Abstract
NRG1-ErbB4 signaling controls inhibitory circuit development in the mammalian cortex through ErbB4 dependent regulation of GABAergic interneuron connectivity. Common genetic variation in ErbB4 (rs7598440) has been associated with ErbB4 messenger RNA levels in the human cortex and risk for schizophrenia. Recent work demonstrates that Erbb4 is expressed exclusively on inhibitory interneurons, where its presence on parvalbumin positive cells mediates the effects of NRG1 on inhibitory circuit formation in the cortex. We therefore hypothesized that genetic variation in ErbB4 at rs7598440 would impact indices of GABA concentration in the human cortex. We tested this hypothesis in 116 healthy volunteers by measuring GABA and GLX (glutamate + glutamine) with proton magnetic resonance spectroscopy (MRS) in the dorsal anterior cingulate gyrus. ErbB4 rs7598440 genotype significantly predicted cortical GABA concentration (p=0.014), but not GLX (p=0.51), with A allele carriers having higher GABA as predicted by their impact on ErbB4 expression. These data establish an association of ErbB4 and GABA in human brain and have implications for understanding the pathogenesis of schizophrenia and other psychiatric disorders.
Keywords: Glutamate, NAA, Creatine, Anterior Cingulate, white matter, gray matter, MRS, 3 Tesla
INTRODUCTION
Neuregulin 1 (NRG1)-ErbB4 signaling plays a critical role in inhibitory circuit development in the mammalian cortex. Studies in rodents have demonstrated that the ErbB4 receptor tyrosine kinase is expressed predominantly by specific subclasses of inhibitory γ-amino-butyric acid (GABA) neurons (Fazzari et al., 2010), including parvalbumin-expressing chandelier cells, where it is localized to synaptic terminals that innervate the axon initial segment of pyramidal neurons. ErbB4 also localizes to the post-synaptic density (Garcia et al., 2000; Huang et al., 2000), with recent data demonstrating this to be the postsynaptic density of GABAergic interneuron dendrites (Fazzari et al, 2010). Signaling via ErbB4 plays a critical role in regulating the development and function of both excitatory input and inhibitory output synapses of parvalbumin positive interneurons (Fazzari et al., 2010; Wen et al., 2010) and its presence on interneurons mediates the effects of neuregulin1 (NRG1) on pyramidal cell activity (Wen et al., 2010). These findings may have important implications for understanding disorders such as schizophrenia, where evidence exists for altered chandelier-pyramidal cell development (Lewis, 2011) and GABA-ergic neurotransmission (Lewis and Gonzalez-Burgos, 2008). Additionally, GABA levels measured in-vivo with MRS have been reported to be altered in schizophrenia, although the directionality at present is unclear (Ongur et al., 2010; Yoon et al., 2010).
In humans, genetic and post-mortem studies have implicated ErbB4 signaling in the pathogenesis of schizophrenia (Hahn et al., 2006; Silberberg et al., 2006; Law et al., 2007; Walsh et al., 2008). In particular, the CYT-1 isoform, which is linked to the PI3K pathway, is over expressed in the prefrontal cortex of patients with schizophrenia (Law et al., 2007) and common genetic variation in a haplotype including single nucleotide polymorphisms (SNPs) rs707284, rs839523 and rs7598440 is associated with increased cortical ErbB4 CYT-1 expression and risk for schizophrenia (Silberberg et al., 2006; Law et al., 2007).
Given the accumulating evidence of the role of ErbB4 in GABA biology, we hypothesized that genetic variation at rs7598440, a SNP that predicts ErbB4 expression in human brain (Law et al., 2007), would impact in-vivo cortical GABA levels in healthy individuals measured with proton MRS. We further predicted that Glx, the metabolite peak in the proton spectrum formed of glutamate and glutamine and most closely related to excitatory transmission, would be less likely to be directly related to ErbB4 genotype. While the Glx peak is partially co-edited in the MRS sequence used herein, resulting in some signal loss, we have documented significant reproducibility of this peak (Geramita et al., 2011), and it has been shown to be altered in psychiatric disorders (Hasler et al., 2007; Stone et al., 2009; Lutkenhoff et al., 2010).
MATERIALS AND METHODS
Participants
We recruited 116 adult healthy volunteers (59 males [mean age 34 ± 10.4 SD], 57 females [mean age 34.3 ± 10.1 SD]; age range 18-55) who had participated in the “CBDB sibling study of the genetics of schizophrenia” (NCT00001486: DRW principal investigator). Subjects were all Caucasian). Exclusion criteria included any history of psychiatric (DSM-IV axis I or II by a modified Structured Clinical Interview) or medical illness affecting the brain, pregnancy, and current psychotropic medication use. All subjects had no first degree relatives with a psychotic disorder according to an assessment performed with the Family Interview for Genetic Studies (https://www.nimhgenetics.org/interviews/figs/figs_interview.php). Participants were informed of the purpose of the study and provided written consent (NIMH protocols 95-M-0150 and 00-M-0085). Only three subjects were smokers. This GABA MRS dataset was the object of a prior report from our group (Marenco et al., 2010).
Genotyping
rs7598440 in ErbB4 was genotyped using the Taqman allelic discrimination assay, as previously described (Nicodemus et al., 2006).
Neuroimaging
Participants were scanned on three different 3T scanners (GE Waukesha, Wisconsin). Two scanners were equipped with a 14m4 software platform (n=79 and 22), while one ran on VH3 software (n=15). A quadrature transmit-receive head coil was used on all scanners (IGC-Medical Advances, Milwaukee, WI). A T1 weighted 3D SPGR sequence (TR/TE, 9.2/3.3 ms, flip angle 170, FOV 24 mm, matrix 256×192, 76 slices, 2 mm thick) was used for voxel placement and for image segmentation with SPM5 (http://www.fil.ion.ucl.ac.uk/spm). This segmentation was used to obtain relative proportions of gray, white matter and CSF present in the voxel by an automated program written in IDL (ITT Visual Information Solutions, Boulder, CO, USA) by ASB.
For MRS, a single voxel of interest (2 × 2 × 4.5 cm: 18cm3) was placed immediately superior to the ventricles, straddling the midline in order to contain the largest proportion of gray matter possible, with the anterior edge of the voxel never exceeding the anterior portion of the genu of the corpus callosum. This maximized coverage of the gray matter in the dorsal anterior cingulate cortex (ACC: Figure 1). One voxel was placed We chose this location because of the ease of measurement (a large amount of gray relative to white matter can be investigated with excellent shimming despite the high magnetic field) and because this region has been shown to function abnormally in schizophrenia.
Figure 1.
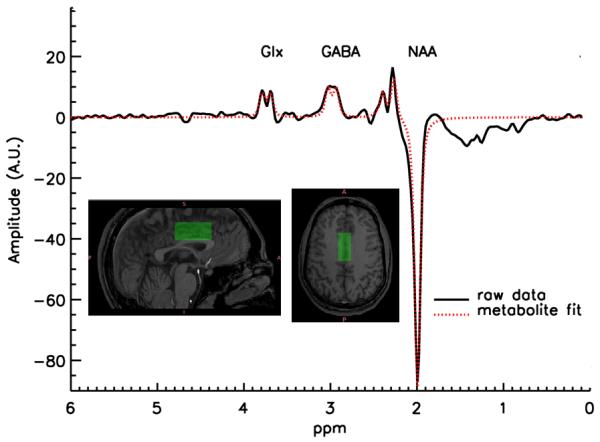
Example of a GABA spectrum and location of the MRS voxel.
An interleaved PRESS-based J editing method (Sailasuta et al., 2001; Hasler et al., 2007) was used for measurement of metabolites (TR/TE 1500/68 ms, NEX=2, 768 acquisitions with water suppression, 8 without, 2048 acquisition points, sampling frequency = 5000 Hz, 20 min scanning time). A fully automated nonlinear fitting program written in IDL by JWvdV was used to calculate metabolite levels, described in more detail in (Geramita et al., 2011). The amplitudes of residual water, N-acetyl aspartate (NAA), Choline, and total Creatine (tCre) were fitted in the unedited spectrum. Subsequently, the unedited spectrum was subtracted from the edited spectrum, resulting in a difference spectrum where the co-edited Glx (glutamate + glutamine) and GABA signals were separated from overlapping resonances. After subtraction of the water baseline, GABA and Glx were fitted automatically, using the line width and positions fit results from the fit of Cho, tCre, and NAA in the unedited spectrum. When the difference in the residual water signal between an “editing-pulse-on” and the alternating “editing-pulse-off” acquisition was greater than 10%, indicating possible movement during the scan (van der Veen et al., 2007), the fitting procedure rejected both acquisitions. The rate of rejection due exclusively to poor fitting is around 4% for healthy volunteers. Two further quality control procedures were put in place: 1) at the time of scanning, the voxel was shimmed to a linewidth for water below 10Hz in all cases (the average linewidth being 7 ± 0.96 Hz); 2) our expert physicist (JWvdV) reviewed all spectra blind to genotype to ensure adequate fitting and lineshapes. The entire process yielded estimates for signal intensities (see Fig. 2 of Geramita et al., 2011) of the main metabolites, which were then corrected for cerebrospinal fluid (CSF) content of the voxel, based on the assumption that no metabolites are present in CSF.
Figure 2.
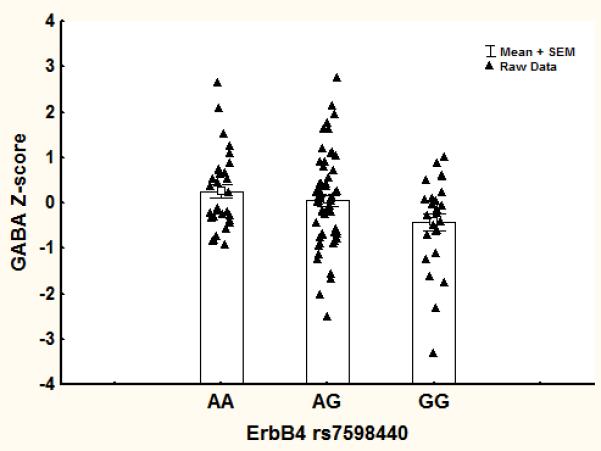
Mean values of GABA for the three genotypes at rs7598440 in ErbB4.
The unsuppressed water signal was used as a reference for all studied metabolites. The raw water signal was corrected based on the voxel composition (gray, white matter and CSF) of each individual subject based on this formula:
Corrected water = water signal / (0.92*%gray + 0.81*%white + %CSF)
This formula was derived taking into account literature values for T1, T2 and proton density of water in gray, white matter and CSF at 3 Tesla (Lin et al., 2001; Stanisz et al., 2005; Aubert-Broche et al., 2006; Piechnik et al., 2009) and was found to be in good agreement with empirical data in our laboratory (Geramita et al., 2011).
Statistics
Our initial hypothesis was limited to GABA and Glx. Glx represents a measure of glutamate metabolism that should be less sensitive than GABA to effects of ErbB4 genetic variation. In the whole sample of 116 subjects, we first transformed all dependent measures (GABA, Glx, NAA and tCre) to a z-score (the mean value for the individual scanner was subtracted from the dependent variable and divided by the standard deviation), thus removing scanner effects prior to further analyses. This procedure was applied to each metabolite separately.
Potential effects of age, sex, and percent gray matter contribution to the voxel were taken into account by testing multiple regression models with a stepwise backward selection procedure where ErbB4 genotype, and the covariates listed above were used as independent variables, with all main effects forced into the model and all two way interactions explored. The covariates were chosen based on prior findings that age (Marenco et al., 2010), gray/white matter partial voluming (Geramita et al., 2011), and sex (Epperson et al., 2005) affect GABA concentration. The p value to include or exclude effects from the model was set to 0.1. One model was run for each independent variable.
A series of secondary analyses were performed to, 1) assess further the biological specificity of the effect of ErbB4 genotype. To this end, N-acetyl-aspartate (NAA) and total creatine (tCre) containing compounds were investigated in the same manner as described above for GABA; 2) to assess the independence of the effects reported here for ErbB4 from GAD1 genotypes at rs1978340 and rs769390, SNPs previously shown to be associated with GABA/tCre ratios in this sample (Marenco et al., 2010), we added GAD1 genotypes to the regression models; 3) to assess robustness of the ratios to water, effects of ErbB4 genotype were also tested on GABA/tCre ratios; 4) to ensure that our initial results were not due to age, gender or percent gray matter differences across scanners, we added the ‘scanner’ variable to the original ErbB4 model as an independent predictor.
RESULTS
We found a significant effect of genotype on GABA levels (F(2,110)=4.4, p=0.014). Individuals homozygous and heterozygous for the (A) allele associated with increased ERBB4 expression had higher cortical GABA concentrations (Figure 2). In contrast, ErbB4 genotype was not significantly associated with Glx (Table 1).
Table 1.
Results of Multiple Regression
| GABA/H2O | dof | F | p |
|---|---|---|---|
| Intercept | 1 | 3.35 | 0.070 |
| Age | 1 | 3.28 | 0.073 |
| %GM/tissue | 1 | 2.34 | 0.129 |
| Sex | 1 | 6.24 | 0.014 |
| Erbb4 | 2 | 4.44 | 0.014 |
| 110 | |||
| Error | |||
| GLX/H2O | |||
|
| |||
| Intercept | 1 | 0.29 | 0.588 |
| Age | 1 | 0.03 | 0.864 |
| %GM/tissue | 1 | 0.35 | 0.557 |
| Sex | 1 | 2.13 | 0.147 |
| Erbb4 | 2 | 0.68 | 0.508 |
| Sex*Age | 1 | 5.67 | 0.019 |
| 109 | |||
| Error | |||
| NAA/H2O | |||
|
| |||
| Intercept | 1 | 3.89 | 0.051 |
| Age | 1 | 5.65 | 0.019 |
| %GM/tissue | 1 | 2.71 | 0.102 |
| Sex | 1 | 5.16 | 0.025 |
| Erbb4 | 2 | 2.36 | 0.099 |
| Sex*%GM/tissue | 1 | 5.77 | 0.018 |
| 109 | |||
| Error | |||
| tCre/H2O | |||
|
| |||
| Intercept | 1 | 6.81 | 0.010 |
| Age | 1 | 36.71 | 0.000 |
| %GM/tissue | 1 | 2.47 | 0.119 |
| Sex | 1 | 0.26 | 0.613 |
| Erbb4 | 2 | 2.42 | 0.094 |
| 110 | |||
| Error | |||
2 way interactions that do not appear in the model were removed.
There were weak, non-significant trends towards association of ErbB4 genotype with tCre and NAA (Table 1). Analysis of GABA/tCre revealed findings for ErbB4 genotype that were also significant (F2,109=5.1, p=0.008). Adding GAD1 rs1978340 and rs769390 to the original model as another independent variable did not impact the main effect of ErbB4 rs7598440 (Table 2). ErbB4 genotype accounted for 7% of the variance in GABA levels and the addition of either GAD1 SNP explained up to 14%. Adding the “scanner” variable to the original model, did not change the results for ErbB4 genotype (F2,105=4.38, p=0.015).
Table 2.
Results after including GAD1 SNPs to the original model
| GABA/H2O | dof | F | p |
|---|---|---|---|
| Intercept | 1 | 3.73 | 0.056 |
| Age | 1 | 4.05 | 0.047 |
| %GM/tissue | 1 | 2.92 | 0.090 |
| Sex | 1 | 5.61 | 0.020 |
|
GAD1
rs1978340 |
2 | 4.68 | 0.011 |
| Erbb4 | 2 | 5.69 | 0.004 |
| 107 | |||
| Error |
| GABA/H2O | dof | F | p |
|---|---|---|---|
| Intercept | 1 | 2.19 | 0.141 |
| Age | 1 | 3.73 | 0.056 |
| %GM/tissue | 1 | 1.70 | 0.194 |
| Sex | 1 | 6.76 | 0.011 |
|
GAD1
rs769390 |
2 | 4.86 | 0.010 |
| Erbb4 | 2 | 5.47 | 0.005 |
| 107 | |||
| Error |
DISCUSSION
Our data provide the first in-vivo evidence of an effect of ErbB4 on GABA concentration in living human brain. The directionality of the effect was consistent with the association of the A allele in rs7598440 with increased cortical ErbB4 expression (Law et al., 2007). At present it is unclear whether the association of cortical GABA levels with ErbB4 genotype in normal subjects reflects functional differences in enzyme activity or neuroanatomical differences in total number or distribution of interneurons or their synapses. It is noteworthy, however, that ErbB4 mutant mice demonstrate changes in the number of GABAergic synapses (Fazzari et al., 2010) but unaltered total number or laminar distribution of GABA neurons.
Weak effects of ErbB4 genotype on NAA and tCre measures were identified in our study and contrary to our expectations, substantial correlations between GABA and these metabolites were observed (GABA was significantly correlated with tCre [r=0.47, p<10−5] and NAA [r=0.53, p<10−5], and tCre was significantly correlated to NAA [r=0.54, p<10−5] and, more weakly, to Glx [r=0.22, p=0.02]). NAA has been used as an index of neuronal integrity and synaptic abundance (De Stefano et al., 2002) and has been indirectly related to glutamate metabolism (Petroff et al., 2002; Egan et al., 2004; Marenco et al., 2006), while tCre represents compounds related to energy metabolism (Miller, 1991) and may be related in some situations to cell density (Rumpel et al., 2001). These analyses were performed as post-hoc surveys and should be viewed with circumspection. It is conceivable that part of the NAA signal reflects the synaptic biology of GABA neurons and is weakly associated with ErbB4 genotype via this link. The relatively high correlations among metabolites reported here may depend on using water as a reference, since small variation in water content or relaxation parameters will result in concomitant variation in the normalized metabolite values, especially those with a higher signal to noise ratio (NAA and tCre). In addition, the correlation between GABA levels and tCre may result from the shared macromolecules underlying both peaks.
The effect of ErbB4 was independent of GAD1 SNPs previously shown to predict GABA levels in this same sample (Marenco et al., 2010). This suggests that, as would be predicted based on their respective biological functions, GAD1 and ErbB4 impact GABA levels through biologically distinct and complementary mechanisms.
In contrast to genetic and cell-based studies of GABAergic function in rodents, assessment of GABA concentration using proton MRS involves a relatively large measurement volume that represents GABA stores beyond just synaptic neurotransmission. Also, the GABA peak is contaminated by macromolecules (Rothman et al., 1993; Kegeles et al., 2007), which are formed by multiple lipoproteins Nevertheless, it is highly unlikely that an ErbB4 genetic effect would systematically impact this diverse pool of molecules. We have further shown that other limitations such as the metabolite used as reference (similar results were obtained using tCre as a reference metabolite rather than water), and residual effects of the different scanners used were inconsequential to the reported genotype effect. Indeed, significant genotype effects were obtained when Cre was used as a reference metabolite, demonstrating that both water and tCre are valid references. However, the interpretation of the GABA/Cre association may be complicated by a weak trend towards significance in the analysis of tCre/water (see Table 1) and the correlation between GABA and tCre.
In sum, our findings establish a link between ErbB4 and GABA in the human brain, and illuminate aspects of the genetic regulation of GABAergic signaling. Our findings also have potential ramifications for elucidating the pathophysiological mechanisms of ErbB4 associated neurodevelopmental disorders, such as schizophrenia.
Acknowledgements
This work was funded entirely from the NIMH IRP. We thank Antonina A. Savostyanova for help in collecting and organizing the data and running preliminary analyses.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
REFERENCES
- Aubert-Broche B, Evans AC, Collins L. A new improved version of the realistic digital brain phantom. Neuroimage. 2006;32:138–145. doi: 10.1016/j.neuroimage.2006.03.052. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Narayanan S, Francis SJ, Smith S, Mortilla M, Tartaglia MC, Bartolozzi ML, Guidi L, Federico A, Arnold DL. Diffuse axonal and tissue injury in patients with multiple sclerosis with low cerebral lesion load and no disability. Arch Neurol. 2002;59:1565–1571. doi: 10.1001/archneur.59.10.1565. [DOI] [PubMed] [Google Scholar]
- Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Mattay VS, Bertolino A, Hyde TM, Shannon-Weickert C, Akil M, Crook J, Vakkalanka RK, Balkissoon R, Gibbs RA, Kleinman JE, Weinberger DR. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci U S A. 2004;101:12604–12609. doi: 10.1073/pnas.0405077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, O’Malley S, Czarkowski KA, Gueorguieva R, Jatlow P, Sanacora G, Rothman DL, Krystal JH, Mason GF. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;57:44–48. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Lerma J, Marin O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geramita M, Van der Veen JW, Barnett AS, Savostyanova AA, Shen J, Weinberger DR, Marenco S. Reproducibility of prefrontal GABA measurements with j-edited spectroscopy. NMR Biomed. 2011 doi: 10.1002/nbm.1662. doi: 10.1002/nbm.1662. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Mao X, Gonsalez R, Shungu DC. Proc. Intl. Soc. Mag. Reson. Med. Berlin, Germany: 2007. Evaluation of Anatomic Variation in Macromolecule Contribution to the GABA Signal using Metabolite Nulling and the J-editing Technique at 3.0 T; p. 1391. [Google Scholar]
- Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- Lewis DA. The chandelier neuron in schizophrenia. Dev Neurobiol. 2011;71:118–127. doi: 10.1002/dneu.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Lin C, Bernstein M, Huston J, Fain S. Proc Intl Soc Mag Reson Med. Glasgow, Scotland: 2001. Measurements of T1 relaxation times at 3.0T: implications for clinical MRA; p. 1391. [Google Scholar]
- Lutkenhoff ES, van Erp TG, Thomas MA, Therman S, Manninen M, Huttunen MO, Kaprio J, Lonnqvist J, O’Neill J, Cannon TD. Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry. 2010;15:308–318. doi: 10.1038/mp.2008.87. [DOI] [PubMed] [Google Scholar]
- Marenco S, Steele SU, Egan MF, Goldberg TE, Straub RE, Sharrief AZ, Weinberger DR. Effect of metabotropic glutamate receptor 3 genotype on N-acetylaspartate measures in the dorsolateral prefrontal cortex. Am J Psychiatry. 2006;163:740–742. doi: 10.1176/appi.ajp.163.4.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenco S, Savostyanova AA, van der Veen JW, Geramita M, Stern A, Barnett AS, Kolachana B, Radulescu E, Zhang F, Callicott JH, Straub RE, Shen J, Weinberger DR. Genetic modulation of GABA levels in the anterior cingulate cortex by GAD1 and COMT. Neuropsychopharmacology. 2010;35:1708–1717. doi: 10.1038/npp.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed. 1991;4:47–52. doi: 10.1002/nbm.1940040203. [DOI] [PubMed] [Google Scholar]
- Nicodemus KK, Luna A, Vakkalanka R, Goldberg T, Egan M, Straub RE, Weinberger DR. Further evidence for association between ErbB4 and schizophrenia and influence on cognitive intermediate phenotypes in healthy controls. Mol Psychiatry. 2006;11:1062–1065. doi: 10.1038/sj.mp.4001878. [DOI] [PubMed] [Google Scholar]
- Ongur D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated Gamma-Aminobutyric Acid Levels in Chronic Schizophrenia. Biol Psychiatry. 2010;68:667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Neuronal and glial metabolite content of the epileptogenic human hippocampus. Ann Neurol. 2002;52:635–642. doi: 10.1002/ana.10360. [DOI] [PubMed] [Google Scholar]
- Piechnik SK, Evans J, Bary LH, Wise RG, Jezzard P. Functional changes in CSF volume estimated using measurement of water T2 relaxation. Magn Reson Med. 2009;61:579–586. doi: 10.1002/mrm.21897. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel H, Khoo JB, Chang HM, Lim WE, Chen C, Wong MC, Tan KP. Correlation of the apparent diffusion coefficient and the creatine level in early ischemic stroke: a comparison of different patterns by magnetic resonance. J Magn Reson Imaging. 2001;13:335–343. doi: 10.1002/jmri.1048. [DOI] [PubMed] [Google Scholar]
- Sailasuta N, LeRoux P, Hurd R, Wang P, Sachs N, Ketter T. Proc. Intl. Soc. Magn. Reson. Med. 2001. Detection of cerebral gamma-aminobutyric acid (GABA) in bipolar disorder patients and healthy volunteers at 3T; p. 1011. [Google Scholar]
- Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med. 2005;54:507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, O’Gorman RL, Barker GJ, McGuire PK. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66:533–539. doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- van der Veen JW, Hattori N, Shen J. Quantification of Co-Edited Macromolecules in GABA J-Editing. Proc Intl Soc Magn Reson Med. 2007;15:1399. [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, Yin DM, Lai C, Terry AV, Jr., Vazdarjanova A, Xiong WC, Mei L. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, Carter CS. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


