Abstract
Most hematopoietic stem progenitor cells (HSPCs) reside in bone marrow, but a small amount of hematopoietic stem progenitor cells have been found to circulate between bone marrow and tissues through blood and lymph. Several lines of evidence suggest that sphingosine-1-phosphate (S1P) gradient triggers HSPC egression to blood circulation after mobilization from bone marrow stem cell niches. Stem cells also visit certain tissues. After a temporary 36 hours short stay in local tissues, HSPCs go to lymph in response to S1P gradient between lymph and tissue and eventually enter the blood circulation. S1P also has a role in the guidance of the primitive HSPCs homing to BM in vivo, as S1P analogue FTY720 treatment can improve HSPC BM homing and engraftment. In stress conditions, various stem cells or progenitor cells can be attracted to local injured tissues and participate in local tissue cell differentiation and tissue rebuilding through modulation the expression level of S1P1, S1P2 or S1P3 receptors. Hence, S1P is important for stem cells circulation in blood system to accomplish its role in body surveillance and injury recovery.
Pathophysiological functions of sphingosine-1-phospahte
Sphingosine-1-phosphate (S1P), a serum-borne bioactive sphingolipid, regulates a variety of biological activities by acting either as an extracellular ligand or intracellular stimulus. As an extracellular ligand, S1P functions are mediated by the S1P family of G-protein-coupled receptors (GPCRs), identified as S1P1, S1P2, S1P3, S1P4, and S1P5 receptors (old nomenclature: EDG-1, EDG-5, EDG-3, EDG-6, EDG-8 respectively). Previously, it was showed that S1P1, a Gi-coupled plasma membrane GPCR abundantly expressed in endothelial cells (ECs), transduces S1P signaling to regulate endothelial survival, adherens junction formation, cytoskeleton architecture, and chemotactic response 1-3. Also, compelling evidences show that S1P can also function as an intracellular stimulus. Three intracellular targets of S1P have recently been identified as HDAC 4, TRAF2 5, and Prohibitin 2 6. The identification of intracellular targets of S1P suggests that S1P may directly regulate gene expression, protein turn-over, and cellular respiratory response 4-6. Moreover, S1P has been elegantly demonstrated to play critical roles in a wide array of pathophysiological functions, including angiogenesis and vasculogenesis, immune modulation, tumorigenesis, rheumatoid arthritis, asthma, inflammation, retinopathy, cardiovascular protection etc 1-6. Several of these S1P-regulated pathophysiological functions are extensively discussed in this review series, entitled “Sphingosine-1-phosphate in health and disease”. Therefore, this review will focus on one emerging area of the S1P-regulated biological activities, i.e. the trafficking of hematopoietic stem progenitor cells (HSPCs).
Hematopoietic stem progenitor cell trafficking
Stem cells, the hope of the future tissue regeneration, reside in specific bone marrow (BM) niches for the long term hematopoietic reconstitution. Bone marrow contains two major populations of stem cells: hematopoietic stem progenitor cells (HSPCs) and mesenchymal stem cells (MSCs). HSPCs can remain in the stem cell niches within the bone marrow for a long time to constantly replenish the various short lived differentiated blood cells such as red blood cell and white blood cells. MSCs, the stroma in BM, provide a supporting role in HSPCs maintenance, survival and differentiation in BM. MSCs also play a key role in the regenerative medicine because its mighty capacities to differentiate into a variety of cell types such as myoblast, fibroblast etc. BM is not the only residential place for the stem cells. Recently, tissue specific stem cells have also been identified in various tissues to support local tissue renewal. Moreover, circulatory stem cells have been found to travel in blood circulation. The continuous trafficking of stem cells between BM and blood is not only to fill the empty distal bone marrow niches, but it may also keep the tissues in surveillance and provide effector cells to foster local tissue regeneration during injury 7-10. Thus, the understanding of the mechanism behind stem cell trafficking is fundamental to both stem cell biology and stem cell transplantation.
The bone marrow microenvironment provides hematopoietic stem cells with an unique capacity for self-renewal, multilineage differentiation and long-term survival 11. Stem cells homing to the bone marrow niches is mediated by a complex array of molecular interactions that include adhesion molecules, proteolytic enzymes, and cytokines, especially chemokines such as stromal cell-derived factor 1 (SDF-1, CXCL12) 12-13. SDF-1 is a key HSPCs chemotactic factor particularly expressed by endothelial cells along the endosteum region in the bone marrow niches. Binding of SDF-1 to its receptor CXCR4 induces migration and activation of human HSPCs, CD34–expressing cells 14-17. Furthermore, in SDF-1–null mice (embryonic lethal), hematopoietic progenitor cells fail to migrate from the fetal liver to the bone marrow 18. Although there is a very significant correlation between migration capacity and the expression of CXCR4, the homing capacity of CXCR4null stem cells are not completely lost 19-20. Recently, Ratajczak et al. found that hematopoietic stem progenitor cells (HSPC) egression from the bone marrow may occur in an SDF independent process 21. These findings suggest that stem cell homing and egression does not appear to depend exclusively on the interaction of CXCR4 and SDF-1; other chemotactic receptors and their ligands also play a role in stem cell migration.
Among the chemokines and inflammatory mediators known to exert potent cellular chemotactic effects, sphingosine-1-phosphate (S1P) is a good candidate for the induction of stem cell trafficking. S1P is an important bioactive lysophospholipid secreted in the blood plasma upon platelet activation. S1P has been demonstrated to induce the chemotaxis of human natural killer cells, immature dendritic cells, and endothelial cells 22,23. Several S1P receptors (S1PRs) appear to be expressed on murine hematopoietic progenitor cells, suggesting a role of the lipid mediator S1P in the hematopoietic microenvironment 24,25. The continuous presence of S1P in the hematopoietic microenvironment and the expression of S1P receptors on the hematopoietic progenitors raised the possibility that S1P might modulate the SDF-1/CXCR4-dependent HSPC homing and lodgment. A clearer understanding of S1P in the physiological regulation of this process may aid in improving the efficiency of stem cell bone marrow transplantation.
S1P gradient triggers cells trafficking
S1P is generated in the process of normal sphingolipid turnover and it is a critical signaling molecule in normal development 26-27 (also see review of Binks W. Wattenberg in this hot-topics review series: The Role of Sphingosine Kinase Localization in Sphingolipid Signaling). It is well known that S1P induces numerous biological responses such as cell proliferation, differentiation, apoptosis, cytoskeletal remodeling and migration 1-6, 23,27,28. The effects of S1P are mediated by the S1P family of G protein coupling receptors, the sphingosine-1-phosphate receptors (S1P1- S1P5) formerly termed endothelial differentiation gene (EDG) 1,28. S1P stimulates distinct pathways such as the Rho, phospholipase C, Ras, MAP kinase and PI3K pathways dependant on the expression patterns of tissue- and cell type-specific S1P receptors 1-3, 23,27-30.
S1P is a circulating bioactive lipid mediator that is abundant in the lymph and blood, largely in forms bound to plasma proteins including HDL and albumin (see review of Koichi Sato and Fumikazu Okajima in this hot-topics review series: Role of sphingosine 1-phosphate in anti-atherogenic actions of high-density lipoprotein). Plasma contains low micromolar concentrations of S1P which is mainly generated by radiation-sensitive hematopoietic cells such as erythrocytes and platelets. Lymph contains S1P in the nano-molar range, which is derived from a radiation-resistant source, possibly endothelial cells 31. S1P lyase, which is abundant in many tissues but absent in circulation, causes rapid interstitial S1P degradation, leading to the establishment of the S1P gradient 32. The S1P gradient is critical for the migration of heart progenitors and directed migration of prechordal plate progenitor cells during zebrafish development 33,34. Recently, the S1P gradient between lymphoid tissue and lymph fluid was reported to be necessary for lymphocyte egression from lymph nodes 32. There is an emerging concept of specific cell type population migrations being triggered by distinct S1P receptors. For example, S1P1 is involved in T cell egression from the thymus, and T and B cell egression from the peripheral lymphoid organs 31,32. However, S1P5 was identified to be responsible for nature killer (NK) cells egression from BM and lymph nodes, and the egression of CD8+ T cells from the lymph nodes 35. Although some S1P receptors promote cell migration, others may play an inhibitory role. In particular, S1P1 and S1P2 are homeostatically involved in S1P-induced chemotaxis 36,37. Expression of high levels of S1P1 promotes chemotaxis 3,23,28,36,37, whereas S1P2 has been shown to inhibit migration 36,37 (also see review of Yoh Takuwa et al. in the series: Roles of sphingosine-1-phosphate signaling in angiogenesis). Therefore, regulation of S1P receptors expression is essential for the trafficking of variety of immune cells during development, in normal physiological conditions and during inflammation.
Bone marrow microenvironments: S1P gradient is essential for bone remodeling
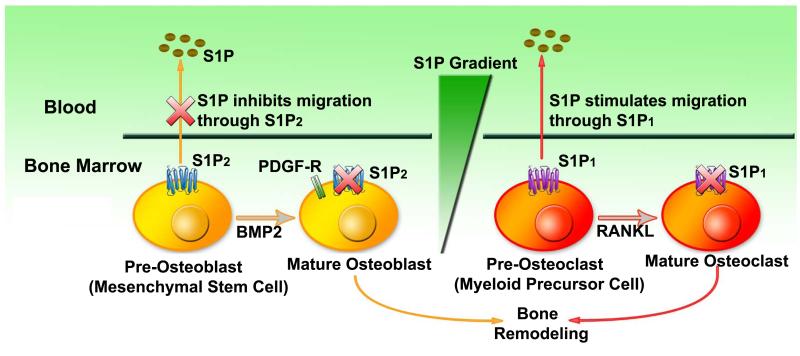
The bone marrow microenvironment provides hematopoietic stem cells with an unique capacity for self-renewal, multilineage differentiation and long-term survival 11. Osteoclasts (OC) and osteoblasts (OB) are derived from HSPC myeloid lineage differentiation and MSC differentiation respectively. Several lines of evidence support that S1P controls the migration and differentiation of osteoclast and osteoblast precursors to dynamically regulate bone mineral homeostasis and bone marrow stem cell mobilization 38,39. During RANKL (Receptor Activator for Nuclear Factor κ B Ligand) stimulated osteoclast differentiation, the expression of S1P1 receptors is suppressed (Figure 1) 38. Osteoclast precursors express functional S1P1 receptors and exhibit positive chemotaxis along a S1P gradient in vitro. Osteoclast /monocyte specific S1P1-deficient (S1P1 −/−) mice were found to be osteoporotic with lower bone tissue density, lower trabecular thickness and density, and higher osteoclast attachment to the bone surface 38. Using intravital two-photon imaging of bone tissues, it was shown that the selective S1P1 agonist SEW2871 (which functionally activates S1P1) stimulated the motility of osteoclast precursor-containing monocytoid populations in vivo. Furthermore, SEW2871 treatment caused a decrease of monocytoids in the bone marrow and an increase of these cells in the peripheral blood circulation. Lastly, S1P analogue FTY720 treatment significantly relieved ovariectomy-induced osteoporosis in mice 38. These results strongly support that S1P gradient plays a critical role in directing the correct localization of the maturing OC cells to the bone surface. Also, the concentration of S1P in the blood is higher than that in tissues. This S1P gradient favors a recirculation of osteoclast precursor monocytes from bone tissues to systemic blood circulation, which could prevent excessive mature osteoclasts from causing bone destruction 38 (Figure 1).
Figure 1. Model of the differentiation and migration of osteoclast precursor myeloid lineage progenitor cells and osteroblast precursor mesenchymal stem cells in response to S1P gradient.
Pre-osteoblast cells express S1P2 to repress it chemotaxis towards PDGF. After BMP2- induced differentiation of pre-osteoblasts to osteoblasts, S1P2 is down-regulated to resume the chemotaxis to PDGF. The migration of osteoclast precursor myeloid linage progenitor cells to blood system is mediated by the S1P1 receptor. During RANKL-induced osteoclast differentiation, S1P1 expression is suppessed to favor the local bone localization and bone remodeling.
Contrary to osteoclast differention, it was shown that the response to S1P during osteoblast differentiation is controlled by the developmental-stage specific expression of the S1P2 receptors 39. Migration of osteoblast precursor mesenchymal stem cells is controlled by a number of growth factors and cytokines such as Platelet Derived Growth Factor (PDGF-BB). The chemotaxis towards PDGF was inhibited by S1P in pre-osteoblasts so S1P acts as a chemorepellent in this scenario. Treatment with a highly selective S1P2 antagonist JTE-013 or ablation of S1P2 expression by RNA interference blocked the inhibitory effect of S1P on PDGF-induced chemotaxis, which suggests that S1P2 is responsible for mediating the inhibitory effect of S1P 39. Treatment of bone morphogenetic protein 2 (BMP2) induces the differentiation of pre-osteoblasts to alkaline phosphatase positive, mature osteoblasts. Strikingly, the inhibitory effect of S1P2 disappeared concomitantly with the down-modulation of S1P2 expression in the mature differentiated osteoblasts. Constitutive S1P2 expression confirmed that the presence or absence of S1P2 receptors is the sole determinant accounting to the change in S1P response during the BMP2-induced pre-osteoblast to osteoblast conversion 39 (Figure 1). Thus, upon conversion to the osteoblast phenotype, S1P2 receptor expression is repressed to favor chemotaxis induced by PDGF in bone tissue for new bone formation; whereas migration of immature pre-osteoblasts is restricted due to high S1P2 expression. This may reflect a mechanism that stem cells use to preserve the progenitor pool, only allowing the more differentiated cells to travel to sites of bone formation.
In summary, the migration of osteoclast precursors from the bone to the circulation is induced by S1P1expression, whereas osteoblast precursors stay in bone for bone remodeling via S1P2 expression. Furthermore, undifferentiated and differentiated OB and OC cells respond differently to the S1P gradient (Figure 1). The differential expression of specific S1P receptor subtypes during bone remodeling may be essential for bone marrow microenvironments, as it reflect a finely-tuned dynamic control of stem cell/progenitor cells trafficking during health and in various physiological conditions.
The role of S1P in HSPC egression from BM
Understanding the process of HSPC mobilization will help a significant number of patients such as those who are poor HSPC mobilizers for the bone marrow transplantation 40,41. It has been found that small amount of HSPCs circulate in the peripheral blood circulation (PB) under steady-state conditions. Significant amounts of HSPCs can be mobilized from the BM into the PB during infection, tissue injury, and after administration of some pharmacological agents 42,43. Mobilization of hematopoietic progenitor cells using granulocyte colony-stimulating factor (G-CSF) is a multifactorial process caused by modulating the activity of granulocytes and the release of proteolytic enzymes to interfere with the major retention signals for HSPC in bone marrow such as SDF–CXCR4, VLA-4–VCAM-1, and cKit ligand–c-Kit receptor axes 42-44.
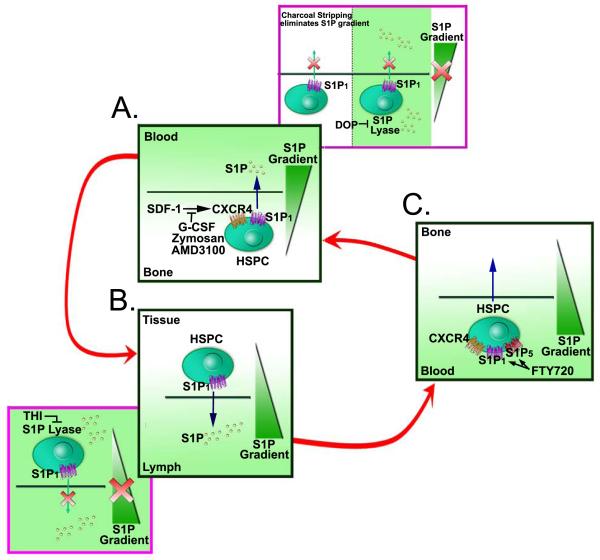
SDF-1 is essential for HSPC anchorage to the stem cell niches in the BM. The plasma concentrations of SDF-1 in either normal or chemical-induced mobilization individuals are low and should be insufficient to chemoattract murine BM HSPCs into circulation 21. Also, plasma-stimulated HSPC chemotactic activity was almost completely abolished after charcoal stripping the plasma, suggesting that bioactive lipids present in the plasma is required to mobilize HSPCs. S1P is a major chemoattractant that is several magnitudes higher in concentration than SDF-1 in normal plasma under steady-state conditions. Thus, S1P at physiologically relevant concentrations may already create a S1P gradient that continuously chemoattracts BM-residing HSPCs (Figure 2A). Erythrocytes are a major source/reservoir of S1P in the PB and form a buffer system that controls S1P levels in the PB as seen during hemolysis 45-48. It has been reported that the complement complex (CC) is activated in the BM during mobilization of HSPCs and erythrocyte lysates resulting from complement activation have a strong chemotactic effect on HSPCs. The S1P gradient is maintained by co-enzyme vitamin B6 dependent S1P lyase. DOP, a vitamin B6 antagonist, decreases S1P lyase activity in tissues. DOP-treated mice are poor mobilizers of HSPCs 21. Stem cells from DOP-treated BM do not respond to a S1P gradient, possibly because of exposure to oversaturation of S1P in the BM environment due to lack of S1P lyase activity (Figure 2A). Interruption of active anchorage of HSPCs in the BM might shift the BM-retention signal towards a plasma S1P gradient that directs the egression of HSPCs into the PB. These results suggest that the S1P gradient is important to stem cell mobilization from BM to peripheral blood circulation and failure in creating a S1P gradient from the BM to the PB greatly affects the HSPC mobilization (Figure 2A). However, it should be noted that the retention of HSPCs in the BM may be primarily regulated by the SDF-1/CXCR4 signaling. The S1P signaling might function in regulating the BM retention of HSPCs only when the SDF-1/CXCR4 signaling is abrupted.
Figure 2. Model of S1P gradient in HSPC trafficking.
(A) Egression of HSPCs from BM to peripheral blood. The molecular interactions of SDF-1/CXCR4 and other adhesion molecules mediate the lodgment of HSPCs in bone marrow niches. Blockage of the key retention signal SDF-1/CXCR4 with G-CSF, Zymosan and CXCR4 antagonist AMD3100 promotes HSPC mobilization from bone morrow and release to peripheral blood. Eliminating S1P gradient either by charcoal stripping or blocking S1P lyase activity inhibits the HSPC mobilization to peripheral blood (pink-colored framed). (B) Egression of HSPCs from tissue to lymph. HSPCs stay in tissue for a short time and then egress to lymph in response to S1P gradient. The disappearance of S1P gradient by the treatment of S1P lyase blocker THI causes the HSPCs be unable to enter lymph (pink-colored framed). (C) Homing of HSPCs from blood to BM. FTY720 may promote primitive HSPC movement to BM through S1P1 and S1P5, or it may synergize with SDF-1/CXCR4 signaling for efficient HSPC homing and engraftment to bone marrow.
S1P1 Regulates the Egression of HSPCs from Tissues into Lymphatics
Increasing evidence supports that circulating HSPCs also visit extramedullary tissues such as the liver 49 and spleen 10. An elegant experiment was performed in GFP and non-GFP parabiotic mice 50. Three days after crosscirculation was established, strong colony formation units (CFUs) chimerism was found in the blood and lymph, indicating that some HSPCs recirculate freely between the lymph and blood. Spleen, lung, liver and kidneys had the highest level of chimerism in the extramedullary tissues of parabiotic mice. The mean time of HSPCs that homed to the peripheral tissues was at least 36 hrs. It was estimated that about 200 clonogenic HSPCs passed through the lymph of the mice every day and at least twice as much HSPCs residing in extramedullary nonlymphoidal tissues 10.
Consistent with the parabiotic experiment, clonogenic progenitors were detected in many tissues, including the lung, liver, kidney, and blood 50. In addition, these tissue-derived clonogenic cells possess a capacity for multilineage reconstitution. Those tissue-derived clonogenic HSPCs were identified as BM derived using chimeric wild-type recipients of GFP+ BM. Lymph-borne HSPCs are also BM derived, and can recirculate back into the BM to maintain blood homeostasis. The primitive clonogenic HSPCs found in the efferent lymphatics possess the capacity for long-term multigenerational reconstitution, which meets the phenotypic and functional criteria for true HSPCs 50. Unlike lymphocytes, HSPCs travel directly from the extramedullary nonlymphoidal tissues to the lymph and do not necessarily need secondary lymphoid organs. Because the mammalian BM lacks lymphatic drainage, BM HSPCs are thought to egress directly into the blood. After BM stem cells traffick out of the BM directly into the blood, they travel constitutively to multiple extramedullary nonlymphoidal tissues, where they reside for at least 36 hrs until entering the draining lymphatics to await return to the bloodstream 50. Hematopoietic progenitors constitutively circulating in extramedullary tissues might provide a role in constitutively replenishing the different populations of old or damaged cells in the tissue microenvironment. For instance, after deposition into the kidney, injected GFP+ HSPCs can locally differentiate into various myeloid lineages 50.
Not much is known about the mechanism of HSPC trafficking from the blood into extramedullary tissues. However, there is evidence suggesting that S1P may play a function in tissue-residing HSPC egression to the lymphatics 50. Lymph-borne HSPCs were markedly reduced in S1P lyase blocker 2-acetyl-4-tetrahydroxybutylimidazole (THI)-treated mice, suggesting that the S1P gradient is required for HSPC egression from tissues and migration to lymphatics. Treatment with Gαi inhibitor pertussis toxin (PTX) caused a dramatic decrease in the number of lymph-borne CFU-Cs in 2–12 hrs, which implies an essential role for Gαi-mediated signaling to direct tissue-residing HSPCs into the draining lymphatics. Both treatment with FTY720 or selective S1P1 agonist SEW2871 depleted HSPCs from the lymph within 6 hrs, indicating S1P1 control over HSPCs exiting from nonlymphoid tissues into the draining lymph vessels 50,51. The treatment of mice with FTY720 over 7 days resulted in a significant increase in the number of HSPCs residing within extramedullary tissues. Hence, the S1P/ S1P1 signaling as well as S1P gradient might serve as a mechanism guiding stem cells and various progenitor cell populations egression from tissues into lymphatics (Figure 2B).
HSPC homing from the blood to the BM
The critical aspects of primitive hematopoietic cell homing to the bone marrow after transplantation are fundamentally important for the self-renewal and development of hematopoietic stem cell. Characterization of the mechanism behind S1P-mediated HSPC migration is of great relevance to the understanding of stem cell transplantation. Nobuaki et al. first found that S1P triggers an invasion of the primitive hematopoietic Lin /Sca-1+/c-Kit+ expressing cell line (THS119) into the stromal cell layers in vitro to form cobblestone areas 24, which reflect the proliferation of primitive hematopoietic cells in the hematopoietic microenvironment. They also found that PTX, an inhibitor of trimeric Gi proteins, partially inhibited THS119 invasive activity 24,52. Therefore, the migratory ability of HSPCs was mediated at least in part by signals from GPCRs eliciting intracellular events that control proliferation and motility. The invasive ability of HSPCs was mediated by the lysophospholipids (LPL) S1P and LPA. LPL receptors are known to activate small-GTPase proteins Rac/Rho/Cdc42 to mediate cell migration. Indeed, C3 exotoxin, an inhibitor of Rho, partially inhibited THS119 invasive activity, which suggests that this is a Rho dependent signaling pathway. Both S1P and LPA induced THS119 invasion may be similar to the homing of hematopoietic stem cells 24. Indeed, Whetton found that LPL synergistically promotes SDF-1 mediated primitive hematopoietic cell chemotaxis 25. Studies with Rac/Rho/Cdc42 inhibitor Clostridium difficile B toxin, Rho GTPase activated kinase inhibitor Y27632, and Rac/Rho/Cdc42 guanyl nucleotide exchange factor Vav1-null mice, indicate a role of these G proteins in LPL and SDF-1–induced migration 25. PI3K inhibitors almost completely inhibit SDF-1 and/or LPL induced migration in Lin−Sca+Kit+ cells. Thus, it has been proposed that S1P receptors expressed in primitive hematopoietic cells bind cognate ligands to activate PI3K and thereby Vav-1, which in turn affect the Rho small-GTPases to control pluripotent cell motility 25.
Supporting the involvement of S1P in stem cell migration, S1P receptors were found in both murine and human HSPCs. S1P1, as well as S1P2, S1P3, and S1P4 have been found to be expressed on murine HSPCs. Among different donors, S1P1 mRNA was consistently expressed in human CD34+ stem cells 53. In another study, S1P5 was found in the more primitive human progenitor CD34+/CD38− cells and S1P2 in the more committed human progenitor CD34+/CD38+ cells 25. S1P analogue FTY720 enhanced SDF-1 mediated transmigration of both primitive and committed progenitor cells, which could be blocked completely by the addition of CXCR4 blocking antibody or pertussis toxin (PTX) 53. An in vivo study showed that S1P receptor agonist FTY720 could increase both short-term homing and long-term engraftment of HSPCs in the xenogeneic NOD/SCID mouse model 53. Pretreatment with FTY720 caused a rapid and significant increase of more primitive, CD34+/CD38− cells homing to the bone marrow 53. This is possibly due to the expression of S1P1 and S1P5 in the more primitive CD34+/CD38− progenitors, which can bind FTY720 (and FTY720-P) most avidly to stimulate migration (Figure 2C). Intriguingly, FTY720 did not affect the expression of CXCR4 or various integrins. Although FTY720 was shown to induce sustained calcium mobilization and actin reorganization which are critical for cellular locomotion, the molecular details for S1P/S1P receptor signaling in the regulation of HSPC homing remain to be elucidated.
To analyze the influence of S1P1 on stem cell chemotaxis and trafficking, S1P1 was over-expressed in mobilized CD34+ peripheral blood progenitor cells (PBPCs) 54. The result showed that S1P1 over-expression sensitized the transfected cells to S1P-mediated migration with the most effective dose being around 10 nM, instead of 100 nM in non-transfected control cells. However, incubation with S1P in CD34+ PBPCs over-expressing S1P1 significantly inhibited in vitro SDF-1-dependent migration. In addition, over-expression of S1P1 receptors caused a significant reduction of HSPCs homing potential to the bone marrow and spleen in vivo. S1P1 over-expression caused a significant reduction of surface CXCR4 expression and completely blocked SDF-1-induced ERK1/2 activation and calcium flux. Inhibition of SDF-1 mediated migration through S1P1 over-expression also occurred in Jurkat cells 54. It has been reported that S1P signaling could transactivate CXCR4. S1P and its synthetic analog FTY720 induced phosphorylation of CXCR4 through the S1P3 receptor to improve blood flow recovery and augment revascularization after hind limb ischemia 55. Thus, signaling cascades mediated by different subtype of S1P receptors might have distinct impact on the homing of hematopoietic progenitors through the stimulation or inhibition of the central SDF-1/CXCR4 axis. However, how various physiological and pathological situations regulating the differential expression of S1P receptor subtypes remains to be determined. These data suggest that the expression of S1P receptor subtypes could be used as a strategy for modulating the SDF-1/CXCR4 axis to regulate HSPC homing and engraftment in the hematopoietic microenvironment.
Stem cell trafficking to injured tissues
The fact that S1P is a multifunctional mediator released by many different cells during inflammation and injury implies that S1P may act as a direct chemoattractant for HSPCs under specific circumstances such as tissue damage and infection.
1. Infection
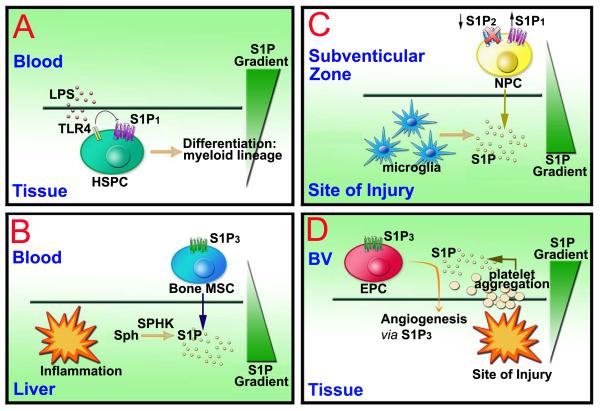
Interestingly, lymph-derived HSPCs have been found to express bacteria recognition pattern receptors TLR2 and TLR4 50. It has been reported that GPCRs play a role in Lipopolysaccharides (LPS) signaling and TLR4 signaling has been shown to cooperate with S1P1 and/or S1P3 to increase cytokine production of IL-6 and IL-8 in oral mucosal epithelial cells 56,57. LPS recognition of TLR4 caused HSPC retention within extramedullary tissues, as TLR stimulation blocked HSPC egression from inflamed tissues and abolished HSPC chemotaxis toward S1P both in vitro and in vivo. A possible explanation for this is that LPS/TLR4 signaling might interfere with S1P/ S1P1-regulated signaling. Indeed, the failure of LPS-treated HSPCs to migrate toward the S1P gradient was caused by the down-regulation of S1P1 in response to LPS treatment 50. The danger signal also induced HSPC proliferation and differentiation to rapidly produce large numbers of innate immune cells in response to tissue damage and infection. In vitro, TLR signaling can trigger HSPC proliferation and drive the differentiation of HSPCs into the myeloid lineage. When LPS-incubated HSPCs were implanted underneath the kidney capsule, clusters of local proliferating GFP+ HSPCs expressing myeloid lineage markers were found in kidney 50. Therefore, the circulation of HSPCs not only replenishes tissue residing hematopoietic cells in the absence of infection, but might also act as an immediate and highly adaptive source of progenitor cells that proliferate locally and generate innate immune effector cells to boost innate immunity to fight off life-threatening infections (Figure 3A).
Figure 3. Stem cell trafficking to injured tissues.
(A) Retention of HSPCs in local tissue. HSPCs can down-regulate the expression of S1P1 in response to LPS and thus stayed in local tissue and could differentiate to multilineage cells to help clear the infection. (B) Trafficking of MSC to injured liver. S1P is elevated in response to liver damage, which attract bone marrow mesenchymal stem cell (MSC) to the injured liver for liver remodeling in a S1P3 dependent manner. Sph, sphingosine; SPHK, sphingosine kinase. (C) Targeting of NPC to the site of brain injury. NPCs (Neural progenitor cells) are found to migrate to the site of injury for local regeneration. This is regulated by the down-regulation of S1P2 receptors. (D) Migration of EPC to sites of vascular injury. EPCs (endothelial progenitor cells) migrate to vascular injury due to high S1P generated from local vessel leakage and platelet clotting. This is probably mediated by the S1P3 receptor subtype. BV, blood vessel.
2. Bone marrow MSC migration to injured Liver
MSCs are a natural regenerative source for damaged tissues in the adult organism, as they can trans-differentiate into various cell types such as myoblasts, hepatocytes, and even neuronal cells 58-61. S1P was found to be the most potent chemoattractant among serum-derived growth factors in inducing MSC mobilization in vitro. S1P is increased after chronic liver injury in both CCl4 treatment and the BDL mice model of liver injury 62,63. The level of S1P in the liver and serum increased significantly by approximately 1.5- and 2-folds, possibly due to the up-regulation of sphingosine kinase expression in liver tissue. The S1P gradient between the damaged liver and the BM is thus established to facilitate the recruitment of MSCs from the BM into circulation and then into the liver. GFP-positive cells of BM origin are positive for the myofibroblast marker α-SMA (α-smooth muscle actin), which is correlated with the progression of liver fibrosis. S1P may mediate the homing of MSCs towards the damaged liver and the differentiation of myofibroblasts to trigger matrix remodeling during acute and chronic liver injury.
To confirm the effect of S1P on MSC migration, a transwell migration assay was performed. S1P induced the migration of MSCs in a dose-dependent manner 62,63. MSCs express three S1P receptor subtypes: S1P1, S1P2 and S1P3; however, only S1P3 was consistently reported to be markedly up-regulated after liver damage. Nonspecific S1P3 receptor antagonist suramin and specific S1P3 siRNA could significantly inhibit in vitro migration of MSCs in a dose-dependent manner. Suramin also markedly blocked the in vivo liver migration of GFP-positive MSCs in a transplantation experiment. Therefore, S1P/ S1P3 signals might play a critical role in mediating the trafficking of MSCs toward the injured liver both in vitro and in vivo 63 (Figure 3B). Also, it was shown that S1P mediates MSCs migration through important signaling pathways such as cytoskeleton remodeling, disassembly of the FAK (focal adhesion kinase)-Paxillin complex, and linkage of the extracellular matrix to the actin cytoskeleton 63. Moreover, it has been found that RhoA/ ROCK and the catalytic activity of matrix metalloproteinases (MMPs) that is involved in the S1P-induced regulation of ERK activation and Paxillin redistribution and FAK phosphorylation 64. The study of the mechanism underlying MSC migration in response to S1P will help optimize the use of bioengineered MSCs as a potent cellular therapeutic tool.
3. Role of S1P in the migration of neural progenitor cells (NPCs) during brain infarction
NPCs are self-renewing stem cells that are important in neurogenesis. Migration of NPCs is important not only for development of the embryonic nervous system, but also for the repair of the nervous system after injury 65. S1P was shown to be a critical mediator for the injury-mediated NPC migration 66,67. It was shown that the neural stem cells of the subventricular zone (SVZ) migrate laterally to sites of brain injury for region-specific neurogenesis. When experimental brain infarction was induced, most glial cells and neurons in the affected brain region were destroyed. Subsequently, microglia and myeloid lineage cells expressing CD11b accumulated 67. S1P was shown to be gradually increased at the site of ischemia at 3 days after insult and peaked at 14 days later. The high S1P level found in the region of microglia accumulation in the infarcted area, suggesting that the local elevation of S1P might be from the release of S1P from the microglia and could be a physiological chemoattractant to enhance the migration of NPCs to induce the subsequent neuroprotective regeneration after a central nervous system injury.
S1P induced NPC migration maximally at 100 nM and it has been previously shown that S1P1 contributed to NPC migration toward areas of high S1P concentration in the injured central nervous system (CNS) 66. NPCs expressed all known S1P receptor subtypes, with S1P1 and S1P2 being the most highly expressed 67. The S1P1-specific agonist failed to enhance NPC migration in the presence of S1P, suggesting that the activation of S1P1 itself could not overcome the inhibitory effect of S1P2 in NPCs 67. Indeed, specific S1P2 antagonist JTE-013 and short interfering RNA against S1P2 significantly enhanced the migration of NPCs induced by S1P in vitro. In vivo, ventricular infusion of JTE-013 promoted dramatic NPC migration towards the ischemic area where S1P increased. Modulation of S1P2, instead of S1P1, could be a more practical strategy to mobilize NPCs to migrate after a brain ischemia (Figure 3C). However, to rule out the effects of JTE-013 unrelated to S1P2 antagonism, the S1P2 gene-deficient mice would be required to confirm the full effects of S1P2 inhibition in the NPC migration.
4. Ischemia/Reperfusion (IR)
In areas of vascular injury, platelet aggregation and activation causes the local release of S1P, which results in high concentrations of S1P as well as SDF-1 to enhance the mobilization of HSPCs to sites of vascular injury for myocardial remodeling. It has been reported that exogenous HDL and its lipid component S1P attenuated the infarction size dramatically and that the level of S1P in the serum is the most reliable marker for predicting the cardiovascular events to follow 68,69 (also see review of Koichi Sato and Fumikazu Okajima in this hot-topics: Role of sphingosine-1-phosphate in anti-atherogenic actions of high-density lipoprotein). S1P-induced recovery of blood flow is due to neovessel formation. The mouse hindlimb model is one of the well-established animal models for ischemia-induced angiogenesis in vivo for evaluating the potential of angiogenic factors as therapeutic agents. The protective effects of HDL and S1P seem to be mediated through S1P3 27,70. S1P may recruit bone marrow-derived circulating endothelial precursor cells (EPCs) to the ischemic tissues 27. EPCs express the S1P3 receptors and stimulation with S1P or FTY720 activates the CXCR4 chemokine receptor which is essential for the EPC mediated angiogenesis 55. Therefore, S1P may stimulate angiogenesis through recruitment of circulating endothelial precursor cells (Figure 3D).
Discussions and Concluding Remarks
There are increasing lines of evidence which show the importance of the previously unrecognized roles of the S1P gradient in the guidance of stem cell trafficking in homeostasis and stressed conditions. However, some discrepancies exist regarding the effect and optimal dosage of S1P on HSPC migration in vitro. Some studies showed that the physiological 100 nanomolar concentration of S1P was enough to simulate HSPC chemotaxis in vitro while others report that micromolar concentrations of S1P are required. These differences might be due to different isolation methods or the status and populations of cells isolated. As shown by Ratajczack’s group, the chemotactic responsiveness of HSPCs to S1P depends on the source of the cells 21. S1P strongly chemoattracted the BM-residing clonogeneic progenitors, but this effect was significantly weaker for those pre-exposed with S1P 21. This finding that the chemotactic responsiveness to S1P is affected by previous exposure may help clear up some previous inconsistent observations. For an instance, S1P agonist had no effect on the spontaneous migration of G-CSF-mobilized human peripheral blood progenitor cells across BMEC cells in vitro, possibly due to the fact that these cells have been exposed to S1P in the peripheral blood before mobilization.
S1P’s function in vivo seems complex as it is related to both HSPC homing and mobilization. The S1P gradient established between bone parachyma and circulation seems to attract stem cells towards the circulation, as indicated by the significant spontaneous HSPC mobilization after AMD3100 blockage or using G-CSF and zymason. Therefore, to stay in specific stem cell niches, stem cells first must overcome this effect which is mediated by various retention signals. The requirement for S1P in the efficient egression of HSPCs from the BM parenchyma into the sinusoids reflects a role for S1P gradient, possibly through S1P1, in spontaneously attracting HSPCs after the retention signaling is overcome. The specific function of S1P in HSPCs traveling to and from the BM are poorly understood, as it still needs to be dissected in the various stages, such as passing in and out of the trans-endothelial barrier. In addition, the differential stages of HSPCs might employ distinct S1P receptor subtype to regulate retention or chemotaxis. There also might be a threshold regulation on the expression level of S1P receptor subtypes in HSPCs in a different tissue microenvironment which helps regulates cellular functions. For example, diminished S1P1 by TLR signaling retains HSPCs to certain extramedullary tissues to differentiate into specific population of cells to fight off the infection 50.
In stressed conditions, S1P as well as SDF-1 released from damaged local tissues might re-establish a new S1P gradient so that injured tissue might attract progenitor cells from circulation. This could be achieved by the up-regulation of sphingosine kinase which would generate more S1P. Alternatively, S1P lyase in tissue could be inactivated to maintain the high S1P concentration compared to the concentration in circulation. If this is the case, S1P may be able to direct chemotaxis to foster the local renewal of damaged cells or the production of tissue-residing innate immune cells in response to stress. It is also possible that under stress conditions, S1P may have a synergic effect with other chemokines to regulate HSPC egression from the BM to migrate to the injured tissues. It was found that the migratory ability of the less motile primitive hematopoietic cells were greatly enhanced (12 fold) when SDF-1 was combined with LPA and S1P 25. Therefore, S1P may play a role in stressed conditions where there is high cytokine production to function with other signals in mobilizing hematopoietic cells. Cytokines such as IL-8 are known to mobilize stem cells into the peripheral blood 67,71. During stressed conditions, bone marrow residing cells, such as osteoclasts, may release lipid mediators like S1P locally to trigger stem cell mobilization from specific niches towards the injury.
The SDF-1/CXCR4 axis is the key retention signal in HSPC anchorage in the bone marrow stem cell niches, and the expression of CXCR4 has been shown to be correlated with success of recovery from bone marrow transplantation. Modulation of the SDF-1/CXCR4 axis through S1P signaling may have therapeutic usage in bone marrow transplantation. It has been shown that CXCR4 inhibition can block FTY720 mediated lymphocyte homing and FTY720 inhibited endothelial cell sprouting 72. Furthermore, S1P3 could transactivate CXCR4 and overexpression of S1P1 reduced CXCR4 expression. Therefore, S1P family of receptors may play an important role in the regulation of the SDF-1/CXCR4-mediated HSPC lodgment to the stem cell niches. Kimura et al. showed that preincubation of CD34+ PBPCs with FTY720 increased SDF-1-dependent in vitro transendothelial migration and in vivo stem cell homing after transplantation 53. Because S1P1 overexpression strongly inhibits expression of CXCR4, CXCR-4 mediated signaling and chemotaxis in human CD34+ PBPCs, FTY720 might be beneficial for bone marrow transplantation as FTY720 treatment leads to the internalization and degradation of S1P1 receptors 73. Moreover, S1P chemical agonists/antagonists can activate or antagonize with various S1P receptor subtypes, and have been widely used to study the specific S1P receptor subtypes involved in cell trafficking. However, caution must be taken when analyzing the effects of S1P agonists/antagonists in a physiologically relevant condition, as small molecules may cause S1P receptors to transduce inappropriate intracellular signaling, and mask the true functions mediated by S1P receptors. Also, undesired non-specific effects are frequently associated with the utilization of pharmacological reagents. Nevertheless, it remains to be determined how FTY720 affects these S1P receptors, from full agonism to functional antagonism, and which receptors are affected. Another issue needed to be noted is that S1P may affect many different steps of HSPC recirculation. For example, FTY720 could induce the disappearance of HSPCs from blood due to the inhibition of HSPC recirculation from the extramedullary tissues 50. Although S1P and its receptors may represent one of several mechanisms that modulate CXCR4-dependent migration in vivo, it should be noted that modulation of S1P signaling in stem cell transplantation might interfere with stem cell activity and long-term engraftment.
It has been reported that the migration of malignant cells underneath the stromal layer depends on the SDF-1/CXCR4 system. Consistent with the notion that S1P increases the migratory capacity of murine and human HSPCs 24,25,74,75, it is very likely that cancer stem cells or cancer initiating cells adopt the same strategy using S1P signaling to transactivate SDF-1/CXCR4 to facilitate their growth and migration. Indeed, S1P has been found at high concentrations in many tumors microenvironments. In addition, locally produced S1P by cancer cells could recruit the MSCs with multi-lineage differentiation potential to promote cancer growth and invasion 76,77. Moreover, S1P and its receptor S1P1 are essential for the recruitment of pericytes and smooth muscle cells to the nascent capillaries, and thus facilitate angiogenesis for building the tumor’s blood supply. Therefore, it has become an urgent need to study how S1P signaling regulates stem cell trafficking and this will provide many insights into the cancer stem cell biology.
Future directions
Mounting evidence suggests that S1P plays a critical role in stem cell development and maintenance. The retaining of primitive cells in the stem cell niches and the release of more mature stem cell into circulation is in part regulated by S1P. Under normal circumstances, the primitive c-Kit+ cells are less motile than the committed Kit− cells, and respond less to SDF-1, LPA or S1P in order to keep the primitive hematopoietic cells dormant. This is supported by an in vitro chemotactic experiment, which showed that S1P does not regulate immature hematopoietic cell migration during steady-state hematopoiesis 24. More light could be shed on this process if the regulation of the expression of S1P receptor subtypes during HSPC development is studied. Certain transcriptional factors are known to regulate the expression of S1P receptors in a different environmental milieu, such as T-bet regulated NK maturation and egression from the BM 35. How and which transcription factors regulate the distinct S1P receptor subtypes in HSPC differentiation and egression from BM remain to be explored.
During HSPC circulation in the peripheral blood stream, while some organs can actively recruit HSPCs, other certain organs lack these circulating HSPCs. For example, donor-derived CFUs are rarely found in the brain. This might be due to the secured blood brain barrier. Perhaps only locally differentiated neural stem cells have the ability to replace the damaged cells. It would be important to investigate the different strategies employed by the different organs in the recruitment of stem cells. S1P1 was first identified in endothelial cells and is critical to endothelial cell function 2,3,26,27. S1P may alter the permissiveness of the endothelium at the egression sites. This is supported by a recent finding that bone marrow progenitor cells could enhance endothelial adherens junction integrity by paracrine S1P release and the following Rac1 and Cdc42 signaling 78. To precisely dissect the roles of S1P receptor subtypes at the site of egression, distinct S1P receptors must be selectively knocked out or blocked in the endothelial cells of the bone marrow.
S1P regulates a wide array of biological activities and physiological functions by functioning as an extracellular ligand or intracellular mediator. In this review, we have summarized recent evidence which strongly suggests the notion that S1P gradient plays a critical role in the mobilization, trafficking and homing of HSPCs. This notion needs to be further confirmed by utilizing sphingosine kinases, rate-limiting enzymes for S1P synthesis, null mice. There are two isoforms of sphingosine kinases, SphK1 and SphK2. No phenotypic alterations have been observed in either SphK1 or SphK2 knockout mice, and SphK1/SphK2 double null mice are embryonic lethal (see reviews: “The Role of Sphingosine Kinase Localization in Sphingolipid Signaling” and “Regulation of Cancer Cell Migration and Invasion by Sphingosine-1-Phosphate” in this hot-topics). Thus, the development of the tet-on/tet-off SphK1/SphK2 conditional double null mice is a need to precisely determine the role of S1P in HSPC mobilization. Also, most of the knowledge for the role of S1P receptor subtypes in stem cell mobilization was obtained by employing pharmacological agonists/antagonists. Similarly, more detailed studies utilizing knockout mice of S1P receptor subtypes are needed. Finally, S1P has been shown to be an important intracellular mediator. Several intracellular targets of S1P have recently been identified, including HDAC 4, TRAP2 5, and Prohibitin 2 6. Therefore, the determination of the intracellular roles of S1P in stem cell mobilization remains to be further explored in the future.
Acknowledgement
This work is supported by NIH HL071071 (MJ Lee).
Abbreviations
- HSPC
hematopoietic stem progenitor cell
- MSC
mesenchymal stem cell
- S1P
sphingosine-1-phosphate
- S1P1-5
S1P family of G-protein coupled receptors
- GPCR
G-protein coupled receptor
- BM
bone marrow
- SDF-1
stromal cell-derived factor 1
- OB
osteoblast
- OC
osteoclast
- PDGF
Platelet Derived Growth Factor
- BMP-2
bone morphogenetic protein 2
- PB
peripheral blood
- G-CSF
granulocyte colony-stimulating factor
- CFU
colony formation units
- PTX
Gαi inhibitor pertussis toxin
- LPS
Lipopolysaccharides
- GFP
green fluorescent protein
- NPC
neural progenitor cell
Reference
- 1.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. PMID: 9488656. [DOI] [PubMed] [Google Scholar]
- 2.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha’afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. PMID: 10555146. [DOI] [PubMed] [Google Scholar]
- 3.Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, Wu M, Morales-Ruiz M, Sessa WC, Alessi DR, Hla T. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell. 2001;8:693–704. doi: 10.1016/s1097-2765(01)00324-0. PMID: 11583630. [DOI] [PubMed] [Google Scholar]
- 4.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. PMID: 19729656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. PMID: 20577214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, Kordula T, Milstien S, Lesnefsky EJ, Spiegel S. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2010 Oct 19; doi: 10.1096/fj.10-167502. [Epub ahead of print]. PMID: 20959514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleming WH, Alpern EJ, Uchida N, Ikuta K, Weissman IL. Steel factor influences the distribution and activity of murine hematopoietic stem cells in vivo. Proc Natl Acad Sci U S A. 1993;90:3760–3764. doi: 10.1073/pnas.90.8.3760. PMID: 7682717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci U S A. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. PMID: 9050878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman JW, Hodgson GS. Evidence for stem cells in the peripheral blood of mice. Blood. 1962;19:702–714. PMID: 13900318. [PubMed] [Google Scholar]
- 10.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. PMID: 11729320. [DOI] [PubMed] [Google Scholar]
- 11.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. PMID: 16550195. [DOI] [PubMed] [Google Scholar]
- 12.Levesque JP, Simmons PJ. Cytoskeleton and integrin-mediated adhesion signaling in human CD34+ hemopoietic progenitor cells. Exp Hematol. 1999;27:579–586. doi: 10.1016/s0301-472x(98)00069-1. PMID: 10210315. [DOI] [PubMed] [Google Scholar]
- 13.Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. PMID: 9933168. [DOI] [PubMed] [Google Scholar]
- 14.Mohle R, et al. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530. PMID: 9616148. [PubMed] [Google Scholar]
- 15.Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood. 2003;102:1249–1253. doi: 10.1182/blood-2003-01-0318. PMID: 12714498. [DOI] [PubMed] [Google Scholar]
- 16.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. PMID: 8996247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petit I, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. PMID: 12068293. [DOI] [PubMed] [Google Scholar]
- 18.Tachibana K, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. PMID: 9634237. [DOI] [PubMed] [Google Scholar]
- 19.Rosu-Myles M, et al. The human hematopoietic stem cell compartment is heterogeneous for CXCR4 expression. Proc Natl Acad Sci U S A. 2000;97:14626–14631. doi: 10.1073/pnas.97.26.14626. PMID: 11121064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. PMID: 10229189. [DOI] [PubMed] [Google Scholar]
- 21.Ratajczak MZ, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. PMID: 20357827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kveberg L, Bryceson Y, Inngjerdingen M, Rolstad B, Maghazachi AA. Sphingosine 1 phosphate induces the chemotaxis of human natural killer cells. Role for heterotrimeric G proteins and phosphoinositide 3 kinases. Eur J Immunol. 2002;32:1856–1864. doi: 10.1002/1521-4141(200207)32:7<1856::AID-IMMU1856>3.0.CO;2-B. PMID: 12115604. [DOI] [PubMed] [Google Scholar]
- 23.Paik JH, Chae S, Lee MJ, Thangada S, Hla T. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vbeta3- and beta1-containing integrins. J Biol Chem. 2001;276:11830–11837. doi: 10.1074/jbc.M009422200. PMID: 11150298. [DOI] [PubMed] [Google Scholar]
- 24.Yanai N, Matsui N, Furusawa T, Okubo T, Obinata M. Sphingosine-1-phosphate and lysophosphatidic acid trigger invasion of primitive hematopoietic cells into stromal cell layers. Blood. 2000;96:139–144. PMID: 10891442. [PubMed] [Google Scholar]
- 25.Whetton AD, Lu Y, Pierce A, Carney L, Spooncer E. Lysophospholipids synergistically promote primitive hematopoietic cell chemotaxis via a mechanism involving Vav 1. Blood. 2003;102:2798–2802. doi: 10.1182/blood-2002-12-3635. PMID: 12829605. [DOI] [PubMed] [Google Scholar]
- 26.Mizugishi K, et al. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. PMID: 16314531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. PMID: 11032855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids--receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. PMID: 11729304. [DOI] [PubMed] [Google Scholar]
- 29.Goetzl EJ, Kong Y, Voice JK. Cutting edge: differential constitutive expression of functional receptors for lysophosphatidic acid by human blood lymphocytes. J Immunol. 2000;164:4996–4999. doi: 10.4049/jimmunol.164.10.4996. PMID: 10799850. [DOI] [PubMed] [Google Scholar]
- 30.Spiegel S, Milstien S. Functions of a new family of sphingosine-1-phosphate receptors. Biochim Biophys Acta. 2000;1484:107–116. doi: 10.1016/s1388-1981(00)00010-x. PMID: 10760461. [DOI] [PubMed] [Google Scholar]
- 31.Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. PMID: 17363629. [DOI] [PubMed] [Google Scholar]
- 32.Schwab SR, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. PMID: 16151014. [DOI] [PubMed] [Google Scholar]
- 33.Kupperman E, An S, Osborne N, Waldron S, Stainier DY. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature. 2000;406:192–195. doi: 10.1038/35018092. PMID: 10910360. [DOI] [PubMed] [Google Scholar]
- 34.Kai M, Heisenberg CP, Tada M. Sphingosine-1-phosphate receptors regulate individual cell behaviours underlying the directed migration of prechordal plate progenitor cells during zebrafish gastrulation. Development. 2008;135:3043–3051. doi: 10.1242/dev.020396. PMID: 18701549. [DOI] [PubMed] [Google Scholar]
- 35.Jenne CN, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. PMID: 19808259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Estrada R, Zeng Q, Lu H, Sarojini H, Lee JF, Mathis SP, Sanchez T, Wang E, Kontos CD, Lin CY, Hla T, Haribabu B, Lee MJ. Up-regulating sphingosine 1-phosphate receptor-2 signaling impairs chemotactic, wound-healing, and morphogenetic responses in senescent endothelial cells. J Biol Chem. 2008;283:30363–3075. doi: 10.1074/jbc.M804392200. PMID: 18765664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JF, et al. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am J Physiol Heart Circ Physiol. 2009;296:H33–42. doi: 10.1152/ajpheart.00097.2008. PMID: 19011048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishii M, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. PMID: 19204730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roelofsen T, et al. Sphingosine-1-phosphate acts as a developmental stage specific inhibitor of platelet-derived growth factor-induced chemotaxis of osteoblasts. J Cell Biochem. 2008;105:1128–1138. doi: 10.1002/jcb.21915. PMID: 18819098. [DOI] [PubMed] [Google Scholar]
- 40.Glaspy JA, et al. Peripheral blood progenitor cell mobilization using stem cell factor in combination with filgrastim in breast cancer patients. Blood. 1997;90:2939–2951. PMID: 9376574. [PubMed] [Google Scholar]
- 41.Chabannon C, et al. Cost-effectiveness of repeated aphereses in poor mobilizers undergoing high-dose chemotherapy and autologous hematopoietic cell transplantation. Leukemia. 2003;17:811–813. doi: 10.1038/sj.leu.2402867. PMID: 12682645. [DOI] [PubMed] [Google Scholar]
- 42.Lee H, Ratajczak MZ. Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells. Arch Immunol Ther Exp (Warsz) 2009;57:269–278. doi: 10.1007/s00005-009-0037-6. PMID: 19578812. [DOI] [PubMed] [Google Scholar]
- 43.Welner RS, Kincade PW. Stem cells on patrol. Cell. 2007;131:842–844. doi: 10.1016/j.cell.2007.11.010. PMID: 18045530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. PMID: 12531874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamming CE, et al. Spontaneous circulation of myeloid-lymphoid-initiating cells and SCID-repopulating cells in sickle cell crisis. J Clin Invest. 2003;111:811–819. doi: 10.1172/JCI15956. PMID: 12639987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bessler M, Hiken J. The pathophysiology of disease in patients with paroxysmal nocturnal hemoglobinuria. Hematology Am Soc Hematol Educ Program. 2008:104–110. doi: 10.1182/asheducation-2008.1.104. PMID: 19074066. [DOI] [PubMed] [Google Scholar]
- 47.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. PMID: 17215483. [DOI] [PubMed] [Google Scholar]
- 48.Ohkawa R, et al. Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Ann Clin Biochem. 2008;45:356–363. doi: 10.1258/acb.2007.007189. PMID: 18583619. [DOI] [PubMed] [Google Scholar]
- 49.Cardier JE, Barbera-Guillem E. Extramedullary hematopoiesis in the adult mouse liver is associated with specific hepatic sinusoidal endothelial cells. Hepatology. 1997;26:165–175. doi: 10.1002/hep.510260122. PMID: 9214466. [DOI] [PubMed] [Google Scholar]
- 50.Massberg S, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. PMID: 18045540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jo E, et al. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005;12:703–715. doi: 10.1016/j.chembiol.2005.04.019. PMID: 15975516. [DOI] [PubMed] [Google Scholar]
- 52.Murayama T, Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983;258:3319–3326. PMID: 6298231. [PubMed] [Google Scholar]
- 53.Kimura T, et al. The sphingosine 1-phosphate receptor agonist FTY720 supports CXCR4-dependent migration and bone marrow homing of human CD34+ progenitor cells. Blood. 2004;103:4478–4486. doi: 10.1182/blood-2003-03-0875. PMID: 14988150. [DOI] [PubMed] [Google Scholar]
- 54.Ryser MF, Ugarte F, Lehmann R, Bornhauser M, Brenner S. S1P(1) overexpression stimulates S1P-dependent chemotaxis of human CD34+ hematopoietic progenitor cells but strongly inhibits SDF-1/CXCR4-dependent migration and in vivo homing. Mol Immunol. 2008;46:166–171. doi: 10.1016/j.molimm.2008.07.016. PMID: 18760838. [DOI] [PubMed] [Google Scholar]
- 55.Walter DH, et al. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler Thromb Vasc Biol. 2007;27:275–282. doi: 10.1161/01.ATV.0000254669.12675.70. PMID: 17158356. [DOI] [PubMed] [Google Scholar]
- 56.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. PMID: 16751103. [DOI] [PubMed] [Google Scholar]
- 57.Eskan MA, et al. TLR4 and S1P receptors cooperate to enhance inflammatory cytokine production in human gingival epithelial cells. Eur J Immunol. 2008;38:1138–1147. doi: 10.1002/eji.200737898. PMID: 18395849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Short B, Brouard N, Occhiodoro-Scott T, Ramakrishnan A, Simmons PJ. Mesenchymal stem cells. Arch Med Res. 2003;34:565–571. doi: 10.1016/j.arcmed.2003.09.007. PMID: 14734097. [DOI] [PubMed] [Google Scholar]
- 59.Gregory CA, Prockop DJ, Spees JL. Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res. 2005;306:330–335. doi: 10.1016/j.yexcr.2005.03.018. PMID: 15925588. [DOI] [PubMed] [Google Scholar]
- 60.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. PMID: 10102814. [DOI] [PubMed] [Google Scholar]
- 61.Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. PMID: 15064130. [DOI] [PubMed] [Google Scholar]
- 62.Li C, et al. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol. 2009;50:1174–1183. doi: 10.1016/j.jhep.2009.01.028. PMID: 19398237. [DOI] [PubMed] [Google Scholar]
- 63.Li C, et al. Involvement of sphingosine 1-phosphate (SIP)/S1P3 signaling in cholestasis-induced liver fibrosis. Am J Pathol. 2009;175:1464–1472. doi: 10.2353/ajpath.2009.090037. PMID: 19729475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meriane M, Duhamel S, Lejeune L, Galipeau J, Annabi B. Cooperation of matrix metalloproteinases with the RhoA/Rho kinase and mitogen-activated protein kinase kinase-1/extracellular signal-regulated kinase signaling pathways is required for the sphingosine-1-phosphate-induced mobilization of marrow-derived stromal cells. Stem Cells. 2006;24:2557–2565. doi: 10.1634/stemcells.2006-0209. PMID: 16931773. [DOI] [PubMed] [Google Scholar]
- 65.Sun L, Lee J, Fine HA. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest. 2004;113:1364–1374. doi: 10.1172/JCI20001. PMID: 15124028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kimura A, et al. Essential roles of sphingosine 1-phosphate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells. 2007;25:115–124. doi: 10.1634/stemcells.2006-0223. PMID: 16990586. [DOI] [PubMed] [Google Scholar]
- 67.Kimura A, et al. Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke. 2008;39:3411–3417. doi: 10.1161/STROKEAHA.108.514612. PMID: 18757288. [DOI] [PubMed] [Google Scholar]
- 68.Deutschman DH, et al. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146:62–68. doi: 10.1016/S0002-8703(03)00118-2. PMID: 12851609. [DOI] [PubMed] [Google Scholar]
- 69.Theilmeier G, et al. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. PMID: 16982942. [DOI] [PubMed] [Google Scholar]
- 70.Means CK, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–2951. doi: 10.1152/ajpheart.01331.2006. PMID: 17293497. [DOI] [PubMed] [Google Scholar]
- 71.Xia P, Wang L, Gamble JR, Vadas MA. Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J Biol Chem. 1999;274:34499–34505. doi: 10.1074/jbc.274.48.34499. PMID: 10567432. [DOI] [PubMed] [Google Scholar]
- 72.Schmid G, et al. The immunosuppressant FTY720 inhibits tumor angiogenesis via the sphingosine 1-phosphate receptor 1. J Cell Biochem. 2007;101:259–270. doi: 10.1002/jcb.21181. PMID: 17203465. [DOI] [PubMed] [Google Scholar]
- 73.Oo ML, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. PMID: 17237497. [DOI] [PubMed] [Google Scholar]
- 74.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94:3658–3667. PMID: 10572077. [PubMed] [Google Scholar]
- 75.Burger JA, Kipps TJ. Chemokine receptors and stromal cells in the homing and homeostasis of chronic lymphocytic leukemia B cells. Leuk Lymphoma. 2002;43:461–466. doi: 10.1080/10428190290011921. PMID: 12002747. [DOI] [PubMed] [Google Scholar]
- 76.Annabi B, Lachambre MP, Plouffe K, Sartelet H, Beliveau R. Modulation of invasive properties of CD133+ glioblastoma stem cells: a role for MT1-MMP in bioactive lysophospholipid signaling. Mol Carcinog. 2009;48:910–919. doi: 10.1002/mc.20541. PMID: 19326372. [DOI] [PubMed] [Google Scholar]
- 77.Annabi B, Naud E, Lee YT, Eliopoulos N, Galipeau J. Vascular progenitors derived from murine bone marrow stromal cells are regulated by fibroblast growth factor and are avidly recruited by vascularizing tumors. J Cell Biochem. 2004;91:1146–1158. doi: 10.1002/jcb.10763. PMID: 15048870. [DOI] [PubMed] [Google Scholar]
- 78.Zhao YD, et al. Bone marrow progenitor cells induce endothelial adherens junction integrity by sphingosine-1-phosphate-mediated Rac1 and Cdc42 signaling. Circ Res. 2009;105:696–704. doi: 10.1161/CIRCRESAHA.109.199778. PMID: 19696411. [DOI] [PMC free article] [PubMed] [Google Scholar]