Abstract
Objective
To estimate the incidence of ADEs associated with health care visits among US adults across all ambulatory settings.
Data Source
We analyzed data from two nationally representative probability sample surveys: the National Ambulatory Medical Care Survey (NAMCS) and the National Hospital and Ambulatory Medical Care Survey (NHAMCS). From 2005–2007, the presence of an ADE was specifically defined, requested and recorded in these surveys.
Study Design
Secondary data analysis.
Principal Findings
An estimated 13.5 million ADE-related visits occurred between 2005–2007 (0.5% of all visits), the large majority (72%) occurring in outpatient practice settings, and the remaining in emergency departments. Older patients (age ≥ 65 years) had the highest age-specific ADE rate, 3.8 ADEs per 10,000 persons per year. In adjusted analyses of outpatient visits, there was an increased odds of an ADE-related visit with increased medication burden (OR for 6–8 medications compared to no medications, (OR 3.83 (2.20, 6.65)), and increased odds of ADEs associated with primary care visits compared to specialty visits (OR 2.22 (1.70, 2.89)).
Conclusions
Approximately 4.5 million ambulatory visits related to adverse drug events occur each year, the majority of these in outpatient office practices. A greater focus on ADE prevention and detection is warranted among patients receiving multiple medications in primary care practices.
Keywords: Patient Safety, Adverse Drug Events, Chronic Illness
Introduction
Ensuring patient safety is a major public health challenge. According to the Institute of Medicine (IOM), in the US, as many as 98,000 deaths per year are attributable to preventable adverse events that occur in the hospital setting, with annual costs (lost income, disability, and health care costs) of between $17 billion and $29 billion(Institute of Medicine 2000). Because the patient safety movement originated in and has focused on acute care settings (Institute of Medicine 2000), less is known about safety outside the hospital setting(Sarkar et al. 2009; Wachter 2006).
Adverse drug events (ADEs), defined as injuries resulting from a medication taken for medical intervention, (Bates et al. 1995; Bourgeois et al. 2009; Gurwitz et al. 2003) constitute an important aspect of patient safety. Not all ADEs are preventable or can be considered medical errors; nevertheless, detection and prevention of ADEs is central to improving safety. Several studies have reported high rates of ADEs among specific populations, such as elderly patients(Gurwitz et al. 2003) and those with chronic diseases,(Zhang et al. 2007) but important gaps in our current understanding of ambulatory ADEs remain. Apart from studies focused exclusively on emergency departments (EDs), (Budnitz et al. 2006; Budnitz et al. 2007) national population estimates for ADEs in the United States are lacking.
Accordingly, we analyzed data from the National Center for Health Statistics (NCHS) to describe the frequency and distribution of ambulatory ADEs among US adults, to estimate age-specific rates for ADEs. In addition, we explored which medication classes are most commonly reported in ADE visits, and whether demographic and clinical characteristics were associated with ADE visits.
Methods
Data Sources
We employed 2005–2007 data from the NCHS, examining the National Ambulatory Medical Care Survey (NAMCS) with the National Hospital and Ambulatory Medical Care Survey (NHAMCS). NAMCS and NHAMCS data collection and process is carried out by the United States Census Bureau. The NAMCS is an annual probability survey and is designed to generate nationally representative estimates of nonfederal, office-based physicians providing direct patient care in the 50 states or District of Columbia, excluding radiologists, anesthesiologists, and pathologists. Similarly, the NHAMCS is also a nationally representative, multi-stage probability-sample of outpatient visits hospital-affiliated outpatient departments (OPD), and EDs. For NAMCS, the data collection is carried out by physicians, who are randomly assigned to 1 of 52 weeks in a year and report information on a systematic random sample of patients treated during that week. Census Bureau representatives are on site to give instructions on how to complete each survey item. For NHAMCS, hospital staff, in conjunction with Census Bureau representatives, conduct data collection using similar systematic random sampling. NHAMCS has two versions, one for the ED and one for the OPD. Details on the sampling and estimation process for both surveys are available at NCHS’s website (http://www.cdc.gov/nchs/). For the ADE question providers are instructed to Mark “Adverse effect of medical/surgical care or adverse effect of medicinal drug if the visit was due to any type of injury, poisoning, or adverse effect of medical treatment.” (Center for Disease Control) We applied techniques utilized by prior studies to merge these data sets (Bourgeois et al. 2009; Burris, and Werler; Schappert, and Rechtsteiner 2008). Visit-level data includes geographic region of Northeast, South, Midwest, West; outpatient versus ED; and primary care vs. non-primary care visit (for outpatient visits only). We defined primary care visits as those visits in which providers responded affirmatively to the item: “Are you the patient’s primary care provider?”. Patient-level data includes socio-demographics (age, race/ethnicity, gender, insurance status); ≤ 3 reasons for visit and symptom manifestations (coded by NCHS classification); and diagnoses (classified by ICD-9 codes). Up to 8 medications initiated or continued at the visit (classified with Lexicon Plus®), and co-morbidity data are captured via the patient record form for outpatient visits only. We restricted analysis to 2005–2007 because these survey years included a specific question about adverse events. We further restricted our analysis to adult visits (≥ 18 years) because pediatric ADEs have been previously described using these data sources.(Bourgeois et al. 2009)
ADE Identification
The question “Is this visit related to: adverse effect of medical/surgical care or adverse effect of medicinal drug?” was used to identify candidate ADE visits. We believe surgical/procedural complications to arise from substantively different causes and to require different approaches for prevention. Therefore, we chose to focus only on adverse drug events. In order to accurately identify ADE-related visits, 2 physician-reviewers (U.S., R.G.) reviewed the diagnostic and reason-for-visit (RFV) codes for all candidate visits. Visits with the RFV code 5905.0, “adverse effect of medication,” which includes allergy to medication, anaphylactic shock, and bad reaction to prescribed medication, were included, as were ICD-9 codes 960–979 which include poisoning by antibiotics, poisoning by sedatives and hypnotics, and poisoning by agents primarily affecting the cardiovascular system as well as 995.2 unspecified adverse effect of unspecified drug, medicinal and biological substance. We also selected previously used ICD-9 codes (Bourgeois et al. 2009) 692.3 dermatitis due to drugs and medications in contact with skin, 693.0, dermatitis due to drugs & medications taken internally, 292.1–292.9 drug induced mental disorders, 708.0 allergic uticaria, 357.6 polyneuropathy due to drugs, 995.0 other anaphylactic shock including allergic shock, anaphylactic reaction or due to adverse effect of correct medicinal substance. In all cases, the reviewers agreed which codes represented ADE visits and should be included. The resulting ADE visits constituted our sample.
Analysis
First, we calculated national estimates of ADE visits using NCHS-provided probability weights. To improve the reliability of the estimates we pooled the data across 2005–2007. Second, to create age-specific annual ADE rates, we divided the 2005–2007 age-stratified estimates of ADE visits by the US age-specific population counts extrapolated by the US Census for 2005–2007(Population Division United States Census Bureau 2008). We were unable to calculate the annual ADE rate for 18–24 year olds because 2005–2007 population extrapolations were not available for this age group. Third, because the majority of events occurred in outpatient settings rather than EDs, we explored possible associations between visit characteristics and patient characteristics and the odds of an ADE visit compared to a non-ADE visit. To identify possible risk factors for ADE visits, we conceptualized ADE risk using a modified version of the Chronic Care Model(Wagner 1998), as previously described(Sarkar et al. 2009). We expected that patient characteristics as well as provider and health system characteristics would affect likelihood of ADEs and aimed to include all available possible predictors. Therefore, we conducted a sequential multivariate analysis, first adjusting for age, sex, race/ethnicity, insurance status, and geographic region (Base Model), seeking to adjust for basic demographic and community factors. We then included practice setting, comparing primary care visits to other visits, as this provides some insight about the role of the health system. Next we added the presence of 1 or more co-morbidities to the model, and finally we added number of medications continued and newly prescribed, both of which suggest the clinical complexity of the individual (Full Model). Analyses were conducted with SAS version 9.2 (Cary, NC) and Sudaan, version 10.0 (Research Triangle, NC).
Results
An estimated 13.5 million ADE-related visits occurred during 2005–2007, or approximately 4.5 million ADE visits per year. ADE visits constituted a significant proportion, 0.5%, of all ambulatory visits. Most of these (9,741,031, 72%) occurred in outpatient practice settings and 28% (3,783,643) in EDs. Among the outpatient visits, 60% occurred in primary care visits (Table 1) and 40% in subspecialty practices.
Table 1.
National Estimates and Demographics Characteristics of Adult Patients with ADEs Treated in US Outpatient Clinics and Emergency Departments 2005–2007
| TOTAL* N (95% CI)-column percent |
Outpatient Clinics† N (95% CI)-column percent |
Emergency Department‡ N (95% CI)-column percent |
|
|---|---|---|---|
| Age, years | |||
| 18–24 | 990,491 (741,263; 1,239,719) 7.3% | 465,452 (233,784; 697,120) 4.8% | 525,039 (409,095; 640,983) 13.9% |
| 25–44 | 3,373,083 (2,673,902; 4,072,264) 24.9% | 2,112,537 (1,452,685; 2,772,389) 21.7% | 1,260,546 (1,017,349; 1,503,743) 33.3% |
| 45–64 | 4,889,476 (3,893,904; 5,885,048) 36.2% | 3,639,759 (2,690,664; 4,588,854) 37.4% | 1,249,717 (1,059,922; 1,439,512) 33.0% |
| 65–74 | 2,248,632 (1,557,269; 2,939,995) 16.6% | 1,854,730 (1,171,639; 2,537,821) 19.0% | 393,902 (285,392; 502,412) 10.4% |
| 75+ | 2,022,992 (1,394,202; 2,651,782) 15.0% | 1,668,553 (1,053,223; 2,283,883) 17.1% | 354,439 (262,927; 445,951) 9.4% |
| Gender | |||
| Female | 8,624,879 (7,294,741; 9,955,017) 63.7% | 6,379,450 (5,114,099; 7,644,801) 65.5% | 2,245,429 (1,931,139; 2,559,719) 59.3% |
| Male | 4,899,795 (3,816,438; 5,983,152) 36.2% | 3,361,581 (2,328,044; 4,395,938) 34.5% | 1,538,214 (1,289,298; 1,787,130) 40.7% |
| Race/Ethnicity | |||
| Non-Hispanic white | 10,249,183 (8,619,880; 11,878,486) 75.8% | 7,598,409 (6,042,880; 9,153,938) 78.0% | 2,650,774 (2,292,116; 3,009,432) 70.1% |
| Non-Hispanic black | 1,224,056 (795,053; 1,653,059) 9.1% | 671,151 (284,835; 1,057,467) 6.9% | 552,905 (414,482; 691,328) 14.6% |
| Hispanic | 1,720,728 (1,053,993; 2,387,463) 12.7% | 1,312,440 (653,486; 1,971,394) 13.5% | 408,288 (304,769; 511,807) 10.8% |
| Other | 330,707 (163,868; 497,546) 2.4% | 159,031§ (18,199; 299,863) 1.6% | 171,676 (78,376; 264,976) 4.5% |
| Practice setting†† | |||
| Primary care visit | 5,891,337 (4,581,685; 7,200,989) 60.5% | ||
| Non-primary care visit | 3,849,694 (2,949,327; 4,750,061) 39.5% | ||
| Insurance | |||
| Private insurance | 6,150,088 (5,035,534; 7,264,642) 45.4% | 4,923,771 (3,860,034; 5,987,508) 50.5% | 1,226,317 (1,012,407; 1,440,227) 32.4% |
| Medicare | 3,763,312 (2,808,692; 4,717,932) 27.8% | 2,867,375 (1,925,971; 3,808,779) 29.4% | 895,937 (728,261; 1,063,613) 23.7% |
| Medicaid | 1,833,343 (1,166,792; 2,499,894) 13.6% | 1,122,134 (472,271; 1,771,997) 11.5% | 711,209 (566,739; 855,679) 18.8% |
| Self-Pay+ charity | 742,941 (564,630; 921,252) 5.5% | 140,582 § (50,616; 230,548) 1.4% | 602,359 (467,852; 736,866) 15.9% |
| Other | 218,951 (105,594; 332,308) 1.6% | 103,514 § (−2,291; 209,319) 1.1% | 115,437 (69,540; 161,334) 3.1% |
| Unknown Source | 816,039 (428,500; 1,203,578) 6.0% | 583,655 (214,673; 952,637) 6.0% | 232,384 (154,270; 310,498) 6.1% |
| Geographical Region | |||
| Northeast | 2,675,425 (1,934,410; 3,416,440) 19.8% | 2,083,732 (1,379,751; 2,787,713) 21.4% | 591,693 (477,531; 705,855) 15.6% |
| Midwest | 2,925,425 (2,197,789; 3,653,823) 21.6% | 2,069,988 (1,371,828; 2,768,148) 21.3% | 855,818 (671,296; 1,040,340) 22.6% |
| South | 4,599,629 (3,464,236; 5,735,022) 34.0% | 3,296,652 (2,299,200; 4,294,104) 33.8% | 1,302,977 (1,032,693; 1,573,261) 34.4% |
| West | 3,323,814 (2,345,605; 4,302,023) 24.6% | 2,290,659 (1,328,068; 3,253,250) 23.5% | 1,033,155 (694,120; 1,372,190) 27.3% |
| Comorbidities | |||
| (NEW+Continued) Medication categories†† | |||
| 0 | 894,680 (479,325; 1,310,035) 9.2% | ||
| 1–3 | 4,734,169 (3,573,334; 5,895,004) 48.6% | ||
| 4–5 | 1,697,979 (1,076,051; 2,319,907) 17.4% | ||
| 6–8 | 2,414,203 (1,668,717; 3,159,689) 24.8% | ||
| Total | 13,524,674 (11,701,300; 15,348,048) 0.5%** | 9,741,031 (8,036,770; 11,445,292) 0.4% | 3,783,643 (3,299,115; 4,268,171) 1.4%** |
Estimates based on total sample size N=1,628
Estimates based on total sample size N=479
Estimates based on total sample size N=1,149
<30 visits-estimate not reliable
percent of total adult visits
Available only for the ambulatory non-ED visits
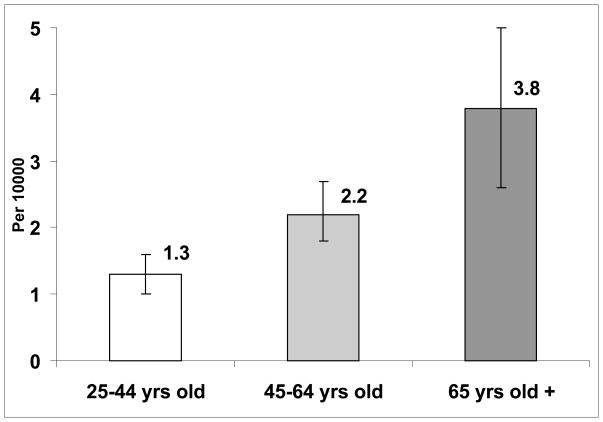
As expected, population-based ADE rates increased with age. Adults aged 25–44 years old had a rate of 1.3 per 10,000 persons per year, those 45–64 had a rate of 2.2 per 10,000 per year; and those ≥ 65 years had the highest rate, at 3.8 ADEs per 10,000 persons per year (Figure 1). For outpatient (non-ED) visits, the sequentially adjusted analyses did not reveal a consistent relationship between age, gender, race/ethnicity, or geographic region and ADE visits (Table 2). We did find that “other” race/ethnicity, representing a small proportion of the population, were less likely to have ADE visits.
Figure 1.
Estimated Annual Age-Specific Ambulatory ADE Rate
Table 2.
Multivariable Regression Analysis Examining Independent Predictors of Ambulatory Adverse Drug Event Visits. (N=148,074)
|
* Base Model Adjusted OR (95% CI) |
† Full Model Adjusted OR (95% CI) |
|
|---|---|---|
| ‡Age | ||
| 18–24 | Ref | Ref |
| 25–44 | 1.22 (0.67, 2.22) | 1.07 (0.59, 1.94) |
| 45–64 | 1.53 (0.86, 2.72) | 1.12 (0.60, 2.10) |
| 65+ | 1.67 (0.80, 3.48) | 1.19 (0.55, 2.59) |
| §Gender | ||
| Male | Ref | Ref |
| Female | 1.20 (0.85, 1.70) | 1.23 (0.87, 1.73) |
| ||Race/ethnicity | ||
| Non-Hispanic white | Ref | Ref |
| Non-Hispanic black | 0.69 (0.38, 1.24) | 0.69 (0.39, 1.22) |
| Hispanic | 1.17 (0.71, 1.93) | 1.19 (0.72, 1.95) |
| Other | 0.30 (0.12, 0.76 | 0.28 (0.11, 0.72) |
| ¶ Insurance | ||
| Private | Ref | Ref |
| Medicare | 0.94 (0.53, 1.65) | 0.88 (0.50, 1.54) |
| Medicaid | 1.12 (0.63, 1.98) | 0.98 (0.56, 1.72) |
| Self-pay/charity | 0.26 (0.13, 0.52) | 0.28 (0.14, 0.55) |
| Other | 0.27 (0.10, 0.77) | 0.33 (0.12, 0.94) |
| Unknown | 1.16 (0.60, 2.24) | 1.25 (0.64, 2.44) |
| **Geographic region | ||
| Northeast | 1.17 (0.79, 1.76) | 1.21 (0.80, 1.82) |
| Midwest | 1.05 (0.69, 1.62) | 1.02 (0.68, 1.53) |
| South | Ref | Ref |
| West | 1.43 (0.89, 2.29) | 1.51 (0.94, 2.42) |
| Primary care vs Specialty care | - | 1.82 (1.40, 2.36) |
| ††Comorbidities | - | |
| 0 | - | Ref |
| ≥1 | - | 1.33 (0.90, 1.96) |
| ‡‡Medications (new & continued) | - | |
| 4a. count 0–8 | - | |
| 4b. 0 (ref) | Ref | |
| 1–3 meds | 2.80 (1.66, 4.74) | |
| 4–5 meds | 3.61 (1.92, 6.78) | |
| 6–8 meds | 3.83 (2.20, 6.65) |
Base Model: Adjusted for age, sex, race/ethnicity, insurance status, geographic region.
Full Model: Adjusted for age, sex, race/ethnicity, insurance status, geographic region, co-morbidities and new and continued medications
Age Reference: 18–24
Gender Reference: Male
Race/ethnicity Reference: Non-Hispanic, White
Insurance Reference: Private
Geographic Region Reference: South
Comorbidities Reference: None
Number of meds Reference: None
Insurance status remained associated with ADE visits even after complete adjustment. Those lacking health insurance, described as “self-pay” or “charity care”, were significantly less likely to have ADE visits compared to those with private insurance (OR 0.28. CI 0.14– 0.55, independent of other factors such as age or number of medications. Similarly, those with “other” insurance similarly experienced fewer ADE visits (OR 0.33, 95% CI 0.12–0.94).
The odds of an ADE visit was greater for primary care visits compared with specialty care visits. The addition of a comorbidity variable to this model, was significantly associated with ADE visits, but this association was primarily driven by medication number. In terms of subsequent health care utilization, 9% of individuals with ADE visits were admitted to the hospital and 22% of patients with ADE visits were scheduled for a follow-up provider visit.
Discussion
This is the first US study to utilize nationally-representative data to examine annual rates of ADEs in the ambulatory setting. We estimate that approximately 4.5 million ambulatory ADE visits occur each year, and that these visits are associated with approximately 400,000 hospitalizations annually. Among outpatient (non-emergency department) ADE visits, the factor most strongly associated with ADE visits was the number of medications recorded for the visit. Although this study cannot elucidate the mechanism by which medication burden leads to ADE visits, we suspect there are multiple contributing causes, as prior regional studies have suggested.(Woods et al. 2007) First, at the physiologic level, the use of multiple prescription and over-the-counter medications(National Center for Health Statistics 2010) increases the potential for drug-drug interactions and difficulties with self-administration(Budnitz et al. 2007; Leendertse et al. 2008). Second, multiple studies have documented the inadequacy of medication counseling in ambulatory medical visits and in pharmacy settings (Cockburn, Reid, and Sanson-Fisher 1987; Makoul, Arntson, and Schofield 1995; Richard, and Lussier 2006; Scherwitz et al. 1985; Stevenson et al. 2000; Svarstad 1974; Tarn et al. 2006; Tarn et al. 2008), Future research explicitly examining medication counseling and ADE risk are needed. Third, prior studies clearly demonstrate that patients often cannot accurately interpret or carry out medication instructions, clearly increasing potential for ADEs (Davis et al. 2006; Persell et al. 2007; Schillinger et al. 2006; Wolf et al. 2007). In-depth, real-time investigation of ambulatory ADEs would shed light on the relative contributions of these possible mechanisms.
Clearly, not all ADEs are preventable. Indeed, a baseline number of ADEs are an expected, and presumably acceptable, aspect of the risk-benefit equation in prescribing medications. However, given the substantial number of ADEs recorded in this nationally-representative sample of ambulatory health care visits, further work to determine the proportion of preventable and ameliorable events must be a priority. This will require not only systematic surveillance for ambulatory ADEs, but also investigation into underlying causes and preventability. As health information-technology becomes more widespread in ambulatory health care delivery,(Blumenthal) automated surveillance for ADEs (Gandhi et al. 2010) will become more feasible, and should be a focus of future research and quality improvement.
Because prior studies have used different ADE detection methods, it is difficult to compare their ADE rates to this visit-based data. However, Gurwitz’s study of older adults (Gurwitz et al. 2000) used multiple detection methods including patient survey and chart review, and uncovered a rate of 5% per year in those 65 and over, compared to our estimate of 3.8 per 10,000 persons per year. A similar study of adults receiving primary care found a rate of 27 per 100 patients (Gandhi et al. 2003), using a combination of patient survey and chart review. The lower rates seen in this study are expected, because all ADEs would not be expected to lead to visits. Moreover, it suggests that providers are not aware of all ambulatory ADEs, as we have found in prior work (Sarkar et al. 2008; Sarkar et al.).
In terms of individual-level ADE risk factors, our data are consistent with prior studies in the emergency department (Budnitz et al. 2006; Budnitz et al. 2007) and in studies of medication reconciliation (Pippins et al. 2008), which suggest that the number of daily medications is the most critical factor in risk of ADEs. The risks of poly-pharmacy have been extensively described, including drug-drug and drug-disease interactions as well as increasing errors in medication self-administration (Chutka et al. 1995; Colley, and Lucas 1993; Hanlon et al. 2001; Salazar, Poon, and Nair 2007).
In these data, the relationship of older age with ADE risk is complex. In consonance with prior emergency department data(Budnitz et al. 2006), older adults experience the highest rates of ADEs per population. However, the largest absolute number of ADE visits occurred among 45–64 year olds, suggesting that ADEs are a clinical and public health concern across the larger age spectrum. Moreover, our multivariate analysis of outpatient ADE visits demonstrates that after adjustment for race/ethnicity, gender and insurance status, older age is no longer significantly associated with ADE visits. Our sequential adjustment strategy further revealed that after adjustment for co-morbidities and number of medications, the effect of increasing age on ADE visits was further attenuated. While we may lack statistical power to capture an age effect, it is clear from this data that ADE prevention strategies must extend beyond geriatric populations to include a focus on medications and co-morbidities.
Surprisingly, ADEs were more likely to be reported in primary care visits, although we expected that patients with multiple medications would be seeing sub-specialists and have multiple prescribers. It is possible that ADEs were more likely to be uncovered and reported by primary care providers than at sub-specialty visits with a narrower focus. An alternative explanation would be that relative ease of access to primary care means that patients experiencing ADEs are more likely to present acutely to their primary care providers than subspecialists.
Among those of ‘other’ ethnicity, ADE visits were less likely. This finding is difficult to interpret in this very small and likely heterogenous group. More detailed race/ethnicity information within these national data sources, as well as more patient safety research among diverse populations, could illuminate this issue. Similarly, the lower odds of ADE visits among those lacking health insurance and those with ‘other’ insurance persisted even after adjustment for all patient and visit characteristics. Although the ‘other’ insurance category is no doubt heterogenous, it is likely to represent under-insurance, including catastrophic health insurance, as most public and private insurance types were separately categorized. As such, we can infer that uninsured and under-insured patients, even when chronically ill and taking multiple medications, may be less likely to seek medical care when they experience ADEs because of costs and access constraints, particularly in non-emergency department settings(McWilliams et al. 2007).
Several limitations of the study should be noted. First, we have only captured ADEs that led to health care utilization; prior studies that surveyed patients would suggest that this underestimates ambulatory ADEs(Gandhi et al. 2000; Gandhi et al. 2003; Gurwitz et al. 2003; Sarkar et al. 2008). Second, use of a large national survey, which has the strength of allowing for reliable national estimates, contains limited data for each visit. From the NAMCS/NHAMCS questionnaire, we cannot determine whether the ADE was the primary reason for the visit, and they do not permit attribution of the ADE to a specific medication or treatment. Moreover, the survey has limited medication information. A maximum of 8 medications can be included, and this likely underestimates the influence of poly-pharmacy on ADE visits. Medications discontinued at the visits are also not captured. We recommend that national surveys consider collecting more comprehensive ADE and medication information to help to fully illuminate the factors involved. Third, in using these estimates to calculate population rates for ADE visits, we cannot account for multiple ADE visits by the same individuals. Finally, our multivariate model does not elucidate underlying causes of ADE visits. Instead, we aimed to identify factors associated with ADE visits in order to characterize those at increased risk, with the goal of devising and testing strategies to prevent and ameliorate ambulatory ADEs. Despite these limitations, these are the first available national estimates for the burden of ADEs in ambulatory health-care settings.
In this analysis, nearly one-third of ADE visits were associated with subsequent health care utilization (compared to 15% of visits overall), with 9% associated with hospitalization. In addition to the harm to patients, ambulatory ADEs are costly to the health care system. A prior study used data from a single academic health care system to estimate that charges for individuals experiencing ambulatory ADEs were $926 more than individual receiving ambulatory care with no ADEs(Burton et al. 2007). The current data should better inform national cost estimates, and certainly underscores the importance of preventing and ameliorating ambulatory ADEs.
We found that ADEs confer a significant burden on ambulatory health systems, and suggest that the consequences and costs of ADEs in ambulatory settings may be comparable to or even greater than those in the inpatient and acute care setting, making ambulatory research and safety promotion all the more pressing.
Acknowledgments
Dr. Sarkar is supported by Agency for Healthcare Research and Quality K08 HS017594, and Dr Gonzales by the National Center for Research Resources. (KL2 RR024130). This project was supported, in part, by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. We thank Dr. Steven Schroeder for his early advice on this project. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. None of the funders had any role in design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
References
- Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. Jama. 1995;274(1):29–34. [PubMed] [Google Scholar]
- Blumenthal D. Launching HITECH. N Engl J Med. 362(5):382–5. doi: 10.1056/NEJMp0912825. [DOI] [PubMed] [Google Scholar]
- Bourgeois FT, Mandl KD, Valim C, Shannon M. Pediatric Adverse Drug Events in the Outpatient Setting: An 11-Year National Analysis. Pediatrics. 2009;124:744–50. doi: 10.1542/peds.2008-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. Jama. 2006;296(15):1858–66. doi: 10.1001/jama.296.15.1858. [DOI] [PubMed] [Google Scholar]
- Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147(11):755–65. doi: 10.7326/0003-4819-147-11-200712040-00006. [DOI] [PubMed] [Google Scholar]
- Burris HH, Werler MM. U.S. Provider Reported Folic Acid or Multivitamin Ordering for Non-Pregnant Women of Childbearing Age: NAMCS and NHAMCS, 2005–2006. Matern Child Health J. doi: 10.1007/s10995-010-0587-6. [DOI] [PubMed] [Google Scholar]
- Burton MM, Hope C, Murray MD, Hui S, Overhage JM. The cost of adverse drug events in ambulatory care. AMIA Annu Symp Proc. 2007:90–3. [PubMed] [Google Scholar]
- Center for Disease Control. [accessed on 08/02/2010];NAMCS/NHAMCS Data Collection and Processing. Available at: http://www.cdc.gov/nchs/ahcd/ahcd_data_collection.htm#namcs_collection.
- Chutka DS, Evans JM, Fleming KC, Mikkelson KG. Symposium on geriatrics--Part I: Drug prescribing for elderly patients. Mayo Clin Proc. 1995;70(7):685–93. doi: 10.4065/70.7.685. [DOI] [PubMed] [Google Scholar]
- Cockburn J, Reid AL, Sanson-Fisher RW. The process and content of general-practice consultations that involve prescription of antibiotic agents. Med J Aust. 1987;147(7):321–4. [PubMed] [Google Scholar]
- Colley CA, Lucas LM. Polypharmacy: the cure becomes the disease. J Gen Intern Med. 1993;8(5):278–83. doi: 10.1007/BF02600099. [DOI] [PubMed] [Google Scholar]
- Davis TC, Fredrickson DD, Potter L, Brouillette R, Bocchini AC, Williams MV, Parker RM. Patient understanding and use of oral contraceptive pills in a southern public health family planning clinic. South Med J. 2006;99(7):713–8. doi: 10.1097/01.smj.0000223734.77882.b2. [DOI] [PubMed] [Google Scholar]
- Gandhi TK, Burstin HR, Cook EF, Puopolo AL, Haas JS, Brennan TA, Bates DW. Drug complications in outpatients. J Gen Intern Med. 2000;15(3):149–54. doi: 10.1046/j.1525-1497.2000.04199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi TK, Seger AC, Overhage JM, Murray MD, Hope C, Fiskio J, Teal E, Bates DW. Outpatient Adverse Drug Events Identified by Screening Electronic Health Records. Journal of Patient Safety. 2010;6(2):91–96. doi: 10.1097/PTS.0b013e3181dcae06. [DOI] [PubMed] [Google Scholar]
- Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, Seger DL, Shu K, Federico F, Leape LL, Bates DW. Adverse drug events in ambulatory care. N Engl J Med. 2003;348(16):1556–64. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- Gurwitz JH, Field TS, Avorn J, McCormick D, Jain S, Eckler M, Benser M, Edmondson AC, Bates DW. Incidence and preventability of adverse drug events in nursing homes. Am J Med. 2000;109(2):87–94. doi: 10.1016/s0002-9343(00)00451-4. [DOI] [PubMed] [Google Scholar]
- Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, Cadoret C, Fish LS, Garber L, Kelleher M, Bates DW. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. Jama. 2003;289(9):1107–16. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- Hanlon JT, Schmader KE, Ruby CM, Weinberger M. Suboptimal prescribing in older inpatients and outpatients. J Am Geriatr Soc. 2001;49(2):200–9. doi: 10.1046/j.1532-5415.2001.49042.x. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. To Err is Human: Building A Safer Health System. Washington, DC: National Academy Press, Institute of Medicine Committee on Quality of Health Care in America; 2000. [Google Scholar]
- Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. 2008;168(17):1890–6. doi: 10.1001/archinternmed.2008.3. [DOI] [PubMed] [Google Scholar]
- Makoul G, Arntson P, Schofield T. Health promotion in primary care: physician-patient communication and decision making about prescription medications. Soc Sci Med. 1995;41(9):1241–54. doi: 10.1016/0277-9536(95)00061-b. [DOI] [PubMed] [Google Scholar]
- McWilliams JM, Meara E, Zaslavsky AM, Ayanian JZ. Use of health services by previously uninsured Medicare beneficiaries. N Engl J Med. 2007;357(2):143–53. doi: 10.1056/NEJMsa067712. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics. Health, United States, 2009: With Special Feature on Medical Technology. Hyattsville, MD: 2010. [PubMed] [Google Scholar]
- Persell SD, Osborn CY, Richard R, Skripkauskas S, Wolf MS. Limited health literacy is a barrier to medication reconciliation in ambulatory care. J Gen Intern Med. 2007;22(11):1523–6. doi: 10.1007/s11606-007-0334-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippins JR, Gandhi TK, Hamann C, Ndumele CD, Labonville SA, Diedrichsen EK, Carty MG, Karson AS, Bhan I, Coley CM, Liang CL, Turchin A, McCarthy PC, Schnipper JL. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414–22. doi: 10.1007/s11606-008-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Population Division United States Census Bureau. Annual Estimates of the Population by Sex and Five-Year Age Groups for the United States: April 1, 2000 to July 1, 2007 2008 [Google Scholar]
- Richard C, Lussier MT. Nature and frequency of exchanges on medications during primary care encounters. Patient Educ Couns. 2006;64(1–3):207–16. doi: 10.1016/j.pec.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Salazar JA, Poon I, Nair M. Clinical consequences of polypharmacy in elderly: expect the unexpected, think the unthinkable. Expert Opin Drug Saf. 2007;6(6):695–704. doi: 10.1517/14740338.6.6.695. [DOI] [PubMed] [Google Scholar]
- Sarkar U, Handley M, Gupta R, Tang A, Murphy E, Seligman HK, Shojania KG, Schillinger D. Use of an interactive, telephone-based self-management support program to identify adverse events among ambulatory diabetes patients. Journal of General Internal Medicine. 2008;23(4):459–65. doi: 10.1007/s11606-007-0398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar U, Handley MA, Gupta R, Tang A, Murphy E, Seligman HK, Shojania KG, Schillinger D. What happens between visits? Adverse and potential adverse events among a low-income, urban, ambulatory population with diabetes. Qual Saf Health Care. 19(3):223–8. doi: 10.1136/qshc.2008.029116. [DOI] [PubMed] [Google Scholar]
- Sarkar U, Wachter RM, Schroeder SA, Schillinger D. Refocusing the Lens: Patient Safety in Ambulatory Chronic Disease Care. Joint Commission Journal on Quality and Patient Safety. 2009;35(7):377–83. doi: 10.1016/s1553-7250(09)35053-9. [DOI] [PubMed] [Google Scholar]
- Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2006. Natl Health Stat Report. 2008;(8):1–29. [PubMed] [Google Scholar]
- Scherwitz L, Hennrikus D, Yusim S, Lester J, Vallbona C. Physician communication to patients regarding medications. Patient Educ Couns. 1985;7(2):121–36. doi: 10.1016/0738-3991(85)90003-5. [DOI] [PubMed] [Google Scholar]
- Schillinger D, Wang F, Rodriguez M, Bindman A, Machtinger EL. The importance of establishing regimen concordance in preventing medication errors in anticoagulant care. J Health Commun. 2006;11(6):555–67. doi: 10.1080/10810730600829874. [DOI] [PubMed] [Google Scholar]
- Stevenson FA, Barry CA, Britten N, Barber N, Bradley CP. Doctor-patient communication about drugs: the evidence for shared decision making. Soc Sci Med. 2000;50(6):829–40. doi: 10.1016/s0277-9536(99)00376-7. [DOI] [PubMed] [Google Scholar]
- Svarstad B. The Doctor-Patient Encounter: An observational study of communication and outcome. University of Wisconsin; 1974. [Google Scholar]
- Tarn DM, Heritage J, Paterniti DA, Hays RD, Kravitz RL, Wenger NS. Physician communication when prescribing new medications. Arch Intern Med. 2006;166(17):1855–62. doi: 10.1001/archinte.166.17.1855. [DOI] [PubMed] [Google Scholar]
- Tarn DM, Paterniti DA, Kravitz RL, Heritage J, Liu H, Kim S, Wenger NS. How much time does it take to prescribe a new medication? Patient Educ Couns. 2008;72(2):311–9. doi: 10.1016/j.pec.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter RM. Is ambulatory patient safety just like hospital safety, only without the “stat”? Ann Intern Med. 2006;145(7):547–9. doi: 10.7326/0003-4819-145-7-200610030-00014. [DOI] [PubMed] [Google Scholar]
- Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1(1):2–4. [PubMed] [Google Scholar]
- Wolf MS, Davis TC, Shrank W, Rapp DN, Bass PF, Connor UM, Clayman M, Parker RM. To err is human: patient misinterpretations of prescription drug label instructions. Patient Educ Couns. 2007;67(3):293–300. doi: 10.1016/j.pec.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Woods DM, Thomas EJ, Holl JL, Weiss KB, Brennan TA. Ambulatory care adverse events and preventable adverse events leading to a hospital admission. Qual Saf Health Care. 2007;16(2):127–31. doi: 10.1136/qshc.2006.021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Holman CD, Preen DB, Brameld K. Repeat adverse drug reactions causing hospitalization in older Australians: a population-based longitudinal study 1980–2003. Br J Clin Pharmacol. 2007;63(2):163–70. doi: 10.1111/j.1365-2125.2006.02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]