Abstract
Prior studies have reported that metallothionein I/II (MT) promote regenerative axonal sprouting and neurite elongation of a variety of central nervous system neurons after injury. In this study, we evaluated whether MT is capable of modulating regenerative axon outgrowth of neurons from the peripheral nervous system. The effect of MT was firstly investigated in dorsal root ganglion (DRG) explants, where axons were scratch-injured in the presence or absence of exogenous MT. The application of MT led to a significant increase in regenerative sprouting of neurons 16 h after injury. We show that the pro-regenerative effect of MT involves an interaction with the low-density lipoprotein receptor megalin, which could be blocked using the competitive antagonist RAP. Pre-treatment with the mitogen-activated protein kinase (MAPK) inhibitor PD98059 also completely abrogated the effect of exogenous MT in promoting axonal outgrowth. Interestingly, we only observed megalin expression in neuronal soma and not axons in the DRG explants. To investigate this matter, an in vitro injury model was established using Campenot chambers, which allowed the application of MT selectively into either the axonal or cell body compartments after scratch injury was performed to axons. At 16 h after injury, regenerating axons were significantly longer only when exogenous MT was applied solely to the soma compartment, in accordance with the localized expression of megalin in neuronal cell bodies. This study provides a clear indication that MT promotes axonal regeneration of DRG neurons, via a megalin- and MAPK-dependent mechanism.
Keywords: Metallothionein, Neuronal injury, Neurite sprouting, Megalin, Dorsal root ganglion neurons
Introduction
Metallothioneins (MTs) are a family of low-molecular-weight (6–7 kDa) intracellular metal-binding proteins (reviewed in [5, 9]). MTs consist of two domains, α- and β- domain, and the protein structure is also characterized by its highly conserved cysteine content (20 cysteine residues across all mammalian isoforms) (reviewed by [5, 9]). There are four isoforms of MT in mammalian species, which have different tissue distribution in the body. Briefly, MT I and II have high sequence homology and appear to be co-expressed throughout the body (e.g., brain, liver, kidney, etc.). In contrast, MT-III is primarily expressed in brain only, while MT-IV is mostly expressed in the epithelium (for further review refer to [5, 9, 11]). In this study, we focus on the action of MT-I and II in promoting the repair of injured neurons.
Metallothionein I/II (hereafter refer to as MT) has been shown in a number of studies to confer protection to injured neurons both in vitro and in vivo. We have previously reported that application of exogenous MT (the major MT-I/II form expressed in mammals) into the lesion site following focal needle stick injury to the adult rat neocortex leads to preservation of tissue damage (characterized by a smaller volume of lesion site) and an increase in regenerative axonal sprouting compared to vehicle-treated animals [3]. The protective effect of MT has also been demonstrated in studies using MT-I/II-deficient mice in which cryolesion injury was performed upon the adult neocortex. In this study, cryolesion led to substantially greater tissue damage in MT-I/II-deficient mice in comparison to wild-type mice [12]. Furthermore, a significant delay in astrogliosis and microgliosis was also observed in the cryolesion-injured MT-I/II-deficient mice when compared to the wild-type mice [12]. To further demonstrate the important role of MT in protecting neurons against trauma, genetic over-expression of MT-II in transgenic mice leads to substantially improved recovery in motor function after focal cerebral ischemic damage in comparison to wild-type mice [14]. In summary, these studies demonstrate that MT is important for neuronal survival and recovery following traumatic injury.
Recently, evidence has emerged identifying a specific molecular pathway through which MT can promote survival and regeneration of injured neurons. In several studies, it has been shown that the low-density lipoprotein receptor megalin (also known as LRP-2) is directly involved in the molecular signaling pathway underlying the neuro-protective effect of MT [4, 6]. Chung et al. [4] reported that megalin is responsible for uptake of MT into cultured cortical neurons. Furthermore, siRNA-mediated knockdown of megalin expression blocked the ability of MT to promote regenerative sprouting after injury in vitro [4]. These results are similar to those published by Fitzgerald et al. [6], in which they reported the co-localization of megalin and MT in the retina in vivo. They also showed that pre-treatment of the cultured retinal ganglion cells with an antibody against megalin blocked the ability of MT to stimulate neurite extension [6]. More recently, an artificial peptide comprised of a small section of the native β-domain of MT-II, named EmtinB, has been shown to promote neurite outgrowth in primary cerebellar granule neuron cultures in a similar manner to native MT via interaction with the megalin receptor [1]. Overall, studies using different neuronal models have shown that megalin is an important component in the signaling pathway through which MT or EmtinB are able to promote regenerative outgrowth of central nervous system (CNS) neurons.
Interestingly, megalin has also recently been reported to be involved in the internalization of another neuritogenic protein, transthyretin [7]. Fleming et al. [7] reported that megalin is expressed in dorsal root ganglion (DRG) neurons in vivo through both immunohistochemistry staining as well as Western blot [7]. Since megalin is expressed by DRG neurons, MT may potentially have an effect on the DRG neurons after injury in a similar manner to its action upon CNS neurons. Therefore, the goal of this study is to investigate whether MT is able to promote axonal sprouting of injured DRG neurons, and whether this involves a megalin-dependent mechanism.
Materials and methods
Primary dorsal root ganglion explants cultures
All animal experimentation was performed under the guidelines stipulated by the University of Tasmania Animal Ethics Committee, which is in accordance with the Australian code of practice for the care and use of animals for scientific purposes. Under a dissecting microscope, spinal cords were slowly dislodged and removed aseptically from decapitated embryonic day 13.5 mice (both wild-type svj129 mice), leaving the DRG explants in between each vertebrate. Fine forceps were then used to pick the DRGs from the root and place them onto the coverslips (coated with polyornithine overnight followed by laminin 1 h prior to use). The explants were incubated in culture media comprised of Dulbecco’s modified Eagle’s medium-F12 (DMEM F-12, Invitrogen) with 10% Nerve growth factor 7S (NGF, Sigma), 1% N2 supplement 100x (Invitrogen) and 1% Gentamicin reagent solution (Gibco) at 37°C, 5% CO2 for 1 week until the axons were fully established.
Once the axons were fully established, rabbit MT-IIA (Bestenbalt; resuspended in 0.7% saline, filter sterilized prior to use) was added to the culture media at 1 μg/ml concentration immediately before the scratch injury. This was performed using a pulled glass capillary under the dissecting microscope to ensure that the injury was only applied to the axons with the cell bodies remaining intact. As a vehicle treatment, culture media was added containing no MT-IIA. The cultures were then fixed with 4% paraformaldehyde for 20 min, 16 h after the application of injury.
Immunohistochemistry on dorsal root ganglion explants
Immediately after fixation, the cultures were washed with phosphate buffered saline (PBS, Medicago, Sweden) three times (5 min incubation per wash at room temperature) followed by an overnight incubation with the primary antibody (Mouse anti-SMI312 from Covance use at 1:1,000, rabbit anti-megalin, Santa Cruz Biotechnology, use at 1:1,000). Three PBS washes (5 min incubation per wash at room temperature) were applied the next day, after which the cultures were incubated with the secondary antibodies (Alexa Flour 488 anti-mouse (Invitrogen) use at 1:1,000, Alexa Flour 594 anti-rabbit (Invitrogen) use at 1:1,000) for 1 h. After the incubation with antibodies, Nuclear yellow (1 μg/ml) was applied for 5 min followed by three PBS washes, after which the coverslips were permanently mounted onto glass slides using fluorescent mounting agent (Dako). The images of the cultures were then captured using a fluorescence microscope (Olympus BX50).
Dissociated dorsal root ganglion cultures for outgrowth assay
The DRGs were removed as stated in the previous sections and collected into a small Petri dish containing the dissecting media (HBSS). The collected tissues were then dissociated using 0.125% trypsin and incubated at 37°C, 5% CO2 for 20 min. The cultures were triturated, counted, and plated either on polyornithine and laminin-coated coverslips in wells containing the culture medium alone (as described previously for the explant culture), or culture medium containing either 1 or 2 μg/ml of MT. The cultures were incubated for 24 h and then fixed with 4% paraformaldehyde for 20 min at room temperature. The cultures were then immunolabeled with SMI312 antibodies using the protocol described previously. The images of the cultures from different treatments were captured using a fluorescence microscope (Olympus BX50) and the lengths of the axons were measured using ImageJ (National Institutes of Health) software.
Addition of inhibitors in both dorsal root ganglion explant culture and dissociated culture for outgrowth assay
To investigate the molecular signaling mechanism involved in the pro-regenerative effect of MT, we used two agents, a competitive antagonist of megalin (RAP, receptor associated protein, Progen Biotechnik, final concentration at 1 μM), and a pharmacological inhibitor of MAPK-signaling (PD98059, Sigma, final concentration at 100 nM) was used. For use in DRG explant cultures, PD98059 or RAP were added 30 min prior to the addition of MT (which was applied immediately after the scratch injury was performed). In the dissociated DRG neuron cultures, PD98059 or RAP were added into the culture wells immediately after plating of neurons into the wells. Thirty minutes later, MT (1 μg/ml) was applied as described previously.
Compartmentalized in vitro injury model for dorsal root ganglion neurons using Campenot chambers
To set up the in vitro injury model, Campenot chambers (Teflon inserts, Tyler Research) were first adhered onto collagen-coated (PureCol®, Advanced BioMatrix) plastic dishes using high vacuum grease (Dow Corning). The dissociated DRG neurons cultures (collected as stated previously) were then plated onto the left compartments and the cultures were maintained at 37°C, 5% CO2 in culture media consisting of Minimum essential medium (MEM, Invitrogen) and N3 supplements for 3 weeks until the axons were fully extended to the other two compartments. Scratch injury was then made at the middle compartments (refer to Fig. 5a, b) using pulled glass capillaries with 1 μg/ml of MT added immediately prior to injury. Culture medium was added instead for the vehicle treatment. Cultures were then incubated for 16 h after injury and then fixed with 4% paraformaldehyde for 30 min.
Fig. 5.
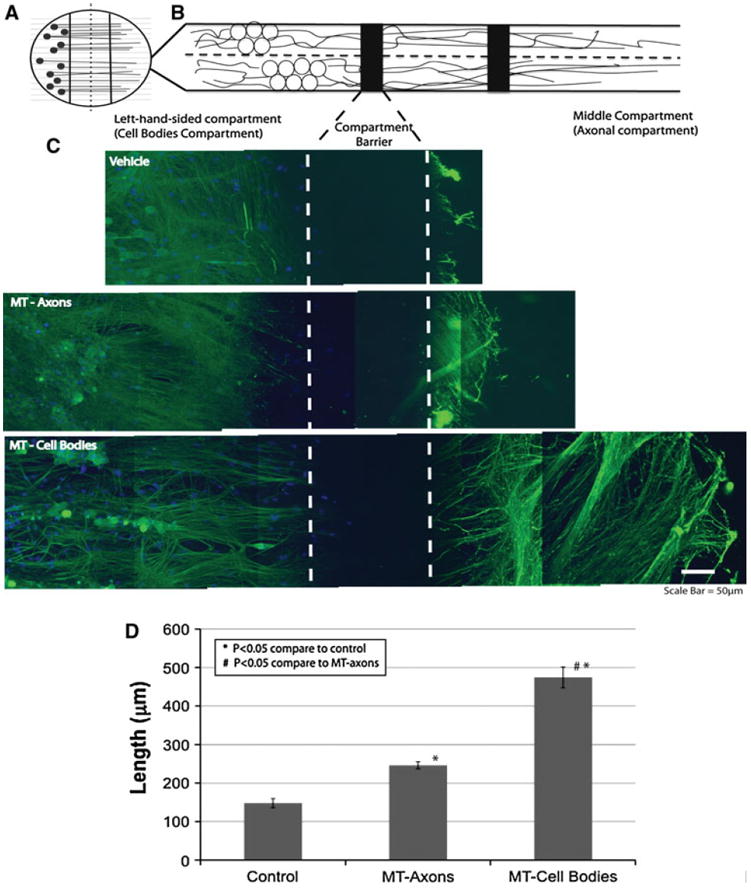
MT promotes a more extensive regenerative response when added selectively to cell bodies of DRGs neurons. DRG neurons were grown in Campenot chambers, allowing the separation of neuronal soma and axons into separate fluid-tight compartments (a and b). Comparison of regenerative neurite outgrowth 24 h after scratch injury between the three treatment conditions (c) revealed that MT treatment specifically at the soma of injured neurons led to significantly longer regenerative neurite outgrowth after injury than when MT was applied directly to injured axons (scale bar = 50 μm). This difference was confirmed when the average length of regenerative sprouts was measured (d). (*p < 0.05 compared to control; #p < 0.05 compared to MT—Axons; n = 9 per treatment group; error bars = standard errors of mean fold changes)
After fixation, the cultures were first washed with 0.1% Triton followed by four PBS washes (5-min incubation per wash) and then blocked with 3% goat serum in PBS for 1 h. Cultures were incubated overnight with primary antibodies (mouse anti-metallothionein (Dako) use at 1:1,000, chicken anti-neurofilament (Millipore) use at 1:1,000, rabbit anti-megalin 1:1,000). Secondary antibodies were then applied for 1 h followed by 5-min incubation with Nuclear yellow. Between each step of the staining process, PBS was used to wash the cultures three times (5 min each). The Campenot chambers were then removed and the cultures were mounted using Vectashield Mounting Media (Vector Laboratories). These immunolabeled cultures were then visualized using a two-photon confocal microscope (LSM 510, Zeiss). The length of axons were measured using ImageJ software.
Results
Metallothionein-IIA promotes axonal sprouting and outgrowth in injured dorsal root ganglion neurons
To examine whether MT-IIA promotes axonal sprouting in dorsal root ganglion (DRG) neurons after injury, DRG explants were scratched with a fine-glass capillary, and the cultures were fixed 16 h after injury. A neurofilament marker, SMI312, was then used to immunolabel axons within the DRG explants. We found that MT-IIA treatment led to extensive regenerative neurite sprouting observed in the injured DRG explants compared to explants receiving vehicle treatment (culture media only) (Fig. 1). This difference was very clear, as the injury tract in vehicle-treated explants (indicated by arrow in Fig. 1a, b) was substantially larger in comparison to MT-IIA-treated DRG explants (indicated by arrow in Fig. 1c, d). In this explant model, it is difficult to accurately track and quantitate the length of individual sprouting axons entering the injury tract. To overcome this issue, a DRG outgrowth assay was established using dissociated DRG neurons. The DRG neurons used in this assay were isolated from E13.5 to E14 embryos, where the cultures were plated into media containing either culture media only (vehicle treatment) or culture media with MT-IIA (1 or 2 μg/ml). After 24 h, the cells were fixed and immunolabeled with the neurofilament marker SMI312. We found a statistically significant increase (t test, p < 0.05) in the length of regenerative neurite growth when the cultures were plated in media containing 1 or 2 μg/ml MT-IIA (of 2.3- and 2.6-fold increase, respectively) compared to the vehicle treatment (Fig. 2).
Fig. 1.
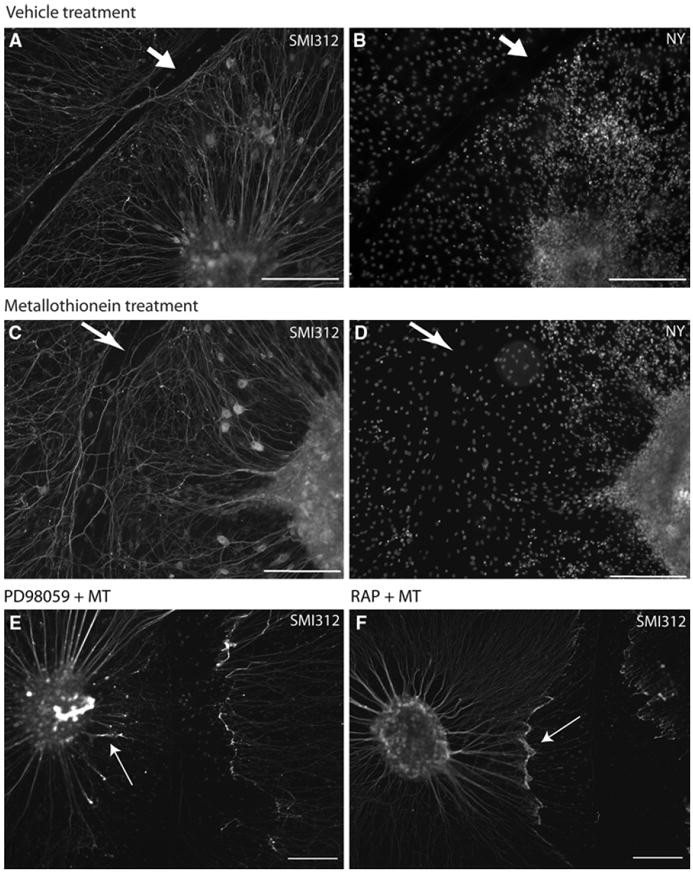
MT promotes regenerative sprouting of scratch-injured DRG explants in vitro. Immunostaining of DRG explants 16 h after scratch injury was performed. The neuronal cytoskeleton of DRG neurons was labeled using the neurofilament antibody SMI312 (a, b, c, and d), while Nuclear yellow was used to identify nuclei (c and d). Results indicate that a more extensive regenerative axonal sprouting was observed in the explants receiving MT treatment (c and d) compared to those receiving vehicle treatment (a and b). The injury tract (indicated by arrow) also appeared to be substantially wider in the explants receiving vehicle treatment compared to those receiving MT-IIA treatment. In some DRG explant cultures, it was found that 30-min pre-treatment with PD98059 (inhibitor of MAPK signaling pathways) or RAP (megalin antagonist) blocked the pro-regenerative effect of MT (marked by arrow in e and f) (scale bar = 200 μm)
Fig. 2.
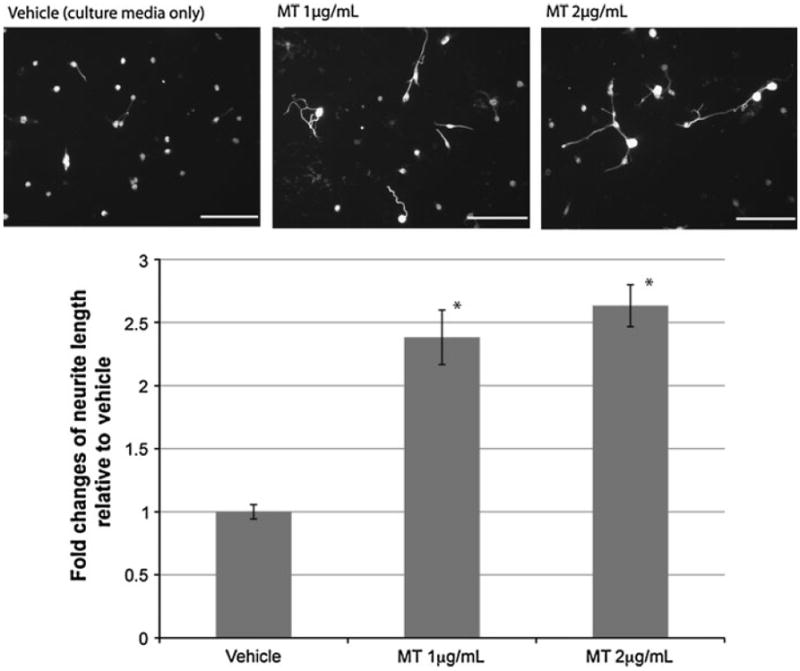
MT promotes neurite outgrowth of DRG in vitro. Dissociated cultures of DRG neurons were immunolabeled with the neurofilament marker SMI312, 24 h after the plating. Results demonstrate that MT-IIA, at both concentrations tested (1–2 mg/ml) promote neurite outgrowth compared to vehicle treatment (a). Quantitative analysis confirmed that MT treatments led to a significant increase in axonal length compared to the vehicle treatment (b). (*p < 0.05 compared to vehicle treatment; n = 15 per treatment group; error bars = standard errors of mean fold changes; scale bar = 100 μm)
Overall, these results demonstrated that application of exogenous MT led to significantly enhanced neurite regeneration after injury in DRG neurons in vitro, and that this involved a direct interaction between MT-IIA and DRG neurons.
Metallothionein promotes regenerative sprouting of injured DRG neurons via a megalin- and MAPK-dependent mechanism
Previous studies have revealed that ligand interactions with megalin activate a MAPK-dependent pathway of neurite outgrowth in CNS neurons [2]. Therefore, we investigated whether MT acts through a similar pathway in DRG neurons. DRG explants were first pre-treated with either RAP (a competitive antagonist of megalin) or the MAPK signaling inhibitor (PD98059) for 30 min prior to the addition of MT (which occurred immediately after injury). It was found that both of these treatments inhibited the pro-regenerative response induced by MT treatment (Fig. 1e, f, respectively).
To confirm these observations in a quantitative manner, dissociated DRG neurons were pre-treated with either RAP or PD98059 for 30 min prior to the addition of MT. We found that PD98059 itself had no effect upon neurite outgrowth of dissociated DRG neurons (Fig. 3). However, PD98059 significantly impaired the neuritogenic effect of MT in promoting neurite outgrowth of DRG neurons (Fig. 3). RAP also significantly blocked the ability of MT to promote neurite outgrowth (Fig. 3). These results indicate that MT acts through a megalin- and MAPK-dependent signaling pathway to promote neurite growth.
Fig. 3.
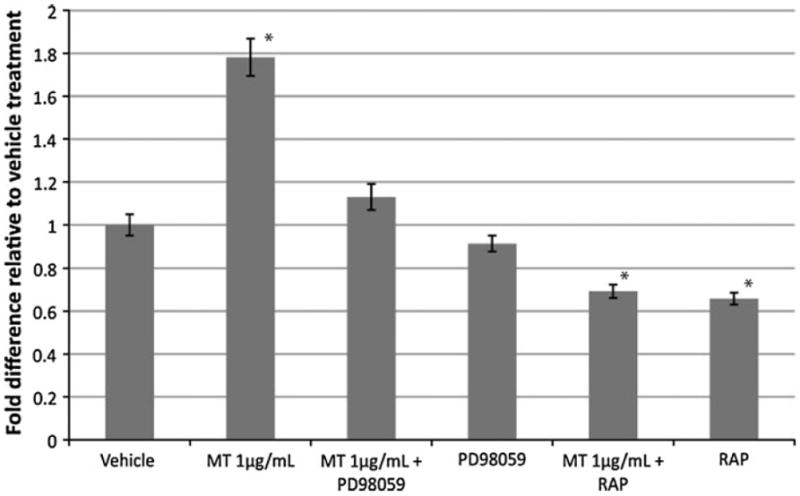
MT-IIA promotes neurite outgrowth through a megalin- and MAPK-dependent pathway. Dissociated DRG neurons were treated with RAP or PD98059 30 min prior to treatment with MT. Quantification of neurite length 24 h after MT treatment found that both RAP and PD98059 blocked the neuritogenic effect of MT-IIA. (*p < 0.05 compared to other treatment group; n = 20–30 per treatment group; error bars = standard errors of mean fold changes)
Expression of megalin in dorsal root ganglion neurons in vitro
Dorsal root ganglion neurons have recently been reported to express megalin in vivo [7], therefore we examined whether DRG explants express megalin in vitro. Immunohistochemistry staining using a megalin antibody upon DRG explants culture (7 DIV) revealed that the majority of megalin expression appeared to be specifically localized within the soma of DRG, with minimal staining observed in the neurofilament stained axons (Fig. 4). Nuclei staining of the DRG explants revealed the presence of non-neuronal cells (SMI312-negative) cells underlying the axons of the DRG neurons (indicated by arrow in Fig. 4c, d), however, these cells were not megalin-positive.
Fig. 4.
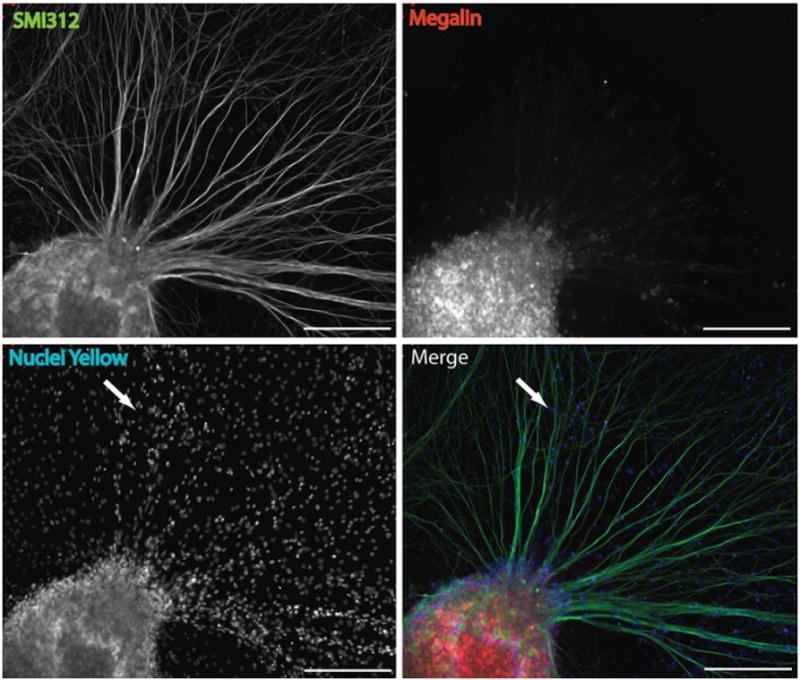
The expression of megalin in DRG explants in vitro. Immunohistochemical staining of DRG explant cultures for megalin (red) and neurofilaments (SMI312; green) revealed that megalin staining was punctate and restricted primarily to the soma of DRG neurons, with limited expression in axons. Nuclei staining (c) indicating the presence of non-neuronal cells (SMI-negative cells) within the culture (arrow in panel c and d); however, these did not express megalin. Therefore, megalin is specifically expressed by neurons in the DRG explant cultures (scale bar = 200 μm)
Metallothionein only promotes axonal sprouting when delivered to the soma of injured DRG neurons, but not injured axons
According to the previous results, megalin was predominantly expressed in the soma of DRG neurons. This suggests that MT might only exert its regenerative effect upon certain cellular compartments of DRG neurons, most likely at the cell bodies. To investigate this, an in vitro model of axonal injury was established in which DRG neurons were cultured within Campenot chambers, allowing the separation of neuronal cell bodies and axons in separate fluid-sealed compartments (Fig. 5a, b). The cultures were grown for 21 days to allow the axons to extend from the soma compartment into the axonal compartment, and then scratch injuries were made using a pulled capillary within the middle compartment (axononly compartment). The MT or vehicle was immediately applied to either the middle axonal chamber or the side soma compartment. At 16 h post-injury, the cultures were fixed and immunostained.
We found that MT treatment led to enhanced regenerative neurite sprouting after injury when added either to axons or cell bodies (Fig. 5c). The action of MT was most effective when delivered to the soma compartment of DRG neurons, resulting in extensive almost fourfold enhancement of regenerative sprouting (Fig. 5d). The intensity of neurofilament staining was substantially elevated in newly sprouting axons, an indicative marker of regenerative sprouting (Fig. 5c). Restricted delivery of MT to only the injured axons of DRG neurons also promoted some regenerative sprouting, although to a far lesser degree compared to when MT was delivered to neuronal cell bodies (Fig. 5d).
Discussion
Overall, this study has demonstrated that MT-IIA can promote regenerative sprouting of DRG neurons after scratch injury. To the best of our knowledge, this is the first report demonstrating the ability of MT-IIA to promote regeneration of injured neurons of the peripheral nervous system. Furthermore, we show that the pro-regenerative effect of MT occurs via a megalin- and MAPK-dependent mechanism. Finally, we show that MT-IIA only promotes regenerative sprouting when applied to the soma of injured DRG neurons, which correlates with the restricted expression of megalin in the cell body. This study provides further evidence that MT-IIA (via a megalin- and MAPK-signaling pathway) represents an important molecular target for promoting peripheral nerve regeneration.
We report that MT can promote regenerative sprouting of DRG neurons using several different culture and injury models. The pro-regenerative effect of MT-IIA was completely blocked by pre-treatment with RAP, indicating that MT interacts with megalin to mediate its pro-regenerative activity. This is in accordance with megalin siRNA knockdown studies that completely blocked the uptake of exogenous MT into the cultured cortical neurons and subsequently blocked the ability of MT to promote neurite outgrowth [4].
The precise mechanism through which ligand binding to megalin results in enhanced neurite outgrowth remains unclear. Megalin is not a classical receptor, but a multi-ligand transporter that encapsulates ligands into vesicles and internalizes them into the cytoplasm (as reviewed in [10]). In this scenario, there are two potential avenues through which MT may interact with megalin to promote regenerative sprouting in DRG neurons. Firstly, megalin has been reported to have classical receptor-like signal transduction properties [2]. Hence, binding of MT to megalin as well as the internalization of MT results in the cleavage of a C-terminal intracellular component of megalin, which subsequently activates a MAPK-dependent signaling pathway. Alternatively however, MT that is internalized by megalin within vesicles may itself activate molecular signaling pathways by the release of zinc into the cytoplasm (MT will lose its bound zinc in the acidic environment of vesicles) [1, 2]. In this study, we show that the MAPK inhibitor, PD98059, completely blocks the pro-regenerative effect of MT, suggesting that the interaction between MT and megalin activates a signal transduction-mediated mechanism. It is also important to note that because of its high cysteine content, MT is able to readily sequester reactive oxygen species (for review see [9]). However, the fact that both RAP and PD98059 completely block the pro-regenerative effect of MT suggests that the free-radical scavenging ability of MT is not involved in MT-mediated regeneration. Interestingly, another megalin ligand, transthyretin (which does not bind zinc or scavenge free radicals), has recently been reported to promote regenerative neurite outgrowth [7]. Therefore, megalin and its ligands appear to represent an important molecular target in promoting peripheral nerve regeneration.
To investigate in greater detail the site of action of MT upon injured DRG neurons, we utilized Campenot chambers to separate different cellular compartments of the neurons (i.e., soma and axons). Campenot chambers have been utilized to study Wallerian degeneration in myelinated axons after cut injury caused by a razor blade [8]. In this study, we utilized a slightly different arrangement of the chambers whereby the DRG neurons were plated into only a single lateral compartment, allowing unidirectional axonal extension through the other two compartments. A pulled fine-glass capillary was used to cause scratch injury to the axons. The advantage of this system is that it allowed us to precisely injure axons and not cell bodies, and also restricted the application of MT specifically to either neuronal soma or injured axons. Using this model, we report that MT significantly enhanced regenerative sprouting when applied directly to the soma of injured DRG neurons, and to a much-lesser effect when applied directly to injured axons. Notably, this site-specific action of MT appears to correlate with the regional distribution of megalin receptors within DRG neurons, since we found megalin was only expressed in DRG neuron soma but not in axons. Future studies using Campenot chambers in combination with megalin siRNA, RAP, or PD98059, could be used to further confirm the involvement of this pathway in MT-mediated regeneration. Overall, these results explain a recent observation that we made in the injured optic nerve where we reported that there was a significant increase in axonal sprouting after injury when MT was injected into the vitreous (MT was internalized by the cell bodies of the retinal ganglion cells that project their axons into the optic nerve), and not when MT was applied directly at the site of injury in the optic nerve [4]. These results are important from a future therapeutic perspective, as they suggest that any future use of MT as an agent to promote regenerative sprouting of injured neurons will require targeted delivery of MT to the cell body of the injured neurons.
In conclusion, this study has demonstrated that exogenous administration of MT led to an increase in regenerative sprouting after axotomy in several different in vitro models of DRG neuronal injury. Furthermore, we report that the regenerative action of MT correlated with the localization of megalin expression in DRG neurons, and that MT acts via a MAPK-dependent signaling pathway. This study provides further evidence that the megalin receptor and its ligands such as MT represent an important molecular target for promoting peripheral nerve regeneration.
Acknowledgments
This research was supported by research grants from the Australian Research Council (DP0984673), the National Health and Medical Research Council of Australia (ID#544913), and the Motor Accident and Insurance Board of Tasmania. JYKL also received a travel fellowship from the Australian Academy of Sciences to visit the NIH (USA) to undertake some of the experiments reported in this study.
Contributor Information
Jacqueline Y. K. Leung, Menzies Research Institute, University of Tasmania, Private Bag 24, Hobart, TAS 7001, Australia
William R. Bennett, Menzies Research Institute, University of Tasmania, Private Bag 24, Hobart, TAS 7001, Australia
Rosalind P. Herbert, Menzies Research Institute, University of Tasmania, Private Bag 24, Hobart, TAS 7001, Australia
Adrian K. West, Menzies Research Institute, University of Tasmania, Private Bag 24, Hobart, TAS 7001, Australia
Philip R. Lee, National Institute of Child Health and Human Development, National Institute of Health, Bethesda, MD 20892, USA
Hiroaki Wake, National Institute of Child Health and Human Development, National Institute of Health, Bethesda, MD 20892, USA.
R. Douglas Fields, National Institute of Child Health and Human Development, National Institute of Health, Bethesda, MD 20892, USA.
Meng Inn Chuah, Menzies Research Institute, University of Tasmania, Private Bag 24, Hobart, TAS 7001, Australia.
Roger S. Chung, Menzies Research Institute, University of Tasmania, Private Bag 24, Hobart, TAS 7001, Australia rschung@utas.edu.au
References
- 1.Ambjorn M, Asmussen JW, Lindstam M, Gotfryd K, Jacobsen C, Kiselyov VV, Moestrup SK, Penkowa M, Bock E, Berezin V. Metallothionein and a peptide modeled after metallothionein, EmtinB, induce neuronal differentiation and survival through binding to receptors of the low-density lipoprotein receptor family. J Neurochem. 2008;104:21–37. doi: 10.1111/j.1471-4159.2007.05036.x. [DOI] [PubMed] [Google Scholar]
- 2.Asmussen JW, Von Sperling ML, Penkowa M. Intraneuronal signaling pathways of metallothionein. J Neurosci Res. 2009;87:2926–2936. doi: 10.1002/jnr.22118. [DOI] [PubMed] [Google Scholar]
- 3.Chung RS, Vickers JC, Chuah MI, West AK. Metallothionein-IIA promotes initial neurite elongation and post-injury reactive neurite growth and facilitates healing after focal cortical brain injury. J Neurosci. 2003;23:3336–3342. doi: 10.1523/JNEUROSCI.23-08-03336.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung RS, Penkowa M, Dittmann J, King CE, Bartlett C, Asmussen JW, Hidalgo J, Carrasco J, Leung YKJ, Walker AK, Fung SJ, Dunlop SA, Fitzgerald M, Beazley LD, Chuah MI, Vickers JC, West AK. Redefining the role of metallothionein within the injured brain: extracellular metallothioneins play an important role in the astrocyte-neuron response to injury. J Biol Chem. 2008;283:15349–15358. doi: 10.1074/jbc.M708446200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald M, Nairn P, Bartlett CA, Chung RS, West AK, Beazley LD. Metallothionein-IIA promotes neurite growth via the megalin receptor. Exp Brain Res. 2007;183:171–180. doi: 10.1007/s00221-007-1032-y. [DOI] [PubMed] [Google Scholar]
- 7.Fleming CE, Milhazes Mar F, Franquinho F, Saraiva MJ, Sousa MM. Transthyretin internalization by sensory neurons is megalin mediated and necessary for its neuritogenic activity. J Neurosci. 2009;29:3220–3232. doi: 10.1523/JNEUROSCI.6012-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guertin AD, Zhang DP, Mak KS, Alberta JA, Kim HA. Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci. 2005;25:3478–3487. doi: 10.1523/JNEUROSCI.3766-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hidalgo J, Aschner M, Zatta P, Vasak M. Roles of the metallothionein family of proteins in the central nervous system. Brain Res Bull. 2001;55:133–145. doi: 10.1016/s0361-9230(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 10.Kozyraki R, Gofflot F. Multiligand endocytosis and congenital defects: roles of cubilin, megalin and amnionless. Curr Pharm Des. 2007;13:3038–3046. doi: 10.2174/138161207782110507. [DOI] [PubMed] [Google Scholar]
- 11.Miles AT, Hawksworth GM, Beattie JH, Rodilla V. Induction, regulation, degradation, and biological significance of mammalian metallothionein. Crit Rev Biochem Mol Biol. 2000;35:35–70. doi: 10.1080/10409230091169168. [DOI] [PubMed] [Google Scholar]
- 12.Penkowa M, Carrasco J, Giralt M, Moos T, Hidalgo J. CNS wound healing is severely depressed in metallothionein I- and II- deficient mice. J Neurosci. 1999;19:2535–2545. doi: 10.1523/JNEUROSCI.19-07-02535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu Z, Hyman BT, Rebeck GW. Apolipoprotein E receptors mediate neurite outgrowth through activation of P44/42 mitogen-activated protein kinase in primary neurons. J Biol Chem. 2004;279:34948–34956. doi: 10.1074/jbc.M401055200. [DOI] [PubMed] [Google Scholar]
- 14.van Lookeren Campagne M, Thibodeaux H, van Bruggen N, Cairns B, Gerlai R, Palmer JT, Williams SP, Lowe DG. Evidence for a protective role of metallothionein-1 in focal cerebral ischemia. PNAS. 1999;96:12870–12875. doi: 10.1073/pnas.96.22.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]


