Abstract
Emotion and cognition have been viewed as largely separate entities in the brain. Within this framework, significant progress has been made in understanding specific aspects of behavior. Research in the past two decades, however, has started to paint a different picture of brain organization, one in which network interactions are key to understanding complex behaviors. From both basic and clinical perspectives, the characterization of cognitive-emotional interactions constitutes a fundamental issue in the investigation of the mind and brain. This review will highlight the interactive and integrative potential that exists in the brain to bring together the cognitive and emotional domains. First, anatomical evidence will be provided, focusing on structures such as hypothalamus, basal forebrain, amygdala, cingulate cortex, orbitofrontal cortex, and insula. Data on functional interactions will then be discussed, followed by a discussion of a dual competition framework, which describes cognitive-emotional interactions in terms of perceptual and cognitive competition mechanisms.
Keywords: emotion, cognition, perception, interaction, integration, amygdala, anterior insula
A century of neuroscience research has yielded evolving views of the organization of the brain in general, and of how emotion and cognition are instantiated in gray matter in particular. Proposals highlighting the importance of specific regions, including the hypothalamus and the amygdala, as well as proposals describing elaborate circuits, such as those by Papez and MacLean, have been advanced. It is undeniable that certain brain regions play an important role in emotion. Yet, it is also apparent that they do not work in isolation and, instead, participate in distributed networks of regions that, collectively, carry out important functions. From both a basic and clinical perspective, an especially challenging problem is to understand the relationship between brain networks that are important for perception and cognition, and those that determine the affective value of stimuli and contexts. In this review, I will highlight the interactive and integrative potential that exists in the brain to bring together the cognitive and emotional domains. Because the backbone for these interactions is anatomical, the first section will describe several examples of how the transfer of information takes place. The second section illustrates some examples of the interaction between perception and emotion, and between cognition and emotion. The final section presents considerations of how to conceptualize cognitive-emotional interactions in terms of perceptual and cognitive competition mechanisms.
Anatomical substrates for cognitive-emotional interactions
This section describes how the architecture of the brain includes multiple avenues for information integration. As described, the substrates for information interaction and integration are plentiful and provide the potential for the coordinated flow of information that characterizes complex behaviors.
Hypothalamus
The importance of the hypothalamus in certain aspects of emotion is well known, as highlighted by the work of Cannon and Bard; the latter showed via “decortication” experiments that emotional expression effects were abolished when the hypothalamus was eliminated, but not when only the neocortex was compromised. Since the 20s and 30s our knowledge of hypothalamic function has been greatly extended and refined, and current understanding concurs with the earlier notion that the hypothalamus is involved in several important survival-related functions. To coordinate these functions, the hypothalamus works in association with a multitude of other sites in the brain stem and spinal cord.
Historically, the role of the hypothalamus has often been conceptualized as “descending”, a view that is summarized in the designation of the hypothalamus as the “head ganglion” of the autonomic nervous system. The importance of the hypothalamus for descending control notwithstanding, a recently recognized fact is the recognition that the cerebral cortex and hypothalamus share massive bidirectional connections. In the rat, which constitutes the best studied case, there are four major routes from the hypothalamus to the cerebral cortex1 (Fig. 1). These include a major direct projection to all parts of the cortical mantle, and three indirect routes by way of the thalamus, basal nuclei (specifically, magnocellular basal forebrain and amygdala), and brainstem (for discussion of the indirect routes, please see ref.1).
Figure 1.
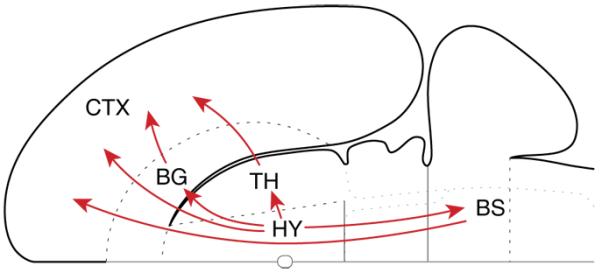
Hypothalamic ascending connectivity. Summary of the four major pathways from the hypothalamus to the cerebral cortex schematized on a flattened representation of the rat brain. The basal ganglia here refer to the magnocellular basal forebrain and the amygdala complex. Note that one of the indirect connections first “descends” to the brainstem. Abbreviations: BG: basal ganglia; BS: brainstem; CTX: cortex; HY: hypothalamus; TH: thalamus. Reprinted from ref.1 with permission from Elsevier.
The direct input to the cortical mantle appears to be the largest source of non-thalamic input to the cortex1, 2. In the rat, some important targets include infralimbic, prelimbic, anterior cingulate, and insular cortices. Interestingly, projections to the lateral prefrontal cortex are also found, and even to primary sensory areas (though both are less prominent). An important indirect system connects the hypothalamus to the cortex via the magnocellular basal forebrain system. Another noteworthy route to the cortex involves several amygdala nuclei, including projections via the basolateral nucleus that reach cingulate, motor, and visual areas. The organization of the connections between prefrontal cortex and hypothalamus has been investigated in non-human primates, too, and are in close concordance with the findings in rats3. Notably, all prefrontal areas investigated received projections from the hypothalamus. In addition to the systems linking the hypothalamus to cortex, conversely, major telencephalic projections to the hypothalamus also exist, including those from the hippocampal formation, amygdala, insular cortex, and prefrontal cortex.
In summary, whereas the hypothalamus is involved in a host of basic control functions, it is part of an extensive bidirectional connective system with cortex and many other subcortical structures in a manner that allows for extensive integration of cognitive and emotional information. Critically, the hypothalamus is linked to other structures that have themselves widespread connectivity, including the magnocellular basal forebrain and the amygdala.
Basal forebrain
The basal forebrain is a heterogeneous set of structures close to the medial and ventral surfaces of the cerebral hemispheres. The magnocellular basal forebrain system is a prominent feature of the primate basal forebrain, involving a continuous collection of large neurons that involve the basal nucleus of Meynert (sometimes called “substantia innominata”), and cell groups within the septum and the horizontal limb of the diagonal band. The magnocellular basal forebrain system originates an “ascending” (i.e., corticopetal) cholinergic and GABAergic projection system that innervates throughout the cortical mantle. Major projections reach several cortical areas, including peristriate, inferotemporal, superior temporal, parahippocampal, temporopolar, posterior parietal, cingulate, frontoparietal opercular, lateral prefrontal, and orbitoinsular regions4. Extensive projections are found to both the hippocampus and amygdala5, too. An important pattern of this projection system is that a connectivity gradient can be identified, such that the densest projections from the basal forebrain to cortex are found for non-isocortical components of temporopolar, insular, and orbitofrontal cortices5 (where isocortical typically refers to cortex with six identifiable layers). This pattern is consistent with the dense innervations (stronger than cortex) observed for both the hippocampus and amygdala, two regions with simplified cytoarchitecture (i.e., pattern of laminar structure).
Given its overall connectivity pattern, the magnocellular basal forebrain system is in a favorable position to influence cortical sites across the brain, including sensory cortex, and thus to influence the flow of information processing. These distributed effects result in increased vigilance, alertness, and attention, and more generally have the potential for widespread impact on cognitive function both in health and mental illness6, 7.
As with other neurotransmitter systems in the brain, the effects of the magnocellular system are at times described as relatively global, or at least unspecific. However, specific effects have also been documented. For instance, visual responses that are conveyed to prefrontal cortex engage the basal forebrain in a polysynaptic way, which then further enhances visual responding8. Direct stimulation of the basal forebrain also enhances the cortical coding of natural scenes in visual cortex by markedly improving the reliability of cell responses9.
Whereas the magnocellular system projects in a widespread, distributed fashion to cortical and subcortical regions, it is noteworthy that afferent fibers originate from a much more circumscribed set of regions. Cortically, inputs originate largely from non-isocortical areas5, 10. Given that these are exactly the regions that receive the densest inputs from the basal forebrain, potent basal forebrain-cortical circuits can be established.
Amygdala
A remarkable property of the primate amygdala is its massive interconnection with cortex. Based on the available data at the time, analysis of amygdala connectivity revealed that this structure was connected to all but 8 of the cortical areas included in the study11 (see also refs.12, 13). These connections involved multiple region clusters, suggesting that the amygdala14 is not only one of the most highly connected regions of the brain, but that its connectivity topology is consistent with that of a “connector” hub15 (where a hub is a region with high degree of connectivity) that links multiple “provincial” hubs15 – where the latter refer to regions of dense connectivity more closely associated with a specific functional group, such as area V4 in visual cortex16. In this manner, the amygdala has strong potential for integrating cognitive and emotional information17.
When whole-brain connectivity data are analyzed, prefrontal areas are among those most distant from the sensory periphery – based on the average number of connections11. Thus, on average, the prefrontal cortex receives highly-processed and integrated sensory information. This structural feature is thought to be important because it provides the prefrontal cortex with relative insulation from the periphery. Indeed, this organization has been proposed to be a key anatomical feature of this region that may confer the primate brain with a greater degree of flexibility4. Highly processed information may also be important in supporting more abstract processing that is required for cognition. It is thus noteworthy that the amygdala (and other regions, such as the hippocampus and entorhinal cortex) was found to be also removed from the sensory periphery11, indicating that this region is well situated to integrate and distribute information, not unlike certain prefrontal cortex territories.
Connections from the sensory periphery to the amygdala that bypass the cortex have been documented, too. For instance, in rodents, the medial geniculate body in the thalamus conveys auditory information to the amygdala and provides a “low road” (i.e., subcortical pathway) for auditory information18. The potential role of subcortical pathways conveying emotional information is discussed at length elsewhere. As described, in primates, it is unlikely that fast, subcortical pathways play a prominent role in affective visual processing19. Instead, it was suggested that fast visual processing of affective stimuli relies on multiple, parallel cortical pathways that rapidly convey information to the amygdala and other evaluative sites, such as the orbitofrontal cortex19, 20.
The pattern of connectivity between the amygdala and prefrontal cortex21 is of particular interest given the latter’s role in cognitive functions. In addition to substantial connections between the amygdala and both medial and orbital aspects of the prefrontal cortex, recent findings indicate that the interconnection between the amygdala and lateral prefrontal cortex extends throughout the lateral surface22. Considered together, the connectivity of the amygdala reveals a substrate for diverse cognitive-emotional interactions that involves the main sectors of the prefrontal cortex – though the anatomical connectivity strength is markedly weaker in the case of the lateral prefrontal cortex.
A further aspect of amygdala connectivity relates to the visual cortex, an aspect that is critical in understanding how amygdala signals modulate visual processing according to an item’s affective significance. Information from visual cortex reaches the amygdala from regions in the anterior ventral visual system, specifically, responses in inferior temporal cortex are conveyed to the lateral and accessory basal nuclei23. In contrast, efferent projections from the amygdala are organized in a completely distinct manner and connect the basal nucleus of the amygdala with nearly all levels of the ventral visual pathway, including primary visual cortex24. Projections from the amygdala to visual cortex terminate preferentially in cortical layers I-II and V-VI (i.e., not in layer IV), a pattern that is typical of feedback-type connections24 (e.g., from V2 to V1). Typically, these connections are unable to drive neuronal activity25 (i.e., independently generate spiking outputs) but have the ability to modulate information processing by enhancing (or decreasing) neural responses26.
I have discussed patterns of amygdala connectivity without closely examining the different components of the amygdala complex. Yet, the connectivity pattern of the central nucleus is quite distinct from the one observed for regions such as the anterior basolateral and lateral nuclei of the amygdala. The latter have been suggested to be part of a frontotemporal association system, in contrast to the central nucleus, which is more directly linked to autonomic structures27. More generally, when discussing the functions of the amygdala, it is thus important to consider how distinct subregions of this structure are anatomically connected.
Prefrontal monitoring and control of visceral and other bodily functions
The idea that the prefrontal cortex is involved in the control of the autonomic nervous system is not new, dating to the turn of the twentieth century (see the historical account by Neafsey28). More recently, the tight interrelation between prefrontal cortex and bodily functions was refined by the work of Damasio, Bechara, and colleagues on the somatic marker hypothesis (i.e., the idea that bodily states function as “marker” signals that influence reasoning and decision making), especially with respect to the orbitofrontal and ventromedial prefrontal cortices29. Likewise, the notion that the anterior insula, a region that is here discussed in conjunction with prefrontal sites, is involved in complex bodily representations has gained visibility30-32.
Cingulate cortex
The functions of the cingulate cortex, which may comprise more than 30-40 sub-areas, are complex33. The anterior sector of the cingulate gyrus is involved in a broad array of functions, including willed action, executive functions, and emotion. A remarkable property of this cortical tissue is that it probably has a more extensive descending projection system than any other cortical region34, including major projections to autonomic regulatory structures, notably the lateral hypothalamus, periaqueductal gray, parabrachial nucleus, and the nucleus of the solitary tract35. This connectivity is consistent with stimulation studies that have documented effects of cingulate electrical stimulation on virtually all autonomic and many endocrine functions33. Conversely, a range of brainstem projections influence cingulate responses36. These include projections from the locus coeruleus to sites throughout the cingulate cortex, as well as from the nucleus of the solitary tract. Several nociceptive circuits also reach anterior- and mid-cingulate areas indirectly via thalamic nuclei. These findings therefore emphasize the notion that the cingulate gyrus is involved in the bidirectional integration of body-related signals – this is true not only for more anterior regions, but also for the posterior cingulate cortex. Given the well-described roles of the cingulate cortex in cognitive functions, this arrangement provides exceptional opportunities for cognitive-emotional interaction and integration.
Orbitofrontal cortex
Based on its connectivity pattern, the orbitofrontal cortex can be divided into “orbital” and “medial” subcomponents37. The orbital network receives extensive sensory information and appears to integrate it, particularly in relation to the assessment of food and reward. The medial network exhibits a distinctive connectivity pattern, and is heavily connected with areas of the medial wall of the brain, including those surrounding the cingulate gyrus, as well as Brodmann areas 9 and 10 medially. Again in contrast to the orbital network, the medial network receives few sensory inputs (with the exception of auditory association areas). Importantly, it projects to the hypothalamus and other visceral-control areas, leading to the suggestion that it is involved in “visceral modulation of emotion”38. Via the hypothalamus, descending medial orbitofrontal influence appears to extend as far as spinal autonomic centers39. In contrast, there are relatively few projections to the hypothalamus from the orbital network.
Anterior insula
The anterior insula is another structure that is critically involved in the processing of bodily signals as it contains a visceral sensory cortex that maps the internal state of the body in a precise fashion31, 32. It has been suggested31 that the anterior insula is more involved in the “afferent representation of ‘feelings’ from the body” (including representation of sensations such as temperature, pain, and visceral ones; see also ref.30), and the cingulate, for instance, is instead involved in the initiation of behaviors (thus more “motor” in function).
More generally, when considering the connectivity of the prefrontal cortex, more differentiated (in terms of laminar structure) regions appear to have restricted connections, whereas the least-differentiated regions have widespread intrinsic connections40. For example, the highly differentiated area 8 on the lateral surface has connections that are more likely to target neighboring regions on the lateral surface of the hemisphere. In contrast, both orbital and medial non-isocortical areas (i.e., areas with poor lamination structure, such as a conjoined layer II/III and/or layer V/VI41) have extensive connections that span the orbital, medial, and lateral surfaces of the hemisphere. Thus, it has been suggested40 that, on the one hand, the widespread connectivity of the less differentiated regions is consistent with a more “global role” in neural processing; on the other hand, the more differentiated regions may have more specific roles in information processing.
Summary on anatomy
Historically, subcortical structures such as the hypothalamus and the amygdala have been implicated in emotion. It is becoming increasingly clear, however, that their connectivity affords them with great potential to interact with many other cortical and subcortical structures that are involved in cognitive functions. As noted in the particularly prescient words by Amaral and Price in the context of the amygdala21: “As our knowledge of the connections of the amygdala has expanded, it has become apparent that the earlier view that it is primarily involved in the control of visceral and autonomic function is incomplete… These widespread interconnections with diverse parts of the brain simply do not fit with a narrow functional role for the amygdaloid complex. They support, rather, the behavioral and clinical observations which suggest that the amygdaloid complex should be included among the structures which are responsible for the elaboration of higher cognitive functions” (pp. 492-493).
The understanding of the anatomy of the prefrontal cortex has also evolved considerably. As described, large sectors of the prefrontal cortex are strongly interconnected with brainstem nuclei that are responsible for controlling autonomic and endocrine function in the service of supporting survival and bodily integrity via homeostasis. The prefrontal and related sectors comprising the cingulate, orbitofrontal, and insula cortices are also strongly interconnected. In addition, they are also strongly interconnected with the amygdala. In all, the vertical integration of information, both ascending and descending, is implemented in an extensive manner. Accordingly, in conceptualizing the function of the prefrontal cortex, not only is horizontal communication (e.g., links between parietal and prefrontal cortices) important, but also vertical communication is of paramount relevance. Finally, given that several prefrontal and insular areas contain less differentiated gray matter, their widespread connectivity amplifies the potential for cognitive-emotional interactions.
Functional interactions between emotion and cognition
Having discussed anatomical substrates for communication, I now turn to functional studies that, when combined with anatomical evidence, further illustrate the interactions between emotion and cognition. The examples will focus on interactions between emotion and i) perception and attention; and ii) executive functions (see also refs. 20, 30, 42-45).
Perception and attention
Viewing emotion-laden visual stimuli is linked to heightened and more extensive visual system activation46, 47. For instance, viewing faces with emotional expressions evokes increased responses relative to viewing neutral faces throughout ventral occipitotemporal visual cortex. Visual responses are also stronger when subjects view emotional scenes (e.g., a war scene) compared do neutral scenes (e.g., a lake scene). Increased visual activation is observed in both “late” visual areas, such as the fusiform gyrus and superior temporal sulcus, and “early” visual cortex in the occipital lobe. Recent studies have shown that, in humans, even retinotopically organized visual cortex, including visual areas V1 and V2 along the calcarine fissure, are modulated by the affective significance of a stimulus48, 49.
Enhanced visual activation when viewing emotional stimuli is consistent with observed improvements in behavioral performance across several tasks. For instance, there is some evidence that angry and happy faces are detected faster in visual search tasks50, and possibly other emotional stimuli, too, such as a snake or spider51 (but see ref.52). Stronger evidence comes from studies of the attentional blink paradigm, in which subjects are asked to report the occurrence of two targets (T1 and T2) among a rapid stream of visual stimuli. When T2 follows T1 by a brief delay, participants are more likely to miss it, as if they had blinked (hence the name). The attentional blink, which is believed to reflect a capacity-limited processing stage, has been shown to be modulated by emotional stimuli, as subjects are significantly better at detecting T2 when it is, for instance, an emotion-laden word (e.g., “rape”) than when it is a neutral word53.
Converging evidence for a link between perception, attention, and emotion comes from additional studies. For example, patients who present with unilateral inattention due to spatial hemineglect (often as a result of right hemisphere parietal lesions) are better at detecting happy or angry faces compared to neutral ones54. These findings are consistent with the notion that emotional faces may direct the allocation of attention. For instance, in one study, emotional faces were flashed at spatial locations that subsequently displayed low-contrast visual stimuli55. Subjects exhibited improved performance for detecting targets shown at those locations, suggesting that attention was deployed to them, thereby facilitating visual detection (see also ref.48).
What are the mechanisms subserving the increase in perceptual processing and attentional capture that are observed during the perception of affective stimuli? Some evidence links the amygdala with these effects. For instance, patients with amygdala lesions do not exhibit improved detection of T2 emotional targets during the attentional blink (i.e., do not show a decrease in the magnitude of the blink)56, and may not exhibit increased responses in visual cortex during the viewing of fearful faces57 (but see ref.58 for evidence that the amygdala is not required for at least some effects). Consistent with the involvement of the amygdala, in a recent study of the attentional blink, we observed that trial-by-trial fluctuations of responses in the amygdala were predictive of behavioral performance in the task – the greater the evoked response, the higher the likelihood that the subject would correctly detect an emotional T2 stimulus59. Thus, it appears that the amygdala may underlie a form of emotional modulation of information that in many ways parallels attentional effects that are observed with non-emotional information47, 60 – the latter is thought to depend on fronto-parietal regions. As discussed in the previous section, given that the amygdala sends projections across nearly all levels of the visual system, it is well situated to modulate sensory processing according to the affective significance of a visual object (see also next section).
Is the perception of emotion-laden stimuli “automatic”, namely independent of attention and awareness? This question has received considerable attention because specific answers (“no” or “yes”) suggest potentially different relationships between emotion and cognition (more or less independence between the two, respectively). Evidence both for and against automaticity has been presented. For instance, emotional faces evoke responses in the amygdala when attention is diverted to other stimuli61, 62. Perhaps even more strikingly, amygdala responses are sometimes observed for emotional faces of which subjects are presumably not conscious63-65. Furthermore, cases of so-called affective blindsight have been reported66. These and other related findings suggest that at least some types of emotional perception occur outside of “cognitive” processing. Other findings have suggested, however, that the perception of emotion-laden items requires attention, as revealed by attentional manipulations that were designed to more strongly consume processing resources, leaving relatively few for the processing of unattended emotional items67-73. It also appears that amygdala responses evoked by “unaware” stimuli depend on the manner by which awareness is operationally defined74, such that unaware responses are not observed when awareness is defined, for instance, via signal detection theory methods75. Overall, the automaticity debate remains unresolved and controversial47, 76-79.
Executive functions
The impact of emotion on cognition is rich and varied and has been documented in a range of tasks. This section will briefly illustrate interactions involving two executive functions. The first examples come from an important dimension of cognitive function that includes inhibiting and controlling behavior. Response inhibition, namely the processes required to cancel an intended action, is believed to involve control regions in medial and lateral prefrontal cortex, including pre-supplementary motor cortex and inferior frontal gyrus80-82. Response inhibition is at times investigated by using so-called go/no-go tasks in which subjects are asked to execute a motor response when shown the “go” stimulus (e.g., “press a key as fast as possible when you see a letter stimulus”), but to withhold the response when shown the “no-go” stimulus (e.g., “do not respond when you see the letter Y”). Typically, the go and no-go stimuli are shown as part of a rapid stream of stimuli (e.g., a sequence of letters). A recent study investigated the interaction between the processing of emotional words and response inhibition83. Response inhibition following negative words (e.g., “worthless”) engaged the dorsolateral prefrontal cortex (although behavioral effects of emotional content were modest, further evidence indicates that response inhibition behavior is affected by stronger emotional stimuli84). Interestingly, this region was not recruited by negative valence or inhibitory task demands per se; instead, the dorsolateral prefrontal cortex was sensitive to the interaction between behavioral inhibition and the processing of negatively valenced words, namely a cognitive-emotional interaction.
Working memory, another important cognitive function, involves the maintenance and updating of information in mind when the information is no longer available to sensory systems. Evidence for cognitive-emotional interaction comes from working memory studies, too. For instance, when participants were asked to keep in mind neutral or emotional pictures, maintenance-related activity in dorsolateral prefrontal cortex was modulated by the valence of the picture, with pleasant pictures enhancing activity and unpleasant pictures decreasing activity relative to neutral ones85. Interestingly, emotional pictures did not affect dorsolateral responses during a second experimental condition during which participants were not required to keep information in mind, indicating that the modulation of sustained activity by emotional valence was particular to the experimental context requiring active maintenance. In another study, participants watched short videos intended to induce emotional states (e.g., clips from uplifting or sad movies), after which they performed challenging working memory tasks86. Lateral prefrontal cortex activity on both hemispheres equally reflected the emotional and working memory task components. In other words, prefrontal activity did not stem from the working memory task alone or by the mood ensuing from the viewing of the video, but resulted from an interaction between emotion and cognition.
In summary, these examples highlight the notion that many of the effects of emotion on cognition are best viewed as interactions between the two such that the resulting processes and signals are neither purely cognitive nor emotional. Instead, the “cognitive” or “emotional” nature of the processes is blurred in a way that highlights the integration of the two domains in the brain.
Dual competition framework
The last two sections described both anatomical and functional evidence for the interaction between emotion and cognition. How do these interactions influence the flow of information processing in the brain14, 43, 87, 88? Several proposals have been advanced in the literature, focusing either on perceptual or cognitive processing. Here, the discussion of the previous sections is extended to further delineate how some of the brain regions discussed may contribute to cognitive-emotional interactions. The presentation refines and extends a conceptual framework described recently89. It was suggested that both emotion and motivation signals are integrated with perception and cognition so as to effectively incorporate value into the unfolding of behavior. The proposed framework was called the dual competition model to reflect the suggestion that affective significance influences competition at both the perceptual and executive levels (Fig. 2) – and because the impact is due to both emotion and motivation, although the latter is not discussed here (but see ref.90).
Figure 2.
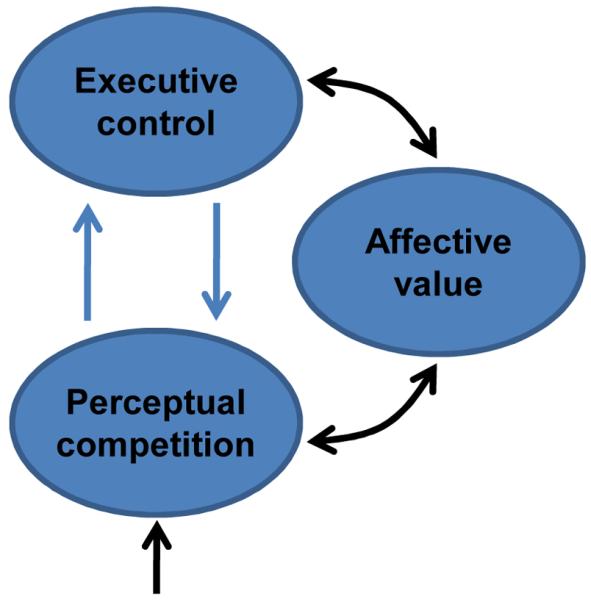
Cognitive-emotional interactions. Affective value interacts with both perceptual and executive processes.
Objects compete for limited perceptual processing capacity and control of behavior91, 92. Because processing capacity is limited, selective attention to one part of the visual field comes at the cost of neglecting other parts. Thus, a popular notion is that there is competition for neural resources91, 93. As described below, to understand the flow of information processing more generally, it is necessary to go beyond the role of perceptual competition, and explicitly incorporate the impact of executive control functions on processing. Behavioral research supports the notion that executive control is not unitary and that different mechanisms may have their own limited processing capacities, or resources94, 95. Neuropsychological research also supports the dissociation of cognitive functions, consistent with the “fractionation” of the central executive96, 97. Yet, ample evidence suggests some unity of executive functions, specifically that certain mechanisms are shared across them98, 99. This capacity sharing has important implications for the understanding of human information processing because it leads to executive competition: subcomponents of executive control are mutually interacting, such that resources devoted to one component will not be available to other functions.
Perceptual competition
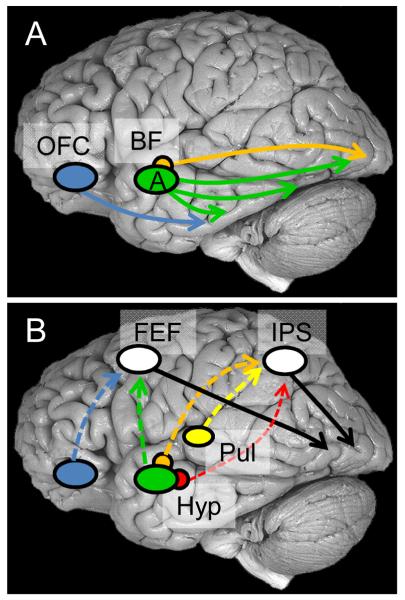
Perceptual competition, which takes place in visual cortex, is affected by emotional content. As discussed, the amygdala is well positioned to implement the enhancement of visual activity given that its efferents reach multiple levels of the visual cortex, including primary visual cortex23. Although the role of the amygdala in the modulation of visual processing is often emphasized in the literature, several other mechanisms likely play important roles, too100. A second modulatory source may involve the orbitofrontal cortex20 (Fig. 3A), a structure that has important roles in the evaluation of sensory stimuli101. The orbitofrontal cortex is reciprocally interconnected with visual cortex, especially the more anterior portions of the ventral stream12, 102 and is thus capable of influencing evoked responses in visual cortex based on affective value.
Figure 3.
Interactions between emotion and perception. (A) Visual processing is suggested to be modulated by affective value via several mechanisms, including those involving projections from the amygdala, basal forebrain, orbitofrontal cortex, and possibly hypothalamus (not shown). (B) Another class of modulatory mechanisms relies on interactions between “evaluative” sites (shown in color) and “control” sites (shown in white), the latter located in fronto-parietal cortex and known to exert top-down influences on visual processing. Dotted lines indicate possibly indirect connections. All locations are approximate, and the positions of the amygdala, basal forebrain, hypothalamus, and pulvinar are shown on the lateral surface for schematic purposes. Abbreviations: A, amygdala; BF, basal forebrain; FEF, frontal eye field; Hyp, hypothalamus; IPS, intraparietal sulcus; OFC, orbitofrontal cortex; Pul, pulvinar.
A third important mechanism involves the basal forebrain (Fig. 3A). The central nucleus of the amygdala has significant projections to several basal forebrain structures, and one mechanism by which the central nucleus influences cortical processing is by engaging magnocellular basal forebrain neurons (see ref.103, 104), whose terminals release acetylcholine onto cortical sensory neurons (GABAergic processes have also been described). Lesions of the basal forebrain have been shown to impair a host of attentional tasks, and together with physiological studies, reveal the importance of the basal forebrain not only for sustained attention, but also for selective aspects of stimulus processing, including the filtering of irrelevant information6, 7.
A final class of modulatory mechanisms relies on the fronto-parietal attentional network (Fig. 3B), including lateral prefrontal cortex, frontal eye field, and parietal cortex, which modulate visual processing according to an item’s behavioral relevance. These regions are believed to be “control sites” that provide the source of top-down attentional signals105, 106. Importantly, both frontal eye field and parietal cortex appear to contain a “priority map”, namely a representation of spatial locations containing information that is rich in terms of salience (e.g., high-contrast stimuli) and/or relevance (e.g., stimuli connected to current goals)107, 108. It is suggested here that the fronto-parietal network works closely with several “evaluative” sites discussed in the first section, such as hypothalamus, amygdala, cingulate cortex, orbitofrontal cortex, and anterior insula, to prioritize processing based on the affective significance of a sensory stimulus (for a related discussion in the case of motivation, see ref.90). In some of these cases, the direct connections between “evaluative” and “control” regions may be relatively weak, and indirect routes involving one or more intermediate steps are probably involved.
An additional modulatory role is proposed for the pulvinar complex of the thalamus (Fig. 3B). Based on anatomical and physiological considerations, it was suggested that the importance of the pulvinar for affective processing is not due to its putative role as part of a subcortical pathway, as often assumed in the literature, but instead because of its connectivity with other cortical regions19. Briefly, the medial nucleus of the pulvinar, which projects to the amygdala, is part of several thalamo-cortical loops that include orbitofrontal, cingulate, and insular cortices (in addition to frontal and parietal sites). Given this broad connectivity pattern, the medial nucleus may be involved in two general functions that directly impact emotional processing: determining behavioral relevance and/or value. Therefore, the role of the pulvinar may extend beyond the well-established roles in attention109 and contribute to affective processing110-112.
In summary, during the past decade, an important role for the amygdala in the emotional modulation of vision has been highlighted in the literature. Yet, as described here, the amygdala is but one of the sources of modulation of visual responses that take into consideration the behavioral and affective significance of sensory stimuli. Future research is needed to establish how these multiple modulatory sources influence visual processing in particular, and other sensory modalities more generally.
Executive control and competition
How does emotional content impact executive function? Because emotion can either enhance or impair performance of executive functions, answering this question has been challenging. At least part of the answer may be related to the level of threat posed by an emotional item. When threat content is relatively low, processing is biased in favor of the emotional item and although emotional items are prioritized, the impact on behavior may be modest. Importantly, emotional content enhances task-relevant processing with relatively minor effects on irrelevant stimuli and other executive functions that may be concurrently needed.
A more dramatic effect of emotional content on behavior is expected when the level of threat is high. In this situation, processing resources are diverted toward the processing of the item at hand and because the mobilization of resources is more extreme, the effects on behavior are considerably more dramatic113, 114. In particular, the impact on behavior may come from the recruitment of attentional/effortful control that is required to prioritize the processing of high-threat information. Attentional/effortful control involves processing resources that are shared across executive functions and because high-threat is expected to recruit some of these resources (see also 78, 115, 116), it will impair other executive functions that are reliant on them (Fig. 4). Consistent with this idea, performance during response inhibition was compromised when participants viewed high- vs. low-arousing pictures84.
Figure 4.
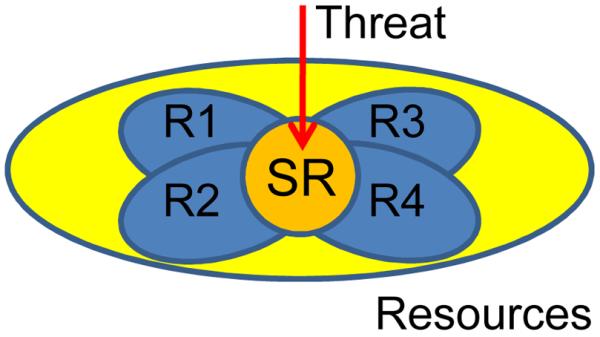
Executive competition and threat. Executive functions can be viewed as relying on multiple mechanisms, also referred to as resources (R1 through R4; e.g., “shifting”, “updating”), that are partly independent but, critically, are also shared (indicated by the orange circle). When threat content is high, these shared resources (“SR”) are engaged, thus detracting from the ability to recruit specific mechanisms at optimal levels. Consequently, behavioral performance relying on those mechanisms will be impaired.
In the past, the notion of resources has been employed in order to account for the limits of human information processing. A potential approach to understanding resource consumption by threat may be to probe the correspondence of brain sites that are sensitive to specific experimental conditions. It is particularly instructive, for instance, to examine the overlap between manipulations of threat level and those involving attention – given that attentional manipulations are sensitive to changes in the distribution of processing resources. The “attentional network” has been extensively researched and is believed to involve fronto-parietal regions, including the middle frontal gyrus, inferior frontal gyrus, anterior cingulate cortex, and anterior insula105, 106. To assess brain regions that are sensitive to high levels of threat, the activation sites of the contrast of CS+ (i.e., aversively conditioned) vs. CS− (i.e., neutral) of 34 aversive conditioning studies were reviewed. Although great emphasis is put on the involvement of the amygdala in the processing of threat, this summary revealed that several frontal activation sites were consistently reported, including middle frontal gyrus, inferior frontal gyrus, anterior cingulate cortex, and anterior insula89. This evaluation thus suggests that processing high-threat items engages key nodes of the attentional network, suggesting that it consumes processing resources.
What are some of the neural substrates of the interactions between emotion and cognition? When items are high in threat, robust interactions between affective processing and executive functions are proposed to take place via several neural mechanisms. First, it is hypothesized that threat processing engages attentional/effortful control mechanisms in several fronto-parietal sites, including lateral prefrontal cortex, anterior cingulate cortex, and parietal cortex. The role of the anterior cingulate cortex may be particularly important because of its role in integrating inputs from multiple sources, including cognitive, affective and motivational inputs117 (Fig. 5). In cognitive studies, the anterior cingulate has been suggested to be involved in conflict detection, error likelihood processing, and error monitoring, among other functions. Anterior cingulate engagement during threat may impair executive function because shared resources required to prioritize threat processing are recruited. In other words, anterior cingulate sites engaged by high-threat are at the intersection of the resources needed for several executive functions (as indicated by the orange region in Fig. 4). Notably, the anterior cingulate engagement includes the dorsal sector, in contrast to the idea that the dorsal anterior cingulate is involved in cognitive function, in opposition to the more rostral, “emotional” sector118.
Figure 5.
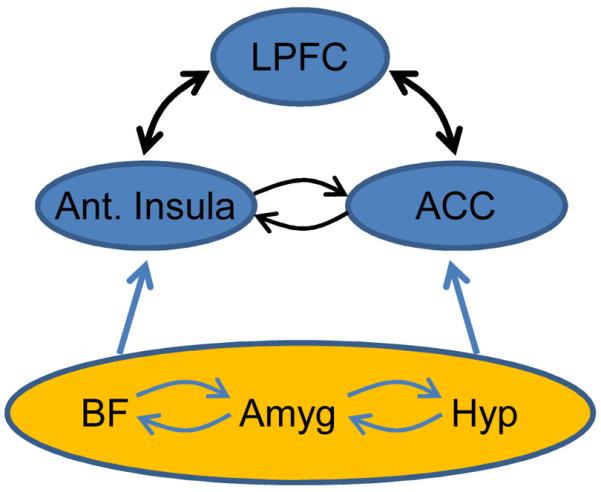
Interactions between emotion and cognition. The anterior insula and the anterior cingulate cortex are important sites involved in determining affective significance and value. In so doing, they are closely affiliated with an extended set of regions, some of which are shown here in the orange ellipse. The anterior insula and anterior cingulate cortex interact closely with the lateral prefrontal cortex, a region involved in several cognitive functions. In this manner, cognitive-emotional interactions occur during complex behaviors. Notably, these interactions can be either beneficial or detrimental to behavior. In the latter case, for instance, when threat level is high, resources required for cognitive operations are partly consumed, thereby compromising behavioral performance. Abbreviations: ACC: anterior cingulate cortex; Amyg, amygdala; Ant. Insula, anterior insula; BF, basal forebrain; Hyp, hypothalamus; LPFC, lateral prefrontal cortex.
As discussed, the anterior insula is critical for interoception, which involves monitoring the sensations that are important for the integrity of the internal body state, and interacting with systems that are important for evaluating context, allocating attention, and planning actions119. Threat, uncertainty, and risk are all potent factors that engage the anterior insula120. Remarkably, the anterior insula also was found to be activated in most cognitive tasks for which Van Snellenberg and Wager121 had meta-analytic data. The anterior insula is thus a site that is engaged during both cognitive and emotional contexts (Fig. 5). Accordingly, recruitment of the anterior insula during high-threat conditions will detract from its ability to assist in executive functions; a concomitant impairment in performance is thus expected. Note that this argument assumes that the engagement of the anterior insula during high-threat conditions substantially intersects with cortical territories that are required for cognitive processes (see “SR” in Fig. 4). Naturally, these and other aspects of the dual competition framework need to be validated by experimental data.
A second effect of threat is to trigger specific executive functions to handle on-going challenges to the organism. For instance, “updating” might be needed to refresh the contents of working memory, “shifting” might be recruited to switch the current task set, and “inhibition” could be called to cancel previously planned actions. Again, this recruitment is suggested to depend, at least in part, on the anterior cingulate cortex and the anterior insula – the former is known to influence activity in other brain regions and to modulate cognitive, motor, and visceral responses117. For instance, the anterior cingulate may work in close cooperation with lateral prefrontal cortex (see also ref.122), a region that is important for the manipulation of information, among other functions. In this manner, additional specific processing resources are coordinated in the service of threat processing (Fig. 5). Affective information conveyed by other brain regions, including the hypothalamus, amygdala, basal forebrain, and orbitofrontal cortex is conveyed (possibly indirectly) to lateral prefrontal cortex and parietal sites, too, further engaging executive power in the function of handling the threat to the organism. In finalizing the discussion of the involvement of fronto-parietal regions in interactions between emotion and executive function, note that these are some of the same regions that were implicated as having an important effect on perceptual competition (Fig. 3B), highlighting the interdependence of perceptual and executive processes – in other words, the sharp distinction between bottom-up and top-down in Fig. 2 is artificial.
A third effect of threat on executive functions involves state changes that are implemented via ascending systems7, 123. The basal forebrain, hypothalamus, and reticular formation have the ability to influence both cortical and subcortical processing via widespread projections. In particular, the overall anatomical arrangement of the basal forebrain (here, more broadly construed) might involve multiple functional-anatomical macrosystems124, 125 with wide-ranging effects on brain computations and important clinical implications6, 14, 124. More generally, the three structures may be viewed as key components of the “behavioral state system”, which has been suggested to be one of the major functional subsystems of the vertebrate nervous system126 (together with “cognitive”, “sensory”, and “motor” systems).
Conclusions
Historically, emotion and cognition have been viewed as largely separate entities. One way in which emotion has been contrasted to cognition has been to link the former with “irrational” or “suboptimal” processes127 that are more “basic”, namely more linked to survival, than cognitive ones. Although much has changed in the past two decades, versions of this viewpoint still are quite frequent in the literature (even if, at times, implicitly). Research in the past decades suggests, however, that such view is likely erroneous and that, in order to understand how complex behaviors are carried out in the brain, an understanding of the interactions between the two is indispensable. Interestingly, neuroimaging in humans may have been one factor contributing to the change in this viewpoint. Because neuroimaging techniques afford whole-brain investigations, it has become increasingly evident that large portions of both cortex and subcortex are engaged during emotional information analyses128.
In many current formulations of how emotion is organized in the brain, a heavy emphasis is found on “special” regions, most notably, the amygdala. In particular, it could be argued that the amygdala is “primitive” (in the sense of being derived from ancestral form), and that it may be better viewed as tied to fear-related functions and as an effective “alarm system” – one that has been evolutionarily conserved for good reasons. Yet, even in rodents important roles for the amygdala in “cognitive” operations, such as attention and decision making, have been documented129, 130. And in primates, as pointed out by Sander and colleagues, the amygdala may have evolved into a less specialized system in order to cope with new environmental problems131. One way in which this may have occurred may be related to an expansion of the connectivity of the amygdala with a wider range of cortical territories132. This may involve new direct connections, such as the connectivity documented between the amygdala and lateral prefrontal cortex22 and, more extensively, indirect connections via other important cortical hubs, such as those involving the anterior cingulate, orbitofrontal, and insular cortices. Altered and enhanced connectivity may be one way in which a system expands the repertoire of functions it is involved in. Although the evolution of the brain is highly constrained, dramatic changes in the pattern of connectivity have been documented – such as those involving the somatosensory cortex and thalamus in several mammals 133, 134. Furthermore, Whereas mice have about 10 cortical fields, and macaque monkeys have more than 50 fields, humans may have more than a hundred fields134. The combinatorial nature of connectivity is such that, in humans, the amygdala, which is extremely highly interconnected, as reviewed here, may be in a position to be an important player in an impressive array of cognitive-emotional functions.
Generally speaking, given the combinatorial connectivity of the brain, it will be important to go beyond simply describing interactions between emotion and cognition, some of which are suggested to be mutually antagonistic135. Instead, future advances will be made by the mechanistic description of how cognition and emotion are effectively integrated in the brain. This is especially pertinent in light of the suggestion that in many cases functional specialization is lost, and emotion and cognition conjointly and equally contribute to the control of mental activities and behavior86. For instance, the affective dimensions of a visual item are reflected at multiple processing stages, from early visual areas to prefrontal sites136. In addition, visual cortical responses reflecting an item’s significance will be a result of simultaneous top-down modulation from fronto-parietal attentional regions and emotional modulation from the amygdala, basal forebrain, orbitofrontal cortex, and other regions. This perspective can also be adopted in the context of executive functioning, such that cognitive and emotional contributions to executive control are difficult to separate. For example, lateral prefrontal cortex signals involved in inhibitory processes may reflect both cognitive variables (e.g., an inhibitory response is required) and affective information (e.g., negative stimuli are viewed before being required to inhibit a response). A key implication of the integration viewpoint is that, in general, it may be simply counterproductive to attempt to separate emotion and cognition. Instead, their interdependence challenges a simple division into separate “cognitive” and “emotional” domains88.
Acknowledgements
The author thanks the National Institute of Mental Health (R01 MH071589) for supporting his research. I would like to thank Jena Wierwille for assistance with figures.
References
- 1.Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Brain Res Rev. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- 2.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain research. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 3.Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. The Journal of comparative neurology. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Mesulam M-M. Behavioral neuroanatomy: Large-scale networks, association cortex, frontal syndromes, the limbic system, and hemispheric specializations. In: Mesulam M, editor. Principles of behavioral and cognitive neurology. Oxford University Press; New York: 2000. pp. 1–120. [Google Scholar]
- 5.Mesulam MM, Hersh LB, Mash DC, Geula C. Differential cholinergic innervation within functional subdivisions of the human cerebral cortex: a choline acetyltransferase study. The Journal of comparative neurology. 1992;318:316–328. doi: 10.1002/cne.903180308. [DOI] [PubMed] [Google Scholar]
- 6.Sarter M, Bruno JP. Abnormal regulation of corticopetal cholinergic neurons and impaired information processing in neuropsychiatric disorders. Trends in neurosciences. 1999;22:67–74. doi: 10.1016/s0166-2236(98)01289-2. [DOI] [PubMed] [Google Scholar]
- 7.Sarter M, Bruno JP. Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents. Neuroscience. 2000;95:933–952. doi: 10.1016/s0306-4522(99)00487-x. [DOI] [PubMed] [Google Scholar]
- 8.Golmayo L, Nunez A, Zaborszky L. Electrophysiological evidence for the existence of a posterior cortical-prefrontal-basal forebrain circuitry in modulating sensory responses in visual and somatosensory rat cortical areas. Neuroscience. 2003;119:597–609. doi: 10.1016/s0306-4522(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 9.Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Annals of the New York Academy of Sciences. 1999;877:339–367. doi: 10.1111/j.1749-6632.1999.tb09276.x. [DOI] [PubMed] [Google Scholar]
- 11.Young MP, Scannell JW, Burns GAPC, Blakemore C. Analysis of connectivity: Neural systems in the cerebral cortex. Reviews in the Neurosciences. 1994;5:227–249. doi: 10.1515/revneuro.1994.5.3.227. [DOI] [PubMed] [Google Scholar]
- 12.Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neuroscience and Biobehavioral Reviews. 1995;19:449–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 13.Swanson LW. The amygdala and its place in the cerebral hemisphere. Annals of the New York Academy of Sciences. 2003;985:174–184. doi: 10.1111/j.1749-6632.2003.tb07081.x. [DOI] [PubMed] [Google Scholar]
- 14.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 15.Guimera R, Nunes Amaral LA. Functional cartography of complex metabolic networks. Nature. 2005;433:895–900. doi: 10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sporns O, Honey CJ, Kotter R. Identification and classification of hubs in brain networks. PLoS ONE. 2007;2:e1049. doi: 10.1371/journal.pone.0001049. doi:1010.1371/journal.pone.00001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pessoa L. Emotion and cognition and the amygdala: From “what is it?” to “what’s to be done?”. Neuropsychologia. 2010 doi: 10.1016/j.neuropsychologia.2010.06.038. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeDoux JE. The emotional brain. Simon & Schuster; New York: 1996. [Google Scholar]
- 19.Pessoa L, Adolphs R. Emotion processing: From a “low road” to “many roads” of evaluating biological significance. Nat Rev Neurosci. 2010 doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett LF, Bar M. See it with feeling: affective predictions during object perception. Philosophical transactions of the Royal Society of London. 2009;364:1325–1334. doi: 10.1098/rstb.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) The Journal of comparative neurology. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 22.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton J, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. pp. 1–66. [Google Scholar]
- 24.Freese JL, Amaral DG. The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. The Journal of comparative neurology. 2005;486:295–317. doi: 10.1002/cne.20520. [DOI] [PubMed] [Google Scholar]
- 25.Girard P, Bullier J. Visual activity in area V2 during reversible inactivation of area 17 in the macaque monkey. J Neurophysiol. 1989;62:1287–1302. doi: 10.1152/jn.1989.62.6.1287. [DOI] [PubMed] [Google Scholar]
- 26.Hupe JM, James AC, Girard P, Bullier J. Response modulations by static texture surround in area V1 of the macaque monkey do not depend on feedback connections from V2. J Neurophysiol. 2001;85:146–163. doi: 10.1152/jn.2001.85.1.146. [DOI] [PubMed] [Google Scholar]
- 27.Swanson LW, Petrovich GD. What is the amygdala? Trends in neurosciences. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 28.Neafsey EJ. Prefrontal cortical control of the autonomic nervous system: anatomical and physiological observations. Progress in brain research. 1990;85:147–165. doi: 10.1016/s0079-6123(08)62679-5. discussion 165-146. [DOI] [PubMed] [Google Scholar]
- 29.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 30.Damasio AR. The feeling of what happens: body and emotion in the making of consciousness. Harcourt Brace; New York: 1999. [Google Scholar]
- 31.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 32.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 33.Vogt BA, editor. Cingulate Neurobiology and Disease. Oxford University Press; Oxford: 2009. [Google Scholar]
- 34.Vogt BA, Vogt LJ. Mu-opiod receptors, placebo map, descending systems, and cingulate-mediated control of vocalization and pain. In: Vogt BA, editor. Cingulate neurobiology and disease. Oxford University Press; Oxford: 2009. pp. 339–364. [Google Scholar]
- 35.Vogt BA, Derbyshire SWG. Visceral circuits and cingulate-mediated functions. In: Vogt BA, editor. Cingulate neurobiology and disease. Oxford University Press; Oxford: 2009. pp. 219–235. [Google Scholar]
- 36.Vogt BA, Aston-Jones G, Vogt LJ. Shared norepinephrinergic and cingulate circuits, nociceptive and allostatic interactions, and models of functional pain and stress disorders. In: Vogt BA, editor. Cingulate neurobiology and disease. Oxford University Press; Oxford: 2009. pp. 467–497. [Google Scholar]
- 37.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. The Journal of comparative neurology. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 39.Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC neuroscience. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. The Journal of comparative neurology. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- 41.Price JL. Architectonic structure of the orbital and medial prefrontal cortex. In: Zald DH, Rauch SL, editors. The orbitofrontal cortex. Oxford University Press; Oxford: 2006. pp. 3–17. [Google Scholar]
- 42.Damasio AR. Descartes’ error: Emotion, reason, and the human brain. G.P. Putnam; New York: 1994. [Google Scholar]
- 43.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual review of psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 44.Dolan R. Emotion, cognition, and behavior. Science (New York, N.Y. 2003;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- 45.Rolls ET. Emotion explained. Oxford University Press; Oxford: 2005. [Google Scholar]
- 46.Pessoa L, Ungerleider LG. Neuroimaging studies of attention and the processing of emotion-laden stimuli. Progress in brain research. 2004;144:171–182. doi: 10.1016/S0079-6123(03)14412-3. [DOI] [PubMed] [Google Scholar]
- 47.Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Padmala S, Pessoa L. Affective learning enhances visual detection and responses in primary visual cortex. J Neurosci. 2008;28:6202–6210. doi: 10.1523/JNEUROSCI.1233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Damaraju E, Huang YM, Barrett LF, Pessoa L. Affective learning enhances activity and functional connectivity in early visual cortex. Neuropsychologia. 2009;47:2480–2487. doi: 10.1016/j.neuropsychologia.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eastwood JD, Smilek D, Merikle PM. Differential attentional guidance by unattended faces expressing positive and negative emotion. Percept Psychophys. 2001;63:1004–1013. doi: 10.3758/bf03194519. [DOI] [PubMed] [Google Scholar]
- 51.Ohman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130:466–478. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- 52.Cave KR, Batty MJ. From searching for features to searching for threat: Drawing the boundary between preattentive and attentive vision. Visual Cognition. 2006;14:629–646. [Google Scholar]
- 53.Anderson AK. Affective influences on the attentional dynamics supporting awareness. J Exp Psychol Gen. 2005;134:258–281. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- 54.Vuilleumier P, Schwartz S. Emotional facial expressions capture attention. Neurology. 2001;56:153–158. doi: 10.1212/wnl.56.2.153. [DOI] [PubMed] [Google Scholar]
- 55.Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychol Sci. 2006;17:292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 57.Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- 58.Tsuchiya N, Moradi F, Felsen C, Yamazaki M, Adolphs R. Intact rapid detection of fearful faces in the absence of the amygdala. Nat Neurosci. 2009;12:1224–1225. doi: 10.1038/nn.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lim SL, Padmala S, Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proc Natl Acad Sci U S A. 2009;106:16841–16846. doi: 10.1073/pnas.0904551106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neutral and emotional stimuli. Cognitive Brain Research. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 61.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 62.Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JD. Neural correlates of the automatic processing of threat facial signals. Journal of Neuroscience. 2003;23:5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whalen PJ, et al. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- 65.Whalen PJ, et al. Human amygdala responsivity to masked fearful eye whites. Science (New York, N.Y. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- 66.de Gelder B, Vroomen J, Pourtois G, Weiskrantz L. Non-conscious recognition of affect in the absence of striate cortex. Neuroreport. 1999;10:3759–3763. doi: 10.1097/00001756-199912160-00007. [DOI] [PubMed] [Google Scholar]
- 67.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences USA. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hsu SM, Pessoa L. Dissociable effects of bottom-up and top-down factors on the processing of unattended fearful faces. Neuropsychologia. 2007;45:3075–3086. doi: 10.1016/j.neuropsychologia.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim SL, Padmala S, Pessoa L. Affective learning modulates spatial competition during low-load attentional conditions. Neuropsychologia. 2008;46:1267–1278. doi: 10.1016/j.neuropsychologia.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. NeuroImage. 2005;28:249–255. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cereb Cortex. 2007;17:1595–1603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- 73.Silvert L, et al. Influence of attentional demands on the processing of emotional facial expressions in the amygdala. Neuroimage. 2007;38:357–366. doi: 10.1016/j.neuroimage.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 74.Merikle PM, Smilek D, Eastwood JD. Perception without awareness: perspectives from cognitive psychology. Cognition. 2001;79:115–134. doi: 10.1016/s0010-0277(00)00126-8. [DOI] [PubMed] [Google Scholar]
- 75.Pessoa L, Japee S, Sturman D, Ungerleider LG. Target visibility and visual awareness modulate amygdala responses to fearful faces. Cerebral Cortex. 2006;16:366–375. doi: 10.1093/cercor/bhi115. [DOI] [PubMed] [Google Scholar]
- 76.Wiens S. Subliminal emotion perception in brain imaging: findings, issues, and recommendations. Progress in brain research. 2006;156:105–121. doi: 10.1016/S0079-6123(06)56006-6. [DOI] [PubMed] [Google Scholar]
- 77.Pessoa L. To what extent are emotional visual stimuli processed without attention and awareness? Curr Opin Neurobiol. 2005;15:188–196. doi: 10.1016/j.conb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 78.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 79.Pessoa L, Oliveira L, Pereira MG. Attention and emotion. Scholarpedia. 2010;5:6314. [Google Scholar]
- 80.Sharp DJ, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci U S A. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 82.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 83.Goldstein M, et al. Neural substrates of the interaction of emotional stimulus processing and motor inhibitory control: an emotional linguistic go/no-go fMRI study. Neuroimage. 2007;36:1026–1040. doi: 10.1016/j.neuroimage.2007.01.056. [DOI] [PubMed] [Google Scholar]
- 84.Verbruggen F, De Houwer J. Do emotional stimuli interfere with response inhibition? Evidence from the stop signal paradigm. Cognition and Emotion. 2007;21:391–403. [Google Scholar]
- 85.Perlstein WM, Elbert T, Stenger VA. Dissociation in human prefrontal cortex of affective influences on working memory-related activity. Proc Natl Acad Sci U S A. 2002;99:1736–1741. doi: 10.1073/pnas.241650598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences USA. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lewis MD. Bridging emotion theory and neurobiology through dynamic systems modeling. The Behavioral and brain sciences. 2005;28:169–194. doi: 10.1017/s0140525x0500004x. discussion 194-245. [DOI] [PubMed] [Google Scholar]
- 88.Duncan S, Barrett LF. Affect is a form of cognition: A neurobiological analysis. Cognition and Emotion. 2007;21:1184–1211. doi: 10.1080/02699930701437931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pessoa L. How do emotion and motivation direct executive function? Trends Cogn Sci. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pessoa L, Engelmann JB. Embedding reward signals into perception and cognition. Front Neurosci. 2010;4 doi: 10.3389/fnins.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Desimone R, Duncan J. Neural mechanisms of selective attention. Annual review of neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 92.Pashler H. The Psychology of Attention. MIT Press; Cambridge, MA: 1998. [Google Scholar]
- 93.Grossberg S. How does a brain build a cognitive code? Psychological review. 1980;87:1–51. doi: 10.1007/978-94-009-7758-7_1. [DOI] [PubMed] [Google Scholar]
- 94.Kahneman D. Attention and effort. Prentice-Hall; Englewood Cliffs, NJ: 1973. [Google Scholar]
- 95.Norman DA, Bobrow DG. On data-limited and resource-limited processes. Cognitive psychology. 1975;7:44–64. [Google Scholar]
- 96.Norman DA, Shallice T. Attention to action: willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and self-regulation. Plenum; New York: 1986. [Google Scholar]
- 97.Stuss D, Knight RT, editors. Principles of frontal lobe function. Oxford University Press; Oxford: 2002. [Google Scholar]
- 98.Miyake A, et al. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognitive psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 99.Duncan J, Emslie H, Williams P, Johnson R, Freer C. Intelligence and the frontal lobe: the organization of goal-directed behavior. Cognitive psychology. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- 100.Barrett LF, Bliss-Moreau E. Affect as a psychological primitive. Advances in Experimental Social Psychology. 2009;41:167–218. doi: 10.1016/S0065-2601(08)00404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zald DH, Rauch SL. The orbitofrontal cortex. Oxford University Press; Oxford: 2007. [Google Scholar]
- 102.Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- 103.Holland PC, Han JS, Gallagher M. Lesions of the amygdala central nucleus alter performance on a selective attention task. J Neurosci. 2000;20:6701–6706. doi: 10.1523/JNEUROSCI.20-17-06701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holland PC, Gallagher M. Different roles for amygdala central nucleus and substantia innominata in the surprise-induced enhancement of learning. J Neurosci. 2006;26:3791–3797. doi: 10.1523/JNEUROSCI.0390-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual review of neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 106.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 107.Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10:38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 108.Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci. 2006;10:382–390. doi: 10.1016/j.tics.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 109.Shipp S. The brain circuitry of attention. Trends Cogn Sci. 2004;8:223–230. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 110.Ward R, Danziger S, Bamford S. Response to visual threat following damage to the pulvinar. Curr Biol. 2005;15:571–573. doi: 10.1016/j.cub.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 111.Ward R, Calder AJ, Parker M, Arend I. Emotion recognition following human pulvinar damage. Neuropsychologia. 2007;45:1973–1978. doi: 10.1016/j.neuropsychologia.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 112.Padmala S, Lim S-L, Pessoa L. Pulvinar and affective significance: responses track moment-to-moment visibility. Frontiers in Human Neuroscience. 2010;4:1–9. doi: 10.3389/fnhum.2010.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Panksepp J. Affective neuroscience: The foundations of human and animal emotions. Oxford University Press; New York: 1998. [Google Scholar]
- 114.Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. Journal of affective disorders. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- 115.Mathews A, Mackinstosh B. A cognitive model of selective processing in anxiety. Cogn Ther Res. 1998;22:539–560. [Google Scholar]
- 116.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 117.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 118.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 119.Paulus MP, Stein MB. An insular view of anxiety. Biological psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 120.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 121.Van Snellenberg JX, Wager TD. Cognitive and motivational functions of the human prefrontal cortex. In: Christensen A-L, Goldberg E, Bougakov D, editors. Luria’s legacy in the 21st century. Oxford University Press; Oxford: 2010. pp. 30–60. [Google Scholar]
- 122.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 123.Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 124.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 125.Zahm DS. The evolving theory of basal forebrain functional-anatomical ‘macrosystems’. Neurosci Biobehav Rev. 2006;30:148–172. doi: 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 126.Swanson LW. Brain architecture: Understanding the basic plan. Oxford University Press; New York: 2003. [Google Scholar]
- 127.Oatley K, Keltner D, Jenkins JM. Understanding emotions. Blackwell Publishing; Malden, MA: 2006. [Google Scholar]
- 128.Kober H, et al. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 130.Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cognitive, affective & behavioral neuroscience. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- 131.Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Rev Neurosci. 2003;14:303–316. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- 132.Barton RA, Aggleton JP. Primate evolution and the amygdala. In: Aggleton JP, editor. The amygdala: A functional analysis. Oxford University Press; Oxford: 2000. pp. 479–508. [Google Scholar]
- 133.Kaas JH. Evolution of somatosensory and motor cortex in primates. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1148–1156. doi: 10.1002/ar.a.20120. [DOI] [PubMed] [Google Scholar]
- 134.Krubitzer L. In search of a unifying theory of complex brain evolution. Annals of the New York Academy of Sciences. 2009;1156:44–67. doi: 10.1111/j.1749-6632.2009.04421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition and Emotion. 1998;12:353–385. [Google Scholar]
- 136.Thielscher A, Pessoa L. Neural correlates of perceptual choice and decision making during fear-disgust discrimination. J Neurosci. 2007;27:2908–2917. doi: 10.1523/JNEUROSCI.3024-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]