Abstract
The RE1-silencing transcription factor (REST) represses the expression of neuronal-specific genes in non-neuronal cells by recruiting histone deacetylases (HDACs) and other histone modifying and chromatin remodeling proteins to the DNA. REST regulation of the expression of neuronal genes is required for the orderly developmental transition from a neuroepithelial stem cell to a functional neuron. Here, we examined the expression and function of REST in neonatal rat oligodendrocyte precursor cells (OPCs). OPCs develop from the same neuroepithelial stem cells as neurons, can be reprogrammed to act as neural stem-like cells in vitro and require HDAC-mediated gene repression to develop into mature oligodendrocytes. We show that OPCs express functional REST protein and that REST interacts with several neuronal-specific genes whose expression is repressed in OPCs. REST transcript and protein expression increased 4-fold during the first 48hrs of oligodendrocyte differentiation. During this differentiation, the expression of RE1 containing neuronal genes further decreased as the transcription of oligodendrocyte specific genes was activated. Expression of a dominant negative form of REST in OPCs prevented the cells from developing into mature MBP-positive oligodendrocytes. Rather, the cells began to develop a neuronal phenotype characterized by increased expression of neuronal proteins, transcription factors, and cell type-specific marker antigens. REST over-expression promoted the development of 04-positive pre-oligodendrocytes from OPCs. Together, these results show that REST function is required for the differentiation of OPCs into oligodendrocytes. By regulating the expression of neuronal genes, REST may also regulate the phenotypic plasticity of OPCs.
Keywords: RE1, REST, oligodendrocyte precursor cell, HDACs, epigenetics
INTRODUCTION
Oligodendrocytes, the myelin forming cells of the CNS, develop from an identified precursor/progenitor cell referred herein as an oligodendrocyte precursor cell or OPC. The differentiation of OPCs occurs in a step or stage-wise progression and is regulated by both genetic and epigenetic mechanisms. Marin-Husstege et al., (2002) first showed that class 1 histone deacetylases (HDACs) are required for the differentiation of OPCs. By removing acetyl residues from histone tails, HDACs help place the chromatin in a condensed state such that transcription activating factors cannot access the DNA and gene expression is repressed. HDACs are recruited to the chromatin as multi-protein complexes by sequence-specific DNA binding proteins and these binding proteins confer cellular specificity to the covalent modifications. Inhibiting HDACs in developing OPCs not only prevents oligodendrocyte differentiation but also confers lineage plasticity on the cells and promotes the development of neuronal properties (Liu et al., 2007; Lyssiotis et al., 2007; Marin-Husstege et al., 2002). Understanding how epigenetic mechanisms regulate this plasticity is important since OPCs can give rise to neurons, astrocytes and oligodendrocytes throughout the life of the organism (Aquirre Gallo, 2004; Belachev et al., 2003; Guo et al., 2009, 2010; Rivers et al., 2008; Zhu et al., 2008).
Principal among the proteins that recruit HDACs and other chromatin and DNA modifying enzymes to DNA in the developing nervous system is the transcriptional regulator REST (RE1 silencing transcription factor, also known as neural restricted silencing factor or NRSF) (Ballas, Mandel, 2005). Through interactions with a 21-base-pair repressor element in DNA (the RE1), REST regulates the expression of a large number of neuronal genes including those that code for transcription factors, neurotransmitter receptors, cytoskeletal proteins, ion channels, and neurotrophins (Bruce et al., 2004; Bruce et al., 2009; Otto et al., 2007). REST recruits the corepressors mSin3 and CoREST to its N- and C- terminal domains respectively to mediate gene repression (Grimes et al., 2000). REST can form complexes with other co-repressors and these diverse complexes regulate different genes in different cell types (Johnson et al., 2008; Mulligan et al., 2008). Lastly, REST can also recruit gene silencing enzymes including MeCP2 and methyltransferase G9a to effectively silence large regions of chromatin, including genes that lack an RE1 (Lunyak et al., 2002).
REST regulates the neuronal differentiation of embryonic and neural stem cells (Ballas et al., 2005), but its role in the oligodendrocyte lineage is unknown. About 10% of the genes whose expression is highly reduced during the OPC to oligodendrocyte transition contain RE1s (Bruce et al., 2004; Cahoy et al., 2008), many of which are occupied by either REST or CoREST (Abrajano et al., 2009). These observations suggest that REST function may be required during oligodendrocyte differentiation. Here, we asked whether REST can regulate OPC differentiation. We used loss-of-function and gain-of-function approaches to show that REST is required for the repression of neuronal properties in OPCs and their development into oligodendrocytes. These results suggest that REST may play important roles in regulating the development and plasticity of OPCs.
MATERIALS AND METHODS
Cell culture
Rat glial cultures were prepared from postnatal day 1–3 rat pups of either sex using a modified shaking method (McCarthy and de Vellis, 1980; Yang et al., 2005). Dissociated cortical cells were grown for 10 days in T-75 flasks and shaken at 37°C at 250rpm overnight (16–20hrs) followed by an additional 30 minutes at 300rpm. Nonadherent cells were filtered through a 20 micron nylon mesh filter (Spectrum Laboratories, Inc.) to remove astrocyte aggregates. The remaining cells underwent two rounds of differential adhesion on tissue culture dishes (Corning, NY) for 30 minutes each at 37°C. The non-adherent cells (OPCs) were plated on 25µg/ml poly-L-lysine (PLL, Sigma) coated tissue culture dishes or 50µg/ml PLL coated glass coverslips and grown in proliferation media (Neurobasal A Medium supplemented with B27 containing 1% Penicillin/Streptomycin (P/S), 1mM L-Glutamine (all from Gibco), 10ng/ml platelet-derived growth factor AA (PDGFAA) and 5ng/ml fibroblast growth factor basic (bFGF) (PreproTech). To initiate oligodendrocyte differentiation, OPCs were switched to DMEM supplemented with N2 (InVitrogen), 1%P/S, 1% fetal bovine serum (FBS, Gemini Bio-products), 40ng/ml thyroxin (T4) and 30ng/ml triidothyronine (T3) (Sigma). For protein analysis, the cells were grown in oligodendrocyte media for 6 days. Type 2 astrocyte (2A) development was initiated by switching the cells to DMEM, 1%P/S and 10% FBS. In some experiments, the cells were grown in a defined media comprising NBA/B27 supplemented with 1% P/S, 1% FBS and 1mM L-Glutamine). Type I astrocytes were prepared by removing the adherent cells from the 75cm2 tissue culture flasks following the overnight shake with trypsin and growing them in DMEM containing 1%P/S and 10% FBS. Primary cultures of rat optic nerve cells and cerebellar granule neurons were grown as described previously (Dou and Levine, 1994; Levine, 1989). Rat embryonic fibroblast (REF) and HEK293 cell lines were grown in DMEM supplemented with 1% P/S and 10% FBS. Rat pheochromocytoma PC12 cells were maintained in DMEM containing 1% P/S, 5% FBS, and 10% horse serum. Plat-E cells were grown in DMEM containing 10% FBS, 1% P/S, 1µg/ml puromycin dihydrochloride (Sigma) and 10µg/ml Blasticidin S HCl (Invitrogen). HCN cells were obtained from Dr. F Gage and maintained as described (Hsieh, et al., 2004). HT22 cells were a gift from Dr. S. Ge. All cell lines were passed 1:5 every 3–5 days.
Nucleofections
Transfections were performed using the Nucleofector electroporation system as per the manufacturer’s instructions (Amaxa). Cells were nucleofected with 1–3µg of DNA and 3×106 cells were used per nucleofection. Prior to nucleofection, the cells were grown overnight in proliferation media with high levels of growth factors (20ng/ml PDGFAA and 20ng/ml bFGF) in uncoated Petri dishes at a concentration of 4–6×106 cells per dish. OPCs were harvested by gentle pipetting, centrifuged, and incubated in DMEM containing 1% P/S and 10% FBS (about 3×106 cells/ml media) for 1hr at 37°C prior to nucleofection. The cells were nucleofected with either pcDNA1-DnREST (Chong et al., 1995, Otto et al., 2007), pcDNA3-REST-VP16 (Immaneni, et al., 2000, a gift from Dr. S. Majumder), pcDNA1.1-REST (a gift from Dr. N.Ballas) or an empty vector (Invitrogen) all mixed with 0.5–1.0µg pMAXeGFP (AMAXA).
Immunofluorescence staining
A list of all primary antibodies can be found at the end of Materials and Methods. Secondary antibodies used for immunocytochemistry include AlexaFluor594 donkey anti-rabbit, AlexaFluor488 donkey anti-rabbit, AlexaFluor594 donkey anti-mouse (all from Molecular Probes, Inc). Live cell staining of cell surface antigens was performed using antibodies against NG2, A2B5, and O4 as described previously (Levine,Stallcup, 1987). Cells (unless being double labeled to visualize REST expression, see below for REST detection) were fixed with 4% buffered paraformaldehyde (PFA) for 10–15min and washed 3x in PBS. To visualize the expression of MBP, GFAP, GFP and TUJ1, cells were fixed in PFA, rinsed as above (in some cases cells were first live stained) and incubated in a permeabilizing solution (PBS containing 5% normal donkey serum (NDS), 0.01% sodium deoxycholate, and 0.02% NP40) for 30 minutes at room temperature. Primary and secondary antibodies were diluted in permeabilizing solution and staining was performed as above.
For immunofluorescence staining to detect REST protein, cells underwent microwave fixation. Coverslips were rinsed 2x’s in PBS, covered in 4% PFA and placed on a glass plate over ice for 20 seconds in a microwave (Pelco 3451 Lab Microwave System with a Pelco 3420 Microwave Load Cooler and a Pelco 3430 Microwave Power Controller). Cells were rinsed 3x’ with PBS, blocked, permeabilized and stained as above. Cells were examined, counted and photographed with a Ziess Axioplan2 microscope.
BrdU labeling
Retroviral infected OPCs were grown in proliferation media for 4d followed by a media switch to either proliferation, oligodendrocyte, defined media, or 2A growth media for 44hrs. 10µM 5-Bromo-2’-deoxyuridine (BrdU) (Sigma) was added to the culture media and the cells were incubated for 4 hours at 37°C. Cells were rinsed in PBS, fixed in 4% PFA for 10 minutes, washed and then immunofluorescently stained to detect GFP and BrdU.
Clonal analysis
OPCs were purified and plated in 25µg /ml PLL coated 100mm tissue culture dishes in proliferation media for 1–2 days and then infected with either pMXsIG or pMXsIG-DNREST (see below). OPCs were grown for 5days post infection in proliferation media and then passed onto coated 18mm glass coverslips (50µg /ml PLL) at a density of approximately 1,000 cells per coverslip. The cells were then grown for 2 additional days in proliferation media, washed with L15, and fed either proliferation, oligodendrocyte, defined, or 2A differentiation media. Nucleofected OPCs were plated directly at clonal density without the intervening proliferation period.
Cell extracts and immunoblot analysis
To isolate optic nerve protein, optic nerves were homogenized in a solution containing 2% sodium dodecyl sulfate (SDS), 10mM Tris (pH 7.5), 5mM EDTA (pH8.0), 200µM phenylmethanesulfonyl fluoride (PMSF), 1µg /ml aprotinin and leupeptin using a Wheaton overhead stirrer (Wheaton Instruments) and glass on glass tissue grinder (Duall 20, Kontes Glass Co). Samples were centrifuged at 30,000 rpm for 15 minutes at 20°C in an Optima TLX ultracentrifuge (Beckman Instruments, Palo Alto, CA). The supernatant was collected and protein concentration determined using a Pierce BCA Protein Assay (Micro BCA Protein Assay Kit, Thermo Scientific). For primary cells and cell lines, whole cell lysates were prepared by rinsing the cells 2x in cold PBS and incubating them on ice in NP-40 lysis buffer (25mM Tris (pH 7.5), 125mM NaCl, 1.0% NP-40, 1mM EDTA, 200uM PMSF, 1µg /ml aprotinin, and leupeptin) for 30 minutes. Samples were centrifuged at 14K for 10 minutes at 4°C and the supernatant was collected. Nuclear extracts were prepared using a modified Dignam method (Grimes et al., 2000). Protein concentrations were measured using a Bradford assay (Bio-Rad). All samples were boiled for 3 minutes in 6X SDS-loading buffer. SDS-polyacrylamide gel electrophoresis and immunoblotting were performed using standard techniques. Proteins were electrophoresed on 6 to 8% polyacrylamide gels, electrophoretically transferred to nitrocellulose (HybondTM-ECL, Amersham Biosciences) and blocked in Tris-buffered saline (TBS) or TBS+0.5% Triton X-100 (TBST) containing 5% non-fat dry milk. Primary antibodies used are listed at the end of the Materials and Methods and detection was carried out with ECL™ anti-rabbit IgG horseradish peroxidase linked whole antibody (from donkey) or ECL™ anti-mouse Ig horseradish peroxidase linked whole antibody (from sheep) secondary antibodies (Amersham Biosciences).
Chromatin immunoprecipitation assays
ChIP assays were performed as described previously (Ballas et al., 2001). The chromatin was immunoprecipitated after diluting the cleared chromatin 1:10 in an IP buffer (0.01% SDS, 1.1% Triton X-100, 1.2mM EDTA, 16.7mM Tris-HCL (pH 8.0), 167mM NaCl, and complete Mini protease inhibitor cocktail) with 4µg /ml of either rabbit anti-REST-P73 (Chong et al., 1995), rabbit anti-REST-C (Ballas et al., 2001), or a non-specific rabbit IgG antibody (Santa Cruz) for 12–16 hours at 4°C. The following primers were used to amplify DNA regions containing an RE-1 sequence:
Scn2a1 left CCAAGATTATATGTCAGCTCGCAG,
right CAACTTCGTCAAGCAGGGTCAGG,
NF-M, left GTTCATTGTGCCAACTCTGCAGTGC,
right GGACATCTGAAGCTTCTAGCAGGTAAGG,
SCG10, left ACAGTCCGAAGTGCAAATGCCAG,
right CTGCCGTTCAGCAAATCATCCAG,
NeuroD2, left GACTGGAGGCATAGGTCACCTTTCTAC,
right GGAAGTCACCACTAGCACATTTCGCG,
Grik3, left CCTGTTAGGAGGGTCGCCCAG,
right CCGCCACAGAAGGCAGAAGACC.
Primers were built around a randomly chosen region of the NG2 gene (CSPG4) that does not contain a RE1 (left CTTCTCTGGACCCCACTCAC, right TGGCCCTCAAATATGTCCAC).
A 50ul PCR reaction contained 2ul of eluted chromatin, 5ul 10X PCR Buffer (Roche), 5ul 10X PCR Enhancer (Invitrogen), 200mM dNTPs (Roche), 200nM forward and reverse primers, and 0.5ul Taq Polymerase (Roche). DNA was amplified using a Gene AMP PCR System 9700 (Applied Biosystems) with an initial denaturation at 94°C for 4min, 40 cycles of 94°C for 30sec, 56–58°C for 30sec, and 72°C for 1min, ending with a final 7min incubation at 72°C. Samples were analyzed on 1.5% agarose gels.
Quantitative real time PCR
Total RNA from primary cells, cell lines, and minced whole optic nerve was isolated by TRIZOL RNA extraction (Invitrogen) and treated with RNase-free DNase (TURBO DNA-free, Ambion) following both manufacturers’ protocols. RNA concentrations were measured using a NanoDrop 1000 spectrophotometer and 250–300ng of total RNA was used for reverse transcription with Superscript II Reverse Transcriptase (Invitrogen) via manufacturer’s protocol. Quantitative real-time PCR was performed using FastStart Universal SYBR Green Master Mix (Roche) in an Applied Biosystems 7300 Real-Time PCR System under the cycling conditions described previously (Otto et al., 2007). The relative abundance of the specific mRNA being examined was normalized to glyceraldehydes 3-phosphate dehydrogenase (GAPDH). All primers were designed using Massachusetts Institute of Technology’s Primer3 software.
Calb1: Left CCGAACAGATCTTGCCCTTA,
Right GCGCACAGTTATGGTTTTAGA,
CelsR3: Left CCATCCTTGCCTCTTTCAAC,
Right TGGAAGGTCCAAGTGCAGA,
CNP: Left AGTGCAAGACGCTGTTCATC,
Right AAGCATCAGCAGACACCATCT,
DCX: Left ACAGAACCAGAACCTTGCAG,
Right AGCGTACACAATCCCCTTGA,
GFAP: Left AGTTACCAGGAGGCACTCG,
Right CGATGTCCAGGGCTAGCTTA,
GFAP: Left AGTTACCAGGAGGCACTCG,
Right CGATGTCCAGGGCTAGCTTA,
GluR2: Left GGGCGCTGATCAAGAATACA,
Right GCGAAACTGTTGGCTACCTC,
Hes1: Left CTGACGGCCAATTTGCTT,
Right GTTAGGACCCACCGAGGTC,
Hes5: Left GAGATGCTCAGTCCCAAGGA,
Right GCAGTTTCAGCTGCTCAATG,
Hes5: Left AAGAGCCTGCACCAGGACTA,
Right CGCTGGAAGTGGTAAAGCAG,
Id2: Left CTCCAAGCTCAAGGAACTGG,
Right CGATCTGCAGGTCCAAGATA,
Id4: Left GTGCGATATGAACGACTGCT,
Right CTGCAGGTCCAGGATGTAGTC,
MAG: Left GTGGGCTCCCTTTCTCTTG,
Right ATGTTGGCAGAGAGGAGCAG,
Mash1/acl1: Left GCCCAGCACTCTCTCACTTC,
Right TGGGATTATTTGGCTGAACC,
MBP Left CACAAGAACTACCCACTACGG,
Right GGGTGTACGAGGTGTCACAA,
MOBP: Left CCCACCCTTCACCTTCCT,
Right CCAGTCCTCCTCCTTCTTCTG,
MYT1 LIKE: Left CGTGACTACTTTGACGGAAATG,
Right AATTCCTCTCACAGCCTGCTT,
Myt1: Left TCATTGCAACTTCCCTCCTAA,
Right GGTCCTTTTCACCCTCATCA,
Nav1.2: Left GATCCTCATCTGCCTCAACA,
Right TGAACAGGACAATGAACACCA,
NF-M: Left GAGAGCAGCCTCGACTTCAG,
Right CCCTGCAGCTGCTCTTTCT
NG2: Left ACCCAGCAGGATGGCTTC,
Right CTCATTCACGTCCCTCACAG,
NeuroD1: Left CCAAATCATACAGCGAGAGC,
Right CTCCTTCTTGTCTGCCTCGT,
NeuroD1: Left AGGAACACGAGGCAGACAAG,
Right TCTTGGGCTTTTGATCATCC,
Nkx2.2: Left GTGATCATCGTTGCCAAATG,
Right AAACCGATGCAACTCAAACA,
PDGFαR: Left CACACCGGATGGTACACTTG,
Right GCAGAATCGTCCTCTTCCAC,
Olig1: Left AACTCCTTGGACCAGTGCAT,
Right CGCCAGTTAAATTCGGCTAC,
Olig2:Left GTGCATCACCCCATCCTC,
Right CCGATGGAGACTTGAGCAG,
PLP: Left GCACTGTTCTGTGGATGTGG,
Right GAAGAAGAAAGAGGCAGTTCCA,
PLP: Left GCTGTGCTAGATGTCTGGTAGG,
Right GCTTCATGTCCACATCCACA
Pou4F3: Left GCTCAAGCCAGTCCTCCA,
Right GTTTGCGCTTACGCTCACT,
POU4F3: Left AGAGAAGCGCTCACTCGAAG,
Right GGACAGCAGAGTACTTCATTCG,
SCG10: Left GGCATTTGTGCCACTGTAAG,
Right CAGCAACCTTCAGGAGTTGG,
SoxII: Left ATGGGCTCTGTGGTCAAGTC,
Right CTGATCATGTCCCGGAGGT,
Sox10:Left AACCTCATCCCTCACCTAACTG,
Right GCAGTGCTAACTGAGGCTGA,
SYN1: Left GAAGCCAGACTTTGTGCTGA,
Right ACTGGGGATCCCAGCATAC,
SYT II: Left GATCTTCGTAGGCAGCAACG,
Right CCTCAGGCTTCAGAGAGTGC,
TrkC: Left ACTATGTGGGCTCCGTGCT,
Right CCAGGAGGGGAAAGAGGTT,
Tubb3: Left CATGAACGACCTGGTGTCTG,
Right CTGGGCTTCCGACTCCTC,
YY1: Left ATGAGAAAGCATCTGCACACC,
Right CCAGCTGGTGGTCGTTTTAGC
Luciferase assay
Luciferase reporter assays were carried out as described previously (Conaco et al., 2006; Otto et al., 2007).
Adenoviral vectors and infection
Adenoviral vectors (pAdTrackCMV vector containing GFP) expressing dominant negative REST (DnREST, lacks the sequences coding for the amino- and carboxy- terminal repressor domains), REST-VP16 (which contains the same region of the REST gene as DnREST fused to the activation domain of the herpes simplex viral protein VP16), or expressing GFP were prepared and used as described previously (Ballas et al., 2005; Chong et al., 1995). OPCs were purified and infected after 2–3 days in proliferation media. For oligodendrocyte and 2A infection, OPCs were purified, grown in proliferation media for 2–3d, and switched to either oligodendrocyte or 2A differentiation media for 3 days prior to infection. Type I astrocytes were isolated as described previously and were infected 3–6 days post purification. All cells were infected for 8–16 hours with adenovirus at an MOI (multiplicity of infection) of 10–25.
Retroviral vectors and infection
DnREST was cloned into the pMXs-IRES-GFP (pMXsIG) vector (Kitamura et al., 2003) between the EcoRI and XhoI sites. PMXsIG and pMXsIG-DnREST retrovirus were assembled in Plat-E cells (Morita et al., 2000). Puromycin dihydrochloride, Blasticidin S HCl, and P/S were removed from Plat-E cell media 24hrs prior to transfection and retroviral vectors were transfected into Plat-E cells using TransIT-293 transfection reagent (MirusBio) following manufacturer's protocol. After 24, 36, and 48 hours the supernatant was collected and stored at −80°C. The retrovirus-containing supernatant was concentrated by ultracentrifugation for 90min at 4°C at 25,000rpm and resuspended in a small volume of PBS. OPCs were purified and infected after 1–2 days in proliferation media. Cells were infected to 60–80% efficiency by incubating the cells with virus for 5–8hrs with 3µg/ml Polybrene (Sigma) (final retroviral concentrations were at least 106 colony forming units (CFU)/ml).
Tissue processing and immunofluorescence staining of optic nerve
Cryostat sections of developing and adult optic nerve were processed and stained as described previously (Tan et al., 2006).
Primary antibodies
Antibodies listed were used for: immunofluorescence staining (IF), Western Blot analysis (WB), cell isolation/purification (CP) and chromatin immunoprecipitation assay (ChIP). Mouse anti-NG2 (Chemicon) IC, Mouse anti-A2B5 (ATCC) IC, CP, Rabbit anti-REST-C (Gail Mandel) IC, IH, WB, ChIP, Rabbit anti-REST P73 (Gail Mandel) ChIP, Mouse anti-TUJ1 (Covance) IC, WB, Mouse anti-O4 (a gift from R. Bansi, U of Conn Health Science Center) IC, Mouse anti-MBP (Millipore) IC, Mouse anti-GFAP (Sigma) IC, Rabbit anti-synapsin I (Millipore) WB, Mouse anti-neurofilament (DSHB) WB, Mouse anti-beta actin (Sigma) WB, Rabbit anti-histone H3 (Cell Signaling) WB, Rabbit anti-CoREST (Gail Mandel) IC, WB, Rabbit anti-Snap25 (Abcam) WB, Rabbit anti-GFP (Molecular Probes) IC, Rabbit anti-SCG10/Stathmin-2 (UC Davis/NIH Neuromab Facility) WB, Mouse anti-CNPase (Sigma) WB,Mouse anti-BrdU (Sigma) IC.
Statistical Methods
The significance of all pair-wise comparisons was determined using Student’s t-test. For multiple comparisons, we used a one-way ANOVA followed by the Holm-Sidak post-hoc test.
RESULTS
Rest is expressed in glial cells
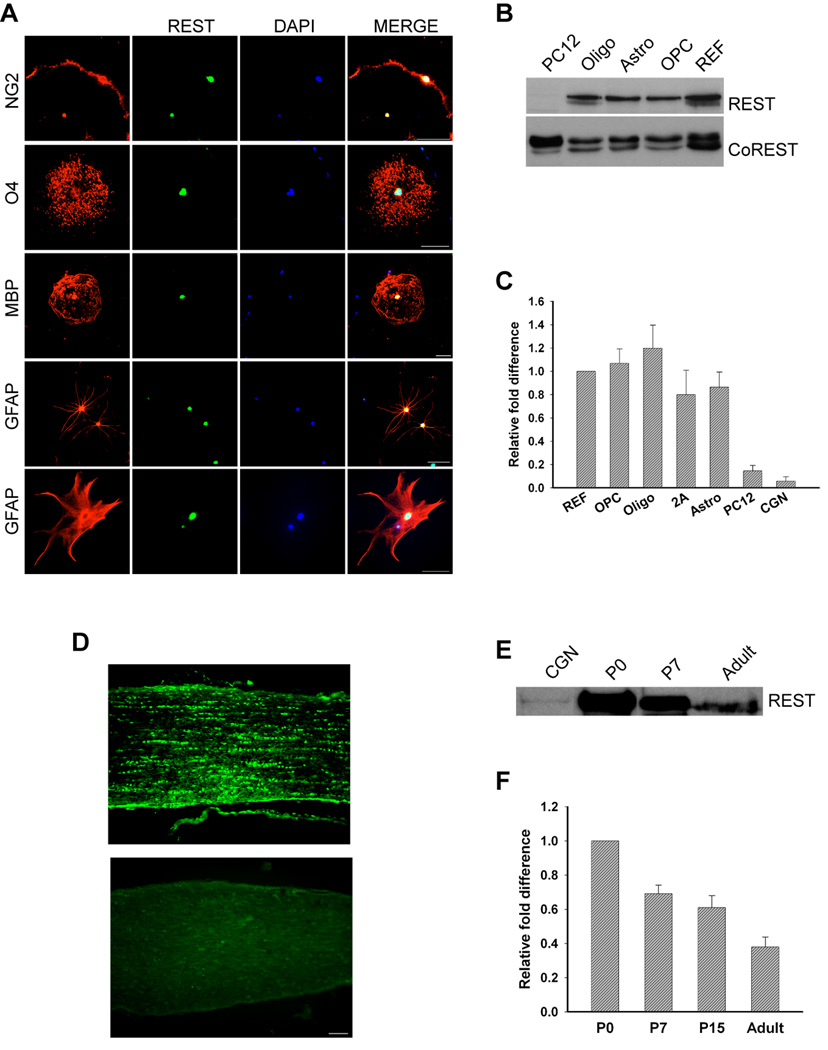
To examine REST expression and function in primary rat glia, we dissociated newborn rat cortices and isolated different types of glia using a modification of the shaking method (Yang et al., 2005). OPCs, oligodendrocytes, type I and type II astrocytes (2As) were grown in different media as described under Materials and Methods. In the astrocyte and OPC cultures, ~95% of the cells expressed the appropriate cell-type speific marker antigens (data not shown). When grown under conditions that foster oligodendrocyte differentiation for 5days, 90% of the cells expressed the O4 antigen and 40% expressed MBP (data not shown). As shown in figure 1A, all glial cells examined expressed REST protein in their nuclei. Immunoblot analysis of nuclear protein extracts confirmed that the different glial types expressed both REST and its co-repressor protein, CO-REST (figure 1B). In these biochemical experiments, rat embryo fibroblasts (REFs) served as positive controls and undifferentiated PC12 cells, which express trace amounts of REST mRNA, served as negative controls. The different types of glia expressed REST transcripts (figure 1C) and protein (figure 1B) at comparable levels. Glial cells also express mSin3a (Ballas et al., 2009) suggesting that a functional repressor complex exists in these cells. Because these glial cells were grown in culture for 10–12 days prior to shaking, we wanted to rule out the possibility that REST expression was induced by the relatively long cell culture period. We therefore dissociated cells from postnatal day 7 rat optic nerve, plated the cells onto coated coverslips for three hours and immunofluorescently stained the cells with anti-REST antibodies. REST protein was detected in greater than 90% of OPCs, pre-oligodendrocytes and astrocytes (figure S1). To confirm REST expression in developing glia in vivo, we stained sections of postnatal day 12 rat optic nerve with anti-REST antibodies. As shown in Figure 1D, the antibodies detected linear arrays of nuclei, a staining pattern typical for developing glia of the oligodendrocyte lineage. Both REST protein and mRNA were present in newborn and P7 optic nerves and levels of both the protein and mRNA declined with increasing age (figure 1E and F). Together these data show that developing glia, including cells of the oligodendrocyte lineage, express REST. The low levels of REST in adult glia suggest that REST function may be required principally during development.
Figure 1. REST is expressed in the nuclear compartment of primary rat glial and in rat optic nerve.
Purified OPCs, astrocytes, and oligodendrocytes were grown as described under Material and Methods for 5–6 days and REST protein and transcript levels measured. A. Immunofluorescence staining detects REST in the nuclei of cultured glial cells. bar=50µm. B. Immunoblot showing the detection of REST and Co-REST protein in nuclear extracts prepared from oligodendrocytes (oligos), astrocytes (astros), OPCs and rat embryo fibroblasts (REFs). REST was not present in extracts of PC12 cells. C. Quantitative real-time PCR analysis showing the fold changes in REST mRNA in rat glia relative to REFs after normalization to GAPDH. REST expression is lower in PC12 cells and cerebellar granular neurons (CGNs). Error bars represent the standard deviation, n=3. D. Immunofluorescence detection of REST in post-natal day 12 rat optic nerve (top). The bottom panel is control staining with secondary antibody alone. bar=50µm. E. Immunoblot analysis of REST protein in P0, P7 and adult rat optic nerve. CGNs serve as a negative control. F. Real-time PCR analysis showing the fold change in REST expression in P7, P15 and adult rat optic nerve relative to P0 after normalization to GAPDH. Error bars represent the standard deviation from six PCR runs; three runs each from two separate experiments.
Rest is a functional gene repressor in developing glia
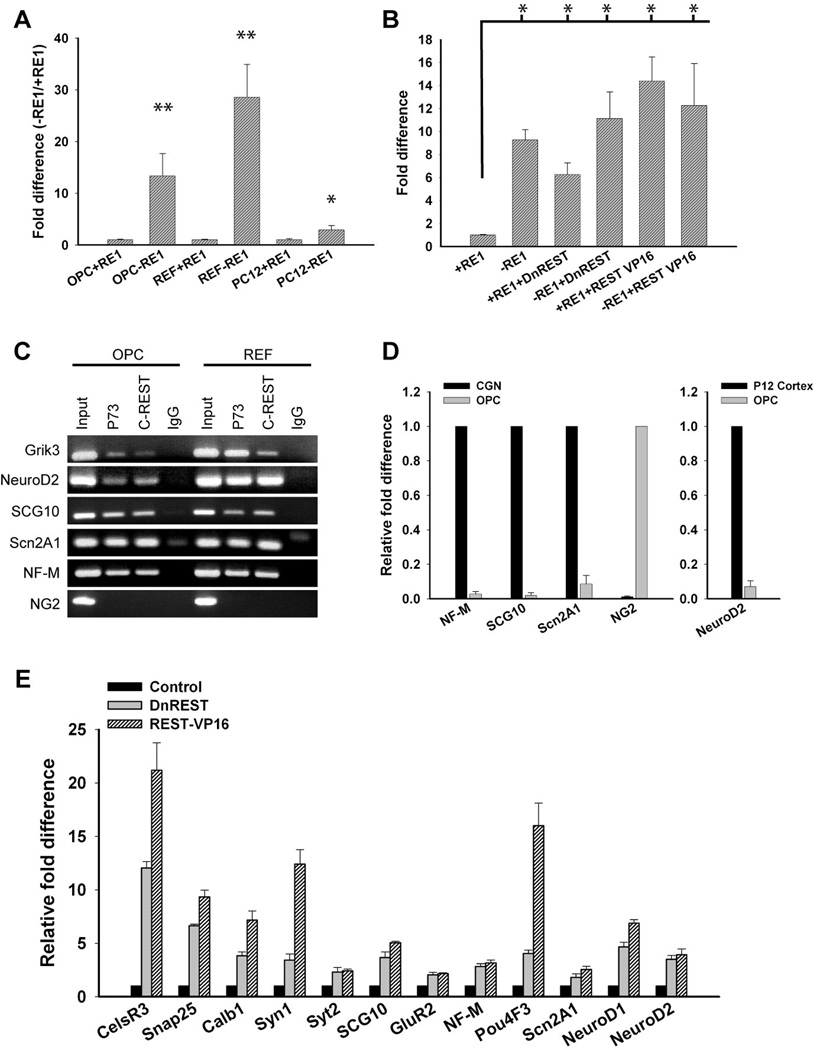
We used a luciferase reporter assay (Otto et al., 2007) to determine whether REST functions as a repressor in oligodendrocyte lineage cells. OPCs, REFs and PC12 cells were nucleofected with a plasmid containing a region of the GAD1 gene promoter with or without an RE1 site upstream of a minimal TK promoter capable of driving Photalis pyralis luciferase expression. If cells express functional REST protein, then luciferase activity is reduced when the RE1 is present. As shown in figure 2A, luciferase activity was 13.4 fold higher in OPCs expressing the RE1-negative construct as compared to cells expressing the RE1-containing construct. To confirm that a REST/RE1 interaction was responsible for the decreased luciferase activity, cells were co-nucleofected with a plasmid expressing DnREST or REST-VP16. The DN-REST construct contains the DNA binding domain but not the N and C-terminal corepressor binding domains (Chong et al., 1995). It competes with endogenous REST for DNA binding but does not repress gene transcription. The REST-VP16 construct has both corepressor domains deleted and the C-terminal domain is replaced by the activator domain of VP-16 (Immaneni et al., 2000). When transfected into cells, this construct activates the transcription of RE1-containing genes and initiates neuronal differentiation (Su et al., 2004). DnREST derepressed luciferase gene expression only when the RE1 was present: REST-VP16 further activated luciferase expression, also in an RE1-dependent manner, although the difference between REST-VP16 and DnREST was not statistically significant (figure 2B). These results demonstrate that REST can act as a functional transcriptional repressor in OPCs.
Figure 2. REST is a functional repressor protein in OPCs.
A. Luciferase reporter assay showing that RE1-containing reporters are repressed in OPCs but not PC12 cells. Error bars represent the standard deviation from three separate experiments. * P value < .03, ** P value <.01. B. Luciferase reporter assay showing that dominant negative REST (DnREST) and REST-VP16 derepressed luciferase gene expression in the presence of a RE1. Error bars represent the standard deviation, n=3. * P value <.01. C. Chip assays showing that REST binds to an RE1 element in Grik3, NF-M, SCG10, Scn2a1 and NeuroD2 but not to a randomly chosen site upstream of the NG2 (CSPG4) gene in both OPCs and REFs. D. Quantitative real-time PCR showing the fold difference in NF-M, SCG10, and Scn2a1 relative to CGNs, NG2 relative to OPCs, and NeuroD2 relative to P12 rat cortex after normalization to GAPDH. Error bars represent the standard deviation, n=3. E. DnREST and RESTVP16 induced changes in the expression of RE1-containing genes. Purified OPCs were infected with either dominant negative REST (DnREST), REST-VP16, or GFP control adenoviruses and grown in proliferation media for 72hrs. Transcript levels of REST regulated neuronal genes were analyzed by real-time PCR. The relative fold change in gene expression is shown relative to control infected cells after normalization to GAPDH. Error bars represent the standard deviation from 6 PCR runs; all gene expression changes had P values < 0.01.
We used chromatin immunoprecipitation assays to determine whether REST interacts with the RE1 element in known REST-regulated genes. REST protein/DNA complexes were immunoprecipitated with polyclonal antibodies against the DNA binding domain of REST (P73) and the C-terminal CoREST binding domain (anti-REST-C). As shown in figure 2C, in OPCs, REST bound to the RE1 elements in Grik3, NeuroD2, SCG10, Scn2A1, and NF-M, but not to a randomly chosen site upstream of the NG2 gene that does not contain an RE1. qRT-PCR demonstrated that these neuronal genes are transcriptionally repressed in OPCs as compared to cerebellar granular neurons (CGNs) or post-natal day 12 rat cortex. Conversely, OPCs contain high levels of transcripts for NG2 which were barely detectable in the CGN cultures (figure 2D). If REST/RE1 interactions are responsible for transcriptional repression in glia, then perturbing REST function should alter the expression of RE1-containing genes. To examine this, we infected OPCs with adenoviruses expressing either DnREST, REST-VP16, or GFP alone and measured gene expression by qRT-PCR 72hrs later (figure 2E). Expression of either DNREST or REST-VP16 was sufficient to derepress or activate the transcription of RE1-containing genes in OPCs (figure 2E). Expression of these and other genes was also derepressed when differentiated astrocytes and oligodendrocytes were infected with the REST-VP16 adenovirus (Table S1). These data show that REST is a functional gene repressor in glia. The variations in the levels of expression of individual genes among different cell types suggest that REST function can vary in a cell-type specific manner.
Loss of REST repression inhibits oligodendrocyte differentiation and induces neuronal gene expression
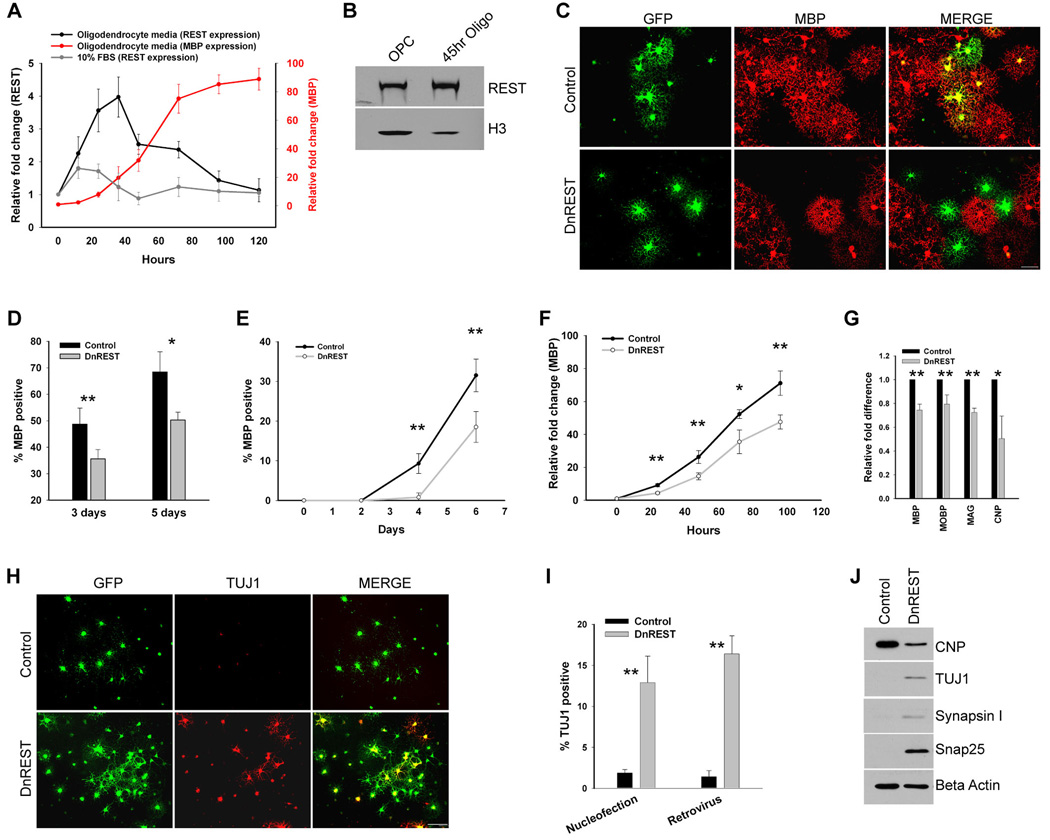
Given that OPCs express functional REST, we next asked whether REST regulates the development of oligodendrocyte lineage cells. We first examined REST expression during the differentiation of OPCs into oligodendrocytes. OPC cultures were expanded in proliferation media for 3 days and then induced to differentiate into oligodendrocytes by plating in serum-free media containing thyroid hormones.. As shown in figure 3A, REST mRNA expression increased 4.0-fold (±0.6, n=3) relative to OPCs 36hrs after inducing differentiation and then returned to baseline as the cells matured into MBP-expressing oligodendrocytes. This increase in mRNA was accompanied by an approximate 4-fold increase in REST protein (Figure 3B). Protein levels remained elevated after 4 days in oligodendrocyte media (data not shown). REST mRNA levels increased modestly but significantly when the cells were induced to develop into 2As by the addition of fetal calf serum to the media. There was no further increase in REST when T3 was added to the serum-containing media. Adding T3 (30ng/ml) did not increase REST transcription in either REFs or HCN neural stem cells but did cause a 2.3-fold increase in the HT22 neuronal progenitor cell line (data not shown). The expression of myelin-specific genes increased and that of RE1-containing genes decreased during oligodendrocyte differentiation (figure S2). This data suggests that REST is active as a gene repressor during the initiation of oligodendrocyte differentiation.
Figure 3. REST repression is necessary for proper oligodendrocyte maturation and to repress neuronal gene and protein expression in OPCs.
A. Real-time PCR of REST mRNA expression after the initiation of oligodendrocyte and 2A differentiation. Purified OPCs were switched to oligodendrocyte or 2A differentiation media at time 0 and total RNA was extracted at the indicated time points. mRNA expression is normalized to GAPDH and fold difference at each time point is relative to time 0. Error bars represent the standard deviation, n=3. B. Immunoblot analysis shows that REST protein levels increase during the onset of oligodendrocyte differentiation. Nuclear protein was isolated from OPCs in proliferation media and OPCs in oligodendrocyte media for 45hrs. Histone H3 is used as a loading control. C–D. Purified OPCs were co-nucleofected with a plasmid expressing GFP and either a plasmid expressing DnREST or an empty vector. C. Immunofluorescence staining for MBP (red) and GFP (green) 5 days post nucleofection. D. The percent of the nucleofected cells expressing MBP. Error bars represent the standard deviation, n=3, ** P value < .007, * P value <.02. E–J. OPCs were infected with control GFP or DnREST retroviruses as described under Materials and Methods and analyzed for the expression of properties associated with either mature oligodendrocyte (E–G) or immature neurons (H–J). E. Quantification of the percent of infected cells expressing MBP at the indicated time points subsequent to the initiation of oligodendrocyte differentiation. Error bars represent the standard deviation, n=3, ** P value < 0.001. F. Real-time PCR of MBP expression subsequent to the initiation of oligodendrocyte differentiation. Fold change is shown relative to time 0. n=3, * P value < 0.01, ** P value < 0.005. G. Real time PCR analysis of myelin gene expression after normalization to GAPDH. RNA was harvested 3 days after initiating oliodendrocyte differentiation. Error bars represent the standard deviation, n=6, *, P value < 0.05, **, P value < 0.001. H. Immunofluorescence staining for TUJ1 5 days post-induction. I. The percent of nucleofected or retroviral infected cells that express TUJ1. Error bars represent the standard deviation, n=3 for the nucleofection experiments, n=4 for the retroviral infection experiments, ** P value <0.0001. J. Immunoblot analysis of the expression of the indicated neuronal proteins 5 days post-infection for cells grown in oligodendrocyte media.
To determine whether REST function is required for oligodendrocyte differentiation, we used a loss-of-function (LOF) approach. OPCs were nucleofected with plasmids expressing either DnREST and GFP or GFP alone, grown in oligodendrocyte media for 3 and 5 days and oligodendrocyte differentiation assayed by staining with antibodies against MBP to identify mature oligodendrocytes. The percent of MBP-positive cells decreased when DnREST was expressed (figure 3C and D). Since protein expression after nucleofection of plasmids declines after 3–4 days, we infected OPCs with retroviruses expressing either DnREST (pMXsIG-DnREST) or control GFP (pMXsIG). The cells were infected, grown in proliferation media for 5–6 days to allow for expression of the virally encoded proteins, plated onto coverslips for two days, and then switched to oligodendrocyte media. As shown in figure 3E, the development of MBP-positive oligodendrocytes was retarded after infection with DnREST viruses. MBP-positive cells were slower to appear, and, after 6 days in oligodendrocyte-inducing media, the percentage of MBP-positive cells was reduced significantly among the DnREST infected cells relative to control infected cells. The number of cells expressing the O4 antigen, a marker for pre-oligodendrocytes, was also reduced in the REST LOF cells (data not shown, see also figure 7H). MBP gene transcription lagged behind that of control cells and never reached the levels of control cells (figure 3F). The transcription of several other myelin genes was also reduced (figure 3G) and the DnREST infected cells also had lower levels of CNP protein (figure 3J). REST LOF led to multiple changes in gene expression during differentiation (Table 1). Genes known to be highly regulated by REST such as Celr3 and Snap25 increased 5–10-fold whereas other genes (with and without identified RE1s) increased more modestly. Several genes involved in the regulation of oligodendrocyte differentiation, including Id2, Id4, olig2, Nkx2.2, and YY1, did not change. The magnitude of any changes in gene transcription is likely under-estimated in table 1 since the infection efficiency was only between 60–80%. These data suggest that REST function is required for oligodendrocyte differentiation.
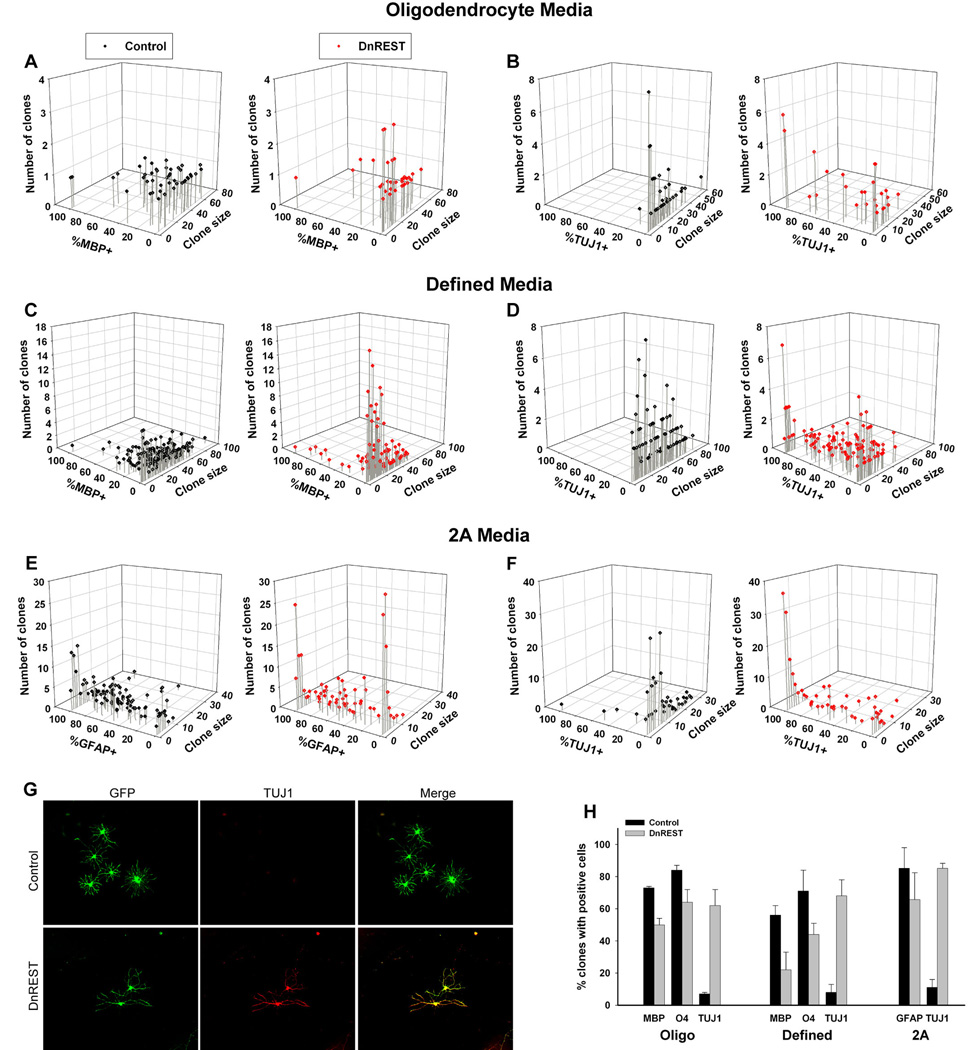
Figure 7. Clonal analysis of OPC differentiation.
OPCs were infected and grown at clonal density as described under Materials and Methods and analyzed for the expression of MBP, TUJ1, or GFAP after 5 days or O4 after 3 days in their indicated media. A–F. The scatter plots show the size and composition of all individual clones for the indicated growth conditions. G. Immunofluorescence staining for TUJ1 in a control and DnREST infected clone grown in 2A differentiation media for 5 days. H. The percent of clones containing cells positive for the indicated marker antigens. Total number of closed analyzed is given in the legend to Figure 6. P<0.05 for all pair-wise comparisons.
Table 1. Gene changes during differentiation.
OPCs were infected with control or DnREST expressing retroviruses and grown in proliferation media for 5 days. Cells were switched to either oligodendrocyte media or 2A differentiation media for 3–4 days. Total RNA was collected and transcript levels were analyzed by real-time RT-PCR. Relative fold difference in gene expression in DnREST infected cells is shown relative to control infected cells. The standard deviation (SD) was taken from 4–6 total PCR runs from 2 separate experiments.
| Oligodendrocyte | 2A | ||
|---|---|---|---|
| Gene Name | Relative Fold Difference |
Gene Name | Relative Fold Difference |
| CelsR3 | 10.48 ± 4.16 ** | SCG10 | 13.73 ± 2.24 ** |
| SCG10 | 6.85 ± 0.25 ** | CelsR3 | 11.33 ± 3.92 ** |
| Snap25 | 5.25 ± 0.35 ** | Snap25 | 4.88 ± 1.09 ** |
| NFM | 4.89 ± 0.98 ** | Nestin | 3.65 ± 2.05 * |
| Myt1 | 3.61 ± 0.12 ** | Myt1L | 2.95 ± 0.78 ** |
| βIIITub | 2.08 ± 0.28 ** | βIIITub | 2.93 ± 0.27 ** |
| Syn1 | 1.96 ± 0.24 ** | ATOH | 2.50 ± 0.08 ** |
| Nestin | 1.83 ± 0.03 ** | DCX | 2.42 ± 1.17 |
| Hes5 | 1.82 ± 0.05 ** | NGN3 | 2.36 ± 0.07 ** |
| Hes1 | 1.76 ± 0.75 | NeuroD1 | 2.29 ± 1.13 |
| Syt2 | 1.75 ± 0.95 | NF-M | 2.03 ± 0.02 ** |
| Calb1 | 1.71 ± 0.08 ** | Myt1 | 1.98 ± 0.15 ** |
| Myt1L | 1.58 ± 0.07 ** | Hes1 | 1.71 ± 0.13 ** |
| Olig2 | 1.49 ± 0.74 | Syn1 | 1.55 ± 0.29 * |
| ASCL1 | 1.40 ± 0.04 ** | Nkx2.2 | 1.48 ± 0.20 ** |
| DCX | 1.35 ± 0.06 ** | NGN2 | 1.48 ± 0.02 ** |
| PLP | NC | Calb1 | 1.47 ± 0.09 ** |
| Sox2 | NC | GluR2 | 1.40 ± 0.07 ** |
| PDGFaR | NC | Olig1 | 1.39 ± 0.03 ** |
| GluR2 | NC | Olig2 | 1.25 ± 0.11 * |
| NG2 | NC | B-actin | NC |
| Olig1 | NC | Sox2 | NC |
| Sox10 | NC | ID4 | NC |
| YY1 | NC | YY1 | NC |
| GFAP | NC | ASCL1 | NC |
| CTNNb1 | NC | Hes5 | NC |
| Id2 | NC | PLP | NC |
| Nkx2.2 | NC | PDGFaR | NC |
| B-actin | NC | ID2 | NC |
| Id4 | NC | CNP | 0.74 ± 0.22 |
| MOBP | 0.79 ± 0.07 ** | NG2 | 0.68 ± 0.36 |
| MBP | 0.74 ± 0.05 ** | MOBP | 0.66 ± 0.38 |
| MAG | 0.72 ± 0.03 ** | MBP | 0.65 ± 0.05 ** |
| CNP | 0.50 ± 0.31 * | MAG | 0.58 ± 0.18 ** |
| GFAP | 0.41 ± 0.11 ** | ||
| Brca1 | 0.39 ± 0.02 ** | ||
NC= no change,
P value < 0.05,
P value < 0.005.
Where not indicated, the change was not statistically significant.
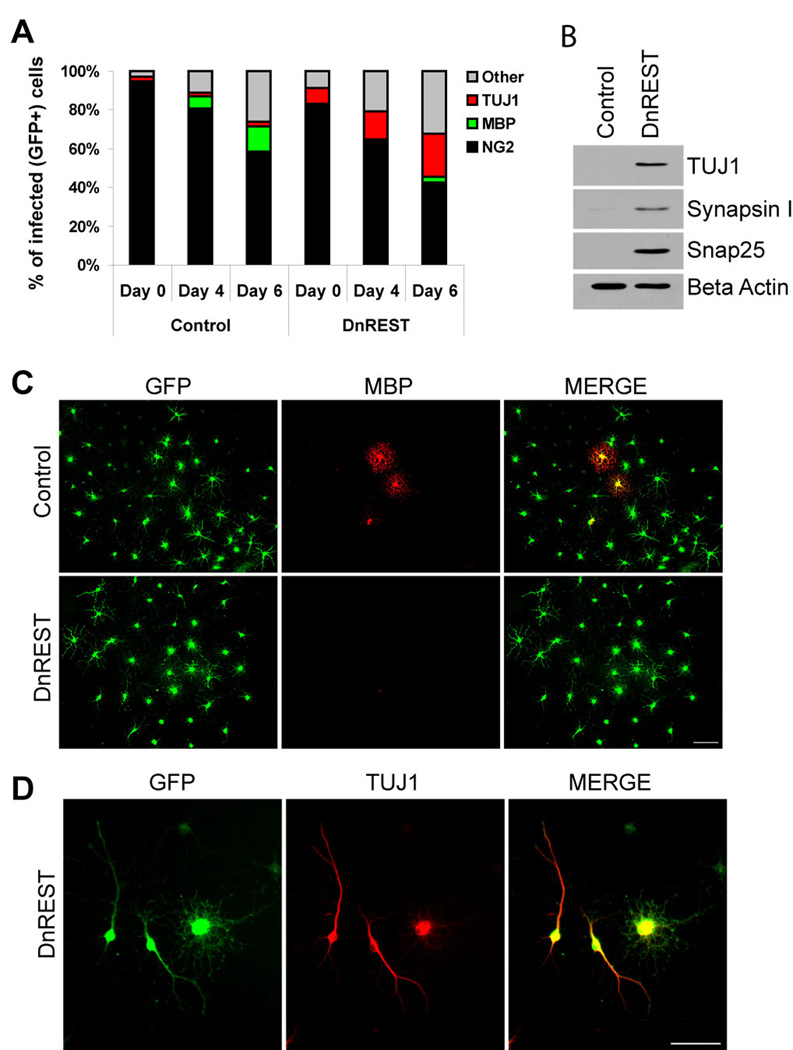
It is possible that the OPCs that did not develop into oligodendrocytes were differentiating along a neuronal lineage (Kondo, Raff, 2000). We therefore stained the nucleofected and infected cells with antibodies that identify young neurons (TUJ1). 12.9±3.2% of the DnREST nucleofected and 16.4±2.2% of the pMSxIG-DnREST infected cells were βIII tubulin (TuJ1) positive whereas only 1.89±0.42% and 1.42±0.75% of the control cells were positive (figures 3H and I). Consistent with the changes in gene expression shown in Table 1, βIII tubulin and other REST-regulated neuronal proteins (Snap25, Synapsin1) were expressed in the DnREST infected cells but not the control infected cells (figure 3J). These data show that REST function normally represses the expression of neuronal genes and proteins in differentiating OPCs.
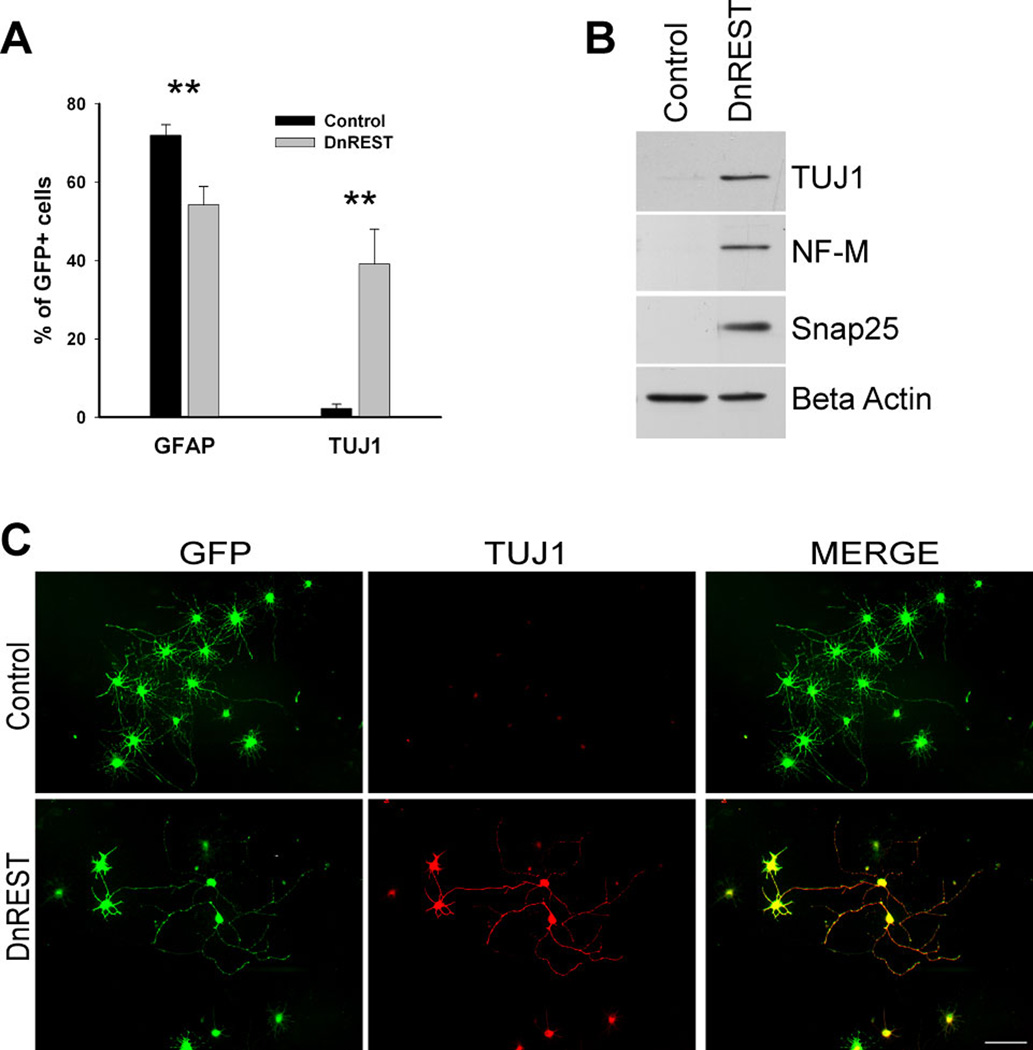
OPCs are highly plastic cells and their development in vitro is responsive to different media conditions (Raff et al., 1983). To rule out the possibility that the expression of neuronal antigens after REST LOF was due to factors found in the oligodendrocyte media used here, we examined the effects of REST LOF on OPCs grown in media containing 10% FBS to promote 2A differentiation. 71.9±2.8 of the control infected cells expressed GFAP as compared to 54.2±7.2% of the DnREST infected cells (figure 4A, n=4). The percent of cells expressing TUJ1 increased (2.2±1.2% in control infected cells, 39.1±8.9% in the DnREST infected cells, figure 4, A and C). Immunoblot analysis detected βIII tubulin, neurofilament-M, and Snap25 protein in the DnREST infected cells (figure 4B). As was the case with cells grown in oligodendrocyte media, there were complex changes in the levels of gene expression with the transcription of RE1-containing genes increasing 3–14-fold while myelin and astrocyte-specific genes decreased (Table 1). Thus, when grow under conditions that foster the appearance of astrocytes, REST LOF reduces astrocytic differentiation and drives the cells towards a neuronal phenotype.
Figure 4. REST loss of function increases neuronal protein expression during astrocytic differentiation.
Purified OPCs were infected with retroviruses expressing either DnREST and/or GFP alone and grown for 5 days in 2A differentiation media. A. The percent of the infected cells that were positively stained with antibodies against GFAP and TUJ1. Error bars represent the standard deviation, n=3, ** P value < 0.001 B. Immunoblot analysis shows the expression of neuronal proteins in the DnREST infected cells after 5 days in 2A differentiation media. C. Immunofluorescence staining showing the expression of TUJ1 (red) in the infected cells (green) bar=50µm.
Lastly, we grew the OPCs in a defined media lacking known growth and trophic factors but that is permissive for neural stem cell differentiation along oligodendrocytic, astrocytic and neuronal pathways (Belachew et al., 2003). In the control cultures, the percent of NG2-positive cells decreased and the MBP-positive population increased slowly and moderately over a 6 day time course (figure 5A and C). A small number of the control cells (2.1%) expressed TUJ1 antigens and this did not change over the time course of the experiment (figure 5A). The percent of cells categorized as “other” (i.e., not identifiable with the antibodies used) in the control cultures also increased by day 6. This pattern of development was altered by REST LOF: 22.1±4.4% of DnREST infected cells became TUJ1-positive but only 2.4±0.6% were MBP-positive (figure 5A, C, and D). Immunoblot analysis detected TUJ1, Synapsin1 and Snap25 protein in DnREST infected cells but not the control cells (figure 5B). In both control and DnREST infected cells, the number of GFAP-positive cells remained low (data not shown). These results show that preventing REST function induces neuronal protein and mRNA expression in OPCs under a variety of media conditions and support the hypothesis that REST repression of neuronal genes is required for the differentiation of oligodendrocyte progenitor cells into either oligodendrocytes or astrocytes.
Figure 5. DnREST infected OPCs grown in a defined media express neuronal proteins.
Purified OPCs were infected with control GFP or DnREST retroviruses and grown in a defined media as described under Materials and Methods. A. The stacked bar graph shows the percent of the cells expressing the indicated marker antigens as determined by immunofluorescence staining. n=4. B. Immunoblot analysis of the expression of neuronal proteins by the infected OPCs. C and D. DnREST infected cells fail to express MBP but express TUJ1. bars= 50µm.
Clonal analysis
One question that arises in the experiments described above is whether all the cells or only a sub-population of the OPCs are responding to REST-LOF. It is possible that the cultures contain undifferentiated neural stem-like cells and that these are the cells that respond to REST LOF by expressing neuronal proteins (Su et al., 2004). To answer this question, we carried out a clonal analysis. If all or most OPCs can respond to REST LOF, we predicted that a large number of clones would express TUJ1, whereas if only a sub-population of stem-like cells responded, the number of clones expressing TUJ1 might be small. Purified OPCs were infected with control or DN-REST retrovirus and grown in proliferation media for 5 days. The cells were plated onto coverslips at clonal density, grown in proliferation media for 2 days, then switched to either oligodendrocyte media, 2A media, or defined media for 3 or 5 days. GFP-expressing clones were identified and cell number and phenotype for each clone determined.
Individual clones were scored for the presence of at least 1 cell expressing either MBP, GFAP, or TUJ1. As shown in figure 7H, regardless of media, between 60–80% of the DnREST clones contained TUJ1-positive cells. This rules out the possibility that REST LOF is affecting only a small sub-population of neural stem-like cells.
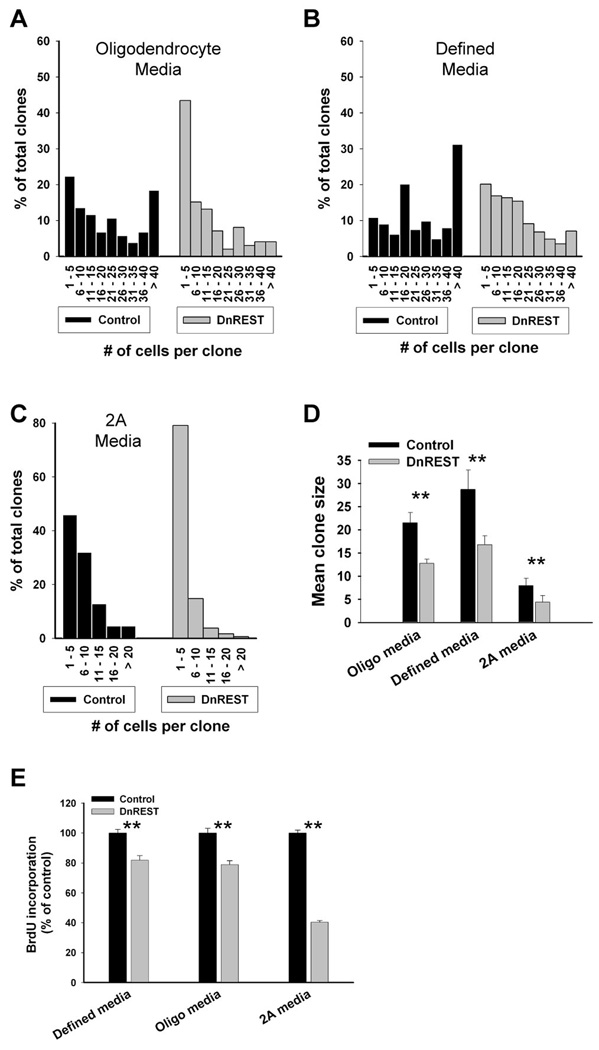
Clonal size varied according to the media conditions, but the mean clonal size of the DnREST infected cells always was smaller than that of control cells when analyzed after 7 days (figure 6D). This reduction was due to an increased number of small sized clones and a reduced number of large sized clones (figure 6A, B, and C). To determine whether REST LOF altered cell proliferation, we pulse-labeled the cells with BrdU as described under Materials and Methods. As shown in figure 6E, there was a small but significant reduction in BrdU incorporation in the REST LOF cells. This effect on cell proliferation may lead to the smaller clone size but we cannot rule out a contribution from either increased cell death or accelerated cell differentiation.
Figure 6. REST LOF decreases clone size and proliferation rate.
OPCs were infected and grown at clonal density as described under Materials and Methods. A,B and C. The histograms show clonal size distribution after growth for 5 days in the indicated media. Total clone number was as follows: oligodendrocyte media, control n=103, DnRest n= 99, defined media control n=387, DnREST n=397, 2A media control n=475, DnREST, 2A media n=473. D. Mean clonal size in the indicated media. Error bars represent the standard deviation, n=4, ** P value <0.001. E. Control and DnREST infected cells were labeled with BrdU as described under Materials and Methods and quantified using immunofluorescence staining. Error bars represent the standard deviation, n=3, ** P value<0.001.
Because OPCs are heterogeneous in terms of their capacity for cell division and since oligodendrocyte differentiation is obligate once an OPC stops dividing (Casaccia-Bonnefil, Lui, 2003), we further analyzed the composition and size of individual clones. The 3-dimensional scatter plots in figure 7 show clearly that the effects of REST LOF on phenotypic differentiation were greater for the smaller sized clones. In all media, the smaller REST-LOF clones contained a lower percent of MBP-positive (or GFAP-positive) cells and a higher percent of TUJ1-positive cells (see also figure S3A–D). For example, in defined media, which had the largest mean clonal size, only 13.5% of the REST LOF clones with 20 or fewer cells contained MBP-positive cells as compared to 34.9% of the control infected clones. Conversely, 4.6% of the smaller control clones contained at least 1 TUJ1-positive cell as opposed to 61% of the LOF clones. LOF lead to a smaller percentage of the cells within any single clone expressing either MBP or GFAP (figure 7) and to more clones where all the cells were 100% positive for the neuronal marker TUJ1 (figure 7G, figure S3). This was especially prominent for the small sized clones: between 60–80% of the 1 and 2 cell clones were 100% positive for TJU1 expression (figure S3). There was also an increase in the number of 04-negative clones (figure S3). This data suggests that REST functions are necessary for the differentiation of OPCs into oligodendrocytes. In its absence, the progeny of both rapidly dividing (ie., founder cells for large clones) and more slowly dividing (ie., founder cells of smaller clones) develop neuronal properties.
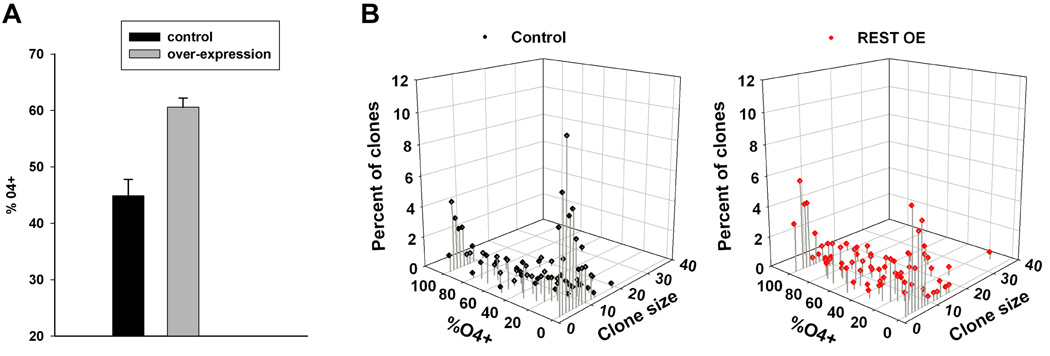
Lastly, we asked whether increased levels of REST can promote oligodendrocyte differentiation. We nucleofected OPCs with a plasmid containing a full length REST cDNA under control of the CMV promoter, grew the cells at clonal density in a serum-free defined media and determined the expression of the 04 antigen after 3 days. As shown in figure 8A, REST over-expression resulted in an increased number of clones containing 04-positive cells. When analyzed on a single clone basis, there were fewer clones with no 04-positive cells (Figure 8B). The average number of 04-positive cells was higher in the over-expressing clones (32.2±.2 for the control clones versus 43.2±10.4 for the over-expressing clones, n=3), although this result was not statistically significant (P<0.09). Nevertheless, these data shows that REST over-expression can promote OPC development to the pre-oligodendrocyte stage.
Figure 8. REST over expression induces 04 expression.
A. OPCs were nucleofected with either pMAXeGFP or a mixture of pMAXeGFP and full-length REST, grown for 3 days at clonal densities in defined media and the percentage of clones containing 04-postive cells determined (n=3, P<0.005). B. 3-dimensional scatter plots of a typical experiment. The number of 04-negative clones decreased and there was a general shift to clones containing higher percentages of 04-positive cells after REST over-expression (REST OE).
DISCUSSION
The RE1 binding protein REST was first discovered on the basis of its ability to repress the expression of neuronal genes in non-neuronal cells (Chong et al., 1995; Schoenherr and Anderson, 1995). In addition to regulating the development of neurons from embryonic stem cells, REST regulates heart and pancreatic development and can function as either a tumor suppressor or an oncogene (Ballas et al., 2005, Kuwahara et al., 2003; Lawinger et al., 2000; Martin et al., 2003; Westbrook et al., 2005). Because REST knock-out mice die by embryonic day 11.5 (Chen et al., 1998), little is known about REST function during the gliogenic stages of neuronal development. The data presented here expand the role of REST by demonstrating that REST represses neuronal gene expression during the differentiation of OPCs into oligodendrocytes. Given the unusual mixture of neuronal and glial properties expressed by OPCs, (for review, see Bakiri et al., 2009, Bergles, et al., 2009), the repression of neuronal genes may be necessary for the initiation of oligodendrocytic development. REST is also required during the BMP-induced astrocytic differentiation of embryonic neural progenitor cells (Kohyama et al., 2010). Together, these studies demonstrate a general requirement for REST function during gliogenesis and suggest that REST is likely to be an important regulator of glial development, differentiation, and phenotypic plasticity.
The oligodendrocyte lineage is one of the most studied cell-specific lineages in the developing CNS (Nishiyama et al., 2009, Trotter et al., 2010). The maturation of OPCs into myelinating oligodendrocytes requires both gene repression, mediated predominantly by class 1 HDACs (Liu, Casaccia, 2010), and gene activation, mediated by transcription factors such as zfp488, MRF, and Nkx2.2 (Emery et al., 2009; Qi et al., 2001; Wang et al., 2006). MicroRNAs also play a role (Dugas et al., 2010; Lau et al., 2008; Zhao et al., 2010). Here, we focused on the functions of REST at the OPC stage of the oligodendrocyte lineage since deleting HDACs prenatally prevents the development of OPCs (He et al., 2007; Ye et al., 2009). In the absence of REST function, OPCs fail to develop into MBP-positive oligodendrocytes. The reduced levels of CNP mRNA and protein (figure 3) and the increase in 04-negative clones (figure S3) suggest that differentiation is arrested prior to the pre-oligodendrocyte stage. Conversely, REST over-expression was able to promote OPC development to an 04-positive pre-oligodendrocyte stage under conditions in which oligodendrocyte differentiation is not especially robust (figure 8). Our clonal analysis shows that REST LOF reduced the expression of MBP and promoted the expression of TUJ1 in almost all the cells within small sized clones whereas the larger sized clones were more heterogeneous in their cellular composition (figure 7). This suggests that REST function is needed at the time when the cells make the decision to differentiate into oligodendrocytes. Since the frequency of clones in which 100% of the cells were positive for either 04, MBP or GFAP did not change after REST LOF, some OPCs must have already been committed to differentiate. Because of this prior commitment, REST LOF reduced but did not entirely eliminate oligodendrocyte development.
REST acts as a functional gene repressor in OPCs and is physically associated with several neuronal genes whose expression, initially low in OPCs, is further reduced during differentiation but up-regulated after LOF (Table 1, figure S2). This is consistent with the known functions of REST as a repressor of the neuronal phenotype in non-neuronal cells (Ballas, Mandel, 2005). While the repression of genes encoding the neuronal phenotype may be necessary for oligodendrocyte differentiation and to prevent the development of cells with mixed phenotypic properties, REST LOF also altered the expression of genes that are not commonly considered targets for REST-mediated regulation (Bruce et al., 2004). Among this group are genes associated with neurogenesis (ASCL1, Myt1L, Olig2) and gliogenesis (GFAP, CNP, MAG, MBP). Thus it is unclear whether the failure to develop into oligodendrocytes is due to the mis-regulated expression of either genes that encode neuronal properties, genes that regulate the decision to develop into a neuron or a glial cell or both. Nevertheless, our findings are consistent with the notion that REST can regulate a wide program of gene expression in cells of the oligodendrocyte lineage (Abrajano et al., 2009) and further show that this regulation is necessary for oligodendrocyte differentiation.
A striking aspect of REST expression during OPCs differentiation is the 4-fold increase in mRNA and protein levels after the initiation of differentiation with thyroid hormones. This increase in REST protein is maintained for at least 45hr. Inducing astrocyte development, either from neural progenitor cells with BMPs (Koyhama et al., 2010), or from OPCs with serum (figure 3A) also leads to increases in REST, albeit smaller increases than during oligodendrocyte differentiation. Based on the manufacturer's specifications, we estimate that the concentration of T3 in serum-containing media is 0.1nM, or about 460-fold less than that in the oligodendrocyte inducing media used here. This suggests that the increase in REST is due to the initiation of a program of glial differentiation rather than a general response to thyroid hormone. Consistent with this idea, the effects of T3 on REST transcription varied in different neural stem cell lines. This increase in REST mRNA and protein levels is in contrast to the proteasome-mediated down-regulation of REST protein that occurs rapidly during retinoic-acid induced neural differentiation of ES cells (Ballas et al., 2005; Westbrook et al., 2008). How this increase in REST expression in differentiating OPCs is regulated is unknown. In cortical neurons, REST is transcriptionally repressed (Ballas et al., 2005) but it can be transcriptionally activated in a neuroblastoma cell line (Jiang et al., 2008). Whether REST is regulated by either proteasomal degradation, gene transcription or both is an area for future study.
Most of the effects of REST on gene transcription result from binding to the 21 base-pair RE1 element. In addition to the canonical RE1, structurally variant RE1 sites have been identified which differ in their affinity for REST (Bruce et al., 2004, Bruce et al., 2009; Otto et al., 2007). These low-affinity REST binding sites are often associated with cell type and tissue-specific genes (Bruce et al., 2009). The transient increase in nuclear REST protein during the initiation of oligodendrocyte differentiation may be necessary to bind to and repress genes with such variant RE1s. For example, the transcription of hes5, which inhibits oligodendrocyte differentiation (Liu et al., 2006), is down-regulated normally during OPC differentiation (Kondo, Raff, 2004, figure S2) and has an expanded RE1 (Otto et al., 2007), was increased approximately 3.5–4 fold when Dn-REST infected cells were differentiated into oligodendrocytes. There were no increases when the cells were differentiated into 2As (Table 1). The changes in REST protein levels demonstrated here may provide a novel mechanism by which REST can differentially modify gene expression during cellular differentiation.
HDAC-mediated changes in chromatin structure are required for oligodendrocyte differentiation and there has been much progress recently in uncovering the mechanisms involved (Shen Casaccia-Bonnefil, 2008, Liu Casaccia, 2010). In general, class1 HDACs are recruited to chromatin and repress the transcription of inhibitors of oligodendrocyte differentiation. For example, the transcription of Id2 and Id4, which inhibit oligodendrocyte differentiation, is activated by Wnt signaling during OPC specification (Ye et al., 2009) and then repressed by Yin Yang1 (YY1)-HDAC complexes during differentiation (He et al., 2007). The Notch pathway is also regulated by class I HDACs and in turn regulates oligodendrocyte development in complex ways (Genoud et al., 2002; Hu et al., 2003; Kao et al., 1998; Wang et al., 1996). Hes5, a downstream target of activated notch, inhibits myelin gene expression and mice null for hes5 display precocious myelination (Lui et al., 2006, Zhang et al., 2009). It seems possible therefore that there may be a division of labor during OPC development where factors such as YY1 repress Id expression after Wnt signaling while REST counteracts Notch-mediated inhibition by repressing hes5. A delicate balance between the activation and repression of transcription mediated by these and other factors (Li et al., 2009) may be necessary to insure that the proper complement of oligodendrocytes develops on an appropriate time schedule (Rosenberg Chan, 2009). Disruption of this timing could be a factor in a wide range of cognitive disorders (Fields, 2008).
Cells with the properties of adult OPCs generate new neurons throughout the life of the organism, but little is known about how this process is regulated (Aguirre Gallo, 2004; Guo et al., 2010; 2009; Rivers et al., 2008). Our data shows that REST can regulate genes associated with neuronal differentiation in developing OPCs suggesting that REST may regulate OPC plasticity. The low levels of REST present in adult glia may be sufficient to repress neuronal and neurogenic genes but allow for their dynamic regulation perhaps in response to environmental stimuli such as depolarization or injury (Ballas et al., 2005, Calderone, et al., 2003). Given the central role of HDACs and chromatin remodeling in regulating the developmental plasticity of OPCs (Kondo, Raff, 2004; Liu et al., 2007; Lyssiotis et al., 2007), it will be important to investigate further the functions of REST in adult glia.
Supplementary Material
Acknowledgements
We thank Dr. H. Sirotkin for his comments on this manuscript and Ds. N. Ballas and G.Mandel for their gifts of reagents. This work was supported by grant #NS21198 from the NIH to JML and a Multiple Sclerosis Society Collaborative Research Center Award.
REFERENCES
- Abrajano JJ, Qureshi IA, Gokhan S, Zheng D, Bergman A, Mehler MF. Differential deployment of REST and CoREST promotes glial subtype specification and oligodendrocyte lineage maturation. PLoS One. 2009;4:e7665. doi: 10.1371/journal.pone.0007665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Gallo V. Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakiri Y, Attwell D, Káradóttir R. Electrical signalling properties of oligodendrocyte precursor cells. Neuron Glia Biol. 2009;5:3–11. doi: 10.1017/S1740925X09990202. [DOI] [PubMed] [Google Scholar]
- Ballas N, Battaglioli E, Atouf F, Andres ME, Chenoweth J, Anderson ME, Burger C, Moniwa M, Davie JR, Bowers WJ, Federoff HJ, Rose DW, Rosenfeld MG, Brehm P, Mandel G. Regulation of neuronal traits by a novel transcriptional complex. Neuron. 2001;31:353–365. doi: 10.1016/s0896-6273(01)00371-3. [DOI] [PubMed] [Google Scholar]
- Ballas N, Mandel G. The many faces of REST oversee epigenetic programming of neuronal genes. Curr Opin Neurobiol 15:500-6. C., Mandel G. (2009) Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2005;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Ballas N, Lloy DT, Grunseich C, Mandel G. Non-cell autonomousinfluence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Jabs R, Steinhäuser C. Neuron-glia synapses in the brain. Brain Res Rev. 2010;63:130–137. doi: 10.1016/j.brainresrev.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AW, Donaldson IJ, Wood IC, Yerbury SA, Sadowski MI, Chapman M, Gottgens B, Buckley NJ. Genome-wide analysis of repressor element 1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) target genes. Proc Natl Acad Sci U S A. 2004;101:10458–10463. doi: 10.1073/pnas.0401827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AW, Lopez-Contreras AJ, Flicek P, Down TA, Dhami P, Dillon SC, Koch CM, Langford CF, Dunham I, Andrews RM, Vetrie D. Functional diversity for REST (NRSF) is defined by in vivo binding affinity hierarchies at the DNA sequence level. Genome Res. 2009;19:994–1005. doi: 10.1101/gr.089086.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone A, Jover T, Noh KM, Tanaka H, Yokota H, Lin Y, Grooms SY, Regis R, Bennett MV, Zukin RS. Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci. 2003;23:2112–2121. doi: 10.1523/JNEUROSCI.23-06-02112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Liu A. Relationship between cell cycle molecules and onset of oligodendrocyte differentiation. J Neurosci Res. 2003;72:1–11. doi: 10.1002/jnr.10565. [DOI] [PubMed] [Google Scholar]
- Chen ZF, Paquette AJ, Anderson DJ. NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat Genet. 1998;20:136–142. doi: 10.1038/2431. [DOI] [PubMed] [Google Scholar]
- Chong JA, Tapia-Ramirez J, Kim S, Toledo-Aral JJ, Zheng Y, Boutros MC, Altshuller YM, Frohman MA, Kraner SD, Mandel G. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell. 1995;80:949–957. doi: 10.1016/0092-8674(95)90298-8. [DOI] [PubMed] [Google Scholar]
- Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci U S A. 2006;103:2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou, Chang-lin, Levine JM. Inhibition of neurite growth by the NG2 chondroitin-sulfate proteoglycan. J.Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas JC, Cuellar TL, Scholze A, Ason B, Ibrahim A, Emery B, Zamanian JL, Foo LC, McManus MT, Barres BA. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65:597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B, Agalliu D, Cahoy JD, Watkins TA, Dugas JC, Mulinyawe SB, Ibrahim A, Ligon KL, Rowitch DH, Barres BA. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell. 2009;138:172–185. doi: 10.1016/j.cell.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud S, Lappe-Siefke C, Goebbels S, Radtke F, Aguet M, Scherer SS, Suter U, Nave KA, Mantei N. Notch1 control of oligodendrocyte differentiation in the spinal cord. J Cell Biol. 2002;158:709–718. doi: 10.1083/jcb.200202002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes JA, Nielsen SJ, Battaglioli E, Miska EA, Speh JC, Berry DL, Atouf F, Holdener BC, Mandel G, Kouzarides T. The co-repressor mSin3A is a functional component of the REST-CoREST repressor complex. J Biol Chem. 2000;275:9461–9467. doi: 10.1074/jbc.275.13.9461. [DOI] [PubMed] [Google Scholar]
- Guo F, Ma J, McCauley E, Bannerman P, Pleasure D. Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci. 2009;29:7256–7270. doi: 10.1523/JNEUROSCI.5653-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L, Vaccarino F, Pleasure D. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci. 2010;30:12036–12049. doi: 10.1523/JNEUROSCI.1360-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Dupree J, Wang J, Sandoval J, Li J, Liu H, Shi Y, Nave KA, Casaccia-Bonnefil P. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 2007;55:217–230. doi: 10.1016/j.neuron.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol. 2004;164:111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu QD, Ang BT, Karsak M, Hu WP, Cui XY, Duka T, Takeda Y, Chia W, Sankar N, Ng YK, Ling EA, Maciag T, Small D, Trifonova R, Kopan R, Okano H, Nakafuku M, Chiba S, Hirai H, Aster JC, Schachner M, Pallen CJ, Watanabe K, Xiao ZC. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell. 2003;115:163–175. doi: 10.1016/s0092-8674(03)00810-9. [DOI] [PubMed] [Google Scholar]
- Immaneni A, Lawinger P, Zhao Z, Lu W, Rastelli L, Morris JH, Majumder S. REST-VP16 activates multiple neuronal differentiation genes in human NT2 cells. Nucleic Acids Res. 2000;28:3403–3410. doi: 10.1093/nar/28.17.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Yao M, Shi J, Shen P, Niu G, Fei J. Yin yang 1 directly regulates the transcription of RE-1 silencing transcription factor. J Neurosci Res. 2008;86:1209–1216. doi: 10.1002/jnr.21595. [DOI] [PubMed] [Google Scholar]
- Johnson R, Teh CH, Kunarso G, Wong KY, Srinivasan G, Cooper ML, Volta M, Chan SS, Lipovich L, Pollard SM, Karuturi RK, Wei CL, Buckley NJ, Stanton LW. REST regulates distinct transcriptional networks in embryonic and neural stem cells. PLoS Biol. 2008;6:e256. doi: 10.1371/journal.pbio.0060256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao HY, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, Kintner CR, Evans RM, Kadesch T. A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev. 1998;12:2269–2277. doi: 10.1101/gad.12.15.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Koshino Y, Shibata F, Oki T, Nakajima H, Nosaka T, Kumagai H. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp. Hematol. 2003;3:1007–1014. [PubMed] [Google Scholar]
- Kohyama J, Sanosaka T, Tokunaga A, Takatsuka E, Tsujimura K, Okano H, Nakashima K. BMP-induced REST regulates the establishment and maintenance of astrocytic identity. J Cell Biol. 2010;189:159–170. doi: 10.1083/jcb.200908048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- Kondo T, Raff M. Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes Dev. 2004;18:2963–2972. doi: 10.1101/gad.309404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara K, Saito Y, Takano M, Arai Y, Yasuno S, Nakagawa Y, Takahashi N, Adachi Y, Takemura G, Horie M, Miyamoto Y, Morisaki T, Kuratomi S, Noma A, Fujiwara H, Yoshimasa Y, Kinoshita H, Kawakami R, Kishimoto I, Nakanishi M, Usami S, Harada M, Nakao K. NRSF regulates the fetal cardiac gene program and maintains normal cardiac structure and function. EMBO J. 2003;22:6310–6321. doi: 10.1093/emboj/cdg601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau P, Verrier JD, Nielsen JA, Johnson KR, Notterpek L, Hudson LD. Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J Neurosci. 2008;28:11720–11730. doi: 10.1523/JNEUROSCI.1932-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawinger P, Venugopal R, Guo ZS, Immaneni A, Sengupta D, Lu W, Rastelli L, Marin Dias Carneiro A, Levin V, Fuller GN, Echelard Y, Majumder S. The neuronal repressor REST/NRSF is an essential regulator in medulloblastoma cells. Nat Med. 2000;6:826–831. doi: 10.1038/77565. [DOI] [PubMed] [Google Scholar]
- Levine J. Neuronal influences on glial progenitor cell development. Neuron. 1989;3:103–113. doi: 10.1016/0896-6273(89)90119-0. [DOI] [PubMed] [Google Scholar]
- Levine JM, Stallcup WB. Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci. 1987;7:2721–2731. doi: 10.1523/JNEUROSCI.07-09-02721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, He Y, Richardson WD, Casaccia P. Two-tier transcriptional control of oligodendrocyte differentiation. Curr Opin Neurobiol. 2009;19:479–485. doi: 10.1016/j.conb.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Li J, Marin-Husstege M, Kageyama R, Fan Y, Gelinas C, Casaccia-Bonnefil P. A molecular insight of Hes5-dependent inhibition of myelin gene expression: old partners and new players. EMBO J. 2006;25:4833–4842. doi: 10.1038/sj.emboj.7601352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Han YR, Li J, Sun D, Ouyang M, Plummer MR, Casaccia-Bonnefil P. The glial or neuronal fate choice of oligodendrocyte progenitors is modulated by their ability to acquire an epigenetic memory. J Neurosci. 2007;27:7339–7343. doi: 10.1523/JNEUROSCI.1226-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Casaccia P. Epigenetic regulation of oligodendrocyte identity. Trends Neurosci. 2010;33:193–201. doi: 10.1016/j.tins.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunyak VV, Burgess R, Prefontaine GG, Nelson C, Sze SH, Chenoweth J, Schwartz P, Pevzner PA, Glass C, Mandel G, Rosenfeld MG. Corepressor-dependent silencing of chromosomal regions encoding neuronal genes. Science. 2002;298:1747–1752. doi: 10.1126/science.1076469. [DOI] [PubMed] [Google Scholar]
- Lyssiotis CA, Walker J, Wu C, Kondo T, Schultz PG, Wu X. Inhibition of histone deacetylase activity induces developmental plasticity in oligodendrocyte precursor cells. Proc Natl Acad Sci U S A. 2007;104:14982–14987. doi: 10.1073/pnas.0707044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Liu A, Casaccia-Bonnefil P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J Neurosci. 2002;22:10333–10345. doi: 10.1523/JNEUROSCI.22-23-10333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Tawadros T, Meylan L, Abderrahmani A, Condorelli DF, Waeber G, Haefliger JA. Critical role of the transcriptional repressor neuron-restrictive silencer factor in the specific control of connexin36 in insulin-producing cell lines. J Biol Chem. 2003;278:53082–53089. doi: 10.1074/jbc.M306861200. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- Mulligan P, Westbrook TF, Ottinger M, Pavlova N, Chang B, Macia E, Shi YJ, Barretina J, Liu J, Howley PM, Elledge SJ, Shi Y. CDYL bridges REST and histone methyltransferases for gene repression and suppression of cellular transformation. Mol Cell. 2008;32:718–726. doi: 10.1016/j.molcel.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes(NG2 cells) multifunctional cells with loineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- Otto SJ, McCorkle SR, Hover J, Conaco C, Han JJ, Impey S, Yochum GS, Dunn JJ, Goodman RH, Mandel G. A new binding motif for the transcriptional repressor REST uncovers large gene networks devoted to neuronal functions. J Neurosci. 2007;27:6729–6739. doi: 10.1523/JNEUROSCI.0091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SS, Chan JR. Modulating myelination: knowing when to say Wnt. Genes Dev. 2009;23:1487–1493. doi: 10.1101/gad.1824009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenherr CJ, Anderson DJ. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science. 1995;267:1360–1363. doi: 10.1126/science.7871435. [DOI] [PubMed] [Google Scholar]
- Shen S, Casaccia-Bonnefil P. Post-translational modifications of nucleosomal histones in oligodendrocyte lineage cells in development and disease. J Mol Neurosci. 2008;35:13–22. doi: 10.1007/s12031-007-9014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Kameoka S, Lentz S, Majumder S. Activation of REST/NRSF target genes in neural stem cells is sufficient to cause neuronal differentiation. Mol Cell Biol. 2004;24:8018–8025. doi: 10.1128/MCB.24.18.8018-8025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci. 2006;26:4729–4739. doi: 10.1523/JNEUROSCI.3900-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J, Karram K, Nishiyama A. NG2 cells: Properties, progeny and origin. Brain Res Rev. 2010;63:72–82. doi: 10.1016/j.brainresrev.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, Weinmaster G, Barres BA. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- Wang SZ, Dulin J, Wu H, Hurlock E, Lee SE, Jansson K, Lu QR. An oligodendrocyte-specific zinc-finger transcription regulator cooperates with Olig2 to promote oligodendrocyte differentiation. Development. 2006;133:3389–3398. doi: 10.1242/dev.02522. [DOI] [PubMed] [Google Scholar]
- Westbrook TF, Martin ES, Schlabach MR, Leng Y, Liang AC, Feng B, Zhao JJ, Roberts TM, Mandel G, Hannon GJ, Depinho RA, Chin L, Elledge SJ. A genetic screen for candidate tumor suppressors identifies REST. Cell. 2005;121:837–848. doi: 10.1016/j.cell.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Westbrook TF, Hu G, Ang XL, Mulligan P, Pavlova NN, Liang A, Leng Y, Maehr R, Shi Y, Harper JW, Elledge SJ. SCFbeta-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature. 2008;452:370–374. doi: 10.1038/nature06780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Watanabe M, Nishiyama A. Optimization of oligodendrocyte progenitor cell culture method for enhanced survival. J Neurosci Methods. 2005;149:50–56. doi: 10.1016/j.jneumeth.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, van Es JH, Clevers H, Hsieh J, Bassel-Duby R, Olson EN, Lu QR. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12:829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Argaw AT, Gurfein BT, Zameer A, Snyder BJ, Ge C, Lu QR, Rowitch DH, Raine CS, Brosnan CF, John GR. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc Natl Acad Sci U S A. 2009;106:19162–19167. doi: 10.1073/pnas.0902834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, He X, Han X, Yu Y, Ye F, Chen Y, Hoang T, Xu X, Mi QS, Xin M, Wang F, Appel B, Lu QR. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron. 2010;65:612–626. doi: 10.1016/j.neuron.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.