48 (4.5) |
| Nasopharyngitis | 135 (4.0) | 92 (4.9) | 43 (2.9) | 56 (3.8) | 21 (3.8) | 15 (2.4) | 63 (5.9) |
| Diarrhea | 40 (1.2) | 17 (0.9) | 23 (1.5) | 24 (1.6) | 10 (1.8) | 9 (1.4) | 11 (1.0) |
| Dizziness | 46 (1.4) | 20 (1.1) | 26 (1.7) | 34 (2.3) | 13 (2.3) | 10 (1.6) | 21 (2.0) |
| Back pain | 34 (1.0) | 19 (1.0) | 15 (1.0) | 21 (1.4) | 6 (1.1) | 10 (1.6) | 20 (1.9) |
| Fatigue | 28 (0.8) | 10 (0.5) | 18 (1.2) | 13 (0.9) | 6 (1.1) | 16 (2.6) | 16 (1.5) |
| Upper respiratory tract infection | 30 (0.9) | 13 (0.7) | 17 (1.1) | 16 (1.1) | 4 (0.7) | 8 (1.3) | 10 (0.9) |
| Bronchitis | 31 (0.9) | 14 (0.7) | 17 (1.1) | 17 (1.2) | 4 (0.7) | 6 (1.0) | 12 (1.1) |
| Nausea | 27 (0.8) | 10 (0.5) | 17 (1.1) | 17 (1.2) | 6 (1.1) | 9 (1.4) | 12 (1.1) |
| Cough | 31 (0.9) | 20 (1.1) | 11 (0.7) | 19 (1.3) | 4 (0.7) | 3 (0.5) | 3 (0.3) |
| Edema peripheral | 21 (0.6) | 11 (0.6) | 10 (0.7) | 13 (0.9) | 6 (1.1) | 1 (0.2) | 5 (0.5) |
| B. Longer-term, active-controlled studies | ||||||
|---|---|---|---|---|---|---|
| Adverse event | Aliskiren monotherapy (150 and 300 mg) n = 2784 | Aliskiren 150 mg n = 1487 | Aliskiren 300 mg n = 1297 | HCTa n = 544 | Aliskiren/losartanb n = 155 | Losartanc n = 154 |
| Most frequent adverse events occurring in ≥ 2% of patients in any group | ||||||
| Headache | 128 (4.6) | 77 (5.2) | 51 (3.9) | 33 (6.1) | 10 (6.5) | 8 (5.2) |
| Dizziness | 63 (2.3) | 37 (2.5) | 26 (2.0) | 19 (3.5) | 8 (5.2) | 3 (1.9) |
| Nasopharyngitis | 64 (2.3) | 25 (1.7) | 39 (3.0) | 16 (2.9) | 11 (7.1) | 13 (8.4) |
| Diarrhea | 62 (2.2) | 28 (1.9) | 34 (2.6) | 8 (1.5) | 7 (4.5) | 9 (5.8) |
| Cough | 49 (1.8) | 21 (1.4) | 28 (2.2) | 14 (2.6) | 2 (1.3) | 3 (1.9) |
| Back pain | 47 (1.7) | 23 (1.5) | 24 (1.9) | 16 (2.9) | 2 (1.3) | 4 (2.6) |
| Upper respiratory tract infection | 48 (1.7) | 26 (1.7) | 22 (1.7) | 2 (0.4) | 1 (0.6) | 1 (0.6) |
| Bronchitis | 38 (1.4) | 7 (0.5) | 31 (2.4) | 15 (2.8) | 3 (1.9) | 3 (1.9) |
| Arthralgia | 36 (1.3) | 15 (1.0) | 21 (1.6) | 13 (2.4) | 1 (0.6) | 3 (1.9) |
| Dyspepsia | 25 (0.9) | 11 (0.7) | 14 (1.1) | 4 (0.7) | 5 (3.2) | 3 (1.9) |
| Influenza | 21 (0.8) | 3 (0.2) | 18 (1.4) | 8 (1.5) | 6 (3.9) | 7 (4.5) |
| Hypercholesterolemia | 18 (0.6) | 15 (1.0) | 3 (0.2) | 14 (2.6) | 1 (0.6) | 0 |
| Asthenia | 17 (0.6) | 11 (0.7) | 6 (0.5) | 5 (0.9) | 2 (1.3) | 4 (2.6) |
Data are number (%) of patients and are summarized according to treatment to received.
Aliskiren/HCT 150/6.25 mg 300/25 mg;
6.25 –25 mg;
aliskiren/valsartan 150/160 mg 300/320 mg;
losartan 50 mg, irbesartan 150 mg, or valsartan 80–320 mg.
ARB, angiotensin receptor blocker; HCT, hydrochlorothiazide.
Data are number (%) of patients and are summarized according to treatment received.
HCT 12.5–25 mg; HCT/amlodipine 25/5–10 mg (in one study, amlodipine was added to HCT if blood pressure was not controlled to < 140/90 mmHg);22
aliskiren/losartan 150/50 mg 300/100 mg;
losartan 50–100 mg.
ARB, angiotensin receptor blocker; HCT, hydrochlorothiazide.
Laboratory Abnormalities
Fewer than 1% of patients in any treatment group had pre-specified laboratory abnormalities of blood urea nitrogen > 40 mg/dL, creatinine > 2.0 mg/dL or estimated glomerular filtration rate < 30 mL/min/1.73 m2. As expected, elevated serum potassium (> 5.5 mEq/L) was found in a greater proportion of patients receiving short-term treatment with aliskiren/valsartan than in those receiving the individual monotherapies (3.4% vs 1.3% for aliskiren and 0.7% for ARBs; Table 3A); elevations in serum potassium levels to ≥ 6.0 mEq/L were observed in similar proportions of patients in these treatment groups (0.3% with aliskiren/valsartan compared with 0.4% with aliskiren monotherapy and 0.5% with ARB [valsartan, irbesartan or losartan] monotherapy). There were no differences between treatments in the rates of serum potassium > 5.5 mEq/L in the longer-term studies: 3.3% of patients receiving aliskiren/losartan compared with 3.6% receiving aliskiren monotherapy and 3.3% receiving losartan monotherapy(Table 3B).
Table 3.
Pre-specified laboratory abnormalities
| A. Short-term, placebo-controlled studies | ||||||
|---|---|---|---|---|---|---|
| Laboratory variable | Aliskiren 150 mg n = 1435 | Aliskiren 300 mg n = 1551 | Aliskiren/HCTa n = 1464 | HCTb n = 555 | Aliskiren/valsartanc n = 624 | ARBd 50–300 mg n = 1069 |
| Potassium level | ||||||
| < 3.5 mEq/L | 13 (1.0) | 19 (1.3) | 21 (1.8) | 14 (3.1) | 13 (2.2) | 26 (2.5) |
| > 5.5 mEql/L | 14 (1.0) | 24 (1.7) | 6 (0.5) | 4 (0.9) | 20 (3.4) | 7 (0.7) |
| ≥ 6.0 mEq/L | 5 (0.4) | 7 (0.5) | 1 (0.1) | 1 (0.2) | 2 (0.3) | 5 (0.5) |
| ≥ 7.0 mEq/L | 0 | 3 (0.2) | 0 | 0 | 2 (0.3) | 2 (0.2) |
| BUN > 40 mg/dL | 0 | 3 (0.2) | 0 | 0 | 0 | 1 (0.1) |
| Creatinine level > 2.0 mg/dL | 0 | 4 (0.3) | 0 | 0 | 4 (0.7) | 2 (0.2) |
| eGFR < 30 mL/min/1.73 m2 | 0 | 1 (0.1) | 0 | 0 | 2 (0.3) | 1 (0.1) |
| B. Longer-term, active-controlled studies | ||||||
|---|---|---|---|---|---|---|
| Laboratory variable | Aliskiren monotherapy (150 and 300 mg) n = 1593 | Aliskiren 150 mg n = 871 | Aliskiren 300 mg n = 722 | HCTa n = 558 | Aliskiren/losartanb n = 155 | Losartanc n = 154 |
| Potassium level | ||||||
| < 3.5 mEq/L | 54 (3.5) | 37 (4.4) | 17 (2.4) | 96 (17.8) | 8 (5.2) | 11 (7.2) |
| > 5.5 mEql/L | 56 (3.6) | 16 (1.9) | 40 (5.7) | 20 (3.7) | 5 (3.3) | 5 (3.3) |
| ≥6.0 mEq/L | 25 (1.6) | 7 (0.8) | 18 (2.6) | 10 (1.9) | 1 (0.7) | 1 (0.7) |
| ≥7.0 mEq/L | 5 (0.3) | 0 | 5 (0.7) | 3 (0.6) | 0 | 1 (0.7) |
| BUN > 40 mg/dL | 10 (0.6) | 6 (0.7) | 4 (0.6) | 4 (0.7) | 0 | 2 (1.3) |
| Creatinine level > 2.0 mg/dL | 4 (0.3) | 2 (0.2) | 2 (0.3) | 0 | 1 (0.7) | 1 (0.7) |
| eGFR < 30 mL/min/1.73 m2 | 5 (0.3) | 3 (0.4) | 2 (0.3) | 0 | 1 (0.7) | 2 (1.3) |
Data are number (%) of patients who had laboratory measurements and are summarized according to the treatment to which patients were randomized.
Aliskiren/HCT 150/6.25 mg 300/25 mg;
6.25–25 mg;
aliskiren/valsartan 75/80 mg 300/320 mg;
losartan 50 mg, irbesartan 150 mg, or valsartan 80–320 mg.
ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; HCT, hydrochlorothiazide.
Data are number (%) of patients who had laboratory measurements and are summarized according to the treatment to which patients were randomized.
HCT 12.5–25 mg; HCT/amlodipine 25/5–10 mg (in one study, amlodipine was added to HCT if blood pressure was not controlled to <140/90 mmHg);22
aliskiren/losartan 150/50 mg 300/100 mg;
losartan 50–100 mg.
ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; HCT, hydrochlorothiazide.
All Adverse Events of Special Interest
There were no statistically significant increases in the relative risk of any of the adverse events of special interest with aliskiren/ARB (aliskiren/valsartan for short-term studies, or aliskiren/losartan for longer-term studies) compared with aliskiren monotherapy or ARB (valsartan, irbesartan or losartan for short-term studies; losartan for longer-term studies) monotherapy (Figure 1). With aliskiren/thiazide diuretic combination therapy, there was a statistically significantly increased risk for hypotension in the short-term studies; this was more frequently reported with aliskiren/thiazide diuretic than with aliskiren monotherapy but not thiazide diuretic monotherapy.
Figure 1. Relative risks of adverse events of special interest with aliskiren/ARB combination compared with ARB and aliskiren monotherapy in the short-term studies.
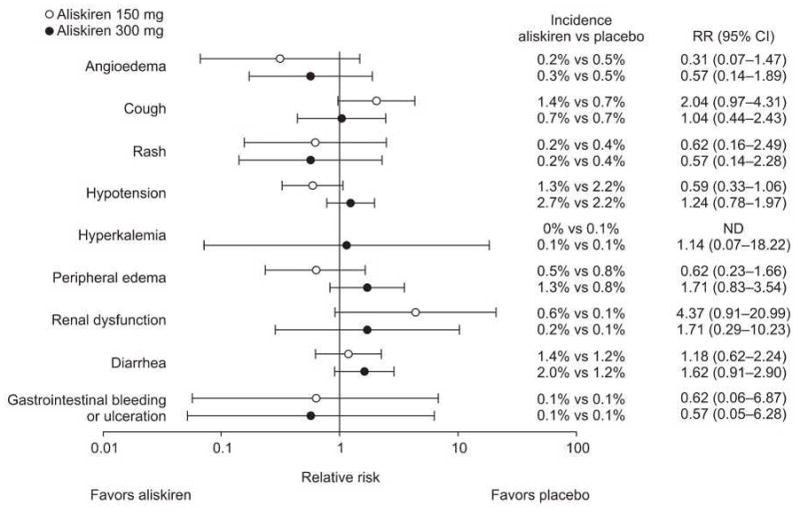
ARB, angiotensin receptor blocker; CI, confidence interval; ND, not determined; RR, relative risk.
Angioedema
Angioedema/urticaria events were experienced by a small number of patients. No patient had angioedema/urticaria while receiving combination therapy with aliskiren/ARB in either the short- or longer-term studies, and no patient had angioedema/urticaria with aliskiren/thiazide diuretic combination therapy in the longer-term studies; one patient receiving the aliskiren/thiazide diuretic combination therapy in the short-term studies had mild facial swelling. Angioedema/urticaria events were experienced by a small proportion of patients receiving monotherapy with aliskiren (0.2% in the short-term studies and 0.5% in the longer-term studies), ARBs (0.3% in the short-term studies and 0% in the longer-term studies), or thiazide diuretic (0.2% in the short-term studies and 0.4% in the longer-term studies; Table 4). Of these events, only two were classed as serious (one in the short-term ARB group and one in the longer-term aliskiren 150 mg group).
Table 4.
Adverse events of special interest
| A. Short-term, placebo-controlled studies | |||||||
|---|---|---|---|---|---|---|---|
| Adverse event of special interest | Aliskiren monotherapy (150 or 300 mg) n = 2985 | Aliskiren 150 mg n = 1435 | Aliskiren 300 mg n = 1550 | Aliskiren/HCTa n = 900 | HCTb n = 555 | Aliskiren/valsartanc n = 564 | ARBd n = 1069 |
| Angioedema/urticaria | 7 (0.2) | 3 (0.2) | 4 (0.3) | 1 (0.1) | 1 (0.2) | 0 | 3 (0.3) |
| Cough | 31 (1.0) | 20 (1.4) | 11 (0.7) | 11 (1.2) | 4 (0.7) | 2 (0.4) | 4 (0.4) |
| Rash | 8 (0.3) | 4 (0.3) | 4 (0.3) | 3 (0.3) | 4 (0.7) | 0 | 1 (0.1) |
| Hypotension | 61 (2.0) | 21 (1.5) | 40 (2.6) | 42 (4.7) | 19 (3.4) | 16 (2.8) | 25 (2.3) |
| Hyperkalemia | 2 (0.1) | 1 (0.1) | 1 (0.1) | 0 | 1 (0.2) | 0 | 0 |
| Peripheral edema | 24 (0.8) | 6 (0.4) | 18 (1.2) | 9 (1.0) | 7 (1.3) | 1 (0.2) | 5 (0.5) |
| Renal dysfunction | 11 (0.4) | 7 (0.5) | 4 (0.3) | 1 (0.1) | 0 | 2 (0.4) | 2 (0.2) |
| Diarrhea | 45 (1.5) | 18 (1.3) | 27 (1.7) | 19 (2.1) | 10 (1.8) | 8 (1.4) | 17 (1.6) |
| Gastrointestinal bleeding or ulceration | 2 (0.1) | 1 (0.1) | 1 (0.1) | 2 (0.2) | 1 (0.2) | 0 | 1 (0.1) |
| B. Longer-term, active-controlled studies | ||||||
|---|---|---|---|---|---|---|
| Adverse event of special interest | Aliskiren monotherapy (150 and 300 mg) n = 1593 | Aliskiren 150 mg n = 871 | Aliskiren 300 mg n = 722 | HCTa n = 558 | Aliskiren/losartanb n = 155 | Losartanc n = 154 |
| Angioedema/urticaria | 8 (0.5) | 5 (0.6) | 3 (0.4) | 2 (0.4) | 0 | 0 |
| Cough | 62 (3.9) | 38 (4.4) | 24 (3.3) | 22 (3.9) | 2 (1.3) | 3 (1.9) |
| Rash | 14 (0.9) | 6 (0.7) | 8 (1.1) | 5 (0.9) | 1 (0.6) | 1 (0.6) |
| Hypotension | 124 (7.8) | 75 (8.6) | 49 (6.8) | 39 (7.0) | 15 (9.7) | 8 (5.2) |
| Hyperkalemia | 2 (0.1) | 1 (0.1) | 1 (0.1) | 0 | 0 | 1 (0.6) |
| Peripheral edema | 75 (4.7) | 41 (4.7) | 34 (4.7) | 35 (6.3) | 3 (1.9) | 2 (1.3) |
| Renal dysfunction | 6 (0.4) | 2 (0.2) | 4 (0.6) | 2 (0.4) | 0 | 1 (0.6) |
| Diarrhea | 74 (4.6) | 52 (6.0) | 22 (3.0) | 17 (3.0) | 7 (4.5) | 9 (5.8) |
| Gastrointestinal bleeding or ulceration | 3 (0.2) | 1 (0.1) | 2 (0.3) | 2 (0.4) | 2 (1.3) | 1 (0.6) |
Data are number (%) of patients and are summarized according to the treatment to which patients were randomized (excluding regimens using the unlicensed 75 mg and 600 mg doses of aliskiren).
Aliskiren/HCT 150/6.25 mg 300/25 mg;
6.25–25 mg;
aliskiren/valsartan 150/160 mg 300/320 mg;
losartan 50 mg, irbesartan 150 mg, or valsartan 80–320 mg.
ARB, angiotensin receptor blocker; HCT, hydrochlorothiazide.
Data are number (%) of patients and are summarized according to the treatment to which patients were randomized (excluding regimens using the unlicensed 75 mg and 600 mg doses of aliskiren).
HCT 12.5–25 mg; HCT/amlodipine 25/5–10 mg (in one study, amlodipine was added to HCT if blood pressure was not controlled to < 140/90 mmHg);22
aliskiren/losartan 150/50 mg 300/100 mg;
losartan 50–100 mg.
ARB, angiotensin receptor blocker; HCT, hydrochlorothiazide.
Cough
In the short- and longer-term studies, cough was no more common in aliskiren/ARB-treated patients than in those treated with the individual monotherapies. In the short-term studies, 0.4% of patients treated with aliskiren/valsartan combination therapy experienced cough, compared with 1.0% of those treated with aliskiren monotherapy and 0.4% of those treated with ARBs (Table 4A). In the longer-term studies, cough was experienced by 1.3% of patients receiving aliskiren/losartan compared with 3.9% of those receiving aliskiren monotherapy and 1.9% of those receiving losartan monotherapy (Table 4B). Cough was experienced by a slightly greater proportion of patients who received the aliskiren/thiazide diuretic combination (1.2%) than in those who received aliskiren monotherapy (1.0%) or thiazide diuretic monotherapy (0.7%) in the short-term studies, but there was no statistically significant increased risk of the event.
Hyperkalemia
No patient receiving aliskiren/ARB or aliskiren/HCT combination therapy had hyperkalemia reported as an adverse event in either the short-term or longer-term studies.
Diarrhea
During the short- and longer-term studies, diarrhea was experienced by a similar proportion of patients receiving combination treatment to those receiving either of the component monotherapies. In the short-term studies, 1.4% of patients treated with aliskiren/valsartan and 2.1% of those treated with aliskiren/thiazide diuretic had diarrhea compared with 1.5% of patients treated with aliskiren monotherapy, 1.6% of those treated with ARB monotherapy and 1.8% of those treated with thiazide monotherapy (Table 4A). In the longer-term studies, 4.5% of patients treated with aliskiren/losartan had diarrhea compared with 4.6% of those treated with aliskiren monotherapy and 5.8% of those treated with losartan monotherapy (Table 4B).
Colorectal Findings
Few patients in any treatment group had colorectal events of interest. In the short-term studies, rectal bleeding or hematochezia was observed in two patients receiving aliskiren monotherapy, one receiving valsartan monotherapy and two receiving aliskiren/thiazide diuretic; in addition, one patient treated with thiazide diuretic monotherapy had erosive gastritis. One of the events (mild rectal bleeding with aliskiren/HCT combination therapy) was suspected possibly to be related to study drug treatment by local investigators; the study drug was not adjusted or discontinued, and no concomitant or non-drug therapies were administered. No patient receiving aliskiren/valsartan therapy had any signs of rectal bleeding or other colorectal events of interest. In the longer-term studies, colorectal events were experienced by three patients in the aliskiren monotherapy group (colon adenoma and rectal ulcer [one patient], diverticular perforation, and erosive duodenitis), two patients in the aliskiren/losartan group (gastric ulcer and erosive gastritis), one patient treated with losartan monotherapy (duodenal ulcer hemorrhage), and two in the thiazide diuretic monotherapy group (colon neoplasm and gastrointestinal carcinoma).
Hypotension
In the short-term studies, the incidence of hypotension with aliskiren/thiazide diuretic combination therapy (4.7%) was similar to that observed with thiazide diuretic monotherapy (3.4%; relative risk = 1.36; 95% CI: 0.80–2.32), but significantly greater than that observed with aliskiren monotherapy (2.0%; relative risk = 2.28, 95% CI 1.55–3.36). In the longer-term studies, there were no differences in the risk of hypotension between aliskiren/losartan combination therapy and either monotherapy.
Subgroup Analyses
No evidence of an increased risk of adverse events with either aliskiren/thiazide diuretic or aliskiren/ARB (valsartan or losartan) combination therapy relative to the individual monotherapies was found for high-risk patient subgroups, including patients ≥ 65 years of age, those with diabetes or those with renal impairment (estimated glomerular filtration rate < 60 mL/min/1.73 m2).
DISCUSSION
Principal Findings
Our analysis shows that combination of the direct renin inhibitor aliskiren with the ARBs valsartan or losartan or a thiazide diuretic has similar tolerability to the component monotherapies. Data from 310 patient-years of aliskiren-based combination treatment in short-term studies showed that the safety and tolerability profile of aliskiren/valsartan combination therapy was similar to that of aliskiren, ARB (losartan, irbesartan or valsartan), and thiazide diuretic monotherapies. These findings were confirmed by data from longer-term studies, which provided an additional 219 patient-years of aliskiren-based combination treatment exposure. In both the short- and longer-term studies, serious adverse events and adverse events leading to study discontinuation were infrequent, and there was no clustering of events within a particular system organ class.
All Adverse Events of Special Interest
This pooled analysis assessed class-specific adverse events previously seen with agents that target the RAS.26, 28–31 In addition, evidence of renal dysfunction has been observed previously when RAS blockers have been used with diuretics and in volume-depleted individuals.24, 25 Special attention was also paid to gastrointestinal adverse events, because diarrhea and symptoms of irritable bowel syndrome have been observed in clinical studies using supratherapeutic aliskiren doses (600 mg or greater).7, 8, 32, 33
The incidence of angioedema overall was low, and thus it is difficult to draw firm conclusions from the present analysis. Data from routine post-marketing surveillance will help to clarify the study findings; however, the results are consistent with our findings from a previous analysis of the pooled database.14 Combination therapy with aliskiren was not associated with increased risk of cough compared with monotherapy, either in the short-term or longer-term studies.
Hyperkalemia is a potential concern with agents that target the RAS, particularly for patients with renal dysfunction or diabetes.34 As in the present analysis, data from a 54-week, open-label study in patients with hypertension show a low incidence of serum potassium elevations with an aliskiren/valsartan combination; the incidence of clinically relevant potassium elevations to ≥ 6 mmol/L was 1.5% and 0% in the subgroups of patients with mild and moderate renal dysfunction, respectively.35
Our analysis suggests that short- and longer-term aliskiren-based combination therapy does not increase the risk of diarrhea compared with aliskiren, ARB (valsartan, irbesartan or losartan), or thiazide diuretic monotherapy. These findings support original study findings7–11 and our previous analysis of aliskiren monotherapy.14
Limitations
A potential limitation of this analysis was that adverse events of special interest were analyzed according to the final treatment regimen to which patients were randomized; thus, patients could have experienced adverse events while on a different regimen, for example during titration periods. However, this factor probably overestimates the incidence of adverse events with combination therapy, hence does not affect our conclusion that combined therapy is well-tolerated.
Conclusions and Implications of the Findings
Single-pill combinations of aliskiren/valsartan and aliskiren/HCT are now approved treatments for hypertension in the USA. The present safety analysis of over 12,000 patients in randomized controlled clinical trials demonstrates that aliskiren in combination with HCT, valsartan, or losartan, is well tolerated and has a good safety profile in patients with hypertension, including older individuals and those with additional risk factors such as diabetes. The safety and tolerability profile of aliskiren in combination with an ARB or thiazide diuretic was similar to that of the component monotherapies. Tolerability is an important contributory factor to patient adherence, which can often present a barrier to the long-term achievement of BP goals.36, 37 Previous studies have suggested that adherence to ARBs is better than to ACE inhibitors,38–40 possibly reflecting the superior tolerability profile of the ARB class.
The results of this analysis and our previous analysis of aliskiren monotherapy14 suggest that the combination of aliskiren with valsartan or losartan, or a thiazide diuretic are safe therapies for the treatment of patients with hypertension likely to need combination therapy to achieve BP control.41, 42
Acknowledgments
The authors acknowledge Dr Anil Rustgi and Dr Stanley Hamilton for their participation on the external safety board, and Dr Annette Keith from Oxford PharmaGenesis Ltd for assistance with preparation of the tables and figures for the article.
Sources of funding: Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA.
Footnotes
DISCLOSURES
WBW, RB, APK, BFP and RHR are members of the Aliskiren Safety Monitoring Committee that meet once or twice annually, and receive financial remuneration for that role. WBW has received research and grant support from National Institutes of Health and Novartis Pharmaceuticals Corporation; and is the president-elect for the American Society of Hypertension. APK has served as a consultant to Novartis Pharmaceuticals Corporation. BFP has received speaker honoraria from Novartis Pharmaceuticals Corporation. RB and RHR declare no further conflicts of interest. AL is an employee of Novartis Pharma AG, Basel, Switzerland and WC and DLK are employees of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA; all three are thus eligible for Novartis stock and stock options.
References
- 1.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 3.Weber MA, Julius S, Kjeldsen SE, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004;363:2049–2051. doi: 10.1016/S0140-6736(04)16456-8. [DOI] [PubMed] [Google Scholar]
- 4.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008;358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 5.McMurray J, Pitt B, Latini R, et al. Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circulation: Heart Failure. 2008;1:17–24. doi: 10.1161/CIRCHEARTFAILURE.107.740704. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, Appelbaum E, Manning WJ, et al. Effect of the direct renin inhibitor aliskiren, the angiotensin receptor blocker Losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009;119:530–537. doi: 10.1161/CIRCULATIONAHA.108.826214. [DOI] [PubMed] [Google Scholar]
- 7.Littlejohn TW, III, Trenkwalder P, Hollanders G, Zhao Y, Liao W. Long-term safety, tolerability and efficacy of combination therapy with aliskiren and amlodipine in patients with hypertension. Curr Med Res Opin. 2009;25:951–959. doi: 10.1185/03007990902785845. [DOI] [PubMed] [Google Scholar]
- 8.Drummond W, Munger MA, Essop MR, Maboudian M, Khan M, Keefe DL. Antihypertensive efficacy of the oral direct renin inhibitor aliskiren as add-on therapy in patients not responding to amlodipine monotherapy. J Clin Hypertens. 2007;9:742–750. doi: 10.1111/j.1524-6175.2007.06614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villamil A, Chrysant SG, Calhoun D, et al. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens. 2007;25:217–226. doi: 10.1097/HJH.0b013e3280103a6b. [DOI] [PubMed] [Google Scholar]
- 10.Andersen K, Weinberger MH, Egan B, et al. Comparative efficacy and safety of aliskiren, an oral direct renin inhibitor, and ramipril in hypertension: a 6-month, randomized, double-blind trial. J Hypertens. 2008;26:589–599. doi: 10.1097/HJH.0b013e3282f3ad9a. [DOI] [PubMed] [Google Scholar]
- 11.Uresin Y, Taylor AA, Kilo C, et al. Efficacy and safety of the direct renin inhibitor aliskiren and ramipril alone or in combination in patients with diabetes and hypertension. J Renin Angiotensin Aldosterone Syst. 2007;8:190–198. doi: 10.3317/jraas.2007.028. [DOI] [PubMed] [Google Scholar]
- 12.Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet. 2007;370:221–229. doi: 10.1016/S0140-6736(07)61124-6. [DOI] [PubMed] [Google Scholar]
- 13.Chrysant SG, Murray AV, Hoppe UC, et al. Long-term safety, tolerability and efficacy of aliskiren in combination with valsartan in patients with hypertension: a 6-month interim analysis. Curr Med Res Opin. 2008;24:1039–1047. doi: 10.1185/030079908x280581. [DOI] [PubMed] [Google Scholar]
- 14.White W, Bresalier R, Kaplan A, et al. Safety and tolerabililty of aliskiren: a pooled analysis of clinical experience in over 12000 patients. J Clin Hypertens. 2010 doi: 10.1111/j.1751-7176.2010.00352.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Study comparing SPP100 (aliskiren) 150mg to placebo and to losartan 50mg in patients with mild to moderate essential hypertension. NCT00344110. www.clinicaltrials.gov.
- 16.Kushiro T, Itakura H, Abo Y, Gotou H, Terao S, Keefe DL. Aliskiren, a novel oral renin inhibitor, provides dose-dependent efficacy and placebo-like tolerability in Japanese patients with hypertension. Hypertens Res. 2006;29:997–1005. doi: 10.1291/hypres.29.997. [DOI] [PubMed] [Google Scholar]
- 17.Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP. Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation. 2005;111:1012–1018. doi: 10.1161/01.CIR.0000156466.02908.ED. [DOI] [PubMed] [Google Scholar]
- 18.Pool JL, Schmieder RE, Azizi M, et al. Aliskiren, an orally effective renin inhibitor, provides antihypertensive efficacy alone and in combination with valsartan. Am J Hypertens. 2007;20:11–20. doi: 10.1016/j.amjhyper.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Oh BH, Mitchell J, Herron JR, Chung J, Khan M, Keefe DL. Aliskiren, an oral renin inhibitor, provides dose-dependent efficacy and sustained 24-hour blood pressure control in patients with hypertension. J Am Coll Cardiol. 2007;49:1157–1163. doi: 10.1016/j.jacc.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 20.Puig JG, Schunkert H, Taylor A, Boye S, Jin J, Keefe D. Evaluation of the dose-response relationship of aliskiren, a direct renin inhibitor, in an 8-week, multicenter, randomized, double-blind, parallel-group, placebo-controlled study in adult patients with stage 1 or 2 essential hypertension. Clin Ther. 2009;31:2839–2850. doi: 10.1016/j.clinthera.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Efficacy and safety of aliskiren 75mg, 150mg and 300mg in elderly patients with essential hypertension when given with a light meal in a 8 week placebo-controlled study. NCT00706134. www.clinicaltrials.gov.
- 22.Schmieder RE, Philipp T, Guerediaga J, et al. Long-term antihypertensive efficacy and safety of the oral direct renin inhibitor aliskiren: a 12-month randomized, double-blind comparator trial with hydrochlorothiazide. Circulation. 2009;119:417–425. doi: 10.1161/CIRCULATIONAHA.107.750745. [DOI] [PubMed] [Google Scholar]
- 23.Duprez DA, Munger MA, Botha J, Keefe DL, Charney AN. Aliskiren for Geriatric Lowering of Systolic Hypertension (AGELESS): a randomized controlled trial. J Hum Hypertens. 2009 doi: 10.1038/jhh.2009.1107. [DOI] [PubMed] [Google Scholar]
- 24.Gavras HP. Issues in hypertension: drug tolerability and special populations. Am J Hypertens. 2001;14:231S–236S. doi: 10.1016/s0895-7061(01)02132-x. [DOI] [PubMed] [Google Scholar]
- 25.Parish RC, Miller LJ. Adverse effects of angiotensin converting enzyme (ACE) inhibitors. An update. Drug Saf. 1992;7:14–31. doi: 10.2165/00002018-199207010-00004. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg AI, Dunlay MC, Sweet CS. Safety and tolerability of losartan potassium, an angiotensin II receptor antagonist, compared with hydrochlorothiazide, atenolol, felodipine ER, and angiotensin-converting enzyme inhibitors for the treatment of systemic hypertension. Am J Cardiol. 1995;75:793–795. doi: 10.1016/s0002-9149(99)80413-5. [DOI] [PubMed] [Google Scholar]
- 27.MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD. Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: a systematic review of the efficacy and safety data. Am J Kidney Dis. 2006;48:8–20. doi: 10.1053/j.ajkd.2006.04.077. [DOI] [PubMed] [Google Scholar]
- 28.Karlberg BE. Cough and inhibition of the renin-angiotensin system. J Hypertens Suppl. 1993;11:S49–52. [PubMed] [Google Scholar]
- 29.Miller DR, Oliveria SA, Berlowitz DR, Fincke BG, Stang P, Lillienfeld DE. Angioedema incidence in US veterans initiating angiotensin-converting enzyme inhibitors. Hypertension. 2008;51:1624–1630. doi: 10.1161/HYPERTENSIONAHA.108.110270. [DOI] [PubMed] [Google Scholar]
- 30.Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–111. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Reardon LC, Macpherson DS. Hyperkalemia in outpatients using angiotensin-converting enzyme inhibitors. How much should we worry? Arch Intern Med. 1998;158:26–32. doi: 10.1001/archinte.158.1.26. [DOI] [PubMed] [Google Scholar]
- 32.Azizi M, Webb R, Nussberger J, Hollenberg NK. Renin inhibition with aliskiren: where are we now, and where are we going? J Hypertens. 2006;24:243–256. doi: 10.1097/01.hjh.0000202812.72341.99. [DOI] [PubMed] [Google Scholar]
- 33.Gradman AH, Traub D. The efficacy of aliskiren, a direct renin inhibitor, in the treatment of hypertension. Rev Cardiovasc Med. 2007;8 (Suppl 2):S22–30. [PubMed] [Google Scholar]
- 34.Weir MR, Rolfe MR. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010 doi: 10.2215/CJN.07821109. [DOI] [PubMed] [Google Scholar]
- 35.Chrysant SG, Murray AV, Hoppe UC, et al. Incidence of hyperkalemia with aliskiren/valsartan combination in hypertensiver patients with mild to moderate renal impairment and patients with normal renal function: a 54 week retrospective analysis. J Clin Hypertens. 2010;12:A30. (Abstract PO-29) [Google Scholar]
- 36.Burnier M, Hess B, Greminger P, Waeber B. Determinants of persistence in hypertensive patients treated with irbesartan: results of a postmarketing survey. BMC Cardiovasc Disord. 2005;5:13. doi: 10.1186/1471-2261-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gregoire JP, Moisan J, Guibert R, et al. Determinants of discontinuation of new courses of antihypertensive medications. J Clin Epidemiol. 2002;55:728–735. doi: 10.1016/s0895-4356(02)00400-6. [DOI] [PubMed] [Google Scholar]
- 38.Wogen J, Kreilick CA, Livornese RC, Yokoyama K, Frech F. Patient adherence with amlodipine, lisinopril, or valsartan therapy in a usual-care setting. J Manag Care Pharm. 2003;9:424–429. doi: 10.18553/jmcp.2003.9.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thaker D, Frech F, Gause D, Zhang W. Patient compliance and persistency with antihypertensive agents: A comparison of agents in different therapeutic classes. Am J Hypertens. 2005;18:222A. (Abstract P-589) [Google Scholar]
- 40.Hoer A, Gothe H, Khan ZM, Schiffhorst G, Vincze G, Haussler B. Persistence and adherence with antihypertensive drug therapy in a German sickness fund population. J Hum Hypertens. 2007;21:744–746. doi: 10.1038/sj.jhh.1002223. [DOI] [PubMed] [Google Scholar]
- 41.Gregoire JP, Moisan J, Guibert R, et al. Tolerability of antihypertensive drugs in a community-based setting. Clin Ther. 2001;23:715–726. doi: 10.1016/s0149-2918(01)80021-7. [DOI] [PubMed] [Google Scholar]
- 42.Black HR, Graff A, Shute D, et al. Valsartan, a new angiotensin II antagonist for the treatment of essential hypertension: efficacy, tolerability and safety compared to an angiotensin-converting enzyme inhibitor, lisinopril. J Hum Hypertens. 1997;11:483–489. doi: 10.1038/sj.jhh.1000482. [DOI] [PubMed] [Google Scholar]


