Abstract
BACKGROUND AND PURPOSE
Knowledge of the anatomic basis of aphasia after stroke has both theoretic and clinical implications by informing models of cortical connectivity and providing data for diagnosis and prognosis. In this study we use diffusion tensor imaging to address the relationship between damage to specific white matter tracts and linguistic deficits after left hemisphere stroke.
MATERIALS AND METHODS
Twenty patients aged 38–77 years with a history of stroke in the left hemisphere underwent diffusion tensor imaging, structural MR imaging, and language testing. All of the patients were premorbidly right handed and underwent imaging and language testing at least 1 month after stroke.
RESULTS
Lower fractional anisotropy (FA) values in the superior longitudinal and arcuate fasciculi of the left hemisphere, an indication of greater damage to these tracts, were correlated with decreased ability to repeat spoken language. Comprehension deficits after stroke were associated with lower FA values in the arcuate fasciculus of the left hemisphere. The findings for repetition were independent of MR imaging ratings of the degree of damage to cortical areas of the left hemisphere involved in language function. There were no findings for homotopic tracts in the right hemisphere.
CONCLUSION
This study provides support for a specific role for damage to the superior longitudinal and arcuate fasciculi in the left hemisphere in patients with deficits in repetition of speech in aphasia after stroke.
Aphasia, or chronic difficulty with communication, may occur in 20% or more of patients after stroke.1,2 Knowledge of the anatomic basis of aphasia has been a matter of research since Paul Broca implicated the left hemisphere in language3 and has both theoretic and clinical implications by informing models of cortical connectivity4 and providing data for diagnosis and prognosis.5 Although much of the information regarding the anatomy of aphasic syndromes was initially gleaned through postmortem examination, the maturation and proliferation of structural MR imaging methodologies have allowed for in vivo characterization of the status of brain structures and the correlation of these data with behavior. Diffusion tenor imaging (DTI) is one such technique that has emerged relatively recently.6
DTI characterizes water molecule mobility in vivo, allowing for the exploration of white matter (WM) tract integrity.7 Highly organized fiber bundles in WM tracts constrain motion of water molecules along a preferred direction parallel to the orientation of the tracts. The degree of facilitation of diffusion of water molecules along their main direction, or diffusion anisotropy, can be quantified, providing a scalar index of the organization of specific WM tracts.8 DTI can characterize reduced fractional anisotropy associated with ischemic stroke months after the event.9 Because disruptions in WM integrity may be present despite normal appearance on MR imaging, DTI provides an important complement to other structural imaging methodologies.
In the current study we use DTI to evaluate the relationship between the status of WM tracts hypothesized to be involved in language function, including the arcuate, superior longitudinal, and uncinate fasciculi, and linguistic deficits in patients who have experienced unilateral stroke, either ischemic or hemorrhagic, affecting the left hemisphere. The arcuate fasciculus (AF) is generally regarded as a subdivision of the superior longitudinal fasciculus (SLF), which provides a pathway connecting receptive (Wernicke) and expressive (Broca) language areas.10 Wernicke area is variably located in the superior temporal and adjacent parietal lobe within the left hemisphere11 in most individuals12 and is thought to be involved in the storage of phonologic representations, or memories of word sounds. Broca area, located in the frontal operculum, including pars opercularis and triangularis, is thought to store the memories of movements needed to produce speech sounds.13 Damage to either the SLF or AF has been associated with difficulty in repetition of spoken language despite relatively intact comprehension.14 A second route connecting temporal lobe language areas and the left frontal lobe via the uncinate fasciculus (UF) has also been proposed. Damage to this pathway may result in deficits in semantic processing, such as dysnomia.10,15,16
We hypothesized that DTI evidence for greater damage to the SLF and the AF would be associated with repetition deficits, whereas damage to the UF would be associated with deficits in naming to visual confrontation and that these relationships would be independent of the effects of damage to cortical areas involved in language function.
Methods
Participants
Twenty individuals (13 men) with a history of left hemisphere stroke, 16 in the distribution of the left middle cerebral artery (15 ischemic and 1 hemorrhagic) and 4 in subcortical regions (3 ischemic and 1 hemorrhagic), participated in the study. There was no history or MR imaging evidence for cortical stroke in the right hemisphere in any of the patients; however, 6 subjects had evidence for WM disease, including multiple lacunar infarcts and/or periventricular WM abnormalities, some of which affected the right hemisphere. For this reason, the presence or absence of WM abnormalities on MR imaging was used as covariates in the analyses presented below. All of the participants were at least 1 month poststroke onset (mean, 22 months; SD, 24 months; range, 1–72 months). Mean age was 58 years (SD, 11 years; range, 38–77 years). All of the participants were given the Western Aphasia Battery (WAB),17 and the composite indices (aphasia quotient [AQ]) for repetition, comprehension, and naming were extracted. Written informed consent was obtained from all of the participants.
MR Imaging Data Acquisition
The MR imaging protocol included dual-echo fast spin-echo (TE1/TE2/TR = 8.2/90/6800 ms), fluid-attenuated inversion recovery (FLAIR; TE/TI/TR = 80/2500/80 ms), dual inversion recovery sequence for suppressing CSF and WM (TE/TI1/TI2/TR = 32/325/3400/15,000 ms), and inversion recovery with phase-sensitive reconstruction (TE/TI/TR = 8/400/4300 ms). The section thickness for both conventional and diffusion-weighted volumes was 3.0 mm with 44 contiguous axial sections covering the entire brain and a square FOV at 240 × 240 mm2.18–20
DTI Acquisition
The diffusion-weighted data were acquired using a single-shot spin-echo diffusion sensitized echo-planar imaging (EPI) sequence with the balanced Icosa21 (21 encoding directions) encoding scheme,18,21 a diffusion sensitization of b = 1000 s/mm−2, TR of 6.1 seconds, and TE of 84 ms. EPI image distortion artifacts were reduced using a sensitivity encoding (SENSE) acceleration factor or k-space under-sampling of R = 2.21–23 The section thickness was 3 mm with 44 axial sections covering the whole brain (foramen magnum to vertex), a FOV of 240 × 240 mm2, and an image matrix of 256 × 256 that matched the 3D spoiled gradient (or field echo) and 2D conventional MR imaging dual spin-echo sequences described above. The number of nondiffusion-weighted or b = 0 magnitude image averages was 8; in addition, each encoding was repeated twice and magnitude averaged to enhance the signal intensity-to-noise ratio (SNR)24; thus, effectively, 50 images were acquired for each of the 44 axial sections to cover the whole brain. The total DTI acquisition time was approximately 7 minutes and resulted in SNR-independent DTI-metric estimation (in brain parenchyma gray and WM tissue); SNR (b = 0) was 50–60 and SNR (DWI) was 20–30, which gave reproducible results.
Structural MR Imaging Ratings
Conventional MR images used for rating the status of specific structures were acquired on a 3T Intera scanner (Philips, Best, The Netherlands). Sequences included the following 1) sagittal 3D T1-weighted turbo field echo with SENSE, 9.9-ms TR, 4.6-ms TE, 256 × 256 matrix, and 1-mm section thickness; 2) axial T2-weighted turbo spin-echo with 4934.4-ms TR, 80-ms TE, 512 × 512 matrix, and 3-mm section thickness without gap; 3) axial FLAIR with SENSE, 10,000-ms TR, 80-ms TE, 256 × 256 matrix, and 3-mm section thickness without gap; and 4) coronal inversion recovery with SENSE, 6246.7-ms TR, 13-ms TE, 400-ms inversion time, 256 × 256 matrix, and 2-mm section thickness without a gap.
Structures within the left hemisphere known to be involved in specific language functions, including the left inferior frontal gyrus, posterior superior and middle temporal gyri, supramarginal gyrus, and anterior temporal lobe, were rated for each patient by a radiologist (W.Z.) who was blind to the DTI and language data by using the above-described images on a 4-point scale: 0 for no evidence of damage; 1 for partial damage, that is, where the partial normal structure can be identified; 2 for almost complete damage, that is, where more than half of the structure is damaged but still some normal appearing structure can be seen; and 3 for complete damage as identified on all of the MR images. The degree of damage in areas involved in speech production (inferior frontal gyrus), comprehension (posterior superior and middle temporal gyri and supramarginal gyrus), and naming (anterior temporal lobe) were determined by summing across the ratings of the structures within these areas.
DTI Analyses
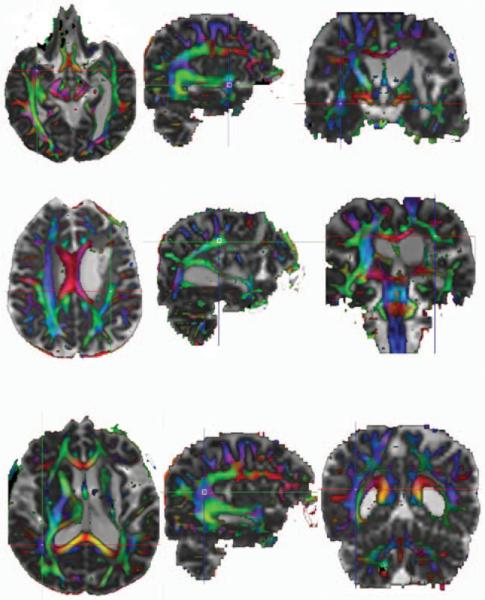
Fractional anisotropy (FA) indices for the left and right SLF, AF, and UF were determined in each hemisphere for each patient by 2 independent raters (J.I.B. and D.M.)who were blind to the language data. We obtained FA values for the AF in the portion that curves around the depth of the caudal portion of the Sylvian fissure, forming an arc. We obtained FA values for the SLF as it runs anteroposterior, deep to the shoulder of the Sylvian fissure. Although the AF and SLF are often regarded as part of the same structure, there is some evidence that they represent independent tracts,25 and we made 2 independent measures at these points to account for the possibility that their relationship to language function may be different.25,26 Regions of interest (ROIs) were selected using color-coded directional diffusion maps. Structures were identified with the help of a standard WM atlas27 and appreciated in all 3 of the planes simultaneously. First the area on the FA maps with the highest FA was determined. A rectangular 12-voxel region was then placed around this point in the sagittal plane and the box moved, if necessary, to produce the highest average FA value possible in that plane (Fig 1). For patients with large lesions, the WM structures were occasionally not identifiable. In those cases, an ROI was placed in a region homotopic to that identified in the right hemisphere, and an FA index was extracted. The correlation between FA indices determined by the 2 raters was r = 0.85 (Pearson correlation coefficient).
Fig 1.
Examples of the 3D visualization for the (A) UF, (B) SLF, and (C) AF. The meeting of the crosshairs in each section represents the point chosen visually as having the greatest intensity of representation for that region in all 3 of the planes. A 12-voxel ROI was then placed around this point in the sagittal plane for all of the structures (see examples).
A DTI scan for a single patient at the level of each structure is presented in Fig 1. The highest FA value is located at the intersection of the crosshairs in each section, and, as an example, an ROI has been placed around this intersection in each structure in the sagittal plane. DTI, structural MR imaging, and language data were acquired within 2 weeks of each other.
Results
To maintain the Familywise error rate at P < .05, the relationships among repetition, comprehension and naming deficits, and the FA index for each of the WM tracts (AF, SLF, and UF) within the left hemisphere were initially evaluated using a multivariate approach to within-subjects analysis of variance.28 The model tested included WAB AQ (repetition AQ, naming AQ, and comprehension AQ) as the dependent variable and the FA value for the specific tract as the independent variable. There were significant WAB AQ × FA value interactions for the AF (F[2,17] = 3.68;p < .05) and the SLF (F[2,17] = 10.45; p < .001). There were no significant findings for the UF (P>/I>.07).
Follow-up analyses evaluated the relationship between specific language functions and FA values within each ROI by using a critical p value of 0.017 (0.05/3). The relationship between the FA value for the AF and the repetition AQ (F[1,18] = 10.64; p < .004) was significant, whereas the relationship with the comprehension AQ (F[1,18] = 6.74; p < .018) was marginally significant. For the SLF, only the relationship between the FA value and repetition was significant (F[1,18] = 15.51; p < .001).
To account for the possibility that lesions that extended into cortical areas involved with language function contributed significantly to the above results, the analyses were repeated with MR imaging ratings of damage to areas of the left inferior frontal gyrus involved in repetition (Broca area) and areas of the left temporal lobe involved in comprehension (posterior superior and middle temporal gyri and supramarginal gyrus) and naming (anterior temporal lobe) as covariates. The findings for the relations between the SLF and AF and repetition AQ remained significant. However, the relationship between comprehension and damage to the AF did not.
The WAB repetition AQ is plotted as a function of the FA for the AF in Fig 2. Poorer repetition is associated with greater damage (lower FA value) to the AF. A similar relationship was obtained for the SLF.
Fig 2.
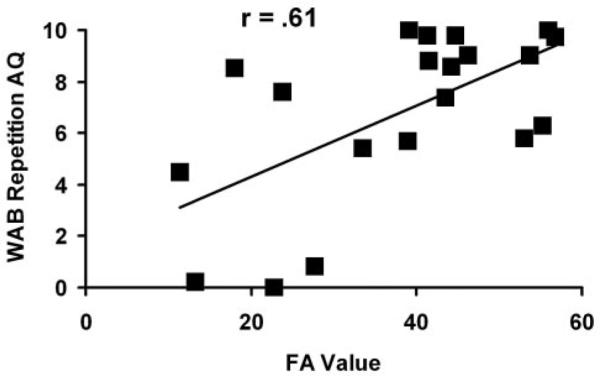
Plot of the WAB repetition AQ score as a function of the fractional anisotropy (FA) index for the AF. A more negative FA score indicates more damage to the tract, and a more negative WAB score indicates poorer repetition.
Repetition of the above analyses using FA values for the homotopic structures within the right hemisphere produced no significant findings, nor were there any significant relationships between DTI variables and month post-stroke onset or sex. When these variables were used as covariates in the above analyses involving the left hemisphere, there were no changes in the results, nor were there any changes in results when the presence of lacunar infarcts and/or periventricular WM abnormalities was used as a covariate. Differences between FA values for the SLF (mean, 0.33; SD, 0.17), AF (mean, 0.38; SD, 0.14), and UF (mean, 0.33; SD, 0.11) within the left hemisphere were evaluated by using a generalized linear model approach to a within-subjects design with ROI as the within-subjects variable. The differences among the 3 ROIs were not significant (p < .22).
Discussion
The current study indicates a specific relationship between the status of the SLF and AF in the left hemisphere and difficulty with repetition of spoken language. These findings were independent of damage to cortical areas potentially involved in these functions, suggesting an independent contribution of damage to these WM tracts to these linguistic deficits after stroke.
The SLF connects areas in the lateral frontal cortex that support expressive language, including Broca area, with areas of the parietal and temporal cortex, including Wernicke area, that support receptive language function.29 The AF is generally thought of as a subdivision of the SLF, forming a large arc around the insula and projecting into the temporal lobe.27 Within the left hemisphere, the fibers of the SLF and AF connect various aspects of the temporal and frontal cortex involved in receptive and expressive language,30 forming part of the distributed language network.31,32 We measured the FA values in the AF in its vertical segment at the point at which it arches around the insula, and we measured the FA values in the SLF where it runs anteroposterior deep to the Sylvian fissur
Damage to the AF has been associated with a specific difficulty in forming the phonologic representations of speech sounds and resultant difficulty with repetition.33 Although there is evidence that repetition deficits can occur with lesions restricted to the AF,34 both the clinical entity itself and the locations of lesions that have been associated with conduction aphasia have been variable.32,33 We found damage to the SLF to be related to repetition only, and damage to the AF to be related to both repetition and comprehension. However, whereas the relationship between damage to both the SLF and AF and repetition was independent of damage to inferior frontal areas within the left hemisphere (Broca area), the relationship between damage to the AF and comprehension was not independent of cortical areas within the left hemisphere associated with language comprehension (posterior superior, middle temporal, and supramarginal gyri). These findings suggest that, whereas damage to the SLF and AF may both contribute independently to repetition deficits after stroke, comprehension deficits may be either mediated through or moderated by cortical damage to left temporal lobe language areas.
It has been suggested that the UF may offer a second route for communication between frontal and temporal language areas10,35 specifically related to semantic analysis, including naming. Lesions restricted to left anterior temporal lobe, which may affect branches of the UF, have been associated with naming deficits.16,36,37 We did not find a relation between damage to the UF and any of the indices of language function. This may have been due to lack of power and/or use of language tests that may not have been sensitive to more subtle semantic deficits. Another possibility is that the UF may be better visualized in a plane other than the sagittal plane, and measurement error related to partial volume averaging and consequent undersampling of the tract may have affected results. Some of the participants exhibited almost complete destruction of a particular structure. Rather than eliminating these individuals from the study, an approach was adopted that allowed the insertion of a low FA rating, presumably indicating greater damage to the structure. The results support this approach as subjects with significant or complete destruction of the SLF and/or the AF within the left hemisphere, as represented by FA values obtained in this manner, exhibited significant repetition deficits independent of the status of MR imaging damage to cortical language areas.
Although we attempted to take the effects of structural damage to cortical areas into account, we did not address cortical functional topography, for example, with blood oxygen level-dependent cortical activation studies. Therefore, it is possible that areas of eloquent cortex in atypical regions that may not have been considered in the analysis may have been responsible for some of the language deficits in addition to the involvement of WM tracts of interest. In addition, the DTI analysis was restricted to FA value measurement. Different contributions to FA decrease may be present other than injury to the tract, thus potentially rendering the FA decreases non-specific. Changes in fiber attenuation, multidirectionality of fibers within sampled voxels, and partial volume averaging because of use of standard rectangular rather than tailored irregular ROIs may all contribute to spuriously decreased FA values. Future research should consider measures such as mean longitudinal diffusivity, transverse diffusivity, volume ratio anisotropy, and fiber tract volumes in correlations with behavioral data.
Given the large range of time since stroke in the study sample, poststroke reorganization is a potential confound in this study. Reorganization after stroke is also potentially affected by a number of variables, including time since stroke, age, and sex. Although there was a good deal of variance on a number of these variables in the current study sample, there were no effects of any of these variables on the findings. There was also a wide range of lesion size in the current study, and in no case was damage limited to specific WM tracts. We included patients with subcortical and cortical damage. Future research might study the relationship between DTI and behavioral data in patients with WM infarcts only to avoid the compounding effects of cortical infarction and wallerian degeneration.
We attempted to account for damage beyond the tracts studied by including ratings of the degree of damage to specific cortical language areas within the left hemisphere as a covariate in analyses, as well as the presence of evidence for WM disease, such as lacunar infarcts and/or periventricular WM abnormalities as covariates. Results suggested that MR imaging and DTI findings explained independent portions of the variance in specific language functions after stroke and that WM abnormalities did not correlate with changes in language function, providing support for a role of the SLF/AF in the repetition of spoken language. The study also highlights the use of DTI as an adjunct to other in vivo imaging modalities in providing data regarding the functional connectivity of distributed cognitive systems and the effects of cerebral insult on these systems.
Acknowledgments
This work was supported by National Institutes of Health grant 5PO1 NS046588 to A.C. Papanicolaou.
References
- 1.Kertesz A. Neurobiological aspects of recovery from aphasia in stroke. Int Rehabil Med. 1984;6:122–27. doi: 10.3109/03790798409165934. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen PM, Jorgensen HS, Nakayama H, et al. Aphasia in acute stroke: incidence, determinants, and recovery. Ann Neurol. 1995;38:659–66. doi: 10.1002/ana.410380416. [DOI] [PubMed] [Google Scholar]
- 3.Broca P. Remarques sur la siege de la faculte du lanage articule suivies d'une observation d'amphemie (perte de la parole). Bulletin et Memoires de la Societe Anatomique de Paris. 1861;36:330–57. [Google Scholar]
- 4.Mesulam M. Imaging connectivity in the human cerebral cortex: the next frontier? Ann Neurol. 2005;57:5–7. doi: 10.1002/ana.20368. [DOI] [PubMed] [Google Scholar]
- 5.Klingberg T, Hedehus M, Temple E, et al. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- 6.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 7.Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39:928–34. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- 8.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–46. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 9.Werring DJ, Toosy AT, Clark CA, et al. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry. 2000;69:269–72. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker GJ, Luzzi S, Alexander DC, et al. Lateralization of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 2005;24:656–66. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 11.Gray H, Standring S, Ellis H, et al. Gray's Anatomy: The Anatomical Basis of Clinical Practice. Elsevier Churchill Livingstone; New York: 2005. [Google Scholar]
- 12.Knecht S, Drager B, Deppe M, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123:2512–18. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- 13.Roth HL, Nadeau SE, Hollingsworth AL, et al. Naming concepts: evidence of two routes. Neurocase. 2006;12:61–70. doi: 10.1080/13554790500502892. [DOI] [PubMed] [Google Scholar]
- 14.Benson DF, Sheremata WA, Bouchard R, et al. Conduction aphasia. A clinicopathological study. Arch Neurol. 1973;28:339–46. doi: 10.1001/archneur.1973.00490230075011. [DOI] [PubMed] [Google Scholar]
- 15.Mandonnet E, Nouet A, Gatignol P, et al. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130:623–29. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- 16.Lu LH, Crosson B, Nadeau SE, et al. Category-specific naming deficits for objects and actions: semantic attribute and grammatical role hypotheses. Neuropsychologia. 2002;40:1608–21. doi: 10.1016/s0028-3932(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 17.Kertesz A. Western Aphasia Battery. The Psychological Corp; San Antonio: 1982. [Google Scholar]
- 18.Hasan KM, Narayana PA. Computation of the fractional anisotropy and mean diffusivity maps without tensor decoding and diagonalization: theoretical analysis and validation. Magn Reson Med. 2003;50:589–98. doi: 10.1002/mrm.10552. [DOI] [PubMed] [Google Scholar]
- 19.Hou P, Hasan KM, Sitton CW, et al. Phase-sensitive T1 inversion recovery imaging: a time-efficient interleaved technique for improved tissue contrast in neuroimaging. AJNR Am J Neuroradiol. 2005;26:1432–38. [PMC free article] [PubMed] [Google Scholar]
- 20.Moran PR, Kumar NG, Karstaedt N, et al. Tissue contrast enhancement: image reconstruction algorithm and selection of TI in inversion recovery MRI. Magn Reson Imaging. 1986;4:229–35. doi: 10.1016/0730-725x(86)91062-3. [DOI] [PubMed] [Google Scholar]
- 21.Hasan KM, Narayana PA. Retrospective measurement of the diffusion tensor eigenvalues from diffusion anisotropy and mean diffusivity in DTI. Magn Reson Med. 2006;56:130–37. doi: 10.1002/mrm.20935. [DOI] [PubMed] [Google Scholar]
- 22.Bammer R, Auer M, Keeling SL, et al. Diffusion tensor imaging using single-shot SENSE-EPI. Magn Reson Med. 2002;48:128–36. doi: 10.1002/mrm.10184. [DOI] [PubMed] [Google Scholar]
- 23.Jaermann T, Crelier G, Pruessmann KP, et al. SENSE-DTI at 3 T. Magn Reson Med. 2004;51:230–36. doi: 10.1002/mrm.10707. [DOI] [PubMed] [Google Scholar]
- 24.Conturo TE, McKinstry RC, Aronovitz JA, et al. Diffusion MRI: precision, accuracy and flow effects. NMR Biomed. 1995;8:307–32. doi: 10.1002/nbm.1940080706. [DOI] [PubMed] [Google Scholar]
- 25.Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–69. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 26.Schmahmann JD, Pandya DN, Wang R, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–53. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 27.Mori S, Wakana S, Magae-Poetscher LM, et al. MRI Atlas of Human White Matter. Elsevier Science Ltd; New York: 2005. [Google Scholar]
- 28.Maxwell SE, Delaney HD. Designing Experiments and Analyzing Data. Lawrence Erlbaum Associates; Mahwah, NJ: 2004. [Google Scholar]
- 29.Catani M, Howard RJ, Pajevic S, et al. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:7–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- 30.Powell HW, Parker GJ, Alexander DC, et al. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32:388–99. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Duffau H, Capelle L, Sichez N, et al. Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain. 2002;125:199–214. doi: 10.1093/brain/awf016. [DOI] [PubMed] [Google Scholar]
- 32.Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- 33.Anderson JM, Gilmore R, Roper S, et al. Conduction aphasia and the arcuate fasciculus: a reexamination of the Wernicke-Geschwind model. Brain Lang. 1999;70:1–12. doi: 10.1006/brln.1999.2135. [DOI] [PubMed] [Google Scholar]
- 34.Alexander MP, Naeser MA, Palumbo CL. Correlations of subcortical CT lesion sites and aphasia profiles. Brain. 1987;110:961–91. doi: 10.1093/brain/110.4.961. [DOI] [PubMed] [Google Scholar]
- 35.Wise RJ. Language systems in normal and aphasic human subjects: functional imaging studies and inferences from animal studies. Br Med Bull. 2003;65:95–119. doi: 10.1093/bmb/65.1.95. [DOI] [PubMed] [Google Scholar]
- 36.Miozzo A, Soardi M, Cappa SF. Pure anomia with spared action naming due to a left temporal lesion. Neuropsychologia. 1994;32:1101–09. doi: 10.1016/0028-3932(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 37.Tippett LJ, Glosser G, Farah MJ. A category-specific naming impairment after temporal lobectomy. Neuropsychologia. 1996;34:139–46. doi: 10.1016/0028-3932(95)00098-4. [DOI] [PubMed] [Google Scholar]