Abstract
We determined the inhibitory effect of dietary atorvastatin, dietary celecoxib and voluntary running wheel exercise (RW) alone or in combination on the formation and growth of androgen-independent LNCaP tumors in castrated SCID mice. Male SCID mice were injected subcutaneously with androgen-dependent prostate cancer LNCaP cells. When the tumors reached a moderate size, the mice were surgically castrated and treated with atorvastatin (0.02% in the diet), celecoxib (0.05% in the diet) or RW alone or in combination for 42 days. RW or celecoxib alone had a moderate inhibitory effect on the androgen-independent growth of LNCaP tumors, but atorvastatin alone had little or no effect on tumor growth. Combinations of atorvastatin and celecoxib had a stronger inhibitory effect on the formation and growth of androgen-independent LNCaP tumors than either drug alone. A combination of RW together with atorvastatin and celecoxib had the most potent inhibitory effect on the progression of LNCaP tumors to androgen independent growth. The serum concentration of atorvastatin after two weeks of oral administration of atorvastatin was 6.1 ng/ml. The serum concentration of celecoxib after treatment with dietary celecoxib for two weeks was 1090 ng/ml. The serum concentration of atorvastatin but not that of celecoxib was substantially reduced when the two drugs were given in combination. The drug concentrations observed in our animal studies are comparable or less than those commonly found in humans treated with atorvastatin or celecoxib. Our results indicate that administration of atorvastatin and celecoxib together with voluntary exercise may be an effective strategy for the prevention of prostate cancer progression from androgen dependence to androgen independence.
Keywords: prostate cancer, androgen depletion, progression, exercise
INTRODUCTION
Prostate cancer is the second leading cause of cancer death among men in the United States (1). Early stage prostate cancer requires androgen for growth and thus responds to androgen deprivation therapy (2, 3). However, the disease progresses to an androgen-independent state and virtually all patients develop hormone-refractory disease. While chemotherapy options are available for patients with androgen-independent prostate cancer, these agents are only temporarily effective (4, 5). Therefore, effective intervention regimens including novel preventive agents and life-style changes that prevent the progression of androgen-dependent prostate cancer to androgen independence would be of great value.
A recent study found that i.p administration of atorvastatin and celecoxib in combination strongly inhibited the progression of androgen-dependent LNCaP tumors to androgen independence in severe combined immunodeficient (SCID) mice (6). In this study, we found that surgical castration of SCID mice with androgen-dependent LNCaP prostate tumors caused temporary tumor regression for about 2 weeks followed by androgen-independent growth of the tumors. Treatment of the mice with i.p injections of atorvastatin or celecoxib alone modestly suppressed the re-growth of LNCaP tumors after castration. A combination of lower doses of atorvastatin and celecoxib together had a more potent effect for inhibiting the progression and growth of LNCaP tumors to androgen independence than a higher dose of either agent alone (6). A separate study found that i.p injections of atorvastatin in combination with celecoxib more potently inhibited the growth of androgen-independent PC-3 prostate tumors in SCID mice than either agent alone (7). In accord with our animal data, recent epidemiological studies suggest that the use of statins (8–12) or non-steroidal anti-inflammatory drugs (NSAIDs) (13, 14) are associated with a reduced risk of prostate cancer.
Although earlier epidemiological studies on the association between physical activity and overall prostate cancer risk were inconclusive (15–17), recent studies suggest that physical exercise is associated with reduced risk of advanced prostate cancer and prostate cancer death (18–21). These results are in accord with our studies that found that voluntary running wheel exercise (RW) for 63 days, starting one week before the subcutaneous injection of androgen independent PC-3 tumor cells into SCID mice, suppressed the formation and growth of these tumors (22). Mechanistic studies showed that RW inhibited proliferation as reflected by decreased mitosis, and the exercise regimen also stimulated apoptosis as reflected by increased caspase-3 (active form) expression in the tumors. RW decreased the ratio of the percent mitotic cells/ apoptotic cells in PC-3 tumors by 32%.
In the present study, we assessed the inhibitory effect of dietary atorvastatin or celecoxib alone or in combination with RW on the progression of androgen-dependent LNCaP xenograft tumors to androgen independence in SCID mice. Our study showed that RW in combination with dietary atorvastatin and celecoxib had the most potent inhibitory effect on the progression of androgen-dependent LNCaP tumors to androgen independence when compared with RW, atorvastatin or celecoxib alone, or for any of the two regimens in combination.
MATERIALS AND METHODS
Cells and reagents
LNCaP cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Atorvastatin and celecoxib were provided by the National Cancer Institute’s Repository. Matrigel was obtained from BD Biosciences (Bedford, MA). RPMI-1640 tissue culture medium, penicillin-streptomycin, L-glutamine and fetal bovine serum (FBS) were from Gibco (Grand Island, NY). LNCaP cells were maintained in RPMI-1640 culture medium containing 10% FBS that was supplemented with penicillin (100 units/ml)-streptomycin (100 µg/ml) and L-glutamine (300 µg/ml). Cultured cells were grown at 37°C in a humidified atmosphere of 5% CO2 and were passaged twice a week. Proliferating LNCaP cells at about 70% confluence were used for the animal experiment as indicated below.
Progression of androgen-dependent prostate LNCaP tumors to androgen independence in immunodeficient mice
Male SCID mice were obtained from Taconic Farms Inc. (Germantown, NY). The animals were housed in sterile filter-capped microisolator cages and were provided with sterilized 5010 rodent diet (WF Fisher & Son Inc, NJ) and water. LNCaP cells (2.5×106 cells/0.1 ml/mouse) suspended in 50% Matrigel (Collaborative Research, Bedford, MA) in RPMI 1640 medium were injected subcutaneously into the right flank of the mice. After 4–6 weeks, mice with LNCaP tumors (0.6-1.0 cm wide and 0.6-1.0 cm long) were surgically castrated to mimic antiandrogen therapy (6). Castrated mice with LNCaP tumors were treated with AIN76A diet containing 0.02% atorvastatin, AIN76A diet containing 0.05% celecoxib or RW alone or in combination. Mice treated with RW have free access to the wheel 24 h/day during the whole treatment period (42 days). The running wheels were connected with digital counters for running wheel revolutions (22). Tumor size (length×width; cm2) and body weight were measured once every third day after surgical castration. The development of androgen independence was monitored by the growth of tumors. The animal experiment was carried out under an Institutional Animal Care and Use Committee (IACUC)-approved protocol.
Serum levels of celecoxib, atorvastatin and their metabolites
Serum samples (100 µl each) were treated with 10 µl of 5% ascorbic acid before storage at −70°C. Extraction of celecoxib and atorvastatin from serum samples was done by treatment with 100 µl of 0.4 mol/L sodium phosphate buffer (pH 5), followed by shaking with 1,000 µl of methyl-tert-butyl ether. After centrifugation, the methyl-tert-butyl ether extract was transferred to another tube and evaporated to dryness. The aqueous residues were dried and consecutively extracted with 1000 µl of ethyl acetate. The ethyl acetate extract was combined with the dried methyl-tert-butyl ether extract and dried. The residue was reconstituted in 100 µl of acetonitrile/water (1:1), and the sample was centrifuged. Twenty microliters of the resulting supernatant were injected into a liquid chromatography tandem mass spectrometry system (LC-MS/MS). The absolute solvent extraction recoveries of celecoxib (1–4100 ng/mL) and atorvastatin (1–4100 ng/mL) from serum were 60% to 67%and 70% to 75%, respectively.
For drug and metabolite analysis, LC/MS was performed on a Thermo LTQ linear ion trap mass detector (ThermoFisher Scientific) interfaced with an electrospray ionization probe to a Surveyor HPLC system (Thermo Fisher Scientific) equipped with a refrigerated (4°C) autosampler. Chromatographic separation was done on a Phenomenex Gemini C18 column (50 × 2.0-mm i.d., 3 µm particle size). The LC mobile phases consisted of acetonitrile/water [10:490 (v/v)], containing 0.2 mmol/L formic acid (solvent A) and acetonitrile/water [450:50 (v/v)], containing 0.2 mmol/L formic acid (solvent B). The mobile phase was delivered at 0.2 mL/min. During 7–29 min after injection of extracted drugs in solvent B:A (20:80), the column was eluted with a linear gradient from B:A (20:80) to B:A (70:30) and then with B:A (70:30) from 29 to 34 min before re-equilibration with B:A (20:80) for 8 min before injection of the next sample. The LC eluent flow after 2 min was introduced into the mass spectrometer for data acquisition. The MS/MS parameters in the negative-ion mode were tuned to maximize the generation of deprotonated drug molecules. All data acquired was processed by Xcalibur software (version 2.0, ThermoFisher, Thermo Electron). Celecoxib and atorvastatin standards in control serum were analyzed side by side with experimental samples and were used for the calculation of serum levels. Because authentic metabolite standards were not available, we used celecoxib as a surrogate standard for the metabolites of celecoxib and atorvastatin for the metabolites of atorvastatin. Therefore, the reported levels of metabolites are estimated values. The identification of metabolites is described later.
Statistical analyses
The analyses of percent change in tumor size from baseline were based on a repeated measurement model (23). Heterogeneous autoregressive correlation was used to account for the within mice correlation. The analysis of variance (ANOVA) model was used to analyze the percent change from baseline for tumor size at day 42 (last time point). Bonferroni’s adjustment was used for comparisons of the triple treatment regimen with any of the double treatment regimens as well as the comparisons of double treatment regimens with any of the single treatment regimens (9 comparisons). ANOVA with Tukey-Kramer multiplicity adjustment was used for the comparison of body weight, food and drinking fluid consumption in different groups. An overall significance level of 5% was used for all multiple tests.
RESULTS
Inhibitory effect of dietary atorvastatin, dietary celecoxib and voluntary running wheel exercise (RW) on androgen independent growth of LNCaP tumors in castrated SCID mice
Male SCID mice were injected subcutaneously with androgen-dependent prostate cancer LNCaP cells as described in Figure 1. When the tumors reached a moderate size (about 0.6–1.0 cm wide and 0.6–1.0 cm long), the mice were assigned into 8 groups. Mice in group 1 were fed regular AIN76A diet, mice in group 2 were fed AIN76A diet containing 0.02% atorvastatin, mice in group 3 were fed AIN76A diet containing 0.05% celecoxib, mice in group 4 were fed regular AIN76A diet and placed in a cage equipped with a running wheel, mice in group 5 were placed in a cage equipped with a running wheel and fed AIN76A diet containing 0.02% atorvastatin, mice in group 6 were placed in a cage equipped with a running wheel and fed AIN76A diet containing 0.05% celecoxib, mice in group 7 were fed AIN76A diet containing both 0.02% atorvastatin and 0.05% celecoxib and mice in group 8 were placed in a cage equipped with a running wheel and fed AIN76A diet containing both 0.02% atorvastatin and 0.05% celecoxib. Each group had 5 mice except that groups 7 and 8 had 4 mice. As shown in Figure 1A, the LNCaP tumors in all groups regressed initially in response to castration, but the tumors then progressed to androgen-independence and started to grow at 2–4 weeks post-castration. Regrowth of the tumors started at 15 days post-castration in the control group and at 18 days post-castration in the atorvastatin, celecoxib or RW group. Regrowth of the tumors in the atorvastatin + celecoxib, atorvastatin + RW, celecoxib + RW or the atorvastatin + celecoxib + RW group started at 18, 21, 15 and 21 days post-castration, respectively (Fig. 1A). The time that it took for the tumors to reach their original size at the time of castration in the control, atorvastatin, celecoxib or RW groups was 24, 27, 27 or 30 days, respectively (Fig.1A). The time that it took for the tumors to reach their original size at the time of castration in the atorvastatin + celecoxib, atorvastatin + RW, celecoxib + RW or the atorvastatin + celecoxib + RW group was 33, 30, 30 and >42 days, respectively (Fig.1A). The growth rate in percent change in tumor size from baseline for the atorvastatin + celecoxib + RW group was significantly smaller than that for any other group (p≤0.0214).
Figure 1.
Effects of atorvastatin (ATOR), celecoxib (CEL) and RW alone or in combination on androgen-independent growth of LNCaP tumors and body weight of SCID mice. Male SCID mice (5 mice/group in groups 1–6; 4 mice/group in groups 7–8) were injected subcutaneously with LNCaP cells in 50% Matrigel (2.0 × 106 cells/0.1 ml). After 4–6 weeks, mice with LNCaP tumors (about 0.6–1.0 cm wide and 0.6–1.0 cm long) were surgically castrated. Castrated mice were treated with atorvastatin (0.02% in the diet), celecoxib (0.05% in the diet) or RW alone or in combination for 42 days. (A) Tumor size (length × width; cm2) was measured and expressed as percent of initial tumor size. (B) Body weights (g) were measured and expressed as percent of initial body weight.
RW or administration of celecoxib alone had a moderate inhibitory effect on the androgen-independent growth of LNCaP tumors, but administration of atorvastatin alone had little or no effect on the tumors (Fig. 1A). A combination of atorvastatin and celecoxib had a stronger inhibitory effect on the growth of androgen-independent LNCaP tumors than either treatment alone (Fig.1A). Treatment with a combination of RW together with atorvastatin and celecoxib had the most potent inhibitory effect on the androgen-independent growth of LNCaP tumors (Fig.1A). The ANOVA model with Bonferroni’s adjustment was used to compare the percent initial tumor size between different treatment groups. The percentage of initial tumor size at day 42 after treatment in the atorvastatin + celecoxib group was significantly smaller than the atorvastatin group or the celecoxib group (p≤0.0026). The percentage of initial tumor size at day 42 after treatment in the atorvastatin + RW group was significantly smaller than the atorvastatin group (p=0.0086). The percentage of initial tumor size at day 42 after treatment in the celecoxib + RW group was significantly smaller than the celecoxib group (p=0.041). The percentage of initial tumor size at day 42 after treatment in the atorvastatin + celecoxib + RW group was significantly smaller than for any of the two-regimen combination groups (p≤0.032).
The average distances ± S.E the mice ran on the running wheel were 1.31±0.22, 1.29±0.23, 1.32±0.14 and 1.28±0.26 miles/mouse/day in the RW, atorvastatin + RW, celecoxib + RW and atorvastatin + celecoxib + RW groups, respectively (Table 1). The difference in miles ran per mouse between any two groups was not statistically significant (p>0.05). The RW group consumed 25% more food and 13% more water when compared with mice in the control group (Table 1). The difference in food consumption between the atorvastatin group and the atorvastatin + RW group, between the celecoxib group and the celecoxib + RW group, and between the atorvastatin + celecoxib group and the atorvastatin + celecoxib + RW group was not statistically significant (p>0.05; Table 1). The results indicate that RW did not significantly alter the intake of atorvastatin and/or celecoxib. The effect of the various treatments on body weight is described in Figure 1B. The mean ± S.E. for the percent of initial body weight after 42 days of treatment was 87.6 ± 5.4 for the control group, 85.4 ± 4.3 for the atorvastatin group, 82. ± 5.2 for the celecoxib group, 90.3 ± 5.4 for the RW group, 86.1 ± 5.8 for the atorvastatin + celecoxib group, 88.6 ± 4.7 for the atorvastatin + RW group, 83.8 ± 5.1 for the celecoxib + RW group and 83.7 ± 4.6 for the atorvastatin + celecoxib + RW group. Statistical analysis with the Tukey-Kramer multiple comparison test showed that the difference in percent of initial body weight between any two groups was not statistically significant (p>0.05).
Table I.
Food and fluid consumption in SCID mice treated with atorvastatin, celecoxib or RW alone or in combination
| Group | Number of mice |
Food consumption (g/mouse/day) |
Fluid consumption (ml/mouse/day) |
Running distance (miles/mouse/day) |
|---|---|---|---|---|
| Control | 5 | 4.31±0.12 | 5.52±0.08 | - |
| Atorvastatin | 5 | 3.99±0.11 | 5.47±0.11 | - |
| Celecoxib | 5 | 3.86±0.12 | 5.53±0.13 | - |
| RW | 5 | 5.39±0.13 | 6.22±0.11 | 1.31±0.22 |
| Atorvastatin + RW | 5 | 4.10±0.14 | 6.03±0.14 | 1.29±0.23 |
| Celecoxib + RW | 5 | 4.02±0.11 | 5.98±0.16 | 1.32±0.14 |
| Atorvastatin + Celecoxib | 4 | 3.98±0.14 | 5.63±0.11 | - |
| Atorvastatin + Celecoxib + RW | 4 | 4.08±0.20 | 6.04±0.12 | 1.28±0.26 |
Male SCID mice were injected subcutaneously with LNCaP cells in Matrigel. After 4–6 weeks, mice with LNCaP tumors (about 0.6–1.0 cm wide and 0.6–1.0 cm long) were surgically castrated and treated with atorvastatin (0.02% in the diet), celecoxib (0.05% in the diet) and RW alone or in combination for 6 weeks. Wheel revolutions and consumption of food and fluid were recorded. Each value represents the mean ± S.E. Statistical analysis was done by using ANOVA with the Tukey-Kramer multiplicity adjustment (adjusted p-values were used in the following). For food consumption, the difference between the RW group and any other group was statistically significant (p<0.001). The differences in food consumption between the atorvastatin group and the atorvastatin + RW group, between the celecoxib group and the celecoxib + RW group, and between the atorvastatin + celecoxib group and the atorvastatin + celecoxib + RW group were not statistically significant (p>0.05). Statistically significant effects on fluid consumption were: control vs RW, p<0.01; atorvastatin vs RW, p<0.01; atorvastatin vs atorvastatin + RW, p<0.05; atorvastatin vs atorvastatin + celecoxib + RW, p<0.05; celecoxib vs RW, p<0.01; atorvastatin + celecoxib vs RW, p<0.05. For running distance, the difference between any two groups was not statistically significant (p>0.05).
Serum levels of atorvastatin and celecoxib and metabolite identification in scid mice
Serum levels
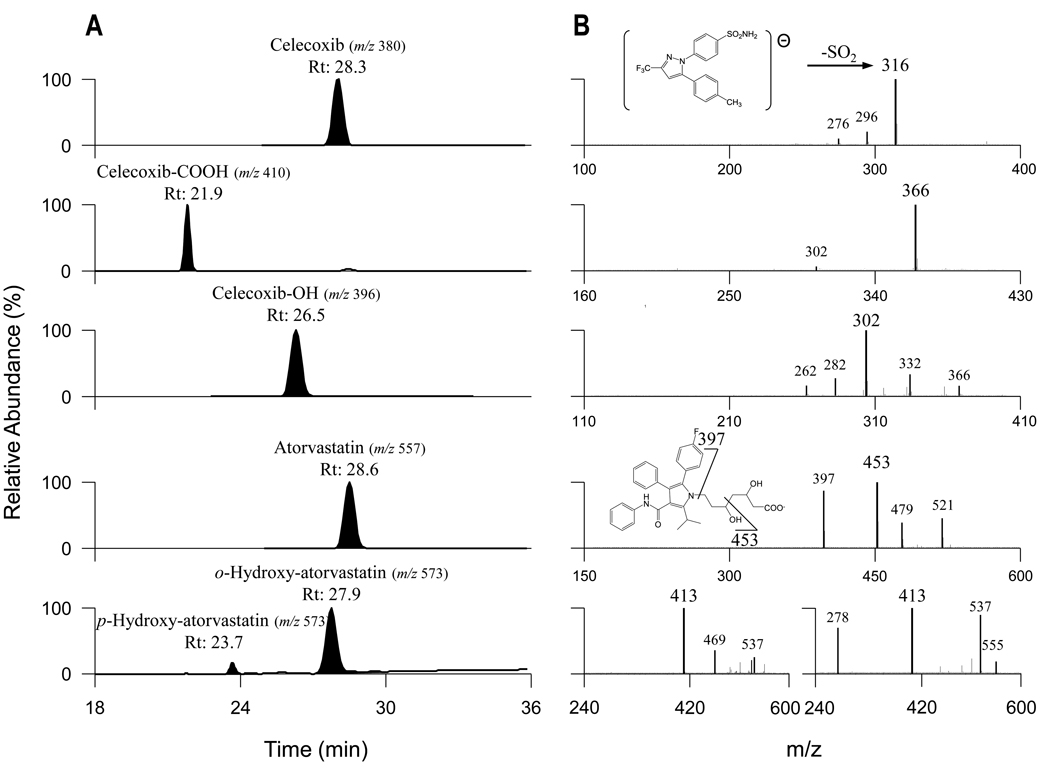
Serum levels of atorvastatin and celecoxib were determined to show the levels associated with biological activity in our animal model. The serum concentration of atorvastatin after two weeks of oral administration (0.02% in diet) was 6.1 ng/ml (Table 2). Figure 2A shows the HPLC chromatograms of serum samples after oral administration of celecoxib and atorvastatin in mice, and Figure 2B shows the fragmentation patterns of [M−H]− from celecoxib, atorvastatin, and their major metabolites. Two atorvastatin metabolites, p-hydroxy-atorvastatin and o-hydroxy-atorvastatin, were also tentatively identified and quantified (described later; structures shown in Fig. 3). As shown in Table 2, the serum level of p-hydroxy-atorvastatin was 6.28 ng/ml and that of o-hydroxy-atorvastatin was 22.6 ng/ml after two weeks of oral administration of atorvastatin (0.02% in the diet). The serum concentration of celecoxib after treatment with celecoxib (0.05% in the diet) for two weeks was 1090 ng/ml (Table 2). After two weeks of oral administration of celecoxib, the serum levels of the hydroxy-celecoxib and carboxy-celecoxib metabolites were 235 and 331 ng/ml, respectively (metabolite identification described below). In mice treated with dietary atorvastatin + celecoxib, the serum levels of atorvastatin and its metabolites were much lower than that in mice treated with atorvastatin alone while the serum levels of celecoxib and its metabolites were similar to those from mice treated with celecoxib alone (Table 2).
Table II.
Average daily level of celecoxib, atorvastatin, and their metabolites in serum from SCID mice treated with celecoxib or atorvastatin alone or in combination.
| Serum concentration (ng/ml) |
|||||||
|---|---|---|---|---|---|---|---|
| Treatment | Atorvastatin | p-Hydroxy-atorvastatin | o-Hydroxy-atorvastatin | Celecoxib | Hydroxy-celecoxib | Carboxy-celecoxib | |
| Atorvastatin | 6.12±2.26 | 6.28±2.44 | 22.6±6.9 | - | - | - | |
| Celecoxib | - | - | - | 1090.4±127.9 | 234.6±26.8 | 331.1±58.4 | |
| Atorvastatin + celecoxib | 0.45±0.30 | 2.03±1.99 | 10.3±2.5 | 963.2±69.8 | 274.8±15.8 | 323.9±52.4 | |
Male SCID mice (10/group) were fed AIN76A diet containing 0.05% celecoxib, 0.02% atorvastatin, or 0.05% celecoxib+0.02% atorvastatin for two weeks. Serum samples were collected at 7 am (5 mice) and 7 pm (5 mice) on the last day of the experiment and analyzed for the levels of celecoxib, atorvastatin, and their metabolites. Since large differences in drug levels at 7 am and 7 pm were not observed, we combined the data from all animals from each group. Values for the mean ± S.E from 10 animals in each group are shown.
Figure 2.
Celecoxib, atorvastatin, and their metabolites in mouse serum. Male SCID mice (10/group) were fed AIN76A diet containing 0.05% celecoxib, 0.02% atorvastatin, or 0.05% celecoxib+0.02% atorvastatin for two weeks. Serum samples were collected on the last day of the experiment and analyzed for the levels of celecoxib, atorvastatin, and their metabolites. (A) HPLC chromatogram of the parent compounds and metabolites. (B) MS2 spectra and structural elucidation of deprotonated ions from celecoxib, atorvastatin, and their metabolites.
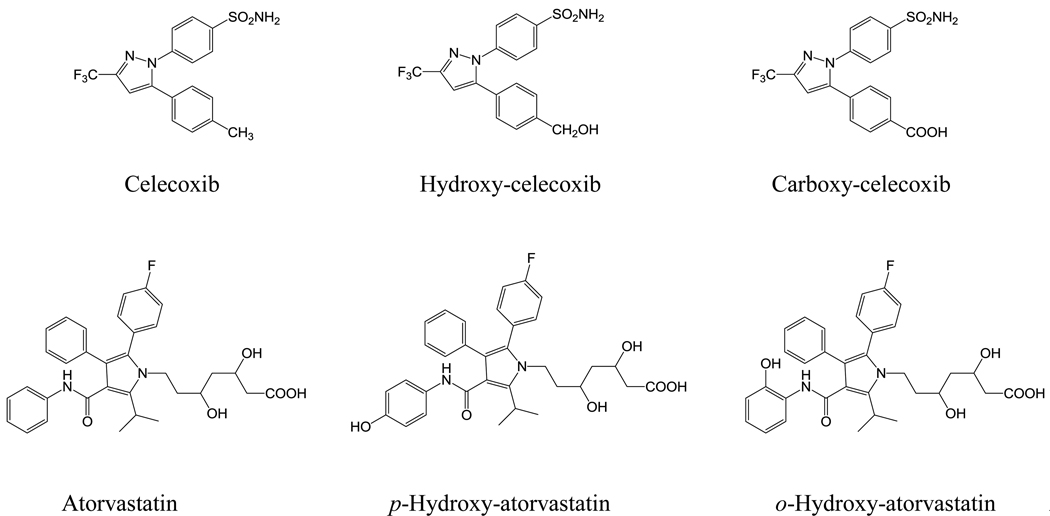
Figure 3.
Structures of atorvastatin, celecoxib and their metabolites.
Metabolite identification
To identify metabolites using LC–MS, the characterization of chromatographic and mass spectrometric properties of candidate compounds were compared to those of the parent compounds and other likely metabolites. Their fragmentation patterns were analyzed based on the MSn fragmentation of the major product ions (Fig. 2B). Moreover, the MS spectra obtained from the samples tested were compared with known control samples (parent drug) so that possible metabolites could be identified.
In this study, the negative-ion ESI mode was more sensitive for the analysis of celecoxib and atorvastatin than the positive-ion ESI mode. The deprotonated ion at m/z 380 for celecoxib with a retention time of 28.2 min generated minor product ions of m/z 296 and 276, as well as a major product ion of m/z 316, designated as the pathway shown in Figure 2B. The product ions at m/z 296 and 276 were generated by two sequential losses of 20 (HF) from the product ion at m/z 316. The product ion at m/z 316 originates from the [M−H]− ion by the loss of 64 (SO2). Two peaks eluted earlier at 21.9 and 26.5 min showed deprotonated ions of m/z 410 and 396, which were 30 and 16 Da higher than that of the parent compound celecoxib, indicating that they were carboxylated and monohydroxylated metabolites of celecoxib. The CID product ion spectrum of the ion at m/z 410 showed a minor product ion at m/z 302 ([M–H–CO2–SO2]–) and a major product ion at m/z 366 ([M–H–CO2]–). Based on these data, the metabolite was identified as carboxy-celecoxib (Fig. 3). The CID product ion spectrum of the ion at m/z 396 showed minor product ions at m/z 366 ([M–H–CH2O]−), 332 ([M–H–SO2]–), 282 ([M–H–CH2O−SO2−HF]–), and 262 ([M–H– CH2O−SO2−2HF]–), as well as a major product ion at m/z 302 ([M–H–CH2O–SO2]–). The loss of 30 (CH2O) suggested that the hydroxylation occurred on the methyl moiety of the 5-(4-methyl) phenyl group. Based on these data, the metabolite was identified as a hydroxymethyl metabolite of celecoxib (Fig. 3). Carboxy-celecoxib and hydroxy-celecoxib were shown to be the major celecoxib metabolites in our mouse serum samples, which was consistent with a previous report in rabbit blood samples (24).
The deprotonated ion at m/z 557 for atorvastatin with a retention time of 28.6 min (Fig. 2A) generated minor product ions of m/z 521 ([M–H–2H2O]−) and 479, as well as major product ions of m/z 453 and 397, designated as a pathway shown in Figure 2B. Cleavage of the side-chain produced product ions of m/z 479, 453 and 397. The ion at m/z 479 is generated by the loss of water and C2H4O2 from the molecule. The ion at m/z 453 was generated by the loss of 104 (C4H8O3). The ion at m/z 397 was generated from the cleavage of the side-chain between the pyrrole nitrogen and C-7 of the side-chain, by the loss of the heptanoic acid side-chain, C7H12O4. Two peaks eluted earlier at 23.7 and 27.9 min and both showed a deprotonated ion of m/z 573, which was 16 Da higher than that of the parent compound atorvastatin, suggesting that they were both monohydroxylated metabolites. The MS2 spectrum of the first peak displayed major product ions of m/z 413 ([M–H–C7H12O4]–) and minor product ions of m/z 469 ([M–H–C4H8O3]–), 537 ([M–H–2H2O]−), and 555 ([M–H–H2O]−). The MS2 spectrum of the second peak displayed major product ions of m/z 278 (C19H17FN–), 413 ([M–H–C7H12O4]–), and 537 ([M–H–2H2O]−, and a minor product ion of m/z 555 ([M–H–H2O]−). The mass difference between the multiple product ions at m/z 537, 469, 413 generated from the metabolites and the respective equivalent product ions at m/z 521, 453, 397 from atorvastatin was 16 Da, suggested that hydroxylation did not occur on the dihydroxyhepanoic acid moiety, and the fragmentation pathways for the metabolites were similar to that of atorvastatin. There are three possible sites for hydroxylation, ortho-, meta- and para-positions on each of the aromatic rings. Based on a previous report (25) and their retention times, our metabolites are p-hydroxy-atorvastatin and o-hydroxy-atorvastatin as shown in Figure 3. Both are pharmacologically active (25, 26).
DISCUSSION
In the present study, we found that the triple combination of RW together with dietary administration of atorvastatin and celecoxib was highly effective at inhibiting the progression and growth of androgen-dependent LNCaP prostate tumors to androgen independence in castrated SCID mice (Fig. 1A). Administration of atorvastatin and celecoxib had a stronger inhibitory effect on the growth of LNCaP tumors than either drug alone. RW significantly increased the inhibitory effect of atorvastatin or celecoxib on the growth of LNCaP tumors. Treatment with RW + atorvastatin + celecoxib had the most potent inhibitory effect on the formation and growth of androgen-independent LNCaP tumors (Fig.1A). We anticipate that this triple treatment regimen will also be effective at inhibiting the formation and growth of other cancers. To the best of our knowledge, the present study is the first example of the use of two drugs and exercise in combination for cancer prevention.
Earlier studies have shown that administration of a combination of atorvastatin and celecoxib was more effective than atorvastatin or celecoxib alone for inhibiting the formation of azoxymethane-induced colon cancer in rats (27), the growth of androgen-independent PC-3 tumors in SCID mice (7), and the progression and growth of androgen-dependent LNCaP tumors to androgen independence in castrated SCID mice (6). In earlier studies, RW inhibited the formation of chemically-induced colon and breast cancer in rats (28–30), UVB-induced skin cancer in mice (31) as well as the formation and growth of androgen-independent PC-3 prostate tumors and Panc-1 pancreatic tumors growing as xenografts in SCID mice (22). In one of these studies, exercise enhanced apoptosis in the tumors (22). The available evidence suggests that voluntary exercise has an anti-inflammatory effect (32, 33).
In the present study, we found that oral administration of 0.02% atorvastatin in AIN76A diet to male SCID mice for two weeks resulted in a serum concentration of 6.1 ng/ml (Table 2). An earlier study showed that oral administration of atorvastatin (20 mg) in humans resulted in a peak plasma level of ~ 7 ng/ml (34). After oral administration of atorvastatin (20 mg) once a day for 14 days to humans, the peak plasma level was 15 ng/ml (35). It was also reported that oral administration of celecoxib (200 mg) to humans resulted in a peak plasma level of 600–1300 ng/ml (36). In the present study, oral administration of celecoxib (0.05% in AIN76A rodent diet) for two weeks in male SCID mice resulted in a plasma level of 1090 ng/ml. The dramatic lowering of the serum level of atorvastatin and the somewhat smaller lowering of the levels of its metabolites in mice that received celecoxib in combination with atorvastatin for two weeks compared with atorvastatin alone suggests that celecoxib administration enhanced the metabolism of atorvastatin and its metabolites. The serum levels of celecoxib and atorvastatin in the present study in male SCID mice were similar or lower than those observed in humans. Our results indicate that the serum levels of atorvastatin and celecoxib associated with preventive efficacy on the progression of prostate tumors to androgen independence in the SCID mouse model are achievable in humans.
In summary, the results of the present study demonstrate that the triple combination of RW combined with oral administration of atorvastatin and celecoxib (regimens that likely work by different mechanisms) has a potent inhibitory effect on the progression and growth of androgen-dependent prostate tumors to androgen independence in a xenograft model in SCID mice. The serum levels of atorvastatin and celecoxib in the present study were similar or lower than the levels obtained in patients taking these drugs. The results of our study suggest a clinical trial to determine the effect of a combination of exercise, atorvastatin and celecoxib on the progression and growth of androgen-dependent prostate tumors to androgen independence in prostate cancer patients as well as to determine the effect of the triple regimen on the progression and growth of other cancers.
Acknowledgments
The present study was supported by NIH grants CA121391 and CA133902. The authors thank Ms. Annette Dionisio for her excellent help in the preparation of this manuscript.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 3.Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, Middleton R, Sharp SA, Smith TJ, Talcott J, Taplin M, Vogelzang NJ, Wade JL, 3rd, Bennett CL, Scher HI. American Society of Clinical Oncology. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol 20. 2007;25(12):1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 4.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Me. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Zheng X, Cui XX, Gao Z, Zhao Y, Lin Y, Shih WJ, Huang MT, Liu Y, Rabson A, Reddy B, Yang CS, Conney AH. Atorvastatin and celecoxib in combination inhibits the progression of androgen-dependent LNCaP xenograft prostate tumors to androgen independence. Cancer Prev Res (Phila Pa) 2010;3(1):114–124. doi: 10.1158/1940-6207.CAPR-09-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng X, Cui XX, Avila GE, Huang MT, Liu Y, Patel J, Kong AN, Paulino R, Shih WJ, Lin Y, Rabson AB, Reddy BS, Conney AH. Atorvastatin and celecoxib inhibit prostate PC-3 tumors in immunodeficient mice. Clin Cancer Res. 2007;13 (18 Pt 1):5480–5487. doi: 10.1158/1078-0432.CCR-07-0242. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2213–2217. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton RJ, Banez LL, Aronson WJ, Terris MK, Platz EA, Kane CJ, Presti JC, Jr, Amling CL, Freedland SJ. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2010;116(14):3389–3398. doi: 10.1002/cncr.25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murtola T, Tammela TLJ, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidemiol Biomarkers Pre. 2007;16(11):2226–2232. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 11.Platz EA, Leitzmann MF, Visvanathan K, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98(24):1819–1825. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 12.Bañez LL, Klink JC, Jayachandran J, Lark AL, Gerber L, Hamilton RJ, Masko EM, Vollmer RT, Freedland SJ. Association between statins and prostate tumor inflammatory infiltrate in men undergoing radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2010;19(3):722–728. doi: 10.1158/1055-9965.EPI-09-1074. [DOI] [PubMed] [Google Scholar]
- 13.Cheng I, Liu X, Plummer SJ, Krumroy LM, Casey G, Witte JS. COX2 genetic variation, NSAIDs, and advanced prostate cancer risk. Br J Cancer. 2007;97(4):557–561. doi: 10.1038/sj.bjc.6603874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stock D, Groome PA, Siemens DR. Inflammation and prostate cancer: a future target for prevention and therapy? Urol Clin North Am. 2008;35(1):117–130. doi: 10.1016/j.ucl.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Cerhan JR, Torner JC, Lynch CF, et al. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States) Cancer Causes and Control. 1997;8:229–238. doi: 10.1023/a:1018428531619. [DOI] [PubMed] [Google Scholar]
- 16.Wannamethee SG, Shaper AG, Walker M, et al. Physical activity and risk of cancer in middle-aged men. British Journal of Cancer. 2001;85:1311–1316. doi: 10.1054/bjoc.2001.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedenreich CM, McGregor SE, Courneya KS, Angyalfi SJ, Elliott FG, et al. Case-control study of lifetime total physical activity and prostate cancer risk. American Journal of Epidemiology. 2004;159:740–749. doi: 10.1093/aje/kwh106. [DOI] [PubMed] [Google Scholar]
- 18.Patel AV, Rodriguez C, Jacobs EJ, Solomon L, Thun MJ, Calle EE. Recreational physical activity and risk of prostate cancer in a large cohort of U.S. men. Cancer Epidemiol Biomarkers Prev. 2005;14(1):275–279. [PubMed] [Google Scholar]
- 19.Nilsen TI, Romundstad PR, Vatten LJ. Recreational physical activity and risk of prostate cancer: A prospective population-based study in Norway (the HUNT study) Int J Cancer. 2006;119(12):2943–2947. doi: 10.1002/ijc.22184. [DOI] [PubMed] [Google Scholar]
- 20.Orsini N, Bellocco R, Bottai M, Pagano M, Andersson SO, Johansson JE, Giovannucci E, Wolk A. A prospective study of lifetime physical activity and prostate cancer incidence and mortality. Br J Cancer. 2009;101(11):1932–1938. doi: 10.1038/sj.bjc.6605404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonelli JA, Jones LW, Bañez LL, Thomas JA, Anderson K, Taylor LA, Gerber L, Anderson T, Hoyo C, Grant D, Freedland SJ. Exercise and prostate cancer risk in a cohort of veterans undergoing prostate needle biopsy. J Urol. 2009;182(5):2226–2231. doi: 10.1016/j.juro.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Zheng X, Cui XX, Huang MT, Liu Y, Shih WJ, Lin Y, Lu YP, Wagner GC, Conney AH. Inhibitory effect of voluntary running wheel exercise on the growth of human pancreatic Panc-1 and prostate PC-3 xenograft tumors in immunodeficient mice. Oncol Rep. 2008;19(6):1583–1588. [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsey JK. Models for Repeated Measurments. Oxford: Claredon Press; 1993. [Google Scholar]
- 24.Zhang JY, Wang Y, Dudkowski C, Yang D, Chang M, Yuan J, Paulson SK, Breau AP. Characterization of metabolites of Celecoxib in rabbits by liquid chromatography/tandem mass spectrometry. J Mass Spectrom. 2000;35(11):1259–1270. doi: 10.1002/1096-9888(200011)35:11<1259::AID-JMS57>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Black AE, Hayes RN, Roth BD, Woo P, Woolf TF. Metabolism and excretion of atorvastatin in rats and dogs. Drug Metab Dispos. 1999;27(8):916–923. [PubMed] [Google Scholar]
- 26.Lins RL, Matthys KE, Verpooten GA, Peeters PC, Dratwa M, Stolear JC, Lameire NH. Pharmacokinetics of atorvastatin and its metabolites after single and multiple dosing in hypercholesterolaemic haemodialysis patients. Nephrol Dial Transplant. 2003;18(5):967–976. doi: 10.1093/ndt/gfg048. [DOI] [PubMed] [Google Scholar]
- 27.Reddy BS, Wang CX, Kong AN, Khor TO, Zheng X, Steele VE, Kopelovich L, Rao CV. Prevention of azoxymethane-induced colon cancer by combination of low doses of atorvastatin, aspirin, and celecoxib in F 344 rats. Cancer Res. 2006;66(8):4542–4546. doi: 10.1158/0008-5472.CAN-05-4428. PubMed PMID: 16618783. [DOI] [PubMed] [Google Scholar]
- 28.Reddy BS, Sugie S, Lowenfels A. Effect of voluntary exercise on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Res. 1988;48:7079–7081. [PubMed] [Google Scholar]
- 29.Cohen LA, Choi K, Backlund JY, Harris R, Wang CX. Modulation of N-nitrosomethylurea induced mammary tumorigenesis by dietary fat and voluntary exercise. In Vivo. 1991;5:333–344. [PubMed] [Google Scholar]
- 30.Thompson HJ. Effects of physical activity and exercise on experimentally-induced mammary carcinogenesis. Breast Cancer Res Treat. 1997;46:135–141. doi: 10.1023/a:1005912527064. [DOI] [PubMed] [Google Scholar]
- 31.Michna L, Wagner GC, Lou YR, Xie JG, Peng QY, Lin Y, Carlson K, Shih WJ, Conney AH, Lu YP. Inhibitory effects of voluntary running wheel exercise on UVB-induced skin carcinogenesis in SKH-1 mice. Carcinogenesis. 2006;27:2108–2115. doi: 10.1093/carcin/bgl057. [DOI] [PubMed] [Google Scholar]
- 32.Beavers KM, Brinkley TE, Nicklas BJ. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010 Feb 25; doi: 10.1016/j.cca.2010.02.069. [Epub ahead of print] PubMed PMID: 20188719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. 2008 doi: 10.1155/2008/109502. 2008:109502. Epub 2009 Jan 11. Review. PubMed PMID: 19148295; PubMed Central PMCID: PMC2615833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cilla DD, Jr, Whitfield LR, Gibson DM, Sedman AJ, Posvar EL. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of atorvastatin, an inhibitor of HMG-CoA reductase, in healthy subjects. Clin Pharmacol Ther. 1996;60(6):687–695. doi: 10.1016/S0009-9236(96)90218-0. [DOI] [PubMed] [Google Scholar]
- 35.Lennernas H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003;42(13):1141–1160. doi: 10.2165/00003088-200342130-00005. [DOI] [PubMed] [Google Scholar]
- 36.Davies NM, McLachlan AJ, Day RO, Williams KM. Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet. 2000;38(3):225–242. doi: 10.2165/00003088-200038030-00003. [DOI] [PubMed] [Google Scholar]