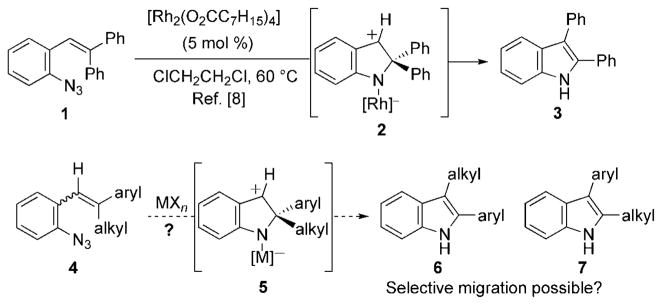
Transition metal-catalyzed migratorial processes that form new carbon–carbon bonds can enable the formation of complex products from readily accessible, simple starting materials. Controlling the selectivity of the migration step is critical to the success of these transformations.[1] Sequential reaction processes that involve metal nitrenes are rare despite their electrophilicity,[2] which enables reaction with carbon–hydrogen bonds or olefins.[3–6] Our mechanistic study of rhodium(II)-catalyzed carbazole formation from biaryl azides which suggested that C–N bond formation preceded C–H bond cleavage through a 4π-electron–5-atom electrocyclization.[7] Consequently, we anticipated that substrates lacking functionalizable C–H bonds might participate in a migratorial process where a new C–C bond is formed in addition to the C–N bond. In support of this hypothesis, rhodium octanoate catalyzed the conversion of β,β-diphenylstryryl azide 1 to 2,3-diphenylindole 3 (Scheme 1).[8] This result, however, does not indicate whether this process can be rendered selective for styryl azides 4 that contain two different β-substituents to form 2,3-disubstituted indoles. Because these N-heterocycles are important pharmaceutical scaffolds,[9] new methods, which streamline their synthesis, remain an ongoing goal.[10,11] Herein, we report our initial studies that resulted in the development of a general method to form 2,3-disubstituted indoles—as single regioisomers—from readily available β,β-disubstituted stryryl azides.
Scheme 1.
Potential for selective 2,3-disubstituted indole formation.
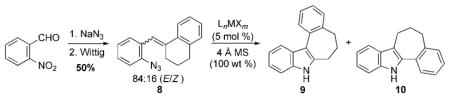
The effect of transition metal complexes on the desired migration was investigated using a mixture of the E- and Z-isomer of β,β-disubstituted aryl azide 8 (Table 1). This azide is readily accessible in two steps from commercially available 2-nitrobenzaldehyde.[12] Examination of a range of dirhodium(II) complexes revealed that selective formation of 9 was obtained using with [Rh2(O2CC3F7)4],[8,13] [Rh2(O2CC7H15)4], or [Rh2(esp)2][14] (Table 1, entries 1–7).[15] Importantly, both the E- and Z-isomer of 8 were converted to indole 9 revealing that the selectivity of the reaction did not depend on the stereochemistry of the starting material. Other rhodium carboxylate complexes provided attenuated selectivities or reduced yields. Other transition metal complexes, such as [(cod)Ir(OMe)2],[16] [Co(tpp)],[17] RuCl3,[18] or copper salts,[19] known to decompose azides or π-Lewis acids,[20] did not promote indole formation (Table 1, entries 8–13). Consequently, the reaction conditions were further optimized using rhodium hexaflourobutyrate, and incomplete conversions were observed when either the catalyst loading or the reaction temperature was lowered (<5 mol%; <70°C). The optimal solvent was found to be either toluene or dichloroethane. Purification proved to be facile: analytically pure indole was obtained by filtering the reaction mixture through a pipette of alumina.
Table 1.
Development of optimal conditions for indole formation.
| ||||
|---|---|---|---|---|
| Entry | LnMXm[a] | T [°C] | Yield [%][b] | 9:10[c] |
| 1 | [Rh2(O2CCH3)4] | 70 | 8 | – |
| 2 | [Rh2(O2CC7H15)4] | 70 | 93 | 96:4 |
| 3 | [Rh2(esp)2] | 70 | 98 | 98:2 |
| 4 | [Rh2(O2CCF3)4] | 70 | 86 | 99:1 |
| 5 | [Rh2(O2CC3F7)4] | 70 | 95 | 100:0 |
| 6[d] | [Rh2(O2CC3F7)4] | 70 | 80[e] | 100:0 |
| 7 | [{(cod)Ir(OMe)}2] | 70 | 0 | – |
| 8 | [Co(tpp)] | 80 | 0 | – |
| 9[f] | RuCl3·n H2O | 65 | trace | – |
| 10 | Cu(OTf)2 | 70 | 0 | – |
| 11 | AgOTf | 65 | 0 | – |
| 12 | AuCl | 65 | 0 | – |
esp =α,α,α′,α′-tetramethyl-1,3-benzenedipropionate; cod = cyclooctadiene; tpp = tetraphenylporphyrin.
Yield after Al2O3 chromatography.
As determined by using 1H NMR spectroscopy.
3 mol% catalyst.
10% aryl azide remained.
No molecular sieve added.
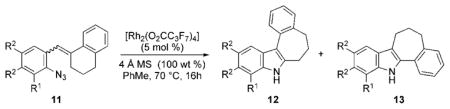
Using these optimized conditions, the scope and limitations of the rhodium(II)-catalyzed formation of 2,3-disubstituted indoles from β,β-disubstituted stryryl azides was examined (Table 2). In every example, only aryl group migration was observed even if the electronic nature of the aryl azide moiety was modulated. High yields were observed with electron-donating substituents such as methoxide (Table 2, entries 1 and 2). Electron-withdrawing groups also did not lower the reaction yield or migration selectivity (Table 2, entries 3–8). Among these, azides bearing potentially reactive bromides, esters, or sulfones were competent substrates in our process. The reaction was also not sensitive to the steric nature around the azide: nearly quantitative yield of 12a was observed with 11a, which contained two ortho-substituents. Purification of every 2,3-disubstituted indole by simple filtration through alumina further underscores the synthetic utility of our reaction.
Table 2.
Scope of Rh2II-catalyzed migratorial reactions.
| ||||||
|---|---|---|---|---|---|---|
| Entry | 11 | R1 | R2 | R3 | Yield [%][a] | 12:13[b] |
| 1 | a | MeO | H | H | 96 | >95:5 |
| 2 | b | H | MeO | H | 97 | >95:5 |
| 3 | c | H | Cl | H | 99 | >95:5 |
| 4[c] | d | H | MeO2C | H | 94 | >95:5 |
| 5 | e | H | F3C | H | 90 | >95:5 |
| 6[d] | f | H | MeO2S | H | 95 | >95:5 |
| 7 | g | H | H | Br | 95 | >95:5 |
| 8[d] | h | H | H | MeO2C | 95 | >95:5 |
Yield after Al2O3 chromatography.
As determined by using 1H NMR spectroscopy.
X-ray structure of product indole obtained.
5 mol% of [Rh2(esp)2] used.
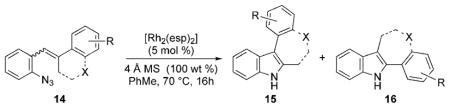
The nature of the migrating group on the aryl azide was subsequently investigated (Table 3). For these substrates, only aryl group migration was observed. While rhodium perfluorobutyrate was a competent catalyst, [Rh2(esp)2] provided the highest yields of the reaction. Only indole 15a was observed when the tether was shortened (Table 3, entry 1). Appending the electron-withdrawing trifluoromethyl group or the electron-donating methoxy group to the migrating arene did not change the outcome of the reaction (Table 3, entries 2 and 3). In both cases, only aryl group migration was observed. High yields and selective formation of indole 15d was obtained when an oxygen atom was incorporated into the tether. The reaction was not limited to ring expansion: despite changing the electronic nature of the migrating aryl group, only indoles 15e and 15 f were formed from azides 14 e and 14 f.
Table 3.
Scope of Rh2II-catalyzed 2,3-disubstituted indole formation.
| ||||
|---|---|---|---|---|
| Entry | 14, 17 | Styryl azide | Indole product | Yield [%][a] |
| 1 | a |
|
|
88 |
| 2 | b |
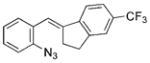
|
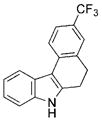
|
78 |
| 3 | c |
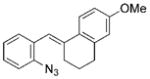
|
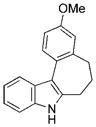
|
92 |
| 4 | d |
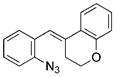
|
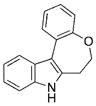
|
94 |
| 5 | e |
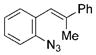
|
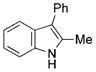
|
91 |
| 6 | f |
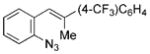
|
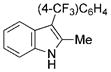
|
93 |
|
| ||||
|
| ||||
| 7 | a |
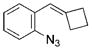
|
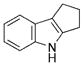
|
98 |
| 8 | b |
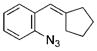
|
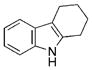
|
93 |
| 9 | c |
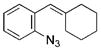
|
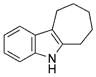
|
93 |
| 10 | d |
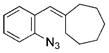
|
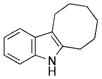
|
18[b],[c] |
| 11 | e |
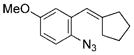
|
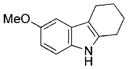
|
quant. |
| 12 | f |
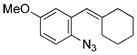
|
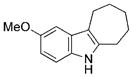
|
quant. |
| 13 | g |
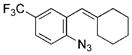
|
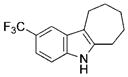
|
88 |
| 14 | h |
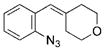
|
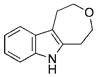
|
97 |
Yield after Al2O3 chromatography.
As determined by using 1H NMR spectroscopy.
35% remaining 17 d. DCE = dichloroethane.
The effect of ring size on the reaction efficiency was further examined using styryl azides 17. For this series of substrates, rhodium octanoate proved to be the most reliable catalyst. While ring-expanded products were formed from 4-, 5-, and 6-membered substrates, poor conversion was observed for 7-membered 17d (Table 3, entries 7–10). Varying the electronic nature of the aryl azide did not attenuate the yield of the reaction (Table 3, entries 11–13). Oxygen atoms were tolerated in the tether without lowering the yield of the ring expansion (Table 3, entry 14).
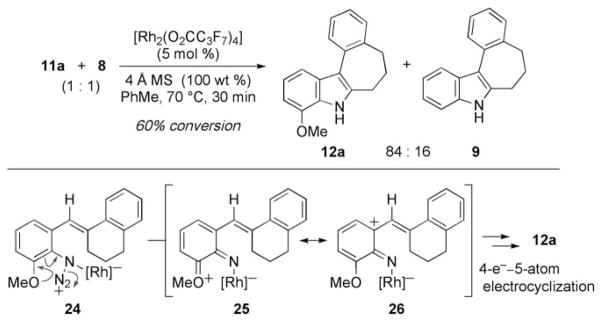
While many mechanisms are possible to explain the reaction outcome, our data suggests that the migration occurs once an intermediate (21) is generated with positive charge on the benzylic carbon. We propose that this intermediate is formed by the mechanism outlined in Scheme 2. Coordination of the rhodium carboxylate complex to the azide produces either α-19 or γ-19.[21] Extrusion of N2 from 19 forms rhodium nitrene 20,[22] which participates in a 4π-electron–5-atom electrocyclization to establish the carbon–nitrogen bond in 21.[7] Aryl migration forms the more stable tertiary iminium ion 22, which tautermizes to produce 9. Alternatively, the ortho-double bond could assist in N2 extrusion to form the intermediate 23, or this intermediate could be formed from [2+1] cycloaddition of the pendant double bond with the electrophilic metallonitrene 20. While 23 is strained,[23] its intermediacy would account for the enhanced reactivity of azides with unsaturated ortho-substituents.
Scheme 2.
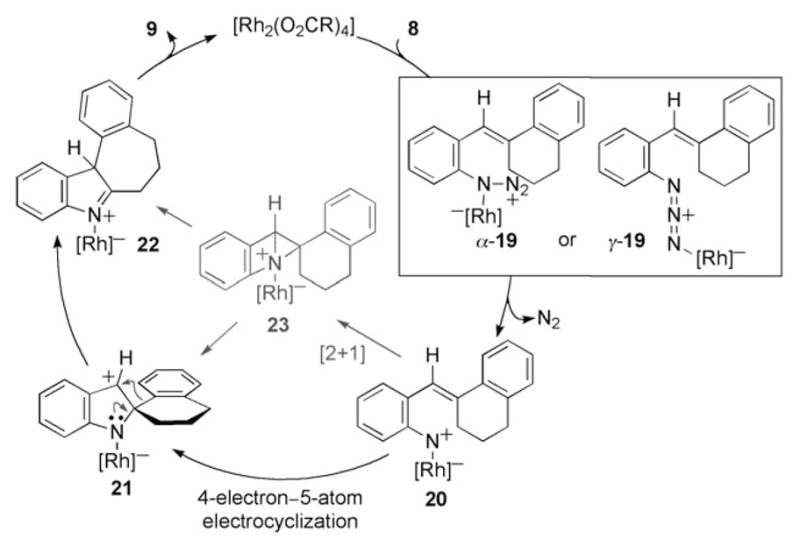
Potential mechanisms for indole formation.
We performed several experiments to test the validity of our mechanism. To examine whether N2 was lost before C–N bond formation, we performed an intermolecular competition experiment between azides 11 a and 8 (Scheme 3). Our previous Hammett correlation study indicated that N2 extrusion occurred faster with electron-rich aryl azides.[7] Acceleration of metallonitrene formation was attributed to the ability of the electron-donating group to assist in N2 loss (24 to 25). In contrast, if N2 loss occurred simultaneously with C–N bond formation, we anticipated that 8 would react faster because the azide moiety was more electrophilic than in 11a. To test these assertions, a 1:1 mixture of styryl azides 11 a and 8 were exposed to reaction conditions. Despite the increased steric pressure around the azide, the more electron-rich substrate reacted faster to produce indole 12a as the major product to support our proposed electrocyclization mechanism.
Scheme 3.
Intermolecular competition experiment.
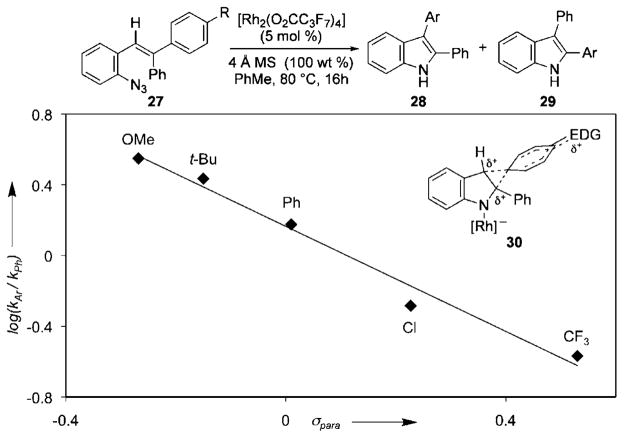
If the migration mechanism involved the formation of a partial positive charge on the α-carbon, we anticipated that electron-rich aryl groups would migrate preferentially. To test this hypothesis, a series of styryl azides, which systematically varied the identity of the para-substituent R, were exposed to reaction conditions (Figure 1). Examination of the product ratios using the Hammett equation revealed that the best linear correlation was obtained with σpara values to give a ρ value of −1.49. The greater propensity of the more electron-rich aryl group to participate in the 1,2-shift was interpreted to suggest that the migration occurs through phenonium ion reactive intermediate 30,[24,25] where the more stable ion leads to the major product.
Figure 1.
Correlation of product ratios with the Hammett equation. y= −1.49x+0.17; R2 =0.98.
In conclusion, we have demonstrated that rhodium carboxylate complexes catalyze cascade reactions of β,β-disubstituted styryl azides to selectively produce 2,3-disubstituted indoles. Our data suggests that the selectivity of the migratorial process is controlled by the formation of a phenonium ion. Future experiments will be aimed at clarifying the mechanism of this reaction as well as determining if the benzylic cation can be intercepted with additional nucleophiles to produce complex, functionalized N-heterocycles from simple, readily available styryl azides.
Supplementary Material
Footnotes
We are grateful to the National Institutes of Health NIGMS (R01M984945) and the University of Illinois at Chicago for their generous support. We also thank Prof. L. Anderson (UIC) for insightful discussions.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201006917.
Contributor Information
Ke Sun, Department of Chemistry, University of Illinois at Chicago 845 W. Taylor St., Chicago (USA).
Sheng Liu, Department of Chemistry, University of Illinois at Chicago 845 W. Taylor St., Chicago (USA).
Patryk M. Bec, Department of Chemistry, University of Illinois at Chicago 845 W. Taylor St., Chicago (USA)
Prof. Tom G. Driver, Department of Chemistry, University of Illinois at Chicago 845 W. Taylor St., Chicago (USA).
References
- 1.For reviews of migratorial processes, see: Crone B, Kirsch SF. Chem Eur J. 2008;14:3514. doi: 10.1002/chem.200701985.Overman LE, Pennington LD. J Org Chem. 2003;68:7143. doi: 10.1021/jo034982c.
- 2.For cascade reactions involving metal nitrenes, see: Thornton AR, Martin VI, Blakey SB. J Am Chem Soc. 2009;131:2434. doi: 10.1021/ja809078d.Thornton AR, Blakey SB. J Am Chem Soc. 2008;130:5020. doi: 10.1021/ja7111788.
- 3.For recent reviews, see: Collet F, Dodd RH, Dauban P. Chem Commun. 2009:5061. doi: 10.1039/b905820f.Dauban P, Dodd RH. In: Amino Group Chemistry. Ricci A, editor. Wiley-VCH; Weinheim: 2008. p. 55.Du Bois J. Chemtracts. 2005;18:1.
- 4.For reviews of metal nitrenes from azides, see: Driver TG. Org Biomol Chem. 2010;8:3831. doi: 10.1039/c005219c.Cenini S, Gallo E, Caselli A, Ragaini F, Fantauzzi S, Piangiolino C. Coord Chem Rev. 2006;250:1234. doi: 10.1002/chem.200801148.Katsuki T. Chem Lett. 2005;34:1304.
- 5.For leading reports of rhodium nitrene chemistry, see: Zalatan DN, Du Bois J. J Am Chem Soc. 2009;131:7558. doi: 10.1021/ja902893u.Kurokawa T, Kim M, Du Bois J. Angew Chem. 2009;121:2815. doi: 10.1002/anie.200806192.Angew Chem Int Ed. 2009;48:2777.Olson DE, DuBois J. J Am Chem Soc. 2008;130:11248. doi: 10.1021/ja803344v.Zalatan DN, Du Bois J. J Am Chem Soc. 2008;130:9220. doi: 10.1021/ja8031955.Huard K, Lebel H. Chem Eur J. 2008;14:6222. doi: 10.1002/chem.200702027.Liang C, Collet F, Robert-Peillard F, Müller P, Dodd RH, Dauban P. J Am Chem Soc. 2008;130:343. doi: 10.1021/ja076519d.Fiori KW, Du Bois J. J Am Chem Soc. 2007;129:562. doi: 10.1021/ja0650450.Lebel H, Huard K, Lectard S. J Am Chem Soc. 2005;127:14198. doi: 10.1021/ja0552850.
- 6.For other metal-mediated nitrene reactions, see: Lu H, Tao J, Jones JE, Wojtas L, Zhang XP. Org Lett. 2010;12:1248. doi: 10.1021/ol100110z.Subbarayan V, Ruppel JV, Zhu S, Perman JA, Zhang XP. Chem Commun. 2009:4266. doi: 10.1039/b905727g.Lu H, Subbarayan V, Tao J, Zhang XP. Organometallics. 2010;29:389.Fantauzzi S, Gallo E, Caselli A, Ragaini F, Casati N, Macchi P, Cenini S. Chem Commun. 2009:3952. doi: 10.1039/b903238j.Ruppel JV, Jones JE, Huff CA, Kamble RM, Chen Y, Zhang XP. Org Lett. 2008;10:1995. doi: 10.1021/ol800588p.Caselli A, Gallo E, Fantauzzi S, Morlacchi S, Ragaini F, Cenini S. Eur J Inorg Chem. 2008:3009.Kawabata H, Omura K, Uchida T, Katsuki T. Chem Asian J. 2007;2:248. doi: 10.1002/asia.200600363.Fantauzzi S, Gallo E, Caselli A, Piangiolino C, Ragaini F, Cenini S. Eur J Org Chem. 2007:6053. doi: 10.1002/chem.200801148.Fujita H, Uchida T, Lrie R, Katsuki T. Chem Lett. 2007;36:1092.Bacci JP, Greenman KL, Van Vranken DL. J Org Chem. 2003;68:4955. doi: 10.1021/jo0340410.Bach T, Schlummer B, Harms K. Chem Eur J. 2001;7:2581. doi: 10.1002/1521-3765(20010618)7:12<2581::aid-chem25810>3.0.co;2-o.
- 7.Stokes BJ, Richert KJ, Driver TG. J Org Chem. 2009;74:6442. doi: 10.1021/jo901224k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen M, Leslie BE, Driver TG. Angew Chem. 2008;120:5134. doi: 10.1002/anie.200800689. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:5056. [Google Scholar]
- 9.For recent reviews, see: Kochanowska-Karamyan AJ, Hamann MT. Chem Rev. 2010;110:4489. doi: 10.1021/cr900211p.Edwankar CR, Edwankar RV, Namjoshi OA, Rallapappi SK, Yang J, Cook JM. Curr Opin Drug Discovery Dev. 2009;12:752.Brancale A, Silvestri R. Med Res Rev. 2007;27:209. doi: 10.1002/med.20080.
- 10.For recent reviews, see: Bandini M, Eichholzer A. Angew Chem. 2009;121:9786.Angew Chem Int Ed. 2009;48:9608.Humphrey GR, Kuethe JT. Chem Rev. 2006;106:2875. doi: 10.1021/cr0505270.Gribble G, Saulnier MG, Pelkey ET, Kishbaugh TLS, Liu Y, Jiang J, Trujillo HA, Keavy DJ, Davis DA, Conway SC, Switzer FL, Roy S, Silva RA, Obaza-Nutaitis JA, Sibi MP, Moskalev NV, Barden TC, Chang L, Habeski nee Simon WM, Pelcman B, Sponholtz WR, III, Chau RW, Allison BD, Garaas SD, Sinha MS, McGowan MA, Reese MR, Harpp KS. Curr Org Chem. 2005;9:1493.Cacchi S, Fabrizi G. Chem Rev. 2005;105:2873. doi: 10.1021/cr040639b.
- 11.For recent reports, see: Pathak TP, Gligorich KM, Welm BE, Sigman MS. J Am Chem Soc. 2010;132:7870. doi: 10.1021/ja103472a.Tan Y, Hartwig JF. J Am Chem Soc. 2010;132:3676. doi: 10.1021/ja100676r.Mochida K, Shimizu M, Hiyama T. J Am Chem Soc. 2009;131:8350. doi: 10.1021/ja901622b.Barluenga J, Jiménez-Aquino A, Aznar F, Valdés C. J Am Chem Soc. 2009;131:4031. doi: 10.1021/ja808652a.Stuart DR, Bertrand-Laperle M, Burgess KMN, Fagnou K. J Am Chem Soc. 2008;130:16474. doi: 10.1021/ja806955s.Leogane O, Lebel H. Angew Chem. 2008;120:356. doi: 10.1002/anie.200703671.Angew Chem Int Ed. 2008;47:350.
- 12.For more information, refer to the Supporting Information.
- 13.a) Stokes BJ, Dong H, Leslie BE, Pumphrey AL, Driver TG. J Am Chem Soc. 2007;129:7500. doi: 10.1021/ja072219k. [DOI] [PubMed] [Google Scholar]; b) Stokes BJ, Jovanović B, Dong H, Richert KJ, Riell RD, Driver TG. J Org Chem. 2009;74:3225. doi: 10.1021/jo9002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espino CG, Fiori KW, Kim M, Du Bois J. J Am Chem Soc. 2004;126:15378. doi: 10.1021/ja0446294. [DOI] [PubMed] [Google Scholar]
- 15.The indole product was verified by X-ray analysis (see Supporting Information).
- 16.Sun K, Sachwani R, Richert KJ, Driver TG. Org Lett. 2009;11:3598. doi: 10.1021/ol901317j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruppel JV, Kamble RM, Zhang XP. Org Lett. 2007;9:4889. doi: 10.1021/ol702265h.b) Ref. [6f].
- 18.Shou WG, Li J, Guo T, Lin Z, Jia G. Organometallics. 2009;28:6847. [Google Scholar]
- 19.a) Chiba S, Zhang L, Lee JY. J Am Chem Soc. 2010;132:7266. doi: 10.1021/ja1027327. [DOI] [PubMed] [Google Scholar]; b) Chiba S, Zhang L, Ang GY, Hui BWQ. Org Lett. 2010;12:2052. doi: 10.1021/ol100522z. [DOI] [PubMed] [Google Scholar]
- 20.cf. Gorin DJ, Davis NR, Toste FD. J Am Chem Soc. 2005;127:11260. doi: 10.1021/ja053804t.
- 21.For crystal structures of metal–azide complexes, see: η2-coordination: Waterman R, Hillhouse GL. J Am Chem Soc. 2008;130:12628. doi: 10.1021/ja805530z.Albertin G, Antoniutti S, Baldan D, Castro J, Garcia-Fontan S. Inorg Chem. 2008;47:742. doi: 10.1021/ic701907y.Nα and Nγ coordination: Dias HVR, Polach SA, Goh SK, Archibong EF, Marynick DS. Inorg Chem. 2000;39:3894. doi: 10.1021/ic0004232.Nγ coordination: Fickes MG, Davis WM, Cummins CC. J Am Chem Soc. 1995;117:6384.Nγ coordination: Proulx G, Bergman RG. J Am Chem Soc. 1995;117:6382.
- 22.For computational studies on the mechanism of copper nitrenoid formation from azides, see: Badiei YM, Dinescu A, Dai X, Palomino RM, Heinemann FW, Cundari TR, Warren TH. Angew Chem. 2008;120:10109. doi: 10.1002/anie.200804304.Angew Chem Int Ed. 2008;47:9961.Cundari TR, Dinescu A, Kazi AB. Inorg Chem. 2008;47:10067. doi: 10.1021/ic801337f.
- 23.For reports of related bicyclo[4.2.0] nonadienes, see: Scholes G, Graham GR, Brookhart M. J Am Chem Soc. 1974;96:5665.Paquette LA, Photis JM. J Am Chem Soc. 1976;98:4936.Warrener RN, Russell RA, Tan RYS. Tetrahedron Lett. 1978;19:1589.
- 24.a) Cram DJ. J Am Chem Soc. 1949;71:3863. [Google Scholar]; b) Cram DJ, Davis R. J Am Chem Soc. 1949;71:3871. [Google Scholar]; c) Cram DJ. J Am Chem Soc. 1949;71:3875. [Google Scholar]; d) Cram DJ. J Am Chem Soc. 1964;86:3767. [Google Scholar]
- 25.a) Olah GA, Head NJ, Rasul G, Prakash GKS. J Am Chem Soc. 1995;117:875. [Google Scholar]; b) del Río E, Menéndez MI, López R, Sordo TL. J Am Chem Soc. 2001;123:5064. doi: 10.1021/ja0039132. [DOI] [PubMed] [Google Scholar]; c) Manet I, Monti S, Grabner G, Protti S, Dondi D, Dichiarante V, Fagnoni M, Albini A. Chem Eur J. 2008;14:1029. doi: 10.1002/chem.200701043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.