Abstract
Biology has inspired solutions to many engineering problems, including chemical sensing. Modern approaches to chemical sensing have been based on the biological principle of combining cross-selective chemical sensors with a pattern recognition engine to identify odors. Here, we review some recent advances made in mimicking biological design and computing principles to develop an electronic nose. The resulting technology will have important applications in fundamental biological research, as well as in industrial, security, and medical domains.
1. Introduction
The olfactory system allows an organism to detect and interpret chemical cues present in its environment. A range of day-to-day functions, including appetite stimulation, food foraging and evaluation, mate recognition, navigation, detection of threats, and even early diagnosis of diseases depend on efficient processing of olfactory cues (1). To perform these essential but complex olfactory tasks most mammalian and insect species have evolved strikingly similar chemosensory systems. This suggests that the iterative process of evolution converged to a common set of design and computing rules for the purpose of odor recognition (2).
Artificial systems for non-invasive chemical sensing, popularly referred to as ‘electronic nose technology’ or ‘e-nose’ for short, have emerged recently (3). Like their biological inspiration, electronic noses typically combine an array of sensitive detectors capable of distinguishing different chemicals with a pattern recognition module to detect and identify odors (4–7). Here, we identify and discuss biological design and computing principles that we believe are particularly relevant for the purpose of artificial olfaction. Second, we review recent progress in engineering approaches inspired by biological principles.
2. The Biological Olfactory System
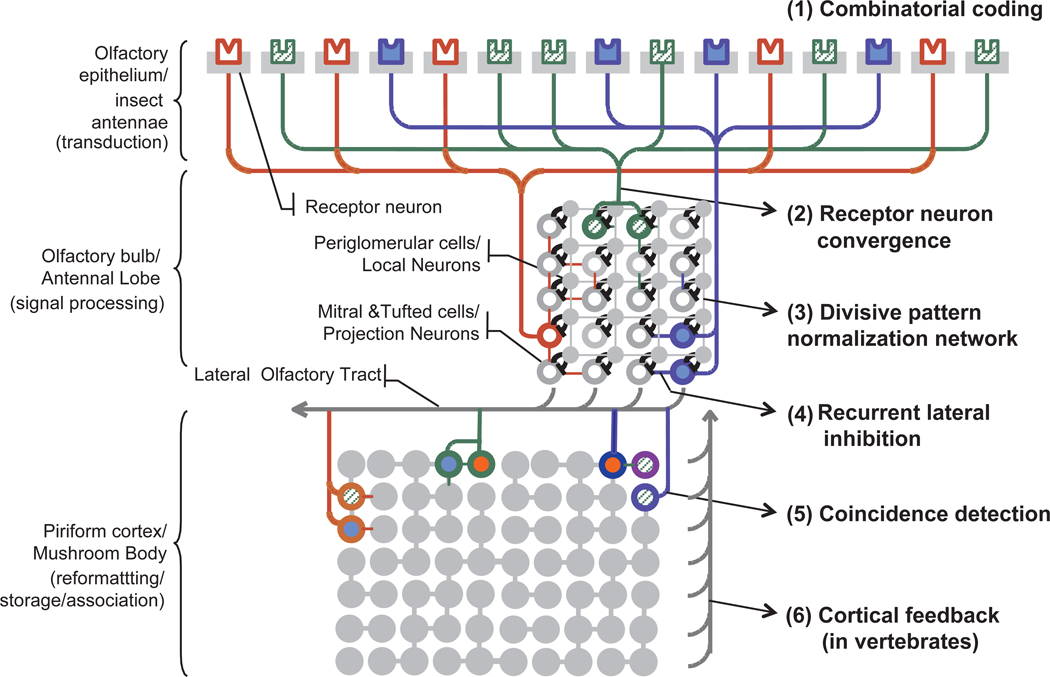
The olfactory system in vertebrates and invertebrates is comprised of three main components: an odor delivery system that transports the odor molecules to the sensory neurons; a large sensor array that transduces the chemical information into electrical signals; and neural circuits that process the sensory inputs to meet various analytical challenges. A schematic diagram of the early stages of the biological olfactory system and its various components are shown in Figure 1. In the following sections we will discuss biological principles that have inspired parallel approaches in artificial analogues.
Figure 1. Anatomical stages and signal-processing primitives in the early olfactory pathway.
(1) odorants are detected by a large population of sensory neurons in the olfactory epithelium (vertebrates)/antenna (invertebrates) that convert the chemical information in to combinatorial activity patterns (2) sensory neuron of the same type (expressing the same receptor gene) converge onto the same location (spherical structure of neurophil called glomeruli) in the next anatomical region (olfactory bulb in vertebrates; antennal lobe in invertebrates). This massive convergence of redundant input serves two computational functions: (i) it allows the system to average out uncorrelated noise to allow reliable detection, and (ii) it enhances the sensitivity of the system over that of individual sensory neurons (3) divisive pattern normalization circuits at the input of the olfactory bulb/antennal lobe allows the system to compress the concentration information (4) neural circuits at the output of the olfactory bulb/antennal lobe decorrelate odor response patterns such that both coarse clustering and fine discrimination can be achieved (5) the output patterns from the olfactory bulb/ antennal lobe is sent to olfactory cortex (vertebrates)/ mushroom body(invertebrates) for further reformatting, storage and association with other sensory modalities. Typically, these projections are both convergent and divergent (many-to-many) allowing detection of combinations of co-occurring molecular features of the odorant (or “coincidence detection”) (6) in vertebrates system cortical feedback modulates the activity in the olfactory bulb. Adapted from (97)
2.1 The Odor Space
To understand the challenges faced by the olfactory system we first need to examine the nature of the odor space. Odorants are volatile chemicals having a low molecular mass (<300 daltons), and are mostly organic (exceptions exist e.g. ammonia, hydrogen sulphide), polar, and water and lipid soluble. A large number of chemicals are odorous to the human nose. The ability of the olfactory system to handle a rather large stimulus set and assign meaningful percepts for the purpose of distinct recognition indicates the high-dimensional nature of olfaction. The spatially and temporally irregular and dynamic nature of the olfactory cues add further complexity that makes the task of reliable detection and encoding of odorants a challenging one.
What are the dimensions of this high-dimensional olfactory space? The problem of identifying the physiochemical properties of odorants that are detected by sensory neurons and their subsequent synthesis into a distinct smell percept is a definitive challenge in the study of olfaction. Historically, three popular theories have been considered in an attempt to relate molecular properties of an odorant with its overall perceptual quality. The vibrational theory of olfaction (8–11) identifies intramolecular vibrations due to stretching, scissoring, rocking, wagging, and twisting of various bonds or groups in the odor molecule as the direct determinants of odor identity and quality. But this idea has garnered little support from empirical studies. The steric theory of olfaction relates odor quality to the overall shape and size of the odor molecules (12–14). This theory also has limitations as chemicals with different molecular shapes and composition could elicit similar overall smell (e.g. benzaldehyde and hydrogen cyanide both have the smell of bitter almonds yet are quite dissimilar in overall shape). Weak-shape theory diminishes the rigid requirement of a lock-key type fit between the overall shape of the ligand and its receptor, and proposes only a partial match, such that a portion of the ligand fits in a receptor’s binding pocket, is required to activate the receptor. According to a version of this theory, molecular features such as carbon chain length, functional groups, and other such properties determine odor quality. Detailed electrophysiological studies of an individual odor receptor have shown that only a subset of the features defining a molecule are sufficient to predict the response of the odor receptor (the rat I7 odor receptor is highly specific to aldehyde group with certain constraints around the carbonyl group but remains insensitive to other systematic variations at the tail of the molecule) (15). But because perception emerges within the brain, not within olfactory receptors, the problem of relating features of a molecule with its percept, popularly known as the ‘Structure-Odor Relationship,’ will only be resolved if the response characteristics of sensory neurons and the subsequent non-linear processing of their outputs are both taken into account.
2.2 Odor Delivery
Olfactory sensory neurons (or olfactory receptor neurons; ORNs) of both vertebrates and insects exist in aqueous media (mucus and sensillar lymph, respectively), and odor molecules present in the external environment must traverse this protein-filled fluid before binding to receptors. The interactions between the odor molecules and the odor delivery interface prior to reaching the sensory neurons are generally referred to as perireceptor events (16).
Two experimental observations present evidence that the perireceptor events allow the olfactory system to use physical variables of space and time to encode odorants. First, sensory neurons are loosely organized into zones in the olfactory epithelium, such that neurons of the same type (expressing the same or the homologous receptor genes) are spatially distributed into regions (referred to as ‘zones’) of the epithelium (17). Second, simultaneous electrophysiological recordings from distinct regions of olfactory epithelium showed that odor molecules were differentially transported across the epithelium, thereby creating a unique spatiotemporal response profile for each odorant (18–23). The latter mechanism has been likened to that of the gas chromatographic column, where constituents of a gas mixture, depending on their physiochemical properties, interact with the liquid stationary phase and elute at different points in time (see Figure 2a).
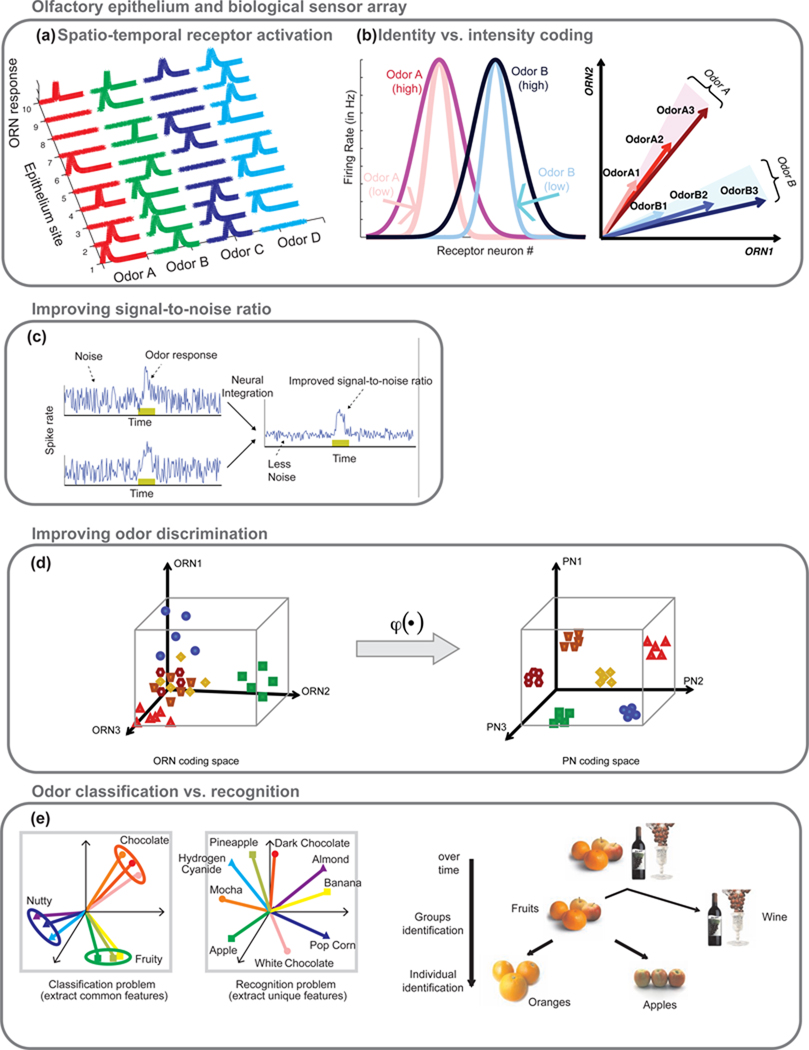
Figure 2. Biological principles that have inspired parallel approaches in artificial olfaction.
(a) Odor molecules traversing the aqueous, protein-filled interface between the environment and the sensory neurons (ORNs) are differentially transported causing different odorants to evoke different spatiotemporal patterns of activation.
(b) Left: The response of the population of receptor neurons to an odor approximates a Gaussian distribution, arranged here by the receptors’ selectivity for Odors A and B (33). Increasing odorant concentrations recruit additional responses from neurons less selective for the odorant, broadening this distribution. Right: Vector representations of odor identity and intensity coding across the population of sensory neurons allow ready comparisons of response characteristics.
(c) Integrating redundant information from multiple copies of a receptor allows the olfactory system to reduce uncorrelated noise. Left: firing rates of two receptor neurons over time. Right: the baseline fluctuations observed in the two independent channels are reduced when the channels are integrated, improving the signal-to-noise ratio. Reprinted with permissions from (98).
(d) Lateral interactions between projection neurons (PNs) non-linearly transform responses originating in sensory neurons and restructure odor patterns to become more uniformly distributed and distinct. Reprinted with permissions from (99). Copyright 2008 Elsevier.
(e) The olfactory system refines odor representation over time such that features common across a set of chemicals are extracted first (odor class information) and finer features required for precise identification are extracted subsequently. Thus a single system, over time, resolves conflicting demands posed by the problems of odor classification and recognition.
Additionally, the complex geometries of olfactory structures and active mechanisms such as sniffing in vertebrates or antennal flicks in invertebrates create complex flow dynamics that could further aid in creation of spatiotemporal distributions across arrays of receptors (24).
2.3 Sensor Array Design
A critical aspect of a biological olfactory system concerns the design of its sensor array. The large stimulus set that the system has to process renders infeasible any approach based on sensors selective for each target ligand. (Separate sub-systems with selective chemoreceptors have been identified in both vertebrate and invertebrate systems for processing small numbers of pheromones and other conspecific odors (25).) The biological approach for dealing with general odors appears to employ a large array of cross-selective sensors (~300 to 1000 receptor types, specified by the genes they express from a family of G-protein coupled receptors (26)). Each olfactory receptor is capable of responding to a number of chemicals, and generates a response containing characteristic temporal features (27–29). The resulting response profile across the sensor arrays provides a chemical fingerprint for each odor.
To accommodate the variability associated with the olfactory stimuli, and to provide sensitive detection at the same time, several hundred thousand copies of each type of olfactory receptor exist in the olfactory epithelium (there are ~10–100 million sensory neurons in the vertebrate nose). Integrating responses from these redundant copies then provides a simple method to average out uncorrelated noise and improve the signal-to-noise ratio (30) (refer Figure 2c). Further, such a scheme serves to enhance the sensitivity of the system as a whole in comparison with those of its individual sensing elements (31).
The next design issue concerns the dynamic range of the chemical sensing system. For this, two design options are possible, and published results suggest both are employed by animals. The first, more straightforward, option is to employ olfactory receptors that individually encompass a large dynamic range (32). While this is certainly the case for some types of receptors, the second design option is to employ individual sensory neurons that have steep dose-response curves spanning only one or two log-units of concentration. Collections of such receptors include members with somewhat different detection thresholds and tunings, so combining them allows the system to sample a wide range of concentrations (33). This option has the advantage of providing an extremely non-linear, input-output relationship suitable for amplifying extremely weak signals.
Another important aspect of the olfactory system that is critical to its ability to function over extended periods of operation without degrading performance is its ability to replace aging olfactory receptor neurons with a complete turnover occurring every 4–8 weeks (34). This feature, which is unique to olfaction among the sensory systems, poses fundamental questions regarding the constancy of the sensory input over time given the changes to the sensor array. At any point in time, the overall distribution of the age of a chemosensor of a particular type in the array must be held constant to shield the pattern recognition circuits from continual changes to the sensory array.
2.4 Computing Principles
A number of computational challenges are to be met for proper functioning of any olfactory system. In this section, we describe biological solutions to various olfactory pattern recognition problems.
2.4.1 Identity vs. Intensity Coding
The biological olfactory system can successfully discriminate among many analytes presented at different concentration levels. This requires a large coding space to encode the stimulus set and an ability to decouple odor identity from intensity (although the latter capability might not be viable for certain chemicals and the overall odor quality could change with concentration (35–37)).
As mentioned earlier, to meet these challenges, the biological system employs an array of broadly-tuned sensors. A single sensory neuron responds to multiple odors, and each odor, whether it is a pure analyte or a complex mixture, evokes a unique response pattern across the population of sensory neurons. The responses of different sensory neurons to a particular odor can be approximated as a Gaussian distribution (33). Increasing odor concentration results in recruitment of responses from a subset of sensory neurons that were not active at lower intensities, thereby expanding the width of the Gaussian distribution (33, 38, 39). The size of the responding population monotonically increases with, and therefore encodes, odor intensity.
This form of encoding automatically allows the receptor array to decouple odor identity from intensity. To better understand this let us consider the response across the population of sensory neurons as a multidimensional vector. Increasing the concentration of the odor would result in a monotonic increase in the length of the response vector, and at the same time displace the vector from its original direction. Since sensory neuron responses that were recruited by increasing concentration are relatively weak, the original vector is displaced, but only by a small amount as shown in Figure 2b. Thus, the responses still cluster according to odor identity, followed by intensity.
To develop a large encoding space for different odorants, the sensory neurons employ time as an additional coding dimension. Both firing rates and rates of change for those firing rates are known to selectively encode odors (27–29, 40–44). Further, the heterogeneity of temporal response properties across sensory neurons has been shown to be particularly important for the following circuits to discriminate a large set of odorants and to recode olfactory information to make better use of the system’s capacity (29, 45–52) (see Figure 2d).
2.4.2 Gain Control
A fundamental requirement for discrimination of a large stimulus set is the ability to amplify small differences (i.e. ‘contrast enhancement’) in neural representations of odors. However, such a signal-processing step could also cause differences in the neural representation arising from noise or concentration differences (i.e. from the recruitment of less selective sensory neurons by higher concentrations of odors) to diverge in a manner similar to representations for different odors. Hence, a preprocessing step to compress the concentration information is necessary in order to retain invariance across different concentrations.
The olfactory circuits at the input of the olfactory bulb (OB; in vertebrates) and antennal lobe (in insects) perform this pattern normalization function. These feed-forward inhibitory circuits are of the divisive-type, and have been hypothesized to serve as a “gain control” mechanism (53). In such a system the overall inhibition scales with the total amount of input from the sensory neurons, i.e. monotonically with concentration. It is important to note that information about the odor’s intensity is not completely removed by this step but only compressed as organisms still need to distinguish concentrations.
2.4.3 Clustering vs. Recognition
Another capability of the biological olfactory systems is the extraordinary ability to demonstrate reliable recognition of odorants to which it has been pre-exposed and to classify new odorants based upon chemical similarity to those that it has previously learned. Correct recognition of a specific chemical requires detection, in some way, of an aspect of the molecular features unique to that analyte. On the other hand, generalization to unknown chemical species requires detection of features that are common across a desired class of analytes. Thus these pattern recognition tasks impose constraints that are opposing in nature.
How does the biological olfactory system deal with these conflicting analytical tasks? The high-dimensional inputs from the sensory neurons are subsequently transformed by neural circuits such that initially coarse odor representation is increasingly refined over time to become more odor-specific (54). The segmentation of odor class and identity information appears to happen in a hierarchical fashion over time (55) as illustrated in Figure 2e. Such a process also allows the system to extract olfactory features at several degrees of resolution (e.g. pleasant->fruity->strawberry).
2.4.4 Processing of Odor Mixtures and Backgrounds
A fundamental characteristic and perhaps limitation of the biological system is its inability to analyze odor mixtures into their constituents. Psychophysical studies in humans reveal that even trained experts have difficulty identifying the constituents of odor mixtures with more than 3 components (56). Since most naturally occurring olfactory stimuli are mixtures of several monomolecular components, olfaction probably evolved as a synthetic sense. Hence, holistic (or configural) processing rather than elemental processing is the demand posed to the system.
Given the need for synthetic or holistic processing, the olfactory system must possess perceptual stability to generalize across minor variations in blend constituents especially since no two odor-objects (e.g. different roses or chocolates) are likely to be identical in chemical composition (57, 58). A simple two-step solution appears to be in place for this computational task. First, the convergent input from multiple sensor types onto a single third-order neuron (Kenyon cells in the mushroom body of the insects; pyramidal neurons in the Piriform cortex of the vertebrates) allows integration of elemental information about various molecular features into a single olfactory percept. Choosing a suitable threshold for these neurons would then allow robustness against minor variations in the overall composition (45). The performance might be further enhanced at the ensemble level by means of auto-associative circuits that could complete partial representations into complete overall percepts (57).
A special case for odor mixtures is the ubiquitous presence of ambient, background odors in an environment. Sensory adaptation can attenuate the contributions of lingering background odors (58–60) relative to those of freshly introduced stimuli, thereby helping to distinguish foreground from background. Additional mechanisms downstream may contribute as well.
3. The Artificial Olfactory System
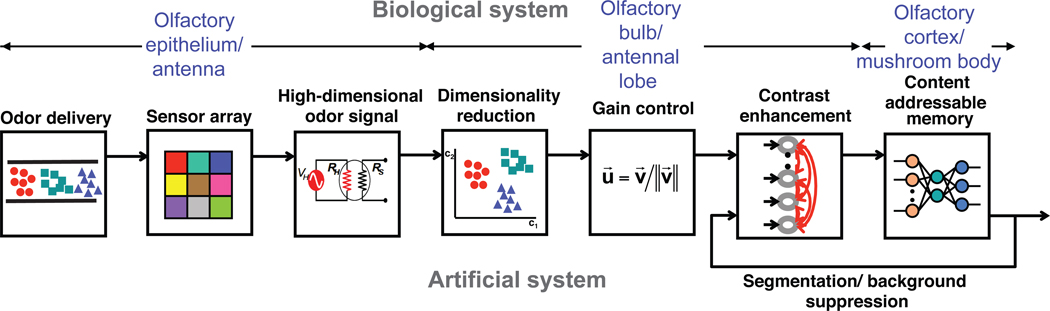
Next we discuss how these biological solutions have inspired parallel solutions in artificial olfaction. Similar to its biological analogue, an artificial olfactory system also has three basic components, namely, the odor delivery system, a cross-selective sensor array, and a pattern recognition engine. A block diagram identifying the important components of a bio-inspired architecture for artificial olfaction is shown in Figure 3. While most attention in artificial olfaction has been directed toward designing sensory arrays employing different transduction mechanisms, the odor delivery system has been largely overlooked. Furthermore, most investigations have focused on the problem of discrimination of multiple analytes. It might be worth noting that applying biological principles to chemical sensing is a fairly novel line of investigation and most important contributions have come from a handful of research groups.
Figure 3.
Building blocks for a biologically-inspired architecture for artificial machine olfaction. The first stage delivers odorants to receptors in a way that mimics peri-receptor events that improve odor separation. The following two stages are related to generation of high-dimensional response patterns across the sensor array similar to the combinatorial activity across a population of olfactory sensory neurons. The sensor array responses are processed first to compress/remove concentration information and then to extract features that allow several levels of abstraction (clustering, discrimination and quantification). Finally, distinct patterns for odors are stored for subsequent recognition during testing phase. Interactions between the recognition and feature extraction steps allow filtering of background signals as well as identification of mixture components. Adapted from (97).
3.1 Odor Delivery System
As noted earlier, discrimination of odorants in the biological system begins with their interactions with the aqueous interface between the sensory neurons and their environment. Motivated by this approach a microfluidic channel coated with a polymeric material was fabricated and mounted to the front end of a chemiresistive microsensor array (61). The internal coating of this ‘artificial mucosa’ allowed differential transport of odor molecules across the linear array with 16 columns of five different sensing elements each (Figure 4b). Placing the sensing columns along the length of the microfluidic channel generated spatiotemporal responses for chemicals that greatly enhanced their discrimination.
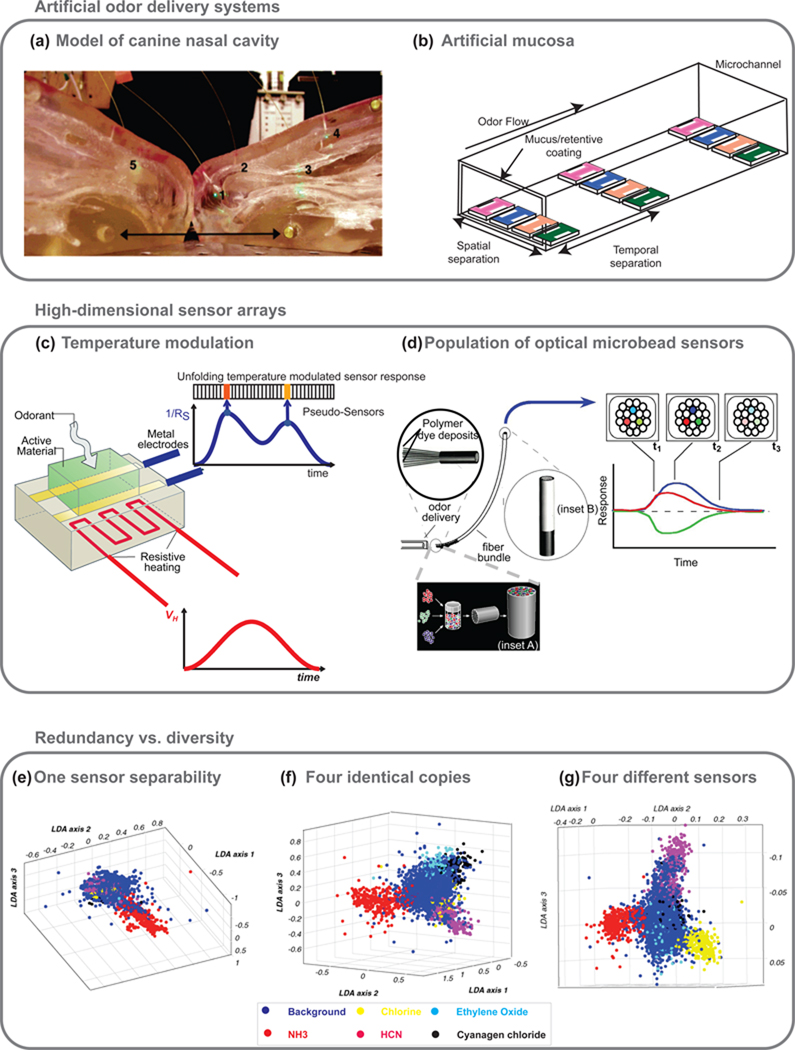
Figure 4. Bio-inspired approaches for odor delivery and chemical sensor array design.
(a) A realistic physical model of the canine nasal cavity. When odorants were introduced through the model’s nares, five identical sensors placed at different spatial locations in the model (numbered 1–5) yielded different spatiotemporal responses. These results show that complex flow dynamics in the nasal cavity could enhance the separation of different chemical species, potentially giving rise to diverse neural responses. Reprinted with permissions from (62). Copyright 2003 American Chemical Society.
(b) An artificial mucosa to simulate fluid found within the nose employs microfluidic channels coated with polymers. This model could separate odor components like the stationary phase of a gas chromatography column. Adapted with permissions from (61). Copyright 2007 The Institution of Engineering Technology.
(c) Temperature modulation of metal-oxide sensors to increase sensor dimensionality. A voltage profile is applied across the resistive heater, and the sensor resistance is continually recorded. Because interactions between metal-oxide chemiresistors and various chemical species are temperature dependent, the response of a sensor at a particular temperature can be treated as a separate “pseudo-sensor” or a ‘virtual sensor’ and used to simulate a large population of ORNs (adapted with Permission from (64). Copyright 1998 IEEE).
(d) Odor sensing by microbead arrays: Odor vapor is delivered to the distal end of the fiber. Exposure to odor vapor induces a change in fluorescence that is recorded and plotted over time Inset A: Microspheres coated with a polymer matrix onto which salvotochromic dye (e.g. Nile red) are immobilized, and randomly filled at the distal end of the fiber. Inset B: The distal end of the optical fiber from which the response is read (adapted with permission from (70). Copyright 1996 Nature Publishing Group).
Benefits of incorporating redundancy or diversity into a sensor array:
(e) Responses of a single temperature modulated metal-oxide sensor to different background conditions and five target toxic industrial chemicals are shown. Each three-dimensional color-coded sphere indicates a sensor measurement after dimensionality reduction.
(f) Responses from four identical copies of the sensor after dimensionality reduction are shown.
(g) Responses from four different chemiresistors are shown for comparison. Both sensor redundancy and diversity improve detection and recognition of target chemicals from the background. Reprinted with permissions from (74). Copyright 2009 Elsevier.
In another elegant effort (62), a simplified model of the canine nasal cavity was used to deliver odorants to optical vapor sensors (Figure 4a). By placing identical sensors at different locations within the model nasal cavity the authors revealed the uneven spatiotemporal distribution of odor molecules to various physical locations, and demonstrated the significance of this distribution for discriminating odors. It is important to note that, unlike the approach taken by Covington and colleagues (61), here no coating material was used to act as a barrier to the transport of odor molecules. Hence these results revealed the existence of multiple flow paths and flow currents that could potentially be exploited by the biological olfactory system to discriminate odors, and that this feature could be also be incorporated to enhance their artificial analogues.
A different approach was taken by Lewis and co-workers (63) to create space- and time-dependent responses across their sensor array. Employing low headspace volume and flow rates, the authors were able to convert upstream sensing elements of a linear sensor array to act akin to the stationary phase of a gas chromatography column, thereby producing a spatiotemporal fingerprint for each analyte introduced.
3.2 Sensor Array
3.2.1. Transduction Mechanisms
A number of sensing technologies have been employed for the purpose of detecting and identifying chemicals, including chemiresistive metal-oxide semiconductors, quartz microbalances, chemiresistive conducting polymer sensors, surface acoustic wave devices, optical-fiber sensors, optical filters, colorimetric sensors, and DNA-based sensors (64–69). However, unlike the biological olfactory system, artificial systems typically use relatively few sensors, commonly one or a few replicates of a small number of different sensor types. This fundamental mismatch between the biological and artificial systems in their input dimensionality must be overcome in order to be able to exploit the processing strategies employed by the biological olfactory system.
3.2.2. Large Sensor Arrays
To reduce the dimensionality mismatch between the two systems and generate a biology-like combinatorial and high-dimensional odor representation from chemical sensor arrays, three different mechanisms have so far been employed. The first method, a direct approach, was taken by researchers at Tufts University to create large chemical sensor arrays with optical microbead sensors (refer Fig 4d). Hundreds of broadly-tuned bead sensors, functionalized such that each belonged to a discrete type, were randomly dispersed across the tip of an optical fiber (70) to create a high-dimensional, combinatorial signal.
The second approach to create a large sensor array involved temperature modulation of metal oxide chemiresistors (7, 71–73). Because the interaction between a metal-oxide sensor and an analyte is a function of the sensor operating temperature, by capturing a given sensor’s response at different temperatures, a large population of ‘pseudo-sensors’ or ‘virtual sensors’ could be generated from a handful of sensor types (as shown in Figure 4c). In this study temperature was used as a modulation parameter, but any means of altering the selectivity of the sensor could be used to generate a population of pseudo-sensors.
A mathematical approach to convert responses from a small set of sensors onto a combinatorial code across a population of simulated olfactory sensory neurons was presented in (7). In this approach, each sensory neuron is modeled as an ‘n’-dimensional vector that randomly samples a certain region of the sensor response space (n is the number of sensors used). The response of this simulated sensory neuron to each analyte is given by: (i) its similarity (cosine of the angle formed) to the vector that describes the n-dimensional sensor array response to that analyte, and (ii) the length of the analyte response vector, which indicates the analyte concentration. Weighting the similarity (raising the cosine of the angle between the vectors to various powers) generates sharply-tuned or broadly-tuned receptor neurons. By sampling the sensor space with a population of such simulated receptors, a high-dimensional odor signal can be obtained that preserves the topology and proximity relationships of the sensor space. This approach has the advantage that it can be combined and used with any sensor array with any number of sensing elements.
3.2.3 Redundancy and Diversity
The biological system employs up to several hundred thousand copies of the same receptor neuron type (those expressing the same receptor gene) and up to hundreds of different types of receptor neurons. Mimicking this approach, arrays of fiber-optic sensors and semiconducting metal-oxide sensors that incorporate both diversity and redundancy have been fabricated and studied (31, 74). Redundant elements in the array allow averaging out uncorrelated noise, and thereby improve the signal-to-noise ratio. Different sensor types, on the other hand, may add additional information about the various chemical species of interest. An illustrative example demonstrating the empirical benefits of incorporating redundant sensors and diverse sensors to an array is shown in Figure 4e–f.
3.3 Information Processing Principles
3.3.1 Preprocessing of Sensory Input
The massive anatomical convergence of redundant information from sensory neurons expressing the same receptor gene has been of great interest to the e-nose signal processing community. Integrating signals from a large population of chemically sensitive microbeads in a manner akin to the convergence mechanism, Pearce et al. (31) demonstrated a sensitivity gain on the order of from n redundant copies can be achieved by this approach.
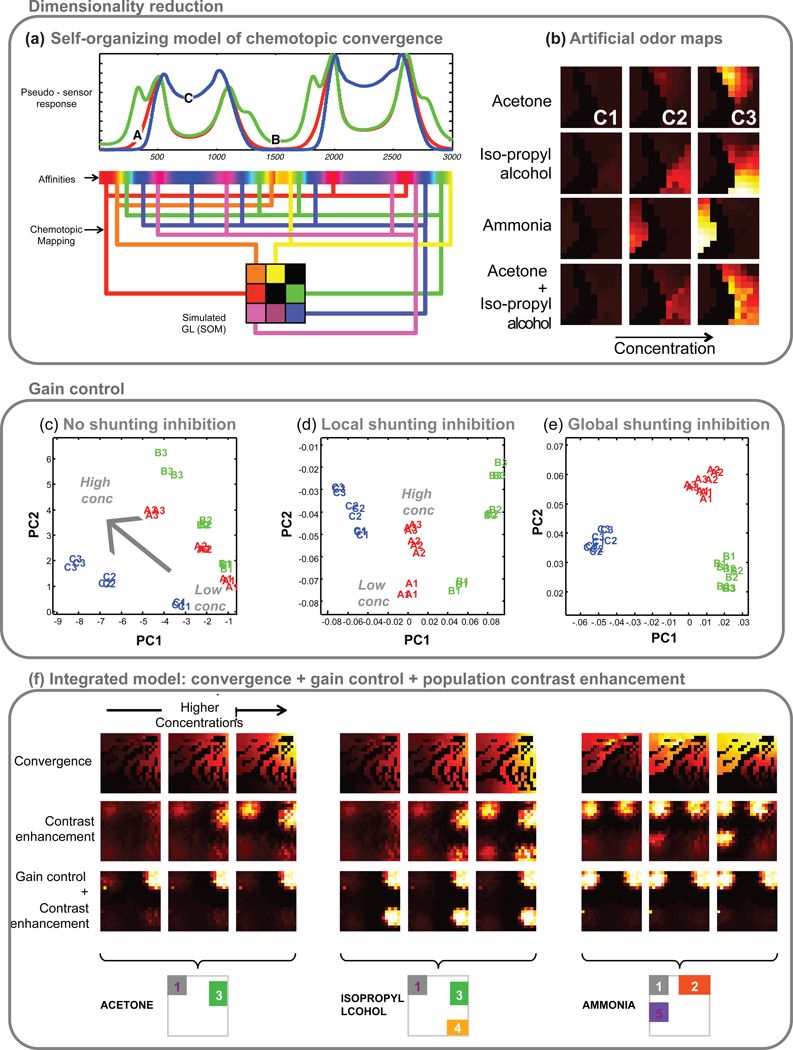
A self-organizing map-based model of receptor neuron convergence onto the olfactory bulb was presented in (7) to reformat high-dimensional sensor input into an organized spatial map that decouples odor identity from intensity. In this model, sensors of the same type converged onto a single node in a 2-d lattice (analogous to the structure of a glomerulus in the olfactory bulb). The authors generated a topographic map by providing neighboring nodes with input from sensors with similar selectivities. Figure 5 a, b provides an illustration of this approach. The resulting activity maps elicited by different concentrations of odors and their binary mixtures were qualitatively similar to published results obtained in vivo. The authors further showed that their model was better able to discriminate odors than competing linear statistical techniques including principal component analysis (PCA) and linear discriminant analysis (LDA).
Figure 5. Neuromorphic approaches for signal processing.
(a) Illustration of chemotopic pseudo-sensor receptor neurons (top) converging onto a simulated glomerular lattice (bottom). The responses of the sensor to three analytes (labeled A, B and C) are used to define the pseudosensor’s affinities (shown as a colorbar). Pseudosensors with similar affinities project to the same artificial glomerulus (a node in the self-organizing map) as a result of chemotopic convergence, as in a biological glomerulus. Activity across the simulated glomerular lattice forms a spatially distributed representation of the odor, and can be considered an artificial odor map. Reprinted from (97).
(b) Artificial glomerular images generated from an experimental database of temperature-modulated metal-oxide semiconductor sensors exposed to acetone, isopropyl alcohol, and ammonia at three different concentrations levels (C1–3). As has been observed in the olfactory bulb, increasing concentrations of odorant expanded the area of activation at the odor-specific locus. Bottom row: odor maps generated from the sensor array response to a mixture of acetone and isopropyl alcohol at three concentrations. The binary mixture had an additive effect and generated odor maps that activated regions of the lattice corresponding to both component odors. More complex mixture responses have been reported in vivo. Reprinted with permissions from (7). Copyright 2006 IEEE.
(c–e) Various forms of shunting inhibition can be employed to either compress or remove concentration information. Principal Component Analysis (PCA) scatterplots showing responses of a temperature-modulated sensor to three analytes presented at three concentrations (A1: lowest concentration of analyte A, C3: highest concentration of analyte C). PCA plots compare the odor identity and intensity distributions following: (c) chemotopic convergence (no shunting inhibition), (d) normalization using a shunting inhibition model with local connections, and (e) normalization using a shunting inhibition model with global connections.
(f) Combining biomimetic processing steps can further enhance odor discrimination. Artificial odor maps after chemotopic convergence (top row), processed with an additive model of the olfactory bulb with center-surround inhibition (middle row), and integrated OB network with shunting inhibition and center-surround inhibition (third row). Outlines of steady-state active regions in the artificial odor maps corresponding to different analytes are highlighted below the plots. Reprinted from (97).
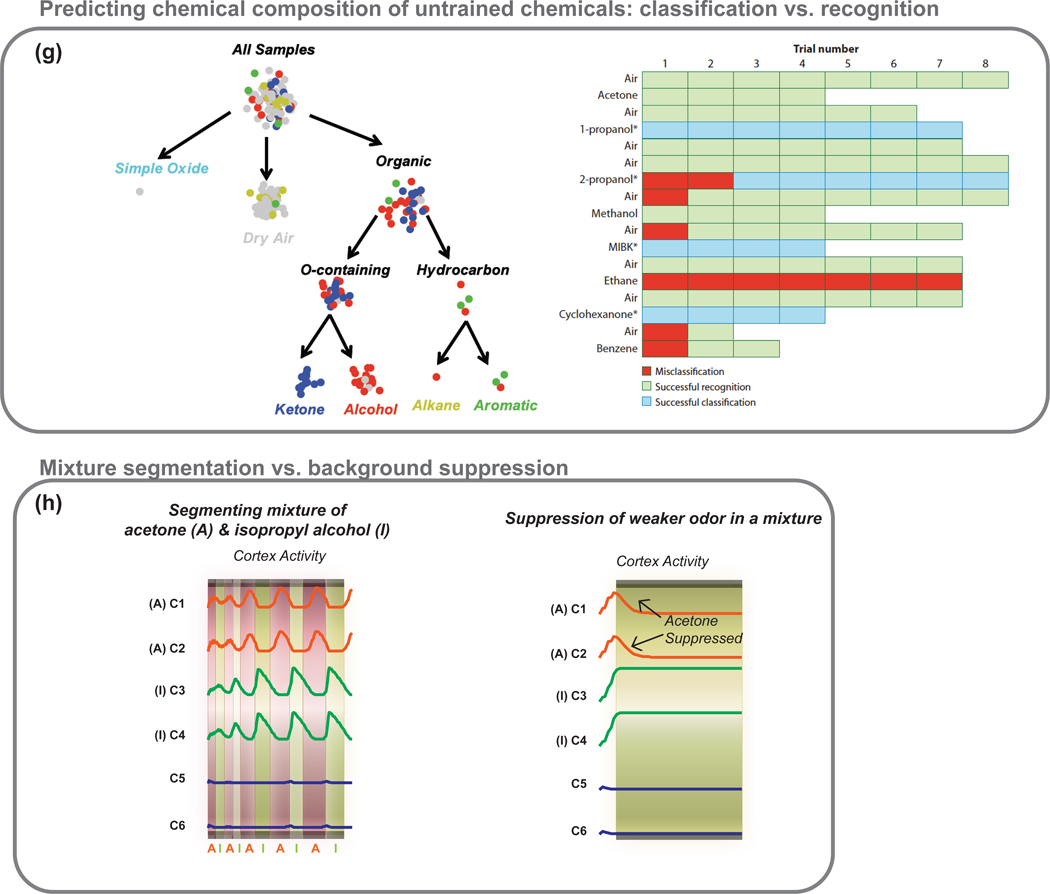
(g) Mimicking the biological approach of refining odor representation over time, a hierarchical scheme that performed initial discrimination between broad chemical classes (e.g. contains oxygen) followed by finer refinements using additional data into sub-classes (e.g. ketones vs. alcohols) and, eventually, specific compositions (e.g. ethanol vs. methanol) is shown. Left panel: Graphical view of the traversal of each measurement through the hierarchy. The chemical family of the analyte present during measurement is color-coded: gray = dry air, cyan = simple oxide, red = alcohol, blue = ketone, yellow = alkane and green = aromatic. Right panel: Chart of the accuracy of placement of each measurement into its proper category: green box = correct recognition, blue box = correct classification and red = incorrect placement. Analytes not included during the training phase are indicated with an asterisk. The order of analyte exposure during the test phase is as shown, progressing from left to right and then top to bottom (Trials 1– 8 were air exposures, trials 9–13 were acetone exposures, and so on). Reprinted with permissions from (88). Copyright 2008 American Chemical Society.
(h) Both mixture segmentation and background suppression can be achieved by a model of olfactory bulb-cortex interactions. In this model, the olfactory bulb sends non-topographic and many-to-many projections to the olfactory cortex such that cortical neurons detect combinations of co-occurring molecular features of the odorant, and therefore function as coincidence detectors. The associational connections within cortex are established through correlative Hebbian learning, such that cortical neurons that respond to at least one common odor have purely excitatory connections between them, and neurons that encode for different odors (no common odor) have purely inhibitory connections between them. The excitatory lateral connections perform pattern-completion of degraded inputs from the bulb, whereas the inhibitory connections introduce winner-take-all competition among cortical neurons.
Two types of feedback connection were investigated: (i) anti-Hebbian update forms feedback connections such that the resulting centrifugal input from the model cortex inhibits bulb neurons responsible for the cortical response, resulting in the temporal segmentation of binary mixtures (left panel). (ii) Hebbian update on the other hand retains only those connections between cortical neurons and bulb neurons that respond to different odors. The resulting feedback from the cortex inhibits bulb neurons other than those responsible for the cortical response, causing cortical activity to resonate with OB activity and lock onto a particular odor and suppress the background/weaker odor (right panel). Reprinted with permissions from (91). Copyright 2005 IEEE.
Perera et al. (75) reformulated the restructuring of receptor neuron inputs as a task of dimensionality reduction. Conventional statistical pattern recognition approaches for clustering operate in the feature space, where each input dimension corresponds to a particular feature (or sensor). However, in this neuromorphic feature-clustering approach, sensors that provide redundant information (respond similarly to a set of target classes) were clustered to improve the signal-to-noise ratio and prevent the washing-out of relevant discriminatory information. The advantage of this approach appears to be its ability to successfully process datasets with very few samples.
3.3.1 Identity and Intensity Coding
A number of biologically inspired models have been used for encoding odor identity and intensity in artificial olfaction, as briefly summarized here.
A distributed neural network of coupled oscillators referred to as the KIII model was proposed by Freeman and colleagues to simulate electro-encephalogram (EEG) activity in the olfactory bulb (76). The nonlinear dynamics of the KIII model had been previously exploited to process chemical data from Fourier Transformed-Infrared spectra (77, 78). More recently, the KIII model has also been used for pattern recognition of chemical sensor responses (79, 80). Incorporating various forms of plasticity and adaptation mechanisms in the KIII model, chemical detection in the presence of pre-trained backgrounds (79), as well as orthogonalization of response patterns (80), have been studied.
Spiking models of the early stages of the olfactory system have also been used for processing sensor array signals. White et al. (81, 82) employed a spiking neuron model inspired by the first two stages of the olfactory system to process signals from an array of fiber-optic sensors. In this model, the response of each sensor is first converted into a pattern of spikes across a population of olfactory receptor neurons. The identity of the odor was encoded by activating different subsets of sensory neurons and intensity was encoded by changes in spike latencies, firing rates, and response durations. An olfactory bulb circuit subsequently processed the sensory neuron input. Different odors produced unique spatiotemporal activation patterns across mitral cells in the model olfactory bulb circuits that were then decoded by a delay line neural network.
Raman et al. investigated spiking (83) as well as firing-rate models (71, 72, 84) of the olfactory bulb circuits for odor identity and intensity encoding. Input data from a pair of temperature-modulated metal-oxide sensors were first reformatted using a self-organizing model of chemotopic convergence of receptor neurons onto a lattice of olfactory bulb neurons to create ordered spatial maps that decoupled odor identity and intensity. Olfactory bulb circuits with additive center-surround type interactions subsequently processed these input odor maps to further enhance their contrast. The evolution of the model olfactory bulb activity over time revealed odor-specific manifolds on which response trajectories (repeatable over trials) that were monotonic with concentration were found to match published results in insect olfaction (45, 85).
Ratton et al. (86) employed a model of olfactory bulb-olfactory cortex interactions (87) to process responses from a chemiresistor sensor array. This biologically inspired model combines a winner-take-all type neural network (for olfactory bulb) with closed-loop cortical feedback (from cortex) to perform hierarchical clustering of the data. Converting their sensor responses to four organic chemicals into binary input to this bulb-cortex model, they found the discrimination performance was relatively poor compared to other conventional signal processing approaches.
3.3.4 Gain control
To understand discrimination of odors across different concentrations in artificial olfaction, Raman et al. (71) proposed a model of the first stage of lateral inhibition mediated by periglomerular interneurons at the input of the olfactory bulb. Sensor responses were first reorganized as spatial odor maps using a self-organizing model of chemotopic convergence. The authors subsequently used a shunting-model of the lateral inhibitory network to compress the concentration information (refer Figure 5c–f). While global shunting inhibition removed the concentration information completely, the spread or width of the lateral inhibitory connections in the model was used as a parameter to provide an appropriate tradeoff between removing and compressing concentration information.
3.3.5 Generalization to unknown odors
Although many workers have investigated recognition of “pretrained” chemicals, the challenges of long-term operation and generalization of training to allow chemical classification of “untrained” analytes remain significant challenges to artificial olfaction. The latter analytical capability is critically important, as it is often not feasible to pre-expose a sensor to every analyte it might encounter. Raman et al. (88) demonstrated a biologically-inspired approach where the recognition and generalization problems were decoupled and resolved in a hierarchical fashion. They validated this approach using a MEMS-based chemiresistive microsensor array with 5600 pseudosensors.
In this approach, analyte composition is refined in a progression from general (e.g., target is an oxygen-containing hydrocarbon) to precise (e.g., target is ethanol), using highly-optimized response features for each step. Using this multi-step process the authors demonstrated the ability to recognize analytes that were exposed to the sensory array in the training phase, and correctly predict the chemical composition of two untrained alchohols and ketones (see Figure 5g). More importantly, they showed that this bioinspired approach is particularly robust to response variability caused by the aging of sensors, thereby allowing sensing devices to remain functional for extended periods of operation.
3.3.6 Processing odor mixtures and backgrounds
Gutierrez-Osuna et al. (79) investigated the use of an adaptation mechanism for processing odor mixtures with chemical sensor arrays. First, responses from temperature modulated metal-oxide commercial chemiresistive sensors were converted into an orthogonal binary pattern that provided input to the KIII model. Adaptation based on local activity of each channel then allowed the model to desensitize itself to previously detected stimuli, thereby facilitating detection of the novel component in subsequent exposures to binary mixtures.
Modeling studies (89) have suggested that cortical feedback to the bulb may play an essential role in recognizing odorants against complex backgrounds and identifying the constituents of an odor mixture. Inspired by this idea, Gutierrez-Osuna et al. (90) presented a statistical pattern recognition model where inhibition triggered by a global feedback signal allowed the system to filter out the previously recognized odor. In another effort (91), a computational model of bulb-cortex interaction was presented to achieve parallel computational functions with gas sensors. Here, the olfactory bulb was modeled with two lateral inhibitory circuits: global shunting gain control circuits (71) followed by local center-surround contrast enhancement circuits (72). Bulbar outputs were then projected in a non-topographic fashion onto the olfactory cortex. Association connections within the cortex obtained through Hebbian learning formed a content addressable memory. Finally, inhibitory feedback from the cortex was used to modulate bulbar activity. Depending on the form of feedback, Hebbian or anti-Hebbian, the model was able to perform background suppression or mixture segmentation (see Figure 5h).
4. The Final Frontier: Structure-Odor Relationships
Relating a sensor or instrument’s odor response to an animal’s perception of the odor remains an elusive goal of neuroscience, and for the neuromorphic approach to artificial olfaction. Several hurdles must be crossed before realizing this goal. Clearly, in the first stage of this process, correlations between odorant molecules and perceptions are only possible if the sensing instrument captures information about physiochemical properties (e.g., functional group, carbon chain-length) to which biological receptors have affinity.
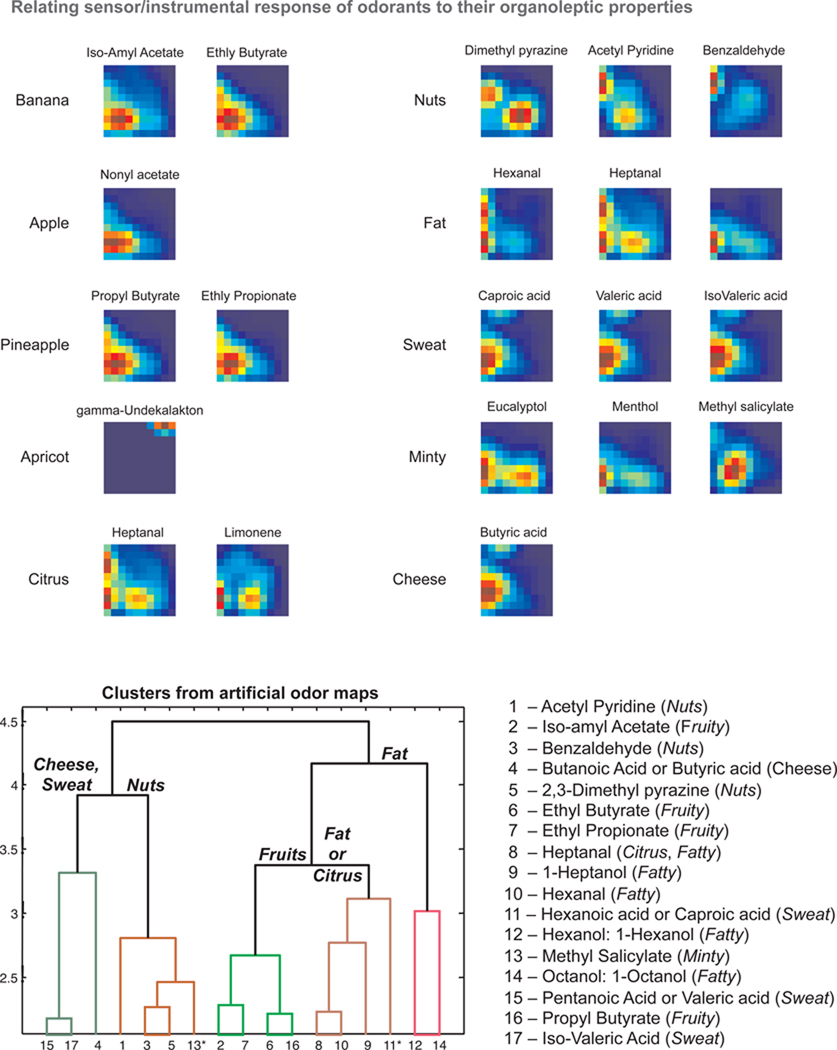
A recent study by Raman et al. (92) has made an attempt to match chemical features with high level descriptors using infrared (IR) spectroscopy. Treating the IR absorption at a particular wavenumber as a ‘pseudo sensor’, the authors created high-dimensional inputs that were subsequently reorganized into compact, spatial odor maps using a feature-clustering scheme that mimics the chemotopic convergence of receptor neurons onto the olfactory bulb (see Figure 6). Cluster analysis of the generated IR odor maps revealed chemical groups with members that have similar perceptual characteristics e.g. fruity, nutty, etc. Further, the generated clusters match those obtained from a similar analysis of olfactory bulb odor maps (36) obtained from rats for the same set of chemicals.
Figure 6.
Artificial odor maps show how sensor responses can be linked to perceptions. Top: Chemicals associated with ten different smell percepts predicted from their infrared absorption spectra were organized into these maps using the chemotopic convergence model (see Figure 5(a)). Dendrograms (complete-linkage) revealed that artificial odor maps formed from their infrared absorption spectra formed clusters determined by organoleptic descriptors provided by human experts (from Flavornet). A cluster analysis of olfactory bulb activation patterns elicited by the same seventeen chemicals (36) revealed qualitatively similar results (not shown). Asterisk identifies a chemical with a smell descriptor different from other members in the cluster. Reprinted with permissions from (92). With kind permission of Springer Science + Business Media.
An approach similar to that proposed by Raman et al. (92) has been independently proposed by Schumaker et al. (93). Here, odor maps were created for a larger set of odorants using 184 molecular descriptors. Given the spatial map of the odor, a panel of two-class naïve Bayes classifiers, one for each odor quality (e.g. fruity vs. non-fruity), predicted the overall percept.
In another effort, Madany and colleagues analyzed the relationships between 851 chemicals based on 278 organoleptic odor descriptors (e.g. fruity, sweet, etc.,) found in the Aldrich Flavor and Fragrances Catalog (94). Using multidimensional scaling and 2-d self-organized maps in series to reduce the dimensionality of the data, they created 2-d topological map of the odor space. This work provides another approach to relate olfactory perception with the physiochemical properties of the molecules.
5. Future Perspectives and Conclusions
To date, the abilities of biological olfaction far outshine the capabilities attained by artificial analogues. The canine olfactory system still remains the state-of-art sensing system for many engineering applications, including homeland security (95) and medical diagnosis (96). Hence, mimicking the design and computing principles of biological olfaction would provide a valuable first step toward developing a non-invasive chemical sensing system.
We have noted important parallels between the biological olfactory system and its artificial analogues, but it is important to point to a number of notable differences between them as well. First, attempts to correlate sensor responses with organoleptic properties have been extremely difficult suggesting that either the physiochemical properties of the molecules sensed by the two types of systems, or their subsequent processing, or both, are fundamentally different. Second, while the inspiration for artificial olfaction has most often come from the general-purpose odor processing system, a biological approach to detect certain highly species-specific ligands, such as pheromones, seems to involve more highly-selective receptors leading to relatively separate channels for further processing. Hence, depending on the target application, an appropriate bioinspired scheme could involve two sub-systems: one with highly selective and another with cross-selective sensors. Third, as mentioned earlier, it appears biological olfaction has evolved as a holistic rather than analytic sense. Hence, for applications requiring the segmentation of mixture signals to identify components, the biology-inspired approach might not be appropriate. Finally, engineering system constraints including array size and the number of types of sensing materials might leave bio-inspired solutions inappropriate for some environmental conditions.
Acknowledgments
The authors would like to thank the following for funding this work: generous start-up funds from the Department of Biomedical Engineering in Washington University and a McDonnell Center for Systems Neuroscience grant to B.R.; an intramural grant from NIH/NICHD to M.S. and partial support from Department of Homeland Security to S.S.
References
- 1.Axel R. The molecular logic of smell. Scientific American. 1995;273:154–159. doi: 10.1038/scientificamerican1095-154. [DOI] [PubMed] [Google Scholar]
- 2.Ache BW, Young JM. Olfaction: diverse species, conserved principles. Neuron. 2005;48:417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Persaud K, Dodd G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature. 1982;299:352–355. doi: 10.1038/299352a0. [DOI] [PubMed] [Google Scholar]
- 4.Pearce TC. Computational parallels between the biological olfactory pathway and its analogue 'the electronic nose': Part II. Sensor-based machine olfaction. Biosystems. 1997;41:69–90. doi: 10.1016/s0303-2647(96)01660-7. [DOI] [PubMed] [Google Scholar]
- 5.Pearce TC. Computational parallels between the biological olfactory pathway and its analogue 'the electronic nose': Part I. Biological olfaction. Biosystems. 1997;41:43–67. doi: 10.1016/s0303-2647(96)01661-9. [DOI] [PubMed] [Google Scholar]
- 6.Kauer JS, White J. Representation of odor information in the olfactory system: from biology to an artificial nose. In: Barth FG, Humphrey JAC, Secomb TW, editors. Sensors and Sensing in Biology and Engineering. Springer; 2002. [Google Scholar]
- 7.Raman B, Sun PA, Gutierrez-Galvez A, Gutierrez-Osuna R. Processing of chemical sensor array with a biologically inspired model of olfactory coding. IEEE Transactions on Neural Networks. 2006;17:1015–1024. doi: 10.1109/TNN.2006.875975. [DOI] [PubMed] [Google Scholar]
- 8.Dyson MG. The scientific basis of odour. Journal of the Society of Chemical Industry. 1938;57:647–651. [Google Scholar]
- 9.Miles WR, Beck LH. Infrared absorption in field studies of olfaction in bees. Science. 1947;106:512. [PubMed] [Google Scholar]
- 10.Wright RH. The sense of smell. Boca Raton: CRC Press; 1982. [Google Scholar]
- 11.Turin L. A spectroscopic mechanism for primary olfactory reception. Chemical Senses. 1996;21:773–791. doi: 10.1093/chemse/21.6.773. [DOI] [PubMed] [Google Scholar]
- 12.Moncrieff RW. What is odor. A new theory. Am. Perfumer. 1948;54:453. [Google Scholar]
- 13.Amoore JE. Current status of the steric theory of odor. Annals of the New York Academy of Sciences. 1964;116:457–476. doi: 10.1111/j.1749-6632.1964.tb45075.x. [DOI] [PubMed] [Google Scholar]
- 14.Rossiter KJ. Structure-odor relationships. Chemical Reviews. 1996;96:3201–3240. doi: 10.1021/cr950068a. [DOI] [PubMed] [Google Scholar]
- 15.Araneda RC, Kini AD, Firestein S. The molecular range of an odorant receptor. Nature Neuroscience. 2000;3:1248–1254. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- 16.Pelosi P. Perireceptor events in olfaction. Journal of Neurobiology. 1996;30:3–19. doi: 10.1002/(SICI)1097-4695(199605)30:1<3::AID-NEU2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- 18.Kent PF, Mozell MM. The recording of odorant-induced mucosal activity patterns with a voltage-sensitive dye. Journal of Neurophysiology. 1992;68:1804–1819. doi: 10.1152/jn.1992.68.5.1804. [DOI] [PubMed] [Google Scholar]
- 19.Mozell MM. The spatiotemporal analysis of odorants at the level of the olfactory receptor sheet. Journal of General Physiology. 1966;50:25–41. doi: 10.1085/jgp.50.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mozell MM. Evidence for sorption as a mechanism of the olfactory analysis of vapours. Nature. 1964;203:1181–1182. doi: 10.1038/2031181a0. [DOI] [PubMed] [Google Scholar]
- 21.Mozell MM, Jagodowics M. Chromatographic separation of odorants by the nose: retention times measured across in vivo mucosa. Science. 1973;181:1247–1249. doi: 10.1126/science.181.4106.1247. [DOI] [PubMed] [Google Scholar]
- 22.Moulton DG. Spatial patterning of response to odors in the peripheral olfactory system. Physiology Reviews. 1976;56:578–593. doi: 10.1152/physrev.1976.56.3.578. [DOI] [PubMed] [Google Scholar]
- 23.Scott JW, Shannon DE, Charpentier J, Davis LM, Kaplan C. Spatially organized response zones in rat olfactory epithelium. Journal of Neurophysiology. 1997;77:1950–1962. doi: 10.1152/jn.1997.77.4.1950. [DOI] [PubMed] [Google Scholar]
- 24.Craven BA, Paterson EG, Settles GS. The fluid dynamics of canine olfaction: unique nasal airflow patterns as an explanation of macrosmia. Journal of Royal Society Interface. 2010;7:933–943. doi: 10.1098/rsif.2009.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo M, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003;299:1196–1201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- 26.Lancet D, Sadovsky E, Seidemann E. Probability model for molecular recognition in biological receptor repertoires: significance to the olfactory system. Proceedings of National Academy of Sciences. 1993;90:3715–3719. doi: 10.1073/pnas.90.8.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 28.Carey RM, Verhagen JV, Wesson DW, Pirez N, Wachowiak M. Temporal structure of receptor neuron input to the olfactory bulb imaged in behaving rats. Journal of Neurophysiology. 2009;101:1073–1088. doi: 10.1152/jn.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raman B, Joseph J, Tang J, Stopfer M. Temporally diverse firing patterns in olfactory receptor neurons underlie spatiotemporal neural codes for odors. Journal of Neuroscience. 2010;30:1994–2006. doi: 10.1523/JNEUROSCI.5639-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nature Neuroscience. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearce TC, Verschure P, White J, Kauer JS. Robust stimulus encoding in olfactory processing: hyperacuity and efficient signal transmission. Emergent neural computational architectures based on neuroscience. 2001:461–479. [Google Scholar]
- 32.Grosmaitre X, Vassalli A, Mombaerts P, Shepherd GM, Ma M. Odorant responses of olfactory sensory neurons expressing the odorant receptor MOR23: a patch clamp analysis in gene-targeted mice. Proceedings of National Academy of Sciences. 2006;103:1970–1975. doi: 10.1073/pnas.0508491103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito I, Bazhenov M, Ong RC, Raman B, Stopfer M. Frequency transitions in odor-evoked neural oscillations. Neuron. 2009;64:692–706. doi: 10.1016/j.neuron.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graziadei PP, Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. Journal of Neurocytology. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- 35.Firestein S. How the olfactory system makes sense of smell. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- 36.Johnson BA, Leon M. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. Journal of Comparative Neurology. 2000;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 37.Wright GA, Thomson MGA, Smith BH. Odour concentration affects odour identity in honeybees. Proceedings of the Royal Society B. 2005;272:2417–2422. doi: 10.1098/rspb.2005.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- 39.Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 40.Gesteland RC, Lettvin JY, Pitts WH. Chemical transmission in the nose of the frog. Journal of Physiology. 1965;181:525–559. doi: 10.1113/jphysiol.1965.sp007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sicard G, Holley A. Receptor cell responses to odorants: similarities and differences amongst odorants. Brain Research. 1984;292:283–296. doi: 10.1016/0006-8993(84)90764-9. [DOI] [PubMed] [Google Scholar]
- 42.Duchamp-Viret P, Chaput MA, Duchamp A. Odor response properties of rat olfactory receptor neurons. Science. 1999;284:2171–2174. doi: 10.1126/science.284.5423.2171. [DOI] [PubMed] [Google Scholar]
- 43.Duchamp A, Revial MF, Holley A, Macleaod P. Odor discrimination by frog olfactory receptors. Chemical Senses and Flavor. 1974;1:213–233. [Google Scholar]
- 44.Getchell TV, Shepherd GM. Responses of olfactory receptor cells to step pulses of odour at different concentrations in salamander. Journal of Physiology. 1978;282:521–540. doi: 10.1113/jphysiol.1978.sp012479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stopfer M, Jayaraman V, Laurent G. Odor identity vs. intensity coding in an olfactory system. Neuron. 2003;39:991–1004. doi: 10.1016/j.neuron.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Laurent G, Wehr M, Davidowitz H. Temporal representations of odors in an olfactory network. Journal of Neuroscience. 1996;16:3837–3847. doi: 10.1523/JNEUROSCI.16-12-03837.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kauer JS. Response patterns of amphibian olfactory bulb neurones to odor stimulation. Journal of Physiology. 1974;243:695–715. doi: 10.1113/jphysiol.1974.sp010772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanzaki R, Arbas EA, Strausfeld NJ, Hildebrand JG. Physiology and morphology of projection neurons in the antennal lobes of the male Manduca sexta. Journal of Comparative Physiology [A] 1989;165:427–453. doi: 10.1007/BF00611233. [DOI] [PubMed] [Google Scholar]
- 49.Meredith M, Moulton DG. Patterned response to odor in single neurons of gold fish olfactory bulb: influence of odor quality and other stimulus parameters. Journal of General Physiology. 1978;71:572–597. doi: 10.1085/jgp.71.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meredith M. The analysis of response similarity in single neurons of the gold fish olfactory bulb using amino acids as odor stimuli. Chemical Senses. 1981;6:277–293. [Google Scholar]
- 51.Adrian ED. Olfactory reactions in the brain of the hedgehog. Journal of Physiology. 1942;100:459–473. doi: 10.1113/jphysiol.1942.sp003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harrison TA, Scott JW. Olfactory bulb responses to odor stimulation: analysis of response pattern and intensity relationship. Journal of Neurophysiology. 1986;56:157–1589. doi: 10.1152/jn.1986.56.6.1571. [DOI] [PubMed] [Google Scholar]
- 53.Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66:287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedrich RW, Laurent G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science. 2001;291:889–894. doi: 10.1126/science.291.5505.889. [DOI] [PubMed] [Google Scholar]
- 55.Stopfer M, Laurent G. Short-term memory in olfactory network dynamics. Nature. 1999;402:664–668. doi: 10.1038/45244. [DOI] [PubMed] [Google Scholar]
- 56.Jinks A, Laing DG. A limit in the processing of components in odour mixture. Perception. 1999;28:395–404. doi: 10.1068/p2898. [DOI] [PubMed] [Google Scholar]
- 57.Barnes DC, Hofacer RD, Zaman AR, Rennaker RL, Wilson DA. Olfactory perceptual stability and discrimination. Nature Neuroscience. 2008;11:1378–1380. doi: 10.1038/nn.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gottfried JA. Central mechanisms of odour object perception. Nature Reviews Neuroscience. 2010;11:628–641. doi: 10.1038/nrn2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kadohisa M, Wilson DA. Olfactory cortical adaptation facilitates detection of odors against background. Journal of Neurophysiology. 2006;95:1888–1896. doi: 10.1152/jn.00812.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Linster C, Henry L, Kadohisa M, Wilson DA. Synaptic adaptation and background segmentation. Neurobiology of learning and memory. 2007;87:352–360. doi: 10.1016/j.nlm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Covington JA, Gardner JW, Hamilton A, Pearce TC, Tan SL. Towards a truly biomimetic olfactory microsystem: an artificial olfactory mucosa. IET Nanobiotechnology. 2007;1:15–21. doi: 10.1049/iet-nbt:20060015. [DOI] [PubMed] [Google Scholar]
- 62.Stitzel SE, Stein DR, Walt DR. Enhancing vapor sensor discrimination by mimicking a canine nasal cavity flow environments. Journal of American Chemical Society. 2003:3684–3685. doi: 10.1021/ja028239y. [DOI] [PubMed] [Google Scholar]
- 63.Woodka MD, Brunschwig BS, Lewis NS. Use of spatiotemporal response information from sorption-based sensor arrays to identify and quantify the composition of analyte mixtures. Langmuir. 2007;23:13232–13241. doi: 10.1021/la7026708. [DOI] [PubMed] [Google Scholar]
- 64.Nagle HT, Gutierrez-Osuna R, Schiffman SS. The how and why of electronic noses. IEEE Spectrum. 1998;35:22–31. [Google Scholar]
- 65.Wilson AD, Baietto M. Applications and advances of electronic nose technologies. Sensors. 2009;9:5099–5148. doi: 10.3390/s90705099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wyszynski B, Nakamoto T. Linking biological and artificial olfaction: biomimetic quartz crystal microbalance odor sensors. IEEJ Transactions on Electrical and Electronic Engineering. 2009;4:334–338. [Google Scholar]
- 67.White J, Truesdell K, Williams LB, Atkisson MS, Kauer JS. Solid-state, dye-labeled DNA detects volatile compounds in vapor phase. PLoS Biology. 6:e9. doi: 10.1371/journal.pbio.0060009. 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lim SH, Feng L, Suslick KS. An optoelectronic nose for detection of toxic gases. Nature Chemistry. 2009;1:562–567. doi: 10.1038/nchem.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng G, Tisch U, Adams U, Hakim M, Shehada N, Broza YY, Billan S, Abdah-Bortnyak R, Kuten A, Haick H. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nature Nanotechnology. 2009;4:669–673. doi: 10.1038/nnano.2009.235. [DOI] [PubMed] [Google Scholar]
- 70.Dickinson TA, White J, Kauer JS, Walt DR. A chemical-detecting system based on a cross-reactive optical sensor array. Nature. 1996;382:697–700. doi: 10.1038/382697a0. [DOI] [PubMed] [Google Scholar]
- 71.Raman B, Gutierrez-Osuna R. Concentration normalization with a model of gain control in the olfactory bulb. Sensors and Actuators B: Chemical. 2006;116:36–42. [Google Scholar]
- 72.Raman B, Yamanaka T, Gutierrez-Osuna R. Contrast enhancement of gas sensor array patterns with a neurodynamics model of the olfactory bulb. Sensors and Actuators B: Chemical. 2006;119:547–555. [Google Scholar]
- 73.Semancik S, Cavicchi RE, Wheeler MC, Tiffany JE, Poirier GE, Walton RM, Suehle JS, Panchapakesan B, DeVoe DL. Microhotplate platforms for chemical sensor research. Sensors and Actuators B: Chemical. 2001;77:579–591. [Google Scholar]
- 74.Raman B, Meier DC, Evju JK, Semancik S. Designing and Optimizing Microsensor Arrays for Recognizing Chemical Hazards in Complex Environments. Sensors and Actuators B: Chemical. 2009;137:617–629. [Google Scholar]
- 75.Perera A, Yamanaka T, Gutierrez-Galvez A, Raman B, Gutierrez-Osuna R. A dimensionality-reduction technique inspired by receptor convergence in the olfactory system. Sensors and Actuators B: Chemical. 2006;116:17–22. [Google Scholar]
- 76.Freeman WJ. Mass action in the nervous system. New York: Academic Press; 1975. [Google Scholar]
- 77.Claussnitzer U, Quarder S, Otto M. Interpretation of analytical patterns from the output of chaotic dynamic memories. Fresenius Journal Analytical Chemistry. 2001;369:698–703. doi: 10.1007/s002160000692. [DOI] [PubMed] [Google Scholar]
- 78.Quarder S, Claussnitzer U, Otto M. Using singular-value decomposition to classify spatial patterns generated by a nonlinear dynamic model of the olfactory system. Chemometrics and Intelligent Laboratory Systems. 2001;59:45–51. [Google Scholar]
- 79.Gutierrez-Osuna R, Gutierrez-Galvez A. Habituation in the KIII olfactory model with chemical sensor arrays. IEEE Transactions on Neural Networks. 2003;14:1565–1568. doi: 10.1109/TNN.2003.820438. [DOI] [PubMed] [Google Scholar]
- 80.Gutierrez-Galvez A, Gutierrez-Osuna R. Increasing the separability of chemosensor array patterns with Hebbian/anti-Hebbian learning. Sensors and Actuators B: Chemical. 2006;116:29–35. [Google Scholar]
- 81.White J, Kauer JS. Odor recognition in an artificial nose by spatiotemporal processing using an olfactory neuronal network. Neurocomputing. 1999;26–27:919–924. [Google Scholar]
- 82.White J, Dickinson TA, Walt DR, Kauer JS. An olfactory neuronal network for vapor recognition in an artificial nose. Biological Cybernetics. 1998;78:245–251. doi: 10.1007/s004220050430. [DOI] [PubMed] [Google Scholar]
- 83.Raman B, Gutierrez-Osuna R. Chemosensory processing in a spiking model of the olfactory bulb: chemotopic convergence and center surround inhibition. In: Saul LK, Weiss Y, Bottou L, editors. Advances in Neural Information Processing Systems. Canada: Vancouver; 2004. pp. 1105–1112. [Google Scholar]
- 84.Raman B, Kotseroglou T, Clark L, Lebl M, Gutierrez-Osuna R. Neuromorphic processing for optical microbead arrays: dimensionality reduction and contrast enhancement. IEEE Sensors Journal. 2007;7:506–514. [Google Scholar]
- 85.Galan RF, Sachse S, Galizia CG, Herz AVM. Odor-driven attractor dynamics in the antennal lobe allow for simple and rapid olfactory pattern classification. Neural Computation. 2004;16:999–1012. doi: 10.1162/089976604773135078. [DOI] [PubMed] [Google Scholar]
- 86.Ratton L, Kunt T, McAvoy T, Fuja T, Cavicchi RE, Semancik S. A comparative study of signal processing techniques for clustering microsensor data (a first step towards an artificial nose) Sensors and Actuators B: Chemical. 1997;41:105–120. [Google Scholar]
- 87.Ambros-Ingerson J, Granger R, Lynch G. Simulation of paleocortex performs hierarchical clustering. Science. 1990;247:1344–1348. doi: 10.1126/science.2315702. [DOI] [PubMed] [Google Scholar]
- 88.Raman B, Hertz JL, Benkstein KD, Semancik S. A bioinspired methodology for artificial olfaction. Analytical Chemistry. 2008;80:8364–8371. doi: 10.1021/ac8007048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Z, Hertz J. Odour recognition and segmentation by a model olfactory bulb and cortex. Network. 2000;11:83–102. [PubMed] [Google Scholar]
- 90.Gutierrez-Osuna R, Powar N. Odor mixtures and chemosensory adaptation in gas sensor arrays. International Journal of Artificial Intelligence Tools. 2003;12:1–16. [Google Scholar]
- 91.Raman B, Gutierrez-Osuna R. International Joint Conference on Neural Networks. Canada: IEEE, Montreal; 2005. Mixture segmentation and background suppression in chemosensor arrays with a model of olfactory bulb-cortex interaction; pp. 121–136. [Google Scholar]
- 92.Raman B, Gutierrez-Osuna R. Responses of odorants to their organoleptic properties by means of a biologically-inspired model of receptor neuron convergence onto olfactory bulb. In: Marco S, Gutierrez-Galvez A, editors. Biologically inspired signal processing for chemical sensing. Springer; 2009. pp. 93–108. [Google Scholar]
- 93.Schmuker M, Schneider G. Processing and classification of chemical data inspired by insect olfaction. Proceedings of National Academy of Science. 2007;104:20285–20289. doi: 10.1073/pnas.0705683104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mamlouk AM, Chee-Ruiter C, Hofmann UG, Bower JM. Quantifying olfactory perception: mapping olfactory perception space by using multidimensional scaling and self-organzing maps. Neurocomputing. 2003;52–54:591–597. [Google Scholar]
- 95.Dunn M, Degenhardt L. The use of drug detection dogs in Sydney, Australia. Drug Alcohol Review. 2009;28:658–662. doi: 10.1111/j.1465-3362.2009.00065.x. [DOI] [PubMed] [Google Scholar]
- 96.McCulloch M, Jeierski T, Broffman M, Hubbard A, Turner K, Janecki T. Diagnostic accuracy of canine scent detection in early and late stage lung and breast cancers. Integrative Cancer Therapy. 2006;5:30–39. doi: 10.1177/1534735405285096. [DOI] [PubMed] [Google Scholar]
- 97.Raman B. Ph. D. Dissertation. College Station: Texas A&M University; 2005. Sensor-based machine olfaction with neuromorphic models of the olfactory system. [Google Scholar]
- 98.Raman B, Ito I, Stopfer M. Bilateral olfaction: two is better than one for navigation. Genome Biology. 2008;9:212. doi: 10.1186/gb-2008-9-3-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raman B, Stopfer M. Olfactory coding: non-linear amplification separates smells. Current Biology. 2008;18:R29–R32. doi: 10.1016/j.cub.2007.10.063. [DOI] [PubMed] [Google Scholar]