Abstract
Inflammatory diseases of the lung are common, cause significant morbidity, and can be refractory to therapy. Airway responses to injury, noxious stimuli, or microbes lead to leukocyte recruitment for host defense. As leukocytes respond, they interact with lung resident cells and can elaborate specific mediators that are enzymatically generated from polyunsaturated fatty acids via transcellular biosynthesis. These bioactive, lipid-derived, small molecules serve as agonists at specific receptors and are rapidly inactivated in the local environment. This review will focus on the biosynthesis, receptors, cellular responses, and in vivo actions of lipoxins, resolvins, and protectins as exemplary molecular signaling circuits in the airway that are anti-inflammatory and proresolving.
Keywords: resolution, anti-inflammatory, polyunsaturated fatty acid, mediator, lipoxin, resolvin, protectin, airway inflammation, asthma, acute lung injury, acute respiratory distress syndrome
INTRODUCTION
Airway inflammation is part of a coordinated host response to infection, injury, or other noxious stimuli and is fundamental to host defense[2]. This process is so common that it is experienced by most individuals frequently throughout their life, such as during a simple community-acquired bronchitis. The natural course of mild airway inflammation is to resolve entirely as the irritation or infection abates[3]. In response to certain stimuli, airway inflammation can be so robust as to lead to bystander tissue injury and contribute to pathophysiology[1]. For example, overexuberant airway inflammation can lead to the acute respiratory distress syndrome (ARDS), a pathologic condition of severe inflammation that is life threatening[4]. In some respiratory conditions, acute inflammation can convert to chronic inflammation, usually with the recruitment of the adaptive immune system, and chronic airway inflammation is part of the pathogenesis of many common lung diseases[2], including asthma[5], which impacts as many as one in 15 adults in the U.S.[6]. The cellular effectors of acute and chronic airway inflammation are evident in sputum, bronchoalveolar lavage fluid (BALF), and lung histology. In general, airway inflammation in acute inflammatory diseases is comprised of cellular effectors that are distinct from those of chronic responses. Acute inflammation, as in acute lung injury (ALI) or ARDS, recruits and activates neutrophils (PMNs)[4]. As this inflammation resolves, PMNs undergo apoptosis[7] and are cleared by macrophages[8]. Allergic airway inflammation consists of eosinophils (EOS) and effector T lymphocytes with modulatory roles for inflammatory macrophages, mast cells, dendritic cells, and structural cells[9].
The molecular signals that initiate acute and provoke chronic inflammation have been the subject of extensive investigation and include cytokines, chemokines, and select lipid mediators. More recently, several anti-inflammatory molecular circuits that also actively promote resolution of tissue inflammation have been uncovered, including the identification of natural small molecules derived from polyunsaturated fatty acids (PUFAs) that are part of a new genus of anti-inflammatory and proresolving mediators (reviewed in [10]). The lipoxins (LXs), resolvins, and protectins are three families of chemical mediators in this genus that are now appreciated to promote the resolution of lung inflammation and they will be the focus of this review on the molecular circuits of resolution of airway inflammation. Information will also be provided on their relationship to both physiologic catabasis and the pathobiology of select lung diseases.
LIPOXINS
Biosynthesis
Lipoxins (LXs) are lipoxygenase (LO)–derived products of arachidonic acid (AA, C20:4) that are predominately generated during cell-cell interactions at sites of vascular or tissue inflammation (reviewed in [11,12]). At sites of inflammation or injury in the lung vasculature, LX formation can occur when platelets interact with activated leukocytes that generate 5-LO–derived leukotriene A4 (LTA4) from AA. Platelet 12-LO, acting as a LX synthase, can then convert LTA4 to LXs. In lung parenchyma, infiltrating leukocytes interact with structural cells to generate LXs via a distinct biosynthetic pathway. In particular, PMN-derived LTA4 can be converted by airway epithelial cell 15-LO to generate LXs (reviewed in [13]). There are additional LX biosynthetic pathways, including the transformation of 15-LO–derived 15-hydroperoxy-eicosatetraenoic acid (15-H(p)ETE) by 5-LO to LXs. 15-epimer-LXs (15-epi-LXs) are also found in respiratory tissues[14]. The 15-epi-LXs are generated by 5-LO–mediated conversion of 15(R)- hydroxy-eicosatetraenoic acid (15(R)-HETE) to 15-epi-LXA4 and 15-epi-LXB4 (reviewed in [11]). Both aspirin-acetylated cyclooxygenase (COX)–2 and cytochrome p450 activities can catalyze the formation of 15(R)-HETE from AA. Of note, statins also demonstrate the ability to trigger 15-epi-LXA4 formation[15,16,17]. Statins and pioglitazone can initiate post-translational modification of COX-2 and 5-LO in rat cardiomyocytes to influence AA conversion to 15-epi-LXA4[15,16]. In addition, cell-cell interactions between PMNs and airway epithelial cells in the presence of statins leads to 15-epi-LXA4 biosynthesis, in which the epoxygenase cytochrome p450 product 14,15-epoxyeicostrienoic acid influences AA metabolism[17].
Signaling
LXA4 and 15-epi-LXA4 are both agonists for a LXA4 receptor termed ALX/FPR2, which is a seven-transmembrane-spanning G protein-coupled receptor (GPCR) that binds these ligands with high affinity (reviewed in [18]). The glucocorticoid-induced protein annexin 1 and related peptides can also bind to ALX, although with lower affinity than LXA4[19]. Of interest to lung biology, ALX is expressed on human airway epithelial cells[20] and leukocytes[20,21,22], and can be induced by select inflammatory mediators[23]. LX signaling is not limited to interactions with ALX. LXs can act as antagonists at CysLT1 receptors[24] and can also signal via the aryl hydrocarbon receptor[25].
LXs interact with ALX to evoke cell type–specific responses that are anti-inflammatory and proresolving (Fig. 1). For example, anti-inflammatory actions for LX signaling through ALX include inhibition of PMN and EOS chemotaxis and activation[26,27,28,29], and proresolving actions include increasing macrophage phagocytosis of apoptotic PMNs to clear inflamed tissue[30]. In human leukocytes, LX-ALX interactions trigger specific intracellular signal events, such as blocking the phosphorylation of leukocyte-specific protein 1 in PMNs and α-fodrin and f-actin in EOS[31,32].
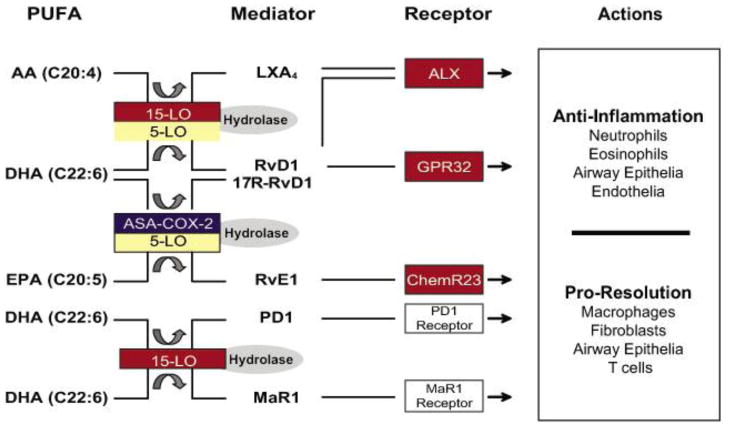
FIGURE 1.
Molecular circuits of resolution for airway inflammation. During airway inflammation, select PUFAs are enzymatically transformed to bioactive mediators in complex biosynthetic circuits. These mediators elicit pathway- and cell type–specific responses that are both anti-inflammatory and proresolving.
ALX signaling can also regulate the apoptotic fate of PMNs. There are several nonlipid ligands for ALX with potent actions on PMNs. Serum amyloid A (SAA) is an acute-phase reactant that binds to ALX and produces proinflammatory signals that delay PMN apoptosis[33]. In contrast, the glucocorticoid-induced protein annexin 1 and related peptides can bind to ALX and promote PMN apoptosis (reviewed in [18,34]). The lipid and protein ligands bind to distinct ALX domains[18] and 15-epi-LXA4 can block the antiapoptotic effects of SAA[33].
LXA4 also blocks polyisoprenyl phosphate remodeling to regulate cell activation[35]. Presqualene diphosphate (PSDP) is a polyisoprenyl diphosphate present in resting cell membranes that serves as an intracellular stop signal for PMNs. Rapid dephosphorylation of this counter-regulatory signaling molecule can facilitate transient cellular responses to provocative stimuli[36]. The addition of exogenous PSDP, but not its related monophosphate, presqualene monophosphate (PSMP), blocks O2- production from human PMNs. In addition, PSDP and PSDP mimetics that resist inactivation can inhibit important signaling checkpoints for cell activation, namely phosphatidylinositol 3-kinase (PI3K) and phospholipase D (PLD)[35,37,38]. Stable LX analogs dramatically block PMN PSDP remodeling in response to proinflammatory agonists[35]. LX-mediated inhibition of PSDP remodeling is linked to its anti-inflammatory effects on PMN functional responses, including O2- generation[35,38]. Recently, polyisoprenyl diphosphate phosphatase 1 (PDP1) (originally identified as CSS2α and PPAPDC2) was characterized as a pivotal phosphatase for PSDP remodeling to PSMP[39,40] and may serve as a target for LXs to prevent PSDP remodeling. Taken together, LX’s role in blocking PSDP remodeling, PSDP’s ability to block enzymatic activities critical to PMN activation, and PDP1’s ability to convert PSDP to PSMP are highly suggestive of an integrated signal transduction pathway that regulates PMN-mediated inflammation and sets the stage for catabasis. Given LX’s cell type–specific actions, it is not surprising that ALX also initiates cell type–specific signaling circuits (reviewed in [18]).
Cellular Responses to LXs
In health, the ability to regulate leukocyte accumulation and activation in the lung is fundamental to homeostatic responses. LXs can inhibit granulocyte locomotion, shape change, transmigration, and degranulation[28,29,35,41,42]. In contrast, LXs stimulate monocytes and macrophages in a nonphlogistic manner to enhance monocyte adherence, locomotion and transmigration, and macrophage phagocytosis of apoptotic PMNs and microbial products[30,43,44]. Together, these LX-mediated cellular responses are both anti-inflammatory for PMNs and EOS, and proresolving for clearance of inflamed tissue by monocytes and macrophages. In addition to these leukocyte-specific actions, LXs promote restitution of injured respiratory epithelia by stimulating bronchial basal epithelial cell proliferation, inhibit release of proinflammatory cytokines IL-6 and IL-8, and block PMN transmigration across differentiated human bronchial cells[20]. ALX receptor expression in both proximal and distal epithelial cells is increased after injury[20]. Consistent with a role in tissue homeostasis, LXs also block inflammatory angiogenesis and endothelial cell migration in response to proinflammatory mediators[45]; IL-1β–mediated synthesis of IL- 6, IL-8, and matrix metalloproteinases by fibroblasts[46]; and leukotriene E4 and IL-13 primed airway smooth muscle migration towards to platelet-derived growth factor[47]. LX’s regulatory actions on this broad array of cell types relevant to lung catabatic responses suggests a pivotal role for this family of mediators in lung physiology.
LXs in Models of Lung Inflammation
To integrate these cellular actions for LXs into more complex settings, the in vivo impact of LXs and LX-stable analogs has been investigated in several experimental models of lung disease (Table 1). These compounds have been extensively studied in experimental asthma. In murine models of asthma, animals are systemically sensitized to allergen and subsequently aerosol challenged in order to direct the allergic inflammation to the airway. Administration of LX analogs prior to aerosol challenge potently blocks the development of allergic airway inflammation and airway hyper-responsiveness, decreases EOS and T-cell accumulation, and dampens Th2 cytokine levels[27,48]. Upon cessation of allergen challenge, the allergic airway responses are self-limited and, within 7 days, EOS and T-cell numbers return to near baseline. During this resolution phase, endogenous generation of LXA4 increases[49]. The administration of a LX- stable analog after the final aerosol challenge accelerates resolution by dramatically decreasing lung leukocyte numbers and selectively regulating airway cytokine levels, including IL-17, IL-23, and IL-6[49]. In mice and humans, IL-17 is generated in inflamed lung and associated with chronic inflammatory diseases[50]. In transgenic mice expressing human ALX receptors, allergic airway responses are blocked and development of allergy (as determined by total IgE levels) is markedly reduced[27]. Of additional note, mice deficient in ALX display a proinflammatory phenotype[51]. LX’s anti-inflammatory effects are not limited to mouse models of allergic lung inflammation. For example, administration of LX-stable analogs also potently blocks edema and antigen-driven recruitment of PMNs and EOS in a rat model of allergic pleurisy[26]. Taken together, these data support the notion that LX signaling through ALX is a potent molecular circuit for the regulation of allergic inflammation.
TABLE 1.
Effects of Anti-Inflammatory and Proresolving Mediators on Human Lung Disease and Murine Models of Lung Disease
| Model or Disease State | Species | Compound | Effect | Ref. |
|---|---|---|---|---|
| Asthma | Human | LXA4 | Blocked LTC4-mediated bronchoconstriction | [99] |
| Allergic airway inflammation | Mouse | LXA4/LX analogs (anti- inflammatory) | Decreased EOS and T cells in BALFs and lungs | [27,48] |
| Decreased TH2 cytokine levels in BALFs | ||||
| Decreased airway hyper-responsiveness | ||||
| LX analogs (proresolving) | Enhanced EOS and T-cell clearance from lungs | [27,48] | ||
| PD1 (anti-inflammatory) | Decreased EOS and T cells in BALFs and lungs | [97] | ||
| Decreased TH2 cytokine levels in BALFs | ||||
| Decreased airway hyper-responsiveness | ||||
| Decreased mucus metaplasia | ||||
| PD1 (proresolving) | Enhanced EOS and T-cell clearance from lungs | [97] | ||
| RvE1 (anti-inflammatory) | Decreased EOS and T-cells in BALFs and lungs | [49,80] | ||
| Decreased TH2 cytokine levels in BALFs | ||||
| Decreased airway hyper-responsiveness | ||||
| Decreased mucus metaplasia | ||||
| RvE1 (proresolving) | Enhanced EOS and T-cell clearance from lungs | [49] | ||
| Improved TH2 cytokine levels in BALFs | ||||
| Improved airway hyper-responsiveness | ||||
| Improved mucus metaplasia | ||||
| Acid-initiated ALI | Mouse | PSDP mimetic | Decreased lung PMNs | [37] |
| Lovastatin | Increased 15-epi-LXA4 in BALFs | [17] | ||
| Decreased lung PMNs | ||||
| Aspirin | Increased 15-epi-LXA4 in BALFs | [54] | ||
| Decreased lung PMNs | ||||
| COX-2 inhibitor | Decreased LXA4 in BALFs | [54] | ||
| Increased lung PMNs | ||||
| RvE1 | Decreased lung PMNs | [81] | ||
| Decreased select proinflammatory mediators | ||||
| Carrageenan-induced lung injury | 15-epi-LXA4 | Decreased lung PMNs | [55] | |
| Increased lung macrophages | ||||
| Promoted PMN apoptosis | ||||
| E. coli peritonitis- associated lung injury | 15-epi-LXA4 | Decreased lung PMNs | [55] | |
| Promoted PMN apoptosis | ||||
| Decreased mortality | ||||
| Pneumonia | Mouse | RvE1 | Enhanced bacterial clearance | [81] |
| Decreased lung PMNs | ||||
| Decreased proinflammatory cytokine levels in BALFs | ||||
| Decreased mortality |
LXA4, lipoxin A4; PD1, protectin D1; RvE1, resolvin E1; PSDP, presqualene diphosphate; BALFs, bronchoalveolar lavage fluids.
In addition to experimental asthma, LX-stable analogs have been used in other models of lung inflammation (Table 1). In a model of pulmonary fibrosis, LXs block bleomycin (BLM)–induced airway inflammation and fibrosis[52,53]. Mice receiving concurrent BLM and LXs, or animals given LXs as a treatment post-BLM exposure, both display decreased cellular infiltration, edema, and collagen deposition in the lung[52]. Moreover, LXs enhance survival from BLM toxicity[52]. In this model, lung collagen deposition is correlated with fibrosis and increased ALX mRNA expression is associated with a decrease in lung collagen[53].
In a self-limited model of ALI by hydrochloric acid, LXs decrease inflammation and promote resolution[54]. Using a nonlethal model of ALI in which acid is selectively instilled into only one lung allows for investigation of catabatic responses during ALI resolution. Using this model, important roles were uncovered for COX-2 in the timely resolution of ALI, in part via generation of LXs[54]. Intratracheal instillation of carrageenan plus myeloperoxidase produces PMN-mediated lung injury. Mice that received 15-epi-LXA4 treatment 24 h postinjury displayed reductions in PMN numbers, total protein amount, and IL-6 levels in BALFs[55]. Promoting PMN apoptosis is a potent proresolving mechanism[56]. 15-epi-LXA4 decreased lung PMNs by enhancing PMN apoptosis, as measured by cytoplasmic histone-associated DNA fragments and PMN caspase-3 activity[55]. In addition, human ALX transgenic mice are protected from ALI[54]. Statins also facilitate resolution in this model of ALI by inducing the production of 15-epi-LXA4[17]. Of note, statins can also block airway inflammation in murine models of allergic asthma[57,58,59].
LXs in Human Airway Disease
The first identification of LXs in human tissues was in BALFs obtained from human subjects with a range of lung diseases[60]. LXA4 is also present in exudative pleural effusions that are typically associated with lung or systemic inflammatory disease[61]. In mild forms of asthma, LX generation is increased in peripheral blood, induced sputum, and BALFs[14,62,63]. In contrast, multiple studies encompassing diverse ethnic backgrounds have now established that severe asthmatics display decreased LX levels, relative to subjects with mild or moderate asthma[14,62,63,64] (Table 2). Of interest, LX biosynthetic capacity is decreased in severe asthmatics[62] and the capacity for LXA4 generation by whole blood is related to lung function[14,62,64], suggesting that decreased LX production may lead to a resolution defect in some individuals with severe asthma. Approximately 5–10% of adult asthmatics experience aspirin-exacerbated respiratory disease (reviewed in [65]) and LX biosynthetic capacity is also decreased in these patients[66]. Moreover, in severe asthma, ALX receptor expression is decreased in peripheral blood PMNs and EOS[63]. Levels of cysteinyl leukotrienes (CysLTs) and LXA4 in both BALFs and peripheral blood demonstrate an increase in the conversion of AA to CysLTs relative to LXA4 in severe compared to nonsevere asthmatics[38,63]. This change in severe asthma is related to both an increase in CysLTs and decrease in LX production[38,63]. Similarly, patients with scleroderma lung disease, marked by leukocyte infiltration and fibrosis of the lung, display enhanced LTB4 and diminished LXA4 levels in BALFs[67]. Cystic fibrosis is a disease of persistent lung inflammation. The nature of the airway inflammation in cystic fibrosis differs from asthma in that it is primarily related to PMN infiltration in response to chronic bacterial infection. Consistent with a theme of underproduction of these protective mediators in chronic lung inflammatory disease, decrements in LX levels are also present in cystic fibrosis[68]. Together, these data point to a role for LXs in the catabasis of human lung disease.
TABLE 2.
Altered Biosynthesis of Proresolving Mediators in Human Disease
| Disease | Defect | Ref. |
|---|---|---|
| Severe asthma | Decreased LXA4 in whole blood, sputum, and BALFs | [14,62,63,64] |
| Increased CysLT to LXA4 ratio in blood and BALF | [62,63] | |
| Asthma exacerbation | Decreased PD1 levels in exhaled breath condensates | [97] |
| Aspirin-intolerant asthma | Decreased LXA4 production from whole blood | [66] |
| Exercise-induced asthma | Decreased LXA4 in plasma | [100] |
| Scleroderma lung disease | Decreased LXA4 in BALFs | [67] |
| Cystic fibrosis | Decreased LXA4 in BALFs | [101] |
RESOLVINS
Biosynthesis
Resolvins are derived from the enzymatic modification of ω-3 PUFAs and were originally identified in murine-resolving exudates[69,70]. Both eicosapentaenoic acid (EPA, C20:5) and docosahexaenoic acid (DHA, C22:6) can be converted to resolvins by murine and human tissues. The E-series resolvins are derived from EPA and are generated by a multistep process involving acetylated COX-2 or cytochrome p450 acting in concert with leukocyte 5-LO[69]. D-series resolvins, which are derived from DHA, occur in both the 17S and 17R configurations (reviewed in [11]). Biosynthesis of D-series resolvins can be catalyzed by 15-LO and 5-LO interactions, and generation of the aspirin-triggered 17R conformers proceeds via aspirin-acetylated COX-2 and 5-LO[71]. The biosynthesis of resolvins is reviewed in detail in Serhan[11] and Seki et al.[72].
Signaling and Cellular Responses to Resolvins
The signaling pathways by which resolvins transduce anti-inflammatory and proresolving actions are rapidly evolving areas of science under active investigation. Current evidence demonstrates that resolvins signal via specific receptors. On PMNs, RvE1 acts as an antagonist and partial agonist at the LTB4 receptor BLT1[73]. By blocking LTB4 signaling, RvE1 decreases PMN accumulation and activation at sites of ongoing inflammation. In addition to BLT1, RvE1 can bind to the chemerin receptor ChemR23[74]. Myeloid dendritic cells express ChemR23[75] and RvE1 blocks proinflammatory responses by dendritic cells, including lipopolysaccharide-induced IL-23 release[49]. ChemR23 is also present on mucosal epithelial cells and when exposed to RvE1, CD55-dependent luminal clearance of PMNs is increased[76]. RvE1 signaling potently increases macrophage phagocytosis of apoptotic PMNs[44] and regulates PMN and T-cell expression of the chemokine receptor CCR5[77], which is an important mechanism for scavenging unwanted proinflammatory signals.
Similar to both RvE1 and LXs, more than one receptor can interact with the D-series resolvin RvD1, namely GPR32, a novel GPCR, and the ALX receptor[78]. Limited information is available for RvDs with respect to intracellular signaling. At the cellular level, RvD1 blocks PMN actin polymerization and migration toward inflammatory stimuli in a microfluidics chamber[79].
Resolvins in Models of Lung Inflammation
RvE1 displays anti-inflammatory and proresolving properties in murine models of allergic airway inflammation, acid-induced ALI, and pneumonia[49,80,81] (Table 1). In experimental asthma, administration of RvE1 (~0.005 mg/kg) prior to aerosol allergen challenge dramatically dampens lung inflammation with decreased airway leukocytes, mucus metaplasia, and hyper-responsiveness, and significant decrements in antigen-specific IgE and IL-13 levels[49,80]. Administration of RvE1 after cessation of allergen challenge promotes resolution, as evident by more rapid decreases in airway leukocytes, airway hyper-responsiveness to inhaled methacholine, and mucus metaplasia[49]. Examination of the chemical mediators present in BALFs from these animals reveals that administration of RvE1 during the resolution phase decreased levels of the proinflammatory cytokines IL-6, IL-23, and IL-17A, thereby blocking the expansion of IL-17–generating cells, such as TH-17 effector lymphocytes[49].
In addition to asthma, RvE1 proved to be anti-inflammatory and proresolving in a murine model of aspiration pneumonia in which enteric bacteria were instilled in the lung 12 h after the establishment of mild ALI[81]. Administration of RvE1 at the onset of the protocol decreases the production of proinflammatory cytokines, blocks PMN infiltration, and improves mortality[81]. RvE1 significantly decreased lung tissue levels of several proinflammatory chemokines and cytokines, including IL-1β, IL-6, HMGB-1, MIP-1α, MIP-1β, KC, and MCP-1, in a manner independent of the anti-inflammatory mediators IL-10 and LXA4. In response to sterile ALI, both LTB4 and KC increase in BALFs and are not significantly decreased by RvE1, despite marked decreases in lung PMNs of approximately 55%[81]. RvE1 has direct regulatory actions for PMNs that are downstream from LTB4 and KC generation. For example, RvE1 can interact with BLT1 as a receptor-level antagonist[82], so functional antagonism for RvE1 at BLT1 would block LTB4-mediated activation of PMNs. During experimental ALI, RvE1 inhibits PMN, but not macrophage, accumulation in the lung and protects the lung from aspiration pneumonia by increased clearance of E. coli infection[81]. RvE1 signals via ChemR23 on macrophages to promote the clearance of apoptotic PMNs and microbial debris[44,83], and mice deficient in ChemR23 display a proinflammatory phenotype[84]. In addition, RvE1 interacts with ChemR23 on mucosal epithelial cells to promote clearance of PMNs from apical surfaces in a CD55-dependent manner[85], and RvE1 prevents destruction of oral mucosal tissues in experimental periodontitis[86]. These findings of direct actions for RvE1 on leukocytes and mucosal epithelial cells are consistent with potent roles for this natural autacoid in regulating airway inflammation and host defense. In experimental pneumonia, the pharmacologically active dose of RvE1 was 100 ng per mouse or ~0.005 mg/kg, providing compelling evidence of this compound’s potent anti-inflammatory and proresolving actions. Thus, even if only present in low amounts in lung tissues, enzymatic conversion of EPA to RvE1 would serve to limit overexuberant tissue responses to injury or infection.
The D-series resolvins also display properties consistent with a role in dampening and resolving lung inflammation. RvD1 provides protection from second-organ injury of the lung in a murine ischemia-reperfusion injury model by blocking PMN infiltration[79]. Administration of RvD2 prior to cecal ligation and puncture leads to decreased bacterial loads, cytokine levels, and neutrophil recruitment in this model of sepsis[87]. In addition, enriching mouse chow with DHA dramatically decreases the severity of an experimental model of bacterial pneumonia[88,89]. C. elegans ω-3 desaturase (fat-1) converts ω-6 PUFAs to ω-3 PUFAs, and transgenic mice expressing the fat-1 gene experience less inflammation in ALI[88].
Resolvins in Human Disease
DHA and EPA are found in significant amounts in fish oils and increased dietary fish ingestion is associated with health benefits. Mucosal tissues of the airway are enriched with DHA, but levels decrease in disease states, such as asthma and cystic fibrosis[90]. In humans, RvE1 is detected in the blood of subjects given EPA and can be significantly increased by ingestion of aspirin[74]. Although not a uniform finding, diets enriched with ω-3 PUFAs can have positive effects on ALI/ARDS outcomes, including decreased length of stay in intensive care units, increased oxygenation, decreased time on mechanical ventilation, and improved mortality[41,91,92]. In addition, the Physicians Health Study uncovered a correlation between increased fish intake and a lower risk of pneumonia[93].
PROTECTINS
Biosynthesis, Signaling, and Cellular Responses to PD1
The lead member of the protectin family is termed protectin D1 (PD1). This potent counter-regulatory lipid mediator is enzymatically derived from DHA via an epoxide-containing intermediate[94,95]. PD1 production, similar to LXs, proceeds via 15-LO[95]. PD1 binds with high affinity to human PMNs (Kd ~25 nM)[94,96], but the molecular identity of the PD1 receptor has yet to be determined. PD1 shares some proresolving counter-regulatory actions with LXs and resolvins, but utilizes distinct signaling circuits. For example, LXA4, RvE1, and PD1 all inhibit PMN migration, yet neither LXA4 nor RvE1 compete for PD1 binding to PMNs[95], suggesting distinct mechanisms and site of interaction.
PD1 in Models of Lung Inflammation
Murine lungs generate PD1 and administration of exogenous PD1 (~0.0001, 0.001, and 0.01 mg/kg) decreases inflammation in a dose-dependent manner in models of allergic airway inflammation (Table 1)[97]. Administration of PD1 prior to peak airway inflammation leads to significantly less leukocyte accumulation and mucus metaplasia. Consistent with decreased lung inflammation, mice given PD1 display decreased levels of proinflammatory mediators in BALFs and decreased airway hyper-responsiveness (ED200 for lung resistance) to inhaled methacholine[97]. Of note, PD1 decreased LXA4 production in vivo, suggestive of independent resolution mechanisms for these two mediators. When PD1 is given as a treatment after airway inflammation is established, the compound, now present in increased amounts at an earlier time point, “jump starts” resolution and enhances the clearance of inflammatory cells (as reviewed in [11]).
PD1 in Human Disease
PD1 is present in human lung and PD1 levels are decreased in human lung disease (Table 2)[97]. The mucosal airway is rich in DHA[90], and both PD1 and its biosynthetic precursor, 17S-hydroxy-DHA, can be detected in human exhaled breath condensates (EBCs). Comparison between healthy subjects and asthmatics experiencing an exacerbation determined that the levels of both PD1 and 17S-HDHA are decreased in EBCs during asthma exacerbations[97]. In addition, the amount of mucosal DHA is also decreased in inflammatory airway diseases, such as asthma and cystic fibrosis[90]. Thus, the properties of PD1 are consistent with protective roles in airway inflammation.
MARESINS
Maresins are the newest family of anti-inflammatory and proresolving mediators. Maresins are 7,14 dihydroxy–containing products that are generated by activated macrophages[98]. DHA is delivered to inflamed or injured tissue by plasma exudation[79] and can be converted by macrophages to maresins to decrease the acute inflammatory response[98]. These novel proresolving compounds also block PMN trafficking and stimulate macrophage clearance of apoptotic PMNs[98]. Roles for maresins in lung biology are areas of active investigation. The presence of large numbers of alveolar macrophages in the lung and their critical role in tissue catabasis and host defense suggest important functions for maresins in the regulation of airway inflammation.
CONCLUSIONS
There are now several lines of evidence to support fundamental roles for PUFA-derived mediators in regulating lung inflammation. In response to airway injury, infection, or noxious stimuli, the acute inflammatory response is initiated. Even at this early stage of acute inflammation, molecular signaling circuits are constructed in the airway in health for the ultimate resolution of the inflammatory response (Fig. 1). These circuits are comprised of specific mediators and receptors that transduce cell type–specific responses for anti-inflammation and resolution. The mediators are enzymatically derived from PUFAs in tightly orchestrated biosynthetic pathways that commonly involve the sequential modification of the PUFA and biosynthetic intermediates by distinct enzymes. Proresolving mediators are generated in human airways during inflammation, and defects in their production exist during severe or uncontrolled airway inflammation. When administered in animal models of inflammatory lung disease, proresolving mediators or their stable analogs display potent protective actions. Only limited information is available for intervention in human disease[99], but this genus of compounds hold promise as disease-modifying agents. With no available medical therapy to promote the resolution of asthma or ARDS, there is a substantial unmet clinical need that serves as a poignant reminder to motivate scientists to develop a more thorough understanding of the endogenous molecular circuits for resolution of airway inflammation, so that this information might be used as a window into the pathobiology of disease and as the foundation for the rational design of novel disease-remitting therapeutics.
Contributor Information
Troy Carlo, Email: Tcarlo@partners.org.
Bruce D. Levy, Email: Blevy@partners.org.
References
- 1.Nathan C, Ding A. Nonresolving inflammation. Cell. 140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Shelhamer JH, Levine SJ, Wu T, Jacoby DB, Kaliner MA, Rennard SI. NIH conference. Airway inflammation. Ann Intern Med. 1995;123:288–304. doi: 10.7326/0003-4819-123-4-199508150-00008. [DOI] [PubMed] [Google Scholar]
- 3.Farver CF. Bacterial diseases. In: Zander DS, Farvarer CF, editors. Pulmonary Pathology. Churchill Livingstone Elsevier; Philadelphia: 2008. pp. 167–203. [Google Scholar]
- 4.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 5.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 6.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ Centers for Disease Control and Prevention. National surveillance for asthma--United States, 1980–2004. MMWR Surveill Summ. 2007;56:1–54. [PubMed] [Google Scholar]
- 7.Matute-Bello G, Liles WC, Radella F, 2nd, Steinberg KP, Ruzinski JT, Hudson LD, Martin TR. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med. 2000;28:1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Haslett C. Resolution of acute inflammation and the role of apoptosis in the tissue fate of granulocytes. Clin Sci. 1992;83:639–648. doi: 10.1042/cs0830639. [DOI] [PubMed] [Google Scholar]
- 9.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN, Chiang N. Endogenous proresolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(Suppl 1):S200–215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 12.Romano M. Lipoxin and aspirin-triggered lipoxins. The Scientific World JOURNAL. 2010;10:1048–1064. doi: 10.1100/tsw.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy BD. Lipoxins and lipoxin analogs in asthma. Prostaglandins Leukot Essent Fatty Acids. 2005;73:231–237. doi: 10.1016/j.plefa.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Vachier I, Bonnans C, Chavis C, Farce M, Godard P, Bousquet J, Chanez P. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. J Allergy Clin Immunol. 2005;115:55–60. doi: 10.1016/j.jaci.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 15.Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Nishi SP, Martinez JD, Huang MH, Uretsky BF, Perez-Polo JR. Augmentation of myocardial production of 15-epi-lipoxin-a4 by pioglitazone and atorvastatin in the rat. Circulation. 2006;114:929–935. doi: 10.1161/CIRCULATIONAHA.106.629907. [DOI] [PubMed] [Google Scholar]
- 16.Ye Y, Lin Y, Perez-Polo JR, Uretsky BF, Ye Z, Tieu BC, Birnbaum Y. Phosphorylation of 5-lipoxygenase at ser523 by protein kinase A determines whether pioglitazone and atorvastatin induce proinflammatory leukotriene B4 or anti-inflammatory 15-epi-lipoxin a4 production. J Immunol. 2008;181:3515–3523. doi: 10.4049/jimmunol.181.5.3515. [DOI] [PubMed] [Google Scholar]
- 17.Planaguma A, Pfeffer MA, Rubin G, Croze R, Uddin M, Serhan CN, Levy BD. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A(4) Mucosal Immunol. 2010;3:270–279. doi: 10.1038/mi.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 19.Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, Solito E, Serhan CN. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol. 2006;168:1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180:253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem. 1997;272:6972–6978. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 23.Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon gamma and inhibits tumor necrosis factor alpha-induced IL-8 release. J Exp Med. 1998;187:1285–1294. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gronert K, Martinsson-Niskanen T, Ravasi S, Chiang N, Serhan CN. Selectivity of recombinant human leukotriene D(4), leukotriene B(4), and lipoxin A(4) receptors with aspirin-triggered 15-epi-LXA(4) and regulation of vascular and inflammatory responses. Am J Pathol. 2001;158:3–9. doi: 10.1016/S0002-9440(10)63937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaldach CM, Riby J, Bjeldanes LF. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry. 1999;38:7594–7600. doi: 10.1021/bi982861e. [DOI] [PubMed] [Google Scholar]
- 26.Bandeira-Melo C, Bozza PT, Diaz BL, Cordeiro RS, Jose PJ, Martins MA, Serhan CN. Cutting edge: lipoxin (LX) A4 and aspirin-triggered 15-epi-LXA4 block allergen-induced eosinophil trafficking. J Immunol. 2000;164:2267–2271. doi: 10.4049/jimmunol.164.5.2267. [DOI] [PubMed] [Google Scholar]
- 27.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 28.Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92:75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soyombo O, Spur BW, Lee TH. Effects of lipoxin A4 on chemotaxis and degranulation of human eosinophils stimulated by platelet-activating factor and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Allergy. 1994;49:230–234. doi: 10.1111/j.1398-9995.1994.tb02654.x. [DOI] [PubMed] [Google Scholar]
- 30.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 31.Ohira T, Bannenberg G, Arita M, Takahashi M, Ge Q, Van Dyke TE, Stahl GL, Serhan CN, Badwey JA. A stable aspirin-triggered lipoxin A4 analog blocks phosphorylation of leukocyte-specific protein 1 in human neutrophils. J Immunol. 2004;173:2091–2098. doi: 10.4049/jimmunol.173.3.2091. [DOI] [PubMed] [Google Scholar]
- 32.Starosta V, Pazdrak K, Boldogh I, Svider T, Kurosky A. Lipoxin A4 counterregulates GM-CSF signaling in eosinophilic granulocytes. J Immunol. 2008;181:8688–8699. doi: 10.4049/jimmunol.181.12.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Kebir D, Jozsef L, Khreiss T, Pan W, Petasis NA, Serhan CN, Filep JG. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. J Immunol. 2007;179:616–622. doi: 10.4049/jimmunol.179.1.616. [DOI] [PubMed] [Google Scholar]
- 34.Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 35.Levy BD, Fokin VV, Clark JM, Wakelam MJ, Petasis NA, Serhan CN. Polyisoprenyl phosphate (PIPP) signaling regulates phospholipase D activity: a ‘stop’ signaling switch for aspirin-triggered lipoxin A4. FASEB J. 1999;13:903–911. doi: 10.1096/fasebj.13.8.903. [DOI] [PubMed] [Google Scholar]
- 36.Levy BD, Petasis NA, Serhan CN. Polyisoprenyl phosphates in intracellular signalling. Nature. 1997;389:985–990. doi: 10.1038/40180. [DOI] [PubMed] [Google Scholar]
- 37.Bonnans C, Fukunaga K, Keledjian R, Petasis NA, Levy BD. Regulation of phosphatidylinositol 3-kinase by polyisoprenyl phosphates in neutrophil-mediated tissue injury. J Exp Med. 2006;203:857–863. doi: 10.1084/jem.20052143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy BD, Hickey L, Morris AJ, Larvie M, Keledjian R, Petasis NA, Bannenberg G, Serhan CN. Novel polyisoprenyl phosphates block phospholipase D and human neutrophil activation in vitro and murine peritoneal inflammation in vivo. Br J Pharmacol. 2005;146:344–351. doi: 10.1038/sj.bjp.0706338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukunaga K, Arita M, Takahashi M, Morris AJ, Pfeffer M, Levy BD. Identification and functional characterization of a presqualene diphosphate phosphatase. J Biol Chem. 2006;281:9490–9497. doi: 10.1074/jbc.M512970200. [DOI] [PubMed] [Google Scholar]
- 40.Carlo T, Petasis NA, Levy BD. Activation of polyisoprenyl diphosphate phosphatase 1 remodels cellular presqualene diphosphate. Biochemistry. 2009;48:2997–3004. doi: 10.1021/bi8020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gewirtz AT, Fokin VV, Petasis NA, Serhan CN, Madara JL. LXA4, aspirin-triggered 15-epi-LXA4, and their analogs selectively downregulate PMN azurophilic degranulation. Am J Physiol. 1999;276:C988–994. doi: 10.1152/ajpcell.1999.276.4.C988. [DOI] [PubMed] [Google Scholar]
- 42.Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, Colgan SP, Madara JL. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- 43.Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fierro IM, Kutok JL, Serhan CN. Novel lipid mediator regulators of endothelial cell proliferation and migration: aspirin-triggered-15R-lipoxin A(4) and lipoxin A(4) J Pharmacol Exp Ther. 2002;300:385–392. doi: 10.1124/jpet.300.2.385. [DOI] [PubMed] [Google Scholar]
- 46.Sodin-Semrl S, Taddeo B, Tseng D, Varga J, Fiore S. Lipoxin A4 inhibits IL-1 beta-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. J Immunol. 2000;164:2660–2666. doi: 10.4049/jimmunol.164.5.2660. [DOI] [PubMed] [Google Scholar]
- 47.Parameswaran K, Radford K, Fanat A, Stephen J, Bonnans C, Levy BD, Janssen LJ, Cox PG. Modulation of human airway smooth muscle migration by lipid mediators and Th-2 cytokines. Am J Respir Cell Mol Biol. 2007;37:240–247. doi: 10.1165/rcmb.2006-0172OC. [DOI] [PubMed] [Google Scholar]
- 48.Levy BD, Lukacs NW, Berlin AA, Schmidt B, Guilford WJ, Serhan CN, Parkinson JF. Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. FASEB J. 2007;21:3877–3884. doi: 10.1096/fj.07-8653com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolls JK, Kanaly ST, Ramsay AJ. Interleukin-17: an emerging role in lung inflammation. Am J Respir Cell Mol Biol. 2003;28:9–11. doi: 10.1165/rcmb.2002-0255PS. [DOI] [PubMed] [Google Scholar]
- 51.Dufton N, Hannon R, Brancaleone V, Dalli J, Patel HB, Gray M, D’Acquisto F, Buckingham JC, Perretti M, Flower RJ. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol. 2010;184:2611–2619. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martins V, Valenca SS, Farias-Filho FA, Molinaro R, Simoes RL, Ferreira TP, e Silva PM, Hogaboam CM, Kunkel SL, Fierro IM, Canetti C, Benjamim CF. ATLa, an aspirin-triggered lipoxin A4 synthetic analog, prevents the inflammatory and fibrotic effects of bleomycin-induced pulmonary fibrosis. J Immunol. 2009;182:5374–5381. doi: 10.4049/jimmunol.0802259. [DOI] [PubMed] [Google Scholar]
- 53.Sato Y, Kitasato H, Murakami Y, Hashimoto A, Endo H, Kondo H, Inoue M, Hayashi I. Down-regulation of lipoxin A4 receptor by thromboxane A2 signaling in RAW246.7 cells in vitro and bleomycin-induced lung fibrosis in vivo. Biomed Pharmacother. 2004;58:381–387. doi: 10.1016/j.biopha.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174:5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 55.El Kebir D, Jozsef L, Pan W, Wang L, Petasis NA, Serhan CN, Filep JG. 15-Epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180:311–319. doi: 10.1164/rccm.200810-1601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, Caldicott A, Martinez-Losa M, Walker TR, Duffin R, Gray M, Crescenzi E, Martin MC, Brady HJ, Savill JS, Dransfield I, Haslett C. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 57.Kim DY, Ryu SY, Lim JE, Lee YS, Ro JY. Anti-inflammatory mechanism of simvastatin in mouse allergic asthma model. Eur J Pharmacol. 2007;557:76–86. doi: 10.1016/j.ejphar.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 58.McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J Immunol. 2004;172:2903–2908. doi: 10.4049/jimmunol.172.5.2903. [DOI] [PubMed] [Google Scholar]
- 59.Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin inhibits airway hyperreactivity: implications for the mevalonate pathway and beyond. Am J Respir Crit Care Med. 2009;180:731–740. doi: 10.1164/rccm.200901-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee TH, Crea AE, Gant V, Spur BW, Marron BE, Nicolaou KC, Reardon E, Brezinski M, Serhan CN. Identification of lipoxin A4 and its relationship to the sulfidopeptide leukotrienes C4, D4, and E4 in the bronchoalveolar lavage fluids obtained from patients with selected pulmonary diseases. Am Rev Respir Dis. 1990;141:1453–1458. doi: 10.1164/ajrccm/141.6.1453. [DOI] [PubMed] [Google Scholar]
- 61.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 62.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Planaguma A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, Bleecker ER, Curran-Everett D, Erzurum SC, Calhoun WJ, Castro M, Chung KF, Gaston B, Jarjour NN, Busse WW, Wenzel SE, Levy BD. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178:574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Celik GE, Erkekol FO, Misirligil Z, Melli M. Lipoxin A4 levels in asthma: relation with disease severity and aspirin sensitivity. Clin Exp Allergy. 2007;37:1494–1501. doi: 10.1111/j.1365-2222.2007.02806.x. [DOI] [PubMed] [Google Scholar]
- 65.Farooque SP, Lee TH. Aspirin-sensitive respiratory disease. Annu Rev Physiol. 2009;71:465–487. doi: 10.1146/annurev.physiol.010908.163114. [DOI] [PubMed] [Google Scholar]
- 66.Sanak M, Levy BD, Clish CB, Chiang N, Gronert K, Mastalerz L, Serhan CN, Szczeklik A. Aspirin-tolerant asthmatics generate more lipoxins than aspirin-intolerant asthmatics. Eur Respir J. 2000;16:44–49. doi: 10.1034/j.1399-3003.2000.16a08.x. [DOI] [PubMed] [Google Scholar]
- 67.Kowal-Bielecka O, Kowal K, Distler O, Rojewska J, Bodzenta-Lukaszyk A, Michel BA, Gay RE, Gay S, Sierakowski S. Cyclooxygenase- and lipoxygenase-derived eicosanoids in bronchoalveolar lavage fluid from patients with scleroderma lung disease: an imbalance between proinflammatory and antiinflammatory lipid mediators. Arthritis Rheum. 2005;52:3783–3791. doi: 10.1002/art.21432. [DOI] [PubMed] [Google Scholar]
- 68.Karp CL, Flick LM, Yang R, Uddin J, Petasis NA. Cystic fibrosis and lipoxins. Prostaglandins Leukot Essent Fatty Acids. 2005;73:263–270. doi: 10.1016/j.plefa.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 69.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 72.Seki H, Sasaki T, Ueda T, Arita M. Resolvins as regulators of the immune system. The Scientific World JOURNAL. 2010;10:818–831. doi: 10.1100/tsw.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 74.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vermi W, Riboldi E, Wittamer V, Gentili F, Luini W, Marrelli S, Vecchi A, Franssen JD, Communi D, Massardi L, Sironi M, Mantovani A, Parmentier M, Facchetti F, Sozzani S. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J Exp Med. 2005;201:509–515. doi: 10.1084/jem.20041310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 77.Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun. 2008;367:509–515. doi: 10.1016/j.bbrc.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 81.Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, Levy BD. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol. 2010;184:836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 83.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, Hendrick AG, Carlton MB, Greaves DR. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med. 2008;205:767–775. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, Colgan SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 86.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 87.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mayer K, Kiessling A, Ott J, Schaefer MB, Hecker M, Henneke I, Schulz R, Gunther A, Wang J, Wu L, Roth J, Seeger W, Kang JX. Acute lung injury is reduced in fat-1 mice endogenously synthesizing n-3 fatty acids. Am J Respir Crit Care Med. 2009;179:474–483. doi: 10.1164/rccm.200807-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tiesset H, Pierre M, Desseyn JL, Guery B, Beermann C, Galabert C, Gottrand F, Husson MO. Dietary (n-3) polyunsaturated fatty acids affect the kinetics of pro- and antiinflammatory responses in mice with Pseudomonas aeruginosa lung infection. J Nutr. 2009;139:82–89. doi: 10.3945/jn.108.096115. [DOI] [PubMed] [Google Scholar]
- 90.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, Weed DA, Gelrud A, Regan MM, Laposata M, Alvarez JG, O’Sullivan BP. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 91.Pacht ER, DeMichele SJ, Nelson JL, Hart J, Wennberg AK, Gadek JE. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:491–500. doi: 10.1097/01.CCM.0000049952.96496.3E. [DOI] [PubMed] [Google Scholar]
- 92.Singer P, Theilla M, Fisher H, Gibstein L, Grozovski E, Cohen J. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med. 2006;34:1033–1038. doi: 10.1097/01.CCM.0000206111.23629.0A. [Erratum appears in Crit. Care Med. (2006) 34(6), 1861.] [DOI] [PubMed] [Google Scholar]
- 93.Merchant AT, Curhan GC, Rimm EB, Willett WC, Fawzi WW. Intake of n-6 and n-3 fatty acids and fish and risk of community-acquired pneumonia in US men. Am J Clin Nutr. 2005;82:668–674. doi: 10.1093/ajcn.82.3.668. [DOI] [PubMed] [Google Scholar]
- 94.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 95.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [Erratum appears in J. Immunol. (2006) 176(6), 3843.] [DOI] [PubMed] [Google Scholar]
- 96.Marcheselli VL, Mukherjee PK, Arita M, Hong S, Antony R, Sheets K, Winkler JW, Petasis NA, Serhan CN, Bazan NG. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot Essent Fatty Acids. 82:27–34. doi: 10.1016/j.plefa.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Christie PE, Spur BW, Lee TH. The effects of lipoxin A4 on airway responses in asthmatic subjects. Am Rev Respir Dis. 1992;145:1281–1284. doi: 10.1164/ajrccm/145.6.1281. [DOI] [PubMed] [Google Scholar]
- 100.Tahan F, Saraymen R, Gumus H. The role of lipoxin A4 in exercise-induced bronchoconstriction in asthma. J Asthma. 2008;45:161–164. doi: 10.1080/02770900701847068. [DOI] [PubMed] [Google Scholar]
- 101.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, Belkaid Y, Xu Y, Whitsett JA, Accurso FJ, Wills-Karp M, Petasis NA. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]