Abstract
Brown adipose tissue (BAT) thermogenesis is critical to maintain homoeothermia and is centrally controlled via sympathetic outputs. Body temperature and BAT activity also impact energy expenditure and obesity is commonly associated with decreased BAT capacity and sympathetic tone. Severely obese mice that lack leptin or its receptor (LepRb) show decreased BAT capacity, sympathetic tone and body temperature, and thus are unable to adapt to acute cold exposure (Trayhurn et al., 1976). LepRb expressing neurons are found in several hypothalamic sites including the dorsomedial hypothalamus (DMH) and median preoptic area (mPOA), both critical sites to regulate sympathetic, thermoregulatory BAT circuits. Specifically, a subpopulation in the DMH/dorsal hypothalamic area (DMH/DHA) is stimulated by fever inducing endotoxins or cold exposure (Dimicco and Zaretsky, 2007;Morrison et al., 2008). Using the retrograde, transsynaptic tracer pseudorabies virus (PRV) injected into the BAT of mice we identified PRV labeled LepRb neurons in the DMH/DHA and mPOA (and other sites), thus indicating their involvement in the regulation of sympathetic BAT circuits. Indeed, acute cold exposure induced cFos (as a surrogate for neuronal activity) in DMH/DHA LepRb neurons, and a large number of mPOA LepRb neurons project to the DMH/DHA. Furthermore, DMH/DHA LepRb neurons (and a subpopulation of LepRb mPOA neurons) project and synaptically couple to rostral raphe pallidus neurons, consistent with the current understanding of BAT thermoregulatory circuits from the DMH/DHA and mPOA (Dimicco and Zaretsky, 2007;Morrison et al., 2008). Thus, these data present strong evidence that LepRb neurons in the DMH/DHA and mPOA mediate thermoregulatory leptin action.
Keywords: leptin receptor, thermoregulation, brown adipose tissue, dorsomedial hypothalamus, median preoptic area, rostral raphe pallidus
Introduction
Leptin acts in the CNS to coordinate metabolism and energy balance. Deficiency of leptin or its receptor (LepRb) results in profound neuroendocrine failure, hyperphagia and autonomic dysfunction (Ahima et al., 2000;Hausberg et al., 2002;Ozata et al., 1999). This results in severe thermogenic defects and the inability to adapt to acute cold exposure due to decreased brown adipose tissue (BAT) function (Himms-Hagen, 1985;Trayhurn et al., 1976;Trayhurn et al., 1977). However, little is known about the neuronal circuits involved in thermogenic leptin action.
Several central components that regulate sympathetic, thermogenic BAT circuits have been unraveled. The median preoptic area (mPOA) plays a key role and a distinct set of mPOA neurons projects directly to premotor neurons in the medullary rostral raphe pallidus (rRPa) to regulate sympathetic BAT inputs (Yoshida et al., 2009). However, the dorsomedial hypothalamus (DMH) is required to mediate mPOA evoked thermoregulatory responses (Dimicco and Zaretsky, 2007). Specifically, neurons bordering the DMH and dorsal hypothalamic area (DMH/DHA) receive inhibitory inputs from the mPOA (Nakamura et al., 2005), and systemic inflammation and cold exposure both activate DMH/DHA neurons due to decreased inhibitory mPOA inputs (Cano et al., 2003;Elmquist et al., 1996;Sarkar et al., 2007).
At least two thermoregulatory circuits originate from DMH neurons, a direct projection to the rRPa from DMH/DHA neurons (Sarkar et al., 2007;Yoshida et al., 2009) and projections to the paraventricular nucleus (PVN) possibly regulating neuroendocrine and/or preautonomic outputs (Hunt et al., 2009;Ter Horst and Luiten, 1986;Thompson et al., 1996;Zaretskaia et al., 2008).
Leptin target neurons that express LepRb are found in several hypothalamic and extra-hypothalamic sites including a large population in the DMH as well as in the mPOA (Myers, Jr. et al., 2009). Thus, we hypothesized that at least a subpopulation of these LepRb neurons represent known thermoregulatory neurons that project to the rRPa or PVN as described above.
Here, we provide clear evidence that LepRb neurons in the DMH/DHA and mPOA (and other sites) are involved in sympathetic, thermoregulatory BAT circuits, by analyzing LepRb neurons that were transsynaptically and retrogradely traced with pseudorabies virus (PRV) injected into the BAT. Further studies revealed that LepRb neurons in the DMH/DHA (but not the mPOA) were stimulated by acute cold exposure (as measured by cFos-induction) and project to the rRPa, but not the PVN.
A large number of mPOA LepRb neurons was found to project directly to the DMH/DHA, suggesting their interaction with LepRb neurons in the DMH/DHA. mPOA LepRb neurons also projected to the rRPa. Furthermore, using LepRb specific expression of an anterograde, transsynaptic tracer we demonstrated that LepRb neurons indeed synaptically couple with neurons in the rRPa. Thus, indicating that leptin acts on several neuronal populations critical for thermoregulatory control.
Therefore, we show strong evidence for an important role of leptin in the control of sympathetic, thermoregulatory BAT circuits. The identification of LepRb neurons in thermoregulatory circuits further raises the important question to what extent thermoregulatory leptin action contributes to the severe obesity in leptin or LepRb deficient mice.
Materials and Methods
Animals
LepRbcre/cre, double homozygous LepRbcre/cre, Gt(ROSA)26Sortm2Sho/tm2Sho (LepRbEGFP) and LepRbWGA mice were generously provided by Dr. Martin G. Myers (University of Michigan, MI) and were described in detail elsewhere (Faouzi et al., 2007;Leinninger et al., 2009;Leshan et al., 2006;Leshan et al., 2009;Leshan et al., 2010). Briefly, LepRbcre/cre mice express an internal ribosomal entry site (IRES)-driven second cistron that encodes cre recombinase from the LepRb specific exon of the Lepr gene. In LepRbEGFP reporter mice LepRb specific cre-recombinase was used to drive the cre-inducible expression of enhanced green fluorescent protein (EGFP) from the rosa locus, thus limiting EGFP expression to LepRb neurons (Leshan et al., 2006). LepRbWGA mice were generated from crossing LepRbcre/cre mice with mice that carry a ubiquitously expressed CMV promoter driven cre-inducible wheatgerm agglutinin (WGA) transgene (iZ/WAP mice), thus resulting in mice that express WGA in LepRb neurons (LepRbWGA mice)(Leshan et al., 2010). LepRbcre/cre mice were used for adenoviral tracing experiments, LepRbEGFP mice for PRV, acute cold exposure and LepRb axonal fiber studies. LepRbWGA mice were used in second order neuron studies and wildtype FVB mice were used for fluorogold tracing studies. Male and female mice were used in studies and no differences between sexes were observed. All animals were in-house bred and group housed at a 12:12h light:dark cycle with ad lib access to food and water unless stated otherwise. All animal experiments were approved by the institutional animal care and use committee.
Pseudorabies virus (PRV) infection of BAT
The retrograde and transsynaptic tracer pseudorabies virus has been extensively used in the literature to visualize neuronal populations that are involved in the autonomic regulation of peripheral organs including the exclusively sympathetically innervated BAT (Bamshad et al., 1999;Kerman et al., 2006;Voss-Andreae et al., 2007). To identify LepRb neurons that are involved in sympathetic BAT regulation, we injected a DsRed expressing PRV614 (Banfield et al., 2003), kindly provided by the National Center for Experimental Neuroanatomy with Neurotropic Viruses) into the BAT of LepRbEGFP reporter mice. Mice were anesthetized with isofluorane/oxygene and BAT was exposed by an intrascapular incision. PRV614 (viral titer: 1×109 pfu/ml) was then injected with a pulled glass tip attached to a 0.5μl Hamilton syringe. Five separate injections each of 50nl were distributed into the right interscapular BAT depot, injection sites were dried off to prevent any systemic leakage of the virus. Mice were single housed post viral infection for 60 (n=2) and 96 hours (n=4) then perfused and processed as described below. Brains and spinal cords were removed, postfixed in formalin, cryoprotected in 30% sucrose until further analysis. The spinal cords of the 60h postinfected mice were immunohistochemically stained for cholinacetyltransferase (ChAT) and PRV; brains were processed for EGFP (LepRb) and PRV (DsRed).
Cold-induced cFos expression in LepRb neurons
To further identify if LepRb neurons in the DMH are involved in thermoregulatory responses we investigated if LepRb neurons are stimulated by acute cold-exposure. To do this, six LepRbEGFP reporter mice were single housed and food was removed to prevent randomized feeding bouts. Three of these mice were then exposed to 4°C for 3 hours and the other 3 mice remained at room temperature (21°C). For cold exposure mice had to be moved to a new room in their home cages, thus the control group was similarly moved to a new room, to control for any handling based cFos induction. Brain sections were processed for cFos (as a surrogate for neuronal activity) and EGFP (to identify LepRb neurons) and the number of LepRbEGFP neurons colocalized with cFos in the DMH/DHA was estimated between groups, as described below.
Stereotaxic injections of Ad-iZ/EGFPf into the DMH
To investigate the projection sites of axonal and dendritic processes from LepRb neurons in the DMH we used a LepRb specific viral tracer (Ad-iZ/EGFPf, kindly provided by Dr. Martin G. Myers, University of Michigan, MI) that expresses farnesylated EGFP upon cre-recombination in LepRbcre/cre mice, as previously described (Leinninger et al., 2009;Leshan et al., 2009). The farnesylation anchors EGFP to the cell membrane and thus increases the visualization of thin axonal processes. For stereotaxic injections LepRbcre/cre mice were anesthetized with isofluorane/oxygen and the head was mounted in a stereotaxic frame (Kopf instruments, Tujunga, CA). An incision was made to expose the skull and an access hole was drilled at coordinates 1.4mm caudal and 0.3mm lateral to Bregma and a guide cannula was inserted 4.2mm ventral to Bregma. An injector filled with Ad-iZ/EGFPf and attached to a 0.5ul Hamilton Syringe was inserted in the guide and a volume of 400nl was slowly infused in the DMH tissue (10nl/30s). Guide and injector were removed, the skull access was sealed with bone wax and the skin closed with wound clips. Analgetics were applied once to the incision site (bupivacaine/lidocaine, 5mg/kg) and subcutaneously during recovery every 12 hours for 2 days (buprenorphine, 10μg/kg). After the surgery mice were single housed for five days to allow for viral EGFPf expression before transcardial perfusion with 10% neutral buffered formalin to collect brains for further analysis.
Retrograde tracing experiments with Fluorogold
To investigate the exact anatomical distribution of LepRb neurons that project to the PVN, rRPa and DMH/DHA we stereotaxically injected mice with the retrograde tracer fluorogold (FG, Hydroxystilbamid, Invitrogen, Carlsbad, CA) into the PVN, the rostral medullary raphe (RMR)(which includes the rRPa) and the DMH/DHA. Surgical procedures were similar as described above; however, for increased stereotaxic precision we used the stereotaxic alignment system (Kopf instrument, Tujunga, CA). In each case we injected 10nl of a 2% FG solution in sterile saline into the PVN (0.8mm caudal, 0.25mm lateral, 5mm ventral to Bregma), RMR/rRPa (6.0mm caudal, 0mm lateral, 5.75mm ventral to Bregma), DMH/DHA (1.8mm caudal, 0.25mm lateral, 5mm ventral to Bregma). After the surgery animals were single housed and FG transport was allowed for 5 days. Animals received an intraperitoneal leptin injection (from National Hormone and Pituitary Program (NHPP) and Dr. Parlow, Harbor-UCLA Medical Center, CA) 1h before perfusion to allow later immunohistochemical identification of LepRb neurons by staining for leptin induced STAT3 phosphorylation (pSTAT3).
Perfusion, Immunohistochemistry (IHC)
For immunohistochemical analysis mice were deeply anesthetized with an overdose of pentobarbital (4mg/kg) and perfused with saline followed by 10% formalin via the left ventricle. Brains or spinal cords were removed, postfixed in formalin and cryoprotected in 30% sucrose. Brains were sliced into 4 series of 30μm sections and processed for free-floating IHC as described earlier (Faouzi et al., 2007). Briefly, sections were pre-treated consecutively with H2O2 in ice cold methanol, 0.3% glycine, 0.1% SDS for pSTAT3-IHC and/or blocked in 3% normal donkey serum then incubated with primary antibodies (rabbit anti-pSTAT3, 1:1000, Cell Signaling; rabbit anti-cFos, 1:5000, EMD; chicken anti-GFP, 1:1000, Abcam; rabbit anti-DsRed, 1:1000, Clonetech, goat anti-beta-galactosidase, 1:3000, Corvance; goat-anti ChAT, 1:1000, Millipore; goat anti-WGA, 1:1000, Vector laboratories, mouse anti-NeuN, 1:1000, Millipore) overnight at room temperature or for 2 days at 4°C. Sections were then washed and incubated in secondary antibodies either labeled with Alexa fluorophores (Invitrogen, Carlsbad, CA) or with Biotin for subsequent visualization with the diaminobenzine procedure.
Analysis of data and quantification of neurons
IHC staining was visualized with a fluorescent microscope (Olympus BX51, Melville, NY) and images were taken with a digital camera (Olympus DP30BW, Melville, NY) using appropriate filters for different fluorophores or brightfield illumination for DAB stain. Identical images were taken for double IHC, overlayed and pseudocolored using Olympus Software (Melville, NY) and Adobe Photoshop (Adobe Systems, San Jose, CA). Contrast and brightness were adjusted for fluorescent signals with Adobe Photoshop (Adobe Systems, San Jose, CA) for better visualization of neurons.
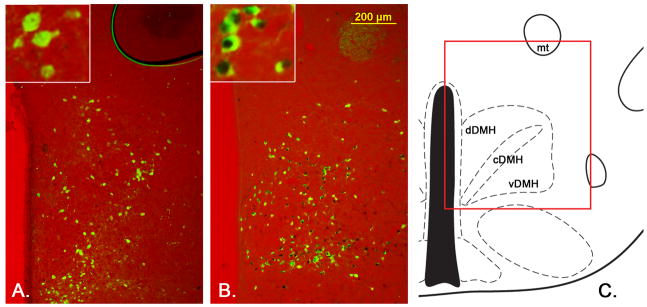
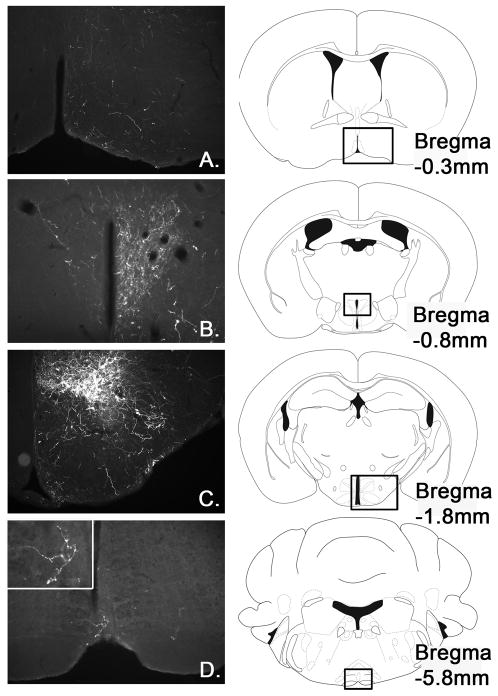
For Ad-iZ/EGFPf tracing from LepRb neurons in the DMH we analyzed 4 mice with correct injections into the DMH and showed representative images of projection sites in the mPOA, DMH, PVN and rRPa in Figure 4.
Figure 4.
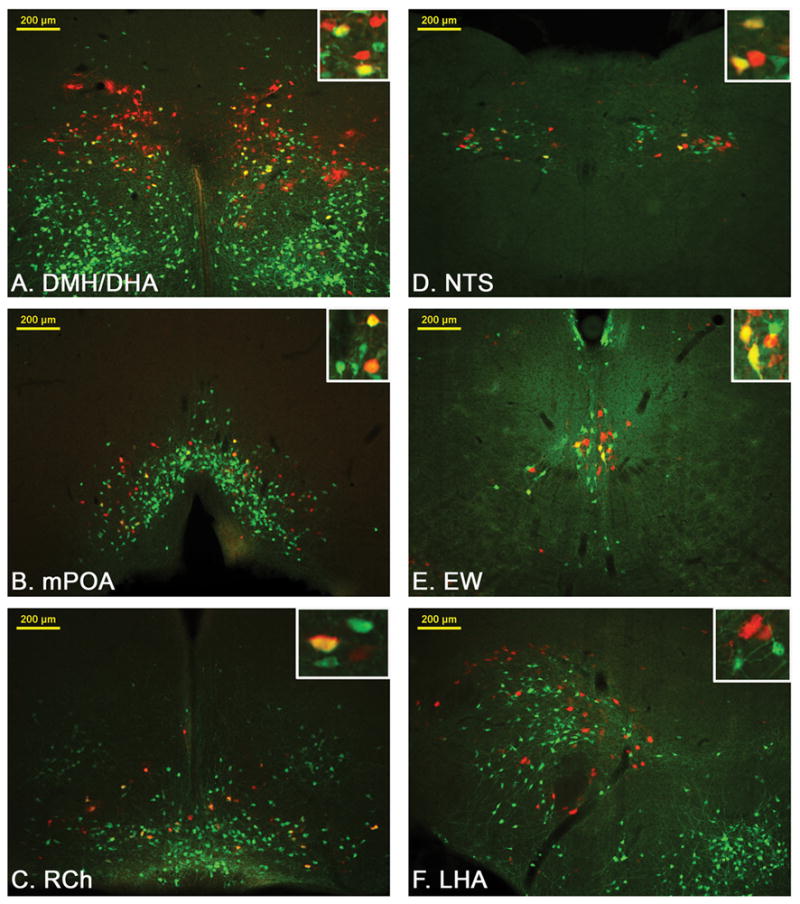
Representative images from LepRbEGFP mice 96hrs after PRV infection of the BAT. Transsynaptically and retrogradely PRV (red stain) traced LepRbEGFP (greed stain) neurons are found in the DMH/DHA (A.), mPOA (B.), RCh (C.), NTS (D.) and EW (E.). Other PRV and LepRb positive sites like the lateral hypothalamic area LHA (F.) did not show co-localized neurons.
For FG tracing experiments we focused our analysis on the mPOA and DMH/DHA to investigate co-localization of LepRb neurons with FG traced neurons from the RMR/rRPa (n=4), DMH/DHA (n=3) or PVN (n=2). Representative images were shown for the DMH/DHA and mPOA and in some cases we generated schematic drawings of the images to emphasize the number and distribution of co-localized LepRb/FG neurons compared to the remaining single labeled LepRb neurons.
Estimates of cell counts were performed as described earlier (Munzberg et al., 2007). Briefly, all images were taken as described above with all settings identical (exposure time, brightness, and contrast) to ensure the comparability between images of different sections and animals.
To quantify the number of leptin induced pSTAT3 neurons that are co-localized with LepRbEGFP in the DMH we counted cells in one LepRbEGFP mouse brain after a 1h leptin stimulation. Images of the DMH were taken between Bregma levels −1.8 mm to −2.0 mm resulting in 3 DMH section and cell counts were performed bilaterally, including the vDMH, cDMH, dDMH and DHA. The mean number of pSTAT3/EGFP double-labeled neurons between the DMH sections was expressed as the percentage of the total number of pSTAT3 positive neurons.
For PRV experiments we analyzed all LepRb expressing sites for their co-localization with PRV. The relative PRV infection, abundance of LepRb expressing neurons as well as co-localized neurons were evaluated in 3 brains (one brain was excluded due to GFP leakage and thus unsufficient GFP staining) and reported in table 1. To further quantify the number of PRV/EGFP co-labeled neurons we took images of three PRV614 injected mouse brains. All sites showing PRV/LepRb labeling were quantified. The following number of sections were taken and quantified for the specific sites: 1–2 sections in the mPOA, 1 section in the DMH/DHA (between Bregma level −1.8mm to −1.9mm), 1 section in the RCh, 3–4 sections in the EW, 1–2 sections in the NTS. The number of PRV/LepRb co-labeled neurons was expressed as percentage of the total number of PRV labeled neurons in those sites.
Table 1.
Rating of PRV infected LepRbEGFP neurons from the BAT
| PRV positive neurons | LepRb positive neurons | LepRb/PRV positive neurons | |
|---|---|---|---|
| Hypothalamus | |||
| Median preoptic area | ++ | +++ | ++ |
| Paraventricular nucleus | +++++ | ++ | −/+ |
| Retrochiasmatic area | ++ | +++ | ++ |
| Arcuate nucleus | ++ | ++++ | + |
| Lateral hypothalamic area | ++ | ++++ | −/+ |
| DMH/DHA | +++ | ++++ | ++ |
| Ventromedial hyp. | + | +++ | − |
| Premammillary nuc., ventral | −/+ | ++++ | −/+ |
| Midbrain | |||
| Edinger westphal | ++ | ++ | ++ |
| Ventral tegmental area | ++ | ++ | − |
| Red nucleus | ++ | − | − |
| Periaqueductal gray | ++ | ++ | −/+ |
| Doral raphe | ++ | ++ | − |
| Medulla | |||
| Parabrachial nuc., lateral | ++ | ++ | −/+ |
| Locus coerelius | +++++ | − | − |
| Rostal ventrolateral medulla | +++ | − | − |
| Raphe pallidus | ++++ | − | − |
| Raphe obscurus | ++++ | − | − |
| Nucleus of the solitary tract | ++ | +++ | ++ |
only scattered neurons on one section,
scattered neurons on several sections or a cluster of neurons on one section,
clusters of neurons on several sections,
heavy clustering on several sections,
very heavy labeling and clustering of neurons on multiple sections
VMH=ventromedial hypothalamus, PMV=ventral premammillary nucleus, VTA=ventral tegmental area, RVLM= rostral ventrolateral medulla, RPa=raphe pallidus, ROb=raphe obscurus
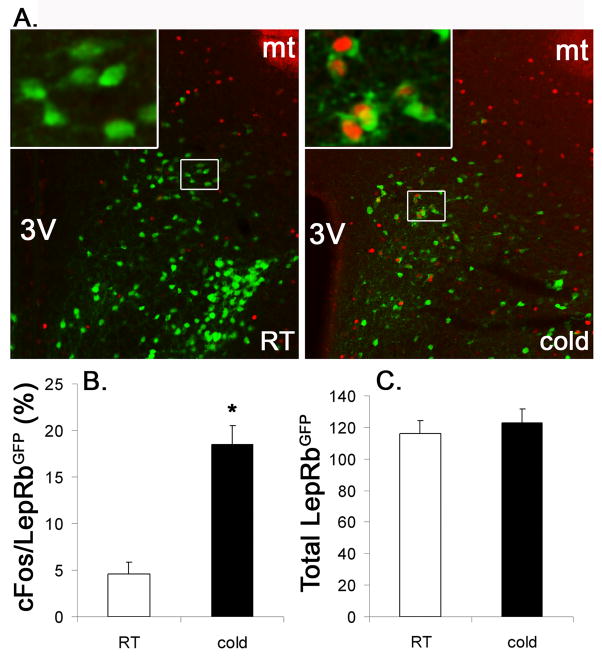
To quantify cold induced cFos in LepRbEGFP neurons images of 5–6 animals per group (room temperature (n=5), 3h cold exposure (n=6)) were taken. For the DMH/DHA area one image per brain was used for cell counts and LepRb neurons were counted in total as well as for their co-localization with cFos. We did not quantify co-localized cFos/LepRb neurons in the mPOA, as we could not identify co-localized cFos/LepRb neurons in either group (data not shown). The total number of counted LepRb neurons was presented to demonstrate the comparison of similar LepRb populations between animals. The number of cFos co-localized LepRb neurons was expressed as percentage of the total number of LepRb neurons in the quantified area. Statistical significance was tested with a Student’s t-test and a statistical significance was accepted at p<0.05. These cell counts were not meant to give precise cell numbers of stimulated LepRb neurons in the DMH/DHA, but served as estimates for cold induced neuronal activity in LepRb neurons in the DMH/DHA compared to the group kept at room temperature.
Results
Anatomical outline of LepRb neurons in the DMH, DHA and mPOA
In this study we describe a population of LepRb neurons at the border of the DMH and DHA (DMH/DHA). In the mouse brain atlas (Paxinos and Franklin, 2004) the DHA is referred to as posterior hypothalamus and describes the area between the DMH and mammillothalamic tract (mt), whereas in the rat brain altas (Paxinos and Watson, 1998) the rostral portion of the PH is specifically described as DHA. In the rat DMH/DHA neurons have been particularly highlighted to project to the raphe pallidus (Hermann et al., 1997;Hosoya et al., 1987;Hosoya et al., 1989) and to be important in thermoregulatory control (Dimicco and Zaretsky, 2007;Morrison et al., 2008). Our findings on LepRb DMH/DHA neurons as outlined below show close similarity to the excitability and connectivity demonstrated in the rat, thus in this manuscript we adopted the rat terminology of DMH/DHA neurons.
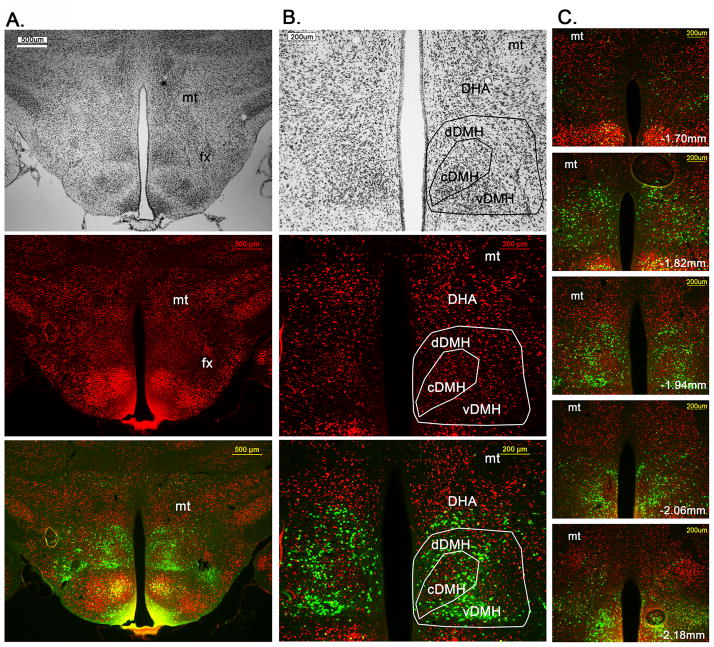
In order to clarify the anatomical localization of LepRb neurons within the mouse DMH and DHA we counterstained brains from LepRbEGFP mice for GFP and the neuronal marker NeuN and further processed adjacent sections for Nissle stain (Figure 1). In an overview (Figure 1A) the distribution of LepRbEGPF neurons compared to common neuroanatomical landmarks such as mamillo-thalamic tract (mt) and fornix (fx) is demonstrated. The middle portion of the DMH can be subdivided into 3 distinct areas based on the mouse brain atlas (Paxinos and Franklin, 2004), whereas the most rostral (Figure 1C, Bregma −1.7mm) and caudal portions (Figure 1C, Bregma −2.18mm) are simply referred to as DMH. The compact DMH is named after its denser neuronal aggregation, which is recapitulated with NeuN and Nissle stain (Figure 1A,B). The ventral and dorsal DMH lay below and above the cDMH, respectively, and show less dense NeuN labeling. Laterally the DMH is bordered by the perifornical area and lateral hypothalamus and the fornix serves as a general landmark to visualize the separation of DMH and lateral hypothalamus. The vDMH is ventrally delimited by the ventromedial hypothalamus, whereas the dDMH is dorsally confined by the DHA. The DHA is described as the area between the mt and the dDMH.
Figure 1.
Neuroanatomical localization of LepRb neurons in the DMH. A and B. Immunohistochemical staining of a LepRbEGFP mouse brain for Nissle (top), NeuN (middle) conterstained with LepRbEGFP (bottom). In the overview (A) the outline of the DMH with regard to the 3V, mt and fx are shown. In the high magnification (B.) the compact DMH is seen by the more cell dense area in the DMH and dDMH and vDMH are located dorsal and ventral to the cDMH, respectively. The DHA is defined as the area between the mt and dDMH. C. Rostral to caudal extend of LepRb neurons in the DMH in relation to the neuronal marker NeuN and mt as a landmark. dDMH=dorsal DMH, cDMH=compact DMH, vDMH=ventral DMH, DHA=dorsal hypothalamic area, mt=mammillothalamic tract, fx=fornix
LepRb neurons are most dense and abundant in the vDMH, but are also found in the dDMH and cDMH, however a noticeable area with less LepRb neurons is found in the cDMH. LepRb neurons in the dDMH extend further into the dorsal portion of the DHA and this population is referred to as DMH/DHA LepRb neurons. In Figure 1C the rostro-caudal extent of LepRb neurons in the DMH is shown including the mt as anatomical landmark. We found that the distribution of LepRb neurons recapitulate the anatomical outline of the DMH sufficiently as defined by NeuN staining. Thus, in the following experiments we use the distribution of LepRb neurons in the DMH/DHA and the mt as anatomical landmarks.
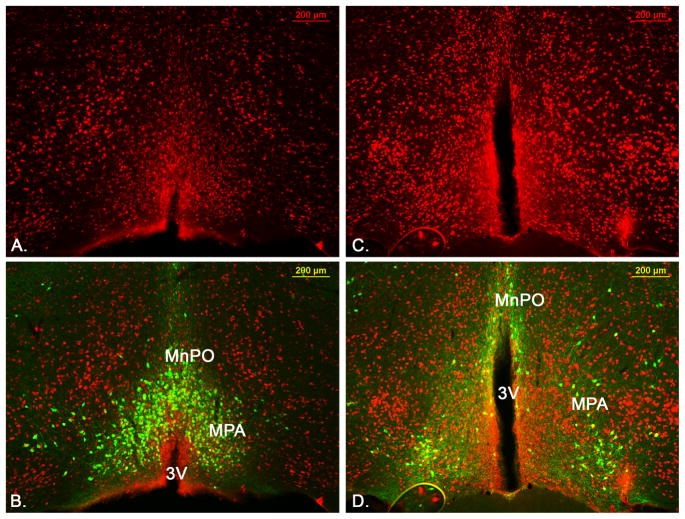
We further investigate the projections of LepRb neurons in the preoptic area, which consists of several specific subregions. The LepRb population investigated in this study is located between Bregma 0.65mm to 0.25mm according to the coordinates in the mouse brain atlas (Paxinos and Franklin, 2004) in the median preoptic nucleus (MnPO) and medial preoptic area (MPA) (Figure 2) that we collectively refer to as median preoptic area (mPOA).
Figure 2.
Neuroanatomical location of LepRb neurons in the mPOA. A and B show two mPOA levels in a LepRbEGFP mouse stained for LepRbEGFP and the neuronal marker NeuN. LepRb neurons are found in the MPA and MnPO (combined referred to as mPOA). MnPO=median preoptic nucleus, MPA=median preoptic area
Detection of LepRb neurons in the DMH and mPOA
Immunohistochemical detection of LepRb with specific antibodies has been difficult in the past and commercially available antibodies either are unspecific or lack sensitivity for immunohistological detection. LepRb signals via the JAK2/STAT3 signaling pathway and leptin induced pSTAT3 has been proven an excellent marker of functional LepRb neurons in the hypothalamus and has been used extensively by us and others (Faouzi et al., 2007;Dhillon et al., 2006;Faouzi et al., 2007;Hosoi et al., 2002;Munzberg et al., 2003). In example, whereas little baseline pSTAT3 is seen in vehicle treated mice, leptin treatment induced pSTAT3 in a typical pattern similar to the expression of LepRb. In contrast, even though pSTAT3 can be induced by many other pathways, e.g. inflammatory cytokines (Rummel et al., 2005), LepRb deficient mice lack baseline and leptin induced pSTAT3 immunoreactivity (Faouzi et al., 2007). Furthermore, neuron-specific deletion of long form leptin receptors resulted accordingly in neuron-specific absence of leptin induced pSTAT3 (Dhillon et al., 2006), thus further indicating that leptin induced pSTAT3 indeed is a correlate of direct LepRb function rather than secondary events. However, in some cases the required leptin stimulation may interfere with the experimental outcome (e.g. induction of neuronal activity). Thus, the use of reporter mice with conditional EGFP expression in LepRb neurons (LepRbEGFP mice) has been a valuable additional approach to detect LepRb in non-treated animals (Leinninger et al., 2009;Leshan et al., 2006;Leshan et al., 2009).
LepRbEGFP reporter mice do not express EGFP in all leptin induced pSTAT3 neurons likely due to cre-recombination efficiency as the number of LepRbEGFP neurons increases with alleles for cre and loxP. Thus, we use double homozygous LepRbEGFP reporter mice in our studies, where about 80% or 96% of all leptin induced pSTAT3 neurons are co-localized with LepRbEGFP as evaluated in the lateral hypothalamus and ventral premammillary nucleus, respectively (Leinninger et al., 2009;Leshan et al., 2009). In order to validate in the DMH that the majority of pSTAT3 neurons recapitulate EGFP reporter expression, we quantified the number of neurons showing LepRbEGFP expression and leptin induced pSTAT3 (after 1h leptin treatment) in the DMH and found as well that the majority (72± 2.4%) of leptin induced pSTAT3 neurons are co-localized with EGFP (Figure 3B). Thus, leptin induced pSTAT3 and LepRbEGFP are both valid methods to detect central LepRb neurons.
Figure 3.
LepRbEGFP mice treated with PBS (A) or leptin (1mg/kg, B) and stained for pSTAT3 (nuclear black stain) and EGFP (green) to demonstrate leptin-induced pSTAT3 in LepRbEGFP neurons (n=3). C. Schematic drawing of the area in A and B.
LepRb neurons in the DMH, mPOA, retrochiasmatic area (RCh), edinger westphal nucleus (EW) and nucleus of the solitary tract (NTS) are involved in sympathetic BAT circuits
PRV has been extensively used in the literature to label neuronal populations in the brainstem and forebrain that are involved in sympathetic BAT circuits (Bamshad et al., 1999;Cano et al., 2003;Voss-Andreae et al., 2007) and this technique has helped to characterize neuronal populations in the DMH/DHA and mPOA as important regulator of thermogenesis (Dimicco and Zaretsky, 2007;Nakamura et al., 2004). While some of these neuronal circuits have been well characterized over the recent years (Dimicco and Zaretsky, 2007;Morrison et al., 2008;Yoshida et al., 2009), much less is known about endogenous regulators of these neuronal circuits.
Leptin has been repeatedly noted to be involved in thermoregulatory events, e.g. leptin deficiency and lack of LepRb results in an inability to adjust to acute cold exposure resulting in severe hypothermia and even death (Trayhurn et al., 1976;Trayhurn et al., 1977). However, very little is known about the neuronal circuits involved.
LepRb neurons are found in the DMH/DHA and mPOA and we thus hypothesized that leptin action in the DMH/DHA and mPOA might be involved in its thermoregulatory functions. To identify LepRb neurons involved in sympathetic BAT circuits we injected PRV into the BAT of LepRbEGFP mice for 60 hours to confirm specificity of PRV infection to the BAT and an extended time of 96 hours to capture the maximal PRV infection of LepRb neurons.
After 60hrs of PRV infection, PRV was found confined to the thoracic portion of the spinal cord (Supplement Figure 1A,B) specifically in the preganglionic intermediolateral bundle (IML), as identified by the expression of cholinacetyl transferase (ChAT). All PRV infected neurons in the spinal cord after 60hrs were co-localized with ChAT indicating exclusive PRV infection to the IML (Supplement Figure 1C).
After 96 hrs of PRV infection we found typical infection patterns in the brain that have been described in detail by others (Bamshad et al., 1999;Cano et al., 2003;Voss-Andreae et al., 2007), generally in the brainstem (raphe nuclei), midbrain (red nucleus, periaqueductal grey) and hypothalamus (PVN, mPOA, DMH/DHA, RCh) and a general rating of most of these areas is reported in Table 1.
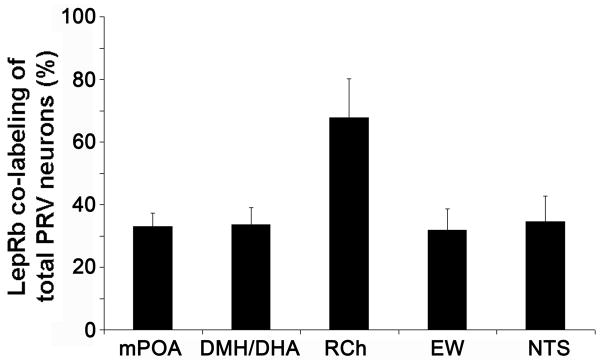
We then analyzed all LepRb expressing brain sites for PRV infections and found retrogradely PRV traced LepRb neurons in the DMH/DHA (34±5 % of total PRV neurons) and mPOA (33±4 % of total PRV neurons)(Figure 4A,B and 5). PRV infection of the DMH/DHA was typically restricted to the rostral DMH/DHA (corresponding to Bregma level −1.82 to −1.94 mm), which has been also reported by others (Cano et al., 2003), and did not extend to the mt, accordingly PRV/LepRb neurons were found in the rostro-dorsal portion of the DMH/DHA (Figure 1A). We also consistently found PRV traced LepRb neurons in the RCh, NTS and EW (68±12 %, 32±7 % and 35±8 % of total PRV neurons, respectively; Figure 4C–F and 5), which have not been specifically investigated as important thermoregulatory sites in the literature and will need further investigation for their role in thermoregulatory leptin action.
Figure 5.
Quantification of PRV/LepRbEGFP positive neurons. Percentage of double labeled neurons compared to total PRV labeled neurons in different brain sites (n=3).
In contrast, several other sites with both LepRb expression and PRV labeling (e.g. the PVN, lateral hypothalamic area, periaqueductal grey, parabrachial nucleus), did not show any co-localization of LepRb and PRV, even though an occasional PRV infected LepRb neuron was found in these sites (Table 1). However, as found by others (Cano et al., 2003) PRV injection from the BAT did vary between animals, e.g. PRV infection of LepRb neurons in the DMH/DHA varied from 15 to 50% in the analyzed brains. Thus, a lack of PRV/LepRb co-localization cannot completely rule out their involvement in thermoregulatory circuits.
Acute cold exposure stimulates LepRb neurons in the DMH/DHA, but not LepRb neurons in the mPOA
Recent work suggests that thermoregulatory neurons in the DMH/DHA receive inhibitory inputs from the mPOA and that DMH/DHA neurons directly project to the raphe pallidus in the medulla (Dimicco and Zaretsky, 2007;Morrison et al., 2008). These neurons are differentially regulated by acute cold exposure, with the mPOA being inhibited, thus reducing GABAergic inputs onto DMH/DHA neurons and consequently leading to a stimulation of DMH/DHA neurons upon cold exposure (Cano et al., 2003;Nakamura et al., 2005;Yoshida et al., 2009).
We used this paradigm to further strengthen our findings that LepRb neurons in the DMH/DHA and mPOA represent these thermoregulatory populations and investigated if LepRb neurons in the DMH/DHA, but not the mPOA, are stimulated by acute cold exposure. Indeed, 3 hrs cold exposure significantly induced cFos (as a surrogate for neuronal activity) in LepRb neurons within the DMH/DHA (Figure 6), but not in LepRb neurons in the mPOA as well as the remaining DMH (data not shown), thus further indicating a significant involvement of leptin signaling in thermoregulatory circuits in the DMH/DHA and mPOA.
Figure 6.
A. Immunohistochemistry of cFos induction (red) in LepRbEGFP neurons (green) after 3hrs acute cold exposure (n=6) compared to room temperature (RT) conditions (n=5). B. Percentage of co-localized cFos/ LepRbEGFP neurons relative to total LepRbEGFP neurons in the DMH/DHA at room temperature versus 3hrs acute cold exposure. C. Total number of LepRbEGFP neurons in the DMH/DHA of mice kept at room temperature or after 3hrs cold exposure. *pt-test<0.00000001
LepRb projections from the DMH
Blocking of GABAergic inputs to the DMH results in increased BAT thermogenesis (Zaretskaia et al., 2002) and inhibition of DMH neurons prevents BAT thermogenesis (Nakamura et al., 2005;Zaretskaia et al., 2003) which involves at least in part signaling via cold responsive neurons in the DMH/DHA that project to the rRPa (Dimicco and Zaretsky, 2007). However, DMH neurons also densely innervate the PVN to regulate neuronal activity (Bailey and Dimicco, 2001;Ter Horst and Luiten, 1986;Thompson et al., 1996;Zaretskaia et al., 2002), even though the involvement of this circuit in thermoregulation is less clear.
Thus, we aimed to investigate the neuronal projections specifically from LepRb neurons in the DMH via targeting a cre-inducible EGFP adenoviral vector to the DMH of LepRbcre/cre mice. The additional farnesylation of EGFP (EGFPf) in this viral construct targeted EGFP to the cell membrane for enhanced visualization of thin axonal projections.
Indeed, we found that LepRb neurons from the DMH densely project to the PVN, as well as sparse but consistent projections to the rRPa. Furthermore, we found terminal fields in the mPOA, bed nucleus of stria terminalis, lateral septum, RCh, arcuate nucleus (ARC), periaqueductal gray as well as within the DMH (Figure 7 and data not shown). Collectively, these data suggest that thermoregulatory LepRb neurons in the DMH indeed may interact via the rRPa and/or PVN, while also interacting with several hypothalamic sites that are likely not all involved in thermoregulatory function.
Figure 7.
Representative images of a LepRbcre/cre mouse with DMH specific injection of cre-inducible Ad-iZ/EGFPf (n=4). Immunohistochemical detection of EGFPf shows projections into the mPOA (A.), PVN (B.), DMH (C.) and rRPa (D).
LepRb neurons in the DMH/DHA and mPOA innervate the raphe pallidus
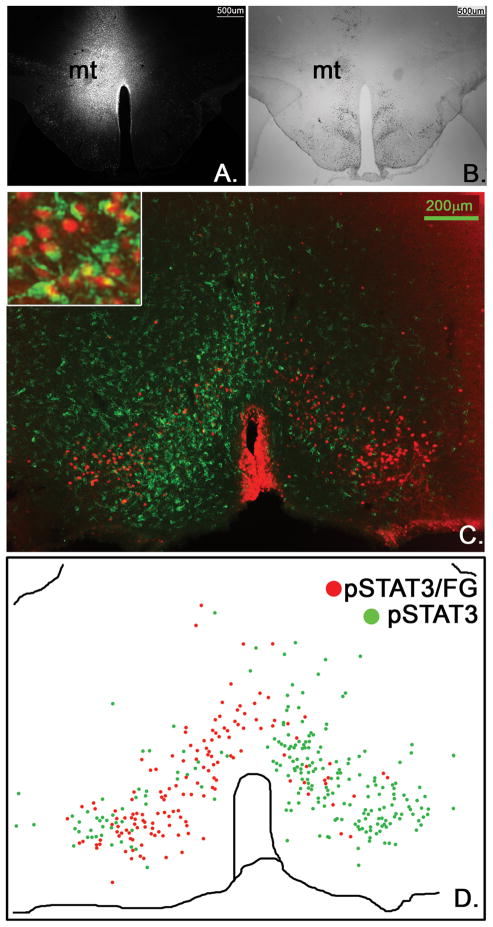
In order to further distinguish if LepRb neurons in the DMH/DHA project to the rRPa or the PVN, we injected the retrograde tracer fluorogold (FG) into the RMR (including the rRPa, Figure 8A) or PVN (Figure 10A).
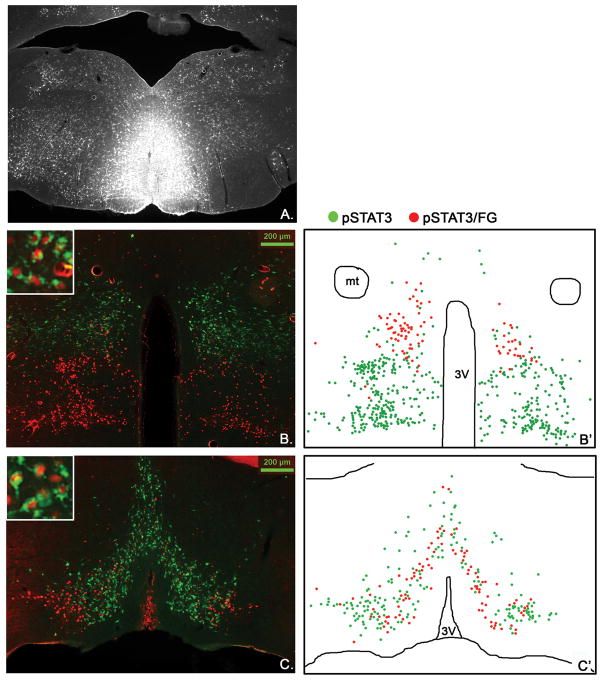
Figure 8.
Stereotaxic injection of the retrograde tracer FG into the RMR (A, n=4) and immunohistochemical detection of retrogradely labeled FG (green) in LepRb neurons (leptin-induced pSTAT3, red) in the DMH/DHA (B.) and mPOA (C.). Schematic drawings of double labeled FG/pSTAT3 neurons (red dots) in contrast to non-FG labeled pSTAT3 neurons (green dots) in the DMH/DHA (B′) and mPOA (C′).
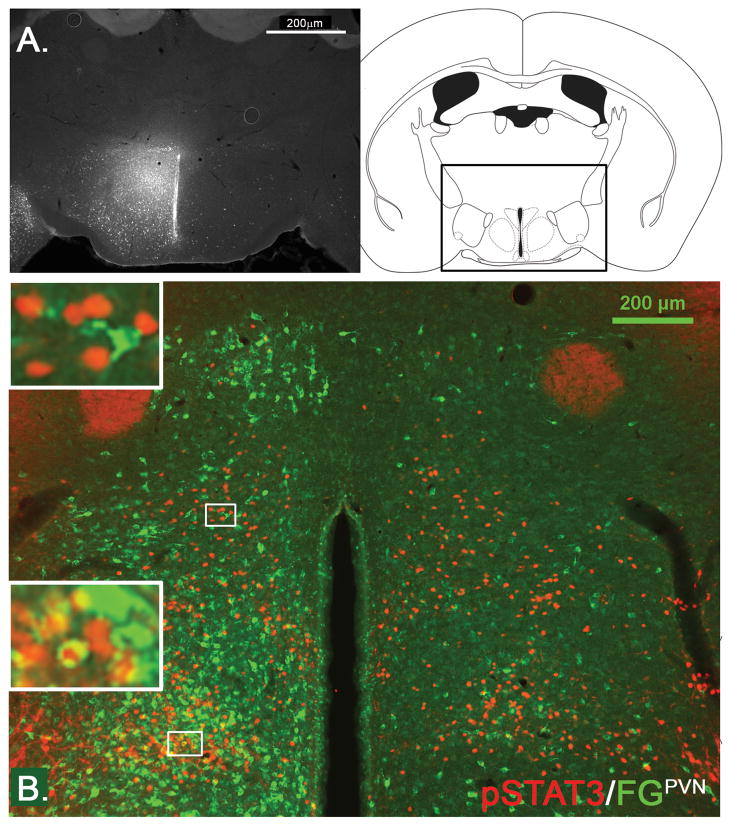
Figure 10.
Stereotaxic injection of the retrograde tracer FG into the PVN (A, n=2) and immunohistochemical detection of retrogradely labeled FG (green) in LepRb neurons (leptin-induced pSTAT3, red) in the DMH/DHA (B.).
We found several LepRb DMH neurons retrogradely labeled from the PVN, however, none were found in the DMH/DHA area (Figure 10B). In contrast, we found the majority of LepRb neurons in the DMH/DHA (Figure 8B, B′) and a large portion of LepRb neurons in the mPOA (Figure 8C and C′) co-localized with FG from the RMR. Due to the small size of the rRPa in the mouse, it is difficult to confine FG injections solely to this site, thus we utilize injections to the RMR to represent neurons projecting into the rRPa (Figure 8A). This is supported by the fact that FG injections into the RMR, excluding the rRPa, showed only a few FG traced neurons in the DMH/DHA or mPOA and only occasionally a co-localization with LepRb neurons (supplement data 2).
These results, together with our adenoviral tracing studies, support that LepRb neurons in the DMH/DHA indeed represent thermoregulatory neurons that act via the rRPa to regulate BAT thermogenesis. Furthermore, we also show that LepRb neurons in the mPOA project to the rRPa, indicating that thermoregulatory leptin action involves several neuronal populations and likely acts differentially on these distinct neuronal populations.
LepRb neurons are synaptically coupled with rRPa neurons
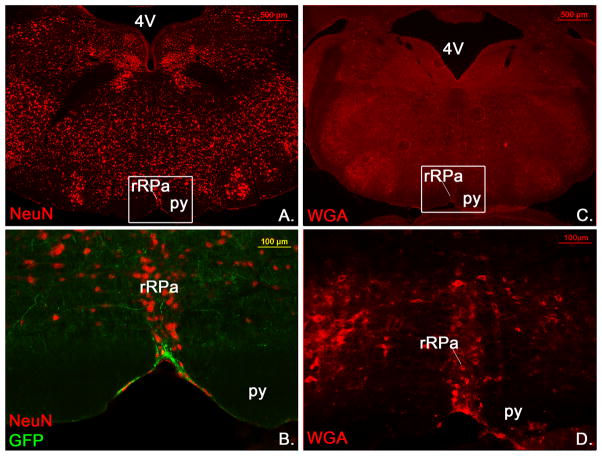
To further demonstrate that LepRb neurons indeed innervate neurons in the rRPa we used LepRbEGPF mice to identify LepRb projections into the rRPa. To visualize rRPa neurons we counterstained with the neuronal marker NeuN. As seen in Figure 9B we found clear LepRb projections intermingling with rRPa neurons (n=10).
Figure 9.
LepRb neurons are synaptically coupled with neurons in the rRPa. In LepRbEGFP reporter mice LepRbEGFP positive fibers (green) are seen in close proximity to neurons in the rRPa (red) (B, n=10). The same section is shown as an overview with NeuN stain in A. Transsynaptically labled WGA positive neurons are found in the rRPa of LepRbWGA mice (D, n=5), indicating synaptic coupling of LepRb neurons with rRPa neurons. An overview of the same sections is shown in C. py=pyramidial tract
We further used LepRbWGA mice, which have been previously described as a new tool to study synaptic targets of LepRb neurons (Leshan et al., 2010). In these mice WGA is produced in LepRb neurons, which is known to be transported anterogradely along the axon and further transsynaptically into second order neurons. Consistent with the identified LepRb projections in the rRPa (Figure 7D and 9B) we were able to find WGA labeled neurons in the rRPa (Figure 9D, n=5), demonstrating that LepRb neurons indeed are synaptically coupled with rRPa neurons.
Even though in these studies we cannot discriminate from which LepRb population these projections originate, we showed additionally that FG tracing from the rRPa intensely labels LepRb neurons in the DMH/DHA and mPOA. Therefore, together these data strongly indicate that LepRb neurons in the DMH/DHA and mPOA innervate rRPa neurons and thus might contribute importantly to the regulation of rRPa neurons.
A population of LepRb neurons in the mPOA projects to the DMH/DHA
Neurons in the mPOA are required for maintainance of basal body temperature, as well as cold or lipopolysaccharide (LPS) induced fever response (Yoshida et al., 2009). These mPOA neurons project directly to the rRPa (mPOA>rRPa neurons), but also to the DMH/DHA (mPOA>DMH/DHA neurons) (Nakamura et al., 2005;Yoshida et al., 2009). Importantly, a functional DMH is required to mediate mPOA evoked thermoregulatory responses (Dimicco and Zaretsky, 2007), further supporting the relevance of mPOA>DMH/DHA projections.
Thus, we hypothesized that LepRb neurons would be also present on mPOA>DMH/DHA neurons and analyzed co-localization of mPOA LepRb with FG labeled neurons from the DMH/DHA (Figure 11A, B). Indeed, we found several mPOA neurons retrogradely labeled with FG from the DMH/DHA and many LepRb neurons in the mPOA (including the MnPO and MPA) were co-localized with FG (Figure 11C, D), demonstrating that they project into the DMH/DHA. Therefore, these data suggest that DMH/DHA and mPOA LepRb neurons represent both inputs to premotor rRPa neurons and that mPOA>DMH/DHA LepRb neurons directly interact with LepRb neurons in the DMH/DHA as schematically summarized in Figure 12, suggesting a cross talk of different LepRb populations in thermoregulatory control.
Figure 11.
Stereotaxic injection of the retrograde tracer FG into the DMH/DHA (A, n=3) and IHC for leptin induced pSTAT3 (B.) to demonstrate the location of the FG injection relative to LepRb neurons in the DMH/DHA. Immunohistochemical detection of retrogradely labeled FG (green) in LepRb neurons (leptin-induced pSTAT3, red) in the mPOA (C.) and schematic drawing of double labeled FG/pSTAT3 neurons (red dots) in contrast to non-FG labeled pSTAT3 neurons (green dots) in the mPOA (D.).
Figure 12.
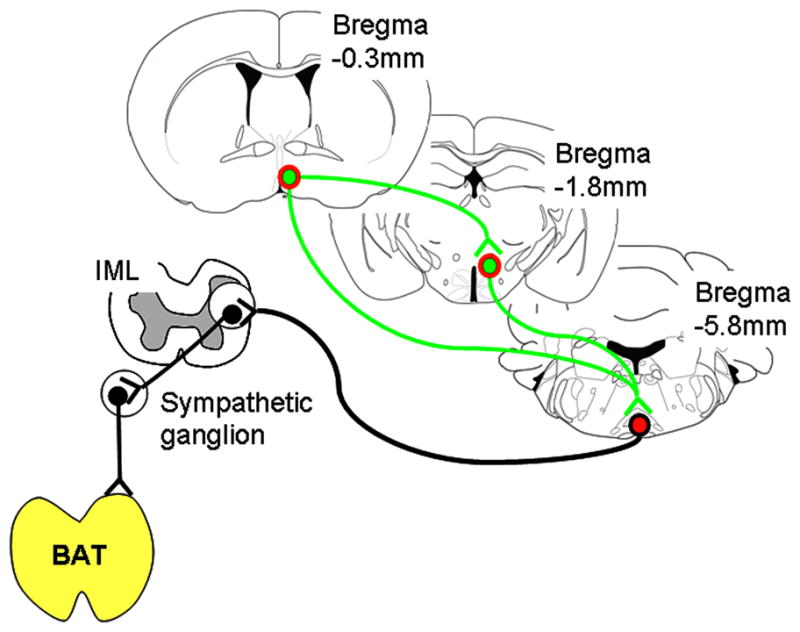
Schematic drawing of identified LepRb connections involved in thermoregulatory circuits.
Discussion
It is well established that leptin deficient mice are hypothermic (about 1°C versus wildtype mice) and are unable to defend their body temperature upon acute cold exposure (Trayhurn et al., 1976;Trayhurn et al., 1977). The thermoregulatory defects in these mice have been attributed to defective BAT thermogenesis (Goodbody and Trayhurn, 1982;Himms-Hagen, 1985), however, little is known about the neuronal circuits involved.
In the thermogenic field, several discoveries over the last years have led to the identification of important neuronal circuits including the mPOA and DMH/DHA and their interaction with the rRPa (Dimicco and Zaretsky, 2007;Morrison et al., 2008), a key component in the thermoregulatory adaptation to cold exposure and febrile responses to endotoxins (Nakamura at al 2005, Nakamura at al 2009).
LepRb neurons represent thermoregulatory circuits in the DMH/DHA and mPOA
In this study we show anatomical evidence that LepRb neurons in the DMH/DHA and mPOA contributed to the regulation of sympathetic BAT outputs. We identified LepRb in the DMH/DHA and mPOA that were labeled with PRV from the exclusively sympathetic innervated BAT. We further demonstrated that LepRb neurons are synaptically coupled with rRPa neurons and that specifically DMH/DHA LepRb neurons project to the rRPa and are stimulated upon acute cold exposure. This strongly suggests that LepRb neurons in the DMH/DHA represent thermoregulatory neurons which play a key role in the regulation of BAT thermogenesis as described by Nakamura et al (Nakamura at al 2005, Nakamura at al 2009).
Furthermore, we found that LepRb neurons in the mPOA project to the DMH/DHA and rRPa, consistent with studies in the rat (Dimicco and Zaretsky, 2007;Nakamura et al., 2005;Nakamura et al., 2009;Yoshida et al., 2009). Thus, leptin action appears to interact on several levels within this thermoregulatory circuit, showing parallel inputs to the rRPa from the mPOA and DMH/DHA and suggesting an interaction of LepRb populations in the mPOA and DMH/DHA.
Leptin targets in thermoregulatory control beyond the DMH/DHA and mPOA
We also found LepRb neurons in the RCh, NTS and EW consistently labeled with PRV from the BAT. Whereas the neuronal circuits of these sites, particularly with regard to thermoregulation, have not been investigated in detail, some data support their involvement in thermoregulatory control.
The RCh lays rostral to the ARC and harbors a large population of proopiomelancortin (POMC)-expressing neurons, which are predominantly co-localized with LepRb in the RCh and rostral ARC (Munzberg et al., 2003). POMC cleavage products stimulate melanocortin 3 and 4 receptors and have been long known to induce thermogenesis along with their important anorexigenic effects (Fan et al., 2005;Raible and Knickerbocker, 1993;Sinha et al., 2004).
The NTS is the major input structure for vagal afferents. NTS neurons are known to project locally within the brainstem, but also to hypothalamic structures (e.g. the DMH and PVN), that have been shown to regulate sympathetic outputs to the BAT (Geerling and Loewy, 2006). Even though leptin alone is unable to regulate BAT thermogenesis via the brainstem, a gating role has been proposed for leptin in combination with thyroid releasing hormone (TRH) which elicited a large increase in body temperature (3.5°C) upon leptin pretreatment compared to a 1°C increase by TRH alone (Hermann et al., 2006;Mark et al., 2009;Rogers et al., 2009)
The EW is a midbrain structure that can be divided into cholinergic pre-ganglionic neurons involved in oculomotor functions and urocortin-1 expressing non-preganglionic neurons (npEW) that are involved in stress and anxiety (Kozicz, 2007). LepRb neurons in the EW have been found to co-localize with anorexigenic urocortin, showing that LepRb neurons are part of the npEW (Xu et al., 2009). The npEW has not been associated with thermoregulation in particular, however, stress induced hyperthermia/fever is well documented and might involve the npEW (Bouwknecht and Paylor, 2002).
Thus, further investigations will be necessary to specify the thermoregulatory role of RCh, NTS and EW as well as the importance of leptin action in these sites.
Leptin action in thermoregulation
Fever
A coordinated rise in body temperature due to inflammation, infection or stress is commonly described as fever and causes vasoconstriction and BAT thermogenesis to raise body temperature (Szekely and Szelenyi, 1979). Bacterial lipopolysaccharides (LPS) have been used experimentally to induce fever, and cytokines like interleukins (IL-1β, IL-6), tumor necrosis factor (TNFα) and prostaglandins (PG) are involved to mediate LPS induced fever (Kluger et al., 1995;Luheshi and Rothwell, 1996).
An involvement of leptin in febrile responses has been discussed controversially. Leptin gene expression and serum leptin levels are induced by LPS, IL-1β, IL-6, TNFα or PG and consistent with this other studies found leptin receptor deficiency associated with a suppressed fever response to LPS (Busbridge et al., 1990;Dascombe et al., 1989;Ivanov and Romanovsky, 2002;Plata-Salaman et al., 1998;Rosenthal et al., 1996). Contrary, leptin receptor deficient rats housed at thermoneutral temperature have normal febrile responses (Ivanov and Romanovsky, 2002). LPS induced fever is largely independent of BAT thermogenesis under thermoneutral conditions, rather utilizing skin vasoconstriction to decrease heat loss and thus increase body temperature (Szekely and Szelenyi, 1979), which might explain this controversy.
Our findings provide a neuroanatomical basis to support a role of leptin action in fever responses. Interestingly, recent findings suggest that specific thermoregulatory systems which regulate fever or basal body temperature involve distinct as well as overlapping neuronal populations in the mPOA (Yoshida et al., 2009). The involvement of leptin action in several of these thermoregulatory circuits as well as other sites, with potentially counteracting functions in the mPOA and DMH/DHA, might further account for some of the controversy of febrile leptin action. The identification of LepRb neurons in known thermoregulatory circuits, however, will help to further dissect the role of leptin in these circuits under specific thermoregulatory conditions.
Cold induced thermogenesis
Leptin deficient mice are unable to adapt to acute cold exposure, whereas exogenous leptin replacement rapidly rescues their ability to survive the cold (Trayhurn et al., 1976;Ukropec et al., 2006). In addition, their BAT capacity is severely decreased and UCP1 transcripts are not induced in response to cold (Himms-Hagen, 1985). However, the mechanisms of leptin action on thermoregulation are not well understood.
Animals with decreased leptin levels due to body fat loss during prolonged fasting or food restriction (as well as obese, but leptin deficient mice) perform daily torpor with a substantial decrease in body temperature to preserve body reserves. Exogenous leptin injections prevent these torpor events (Gavrilova et al., 1999), possibly by increasing UCP1 expression in response to leptin (Commins et al., 1999;Scarpace et al., 1997), which would involve an increased sympathetic tone to the BAT. Therefore, this suggests that leptin would have a stimulatory effect on DMH/DHA neurons.
Contrary, in humans daily fluctuations of body temperature underlying the sleep:wake cycles is associated with high circulating leptin levels during sleep cycles and thus low body temperature (Simon et al., 1998). In addition, cold exposure generally decreases leptin mRNA expression in adipose tissue (Evans et al., 1999), rather supporting an inhibitory action of leptin on DMH/DHA neurons.
Furthermore, even though leptin deficient mice can die from hypothermia by acute cold exposure (4°C), they are capable to adjust to the same cold environment by gradual adaptation to the cold (reduction of temperature by 2–3°C/day), which involves a UCP1 independent mechanism that requires TRH and leptin for proper function (Ukropec et al., 2006).
Therefore, complex interactions of diverse systems collectively regulate body temperature and leptin appears to plays a key role in orchestrating these diverse systems. Further studies will be necessary to investigate the effects of leptin on DMH/DHA and mPOA neurons in specific thermoregulatory situations.
Thermoregulation and body weight
A role of BAT function in the development of obesity has been speculated, even though the presence and functionality of BAT in adult humans has been doubted. However, recent studies clearly demonstrated the presence of large amounts of functional BAT in adult humans and revived the interest in thermogenic BAT function, their underlying neuronal circuits and their endogenous regulators as potential targets for obesity drugs (Fruhbeck et al., 2009;Garrow, 1983).
It has been long known that the ingestion of high caloric food is associated with an increase in body temperature, sympathetic tone and BAT capacity (Rothwell and Stock, 1979). However, if these effects indeed involve controlled adaptive thermogenesis (via centrally controlled BAT thermogenesis) or if simply thermic effects are involved (due to increased digestive function and metabolizing additional fuels) has not been resolved (Garrow, 1983;Nedergaard et al., 2007;Ravussin and Kozak, 2009).
Our data support the possibility that leptin’s action on energy homeostasis utilizes several thermoregulatory circuits and further studies are necessary to specifically address the importance of thermoregulatory control by leptin for whole body energy balance and the development of obesity.
Supplementary Material
Acknowledgments
This work was supported by AHA053298N, P/F DK020572-30 and NIH P20 RR02195 (HM); NARSAD Young Investigator Award and NIMH 5K99MH081927-02 (IAK); DK055267 (CJR). Pseudorabies virus strains were provided by Dr. L.W. Enquist, Princeton University as a service of the National Center for Experimental Neuroanatomy with Neurotropic Viruses: NCRR P40 RRO118604 and the National Science Foundation under agreement No. IBN-9876754. This work utilized the facilities of the Cell Biology and Bioimaging Core, supported in part by COBRE (NIH P20-RR021945) and CNRU (NIH 1P30-DK072476) center grants from the National Institutes of Health. Partial support was provided through the Animal Phenotyping Core supported through NORC Center Grant #1P30 DK072476 entitled ‘Nutritional Programming: Environmental and Molecular Interactions’ sponsored by NIDDK.
Reference List
- Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- Bailey TW, Dimicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R8–15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- Banfield BW, Kaufman JD, Randall JA, Pickard GE. Development of pseudorabies virus strains expressing red fluorescent proteins: new tools for multisynaptic labeling applications. J Virol. 2003;77:10106–10112. doi: 10.1128/JVI.77.18.10106-10112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002;136:489–501. doi: 10.1016/s0166-4328(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Busbridge NJ, Carnie JA, Dascombe MJ, Johnston JA, Rothwell NJ. Adrenalectomy reverses the impaired pyrogenic responses to interleukin-beta in obese Zucker rats. Int J Obes. 1990;14:809–814. [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Commins SP, Watson PM, Padgett MA, Dudley A, Argyropoulos G, Gettys TW. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology. 1999;140:292–300. doi: 10.1210/endo.140.1.6399. [DOI] [PubMed] [Google Scholar]
- Dascombe MJ, Hardwick A, Lefeuvre RA, Rothwell NJ. Impaired effects of interleukin-1 beta on fever and thermogenesis in genetically obese rats. Int J Obes. 1989;13:367–373. [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R47–R63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Jacobson CD, Saper CB. Distribution of Fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J Comp Neurol. 1996;371:85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Evans BA, Agar L, Summers RJ. The role of the sympathetic nervous system in the regulation of leptin synthesis in C57BL/6 mice. FEBS Lett. 1999;444:149–154. doi: 10.1016/s0014-5793(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Fan W, Voss-Andreae A, Cao WH, Morrison SF. Regulation of thermogenesis by the central melanocortin system. Peptides. 2005;26:1800–1813. doi: 10.1016/j.peptides.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Faouzi M, Leshan R, Bjornholm M, Hennessey T, Jones J, Munzberg H. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology. 2007;148:5414–5423. doi: 10.1210/en.2007-0655. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G, Becerril S, Sainz N, Garrastachu P, Garcia-Velloso MJ. BAT: a new target for human obesity? Trends Pharmacol Sci. 2009;30:387–396. doi: 10.1016/j.tips.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Garrow JS. Luxuskonsumption, brown fat, and human obesity. Br Med J (Clin Res Ed) 1983;286:1684–1686. doi: 10.1136/bmj.286.6379.1684-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova O, Leon LR, Marcus-Samuels B, Mason MM, Castle AL, Refetoff S, Vinson C, Reitman ML. Torpor in mice is induced by both leptin-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 1999;96:14623–14628. doi: 10.1073/pnas.96.25.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary: efferent projections. J Comp Neurol. 2006;498:223–250. [PubMed] [Google Scholar]
- Goodbody AE, Trayhurn P. Studies on the activity of brown adipose tissue in suckling, pre-obese, ob/ob mice. Biochim Biophys Acta. 1982;680:119–126. doi: 10.1016/0005-2728(82)90002-0. [DOI] [PubMed] [Google Scholar]
- Hausberg M, Morgan DA, Mitchell JL, Sivitz WI, Mark AL, Haynes WG. Leptin potentiates thermogenic sympathetic responses to hypothermia: a receptor-mediated effect. Diabetes. 2002;51:2434–2440. doi: 10.2337/diabetes.51.8.2434. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Barnes MJ, Rogers RC. Leptin and thyrotropin-releasing hormone: cooperative action in the hindbrain to activate brown adipose thermogenesis. Brain Res. 2006;1117:118–124. doi: 10.1016/j.brainres.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J. Defective brown adipose tissue thermogenesis in obese mice. Int J Obes. 1985;9(Suppl 2):17–24. [PubMed] [Google Scholar]
- Hosoi T, Kawagishi T, Okuma Y, Tanaka J, Nomura Y. Brain stem is a direct target for leptin’s action in the central nervous system. Endocrinology. 2002;143:3498–3504. doi: 10.1210/en.2002-220077. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Ito R, Kohno K. The topographical organization of neurons in the dorsal hypothalamic area that project to the spinal cord or to the nucleus raphe pallidus in the rat. Exp Brain Res. 1987;66:500–506. doi: 10.1007/BF00270682. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Sugiura Y, Zhang FZ, Ito R, Kohno K. Direct projection from the dorsal hypothalamic area to the nucleus raphe pallidus: a study using anterograde transport with Phaseolus vulgaris leucoagglutinin in the rat. Exp Brain Res. 1989;75:40–46. doi: 10.1007/BF00248528. [DOI] [PubMed] [Google Scholar]
- Hunt JL, Zaretsky DV, Sarkar S, Dimicco JA. Dorsomedial hypothalamus mediates autonomic, neuroendocrine, and locomotor responses evoked from the medial preoptic area. Am J Physiol Regul Integr Comp Physiol. 2009 doi: 10.1152/ajpregu.00574.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Romanovsky AA. Fever responses of Zucker rats with and without fatty mutation of the leptin receptor. Am J Physiol Regul Integr Comp Physiol. 2002;282:R311–R316. doi: 10.1152/ajpregu.00376.2001. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Akil H, Watson SJ. Rostral elements of sympatho-motor circuitry: a virally mediated transsynaptic tracing study. J Neurosci. 2006;26:3423–3433. doi: 10.1523/JNEUROSCI.5283-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger MJ, Kozak W, Leon LR, Soszynski D, Conn CA. Cytokines and fever. Neuroimmunomodulation. 1995;2:216–223. doi: 10.1159/000097199. [DOI] [PubMed] [Google Scholar]
- Kozicz T. On the role of urocortin 1 in the non-preganglionic Edinger-Westphal nucleus in stress adaptation. Gen Comp Endocrinol. 2007;153:235–240. doi: 10.1016/j.ygcen.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, Jones JC, Rhodes CJ, Chua S, Jr, Diano S, Horvath TL, Seeley RJ, Becker JB, Munzberg H, Myers MG., Jr Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshan RL, Bjornholm M, Munzberg H, Myers MG., Jr Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring) 2006;14(Suppl 5):208S–212S. doi: 10.1038/oby.2006.310. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Louis GW, Jo YH, Rhodes CJ, Munzberg H, Myers MG., Jr Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci. 2009;29:3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, Munzberg H, Myers MG., Jr Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci. 2010;30:5713–5723. doi: 10.1523/JNEUROSCI.1001-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luheshi G, Rothwell N. Cytokines and fever. Int Arch Allergy Immunol. 1996;109:301–307. doi: 10.1159/000237256. [DOI] [PubMed] [Google Scholar]
- Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53:375–380. doi: 10.1161/HYPERTENSIONAHA.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Jobst EE, Bates SH, Jones J, Villanueva E, Leshan R, Bjornholm M, Elmquist J, Sleeman M, Cowley MA, Myers MG., Jr Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27:69–74. doi: 10.1523/JNEUROSCI.3168-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Morrison SF. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience. 2009;161:614–620. doi: 10.1016/j.neuroscience.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–3695. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Elsevier Science; USA: 2004. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Plata-Salaman CR, Peloso E, Satinoff E. Cytokine-induced fever in obese (fa/fa) and lean (Fa/Fa) Zucker rats. Am J Physiol. 1998;275:R1353–R1357. doi: 10.1152/ajpregu.1998.275.4.R1353. [DOI] [PubMed] [Google Scholar]
- Raible LH, Knickerbocker D. Alpha-melanocyte-stimulating hormone (MSH) and [Nle4,D-Phe7]-alpha-MSH: effects on core temperature in rats. Pharmacol Biochem Behav. 1993;44:533–538. doi: 10.1016/0091-3057(93)90163-n. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Kozak LP. Have we entered the brown adipose tissue renaissance? Obes Rev. 2009;10:265–268. doi: 10.1111/j.1467-789X.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RC, Barnes MJ, Hermann GE. Leptin “gates” thermogenic action of thyrotropin-releasing hormone in the hindbrain. Brain Res. 2009;1295:135–141. doi: 10.1016/j.brainres.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M, Roth J, Storr B, Zeisberger E. Fever response in lean (Fa/−) and obese (fa/fa) Zucker rats and its lack to repeated injections of LPS. Physiol Behav. 1996;59:787–793. doi: 10.1016/0031-9384(95)02158-2. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Rummel C, Voss T, Matsumura K, Korte S, Gerstberger R, Roth J, Hubschle T. Nuclear STAT3 translocation in guinea pig and rat brain endothelium during systemic challenge with lipopolysaccharide and interleukin-6. J Comp Neurol. 2005;491:1–14. doi: 10.1002/cne.20653. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Zaretskaia MV, Zaretsky DV, Moreno M, Dimicco JA. Stress- and lipopolysaccharide-induced c-fos expression and nNOS in hypothalamic neurons projecting to medullary raphe in rats: a triple immunofluorescent labeling study. Eur J Neurosci. 2007;26:2228–2238. doi: 10.1111/j.1460-9568.2007.05843.x. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Pollock BH, Tumer N. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol. 1997;273:E226–E230. doi: 10.1152/ajpendo.1997.273.1.E226. [DOI] [PubMed] [Google Scholar]
- Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: relationship to sleep and body temperature. J Clin Endocrinol Metab. 1998;83:1893–1899. doi: 10.1210/jcem.83.6.4864. [DOI] [PubMed] [Google Scholar]
- Sinha PS, Schioth HB, Tatro JB. Roles of the melanocortin-4 receptor in antipyretic and hyperthermic actions of centrally administered alpha-MSH. Brain Res. 2004;1001:150–158. doi: 10.1016/j.brainres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Szekely M, Szelenyi Z. Endotoxin fever in the rat. Acta Physiol Acad Sci Hung. 1979;53:265–277. [PubMed] [Google Scholar]
- Ter Horst GJ, Luiten PG. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Res Bull. 1986;16:231–248. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J Comp Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Thurlby PL, James WP. A defective response to cold in the obese (obob) mouse and the obese Zucker (fafa) rat [proceedings] Proc Nutr Soc. 1976;35:133A. [PubMed] [Google Scholar]
- Trayhurn P, Thurlby PL, James WP. Thermogenic defect in pre-obese ob/ob mice. Nature. 1977;266:60–62. doi: 10.1038/266060a0. [DOI] [PubMed] [Google Scholar]
- Ukropec J, Anunciado RV, Ravussin Y, Kozak LP. Leptin is required for uncoupling protein-1-independent thermogenesis during cold stress. Endocrinology. 2006;147:2468–2480. doi: 10.1210/en.2005-1216. [DOI] [PubMed] [Google Scholar]
- Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology. 2007;148:1550–1560. doi: 10.1210/en.2006-1389. [DOI] [PubMed] [Google Scholar]
- Xu L, Bloem B, Gaszner B, Roubos EW, Kozicz T. Sex-specific effects of fasting on urocortin 1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 expression in the rat Edinger-Westphal nucleus. Neuroscience. 2009;162:1141–1149. doi: 10.1016/j.neuroscience.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009;29:11954–11964. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Dimicco JA. Role of the dorsomedial hypothalamus in thermogenesis and tachycardia caused by microinjection of prostaglandin E2 into the preoptic area in anesthetized rats. Neurosci Lett. 2003;340:1–4. doi: 10.1016/s0304-3940(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Sarkar S, Shekhar A, Dimicco JA. Induction of Fos-immunoreactivity in the rat brain following disinhibition of the dorsomedial hypothalamus. Brain Res. 2008;1200:39–50. doi: 10.1016/j.brainres.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Shekhar A, Dimicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.