Abstract
Sarcomere assembly in striated muscles has long been described as a series of steps leading to assembly of individual proteins into thick filaments, thin filaments and Z-lines. Decades of previous work focused on the order in which various structural proteins adopted the striated organization typical of mature myofibrils. These studies led to the view that actin and α-actinin assemble into premyofibril structures separately from myosin filaments, and that these structures are then assembled into myofibrils with centered myosin filaments and actin filaments anchored at the Z-lines. More recent studies have shown that particular scaffolding proteins and chaperone proteins are required for individual steps in assembly. Here, we review the evidence that N-RAP, a LIM domain and nebulin repeat protein, scaffolds assembly of actin and α-actinin into I-Z-I structures in the first steps of assembly; that the heat shock chaperone proteins Hsp90 & Hsc70 cooperate with UNC-45 to direct the folding of muscle myosin and its assembly into thick filaments; and that the kelch repeat protein Krp1 promotes lateral fusion of premyofibril structures to form mature striated myofibrils. The evidence shows that myofibril assembly is a complex process that requires the action of particular catalysts and scaffolds at individual steps. The scaffolds and chaperones required for assembly are potential regulators of myofibrillogenesis, and abnormal function of these proteins caused by mutation or pathological processes could in principle contribute to diseases of cardiac and skeletal muscles.
Keywords: Myofibrillogenesis, N-RAP, Krp1, Hsp90, Hsc70, UNC-45
Myofibrils and myofibril assembly
Myofibrils are the organelles responsible for generating force and movement in skeletal and cardiac muscles. They are an archetypal example of organization generated by a specific cell type to perform a specialized function, and as such are one of the most well-studied structures in cell biology. Sarcomeres are the longitudinally repeating subunit of myofibrils, with every sarcomere bounded by a Z-disk at each end. The sarcomeric actin filaments are anchored at the Z-disks via their barbed ends, which are crosslinked by α-actinin. The pointed ends of the actin filaments extend toward the middle of the sarcomere, where they interdigitate with the myosin-containing thick filaments. The thick filaments are aligned at the center of the sarcomere by titin filaments that bind along their length and extend elastically between the ends of the thick filaments and the Z-disks. This organization allows for efficient shortening and force generation by the coordinated interaction of the organized array of actin and myosin filaments.
The development of sarcomeres and their organization into mature myofibrils requires the coordinated assembly of numerous proteins, and a number of models have been proposed to describe this complex series of events. There is broad agreement that the earliest myofibril precursors contain punctate α-actinin Z-bodies, α-actin and muscle tropomyosin (Rhee et al. 1994; Dabiri et al. 1997; Imanaka-Yoshida 1997; Ehler et al. 1999; Rudy et al. 2001; Lu et al. 2005; Dlugosz et al. 1984; Handel et al. 1991; Schultheiss et al. 1990; Wang et al. 1988). Although these structures appear near the cell periphery as immature fibrils, actual anchoring of these structures to the cell membrane has not been demonstrated. Nevertheless, integrins appear to be necessary for myofibril assembly (Volk et al. 1990; Bloor and Brown 1998; Schwander et al. 2003), and a model has been proposed in which integrin adhesion sites promote the initial actin-polymerizing step in myofibril assembly (Sparrow and Schock 2009). The muscle myosin filaments assemble separately and are subsequently interdigitated with the I-Z-I complexes (symmetrical actin filaments with their barbed ends anchored at a central Z-body or Z-line containing α-actinin) to form full-fledged sarcomeres (Ehler et al. 1999; Holtzer et al. 1997; Rudy et al. 2001; Srikakulam and Winkelmann 2004).
Other aspects of sarcomere assembly remain controversial. Assembling myofibrils with Z-line spacings shorter than found in mature myofibrils have been observed in many experimental sytems, including spreading cardiomyocytes (Dabiri et al. 1997; Rhee et al. 1994), precardiac mesoderm explants (Du et al. 2003; Imanaka-Yoshida et al. 1998), intact embryonic hearts (Du et al. 2008a), and intact embryonic skeletal muscle (Sanger et al. 2009). However, some reports indicate that these are less abundant in embryos than in cultured cells (Ehler et al. 1999; Lu et al. 2005). Nevertheless, time-lapse microscopy of cardiomyocytes expressing α-actinin as a GFP fusion protein demonstrated the assembly of closely spaced Z-bodies that increased their longitudinal spacing and fused laterally to form Z-lines (Dabiri et al. 1997; Manisastry et al. 2009). A requirement for nonmuscle myosin IIB in the assembly of premyofibrils has also been proposed (Du et al. 2003; Du et al. 2008a; Rhee et al. 1994), but subsequently questioned. Knockout of nonmuscle myosin IIB by gene targeting shows that myofibrils can assemble in the absence of this protein in vivo (Tullio et al. 1997), and experiments in which nonmuscle myosin IIB levels were decreased in cultured cardiomyocytes by RNA interference also suggest that this protein is dispensable for myofibril assembly (Lu and Horowits 2008).
Possible roles for scaffolds in myofibril assembly have long been discussed. These include the suggestion that actin-based stress fibers might direct the assembly of nascent myofibrils (Dlugosz et al. 1984) or that the giant structural proteins, titin and nebulin, may control actin and myosin filament lengths and assembly (Kontrogianni-Konstantopoulos et al. 2009; Wang 1985). However, titin and nebulin are structural components that are present in the final assembled structures, and they may more accurately be termed putative template molecules (Clark et al. 2002; Gregorio et al. 1999; Wang et al. 1996). This review is designed to provide a brief introduction to true scaffolding proteins involved in myofibril assembly, i.e. proteins that are temporarily associated with the assembling structures, but that are not structural components in the fully assembled sarcomeres. We focus on N-RAP, a scaffolding protein for I-Z-I assembly; Hsp90, Hsc70, and UNC-45, chaperone proteins involved in myosin assembly; and Krp1, a putative scaffold for lateral fusion of premyofibril structures. Figure 1 schematically illustrates the steps in myofibril assembly and emphasizes the role of these scaffolding proteins at particular stages.
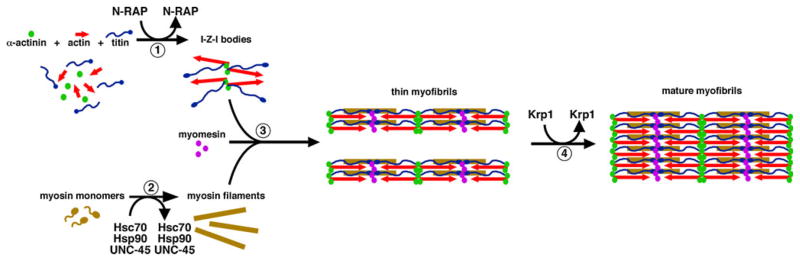
Fig. 1.
A putative pathway for myofibril assembly highlighting the role of transiently associated proteins in organizing the major structural components (modified from Greenberg et al. 2008). (1) N-RAP promotes assembly of the I-Z-I structures containing actin, α-actinin, and N-terminal titin. (2) Myosin filaments form separately, with appropriate folding and assembly promoted by the Hsc70 and Hsp90 chaperone proteins and the UNC-45 co-chaperone. (3) Titin associates with the myosin filaments along their length, helping to integrate the thick filaments with the I-Z-I structures. In addition, myomesin interacts with titin and myosin to crosslink the array of myosin filaments at the center of the sarcomere. This gives rise to thin myofibrils. (4) Finally, Krp1 promotes the lateral fusion of thin myofibrils to form mature myofibrils
N-RAP: a scaffolding protein for assembly of sarcomeric actin and α-actinin
N-RAP is a multi-domain protein specifically expressed in striated muscles and with a range of potential binding partners identified by in vitro assays (Fig. 2a), making it an ideal candidate for a scaffolding protein involved in myofibril assembly. The protein consists of an N-terminal LIM domain, a C-terminal domain composed of five nebulin-related super repeats, and a linker region with nebulin-related single repeats (Mohiddin et al. 2003). The LIM domain has been shown to interact with talin and α-actinin (Zhang et al. 2001; Luo et al. 1999), while the C-terminal super repeats can bind actin, vinculin, filamin, and the BTB-Kelch protein, Krp1 (Lu et al. 2003; Luo et al. 1999). The nebulin-related single repeats bind actin, α-actinin, MLP and Krp1 (Lu et al. 2003; Zhang et al. 2001; Ehler et al. 2001; Luo et al. 1999).
Fig. 2.
Domain organization of a N-RAP and b Krp1. The hatched N-RAP single repeat is encoded by an alternatively spliced exon that is not expressed in cardiac muscle (Mohiddin et al. 2003). The hatched region in the C-terminal super repeat is encoded by an exon reported to be alternatively spliced in skeletal muscle (Gehmlich et al. 2004). Boxes below the diagrams list the known binding partners for each region. See text for details
Two distinct splice variants of N-RAP have been identified and have been given the names N-RAP-s and N-RAP-c based on their primary expression in either skeletal muscle or cardiac muscle, respectively. N-RAP-c is the only isoform present in cardiomyocytes, while both N-RAP-s and N-RAP-c are expressed in skeletal muscle, with the expression of the former predominant (Lu et al. 2008; Mohiddin et al. 2003). The difference between the two isoforms arises from alternative splicing of exon 12, resulting in an additional nebulin simple repeat in the skeletal isoform that is not expressed in cardiac muscle. The complete absence of the skeletal isoform in cardiac muscle and the conservation of these tissue-specific isoforms from mice to humans suggests some functional importance, although this remains to be demonstrated. Interestingly, the balance between the two N-RAP isoforms in skeletal muscle is perturbed in myotonic dystrophy, a disease characterized by aberrant alternative splicing of many proteins in striated muscle (Lin et al. 2006).
In mature tissues, N-RAP is localized at the longitudinal ends of the striated muscle cells (Lu et al. 2005, 2008; Mohiddin et al. 2003; Luo et al. 1997). In skeletal muscle, this is the myotendinous junction, and in the heart, it is the intercalated disk. In each case, N-RAP colocalizes with actin bundles that link the terminal myofibrils to protein complexes associated with the cell membrane in these regions (Herrera et al. 2000; Zhang et al. 2001). Since N-RAP binds actin as well as several proteins found in the membrane complexes in these regions, it was hypothesized that N-RAP might physically link the terminal actin bundles to the membrane complexes and transmit tension produced by the myofibrils to the ends of the cells (Luo et al. 1997, 1999). This view was also consistent with the finding that N-RAP remained tightly associated with the ends of myofibrils as well as with cardiac intercalated disks during biochemical purification (Zhang et al. 2001).
Although it is not a component of fully assembled sarcomeres in mature muscle cells, N-RAP is associated with assembling and newly formed sarcomeres during embryonic development. During heart and skeletal muscle development, N-RAP is found in assembling premyofibril structures containing α-actinin, as well as in Z-lines and M-lines of newly formed myofibrils (Lu et al. 2005, 2008). N-RAP was also observed in premyofibril structures found in spreading cardiomyocytes and cultured myotubes as well as at the periphery of the cultured cells, although once again it was absent from mature sarcomeres (Carroll and Horowits 2000; Lu et al. 2008).
The first functional data implicating N-RAP in myofibril assembly came from a series of experiments utilizing fusion proteins tagged with green fluorescent protein (GFP) to determine the targeting behavior and functional effects of expressing individual regions of N-RAP in embryonic avian cardiomyocytes (Carroll et al. 2004; Carroll et al. 2001). The individual regions of N-RAP displayed distinct localization patterns that were consistent with the known binding partners of each region. Since the N-RAP LIM domain associated with the cell periphery but did not appear in myofibril precursors (Carroll et al. 2001), it was proposed that its interaction with talin (Luo et al. 1999), an integrin-binding protein found at the cytoplasmic face of focal contacts (Critchley 2000), might be responsible for initiating myofibril assembly at the cell periphery. This model is consistent with the proposal that integrin-dependent adhesion sites are the starting point for myofibril assembly (Sparrow and Schock 2009). In addition, individually expressing the N-RAP LIM domain, single repeat region or super repeat region inhibited the assembly of α-actinin into Z-lines (Carroll et al. 2001). Experiments utilizing N-RAP deletion mutants showed that the single repeat region was critical for Z-line assembly, while the super repeats were essential for organizing sarcomeric actin filaments (Carroll et al. 2004). These results led to the proposal that N-RAP scaffolds I-Z-I assembly, with the single repeats binding α-actinin, the super repeats binding actin, and the intact N-RAP protein organizing the assembly of these components into the organized structure found in the sarcomere (Fig. 1, step 1). Consistent with this model, N-RAP knockdown by RNA interference in mouse cardiomyocytes demonstrated that α-actinin organization into myofibrils is closely linked to N-RAP levels (Dhume et al. 2006). Interestingly, N-RAP and α-actinin levels are both regulated by the transcription factor Prox1, and a cardiac-specific knockout of Prox1 leads to disorganization of sarcomeric proteins (Risebro et al. 2009).
In a recent study, the time-course and dynamics of N-RAP’s association with assembling actin and α-actinin during myofibrillogenesis was explored using time-lapse confocal microscopy (Manisastry et al. 2009). In these experiments, avian cardiomyocytes were transfected with plasmids encoding N-RAP fused to mCherry and either actin or α-actinin fused to yellow fluorescent protein (YFP), and then imaged for up to 24 h at intervals of 5–10 min. The results showed that N-RAP was incorporated into previously polymerized actin fibrils, after which α-actinin was recruited to the structure in the form of closely spaced dots. The α-actinin dots then broadened to Z-lines that were wider than the underlying N-RAP fibril, and N-RAP gradually left the assembled structure. Dynamic measurements using fluorescence recovery after photobleaching (FRAP) demonstrated that most of the N-RAP in premyofibrils was immobile, being more stably bound than the assembling α-actinin. However, the bound N-RAP was mobilized as the myofibrils matured.
Although the precise mechanism by which N-RAP may scaffold actin and α-actinin assembly into I-Z-I structures is unknown, a recently proposed hypothetical model is shown in Fig. 3 (Manisastry et al. 2009). In particular, this model is consistent with the transient association of N-RAP with developing myofibrils (Carroll and Horowits 2000; Lu et al. 2003, 2005, 2008; Lu and Horowits 2008), the targeting and phenotypic effects of individual N-RAP domains (Carroll et al. 2001, 2004), the effect on myofibril assembly of N-RAP knockdown by RNA interference (Dhume et al. 2006), and the order in which actin, α-actinin and N-RAP are recruited to assembling myofibrils (Manisastry et al. 2009). In order to explain a periodic substructure observed in the premyofibril before recruitment of α-actinin (Manisastry et al. 2009), the model includes an antiparallel dimerization of N-RAP via its LIM domain, consistent with previously observed dimerization of LIM domains in other proteins (Feuerstein et al. 1994; Sanchez-Garcia et al. 1995). The antiparallel organization of actin filaments at the Z-line is then controlled by actin binding to the N-RAP super repeats, which are essential for appropriate sarcomeric actin assembly (Carroll et al. 2004).
Fig. 3.
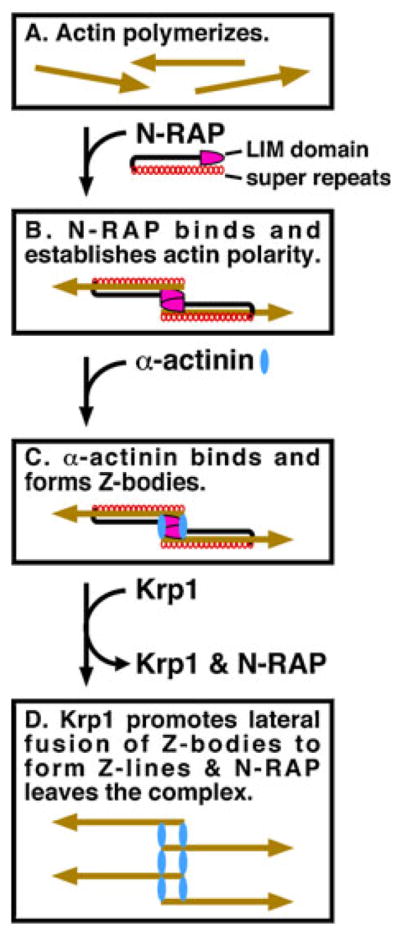
Schematic model illustrating N-RAP scaffolding during assembly of the I-Z-I complex (from Manisastry et al. 2009). a Actin filaments form. b Antiparallel N-RAP dimers cross-link actin filaments in an antiparallel orientation. The orientation of each actin filament is indicated by an arrowhead at the pointed end. c α-actinin binds to N-RAP and cross-links the actin filaments. d Krp1 promotes lateral fusion of the Z-bodies to form Z-lines, and N-RAP leaves the complex
The concentration of N-RAP at the ends of myofibrils in mature muscle and cardiac cells is consistent with its role in de novo sarcomere formation, if it is assumed that these are sites where new sarcomeres would be added in response to stretch or growth signals. The accumulated N-RAP in these regions would then be poised to quickly initiate assembly.
Hsp90, Hsc70, and UNC-45: chaperone proteins for myosin assembly
Compared to actin and other muscle proteins, functional muscle myosin has been difficult to express outside muscle cells (Chow et al. 2002; Kinose et al. 1996). As the role of other proteins in myosin folding was investigated, the reasons for this limitation became clear. Appropriate folding and assembly of myosin in striated muscles is mediated by a series of molecular chaperones (Fig. 1, step 2). These include the general chaperone proteins Hsp90 and the Hsc70 isoform of the Hsp70 family (Srikakulam and Winkelmann 1999, 2004; Willis et al. 2009). Hsp90 contains an N-terminal ATP binding domain, a middle domain that activates the ATPase and binds to client proteins and co-chaperones, and a C-terminal domain that also binds client and co-chaperones, but further contains a site for homodimerization (Whitesell and Lindquist 2005; Zhao and Houry 2007). Hsp70 also contains an N-terminal ATPase domain and a more variable C-terminal substrate-binding domain (Morano 2007). A complex containing Hsp70 appears to efficiently capture unfolded protein molecules and transfer them to Hsp90 for completion of the folding reaction (Wegele et al. 2006).
A key feature in the C-terminal domain of Hsp90 is the presence of a conserved motif that binds to tetratricopeptide repeats (TPR) present in many co-chaperones (Whitesell and Lindquist 2005). One such co-chaperone involved in folding of myosin is UNC-45. UNC-45 contains an N-terminal TPR region that binds the conserved motif in Hsp90 (Barral et al. 2002). A UCS domain (UNC45-Cro1p-She4p) is located at the C-terminus of UNC-45 and associates with the myosin head domain. UNC-45 also contains a central conserved region, but its role has yet to be identified. C. elegans and D. malonogaster possess a single isoform of UNC-45 (Hutagalung et al. 2002). However, in vertebrates, there are two isoforms, a general cellular form designated UNC-45a (formerly GC UNC-45) and a striated muscle form designated UNC-45b (formerly SM UNC-45) (Price et al. 2002).
Expressing GFP-tagged myosin in C2C12 muscle cells, Srikakulam and Winkelmann (2004) demonstrated that Hsp90 and Hsc70 form complexes with the myosin motor domains. Furthermore, inhibiting Hsp90 activity blocked myofibril assembly and led to accumulation of myosin folding intermediates. The role of UNC-45 was suggested by the discovery of mutants in C. elegans characterized by decreased body movement and disorganized myofilaments (Epstein and Thomson 1974). The interaction between Hsp90 and UNC-45 has also been confirmed in zebrafish, with similar muscle defects resulting from mutation or knockdown of these components (Wohlgemuth et al. 2007; Du et al. 2008b; Etard et al. 2007). UNC-45 was subsequently shown to target unfolded myosin to Hsp90 and promote folding of the myosin motor domain (Barral et al. 2002; Srikakulam et al. 2008).
Using fluorescently tagged proteins in the zebrafish model, Etard et al. (2008) provided evidence that the role of these chaperones is not limited to the initial stages of myofibrillogenesis. In their experiments, UNC-45 and Hsp-90 were present at the Z-disk in mature myofibrils but relocalized to the A-bands when stressed. The Z-line associated chaperones may thus provide a reserve of proteins necessary for rapid assembly of sarcomeric components as a quick response to cell stress or damage.
Krp1: a scaffolding protein for myofibril maturation
Krp1 (kelch-related protein 1, also called sarcosin) is a muscle-specific member of the BTB-kelch family of proteins (Spence et al. 2000; Taylor et al. 1998) (Fig. 2b), which are characterized by a C-terminal kelch domain and an N-terminal BTB/POZ domain (Adams et al. 2000). Kelch motifs consist of repeats of 44–56 amino acids that form four anti-parallel β-strands (Adams et al. 2000). In some kelch family proteins, between four and seven of these repeats organize into a larger β-propeller structure with individual kelch motifs forming individual blades of the larger structure. However, the recently reported crystal structure of the Krp1 kelch region demonstrates that the previously assigned roles of conserved kelch residues are not preserved in all blades; furthermore, the β-propeller in Krp1 includes a sixth blade that was not predicted to be a kelch motif from the primary sequence (Gray et al. 2009). Many kelch repeat proteins associate with the actin cytoskeleton, and some of these have been shown to bind actin directly through their kelch motifs (Adams et al. 2000; Kim et al. 1999; Soltysik-Espanola et al. 1999). However, direct binding of actin by Krp1 has not been demonstrated; instead, the Krp1 kelch repeats have been shown to directly interact with a variety of actin-binding nebulin-repeat proteins, including Lasp1, nebulin, and N-RAP (Lu et al. 2003; Spence et al. 2006) (Fig. 2b).
BTB domains in other kelch proteins can act as adaptors for ubiquitination by cullin based E3 ubiquitin-ligase complexes (Zhang et al. 2005; Geyer et al. 2003; Xu et al. 2003), while the precise function of BACK domains remains speculative (Stogios and Prive 2004). Binding partners for the BTB/POZ and BACK regions of Krp1 have not yet been identified.
The first functional evidence for Krp1’s role as a cytoskeletal scaffolding protein came from oncogene-transformed rat fibroblasts. Krp1 is upregulated in these cells, and most of the Krp1 remains in the cytoplasm; however, some Krp1 associates with LASP-1 at the tips of pseudopodia, and this interaction promotes pseudopod elongation and invasion (Spence et al. 2000, 2006).
The first indication of Krp1’s role in striated muscles, where it is normally expressed, came as the result of a yeast two-hybrid screen for N-RAP binding partners (Lu et al. 2003). These experiments showed that Krp1 bound the nebulin-related simple repeats and super repeats of N-RAP. Much of the Krp1 in cultured cardiomyocytes was diffusely distributed in the cytoplasm, but some of the protein was found in premyofibril areas, particularly in the space between narrow myofibrils that were fusing laterally into more mature structures (Greenberg et al. 2008; Lu et al. 2003). Upon knockdown of Krp1 by siRNA, morphometric analysis of α-actinin-stained cardiomyocytes revealed a decrease in mature myofibril area and an increase in periodic Z-bodies. Staining for thick and thin filament marker proteins demonstrated that these structures were actually very thin myofibrils containing periodically organized α-actinin, actin and myosin typical of otherwise normal sarcomeres (Greenberg et al. 2008). The results showed that Krp1 is a scaffolding protein that plays a role in the transition of nascent myofibrils to mature myofibrils by promoting lateral fusion of assembly intermediates (Fig. 1, step 4). Additional information regarding Krp1 dynamics and binding partners will be essential for elucidating the molecular mechanism of Krp1 function.
Conclusions
The evidence shows that myofibril assembly is a complex process that requires the action of particular catalysts and scaffolds at individual steps. The scaffolds and chaperones required for assembly are potential regulators of myofibrillogenesis, and in principle these proteins could contribute to pathologic responses in diseases involving cardiac and skeletal muscles. Indeed, cardiac N-RAP levels are increased in patients with dilated cardiomyopathy as well as in two genetic mouse models of this disease (Ehler et al. 2001; Perriard et al. 2003). In the mouse models, the N-RAP upregulation occurs early, preceding remodeling of the intercalated disks and other morphologic changes associated with the dilated phenotype (Ehler et al. 2001). Likewise, heat shock chaperone proteins are generally involved in responses to cellular stress, and their role in the response of muscle to physiologic stresses and myopathies has been reviewed (Liu et al. 2006; Liu and Steinacker 2001). Mutations in chaperones and scaffolding proteins involved in myofibril assembly may yet be discovered to be the primary cause of particular diseases of striated muscles.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health.
Contributor Information
Garland L. Crawford, Department of Chemistry and Biochemistry, Bloomsburg University, Bloomsburg, PA, USA
Robert Horowits, Email: horowits@helix.nih.gov, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Department of Health and Human Services, Bethesda, MD 20892, USA.
References
- Adams J, Kelso R, Cooley L. The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol. 2000;10:17–24. doi: 10.1016/s0962-8924(99)01673-6. [DOI] [PubMed] [Google Scholar]
- Barral JM, Hutagalung AH, Brinker A, Hartl FU, Epstein HF. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science. 2002;295:669–671. doi: 10.1126/science.1066648. [DOI] [PubMed] [Google Scholar]
- Bloor JW, Brown NH. Genetic analysis of the Drosophila alphaPS2 integrin subunit reveals discrete adhesive, morphogenetic and sarcomeric functions. Genetics. 1998;148:1127–1142. doi: 10.1093/genetics/148.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SL, Horowits R. Myofibrillogenesis and formation of cell contacts mediate the localization of N-RAP in cultured chick cardiomyocytes. Cell Motil Cytoskeleton. 2000;47:63–76. doi: 10.1002/1097-0169(200009)47:1<63::AID-CM6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Carroll SL, Herrera AH, Horowits R. Targeting and functional role of N-RAP, a nebulin-related LIM protein, during myofibril assembly in cultured chick cardiomyocytes. J Cell Sci. 2001;114:4229–4238. doi: 10.1242/jcs.114.23.4229. [DOI] [PubMed] [Google Scholar]
- Carroll S, Lu S, Herrera AH, Horowits R. N-RAP scaffolds I-Z-I assembly during myofibrillogenesis in cultured chick cardiomyocytes. J Cell Sci. 2004;117:105–114. doi: 10.1242/jcs.00847. [DOI] [PubMed] [Google Scholar]
- Chow D, Srikakulam R, Chen Y, Winkelmann DA. Folding of the striated muscle myosin motor domain. J Biol Chem. 2002;277:36799–36807. doi: 10.1074/jbc.M204101200. [DOI] [PubMed] [Google Scholar]
- Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- Critchley DR. Focal adhesions - the cytoskeletal connection. Curr Opin Cell Biol. 2000;12:133–139. doi: 10.1016/s0955-0674(99)00067-8. [DOI] [PubMed] [Google Scholar]
- Dabiri GA, Turnacioglu KK, Sanger JM, Sanger JW. Myofibrillogenesis visualized in living embryonic cardiomyocytes. Proc Natl Acad Sci USA. 1997;94:9493–9498. doi: 10.1073/pnas.94.17.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhume A, Lu S, Horowits R. Targeted disruption of N-RAP gene function by RNA interference: a role for N-RAP in myofibril organization. Cell Motil Cytoskeleton. 2006;63:493–511. doi: 10.1002/cm.20141. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Antin PB, Nachmias VT, Holtzer H. The relationship between stress fiber-like structures and nascent myofibrils in cultured cardiac myocytes. J Cell Biol. 1984;99:2268–2278. doi: 10.1083/jcb.99.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du A, Sanger JM, Linask KK, Sanger JW. Myofibrillogenesis in the first cardiomyocytes formed from isolated quail precardiac mesoderm. Dev Biol. 2003;257:382–394. doi: 10.1016/s0012-1606(03)00104-0. [DOI] [PubMed] [Google Scholar]
- Du A, Sanger JM, Sanger JW. Cardiac myofibrillogenesis inside intact embryonic hearts. Dev Biol. 2008a;318:236–246. doi: 10.1016/j.ydbio.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du SJ, Li H, Bian Y, Zhong Y. Heat-shock protein 90alpha1 is required for organized myofibril assembly in skeletal muscles of zebrafish embryos. Proc Natl Acad Sci USA. 2008b;105:554–559. doi: 10.1073/pnas.0707330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehler E, Rothen BM, Hämmerle SP, Komiyama M, Perriard J-C. Myofibrillogenesis in the developing chicken heart: assembly of the z-disk, m-line and thick filaments. J Cell Sci. 1999;112:1529–1539. doi: 10.1242/jcs.112.10.1529. [DOI] [PubMed] [Google Scholar]
- Ehler E, Horowits R, Zuppinger C, Price RL, Perriard E, Leu M, Caroni P, Sussman M, Eppenberger HM, Perriard JC. Alterations at the intercalated disk associated with the absence of muscle LIM protein. J Cell Biol. 2001;153:763–772. doi: 10.1083/jcb.153.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein HF, Thomson JN. Temperature-sensitive mutation affecting myofilament assembly in Caenorhabditis elegans. Nature. 1974;250:579–580. doi: 10.1038/250579a0. [DOI] [PubMed] [Google Scholar]
- Etard C, Behra M, Fischer N, Hutcheson D, Geisler R, Strahle U. The UCS factor Steif/Unc-45b interacts with the heat shock protein Hsp90a during myofibrillogenesis. Dev Biol. 2007;308:133–143. doi: 10.1016/j.ydbio.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Etard C, Roostalu U, Strahle U. Shuttling of the chaperones Unc45b and Hsp90a between the A band and the Z line of the myofibril. J Cell Biol. 2008;180:1163–1175. doi: 10.1083/jcb.200709128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein R, Wang X, Song D, Cooke NE, Liebhaber SA. The LIM/double zinc-finger motif functions as a protein dimerization domain. Proc Natl Acad Sci USA. 1994;91:10655–10659. doi: 10.1073/pnas.91.22.10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehmlich K, Geier C, Osterziel KJ, Van Der Ven PF, Furst DO. Decreased interactions of mutant muscle LIM protein (MLP) with N-RAP and alpha-actinin and their implication for hypertrophic cardiomyopathy. Cell Tissue Res. 2004;317:129–136. doi: 10.1007/s00441-004-0873-y. [DOI] [PubMed] [Google Scholar]
- Geyer R, Wee S, Anderson S, Yates J, Wolf DA. BTB/POZ domain proteins are putative substrate adaptors for cullin 3 ubiquitin ligases. Mol Cell. 2003;12:783–790. doi: 10.1016/s1097-2765(03)00341-1. [DOI] [PubMed] [Google Scholar]
- Gray CH, McGarry LC, Spence HJ, Riboldi-Tunnicliffe A, Ozanne BW. Novel beta-propeller of the BTB-Kelch protein Krp1 provides a binding site for Lasp-1 that is necessary for pseudopodial extension. J Biol Chem. 2009;284:30498–30507. doi: 10.1074/jbc.M109.023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg CC, Connelly PS, Daniels MP, Horowits R. Krp1 (Sarcosin) promotes lateral fusion of myofibril assembly intermediates in cultured mouse cardiomyocytes. Exp Cell Res. 2008;314:1177–1191. doi: 10.1016/j.yexcr.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorio CC, Granzier H, Sorimachi H, Labeit S. Muscle assembly: a titanic achievement? Curr Opin Cell Biol. 1999;11:18–25. doi: 10.1016/s0955-0674(99)80003-9. [DOI] [PubMed] [Google Scholar]
- Handel SE, Greaser ML, Schultz E, Wang SM, Bulinski JC, Lin JJ, Lessard JL. Chicken cardiac myofibrillogenesis studied with antibodies specific for titin and the muscle and nonmuscle isoforms of actin and tropomyosin. Cell Tissue Res. 1991;263:419–430. doi: 10.1007/BF00327276. [DOI] [PubMed] [Google Scholar]
- Herrera AH, Elzey B, Law DJ, Horowits R. Terminal regions of mouse nebulin: sequence analysis and complementary localization with N-RAP. Cell Motil Cytoskeleton. 2000;45:211–222. doi: 10.1002/(SICI)1097-0169(200003)45:3<211::AID-CM4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Holtzer H, Hijikata T, Lin ZX, Zhang ZQ, Holtzer S, Protasi F, Franzini-Armstrong C, Sweeney HL. Independent assembly of 1.6 microns long bipolar MHC filaments and I-Z-I bodies. Cell Struct Funct. 1997;22:83–93. doi: 10.1247/csf.22.83. [DOI] [PubMed] [Google Scholar]
- Hutagalung AH, Landsverk ML, Price MG, Epstein HF. The UCS family of myosin chaperones. J Cell Sci. 2002;115:3983–3990. doi: 10.1242/jcs.00107. [DOI] [PubMed] [Google Scholar]
- Imanaka-Yoshida K. Myofibrillogenesis in precardiac mesoderm explant culture. Cell Struct Funct. 1997;22:45–49. doi: 10.1247/csf.22.45. [DOI] [PubMed] [Google Scholar]
- Imanaka-Yoshida K, Knudsen KA, Linask KK. N-cadherin is required for the differentiation and initial myofibrillogenesis of chick cardiomyocytes. Cell Motil Cytoskeleton. 1998;39:52–62. doi: 10.1002/(SICI)1097-0169(1998)39:1<52::AID-CM5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kim IF, Mohammadi E, Huang RC. Isolation and characterization of IPP, a novel human gene encoding an actin-binding, kelch-like protein. Gene. 1999;228:73–83. doi: 10.1016/s0378-1119(99)00006-2. [DOI] [PubMed] [Google Scholar]
- Kinose F, Wang SX, Kidambi US, Moncman CL, Winkelmann DA. Glycine 699 is pivotal for the motor activity of skeletal muscle myosin. J Cell Biol. 1996;134:895–909. doi: 10.1083/jcb.134.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A, Ackermann MA, Bowman AL, Yap SV, Bloch RJ. Muscle giants: molecular scaffolds in sarcomerogenesis. Physiol Rev. 2009;89:1217–1267. doi: 10.1152/physrev.00017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Miller JW, Mankodi A, Kanadia RN, Yuan Y, Moxley RT, Swanson MS, Thornton CA. Failure of MBNL1-dependent post-natal splicing transitions in myotonic dystrophy. Hum Mol Genet. 2006;15:2087–2097. doi: 10.1093/hmg/ddl132. [DOI] [PubMed] [Google Scholar]
- Liu Y, Steinacker JM. Changes in skeletal muscle heat shock proteins: pathological significance. Front Biosci. 2001;6:D12–D25. doi: 10.2741/liu. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gampert L, Nething K, Steinacker JM. Response and function of skeletal muscle heat shock protein 70. Front Biosci. 2006;11:2802–2827. doi: 10.2741/2011. [DOI] [PubMed] [Google Scholar]
- Lu S, Horowits R. Role of nonmuscle myosin IIB and N-RAP in cell spreading and myofibril assembly in primary mouse cardiomyocytes. Cell Motil Cytoskeleton. 2008;65:747–761. doi: 10.1002/cm.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Carroll SL, Herrera AH, Ozanne B, Horowits R. New N-RAP-binding partners α-actinin, filamin and Krp1 detected by yeast two-hybrid screening: implications for myofibril assembly. J Cell Sci. 2003;116:2169–2178. doi: 10.1242/jcs.00425. [DOI] [PubMed] [Google Scholar]
- Lu S, Borst DE, Horowits R. N-RAP expression during mouse heart development. Dev Dyn. 2005;233:201–212. doi: 10.1002/dvdy.20314. [DOI] [PubMed] [Google Scholar]
- Lu S, Borst DE, Horowits R. Expression and alternative splicing of N-RAP during mouse skeletal muscle development. Cell Motil Cytoskeleton. 2008;65:945–954. doi: 10.1002/cm.20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Herrera AH, Horowits R. Molecular interactions of N-RAP, a nebulin-related protein of striated muscle myotendon junctions and intercalated disks. Biochemistry. 1999;38:6135–6143. doi: 10.1021/bi982395t. [DOI] [PubMed] [Google Scholar]
- Luo G, Zhang JQ, Nguyen TP, Herrera AH, Paterson B, Horowits R. Complete cDNA sequence and tissue localization of N-RAP, a novel nebulin-related protein of striated muscle. Cell Motil Cytoskeleton. 1997;38:75–90. doi: 10.1002/(SICI)1097-0169(1997)38:1<75::AID-CM7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Manisastry SM, Zaal KJ, Horowits R. Myofibril assembly visualized by imaging N-RAP, alpha-actinin, and actin in living cardiomyocytes. Exp Cell Res. 2009;315:2126–2139. doi: 10.1016/j.yexcr.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohiddin SA, Lu S, Cardoso J-P, Carroll SL, Jha S, Horowits R, Fananapazir L. Genomic organization, alternative splicing, and expression of human and mouse N-RAP, a nebulin-related LIM protein of striated muscle. Cell Motil Cytoskeleton. 2003;55:200–212. doi: 10.1002/cm.10123. [DOI] [PubMed] [Google Scholar]
- Morano KA. New tricks for an old dog: the evolving world of Hsp70. Ann NY Acad Sci. 2007;1113:1–14. doi: 10.1196/annals.1391.018. [DOI] [PubMed] [Google Scholar]
- Perriard JC, Hirschy A, Ehler E. Dilated cardiomyopathy: a disease of the intercalated disc? Trends Cardiovasc Med. 2003;13:30–38. doi: 10.1016/s1050-1738(02)00209-8. [DOI] [PubMed] [Google Scholar]
- Price MG, Landsverk ML, Barral JM, Epstein HF. Two mammalian UNC-45 isoforms are related to distinct cytoskeletal and muscle-specific functions. J Cell Sci. 2002;115:4013–4023. doi: 10.1242/jcs.00108. [DOI] [PubMed] [Google Scholar]
- Rhee D, Sanger JM, Sanger JW. The premyofibril: evidence for its role in myofibrillogenesis. Cell Motil Cytoskeleton. 1994;28:1–24. doi: 10.1002/cm.970280102. [DOI] [PubMed] [Google Scholar]
- Risebro CA, Searles RG, Melville AA, Ehler E, Jina N, Shah S, Pallas J, Hubank M, Dillard M, Harvey NL, Schwartz RJ, Chien KR, Oliver G, Riley PR. Prox1 maintains muscle structure and growth in the developing heart. Development. 2009;136:495–505. doi: 10.1242/dev.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy DE, Yatskievych TA, Antin PB, Gregorio CC. Assembly of thick, thin, and titin filaments in chick precardiac explants. Dev Dyn. 2001;221:61–71. doi: 10.1002/dvdy.1125. [DOI] [PubMed] [Google Scholar]
- Sanchez-Garcia I, Axelson H, Rabbitts TH. Functional diversity of LIM proteins: amino-terminal activation domains in the oncogenic proteins RBTN1 and RBTN2. Oncogene. 1995;10:1301–1306. [PubMed] [Google Scholar]
- Sanger JW, Wang J, Holloway B, Du A, Sanger JM. Myofibrillogenesis in skeletal muscle cells in zebrafish. Cell Motil Cytoskeleton. 2009;66:556–566. doi: 10.1002/cm.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss T, Lin ZX, Lu MH, Murray J, Fischman DA, Weber K, Masaki T, Imamura M, Holtzer H. Differential distribution of subsets of myofibrillar proteins in cardiac nonstriated and striated myofibrils. J Cell Biol. 1990;110:1159–1172. doi: 10.1083/jcb.110.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Muller U. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4:673–685. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- Soltysik-Espanola M, Rogers RA, Jiang S, Kim TA, Gaedigk R, White RA, Avraham H, Avraham S. Characterization of Mayven, a novel actin-binding protein predominantly expressed in brain. Mol Biol Cell. 1999;10:2361–2375. doi: 10.1091/mbc.10.7.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JC, Schock F. The initial steps of myofibril assembly: integrins pave the way. Nat Rev Mol Cell Biol. 2009;10:293–298. doi: 10.1038/nrm2634. [DOI] [PubMed] [Google Scholar]
- Spence HJ, Johnston I, Ewart K, Buchanan SJ, Fitzgerald U, Ozanne BW. Krp1, a novel kelch related protein that is involved in pseudopod elongation in transformed cells. Oncogene. 2000;19:1266–1276. doi: 10.1038/sj.onc.1203433. [DOI] [PubMed] [Google Scholar]
- Spence HJ, McGarry L, Chew CS, Carragher NO, Scott-Carragher LA, Yuan Z, Croft DR, Olson MF, Frame M, Ozanne BW. AP-1 differentially expressed proteins Krp1 and fibronectin cooperatively enhance Rho-ROCK-independent mesenchymal invasion by altering the function, localization, and activity of nondifferentially expressed proteins. Mol Cell Biol. 2006;26:1480–1495. doi: 10.1128/MCB.26.4.1480-1495.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikakulam R, Winkelmann DA. Myosin II folding is mediated by a molecular chaperonin. J Biol Chem. 1999;274:27265–27273. doi: 10.1074/jbc.274.38.27265. [DOI] [PubMed] [Google Scholar]
- Srikakulam R, Winkelmann DA. Chaperone-mediated folding and assembly of myosin in striated muscle. J Cell Sci. 2004;117:641–652. doi: 10.1242/jcs.00899. [DOI] [PubMed] [Google Scholar]
- Srikakulam R, Liu L, Winkelmann DA. Unc45b forms a cytosolic complex with Hsp90 and targets the unfolded myosin motor domain. PLoS ONE. 2008;3:e2137. doi: 10.1371/journal.pone.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stogios PJ, Prive GG. The BACK domain in BTB-kelch proteins. Trends Biochem Sci. 2004;29:634–637. doi: 10.1016/j.tibs.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Taylor A, Obholz K, Linden G, Sadiev S, Klaus S, Carlson KD. DNA sequence and muscle-specific expression of human sarcosin transcripts. Mol Cell Biochem. 1998;183:105–112. doi: 10.1023/a:1006824331819. [DOI] [PubMed] [Google Scholar]
- Tullio AN, Accili D, Ferrans VJ, Yu ZX, Takeda K, Grinberg A, Westphal H, Preston YA, Adelstein RS. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc Natl Acad Sci USA. 1997;94:12407–12412. doi: 10.1073/pnas.94.23.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T, Fessler LI, Fessler JH. A role for integrin in the formation of sarcomeric cytoarchitecture. Cell. 1990;63:525–536. doi: 10.1016/0092-8674(90)90449-o. [DOI] [PubMed] [Google Scholar]
- Wang K. Sarcomere-associated cytoskeletal lattices in striated muscle. Review and hypothesis. Cell Muscle Motil. 1985;6:315–369. doi: 10.1007/978-1-4757-4723-2_10. [DOI] [PubMed] [Google Scholar]
- Wang SM, Greaser ML, Schultz E, Bulinski JC, Lin JJ, Lessard JL. Studies on cardiac myofibrillogenesis with antibodies to titin, actin, tropomyosin, and myosin. J Cell Biol. 1988;107:1075–1083. doi: 10.1083/jcb.107.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Knipfer M, Huang QQ, van Heerden A, Hsu LC, Gutierrez G, Quian XL, Stedman H. Human skeletal muscle nebulin sequence encodes a blueprint for thin filament architecture: sequence motifs and affinity profiles of tandem repeats and terminal SH3. J Biol Chem. 1996;271:4304–4314. doi: 10.1074/jbc.271.8.4304. [DOI] [PubMed] [Google Scholar]
- Wegele H, Wandinger SK, Schmid AB, Reinstein J, Buchner J. Substrate transfer from the chaperone Hsp70 to Hsp90. J Mol Biol. 2006;356:802–811. doi: 10.1016/j.jmb.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- Willis MS, Schisler JC, Portbury AL, Patterson C. Build it up-Tear it down: protein quality control in the cardiac sarcomere. Cardiovasc Res. 2009;81:439–448. doi: 10.1093/cvr/cvn289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth SL, Crawford BD, Pilgrim DB. The myosin co-chaperone UNC-45 is required for skeletal and cardiac muscle function in zebrafish. Dev Biol. 2007;303:483–492. doi: 10.1016/j.ydbio.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Xu L, Wei Y, Reboul J, Vaglio P, Shin TH, Vidal M, Elledge SJ, Harper JW. BTB proteins are substrate-specific adaptors in an SCF-like modular ubiquitin ligase containing CUL-3. Nature. 2003;425:316–321. doi: 10.1038/nature01985. [DOI] [PubMed] [Google Scholar]
- Zhang JQ, Elzey B, Williams G, Lu S, Law DJ, Horowits R. Ultrastructural and biochemical localization of N-RAP at the interface between myofibrils and intercalated disks in the mouse heart. Biochemistry. 2001;40:14898–14906. doi: 10.1021/bi0107445. [DOI] [PubMed] [Google Scholar]
- Zhang DD, Lo SC, Sun Z, Habib GM, Lieberman MW, Hannink M. Ubiquitination of Keap1, a BTB-Kelch substrate adaptor protein for Cul3, targets Keap1 for degradation by a proteasome-independent pathway. J Biol Chem. 2005;280:30091–30099. doi: 10.1074/jbc.M501279200. [DOI] [PubMed] [Google Scholar]
- Zhao R, Houry WA. Molecular interaction network of the Hsp90 chaperone system. Adv Exp Med Biol. 2007;594:27–36. doi: 10.1007/978-0-387-39975-1_3. [DOI] [PubMed] [Google Scholar]