Abstract
The itch field has made great advances in recent years, building upon earlier work to form a clearer picture of the biology behind this important sensory modality. Models for how itch is encoded have emerged that fit with physiological, molecular, and behavioral data. The molecular mechanisms of itch, both peripherally and centrally, are being revealed with the aid of newer animal models. Future work must address shortcomings in our current understanding of itch including limitations of current experimental methods. Here we review what is known about the cells, molecules, and circuits involved in itch and highlight key questions that remain to be answered.
Keywords: Itch, pain, DRG neurons, skin, spinal cord, Mrgpr, GRPR, histamine, TRP
The definition of itch, or pruritus, as an “unpleasant sensation that elicits the desire or reflex to scratch” was coined 350 years ago by the German physician Samuel Hafenreffer (1). However, this simple description belies the complexity of a sensory modality that becomes more complicated upon further investigation. As the study of the neuroscience of itch has blossomed in recent years, our understanding of this biologically interesting and clinically relevant phenomenon has led to exciting discoveries about the nature of pruritoception while generating a new set of questions.
Itch is manifested in both acute and chronic forms, and in the clinic there are several varieties arising from different or mixed etiologies. Poison ivy produces pruritoceptive itch via peripheral fiber activation, while neurogenic itch, which occurs in cholestasis (2, 3), may include central mechanisms. Nerve damage as in the case of shingles causes neuropathic itch, and psychiatric conditions such as obsessive–compulsive disorder can generate psychogenic itch (4).
Any discussion of itch is incomplete without mentioning pain, as the two are closely related. The requisite example of this is how the mild pain of scratching inhibits the perception of itch. Itch and pain share certain molecular mechanisms and are transduced peripherally by similar neuronal populations. Unlike other sensory modalities, both can be elicited by more than one type of stimulus, including mechanical and chemical stimuli. Many compounds are capable of producing both itch and pain (5), and some medical conditions can invoke both symptoms (6).
The neural circuitry that transmits itch, and in particular how it relates to that which conveys pain, has yet to be clearly elucidated, although critical components of the itch pathway have been identified. Also unclear is how itch is encoded both peripherally and centrally with several theories proposed to explain this process. We will address progress in these areas and identify what remains to be uncovered.
A great deal of recent work has focused on investigating the molecular mechanisms behind the transduction of itch by peripheral neurons. These have been important in explaining how myriad pruritogens are able to generate itch including those that act in a nonhistaminergic manner. Histamine is by far the most closely studied pruritogen. Antihistamines serve as a standard treatment for clinical itch, although they are ineffective at alleviating itch in many medical conditions (7). Molecular, cellular, physiological, and behavioral experiments have confirmed the existence of nonhistaminergic itch and shed light on how this can be separate from or overlap with the histaminergic variety. Establishment of the mouse as an important model for studying pruritoception with its amenability to genetic manipulation has further clarified how itch is detected.
There remains, however, room for improvement in the approaches used to study itch experimentally, and we draw attention to the limitations of current methods and indicate what must be dealt with in the future. We will also speak to the important unanswered questions and suggest further studies that will help advance the field.
Neural Pathways and Circuitry for Itch
Theories to Explain Encoding of Itch
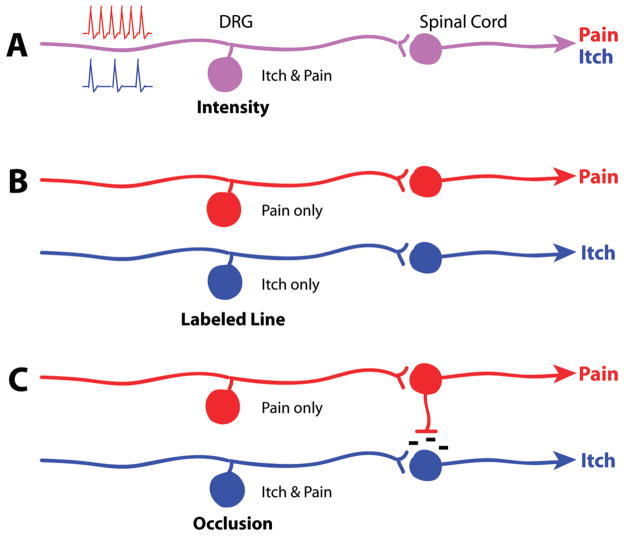
As itch has historically been defined in relation to pain because of its relief by scratching, explanations for the neural circuitry of itch derive from this interaction. Several hypotheses have been proposed to explain the coding of itch beginning with intensity and labeled line theories.
Intensity theory was initially invoked to describe coding for itch based on neurons that respond to both itch and pain. This hypothesis came from work in the early 20th century (8, 9) that supposedly identified coinciding itch and pain “spots” in human skin. These two modalities would be differentiated by way of intensity: itchy stimuli generate weaker neuronal responses, while painful stimuli produce stronger activation within the same neuronal population (10), but evidence against the theory has accumulated. Later studies with human subjects show that increased frequency of electrical stimulation produces itch of increasing intensity without a transition from itch to pain perception (11), although it is not certain that intensifying the stimulus raised the number of action potentials elicited. Painful stimulation does not become itch at lower frequencies (12, 13).
In 1997, Schmelz et al. (14) identified a population of human C fibers activated by histamine. These mechano-insensitive, high threshold fibers of low conduction velocity were distinct from other nociceptive populations, providing support for labeled line or specificity theory. This postulates distinct sets of afferent fibers dedicated solely to detecting either itch or pain with no overlap between the two populations (Figure 1B). Labeled line is a common explanation for coding in sensory biology, perhaps best exemplified in the auditory system, which delineates the frequencies of sound stimuli based on the particular regions activated along the length of the cochlea (15).
Figure 1.
Itch coding models. (A) Intensity theory hypothesizes individual neurons that signal either itch or pain through lower or higher intensity stimuli (represented by low and high frequency spiking), respectively. (B) Labeled line theory hypothesizes entirely separate neurons and circuitry for detecting pain versus itch. (C) Occlusion theory hypothesizes dual itch/pain-sensing neurons that signal itch when they are selectively activated. Noxious stimuli will activate both this and the pain-only population, leading to inhibition of the itch circuit.
Later work from Schmelz and colleagues (16) found the purported itch-specific C fibers also responded to nociceptive compounds such as capsaicin. This was confirmed by another group (17) that demonstrated the nonhistaminergic form of itch induced by cowhage involves a population that is distinct from histamineactivated fibers but still capsaicin-responsive. These results establish that so-called pruritoceptors can also detect nociceptive stimuli, arguing against the labeled line hypothesis. It remains possible, however, that an itch-specific subset of dorsal root ganglion (DRG) neurons has yet to be found.
Consequently, the explanation might lie somewhere between the intensity and labeled line theories, allowing for cells that are tuned to detect both itchy and painful stimuli but are ultimately able to distinguish between modalities. At the periphery, nociceptors that do not respond to histamine or other pruritogens have been observed, though pruritoceptive neurons tend to also respond to noxious stimuli (16–20). When nociceptors in the form of TRPV1+ primary sensory fibers are ablated, mice exhibit a severe reduction in various itch responses (21). These data are compatible with some form of pattern-based coding, whereby the particular arrangement of neurons activated produces a sensory percept (10). In human color vision, for example, different colors are encoded by the pattern of relative activity among the three classes of cones (22).
The pruritoceptors within the nociceptive neurons may thus constitute an itch-selective subset. This subgroup could generate the sensation of itch when activated, but if a larger group of cells including nociceptors is recruited, that is, by a noxious stimulus, this would occlude the itch response to produce pain. This occlusion model (10, 23, 24) (Figure 1C) accounts for the similarities between itch and pain as well as the antagonistic relationship between them.
Encoding Itch at the Spinal Level
As a subset of C fibers, pruritoceptors ostensibly synapse onto dorsal horn neurons in the upper laminae of the spinal cord. This population has been studied in several animal models to investigate itch-responsive second order neurons.
Experiments in the cat spinal cord identified spino-thalamic tract (STT) neurons activated by histamine (25). This report endorsed the labeled line hypothesis, but it was based on a limited sample size and the histamine-responsive cells were not demonstrably itch-specific as they were not tested with capsaicin. Conversely, the purported nociceptor population was not evaluated for responsiveness to histamine.
A pair of studies by Sun and colleagues (26, 27) high-light the vital role of gastrin-releasing peptide receptor (GRPR), found in the spinal cord, in detecting itch. Their initial work showed GRPR is expressed in dorsal horn neurons while its ligand, gastrin-releasing peptide (GRP), is found in a subset of small-diameter DRG neurons that may include the pruritoceptors. GRPR mutant mice show deficits in itch behavior, whereas pain responses are normal. Remarkably, ablating the GRPR+ population leads to a severe deficit in the scratching response to a variety of pruritogens, both histaminergic and nonhistaminergic, while leaving pain behavior unchanged. This makes them candidates for itch-specific neurons in the spinal cord as predicted by labeled line theory. However, GRPR+ neurons may also be involved in pain signaling but dispensable for pain behavior, which is in agreement with the occlusion model. Therefore, recording from GRPR+ neurons should be performed to confirm the behavioral data at a physiological level. It will also be important to identify the GRPR+ population in other species.
Rat dorsal horn recordings identified a population that responds to the pruritogen serotonin (5HT) but is also capsaicin-sensitive (28). This overlap rejects the labeled line theory at the spinal level as well as peripherally. Davidson and colleagues (29) found that monkey STT neurons activated by either histamine or cowhage show decreased responses upon scratching in their receptive fields. Later work from the same group (30) uncovered a neuronal population that was inhibited by scratching during activation by histamine but not capsaicin. Collectively, these experiments demonstrate the well-known inhibition of itch by pain while confirming that algogenic stimuli can activate most itch-selective spinal neurons. Mouse dorsal horn neurons activated by the pruritogens histamine, 5HT, and the protease-activated receptor 2 (PAR2) agonist SLIGRL-NH2 largely respond to noxious stimuli as well, for example, mustard oil and heat (31, 32).
Work in rats has shown that neurokinin-1-expressing (NK-1) cells of the dorsal horn play a role in 5HTinduced itch (33). Ablation of these neurons also reduces chronic pain and hyperalgesia (34, 35). Thus, the rat NK-1+ population appears to include, but is not restricted to, the pruritoceptive subset of neurons predicted by the occlusion model.
Further experiments can shed light on the itch coding model. Will ablation of itch-selective DRG neurons, for example, via the GRP promoter, produce a loss-of-function phenotype that leaves pain sensation unchanged? Is there a minimum number of neurons needed to generate the perception of itch? One way to test the occlusion model is to eliminate the “nociceptor-only” population, that is, those nociceptive neurons that do not also respond to pruritogens: will activation of the remaining cells produce the sensation of itch even if a noxious stimulus is applied because there is no occlusion or competition from the eliminated nociceptors?
Molecular Mechanisms of Itch in the DRG and Spinal Cord
Early experiments in the itch field used human subjects to provide important psychophysical and physiological data, building the foundation for future studies. The establishment of a mouse behavioral model of itch (36) coupled with advances in techniques for genetic manipulation has opened the door to uncovering the molecular mechanisms of itch. These have been particularly fruitful in characterizing pruritoceptor populations, especially in regard to the exploration of nonhistaminergic forms of itch and how signaling works at the periphery.
Pruritogens are thought to activate primary sensory neurons, producing the sensation of itch either directly, that is, by activation of a receptor expressed by these afferents, or indirectly via activation of a secondary cell type, for example, mast cells, which release one or more pruritogens that then activate sensory afferents. Although pruritogens often act through both routes, research has generally focused on the specific contribution of the former, that is, pruritogens directly activating primary fibers, in order to pinpoint the appropriate receptors for each pruritogen and elucidate the relevant transduction pathways.
Itch Receptors at the Periphery
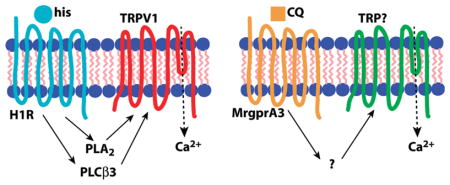
Histamine-induced itch in the mouse is thought to act primarily through direct activation of the histamine H1 receptor (H1R), although the H4 receptor has also been implicated (37). In the context of pruritoception, the G protein-coupled receptor (GPCR) H1R is coupled to phospholipase C β3 (PLCβ3), which is required for H1R-dependent itch (38). Other components of this signal transduction pathway include TRPV1 (18, 21), better known for its role in thermal nociception (39, 40), and phospholipase A2, which hydrolyzes lipids that activate TRPV1 (18). Thus, the pruritogen histamine acts as a GPCR ligand in primary sensory neurons, leading to Gq-coupled phospholipase activation upstream of TRP channel opening (Figure 2A).
Figure 2.
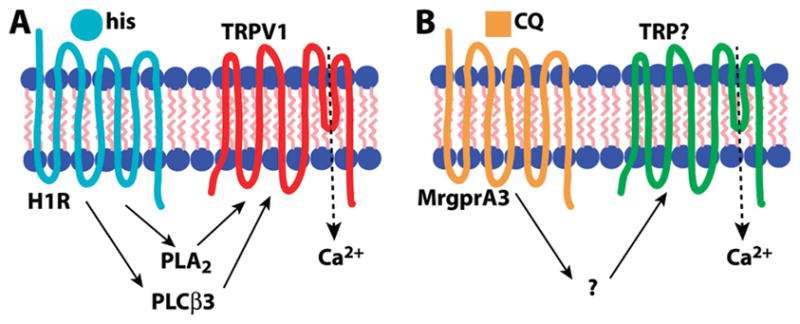
Comparison of signal transduction pathways for itch. (A) Histamine (his) is a ligand for H1R that activates TRPV1 via phoszpholipase intermediaries PLCβ3 and PLA2. (B) Chloroquine (CQ) activates MrgprA3 leading to TRP channel opening, but the intermediate steps in the pathway are unknown. In the case of both pruritogens, TRPs permit Ca2+ influx, which may also have a signaling role.
5HT and its derivative alpha-methyl-serotonin (αMe-5HT) target 5HTRs to induce itch (41) in a manner that also utilizes PLCβ3 though not TRPV1 (21). Another potent pruritogen, the peptide endothelin-1 (ET-1), acts via aGPCR, the ET-Areceptor, but signals itch independently of either PLCβ3 orTRPV1 (21). The antimalarial chloroquine (CQ), which often produces severe itch as a side effect (42, 43), acts through the MrgprA3GPCR(20). Interestingly, although MrgprA3 is coupled to TRP channels, it uses a TRPV1-independent mechanism (our unpublished observations) and does not require PLCβ3 (21), making it distinct from the H1R signaling pathway (Figure 2B). The active ingredient in cowhage is mucunain, a protease shown to activate PAR2 and PAR4 (44). PAR2 activators tryptase (45) and the peptide SLIGRL-NH2 (46) are capable of inducing itch, and this GPCR may also be coupled to TRPV1, which can be sensitized by PAR2 activation (47–49). Together these data suggest GPCR coupling to TRP channels may be a common theme among various itch signaling pathways.
One unresolved question is how pruritogens signal independently of one another. For example, CQ-induced itch is not amenable to antihistamine treatment in humans (42) and its murine target, MrgprA3, is not involved in histaminergic itch (20). However, CQ-responsive DRG neurons are wholly contained within the histamine-responsive population (20). The most parsimonious explanation is separable signal transduction pathways that segregate activation by one pruritogen from another one, which could be accomplished through distinct G protein and/or TRP targets. It also begs the question of whether different pruritogens are capable of producing qualitatively different kinds of itch due to distinct peripheral mechanisms or perhaps unique central projections.
Itch by Indirect Means
The above results address potential mechanisms for direct activation by pruritogens, that is, through primary afferents expressing target receptors. However, there is less data on how pruritogens produce itch indirectly. The best example of this is through activation of mast cells, which release pruritogens including histamine, 5HT, tryptase, and cytokines among others (50) that then act upon peripheral targets. Compound 48/80, a mast cell degranulator, is the pruritogen most commonly used to produce mast cell-dependent itch (51). However, nearly every pruritogenic agent mentioned, including histamine (52, 53), CQ (54, 55), and 5HT (56, 57), can act upon mast cells, which express a number of prospective targets for pruritogenic ligands. Mast cells can potentially contribute to nearly every pruritoceptive response both acute and chronic. One allergy model of itch that uses ovalbumin is also mast-cell-dependent (58). It will be important to determine the exact role of mast cells in different types of itch, and this can be facilitated by the use of W-sash, a naturally occurring mutation producing mice that lack mast cells (59, 60).
There are additional indirect mechanisms of itch besides mast cells. Other immune cells that release compounds such as cytokines appear frequently in clinical cases (1). One cytokine, interleukin-31, is pruritogenic and its expression in the immune system produces a dermatitis phenotype in mice (61, 62). Also present in the skin are a variety of cell types that may contribute to itch. For example, keratinocytes are an attractive candidate because of their localization as well as expression of potential itch mediators such as TRPV3 and TRPV4 (63, 64). There is a paucity of research pertaining to the contribution of non-neuronal cells to pruritoception. As these are likely to play a key role in regulation of itch in both innocuous and clinical states, it is crucial to study them further. This will be made easier by the use of conditional knockout mice to determine the specific input of different cell populations to pruritoception.
While significant progress has been made in our understanding of itch mediators at the periphery, less is known about molecular mechanisms in the spinal cord or how peripheral fibers interact with second order neurons. GRPR is an attractive target for labeling this population for further study, and other markers should be sought as well. Trans-synaptic markers and gene expression screens are two ways to begin investigating the molecular identity of these neurons. As for itch-selective neurons in the DRG, these cells and their projection patterns can be studied by using markers such as MrgprA3 and GRP to label them. Additionally, spinal interneurons, which likely play an essential role at the critical first synapse in the itch circuit, have been the subject of limited study to this point (65). A first step would be to identify interneuron populations that synapse with itch-selective DRG and dorsal horn neurons.
How to Study Itch in the Laboratory
The recent explosion of interest in the itch field speaks to its growing importance and relevance. It also clarifies some questions that should be addressed to continue moving forward. Possibly the most important question in the field, and seemingly the most trivial, remains: what exactly constitutes itch? There is also its corollary: how is itch separable from pain? Data from human studies is ambiguous because of the dual action, both algogenic and pruritogenic, of many compounds. Animal models, while allowing a reductionist approach to study molecular and cellular mechanisms, are in some ways even more opaque.
Histamine is a well-established pruritogen that is considered to produce a “pure” form of itch (66). Yet histamine is known to produce a complex reaction including wheal and flare that does not necessarily correlate with the perceived level of itch (67). It can also produce the sensation of pain in addition to itch (68), as can cowhage (69, 70), the best described initiator of nonhistaminergic itch. Capsaicin is considered a noxious stimulus, but it can generate itch in combination with burning pain (71, 72).
Strikingly, if an inactivated cowhage spicule is loaded with any one of the above three compounds, each produces a combined itch, pricking/stinging, and burning sensation (5). Of note, the spicules deliver the compound superficially relative to injection methods. This suggests pruritoceptive fibers may be spatially segregated from nociceptive ones, potentially identifiable by amore apical termination in the epidermis. One must pay close attention to the method of delivery and how that may affect the perceived stimulus. It is likely that different populations of neurons are being activated and comparison of these types of experiments may provide insight as to how histamine, for example, can signal itch versus pain.
Animal Models of Itch
It becomes even more difficult to address the issue of perception with animal models. One approach is to identify purely algogenic or pruritogenic compounds and develop an assay that can distinguish between the two. Shimada and LaMotte (73) did this for the mouse model using a cheek injection, which ostensibly leads to forelimb wiping for painful stimuli and hindlimb scratching for itchy stimuli. This produces the anticipated results for histamine and capsaicin, and a study by Ross et al. (65) also tested formalin, long established for its use in a pain assay (74). Unexpectedly, formalin generated a scratching response upon injection into the cheek. This brings up the issue of what sensation exactly formalin produces (itchy pain? painful itch?), which is hard to answer definitively because its toxicity prevents its use in human studies. It calls for analyzing other compounds that have yet to be tested using human psychophysics or microneurography to better clarify the itchy and/or painful nature of these stimuli. These include CQ, α-Me-5HT, and even synthetic compounds that may activate particular receptors, for example, specific agonists for MrgprA3 or PAR2 (75, 76).
A parallel line of experiments involves behavioral assays in monkeys. Histamine and cowhage can induce a scratch response (77) as can opioids such as morphine (78). Extending these studies to test other pruritogens is vital for comparison with human and mouse behavioral data. The monkey is of particular interest as the animal model most closely related to humans, promising considerable potential for overlap with respect to both circuitry and molecular mechanisms.
At the same time, there is a great deal of diversity among the animal models that may be enlightening. Among humans and nonhuman primates, there seem to be distinct neuronal populations that detect histamine versus cowhage (19, 77, 79), but in mice the neurons responding to either pruritogen overlap substantially (32). Rats, despite their relatedness to mice, do not appear to perceive histamine as pruritogenic (28), although 5HT does induce itch. A comparison of how rats versus mice detect histamine and 5HT (do they utilize different receptors? different DRG and/or spinal neuron populations? what meaning do histaminergic and nonhistaminergic itch have in this context?) will shed light on how itch can be detected in distinct ways and what principles are common across species.
Another intriguing rodent species is the naked mole-rat, which containsNK-1-expressing spinal neurons but does not produce substance P (SP), a neuropeptide ligand for NK-1 that is generated by DRG neurons in other animals. Histamine can only induce itch if exogenous SP is first administered intrathecally (80). Deep dorsal horn projections, present in addition to the typical, more superficial dorsal horn projections, appear to be responsible. The deep projections may inhibit the superficial fibers unless SP “overexcites” the latter to overcome this inhibition. These projections are alsoTRPV1+, and a situation analogous to histamine-dependent itch occurs with capsaicin and nocifensive behavior, which is only inducible after SP injection (81). The naked mole-rat has lost its customary capacity for itch and thermal hyperalgesia. Its evolutionary niche has created an intriguing creature worthy of further study to characterize what “normal” itch looks like.
Conclusion
Itch is familiar to everyone, yet we lack a fundamental understanding of this biologically and clinically important sensation. Early support in favor of first the intensity and then labeled line theories has waned as experiments utilizing human subjects and many animal models have made these descriptions less viable. The discovery of a histamine-responsive, itch-selective C fiber population in humans has been extended to other species and other pruritogens, notably cowhage, which produces a nonhistaminergic form of itch. Electrophysiological and behavioral studies focused on the spinal cord have unearthed a group of dorsal horn neurons that may be the second order cells of the itch pathway.
Occlusion theory posits a pruritoceptive subset of nociceptive neurons that is selective for itch but can be overridden by activation of the larger nociceptive group. Further experiments are called for to pin down this cell population across species and determine how it interacts with other participants in the circuit including modulatory cells such as interneurons. Moreover, other critical features such as thalamic and cortical components have yet to be fully integrated into the itch pathway (82, 83).
Experiments to identify molecular and cellular mechanisms, spurred by adoption of the mouse as a model system, have honed in on key mediators of itch. It appears that GPCRs and TRP channels play important roles in signaling itch just as they do in other sensory transduction pathways (84, 85). At the same time, the indirect activation of itch pathways, through mast cells or other non-neuronal types, signifies a serious gap in our understanding of how pruritogens are capable of generating itch. The complete molecular picture has yet to be revealed, and there is a particular need to explain how various pruritogens, acting in part through the same peripheral neurons, are able to differentially signal their particular form of itch.
The current set of approaches, spanning a number of techniques and model organisms, has proven to be successful for investigating itch. We feel the development and adoption of new methods, however, is important to extend previous work in humans, monkeys, and rodents. In particular, defining the true qualities of itch versus pain, the character of histaminergic and nonhistaminergic forms of itch, and the differences in how itch is sensed and encoded across the animal kingdom is necessary.
The field has flourished in recent years with a number of new scientists, ourselves among them, joining this exciting area of research. We look forward to new developments in this realm as we proceed in our exploration of the fascinating but poorly characterized sensation of itch.
References
- 1.Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 2.Bergasa NV. The pruritus of cholestasis. J Hepatol. 2005;43:1078–1088. doi: 10.1016/j.jhep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Yosipovitch G, Greaves MW, Schmelz M. Itch. Lancet. 2003;361:690–694. doi: 10.1016/S0140-6736(03)12570-6. [DOI] [PubMed] [Google Scholar]
- 4.Yosipovitch G, Samuel LS. Neuropathic and psychogenic itch. Dermatol Ther. 2008;21:32–41. doi: 10.1111/j.1529-8019.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- 5.Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine, and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oaklander AL, Bowsher D, Galer B, Haanpa M, Jensen MP. Herpes zoster itch: preliminary epidemiologic data. J Pain. 2003;4:338–343. doi: 10.1016/s1526-5900(03)00637-0. [DOI] [PubMed] [Google Scholar]
- 7.Twycross R, Greaves MW, Handwerker H, Jones EA, Libretto SE, Szepietowski JC, Zylicz Z. Itch: scratching more than the surface. QJM. 2003;96:7–26. doi: 10.1093/qjmed/hcg002. [DOI] [PubMed] [Google Scholar]
- 8.von Frey M. Zur physiologie der juckempfindung. Arch Neerl Physiol. 1922;7:142–145. [Google Scholar]
- 9.Lewis T, Grant RT, Marvin HM. Vascular reactions of the skin to injury. X The intervention of a chemical stimulus illustrated especially by the flare: the response to faradism. Heart. 1927;14:139–160. [Google Scholar]
- 10.McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497–501. doi: 10.1016/0166-2236(92)90102-e. [DOI] [PubMed] [Google Scholar]
- 11.Tuckett RP. Itch evoked by electrical stimulation of the skin. J Invest Dermatol. 1982;79:368–373. doi: 10.1111/1523-1747.ep12529734. [DOI] [PubMed] [Google Scholar]
- 12.Ochoa J, Torebjörk E. Sensations evoked by intraneural microstimulation of C nociceptor fibres in human skin nerves. J Physiol. 1989;415:583–599. doi: 10.1113/jphysiol.1989.sp017737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handwerker HO, Forster C, Kirchhoff C. Discharge patterns of human C-fibers induced by itching and burning stimuli. J Neurophysiol. 1991;66:307–315. doi: 10.1152/jn.1991.66.1.307. [DOI] [PubMed] [Google Scholar]
- 14.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenwood DD. Acochlear frequency-position function for several species--29 years later. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 16.Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- 17.Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci USA. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Covey E. Neural population coding and auditory temporal pattern analysis. Physiol Behav. 2000;69:211–220. doi: 10.1016/s0031-9384(00)00203-1. [DOI] [PubMed] [Google Scholar]
- 23.Handwerker HO. Pain and allodynia, itch and alloknesis: an alternative hypothesis. APSJ. 1992;1:135–138. [Google Scholar]
- 24.Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol. 2004;91:213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- 25.Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- 26.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 27.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. J Neurophysiol. 2002;87:1280–1289. doi: 10.1152/jn.00431.2001. [DOI] [PubMed] [Google Scholar]
- 29.Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Responses and modulation of monkey spinothalamic tract neurons to itch-producing and itch-inhibiting stimuli. Acta Derm-Venereol. 2007;87:475. [Google Scholar]
- 30.Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akiyama T, Merrill AW, Carstens MI, Carstens E. Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role in itch. J Neurosci. 2009;29:6691–6699. doi: 10.1523/JNEUROSCI.6103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akiyama T, Carstens MI, Carstens E. Excitation of mouse superficial dorsal horn neurons by histamine and/or PAR-2 agonist: potential role in itch. J Neurophysiol. 2009;102:2176–2183. doi: 10.1152/jn.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carstens EE, Carstens MI, Simons CT, Jinks SL. Dorsal horn neurons expressing NK-1 receptors mediate scratching in rats. NeuroReport. 2010;21:303–308. doi: 10.1097/WNR.0b013e328337310a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 35.Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, et al. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- 36.Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- 37.Bell JK, McQueen DS, Rees JL. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br J Pharmacol. 2004;142:374–380. doi: 10.1038/sj.bjp.0705754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 39.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 40.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 41.Kim DK, Kim HJ, Kim H, Koh JY, Kim KM, Noh MS, Kim JJ, Lee CH. Involvement of serotonin receptors 5-HT1 and 5-HT2 in 12(S)-HPETE-induced scratching in mice. Eur J Pharmacol. 2008;579:390–394. doi: 10.1016/j.ejphar.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Mnyika KS, Kihamia CM. Chloroquine-induced pruritus: its impact on chloroquine utilization in malaria control in Dar es Salaam. J Trop Med Hyg. 1991;94:27–31. [PubMed] [Google Scholar]
- 43.Sowunmi A, Fehintola FA, Adedeji AA, Falade AG, Falade CO, Akinyinka OO, Oduola AM. Comparative efficacy of chloroquine plus chlorpheniramine alone and in a sequential combination with sulfadoxine-pyrimethamine, for the treatment of acute, uncomplicated, falciparum malaria in children. Ann Trop Med Parasitol. 2000;94:209–217. doi: 10.1080/00034980050006375. [DOI] [PubMed] [Google Scholar]
- 44.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ui H, Andoh T, Lee JB, Nojima H, Kuraishi Y. Potent pruritogenic action of tryptase mediated by PAR-2 receptor and its involvement in anti-pruritic effect of nafamostat mesilate in mice. Eur J Pharmacol. 2006;530:172–178. doi: 10.1016/j.ejphar.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 46.Shimada SG, Shimada KA, Collins JG. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol. 2006;530:281–283. doi: 10.1016/j.ejphar.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai Y, Moriyama T, Higashi T, Togashi K, Kobayashi K, Yamanaka H, Tominaga M, Noguchi K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J Neurosci. 2004;24:4293–4299. doi: 10.1523/JNEUROSCI.0454-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harvima IT, Nilsson G, Suttle MM, Naukkarinen A. Is there a role for mast cells in psoriasis? Arch Dermatol Res. 2008;300:461–478. doi: 10.1007/s00403-008-0874-x. [DOI] [PubMed] [Google Scholar]
- 51.Sugimoto Y, Umakoshi K, Nojiri N, Kamei C. Effects of histamine H1 receptor antagonists on compound 48/80-induced scratching behavior in mice. Eur J Pharmacol. 1998;351:1–5. doi: 10.1016/s0014-2999(98)00288-x. [DOI] [PubMed] [Google Scholar]
- 52.Wescott SL, Hunt WA, Kaliner M. Histamine H-1 receptors on rat peritoneal mast cells. Life Sci. 1982;31:1911–1919. doi: 10.1016/0024-3205(82)90029-7. [DOI] [PubMed] [Google Scholar]
- 53.Antohe F, Dobrila LN, Heltianu C. Histamine receptor on mast cells. Microcirc, Endothelium, Lymphatics. 1988;4:469–488. [PubMed] [Google Scholar]
- 54.Green KB, Lim HW. Effects of chloroquine on release of mediators from mast cells. Skin Pharmacol. 1989;2:77–85. doi: 10.1159/000210804. [DOI] [PubMed] [Google Scholar]
- 55.Nosal R, Drabikova K, Pecivova J. Effect of chloroquine on isolated mast cells. Agents Actions. 1991;33:37–40. doi: 10.1007/BF01993121. [DOI] [PubMed] [Google Scholar]
- 56.Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, Brown JM, Bruening S, Toth M, Metcalfe DD. 5-hydroxytryptamine induces mast cell adhesion and migration. J Immunol. 2006;177:6422–6432. doi: 10.4049/jimmunol.177.9.6422. [DOI] [PubMed] [Google Scholar]
- 57.Walther A, Peter C, Yilmaz N, Schmidt W, Martin E, Schmidt H. Influence of serotonin-receptor antagonism on mast cell activation during endotoxemia. Pathophysiology. 2002;8:161–165. doi: 10.1016/s0928-4680(02)00006-8. [DOI] [PubMed] [Google Scholar]
- 58.Hashimoto Y, Arai I, Tanaka M, Nakaike S. Prostaglandin D2 inhibits IgE-mediated scratching by suppressing histamine release from mast cells. J Pharmacol Sci. 2005;98:90–93. doi: 10.1254/jphs.sc0040209. [DOI] [PubMed] [Google Scholar]
- 59.Duttlinger R, Manova K, Chu TY, Gyssler C, Zelenetz AD, Bachvarova RF, Besmer P. W-sash affects positive and negative elements controlling c-kit expression: ectopic c-kit expression at sites of kit-ligand expression affects melanogenesis. Development. 1993;118:705–717. doi: 10.1242/dev.118.3.705. [DOI] [PubMed] [Google Scholar]
- 60.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, Haugen HS, Maurer M, Harder B, Johnston J, Bort S, Mudri S, Kuijper JL, Bukowski T, Shea P, Dong DL, Dasovich M, Grant FJ, Lockwood L, Levin SD, LeCiel C, Waggie K, Day H, Topouzis S, Kramer J, Kuestner R, Chen Z, Foster D, Parrish-Novak J, Gross JA. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 62.Takaoka A, Arai I, Sugimoto M, Honma Y, Futaki N, Nakamura A, Nakaike S. Involvement of IL-31 on scratching behavior in NC/Nga mice with atopic-like dermatitis. Exp Dermatol. 2006;15:161–167. doi: 10.1111/j.1600-0625.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 63.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem. 2004;279:21569–21575. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- 64.Yoshioka T, Imura K, Asakawa M, Suzuki M, Oshima I, Hirasawa T, Sakata T, Horikawa T, Arimura A. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol. 2009;129:714–722. doi: 10.1038/jid.2008.245. [DOI] [PubMed] [Google Scholar]
- 65.Ross SE, Mardinly AR, McCord AE, Zurawski J, Cohen S, Jung C, Hu L, Mok SI, Shah A, Savner EM, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simone DA, Ngeow JY, Whitehouse J, Becerra-Cabal L, Putterman GJ, LaMotte RH. The magnitude and duration of itch produced by intracutaneous injections of histamine. Somatosens Res. 1987;5:81–92. doi: 10.3109/07367228709144620. [DOI] [PubMed] [Google Scholar]
- 67.Darsow U, Ring J, Scharein E, Bromm B. Correlations between histamine-induced wheal, flare and itch. Arch Dermatol Res. 1996;288:436–441. doi: 10.1007/BF02505231. [DOI] [PubMed] [Google Scholar]
- 68.Broadbent JL. Observations on histamine-induced pruritus and pain. Br J Pharmacol Chemother. 1955;10:183–185. doi: 10.1111/j.1476-5381.1955.tb00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Graham DT, Goodell H, Wolff HG. Neural mechanisms involved in itch, itchy skin, and tickle sensations. J Clin Invest. 1951;30:37–49. doi: 10.1172/JCI102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shelley WB, Arthur RP. Studies on cowhage (Mucuna pruriens) and its pruritogenic proteinase, mucunain. AMA Arch Derm. 1955;72:399–406. doi: 10.1001/archderm.1955.03730350001001. [DOI] [PubMed] [Google Scholar]
- 71.Green BG. Spatial summation of chemical irritation and itch produced by topical application of capsaicin. Percept Psychophys. 1990;48:12–18. doi: 10.3758/bf03205007. [DOI] [PubMed] [Google Scholar]
- 72.Green BG, Shaffer GS. The sensory response to capsaicin during repeated topical exposures: differential effects on sensations of itching and pungency. Pain. 1993;53:323–334. doi: 10.1016/0304-3959(93)90228-H. [DOI] [PubMed] [Google Scholar]
- 73.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 75.Scarborough RM. Protease-activated receptor-2 antagonists and agonists. Curr Med Chem: Cardiovasc Hematol Agents. 2003;1:73–82. doi: 10.2174/1568016033356698. [DOI] [PubMed] [Google Scholar]
- 76.Gardell LR, Ma JN, Seitzberg JG, Knapp AE, Schiffer HH, Tabatabaei A, Davis CN, Owens M, Clemons B, Wong KK, Lund B, Nash NR, Gao Y, Lameh J, Schmelzer K, Olsson R, Burstein ES. Identification and characterization of novel small-molecule protease-activated receptor 2 agonists. J Pharmacol Exp Ther. 2008;327:799–808. doi: 10.1124/jpet.108.142570. [DOI] [PubMed] [Google Scholar]
- 77.Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee H, Naughton NN, Woods JH, Ko MC. Effects of butorphanol on morphine-induced itch and analgesia in primates. Anesthesiology. 2007;107:478–485. doi: 10.1097/01.anes.0000278876.20263.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith ES, Blass GR, Lewin GR, Park TJ. Absence of histamine-induced itch in the African naked mole-rat and “rescue” by Substance P. Mol Pain. 2010;6:29. doi: 10.1186/1744-8069-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park TJ, Lu Y, Jüttner R, Smith ES, Hu J, Brand A, Wetzel C, Milenkovic N, Erdmann B, Heppenstall PA, Laurito CE, Wilson SP, Lewin GR. Selective inflammatory pain insensitivity in the African naked mole-rat (Heterocephalus glaber) PLoS Biol. 2008;6:e13. doi: 10.1371/journal.pbio.0060013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Drzezga A, Darsow U, Treede RD, Siebner H, Frisch M, Munz F, Weilke F, Ring J, Schwaiger M, Bartenstein P. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15H2Opositron emission tomography studies. Pain. 2001;92:295–305. doi: 10.1016/s0304-3959(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 83.Craig AD, Andrew D. Responses of spinothalamic lamina I neurons to repeated brief contact heat stimulation in the cat. J Neurophysiol. 2002;87:1902–1914. doi: 10.1152/jn.00578.2001. [DOI] [PubMed] [Google Scholar]
- 84.Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci. 2004;5:263–278. doi: 10.1038/nrn1365. [DOI] [PubMed] [Google Scholar]
- 85.Damann N, Voets T, Nilius B. TRPs in our senses. Curr Biol. 2008;18:R880–889. doi: 10.1016/j.cub.2008.07.063. [DOI] [PubMed] [Google Scholar]