Summary
Plants respond to a reduction in the red:far-red ratio (R:FR) of light caused by the proximity of other plants by initiating morphological changes that improve light capture. In Arabidopsis, this response (the shade avoidance syndrome, SAS) is controlled by the phytochromes (particularly phyB) and dependent on the TAA1 pathway of auxin biosynthesis. However, when grown in real canopies, we found that phyB mutants and mutants deficient in TAAI (sav3) still display robust SAS responses to increased planting density and leaf shading. The SAS morphology (leaf hyponasty and reduced lamina:petiole ratio) could be phenocopied by exposing plants to blue (B) light attenuation. These responses to B light attenuation required the UV-A/blue light photoreceptor cry1. Moreover, they were mediated through mechanisms that showed only limited overlap with the pathways recruited by phyB inactivation. In particular, pathways for polar auxin transport, auxin biosynthesis and gibberellin signaling that are involved in SAS responses to low R:FR were not required for the SAS responses to B light depletion. By contrast, brassinosteroid response appeared to be required for full expression of the SAS phenotype under low B light. The phyB and cry1 inactivation pathways appeared to converge in their requirement for the bHLH transcription factors PHYTOCHROME INTERACTING FACTOR 4 and 5 (PIF4 and 5) to elicit the SAS phenotype. Our results suggest that B light is an important control of SAS responses, and that PIF4 and PIF5 are critical hubs for a diverse array of signaling routes that control plant architecture in canopies.
Keywords: Blue light, Brassinosteroid, Phytochrome Interacting Factors (PIFs), PIN3, TAA1, DELLA
Introduction
Competition is a critical determinant of plant fitness in dense populations. One strategy used by plants to improve their competitive success is based on morphological plasticity, where the shape of the plant body is constantly remodeled to optimize the capture of light and other resources (Aphalo and Ballaré 1995; Ballaré 1999; Dorn et al. 2000; De Kroon et al. 2009; Novoplansky 2009; Sultan 2010). A prime example of morphological plasticity is the shade avoidance syndrome (or SAS) (Smith 1982). SAS responses typically include increased elongation of the stem and petioles, leaf hyponasty, reduced branching, and phototropic orientation of the plant shoot toward canopy gaps (Ballaré 1999).
SAS responses are triggered and controlled by multiple canopy signals, particularly signals in the light environment (reviewed in Ballaré 1999; Vandenbussche et al. 2005; Ballaré 2009; Keuskamp et al. 2010b). The best characterized of these signals is the R:FR ratio (660-670 nm:725-735 nm), which decreases in response to canopy density owing to the strong absorption of R light by chlorophyll and scattering of FR photons by cell walls and other plant constituents. Other potentially important light signals controlling SAS responses include variations in total irradiance, and specific changes in the blue (B) light component caused by absorption of visible wavelengths by chlorophyll and other leaf pigments (reviewed in Ballaré 2009; Keuskamp et al. 2010b)
Phytochrome B (phyB) is the major photoreceptor that senses a reduction in R:FR ratio, and controls the initial appearance of SAS phenotypes (reviewed in Franklin 2008; Ballaré 2009; Jaillais and Chory 2010; Martínez-García et al. 2010). In fully de-etiolated Arabidopsis plants in the rosette stage, SAS responses to low R:FR (increased petiole elongation and leaf hyponasty) depend on increased auxin biosynthesis through the TAA1 (TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1) pathway (Tao et al. 2008; Moreno et al. 2009), and polar auxin transport by the auxin efflux carrier PIN3 (Keuskamp et al. 2010a). Increased gibberellin (GA) production, perhaps in response to increased auxin (Frigerio et al. 2006), is also required for expression of petiole elongation responses to low R:FR in Arabidopsis plants at the rosette stage (Djakovic-Petrovic et al. 2007). Increased GA production triggers the degradation by the 26S proteasome of DELLA proteins, a group of five nuclear proteins that redundantly repress growth (Djakovic-Petrovic et al. 2007). DELLA proteins bind to and inactivate PHYTOCHROME INTERACTING FACTORS (PIFs) (de Lucas et al. 2008; Feng et al. 2008), which are growth-promoting transcription factors involved in the elicitation of SAS responses to low R:FR (Lorrain et al. 2008; Hornitschek et al. 2009). Brassinosteroids (BR) (Kozuka et al. 2010) and ethylene (Pierik et al. 2009) are additional hormones that have been implicated as playing a role during the elicitation of SAS responses to low R:FR ratios.
Reduction in B light fluence rate may also provide plants with information on the proximity of competitors and trigger adaptive SAS responses. B light inhibits hypocotyl growth in recently-germinated seedlings, and this effect is mediated by the cryptochrome photoreceptors (Cashmore et al. 1999; Folta and Spalding 2001; Pierik et al. 2009; Sellaro et al. 2010). Blue light gradients can drive phototropic responses of seedlings in patchy canopies (Ballaré et al. 1992), an effect likely mediated by the phototropins (Briggs and Christie 2002; Takemiya et al. 2005). Even fully de-etiolated plants can present strong SAS-like responses to B light attenuation, including increased main-stem elongation (Ballaré et al. 1991), petiole elongation (Kozuka et al. 2005), and leaf hyponasty (Pierik et al. 2004; Millenaar et al. 2009).
The importance of B light signals in the control of morphological plasticity in plant canopies has not been clearly established. Furthermore, it is not known whether the hormone signaling circuits activated by phyB Pfr depletion (in response to low R:FR) are also recruited to elicit SAS responses to B light attenuation. These questions are addressed in this manuscript, using a combination of canopy and physiological experiments with Arabidopsis plants at the rosette stage. We evaluated variations in leaf morphology (lamina-to-petiole length ratio, L:P) and leaf angle (hyponasty) as the principal readouts of SAS. We found that mutants deficient in R:FR responses show robust morphological responses to increased canopy density or leaf shading, suggesting that additional light-regulated pathways are involved in SAS. The high-density/shade morphology could be phenocopied by exposing plants to light in which the B light component was attenuated. These B light responses required cry1, and were mediated through hormonal pathways that showed only limited overlap with the pathways activated in response to phyB Pfr depletion by low R:FR ratios. Interestingly, our results demonstrate that PIF4 andPIF5, which are bHLH transcription factors known to mediate morphological responses to phyB inactivation, are also required for the elicitation of the SAS phenotype in response to B light attenuation.
Results
Mutants deficient in R:FR responses display SAS-like responses in canopies
PhyB and increased auxin biosynthesis through the TAA1 pathway are essential for plants to respond to variations in the R:FR ratio caused by the proximity of other plants (Tao et al. 2008; Moreno et al. 2009). We tested the effects of the phyB-9 and sav3-2 mutations, which affect the genes encoding for the PHYB apoprotein and the TAA1 enzyme, respectively, on the responses of Arabidopsis plants to real plant neighbors in a series of greenhouse experiments under the full solar spectrum. When wild-type (Col-0) Arabidopsis plants were grown in monocultures of different densities they responded to crowding with characteristic SAS responses (Figure 1). These responses included increased leaf angles (hyponasty) and reduced growth of the leaf lamina relative to the petiole (reduced L:P ratio). Compared to Col-0 plants, phyB mutants had constitutively hyponastic leaves and low L:P ratios. sav3-2 plants showed significantly attenuated morphological responses to crowding, but only during the early part of the experiment, when canopy cover was small (Figure 1a, b; 25 d). As the plants grew bigger and the canopies closed (greater canopy cover), both sav3-2 and phyB plants displayed clear morphological responses to increased levels of crowding (Figure 1c, d; 45 d).
Figure 1.
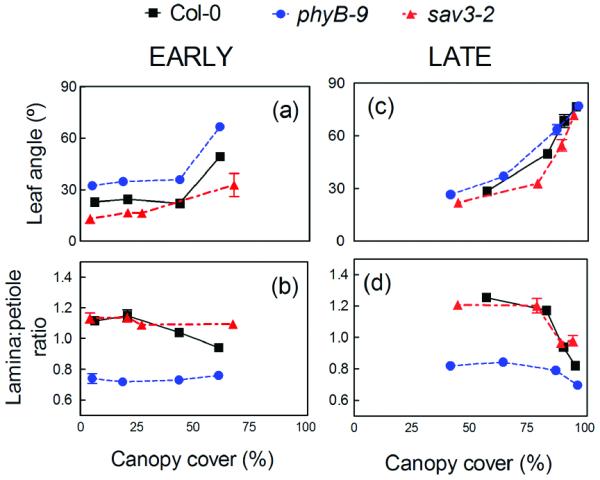
The sav3 and phyB mutations reduce SAS responses to crowding at low, but not at high canopy cover. Variations leaf angle and lamina-to-petiole length ratio (L:P) of Arabidopsis plants were measured in response to crowding (increased canopy density) in a density gradient experiment. The experiment was carried out in a glasshouse, under natural radiation during the autumn in Buenos Aires (see Experimental procedures for details). There were 4 trays for each genotype and canopy density combination; the 9 central plants were measured in each tray. Bars indicate ±1 S.E (n=4). (a-b) Measurements of leaf angle and leaf morphology 25 d after germination. (c-d) Measurements of leaf angle and leaf morphology 45 d after germination.
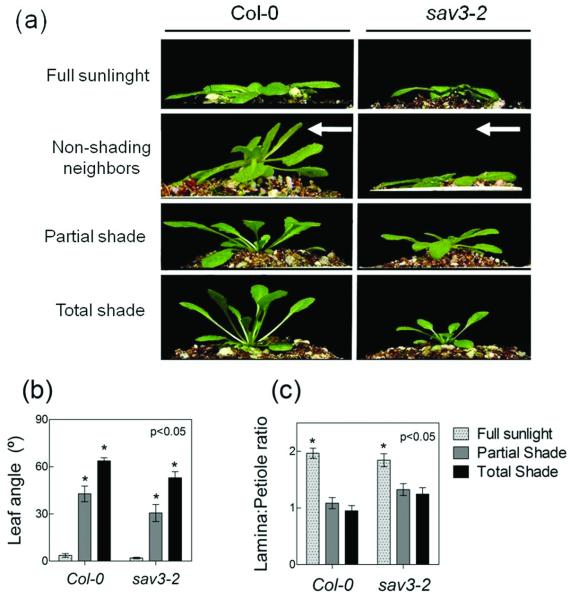
Neighbor proximity was further manipulated by growing Arabidopsis plants in front of or underneath canopies of annual ryegrass (Lolium multiflorum). Rosettes of the Col-0 plants responded to FR radiation reflected from the grass canopy with a typical SAS phenotype, even though the canopy did not shade the target plants; this response to reflected FR was missing in sav3-2 (Figure 2a, 2nd row; non-shading neighbors). In contrast, when plants were placed under the ryegrass canopy and actually shaded by the grass leaves, Col-0 and sav3-2 mutant displayed very similar SAS responses (Figure 2a, 3rd and 4th rows; see quantitative data in Figure 2b, c).
Figure 2.
The sav3 mutation eliminates SAS responses to the proximity of non-shading neighbors, but does not prevent SAS responses to canopy shading. Plants grown in a glasshouse under natural radiation under the following conditions: with no neighbors (full sunlight); on the north side of a thick ryegrass canopy (non-shading neighbors); or under the ryegrass canopy and two levels of leaf shading (partial shade and total shade). The experiment was carried out during the autumn in Buenos Aires (see Experimental procedures for details). (a) Representative photographs of the rosettes taken after 7 days of treatment. The arrow in the second row indicates the predominant direction of the radiation reflected from the grass leaves, and phototropic bending away from this radiation in Col-0, but not in sav3-2. (b and c) Quantitative data for the SAS response in the two shading treatments. Thin bars indicate ±1 S.E. (n=6-10). Asterisks indicate significant effects of the shading treatments (p<0.05).
SAS responses to B light attenuation are retained in sav3 and pin3 mutants
It is well established that reflected FR is the principal signal of canopy density in open canopies (low Leaf Area Index, LAI), but at high LAI other signals are thought to play an important role, including the amount of B light received per plant, which decreases as the density of the canopy increases (references in Ballaré 1999). Therefore, we reasoned that the responses of sav3-2 to crowding in the high %-cover range (Figure 1), and to shading by the ryegrass canopy (Figure 2), could reflect a conserved response to B light attenuation.
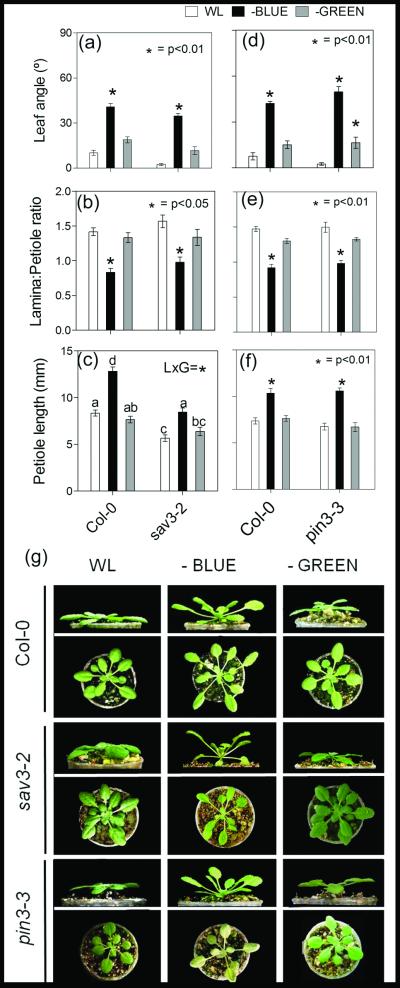
We directly tested the effect of B light attenuation on plants at the rosette stage, similar to the ones used in the canopy experiments described above, by using selective light filters. Plants responded to B light attenuation with strong hyponasty and reduced L:P ratio (Figure 3). The SAS response to B light attenuation was likely mediated by a specific B light photoreceptor, as we found little or no response to green light attenuation, where a control filter was used to test for the effects of reducing photosynthetically active radiation (PAR). Importantly, the SAS responses to B light depletion were clearly conserved in the sav3-2 mutant (Figure 3a-c, g).
Figure 3.
Arabidopsis plants at the rosette stage show robust SAS responses to B light attenuation, which are conserved in sav3 and pin3 mutants. Plants were grown under white light (WL) or WL filtered through a yellow filter (-BLUE) or a pink filter (-GREEN), which was used as a control for the PAR reduction caused by B light attenuation. Morphological measurements and photographs were taken after 7 d of treatment. Thin bars indicate ±1 S.E. (n=10-16 individual plants). Asterisks indicate significant effects of the light treatments at the indicated p value. Different letters indicate significant differences between means in cases in which the light x genotype interaction term (LxG) was significant. (a-c) Comparison of morphological responses between Col-0 and sav3 plants. (d-f) Comparison of morphological responses between Col-0 and pin3 plants. (g) Representative photographs of the plants after exposure to the indicated light treatments.
Recent work showed that, in addition to TAA1, the auxin efflux carrier PIN3 is required for petiole elongation triggered by low R:FR ratios in rosette-stage plants (Keuskamp et al. 2010a). This suggests that auxin produced in the leaves by TAA1 in response to low R:FR is actively transported to its site of action by PIN3. However, in the case of SAS responses elicited by B light attenuation, we found a completely wild-type-response in the pin-formed3 (pin3-3) mutant (Figure 3d-f, h).
Next, we addressed whether other auxin biosynthesis or transport pathways are involved in B light attenuation responses in rosette plants. First, we tested the requirement for auxin biosynthesis catalyzed by YUCCA proteins. YUCCA proteins are flavin monooxygenases and form a family of 11 members in Arabidopsis (Zhao et al. 2001). Quintuple yucca mutant, (lacking yuc3, 5, 7, 8 and 9- Tao et al. 2008) displayed normal responses to B light attenuation (Supplementary Figure S1). We also tested the effect of NPA, an inhibitor of polar auxin transport (Okada et al. 1991), on SAS responses to attenuated B light. The NPA experiments produced results that were difficult to interpret, because, as found in previous studies (reviewed in van Zanten et al. 2010), low doses of NPA per-se induced leaf hyponasty in the control plants grown under white light (Supplementary Figure S2). For the other SAS responses characterized in our experiments (reduction of L:P ratio), 5 μM NPA tended to cancel the effect of B light depletion, mainly by reducing petiole elongation (Supplementary Figure S2). These results suggest that polar auxin transport may play a role in the activation of the SAS response to B light attenuation, but the auxin biosynthesis and transport genes that are critical for activating the SAS response to low R:FR (SAV3 and PIN3) are not essential for B light responses.
B light attenuation does not induce DELLA turnover in rosette plants
Petiole elongation responses induced by low R:FR in mature plants correlate with DELLA turnover induced by gibberellins, and are eliminated by enhanced DELLA stability in the gain-of-function gai-1 mutant (Djakovic-Petrovic et al. 2007). Therefore, we wanted to determine whether the B light attenuation effects inducing SAS responses are associated with increased DELLA degradation. We tested the effect of B light attenuation on DELLA stability using transgenic lines expressing RGA fused to the fluorescent protein mCITRINE at its N-terminus. To circumvent the potential transcriptional regulation of RGA by B light attenuation, we used the mild constitutive promoter of the POLYUBIQUITIN10 gene (pUBQ10). Our pUBQ10::mCITRINE-RGA line did not harbor any developmental phenotypes (data not shown) and GA treatment triggered rapid mCITRINE-RGA turnover (Supplementary Figure S3). We found that B light attenuation treatments that are highly effective at inducing the SAS phenotype (Figure 3) failed to increase DELLA degradation in both Col-0 and sav3 mutant (Figure 4). These results indicate that B light attenuation and phyB inactivation by low R:FR have very different effects on DELLA turnover in Arabidopsis plants at the rosette stage.
Figure 4.
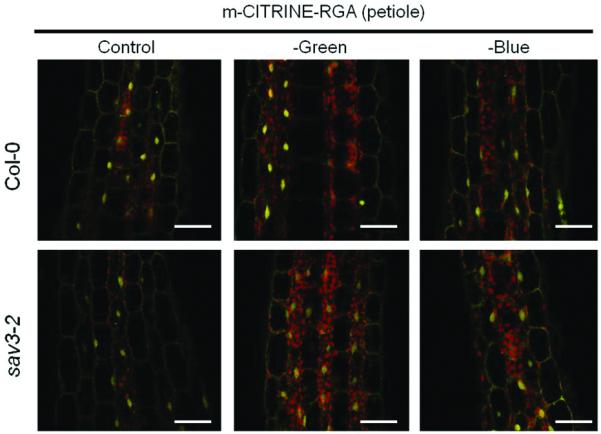
B light attenuation fails to trigger DELLA degradation in Arabidopsis petioles. Plants were grown for 2 weeks under white light ant then exposed to the indicated light treatments for 7 d. Control = clear polyester; -Green = pink filter; -Blue = yellow filter (-Blue). Scale bar = 25 μm.
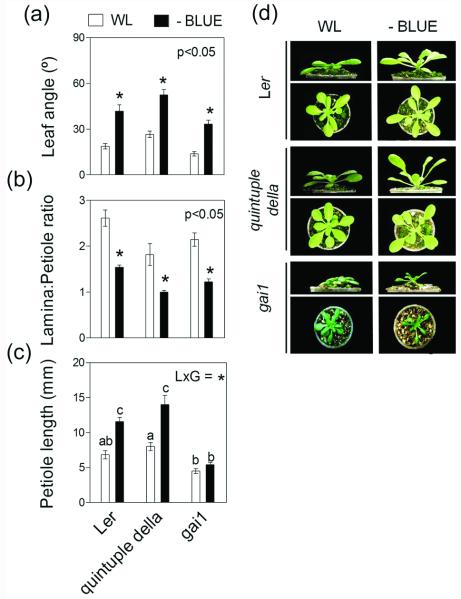
We directly tested the role of DELLA turnover in SAS responses induced by B light attenuation by using a quintuple (global) della mutant (Feng et al. 2008) and the gai-1 gain-of-function mutant (Koorneef et al. 1985), which is impaired in DELLA degradation (all in the Landsberg erecta background, Ler). Ler plants are known to be less responsive than those of the Col-0 accession in terms of their hyponastic response, when the response is induced by ethylene (Benschop et al. 2007). However, in our filter experiments, Ler plants showed a clear hyponastic response to B light attenuation (Figure 5), which is consistent with the idea that ethylene and light attenuation trigger hyponastic responses through independent pathways (Millenaar et al. 2009). The global della mutant clearly retained the capacity to respond to B light attenuation with a typical SAS repertoire (leaf hyponasty and reduced L:P ratio) (Figure 5). Plants of gai-1 mutant were notoriously dwarfed compared with the Ler WT, as expected (Koorneef et al. 1985). This phenotype is consistent with the persistence of the growth inhibitory effects of the stable version of the GAI protein (Harberd et al. 2009). However, the effects of B light depletion promoting leaf hyponasty and reducing the L:P ratio were retained, although the expression of the response was not as intense as in the Ler wild type. Absolute petiole elongation was not promoted by low B light in gai-1 (Figure 5c). Taken together, these results indicate that DELLA turnover is neither induced by B light attenuation (Figure 4) nor directly involved in the elicitation by B light attenuation of specific growth remodeling components of the SAS phenotype (hyponasty and reduced L:P ratio) (Figure 5). Nevertheless, abnormal DELLA stability may reduce the L:P response, probably as a consequence of persistent inhibition of petiole elongation (Figure 5c).
Figure 5.
SAS responses of Arabidopsis rosettes to B light attenuation are conserved in global della mutants and in the gai-1 gain-of-function mutant, which is impaired in DELLA turnover. Plants were grown under white light (WL) or WL filtered through a yellow filter (-BLUE). Morphological measurements and photographs were taken after 7 d of treatment. Thin bars indicate ±1 S.E. (n=8-12 individual plants). Asterisks indicate significant effects of the light treatment at the indicated p value. Different letters indicate significant differences between means in cases in which the light x genotype interaction term (LxG) was significant. (a-c) Comparison of morphological responses between Ler and the mutants impaired in DELLA function. (d) Representative photographs of the plants after exposure to the indicated light treatments.
BR signaling is required for elongation responses to B light depletion
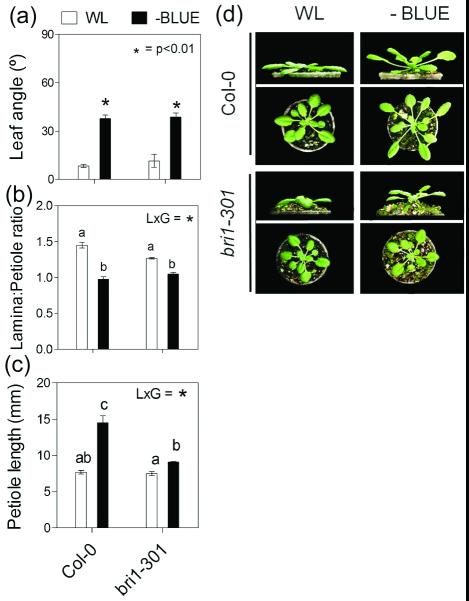
Studies with Arabidopsis plants at the rosette stage (Kozuka et al. 2010) showed that BR (along with auxin) are involved in controlling petiole elongation and leaf morphology responses to end-of-day low R:FR treatments. Furthermore, studies with recently-germinated Arabidopsis seedlings established that the auxin (from the TAA1 pathway) and BR signaling are both required for the effects of B light attenuation in the promotion of hypocotyl elongation (Keuskamp et al. 2011). We tested the response to B light attenuation at the rosette stage in mutants impaired in BR synthesis and perception. The det2-1 plants (impaired in BR biosynthesis) have a strong phenotype and very compact rosettes. Their growth is severely impaired, and no morphological responses to B light attenuation were evident in this mutant (Supplementary Figure S4). Using a mutant that carries a weak allele of BRASSINOSTEROID INSENSITIVE1, bri1-301, we found that the hyponastic response to B light attenuation was completely retained. However, the effect of B light attenuation reducing L:P ratio was impaired in bri1-301, mainly because petiole elongation was inhibited (Figure 6).
Figure 6.
SAS responses of Arabidopsis rosettes to B light attenuation are partially dependent on BR sensitivity. Plants were grown under white light (WL) or WL filtered through a yellow filter (-BLUE). Morphological measurements and photographs were taken after 7 d of treatment. Thin bars indicate ±1 S.E. (n=8-12 individual plants). Asterisks indicate significant effects of the light treatment at the indicated p value. Different letters indicate significant differences between means in cases in which the light x genotype interaction term (LxG) was significant. (a-c) Comparison of morphological responses between Col-0 and the bri1-301 mutant. (d) Representative photographs of the plants after exposure to the indicated light treatments.
SAS responses to B light attenuation in rosette plants are likely mediated by CRY1
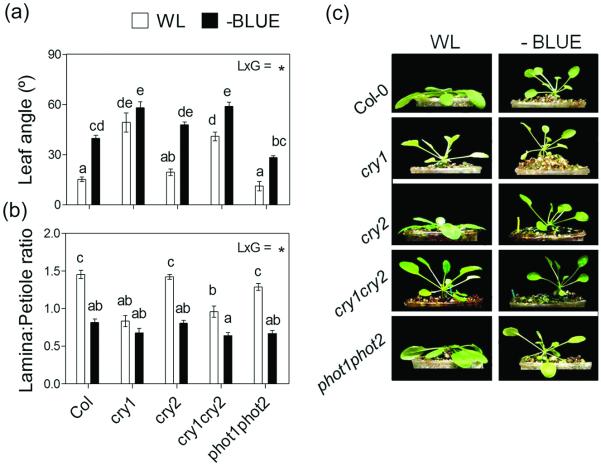
Cryptochromes are known to be involved in the leaf hyponastic response of Arabidopsis rosettes to neutral shade (Millenaar et al. 2009), and both cry1 and cry2 were found to be required for the inhibitory effect of monochromatic B light on petiole elongation (Kozuka et al. 2005). We tested B light receptor mutants for their SAS responses to B light attenuation. In the cry1 single mutants and cry1cry2 double mutants, the leaves were constitutively hyponastic and had reduced L:P ratios, compared to the Col wild type (Figure 7). cry2 had a normal phenotype, whereas the phot1phot2 phototropin double mutant showed a slightly attenuated response in terms of hyponasty and normal L:P response (Figure 7). These results suggest that the SAS-like response to B light attenuation in rosette plants of the Col-0 ecotype is principally mediated by cry1 inactivation. Differences among accessions may be present, however, as previous studies in the Ler background have indicated that plants of the cry1 single mutant are not hyponastic under white light (Ballaré and Scopel 1997; Mullen et al. 2006).
Figure 7.
Cry1 is the photoreceptor that mediates SAS responses of rosette plants to B light attenuation. Plants were grown under white light (WL) or WL filtered through a yellow filter (-BLUE). Morphological measurements and photographs were taken after 7 d of treatment. Thin bars indicate ±1 S.E. (n=8-16 individual plants). The light x genotype interaction term (LxG) was significant in panels a and b (p<0.01). Different letters indicate significant differences between means. (a-b) Morphological responses to B light attenuation in Col-0 plants and in the various B light photoreceptor mutants. (c) Representative photographs of the plants after exposure to the indicated light treatments.
The response to B light attenuation requires PIF4 or PIF5
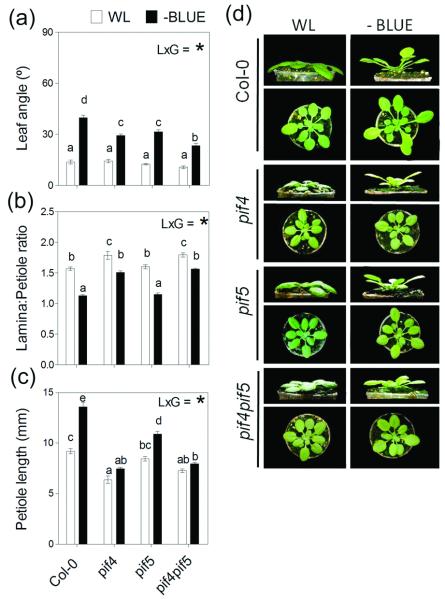
In seedlings, hypocotyl elongation responses triggered by low R:FR (or the phyB mutation) are partially dependent on the bHLH transcription factors PIF4 and PIF5 (Lorrain et al. 2008; Hornitschek et al. 2009). We found that, compared to Col-0, the hyponastic response to B light attenuation was reduced in pif4 and pif5 single mutants, and more so in the pif4 pif5 double mutant (Figure 8). In terms of leaf morphology pif4 had a greater impact than pif5 in reducing the L:P ratio and petiole elongation response to B light attenuation (Figure 8). These results suggest that both of these bHLH transcription factors, but particularly PIF4, are required for full activation of SAS phenotype in response to low B light levels.
Figure 8.
PIF4 and PIF5 are required for full expression of the SAS phenotype to B light attenuation. Plants were grown under white light (WL) or WL filtered through a yellow filter (-BLUE). Morphological measurements and photographs were taken after 7 d of treatment. Thin bars indicate ±1 S.E. (n=8-20 individual plants). The light x genotype interaction term (LxG) was significant (p<0.05) in all panels; different letters indicate significant differences between means. (a-c) Morphological responses to B light attenuation in Col-0 plants and in the various pif mutants. (d) Representative photographs of the plants after exposure to the indicated light treatments.
Discussion
Plants perceive the proximity of neighboring plants using specific photoreceptors, which modulate the expression of growth and tropic responses and allow the plant to forage for photons in heterogeneous light environments. A critical signal of neighbor proximity is a low R:FR ratio, which reduces the levels of the active form of phyB and other stable phytochromes and leads to the appearance of SAS (reviewed in Franklin 2008; Ballaré 2009; Keuskamp et al. 2010b). Two hallmarks of SAS in Arabidopsis and other rosette plants are leaf hyponasty and reduced growth of the leaf lamina relative to petiole growth (reduced L:P ratio). These responses help the plant to move its photosynthetic organs upward and reach canopy strata with improved light conditions. In even-aged plant stands, both responses are triggered early on during canopy development, well before mutual shading among neighboring plants becomes intense. These responses are (1) triggered by the low R:FR ratio of the light reflected by neighboring plants, (2) mediated by inactivation of phyB, and (3) completely dependent of the TAA1 pathway of auxin biosynthesis (Tao et al. 2008; Moreno et al. 2009). In our canopy experiments, we found reduced SAS responses in the sav3 mutant at low canopy cover (Figure 1a, b) or to the proximity on non-shading neighbors (Figure 2a). In contrast, later in the density gradient experiment, when leaf cover was 70 % or more (Figure 1c, d), or when the plants were placed underneath a dense grass canopy (Figure 2), sav3 displayed robust hyponasty and L:P responses. Furthermore, even phyB plants showed exacerbation of their constitutively-expressed SAS phenotype at high LAI (Figure 1c, d). These observations demonstrate that phyB-independent light and hormonal signals participate in activating the SAS phenotype in Arabidopsis plants grown in real canopies.
A signal that may activate SAS responses to crowding or leaf shading is B light attenuation, which results from strong absorption of blue photons by chlorophyll (Ballaré et al. 1991). Hypocotyl growth is inhibited by B light (Cashmore et al. 1999; Folta and Spalding 2001; Pierik et al. 2009; Sellaro et al. 2010; Keuskamp et al. 2011), and in fully de-etiolated plants grown under white light, B light attenuation has been shown to cause increased internode elongation in mustard and Datura ferox (Ballaré et al. 1991), and leaf hyponasty in tobacco (Pierik et al. 2004). Previous studies have shown that Arabidopsis plants at the rosette stage respond to treatments that reduce total light intensity with leaf hyponasty, and that this response can be partially reversed by adding back B light to the low light treatment (Millenaar et al. 2009). Our results confirm that Arabidopsis rosettes can display very marked SAS responses to B light attenuation, including pronounced hyponasty and reduced L:P ratio (Figure 3). Our data also suggest that, at least in Col-0, this response is predominantly mediated by cry1, with no obvious participation of cry2 or phototropins (Figure 7). Thus, cry1 is likely to participate in the SAS responses of Arabidopsis plants to conditions of leaf shading by competitors (Figures 1 and 2).
The two SAS responses characterized in this study (hyponasty and altered leaf morphology), although concomitantly displayed in response to competition signals (low R:FR or low B light), appeared to be controlled by different mechanisms. This difference between responses is apparent from the differential effects of the mutations tested in our experiments, which are summarized in Table 1. Therefore, L:P and leaf hyponasty responses will be considered separately in the discussion below.
Table 1.
Summary of effects of the various mutations on the expression of different components of the SAS phenotype in response to B light attenuation in rosette plants.
| Response | cry1 | sav3 | pin3 | 5xdella | gai1 | bri1 | pif4 | pif5 |
pif4 pif5 |
|---|---|---|---|---|---|---|---|---|---|
| Hyponasty | constitutive | normal | normal | normal | reduced | normal | reduced | reduced | strongly reduced |
| Reduction of L:P |
constitutive | normal | normal | normal | normal | reduced | reduced | normal | reduced |
| Petiole elongation |
constitutive | reduced | normal | normal | strongly reduced |
strongly reduced |
strongly reduced |
reduced | strongly reduced |
Leaf morphology (L:P ratio)
Our results suggest that, at the rosette stage, the auxin and GA action pathways that are recruited by phyB inactivation are not engaged by cry1 inactivation, even though inactivation of both photoreceptors produce similar L:P phenotypes. The L:P response to B light attenuation was completely independent of SAV3 and PIN3 (Figure 3). In contrast, SAV3 and PIN3 are required for the expression of the leaf morphology responses elicited by low R:FR ratios in rosette plants (Tao et al. 2008; Moreno et al. 2009; Keuskamp et al. 2010a), and are also known to play important roles in the hypocotyl elongation responses to low B light and R:FR in young Arabidopsis seedlings (Tao et al. 2008; Keuskamp et al. 2010a; Keuskamp et al. 2011). Moreover, whereas DELLA degradation appears to play a significant role mediating SAS responses to low R:FR ratios in seedlings and rosette plants (Djakovic-Petrovic et al. 2007), it was not activated by B light attenuation (Figure 4) and not directly involved in the production of the SAS response, because B light attenuation causes robust L:P responses in global della mutants (Figure 5). It is apparent, however, that DELLA degradation is somehow required for the full expression of the SAS phenotype in low B light, presumably because L:P responses and hyponasty ultimately depend on elongation, which is repressed in the mutants carrying stable DELLAs (gai-1 in Figure 5c).
BR were also appeared to be partially involved in the L:P response to B light attenuation (Figure 6). Although BR are known to participate in light-controlled signaling networks (Szekeres et al. 1996; Neff et al. 1999; Luccioni et al. 2002; Luo et al. 2010), very little is known about BR involvement in SAS responses. A recent study (Kozuka et al. 2010) demonstrated that BR are required for petiole elongation and lamina growth inhibition responses to end-of-day FR pulses. Experiments reported in a companion paper (Keuskamp et al. 2011) demonstrate that BR are required, along with auxin, for hypocotyl elongation responses of Arabidopsis seedlings to B light attenuation. Collectively, these studies suggest that BR may have a more important role than previously thought in the fine tuning of SAS responses elicited by multiple canopy signals. The mechanism of BR action in SAS responses remains to be elucidated. The study of Keuskamp et al. (2011) suggests that, in Arabidopsis hypocotyls, BR regulate elongation responses to B light depletion via regulation of specific sets of XTH genes.
Leaf hyponasty
None of our experiments revealed a specific connection between the hyponastic response to B light attenuation and hormonal action, as the leaf angle response to low B light was present in all the hormone-related mutants that we tested (Table 1). Furthermore, previous work has shown that ethylene, which is known to cause leaf hyponasty when exogenously applied to plants, does not play a significant role mediating the hyponastic response to low light intensity in Arabidopsis (Millenaar et al. 2009). The same study concluded that auxin perception and PIN3 are necessary for short-term (hours) leaf angle responses to reduced total irradiance (neutral shade). However, although we cannot completely rule out a requirement for auxin action, it is clear that the hyponastic response elicited by B light attenuation is independent of SAV3 and PIN3. This is most remarkable, particularly because when exposed to low R:FR ratios, the hyponastic response is completely absent in sav3 (Moreno et al. 2009) and clearly attenuated in pin3 mutants (Keuskamp et al. 2010a). Therefore, as discussed for the L:P response, we are forced to conclude that the auxin synthesis and transport pathways that are recruited by phyB inactivation are not used to mount responses to BL attenuation, even though the ultimate responses (hyponasty) are very similar.
The mechanism that controls the hyponastic response elicited by cry1 inactivation requires further investigation. Some morphological responses to B light attenuation have been attributed to hydraulic signals, activated by stomatal closure (Barillot et al. 2010). B light promotes stomatal opening though the combined action of cryptochromes and phototropins. It has been shown that cry1 single and cry1 cry2 double mutants were more tolerant to water deprivation than wild-type plants, and this effect was correlated with a reduced stomatal sensitivity to B light (Mao et al. 2005). Further work is needed to test whether the effect of B light attenuation producing leaf hyponasty is functionally connected with stomatal responses.
PIF4 and PIF5: integrators for multiple signals in the plant canopy?
A key point of convergence between the phyB and cry1 inactivation signals uncovered by this study was the requirement for the bHLH transcription factors PIF4 and PIF5 (Figure 8). Previous work has shown that PIF4 or PIF5 are required for normal elongation responses of hypocotyls to light of low R:FR (Lorrain et al. 2008). The role of PIFs in SAS responses of plants at the rosette stage has been less well characterized (Lorrain et al. 2008), and no previous study has demonstrated involvement of these transcription factors in photomorphogenic responses activated by specific B light photoreceptors (Leivar and Quail 2011). Our experiments show that PIF4 is required for normal L:P and petiole elongation responses, and that both PIF4 and PIF5 need to be present for full expression of the hyponastic response to B light attenuation (Figure 8). Interestingly, PIF4 was also found to be essential for hyponastic responses induced by high temperatures in Arabidopsis, with no obvious redundancy with other PIFs (Koini et al. 2009).
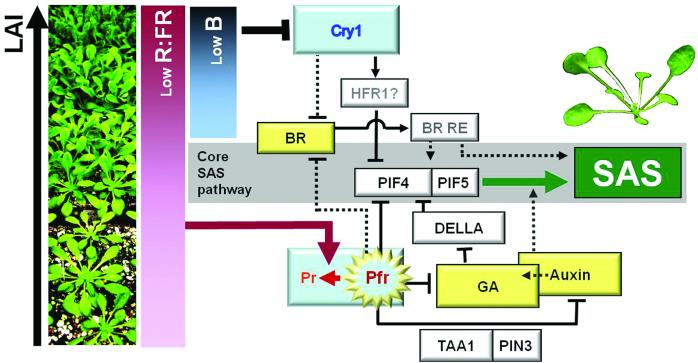
Collectively, these results suggest a model in which PIFs integrate the effects of several environmental and hormonal pathways in the control of leaf angle and morphology in canopies (Figure 9). The mechanisms of interaction between cry1 and PIF proteins need to be investigated in future studies. In the case of phyB, there is direct interaction between PIF and photo-activated phyB in the nucleus, and indirect interactions mediated by the phyB control of GA synthesis and DELLA stability (de Lucas et al. 2008; Feng et al. 2008; Lorrain et al. 2008; Kami et al. 2010). There is no evidence of cry-induced degradation of PIFs or of physical interactions between crys and PIFs (Leivar and Quail 2011), and our data show that B light attenuation treatments do no result in increased DELLA turnover (Figure 4). A potential mechanism whereby B light attenuation activates PIF4 and PIF5 might involve a related bHLH factor, HFR1 (for long Hypocotyl in FR light) (Fairchild et al. 2000). Previous work has established that HFR1 is a positively-acting component in cry1 signaling (Duek and Fankhauser 2003) and recent studies demonstrate that HFR1 directly inhibits the action of growth-promoting PIFs by forming trascriptionally-inactive, non-DNA-binding heterodimers (Hornitschek et al. 2009).
Figure 9.
Proposed model for signal integration during SAS responses of Arabidopsis rosettes to increased canopy density (indicated by increased leaf area index, LAI). At low LAI, only the phyB pathway is triggered in response to FR radiation reflected from neighboring plants. At high LAI, when mutual shading among neighbors becomes intense, the reduction in B light activates the cry1 response pathway, which converges with the phyB pathway at the level of BR, PIF4 and PIF5, thereby boosting the SAS response. The mechanisms and signaling elements (BR RE) connecting BR with SAS are not known.
Conclusions
Understanding how plants detect their neighbors is a requisite for intelligent modification of plant responses to population density in order to increase crop yield per unit area. The data reported in this paper show that mutations that knock down key players involved in phyB signaling (phyB, sav3) do not eliminate the SAS responses of Arabidopsis plants to increased canopy density. This conserved SAS phenotype in mutants impaired in phyB signaling indicates the presence of additional signaling circuits. Communication theory maintains that redundancy is a requirement for reliable transmission and processing of information in noisy environments (Lesne 2008). Our results suggest plants use low R:FR and B light as partially redundant signals of the proximity of competitors. Low R:FR is the main signal at low canopy LAI, where plants can sense FR radiation reflected from neighbors, and both low R:FR and reduced B light irradiance become important indicators of competition at high LAI (Ballaré 1999). Our experiments demonstrate that these signals, perceived by phyB and cry1, respectively, also activate separate signaling networks that show limited overlap with regard to the molecular players involved (Figure 9). Parallel control pathways frequently converge in major regulatory nodes, sometimes characterized as phenotypic capacitors (Levy and Siegal 2008), which are responsible for ensuring that a robust, adaptive phenotype is produced in response to a diverse array of input signals. Our data suggest that PIF4 and PIF5 are critical hubs in the core SAS pathway, which integrate information from the principal light signaling routes that control adaptive plasticity in plant canopies.
Experimental procedures
Plant material
Arabidopsis thaliana (L.) Heynh accessions used as the WT controls were Columbia (Col-0) and Landsberg erecta (Ler). The mutants phyB-9 (Reed et al. 1993), sav3-2 (Tao et al. 2008), pin3-3 (Friml et al. 2002), quintuple yucca yuc3,5,7,8,9 (donated by Y. Zhao), bri1-301 (weak allele of bri1; donated by S. Trupkin), det2-1 (Chory et al. 1991), cry1-301 (Mockler et al. 1999), cry2-1 (Guo et al. 1998), cry1cry2 (cry1-hy4-b104 cry2-1) (Buchovsky et al. 2008), phot1-5phot2-1 (both donated by H. Boccalandro), pif4-101 and pif4pif5 (de Lucas et al. 2008) were all in the Col background. The GA-insensitive gain-of-function gai1 mutant (Koorneef et al. 1985) and gai-t6 rga-t2 rgl1-1 rgl3-1 SGT625-5-2 quintuple della (CS 16298-ABRC) mutants were all in the Ler background.
Growth conditions
For all the experiments, seeds were sown on Petri dishes onto solid agar (0.8%, w/v), and stratified at 4°C in darkness for 48 h. They were then irradiated for 1 h with R light and transferred to white light (120 μmol m−2 s−1). Seedlings were grown for 5 to 6 d, until they were transplanted to individual pots or trays with a 1:1:1 vermiculite:perlite:peat mixture. For the density experiments, seedlings were transplanted to 20 × 16 × 4.5 cm trays in monocultures of four densities: 394; 701; 1315 and 2456 plants per m2 (9, 20, 42 and 81 plants per tray in a square planting arrangement), and placed in glasshouse conditions. For each genotype tested (Col-0, phyB-9 and sav3-2) there were four replicates per density. Plants were allowed to compete above and below ground. For the Lolium canopy shade experiments, Arabidopsis Col-0 and sav3-2 seedlings were transplanted to individual plastic pots (0.2 L) and grown in a glasshouse under full sunlight, until they were 21 d old. The non-shading grass neighbor condition was obtained by placing the plants on the north side of a thick Lolium multiflorum canopy (the experiment was performed in the southern hemisphere; therefore, Arabidopsis seedlings were not shaded by grass leaves). To produce the two levels of shading (partial shade and total shade), the plants were placed under the L. multiflorum canopy at two different heights, which resulted in attenuation of photosynthetically active radiation (PAR) of 75 or 92%, respectively. All the glasshouse experiments were carried out in the autumn, under short photoperiods (10-11 h per day); daily temperatures fluctuated between 11 and 25°C. Peak levels of PAR were between 700 and 1000 μmol m−2 s−1. Trays and individual pots were watered daily with tap water until the appearance of the 6th rosette leaf, and then twice a week with a solution (1X) of HAKAPHOS Rojo 18-18-18 (Compo).
For the light manipulation experiments, seedlings were transplanted to soil in individual pots and placed in a growth chamber (photoperiod = 10 h; PAR = 120 μmol m−2 s−1, standard fluorescent bulbs; temperature = 21°C). At the start of the light manipulation experiment, the plants had between 14 and 16 d after germination (between 5 and 7 true leaves).
Light treatments and measurements
In the growth chamber, B light attenuation was achieved with a yellow filter (Code 101, Lee Filters USA); a pink filter (Lee Filters Code 794) was used to attenuate PAR with minimal reduction of the B light component, and a clear film (clear polyester 0.75 mm, Oeste Aislante, Buenos Aires) was used as a control (see spectral scans in Supplementary Figure S5). Light measurements were taken with a SKL 904/I SpectroSense2 Meter fitted with a SKR 1850 4-channel sensor (Skye Instruments). Light spectra were scanned with an USB4000-UV-VIS Spectrometer, pre-configured with a DET4-200-850 detector and a QP600-2-SR optical fiber (Ocean Optics, Inc.), and processed with the SpectraSuite Software (www.oceanoptics.com/Products/spectrasuite.asp).
Measurements of leaf angle and leaf morphology
In the light attenuation experiments, plants were measured and photographed at the end of the photoperiod of the 7th day after the initiation of the light treatments, unless otherwise stated. Because leaf inclination in Arabidopsis displays diurnal variation (Mullen et al. 2006), plants of all genotypes and light treatments were imaged at the same time of the day. Leaf blade angle relative to the horizontal plane was measured in the tallest (petiolated) leaf of the rosette with a protractor. Petiole and lamina lengths were measured with a digital caliper in the 5th-6th leaf, which sometimes corresponded to the same leaf used to measure the leaf angle.
In the density experiment, plant morphological measurements were taken every week. Measurements taken 25 and 45 d after germination were used to construct Figure 1. Because the initial rate of leaf area expansion varied among genotypes, we used relative canopy cover (instead of the actual plant densities) to compare the different genotypes with regard to their morphological responses to crowding, essentially as described (Ballaré and Scopel 1997). In the canopy shading experiment, plants were measured and photographed as indicated for the light attenuation experiments.
DELLA abundance
RGA coding sequence was amplified from Col-0 genomic DNA with the following primers (RGA-B2R: GGGGACAGCTTTCTTGTACAAAGTGGCTATGAAGAGAGATCATCAC and RGA-B3wSTOP GGGGACAACTTTGTATAATAAAGTTGCTCAGTACGCCGCCGTCGA) and cloned into pDONR-P2RP3 using gateway recombination (Invitrogen). mCITRINE was amplified with the following primers (mCITRINE-B1wKOZAK GGGGACAAGTTTGTACAAAAAAGCAGGCTTAACCATGGTGAGCAAGGGCGAG and mCITRINE-B2noSTOP GGGGACCACTTTGTACAAGAAAGCTGGGTACTTGTACAGCTCGTCCATGCC) and cloned into pDONR221 using gateway recombination as well (Invitrogen). The final destination vector was obtained by using three fragment recombination system using pUBQ10/pDONRP4P1R (Jaillais et al. 2011), mCITRINE/pDONR221, RGA/pDONRP2RP3 entry vectors and pB7m34GW destination vector (Karimi et al. 2007). The pUBQ10::mCITRINE-RGA was transformed into Col-0 and selected using its glyfosinate (Basta) resistance. Confocal microscopy was carried out with a Leica SP/2 inverted microscope. The same confocal settings were used in all conditions and each image is representative of 3 independent experiments (8 plants per genotype and experiment).
Pharmacological experiments
To investigate the involvement of auxin polar transport, intact Col-0 rosette plants were sprayed at 15 d after germination with an aqueous solution of the polar auxin transport inhibitor NPA (Sigma Aldrich). NPA stock solutions (1000X) were prepared in DMSO. Working solutions at final concentrations of 0.5, 5 and 50 μM of NPA and 0.1% Tween 20 were sprayed onto the plants. Control plants were sprayed with solutions containing equivalent quantities of DMSO and Tween 20, without NPA. After the NPA application, plants were immediately placed under the light treatments. Morphological data were obtained 4 d after NPA application.
Statistical analyses
Experimental data were analyzed using INFOSTAT (InfoStat Professional version 1.1; http://www.infostat.com.ar) by means of factorial analysis of variance (ANOVA). In the figures, we report the significance of the main effect of the light treatments. If the light effect varied with genotype (i.e., in cases of a significant light x genotype interactions; LxG), treatment means were further separated using Tukey comparisons. In this case, significant differences between treatment means are indicated by different letters within a given genotype.
Supplementary Material
Acknowledgments
We thank Ronald Pierik and Diedrik Keuskamp for discussions and many useful comments on early versions of this manuscript, Carlos Mazza, Miriam Izaguirre, Patricia Demkura and Miriam Cargnel for discussions and technical assistance, and Hernán Boccalandro, Romina Sellaro and Santiago Trupkin for seed stocks. Supported by grants from ANPCyT and UBACyT (to C.L.B.), and the National Institute of Health (R01 GM52413 to J.C.). J.C. is an investigator of the Howard Hughes Medical Institute. Y.J. was supported by a postdoctoral fellowships from the European Molecular Biology Organization.
References
- Aphalo PJ, Ballaré CL. On the importance of information-acquiring systems in plant-plant interactions. Funct Ecol. 1995;9:5–14. [Google Scholar]
- Ballaré CL. Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci. 1999;4:97–102. doi: 10.1016/s1360-1385(99)01383-7. [DOI] [PubMed] [Google Scholar]
- Ballaré CL. Illuminated behaviour: phytochrome as a key regulator of light foraging and plant anti-herbivore defence. Plant Cell Environ. 2009;32:713–725. doi: 10.1111/j.1365-3040.2009.01958.x. [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL. Phytocrome signalling in plant canopies. Testing its population-level consequences using photoreceptor mutants of Arabidopsis. Funct Ecol. 1997;11:441–450. [Google Scholar]
- Ballaré CL, Scopel AL, Radosevich SR, Kendrick RE. Phytochrome-mediated phototropism in de-etiolated seedlings. Plant Physiol. 1992;100:170–177. doi: 10.1104/pp.100.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Sanchez RA. Photocontrol of stem elongation in plant neighbourhoods: effects of photon fluence rate under natural conditions of radiation. Plant Cell Environ. 1991;14:57–65. [Google Scholar]
- Barillot R, Frak E, Combes D, Durand JL, Escobar-Gutiérrez AJ. What determines the complex kinetics of stomatal conductance under blueless PAR in Festuca arundinacea? Subsequent effects on leaf transpiration. J Expt Bot. 2010;61:2795–2806. doi: 10.1093/jxb/erq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benschop JJ, Millenaar FF, Smeets ME, Van Zanten M, Voesenek LACJ, Peeters AJM. Abscisic acid antagonizes ethylene-induced hyponastic growth in Arabidopsis. Plant Physiol. 2007;143:1013–1023. doi: 10.1104/pp.106.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR, Christie JM. Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci. 2002;7:204–210. doi: 10.1016/s1360-1385(02)02245-8. [DOI] [PubMed] [Google Scholar]
- Buchovsky AS, Strasser B, Cerdán PD, Casal JJ. Suppression of pleiotropic effects of functional CRYPTOCHROME genes by TERMINAL FLOWER 1. Genetics. 2008;180:1467–1474. doi: 10.1534/genetics.108.088096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore AR, Jarillo JA, Wu Y-J, Liu D. Cryptochromes: blue light receptors for plants and animals. Science. 1999;284:760–765. doi: 10.1126/science.284.5415.760. [DOI] [PubMed] [Google Scholar]
- Chory J, Nagpal P, Peto CA. Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell. 1991;3:445–459. doi: 10.1105/tpc.3.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kroon H, Visser EJW, Huber H, Mommer L, Hutchings MJ. A modular concept of plant foraging behaviour: The interplay between local responses and systemic control. Plant Cell Environ. 2009;32:704–712. doi: 10.1111/j.1365-3040.2009.01936.x. [DOI] [PubMed] [Google Scholar]
- de Lucas M, et al. A molecular framework for light and gibberellin control of cell elongation. Nature. 2008;451:480–484. doi: 10.1038/nature06520. [DOI] [PubMed] [Google Scholar]
- Djakovic-Petrovic T, Wit M.d., Voesenek LACJ, Pierik R. DELLA protein function in growth responses to canopy signals. Plant J. 2007;51:117–126. doi: 10.1111/j.1365-313X.2007.03122.x. [DOI] [PubMed] [Google Scholar]
- Dorn LA, Pyle EH, Schmitt J. Plasticity to light cues and resources in Arabidopsis thaliana: testing for adaptive value and costs. Evolution. 2000;54:1982–1994. doi: 10.1111/j.0014-3820.2000.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Duek PD, Fankhauser C. HFR1, a putative bHLH transcription factor, mediates both phytochrome A and cryptochrome signalling. Plant J. 2003;34:827–836. doi: 10.1046/j.1365-313x.2003.01770.x. [DOI] [PubMed] [Google Scholar]
- Fairchild CD, Schumaker MA, Quail PH. HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev. 2000;14:2377–2391. [PMC free article] [PubMed] [Google Scholar]
- Feng SH, et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature. 2008;451:475–479. doi: 10.1038/nature06448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Spalding EP. Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J. 2001;26:471–478. doi: 10.1046/j.1365-313x.2001.01038.x. [DOI] [PubMed] [Google Scholar]
- Franklin KA. Shade avoidance. New Phytol. 2008;179:930–944. doi: 10.1111/j.1469-8137.2008.02507.x. [DOI] [PubMed] [Google Scholar]
- Frigerio M, Alabadí D, Pérez-Gómez J, García-Cárcel L, Phillips AL, Hedden P, Blázquez MA. Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiol. 2006;142:553–563. doi: 10.1104/pp.106.084871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature. 2002;415:806–809. doi: 10.1038/415806a. [DOI] [PubMed] [Google Scholar]
- Guo H, Yang H, Mockler TC, Lin C. Regulation of flowering time by Arabidopsis photoreceptors. Science. 1998;279:1360–1363. doi: 10.1126/science.279.5355.1360. [DOI] [PubMed] [Google Scholar]
- Harberd NP, Belfield E, Yasumura Y. The Angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: How an “inhibitor of an inhibitor” enables flexible response to fluctuating environments. Plant Cell. 2009;21:1328–1339. doi: 10.1105/tpc.109.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornitschek P, Lorrain S, Zoete V, Michielin O, Fankhauser C. Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J. 2009;28:3893–3902. doi: 10.1038/emboj.2009.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Chory J. Unraveling the paradoxes of plant hormone signaling integration. Nat Struct Mol Biol. 2010;17:642–645. doi: 10.1038/nsmb0610-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Hothorn M, Belkhadir Y, Dabi T, Nimchuk ZL, Meyerowitz EM, Chory J. Tyrosine phosphorylation controls brassinosteroid receptor activation by triggering membrane release of its kinase inhibiton. Genes Dev. 2011;25:232–237. doi: 10.1101/gad.2001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami C, Lorrain S, Hornitschek P, Fankhauser C, Marja CPT. Current Topics in Developmental Biology. Academic Press; 2010. Light-regulated plant growth and development; pp. 29–66. [DOI] [PubMed] [Google Scholar]
- Karimi M, Depicker A, Hilson P. Recombinational cloning with plant gateway vectors. Plant Physiol. 2007;145:1144–1154. doi: 10.1104/pp.107.106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp DH, Pollmann S, Voesenek LACJ, Peeters AJM, Pierik R. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc Natl Acad Sci USA. 2010a;107:22740–22744. doi: 10.1073/pnas.1013457108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp DH, Sasidharan R, Pierik R. Physiological regulation and functional significance of shade avoidance responses to neighbors. Plant Signal Behav. 2010b;5:1–8. doi: 10.4161/psb.5.6.11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuskamp DH, Sasidharan R, Vos I, Peeters AJM, Voesenek LACJ, Pierik R. Blue light-mediated shade avoidance requires combined auxin and brassinosteroid action. 2011 doi: 10.1111/j.1365-313X.2011.04597.x. in press. [DOI] [PubMed] [Google Scholar]
- Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, Franklin KA. High Temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol. 2009;19:408–413. doi: 10.1016/j.cub.2009.01.046. [DOI] [PubMed] [Google Scholar]
- Koorneef M, Elgersma A, Hanhart CJ, van Loenen-Martinet EP, van Rijn L, Zeevaart JAD. A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol Plant. 1985;65:33–39. [Google Scholar]
- Kozuka T, Horiguchi G, Kim G-T, Ohgishi M, Sakai T, Tsukaya H. The different growth responses of the Arabidopsis thaliana leaf blade and the petiole during shade avoidance are regulated by photoreceptors and sugar. Plant Cell Physiol. 2005;46:213–223. doi: 10.1093/pcp/pci016. [DOI] [PubMed] [Google Scholar]
- Kozuka T, Kobayashi J, Horiguchi G, Demura T, Sakakibara H, Tsukaya H, Nagatani A. Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol. 2010;153:1608–1618. doi: 10.1104/pp.110.156802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Quail PH. PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne A. Robustness: Confronting lessons from physics and biology. Biol Rev. 2008;83:509–532. doi: 10.1111/j.1469-185X.2008.00052.x. [DOI] [PubMed] [Google Scholar]
- Levy SF, Siegal ML. Network hubs buffer environmental variation in Saccharomyces cerevisiae. PLoS Biology. 2008;6:2588–2604. doi: 10.1371/journal.pbio.0060264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C. Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 2008;53:312–323. doi: 10.1111/j.1365-313X.2007.03341.x. [DOI] [PubMed] [Google Scholar]
- Luccioni LG, Oliverio KA, Yanovsky MJ, Boccalandro HE, Casal JJ. Brassinosteroid mutants uncover fine tuning of phytochrome signaling. Plant Physiol. 2002;128:173–181. [PMC free article] [PubMed] [Google Scholar]
- Luo XM, et al. Integration of light- and brassinosteroid-signaling pathways by a GATA transcription factor in Arabidopsis. Dev Cell. 2010;19:872–883. doi: 10.1016/j.devcel.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Zhang YC, Sang Y, Li QH, Yang HQ. A role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc Natl Acad Sci USA. 2005;102:12270–12275. doi: 10.1073/pnas.0501011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García JF, Galstyan A, Salla-Martret M, Cifuentes-Esquivel N, Gallemí M, Bou-Torrent J, Jean-Claude Kader, Michel D. Advances in Botanical Research. Academic Press; 2010. Regulatory components of shade avoidance syndrome; pp. 65–116. [Google Scholar]
- Millenaar FF, Van Zanten M, Cox MCH, Pierik R, Voesenek LACJ, Peeters AJM. Differential petiole growth in Arabidopsis thaliana: photocontrol and hormonal regulation. New Phytol. 2009;184:141–152. doi: 10.1111/j.1469-8137.2009.02921.x. [DOI] [PubMed] [Google Scholar]
- Mockler TC, Guo H, Yang H, Duong H, Lin C. Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development. 1999;126:2073–2082. doi: 10.1242/dev.126.10.2073. [DOI] [PubMed] [Google Scholar]
- Moreno JE, Tao Y, Chory J, Ballaré CL. Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc Natl Acad Sci USA. 2009;106:4935–4940. doi: 10.1073/pnas.0900701106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JL, Weinig C, Hangarter RP. Shade avoidance and the regulation of leaf inclination in Arabidopsis. Plant Cell Environ. 2006;29:1099–1106. doi: 10.1111/j.1365-3040.2005.01484.x. [DOI] [PubMed] [Google Scholar]
- Neff MM, et al. BAS1: a gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc Natl Acad Sci USA. 1999;96:15316–15323. doi: 10.1073/pnas.96.26.15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoplansky A. Picking battles wisely: Plant behaviour under competition. Plant Cell Environ. 2009;32:726–741. doi: 10.1111/j.1365-3040.2009.01979.x. [DOI] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Djakovic-Petrovic T, Keuskamp DH, de Wit M, Voesenek LACJ. Auxin and ethylene regulate elongation responses to neighbor proximity signals independent of gibberellin and DELLA proteins in Arabidopsis. Plant Physiol. 2009;149:1701–1712. doi: 10.1104/pp.108.133496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Whitelam GC, Voesenek LACJ, de Kroon H, Visser EJW. Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant-plant signalling. Plant J. 2004;38:310–319. doi: 10.1111/j.1365-313X.2004.02044.x. [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellaro R, Crepy M, Trupkin SA, Karayekov E, Buchovsky AS, Rossi C, Casal JJ. Cryptochrome as a sensor of the blue/green ratio of natural radiation in Arabidopsis. Plant Physiol. 2010;154:401–409. doi: 10.1104/pp.110.160820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Light quality, photoperception, and plant strategy. Annu Rev Plant Physiol. 1982;33:481–518. [Google Scholar]
- Sultan SE. Plant developmental responses to the environment: eco-devo insights. Curr Opin Plant Biol. 2010;13:96–101. doi: 10.1016/j.pbi.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Szekeres M, et al. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Takemiya A, Inoue S.-i., Doi M, Kinoshita T, Shimazaki K.-i. Phototropins promote plant growth in response to blue light in low light environments. Plant Cell. 2005;17:1120–1127. doi: 10.1105/tpc.104.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zanten M, Pons TL, Janssen JAM, Voesenek LACJ, Peeters AJM. On the Relevance and Control of Leaf Angle. Critical Rev Plant Sci. 2010;29:300–316. [Google Scholar]
- Vandenbussche F, Pierik R, Millenaar FF, Voesenek LACJ, Van Der Straeten D. Reaching out of the shade. Curr Opin Plant Biol. 2005;8:462–468. doi: 10.1016/j.pbi.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science. 2001;291:306–309. doi: 10.1126/science.291.5502.306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.