Abstract
We have identified several ligands with high binding affinities to the dopamine D3 receptor and excellent selectivity over the D2 and D1 receptors. CJ-1639 (17) binds to the D3 receptor with a Ki value of 0.50 nM and displays a selectivity of >5,000 times over D2 and D1 receptors in binding assays using dopamine receptors expressed in the native rat brain tissues. CJ-1639 binds to human D3 receptor with a Ki value of 3.61 nM and displays over >1000-fold selectivity over human D1 and D2 receptors. CJ-1639 is active at 0.01 mg/kg at the dopamine D3 receptor in the rat and only starts to show a modest D2 activity at doses as high as 10 mg/kg. CJ-1639 is the most potent and selective D3 full agonist reported to date.
Keywords: Dopamine receptors, ligands, agonists, drug abuse
Dopamine is an essential neurotransmitter in the central nervous system and exerts its effects through activation of five distinct dopamine receptor subtypes that belong to the G protein-coupled receptor superfamily. The receptors are grouped into the D1-like (D1 and D5) and D2-like (D2, D3, and D4) receptor subtypes. Numerous studies have provided strong evidence that the D3 receptor is a promising therapeutic target for a variety of conditions, including drug abuse, restless legs syndrome, schizophrenia, Parkinson’s disease, and depression.(1)-(3) Extensive efforts have been devoted to the discovery and development of potent and selective D3 ligands, including agonists, partial agonists and antagonists.(5)-(6)
Due to the high degree of sequence identity within the transmembrane helices between D2 and D3 receptors, and the near-identity of the residues inferred to form the binding site in these receptors,(4) it has been a challenge to design highly selective D3 ligands with excellent aqueous solubility and bioavailability.(5),(6) Pramipexole (1), Quinpirole (2) and 7-hydroxy dipropylaminotetralin (7-OH-DPAT, 3), three widely used D3 agonists, all have modest selectivity (<100-fold) over the D2 receptor based upon their in vitro binding data(4) and a narrow range of selectivity at the D3 receptor over the D2 receptor in vivo.(7),(8) SB-277011A (4),(9) SB-414796 (5),(10) NGB 2904 (6) (11) and BP 897(7)(12) are four commonly used D3 antagonists but they also have a selectivity of 100-fold or less for D3 over D2. Furthermore, these four ligands have limited aqueous solubility. R-PG648 (8), whose design is based upon the core structure of NGB 2904, is a potent D3 antagonist with a selectivity of >400-fold over D2 and also has a much improved aqueous solubility compared to NGB 2904.(13) Recently, compound 9 was reported to be a potent and orally active D3 antagonist with a 500-fold selectivity over D2 in in vitro functional assays. (14) The in vivo selectivity for compounds 8 and 9 at the D3 receptor over the D2 receptor has not been reported.
In our effort to identify D3 ligands with high selectivity and good solubility, we have previously reported the design of a set of new compounds based upon pramipexole (1),(15) which included CJ-1037 (10) and CJ-998 (11) as the two best compounds. Both 10 and 11 bind to D3 with high affinities, have a good selectivity for D3 over D2 and excellent aqueous solubility (>50 mg/ml). Unlike pramipexole, which is a D3 full agonist, compounds 10 and 11 appear to behave as partial agonists with low intrinsic agonist activity in rats.(15) However, despite their high binding affinities to D3, both compounds 10 and 11 only show in vivo activity at a very high dose (32 mg/kg), indicating their poor bioavailability in the brain. Here we report further modifications based upon compound 11. These efforts have now yielded a set of new D3 ligands that are highly potent and selective, not only in vitro, but also in vivo.
A recent publication by Newman’s group showed that introduction of a hydroxyl group in the linker region in their D3 ligands improves D3 selectivity and aqueous solubility.(13) Accordingly, we designed and synthesized compounds 12 and 13 by placing a hydroxyl group in the cyclohexane ring of compound 11 to improve its solubility and to also explore the effect of this hydroxyl group for binding and selectivity at the D3 receptor (Figure 2). Compound 12 with a cis-hydroxycyclohexyl group and compound 13, the corresponding trans isomer, have Ki values of 1.6 nM and 0.9 nM to D3, respectively, in our in vitro binding assays using dopamine receptors expressed in the native rat brain tissues. Thus, they are as potent as compound 11, indicating that introduction of a hydroxyl group at the bridge carbon atom of the cyclohexyl group is not detrimental to D3 binding. Both compounds 12 and 13, however, have a reduced binding affinity to D2 as compared to pramipexole. As a result, 12 and 13 display a selectivity of >1000-times and >3000-times, respectively, for D3 over D2.
Figure 2.
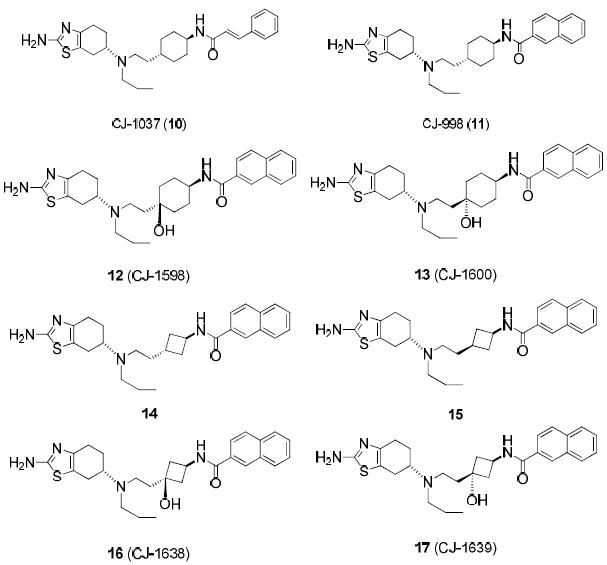
Chemical structures of two previously reported D3 ligands, 10 and 11, and designed new analogues 12-17.
We next synthesized compounds 14 and 15 with a 4-membered ring in the linker region to replace the 6-membered ring in compound 11. Compound 14, with a trans-cyclobutyl group, has a Ki value of 0.41 nM to D3 and is thus 2 times more potent than 11. Compound 14 binds to D2 with a Ki value of 584 nM and is 6 times less potent than 11. Hence, compound 14 is a highly potent D3 ligand and displays >1000-fold selectivity for D3 over D2. Compound 15 with a cis-cyclobutyl group in the linker region binds to D3 with a Ki value of 0.35 nM, very similar to that for compound 14. Compound 15 also shows a 1000-fold selectivity for D3 over D2. Hence, replacement of the trans-cyclohexyl group in compound 11 with either a trans- or cis-cyclobutyl group improves both the binding affinity for D3 and selectivity over D2.
Encouraged by the promising binding and selectivity data observed for compounds 12-15, we next synthesized compounds 16 and 17, in which a hydroxyl group is introduced to the bridge carbon atom of the 4-membered ring in compounds 14 and 15, respectively. Compound 16 binds to D3 with a Ki value of 0.40 nM and displays a selectivity of 1827-times over D2. Compound 17 binds to D3 with a Ki of 0.50 nM and displays a selectivity of >5000-times over D2. In addition to their high binding affinities to D3 and excellent selectivity over D2, compounds 16 and 17 also have excellent aqueous solubility (>100 mg/ml) in their HCl salt form.
The synthesis of compounds 12 and 13 were outlined in Scheme 1. Basically 2-naphthoyl chloride was reacted with trans-4-aminocyclohexanol in the presence of triethylamine, followed by oxidation with PCC in DCM to give ketone 18. Ketone 18 was treated with allylmagnesium bromide at - 78 °C to afford cis-cyclohexanol 20 and trans-cyclohexanol 21. Compounds 20 and 21 were separated by silica gel chromatography (Hexane:Ethyl acetate=1:1). Cis-aldehyde 22 was obtained by the treatment of cis-cyclohexanol 20 with Osmium tetraoxide followed by sodium periodate. Trans-aldehyde 23 was obtained in a similar manner. Reductive amination of pramipexole 1 with 22 and 23 gave designed compounds 12 and 13 respectively. Details of the synthesis of compounds 12-17 are provided in Supporting Information (SI).
Scheme 1.
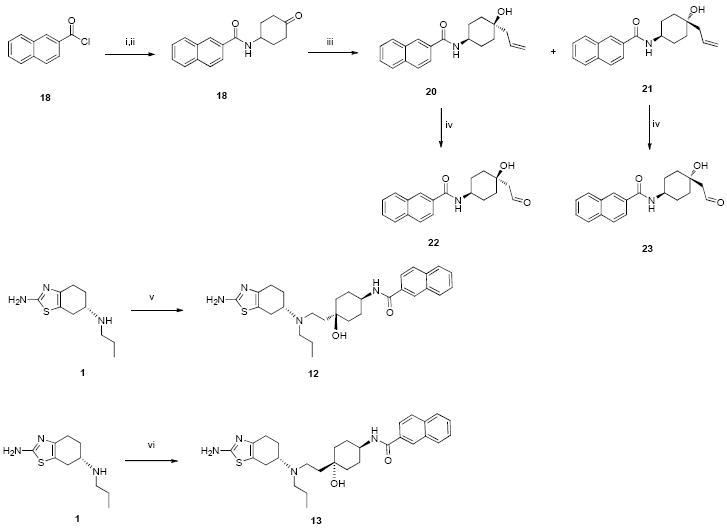
Synthesis of compounds 12 and 13. Conditions and reagents: (i) 2-naphthoyl chloride, trans-4-aminocyclohexanol, triethylamine, DCM; (ii) PCC, DCM, RT, 12 hr; (iii) allylmagnesium bromide, THF, -78 °C, 2 hr; (iv) OsO4, NaIO4, THF-H2O, RT, 30 min; (v) 22, NaBH(OAc)3, CH3CO2H, DCM; (vi) 23, NaBH(OAc)3, CH3CO2H, DCM.
The stereochemistry of cis-conformation of compound 12 was confirmed by x-ray crystallographic analysis of key intermediate 20 (Figure 3). The stereochemistry of cis-conformation of compound 16 was confirmed by x-ray crystallographic analysis of a key intermediate (see supporting information).
Figure 3.
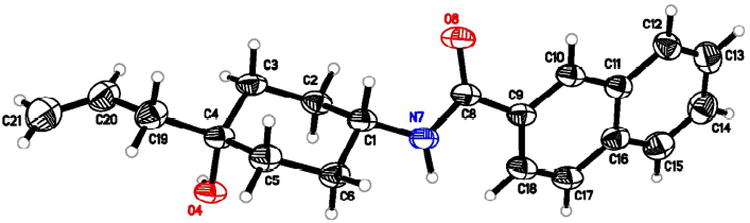
Crystal structure of intermediate 20.
Our previous studies have shown that the induction of yawning by D2-like agonists is a sensitive and well validated measure of agonist activity at the dopamine D3 receptor,(7),(8),(17) whereas the inhibition of yawning, and induction of hypothermia by D2-like agonists is indicative of an agonist activation at the D2 receptor.(17)-(19) We have evaluated compounds 16 and 17 for their in vivo function in the yawning and hypothermia assays in the rat and included pramipexole as the reference compound in the evaluations. The results are shown in Figure 4.
Figure 4.
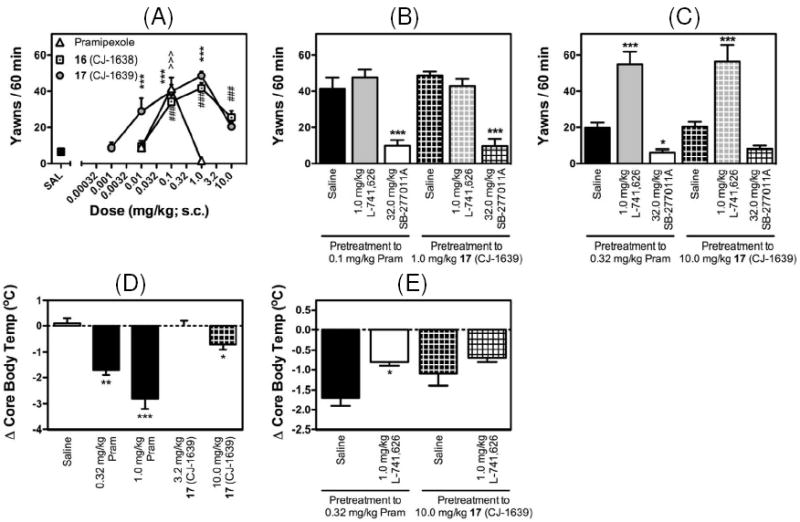
Functional evaluations of the D3 and D2 activity of pramipexole and compounds 16 and 17 in yawning and hypothermia assays in rats. *, P <0.05; **, P<0.005; ***, P<0.001.
Pramipexole produced dose-dependent increases in yawning at low doses and inhibition of yawning and induction of hypothermia at higher doses (Figure 4A, and 4D). The data is consistent with its full agonist activity profile at the D3 and D2 receptors and a narrow in vivo selectivity at the D3 receptor.(7),(8) Like pramipexole, both 16 and 17 produced a dose-dependent increase in yawning (Figure 4A). Unlike pramipexole, which induced yawning over a very narrow dose range, compound 16 and 17 produced increases in yawning over a very wide range of doses, with significant increases in yawning observed at a dose range of 0.1 to 10.0 mg/kg for compound 16 (CJ-1638), and 0.01 to 10.0 mg/kg for compound 17 (CJ-1639) (Figure 4A). Although both compounds have very similar binding affinities to D3 based upon our in vitro binding data, our yawning assay showed that compound 17 is more active than 16 in activation of the D3 receptor in the rat and has a significant yawning activity at a dose of 0.01 mg/kg. These data suggest that compound 17 has a better bioavailability in the brain than compound 16. Both compounds produced peak levels of yawning at a dose of 1.0 mg/kg (Figure 4A).
Because 17 is more potent in vivo in the yawning assay, we focused our further evaluation on this compound. Consistent with previous reports, significant decreases in core body temperature were observed with doses of 0.32 and 1.0 mg/kg pramipexole (Figure 4D). In comparison, compound 17 had no significant effect on core body temperature at 3.2 mg/kg and had a significant but a modest effect at 10 mg/kg (Figure 4D). These data suggest that compound 17 readily crosses the blood-brain barrier, and functions as a highly selective, full efficacy agonist at the D3 receptor.
The in vivo profiles of activity for compound 17 and pramipexole were assessed by the antagonist interaction studies with the known D3 antagonist SB-277011A(9) and the D2 antagonist L-741,626(20) in both yawning and hypothermia assays. The selective D3 antagonist SB-277011A effectively inhibited the induction of yawning by both 0.1 and 0.32 mg/kg of pramipexole and 1 and 10 mg/kg of 17 (Figure 4B and 4C). In comparison, although the selective D2 antagonist L-741,626 failed to modulate the yawning effect induced by the lower doses of pramipexole and 17 (0.1 and 1.0 mg/kg, respectively), a significant increase in the yawning effect was observed when L-741,626 was administered prior to the higher doses of pramipexole and 17 (0.32 and 10.0 mg/kg, respectively; Figure 4B and 4C). These data further confirm the agonist activity for pramipexole and compound 17 at the D3 receptor, and suggest that D2 agonist effects may be observed with high doses of pramipexole (~1.0 mg/kg) and 17 (~10.0 mg/kg). In the hypothermia assay, although the D2 antagonist L-741,626 significantly reversed the hypothermia effect induced by 0.32 mg/kg of pramipexole, it did not have a significant effect on the modest hypothermia induced by 10 mg/kg of compound 17 (Figure 4E). Of note, both pramipexole at 0.32 mg/kg and compound 17 at 10 mg/kg produced similar modest decreases in core body temperature (approximately 0.75°C) with pretreatment of L-741,626 at 1 mg/kg (Figure 4E). Taken together, these in vivo data show that 17 functions as a potent, highly selective, full efficacy agonist at the D3 receptor, with the onset of modest D2 agonist activity observed at 10 mg/kg for compound 17 and at 0.32 mg/kg for pramipexole. Pramipexole was one of the most selective D3 agonists identified to date(7) and has been extensively used in in vitro and in vivo studies. The profiles of activity obtained in the current studies have shown that compound 17 functions as a full agonist at the D3 receptor at a dose of 0.01 mg/kg, but has minimal D2 activity at a dose of 10.0 mg/kg, displaying not only higher potency but also much better selectivity than pramipexole.
To further assess the binding affinity, selectivity and functional activity of compound 17, we evaluated this compound in the Addiction Treatment Discovery Programs of the National Institute on Drug Abuse (NIDA) using cloned human dopamine receptors (SI). Compound 17 binds to human D3 receptor with a Ki value of 3.61 ± 0.53 nM and to human D2 receptor with a Ki value of >4000 nM. Furthermore, 17 shows no detectable binding to human D1 receptor at 10,000 nM. Functional testing using a mitogenesis functional assay for the D3 receptor in CHO cells showed that 17 is a potent and full agonist with an EC50 value of 1.70 ± 0.53 nM and 103.4% of maximum stimulation of quinpirole as the standard D3 agonist control. Therefore, compound 17 is also a highly potent D3 full agonist in the functional assays using the cloned human D3 receptor and displays a >1000-fold selectivity for D3 over D2 and D1 in the binding assays using cloned human dopamine receptors.
In summary, through exploration of the linker region of our previously reported D3 ligand 11, we have identified a number of new D3 ligands with high binding affinities to the D3 receptor and a selectivity of >1000-times over the D2 and D1 receptors based upon our in vitro binding data. Compound 17 binds to D3 with a Ki value of 0.50 nM and shows a selectivity of >5000-times over D2 and >10,000 over D1 in our binding assays using rat brain. The high binding affinity and selectivity of 17 for the D3 receptor was further confirmed using cells transfected with cloned human dopamine receptors. Our in vivo functional evaluation demonstrated that compound 17 is a highly potent and selective D3 full agonist. While it is active at the D3 receptor at doses as low as 0.01 mg/kg, it has a minimal activity at the D2 receptor in the rat at doses as high as 10 mg/kg. Its full agonist profile at the D3 receptor was also confirmed using cells transfected with cloned human D3 receptor in the mitogenesis assay. To the best of our knowledge, compound 17 is the most active and selective D3 full agonist reported to date. Furthermore, compound 17 also has good aqueous solubility. Taken together, our data show that compound 17 (CJ-1639) is an excellent pharmacological tool with which to elucidate the role of the D3 receptor in different neurological conditions in animal models. Extensive in vivo studies are in progress to evaluate its therapeutic potential for the treatment of drug abuse and other neurological conditions in which the D3 receptor may play a role, and the results will be reported in due course.
Supplementary Material
Figure 1.
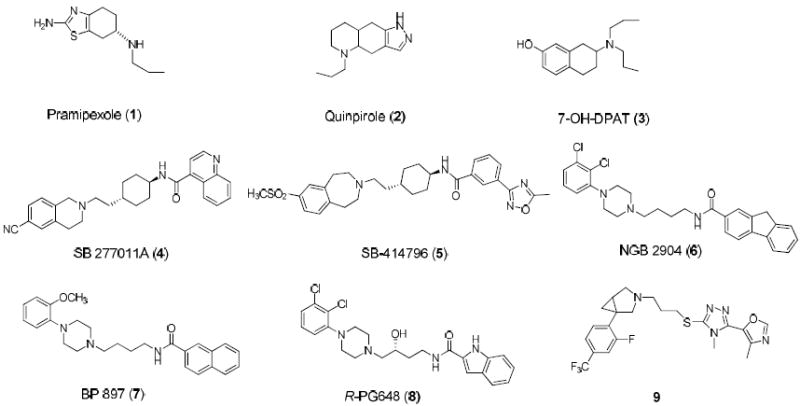
Chemical structures of representative known D3 agonists (1-3) and antagonists (4-9).
Table 1.
Binding Affinities at the D1-like, D2-like, and D3 Receptors in Binding Assays Using Rat Brain. Binding affinities for all the compounds in this Table were evaluated under the same assay conditions.
| Ki ± SEM (nM) | Selectivity | ||||
|---|---|---|---|---|---|
| ligand | D3 ([3H]-R(+)-7-OH-DPAT) | D2-like [3H]Spiperone | D1-like [3H]SCH23390 | D2-like/D3 | D1-like/D3 |
| 1 | 0.45±0.034 | 39± 4 | >10,000 | 87 | >10,000 |
| 11 | 0.80 ± 0.05 | 92 ± 9 | >5,000 | 115 | >5,000 |
| 12 | 1.6±0.25 | 1,628±234 | 11,557±996 | 1,039 | 7,373 |
| 13 | 0.93±0.094 | 3,549±421 | 14,057±1,095 | 3,816 | 15,115 |
| 14 | 0.41±0.038 | 584±72 | 6,125±271 | 1,416 | 14,848 |
| 15 | 0.35±0.019 | 564±52 | 3,058±198 | 1,627 | 8,822 |
| 16 | 0.40±0.087 | 725±45 | 1,616±167 | 1,827 | 4,074 |
| 17 | 0.50±0.080 | 2,568±302 | 10,127±1,002 | 5,136 | 20,254 |
Acknowledgments
This work was supported by a grant from the NIDA, NIH (R01DA020669). Crystallographic studies were supported by a grant from NIDA under contract Y1-DA-1101. We are grateful to the Addiction Treatment Discovery Programs at the NIDA, NIH for evaluation of CJ-1639 for its binding affinities and functional activity in cells transfected with cloned human dopamine receptors under the contract NIDA Y1-DA-0101, performed by Dr. Aaron Janowsky at the Oregon Health & Science University, Portland, Oregon.
Footnotes
Supporting Information Available: Experimental details of chemical synthesis, chemical data, in vitro and in vivo characterizations of the ligands. This material is free of charge via the Internet at http://pubs.acs.org.
References
- 1.Joyce JN. Dopamine D3 receptor as a therapeutic target for antipsychotic and antiparkinsonian drugs. Pharmacol Ther. 2001;90:231–259. doi: 10.1016/s0163-7258(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 2.Crocg MA, Mant R, Asherson P, Williams J, Hode Y, Shimohama S, Sawada H, Kitamura Y, Taniguchi T. Disease model: Parkinson’s disease. Trends Mol Med. 2003;9:360–365. doi: 10.1016/s1471-4914(03)00117-5. [DOI] [PubMed] [Google Scholar]
- 3.Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- 5.Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- 6.Heidbreder CA, Newman AH. Current perspectives on selective dopamine D(3) receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann N Y Acad Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology (Berl) 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. Dopamine Agonist-Induced Yawning in Rats: A Dopamine D3 Receptor-Mediated Behavior. J Pharmacol Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, Boyfield I, Branch CL, Cilia J, Coldwell MC. Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2000;294:1154–1165. [PubMed] [Google Scholar]
- 10.Macdonald GJ, Branch CL, Hadley MS, Johnson CN, Nash DJ, Smith AB, Stemp G, Thewlis KM, Vong AK, Austin NE, Jeffrey P, Winborn KY, Boyfield I, Hagan JJ, Middlemiss DN, Reavill C, Riley GJ, Watson JM, Wood M, Parker SG, Ashby CR., Jr Design and synthesis of trans-3-(2-(4-((3-(3-(5-methyl-1,2,4-oxadiazolyl))-phenyl)carboxamido)cyclohexyl)ethyl)-7-methylsulfonyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SB-414796): a potent and selective dopamine D3 receptor antagonist. J Med Chem. 2003;46:4952–4964. doi: 10.1021/jm030817d. [DOI] [PubMed] [Google Scholar]
- 11.Yuan J, Chen X, Brodbeck R, Primus R, Braun J, Wasley JWF, Thurkauf A. NGB 2904 and NGB 2849: Two highly selective dopamine D3 receptor antagonists. Bioorg Med Chem Lett. 1998;8:2715–2718. doi: 10.1016/s0960-894x(98)00469-7. [DOI] [PubMed] [Google Scholar]
- 12.Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P. Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- 13.Newman AH, Grundt P, Cyriac G, Deschamps JR, Taylor M, Kumar R, Ho D, Luedtke RR. N-(4-(4-(2,3-Dichloro- or 2-methoxyphenyl)piperazin-1-yl)butyl)heterobiarylcarboxamides with Functionalized Linking Chains as High Affinity and Enantioselective D3 Receptor Antagonists. J Med Chem. 2009;52:2559–2570. doi: 10.1021/jm900095y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micheli F, Arista L, Bonanomi G, Blaney FE, Braggio S, Capelli AM, Checchia A, Damiani F, Di-Fabio R, Fontana S, Gentile G, Griffante C, Hamprecht D, Marchioro C, Mugnaini M, Piner J, Ratti E, Tedesco G, Tarsi L, Terreni S, Worby A, Ashby CR, Jr, Heidbreder C. 1,2,4-Triazolyl azabicyclo[3.1.0]hexanes: a new series of potent and selective dopamine D(3) receptor antagonists. J Med Chem. 2010;53:374–391. doi: 10.1021/jm901319p. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Collins GT, Zhang J, Yang C-Y, Levant B, Woods J, Wang S. Design, synthesis and evaluation of potent and highly selective dopamine 3 receptor ligands with a novel profile of pharmacologic and behavioral activity. J Med Chem. 2008;51:5905–5908. doi: 10.1021/jm800471h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi J-K, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic Analogues of N-(4-(4-(2,3-Dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with Functionalized Linking Chains as Novel Dopamine D3 Receptor Ligands: Potential Substance Abuse Therapeutic Agents. J Med Chem. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- 17.Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH. Food restriction alters N’-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride (pramipexole)-induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor-mediated effects. J Pharmacol Exp Ther. 2008;325:691–697. doi: 10.1124/jpet.107.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulay D, Depoortere R, Perrault G, Borrelli E, Sanger DJ. Dopamine D2 receptor knock-out mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D2/D3 receptor agonists. Neuropharmacology. 1999;38:1389–1396. doi: 10.1016/s0028-3908(99)00064-7. [DOI] [PubMed] [Google Scholar]
- 19.Chaperon F, Tricklebank MD, Unger L, Neijt HC. Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology. 2003;44:1047–1053. doi: 10.1016/s0028-3908(03)00113-8. [DOI] [PubMed] [Google Scholar]
- 20.Kulagowski JJ, Broughton HB, Curtis NR, Mawer IM, Ridgill MP, Baker R, Emms F, Freedman SB, Marwood R, Patel S, Ragan CI, Leeson PD. 3-[[4-(4-Chlorophenyl)piperazin-1-yl]methyl]-1H-pyrrolo[2,3-b]pyridine: An Antagonist with High Affinity and Selectivity for the Human Dopamine D4 Receptor. J Med Chem. 1996;39:1941–1942. doi: 10.1021/jm9600712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


