Abstract
Treatment of dentin hypersensitivity with oxalates is common, but their efficacy remains unclear. Our objective was to systematically review clinical trials reporting an oxalate treatment compared to no treatment or placebo with a dentin hypersensitivity outcome. Risk-of-bias assessment and data extraction were performed independently by two reviewers. Standardized mean differences (SMD) were estimated using random-effects meta-analysis. Of 677 unique citations, 12 studies with high risk-of-bias were included. The summary SMD for 3% monohydrogen-monopotassium oxalate(n=8 studies) was −0.71 [95% Confidence Interval: −1.48, 0.06]. Other treatments, including 30% dipotassium oxalate (n=1), 30% dipotassium oxalate plus 3% monohydrogen monopotassium oxalate (n=3), 6% monohydrogen monopotassium oxalate (n=1), 6.8% ferric oxalate (n=1) and oxalate-containing resin (n=1), also were not statistically significant different from placebo treatments. With the possible exception of 3% monohydrogen monopotassium oxalate, available evidence currently does not support the recommendation of dentin hypersensitivity treatment with oxalates.
Keywords: dentin sensitivity, oxalates, systematic review, meta-analysis
INTRODUCTION
Dentin hypersensitivity (DH) is defined as brief, sharp pain elicited when dentin is exposed to thermal, tactile, osmotic, chemical, or evaporative stimuli (Canadian Advisory Board on Dentin Hypersensitivity, 2003). Most data to-date support a theory that these stimuli induce fluid flow within dental tubules, which triggers baroreceptors near the pulp, leading to pain (Pashley, 1994). This so-called hydrodynamic theory of pain generation assumes an exposed dentin surface and patent tubules that allow fluid flow to reach the pulp where the baroreceptors reside (Brannstrom et al., 1967). Up to 40 million American adults report DH symptoms each year (Addy, 1990), but reported prevalence rates range widely. Some research has placed the incidence as high as 74 percent, but in most populations, it appears to range between 10 and 30 percent, depending on the population studied, study setting, and study design (Rees and Addy, 2004).
A panoply of current diagnostic and treatment strategies for DH suggests considerable uncertainty among dental practitioners about how to manage this condition (Cunha-Cruz et al., 2010). The diagnosis of DH remains by exclusion of other dental and periodontal conditions that might cause pain (Holland et al., 1997), and no less than a dozen of methods are currently used for diagnosis (Cunha-Cruz et al., 2010). Most contemporary treatments seek to occlude the exposed dentin using restorative materials, laser treatment, resin-based sealants, or pharmacological agents. Among the latter group, oxalates have a particularly long history of use and acceptance by practitioners. For example, a recent survey of practicing dentists suggests that 40% use oxalates to treat DH (Cunha-Cruz et al., 2010).
Oxalates were introduced as agents to treat DH in the late 1970’s to mid 1980’s based on work done primarily in vitro. Several studies reported significant decreases in hydraulic conductance across dentin disks treated with oxalates, suggesting that oxalates limit fluid flow in exposed dentin in vivo, thereby reducing pain (Greenhill and Pashley, 1981; Pashley et al., 1978; Pashley et al., 1984; Pashley and Galloway, 1985). Subsequent work showed that oxalates formed precipitates within dentin tubules that blocked dentinal fluid flow (Cuenin et al., 1991). Oxalates reportedly have the added advantage of relative insolubility in acid, making them resistant to dissolution after treatment (Pereira et al., 2005).
In spite of the compelling in vitro work supporting the use of oxalates and relatively wide acceptance by practitioners, few controlled studies have shown their efficacy clinically, and a rigorous systematic evaluation of existing studies has not been reported. Yet, controlled data on the efficacy of oxalate treatment is essential to guide both dental practice and further research in the treatment of DH. Thus, our objective was to present a systematic review of controlled trials on humans with DH comparing an oxalate intervention to a placebo or no treatment group to reduce DH.
METHODS
Study Selection Criteria
Participants: Humans with dentin hypersensitivity. Post-restorative hypersensitivity studies were excluded.
Intervention: Oxalates
Comparison: Placebo or no treatment.
Outcomes: DH pain response to routine activities, thermal, tactile, evaporative, or electrical stimuli. Because of the heterogeneity of methods used to assess DH, no a priori outcome measure was required.
Studies: randomized controlled trial (RCT) or clinical controlled trial (CCT)
Search Methods to Identify Studies
After the development of a protocol, article citations were obtained through an electronic search of databases (to July 2009) and hand searching of bibliographic reference listings of published primary and review studies (for a complete list of databases, see Appendix 1). The MEDLINE and CENTRAL search strategy included the terms “dentin sensitivity”[MeSH Term] OR “dentin hypersensitivity”. Additional electronic searches were performed by two students using the terms “dentin hypersensitivity” OR “dentin sensitivity”. The Cochrane highly sensitive search strategy for identifying randomized trials (revision 2008) (Higgins and Green, 2009) was applied to restrict studies to clinical trials in MEDLINE; no language restrictions were employed. Reports identified through electronic searches of MEDLINE and CENTRAL were coded according to participants and interventions by two independent reviewers, and agreement was calculated using the Kappa statistic.
Study Description and Risk-of-bias Assessment
Two reviewers independently performed study description and risk-of-bias assessments; disagreements were resolved by discussion among the two reviewers and a third reviewer. Where needed, authors of studies were contacted for additional information to resolve ambiguities, and their responses were accepted until April 20, 2010. Risk-of-bias was assessed using the Cochrane Collaboration tool (Higgins and Green, 2009) (for a detailed description, see Appendix 2).
Synthesis of Results
Three reviewers performed data extraction. The number of participants, means and standard deviations were extracted from the reports (for a detailed description, see Appendix 2). Based on random effect models (DerSimonian and Laird, 1986), standardized weighted-mean differences (SMD), reported in units of standard deviation, were calculated for each oxalate treatment after calculating the SMD of all outcomes for each study. For split-mouth trials, the reviewers assumed a within-patient correlation coefficient equal to 0. Heterogeneity between studies, quantified using the I2-statistic (Higgins and Thompson, 2002), was considered high if statistical heterogeneity levels were higher than 70%. Data were analyzed using RevMan 4.2.7.
RESULTS
Study selection
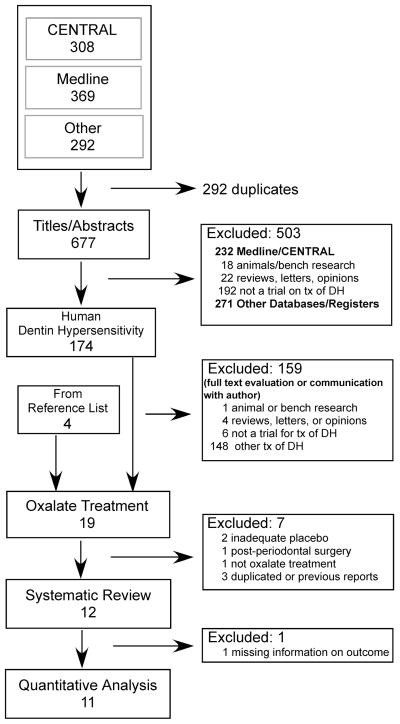
Electronic searches from all sources retrieved 677 unique citations (Fig. 1). Using titles and abstracts to screen content, 503 citations were excluded because they were not clinical studies of DH in humans, or were reviews or opinion papers. Agreement between reviewers was good (Kappa = 0.79). The remaining 174 citations were pared to 15 by evaluating full article content with information provided by correspondence with authors as needed. Four articles not previously found through electronic search were discovered in the references of citations. Of the 19 human clinical trials on oxalates, 4 did not meet the inclusion criteria and 3were previous reports of included studies (see Appendix 3 for list of excluded trials and reasons). The remaining 12 reports (Camps and Pashley, 2003; Cooley and Sandoval, 1989; Cuenin et al., 1991; Gillam et al., 1997; Gillam et al., 2004; Hansson, 1987; Holborow, 1994; Morris et al., 1999; Muzzin and Johnson, 1989; Pamir et al., 2007; Pereira et al., 2001; Pillon et al., 2004) were subjected to detailed analysis (Table 1).
Figure 1.
Flow-chart of the selection of studies for the systematic review of the effects of oxalates on dentin hypersensitivity.
Table 1.
Characteristics of the studies included in the systematic review of the effects of oxalates on dentin hypersensitivity
| First author, year of publication | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pamir, 2007 | Gillam, 2004 | Pillon, 2004 | Camps, 2003 | Pereira, 2001 | Morris, 1999 | Gillam, 1997 | Holborow, 1994 | Cuenin, 1991 | Cooley, 1989 | Muzzin, 1989 | Hansson, 1987 | |
| Design | RCT | RCT, split mouth | RCT, split mouth | RCT, split mouth | RCT, split mouth | RCT, split mouth | RCT, split mouth | RCT, split mouth | RCT | RCT, split mouth | RCT, split mouth | RCT |
| Follow-up duration | 4 wk | 4 wk | 3 wk | 2–3 min | 1 y | 3 mon | 3 mon | 4 wk | Immediate | 3 mon | 4 wk | 4 wk |
| Country | Turkey | England (UK) | Brazil | France | Brazil | United States | UK | New Zealand | United States | United States | United States | United States |
| Setting | University | University | University | University | University | Military | University | NR | Military | University | University | University |
| Funding | Manufacturers (products only) | Block Drug Co., Inc. (now GlaxoSmithKline) | None | University | CAPES | NR | None | J.O. Butler Co. | US Military, NIH | NR | Baylor University | NR |
| N of participants by group | 15 / 15 | 13 | 15 | 87 | 19 | 10 | 10 | 30 | 6 / 7 | 28 | 17 | 14 / 14 |
| N of teeth by group | 15 / 15 | 13 / 13 | 15 / 15 | 87 / 87 | 23 / 21 / 24 / 14 | 10 / 10 | 11 / 33 | 30 / 30 | 6 / 7 | 28 / 28 | 17 / 17 / 17 / 17 | mean 6 / 9.8 per patient |
| Age | 18–57 | Mean 46.2 (SD 4.2) | NR | Mean 32 (SD 11), 17–52 | 21–45 | Mean 32.9, 21–43 | Mean 45.1 (SD 8.8) | NR | Mean 45, 29–68 | NR | Mean 40.5, 23–68 | Mean 48, 23–61 Mean 51, 30–63 |
| Gender (% female) | 70 | 62 | NR | 72 | 58 | 50 | 60 | NR | NR | NR | 59 | 71 /79 |
| Experimental Intervention | *3% monohydrogen-monopotassium oxalate gel, pH 2 | *6.8% ferric oxalate in 0.9% saline | *3% monohydrogen-monopotassium oxalate gel, pH 4 | *3% monohydrogen-monopotassium oxalate gel, pH 2 | *3%, 6% monohydrogen-monopotassium oxalate gel, pH 4 + carboxymethyl cellulose *3% monohydrogen-monopotassium oxalate gel, pH 2.5 + carbopol |
*Oxalate-containing pre-polymerized resin solution | *3% monohydrogen-monopotassium oxalate | *3% monohydrogen-monopotassium oxalate pH 2 | *3% monohydrogen-monopotassium oxalate pH 2.4 | *30% dipotassium oxalate pH 5.6 + 3% monohydrogen-monopotassium oxalate | *distilled water, then 30% dipotassium oxalate pH 5.6 *distilled water, then 3% monohydrogen-monopotassium oxalate pH 2 *30% dipotassium oxalate pH 5.6, then 3% monohydrogen-monopotassium oxalate pH 2 |
*30% dipotassium oxalate pH 5.6, then 3% monohydrogen-monopotassium oxalate pH 2 |
| Control Intervention | placebo (distilled water) | placebo (undisclosed) | placebo (100 mg carbopol, 30 mg 1% green tincture, 30 mg blue tincture | placebo (4% glucose solution) | placebo (water + carboxymethyl cellulose | placebo (distilled water) | No treatment | placebo (water + non-staining dye) | placebo (3% sodium chloride, pH 2.4) | placebo (distilled water) | placebo (2 applications of distilled water | placebo (2 applications of distilled water) |
| Pain stimuli | Thermal, Evaporative | Thermal, Tactile, Evaporative | Routine activities of eating, drinking, and tooth cleaning | Tactile, Evaporative | Tactile, Evaporative | Tactile, Evaporative | Thermal, Tactile, Evaporative | Thermal, Tactile | Evaporative | Thermal | Thermal | Thermal, Routine Stimuli |
| Outcomes | *Pain visual analog scale (cm) | *Temperature eliciting pain (°C) *Force eliciting pain (g) *Pain visual analog scale (cm) |
*Pain visual analog scale | *Force eliciting pain (cN) *Pain (+/−) |
*Pain verbal rating scale | *Pain visual analog scale | *Temperature eliciting pain (°C) *Force eliciting pain (g) *Pain visual analog scale |
*Pain scale | *Pain numeric rating scale | *Pain verbal rating scale | *Temperature eliciting pain (°C) | *Temperature eliciting pain (°C) *Pain visual analog scale *Pain verbal description scale *Pain numerical rating scale *McGill pain questionnaire |
| Adverse Events | None | None | None | None | None | NR | None | None | None | NR | None | |
RCT: Randomized clinical trial, SD: standard deviation, NR: Not reported
Study description
Studies included in the systematic review were diverse (Table 1), with five conducted in the United States, two each in Brazil and the United Kingdom, and one each in Turkey, France, and New Zealand. Nine of the studies were conducted in university settings, two at military installations, and one study did not report a location. Four of the studies were funded by university or government grants, three by product manufacturers, two were without external funding, and three did not report a funding source. All studies were full reports published in English between 1987 and 2007, except one that was a short communication (Holborow, 1994).
Nine of the 12 studies were split mouth trials that took various approaches to assessing oxalate efficacy (Table 1). Eight of the 12 studies evaluated some form of monohydrogen-monopotassium oxalate. Other studies included ferric oxalate, di-potassium oxalate, or oxalate-containing pre-polymerized resin; two of the studies used combinations of monohydrogen-monopotassium and di-potassium oxalate. The placebo groups were diverse as well. Several studies used distilled water, some with dyes for blinding purposes. Two studies used the thickeners carboxymethyl cellulose or carbopol for blinding; one of these used a dye. Other studies used 4% glucose or 3% sodium chloride as placebo solutions. One study used no treatment and one did not disclose the placebo procedure. Even more diverse were the follow-up intervals, which ranged from immediate to 1 year; 4 weeks was the most common follow-up time (5 studies).
DH pain was elicited by tactile, evaporative, or thermal stimuli (11 of 12 studies), although 2 studies used reports of pain elicited by routine activities (Table 1). Eight of the 12 studies used more than one stimulator to elicit pain. DH was commonly quantified using a pain scale, with either verbal or numeric descriptors; 10 of 12 studies used at least this method to quantify DH. Other outcome measures included the force of tactile pressure or temperature of applied liquid which elicited pain. Adverse events during the studies were not observed; 1 study did not report information about adverse events and author follow-up was not successful.
Risk-of-bias assessment
Most studies reported use of random assignment of interventions. However, close scrutiny and author follow-up revealed that only 4 of the 12 studies had followed procedures to assure random sequence generation and concealed allocation strategy (Table 2). Four studies did not report sufficient information to assess these factors. Similarly, only 4 of the 12 studies took measures to assure blinding of participants, care providers, and assessors. Most studies reported blinding participants (10 of 12). The information published or provided via author communication was not sufficient to determine blinding in 2 studies.
Table 2.
Assessment of risk-of-bias of the studies included in the systematic review of the effects of oxalates on dentin hypersensitivity
| First author, Year of Publication | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Domain |
Pamir 2007 |
Gillam 2004 |
Pillon 2004 |
Camps 2003 |
Pereira 2001 |
Morris 1999 |
Gillam 1997 |
Holborow 1994 |
Cuenin 1991 |
Cooley 1989 |
Muzzin 1989 |
Hansson 1987 |
|
| Sequence generation | Assignment really random | Yes | Yes | Yes | No | No | Unclear | Unclear | Unclear | No | Unclear | Yes | No |
|
| |||||||||||||
| Allocation concealment | Allocation concealed | Yes | Yes | Yes | No | No | Unclear | No | No | Yes | No | Yes | No |
|
| |||||||||||||
| Blinding of participants, care providers and outcome assessors | Patient blinded | Yes | Yes | Yes | Yes | Yes | Unclear | No | Yes | Yes | Yes | Yes | Yes |
| Care provider blinded | No | Yes | Yes | No | No | No | No | Unclear | Yes | No | Yes | Yes | |
| Outcome assessor blinded | Yes | Yes | Yes | Yes | No | No | Yes | Unclear | Yes | No | Yes | Yes | |
|
| |||||||||||||
| Incomplete outcome data | Point estimate and measure of variability presented | Yes | Yes | Yes | Yes | No | Yes | No | No | Yes | No | Yes | Yes |
| Intention to treat analysis | NA | Unclear | NA | NA | No | Unclear | NA | Unclear | NA | Unclear | NA | NA | |
|
| |||||||||||||
| Selective outcome reporting | Free of selective outcome reporting | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Unclear | Yes | Yes | Yes |
|
| |||||||||||||
| Other sources of bias | Eligibility criteria specified | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Groups similar at baseline | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | |
| Split mouth (cross-over design) appropriate | NA | Unclear | Yes | No | Yes | No | No | No | NA | Yes | Yes | NA | |
NA: Not applicable
Other factors increased the risk-of-bias. Four studies did not report point estimates, number of observations, or variability measures (Table 2). One study had a risk-of-bias via selective reporting and in another one selective reporting was unclear. One study did not specify eligibility criteria; another reported a non-equivalence of treatment and placebo groups at baseline. Of the 9 split mouth designs, five studies tried to limit cross-over effects by enrolling teeth from different areas of the mouth or isolating teeth with cotton rolls or rubber dam.
Synthesis of results
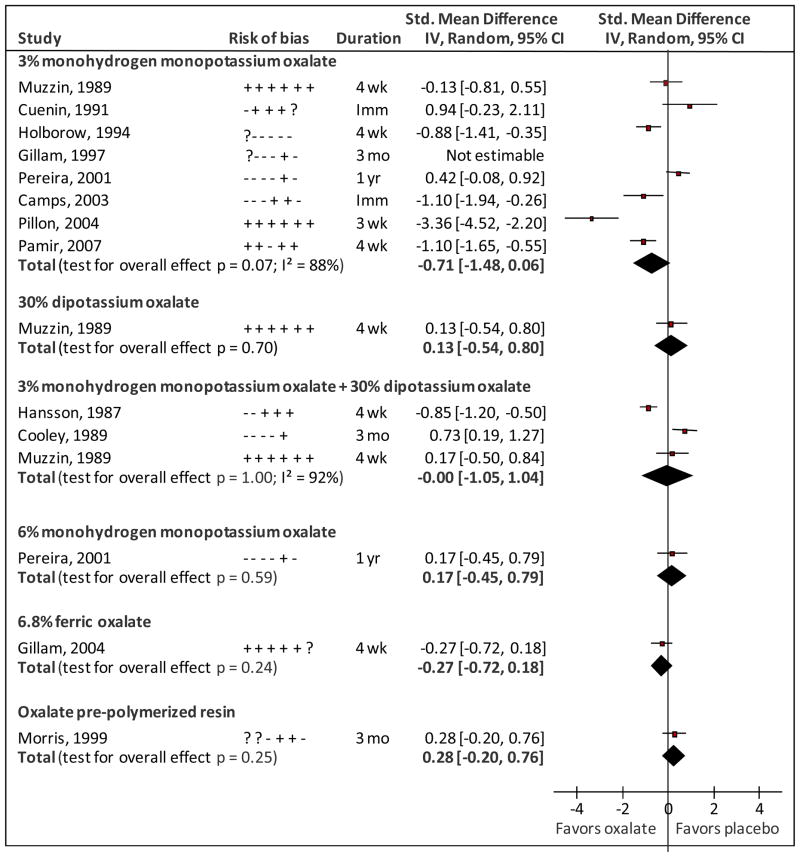
Treatment with 3% monohydrogen-monopotassium oxalate resulted in less DH as measured by thermal stimuli, tactile stimuli, and routine activities; the most favorable results were obtained when using routine activities as a measure. For other outcomes, interventions with 3% monohydrogen-monopotassium oxalate produced no change or made DH worse (for results by outcome, see Appendix 2). Data were not available for three outcomes of one study (Gillam et al., 1997), and one outcome of another study (Holborow, 1994). Based on 187 and 179 units (patients or teeth) in the intervention and placebo groups from seven studies the summary SMD for 3% monohydrogen-monopotassium oxalate was −0.71 [95% Confidence Interval (CI): −1.48, 0.06] with high statistical heterogeneity (88%) (Fig. 2).
Figure 2.
Forest plot of standardized mean differences between oxalate and placebo treatments for dentin hypersensitivity. Risk-of-bias indicate criteria met (+), not met (−) or unclear (?) in sequence generation; allocation concealment; blinding of participants, care providers and outcome assessors; completeness of outcome data; selective outcome reporting; and cross-over effects (for split mouth trials only). Duration Imm, wk, mo, yr indicate Immediate assessment, weeks, months and years, respectively.
A combination of 3% monohydrogen monopotassium oxalate and 30% dipotassium oxalate was the second most common intervention, and three studies evaluated its efficacy (Fig. 2). When daily activities were used as an outcome, this intervention reduced DH. However, when thermal stimuli were used, the DH was either similar or higher than the placebo group (Appendix 2). Based on 59 units in the intervention and placebo groups from three studies, the summary SMD for 30% dipotassium oxalate followed by 3% monohydrogen-monopotassium oxalate was 0.00 (95%CI: −1.05, 1.04) with high statistical heterogeneity (92%) (Fig. 2).
Other interventions were evaluated only by single studies (Fig. 2). SMD ranged from −0.28 to +0.17, but results were not statistically significant.
DISCUSSION
This systematic review suggests no benefit from treating dentin hypersensitivity with oxalates beyond a placebo effect. Dentin hypersensitivity has an intermittent nature, and strong placebo effects have been observed which makes definitive demonstration of clinical efficacy of any treatment difficult (Pashley et al., 2008). Yet, the data suggested that 3% monohydrogen-monopotassium oxalate may have some beneficial effect; this treatment appears to be a rationale first line of oxalate treatment. Taken together, the current data cannot dissect if a lack of effect was from a truly ineffective treatment or study design limitations.
A combination of 3% monohydrogen-monopotassium oxalate and 30% dipotassium oxalate was the second-most common DH treatment assessed by clinical studies, yet its efficacy relied heavily on the outcome measure examined. This lack of significant effect was curious considering the more favorable results observed for 3% monohydrogen-monopotassium oxalate contained in the treatment regimen, and the lack of effect observed for 30% dipotassium oxalate alone. This paradox suggests some sort of interference or competition between these treatments, or important and yet unidentified factors in how the two treatments are applied. At present, there appears little motivation to use this more complex treatment regimen clinically.
The studies included in this systematic review had several design limitations. With the exception of 3% monohydrogen-monopotassium oxalate, most of the treatments were evaluated by only one study. Additionally, most studies involved small sample sizes (range 6–87 units). Further, the extent to which studies employed strict randomization, concealment, and blinding procedures varied considerably. For split-mouth trials, we considered use of isolation during product placement as a reasonable strategy to avoid cross-over effects. However, patient response to pain on different teeth is not necessarily independent, and the outcome assessment may be confounded by pain response in adjoining or distant teeth.
Lack of standardization when measuring pain and poor diagnostic criteria are difficulties facing research in the area of DH; (Markowitz and Pashley, 2008). Studies in this review used different stimuli to elicit pain and treatments decreased DH pain for some stimuli, but not others (see Appendix 2). In addition, numerical and verbal rating scales, 10-cm visual analog scales, and pain threshold tasks were used to measure pain intensity. Utility of pain scales depends on patients understanding the correct way to use the scales, and can be impacted by use of instruction sets (Dixon et al., 2004). In all, the effectiveness of DH treatments remains unclear because of diverse and sometimes lax methods that have been employed to assess efficacy to this point in time.
Taking the lack of standardized measures into account, we completed a meta-analysis using the standardized mean difference (SMD) across studies evaluating the same interventions. Whereas this method of analysis provides a clear way of assessing one overall outcome per study, the statistical heterogeneity across studies suggests that clinical and design differences among the studies were present and summary estimates may not have been presented. For instance, number of outcomes assessed, follow-up length, and criteria met on the risk of bias assessment differed among the studies. In addition, due to poor reporting of the results in the included studies assumptions were made to extract the data. Finally, no adjustment was made for within-study correlation. How liberal or conservative the overall analysis is depends on if this correlation is negative or positive, respectively, but we could not reliably estimate or find such a correlation published in other clinical trials of DH.
In conclusion, available evidence suggests that oxalates are not effective in decreasing DH when compared to placebo; with a possible exception for 3% monohydrogen-monopotassium oxalate. These data should be considered when treating DH until further evidence is available. The great variability observed across clinical trials illuminated the need for strict study protocols including appropriate randomization and allocation concealment. Standardized use of pain stimuli and scales, both across and within studies, also would aid meta-analysis.
Acknowledgments
We would like to thank the authors of the studies included in this systematic review who answered our requests for additional information, Dr. Lei Li for translation of an article in Chinese, and Dr. D.J. Olson for helping with the electronic searches.
This work was supported by the National Institute of Dental and Craniofacial Research [grant numbers DE016750, DE016752, DE018436, and DE019202].
Appendix 1. Details on search methods to identify studies
The MEDLINE search strategy (searched on 03/25/2009) was:
| Queries | Result |
|---|---|
| #3 Search (#1) AND (#2) | 369 |
| #2 Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] not humans [mh]) | 596752 |
| #1 Search “dentin sensitivity”[MeSH Terms] OR “dentin hypersensitivity” | 1632 |
Additional search strategy on MEDLINE including other terms for dentin hypersensitivity (searched on 10/05/2010) did not retrieve any additional study meeting the inclusion criteria.
| Queries | Result |
|---|---|
| #4 Search (((#1) AND #2) AND #3) AND “1”[Entrez Date] : “2009/03/25”[Entrez Date] | 19 |
| #3 Search (“Oxalic Acids”[Mesh] OR oxalate[tiab] OR oxalates[tiab]) | 14430 |
| #2 Search “dentin sensitivity”[MeSH] OR ((teeth[tiab] OR tooth[tiab] OR dentin[tiab] OR dentine[tiab] OR dentinal[tiab]) AND (sensitive[tiab] OR sensitivity[tiab] OR hypersensitive OR hypersensitivity[tiab])) | 4332 |
| #1 Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized 4 [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] not humans [mh]) | 65971 |
List of websites searched for the systematic review of the oxalate treatment for dentin hypersensitivity (source: Higgins, 2009). Searches were performed through July 2009, unless otherwise stated.
| MEDLINE (from 1966 through 03/25/2009) | University hospital Medical Information Network (UMIN) Clinical Trials Registry (for Japan) – UMIN CTR |
| Cochrane CENTRAL1 (from 1948 through 03/25/2009) | |
| Cochrane Database of Systematic Reviews (CDSR) (through 03/25/2009) | AstraZeneca Clinical Trials web site |
| Database of Abstracts of Reviews of Effects (DARE) (through 03/25/2009) | Bristol-Myers Squibb Clinical Trial Registry |
| Eli Lilly and Company Clinical Trial Registry | |
| Health Technology Assessment (HTA) (through 03/25/2009) | GlaxoSmithKline clinical trial register |
| African Index Medicus | |
| LILACS | Australasian Medical Index |
| Index Medicus for the South-East Asia Region (IMSEAR) | World Health Organization, Regional Office for the Eastern Mediterranean |
| Ukraine and the Russian Federation Panteleimon | The Institute for Scientific and Technical Information (Institut de l’Information Scientifique et Technique) of the French National Center for Scientific Research |
| Western Pacific Western Pacific Region Index Medicus (WPRIM) | |
| Turning Research into Practice (TRIP) database | IndMED |
| ProQuest Dissertation & Theses Database | KoreaMed |
| National Technical Information Service (NTIS) | OpenSIGLE |
| Conference abstracts Biosis.org | NHS Evidence - National Library of Guidelines |
| Australian National Health and Medical Research Council Clinical Practice Guidelines | New Zealand Guidelines Group |
| Canadian Medical Association – Infobase Clinical Practice Guidelines | NICE; National Institute for Health and Clinical Excellence (UK) |
| National Guideline Clearinghouse (US) | Pharmaceutical Industry Clinical Trials Database (ABPI/CMR) |
| Hong Kong clinical trials register - HKClinicalTrials.com | The Australian New Zealand Clinical Trials Registry |
| CenterWatch | |
| Indian clinical trials registry - Clinical Trials Registry-India (CTRI) | CenterWatch |
| ClinicalTrials.gov | |
| International Clinical Trials Registry Platform Search Portal | CORDIS: Community Research and Development Information Service |
| International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) Clinical Trials Portal | Current Controlled Trials |
| Platform Search Portal | |
| International Standard Randomised Controlled Trial Number Register | European Medicines Agency |
| Netherlands trial register | World Health Organization (WHO) International Clinical Trials Registry |
| South African National Clinical Trial Register | GlaxoSmithKline (GSK) Clinical Study Register |
| UK Clinical Research Network Portfolio Database | Novartis Clinical Trials |
| UK Clinical Trials Gateway | Roche trials database |
| UK National Research Register (NRR) | Wyeth (formerly Pfizer) clinical trials registry |
| Clinical study results for U.S.-marketed pharmaceuticals | |
| Bristol-Myers Squibb clinical trials database |
CENTRAL includes results of systematic searches of clinical trials from several databases such as Medline and EMBASE, as well as from handsearching journals and conference proceedings.
Appendix 2. Details on the methods of the systematic review on oxalate treatments for dentin hypersensitivity
Study Selection Criteria
Clinical controlled trials are defined as trials that definitely or possibly assigned participants to one of two (or more) alternative forms of intervention using either random allocation or some quasi-random method of allocation (such as alternation, date of birth or medical record number) (Worthington et al., 2009).
Risk-of-bias
The domains of the Cochrane Collaboration tool to assess risk-of-bias (Higgins and Green, 2009) include:
sequence generation (identified method used to generate the random allocation sequence);
allocation concealment (identified method used to conceal the allocation sequence before allocation);
blinding of participants, care providers and outcome assessors (employed measures to blind participants and personnel from knowledge of which intervention a participant received);
incomplete outcome data (provided number of observations, point estimates and measures of variability for the outcome measures and adequately addressed any incomplete outcome data, e.g. by intention-to-treat or sensitivity analyses);
selective outcome reporting (reported study’s pre-specified primary and secondary outcomes of interest); and
other sources of bias (e.g., extreme baseline imbalance, study stopped early, study claimed to be fraudulent, or design-specific bias). Design-specific bias was assessed for split-mouth trials and included the potential for carry-over effects.
Reviewers judged the studies for each item assigning a “Yes” or “No” if the criterion was met or not (low and high risk-of-bias) or “Unclear” if there were insufficient information to permit a judgment of ‘Yes’ or ‘No’.
Data Extraction and Analysis
Number of observations. mean and standard deviations of each outcome were extracted for the intervention and placebo groups. When the means and standard deviations were not reported, this information was solicited to the authors and/or estimated based on the reported data. Final results were preferred, but change from baseline was also included in the data analysis. Data was calculated for each study as follows:
Pamir, 2007: data was obtained from the author.
Gillam, 2004: standard deviations were calculated from 95% confidence intervals.
Pillon, 2004: standard deviations for the change from baseline were calculated from the 95% confidence intervals extracted from a figure.
Camps, 2003: standard deviations of the percentages were estimated based on an independent sample. Data for one outcome not reported or possible to estimate.
Pereira, 2001: the numbers of observations for each treatment group at one year were estimated by assuming a 1-yr attrition rate as the same as the 6-month attrition rate published in a thesis (Martineli, 1999).
Cooley, 1989: standard deviations for each treatment group were assumed to be equal to the standard deviation of the mean difference between treatments. The latter was calculated assuming a p-value equal to 0.01 and independent samples.
Hansson, 1987: the different pain scales used in response to the same stimulus were combined in a single measure for that stimulus.
After obtaining the number of observations, means and standard deviations for each pain stimulus outcome, a weighted standardized mean difference (SMD) was calculated for each study summarizing the different stimuli into a single study measure. After calculating a summary SMD for each study using all outcomes from that study, an overall SMD was calculated for each oxalate treatment. For split-mouth trials, we assumed a within-patient correlation coefficient equal to 0 because this coefficient was unreported by any study, and attempts to estimate it using reported data failed to calculate a reliable estimate.
The standardized weighted-mean differences were calculated based on random effect models (DerSimonian and Laird, 1986). The SMD estimated the size of the intervention effect in each study relative to the variability observed in that study (Higgins and Green, 2009). This measure of association was selected because studies used a variety of stimuli and scales to assess DH outcomes. The summary estimate was reported in units of standard deviation. In other words, the SMD is the number of SD by which the oxalate treatment changes the outcome and can be re-expressed in the original scale of a particular instrument. As an example, let us consider the standard deviation of 2.7 for the pain elicited by the thermal stimulus and measured with a 10 cm VAS scale for the control group at baseline in one study (Pamir, 2007). A SMD of 0.7 (95%CI=−1.5, 0.06) would be equivalent to a decrease of 1.9 points on the VAS pain scale, ranging from −4 to +0.2. However, these back-transformations should be interpreted with caution because they could yield different sizes of effect for the same scale and the same effect depending on the different standard deviations used (Higgins and Green, 2009).
Appendix table presents the results by outcomes. Because of the small number of studies available for each oxalate treatment, the meta-analysis summary estimate should be interpreted with caution. In addition, influence analysis, publication bias assessment and meta-regression could not be performed.
Appendix table.
Results of clinical studies on dentin hypersensitivity by stimuli and oxalate treatments.
| Experimental | Placebo | Standardized mean difference (95%CI) | |||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
|
3% monohydrogen monopotassium oxalate
| |||||||
| Pain elicited by routine stimuli | |||||||
| Gillam, 1997 | - | - | - | ||||
| Pillon, 2004 | 15 | −81.00 | 14.82 | 15 | −34.70 | 11.86 | −3.36 (−4.52; −2.20) |
| Pain elicited by thermal stimulus | −1.00 (−1.44; −0.56) | ||||||
| Gillam, 1997 | |||||||
| Holborow, 1994 | 30 | 1.00 | 1.00 | 30 | 2.27 | 1.75 | −0.88 (−1.41; −0.35) |
| Pamir, 2007 | 15 | 1.60 | 1.84 | 15 | 4.60 | 2.69 | −1.27 (−2.06; −0.47) |
| Pain elicited by tactile stimulus | 0.88 (0.21; 1.54) | ||||||
| Gillam, 1997 | - | - | - | ||||
| Holborow, 1994 | - | - | - | ||||
| Pereira, 2001 | 17 | 1.46 | 1.18 | 7 | 0.66 | 0.86 | 0.70 (−0.21; 1.61) |
| Pereira, 2001 | 14 | 1.38 | 0.50 | 7 | 0.66 | 0.86 | 1.09 (0.11; 2.06) |
| Pain elicited by evaporative stimulus | −0.27 (−0.81; 0.28) | ||||||
| Camps, 2003 | 87 | 0.38 | 0.49 | 87 | 0.70 | 0.46 | −0.68 (−0.98; −0.37) |
| Cuenin, 1991 | 6 | 1.50 | 1.05 | 7 | 0.57 | 0.79 | 0.94 (−0.23; 2.12) |
| Pamir, 2007 | 15 | 1.40 | 1.92 | 15 | 3.33 | 2.06 | −0.94 (−1.70; −0.18) |
| Pereira, 2001 | 15 | 0.53 | 0.64 | 8 | 0.55 | 1.13 | −0.02 (−0.88; 0.84) |
| Pereira, 2001 | 13 | 0.61 | 0.65 | 8 | 0.55 | 1.13 | 0.07 (−0.81; 0.95) |
| Temperature at which patient report pain (thermal stimulus) | |||||||
| Muzzin, 1989 | 17 | 3.35 | 2.35 | 17 | 3.76 | 3.59 | −0.13 (−0.81; 0.54) |
| Force at which patient report pain (tactile stimulus) | |||||||
| Camps, 2003 | 87 | −85.00 | 24.00 | 87 | −53.00 | 17.00 | −1.53 (−1.87; −1.19) |
|
| |||||||
|
3% monohydrogen monopotassium oxalate + 30% dipotassium oxalate
| |||||||
| Pain elicited by routine activities | |||||||
| Hansson, 1987 | 14 | 11.49 | 9.63 | 14 | 18.60 | 7.69 | −0.79 (−1.57; −0.02) |
| Pain elicited by thermal stimulus | |||||||
| Cooley, 1989 | 28 | 2.70 | 0.81 | 28 | 2.10 | 0.81 | 0.73 (0.19; 1.27) |
| Temperature at which patient report pain (thermal stimulus) | −0.50 (−1.84; 0.85) | ||||||
| Hansson, 1987 | 14 | 2.34 | 1.07 | 14 | 3.50 | 0.78 | −1.20 (−2.02; −0.39) |
| Muzzin, 1989 | 17 | 4.29 | 2.33 | 17 | 3.76 | 3.59 | 0.17 (−0.50; 0.84) |
|
| |||||||
|
6% monohydrogen monopotassium oxalate
| |||||||
| Pain elicited by tactile stimulus | |||||||
| Pereira, 2001 | 15 | 1.06 | 0.79 | 7 | 0.66 | 0.86 | 0.47 (−0.44; 1.38) |
| Pain elicited by evaporative stimulus | |||||||
| Pereira, 2001 | 16 | 0.46 | 0.74 | 8 | 0.55 | 1.13 | −0.10 (−0.95; 0.75) |
|
| |||||||
|
30% dipotassium oxalate
| |||||||
| Temperature at which patient report pain (thermal stimulus) | |||||||
| Muzzin, 1989 | 17 | 4.24 | 3.59 | 17 | 3.76 | 3.59 | 0.13 (−0.54; 0.80) |
|
| |||||||
|
6.8% ferric oxalate
| |||||||
| Pain elicited by evaporative stimulus | |||||||
| Gillam, 2004 | 13 | 5.04 | 3.04 | 13 | 5.65 | 2.89 | −0.20 (−0.97; 0.57) |
| Temperature at which patient report pain (thermal stimulus) | |||||||
| Gillam, 2004 | 13 | 7.41 | 7.67 | 13 | 10.55 | 3.60 | −0.51 (−1.29; 0.28) |
| Force at which patient report pain (tactile stimulus) 13 | |||||||
| Gillam, 2004 | 13 | −17.25 | 20.79 | 13 | −15.42 | 10.49 | −0.11 (−0.88; 0.66) |
|
| |||||||
|
Oxalate pre-polymarized resin
| |||||||
| Pain elicited by tactile stimulus | |||||||
| Morris, 1999 | 18 | 19.60 | 30.40 | 16 | 16.80 | 22.60 | 0.10 (−0.57; 0.78) |
| Pain elicited by evaporative stimulus | |||||||
| Morris, 1999 | 18 | 28.30 | 32.20 | 16 | 15.10 | 21.30 | 0.47 (−0.22; 1.15) |
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] In: Higgins JPT, Green S, editors. The Cochrane Collaboration. 2009. [Google Scholar]
- Martineli ACBF. Avaliação da hiperestesia dentinária após tratamento com diferentes formulações à base de oxalato de potássio utilizando-se um placebo como controle. São Paulo: Universidade de São Paulo; 1999. [Google Scholar]
- Worthington HV, Clarkson JE, Coulthard P, Esposito M, Fernandez-Mauleffinch LM, Floate R, et al. Cochrane Oral Health Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 4. 2009. Art. No.: ORAL. [Google Scholar]
Appendix 3. List of studies excluded after further evaluation and reasons for exclusion
| Excluded studies | Reason for exclusion |
| Brough KM, Johnson, R (1988). Effects of Potassium Oxalate on Dentin Hypersensitivity in vivo [Abstract 1075]. J Dent Res 67(Spec Iss): 247. | Other report of included study (Muzzin, 1989) |
| Martineli ACBF, Pereira JC (2000). Treating dentin hypersensitivity with potasssium oxalate: a one-year evaluation [Abstract 1740]. J Dent Res 80 (Spec Iss): 361. | Other report of included study (Pereira, 2001) |
| Merika K, HeftitArthur F, Preshaw PM (2006). Comparison of two topical treatments for dentine sensitivity. Eur J Prosthodont Restor Dent 14(1):38–41. | No placebo or no treatment comparison group |
| Santos RL, Gusmão ES, Jovino-Silveira RC, Tenório SB, Barbosa RFS (2006). Avaliação clínica de dessensibilizantes obliteradores após raspagem periodontal / Clinical avaluation of obliterative desensitizing after periodontal scaling. Rev. Bras. Ciênc. Saúde;10(2):123–132. | No placebo or no treatment comparison group |
| Smith BA, Hansson RE, Caffesse RG, Bye FL (1988). Evaluation of dipotassium oxalate in the treatment of root hypersensitivity [Abstract 1733]. J Dent Res 67:329. | Other report of included study (Hansson, 1987) |
| Stewardson DA, Crisp RJ, McHugh S, Lendenmann U, Burke FJ (2004). The Effectiveness of Systemp.desensitizer in the treatment of dentine hypersensitivity. Prim Dent Care 11(3):71–6. | No oxalate treatment |
| Wang HL, Yeh CT, Smith F, Burgett FG, Richards P, Shyr Y, et al. (1993). Evaluation of ferric oxalate as an agent for use during surgery to prevent post-operative root hypersensitivity. J Periodontol 64(11):1040–4. | Post-periodontal surgery patients |
References
- Addy M. Etiology and clinical implications of dentine hypersensitivity. Dent Clin North Am. 1990;34(3):503–14. [PubMed] [Google Scholar]
- Brannstrom M, Linden LA, Astrom A. The hydrodynamics of the dental tubule and of pulp fluid. A discussion of its significance in relation to dentinal sensitivity. Caries Res. 1967;1(4):310–7. doi: 10.1159/000259530. [DOI] [PubMed] [Google Scholar]
- Camps J, Pashley D. In vivo sensitivity of human root dentin to air blast and scratching. J Periodontol. 2003;74(11):1589–94. doi: 10.1902/jop.2003.74.11.1589. [DOI] [PubMed] [Google Scholar]
- Canadian Advisory Board on Dentin Hypersensitivity . Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69(4):221–6. [PubMed] [Google Scholar]
- Cooley RL, Sandoval VA. Effectiveness of potassium oxalate treatment on dentin hypersensitivity. Gen Dent. 1989;37(4):330–3. [PubMed] [Google Scholar]
- Cuenin MF, Scheidt MJ, O’Neal RB, Strong SL, Pashley DH, Horner JA, et al. An in vivo study of dentin sensitivity: the relation of dentin sensitivity and the patency of dentin tubules. J Periodontol. 1991;62(11):668–73. doi: 10.1902/jop.1991.62.11.668. [DOI] [PubMed] [Google Scholar]
- Cunha-Cruz J, Wataha J, Zhou L, Manning W, Trantow M, Bettendorf MM, et al. Dentin hypersensitivity: Choice of treatments by dentists of the Northwest PRECEDENT network. J Am Dent Assoc. 2010 doi: 10.14219/jada.archive.2010.0340. Accepted( [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: a path analytic description. Pain. 2004;112(1–2):188–96. doi: 10.1016/j.pain.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Gillam DG, Coventry JF, Manning RH, Newman HN, Bulman JS. Comparison of two desensitizing agents for the treatment of cervical dentine sensitivity. Endod Dent Traumatol. 1997;13(1):36–9. doi: 10.1111/j.1600-9657.1997.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Gillam DG, Newman HN, Davies EH, Bulman JS, Troullos ES, Curro FA. Clinical evaluation of ferric oxalate in relieving dentine hypersensitivity. J Oral Rehabil. 2004;31(3):245–50. doi: 10.1046/j.0305-182X.2003.01230.x. [DOI] [PubMed] [Google Scholar]
- Greenhill JD, Pashley DH. The effects of desensitizing agents on the hydraulic conductance of human dentin in vitro. J Dent Res. 1981;60(3):686–98. doi: 10.1177/00220345810600030401. [DOI] [PubMed] [Google Scholar]
- Hansson RE. The assessment of the subjective nature of pain associated with cervical root dentin hypersensitivity and the evaluation of the effectiveness of dipotassium oxalate in the reduction of cervical root dentin hypersensitivity. Ann Arbor: University of Michigan; 1987. [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] In: Higgins JPT, Green S, editors. The Cochrane Collaboration. 2009. [Google Scholar]
- Holborow DW. A clinical trial of a potassium oxalate system in the treatment of sensitive root surfaces. Arch Oral Biol. 1994;39:S134. [Google Scholar]
- Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24(11):808–13. doi: 10.1111/j.1600-051x.1997.tb01194.x. [DOI] [PubMed] [Google Scholar]
- Markowitz K, Pashley DH. Discovering new treatments for sensitive teeth: the long path from biology to therapy. J Oral Rehabil. 2008;35(4):300–15. doi: 10.1111/j.1365-2842.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- Morris MF, Davis RD, Richardson BW. Clinical efficacy of two dentin desensitizing agents. Am J Dent. 1999;12(2):72–6. [PubMed] [Google Scholar]
- Muzzin KB, Johnson R. Effects of potassium oxalate on dentin hypersensitivity in vivo. J Periodontol. 1989;60(3):151–8. doi: 10.1902/jop.1989.60.3.151. [DOI] [PubMed] [Google Scholar]
- Pamir T, Dalgar H, Onal B. Clinical evaluation of three desensitizing agents in relieving dentin hypersensitivity. Oper Dent. 2007;32(6):544–8. doi: 10.2341/07-5. [DOI] [PubMed] [Google Scholar]
- Pashley D, Tay FR, Haywood VB, Collins MA, Drisko CL. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. Comp Contin Edu Dent. 2008;29(8 Spec Iss):1–35. [Google Scholar]
- Pashley DH, Livingston MJ, Reeder OW, Horner J. Effects of the degree of tubule occlusion on the permeability of human dentine in vitro. Arch Oral Biol. 1978;23(12):1127–33. doi: 10.1016/0003-9969(78)90119-x. [DOI] [PubMed] [Google Scholar]
- Pashley DH, O’Meara JA, Kepler EE, Galloway SE, Thompson SM, Stewart FP. Dentin permeability. Effects of desensitizing dentifrices in vitro. J Periodontol. 1984;55(9):522–5. doi: 10.1902/jop.1984.55.9.522. [DOI] [PubMed] [Google Scholar]
- Pashley DH, Galloway SE. The effects of oxalate treatment on the smear layer of ground surfaces of human dentine. Arch Oral Biol. 1985;30(10):731–7. doi: 10.1016/0003-9969(85)90185-2. [DOI] [PubMed] [Google Scholar]
- Pashley DH. Dentine permeability and its role in the pathobiology of dentine sensitivity. Arch Oral Biol. 1994;39(Suppl):73S–80S. doi: 10.1016/0003-9969(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Pereira JC, Martineli ACBF, Santiago SL. Tratamento da hipersensibilidade dentinária com três diferentes formulações à base de oxalato de potássio: estudo clínico / Treating hypersensitive dentin with three different potassium oxalate-based gel formulations: a clinical study. Rev Fac Odontol Bauru. 2001;9(3/4):7. [Google Scholar]
- Pereira JC, Segala AD, Gillam DG. Effect of desensitizing agents on the hydraulic conductance of human dentin subjected to different surface pre-treatments--an in vitro study. Dent Mater. 2005;21(2):129–38. doi: 10.1016/j.dental.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Pillon FL, Romani IG, Schmidt ER. Effect of a 3% potassium oxalate topical application on dentinal hypersensitivity after subgingival scaling and root planing. J Periodontol. 2004;75(11):1461–4. doi: 10.1902/jop.2004.75.11.1461. [DOI] [PubMed] [Google Scholar]
- Rees JS, Addy M. A cross-sectional study of buccal cervical sensitivity in UK general dental practice and a summary review of prevalence studies. Int J Dent Hyg. 2004;2(2):64–9. doi: 10.1111/j.1601-5029.2004.00068.x. [DOI] [PubMed] [Google Scholar]