Abstract
Peroxisome proliferator activated receptor gamma (PPARγ) regulates metabolic homeostasis and is a molecular target for antidiabetic drugs. We report here the identification of a steroid receptor ligand, RU-486, as an unexpected PPARγ agonist, thereby uncovering a novel signaling route for this steroid drug. Similar to rosiglitazone, RU-486 modulates the expression key PPARγ target genes and promotes adipocyte differentiation but with lower adipogenic activity. Structural and functional studies of receptor-ligand interactions reveal the molecular basis for a unique binding mode for RU-486 in the PPARγ ligand binding pocket with distinctive properties and epitopes, providing the molecular mechanisms for the discrimination of RU-486 from thiazolidinediones (TZDs) drugs. Our findings together indicate that steroid compounds may represent an alternative approach for designing non-thiazolidinedione PPARγ ligands in the treatment of insulin resistance.
Keywords: PPARγ, nuclear receptor, diabetes, crystal structure, steroid compound
Introduction
Peroxisome proliferator activated receptor gamma (PPARγ) is a nuclear receptor that plays key roles in regulating glucose homeostasis and adipocyte differentiation 1, 2. The synthetic PPARγ ligands rosiglitazone (Avandia™) and pioglitazone (Actos™) are thiazolidinedione derivatives (TZDs) available currently for the treatment of type 2 diabetes. While improving insulin sensitivity and lowering plasma glucose levels, the clinical use of TZDs has been tempered by side effects such as edema, weight gain, and increased incidence of heart attack 3,4. These adverse side effects may limit further development and clinical application of TZD-based PPARγ ligands. Indeed, the TZD drug Avandia was recently banned in Europe and was also restricted by FDA due to its cardiovascular risks. As such, a new drug design strategy for PPARγ ligands is needed to yield more efficacious PPARγ-targeted drugs with less adverse effect 5.
The pharmacological actions of PPARγ ligands are mediated through the ligand-binding domain (LBD) of PPARγ that recruits a variety of nuclear receptor coactivators (or co-repressors)to regulate its downstream target genes depending on the nature of ligands 6-10. The large pocket seen in all three PPARs has a distinct three-arm Y-shape, allowing PPARs to bind singly branched ligands or ligands with multiple branches 11. Aside from the size and shape, the overall hydrophobic nature of the ligand-binding pocket also plays a role in controlling the binding promiscuity of nuclear receptors to various lipid soluble ligands, which also raises challenging questions on ligand selectivity among many other nuclear receptor members 12, 13. Indeed, many undesired side effects of drugs targeting nuclear receptors are associated with the cross-reactivity of these ligands with other members in the nuclear receptor family. On the other hand, cross-reactivity may also offer opportunities to improve therapeutic efficacy of the ligands by providing additive or complementary effects through simultaneously regulating several related targets 14, 15.
We report here the identification of the steroid compound RU-486 as an unexpected PPARγ agonist and also the structural basis for its binding selectivity. RU-486 (Mifeprstone) is a steroid compound with many medical uses such as treatment of hypercortisolism 16, 17. Remarkably, RU-486 also displays anti-diabetic activities by reducing blood glucose levels and improving insulin sensitivity 18-21. However, the molecular signaling pathway of RU-486 is not clear except for its antagonist properties for steroid receptors. In this report, we attributed the medical effects of RU486, at least in part, to its interaction with the nuclear receptor PPARγ, revealing a hitherto unidentified signaling route for this multifaceted drug. The structure of PPARγ complexed with RU-486 shows a unique binding mode of RU-486 in the PPARγ ligand binding pocket, with the receptor adopting the canonical active conformation. Directed mutations in the PPARγ pocket reveal a strong correlation of RU-486 binding and PPARγ activation. Moreover, RU-486 modulates key PPARγ target gene expression in adipocyte differentiation but with lower adipogenic activity than TZDs. Our observations therefore indicate that RU-486 may represent an alternative approach for designing non-thiazolidinedione PPARγ ligands for the treatment of insulin resistance.
Results
Identification of RU-486 as a potent PPARγ ligand
To identify new PPARγ agonists, we screened chemical libraries based on AlphaScreen biochemical assay, which is widely used for detecting ligand-dependent interaction between nuclear receptors and their coactivators 22, 23. As expected, results from Biomol FDA drug library revealed that TZD drugs pioglitazone and troglitazone as positive PPARγ activators together with rosiglitzazone which was used as positive control (Supplementary information, Figure S1). Strikingly, the steroid compound RU-486 also substantially induced the coactivator binding activity by PPARγ. To further attain characteristics of RU-486 in activating nuclear receptors, Cos7 cells were cotransfected with a Gal4-driven reporter together with plasmids encoding various nuclear receptor LBDs fused with the Gal4 DNA-binding domain. Consistent with previous observations, RU-486 showed weak agonist activity on glucocorticoid receptors (GR) in the absence of endogenous or exogenous glucocorticoids (Fig. 1A) 24. In agreement with the results from AlphaScreen biochemical assay, treatment of RU-486 significantly induced the transactivational activity of PPARγ, with weak cross-reactivity for PPARα and δ (Fig. 1A). In contrast, treatment of RU-486 had no impact on a variety of other nuclear receptors tested, including retinoic acid receptors α and γ, throid hormone receptor β, liver X receptor β, or pregnane X receptor (Fig. 1A). Unlike the antagonistic properties of RU-486 on PR and GR, RU-486 did not show inhibitory effects on PPARγ activity when tested with receptor-specific agonists (Supplementary information, Figure S2). Moreover, RU-486-induced PPARγ activity was also observed in the context of the full-length receptor and a PPARγ response reporter (Fig. 1B). In addition, full dose curves in vivo (Fig. 1B) revealed that RU-486 activated PPARγ in a concentration-dependent manner. Further, RU-486-mediated PPARγ activity was inhibited by the PPARγ-specific antagonist GW9662 in cell-based reporter assays (Fig. 1C) 25, all indicating that RU-486 is a bona fide PPARγ ligand.
Figure 1. RU-486 is a high affinity PPARγ agonist.
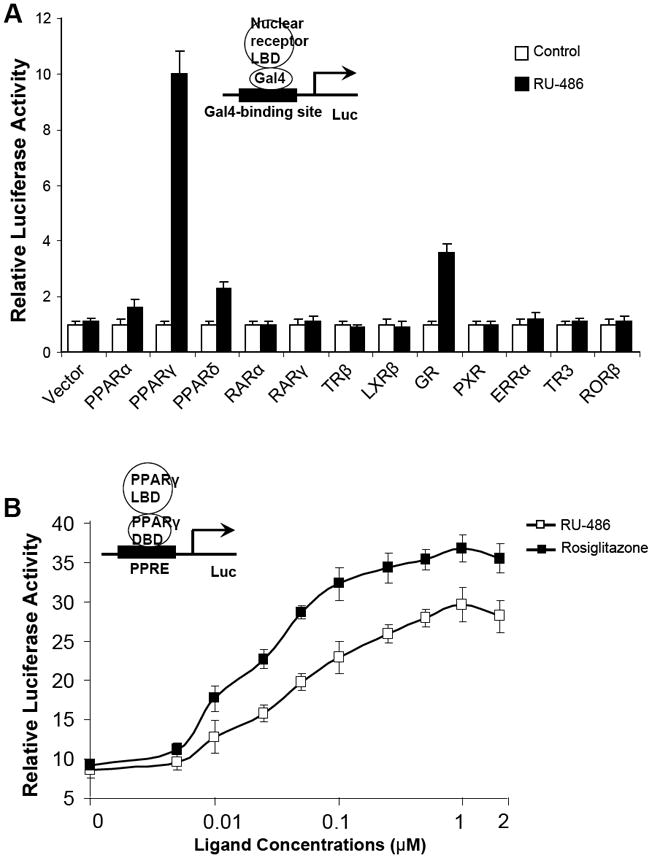
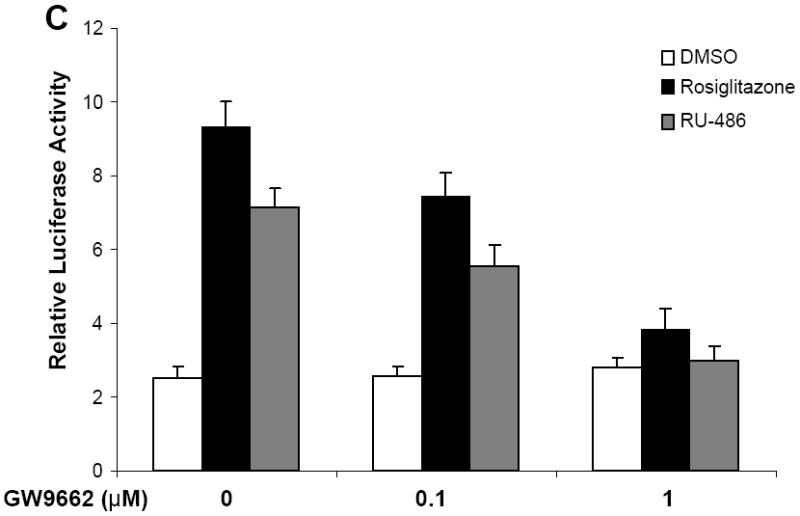
(A) Receptor-specific transactivation by RU-486. Cos7 cells were cotransfected with pG5Luc reporter together with the plasmids encoding various nuclear receptors LBDs fused with the Gal4 DNA-binding domain. After transfection, cells were treated with DMSO or 1 μM RU-486. (B) Dose responses of RU-486 in transactivating PPARγ. Cos7 cells were cotransfected with plasmids encoding full-length PPARγ and a PPRE luciferase reporter. After transfection, cells were treated with DMSO or various concentrations of RU-486 and rosiglitazone. (C) PPARγ activation by RU-486 was inhibited by PPARγ antagonist GW9662. Cos7 cells were cotransfected with plasmids encoding full-length PPARγ and a PPRE luciferase reporter. After transfection, cells were treated with RU-486 together with various concentrations of GW9662.
To unravel the biochemical mechanism of PPARγ activation by RU-486, we determined the ability of RU-486 in promoting recruitment of coregulator motifs by PPARγ using AlphaScreen biochemical assay. As shown in Fig. 2, both RU-486 and rosiglitazone strongly enhanced the interaction of PPARγ with various coactivator LXXLL motifs from the family of steroid receptor coactivators (SRC1-2, SRC1-4, SRC2-3 and SRC3-3), CBP and PGC-1α but not a corepressor motif from NcoR (NcoR-2), reaffirming that RU-486 functions as an agonist through directly binding to PPARγ.
Figure 2. The transcriptional properties of PPARγ in response to RU-486 ligand.
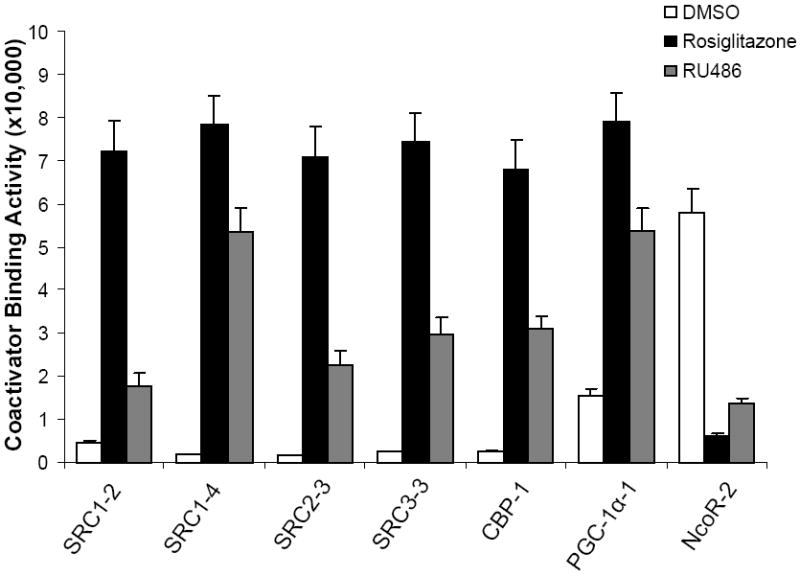
RU-486 promotes the interaction coactivator LXXLL motifs with PPARγ. Modulation of the interaction of PPARγ LBD with various coactivator LXXLL motifs and co-repressor motifs in response to 1 μM RU-486 was shown by AlphaScreen assays. The peptide sequences are listed in experimental procedures.
RU-486 regulates PPARγ-target genes in adipocytes
To further assess the roles of RU-486 in the physiological function of PPARγ, we studied the effects of RU-486 on PPARγ-activated adipogenesis in 3T3-L1 preadipocyte cells. Compared to rosiglitazone, RU-486 induced weaker adipocyte differentiation, as indicated by oil red O staining (Fig. 3A). We further performed PPARγ-target gene analysis on 3T3-L1 cells treated with RU-486. Similar to rosiglitazone, RU-486 also auto-regulated the expression of PPARγ2 gene 26 (Fig. 3B). As shown in Fig. 3B, the expression of differentiation-dependent PPARγ-target genes, like aP2 27, LPL 28, CD36 29 and adiponectin 30, were also induced by RU-486, but to a lower extent than rosiglitazone. As such, the gene profile analysis is consistent with oil red O staining. Further, RU-486-mediated PPARγ-target gene regulation was reduced by the PPARγ-specific antagonist GW9662, reaffirming that RU-486 affects gene expression at least in part by directly activating PPARγ (Supplementary information, Figure S3). Overall, these results indicate that RU-486 is a potent activator of PPARγ-responsive genes during adipocyte differentiation but with lesser effect on adipogenesis, suggesting a potential advantage over TZD drugs.
Figure 3. RU-486 regulates PPARγ-target genes in adipogenesis and adipocytes.
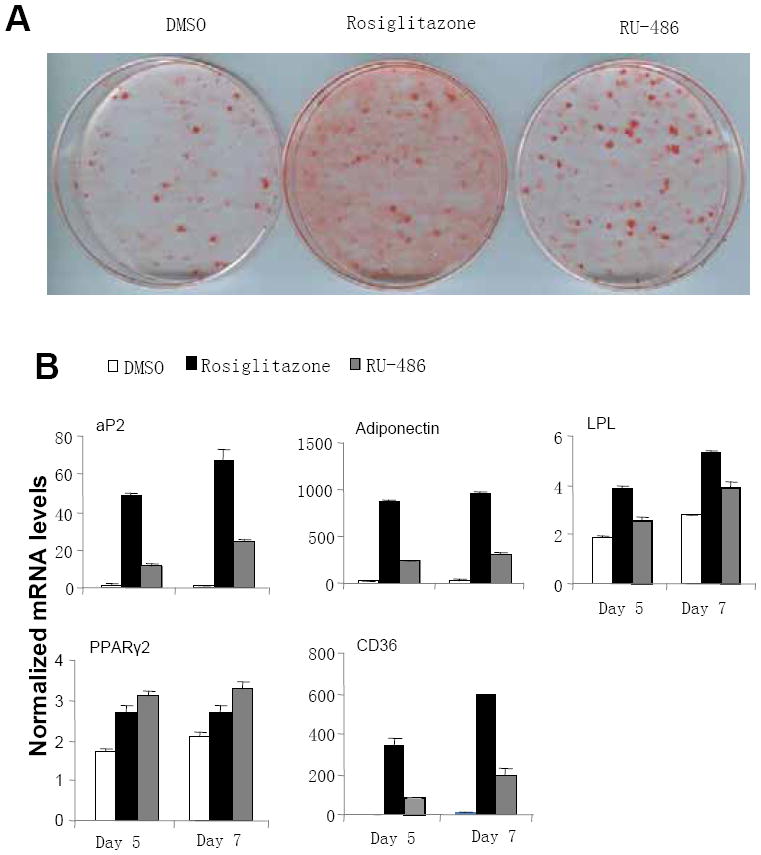
(A) Oil red O staining of 3T3-L1 cells after treatment with 1 μM ligands indicated for 7 days. (B) Gene expression profile during the adipocyte differentiation of 3T3-L1 cells induced by 1 μM ligands indicated.
Structural determination of the PPARγ LBD in complex with RU-486
To determine the molecular basis for the high binding affinity of RU-486 by PPARγ, we solved the crystal structure of PPARγ complexed with RU-486 and the SRC1-2 LXXLL motif at 2.5 Ǻ resolution. The data statistics and the refined structures are summarized in Table 1. The structure reveals that the RU-486-bound PPARγ LBD adopts a canonical active conformation that resembles the rosiglitazone-bound PPARγ structure (Fig. 4A) 31, 32. Specifically, the PPARγ LBD is composed of 13 α helices and four short β strands that are folded into a three-layer helical sandwich. The C-terminal AF-2 helix is positioned in the active conformation by packing tightly against the main domain of the LBD.
Table 1.
Data collection and refinement statistics
| PPARγ/RU-486 | |
|---|---|
| Data collection | |
| Space group | C 2 2 21 |
| Cell dimensions | |
| a, b, c (Å) | 53.22, 95.78, 125.29 |
| α, β, γ (°) | 90, 90, 90 |
| Resolution (Å) | 50.0-2.5(2.54-2.50) * |
| Rsym | 0.068(0.482) |
| I / σ | 65.4(2.5) |
| Completeness (%) | 99.6(97.1) |
| Redundancy | 8.6(4.7) |
| Refinement | |
| Resolution (Å) | 31.4-2.5 |
| No. reflections | 11435 |
| Rwork / Rfree | 19.6/27.7 |
| No. atoms | |
| Protein | 2207 |
| Ligand/ion | 32 |
| Water | 5 |
| B-factors | |
| Protein | 67.8 |
| Ligand/ion | 67.1 |
| Water | 53.3 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.009 |
| Bond angles (°) | 1.4 |
Values in parentheses are for highest-resolution shell.
r.m.s.d is the root mean square deviation from ideal geometry of protein
Rsym =Σ | Iavg - Ii | / Σ Ii
Rfactor = Σ | FP - FPcalc | / Σ Fp , where Fp and Fpcalc are observed and calculated structure factors, R free was calculated from a randomly chosen 8% of reflections excluded from refinement and R factor was calculated for the remaining 92% of reflections.
Figure 4. Recognition of RU-486 by PPARγ.
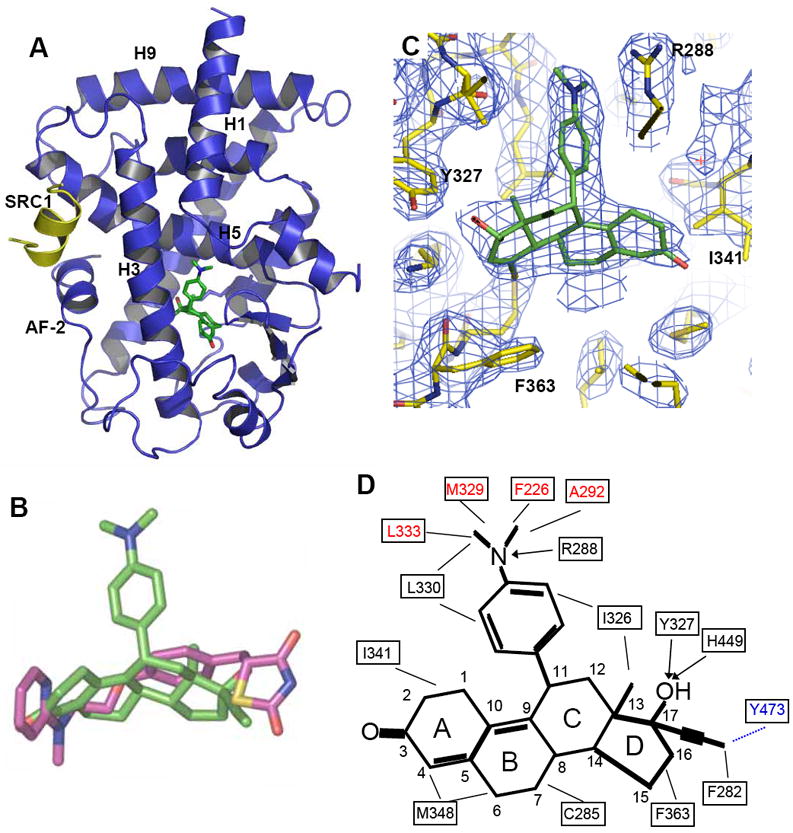
(A) The structures of RU-486 bound with PPARγ LBD in ribbon representation. PPARγ LBD is colored in blue and the SRC1 motif is in yellow. The bound RU-486 is shown in stick representation with carbon and oxygen atoms depicted in green and red, respectively. (B) Superposition of RU-486 (green) with rosiglitazone (purple). (C) 2Fo-Fc electron density map (1.0σ) showing bound RU-486 to the PPARγ LBD. The bound RU-486 is shown in stick representation with carbon and oxygen atoms depicted in green and red, respectively. (D) Schematic representation of PPARγ-RU-486 interaction. The residues labeled red are unique contacts for RU-486 not observed for rosiglitazone, while the residues in blue are the ones lost contacts with RU-486. Key hydrocarbon positions of RU-486 molecule are indicated. Hydrophobic interactions are indicated by lines and hydrogen bonds are indicated by arrows from proton donors to acceptors.
PPARγ/RU-486 and PPARγ/rosiglitazone displayed a similar LBD structure with both ligands docking the similar binding site in the PPARγ pocket (Fig. 5A & 5B). Superposition of their ligand-bound structures revealed that the steroid core of RU-486 aligned well with rosigltazone, suggesting that the steroid core occupies a well conserved ligand-binding site in PPARγ (Fig. 4B). In fact, this is also the very location that accommodates various endogenous PPARγ ligands 33, 34. In addition to sitting into the conserved binding mode by the steroid core, the distinct dimethylaniline side chain of RU-486 was apparent from the highly revealing electron density map shown in Fig. 4C. The binding of RU-486 to PPARγ was stabilized by a combination of hydrogen bonds and hydrophobic interactions (Fig. 4D). Compared with rosiglitazone, the shorter steroid core of RU-486 resulted in fewer contacts with residues that outline the cavity (Fig. 4B). However, the loss of these hydrophobic contacts was compensated by several hydrogen bonds and hydrophobic interactions created by the dimethylaniline side chain of RU-486, which were not observed in rosiglitazone-bound PPARγ structure (Fig. 4D).
Figure 5. Superposition of the RU-486-bound PPARγ to rosiglitazone-bound PPARγ and RU-486-bound GR LBD.
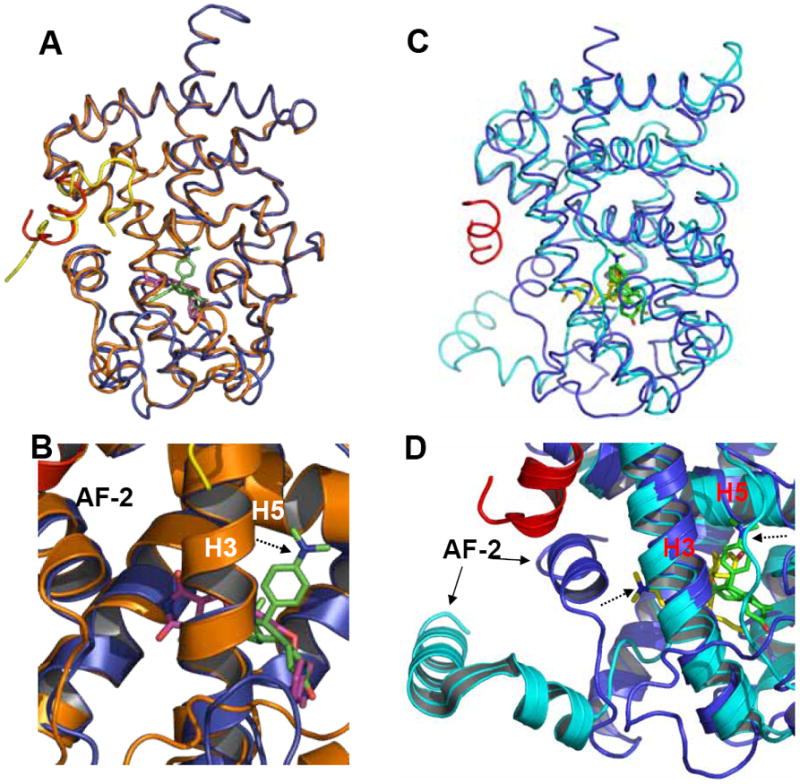
(A &B) Overlays of the PPARγ-RU486 structure (blue) with the PPARγ-rosiglitazone (gold) structure, where ligand RU-486 is in green and rosiglitazone is in purple. (C &D) Overlays of the PPARγ-RU486 structure (blue) with the GR-RU-486 structure (cyan), where ligand RU-486 is in green for PPARγ and the GR-bound RU-486 is in yellow. The dashed arrows indicate the positions of the dimethylaniline side chain of RU486 .
RU-486 possesses antagonist activity for steroid receptors such as glucocorticoid receptor (GR). The opposing roles of RU-486 in GR and PPARγ can be possibly explained by the specific position of the RU-486 dimethylaniline side chain in the PPARγ pocket. Superposition of the RU-486/PPARγ structure with RU-486/GR structure revealed two distinct orientations of RU-486 binding (Fig.5C & 5D). In RU-486/GR structure, the dimethylaniline side chain of RU-486 points to helix 12 (AF-2), thus interfering the configuration of the active conformation of GR 35. Interestingly, the binding mode of RU-486 to progesterone receptor (PR) was similar to that of GR 36. In contrast to the binding mode of RU-486 to the steroid receptors GR and PR, the dimethylaniline side chain of RU-486 adapts to the specific microenvironment in PPARγ composed of residues from helix 3, helix 5 and a loop between helix1 and helix 2 (Fig. 4A and Fig. 5B). Instead of pointing to helix 12 (AF-2) observed in steroid receptors, the dimethylaniline side chain orientates toward helix 5 of PPARγ (Fig. 4A, Fig.5 C & 5D). Overall, the three-dimensional structure provides critical insights into the molecular basis for the formation of potent PPAR activators and informs the design of RU-486 derivatives with improved selectivity to PPARγ.
The unique binding mode of RU-486 in the PPARγ pocket
To validate the roles of pocket residues in RU-486 binding and PPARγ activation, we mutated several key residues that contact different groups of RU-486 and tested the transcriptional activity of these mutated PPARγ in response to RU-486 in cell-based reporter assays using full-length PPARγ and a PPARγ response reporter. R288, A292 and I326 are three PPARγ pocket residues that form contacts with the dimethylaniline side chain of RU-486 (Fig. 6A). Three mutations were designed to reduce the size of the pocket arm in PPARγ that accommodates the dimethylaniline side chain by changing the corresponding residue to a tryptophan, thereby preventing the binding of the bulky RU-486 ligand. In contrast to interfering with RU-486 binding, these mutations were predicted to stabilize the rosiglitazone-bound PPARγ receptor by filling up the empty arm not occupied by the rosiglitazone with bulky tryptophan side chains. Accordingly, all three mutations abolished or substantially reduced RU-486-mediated PPARγ transcriptional activity but they retained the ability to be activated by rosiglitazone (Fig. 6E). In addition, the nitrogen on the dimethylaniline side chain of RU-486 forms a hydrogen bond with R288 (Fig. 6A). Mutation of this residue to hydrophobic leucine decreased PPARγ activation by RU-486 but enhanced PPARγ activation by rosiglitazone (Fig. 6E). Together, these data suggest that interactions between the dimethylaniline side chain of RU-486 and PPARγ are required for stable binding of RU-486.
Figure 6. Functional correlation of the RU-486/ PPARγ interactions.
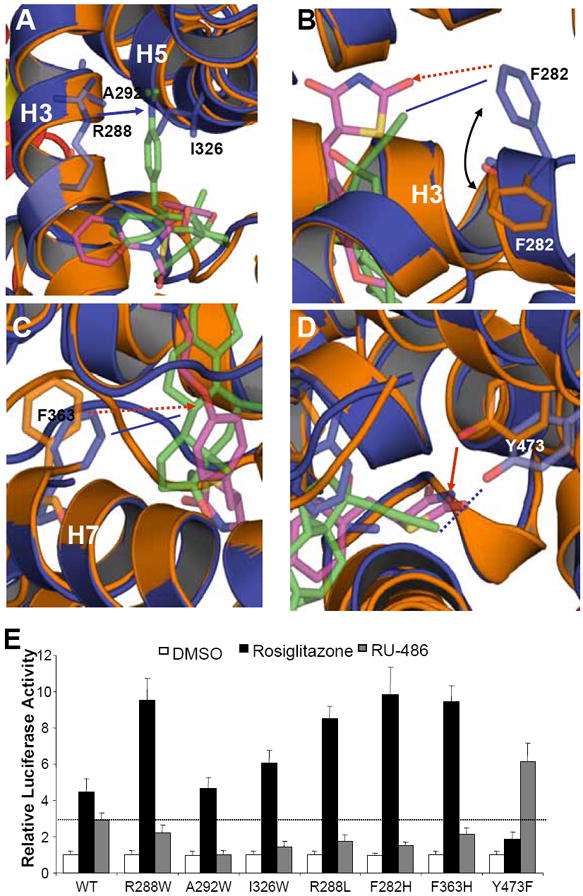
(A-D) Molecular determinants of the interaction between PPARγ with ligand RU-486. Overlays of the PPARγ-RU-486 structure (blue) with the PPARγ-rosiglitazone (gold) structure, where ligand RU-486 is in green and rosiglitazone is in purple. The hydrophobic interactions and hydrogen bonds are shown with lines and arrows, respectively. The potential hydrophobic interactions and hydrogen bonds, if the corresponding mutations are made as indicated in Figure 5, are shown in dashed lines and dashed arrows, respectively. The blue lines indicate the interactions between PPARγ and RU-486, while the gold lines indicate the interaction between PPARγ and rosiglitazone. (E) Effects of mutations of key PPARγ residues on its transcriptional activity in response to RU-486 in cell-based reporter gene assays. Cos7 cells were cotransfected with plasmids encoding full-length PPARγ or PPARγ mutants as indicated in the figure together with a PPRE luciferase reporter. The cells were treated with 1 μM RU-486 and rosiglitazone, respectively. The dashed line indicates the activation level of wild-type PPARγ by RU-486.
The 17β-hydroxy group of the steroid core of RU-486 forms several hydrogen bonds with the surrounding PPARγ residues, including Y327 from helix 5 and H449 from helix 10 (Fig. 6D). These interactions were observed for both RU-486- and rosiglitazone-bound PPARγ, thereby supporting a critical conserved mechanism for ligand-mediated activation of PPARγ. Interestingly, the 17-(1-propynyl) group and the D ring of RU-486 formed a stronger hydrophobic environment instead of hydrophilic for the corresponding position of rosiglitazone (Fig. 6B & 6D). Accordingly, PPARγ displayed a great conformational flexibility to accommodate different bound ligands. Conformational changes in two surrounding pocket residues (F282 and F363) were evidenced when the RU-486/PPARγ complex was overlaid on the rosiglitazone/PPARγ structure (Fig. 6B & 6C). The hydrophobic side chains of F282 and F363 shifted from their rosiglitazone-bound conformation toward the hydrophobic group of RU-486, thus stabilizing RU-486 binding by making additional hydrophobic interactions with RU-486. The mutations disrupting these interactions showed distinct results on the activation of PPARγ by RU-486 and rosiglitazone, highlighting the differential roles of these residues in recognizing RU-486 and rosiglitazone (Fig. 6E).
Notably, while a hydrogen bond between ligand and Y473 on helix 12 is critical in rosiglitazone-bound PPARγ, it is not seen in the RU-486-occupied receptor structure due to the hydrophobic nature of the 17-(1-propynyl) group at this position (Fig. 6D). As such, an Y473F mutation should favor the hydrophobic interaction between PPARγ and RU-486 (Fig. 6D). Indeed, the RU-486-mediated PPARγ transcriptional activity was substantially increased by this mutation while rosiglitazone-mediated activity was decreased (Fig. 6E), further affirming that the PPARγ ligand binding pocket has unique properties that dictate the discrimination between RU-486 and rosiglitazone.
Discussion
RU-486 (Mifeprstone) was developed as a GR antagonist that also shows anti-progestin and anti-androgen activities. In this paper, we have used structural studies in combination with biochemical and gene expression analysis to provide strong evidence that the steroid compound RU-486 is a unique PPARγ ligand. First, RU-486 is able to activate the transcriptional activity of PPARγ LBD and PPARγ full-length in cell-based reporter assays. In addition, RU-486-mediated PPARγ activity was inhibited by the PPARγ-specific antagonist GW9662. Second, the interaction of PPARγ with coactivators was enhanced by the binding of this ligand. Further, the crystal structure of PPARγ complexed with RU-486 has revealed a clear binding mode of RU-486 ligand, in which the steroid core occupies the conserved position and the unique side chain adopts a distinct binding mode with new epitopes on PPARγ. The RU-486-bound PPARγ adopts an active conformation, further underscoring the agonist nature of this ligand. Moreover, the biological significance of RU-486 binding to PPARγ was further supported by the adipocyte gene analysis in 3T3-L1 cells. Taken together, we have provided coherent evidence that RU-486 regulates the transcriptional activity of PPARγ by binding to its LBD.
The specific interactions between the critical LBD residues of PPARγ and RU-486 provide critical perspectives regarding the recognition of RU-486 by PPARγ. Our structural observations indicate that the PPARγ ligand binding pocket has a unique shape, carrying both hydrophilic and hydrophobic properties that allow for discrimination of various ligands. Compared with the rosiglitazone/PPARγ structure, RU-486 initiates several new contacts with PPARγ through its dimethylaniline side chain to offset the loss of interactions with a few residues on PPARγ including one critical epitope on AF-2 helix, which is used by both natural PPARγ ligands and synthetic TZDs 33, 34. The cavity occupied by the dimethylaniline side chain belongs to the one arm of the Y-shaped pocket, which is highly conserved among all three PPARs. However, the epitopes on this arm cavity have not been entirely used by previously identified PPARγ ligands like rosiglitazone, despite that these residues are known to align around the PPARγ pocket. The differential binding modes of RU-486 vs therapeutic drug TZDs may subsequently impart the differential modulation of overall structure of PPARγ, thereby affecting target gene recognition and physiological outcomes induced by various PPARγ ligands. Thus, the unique characteristics of the dimethylaniline side chain may represent a new pharmacophore that can be optimized for selectively targeting PPARγ.
The physiological function of RU-486 has been linked to steroid hormone receptor signaling pathways. Indeed RU-486 was initially developed as an antagonist targeting GR and has many clinical values including therapeutic potentials for hypercortisolism in Cushing’s syndrome. Notably, RU-486 has also been shown to improve insulin sensitivity in rodents 21. In fact, the glucocorticoid antagonism regulates both hepatic and adipocyte gene expression that improve glucose control in diabetic animal models 21, 37. Our results indicate that at least part of the RU-486 effects are in fact through targeting nuclear receptor PPARγ and PPARγ-target genes, thus uncovering a novel signaling route for RU-486. Of note, the pharmacologic effects of PPARγ activation by various ligands are involved in both adipogenic and the antidiabetic regulation in the adipocyte 38, 39. Interestingly, it has been reported that RU-486 treatment did not cause the body weight gain, which is a side effect of rosiglitazone 18, 19. In agreement with this beneficial role of RU-486, our adipocyte gene expression analysis indicates weaker adipogenic activity of RU-486 compared to rosiglitazone. Intriguingly, the extent of PPARγ activation does not correlate with insulin sensitivity. In fact, mice with heterozygous loss of the PPARγ gene showed improved insulin sensitivity 40. Several PPARγ agonists with weaker or partial activity display greater antidiabetic potency 41, 42. Similarly, RU-486 may possess distinct properties that provide a novel method for the activation of PPARγ or the treatment of PPARγ-mediated diseases including insulin resistance.
The discovery of RU-486 as a potent PPARγ ligand suggests further functional linkages between the roles of steroid receptor and PPARγ signaling in adipocyte differentiation and glucose metabolism. Since RU-486 interacts with both PPARγ and steroid receptors, the structural mechanism may provide an RU-486 based drug design for compounds that can be used more specifically either for PPARγ-mediated diseases or GR-mediated diseases, or for a combinatorial therapy. However, the antagonistic activity of RU-486 towards PR and GR has been associated with many undesired side effects. The beneficial and side effects arising from cross-interacting with each receptor can be optimized by designing new RU-486-based compounds with more selectivity toward PPARγ or steroid receptors. Apart from the drug design, further elucidation of these two disparate signaling pathways of PPARγ and GR receptors by using RU-486 based compounds should reveal specific molecular basis for their roles in glucose homeostasis.
Materials and Methods
Protein Preparation
The human PPARγ LBD (residues 206-477) was expressed as N-terminal 6xHis fusion protein from the expression vector pET24a (Novagen). BL21 (DE3) cells transformed with each expression plasmid were grown in LB broth at 25 °C to an OD600 of ~1.0 and induced with 0.1 mM isopropyl 1-thio-β-D-galactopyranoside (IPTG) at 16 °C. Cells were harvested and sonicated in 200 ml extract buffer (20 mM Tris pH8.0, 150 mM NaCl, 10% glycerol, and 25 mM imadazole) per 6 liters of cells. The lysate was centrifuged at 20,000 rpm for 30 minutes and the supernatant was loaded on a 5 ml NiSO4-loaded HisTrap HP column (GE Healthcare). The column was washed with extract buffer and the protein eluted with a gradient of 25-500 mM imidazole. The PPARγ LBD was further purified with a Q-Sepharose column (Amersham Biosciences. To prepare the protein-ligand complex, we added a 5-fold excess of the RU-486 ligand (11β-(4-Dimethylamino)phenyl-17β-hydroxy-17-(1-propynyl)-estra-4,9-dien-3-one) to the purified protein, followed by filter concentration to 10 mg/ml. The PPARγ LBD was complexed with 2-fold of SRC1 peptide (SLTERHKI LHRLL QEGSP) before filter concentration.
Crystallization, Data Collection and Structure Determination
The crystals of PPARγ/RU-486 complex were grown at room temperature in hanging drops containing 1.0 μl of the above protein-peptide solutions and 1.0 μl of well buffer containing 0.2 M Succinic acid, pH 7.0, and 25% PEG 3350. The crystals were directly flash frozen in liquid nitrogen for data collection. Diffraction data were collected with a MAR300 CCD detector at the ID line of sector-21 at the Advanced Photon Source. The observed reflections were reduced, merged and scaled with DENZO and SCALEPACK in the HKL2000 package 43. The structures were determined by molecular replacement in the CCP4 suite (http://www.ccp4.ac.uk). Manual model building was carried out with Coot 44, followed by REFMAC refinement in the CCP4 suite.
Cofactor Binding Assays
The binding of the various peptide motifs to PPARγ LBD in response to ligands was determined by AlphaScreen™ assays using a hexahistidine detection kit from Perkins-Elmer as described 23. The experiments were conducted with approximately 20-40 nM receptor LBD and 20 nM biotinylated cofactor peptides in the presence of 5 μg/ml donor and acceptor beads in a buffer containing 50 mM MOPS, 50 mM NaF, 0.05 mM CHAPS, and 0.1 mg/ml bovine serum albumin, all adjusted to a pH of 7.4.
The peptides with an N-terminal biotinylation are listed below.
SRC1-2, SPSSHSSLTERHKILHRLLQEGSP;
SRC1-4, QKPTSGPQTPQAQQKSLLQQLLTE;
SRC2-3, QEPVSPKKKENALLRYLLDKDDTKD;
SRC3-3, PDAASKHKQLSELLRGGSG
PGC-1α-1, AEEPSLLKKLLLAPA;
CBP-1, SGNLVPDAASKHKQLSELLRGGSG;
NCOR-2, GHSFADPASNLGLEDIIRKALMGSF.
Transient Transfection Assay
Cos-7 cells were maintained in DMEM containing 10% fetal bovine serum (FBS) and were transiently transfected using Lipofectamine 2000 (Invitrogen) 23. All mutant PPARγ plasmids were created using the Quick-Change site-directed mutagenesis kit (Stratagene). 24-Well plates were plated 24 h prior to transfection (5 × 104 cells per well). For Gal4-driven reporter assays, the cells were transfected with 200 ng Gal4- LBDs of various nuclear receptors and 200 ng of pG5Luc reporter (Promega). For native promoter reporter assays, the cells were cotransfected with plasmids encoding full-length nuclear receptors and their cognate luciferase reporters as follows: three human PPAR subtypes (α, δ, and γ) and PPRE-Luc. Ligands were added five hours after transfection. Cells were harvested 24 h later for the luciferase assays. Luciferase activities were normalized to Renilla activity cotransfected as an internal control.
3T3-L1 Differentiation and RNA Analysis
3T3-L1 preadipocytes were maintained in growth media containing 10% FBS and differentiated as described previously (Reginato et al., 1998). Briefly, for ligand-induced adipocyte differentiation, cells were treated with 1 μM rosiglitazone or RU-486 two days after confluence. The cells were then stained with a filtered Oil Red O stock solution [0.5 g of Oil Red O (Sigma, St. Louis, MO) in 100 ml of isopropyl alcohol] for 15 min at room temperature.
Total RNA was extracted from 3T3-L1 cells with Trizol reagent (Life Technologies, Inc.). RNA was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad). Real-time quantitative PCR analysis was performed using Power SYBR® Green PCR Master Mix (Applied Biosystems). The primer sequences of all genes are reported before (Waki et al., 2007). The mRNA expression was normalized to 36B4.
Supplementary Material
Acknowledgments
We thank the staff at BL17U of Shanghai Synchrotron Radiation Source and sector 21 (LS-CAT) of the Advance Photo Source for assistance in data collection. Use of the Advanced Photon Source was supported by the Office of Science of the U. S. Department of Energy. This work was supported by the National Institutes of Health Grant DK081757, and grants from the National Natural Science Foundation of China (31070646 and 30730025) and the Science Planning Program of Fujian Province (2009J1010).
Footnotes
Accession Numbers Coordinates and structure factors for the PPARγ/RU-486 complex are available in the Protein Data Bank (http://www.rcsb.org/) under ID code 3QT0.
References
- 1.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123(6):993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Wang YX. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell research. 2010;20(2):124–137. doi: 10.1038/cr.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yki-Jarvinen H. Thiazolidinediones. The New England journal of medicine. 2004;351(11):1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 4.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. The New England journal of medicine. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 5.Waki H, Park KW, Mitro N, et al. The small molecule harmine is an antidiabetic cell-type-specific regulator of PPARgamma expression. Cell Metab. 2007;5(5):357–370. doi: 10.1016/j.cmet.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Feige JN, Gelman L, Michalik L, Desvergne B, Wahli W. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res. 2006;45(2):120–159. doi: 10.1016/j.plipres.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Glass CK, Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol. 2006;6(1):44–55. doi: 10.1038/nri1748. [DOI] [PubMed] [Google Scholar]
- 8.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10(4):355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 9.Moras D, Gronemeyer H. The nuclear receptor ligand-binding domain: structure and function. Curr Opin Cell Biol. 1998;10(3):384–391. doi: 10.1016/s0955-0674(98)80015-x. [DOI] [PubMed] [Google Scholar]
- 10.Tsukahara T, Tsukahara R, Fujiwara Y, et al. Phospholipase D2-dependent inhibition of the nuclear hormone receptor PPARgamma by cyclic phosphatidic acid. Mol Cell. 2010;39(3):421–432. doi: 10.1016/j.molcel.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Lambert MH, Xu HE. Activation of nuclear receptors: a perspective from structural genomics. Structure (Camb) 2003;11(7):741–746. doi: 10.1016/s0969-2126(03)00133-3. [DOI] [PubMed] [Google Scholar]
- 12.Jin L, Li Y. Structural and functional insights into nuclear receptor signaling. Advanced drug delivery reviews. doi: 10.1016/j.addr.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin L, Li Y. Structural and functional insights into nuclear receptor signaling. Adv Drug Deliv Rev. 2010;62(13):1218–1226. doi: 10.1016/j.addr.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26(5):244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Michalik L, Wahli W. Guiding ligands to nuclear receptors. Cell. 2007;129(4):649–651. doi: 10.1016/j.cell.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129–156. doi: 10.1146/annurev.med.48.1.129. [DOI] [PubMed] [Google Scholar]
- 17.Johanssen S, Allolio B. Mifepristone (RU 486) in Cushing’s syndrome. Eur J Endocrinol. 2007;157(5):561–569. doi: 10.1530/EJE-07-0458. [DOI] [PubMed] [Google Scholar]
- 18.Dubuc PU, Peterson CM. Ineffectiveness of parenteral fluoxetine or RU-486 to alter long-term food intake, body weight or body composition of genetically obese mice. The Journal of pharmacology and experimental therapeutics. 1990;255(3):976–979. [PubMed] [Google Scholar]
- 19.Langley SC, York DA. Effects of antiglucocorticoid RU 486 on development of obesity in obese fa/fa Zucker rats. Am J Physiol. 1990;259(3 Pt 2):R539–544. doi: 10.1152/ajpregu.1990.259.3.R539. [DOI] [PubMed] [Google Scholar]
- 20.Gettys TW, Watson PM, Taylor IL, Collins S. RU-486 (Mifepristone) ameliorates diabetes but does not correct deficient beta-adrenergic signalling in adipocytes from mature C57BL/6J-ob/ob mice. Int J Obes Relat Metab Disord. 1997;21(10):865–873. doi: 10.1038/sj.ijo.0800479. [DOI] [PubMed] [Google Scholar]
- 21.Taylor AI, Frizzell N, McKillop AM, Flatt PR, Gault VA. Effect of RU486 on hepatic and adipocyte gene expression improves diabetes control in obesity-type 2 diabetes. Horm Metab Res. 2009;41(12):899–904. doi: 10.1055/s-0029-1234071. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Choi M, Cavey G, et al. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol Cell. 2005;17(4):491–502. doi: 10.1016/j.molcel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Suino K, Daugherty J, Xu HE. Structural and biochemical mechanisms for the specificity of hormone binding and coactivator assembly by mineralocorticoid receptor. Mol Cell. 2005;19(3):367–380. doi: 10.1016/j.molcel.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Laue L, Chrousos GP, Loriaux DL, et al. The antiglucocorticoid and antiprogestin steroid RU 486 suppresses the adrenocorticotropin response to ovine corticotropin releasing hormone in man. J Clin Endocrinol Metab. 1988;66(2):290–293. doi: 10.1210/jcem-66-2-290. [DOI] [PubMed] [Google Scholar]
- 25.Leesnitzer LM, Parks DJ, Bledsoe RK, et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41(21):6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- 26.Shao D, Lazar MA. Peroxisome proliferator activated receptor gamma, CCAAT/enhancer-binding protein alpha, and cell cycle status regulate the commitment to adipocyte differentiation. J Biol Chem. 1997;272(34):21473–21478. doi: 10.1074/jbc.272.34.21473. [DOI] [PubMed] [Google Scholar]
- 27.Bernlohr DA, Angus CW, Lane MD, Bolanowski MA, Kelly TJ., Jr Expression of specific mRNAs during adipose differentiation: identification of an mRNA encoding a homologue of myelin P2 protein. Proc Natl Acad Sci U S A. 1984;81(17):5468–5472. doi: 10.1073/pnas.81.17.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, et al. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. Embo J. 1996;15(19):5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 29.Tontonoz P, Singer S, Forman BM, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proc Natl Acad Sci U S A. 1997;94(1):237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banga A, Unal R, Tripathi P, et al. Adiponectin translation is increased by the PPARgamma agonists pioglitazone and omega-3 fatty acids. Am J Physiol Endocrinol Metab. 2009;296(3):E480–489. doi: 10.1152/ajpendo.90892.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gampe RT, Jr, Montana VG, Lambert MH, et al. Asymmetry in the PPARgamma/RXRalpha crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol Cell. 2000;5(3):545–555. doi: 10.1016/s1097-2765(00)80448-7. [DOI] [PubMed] [Google Scholar]
- 32.Chandra V, Huang P, Hamuro Y, et al. Structure of the intact PPAR-gamma-RXR-nuclear receptor complex on DNA. Nature. 2008;456(7220):350–356. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itoh T, Fairall L, Amin K, et al. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol. 2008;15(9):924–931. doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Zhang J, Schopfer FJ, et al. Molecular recognition of nitrated fatty acids by PPAR gamma. Nat Struct Mol Biol. 2008;15(8):865–867. doi: 10.1038/nsmb.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kauppi B, Jakob C, Farnegardh M, et al. The three-dimensional structures of antagonistic and agonistic forms of the glucocorticoid receptor ligand-binding domain: RU-486 induces a transconformation that leads to active antagonism. J Biol Chem. 2003;278(25):22748–22754. doi: 10.1074/jbc.M212711200. [DOI] [PubMed] [Google Scholar]
- 36.Raaijmakers HC, Versteegh JE, Uitdehaag JC. The X-ray structure of RU486 bound to the progesterone receptor in a destabilized agonistic conformation. J Biol Chem. 2009;284(29):19572–19579. doi: 10.1074/jbc.M109.007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobson PB, von Geldern TW, Ohman L, et al. Hepatic glucocorticoid receptor antagonism is sufficient to reduce elevated hepatic glucose output and improve glucose control in animal models of type 2 diabetes. J Pharmacol Exp Ther. 2005;314(1):191–200. doi: 10.1124/jpet.104.081257. [DOI] [PubMed] [Google Scholar]
- 38.de Souza CJ, Eckhardt M, Gagen K, et al. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes. 2001;50(8):1863–1871. doi: 10.2337/diabetes.50.8.1863. [DOI] [PubMed] [Google Scholar]
- 39.Chao L, Marcus-Samuels B, Mason MM, et al. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J Clin Invest. 2000;106(10):1221–1228. doi: 10.1172/JCI11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miles PD, Barak Y, He W, Evans RM, Olefsky JM. Improved insulin-sensitivity in mice heterozygous for PPAR-gamma deficiency. J Clin Invest. 2000;105(3):287–292. doi: 10.1172/JCI8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reginato MJ, Bailey ST, Krakow SL, et al. A potent antidiabetic thiazolidinedione with unique peroxisome proliferator-activated receptor gamma-activating properties. J Biol Chem. 1998;273(49):32679–32684. doi: 10.1074/jbc.273.49.32679. [DOI] [PubMed] [Google Scholar]
- 42.Rocchi S, Picard F, Vamecq J, et al. A unique PPARgamma ligand with potent insulin-sensitizing yet weak adipogenic activity. Mol Cell. 2001;8(4):737–747. doi: 10.1016/s1097-2765(01)00353-7. [DOI] [PubMed] [Google Scholar]
- 43.Otwinowski Z, Minor W. Processing of x-ray diffraction data collected in oscillation mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 44.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


