Abstract
Piezoelectric microcantilever sensors (PEMS) can be sensitive tools for the detection of proteins and cells in biological fluids. However, currently available PEMS can only be used a single time or must be completely stripped and refunctionalized prior to subsequent uses. Here we report the successful use of an alternative regeneration protocol employing high salt concentrations to remove the target, leaving the functional probe immobilized on the microcantilever surface. Our model system employed the extracellular domain (ECD) of recombinant human Epidermal Growth Factor Receptor (EGFR) as the probe and anti-human EGFR polyclonal antibodies as the target. We report that high concentrations of MgCl2 dissociated polyclonal antibodies specifically bound to EGFR ECD immobilized on the sensor surface without affecting its bioactivity. This simple regeneration protocol both minimized the time required to re-conjugate the probe and preserved the density of probe immobilized on PEMS surface, yielding identical biosensor sensitivity over a series of assays.
Keywords: Piezoelectric microcantilever, biosensor, regeneration, antibody, antigen, biomarker
1. Introduction
Label-free microcantilever biosensors have recently been the focus of significant developmental efforts for use in biomedical applications including disease screening [1], blood glucose monitoring [2], and detection of cancer biomarkers such as prostate specific antigen [3]. Piezoelectric microcantilever sensors (PEMS) consisting of a piezoelectric layer, lead magnesium niobate–lead titanate [4; 5], (PbMg1/3Nb2/3O3)0.63–(PbTiO3)0.37(PMN-PT) bonded to a nonpiezoelectric layer such as tin have shown particular promise for label-free detection in real time. When a PEMS probe (e.g., an antibody) binds to its target (e.g., an antigen) in a biological fluid a shift occurs in the PEMS resonance frequency, which can be measured in real time [6; 7]. PEMS are particularly attractive for these applications as they undergo electric self-excitation and self-sensing and are capable of withstanding liquid damping, thereby allowing highly sensitive measurements to be performed in biological fluids. They can be functionalized by immobilizing receptors or target biomolecules to their surface to facilitate the highly specific binding of target molecules such as antigens or even intact cells. The resonance frequency shifts can then be detected by electrical means for further analysis. Using this approach, we have detected extremely low concentrations of human HER2, a breast cancer associated biomarker, in serum using PEMS functionalized with anti-HER2 single chain Fv (scFv) engineered antibody-based molecules [6].
An ideal biosensor would embody basic features such as selectivity, specificity and reproducibility. Specificity and selectivity are readily obtained by functionalizing the surface of the PEMS with probes (e.g., anti-HER2 antibodies) that selectively capture the desired analytes (e.g., HER2). However, the reuse of functionalized PEMS sensors in a reproducible manner has not yet been reported. Typically harsh techniques, such as glycine-HCl mixture at pH 2.5 [4] [11] or a “Piranha Solution” composed of sulfuric acid and hydrogen peroxide [8; 9; 10], have been employed to strip both the analyte and probe from the PEMS surface, often damaging or removing the insulation layer in the process. These procedures require the PEMS to be completely refunctionalized via the addition of entirely new insulation layers and biomolecule probes. As a result, the sensitivity of the probe is often altered [12; 13]. Additional drawbacks to these techniques are that they are extremely time consuming in nature and they use significant quantities of often valuable and limited resources.
Interactions between antibodies and their cognate antigens involve a variety of factors: electrostatic, hydrogen bonds, Van der Waals and hydrophobic forces. These forces can be weakening/disrupted by using high salt and/or low pH buffer solutions. When employed properly, this process is reversible with little or no damage to the antibody or antigen. This principle has been applied in a wide variety of immunological studies including affinity-based target antigen-capture and release assays [14; 15]. These approaches are commonly used to regenerate the surface plasmon resonance (Biacore) chips or Quartz Crystal Microbalance (QCM)[16; 17]. However, to the best of our knowledge, this approach has not been previously employed with piezoelectric sensors. Our goal in the current study was to examine the ability of high salt concentrations to dissociate the target-probe complex, removing the analyte but leaving intact probe immobilized on PEMS surface. Our proof-of-concept model system employed the extracellular domain (ECD) of recombinant human Epidermal Growth Factor Receptor (EGFR) as the probe and goat anti-human EGFR polyclonal antibodies as the targets, but the procedures can be employed for all antibody-antigen interactions. After utilizing the PEMS biosensor to detect a target, the PEMS/EGFR ECD/anti-EGFR polyclonal antibody complex was immersed in MgCl2 to dissociate the probe/target complex. The target was stripped from the PEMS leaving the intact functional probe (EGFR ECD) on the surface for the next measurement. This straightforward regeneration protocol both minimized the time required to re-conjugate the probe and preserved the density of probe immobilized on PEMS surface, allowing identical sensitivity over a series of assays.
2. Experimental Section
PEMS preparation for assay
PEMS were constructed by bonding an 8 μm thick lead magnesium niobate-lead titanate (PMN-PT) freestanding film piezoelectric layer to a gold/copper nonpiezoelectric layer as previously described [5]. The PMN-PT/Cu was cut to the cantilever shape, a dimension of 560μm long and 720μm width, with a wire saw (Princeton Scientific Precision, Princeton, NJ) after embeding in wax. Wires were attached to the top and bottom electrodes using conductive glue (XCE 3104XL Emerson and Cuming Company, Billerice, MA), followed by using glue to attach the PMN-PT/Cu strips to a glass substrate. To insulate PEMS with mercaptopropyltrimethoxysilane (MPS), the PEMS was first soaked in a piranha solution, which contains two parts of 98% sulfuric acid (Fisher, Fair Lawn, NJ) with one part of 30% hydrogen peroxide (FisherBiotech, Fair Lawn, NJ) at 20°C for 1 min. The PEMS was submerged in 0.1 mM MPS(Sigma Aldrich) diluted in ethanol for 30 minutes before drying the PEMS in a vacuum-oven (Model 1400E, VWR International) at 762 mm Hg overnight. Next, the PEMS was submerged in a 1% (volume) of MPS diluted in ethanol (titrated to a pH 4.5 with acetic acid) for 36 hours with the solution being changed every 12 hours. The PEMS was then dried in a vacuum-oven (Model 1400E, VWR International) overnight at 762 mm Hg.
Immobilization of extracellular domain (ECD) of recombinant human Epidermal Growth Factor Receptor (EGFR) on PEMS
Recombinant human EGFR ECD was expressed from stably transfected HEK293 cells and purified as we have previously described [18]. 1μM EGFR ECD was conjugated to 80uM Sulfosuccinimidyl 4-N-maleimidomethyl cyclohexane-1-carboxylate (sulfo- SMCC) (Pierce) in 1ml 1xPBS (Gibco) mixed with 5mM EDTA, pH 7.4, for 30 minutes at room temperature. Excess sulfo-SMCC was then removed by filtration in a Microcon-10k (Millipore). The PEMS were then immersed in sulfo-SMCC-linked EGFR ECD solution for 30 minutes. Subsequently, PEMS functionalized with EGFR ECD were immersed in 3% BSA for 30 minutes to block the PEMS surface.
Detection of anti-human EGFR polyclonal antibody
After BSA treatment, PEMS functionalized with EGFR ECD were incubated in a 3.5ml custom-built flow cell containing 3ml PBS-EDTA. A peristaltic pump (model 77120-62, Cole-Parmer’s Master Flex, Vernon Hills, IL), was then used to flow PBS-EDTA at the low rate of 0.7ml/min in a perpendicular direction relative to the PEMS surface. Once a stable baseline was obtained for a period of at least 20 minutes, anti-human EGFR polyclonal antibodies diluted in PBS were injected into the system and the frequency shift was measured for 90 minutes with an electrical impedance analyzer (Agilent 4294A, Agilent). Two formulations of anti-human EGFR polyclonal antibodies were used in this study; anti-EGFR (1005) polyclonal antibodies, PAbpeptide, raised against a peptide located at the C-terminus of human EGFR (Santa Cruz Biotechnology Inc) and polyclonal antibodies directed against recombinant full length human EGFR, PAbfull-length, (Abnova). An anti-rabbit polyclonal antibody (Santa Cruz Biotechnology, sc-2030) was used as a negative control to study the specificity binding of PEMS. This anti-rabbit polyclonal antibody, referred as the non-binding polyclonal antibody in Figure 1C, recognized rabbit polyclonal antibody and not the probe (EGFR ECD) immobilized on PEMS.
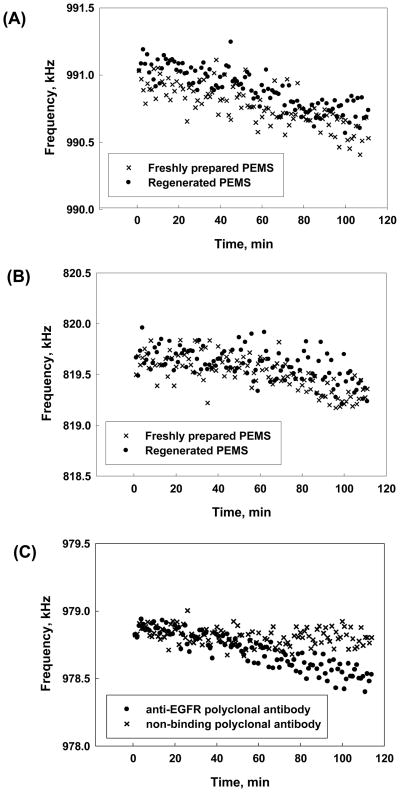
Figure 1.
Comparison of frequency shifts observed when (A) 1ng/ml and (B) 4ng/ml of PAbpeptide were administered to a freshly prepared EGFR ECD conjugated PEMS (crosses) and a regenerated PEMS (filled circles). For both concentrations, the regenerated PEMS yields a frequency shift that was similar to the freshly prepared PEMS with the recovery rate described in text. (C) Comparison of frequency shifts resulting from the binding of 4ng/ml of specific PAbpeptide anti-EGFR polyclonal antibodies (filled circles) and 4ng/ml of non-binding, non-reactive polyclonal antibodies (crosses) to a regenerated EGFR ECD conjugated PEMS. The injection of non-binding antibody yielded a minimal frequency shift, suggesting that non-specific binding was not a major factor.
Piranha solution stripping and refunctionalization
Stripping of PEMS with piranha solution was performed as previously described [5]. Briefly, after each detection run, the PEMS were immersed in a 1:100 diluted piranha solution (two parts of 98% sulfuric acid and one part of 30% hydrogen peroxide) for one minute, followed by immersion in water and ethanol for 30 seconds each. The PEMS were then incubated in 40mM solution of MPS in ethanol overnight, before drying in a vacuum-oven. The PEMS were then insulated and refunctionalized as described above for new sensors.
Rapid PEMS regeneration by high salt solution
After detection runs, PEMS were immersed in 2M MgCl2 for 30 seconds, followed by 1.5M Tris, pH 8.8 for an additional 30 seconds. The regenerated PEMS were incubated in 3% BSA for 30 minutes before being employed for a subsequent detection run.
Data analysis
The longitudinal extension mode of PEMS vibration in the range of 200–1000 kHz was used for data analysis as previously described [5]. Longitudinal mode was selected as liquid damping has the least effect on this mode. The relative frequency shift (Df/f) was calculated based on equation (1) as below:
| (1) |
where Fbaseline is the average 20 points of the baseline (before sample injection) and Fsample is calculated by averaging the last 20 points of the detection period. To compare the recovery of a regenerated PEMS-functionalized with EGFR ECD, an equation as below was used:
| (2) |
The significance of the differences in analyte detection between PEMS (freshly prepared, stripped and refunctionalized or regenerated) were determined using a two-tailed student’s t-test (GraphPad). P values <0.05 were considered to indicate the presence of statistically significant differences.
3. Results and Discussion
3.1. Comparison of Freshly prepared PEMS and Regenerated PEMS
Initial studies were performed examining the regeneration of EGFR ECD conjugated PEMS following their use in the detection of polyclonal antibodies, PAbpeptide, directed against a single peptide sequence on EGFR ECD protein. As the antibodies used in this experiment were focused on a small region of the ECD, the quantity of antibodies capable of binding would be expected to be limited, potentially leading to a more straightforward regeneration process. A typical spectrum (frequency vs. time) observed for 1ng/ml of PAbpeptide binding to a freshly prepared EGFR ECD-conjugated PEMS is shown in Figure 1A (crosses). The same PEMS was then regenerated using MgCl2 as described in the methods section and equilibrated with PBS buffer to reach a stable baseline. The regenerated EGFR ECD-conjugated PEMS was then reassayed with PAbpeptide at 1ng/ml (filled circles), yielding a frequency shift that was very similar to that observed with the freshly prepared PEMS (crosses) (Figure 1A). The same PEMS was then cleaned with piranha solution, refunctionalized with new insulation and EGFR ECD and employed in the detection of a higher concentration (4ng/ml) of PAbpeptide. The resulting frequency shift is presented as filled circles in Figure 1B. The PEMS was regenerated with MgCl2 and employed to detect the binding of 4ng/ml of PAbpeptide (filled circles), again yielding a frequency shift that was similar to that seen with the freshly prepared PEMS. Each experiment was repeated three times and the average binding to the regenerated PEMS was highly comparable to that seen with the original freshly prepared or refunctionalized PEMS (101.80+/−10.82% and 101.67+/−7.50% at PAbpeptide concentrations of 1 ng/ml and 4 ng/ml, respectively). In summary, assays performed with both concentrations of PAbpeptide revealed that the regenerated EGFR ECD-conjugated PEMS exhibited no loss in ability to bind polyclonal anti-EGFR antibodies.
To eliminate the possibility that the frequency shifts observed in these assays were due to non-specific interactions, regenerated EGFR ECD conjugated PEMS were assayed with 4ng/ml of non-binding polyclonal antibody (anti-rabbit polyclonal antibody) that was incapable of binding specifically to the EGFR ECD. The non-binding antibody yielded a minimal frequency shift (13.3% of the value observed with the specific PAbpeptide) at the conclusion of the 90-minute measurement period (Figure 1C). This suggested that the binding of PAbpeptide to EGFR ECD immobilized on the PEMS surface was specific.
The ability of the regeneration procedure to remove a more complex mixture of polyclonal antibodies was examined next. For these studies, we employed polyclonal antibodies generated against the entire EGFR ECD. As the targeted sequence on the protein was significantly larger than that used in the studies described above, the mixture of antibodies likely contained a broader range of affinities and could potentially target multiple epitopes on each EGFR ECD molecule simultaneously. A range of different dilutions of PAbfull-length were assayed on regenerated PEMS-functionalized with EGFR ECD and compared to a freshly prepared PEMS-functionalized with EGFR ECD. As shown in Table 1, the observed Df/f values at the conclusion of the 90 minutes measurement period were not significantly different for the freshly prepared and regenerated PEMS, indicating that the regenerated PEMS were capable of binding the same quantity of polyclonal antibody. This demonstrated that PEMS regeneration with MgCl2 was capable of stripping a complex mixture of antibodies from the immobilized antigen yet gentle enough to leave the antigen probe intact.
Table 1.
Comparison between freshly prepared PEMS and regenerated PEMS.
| Dilutions of PAbfull-length | Df/f (× 10−4) | P value | |
|---|---|---|---|
| Freshly prepared PEMS | Regenerated PEMS | ||
| 10−6 | 7.54 ±1.29 | 6.05 ± 1.15 | 0.437 |
| 10−9 | 4.55 ± 0.79 | 5.13 ± 0.45 | 0.558 |
| 10−11 | 2.04 ± 0.38 | 1.50 ± 0.14 | 0.253 |
| 10−12 | 0.56 ± 0.08 | 0.56 ± 0.03 | 0.957 |
Table 1 compares the full-length anti-EGFR polyclonal antibody (PAbfull-length) detection by PEMS prepared by stripping with piranha solution and refunctionalizing with new insulation and EGFR ECD (freshly prepared) and PEMS treated with MgCl2 leaving the EGFR ECD intact on the sensor surface (Regenerated). The experiments were performed in triplicate and the Df/f were reported as average standard error. P values were >0.05 suggesting that the measurements between the freshly prepared PEMS and regenerated PEMS were in accordance.
3.2. Determination of the reusability of MgCl2 regenerated PEMS
An important requirement for assays employed in the development and validation of biosensors is the availability to reproducibly reuse the sensors without significant changes in sensitivity. This is particularly critical when small variations in the dimensions of manually prepared sensors can lead to disparate results. To address this, EGFR ECD conjugated PEMS were sequentially employed in a series of detection and regeneration cycles. In each cycle, a stable baseline was obtained, 2ng/ml of PAbpeptide was applied to the sensor and the change in frequency was measured 90 minutes later. The PEMS was then regenerated as described above and the process was repeated. As shown in Table 2, we observed that the regeneration procedure was gentle enough to remove the bound polyclonal antibodies while still maintaining the quality of the EGFR ECD. The minimal frequency changes suggested that probe remained intact on the cantilever surface for at least three cycles (four consecutive measurements), after which the change in frequency was significantly altered. A typical PEMS can be stripped with piranha, refunctionalized and reused for more than five cycles as shown in Figure 1. Therefore, the loss of PEMS’s recovery after three cycles of regeneration protocol is more likely due to the damage of the probe, and not the damage of the PEMS. One possible explanation to the probe damage is due to the long exposure of the probe at room temperature during the repetitive measurements (>6 hr), thus resulting in the probe degradation and loss of binding ability.
Table 2.
Recovery rate of regenerated PEMS.
| Regeneration cycle | Recovery (%) | P value |
|---|---|---|
| 1 | 100 | 0.9336 |
| 2 | 95.90 ± 2.37 | 0.1587 |
| 3 | 93.93 ± 4.33 | 0.7102 |
| 4 | 87.76 ± 10. 91 | 0.6269 |
| 5 | 4.984 ± 2.879 | 0.0018 |
Table 2 compares the recovery rate of regeneration cycle for the detection of 2ng/ml anti-EGFR peptide polyclonal antibody. P values were calculated to compare the statistically difference between the indicated regeneration cycle and the previous regeneration cycle.
4. Conclusions
We have described a rapid and reliable regeneration procedure for the gentle dissociation of target proteins (e.g., antibody) from probes immobilized (e.g., antigen) on the surface of PEMS. This regeneration protocol allows PEMS-based sensors to be used sequentially with minimal loss of sensitivity. Further experiments are needed to validate the versatility of this regeneration protocol on PEMS for other antigen-antibody detections. However, we believe that the method can be widely extended to biosensors that rely on probe immobilization including receptor-ligand, antibody-antigen and enzyme-substrate interactions. As PEMS technology matures, reproducibility will likely be achieved through the use of standardized production of arrayed sensors that include internal controls, negating the need for regeneration. However, in the meantime, the availability of reliable regeneration procedures will be critical for the evaluation of new PEMS. In conclusion, the regeneration protocol described here gently removes target proteins from probes on the PEMS surface and facilitates the repetitive use of these biosensors.
Acknowledgments
This work was supported by the Nanotechnology Institute, a University Grant program of the Commonwealth of Pennsylvania’s Ben Franklin Technology Development Authority through Ben Franklin Technology Partners of Southeast Pennsylvania, by NCI grant R01 CA118159 (GPA), the Bernard A. & Rebecca S. Bernard Foundation and an appropriation from the Commonwealth of Pennsylvania. We would like to thank Dr. Anthony Green of the Nanotechnology Institute, Dr. Matthew Robinson of the Fox Chase Cancer Center and Dr. Eric Borguet and Aseem Malhotra of Temple University for helpful discussions and Heidi Simmons of the Fox Chase Cancer Center for expert technical assistance.
References and Notes
- 1.Hwang KS, Jeon HK, Lee SM, Kim SK, Kim TS. Quantification of disease marker in undiluted serum using an actuating layer-embedded microcantilever. Journal of Applied Physics. 2009;105 [Google Scholar]
- 2.Subramanian A, Oden PI, Kennel SJ, Jacobson KB, Warmack RJ, Thundat T, Doktycz MJ. Glucose biosensing using an enzyme-coated microcantilever. Applied Physics Letters. 2002;81:385–387. [Google Scholar]
- 3.Wu GH, Datar RH, Hansen KM, Thundat T, Cote RJ, Majumdar A. Bioassay of prostate-specific antigen (PSA) using microcantilevers. Nature Biotechnology. 2001;19:856–860. doi: 10.1038/nbt0901-856. [DOI] [PubMed] [Google Scholar]
- 4.McGovern JP, Shih WY, Shih WH. In situ detection of Bacillus anthracis spores using fully submersible, self-exciting, self-sensing PMN-PT/Sn piezoelectric microcantilevers. Analyst. 2007;132:777–783. doi: 10.1039/b704579d. [DOI] [PubMed] [Google Scholar]
- 5.Capobianco JA, Shih WY, Shih WH. 3-mercaptopropyltrimethoxysilane as insulating coating and surface for protein immobilization for piezoelectric microcantilever sensors. Review of Scientific Instruments. 2007;78:046106. doi: 10.1063/1.2727466. [DOI] [PubMed] [Google Scholar]
- 6.Capobianco JA, Shih WY, Yuan QA, Adams GP, Shih WH. Label-free, all-electrical, in situ human epidermal growth receptor 2 detection. Review of Scientific Instruments. 2008;79:076101. doi: 10.1063/1.2949831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Q, Shih WY, Shih WH. Real-time, label-free, all-electrical detection of Salmonella typhimurium using lead titanate zirconate/gold-coated glass cantilevers at any relative humidity. Sensors and Actuators B-Chemical. 2007;125:379–388. doi: 10.1016/j.snb.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang KS, Lee SM, Eom K, Lee JH, Lee YS, Park JH, Yoon DS, Kim TS. Nanomechanical microcantilever operated in vibration modes with use of RNA aptamer as receptor molecules for label-free detection of HCV helicase. Biosensors & Bioelectronics. 2007;23:459–465. doi: 10.1016/j.bios.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Xu XH, Thundat TG, Brown GM, Ji HF. Detection of Hg2+ using microcantilever sensors. Analytical Chemistry. 2002;74:3611–3615. doi: 10.1021/ac0255781. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Q, Shih WY, Shih WH. In situ, in-liquid, all-electrical detection of Salmonella typhimurium using lead titanate zirconate/gold-coated glass cantilevers at any dipping depth. Biosensors & Bioelectronics. 2007;22:3132–3138. doi: 10.1016/j.bios.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandimalla VB, Neeta NS, Karanth NG, Thakur MS, Roshini KR, Rani BEA, Pasha A, Karanth NGK. Regeneration of ethyl parathion antibodies for repeated use in immunosensor: a study on dissociation of antigens from antibodies. Biosensors & Bioelectronics. 2004;20:903–906. doi: 10.1016/j.bios.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Backmann N, Zahnd C, Huber F, Bietsch A, Pluckthun A, Lang HP, Guntherodt HJ, Hegner M, Gerber C. A label-free immunosensor array using single- chain antibody fragments. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14587–14592. doi: 10.1073/pnas.0504917102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grogan C, Raiteri R, O’Connor GM, Glynn TJ, Cunningham V, Kane M, Charlton M, Leech D. Characterisation of an antibody coated microcantilever as a potential immunobased biosensor. Biosensors & Bioelectronics. 2002;17:201–207. doi: 10.1016/s0956-5663(01)00276-7. [DOI] [PubMed] [Google Scholar]
- 14.Gagnon J, Ramanathan S, Leblanc C, Cloutier A, McDonald PP, Ilangumaran S. IL-6, in synergy with IL-7 or IL-15, stimulates TCR-independent proliferation and functional differentiation of CD8(+) T lymphocytes. Journal of Immunology. 2008;180(12):7958–7968. doi: 10.4049/jimmunol.180.12.7958. [DOI] [PubMed] [Google Scholar]
- 15.Muerhoff AS, Rupprecht K, Ruan QQ, Zeck B, Ramsay C, Zhao C, Desai SM. Microheterogeneous monoclonal antibody subspecies with differential hepatitis C virus core antigen binding properties identified by SEC-HPLC. J Immunol Methods. 2009;345(1–2):60–69. doi: 10.1016/j.jim.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Andersson K, Hamalainen M, Malmqvist M. Identification and optimization of regeneration conditions for affinity-based biosensor assays. A multivariate cocktail approach. Analytical Chemistry. 1999;71(13):2475–2481. doi: 10.1021/ac981271j. [DOI] [PubMed] [Google Scholar]
- 17.Safsten P, Klakamp SL, Drake AW, Karlsson R, Myszka DG. Screening antibody-antigen interactions in parallel using Biacore A100. Analytical Biochemistry. 2006;353(2):181–190. doi: 10.1016/j.ab.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 18.Horak E, Heitner T, Robinson MK, Simmons HH, Garrison J, Russeva M, Furmanova P, Lou JL, Zhou Y, Yuan QA, Weiner LM, Adams GP, Marks JD. Isolation of scFvs to in vitro produced extracellular domains of EGFR family members. Cancer Biotherapy and Radiopharmaceuticals. 2005;20:603–613. doi: 10.1089/cbr.2005.20.603. [DOI] [PubMed] [Google Scholar]