Abstract
The thyrotropin receptor (TSHR) exhibits elevated cAMP signaling in the basal state and becomes fully activated by thyrotropin. Previously we presented evidence that small-molecule ligands act allosterically within the transmembrane region in contrast to the orthosteric extra-cellular hormone-binding sites. Our goal in this study was to identify positions that surround the allosteric pocket and that are sensitive for inactivation of TSHR. Homology modeling combined with site-directed mutagenesis and functional characterization revealed seven mutants located in the allosteric binding site that led to a decrease of basal cAMP signaling activity. The majority of these silencing mutations, which constrain the TSHR in an inactive conformation, are found in two clusters when mapped onto the 3D structural model. We suggest that the amino acid positions identified herein are indicating locations where small-molecule antagonists, both neutral antagonists and inverse agonists, might interfere with active TSHR conformations.
Keywords: G-protein-coupled receptor (GPCR), Thyrotropin receptor, Signal transduction, Allosteric binding pocket, Antagonist, Silencing mutations
Introduction
The thyrotropin (thyroid-stimulating hormone, TSH) receptor (TSHR), the lutropin receptor (luteinizing hormone/choriogonadotropin receptor, LHCGR) and the follitropin receptor (follicle-stimulating hormone receptor, FSHR) are glycoprotein hormone receptors (GPHRs) that constitute subfamily 1C of the rhodopsin-like (family 1) G-protein-coupled receptors (GPCRs) with seven transmembrane helices (seven-transmembrane helix receptors, 7TMRs) [1]. The TSHR and the endogenous agonist TSH are pivotal proteins with respect to a variety of physiological functions including control of thyroid growth and regulation of metabolic cycles [2–4]. The TSHR is also known to be expressed in multiple extrathyroidal tissues, including bone, ovary, kidney, testis, and cells of the immune system [5–14], but the regulatory role of the TSHR in these tissues is not understood.
A functional feature of the TSHR is an elevated level of cAMP accumulation in the basal state (in absence of thyrotropin). Malfunction of the TSHR can cause non-autoimmune diseases or participate in autoimmune diseases characterized by a hyper- or hypo- functioning thyroid gland. Antibodies directed at TSHR and naturally occurring mutations in TSHR are determinants of several thyroid-malfunctions [15–26]. In general, such mutations and antibodies can be distinguished by either inactivating (loss-of-function, hypothyroidism) or activating (gain-of-function, hyperthyroidism) effects. Numerous pathogenic constitutively activating mutations (CAMs) that increase basal TSHR signaling independently from the hormone have been found [27]. The identification of antagonistic molecules that might be able to suppress constitutive activity is a special focus of TSHR research. Such compounds might have therapeutic potential for pharmacological intervention of TSHR-mediated diseases [24, 28, 29]. Several strategies to develop antagonists—both neutral antagonists that inhibit TSH or antibody-mediated activation of the TSHR, and inverse agonists that inhibit constitutive signaling by TSHR—for direct therapeutic intervention have been pursued (reviewed in [30–32]). Thyroid blocking antibodies (TBAbs) have been shown to exhibit neutral antagonist or inverse agonist properties at TSHR [24, 28, 29]. Recently, the first low to moderate affinity antagonistic small-molecule ligands for the TSHR were identified [33, 34].
Approaches of combined site-directed mutagenesis studies and homology modeling of the TSHR have provided evidence that in contrast to the orthosteric hormone binding site, such drug-like small molecules bind allosterically in the transmembrane region [33, 35, 36]. We found points of interaction for TSHR agonists and antagonists. In particular, recent site-directed mutagenesis at residues surrounding the allosteric binding pocket led to constitutive TSHR activation [37]. These CAMs are indicators for signaling-sensitive points of activation and might also be potential interaction partners for small-molecule agonists to induce TSHR activation.
Here we hypothesized that the transmembrane region in close proximity to the small ligand binding site may also harbor amino acids which are sensitive for silencing effects on constitutive receptor activity. Indeed, we report the identification of several silencing mutations flanking the allosteric binding pocket. These amino acid positions might play an important role as points of small-molecule antagonist interactions that can inhibit TSHR signaling. Our long-term goal is to understand the diverse intramolecular events that occur in or adjacent to the allosteric binding site. This knowledge will be important for the development of small-molecule ligands for pharmacological interventions at the TSHR. Based on the conservation of structural features and amino acid composition among the family A GPCRs, our study also provides interesting information regarding a ligand binding region that is of importance also for other receptors.
Materials and methods
Construction of vectors and site-directed mutagenesis
The restriction site KpnI was introduced into hTSHR-pSVL by use of QuikChange Site-Directed Mutagenesis Kit (Stratagene). Wild-type (wt) TSHR sequence was cloned into pcDNA3 vector (Invitrogen) via KpnI and BamHI restriction sites. Mutations were introduced into wt TSHR in vector pcDNA3 using QuikChange Site-Directed Mutagenesis Kit (Stratagene). The sequences of all constructs were verified by nucleotide sequencing.
Cell culture and transfection
Wild-type and mutated TSHRs were expressed in HEK 293 cells (DSMZ). They were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (Biochrom) at 37°C in a humidified 5% CO2 incubator. For measurement of intracellular cAMP and IP accumulation, cells were seeded in 24-well plates (7.5 × 104 cells per well) and transfected with 0.6 µg DNA/well. For determination of receptor expression at the cell surface, cells were seeded in six-well plates (3 × 105 cells per well) and transfected with 2.4 µg DNA/well. The cells were transfected using Lipofectamine 2000 reagent (Invitrogen) 24 h after seeding.
Determination of cell surface expression by FACS
The expression levels of wt TSHR and TSHR mutants were quantified on a FACS flow cytometer (FACSCalibur, Becton–Dickinson). All steps were performed at 4°C or on ice. 48 h after transfection, cells were harvested using 1 mM EDTA and 1 mM EGTA in PBS. After detachment, cells were spun at 300 × g for 3 min and the supernatant was discarded. Cells were incubated for 15 min in FACS buffer (PBS containing 0.5% BSA), spun and subsequently, they were incubated for 30 min with a 1:200 dilution of a mouse anti-extracellular human TSHR antibody (2C11, Serotec) in FACS buffer. Cells were washed twice in FACS buffer and incubated for 30 min with a 1:400 dilution of a DyLight488-conjugated goat anti-mouse IgG antibody (Serotec). Subsequently, cells were washed twice and resuspended in FACS buffer. For exclusion of damaged cells from analysis, 7-AAD (Becton–Dickinson) was added. The fluorescence of at least 10,000 cells per tube was assayed. Receptor expression was determined by the mean fluorescence intensity by comparison to wt TSHR.
Determination of intracellular cyclic AMP accumulation by RIA
After transfection, cells were cultured for 48 h and stimulated for 1 h at 37°C with stimulation buffer (DMEM supplemented with 10 mM HEPES, 0.5% BSA, 0.25 mM 3-isobutyl-1-methylxanthine) alone or stimulation buffer containing increasing concentrations of bTSH (1 µIU/ml to 0.1 IU/ml, Sigma-Aldrich). Stimulation medium was aspirated and reactions were stopped by lysis of the cells with 0.1% trifluoroacetic acid, 0.005% Triton X-100 for 30 min at 4°C. Supernatants were collected and heated at 95°C for 10 min, dried in a rotation vacuum concentrator (Alpha-RCV, Christ) and finally stored at –20°C until use. After reconstitution and acetylation of the samples [38], the cAMP content was determined using cAMP-125I-tyro-sylmethylester (10,000 cpm, IBL) and polyclonal rabbit anti cAMP-antibody (final dilution 1:1,60,000). Samples were incubated overnight at 4°C. The antibody-bound fraction was precipitated using 50 µl of Saccel (IBL). The radioactivity of the precipitate was determined in a γ-counter.
Determination of [3H]Inositol Phosphates
After transfection cells were cultivated for 24 h. The DMEM was supplemented with 74 kBq/ml myo-[2-3H]-inositol (Amersham Biosciences) and cells were incubated for additional 24 h. Cells were washed with stimulation buffer (culture medium with 10 mM HEPES, 0.5% BSA and 10 mM LiCl) and then stimulated for 60 min at 37°C with bTSH in stimulation buffer. Stimulation medium was removed and cells were lysed with 0.1 M NaOH. By subsequent addition of 0.2 M formic acid and dilution buffer (5 mM sodium tetraborate, 0.5 mM EDTA) the appropriate conditions (pH, ion strength) were adjusted. The lysates were spun and supernatants were subjected to anion exchange chromatography on Sep-Pak Accell™ Plus QMA Cartridges (Waters). [3H]Inositol 1,4,5-triphosphate was eluted with 0.1 M formic acid and 0.4 M ammonium formate. Radioactivity was determined in a liquid scintillation counter. For normalization, the individual counting results were related to the total amount of myo-[2-3H]-inositol incorporated into the HEK293 cells.
Generation of a TSHR serpentine domain model by molecular homology modeling procedures
The available GPCR crystal structures for generation of GPCR homology models in the inactive state are the β2-adrenergic receptor, rhodopsin, and adenosine-receptor (reviewed in [39–42]). These structures are silenced in their signaling activity by keeping the receptors in a rigid conformation due to different methods: (a) inverse agonists as ligands, (b) modifications by mutations (silencing or thermostabilizing mutations), or (c) fusion with proteins like lysozyme (reviewed in [43]).
Here, we used previously described homology models of the inactive TSHR based on rhodopsin [44] and docking models of TSHR with allosterically bound drug-like antagonist or agonists [33, 45] to estimate potentially participating and signaling sensitive residues of the allosteric binding region. The amino acids for mutagenesis were chosen according to the following criteria: (1) they are in spatial proximity to the known allosteric binding region, (2) they have not been characterized previously by mutagenesis to our knowledge.
To facilitate comparison of different GPCRs, we used both the amino acid numbering scheme of the entire TSHR with its putative signal peptide and the Ballesteros–Weinstein nomenclature [46]. All structure images were produced using PyMOL software (DeLano WL, version 099, San Carlos, CA, USA).
Results
Previously, several mutations of residues covering the allosteric ligand binding pocket in the TSHR (Table 1) were identified, where side-chain variations lead to TSHR activation in the serpentine domain [37]. Our goal in this study was to identify those positions in this region that are sensitive for impairment of TSHR signaling and we mutated amino acids which have not been investigated so far. It is of note, that the TSHR exhibits elevated basal signaling activity, which can be impaired or nullified by in vitro and in vivo mutations. These mutations are termed ‘silencing mutations’ and they are known to decrease also constitutive activation induced by CAMs [44].
Table 1.
Summary of amino acids constituting the transmembrane binding pocket for small-molecule ligands of the TSHR
| Location | Ballesteros and Weinstein numbering |
Amino acid hTSHR |
Contacts to org41841 (agonistic) |
Contacts to c52 (antagonistic) |
|---|---|---|---|---|
| TMH1 | 1.35 | L417 | ||
| 1.39 | V421 | |||
| 1.42 | V424* | |||
| TMH2 | 2.53 | M463 | ||
| 2.56 | Y466 | |||
| 2.57 | L467* | |||
| 2.60 | I470 | |||
| 2.64 | D474 | |||
| TMH3 | 3.32 | T501 | ||
| 3.33 | V502* | + | + | |
| 3.36 | S505 | + | − | |
| 3.37 | E506 | |||
| 3.40 | V509 | |||
| TMH4 | 4.56 | L552* | − | + |
| ECL2 | I568 | − | + | |
| L570 | ||||
| P571 | ||||
| M572 | − | + | ||
| TMH5 | 5.39 | Y582* | − | + |
| 5.40 | I583 | |||
| 5.43 | V586* | + | + | |
| 5.44 | L587 | |||
| 5.46 | L589* | |||
| 5.47 | N590 | |||
| TMH6 | 6.48 | M637 | + | − |
| 6.50 | P639 | |||
| 6.51 | I640 | + | + | |
| 6.52 | S641 | |||
| 6.54 | Y643* | |||
| 6.56 | L645 | |||
| 6.55 | A644 | |||
| 6.59 | I648 | |||
| TMH7 | 7.39 | V664 | ||
| 7.40 | L665* | |||
| 7.42 | Y667 | + | + |
This overview is generated from observations at the TSHR homology model (Fig. 3) and by previous studies [33, 36, 45] concerning potential participation of amino acids that cover the allosteric small ligand binding pocket in the transmembrane region. The numbers of positions are provided according to the Ballesteros and Weinstein numbering system for GPCRs [46] and in parallel the TSHR specific residue and number starting with the first amino acid of the signal-peptide. Amino acids investigated in this study are bold and marked with asterisk, for all other amino acids experimental data were published previously (see GPHR information system: http://www.ssfa-gphr.de [53, 54]). We also assigned contacts (+) between the TSHR and the small ligands bound to the allosteric binding site as shown in Fig. 4
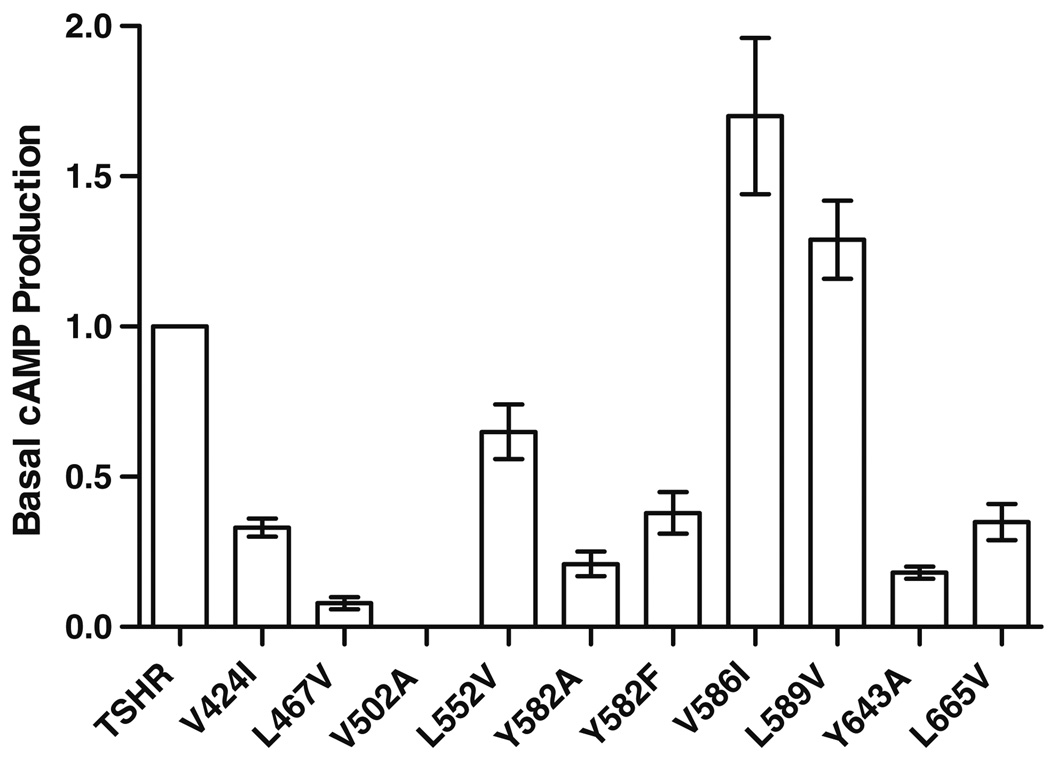
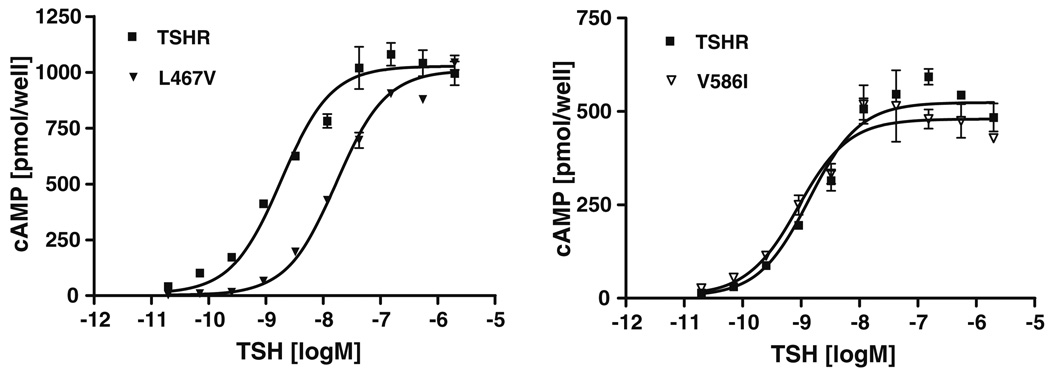
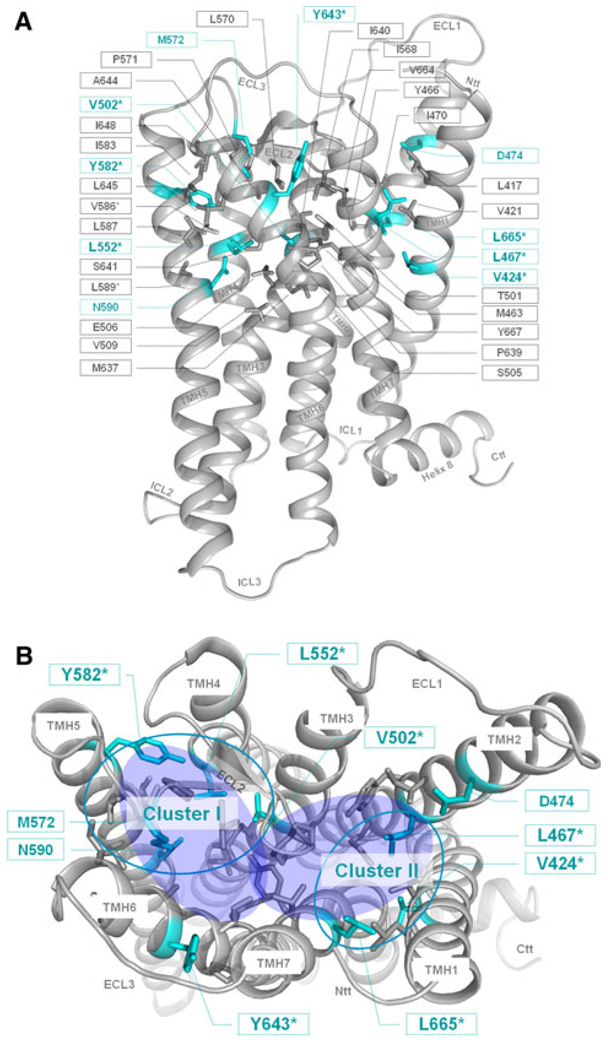
We determined basal and TSH-induced cAMP accumulation to characterize signaling properties of mutant TSHRs (Table 2; Fig. 1). Most of the side-chain variations led to cell surface expression levels comparable to wild-type, with the exception of mutation V502A in TMH3 with around 40% of wild-type receptor expression. Each of the ten mutants reached a maximum level of TSH induced cAMP accumulation of about 80–110% compared to wild-type (set at 100%). Mutation V586I lead to a slight increase in basal activity of 1.7 (±0.26) and substitution L589V showed a basal activity of 1.29 (±0.13) compared to wild-type, respectively. Although they are well expressed at the cell surface, eight of the ten tested TSHR variants, V424I (TMH1), L467V TMH2), V502A (TMH3), L552V (TMH4), Y582A/F (TMH5), Y643A (TMH6), and L665V (TMH7) are characterized by impaired or even nullified basal cAMP values compared to wild-type (Fig. 1). Two of them, L467V (TMH2) and Y643A (TMH6), showed a 5- and 9-fold decreased potency for TSH compared to the wild-type TSHR, respectively (Table 2; Fig. 2). This indicates an influence on TSH-induced cAMP signaling in addition to the effect on basal signaling. Furthermore, these silencing mutants are simultaneously characterized by decreased maximum inositol phosphate second messenger accumulations (between 18 and 70% compared to the wild-type) stimulated by TSH. In conclusion, the here identified sensitive side-chain alterations do selectively affect the maximum of Gq-protein-mediated signaling of TSHR, but not the maximum of signaling capacity mediated by Gs. Six out of the seven positions of silencing mutations are arranged spatially in two different clusters (Fig. 3b, clusters I and II).
Table 2.
Functional characterization of TSHR mutations
| Location | Transfected construct |
Ballesteros and Weinstein numbering |
Cell surface expression | cAMP accumulation | IP accumulation | |
|---|---|---|---|---|---|---|
| FACS% of TSHR wt |
EC50 nM | TSH stimulated % of TSHR |
TSH stimulated % of TSHR |
|||
| wt TSHR | 100 | 1.2 (0.8–1.7) | 100 | 100 | ||
| TMH1 | V424I | 1.43 | 102 ± 10 | 3.0 (2.2–4.1) | 98 ±8 | 53 ± 4 |
| TMH2 | L467V | 2.57 | 85 ± 8 | 11.2 (10.4–12.1) | 105 ± 14 | 19 ± 1 |
| TMH3 | V502A | 3.33 | 44 ± 4 | 4.3 (3.9–4.7) | 84 ± 10 | 13 ± 0 |
| TMH4 | L552V | 4.56 | 90 ± 5 | 1.0 (0.8–1.4) | 84 ± 19 | 79 ± 11 |
| TMH5 | Y582A | 5.39 | 71 ± 5 | 3.7 (2.6–5.2) | 106 ± 19 | 45 ± 17 |
| TMH5 | Y582F | 5.39 | 86 ± 8 | 1.9 (1.3–2.8) | 108 ± 12 | 114 ± 18 |
| TMH5 | V586I | 5.43 | 107 ± 6 | 1.1 (0.7–1.7) | 82 ± 7 | 120 ± 6 |
| TMH5 | L589V | 5.46 | 100 ± 12 | 1.1 (1.0–1.2) | 90 ± 9 | 110 ± 11 |
| TMH6 | Y643A | 6.54 | 99 ± 9 | 6.0 (4.5–8.0) | 93 ± 9 | 47 ± 4 |
| TMH7 | L665V | 7.40 | 95 ± 11 | 2.0 (2.0–2.0) | 100 ± 10 | 71 ± 6 |
HEK 293 cells were transfected with wt TSHR and mutated TSHRs. The TSHR cell surface expression level was quantified on a FACS flow cytometer. Receptor expression was determined by the mean fluorescence intensity by comparison to wt TSHR. Stimulation was carried out with increasing concentrations of TSH. Results for cAMP accumulation were measured by RIA. Quantification of IP accumulation was done by [3H]Inositol incorporation studies. Data are given as mean ± SD of at least two independent experiments, each carried out in duplicate. EC50 values are shown as geometric mean (95% confidence limits). The pcDNA3 vector was used as a control
Fig. 1.
Basal cAMP production of TSHRs harboring mutations in the allosteric ligand binding site. HEK 293 cells were transiently transfected with wt TSHR and mutated TSHRs. cAMP production was measured by RIA as described in Materials and methods. The basal activity of wt TSHR was set at 1 and the basal activity of the mutants is expressed as fold of wt TSHR. Data are given as mean ± SD of at least two independent experiments, each carried out in duplicate
Fig. 2.
Effect of mutations L467 V and V586I on TSH stimulated cAMP accumulation. TSHR mutants L467V and V586I are shown as examples for mutants with impaired and unaffected TSH stimulation. cAMP concentration as a function of TSH stimulation was determined in HEK 293 cells transiently transfected either with wild-type or mutated TSHR. Measurement was done by RIA as described in the Materials and methods section. Data are given as mean ± SD. Curves are representative for one out of two independent experiments each done in duplicates
Fig. 3.
Amino acids of the potential allosteric binding pocket for small-molecule ligands in the thyrotropin receptor. The homology model of the transmembrane TSHR serpentine domain visualizes the spatial localization of 35 amino acids (sticks) that participate in the constitution of the transmembrane binding region for small drug-like molecules (TSHR specific numbering starting with the signal-peptide). Colored in cyan are residues whose mutations lead to an impaired signaling activity. Amino acids marked with the symbol asterisk were investigated in this study. For all other amino acids functional data from mutagenesis studies are published previously. a Side view (lateral to the membrane) of the transmembrane homology model. b In the top-view (from the extracellular side) only positions of inverse agonistic mutations are labeled and the potential allosteric binding region is highlighted with two translucent blue fully colored circles. The accumulation of inverse agonistic mutations (cyan circles) occurs between TMHs 3, 4, 5, ECL2 (Cluster I), and between TMHs 1, 2 and 7 (Cluster II)
Discussion
The allosteric binding region for drug-like small-molecule ligands of the TSHR is comprised of 35 amino acids (Table 1). Evidence for small-molecule binding in this spatial region of the TSHR between the transmembrane helices (TMHs) and the extracellular loop (ECL) 2 was provided in several previous studies [33, 35, 45]. Interestingly, this transmembrane binding pocket region is generally known for family A GPCRs to be sensitive for ligand binding [47]. Our goal in this study was to identify those positions in this region that are sensitive for inactivation of the receptor in order to support the development of low-molecular weight (LMW) antagonists that are able to block pathogenic TSHR activation. Indeed, we here report the identification of mutations characterized by impairment of constitutive signaling activity (silencing) flanking the allosteric binding pocket within two spatial clusters.
Based on the decreased basal activity of the mutants we hypothesize that their wild-type amino acids are important to maintain the basally active wild-type TSHR conformation. Our TSHR homology model predicts that the wild-type amino acids V502 (TMH3), L552 (TMH4), and Y582 (TMH5) are in close proximity to each other. Moreover, amino acid M572 at ECL2 contributes to cluster I of signaling-sensitive amino acids (Fig. 3b). Silencing mutations at this position were previously reported [48]. In particular, the hydrophobic side-chains of V502 and L552 interact with each other between TMH3 and 4, while Y582 from TMH5 interacts with M572 from ECL2 and completes this cluster arrangement. Mutations at these positions indicating that reduction of bulkiness of the side-chains led to silencing of basal signaling. The second significant accumulation of silencing mutations is arranged in a microdomain between TMHs 1, 2, and 7 (cluster II in Fig. 3b). Amino acid mutations at V424 (TMH1), L467 (TMH2), and L665 (TMH7) are characterized by slight variations of bulk of side-chains (Table 2) modifying hydrophobic interactions mandatory for basal signaling activity.
Noteworthy, in contrast to the herein identified silencing mutant Y643A, the substitution Y643F is a CAM [37]. This indicates that dependent on the side-chain substitution, in this case a small alanine versus a bulky aromatic phenylalanine, the TSHR activity can be tuned up or down at position 643. This sensitivity with respect to modification of the bulkiness of the side group also shows that Y643 must be tightly packed between surrounding amino acids in a signaling sensitive region between TMH6, TMH7 and ECL3. Since the Y643F mutant is constitutively active it can be assumed that the hydroxy-group of the tyrosine side chain is of importance for maintaining the basally active conformation (it likely interacts with the backbone of ECL3) of the TSHR. In contrast, mutations of Y582 at TMH5 to small alanine and bulky phenylalanine revealed no reversed effects, both side-chain variations lead to decreased basal activity (Fig. 1). All these findings highlight Y643 as a potential trigger point for changing a small molecule from an agonist to an antagonist or vice versa by chemical modifications. We conclude from our observations that sensitive positions for inhibition of constitutive TSHR activity are located between TMHs 1, 2, and 7 (cluster II, Fig. 3b), and they are also cumulated at the interfaces between TMHs 3, 4, and 5 close to the small ligand binding site (cluster I, Fig. 3b). This is supported by previous mutagenesis data at TMH3 of the TSHR where mutations that are located between the interfaces of TMH3 to TMH4 and TMH3 to TMH5 caused impaired basal activity [49].
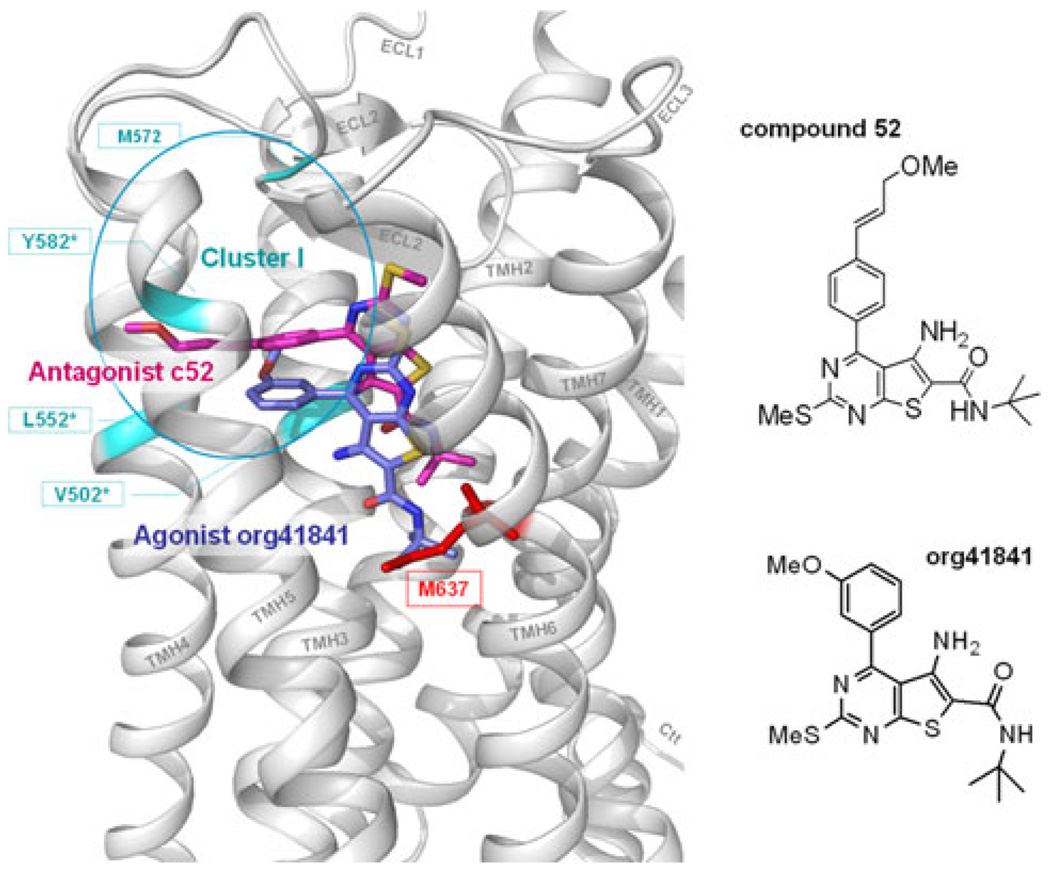
Furthermore, the small-molecule ligand compound 52 (Fig. 4) was previously described to antagonize TSHR activation [33]. It is a neutral antagonist as it inhibits TSH-induced TSHR activity only. Compound 52 shares the same basic thienopyrimidine scaffold with the agonist compound org41841 [35, 36, 50, 51] but differs by the presence of an enlarged substituent on the phenyl-group (Fig. 4). Based on molecular docking studies using a 3D model of the TSHR we predicted that both compounds are localized between the TMHs 3, 4, 5, 6, and ECL2 (Fig. 4). However, the antagonist is located higher in the binding pocket more towards the extracellular region compared to the agonist org41841 (33). Interestingly, the extended substituent of c52 points between TMHs 4, 5, and ECL2 [33] and matches the spatial location where we found the inactivating mutations of residues within cluster I. We conclude that c52 interacts at cluster I and induces a conformation of decreased capability for signaling. Thus both c52 and our mutations in cluster I, are decreasing the signaling capability of the TSHR at the same spatial region. However, the molecular effects of the antagonist and the mutation-induced changes in this region can not completely overlap, because c52 is not characterized as inverse agonist. It should be mentioned that the partial agonist org41841 interacts with residue M637 at TMH6 (Fig. 4) while antagonist c52 can not reach and interact with M637. This is in agreement with the recently reported finding that residue M637 is sensitive for TSHR activation [37] and might explain the agonistic effect of org41841.
Fig. 4.
Comparison of poses and spatial location between the small-molecule antagonist c52 and the agonist org41841 within the allosteric binding pocket of TSHR. Both compounds, antagonist c52 (magenta) [33] and agonist org41841 (blue) [35, 36, 50, 51], are predicted to bind between the helices 3, 4, 5, 6, and ECL2. The chemical structure of c52 differs by the presence of an enlarged substituent on the phenyl-group. This causes c52 to be located spatially higher towards the extracellular region compared to the agonist org41841. The extended substituent of c52 points between helices 4, 5, and ECL2 and matches the spatial location where we found inverse agonistic mutations (cyan) of residues within Cluster I. Thus both c52 and our newly identified mutations in cluster I, are decreasing the signaling capability of the TSHR at the same spatial region. However, the molecular effects of the antagonist and the mutation induced changes can not completely overlap, because c52 is not an inverse agonist. It should be mentioned that the agonist org41841 is interacting with residue M637 at TMH6 (red stick) while c52 can not reach this location. This is in agreement with the recently reported finding that residue M637 is an important sensitive trigger point for TSHR activation [37] and might explain the agonistic effect of org41841
In summary, we show that side-chain alterations at seven positions located in close proximity to the potential binding pocket of small-molecule ligands lead to impairment of basal signaling and in particular cases also to a decreased TSH induced activity (both cAMP und IP accumulation) of the TSHR. Interestingly, the majority of identified positions are cumulated in two distinct spatial clusters between TMHs 3, 4, and 5, and TMHs 1, 2, and 7. The here identified amino acids indicate locations where small-molecule antagonists could impair signaling activity. Thus, these results are the base for refined structure–function knowledge that might support rational development of small-molecule inverse agonists or of neutral antagonists for the TSHR in the future. Our new findings are of interest for homologous GPHRs [51] and might have implications for other GPCRs with elevated basal signaling activity [44, 52]. Finally, our study provides interesting information regarding a ligand-binding region that is conserved among the family A GPCRs.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (KR1273/4-1) and by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Abbreviations
- GPHR
Glycoprotein hormone receptor
- LHCGR
Lutropin/choriogonadotropin receptor
- FSHR
Follicle stimulating hormone receptor
- TSHR
Thyroid stimulating hormone receptor
- bTSH
Bovine thyroid stimulating hormone
- LH
Luteinizing hormone
- CG
Choriogonadotropin
- FSH
Follicle stimulating hormone
- GPCR
G-protein-coupled receptor
- TMH
Transmembrane helix
- ECL1/2/3
Extracellular loops 1/2/3
- ICLs 1/2/3
Intracellular loops 1/2/3
- LRRD
Leucine-rich repeat domain
- SD
Serpentine domain
- CAM
Constitutively activating mutation
- LGRs
Leucine-rich repeat containing GPCRs
- 7TMRs
Seven-transmembrane spanning receptors
- c52
Compound 52
- org41841
Organon 41841
- DMEM
Dulbecco’s modified Eagle’s medium
- PBS
Phosphate buffered saline
- IP
Inositol phosphate
Contributor Information
Ann-Karin Haas, Email: haas@fmp-berlin.de, Leibniz-Institut für Molekulare Pharmakologie, Robert-Rössle-Str.10, 13125 Berlin, Germany.
Gunnar Kleinau, Email: Kleinau@fmp-berlin.de, Leibniz-Institut für Molekulare Pharmakologie, Robert-Rössle-Str.10, 13125 Berlin, Germany.
Inna Hoyer, Email: hoyer@fmp-berlin.de, Leibniz-Institut für Molekulare Pharmakologie, Robert-Rössle-Str.10, 13125 Berlin, Germany.
Susanne Neumann, Email: susanneN@intra.niddk.nih.gov, NIDDK, National Institutes of Health Clinical Endocrinology Branch, Bethesda, MD 20892, USA.
Jens Furkert, Email: furkert@fmp-berlin.de, Leibniz-Institut für Molekulare Pharmakologie, Robert-Rössle-Str.10, 13125 Berlin, Germany.
Claudia Rutz, Email: rutz@fmp-berlin.de, Leibniz-Institut für Molekulare Pharmakologie, Robert-Rössle-Str.10, 13125 Berlin, Germany.
Ralf Schülein, Email: schuelein@fmp-berlin.de, Leibniz-Institut für Molekulare Pharmakologie, Robert-Rössle-Str.10, 13125 Berlin, Germany.
Marvin C. Gershengorn, Email: MarvinG@intra.niddk.nih.gov, NIDDK, National Institutes of Health Clinical Endocrinology Branch, Bethesda, MD 20892, USA.
Gerd Krause, Email: gkrause@fmp-berlin.de, Leibniz-Institut für Molekulare Pharmakologie, Robert-Rössle-Str.10, 13125 Berlin, Germany.
References
- 1.Foord SM. Receptor classification: post genome. Curr Opin Pharmacol. 2002;2:561–566. doi: 10.1016/s1471-4892(02)00214-x. [DOI] [PubMed] [Google Scholar]
- 2.Kosugi S, Sugawa H, Mori T. TSH receptor and LH receptor. Endocr J. 1996;43:595–604. doi: 10.1507/endocrj.43.595. [DOI] [PubMed] [Google Scholar]
- 3.Rapoport B, Chazenbalk GD, Jaume JC, McLachlan SM. The thyrotropin (TSH) receptor: interaction with TSH and auto-antibodies. Endocr Rev. 1998;19:673–716. doi: 10.1210/edrv.19.6.0352. [DOI] [PubMed] [Google Scholar]
- 4.Vassart G, Dumont JE. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992;13:596–611. doi: 10.1210/edrv-13-3-596. [DOI] [PubMed] [Google Scholar]
- 5.De Lloyd A, Bursell J, Gregory JW, Rees DA, Ludgate M. TSH receptor activation and body composition. J Endocrinol. 2010;204:13–20. doi: 10.1677/JOE-09-0262. [DOI] [PubMed] [Google Scholar]
- 6.Eckstein AK, Johnson KT, Thanos M, Esser J, Ludgate M. Current insights into the pathogenesis of Graves’ orbitopathy. Horm Metab Res. 2009;41:456–464. doi: 10.1055/s-0029-1220935. [DOI] [PubMed] [Google Scholar]
- 7.Balzan S, Nicolini G, Forini F, Boni G, Del Carratore R, Nicolini A, Carpi A, Iervasi G. Presence of a functional TSH receptor on human erythrocytes. Biomed Pharmacother. 2007;61:463–467. doi: 10.1016/j.biopha.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Agretti P, Chiovato L, De Marco G, Marcocci C, Mazzi B, Sellari-Franceschini S, Vitti P, Pinchera A, Tonacchera M. Real-time PCR provides evidence for thyrotropin receptor mRNA expression in orbital as well as in extraorbital tissues. Eur J Endocrinol. 2002;147:733–739. doi: 10.1530/eje.0.1470733. [DOI] [PubMed] [Google Scholar]
- 9.Busuttil BE, Frauman AG. Extrathyroidal manifestations of Graves’ disease: the thyrotropin receptor is expressed in extraocular, but not cardiac, muscle tissues. J Clin Endocrinol Metab. 2001;86:2315–2319. doi: 10.1210/jcem.86.5.7474. [DOI] [PubMed] [Google Scholar]
- 10.Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves’ orbital adipose/connective tissues: potential autoantigen in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002. doi: 10.1210/jcem.83.3.4676. [DOI] [PubMed] [Google Scholar]
- 11.Dutton CM, Joba W, Spitzweg C, Heufelder AE, Bahn RS. Thyrotropin receptor expression in adrenal, kidney, and thymus. Thyroid. 1997;7:879–884. doi: 10.1089/thy.1997.7.879. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Baker G, Janus D, Paddon CA, Fuhrer D, Ludgate M. Biological effects of thyrotropin receptor activation on human orbital preadipocytes. Invest Ophthalmol Vis Sci. 2006;47:5197–5203. doi: 10.1167/iovs.06-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun SC, Hsu PJ, Wu FJ, Li SH, Lu CH, Luo CW. Thyrostimulin, but not thyroid-stimulating hormone (TSH), acts as a paracrine regulator to activate the TSH receptor in mammalian ovary. J Biol Chem. 2010;285:3758–3765. doi: 10.1074/jbc.M109.066266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aghajanova L, Lindeberg M, Carlsson IB, Stavreus-Evers A, Zhang P, Scott JE, Hovatta O, Skjoldebrand-Sparre L. Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod Biomed Online. 2009;18:337–347. doi: 10.1016/s1472-6483(10)60091-0. [DOI] [PubMed] [Google Scholar]
- 15.Szkudlinski MW, Fremont V, Ronin C, Weintraub BD. Thyroid-stimulating hormone and thyroid-stimulating hormone receptor structure–function relationships. Physiol Rev. 2002;82:473–502. doi: 10.1152/physrev.00031.2001. [DOI] [PubMed] [Google Scholar]
- 16.Schoneberg T, Schulz A, Biebermann H, Hermsdorf T, Rompler H, Sangkuhl K. Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol Ther. 2004;104:173–206. doi: 10.1016/j.pharmthera.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Rodien P, Ho SC, Vlaeminck V, Vassart G, Costagliola S. Activating mutations of TSH receptor. Ann Endocrinol (Paris) 2003;64:12–16. [PubMed] [Google Scholar]
- 18.Michalek K, Morshed SA, Latif R, Davies TF. TSH receptor autoantibodies. Autoimmun Rev. 2009;9:113–116. doi: 10.1016/j.autrev.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latif R, Morshed SA, Zaidi M, Davies TF. The thyroid-stimulating hormone receptor: impact of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on multimerization, cleavage, and signaling. Endocrinol Metab Clin North Am. 2009;38:319–341. doi: 10.1016/j.ecl.2009.01.006. viii. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Jimenez C, Santisteban P. TSH signalling and cancer. Arq Bras Endocrinol Metabol. 2007;51:654–671. doi: 10.1590/s0004-27302007000500003. [DOI] [PubMed] [Google Scholar]
- 21.Duprez L, Parma J, Van Sande J, Rodien P, Sabine C, Abramowicz M, Dumont JE, Vassart G. Pathology of the TSH receptor. J Pediatr Endocrinol Metab. 1999;12 Suppl 1:295–302. [PubMed] [Google Scholar]
- 22.Davies TF, Ando T, Lin RY, Tomer Y, Latif R. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest. 2005;115:1972–1983. doi: 10.1172/JCI26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corvilain B, Van Sande J, Dumont JE, Vassart G. Somatic and germline mutations of the TSH receptor and thyroid diseases. Clin Endocrinol (Oxf) 2001;55:143–158. [PubMed] [Google Scholar]
- 24.Chen CR, McLachlan SM, Rapoport B. Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology. 2007;148:2375–2382. doi: 10.1210/en.2006-1754. [DOI] [PubMed] [Google Scholar]
- 25.Ando T, Davies TF. Monoclonal antibodies to the thyrotropin receptor. Clin Dev Immunol. 2005;12:137–143. doi: 10.1080/17402520500078238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alberti L, Proverbio MC, Costagliola S, Romoli R, Boldrighini B, Vigone MC, Weber G, Chiumello G, Beck-Peccoz P, Persani L. Germline mutations of TSH receptor gene as cause of nonautoimmune subclinical hypothyroidism. J Clin Endocrinol Metab. 2002;87:2549–2555. doi: 10.1210/jcem.87.6.8536. [DOI] [PubMed] [Google Scholar]
- 27.Kleinau G, Krause G. Thyrotropin and homologous glycoprotein hormone receptors: structural and functional aspects of extracellular signaling mechanisms. Endocr Rev. 2009;30:133–151. doi: 10.1210/er.2008-0044. [DOI] [PubMed] [Google Scholar]
- 28.Moriyama K, Okuda J, Saijo M, Hattori Y, Kanamoto N, Hataya Y, Matsuda F, Mori T, Nakao K, Akamizu T. Recombinant monoclonal thyrotropin-stimulation blocking antibody (TSBAb) established from peripheral lymphocytes of a hypothyroid patient with primary myxedema. J Endocrinol Invest. 2003;26:1076–1080. doi: 10.1007/BF03345253. [DOI] [PubMed] [Google Scholar]
- 29.Sanders J, Evans M, Betterle C, Sanders P, Bhardwaja A, Young S, Roberts E, Wilmot J, Richards T, Kiddie A, Small K, Platt H, Summerhayes S, Harris R, Reeve M, Coco G, Zanchetta R, Chen S, Furmaniak J, Smith BR. A human monoclonal autoantibody to the thyrotropin receptor with thyroid-stimulating blocking activity. Thyroid. 2008;18:735–746. doi: 10.1089/thy.2007.0327. [DOI] [PubMed] [Google Scholar]
- 30.Beck-Peccoz P. Antithyroid drugs are 65 years old: time for retirement? Endocrinology. 2008;149:5943–5944. doi: 10.1210/en.2008-1349. [DOI] [PubMed] [Google Scholar]
- 31.Smith BR, Sanders J, Furmaniak J. TSH receptor antibodies. Thyroid. 2007;17:923–938. doi: 10.1089/thy.2007.0239. [DOI] [PubMed] [Google Scholar]
- 32.Neumann S, Raaka BM, Gershengorn MC. Human TSH receptor ligands as pharmacological probes with potential clinical application. Expert Rev Endocrinol Metabol. 2009;4:669–679. doi: 10.1586/eem.09.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann S, Kleinau G, Costanzi S, Moore S, Jiang JK, Raaka BM, Thomas CJ, Krause G, Gershengorn MC. A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology. 2008;149:5945–5950. doi: 10.1210/en.2008-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann S, Huang W, Eliseeva E, Titus S, Thomas CJ, Gershengorn MC. A small molecule inverse agonist for the human TSH receptor. Endocrinology. 2010 doi: 10.1210/en.2010-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaschke H, Neumann S, Moore S, Thomas CJ, Colson AO, Costanzi S, Kleinau G, Jiang JK, Paschke R, Raaka BM, Krause G, Gershengorn MC. A low molecular weight agonist signals by binding to the transmembrane domain of thyroid-stimulating hormone receptor (TSHR) and luteinizing hormone/chorionic gonadotropin receptor (LHCGR) J Biol Chem. 2006;281:9841–9844. doi: 10.1074/jbc.C600014200. [DOI] [PubMed] [Google Scholar]
- 36.Moore S, Jaeschke H, Kleinau G, Neumann S, Costanzi S, Jiang JK, Childress J, Raaka BM, Colson A, Paschke R, Krause G, Thomas CJ, Gershengorn MC. Evaluation of small-molecule modulators of the luteinizing hormone/choriogonadotropin and thyroid stimulating hormone receptors: structure-activity relationships and selective binding patterns. J Med Chem. 2006;49:3888–3896. doi: 10.1021/jm060247s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleinau G, Haas AK, Neumann S, Worth CL, Hoyer I, Furkert J, Rutz C, Gershengorn MC, Schulein R, Krause G. Signaling-sensitive amino acids surround the allosteric ligand binding site of the thyrotropin receptor. FASEB J. 2010 doi: 10.1096/fj.09-149146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith BJ, Wales MR, Perry MJ. Assays of cyclic nucleotides: a review of current techniques. Appl Biochem Biotechnol. 1993;41:189–218. doi: 10.1007/BF02916422. [DOI] [PubMed] [Google Scholar]
- 39.Hanson MA, Stevens RC. Discovery of new GPCR biology: one receptor structure at a time. Structure. 2009;17:8–14. doi: 10.1016/j.str.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobilka B, Schertler GF. New G-protein-coupled receptor crystal structures: insights and limitations. Trends Pharmacol Sci. 2008;29:79–83. doi: 10.1016/j.tips.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Worth CL, Kleinau G, Krause G. Comparative sequence and structural analyses of G-protein-coupled receptor crystal structures and implications for molecular models. PloS One. 2009;4:e7011. doi: 10.1371/journal.pone.0007011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tate CG, Schertler GF. Engineering G protein-coupled receptors to facilitate their structure determination. Curr Opin Struct Biol. 2009;19:386–395. doi: 10.1016/j.sbi.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Kleinau G, Jaeschke H, Mueller S, Worth CL, Paschke R, Krause G. Molecular and structural effects of inverse agonistic mutations on signaling of the thyrotropin receptor—a basally active GPCR. Cell Mol Life Sci. 2008;65:3664–3676. doi: 10.1007/s00018-008-8450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neumann S, Huang W, Titus S, Krause G, Kleinau G, Alberobello AT, Zheng W, Southall NT, Inglese J, Austin CP, Celi FS, Gavrilova O, Thomas CJ, Raaka BM, Gershengorn MC. Small-molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc Natl Acad Sci USA. 2009;106:12471–12476. doi: 10.1073/pnas.0904506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ballesteros JA, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure–function relationships in G-protein coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 47.Gloriam DE, Foord SM, Blaney FE, Garland SL. Definition of the G protein-coupled receptor transmembrane bundle binding pocket and calculation of receptor similarities for drug design. J Med Chem. 2009;52:4429–4442. doi: 10.1021/jm900319e. [DOI] [PubMed] [Google Scholar]
- 48.Kleinau G, Claus M, Jaeschke H, Mueller S, Neumann S, Paschke R, Krause G. Contacts between extracellular loop two and transmembrane helix six determine basal activity of the thyroid-stimulating hormone receptor. J Biol Chem. 2007;282:518–525. doi: 10.1074/jbc.M606176200. [DOI] [PubMed] [Google Scholar]
- 49.Kosugi S, Hai N, Okamoto H, Sugawa H, Mori T. A novel activating mutation in the thyrotropin receptor gene in an autonomously functioning thyroid nodule developed by a Japanese patient. Eur J Endocrinol. 2000;143:471–477. doi: 10.1530/eje.0.1430471. [DOI] [PubMed] [Google Scholar]
- 50.van Straten NC, Schoonus-Gerritsma GG, van Someren RG, Draaijer J, Adang AE, Timmers CM, Hanssen RG, van Boeckel CA. The first orally active low molecular weight agonists for the LH receptor: thienopyr(im)idines with therapeutic potential for ovulation induction. Chembiochem. 2002;3:1023–1026. doi: 10.1002/1439-7633(20021004)3:10<1023::AID-CBIC1023>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 51.Heitman LH, Ijzerman AP. G protein-coupled receptors of the hypothalamic-pituitary-gonadal axis: a case for Gnrh, LH, FSH, and GPR54 receptor ligands. Med Res Rev. 2008;28:975–1011. doi: 10.1002/med.20129. [DOI] [PubMed] [Google Scholar]
- 52.Tao YX. Inactivating mutations of G protein-coupled receptors and diseases: structure-function insights and therapeutic implications. Pharmacol Ther. 2006;111:949–973. doi: 10.1016/j.pharmthera.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Kleinau G, Brehm M, Wiedemann U, Labudde D, Leser U, Krause G. Implications for molecular mechanisms of glycoprotein hormone receptors using a new sequence-structure–function analysis resource. Mol Endocrinol. 2007;21:574–580. doi: 10.1210/me.2006-0309. [DOI] [PubMed] [Google Scholar]
- 54.Kleinau G, Kreuchwig A, Worth CL, Krause G. An interactive web-tool for molecular analyses links naturally occurring mutation data with three-dimensional structures of the rhodopsin-like glycoprotein hormone receptors. Hum Mutat. 2010;31:E1519–E1525. doi: 10.1002/humu.21265. [DOI] [PubMed] [Google Scholar]