Abstract
Dimethylarginine dimethylaminohydrolase (DDAH) is an endogenous regulator of nitric oxide production and represents a potential therapeutic target. However, only a small number of biologically useful inhibitors have been reported, and many of these are substrate analogs. To seek more diverse scaffolds, we developed a high-throughput screening (HTS) assay and queried two small libraries totaling 2446 compounds. The HTS assay proved to be robust, reproducible and scalable, with Z' factors ≥ 0.78. One inhibitor, ebselen, is structurally divergent from substrate and was characterized in detail. This selenazole covalently inactivates DDAH in vitro and in cultured cells. The rate constant for inactivation of DDAH (44,000 ± 2,400 M−1s−1) is greater than those reported for any other target, suggesting this pathway is an important aspect of ebselen's total pharmacological effects.
Keywords: Dimethylarginine dimethylaminohydrolase, high-throughput screening, ebselen, covalent inhibitors, nitric oxide
Small molecule tools to monitor and control nitric oxide (NO) production have greatly facilitated our understanding of this signaling molecule. Nature also uses a small-molecule approach by constantly producing, catabolizing and excreting Nω, Nω-dimethyl-l-arginine (dimethylarginine), which serves as an endogenous inhibitor of all three isoforms of nitric oxide synthase in humans. One of the key control points for regulation of dimethylarginine concentrations in vivo is the catabolic enzyme dimethylarginine dimethylaminohydrolase (DDAH), which catalyzes the hydrolytic formation of citrulline and dimethylamine products, thus relieving the inhibition of NO production (Figure 1). Conversely, inhibitors of DDAH can indirectly block NO production via the resulting accumulation of dimethylarginine.1
Figure 1.
Role of DDAH and DDAH inhibitors. A) NO Synthase isoforms convert arginine (1) to citrulline (2) and NO. Dimethylarginine (3) inhibits all isoforms of NO synthase, and is catabolized to (2) and dimethylamine by DDAH. B) Structures of selected substrate-based DDAH inhibitors. (4): 4124W; (5): L-257; (6): N5-(1-iminopentyl)-l-ornithine; (7): N5-(1-iminobut-3-enyl)-l-ornithine; (8): 2-chloroacetamidine; (9): N-but-3-ynyl-2-chloroacetamidine.
There are a number of clinical indications for drugs that block NO production, including septic shock, ischemic stroke and some cancers. Interest in developing DDAH inhibitors has been fueled by their possible applications as therapeutics and research tools, but only a few structural categories of inhibitors have been reported, among them indolylthiobarbituric acids and pentafluorophenyl sulfonates.2 Most of the other inhibitors can be classified as substrate analogs (Figure 1).3–6 Although the similarity of these inhibitors to arginine could be exploited for dual targeting of DDAH and NO synthase,3,6 it may also be a drawback due to off-target interactions with other arginine handling enzymes. Also, the reliance of these highly-charged inhibitors on the y+ cationic transporter will further limit what substitutions can be tolerated. Therefore, we sought to use high-throughput screening (HTS) for the discovery of structurally divergent DDAH inhibitors as alternative scaffolds for drug and probe development.
To develop a HTS assay, we replaced the natural DDAH substrate, dimethylarginine, with a synthetic substrate, S-methyl-l-thiocitrulline, that yields citrulline and methanethiol as products.7 This reaction was converted into a 384-well microplate assay format in which methanethiol production is continuously monitored through its reaction with 5,5'-dithio-bis(2-nitrobenzoic acid) (DTNB) and the ensuing increase of absorbance at 405 nm. The observed rates are linearly proportional to DDAH concentrations (10 – 200 nM). This assay yields steady-state constants (kcat = 55.8 ± 0.6 min−1; KM = 22 ± 1 µM) similar to those previously reported for both the DTNB-based microcuvette assay (kcat = 48.6 ± 0.6 min−1; KM = 23 ± 3 µM)7 and a discontinuous citrulline derivatization assay (kcat = 72 ± 0.6 min−1; KM = 19 ± 1 µM).7 To enable the identification of a variety of inhibitor types (competitive, not-competitive, time-dependent), HTS was completed using an enzyme + inhibitor pre-incubation step (≥ 10 min) and a substrate concentration equal to KM (22 µM). Because of the significantly lower kcat and KM values of human DDAH-1 (kcat = 2.51 ± 0.02 min−1; KM = 3.1 ± 0.3 µM)3 and the limits of DTNB detection, we used the better-suited P. aeruginosa DDAH isoform, which contains a nearly identical active site to DDAH-1, for the primary HTS. Both isoforms are fully active in 1 % DMSO, the maximum amount of co-solvent used.
After optimization, this assay proved to be robust, reproducible, and compatible with liquid-handling robots (Figure 2). A test plate containing positive (with enzyme) and negative (without enzyme) controls was prepared on two consecutive days and the assay results were used to calculate a Z’ factor of 0.87 for each, indicating excellent suitability for HTS.8 A small decrease in the observed rate is observed in successive rows when moving down a column. The origin of this slight row effect was not determined, but the calculated Z’ factors include this variation because controls were spread across all rows.
Figure 2.
High-throughput screening results. A) Single-well positive controls containing DDAH (20 nM, ●) for all plates screened are compared with negative controls containing no enzyme (□). B) Mean percent inhibition of duplicates for all library compounds screened. The dashed line represents the cutoff for “hits” (50 %). C) Per-plate Z’ factors. D) Per-plate Z factors. See Supporting Information for details.
The mean vo/[E] rates for positive (26.6 ± 0.9 min−1) and negative (0.27 ± 0.14 min−1) controls were in agreement with the values expected from kcat and KM values. Enzyme and reagents were stable when stored in plates at 25 °C, showing only a 12 % decrease in initial rates over 8 h, which did not significantly affect Z' factors.
As described, this HTS is capable of screening approx one plate containing 320 library compounds every 10 min. The majority of the elapsed time is spent collecting 10 different time points for each well, which allows for measurement of more accurate rates, and for “single-well”-based corrections for compounds that absorb at the assay wavelength. Based on these parameters, this assay is highly extensible. To date, we have screened over 13,000 compounds and larger library sizes can likely be screened by decreasing the sampling to only two time points to increase throughput without significant compromise of discriminating power.
With a novel HTS assay in hand, we screened two commercial libraries: the 446 compound NIH Clinical Collection (NCC, BioFocus) as a proof of principle, and the 2,000 compound Spectrum Collection (MicroSource) as a test of scalability. Each assay plate contained 320 test compounds, along with 32 positive and 32 negative control wells, and was screened in duplicate, bringing the total plates for both libraries to 18. The Z' factors for each plate ranged from 0.78 to 0.91, indicating good discriminating power (Figure 2).8 “Hits” were identified as compounds showing greater than 50 % inhibition when assayed at 10 µM.
Primary HTS of the NCC library gave three hits (% inhibition): ebselen (101 %), disulfiram (82 %) and cefatrizine (55 %). For verification, these hits were assayed using an orthogonal assay with the native substrate dimethylarginine and a colormetric citrulline derivatization procedure.9 DDAH inhibition was confirmed for ebselen and cefatrizine, but not for disulfiram, suggesting that it interfered with the DTNB assay. Using freshly prepared stock solutions, inhibition by ebselen was confirmed, but inhibition by cefatrizine was no longer observed, suggesting that a degradation product was responsible for the original hit.
Primary HTS of the Spectrum Collection gave 15 hits (Table S2). Ebselen was reidentified as a hit, along with several other structural classes: quinone-containing, disulfiram-like, nitrogen mustard-like, disulfide, and metal-containing compounds. Quinone derivatives have recently been identified via HTS as covalent inactivators of peptidylarginine deiminase,10 an enzyme in the same superfamily. Many of the other compounds likely react non-selectively with most thiols and may interfere with the HTS assay due to direct reaction with the methanethiol product.
Therefore, the assay was successful in screening compound libraries to identify and verify ebselen as a previously unreported DDAH inhibitor that is structurally divergent from the natural substrate. This compound can react with many different thiols, but its covalent adducts are often reversible, and the resulting products can be recycled back into the parent compound in vivo.11 Most notably, ebselen has undergone extensive testing in cultured cells, animals and humans for many of the same indications as nitric oxide-blocking drugs: septic shock, ischemic stroke, and cancer. Since DDAH inhibition may be an unrecognized mechanism of action for this compound, we pursued further characterization.
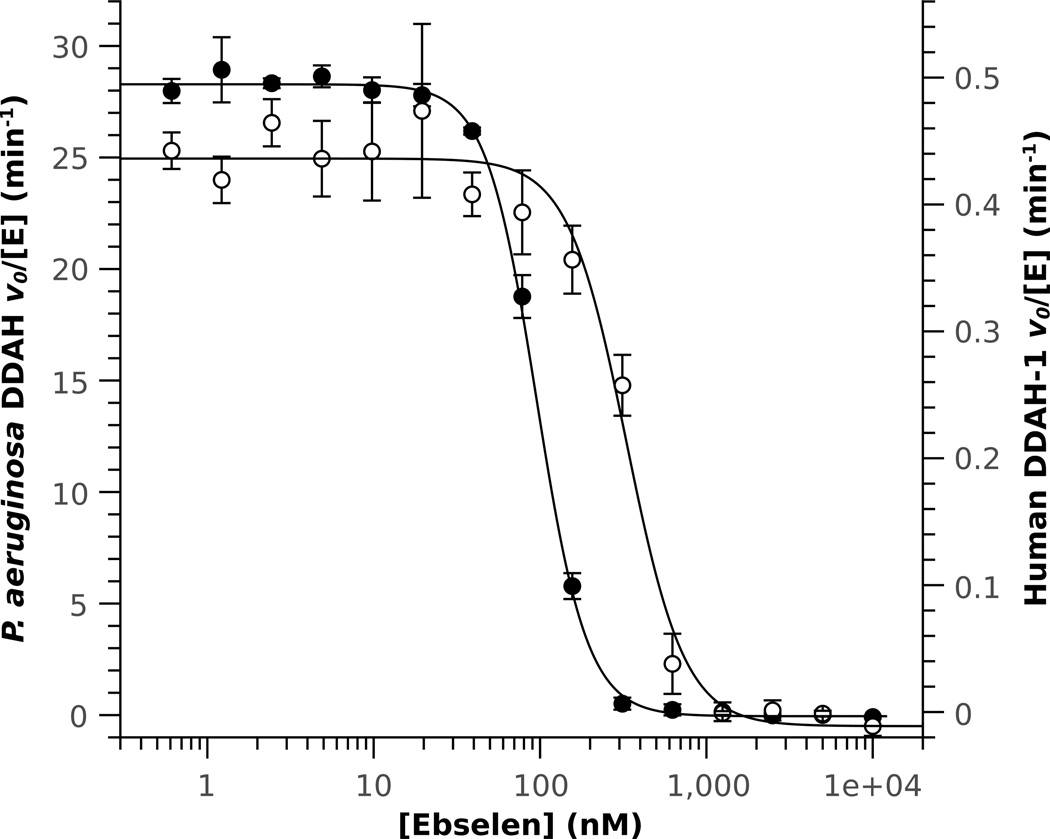
Dose dependence curves show very potent inhibition of both P. aeruginosa (IC50 = 96 ± 2 nM) and human DDAH-1 (IC50 = 330 ± 30 nM) isoforms at ebselen concentrations close to the enzyme concentrations used in the assay (Figure 2). The near-stoichiometric potency, the Hill coefficients greater than one, and the known reactivity of ebselen are all consistent with a covalent mode of inhibition. Therefore, reversibility was tested by gel filtration, after which the enzyme only regained ≤ 3% of its original activity. After DDAH was incubated with ebselen (MW = 274 Da), ESI-MS showed a 273 Da covalent adduct (Figure S4). Excess substrate can protect against inactivation, indicating that modification occurs at the active site (see below). Ebselen selectively reacts with Cys sidechains,12 and docking of ebselen to the active site puts its reactive Se-N bond next to the only active-site Cys residue (Figure S5). These results are consistent with a covalent mode of inhibition in which ebselen reacts with the active-site Cys of DDAH to open the selenazol ring and form a selenosulphide bond (Figure 4). Treatment with excess glutathione can only partially recover activity (< 20 %), suggesting that the ebselen adduct is well protected in the active-site cavity.
Figure 4.
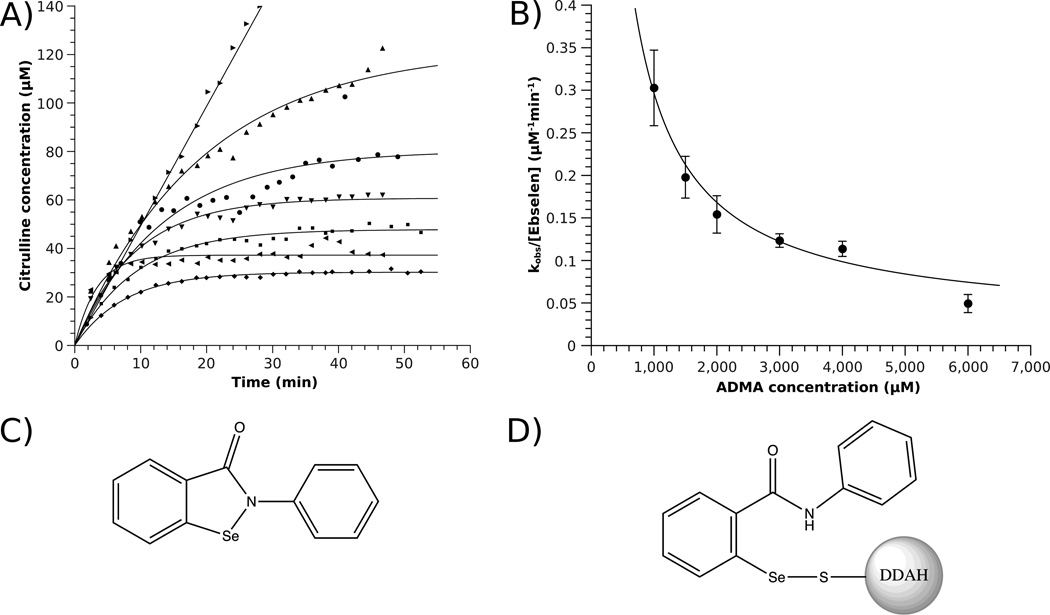
Covalent DDAH inactivation by ebselen. A) Product formation of P. aeruginosa DDAH incubated in the absence (▶, 1 mM dimethylarginine) and presence of ebselen (0.64 µM) at varying dimethylarginine (ADMA) concentrations (1 mM (◀), 1.5 mM (◆), 2 mM (■), 3 mM (▼), 4 mM (●), 6 mM (▲) ). B) Dependence of observed inactivation rates kobs/[I] fits to a hyperbola with inactivation rates of 43,000 ± 7,000 M−1s−1 for free enzyme and 400 ± 300 M−1s−1 for substrate-bound enzyme. C) Structure of ebselen. D) Structure of proposed inhibitory adduct with the active-site Cys.
Because ebselen is a time-dependent inhibitor, IC50 values do not truly reflect relative potencies. Therefore we determined the inactivation rate constant of ebselen with P. aeruginosa DDAH. To slow the rapid inactivation rates, these experiments were carried out in the presence of competing substrate (dimethylarginine, 4 mM ≈ 33×KM) and showed second order dependence on ebselen concentration with an estimated rate constant of 44,000 ± 2,400 M−1s−1 for reaction with the free form of the enzyme (Figure S2). A second set of experiments with a fixed ebselen concentration and varying substrate concentrations (dimethylarginine, 1–6 mM) were fitted with inactivation rates of 43,000 ± 7,000 M−1s−1 for free enzyme and 400 ± 300 M−1s−1 for substrate-bound enzyme, indicating that ebselen is competitive with substrate and that modification occurs predominantly with free enzyme at the active site (Figure 4). To our knowledge, DDAH has the highest inactivation rate constant for any ebselen-inhibited enzyme, including papain and glutathione-S-transferases, which have reported inactivation rate constants from 20 to 2,250 M−1s−1.13 This is a surprising result considering that the resting pKa of the active-site Cys in DDAH is > 8.7,14 but those of papain15 and glutathione-S-transferases16 are ≤ 4.2, and suggests that active-site determinants other than thiol reactivity contribute to the increased susceptibility of DDAH toward inactivation by ebselen. This conclusion is supported by the docking results, which place the phenyl ring of ebselen in a π-stacking interaction with Phe76 of DDAH and the benzoisoselenazol ring in the hydrophobic pocket where the Nω-methyl groups of the dimethylarginine substrate bind. Also, His162, which acts as a general acid during substrate turnover, is positioned to possibly aid the Se-N bond cleavage. The observed kinetic preference for DDAH inactivation suggests that this enzyme might be preferentially inactivated in cells, even in the presence of other thiols.
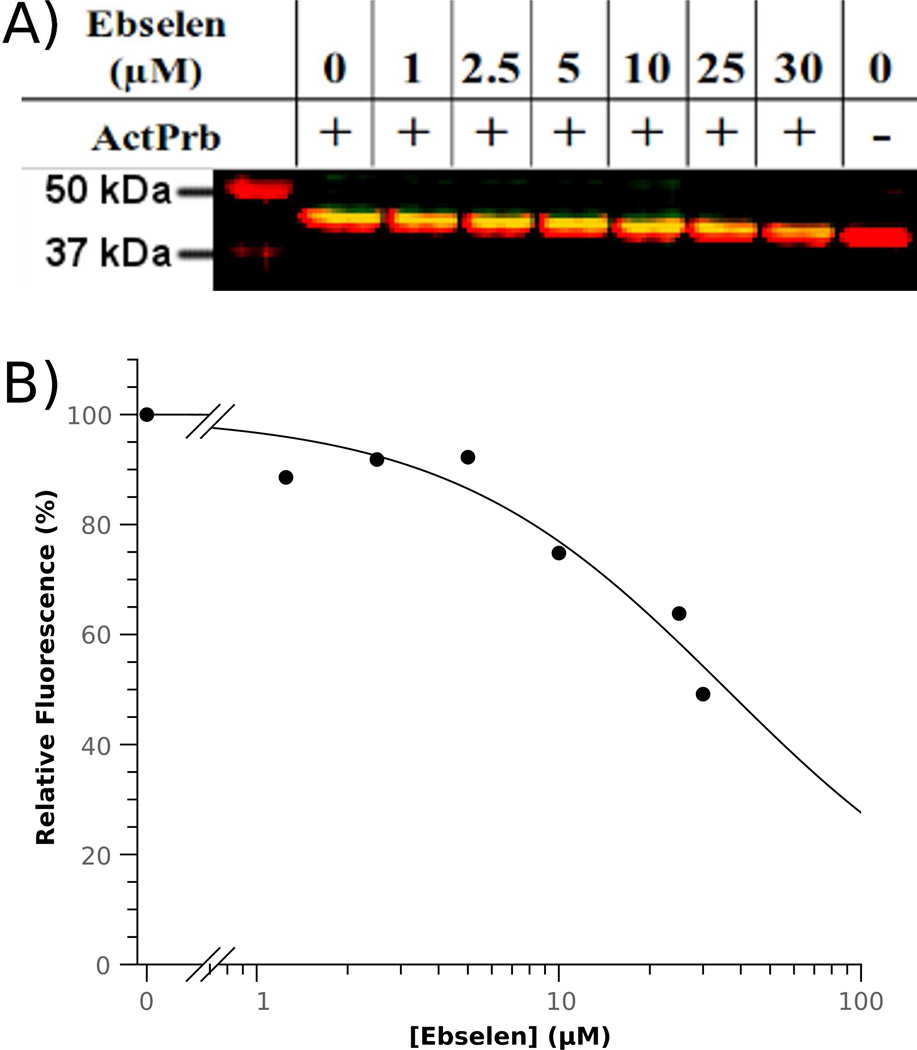
To determine whether ebselen could inhibit human DDAH-1 expressed within cultured HEK 293T cells, we used a two-color ratiometric Western blot. Protein expression levels were monitored by response to a genetically encoded myc-tag and catalytic activity levels were monitored by using a previously described two-part click chemistry-mediated activity probe that selectively biotinylates the active-site Cys.17 Addition of increasing doses of ebselen prior to addition of the activity probe led to a decreasing fraction of DDAH active-sites that could be labeled by the probe (Figure 5). Using these short incubation periods (30 min), an “in cell” IC50 value of 36 ± 7 µM was estimated. For comparison, the concentration range for in vivo ebselen studies has been estimated to be 0.5 – 109 µM.18 These experiments are consistent with DDAH-1 serving as an in vivo target of ebselen inhibition.
Figure 5.
“In cell” dose-dependent inhibition of human DDAH-1. A) HEK 293T cells treated with the DDAH activity probe N-but-3-ynyl-2-chloroacetamidine (ActPrb, 154 µM) and increasing ebselen concentrations are washed, lysed, derivatized with azide-labeled biotin, and analyzed by a two color Western blot to show equal loading of DDAH (red), and a decreasing number of active-sites available for biotinylation by the activity probe (yellow). The left-most lane contains molecular weight markers (also in red). B) Dependence of relative fluorescence of biotin-tagged DDAH on ebselen concentration gives an IC50 value of 36 ± 7 µM and Hill coefficient of 0.9 ± 0.2.
Ebselen is a hydrophobic, selenium-containing, promiscuously-targeted, thiol-reactive compound. These are typically undesirable features in a drug lead compound and we nearly chose to exclude it from our HTS hits based on “chemical intuition.” However, ebselen is surprisingly well tolerated in humans19–21 at doses up to 300 mg/d and is currently listed in the Stroke Trials Registry as ongoing a Phase III trial for acute ischemic stroke.22 One key facet of ebselen’s biological efficacy is its ability to undergo a redox reaction cycle that is catalyzed by thioredoxin in vitro,11 and likely enables covalent adducts to exist in equilibrium amongst accessible cellular thiols,23,24 as well as recycling of the parent structure. A second facet is its polypharmacology. A number of different enzymes are reportedly inhibited by ebselen in vitro (although not all have been confirmed in vivo). However, inhibition of many of these targets could contribute synergistically to ebselen’s pharmacological profile. For example, ebselen inhibits lipoxygenase, NADPH oxidase, protein kinase C, and H+/K+ ATPase. It also inhibits NO production in cell lysates, although it is not clear whether this is a result of direct NO synthase inhibition or the DDAH inactivation discovered here. In addition to its effects on proteins, ebselen also scavenges peroxides and peroxynitrite.25 All of these effects would be beneficial for treating conditions such as ischemic stroke and some types of cancer. The ability of ebselen and similar compounds to target both proteins and metabolites may provide new ammunition for the “magic shotgun” strategy of targeted polypharmacology, which has become attractive for design of therapeutic agents for diseases with complex molecular causes. Here, the demonstrated sensitivity and relative irreversibility of DDAH inhibition strongly suggests that it is an important part of ebselen’s NO-blocking abilities and possibly of its overall pharmacological effects.
In summary, a HTS assay to discover novel DDAH inhibitors is designed, optimized and validated. Screens of two small libraries identified ebselen as a bioavilable, active-site directed, covalent inactivator, with a kinetic preference for DDAH and a demonstrated utility for inhibiting DDAH in cells. These results will facilitate the discovery of novel DDAH inhibitors for therapeutic control of NO production, and help to elucidate the multifactorial mechanism of action of the selenazole drug ebselen.
Supplementary Material
Figure 3.
Dose-dependence of inhibition by ebselen. Inhibition of P. aeruginosa (●) and human (○) DDAH give IC50 values (and Hill coefficients) of 96 ± 2 nM (2.9 ± 0.1) and 330 ± 30 nM (2.5 ± 0.4), respectively. All data points were collected in triplicate.
ACKNOWLEDGMENT
We thank Dr. Eun Jeong Cho and the Automation and High Throughput Screening Facility at the Texas Institute for Drug and Diagnostic Development (TI-3D, University of Texas, Austin) for assistance with the HTS. This work was supported in part by grants from the National Institutes of Health (GM69754), the Robert A. Welch Foundation (F-1572), and a seed grant from the CTT / TI-3D chemistry & molecularly-targeted therapeutic development grant program.
Footnotes
SUPPORTING INFORMATION AVAILABLE Details on DDAH production, HTS assay, hits and their characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O’Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat. Med. 2007;13:198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- 2.Hartzoulakis B, Rossiter S, Gill H, O’Hara B, Steinke E, Gane PJ, Hurtado-Guerrero R, Leiper JM, Vallance P, Rust JM, Selwood DL. Discovery of inhibitors of the pentein superfamily protein dimethylarginine dimethylaminohydrolase (DDAH), by virtual screening and hit analysis. Bioorg. Med. Chem. Lett. 2007;17:3953–3956. doi: 10.1016/j.bmcl.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Monzingo AF, Hu S, Schaller TH, Robertus JD, Fast W. Developing dual and specific inhibitors of dimethylarginine dimethylaminohydrolase-1 and nitric oxide synthase: Toward a targeted polypharmacology to control nitric oxide. Biochemistry. 2009;48:8624–8635. doi: 10.1021/bi9007098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lluis M, Wang Y, Monzingo AF, Fast W, Robertus JD. Characterization of C-alkyl amidines as bioavailable covalent reversible inhibitors of human DDAH-1. ChemMedChem. 2011;6:81–88. doi: 10.1002/cmdc.201000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossiter S, Smith CL, Malaki M, Nandi M, Gill H, Leiper JM, Vallance P, Selwood DL. Selective substrate-based inhibitors of mammalian dimethylarginine dimethylaminohydrolase. J. Med. Chem. 2005;48:4670–4678. doi: 10.1021/jm050187a. [DOI] [PubMed] [Google Scholar]
- 6.Kotthaus J, Schade D, Muschick N, Beitz E, Clement B. Structure-activity relationship of novel and known inhibitors of human dimethylarginine dimethylaminohydrolase-1: Alkenyl-amidines as new leads. Bioorg. Med. Chem. Lett. 2008;16:10205–10209. doi: 10.1016/j.bmc.2008.10.058. [DOI] [PubMed] [Google Scholar]
- 7.Stone EM, Fast W. A continuous spectrophotometric assay for dimethylarginine dimethylaminohydrolase. Anal. Biochem. 2005;343:335–337. doi: 10.1016/j.ab.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Zhang, Chung Oldenburg A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 9.Knipp M, Vasák M. A colorimetric 96-well microtiter plate assay for the determination of enzymatically formed citrulline. Anal. Biochem. 2000;286:257–264. doi: 10.1006/abio.2000.4805. [DOI] [PubMed] [Google Scholar]
- 10.Knuckley B, Jones JE, Bachovchin DA, Slack J, Causey CP, Brown SJ, Rosen H, Cravatt BF, Thompson PR. A fluopol-ABPP HTS assay to identify PAD inhibitors. Chem. Commun. 2010;46:7175. doi: 10.1039/c0cc02634d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao R, Masayasu H, Holmgren A. Ebselen: A substrate for human thioredoxin reductase strongly stimulating its hydroperoxide reductase activity and a superfast thioredoxin oxidant. Proc. Natl. Acad. Sci. USA. 2002;99:8579–8584. doi: 10.1073/pnas.122061399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu K, Zhang Y, Tang B, Laskin J, Roach PJ, Chen H. Study of highly selective and efficient thiol derivatization using selenium reagents by mass spectrometry. Anal. Chem. 2010;82:6926–6932. doi: 10.1021/ac1011602. [DOI] [PubMed] [Google Scholar]
- 13.Nikawa T, Schuch G, Wagner G, Sies H. Interaction of ebselen with glutathione S-transferase and papain in vitro. Biochem. Pharmacol. 1994;47:1007–1012. doi: 10.1016/0006-2952(94)90411-1. [DOI] [PubMed] [Google Scholar]
- 14.Stone EM, Costello AL, Tierney DL, Fast W. Substrate-assisted cysteine deprotonation in the mechanism of dimethylargininase (DDAH) from Pseudomonas aeruginosa. Biochemistry. 2006;45:5618–5630. doi: 10.1021/bi052595m. [DOI] [PubMed] [Google Scholar]
- 15.Pinitglang S, Watts AB, Patel M, Reid JD, Noble MA, Gul S, Bokth A, Naeem A, Patel H, Thomas EW, Sreedharan SK, Verma C, Brocklehurst K. A classical enzyme active center motif lacks catalytic competence until modulated electrostatically. Biochemistry. 1997;36:9968–9982. doi: 10.1021/bi9705974. [DOI] [PubMed] [Google Scholar]
- 16.Lo Bello M, Parker MW, Desideri A, Polticelli F, Falconi M, Del Boccio G, Pennelli A, Federici G, Ricci G. Peculiar spectroscopic and kinetic properties of Cys-47 in human placental glutathione transferase. Evidence for an atypical thiolate ion pair near the active site. J. Biol. Chem. 1993;268:19033–19038. [PubMed] [Google Scholar]
- 17.Wang Y, Hu S, Fast W. A click chemistry mediated in vivo activity probe for dimethylarginine dimethylaminohydrolase. J. Am. Chem. Soc. 2009;131:15096–15097. doi: 10.1021/ja906432e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi H, Liu S, Miyake M, Liu KJ. Ebselen induced C6 glioma cell death in oxygen and glucose deprivation. Chem. Res. Toxicol. 2006;19:655–660. doi: 10.1021/tx0502544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parnham M, Sies H. Ebselen: prospective therapy for cerebral ischaemia. Expert Opin. Investig. Drugs. 2000;9:607–619. doi: 10.1517/13543784.9.3.607. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H. Ebselen in acute ischemic stroke: A placebo-controlled, double-blind clinical trial. Stroke. 1998;29:12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa A, Yoshimoto T, Kikuchi H, Sano K, Saito I, Yamaguchi T, Yasuhara H. Ebselen in acute middle cerebral artery occlusion: A placebo-controlled, double-blind clinical trial. Cerebrovascular Diseases. 1999;9:112–118. doi: 10.1159/000015908. [DOI] [PubMed] [Google Scholar]
- 22.Stroke Trials Registry. http://www.strokecenter.org/trials/
- 23.Ullrich V, Weber P, Meisch F, von Appen F. Ebselen-binding equilibria between plasma and target proteins. Biochem. Pharmacol. 1996;52:15–19. doi: 10.1016/0006-2952(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 24.Wagner G, Schuch G, Akerboom TP, Sies H. Transport of ebselen in plasma and its transfer to binding sites in the hepatocyte. Biochem. Pharmacol. 1994;48:1137–1144. doi: 10.1016/0006-2952(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 25.Schewe T. Molecular actions of ebselen--an antiinflammatory antioxidant. Gen. Pharmacol. 1995;26:1153–1169. doi: 10.1016/0306-3623(95)00003-j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.