Abstract
Women with ductal carcinoma in situ (DCIS) need to comprehend the meaning of the diagnosis and the potential benefits and harms of treatment options. Full and understandable information is a requirement, not an option. However, with DCIS, as with many areas of medicine, a high level of uncertainty about the disease remains. In this article, we define informed medical decision making, review challenges to its implementation, and provide suggestions on how to improve communication with women about the diagnosis and treatment of DCIS.
Defining Informed Medical Decision Making
A growing body of research shows that both patients and providers benefit when patients are well informed and play a significant role in deciding how to manage their health conditions. Informed medical decision making occurs when patients understand both the disease or condition under consideration and the implications of the related clinical care, including benefits, harms, limitations, alternatives, and uncertainties. Patients should participate in the decision-making process to the degree they desire and arrive at a decision consistent with their preferences (1,2). Medical decisions have two components: a technical component, which requires knowledge of the risks, benefits, and side effects associated with each treatment option, and a value component, which requires input from patients about their values and preferences. Incorporating patient input is important for all medical decisions and is essential for decisions about preference-sensitive conditions—those for which two or more valid treatment choices are available to most patients, even if one of those choices is to forgo treatment.
A recent survey of patients in the United States found that the decision-making process is far from ideal (3). Fewer than one in five respondents reported that their health-care provider asked them about their preferences for care, and fewer than 50% were able to answer basic questions about their condition and its treatment—information that a truly informed patient needs to grasp before making a treatment decision. The survey also found that providers often neglected to tell patients about the potential disadvantages of treatments or tests that they recommended.
Barriers to Effective Communication and Informed Medical Decision Making
Unfortunately, many barriers to informed decision making exist in clinical practice, especially in the area of breast health. Informing a woman about her new diagnosis of DCIS and discussing treatment options may be challenging for both physicians and patients for many reasons including a widespread fear of breast cancer. In this article, we review the barriers to effective communication and informed medical decision making in the specific context of ductal carcinoma in situ (DCIS).
The term DCIS includes the anxiety-producing term “carcinoma,” which may add to the challenge of effective communication as concluded by the State of the Science panel (4). Indeed, a heightened sense of risk with regard to breast cancer has been noted in studies of women, even in the absence of a diagnosis of DCIS. One survey found that women in their 40s overestimated their risk of a breast cancer diagnosis within the next 10 years by a factor of 6, and their risk of dying of breast cancer by a factor of 20 (5). In addition, it can also be difficult to explain the nature of DCIS as a preinvasive lesion that is distinct from invasive cancer. This concept may be hard for clinicians to describe and for patients and their families to comprehend. Among women with DCIS, uncertainty regarding the relationship of DCIS to future invasive cancer often leads to anxiety (6–8). Even after receiving treatment, women with DCIS overestimate their future breast cancer risk (6,9–11).
Some women might be so alarmed by a diagnosis of DCIS that they just want to “take it off”—meaning to undergo mastectomy, the most aggressive and complete treatment possible. Preferences for cancer treatment lean strongly in the direction of extensive treatment, even if significant potential harms are associated with the treatment. The thought of living with uncertainty after a less aggressive treatment such as a lumpectomy with radiation therapy might be unbearably stressful to some women, and thus they would prefer to do everything possible to treat DCIS. One study, entitled “Cure me even if it kills me,” found that individuals would prefer to undergo invasive surgery for cancer even if the treatment might be more harmful than beneficial (12).
The stress caused by uncertainty for some women is so acute that they even want to have the contralateral breast removed. The use of contralateral prophylactic mastectomy in the United States has markedly increased over time (13). Among patients who underwent mastectomy to treat DCIS, the contralateral prophylactic mastectomy rate increased by 188% from 1998 (6.4%) to 2005 (18.4%).
Mass media can influence women’s decisions about DCIS treatment. The media typically sensationalize medical information about breast cancer (14), and numerous individuals and groups publicize their “war” against cancer (15). One study suggests a relationship between fear of breast cancer and exposure to breast cancer coverage in television news programs (16). The medical care received by female celebrities can also influence the behavior of the general population. For example, immediately after Nancy Reagan decided against breast-conserving surgery and underwent a mastectomy, a significant reduction was noted in the percentage of women with early-stage breast cancer who received breast-conserving surgery (17).
Patients may not understand the numeric information provided during clinical encounters. For example, Lipkus et al. (18) found that 16% of highly educated individuals incorrectly answered straightforward questions about risk magnitudes (eg, Which represents the larger risk: 1%, 5%, or 10%?). One study of adults with a high school diploma or less reported that only 54% correctly answered the question “How many heads in 1000 coin flips?”, and only 54% could correctly convert 1% to the number of patients in 1000. Sadly, only about one in four adults, including those with postgraduate degrees, correctly converted 1 in 1000 to a percentage (19).
While clinicians may have a better understanding of the numeric information, they rarely have the numbers at hand when meeting with patients. The challenge of understanding cancer risk is also not unique to patients; clinicians are similarly challenged (20,21). For example, nine out of ten radiologists working in breast imaging overestimated a women’s 5-year risk of breast cancer (21).
Other barriers to effective communication include physicians’ lack of time with patients in the busy outpatient medical setting. Our current reimbursement system in the United States provides fewer incentives for physicians to spend time talking with patients and more incentives to perform diagnostic tests (22).
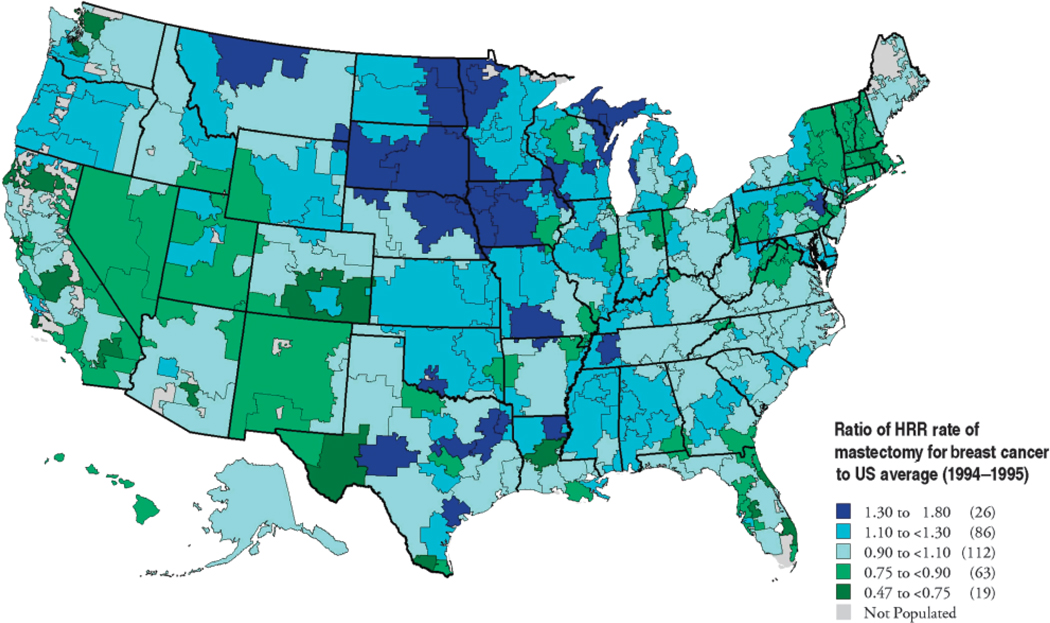
Geographic variation in physician recommendation and practice patterns also may affect women’s treatment decisions. Extensive medical practice variation has been documented by investigators in the Dartmouth Atlas Project (23). For example, mastectomy for breast cancer is a high variation procedure. In one study, 26 US hospital regions had mastectomy rates that were 30% or more higher than the national average (blue), and 19 regions had rates more than 25% below the national average (green). Rates were higher in the Midwest than on the East or West Coasts (Figures 1 and 2). While data are not available from these investigators on the current variations in treatments received by women with DCIS, we suspect that similar differences would be noted.
Figure 1.
(1994–1995). Data shown are the ratio of hospital referral region (HRR) rates of mastectomy for breast cancer to the US average (This figure reproduced with permission from Kristen Bronner, Dartmouth Atlas Project).
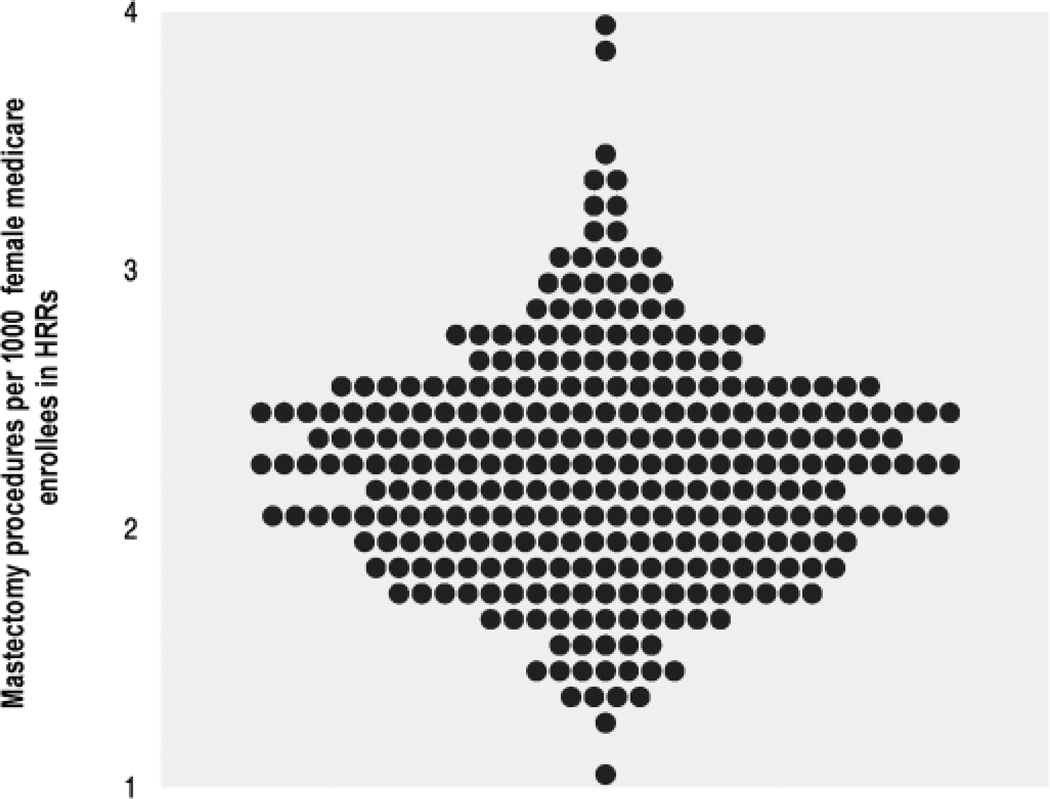
Figure 2.
Mastectomy among hospital referral regions (HRRs) (1994–1995). The rates varied from 1.1 to 4.0 per thousand female Medicare enrollees, after adjustment. Each point represents one of the 306 HRRs in the United States (This figure reproduced with permission from Kristen Bronner, Dartmouth Atlas Project).
Finally, some women might be uninterested in participating in the decision-making process and just want the doctor to “tell me what to do.” While most patients report wanting to be involved in their medical treatment decisions, Bruera et al. (24) reported that 11% of women did not want to participate in decisions about breast cancer treatment.
Once DCIS is diagnosed, clinicians face the challenge of explaining the diagnosis, the various treatment options, their risks and benefits, and the prognosis. An example of the difficulties encountered during the discussion between a clinician and a patient with a new diagnosis of DCIS is illustrated in a video just over 7 minutes long found at: http://faculty.washington.edu/jelmore/Videos
This video shows how a woman might react to a new diagnosis with the term “carcinoma,” the impact of the media and friends on women’s treatment decisions, the confusion regarding numbers and what is meant by “hormone treatment,” and the desire for overtreatment. The video concludes with brief suggestions for ways to improve the discussion and help women make informed decisions about their care.
General Suggestions for Communication
Table 1 outlines general suggestions for communication with women during a clinical encounter where they are being informed of a new diagnosis of DCIS. Because time is often limited in clinical encounters, and the information related to medical care can often be hard for patients to understand, use of decision aids has been suggested. Decision aids such as educational pamphlets, DVDs and Web sites can be used to help patients understand their prognosis and the benefits and risks of different treatment options. Decision aids are tools designed to help people participate in decision making about health-care options. They provide information on the different treatment options and help patients clarify and communicate the personal value they associate with different features of the options. Decision aids do not advise people to choose one option over another. They prepare patients to make informed, values-based decisions with their practitioner (25).
Table 1.
General suggestions for communicating with women regarding their new diagnosis of ductal carcinoma in situ (DCIS)
| Use decision aids | Decision aids such as educational pamphlets, DVDs and web sites can be used to help patients understand their prognosis and the benefits and risks of different treatment options |
| Use positive and negative framing and express logically equivalent information in different forms | Positive framing emphasizes healthy outcomes while negative framing emphasizes the presence of disease |
| Use absolute risks during the discussion | Relative risk data may sound more threatening than absolute risks |
| Put risk into context | Place the patient’s risk of developing invasive breast cancer in context of developing other diseases |
| Listen carefully to patients values | Understand expectations and past experiences that may be relevant to the decision |
| Recognize/validate her emotions | Acknowledge that a diagnosis of DCIS can be frightening and confusing |
| Use nonmedical language | Use simple words that are easy to understand |
| Address uncertainty related to the diagnosis | Explain that more research is needed before we can accurately assess the risks and potential harms of DCIS and treatment options |
| Identify and deal with misconceptions | Separate the scientific data that supports our current understanding of DCIS from sensational factoids |
| Do not rush decisions | Let your patient know there is time to make a treatment decision |
| Arrange follow-up | Encourage her to look through her decision aids and write down any questions she has to bring to a follow-up appointment |
The DCIS State of the Science panel recommended that future research focus on the impact of decision aids for DCIS (4). Unfortunately, there have been no trials of decision aids for DCIS. A Cochrane systematic review evaluated 55 trials of decision aids addressing 23 different screening or treatment decisions and found that using such aids led to greater knowledge, more accurate risk perceptions, greater comfort with decisions, greater participation in decision making, fewer people remaining undecided, and fewer patients choosing major surgery (2). It is unclear if the results noted in trials of decision aids on topics other than DCIS are applicable to the clinical situation encountered by women facing a new diagnosis of DCIS. The heightened sense of anxiety among women with DCIS and the limited data on the natural history of DCIS make this clinical encounter particularly challenging (26,27).
Patients may choose a different treatment option after using decision aids, suggesting that standard practice may not sufficiently educate patients in the complexities of their medical decisions (2). Interestingly, patients whose physicians use decision aids are more likely to choose a less invasive treatment option (28), and patients who engage in shared decision making are more likely to disagree with recommendations for more invasive options. This suggests that using decision aids helps to clarify patients’ values and preferences, even when their values differ from those of their physicians. Patients who use decision aids also report less decisional conflict and greater satisfaction with their treatment decision than patients who do not, suggesting that shared decision making empowers these patients to choose a treatment option that best fits their values (28).
To implement decision aids, we need patients who are interested in being informed and activated to participate in their health decisions, as well as clinicians and hospitals that are receptive to patient participation. Practical systems and protocols for the routine use of decision aids are also needed, as is a health-care environment that rewards good decision-making processes rather than simply a high volume of tests and procedures.
Because so many people have trouble grasping the difference between relative and absolute risks, providers need to exercise special care when they talk to patients. Some studies find that natural frequencies are the easiest way for women to understand risk (20,29).
One additional strategy in communicating with patients about a new diagnosis is to place the patient’s risk of developing an invasive breast cancer in the context of developing other diseases (20). Women rarely die of breast cancer after a diagnosis of DCIS; they are more likely to die of many other causes. For example, among 1000 women 65 years of age who never smoked, eight may die of breast cancer in the next decade and 25 may die of coronary disease (30). Any information provided to patients should be suitable for communication in verbal, numeric, and also visual formats (31–33). A considerable body of research suggests that visual displays aid in understanding risk (31,32,34–37), although more study in the general area of decision support tools is warranted.
The way risk information is presented, including the framing of the discussion and the choice of words, can affect how patients interpret this information. Providers need to decide how to approach the discussion, and they must be able to express logically equivalent information in different forms. Positive framing emphasizes healthy outcomes and the absence of disease, while negative framing emphasizes the presence of disease. A positive frame seems preferable for most patients. For example, after 10 years, more than 98% of women diagnosed with DCIS will not die of breast cancer (38). We can also describe this as 980 of 1000 women diagnosed with DCIS will not die of breast cancer in 10 years (27).
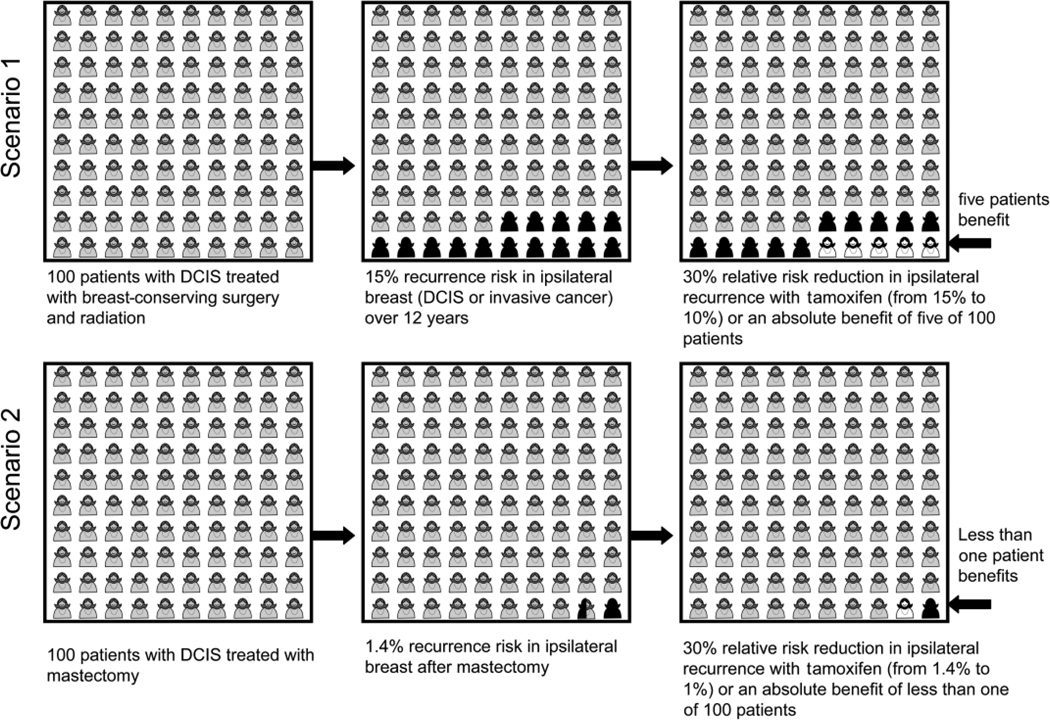
Much of the literature about cancer risk deals with relative risks, which sound more threatening than absolute risks (39). A hypothetical example of 100 patients with DCIS undergoing two different treatments, breast-conserving surgery with radiation vs mastectomy, is provided in Figure 3 for illustration purposes. Both groups of women here are assumed to have a 30% relative risk reduction in ipsilateral recurrence by taking tamoxifen for 5 years (1). However, while this risk reduction sounds impressive, the figure shows that the absolute number of women who benefit in each group is quite different. This is because the baseline risk of recurrence is different: approximately 15% among women who had breast-conserving surgery with radiation (scenario 1) and approximately 1.4% among women who had a mastectomy (scenario 2) (1,2). In scenario 1, 15 of the 100 patients are at risk of recurrence of the disease without tamoxifen. If all 100 women take the medication, a 30% relative risk reduction will mean that five women will benefit from the treatment. In scenario 2, the baseline risk of recurrence is much smaller, at 1.4%, but with the same 30% relative risk reduction, thus out of 100 women who take tamoxifen after a mastectomy, the number who will benefit from the treatment is less than 1. As shown in Figure 3, if the recurrence rate is 15% vs 1.4%, the absolute numbers of patients benefiting from the treatment are quite different, even with the same 30% relative risk reduction. It is important to note that this example only depicts the benefits of tamoxifen on ipsilateral recurrence and does not include the effect on contra-lateral breast cancer risk for either group of women with DCIS (40,41).
Figure 3.
Two clinical scenarios, both with a 30% relative risk reduction in ipsilateral breast tumor recurrence using tamoxifen after surgical treatment for DCIS, but with two different reductions in absolute risk because of differences in baseline recurrence risks. Scenario 1 has a 15% risk of recurrence after breast-conserving surgery with radiation, with five of 100 women benefiting from tamoxifen treatment. Scenario 2 has a 1.4% risk of recurrence after mastectomy, with less than one in 100 women treated with mastectomy benefiting from tamoxifen treatment. This figure represents recurrence risk. These scenarios do not take into account the effects of tamoxifen on contralateral breast cancer risk.
The State of the Science DCIS panel identified patient communication for the diagnosis and treatment of DCIS as a critical area for future research. Included in this recommendation was research involving the use of an informed consent process that takes place when a woman is considering undergoing screening mammography. Screening invitations should present both benefits and harms in a balanced fashion and should offer, not encourage, participation (42–44). As it relates to DCIS, introduction of an informed consent at the time of mammography invitation may provide opportunities to introduce the concept of carcinoma in situ and the potential harms from overdiagnosis and overtreatment of otherwise indolent DCIS. The concern of overdiagnosis of DCIS stems from the marked increase in the incidence of DCIS since the 1970s coinciding with an increased use of screening mammography (26,45). The most rapid increases were among women aged 50 years and older. In 1975, approximately six per 100 000 women were diagnosed with DCIS, the current age-adjusted incidence rate of DCIS is 32.5 per 100 000 women, and at age 50–64 years, the incidence is approximately 88 per 100 000 women (4). Knowing this information before screening may have implications not only on the decisions to screen but also on women’s anxiety levels and the treatment decisions once a diagnosis of DCIS is made.
One additional strategy in communicating with patients about a new diagnosis is to place the patient’s risk of developing an invasive breast cancer in the context of developing other diseases (20). Women rarely die of breast cancer after a diagnosis of DCIS; they are more likely to die of many other causes.
Patients’ values are also fundamental in treatment decisions, so listen carefully to what they say (eg, “I hate being on medications”). Ask them how they would like to participate in these decisions, and what they want to know (46). Make sure you understand any expectations and past experiences that may be relevant to the decision.
Recognize and validate her emotions during the clinical encounter. Acknowledge that a diagnosis of DCIS can be frightening and confusing. Check frequently to see if she understands what you have said or if she has any questions.
We encourage the use of nonmedical language. Medical terminology can be confusing and often holds little meaning for patients. When communicating a diagnosis of DCIS, use simple words that are easy to understand. Consider the patient’s frame of reference, and use concepts that are inclusive and culturally appropriate. Explain treatment options in lay language and describe how the patient herself may experience the treatment.
During the clinical encounter, address the uncertainty related to this diagnosis. Explain that, although we know a good deal about DCIS, we need to do more work before we can accurately assess the risks and potential harms. Make sure to discuss the uncertainties of treatment options in addition to the uncertainties about the natural progression of the disease if it is left untreated.
If possible, identify and deal with misperceptions. Breast cancer and related conditions such as DCIS have been regularly featured in health-care news, and a great deal of sensationalized and often inaccurate information has been disseminated (14). Talk with patients about separating the scientific data that support our current understanding of DCIS from the sensational factoids.
Use both positive and negative framing when describing clinical outcomes. It is important for physicians to emphasize the potential for positive outcomes. This approach can greatly reduce patients’ anxiety when they hear the initial diagnosis of DCIS.
Make certain that the patient does not rush to make her decision about treatment. Remember to let your patient know that there is time to make a treatment decision and that she does not need to make the decision that day. Encourage her to come back to discuss options after she has had time to think about and discuss them with family and friends.
At the end of the initial clinic visit where the diagnosis of DCIS is disclosed, arrange for a follow-up appointment. Ask if she has what she needs to think about her decision, and remind her that she can come back with more questions. Encourage her to look through decision aids, and write down her questions.
Summary
Patients should understand their choices and have the information and guidance they need to make sound decisions that affect their health and well-being. Although we have high-tech medical programs and decades of research on cancer, our ability to communicate with patients regarding DCIS is far from optimal. Not only do we need to develop better risk prediction methods, we must also learn how to communicate the uncertainty in our knowledge base, as well as the risks and benefits associated with specific treatment options, in ways that make sense to our patients. High-quality health-care demands no less.
Acknowledgments
Funding
National Cancer Institute at the National Institutes of Health (KO5 CA 104699 to J.G.E.).
Footnotes
Dr Elmore serves as a medical editor for the nonprofit Foundation for Informed Medical Decision Making (FIMDM). Support for the video referred to in this article was made possible by an educational grant from FIMDM.
References
- 1.Rimer BK, Briss PA, Zeller PK, Chan EC, Woolf SH. Informed decision making: what is its role in cancer screening? Cancer. 2004;101(5 suppl):1214–1228. doi: 10.1002/cncr.20512. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor AM, Bennett CL, Stacey D, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2009;8(3):CD001431. doi: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- 3.The Foundation for Informed Medical Decision Making (FIMDM) [Accessed January 6, 2010];Problems With Medical Decision Making—Ethical Concerns. 2010 http://www.informedmedicaldecisions.org/problems_with_medical_decision_making.html.
- 4.Allegra CJ, Aberle DR, Ganschow P, et al. NIH state-of-the-science conference statement: diagnosis and management of ductal carcinoma in situ (DCIS) NIH Consens State Sci Statements. 2009;26(2):1–27. [PubMed] [Google Scholar]
- 5.Black WC, Nease RF, Jr, Tosteson AN. Perceptions of breast cancer risk and screening effectiveness in women younger than 50 years of age. J Natl Cancer Inst. 1995;87(10):720–731. doi: 10.1093/jnci/87.10.720. [DOI] [PubMed] [Google Scholar]
- 6.Partridge A, Adloff K, Blood E, et al. Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. J Natl Cancer Inst. 2008;100(4):243–251. doi: 10.1093/jnci/djn010. [DOI] [PubMed] [Google Scholar]
- 7.Welch HG, Woloshin S, Schwartz LM. The sea of uncertainty surrounding ductal carcinoma in situ—the price of screening mammography. J Natl Cancer Inst. 2008;100(4):228–229. doi: 10.1093/jnci/djn013. [DOI] [PubMed] [Google Scholar]
- 8.Welch HG. Should I Be Tested for Cancer? Maybe Not and Here’s Why. Berkeley, CA: University of California Press; 2004. [Google Scholar]
- 9.Bluman LG, Borstelmann NA, Rimer BK, Iglehart JD, Winer EP. Knowledge, satisfaction, and perceived cancer risk among women diagnosed with ductal carcinoma in situ. J Womens Health Gend Based Med. 2001;10(6):589–598. doi: 10.1089/15246090152543175. [DOI] [PubMed] [Google Scholar]
- 10.Rakovitch E, Franssen E, Kim J, et al. A comparison of risk perception and psychological morbidity in women with ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res Treat. 2003;77(3):285–293. doi: 10.1023/a:1021853302033. [DOI] [PubMed] [Google Scholar]
- 11.van Gestel YR, Voogd AC, Vingerhoets AJ, et al. A comparison of quality of life, disease impact and risk perception in women with invasive breast cancer and ductal carcinoma in situ. Eur J Cancer. 2007;43(3):549–556. doi: 10.1016/j.ejca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Cure me even if it kills me: preferences for invasive cancer treatment. Med Decis Making. 2005;25(6):614–619. doi: 10.1177/0272989X05282639. [DOI] [PubMed] [Google Scholar]
- 13.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27(9):1362–1367. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 14.Burke W, Olsen AH, Pinsky LE, Reynolds SE, Press NA. Misleading presentation of breast cancer in popular magazines. Eff Clin Pract. 2001;4(2):58–64. [PubMed] [Google Scholar]
- 15.Lerner BH. The Breast Cancer Wars: Hope, Fear, and the Pursuit of a Cure in Twentieth-Century America. New York, NY: Oxford University Press; 2001. [PubMed] [Google Scholar]
- 16.Lemal M, Van den Bulck J. Television news exposure is related to fear of breast cancer. Prev Med. 2009;48(2):189–192. doi: 10.1016/j.ypmed.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Nattinger AB, Hoffmann RG, Howell-Pelz A, Goodwin JS. Effect of Nancy Reagan’s mastectomy on choice of surgery for breast cancer by US women. JAMA. 1998;279(10):762–766. doi: 10.1001/jama.279.10.762. [DOI] [PubMed] [Google Scholar]
- 18.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. doi: 10.1177/0272989X0102100105. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med. 1997;127(11):966–972. doi: 10.7326/0003-4819-127-11-199712010-00003. [see comment] [DOI] [PubMed] [Google Scholar]
- 20.Schwartz LM, Woloshin S, Welch HG. Risk communication in clinical practice: putting cancer in context. J Natl Cancer Inst Monogr. 1999;25:124–133. doi: 10.1093/oxfordjournals.jncimonographs.a024187. [DOI] [PubMed] [Google Scholar]
- 21.Egger JR, Cutter GR, Carney PA, et al. Mammographers’ perception of women’s breast cancer risk. Med Decis Making. 2005;25(3):283–289. doi: 10.1177/0272989X05276857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Foundation for Informed Medical Decision Making (FIMDM) [Accessed January 12, 2010];Informing and Involving Patients in Medical Decisions: The Primary Care Physicians Perspective. www.informedmedicaldecisions.org/pdfs/FinalwhitepaperPCPSurvey.pdf.
- 23.Wennberg JE, Cooper MM, Bubolz TA, et al. The Dartmouth Atlas of Health Care in the United States. Chicago, IL: American Hospital Publishing; 1996. [PubMed] [Google Scholar]
- 24.Bruera E, Willey JS, Palmer JL, Rosales M. Treatment decisions for breast carcinoma: patient preferences and physician perceptions. Cancer. 2002;94(7):2076–2080. doi: 10.1002/cncr.10393. [DOI] [PubMed] [Google Scholar]
- 25.Elwyn G, O’Connor A, Stacey D, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerlikowske K, Molinaro AM, Gauthier ML, et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst. 2010;102(9):627–637. doi: 10.1093/jnci/djq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102(3):170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 28.Whelan T, Levine M, Willan A, et al. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial. JAMA. 2004;292(4):435–441. doi: 10.1001/jama.292.4.435. [DOI] [PubMed] [Google Scholar]
- 29.Schapira MM, Nattinger AB, McHorney CA. Frequency or probability? A qualitative study of risk communication formats used in health care. Med Decis Making. 2001;21(6):459–467. doi: 10.1177/0272989X0102100604. [DOI] [PubMed] [Google Scholar]
- 30.Woloshin S, Schwartz LM, Welch HG. The risk of death by age, sex, and smoking status in the United States: putting health risks in context. J Natl Cancer Inst. 2008;100(12):845–853. doi: 10.1093/jnci/djn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making. 2007;27(5):696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- 32.Edwards A, Gray J, Clarke A, et al. Interventions to improve risk communication in clinical genetics: systematic review. Patient Educ Couns. 2008;71(1):4–25. doi: 10.1016/j.pec.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 33.O’Connor A, Llewellyn-Thomas H, Stacey D. [Accessed January 14, 2010];International Patient Decision Aid Standards (IPDAS) Collaboration Background Document. 2005 http://ipdas.ohri.ca/IPDAS_background.pdf.
- 34.Ancker JS, Senathirajah Y, Kukafka R, Starren JB. Design features of graphs in health risk communication: a systematic review. J Am Med Inform Assoc. 2006;13(6):608–618. doi: 10.1197/jamia.M2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipkus IM, Hollands JG. The visual communication of risk. J Natl Cancer Inst Monogr. 1999;25:149–163. doi: 10.1093/oxfordjournals.jncimonographs.a024191. [DOI] [PubMed] [Google Scholar]
- 36.Peters E, Hibbard J, Slovic P, Dieckmann N. Numeracy skill and the communication, comprehension, and use of risk-benefit information. Health Aff (Millwood) 2007;26(3):741–748. doi: 10.1377/hlthaff.26.3.741. [DOI] [PubMed] [Google Scholar]
- 37.Trevena LJ, Davey HM, Barratt A, Butow P, Caldwell P. A systematic review on communicating with patients about evidence. J Eval Clin Pract. 2006;12(1):13–23. doi: 10.1111/j.1365-2753.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 38.Fisher ER, Dignam J, Tan-Chiu E, et al. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer. 1999;86(3):429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 39.Gigerenzer G. Calculated Risks: How to Know When Numbers Deceive You. New York, NY: Simon and Schuster; 2002. [Google Scholar]
- 40.Boyages J, Delaney G, Taylor R. Predictors of local recurrence after treatment of ductal carcinoma in situ: a meta-analysis. Cancer. 1999;85(3):616–628. [PubMed] [Google Scholar]
- 41.Fisher B, Land S, Mamounas E, Dignam J, Fisher ER, Wolmark N. Prevention of invasive breast cancer in women with ductal carcinoma in situ: an update of the National Surgical Adjuvant Breast and Bowel Project experience. Semin Oncol. 2001;28(4):400–418. doi: 10.1016/s0093-7754(01)90133-2. [DOI] [PubMed] [Google Scholar]
- 42.Jorgensen KJ, Brodersen J, Hartling OJ, Nielsen M, Gotzsche PC. Informed choice requires information about both benefits and harms. J Med Ethics. 2009 Apr;35(4):268–269. doi: 10.1136/jme.2008.027961. [DOI] [PubMed] [Google Scholar]
- 43.Zapka JG, Geller BM, Bulliard JL, Fracheboud J, Sancho-Garnier H, Ballard-Barbash R. Print information to inform decisions about mammography screening participation in 16 countries with population-based programs. Patient Educ Couns. 2006;63(1–2):126–137. doi: 10.1016/j.pec.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Geller BM, Zapka J, Hofvind SS, et al. Communicating with women about mammography. J Cancer Educ. 2007;22(1):25–31. doi: 10.1007/BF03174371. [DOI] [PubMed] [Google Scholar]
- 45.Li CI, Malone KE, Saltzman BS, Daling JR. Risk of invasive breast carcinoma among women diagnosed with ductal carcinoma in situ and lobular carcinoma in situ, 1988–2001. Cancer. 2006;106(10):2104–2112. doi: 10.1002/cncr.21864. [DOI] [PubMed] [Google Scholar]
- 46.Epstein RM, Alper BS, Quill TE. Communicating evidence for participatory decision making. JAMA. 2004;291(19):2359–2366. doi: 10.1001/jama.291.19.2359. [DOI] [PubMed] [Google Scholar]