Abstract
Hap1 was originally identified as a neuronal protein that interacts with huntingtin, the Huntington’s disease (HD) protein. Later studies revealed that Hap1 participates in intracellular trafficking in neuronal cells and that this trafficking function can be adversely affected by mutant huntingtin. Hap1 is also present in pancreatic β-cells and other endocrine cells; however, the role of Hap1 in these endocrine cells remains unknown. Using the Cre-loxP system, we generated conditional Hap1 knockout mice to selectively deplete the expression of Hap1 in mouse pancreatic β-cells. Mutant mice with Hap1 deficiency in pancreatic β-cells had impaired glucose tolerance and decreased insulin release in response to intraperitoneally injected glucose. Using cultured pancreatic β-cell lines and isolated mouse pancreatic islets, we confirmed that decreasing Hap1 could reduce glucose-mediated insulin release. Electron microscopy suggested that there was a reduced number of insulin-containing vesicles docked at the plasma membrane of pancreatic islets in Hap1 mutant mice following intraperitoneal glucose injection. Glucose treatment decreased the phosphorylation of Hap1A in cultured β-cells and in mouse pancreatic tissues. Moreover, this glucose treatment increased Hap1’s association with kinesin light chain and dynactin p150, both of which are involved in microtubule-dependent trafficking. These studies suggest that Hap1 is important for insulin release from β-cells via dephosphorylation that can regulate its intracellular trafficking function.
Keywords: Huntingtin, Trafficking, Insulin, Pancreas, Phosphorylation
Introduction
Huntingtin-associated protein 1 (Hap1) was first identified as a neuronal protein that interacts with huntingtin (htt), the Huntington’s disease protein [1]. HAP1 consists of two isoforms, Hap1A and Hap1B, which differ at their C-terminal sequences [1]. Unlike htt, which is expressed ubiquitously throughout the brain and body, Hap1 is enriched in the brain, with abundant expression in the hypothalamus, amygdala, and brain stem [2–4]. The critical role of Hap1 in neuronal function is clear from the fact that loss of Hap1 leads to neurodegeneration [5–7], as well as a feeding defect, retarded growth, and early postnatal death in mice [6, 8]. Growing evidence indicates that Hap1 is involved in microtubule-dependent trafficking in neurons [9–11].
Microtubule-based molecular trafficking is essential for neuronal survival and function. Proper distribution of cargos depends on the ATP-dependent molecular motor complexes kinesin and dynein, which drive anterograde and retrograde movement, respectively [12, 13]. These motor systems transport a plethora of membrane-bound cargos from the cell body to the nerve terminals. Hap1 is known to bind the microtubule motor-associated proteins dynactin p150Glued (p150) and kinesin light chain (KLC) [14–16]. When Hap1 protein expression is reduced, microtubule-dependent transport of amyloid protein-containing vesicles is inhibited [16]. Consistently, Hap1 is involved in the intracellular transport of several membrane receptors [17–19], and importantly, mutant htt can affect Hap1-mediated BDNF trafficking [20, 21] and the transport of GABAA receptors to synapses [22].
Endocytosis and exocytosis, which require numerous trafficking proteins, function in many common ways in a variety of cell types, including neurons and endocrine cells. In addition to the brain, Hap1 is also expressed in the peripheral nervous system and in the endocrine cells of the pituitary, thyroid, adrenal glands, and pancreas [3, 23, 24]. In particular, the expression of Hap1 in the pancreas is restricted to insulin secreting β-cells [23, 24]. Interestingly, impaired glucose tolerance and decreased insulin secretion from β-cells are features of the R6/2 HD mouse model [25–27], and patients with HD have a higher prevalence of diabetes and impaired glucose tolerance [28–32]. However, whether and how Hap1 functions in endocrine cells remain unclear.
Here, we report that Hap1 is functionally required for insulin secretion from pancreatic β-cells. Both in vitro and in vivo data confirm that loss of Hap1 impairs glucose tolerance and reduces glucose-stimulated insulin release. In addition, our data suggest a trafficking defect in insulin-containing vesicles without morphologic abnormalities in Hap1-deficient β-cells. Mechanistic data demonstrate that glucose-mediated dephosphorylation of Hap1A functions as a molecular switch allowing more Hap1A to associate with microtubule transport proteins. Our findings offer a novel research and therapeutic target for ameliorating metabolic disorders due to defective insulin release from pancreatic β-cells.
Materials and methods
Animals
Germline Hap1 knockout mice were generated in our early study [6]. Generation of conditional Hap1 knockout mice, in which exon1 of the mouse Hap1 gene is flanked by two loxP sites to allow the Cre-mediated deletion of exon1, was described in our recent study [7]. β-cell conditional Hap1 knockout mice were generated by crossing INS2-Cre transgenic mice [B6.Cg-Tg(Ins2-cre)25Mgn/J; The Jackson Laboratory] with Hap1flox/flox mice. All mice were generated on a C57BL/6 J background. The resulting heterozygous mice were used to generate homozygous conditional knockout mice (Ins2-Cre+/Hap1loxp/loxp, referred to as Ins2-Hap1−/−) or heterozygous mice (Ins2-Cre+/Hap1+/loxp, referred to as Ins2-Hap1+/−). Genotyping of these mice was performed with genomic DNA extracted from the tails via PCR to amplify the mouse Hap1 DNA fragment (from 4,929 to 5,003 nt) using the forward (5′-TTT TTC TGG GGA GCA TAC GTC-3′) and reverse (5′-ATC CGT TAT CCC AGG GTC TGA-3′) primers. Primers (forward 5′-GCG GTC TGG CAG TAA AAA CTA TC-3′and reverse 5′-TGT TTC ACT ATC CAG GTT ACG G-3′) that amplify Cre recombinase were also used to determine the presence of Cre.
Expression analysis of Hap1 and behavioral tests of heterozygous Ins2-Hap1+/− and wild-type mice revealed no differences between these two groups. Because homozygous (Ins2-Hap1−/−) and heterozygous (Ins2-Hap1+/−) mice share the same mixed genetic background, mice of these two genotypes were used mainly to reveal differences related to Hap1 deficiency in β-cells. Mice were housed in a dark [12 h dark (1900–0700 hours)] and light [12 h light (0700–1900 hours)] cycle, temperature (22°C)-controlled chamber. Animals were fed ad libitum (Lab Diet 5001) and maintained in the animal facility at Emory University in accordance with institutional animal care and use guidelines.
Antibodies
Guinea pig antibody (EM78) to Hap1, rabbit antibody to kinesin light chain 2 (EM41A), and rabbit antibody to pHap1A (EM41a) have been described previously [16, 19]. Rabbit serum antibody to phogrin was a gift from Dr. John Hutton, Barbara Davis Center for Childhood Diabetes, University of Colorado Health Sciences Center. Other antibodies used were rat anti-insulin (R&D systems), mouse dynactin p150Glued (BD Biosciences), mouse anti-gamma tubulin (Sigma), anti-pAKT (Ser473), and anti-pERK (Cell Signaling Technology).
Glucose tolerance test
After an overnight fast, mice were given an i.p. injection of glucose at 2.0 g/kg body weight. Blood glucose levels were measured with a Lifescan SureStep glucose monitor. Each time point reading was performed twice for each mouse.
Insulin tolerance test
After an overnight fast, 3-month-old mice were injected intraperitoneally (i.p.) with 1.0 unit/kg body weight human insulin (Sigma 12767). Blood plasma glucose levels were measured at various time points from tail blood samples analyzed by a Lifescan SureStep glucose monitor. Each time point reading was performed twice for each mouse.
Cell culture and Hap1 shRNA treatment
Adenoviral Hap1 shRNA was generated in our previous studies [36]. Min6 and NIT-1 cells were grown in standard media (DMEM, Sigma D5671) with 15% FCS (Invitrogen 10437-028), 71 μM 2-mercaptoethanol (Sigma M7522), 2 mM glutamine (Sigma G6392), 2.8 mM glucose, and 100 U/ml penicillin/100 μg/ml streptomycin (Invitrogen 15070-063) in 12-well plates to 60% confluency. Min6 cells were treated with adenoviral Hap1 shRNA or scramble shRNA for 12 h in standard culture media. NIT-1 cells were transfected for 12 h with the pGenesil-1 vector that can independently express GFP and Hap1 shRNA or scrambled shRNA.
Insulin secretion
Insulin secretion was measured using an ELISA Kit (Crystal Chemicon). For in vivo measurements, mice were fasted overnight and then i.p. injected with 2.0 g/kg glucose. Plasma insulin levels were measured using tail blood collected at different times. For measuring insulin release from Min6 cells, Min6 cells were plated in 12-well plates and maintained in DMEM (Sigma D5671) with 15% FCS (Invitrogen), 71 μM 2-mercaptoethanol (Sigma M7522), 2 mM glutamine (Sigma G6392), 100 U/ml penicillin/100 μg/ml streptomycin (Sigma P0781). Cells were kept in a 37°C tissue culture incubator with 5% CO2 and plated in 12-well plates for 48 h. Culture media was removed and replaced with low glucose (2.8 mM) Krebs buffer for 1 h. Cells were then treated with Krebs buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 4.2 mM NaHCO3, 2 mM CaCl2, 200 mM sulphinpyrazone and 10 mM Hepes, pH 7.4) containing 20 mM glucose for stimulation. For measuring insulin release from NIT-1 cells, the medium in 24-well plates was collected for measuring released insulin and the cell lysates for measuring the total content of insulin using radioimmunoassay (RIA) with the DFM 96-type multi-tube radioimmunoassay Counter. Each group contained 10–20 samples.
For measuring insulin release from pancreatic islets isolated from mice, isolation of intact islets and assay of static insulin release were performed according to the previously established protocol [33–35]. Briefly, pancreatic tissues were digested in collagenase A (Sigma) for 20 min at 37°C. Islets were purified using Ficoll gradient centrifugation and then hand-picked under microscope. Islets were cultured for 1 h RPMI 1640 (Cellgro) media containing 2.8 mM glucose, 10% FBS, 100 IU/ml pen/strep in 5% CO2 at 37°C. For insulin release assay, 20 hand-picked islets were placed in 500 μL KRBH with either 2.8 mM or 20 mM glucose and incubated at 5% CO2 at 37°C for 60 min. After centrifugation, culture media was collected for measuring secreted insulin by ELISA assay (Crystal Chem). Cellular insulin was extracted using acid–ethanol technique (Alonso-Magdalena) and also measured by ELISA. Protein concentration of collected islets was determined using BCA protein assay (Bio-Rad).
Coimmunoprecipitation
Mice were injected i.p. with glucose at 2.0 g/kg body weight and sacrificed by cervical dislocation. Pancreas tissue was quickly removed and stored in lysis buffer on ice, followed immediately by hand homogenization, sonication, and centrifugation, all done on ice or at 4°C. Lysates (500 μg) were precleared with Protein-A-conjugated Sepharose beads at 4°C for 1 h. Samples were incubated with 10 μl of anti-Hap1A antibody at 4°C overnight and then precipitated with protein-A beads. The beads were washed two times with lysis buffer and eluted by boiling in loading buffer. The eluted protein samples were subjected to western blotting with various antibodies.
Immunofluorescent labeling
Mice were sacrificed by cervical dislocation, and the pancreas was removed. Pancreatic tissues were submerged in Bouin’s fixative (30 ml saturated picric acid, 10 ml formaldehyde, 2 ml glacial acetic acid) overnight. Tissue was washed in water until the yellow color faded and then placed in neutral buffered formalin for storage until processed by the WCI Pathology Core Lab at Emory University. The paraffin embedded slides were dewaxed in xylene (two times, 5 min/each time), hydrolyzed with EtOH (100% for 3 min twice, 95% for 1 min, and 80% for 1 min), and rinsed with distilled water. Antigens were unmasked in sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) for 3 min in microwave at low heat. Slides were washed three times with PBS and blocked in 2% normal goat serum, 0.5% Triton X-100, 1% BSA in PBS for 30 min. Slides were then incubated with primary antibodies to insulin or/and Hap1 (EM 78), overnight at 4°C. Alexa-488 conjugated goat anti-guinea pig or rhodamine red-X conjugated donkey anti-rat secondary antibodies were used. Fluorescent micrographs were taken with a Zeiss microscope (Axiovert 200 M) and a digital camera (Hamamatsu ORCA-100). Images were processed with Openlab software (Improvision, Inc).
Electron microscopy
Mice were intracardially perfused with 4% paraformaldehdye and 0.1% glutaraldehyde, pancreas tissue was removed from animal and further dissected into 1- to 1.5-mm-thick pieces. Tissues were continuously fixed in 4% paraformaldehdye and 0.2% glutaraldehyde overnight at 4°C. After washing with 0.1 M phosphate buffered (pH 7.2) twice, the tissue blocks were dehydrated in ethanol series to 100%, and then infiltrated overnight with 50% L.R. White resin (Electron Microscopy Sciences, Hatfield, PA, USA) in 100% ethanol followed by pure L.R. White resin overnight. Embedding of the tissue blocks was carried out by placing tissue pieces in capped gelatin capsule and letting resin polymerizing in a 50°C oven for 24 h. Ultra-thin sections were cut at 70–80 nm and placed on Formvar-coated nickel grids for immunogold labeling.
For immunogold labeling, ultrathin sections on nickel grids were treated with 1% hydrogen peroxide for 5 min and washed three times with double distilled water. Sections were blocked for 30 min in phosphate buffered saline (PBS) containing 5% normal goat serum, 5% BSA, and 0.1% gelatin. After blocking, sections were incubated at 4°C overnight with mouse anti-insulin (Fitzgerald Industries, Concord, MA, USA) primary antibody (diluted 1:100) in PBS containing 0.1% acetylated BSA (Aurion, The Netherlands). Sections were subsequently washed three times with 1 × PBS and incubated with 10-nm gold particles conjugated goat anti-mouse secondary antibody (Aurion) at 1:20 dilution in the same solution for 2 h at room temperature. After washing off excess antibody, sections were fixed with 2.5% glutaraldehyde in 0.1 M PB, and then counter-stained with 4% aqueous uranyl acetate and 2% lead citrate. Insulin immunogold labeled sections were examined using a Hitachi H-7500 transmission electron microscope (Hitachi, Pleasanton, CA, USA) equipped with a Gatan BioScan CCD camera.
Images were analyzed using Image J software (NIH). The distance (<0.2 μm) of granule center to plasma membrane has been used to reflect morphologically docked granules [37]. The number of these insulin granules per μm distance along capillary membrane periphery was counted. Three β cell images each for control and KO were used, and ten different distances along capillary membranes were compared and averaged to display results as vesicles per μm distance.
Western immunoblots
Min6 cells or dissected mouse pancreas was homogenized in NP40 buffer (50 mM Tris pH 7.4. 50 mM NaCl, 0.1% Triton X-100, 1% NP40, and protease inhibitor cocktail Pierce 78430 and 1 mM PMSF, Sigma P-7626). For phosphorylation studies, phosphatase inhibitors (2 mM sodium orthovanadate, Sigma S-6508; 50 mM sodium fluoride, Sigma S-7920) were also added. Samples were sonicated for 10 s, centrifuged at 16,000g at 4°C for 20 min. Equal amounts of protein were loaded on Invitrogen Tris–Glycine (4–12%) gels for SDS-PAGE. Proteins transferred to nitrocellulose were blocked in 5% non-fat dry milk (Nestle) in PBS for 30 min then incubated in primary antibodies in 3% BSA/PBS overnight at 4°C. Following incubation, blots were washed and secondary HRP-conjugated antibodies (Jackson Immuno-Resaerch) were added in 5% milk for 1 h. Blots were visualized using SuperSignal ECL (Pierce).
Statistical analysis
All the values are presented as mean ± SE and analyzed by Student’s t test. Differences were considered statistically significant with a p value <0.05.
Results
Conditional knockout of Hap1 in β-cells in mouse pancreas
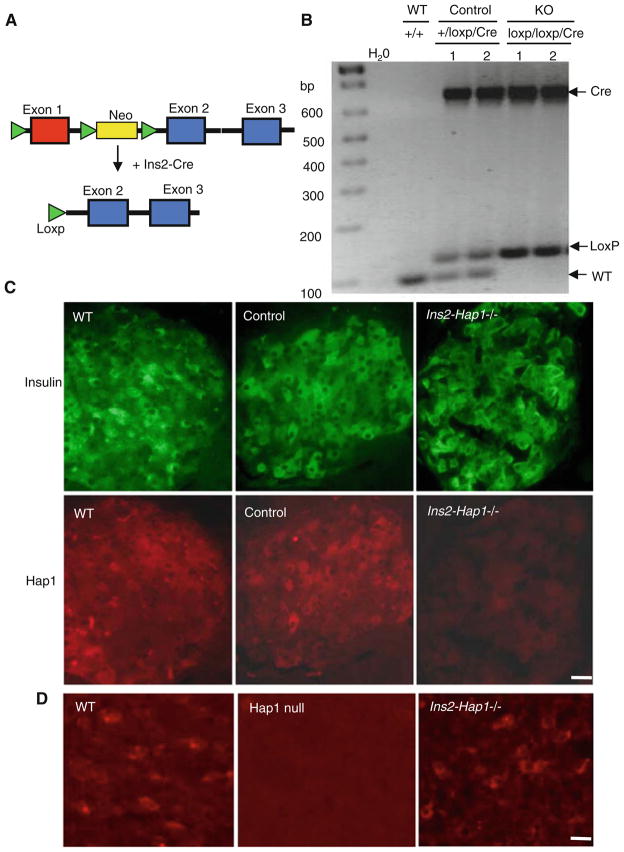
To investigate the role of Hap1 in endocrine cells, we selectively deleted exon1 of the Hap1 gene in mouse pancreatic β-cells by crossing floxed Hap1 mice (C57BL/6/SV129) carrying homozygous loxP-flanked Hap1 exon1 (Hap1Flox/Flox) to Ins2-Cre C57BL/6 mice (Fig. 1a), which express Cre in pancreatic β-cells under the control of the rat insulin II promoter [38]. Heterozygous mice carrying the Loxp-Hap1 and Cre transgenes were mated to obtain homozygous floxed Hap1 mice that also carry the Cre transgene (Fig. 1b). Because deletion of the Hap1 gene selectively occurs in a few β-cells in the pancreas, we analyzed the expression of Hap1 via immunohistochemistry. Our early studies have demonstrated that Hap1 is only present in β cells, but not in α and δ cells [24]. We did not find any significant difference in Hap1 expression in β-cells between wild-type and heterozygous floxed Hap1 (Ins2-Hap1+/−) mice that also express Cre (Fig. 1c). However, immunohistochemistry staining of Hap1 signal in β-cells of homozygous floxed mice with Cre (Ins2-Hap1−/− or KO) is markedly reduced when compared with β-cells of the heterozygous Ins2-Hap1+/− mice (Fig. 1c) and the hypothalamus of Ins2-Hap1−/− mice (Fig. 1d). Since heterozygous Ins2-Hap1+/− mice also carry the Cre transgene, a genetic background similar to homozygous Ins2-Hap1−/− mice, we used Ins2-Hap1+/− (referred to as control) mice as a control for comparison with Ins2-Hap1−/− (referred to as KO) mice to define the specific effects caused by Hap1 deficiency in β-cells.
Fig. 1.
Generation of conditional Hap1 knockout mice that deplete Hap1 in pancreatic β-cells. a Exon1 of the mouse Hap1 gene was flanked by loxP sites in the targeted allele. Mice carrying this targeted gene were mated with transgenic mice that express Cre in pancreatic β-cells driven by the mouse preproinsulin 2 (Ins2) promoter, resulting in offspring mice that have exon1 of the Hap1 gene deleted. b PCR genotyping of crossed mice showing the presence of the Cre and loxP alleles in two homozygous loxp/loxp/Cre and two heterozygous (+/loxp/Cre) mice, but not in wild-type (+/+) mice. For simplicity, homozygous loxp/loxp/Cre mice are referred to as Ins2-Hap1−/− or KO, and heterozygous (+/loxp/Cre) as Ins2-Hap1± or control. c Double immunofluorescence staining of insulin (green) and Hap1 (red) showing Hap1 depletion in insulin-positive β-cells in KO mice. d Immunofluroescent Hap1 staining of the hypothalamic tissues of Ins2-Hap1−/− and WT mice at the age of 2 months. The hypothalamus of Hap1 germline knockout pup (Hap1 null) at postnatal day 1 served as a control to show the specific labeling of Hap1 by the anti-Hap1 antibody. Scale bars 10 μm
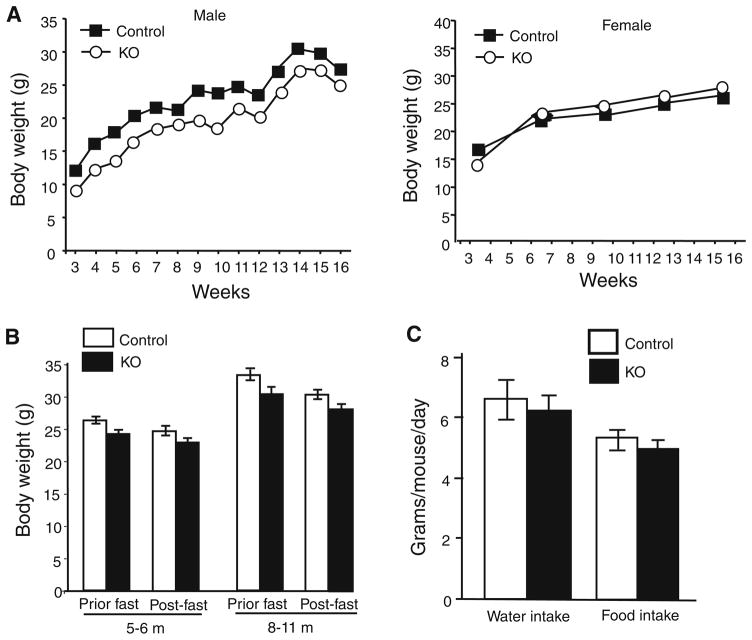
Since β-cells in the pancreas are important for the body’s metabolism, we wondered whether selective elimination of Hap1 in β-cells would cause any alteration of body weight. We monitored the body weight of Ins2-Hap1−/− (KO) mice along with control (Ins2-Hap1+/−) mice and found that male control mice gained more weight than KO male mice (Fig. 2a). There was no significant difference in body weight between female KO mice and female control mice. We did not see any significant difference in body weight and food intake between female KO and control mice after fasting for 16 h. However, a slight decrease in body weight was also seen in male KO mice compared with control mice, regardless of prior fasting or post-fasting conditions (Fig. 2b). There was also a slight reduction, though not statistically significant, in food intake and water consumption in male KO mice compared with control mice (Fig. 2c). Although the mechanism for the sex-dependent difference in body weight, which also occurs in mice null for other genes [39], remains to be investigated, depletion of Hap1 in β-cells in the pancreas seems to have more impact on the body weight of male mice.
Fig. 2.
Body weight of conditional mutant mice with Hap1 deficiency in β-cells. a Body weight in Hap1 KO (Ins2-Hap1−/−) mice compared with the littermate control (Ins2-Hap1±) mice under normal feeding condition (n = 10/each group). b Body weight of male KO and control mice after fasting for 16 h. n = 10/each group. c Water intake and food intake of male control and KO mice (n = 6/each group). Mice at the age of 3–12 months were examined
Reduced glucose tolerance and insulin secretion in Ins2-Hap1−/− mice
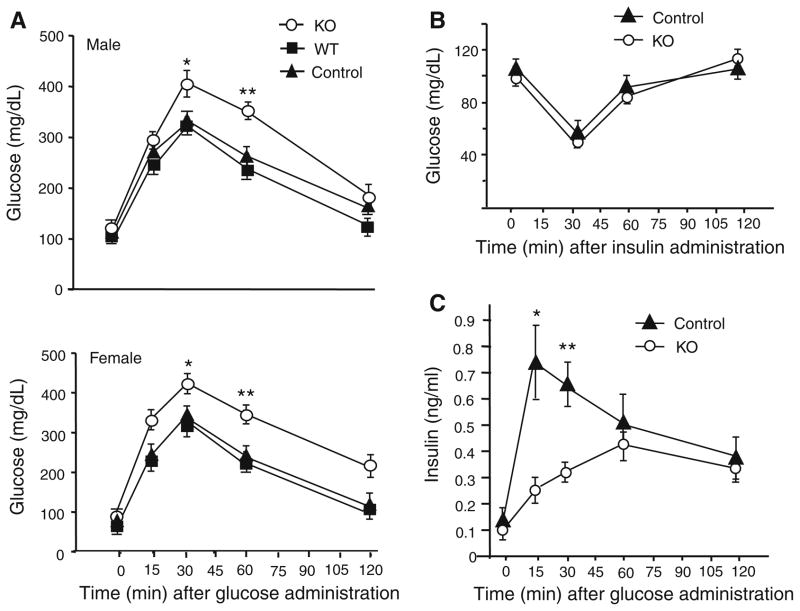
Since β-cells in the pancreas secrete insulin to regulate blood glucose levels, we performed glucose tolerance experiments on Ins2-Hap1−/− mice and their controls. Following intraperitoneal (i.p.) injection of glucose at 2.0 g/kg body weight, there was a marked increase of plasma glucose at 15–30 min, which then declined within 120 min. Ins2-Hap1−/− mice showed a significantly higher level of plasma glucose compared with wild-type (WT) or control (Ins2-Hap1+/−) mice (Fig. 3a), suggesting a defect in insulin release. To verify that this defective glucose tolerance is not due to impairment of insulin receptor function in peripheral tissues, we injected insulin (1.0 unit/kg body weight) intraperitoneally into Ins2-Hap1−/− mice and their controls. There was no significant difference in plasma glucose levels between Ins2-Hap1−/− and control mice after insulin injection (Fig. 3b), indicating insulin signaling is unaffected in KO mice. To examine the levels of insulin secreted from β-cells, we then measured plasma insulin levels following intraperitoneal injection of glucose at 2.0 g/kg body weight. We observed a significant decrease of plasma insulin levels in Ins2-Hap1−/− mice compared with control mice (Fig. 3c). These findings suggest that loss of Hap1 in β-cells can affect the release of insulin into blood in response to elevated blood glucose.
Fig. 3.
Hap1 deficiency in β-cells leads to impaired glucose tolerance and insulin release. a A glucose tolerance test was performed on overnight-fasted male mice (n = 10/each group) at the age of 3 months. Hap1 KO mice displayed glucose clearing impairment measured by blood glucose monitor following intraperitoneal injection (i.p.) of glucose (2 g/kg bodyweight). *p <0.05, **p <0.01 compared with control. b An insulin tolerance test on 3- to 4-month- old male mice fasted overnight. Blood glucose levels were measured after i.p. injection of insulin (1 unit/kg body weight). n = 10/each group. c ELISA results showing that insulin secretion is reduced in Ins2-Hap1−/− (KO) mice. Blood plasma from male mice fasted overnight was collected at various time points after i.p. injection of glucose (2 g/kg body weight). n = 8 for each group, *p <0.05 compared to control
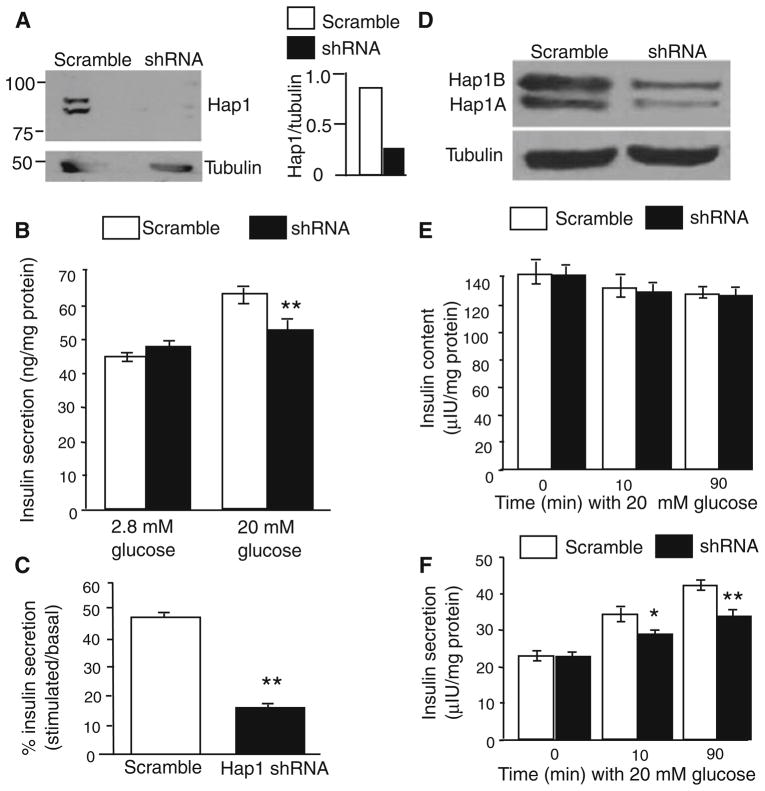
To provide additional evidence for the idea that Hap1 is important for insulin release from β-cells, we investigated the effect of Hap1 deficiency on cultured β-cell lines, as these cultured cells have been widely used to investigate the mechanisms underlying insulin release. We cultured Min6 cells to measure insulin released in the medium via an ELISA assay. Min6 cells are derived from mouse β-cells and are capable of releasing insulin in a physiologically similar manner to mouse glucose stimulation [40]. Min6 cells were infected with adenoviral Hap1 shRNA [36] to reduce the level of Hap1 (Fig. 4a). Glucose triggers insulin release from Min6 cells by regulating gene transcription and protein function such as phosphorylation [41]. We measured insulin release at 10 min after 20 mM glucose stimulation to better define if the loss of Hap1 can directly affect insulin release. We found that Hap1 shRNA-treated cells showed smaller amounts of insulin released into the medium as compared with that in scramble shRNA-treated cells (Fig. 4b, c). We also tested the insulin release from NT-1 cells, another insulin secretion cell line. When Hap1 expression is inhibited via shRNA (Fig. 4d), the total cellular contents of insulin were not significantly different between control and Hap1 shRNA-treated cells (Fig. 4e). However, we also observed a decrease in insulin release from Hap1 shRNA-treated cells into the medium at 10 and 90 min following glucose stimulation (Fig. 4f).
Fig. 4.
Suppressing Hap1 expression decreases insulin release in β-cell lines. a Western blotting of Min6 cells showing that Hap1 protein level was reduced by adenoviral Hap1 shRNA treatment, but not by scrambled shRNA control (left panel). The ratio of Hap1 to tubulin is also presented (right panel). b Min6 cells treated with Hap1 shRNA displayed a significant reduction in insulin (ng/mg protein) release after 20 mM glucose stimulation for 10 min. The basal level of glucose is 2.8 mM. c The percentage increase of insulin release relative to the unstimulated in Min6 cells treated with scrambled or Hap1 shRNA. (n = 9, **p <0.01). d Western blots of NIT-1 cells treated with Hap1 shRNA or scrambled shRNA. e, f Insulin levels in lysates (total insulin) of NIT-1 cells (e) and medium (released insulin) (f) at 0, 10, and 90 min after 20 mM glucose stimulation. (*p <0.05, **p <0.01 n = 10–20)
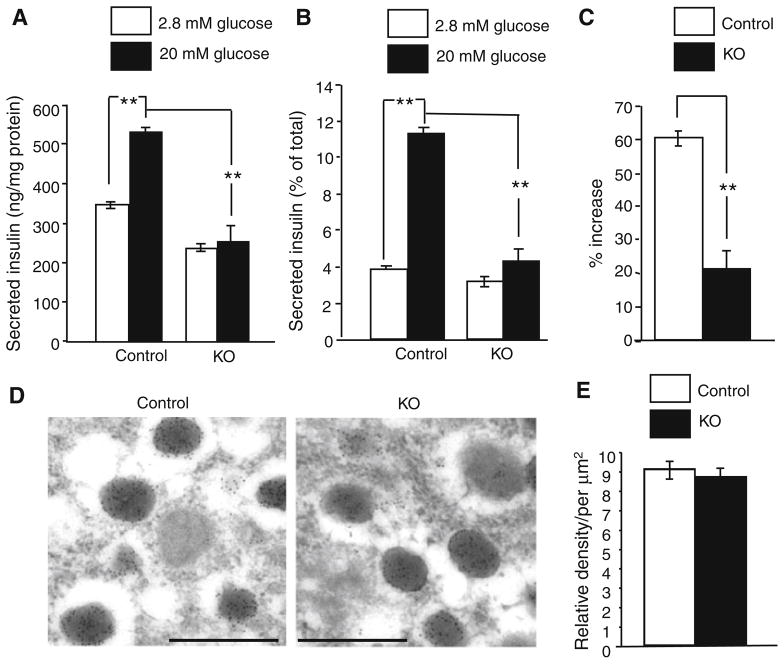
It would be more important to examine the direct effect of loss of Hap1 on insulin release from pancreatic tissues. Thus, we examined pancreatic islets isolated from Hap1 KO and control mice to measure static insulin release using the established assays [33–35]. Insulin secretion from the KO islets was decreased as compared with that from the control islets (Fig. 5a). Comparing the released insulin relative to the total insulin contents or the basal level of insulin can reduce variations in the total number of β-cells examined in each sample. This comparison also revealed a significant reduction of insulin release from the KO islets (Fig. 5b, c). Because inhibiting Hap1 via shRNA did not significantly change the content of insulin in cultured NT-1 cells (Fig. 4e), it is likely that lack of Hap1 does not affect the synthesis of insulin but can reduce its release from β-cells. Electron microscopic examination did not reveal any significant difference in the morphology and density of insulin-containing granules between the KO and control mouse pancreatic islets (Fig. 5d, e). Taken together, these findings indicate that Hap1 deficiency negatively affects insulin release from β-cells.
Fig. 5.
Reduced insulin release from pancreatic islets isolated from Ins2-Hap1−/− (KO) mice. a–c Secreted insulin is presented as ng/mg protein in (a) the percentage of total insulin content (b), and the increased percentage of the basal level in (c). Control mice (Ins2-Hap1 ±) mice were used for comparison (n = 6, **p <0.01). d, e Immunogold electron microscopic examination of the pancreatic islets from control and KO mice. The morphology (d) and the density (e) of insulin-containing granules are not significantly different between control and KO mice. Scale bars 1.0 μm
Density of docked insulin granules are decreased in Hap1-deficient β-cells
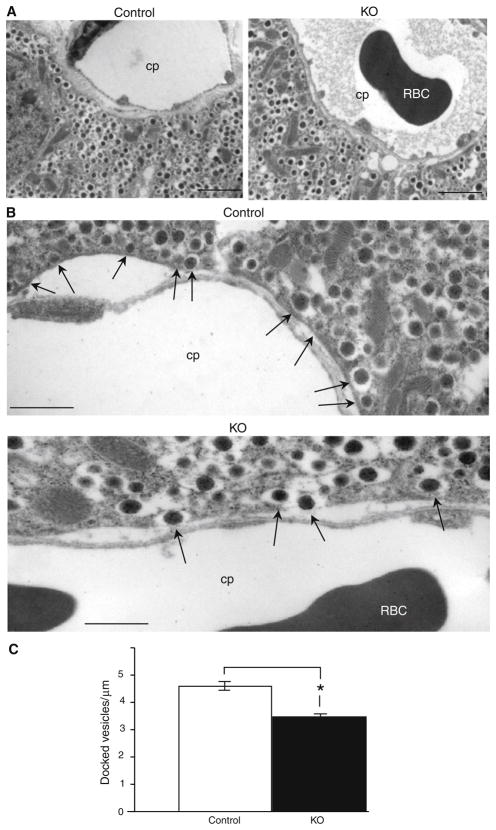
Insulin secretion involves the trafficking of insulin-containing vesicles and their docking to the plasma membrane of β-cells. We performed electron microscopic examination of the pancreas of Ins2-Hap1−/− and control mice after they had been stimulated by i.p. glucose injection. We observed a decreased number of vesicles lining the plasma membrane of mutant β-cells compared with controls (Fig. 6a). Vesicles in Hap1 mutant β-cells were largely scattered in the region proximate to the plasma membrane, with a few docked vesicles at the plasma membrane (Fig. 6b). In contrast, more vesicles were docked or fused to the plasma membrane in control β-cells. Granules with the distance (<0.2 μm) of their center to plasma membrane are defined as morphologically docked granules in β-cells [37]. To quantitatively analyze these docked vesicles, we used imaging software ImageJ (NIH) to count the relative numbers of these vesicles at the capillary plasma membrane per μm. This quantification suggested that there was a decrease in the number of docked vesicles in Hap1 mutant β-cells (3.4 ± 0.08, n = 10, p <0.05) versus control cells (4.64 ± 0.16; Fig. 6c). Because this decrease is not substantial and because lack of Hap1 has no effect on the morphology of insulin-containing granules, loss of Hap1 is likely to functionally affect in the release of insulin.
Fig. 6.
Reduced number of insulin-containing vesicles at the plasma membrane of pancreatic islets in Ins2-Hap1−/− mice. Electron microscopic images of the pancreas of Ins2-Hap1−/−(KO) and Ins2-Hap1 ± (control) mice that had been i.p.-injected with glucose (2 g/kg body weight) for 10 min. a Micrographs at ×5,000 magnification showing fewer vesicles lining the plasma membrane in KO β-cells than control cells. Scale bars 2.5 μm. b High-magnification (×15,000) micrographs showing the reduced number of insulin vesicles that are docked on the plasma membrane in the KO pancreatic tissue. Arrows indicate docking insulin vesicles. cp Capillary, RBC red blood cell. Scale bars 1.0 μm. c Quantification of the number of insulin vesicles lining the plasma membrane per μm capillary membrane distance in β cells of control (Ins2-Hap1 ±) and KO (Ins2-Hap1−/−) mice. (n = 10, *p <0.05)
Glucose stimulation reduces phosphorylation of Hap1A and increases its association with trafficking proteins in β-cells
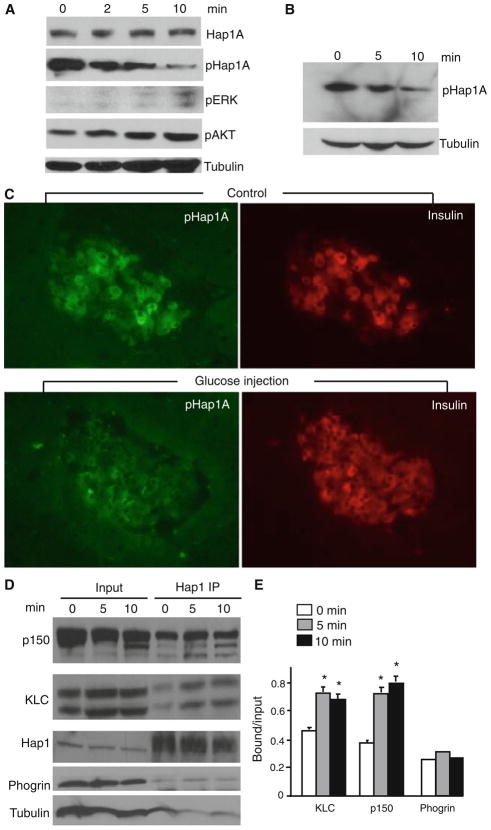
Hap1 consists of two isoforms, Hap1A and Hap1B, which are alternatively spliced forms with different C-terminal sequences, both of which can form heterodimers [42]. Our early studies identified a unique phosphorylation site (T598) at the C-terminal region of Hap1A, which can be phosphorylated by PKA, and phosphorylation of this site can regulate the association of Hap1A with trafficking proteins [19]. We thus examined the phosphorylation of Hap1A in Min6 cells that had been stimulated with 20 mM of glucose. We saw a marked decrease of phosphorylated Hap1A at 5–10 min after glucose stimulation, despite the expected increase of phosphorylation in positive controls (Erk and Akt) (Fig. 7a). This rapid decrease in Hap1A phosphorylation is consistent with the initial increase of insulin release, which peaks near 5 min post glucose stimulation [43]. To explore whether this change also occurs in vivo, we treated wild-type mice with i.p. injections of glucose and then isolated their pancreatic tissues for analysis. Western blotting also demonstrated a decrease in Hap1A phosphorylation in pancreatic tissues at 5–10 min following glucose challenge (Fig. 7b). Immunocytochemical staining verified decreased labeling of phosphorylated Hap1A (pHap1A) in insulin-containing β-cells after glucose stimulation (Fig. 7c).
Fig. 7.
Glucose stimulation of β-cells reduces Hap1A phosphorylation and increases its association with trafficking proteins. a Western blots probed with antibodies to Hap1A and phosphorylated Hap1A (pHap1A) showing reduction of pHap1A in Min6 cells after treatment with 20 mM glucose for different times (min). The blots were also probed with antibodies to phosphorylated ERK (pERK) and AKT (pAKT), which are positive controls known to be upregulated after glucose stimulation. b Western blot of phosphorylated Hap1A (pHap1A) levels in the pancreas from wild-type mice stimulated with i.p. injection of glucose (2 g/kg) for 5 or 10 min. c Double immunofluorescence of pancreatic tissues showing that phosphorylated Hap1A (pHap1A, green) was reduced after glucose challenge for 10 min in wild-type mice compared with those without glucose treatment. d Immunoprecipitation of Hap1A from pancreatic tissues of wild type mice that had been i.p. injected with glucose (2 g/kg body weight) for 5 or 10 min. The precipitates were probed with antibodies to dynactin p150, kinesin light chain (KLC), Hap1A, tubulin, and phogrin, which is an insulin granule protein. e Quantification of the relative precipitated proteins (ratio of precipitated or bound to input, mean ± SE, n = 3) showing a significant increase of KLC and dynactin p150 associated with Hap1A in mouse pancreatic tissues after glucose stimulation. *p <0.05 compared to the samples without glucose stimulation (0 min)
Since soluble Hap1 binds KLC and dynactin p150 in the cytoplasm, investigation of their diffuse co-localization may not be able to reveal changes of their association after glucose stimulation. Thus, we performed immunoprecipitation, which can better detect the interactions of soluble proteins. Hap1 was immunoprecipitated to assess its association with KLC and dynactin p150 in the pancreas of mice. There was increased precipitation of KLC and dynactin p150 with Hap1A at 5 and 10 min after i.p. injection of glucose as compared to tubulin (Fig. 7d). Phogrin, an insulin granule membrane protein [44], was not significantly increased in the precipitates after glucose stimulation, suggesting that glucose only enhances the association of the Hap1 with microtubule-dependent transporters. Quantitative analysis of the ratios of precipitated proteins to the input also showed that more KLC and dynactin p150 were associated with Hap1A in pancreatic tissues after glucose stimulation than that without glucose treatment (Fig. 7e). Taken together, these findings suggest that glucose stimulation can alter the phosphorylation of Hap1A and increase its association with trafficking proteins, which may contribute to the release insulin from β-cells.
Discussion
Although Hap1 is enriched in the brain, it is also present in endocrine cells [3, 23, 24], the in vivo role of Hap1 in endocrine cells remains unknown. By selectively eliminating Hap1 expression in β-cells in the mouse pancreas, we have provided evidence that Hap1 deficiency in β-cells can impair insulin release and glucose tolerance. We have further demonstrated that glucose stimulation alters Hap1A’s phosphorylation and increases its association with microtubule-dependent trafficking proteins. These findings not only support the role of Hap1 in intracellular trafficking, but also reveal its critical function for vesicular release of hormones from endocrine cells. Given the vital roles hormones play in a variety of the body’s functions, our findings have implications for the pathogenesis of metabolic conditions that result from impaired vesicular trafficking or exocytosis in endocrine cells, offering new therapeutic targets for the treatment of such conditions.
The process of insulin release from β-cells shares many common characteristics with synaptic vesicle docking, fusion, and release of neurotransmitters in neurons [43, 45, 46]. For example, insulin release involves the packaging of insulin in small secretory vesicles, the trafficking of these vesicles to the plasma membrane, the exocytotic fusion of the vesicles with the plasma membrane, and eventually the secretion of insulin. These vesicles are known to be transported on microtubules to the proximity of the release site just below the plasma membrane [45, 46]; however, how microtubule-dependent transport is involved in vesicle movement, docking, and fusion in neuronal and endocrine cells remains unclear.
Hap1 is found to associate with synaptic vesicles in neuronal cells [2, 5]; by demonstrating that Hap1 participates in the process of vesicular insulin release in β-cells, our studies suggest that Hap1 also plays a critical role in exocytosis in endocrine cells. Given that glucose-stimulated insulin release can reduce the phosphorylation of Hap1A and increase the association of Hap1 with kinesin light chain and dynactin p150, which link microtubule motor proteins to various types of cargos, Hap1 is likely to regulate insulin release via its trafficking function. Although how the phosphorylation of Hap1A is regulated remains to be investigated, it has been well documented that glucose stimulation can trigger multiple second messenger signaling pathways that can alter protein phosphorylation [47]. For example, glucose also triggers Akt activation, which could also alter Hap1 phosphorylation. There are several possibilities for the regulatory role of Hap1 in insulin release from β-cells. First, intracellular cargo transport requires microtubule-based motors, kinesin and cytoplasmic dynein, and the actin-based myosin motors. These molecular motors exchange cargo while traveling between filamentous tracks and deliver it to its destination [12, 13]. Kinesin and cytoplasmic dynein have been found to be involved in vesicle trafficking in β-cells [48, 49]. Hap1 may function as an adaptor involved in targeting cargos to different motor proteins or transporters during the trafficking of these cargos. Second, Hap1 interacts with type 1 inositol (1, 4, 5)-triphosphate receptor (InsP3R1) IP3 receptors to regulate intracellular calcium release in neuron [17]. As intracellular calcium release triggers insulin secretion from β-cells, it remains to be investigated whether loss of Hap1 can affect intracellular calcium release to impair insulin secretion from β-cells. Third, Hap1 is involved in intracellular trafficking and recycling of membrane receptors [18, 22]. In β-cells, membrane receptor recycling could regulate membrane potential and calcium influx to influence insulin release. Thus, it would also be important to investigate whether loss of Hap1 affects membrane calcium channel activity and calcium influx. Our findings suggest that Hap1A phosphorylation could be a new target for the future therapeutic regulation of insulin release.
The essential element in the progression and development of impaired glucose tolerance in type 2 diabetes is abnormal insulin secretion from dysfunctional β-cells [50]. It would be interesting to investigate whether Hap1 dysfunction is involved in type 2 diabetes, or conversely, whether the addition of Hap1 could ameliorate insulin-secretion defects. Our studies also raise the possibility that Hap1 dysfunction could be involved in the abnormal metabolism seen in Huntington’s disease (HD), as abnormal glucose tolerance and insulin release are found in both HD mouse models and patients [25–32]. In addition, there is impaired secretion of various hormones in HD patients [51–53]. Given that mutant htt can affect Hap1-mediated transport of BDNF [20] and membrane receptors [19, 22], it would be interesting to investigate whether mutant htt affects hormone release via its abnormal interaction with Hap1. Generation of conditional Hap1 knockout mice would allow for further investigation into the roles Hap1 plays in different types of endocrine cells.
Acknowledgments
This work was supported by NIH Grants NS036232 (X.J.L.) and NS045016 (S.H.L.). We thank Cheryl T. Strauss for her critical reading of the manuscript.
Footnotes
Conflict of interest The authors report no conflicts of interest.
Contributor Information
Austin Cape, Department of Human Genetics, Emory University School of Medicine, 615 Michael Street, Atlanta, GA 30322, USA.
Xingxing Chen, Division of Histology and Embryology, Department of Anatomy, Tongji Medical College, Huazhong University of Science Technology, Wuhan 430030, China.
Chuan-En Wang, Department of Human Genetics, Emory University School of Medicine, 615 Michael Street, Atlanta, GA 30322, USA.
Ashley O’Neill, Department of Human Genetics, Emory University School of Medicine, 615 Michael Street, Atlanta, GA 30322, USA.
Yung-Feng Lin, Department of Human Genetics, Emory University School of Medicine, 615 Michael Street, Atlanta, GA 30322, USA.
Jun He, Division of Histology and Embryology, Department of Anatomy, Tongji Medical College, Huazhong University of Science Technology, Wuhan 430030, China.
Xing-Shun Xu, Department of Human Genetics, Emory University School of Medicine, 615 Michael Street, Atlanta, GA 30322, USA.
Hong Yi, A Core Facility of Emory University, Suite E106, Cherry L. Emerson Hall, 1521 Dickey Drive, Atlanta, GA 30322, USA.
He Li, Division of Histology and Embryology, Department of Anatomy, Tongji Medical College, Huazhong University of Science Technology, Wuhan 430030, China.
Shihua Li, Email: sli@emory.edu, Department of Human Genetics, Emory University School of Medicine, 615 Michael Street, Atlanta, GA 30322, USA.
Xiao-Jiang Li, Email: xli2@emory.edu, Department of Human Genetics, Emory University School of Medicine, 615 Michael Street, Atlanta, GA 30322, USA.
References
- 1.Li XJ, Li SH, Sharp AH, Nucifora FC, Jr, Schilling G, Lanahan A, Worley P, Snyder SH, Ross CA. A huntingtin-associated protein enriched in brain with implications for pathology. Nature. 1995;378:398–402. doi: 10.1038/378398a0. [DOI] [PubMed] [Google Scholar]
- 2.Gutekunst CA, Li SH, Yi H, Ferrante RJ, Li XJ, Hersch SM. The cellular and subcellular localization of huntingtin-associated protein 1 (HAP1): comparison with huntingtin in rat and human. J Neurosci. 1998;18:7674–7686. doi: 10.1523/JNEUROSCI.18-19-07674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dragatsis I, Dietrich P, Zeitlin S. Expression of the Huntingtin-associated protein 1 gene in the developing and adult mouse. Neurosci Lett. 2000;282:37–40. doi: 10.1016/s0304-3940(00)00872-7. [DOI] [PubMed] [Google Scholar]
- 4.Fujinaga R, Kawano J, Matsuzaki Y, Kamei K, Yanai A, Sheng Z, Tanaka M, Nakahama K, Nagano M, Shinoda K. Neuro-anatomical distribution of Huntingtin-associated protein 1-mRNA in the male mouse brain. J Comp Neurol. 2004;478:88–109. doi: 10.1002/cne.20277. [DOI] [PubMed] [Google Scholar]
- 5.Li SH, Li H, Torre ER, Li XJ. Expression of huntingtin-associated protein-1 in neuronal cells implicates a role in neuritic growth. Mol Cell Neurosci. 2000;16:168–183. doi: 10.1006/mcne.2000.0858. [DOI] [PubMed] [Google Scholar]
- 6.Li SH, Yu ZX, Li CL, Nguyen HP, Zhou YX, Deng C, Li XJ. Lack of huntingtin-associated protein-1 causes neuronal death resembling hypothalamic degeneration in Huntington’s disease. J Neurosci. 2003;23:6956–6964. doi: 10.1523/JNEUROSCI.23-17-06956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin YF, Xu X, Cape A, Li S, Li XJ. Huntingtin associated protein-1 deficiency in orexin-producing neurons impairs neuronal process extension and leads to abnormal behavior in mice. J Biol Chem. 2010;285:15941–15949. doi: 10.1074/jbc.M110.107318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan EY, Nasir J, Gutekunst CA, Coleman S, Maclean A, Maas A, Metzler M, Gertsenstein M, Ross CA, Nagy A, Hayden MR. Targeted disruption of Huntingtin-associated protein-1 (Hap1) results in postnatal death due to depressed feeding behavior. Hum Mol Genet. 2002;11:945–959. doi: 10.1093/hmg/11.8.945. [DOI] [PubMed] [Google Scholar]
- 9.Li XJ, Li SH. HAP1 and intracellular trafficking. Trends Pharmacol Sci. 2005;26:1–3. doi: 10.1016/j.tips.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Borrell-Pages M, Zala D, Humbert S, Saudou F. Huntington’s disease: from huntingtin function and dysfunction to therapeutic strategies. Cell Mol Life Sci. 2006;63:2642–2660. doi: 10.1007/s00018-006-6242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong J, Li SH, Li XJ. Regulation of intracellular HAP1 trafficking. J Neurosci Res. 2007;85:3025–3029. doi: 10.1002/jnr.21326. [DOI] [PubMed] [Google Scholar]
- 12.Ross JL, Ali MY, Warshaw DM. Cargo transport: molecular motors navigate a complex cytoskeleton. Curr Opin Cell Biol. 2008;20:41–47. doi: 10.1016/j.ceb.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gennerich A, Vale RD. Walking the walk: how kinesin and dynein coordinate their steps. Curr Opin Cell Biol. 2009;21:59–67. doi: 10.1016/j.ceb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelender S, Sharp AH, Colomer V, Tokito MK, Lanahan A, Worley P, Holzbaur EL, Ross CA. Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum Mol Genet. 1997;6:2205–2212. doi: 10.1093/hmg/6.13.2205. [DOI] [PubMed] [Google Scholar]
- 15.Li SH, Gutekunst CA, Hersch SM, Li XJ. Interaction of huntingtin-associated protein with dynactin P150Glued. J Neurosci. 1998;18:1261–1269. doi: 10.1523/JNEUROSCI.18-04-01261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuire JR, Rong J, Li SH, Li XJ. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem. 2006;281:3552–3559. doi: 10.1074/jbc.M509806200. [DOI] [PubMed] [Google Scholar]
- 17.Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1, 4, 5) triphosphate receptor type 1. Neuron. 2003;39:227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-amino-butyric acid type A receptor membrane trafficking. Proc Natl Acad Sci USA. 2004;101:12736–12741. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rong J, McGuire JR, Fang ZH, Sheng G, Shin JY, Li SH, Li XJ. Regulation of intracellular trafficking of huntingtin-associated protein-1 is critical for TrkA protein levels and neurite outgrowth. J Neurosci. 2006;26:6019–6030. doi: 10.1523/JNEUROSCI.1251-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, Saudou F. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Wu LL, Fan Y, Li S, Li XJ, Zhou XF. Huntingtin-associated protein-1 interacts with pro-brain-derived neurotrophic factor and mediates its transport and release. J Biol Chem. 2010;285:5614–5623. doi: 10.1074/jbc.M109.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Twelvetrees AE, Yuen EY, Arancibia-Carcamo IL, MacAskill AF, Rostaing P, Lumb MJ, Humbert S, Triller A, Saudou F, Yan Z, Kittler JT. Delivery of GABAARs to synapses is mediated by HAP1-KIF5 and disrupted by mutant huntingtin. Neuron. 2010;65:53–65. doi: 10.1016/j.neuron.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao M, Shen J, Zhang Y, Li SH, Li XJ, Li H. Immunohistochemical localization of huntingtin-associated protein 1 in endocrine system of the rat. J Histochem Cytochem. 2005;53:1517–1524. doi: 10.1369/jhc.5A6662.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao M, Chen X, Han J, Yang S, Peng T, Li H. Selective expression of Huntingtin-associated protein 1 in {beta}-cells of the rat pancreatic islets. J Histochem Cytochem. 2010;58:255–263. doi: 10.1369/jhc.2009.954479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurlbert MS, Zhou W, Wasmeier C, Kaddis FG, Hutton JC, Freed CR. Mice transgenic for an expanded CAG repeat in the Huntington’s disease gene develop diabetes. Diabetes. 1999;48:649–651. doi: 10.2337/diabetes.48.3.649. [DOI] [PubMed] [Google Scholar]
- 26.Bjorkqvist M, Fex M, Renstrom E, Wierup N, Petersen A, Gil J, Bacos K, Popovic N, Li JY, Sundler F, Brundin P, Mulder H. The R6/2 transgenic mouse model of Huntington’s disease develops diabetes due to deficient beta-cell mass and exocytosis. Hum Mol Genet. 2005;14:565–574. doi: 10.1093/hmg/ddi053. [DOI] [PubMed] [Google Scholar]
- 27.Smith R, Bacos K, Fedele V, Soulet D, Walz HA, Obermuller S, Lindqvist A, Bjorkqvist M, Klein P, Onnerfjord P, Brundin P, Mulder H, Li JY. Mutant huntingtin interacts with {beta}-tubulin and disrupts vesicular transport and insulin secretion. Hum Mol Genet. 2009;18:3942–3954. doi: 10.1093/hmg/ddp336. [DOI] [PubMed] [Google Scholar]
- 28.Podolsky S, Leopold NA, Sax DS. Increased frequency of diabetes mellitus in patients with Huntington’s chorea. Lancet. 1972;1:1356–1358. doi: 10.1016/s0140-6736(72)91092-6. [DOI] [PubMed] [Google Scholar]
- 29.Podolsky S, Leopold NA. Abnormal glucose tolerance and arginine tolerance tests in Huntington’s disease. Gerontology. 1977;23:55–63. doi: 10.1159/000212174. [DOI] [PubMed] [Google Scholar]
- 30.Schubotz R, Hausmann L, Kaffarnik H, Zehner J, Oepen H. Fatty acid patterns and glucose tolerance in Huntington’s chorea (author’s transl) Res Exp Med (Berl) 1976;167:203–215. doi: 10.1007/BF01851645. [DOI] [PubMed] [Google Scholar]
- 31.Farrer LA. Diabetes mellitus in Huntington disease. Clin Genet. 1985;27:62–67. doi: 10.1111/j.1399-0004.1985.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 32.Kremer HP, Roos RA, Frolich M, Radder JK, Nieuwenhuijzen Kruseman AC, Van der Velde A, Buruma OJ. Endocrine functions in Huntington’s disease. A two-and-a-half years follow-up study. J Neurol Sci. 1989;90:335–344. doi: 10.1016/0022-510x(89)90120-2. [DOI] [PubMed] [Google Scholar]
- 33.Cantley J, Selman C, Shukla D, Abramov AY, Forstreuter F, Esteban MA, Claret M, Lingard SJ, Clements M, Harten SK, Asare-Anane H, Batterham RL, Herrera PL, Persaud SJ, Duchen MR, Maxwell PH, Withers DJ. Deletion of the von Hippel-Lindau gene in pancreatic beta cells impairs glucose homeostasis in mice. J Clin Invest. 2009;119:125–135. doi: 10.1172/JCI26934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huising MO, van der Meulen T, Vaughan JM, Matsumoto M, Donaldson CJ, Park H, Billestrup N, Vale WW. CRFR1 is expressed on pancreatic beta cells, promotes beta cell proliferation, and potentiates insulin secretion in a glucose-dependent manner. Proc Natl Acad Sci USA. 2010;107:912–917. doi: 10.1073/pnas.0913610107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown JE, Onyango DJ, Ramanjaneya M, Conner AC, Patel ST, Dunmore SJ, Randeva HS. Visfatin regulates insulin secretion, insulin receptor signalling and mRNA expression of diabetes-related genes in mouse pancreatic beta-cells. J Mol Endocrinol. 2010;44:171–178. doi: 10.1677/JME-09-0071. [DOI] [PubMed] [Google Scholar]
- 36.Sheng G, Chang GQ, Lin JY, Yu ZX, Fang ZH, Rong J, Lipton SA, Li SH, Tong G, Leibowitz SF, Li XJ. Hypothalamic huntingtin-associated protein 1 as a mediator of feeding behavior. Nat Med. 2006;12:526–533. doi: 10.1038/nm1382. [DOI] [PubMed] [Google Scholar]
- 37.Speidel D, Salehi A, Obermueller S, Lundquist I, Brose N, Renstrom E, Rorsman P. CAPS1 and CAPS2 regulate stability and recruitment of insulin granules in mouse pancreatic beta cells. Cell Metab. 2008;7:57–67. doi: 10.1016/j.cmet.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knockouts using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 39.Harslund J, Nielsen OL, Brunner N, Offenberg H. Gender-dependent physiological implications of combined PAI-1 and TIMP-1 gene deficiency characterized in a mouse model. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1630–R1639. doi: 10.1152/ajpregu.00380.2007. [DOI] [PubMed] [Google Scholar]
- 40.Lilla V, Webb G, Rickenbach K, Maturana A, Steiner DF, Halban PA, Irminger JC. Differential gene expression in well-regulated and dysregulated pancreatic beta-cell (MIN6) sublines. Endocrinology. 2003;144:1368–1379. doi: 10.1210/en.2002-220916. [DOI] [PubMed] [Google Scholar]
- 41.Dalle S, Longuet C, Costes S, Broca C, Faruque O, Fontes G, Hani EH, Bataille D. Glucagon promotes cAMP-response element-binding protein phosphorylation via activation of ERK1/2 in MIN6 cell line and isolated islets of Langerhans. J Biol Chem. 2004;279:20345–20355. doi: 10.1074/jbc.M312483200. [DOI] [PubMed] [Google Scholar]
- 42.Li SH, Gutekunst CA, Hersch SM, Li XJ. Association of HAP1 isoforms with a unique cytoplasmic structure. J Neurochem. 1998;71:2178–2185. doi: 10.1046/j.1471-4159.1998.71052178.x. [DOI] [PubMed] [Google Scholar]
- 43.Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- 44.Wasmeier C, Hutton JC. Secretagogue-dependent phosphorylation of phogrin, an insulin granule membrane protein tyrosine phosphatase homologue. Biochem J. 1999;341:563–569. [PMC free article] [PubMed] [Google Scholar]
- 45.Wang S, Hsu SC. The molecular mechanisms of the mammalian exocyst complex in exocytosis. Biochem Soc Trans. 2006;34:687–690. doi: 10.1042/BST0340687. [DOI] [PubMed] [Google Scholar]
- 46.Park JJ, Loh YP. How peptide hormone vesicles are transported to the secretion site for exocytosis. Mol Endocrinol. 2008;22:2583–2595. doi: 10.1210/me.2008-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mears D. Regulation of insulin secretion in islets of Langerhans by Ca(2+)channels. J Membr Biol. 2004;200:57–66. doi: 10.1007/s00232-004-0692-9. [DOI] [PubMed] [Google Scholar]
- 48.Varadi A, Ainscow EK, Allan VJ, Rutter GA. Involvement of conventional kinesin in glucose-stimulated secretory granule movements and exocytosis in clonal pancreatic beta-cells. J Cell Sci. 2002;115:4177–4189. doi: 10.1242/jcs.00083. [DOI] [PubMed] [Google Scholar]
- 49.Varadi A, Tsuboi T, Johnson-Cadwell LI, Allan VJ, Rutter GA. Kinesin I and cytoplasmic dynein orchestrate glucose-stimulated insulin-containing vesicle movements in clonal MIN6 beta-cells. Biochem Biophys Res Commun. 2003;311:272–282. doi: 10.1016/j.bbrc.2003.09.208. [DOI] [PubMed] [Google Scholar]
- 50.Del Prato S, Tiengo A. The importance of first-phase insulin secretion: implications for the therapy of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2001;17:164–174. doi: 10.1002/dmrr.198. [DOI] [PubMed] [Google Scholar]
- 51.Keogh HJ, Johnson RH, Nanda RN, Sulaiman WR. Altered growth hormone release in Huntington’s chorea. J Neurol Neurosurg Psychiatry. 1976;39:244–248. doi: 10.1136/jnnp.39.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lavin PJ, Bone I, Sheridan P. Studies of hypothalamic function in Huntington’s chorea. J Neurol Neurosurg Psychiatry. 1981;44:414–418. doi: 10.1136/jnnp.44.5.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saleh N, Moutereau S, Durr A, Krystkowiak P, Azulay JP, Tranchant C, Broussolle E, Morin F, Bachoud-Levi AC, Maison P. Neuroendocrine disturbances in Huntington’s disease. PLoS One. 2009;4:e4962. doi: 10.1371/journal.pone.0004962. [DOI] [PMC free article] [PubMed] [Google Scholar]