Abstract
Although fundamentally similar to other bones, the jaws demonstrate discrete responses to developmental, mechanical, and homeostatic regulatory signals. Here, we hypothesized that rat mandible vs. long-bone marrow-derived cells possess different osteogenic potential. We established a protocol for rat mandible and long-bone marrow stromal cell (BMSC) isolation and culture. Mandible BMSC cultures formed more colonies, suggesting an increased CFU-F population. Both mandible and long-bone BMSCs differentiated into osteoblasts. However, mandible BMSCs demonstrated augmented alkaline phosphatase activity, mineralization, and osteoblast gene expression. Importantly, upon implantation into nude mice, mandible BMSCs formed 70% larger bone nodules containing three-fold more mineralized bone compared with long-bone BMSCs. Analysis of these data demonstrates an increased osteogenic potential and augmented capacity of mandible BMSCs to induce bone formation in vitro and in vivo. Our findings support differences in the mechanisms underlying mandible homeostasis and the pathophysiology of diseases unique to the jaws.
Keywords: marrow stromal cells, BMSCs, mandible, long bone, osteogenic
INTRODUCTION
Despite being seemingly similar to other bones in the body, the maxilla and mandible serve distinct functions and demonstrate discrete responses to developmental, mechanical, and homeostatic stimuli (Sodek and McKee, 2000). Developmentally, the jaws, similar to other craniofacial bones, but distinct from the axial and appendicular skeleton, arise from neural crest cells of the neuroectoderm germ layer, and not from the mesoderm (Chai and Maxson, 2006), and undergo intramembranous instead of endochondral ossification (Karaplis, 2002). The mandible in particular is formed primarily by intramembranous ossification, while secondary cartilage at its proximal end contributes endochondral components at later stages. Meckel’s cartilage, which precedes mandible formation but mainly disappears as the mandible develops, plays an important role in mandibular morphogenesis (Ramaesh and Bard, 2003; Tsutsui et al., 2008). Although the same key regulators of osteoblastic differentiation, such as Runx2 and osterix, determine precursor commitment in intramembranous and endochondral bones (Ducy et al., 1997; Komori et al., 1997; Otto et al., 1997; Nakashima et al., 2002), several growth factors, receptors, and associated signaling cascades play distinct roles in the craniofacial vs. axial and appendicular skeleton (Abzhanov et al., 2007; De Coster et al., 2007; Kimmel et al., 2007). Systemic diseases, such as osteoporosis, hyperparathyroidism, or Paget’s disease, affect all bones, including the jaws (White and Pharoah, 2004). Indeed, there appears to be a correlation between mandibular bone density and osteoporosis (Kribbs et al., 1989; Jeffcoat et al., 2000; Lerner, 2006; Mavropoulos et al., 2007). However, rat mandibles lose significantly less trabecular bone and bone mineral density at a lower rate than tibiae primary spongiosa after ovariectomy and malnutrition (Mavropoulos et al., 2007), suggesting different homeostatic mechanisms of the two bones.
The small size and anatomic complexity of the maxilla and mandible render bone cell isolation a challenge (Sodek and McKee, 2000). Thus, little is known about the cellular and particularly molecular basis for mandibular bone vs. long-bone divergent homeostasis. Most of our knowledge on mandible cell function and differentiation is derived from experimental models with cells from other skeletal sites (Lerner, 2006). However, caution should be exercised in the extrapolation of such data to mandibular cell function. Indeed, human mandibular or maxillary marrow stromal cells demonstrate increased cell proliferation, delayed senescence, and stronger expression of osteoblastic markers compared with iliac-crest-derived marrow cells from the same patients (Akintoye et al., 2006), suggesting distinct functions and differentiation potential.
Here, we sought to compare the in vitro osteoblastic differentiation and capacity for in vivo bone formation of bone marrow stromal cells (BMSCs) derived from rat mandible vs. BMSCs derived from rat tibiae. We hypothesized that these marrow populations, derived from two distinct skeletal sites in the rat, would display diverse osteogenic potential. Indeed, mandible BMSCs demonstrated a more robust osteoblastic differentiation and induced significantly greater bone formation than their long-bone counterparts. Our findings demonstrate differences in osteogenic potential of mandible vs. long-bone BMSCs, and suggest an increased capacity of mandible BMSCs to induce bone formation in vitro and in vivo.
MATERIALS & METHODS
Isolation and Culture of Mandible and Long-bone BMSCs
All animals were handled in accordance with guidelines of the Chancellor’s Animal Research Committee at UCLA. Soft tissue was dissected from one-month-old Sprague-Dawley rat mandibles, and the third molar was extracted. Using a 26-gauge needle on the buccal cortex, we flushed bone marrow from the superior alveolar ridge with α-MEM (Mediatech, Herndon, VA, USA) and collected it through the extraction socket. Long-bone BMSCs were isolated from the tibiae of the same animals (Javazon et al., 2001; Yoshimura et al., 2007). Harvested cells were pooled from 10 rats, filtered through a 40-μm strainer, counted (Vi-CELL™ cell viability analyzer, Beckman Coulter, Fullerton, CA, USA), and plated at 1 × 106 cells/mL (2.6 × 105 cells/cm2). Confluent cells were supplemented with osteogenic differentiation media (α-MEM+10% FBS with 50 μg/μL ascorbic acid and 4 mM β-glycerophosphate), which was replaced every 3-4 days.
Osteoblast Phenotype Assays
To evaluate CFU-F, we counted colonies (at least 50 cells per group) on BMSC cultures stained with Giemsa (Ladd Research, Williston, VT, USA). Alkaline phosphatase (ALP) staining and activity and von Kossa staining were measured (Pirih et al., 2008; Liu et al., 2009). To evaluate 45Ca incorporation, we added 1 μCi/mL 45CaCl2 (ICN Biomedicals, Irvine, CA, USA) for 48 hrs. Then, cells were collected, and 45Ca was measured by scintillation counting. To assess gene expression, we collected total RNA at days 3 and 7 using Trizol (Invitrogen, Carlsbad, CA, USA). qPCR was performed in triplicate for at least 3 independent experiments, with iQ SYBR Green supermix (Bio-Rad, Hercules, CA, USA) and rat gene-specific primers, including: ALP (NM_013059) 5′-GGACGGTGAACGGGAGAAC-3′ (forward, 1424–1442), 5′-TGAAGCAGGTGAGCCATAGG-3′ (reverse, 1548–1567); osteocalcin (NM_013414) 5′-GGACCCT-CTCTCTGCTCACTC TG-3′ (forward, 54–80), 5′-ACCTTACTGC-CCTCCTGCTTGG-3′ (reverse, 156–177); DSPP (NM_ 012790) 5′-CGGTCCCTCAGT-TAGT-3′ (forward, 64–80), 5′-TACGTCCTCGCGTTCT-3′ (reverse, 336–355); and GAPDH (NM_017008) 5′-CGGCAAGTTCAA-CGGCACAGTCAAGG-3′ (forward, 229–254) and 5′-ACGACA-TACTCAGCACCAGCATCACC-3′ (reverse, 332–357). Gene expression normalized to GAPDH was expressed as fold induction.
Surgical BMSC Implantation
We collected 20 × 106 long-bone or mandible BMSCs, then centrifuged and incubated them with gelatin sponges (Gelfoam, Pfizer, New York, NY, USA) for 3 days. Sponges alone were utilized as controls. Sponges were subcutaneously implanted into nude mice at the intrascapular area (Pettway et al., 2005). After 6 wks, transplants were placed in 10% formalin for 48 hrs and stored in 70% ethanol. μCT imaging was performed at 12 μM isotropic voxel resolution, and tissue and bone volume analysis was performed. We used Dolphin Software (Dolphin Imaging, Chatsworth, CA, USA) to generate 3D and multiplanar reconstructed images. Then, transplants were decalcified (Fisher, Pittsburgh, PA, USA), paraffin-embedded, sectioned, and H&E-stained. Photomicrographs were taken with a Leica DMLB microscope (Leica Microsystems, Wetzlar, Germany) with BioQuant software (R&M Biometrics, Nashville, TN, USA). Two independent experiments, utilizing cells from different animals and performed at a different time, were performed, for a total of 8 transplants for each group.
Statistical Analysis
Data are expressed as mean ± standard error of the mean (SEM) from at least 3 independent experiments. One-way ANOVA and unpaired t tests were used to determine statistical significance between 3 and 2 groups, respectively. A p < 0.05 was considered as significant.
RESULTS
Isolation, Culture, and Characterization of Mandible BMSCs
To avoid contamination of bone marrow cells with incisor pulp, we inserted a 26-gauge needle into the buccal cortex, at the ret-romolar area and above the external oblique ridge, away from the central incisor root, and directed toward the alveolar ridge. A plain radiograph demonstrates the needle position, superior to the incisor root and inferior to the molars (Fig. 1A). Marrow was flushed from the mandible and collected through the extraction socket.
Figure 1.
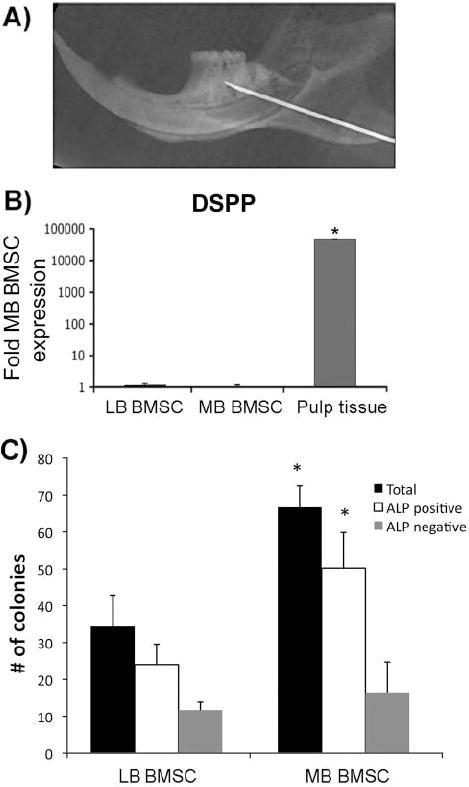
Isolation and culture of mandible BMSCs. (A) Radiograph of hemimandible showing the final position of the needle in the alveolar ridge superior to the incisor. The third molar has been extracted. (B) DSPP expression of long-bone (LB) BMSCs, mandible (MB) BMSCs, and pulp tissue by qPCR representative of more than 6 independent experiments. (C) Quantification of total, ALP-positive, and ALP-negative colonies formed by long-bone (LB) vs. mandible (MB) BMSC cultures (average of 3 independent experiments). *p < 0.05; error bars represent standard error of the mean.
To confirm the absence of contamination from dental pulp/ odontoblasts, we extracted total RNA from confluent BMSC cultures, and determined dentin sialophosphoprotein (DSPP) expression by qPCR using incisor pulp RNA as a positive control. DSPP, a precursor of dentin phosphoprotein (DPP) and dentin sialoprotein (DSP), is highly expressed by odontoblasts and has been used widely to demonstrate odontogenic differentiation (Iohara et al., 2006; Yang et al., 2007). Bone cells express DSPP, but at a much lower level than odontoblasts (Qin et al., 2002). Pulp tissue expressed high DSPP levels that were > 42,000-fold than those in mandible BMSC cultures. In contrast, long-bone BMSCs expressed DSPP levels (1.12-fold) very similar to those in mandible BMSCs (Fig. 1B). Analysis of these data suggests that mandible BMSC contamination with pulp cells, if any, was minimal. For all remaining experiments, DSPP levels were determined.
To begin characterizing CFU-F numbers in BMSCs from mandible vs. long bone, we grew cells for 1 wk in α-MEM and counted colonies. Mandible BMSC cultures displayed significantly more total colonies and more ALP-positive colonies than their long-bone counterparts (Fig. 1C), suggesting increased CFU-F numbers in mandible marrow.
Mandible BMSCs Have a Higher Osteoblastic Potential Compared with Long-bone BMSCs
To evaluate the osteogenic differentiation potential of mandible vs. long-bone BMSCs, we cultured confluent cells in osteogenic media for 0-21 days. ALP staining was stronger in mandible BMSCs from the beginning of the culture (3 days), continuing through the entire experiment (21 days; Fig. 2A). Von Kossa assay showed significant mineral deposition in mandible BMSCs compared with long-bone BMSCs (Fig. 2B). ALP activity paralleled ALP staining, demonstrating significantly higher levels for mandible vs. long-bone BMSCs (Fig. 2C). Mineralization quantification was also tested by [45]Ca assays that, similar to von Kossa, showed higher calcium deposition in mandible BMSC cultures (Fig. 2D). For further investigation of BMSC osteoblastic potential, ALP and osteocalcin (OCN) mRNA levels were examined. Mandible BMSCs demonstrated significantly higher ALP and OCN expression at 3 and 7 days (Figs. 2E, 2F). Analysis of these data, collectively, demonstrates that mandible BMSCs possess higher osteogenic potential compared with their long-bone counterparts.
Figure 2.
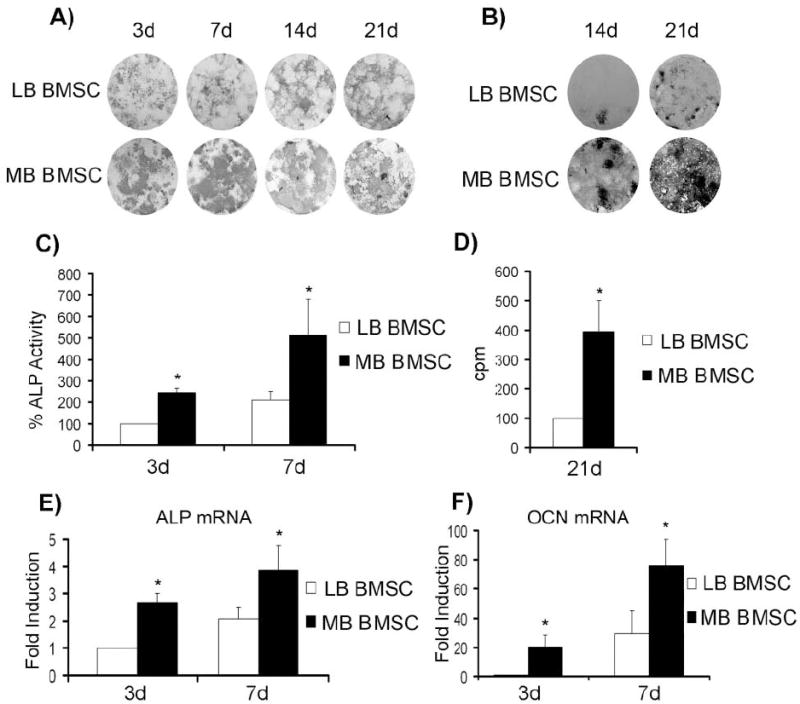
In vitro characterization of mandible vs. long-bone BMSCs. (A) Representative of 5 independent experiments of ALP staining at 3-, 7-, 14-, and 21-day cultures of long-bone (LB) vs. mandible (MB) BMSCs in osteogenic media. (B) Representative of 4 independent experiments of von Kossa staining at 14- and 21-day cultures of long-bone (LB) vs. mandible (MB) BMSCs in osteogenic media. (C) ALP activity assay at 3- and 7-day cultures of long-bone (LB) vs. mandible (MB) BMSCs in osteogenic media (average of 3 independent experiments). (D) [45]Ca assay at 21 days’ culture of long-bone (LB) vs. mandible (MB) BMSCs in osteogenic media (average of 3 independent experiments). (E) ALP and (F) OCN mRNA expression determined by qPCR of long-bone (LB) vs. mandible (MB) BMSCs cultured in osteogenic media for 3 and 7 days (average of 4 independent experiments). *p < 0.05; error bars represent standard error of the mean.
In vivo-increased Osteogenic Potential of Mandible vs. Long-bone BMSCs
For determination of whether in vitro differences are recapitulated in vivo, BMSCs seeded on gelatin sponges for 3 days or gelatin sponges alone were implanted subcutaneously into nude mice for 6 wks (Pettway et al., 2005). At the end of the experiment, sponges alone did not produce a radiographic image (data not shown), suggesting absence of mineralization. Representative 3D-reconstructed microCT images of long-bone (Fig. 3A) and mandible (Fig. 3B) BMSC-seeded sponges are shown. Mandible BMSC sponges were consistently larger and more calcified than long-bone BMSC sponges. Tissue volume (TV) and bone volume (BV) quantification further demonstrate the significantly greater ability of mandible BMSCs to form larger and more calcified nodules than long-bone BMSCs in vivo (Figs. 3C, 3D).
Figure 3.
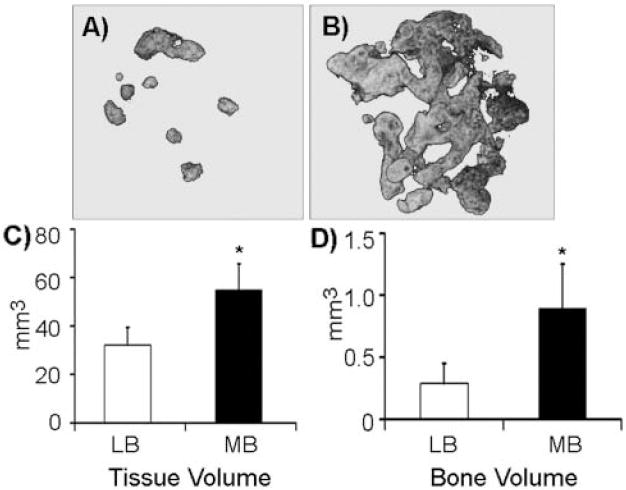
3D-reconstructed (A,B) microCT images of representative gelatin sponges seeded with (A) long-bone- or (B) mandible-derived marrow cells. (C) Tissue volume (TV) and (D) bone volume (BV) of long-bone (LB) vs. mandible (MB) marrow cell-seeded sponges, quantified by μCT (average of 8 individual transplants from 2 independent experiments). *p < 0.05; error bars represent standard error of the mean.
Then, transplants were decalcified, and H&E sections were performed (Fig. 4). Sponges without BMSCs demonstrated a thin fibrous capsule only, without any indication of bone formation (data not shown). All sponges seeded with long-bone or mandible marrow cells supported bone formation. Mandible BMSC sponges showed increased and more mature lamellar bone (Fig. 4D) compared with long-bone BMSC sponges (Fig. 4A). Marked osteoblastic rimming of bony trabeculae and bone marrow was seen only in mandible BMSC sponges (Fig. 4E). On high magnification, orderly lines of lamellar bone formation with osteocytes were also observed (Fig. 4F). In contrast, long-bone BMSC sponges showed a primarily cartilaginous matrix (purple in Figs. 4B, 4C), with only peripheral bone formation. Analysis of these in vivo data further complements the in vitro data demonstrating that mandible BMSCs have higher osteoblastic potential compared with long-bone BMSCs.
Figure 4.
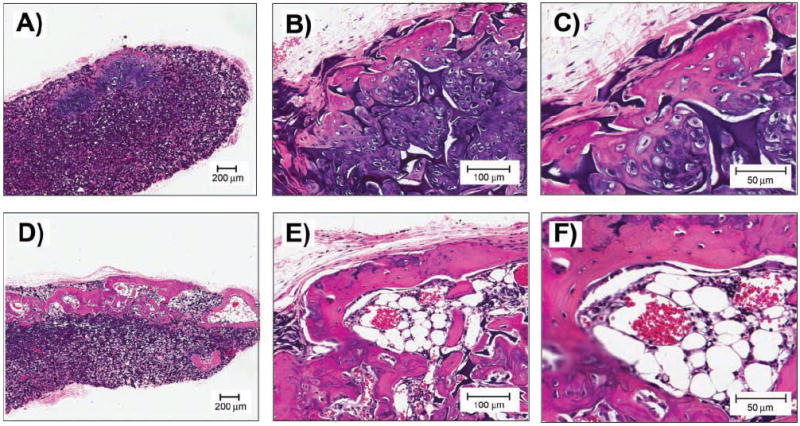
H&E sections at 2X (A,D), 10X (B,E), and 20X (C,F) of sponges seeded with long-bone marrow cells (A,B,C) or sponges seeded with mandible marrow cells (D,E,F) and implanted at the intrascapular area of nude mice.
DISCUSSION
Systemic diseases, such as osteoporosis, hyperparathyroidism, and Paget’s disease, affect all bones, including the jaws (White and Pharoah, 2004). Oral bone metabolism is affected in ovariectomized rodent models of osteoporosis and osteopenia, showing parallel responses in long bones and mandible (Hsieh et al., 1995; Miller et al., 1997). But in response to external stimuli, including ovariectomy and malnutrition, the mandible loses significantly less bone than the proximal tibia (Mavropoulos et al., 2007). In addition, cherubism (Ueki et al., 2001), hyperparathyroid jaw tumor syndrome (Simonds et al., 2002), and, more recently, bisphosphonate-related osteonecrosis of the jaws (BRONJ) (Ruggiero et al., 2004) affect only the maxilla and mandible, suggesting different homeostatic mechanisms between the jaws and long bones. This is further demonstrated by differences in mandibular mechanical loading during mastication (Mavropoulos et al., 2004). In fact, forces generated during walking are almost half those to which alveolar bone is exposed during mastication (Knoell, 1977; Daegling and Hylander, 1997).
Although craniofacial and long bones are similar histologically (Leucht et al., 2008), the craniofacial skeleton arises from neural crest cells that migrate ventrolaterally to the branchial arches (Chai et al., 2000). During neural crest cell migration, growth factors and their signaling pathways determine neural crest differentiation into functional cells forming craniofacial structures primarily via intramembranous ossification (Chai et al., 2000). In contrast, the axial and appendicular skeleton arises from mesenchymal condensations of mesoderm that undergo chondrocytic differentiation (Mackie et al., 2008). Taken together, in vivo evidence of varied responses to external stimuli, coupled with development of the mandible vs. long bones from different germ layers, encourages study to evaluate differences between mandible and long bones.
A better understanding of the diseases and processes affecting the jaws would be significantly improved by an animal system to study cellular and molecular processes in vitro. Here, we established a protocol for isolation and culture of rat mandible BMSCs, to test our hypothesis that mandible vs. long-bone BMSCs possess distinct osteogenic differentiation potential. Isolated mandible BMSCs differentiate, under appropriate conditions, toward an osteoblastic phenotype, expressing osteoblastic markers and forming mineralized nodules. Interestingly, ALP staining and activity were stronger in mandible BMSCs compared with long-bone BMSCs. Von Kossa and [45]Ca also showed increased mineral deposition in mandible vs. long-bone BMSCs. Furthermore, mandible BMSCs demonstrated significantly increased ALP and OCN mRNA expression. Analysis of these data, collectively, demonstrates that mandible BMSCs possess higher osteogenic potential compared with their long-bone counterparts. Our findings are consistent with those of studies utilizing trabecular bone from patient extraction sites, and showing that orofacial BMSCs demonstrate increased osteogenic differentiation compared with iliac-crest-derived BMSCs from the same individuals (Akintoye et al., 2006). Furthermore, a recent study with labeled neural-crest- and mesoderm-derived cells demonstrated bone defect healing through selective recruitment of cells from their specific embryonic origin (Leucht et al., 2008), reinforcing site-specific differences in BMSCs from the craniofacial vs. appendicular skeleton.
To investigate whether in vitro-observed differences between mandible vs. long-bone BMSCs osteogenic differentiation is reproducible in vivo, we used an ectopic bone formation model (Krebsbach et al., 1997; Kuznetsov et al., 1997; Pettway et al., 2005; Akintoye et al., 2006). Differences between mandible and long-bone BMSCs in vitro were recapitulated in vivo. BMSCs implanted into nude mice underwent osteogenic differentiation and developed mineralized nodules. MicroCT qualitatively and quantitatively showed larger and more calcified structures from mandible vs. long-bone BMSCs. These data were confirmed by histology, which revealed increased and more mature lamellar bone derived from mandible BMSC sponges.
Analysis of our data strongly supports increased osteogenic potential of mandible vs. long-bone BMSCs both in vitro and in vivo. The diverse osteogenic potential between mandible- and long-bone-derived cells could be due to inherent differences of BMSCs between these two sites. However, marrow cells consist of a variety of cell lineages. Thus, some of the observed differences between these two cell populations could be due to the differential composition of marrow from mandible vs. long bone. Utilizing this in vitro mandible BMSC model system, we detected baseline differences and can now explore detailed characterization of mandible marrow composition, as well as evaluate responses to various external stimuli including hormones, growth factors, and signaling cascades as well as potential differences in bone remodeling and healing.
Acknowledgments
This work was funded by R01 DE019465, the Oral and Maxillofacial Surgery Foundation, and the Stein-Oppenheimer endowment award. TC was supported by T32 DE007296.
References
- Abzhanov A, Rodda SJ, McMahon AP, Tabin CJ. Regulation of skeletogenic differentiation in cranial dermal bone. Development. 2007;134:3133–3144. doi: 10.1242/dev.002709. [DOI] [PubMed] [Google Scholar]
- Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–768. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Chai Y, Maxson RE., Jr Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235:2353–2375. doi: 10.1002/dvdy.20833. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Daegling DJ, Hylander WL. Occlusal forces and mandibular bone strain: is the primate jaw “overdesigned”? J Hum Evol. 1997;33:705–717. doi: 10.1006/jhev.1997.0164. [DOI] [PubMed] [Google Scholar]
- De Coster PJ, Mortier G, Marks LA, Martens LC. Cranial suture biology and dental development: genetic and clinical perspectives. J Oral Pathol Med. 2007;36:447–455. doi: 10.1111/j.1600-0714.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Hsieh YD, Devlin H, McCord F. The effect of ovariectomy on the healing tooth socket of the rat. Arch Oral Biol. 1995;40:529–531. doi: 10.1016/0003-9969(94)00197-j. [DOI] [PubMed] [Google Scholar]
- Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells. 2006;24:2493–2503. doi: 10.1634/stemcells.2006-0161. [DOI] [PubMed] [Google Scholar]
- Javazon EH, Colter DC, Schwarz EJ, Prockop DJ. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19:219–225. doi: 10.1634/stemcells.19-3-219. [DOI] [PubMed] [Google Scholar]
- Jeffcoat MK, Lewis CE, Reddy MS, Wang CY, Redford M. Post-menopausal bone loss and its relationship to oral bone loss. Periodontol 2000. 2000;23:94–102. doi: 10.1034/j.1600-0757.2000.2230109.x. [DOI] [PubMed] [Google Scholar]
- Karaplis A. Embryonic development of bone and the molecular regulation of intramembranous and endochondral bone formation. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of bone biology. Chapter 3. San Diego: Academic Press; 2002. pp. 33–58. [Google Scholar]
- Kimmel CB, Walker MB, Miller CT. Morphing the hyomandibular skeleton in development and evolution. J Exp Zoolog B Mol Dev Evol. 2007;308:609–624. doi: 10.1002/jez.b.21155. [DOI] [PubMed] [Google Scholar]
- Knoell AC. A mathematical model of an in vitro human mandible. J Biomech. 1977;10:159–166. doi: 10.1016/0021-9290(77)90054-9. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RV, Rowe DW, Robey PG. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- Kribbs PJ, Chesnut CH, 3rd, Ott SM, Kilcoyne RF. Relationships between mandibular and skeletal bone in an osteoporotic population. J Prosthet Dent. 1989;62:703–707. doi: 10.1016/0022-3913(89)90596-9. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- Lerner UH. Inflammation-induced bone remodeling in periodontal disease and the influence of post-menopausal osteoporosis. J Dent Res. 2006;85:596–607. doi: 10.1177/154405910608500704. [DOI] [PubMed] [Google Scholar]
- Leucht P, Kim JB, Amasha R, James AW, Girod S, Helms JA. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development. 2008;135:2845–2854. doi: 10.1242/dev.023788. [DOI] [PubMed] [Google Scholar]
- Liu Q, Cen L, Zhou H, Yin S, Liu G, Liu W, et al. The role of ERK signaling pathway in osteogenic differentiation of human adipose-derived stem cells and in adipogenic transition initiated by dexamethasone. Tissue Eng Part A. 2009;15:3487–3497. doi: 10.1089/ten.TEA.2009.0175. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 2008;40:46–62. doi: 10.1016/j.biocel.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Mavropoulos A, Kiliaridis S, Bresin A, Ammann P. Effect of different masticatory functional and mechanical demands on the structural adaptation of the mandibular alveolar bone in young growing rats. Bone. 2004;35:191–197. doi: 10.1016/j.bone.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Mavropoulos A, Rizzoli R, Ammann P. Different responsiveness of alveolar and tibial bone to bone loss stimuli. J Bone Miner Res. 2007;22:403–410. doi: 10.1359/jbmr.061208. [DOI] [PubMed] [Google Scholar]
- Miller SC, Hunziker J, Mecham M, Wronski TJ. Intermittent parathyroid hormone administration stimulates bone formation in the mandibles of aged ovariectomized rats. J Dent Res. 1997;76:1471–1476. doi: 10.1177/00220345970760080901. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Pettway GJ, Schneider A, Koh AJ, Widjaja E, Morris MD, Meganck JA, et al. Anabolic actions of PTH (1–34): use of a novel tissue engineering model to investigate temporal effects on bone. Bone. 2005;36:959–970. doi: 10.1016/j.bone.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Pirih FQ, Abayahoudian R, Elashoff D, Parhami F, Nervina JM, Tetradis S. Nuclear receptor profile in calvarial bone cells undergoing osteogenic versus adipogenic differentiation. J Cell Biochem. 2008;105:1316–1326. doi: 10.1002/jcb.21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, et al. The expression of dentin sialophosphoprotein gene in bone. J Dent Res. 2002;81:392–394. doi: 10.1177/154405910208100607. [DOI] [PubMed] [Google Scholar]
- Ramaesh T, Bard JB. The growth and morphogenesis of the early mouse mandible: a quantitative analysis. J Anat. 2003;203:213–222. doi: 10.1046/j.1469-7580.2003.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Simonds WF, James-Newton LA, Agarwal SK, Yang B, Skarulis MC, Hendy GN, et al. Familial isolated hyperparathyroidism: clinical and genetic characteristics of 36 kindreds. Medicine (Baltimore) 2002;81:1–26. doi: 10.1097/00005792-200201000-00001. [DOI] [PubMed] [Google Scholar]
- Sodek J, McKee MD. Molecular and cellular biology of alveolar bone. Periodontol 2000. 2000;24:99–126. doi: 10.1034/j.1600-0757.2000.2240106.x. [DOI] [PubMed] [Google Scholar]
- Tsutsui TW, Riminucci M, Holmbeck K, Bianco P, Robey PG. Development of craniofacial structures in transgenic mice with constitutively active PTH/PTHrP receptor. Bone. 2008;42:321–331. doi: 10.1016/j.bone.2007.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Tiziani V, Santanna C, Fukai N, Maulik C, Garfinkle J, et al. Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause cherubism. Nat Genet. 2001;28:125–126. doi: 10.1038/88832. [DOI] [PubMed] [Google Scholar]
- White SC, Pharoah MI. Oral radiology: principles and interpretation. 5. St Louis: Mosby; 2004. [Google Scholar]
- Yang X, van den Dolder J, Walboomers XF, Zhang W, Bian Z, Fan M, et al. The odontogenic potential of STRO-1 sorted rat dental pulp stem cells in vitro. J Tissue Eng Regen Med. 2007;1:66–73. doi: 10.1002/term.16. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]


