Abstract
Contact hypersensitivity (CHS) is a delayed-type-hypersensitivity that can be induced by haptens, such as 2,4-dinitrofluorobenzene (DNFB). Innate and adaptive immunity are both important for CHS development. To treat CHS related disease, such as allergic contact dermatitis (ACD) prevalent in industrialized countries, ways to interfere with immune function during CHS response need to be identified. Transforming growth factor-β activated kinase-1 (TAK1), a member of mitogen-activated protein kinase kinase kinase (MAPKKK) family, is important for both innate and adaptive immunity. We thus hypothesized that CHS response could be inhibited by interfering with TAK1 activity. Using a mouse model where TAK1 deletion can be locally induced, we observed that TAK deficiency led to impaired CHS response associating with defective T cell expansion, activation and IFN-γ production. In addition, we investigated the effect of TAK1 deletion specifically in dendritic cells (DC) on CHS response. We found that, when TAK1 is deficient in DC, CHS response was abolished and hapten elicited T cell response was defective. Collectively, this study demonstrates an essential role of TAK1 in the induction of CHS and suggests that targeting TAK1 could be a viable approach to treat CHS.
Keywords: CHS; TAK1; DC; 2,4-dinitrofluorobenzene (DNFB)
Introduction
Allergic contact dermatitis (ACD) is one of the most common occupational diseases in industrialized countries with a great socioeconomic impact (1, 2). Knowledge of the pathophysiology of human ACD is obtained mainly from studies using murine models of contact hypersensitivity (CHS) to strong haptens, such as 2,4-dinitrofluorobenzene (DNFB). With a single contact, DNFB is able to elicit CHS response in the skin. Development of CHS occurs in two phases (3). The first phase is the sensitization phase that is induced by painting a small area of abdominal or back skin with hapten. During the sensitization phase, hapten binds to endogenous proteins in the skin, alters antigenic specificity of these proteins, and activates professional antigen presenting dendritic cells (DC) residing in the skin. Proteins with newly arisen antigenic specificities are processed by epidermal DCs, Langerhans cells, and dermal DCs (4, 5). Activated hapten-bearing DCs migrate to the draining lymph nodes (DLN) where specific CD4 and CD8 T cells are primed (6). The second phase is the elicitation phase that is induced by epicutaneous hapten challenge 5 days after the initiation phase at a previously unexposed site, usually the ear skin. At the elicitation phase, previously primed T lymphocytes during the initiation phase are activated in the dermis and the epidermis in response to the challenge to trigger inflammatory process through IFN-γ production and cytotoxicity towards keratinocytes (7, 8). Both DCs and T cells play critical roles for the development of CHS. Therefore, in order to treat CHS, efficient ways of inhibiting DC and T cell function need to be developed.
Transforming growth factor-β activated kinase-1 (TAK1) is a serine/threonine kinase belonging to the mitogen-activated protein kinase kinase kinase (MAPKKK) family. Various stimuli could activate TAK1 (9, 10). Once activated, TAK1 in turn promotes downstream signaling cascades including NF-κB (11, 12), c-Jun N-terminal kinase MAPK and p38 MAPK (13). TAK1 is essential to control the survival, differentiation and function of innate and adaptive immune cells and thus related responses (9, 12, 14). Therefore, we hypothesize that, by interfering with TAK1 function, we can inhibit CHS response.
In this study, we tested this hypothesis by disrupting TAK1 function both locally and specifically in DCs. We demonstrated that normal function of TAK1 during the initiation phase is required for CHS response and corresponding T cell activation, expansion and effector function. In addition, we showed that TAK1 function specifically in DC is critical for the development of CHS and T cell activation. Therefore, we have provided evidence that TAK1 is essential for CHS response and local or DC-specific targeting of TAK1 may be a viable approach to treat CHS and related delayed-type-hypersensitivity.
Methods
Mice
TAK1fl/fl was as described previously (12). CD11c-Cre mice were kindly provided by B. Reizis (Columbia University). ERCre mice were kindly provided by T. Ludwig (Columbia University). All mice are on C57BL/6 background and were kept under specific pathogen-free conditions in the animal care facility at the University of North Carolina at Chapel Hill. All mouse experiments were approved by Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Hapten sensitization and elicitation of CHS
Mice were sensitized to DNFB (Sigma-Aldrich) on day 1 by applying 25µl 0.5% DNFB topically on de-haired back skin (sensitization phase). On day 6, sensitized mice were challenged topically with 10µl 0.25% DNFB on the left ear (elicitation phase). To delete TAK1 gene in ER-TAK1fl/fl mice, 50µg tamoxifen (Sigma-Aldrich) was painted together with DNFB solution during the sensitization phase. Ear thickness was assessed with an electronic digital caliper (VWR, USA) before challenge and 48 h after challenge. The degree of CHS response was determined by the increase of the ear thickness 48 hours post challenge.
Staining for surface markers and Foxp3
Lymphocytes were isolated from the auricular DLN. Different surface markers and intracellular protein Foxp3 were stained with the cocktails of fluorescence-conjugated antibodies per manufactured protocols (eBioscience).
Stimulation of cytokine production and intracellular cytokine staining
Lymphocytes were isolated from DLN and stimulated with 50 ng/ml PMA (Sigma-Aldrich) and 1 µM of ionomycin (Sigma-Aldrich) for 4 hours in the presence of 2 µg/ml Brefeldin A (Sigma-Aldrich). The cells were stimulated in Bruff’s T cell medium (Sigma-Aldrich) supplemented with 10% FBS and 1% penicillin/streptomycin under cell culturing conditions (37°C, 5% CO2). Different surface markers and intracellular cytokines were stained with the cocktails of fluorescence-conjugated antibodies per manufactured protocols (BD Bioscience).
Flow cytometry analysis
Fluorescence-conjugated antibodies, CD4-Pacific blue, CD8-PE-CY7, CD44-FITC, IFN-γ-FITC, IL-4-APC, CD62L-APC, Foxp3-PE and IL-17-PE, were purchased from eBioscience. Flow cytometry analysis of labeled cells was performed on LSRII (Becton Dickinson) or CyAn (Dako Cytomation, Beckman Coulter).
Statistical analysis
Data from at least three sets of samples were used for statistical analysis. Statistical significance was calculated by Student's t-test. A P value of less than 0.05 was considered significant.
Results
Local disruption of TAK1 inhibited the development of CHS
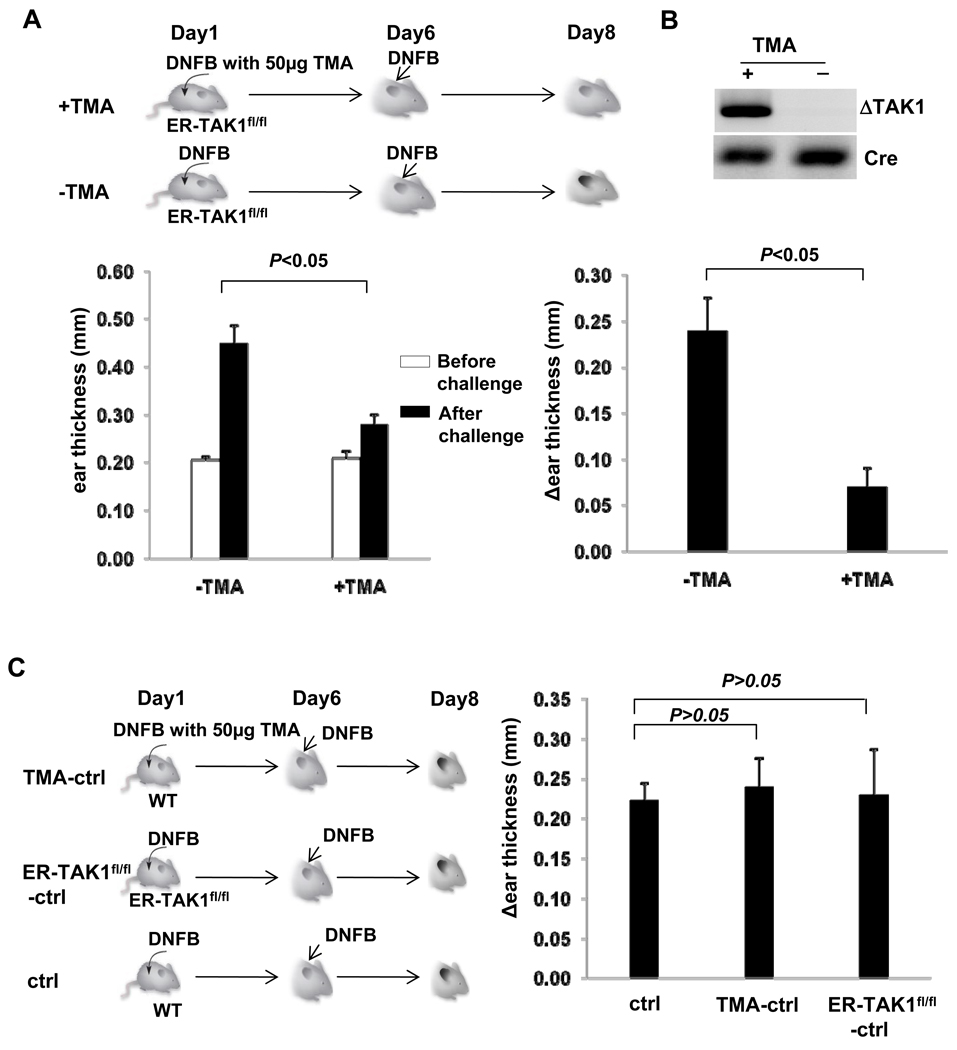
While TAK1 has been shown to be important for the generation and function of various cell types mediating innate and adaptive immunity (15), it remains poorly understood as how TAK1 is involved in the development of inflammatory diseases. We hypothesized that TAK1 is critical in the development of CHS response and CHS response can be inhibited by interfering with TAK1 function. We thus investigated whether TAK1 is required for the CHS induction by DNFB. To this end, we crossed mice bearing loxP-flanked TAK1 alleles (12) with ER-Cre mice to generate ER-TAK1fl/fl mice. In ER-Cre mice, constitutively expressed Cre protein is activated only when tamoxifen was provided (16). Therefore, ER-TAK1fl/fl mice allow us to delete TAK1 gene at the time of our choosing by providing tamoxifen as described previously (17). We topically applied DNFB mixed with 50µg tamoxifen on the de-haired back of ER-TAK1fl/fl mice to delete TAK1 at the sensitization phase of CHS response (Fig. 1A, top panel). Following DNFB treatment on the ear skin, CHS response was elicited. The degree of CHS response was assessed by the ear thickness. In response to this treatment, the ear thickness of mock-treated ER-TAK1fl/fl mice increased substantially, indicating severe skin inflammation. However, the ears of tamoxifen-treated ER-TAK1fl/fl mice failed to swell (Fig. 1A, lower panel). TAK1 deletion is detected in the skin of ER-TAKfl/fl mice treated with TMA but not the ones treated with mock. (Fig. 1B). Therefore, local disruption of TAK1 at the initiation phase inhibited the development of CHS.
Figure 1. Defective CHS response to DNFB in tamoxifen treated ER-TAK1fl/fl mice.
(A) On Day 1, ER-TAK1fl/fl mice were painted with DNFB mixed with (+) or without (−) tamoxifen on the de-haired back skin. Mice were challenged with DNFB on the left ear skin on Day 6. Ear thickness was measured before (open bar) and 48-hour after (solid bar) the challenge and plotted (lower left panel). The changes of ear thickness (Δear thickness) were also calculated and plotted (lower right panel). Mean values of four mice ±SD are shown and the P value is indicated. (B) ER-TAK1fl/fl mice were painted with TMA (+) or not (−). The painted skin were excised and subjected to PCR analysis for TAK deleted alleles (ΔTAK1) and Cre (served as internal control). (C) CHS response was induced in indicated mouse strains with or without tamoxifen (TMA) as shown in the scheme (left panel). The ear thickness was measured before and after challenge as described in (A). And the changes of ear thickness (Δear thickness) were calculated and plotted (right panel). Mean values of three mice ±SD are shown and the P value is indicated.
To confirm that above finding was not due to a defect in ER-TAK1fl/fl mouse itself or tamoxifen application alone, we elicited DNFB-induced CHS response in ER-TAK1fl/fl mice without tamoxifen treatment and in WT mice with or without tamoxifen treatment. DNFB efficiently induced CHS response in both ER-TAK1fl/fl mice and in WT mice treated with tamoxifen (Fig. 1C). Therefore, defective CHS response observed in tamoxifen treated ER-TAK1fl/fl mice was resulted from TAK1 deficiency.
T cell activation, expansion and effector function required intact TAK1 during CHS response
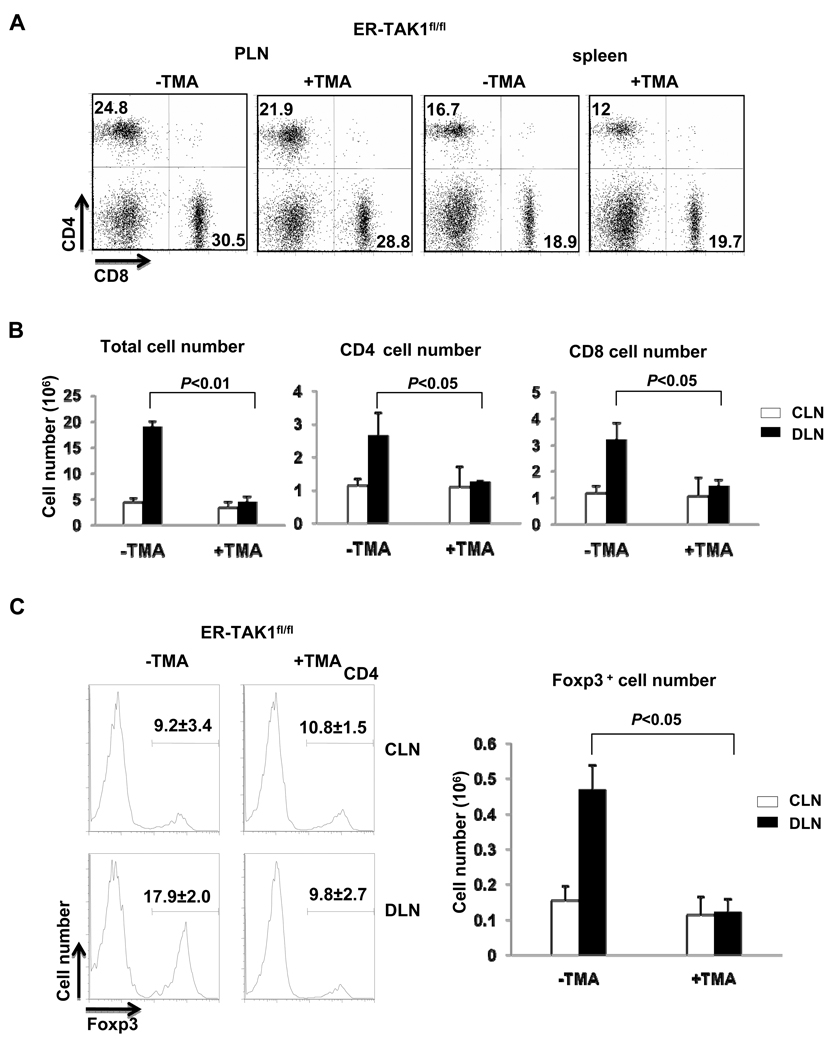
To elucidate the cellular mechanisms of the requirement of TAK1 for CHS response, we investigated whether and how T cell function was affected, as T cells are known to be the critical cell type mediating CHS response (18). There was no significant difference in the percentages of CD4 and CD8 T cells from peripheral lymph node (PLN) and spleen between TMA and mock treated ER-TAK1fl/fl mice, suggesting that application of 50µg TMA painting on skin did not cause general defect in T cell (Fig. 2A). Auricular lymph nodes of both draining side (DLNs) and non-draining side (CLN) for DNFB-treated ear were collected. The numbers of total lymphocytes in these lymph-nodes were determined. The total numbers of lymphocytes, CD4 and CD8 T cells in CLN did not increase in tamoxifen- and mock- treated ER-TAK1fl/fl mice (Fig. 2B). Nevertheless, the total numbers of lymphocytes, CD4 and CD8 T cells in DLN for DNFB-treated ear were increased in mock-treated ER-TAK1fl/fl mice (Fig. 2B), in accordance with observed ear inflammation and swelling. In contrast, such an increase did not occur in tamoxifen-treated ER-TAK1fl/fl mice (Fig. 2B). Similarly, we observed that the percentage and the numbers of Foxp3+ CD4 T cells increased in mock-treated but not in tamoxifen-treated ER-TAK1fl/fl mice (Fig. 2C). Therefore, interfering with TAK1 function during the initiation phase abrogated T cell number increase associated with inflammation during CHS response.
Figure 2. T cells failed to expand in tamoxifen treated ER-TAK1fl/fl mice during CHS response.
(A) Flow cytometry analysis of CD4 and CD8 T cell in PLN and spleen in ER-TAK1fl/fl mice with or without TMA treatment. (B) 48 hours after challenge, auricular lymph nodes of draining side (DLN, solid bar) and non-draining side (CLN, open bar) for DNFB-treated ear were collected and the numbers of total lymphocytes were counted and plotted. CD4 and CD8 T cell numbers were calculated and plotted based on the percentages of each population determined by flow-cytometry analysis and the total numbers of lymphocytes. (C) Foxp3 expression was detected by intracellular staining and flow cytometry. Numbers above the bracketed lines indicate the percent of Foxp3+ cells among CD4 population (left panel). The absolute number of Foxp3+ cells from DLN (solid bar) and CLN (open bar) were calculated and plotted. Mean values of four mice ±SD are shown and the P value is indicated.
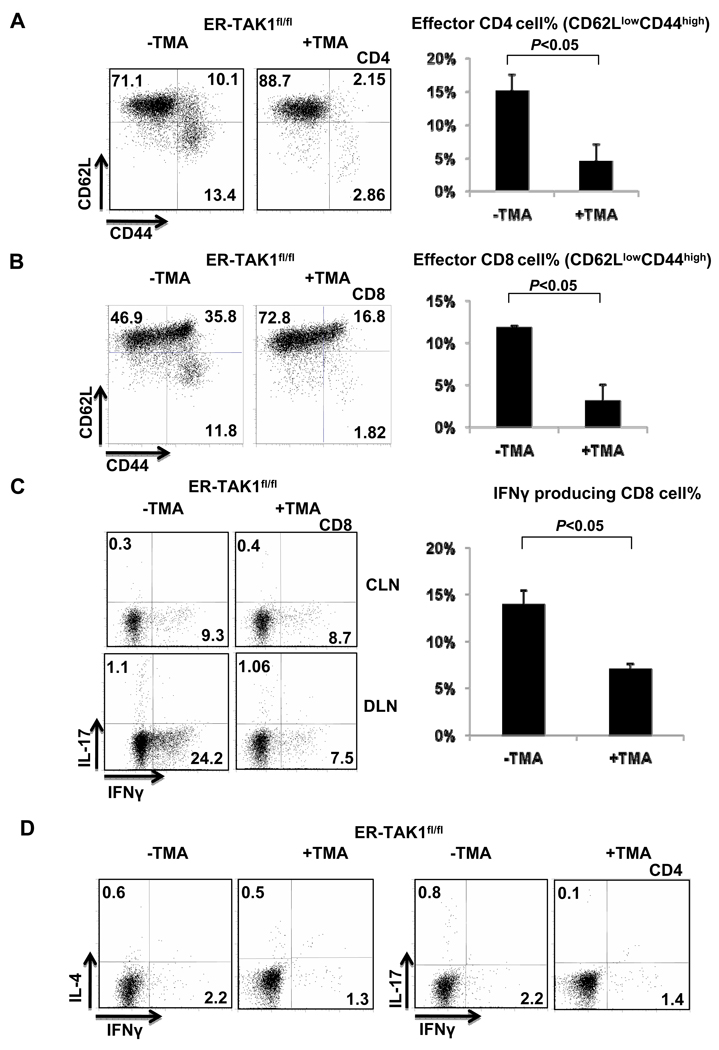
Hapten-specific CD8 T cells are recognized as the main effector cells mediating CHS (19, 20). CD4 T cells cooperate with CD8 T cells (21) and, in certain instance, mediate mouse CHS response to strong haptens (22). We further investigated whether T cell activation and effector cytokine production were affected by TAK1 deletion during CHS response. We found that while CD4 and CD8 T cells displayed activated phenotype (CD62LlowCD44high) in mock-treated ER-TAK1fl/fl mice (Fig. 3A and 3B), such an activation did not occur in tamoxifen-treated ER-TAK1fl/fl mice (Fig. 3A and 3B), suggesting that TAK1 is required for T cell activation during CHS response.
Figure 3. Lack of activation and cytokine production of CD4 and CD8 T cells in tamoxifen treated ER-TAK1fl/fl mice.
Flow cytometry analysis of CD62L and CD44 expression on CD4 (A) and CD8 (B) T cells in DLN of indicated mice 48 hours after challenge (left panels). The percentages of CD62Llow CD44high effector T cell population were plotted (right panels). Mean values of four mice ±SD are shown and the P value is indicated. (C) IFN-γ and IL-17 production by CD8 cells in the DLN of indicated mice (left panels). The frequencies (%) of IFN-γ producing CD8 cells from results of four independent experiments were plotted. Mean values ±SD are shown and the P value is indicated. (D) IFN-γ, IL-4 and IL-17 production in CD4 cells from DLN of indicated mice were detected by intracellular staining. Representative results of four independent experiments are shown.
CHS response induced by DNFB is controlled by CD8 T cell-produced IFN-γ and, to a lesser extent, by Th1, Th2 and Th17 produced IFN-γ, IL-4 and IL-17 respectively. We assessed whether interfering with TAK1 function impacted cytokine production by T cells. Indeed, while a great number of IFN-γ producing CD8 T cells was detected in mock-treated ER-TAK1fl/fl mice with ongoing CHS (Fig. 3C), smaller numbers of IFN-γ producing CD8 T cells were found in tamoxifen-treated ER-TAK1fl/fl mice (Fig. 3C). Similarly, smaller percentages of IFN-γ producing Th1 cells were found in tamoxifen-treated than in mock-treated ER-TAK1fl/fl mice (Fig. 3D). Therefore, TAK1 is required for the effector function of T cells during CHS response.
TAK1 function in DC is essential for CHS response
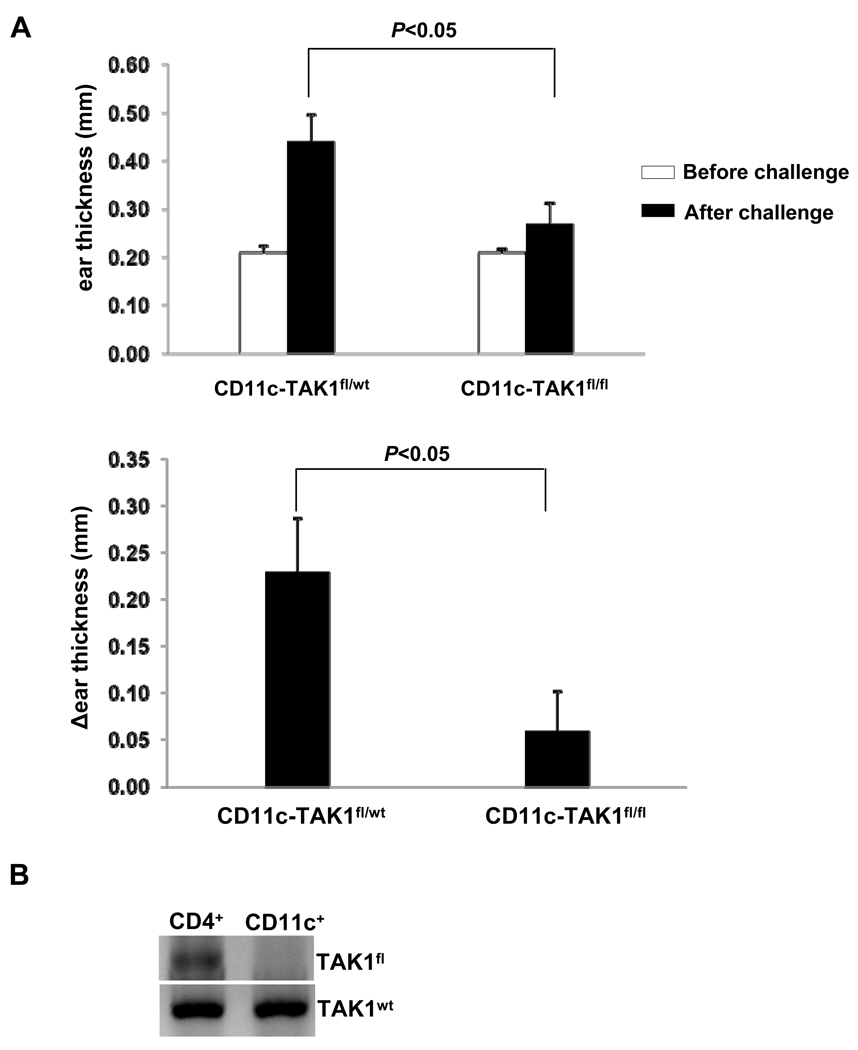
Hapten induced T cell activation and CHS requires intact function of innate cells, such as cutaneous DC (4, 23). We therefore hypothesized that the requirement of TAK1 in CHS response can be attributed, at least in part, to its essential role in regulating DC function. To test this hypothesis, we crossed TAK1fl/fl mice with CD11c-Cre mice to generate CD11c-TAK1fl/fl mice to delete TAK1 gene specifically in CD11c+ DCs and elicited DNFB-induced CHS response in CD11c-TAK1fl/fl and CD11c-TAK1fl/wt mice. While CHS response was efficiently induced in CD11c-TAK1fl/wt mice with ear swelling upon challenge, CD11c-TAK1fl/fl mice failed to develop CHS (Fig. 4A). Floxed TAK1 allele was efficiently deleted in sorted CD11c+ cells but not in CD4+ cells (Fig. 4B), indicating an efficient TAK1 deletion specifically in DC population.
Figure 4. Abolished CHS response in CD11c-TAK1fl/fl mice.
(A) On Day1, CD11c-TAK1fl/fl and CD11c-TAK1fl/wt mice were painted with DNFB on the de-haired back skin. Mice were then challenged with DNFB on Day 6. Before (open bar) and 48-hour post (solid bar) challenge, ear thickness was measured and plotted (top panel). The changes of ear thickness (Δear thickness) were also calculated and plotted (lower panel). Mean values of four mice ±SD are shown and the P value is indicated. (B) CD11c+, CD4+ cells were purified from CD11c-TAK1fl/wt mice. PCR analysis for floxed-TAK allele (TAK1fl) and wt TAK1 allele (TAK1wt) (served as internal control) were performed.
Intact TAK1 in DC is critical for T cell function during CHS development
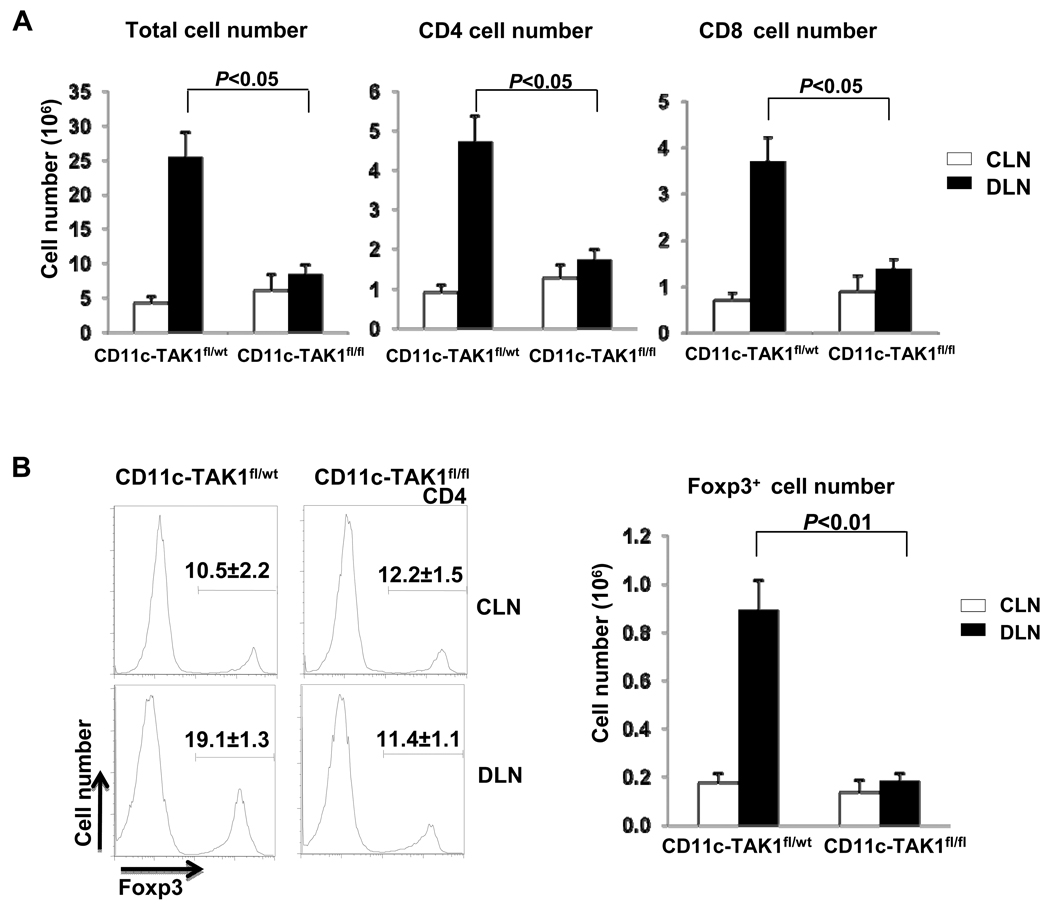
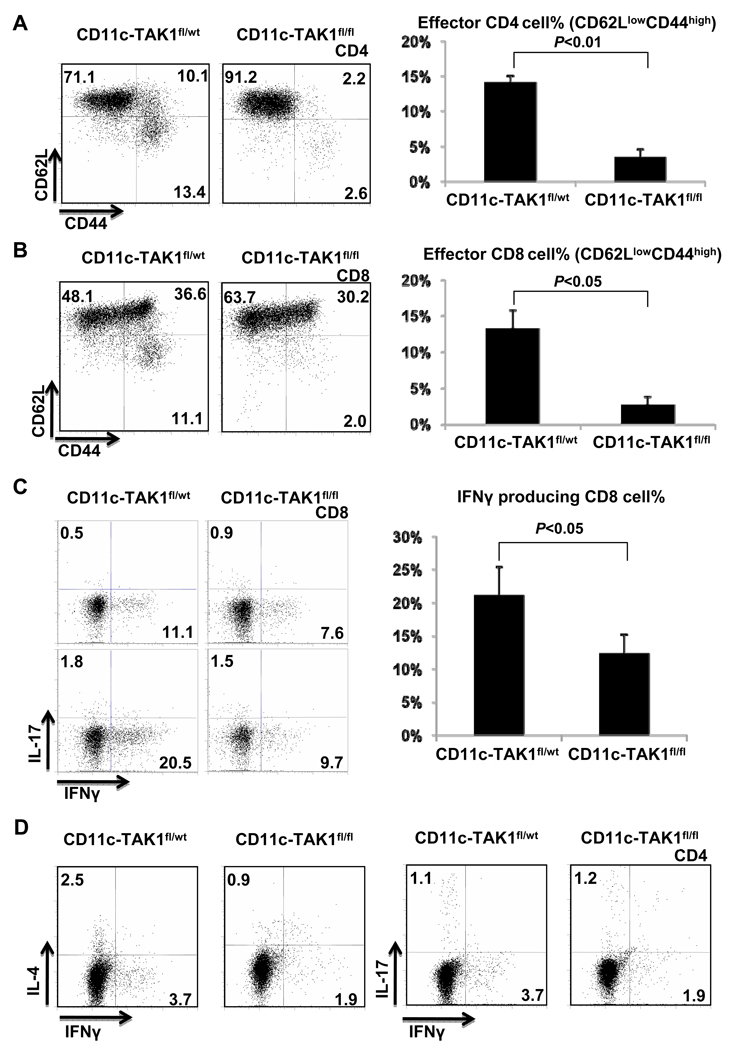
We further investigated whether and how T cell function was affected in CD11c-TAK1fl/fl mice during CHS development. Our analysis revealed that the numbers of CD4, CD8 and Foxp3-expressing CD4 cells were elevated in CD11c-TAK1fl/wt mice with ongoing CHS response. However, such a cell number increase was abolished in CD11c-TAK1fl/fl mice with the same treatment (Fig. 5A and 5B). Therefore, intact function of TAK1 in CD11c+ DC population is important to promote T cell expansion during CHS response. In addition, we observed that T cell activation was abolished in CD11c-TAK1fl/fl mice following CHS induction. The percentages of activated (CD62LlowCD44high) CD4 and CD8 T cells of the DLN from CD11c-TAK1fl/wt mice increased significantly after CHS induction. However, such an increase did not occur in similarly treated CD11c-TAK1fl/fl mice, (Fig. 6A and 6B), suggesting an important role of TAK1 in DC for T cell activation during CHS. Furthermore, we assessed the cytokine production of T cells in CD11c-TAK1fl/wt mice and CD11c-TAK1fl/fl mice during CHS response. Consistent with T cell activation status, while a large percentage of IFN-γ producing CD8 T cells was found in the DLN of CD11c-TAK1fl/wt mice, CD8 T cells from CD11c-TAK1fl/fl mice failed to upregulate IFN-γ production (Fig. 6C). Similarly, CD4 T cells in CD11c-TAK1fl/fl mice did not produce elevated levels of IFN-γ upon CHS induction (Fig. 6D).
Figure 5. T cells failed to expand during CHS response in CD11c TAK1fl/fl mice.
(A) 48 hours after challenge, lymphocytes of DLN (solid bar) and CLN (open bar) were collected from indicated mouse strains and the numbers of total lymphocytes were counted. CD4 and CD8 T cell numbers were calculated and plotted based on the percentage of each population determined by flow-cytometry analysis and the total numbers of lymphocytes. (B) Foxp3 expression was detected by intracellular staining and flow cytometry. Numbers above the bracketed lines indicate the percentage of Foxp3+ cells among CD4 population (left panels). The absolute number of Foxp3+ cells from DLN (solid bar) and CLN (open bar) were calculated and plotted (right panel). Mean values of four mice ±SD are shown and the P value is indicated.
Figure 6. T cell activation and effector function during CHS response was abrogated in CD11c TAK1fl/fl mice.
Flow cytometry analysis of CD62L and CD44 expression on CD4 (A) and CD8 (B) T cells in DLN of indicated mice 48 hours after challenge (left panels). The percentages of CD62Llow CD44high effector T cells were plotted (right panels). Mean values of four mice ±SD are shown and the P value is indicated. (C) IFN-γ and IL-17 production by CD8 cells in the DLN of indicated mice was assessed by flow-cytometry (left panels). The frequencies (%) of IFN-γ producing CD8 cells from results of four independent experiments were plotted. Mean values ±SD are shown and the P value is indicated. (D) IFN-γ, IL-4 and IL-17 production in CD4 cells from DLN of indicated mice were detected by intracellular staining. Representative results of four independent experiments are shown.
Collectively, this study demonstrated that intact function of TAK1 is critical for hapten elicited activation and effector function of T cells and the development CHS response. And such a function of TAK1 owes, at least in part, to its critical role in regulating DC function.
Discussions
The relationship between TAK1 and immune function was originally revealed by the studies on Drosophila (24). Subsequently, TAK1 was shown to be required for both innate and adaptive immunity in mammals (9, 12, 14). Former studies indicated that TAK1 is involved in the development of inflammatory diseases, such as rheumatoid arthritis (25, 26). Targeting TAK1 function therefore may be a viable approach to treat inflammatory diseases. CHS, a murine model for studying ACD, is characterized as a classic T cell mediated immune response (18). Whether TAK1 is functionally involved in CHS development was not previously addressed. Our current study showed for the first time that CHS induction and T cell activation by DNFB are dependent on TAK1.
During the elicitation phase of CHS, re-exposure of DNFB in sensitized mice induces quantitative and qualitative alteration in T lymphocyte responses. Quantitatively, T cells proliferate and increase in numbers in DLN (27). Qualitively, T cells display activated phenotype by downregulating CD62L expression and upregulating CD44 expression (28) as well as by producing IFN-γ (6). We found that interfering with TAK1 function affected T cell function both quantitatively and qualitatively. Firstly, T cell number failed to increase in response to CHS response when TAK1 is disrupted (Fig. 2B). Peripheral T cell survival has been shown to be dependent on TAK1 (12). However, the percentages of CD4 and CD8 T cell from PLN and spleen and T cell number in CLNs did not decrease in tamoxifen-treated ER-TAK1fl/fl mice during CHS response (Fig. 2A and 2B), indicating that local administration of tamoxifen did not cause systemic depletion of T cells. And the lack of T cell expansion in DLN is likely due to unresponsiveness to DNFB elicitation rather than a defect in T cell survival caused by TAK1 deletion. In agreement with this notion, our study showed that T cell failed to be activated and to produce IFN-γ when TAK1 is absent. Multiple mechanisms may account for this observation. The requirement of TAK1 for T cell function as described previously (12) could explain our findings. In addition, defective innate function due to TAK1 disruption may also attribute to the T cell unresponsiveness.
The induction of CHS response relies on the activation, maturation, antigen presentation function and migration of cells of innate immunity (29). TAK1 may be important for innate function during the initiation of CHS. In ER-TAK1fl/fl mice, tamoxifen application on local skin could induce TAK1 deletion in various cell types including stromal cells, particularly cutaneous DC that are considered to play a central role for initiating response to haptens (29). In order to specifically study the influence of TAK1 on DCs, we generated CD11c-TAK1fl/fl to knockout TAK1 gene specific in CD11c+ cells (mainly DCs). Using this mice model, we found that TAK1 function in DC is critical for the induction of CHS and T cell response (Fig. 4A, 5A and 6). While we have provided convincing genetic evidence to support the notion that TAK1 is essential for DC function during hapten-elicited CHS response, the underlying mechanisms remain to be addressed. One of the explanations could be that TAK1 plays an important role in Toll-like receptor induced NF-κB activation (30, 31) that is critical for DC functions, including migration, antigen processing and presentation, and survival (32). As TLR is suggested to be indispensable for CHS development (23), a mechanism by which TAK1 regulates DC function during CHS may be through TLR pathway. In addition, the migratory ability of DC might also be affected by TAK1 deletion as the expression of chemokine ligands, including ccl2, ccl7, ccl5, were strongly suppressed in TAK1 null cells (33). Furthermore, whether TAK1 deficiency affects the maturation, antigen presentation ability or cytokine production of DC warrants to be studied in the future.
Regulatory T cells potently suppress innate and adaptive immunity (34) to quench immune response and to maintain homeostasis. Indeed, regulatory T cell could be induced by foreign antigen (35) to limit the inflammatory response in the skin (36). It is possible that impaired CHS response in mice with defective TAK1 function was due to increased immune suppression mediated by Foxp3-expressing Treg cells. However, our findings suggested that it may not be the case because the percentage and numbers of Foxp3+ cell in tamoxifen-treated ER-TAK1fl/fl mice and in CD11c-TAK1fl/fl mice failed to be upregulated during CHS response, unlike in the control mice (Fig 2C and 4B). This finding suggests that TAK1 is critically involved in CHS response through a regulatory T cell-independent mechanism.
In conclusion, our study showed for the first time that TAK1 is critical for CHS development. Mechanistically, TAK1 is essential for the functions of both innate immunity mediated by DC and adaptive immunity mediated by T cells for the elicitation of CHS response. Therefore, local or DC-specific targeting of TAK1 may serve as a viable approach to treat CHS type immune disease.
Acknowledgments
Funding
This work was supported by National Institutes of Health [R00AI072956 to Y.Y.W]; Beijing Natural Science Foundation [7082054] and National Science and Technology Major Specific Project of the People’s Republic of China [2009ZX09301-010 to W.H.] and China Scholarship Council [Y.G.Z].
Abbreviations
- ACD
allergic contact dermatitis
- CHS
contact hypersensitivity
- DC
dendritic cells
- DLN
draining lymph node
- CLN
Non-draining, control lymph node
- DNFB
2,4-dinitrofluorobenzene
- PLN
peripheral lymph node
- TAK1
transforming growth factor-β activated kinase-1
- WT
wild type
- TMA
Tamoxifen
Footnotes
Conflict of interest
The authors have no conflicting interests.
References
- 1.Coenraads PJ, Goncalo M. Skin diseases with high public health impact. Contact dermatitis. Eur J Dermatol. 2007;17:564–565. doi: 10.1684/ejd.2007.0299. [DOI] [PubMed] [Google Scholar]
- 2.Asano Y, Makino T, Norisugi O, Shimizu T. Occupational cobalt induced systemic contact dermatitis. Eur J Dermatol. 2009;19:166–167. doi: 10.1684/ejd.2008.0581. [DOI] [PubMed] [Google Scholar]
- 3.Saint-Mezard P, Rosieres A, Krasteva M, Berard F, Dubois B, Kaiserlian D, et al. Allergic contact dermatitis. Eur J Dermatol. 2004;14:284–295. [PubMed] [Google Scholar]
- 4.Martin SF, Jakob T. From innate to adaptive immune responses in contact hypersensitivity. Curr Opin Allergy Clin Immunol. 2008;8:289–293. doi: 10.1097/ACI.0b013e3283088cf9. [DOI] [PubMed] [Google Scholar]
- 5.Jakob T, Ring J, Udey MC. Multistep navigation of Langerhans/dendritic cells in and out of the skin. J Allergy Clin Immunol. 2001;108:688–696. doi: 10.1067/mai.2001.118797. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (Il) 4/Il-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trautmann A, Akdis M, Schmid-Grendelmeier P, Disch R, Brocker EB, Blaser K, et al. Targeting keratinocyte apoptosis in the treatment of atopic dermatitis and allergic contact dermatitis. J Allergy Clin Immunol. 2001;108:839–846. doi: 10.1067/mai.2001.118796. [DOI] [PubMed] [Google Scholar]
- 8.Akiba H, Kehren J, Ducluzeau MT, Krasteva M, Horand F, Kaiserlian D, et al. Skin inflammation during contact hypersensitivity is mediated by early recruitment of CD8+ T cytotoxic 1 cells inducing keratinocyte apoptosis. J Immunol. 2002;168:3079–3087. doi: 10.4049/jimmunol.168.6.3079. [DOI] [PubMed] [Google Scholar]
- 9.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 10.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skaug B, Jiang X, Chen ZJ. The role of ubiquitin in NF-kappaB regulatory pathways. Annu Rev Biochem. 2009;78:769–796. doi: 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- 12.Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol. 2006;7:851–858. doi: 10.1038/ni1355. [DOI] [PubMed] [Google Scholar]
- 13.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 14.Schuman J, Chen Y, Podd A, Yu M, Liu HH, Wen R, et al. A critical role of TAK1 in B-cell receptor-mediated nuclear factor kappaB activation. Blood. 2009;113:4566–4574. doi: 10.1182/blood-2008-08-176057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landstrom M. The TAK1-TRAF6 signalling pathway. Int J Biochem Cell Biol. 2010;42:585–589. doi: 10.1016/j.biocel.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro-Shelef M, Lin KI, Savitsky D, Liao J, Calame K. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. J Exp Med. 2005;202:1471–1476. doi: 10.1084/jem.20051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vooijs M, Jonkers J, Berns A. A highly efficient ligand-regulated Cre recombinase mouse line shows that LoxP recombination is position dependent. EMBO Rep. 2001;2:292–297. doi: 10.1093/embo-reports/kve064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nosbaum A, Vocanson M, Rozieres A, Hennino A, Nicolas JF. Allergic and irritant contact dermatitis. Eur J Dermatol. 2009;19:325–332. doi: 10.1684/ejd.2009.0686. [DOI] [PubMed] [Google Scholar]
- 19.Vocanson M, Hennino A, Cluzel-Tailhardat M, Saint-Mezard P, Benetiere J, Chavagnac C, et al. CD8+ T cells are effector cells of contact dermatitis to common skin allergens in mice. J Invest Dermatol. 2006;126:815–820. doi: 10.1038/sj.jid.5700174. [DOI] [PubMed] [Google Scholar]
- 20.Martin S, Lappin MB, Kohler J, Delattre V, Leicht C, Preckel T, et al. Peptide immunization indicates that CD8+ T cells are the dominant effector cells in trinitrophenyl-specific contact hypersensitivity. J Invest Dermatol. 2000;115:260–266. doi: 10.1046/j.1523-1747.2000.00038.x. [DOI] [PubMed] [Google Scholar]
- 21.Traidl C, Sebastiani S, Albanesi C, Merk HF, Puddu P, Girolomoni G, et al. Disparate cytotoxic activity of nickel-specific CD8+ and CD4+ T cell subsets against keratinocytes. J Immunol. 2000;165:3058–3064. doi: 10.4049/jimmunol.165.6.3058. [DOI] [PubMed] [Google Scholar]
- 22.Vocanson M, Hennino A, Chavagnac C, Saint-Mezard P, Dubois B, Kaiserlian D, et al. Contribution of CD4(+)and CD8(+) T-cells in contact hypersensitivity and allergic contact dermatitis. Expert Rev Clin Immunol. 2005;1:75–86. doi: 10.1586/1744666X.1.1.75. [DOI] [PubMed] [Google Scholar]
- 23.Martin SF, Dudda JC, Bachtanian E, Lembo A, Liller S, Durr C, et al. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J Exp Med. 2008;205:2151–2162. doi: 10.1084/jem.20070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverman N, Zhou R, Erlich RL, Hunter M, Bernstein E, Schneider D, et al. Immune activation of NF-kappaB and JNK requires Drosophila TAK1. J Biol Chem. 2003;278:48928–48934. doi: 10.1074/jbc.M304802200. [DOI] [PubMed] [Google Scholar]
- 25.Hammaker DR, Boyle DL, Chabaud-Riou M, Firestein GS. Regulation of c-Jun N-terminal kinase by MEKK-2 and mitogen-activated protein kinase kinase kinases in rheumatoid arthritis. J Immunol. 2004;172:1612–1618. doi: 10.4049/jimmunol.172.3.1612. [DOI] [PubMed] [Google Scholar]
- 26.Hammaker DR, Boyle DL, Inoue T, Firestein GS. Regulation of the JNK pathway by TGF-beta activated kinase 1 in rheumatoid arthritis synoviocytes. Arthritis Res Ther. 2007;9:R57. doi: 10.1186/ar2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimber I, Dearman RJ. Investigation of lymph node cell proliferation as a possible immunological correlate of contact sensitizing potential. Food Chem Toxicol. 1991;29:125–129. doi: 10.1016/0278-6915(91)90167-6. [DOI] [PubMed] [Google Scholar]
- 28.Gerberick GF, Cruse LW, Miller CM, Sikorski EE, Ridder GM. Selective modulation of T cell memory markers CD62L and CD44 on murine draining lymph node cells following allergen and irritant treatment. Toxicol Appl Pharmacol. 1997;146:1–10. doi: 10.1006/taap.1997.8218. [DOI] [PubMed] [Google Scholar]
- 29.Vocanson M, Hennino A, Rozieres A, Poyet G, Nicolas JF. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699–1714. doi: 10.1111/j.1398-9995.2009.02082.x. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Z, Zamanian-Daryoush M, Nie H, Silva AM, Williams BR, Li X. Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J Biol Chem. 2003;278:16713–16719. doi: 10.1074/jbc.M300562200. [DOI] [PubMed] [Google Scholar]
- 31.Irie T, Muta T, Takeshige K. TAK1 mediates an activation signal from toll-like receptor(s) to nuclear factor-kappaB in lipopolysaccharide-stimulated macrophages. FEBS Lett. 2000;467:160–164. doi: 10.1016/s0014-5793(00)01146-7. [DOI] [PubMed] [Google Scholar]
- 32.Zanoni I, Granucci F. Regulation of antigen uptake, migration, and lifespan of dendritic cell by Toll-like receptors. J Mol Med. 88:873–880. doi: 10.1007/s00109-010-0638-x. [DOI] [PubMed] [Google Scholar]
- 33.Thiefes A, Wolter S, Mushinski JF, Hoffmann E, Dittrich-Breiholz O, Graue N, et al. Simultaneous blockade of NFkappaB, JNK, and p38 MAPK by a kinase-inactive mutant of the protein kinase TAK1 sensitizes cells to apoptosis and affects a distinct spectrum of tumor necrosis factor [corrected] target genes. J Biol Chem. 2005;280:27728–27741. doi: 10.1074/jbc.M411657200. [DOI] [PubMed] [Google Scholar]
- 34.Wan YY, Flavell RA. TGF-beta and regulatory T cell in immunity and autoimmunity. J Clin Immunol. 2008;28:647–659. doi: 10.1007/s10875-008-9251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 36.Dubois B, Chapat L, Goubier A, Papiernik M, Nicolas JF, Kaiserlian D. Innate CD4+CD25+ regulatory T cells are required for oral tolerance and inhibition of CD8+ T cells mediating skin inflammation. Blood. 2003;102:3295–3301. doi: 10.1182/blood-2003-03-0727. [DOI] [PubMed] [Google Scholar]