SUMMARY
Background
Limitations of the current annual influenza vaccine have led to ongoing efforts to develop a “universal” influenza vaccine, i.e., one that targets a ubiquitous portion of the influenza virus so that the coverage of a single vaccination can persist for multiple years.
Objectives
To estimate the economic value of a “universal” influenza vaccine compared to the standard annual influenza vaccine, starting vaccination in the pediatric population (2–18 year olds), over the course of their lifetime.
Patient/methods
Monte Carlo decision analytic computer simulation model
Results
Universal vaccine dominates (i.e., less costly and more effective) the annual vaccine when the universal vaccine cost ≤$100/dose and efficacy ≥75% for both the 5 and 10 year duration. The universal vaccine is also dominant when efficacy is ≥50% and protects for 10 years. A $200 universal vaccine was only cost-effective when ≥75% efficacious for a 5 year duration when annual compliance was 25% and for a 10 year duration for all annual compliance rates. A universal vaccine is not cost-effective when it cost $200 and when its efficacy is ≤50%. The cost-effectiveness of the universal vaccine increases with the duration of protection.
Conclusions
Although development of a universal vaccine requires surmounting scientific hurdles, our results delineate the circumstances under which such a vaccine would be a cost-effective alternative to the annual influenza vaccine.
Keywords: Influenza Vaccine; Universal Vaccine; Economics; Pediatrics, Cost-effectiveness
Background
The following limitations of the current annual influenza vaccine have led to ongoing efforts to develop a “universal” influenza vaccine, i.e., one that targets a ubiquitous portion of the influenza virus so that the coverage of a single vaccination can persist for multiple years:
Annual vaccine administration: Administering influenza vaccine to the same patients each year incurs substantial costs and efforts. Persons must miss work. Maintaining influenza vaccination clinics and sites requires personnel time.
Annual vaccine manufacturing: Every year influenza vaccine manufacturers must allocate significant resources to produce influenza vaccines. Due to varying viral strains every season and the limited production period, the timing and preparation of vaccine development might cause unnecessary delays.
Patient compliance: Even when a person is recommended to be vaccinated, he or she may miss getting immunized certain years. According to the National Health Interview Survey and National Immunization Survey of United States for seasons 2005–2006, 2006–2007, and 2007–2008 and National Immunization Survey, influenza vaccination coverage levels ranged 31.8%–32.2% for ages 6–23 months, 26.4%–40.3% for ages 2–4 years, and 12.4%–21.1% for ages 5–17 years.[1] Estimation from the Behavioral Risk Factor Surveillance System (BRFSS) for influenza season 2008–2009 was 26.0%–38.7% for ages 2–4 year olds and 18.4%–23.4% for ages 5 to 17 year olds.[2]
Changing influenza strains: Each year, different influenza strains emerge as the dominant circulating strains. Although each year, scientists attempt to predict these strains, their predictions are not always accurate.[3] Mutations may cause major antigenic drift every 2 to 5 years.[4]
Emergence of novel influenza strain: As the 2009 influenza pandemic demonstrated, the annual vaccine may not cover new emergent strains.
Better understanding of the potential economic value of a “universal” vaccine can help guide investment and development for policy makers, manufacturers, insurance companies, investors, scientists, and other decision makers. Forecasting the impact of a vaccine early in its development when changes can still be made can increase the chances of a vaccine's success.[5]
Objectives
We developed a computational model to estimate the potential economic value of a “universal” influenza vaccine compared to the standard annual influenza vaccine in the pediatric population (ages 2 to 18 years), one of the Advisory Committee on Immunization Practices (ACIP) recommended high risk groups.[6]
Patients/methods
Model Structure
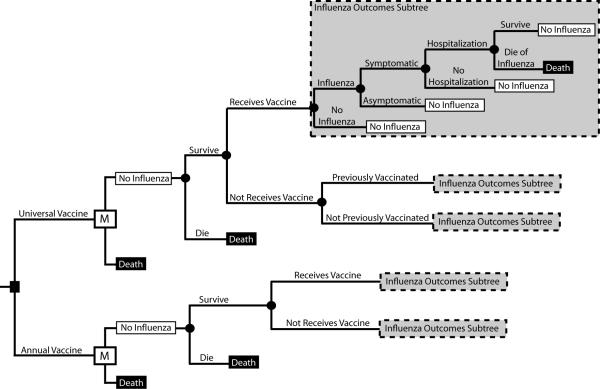
Figure 1 presents the general structure of the Markov decision analytic computer simulation model constructed using TreeAge Pro 2009 (TreeAge Software, Williamstown, Massachusetts). The model represents the decision from the societal perspective of whether a child (age 2 to 18 years old) should begin receiving a hypothetical universal influenza vaccine or the standard annual influenza vaccine The universal vaccine would have a certain duration of protection, therefore necessitating a periodic booster, and is assumed to be a single immunization. Each year the individual is scheduled to receive a vaccine, the individual had a probability of complying. Additionally, we looked at the effects of vaccinating high-risk children. For these scenarios we assumed individuals were high-risk throughout their lifetime and had a twofold risk of hospitalization and mortality.
Figure 1.
Model Structure State Diagram
The time horizon for the model is the child's lifetime. The model has a cycle length of 1 year. The Markov states are mutually exclusive; an individual can only be in one state in a given year. Each year, an individual had a probability of becoming infected with influenza. Vaccination attenuates this probability by the vaccine-related efficacy. Each time an individual is vaccinated, he or she has a probability of developing vaccine side effects.[7] Individuals who contract influenza have probabilities of developing symptoms or remaining asymptomatic. Symptomatic individuals then have a probability of visiting an outpatient setting and a probability of requiring hospitalization. Each individual with influenza has a probability of surviving or dying from influenza. Those who die from influenza or other unrelated causes enter the death state. The model concludes its run when an individual enters this state, otherwise known as the absorptive state.
Each simulation run sends 1,000 individuals 1,000 times through the model for a total of 1,000,000 trials of an individual's lifetime. For each simulation, the following equation calculates the incremental cost-effectiveness ratio (ICER) of the “universal” vaccine versus the annual vaccine:
where effectiveness is expressed in quality-adjusted life-years (QALYs). ICER values less than $50,000 per quality-adjusted life-year (QALY) identified the strategy as cost-effective.[8–9] The model was from the societal perspective, and therefore accounted for both direct (i.e., outpatient and hospitalization costs) and indirect costs (i.e., cost of productivity losses due to missed work (e.g., parent losses for child care) and influenza-attributable mortality of expected lifetime earnings).
Budget Impact Analysis
We also calculated the potential economic value of a universal influenza vaccine from the societal perspective for the U.S. pediatric population. The U.S. Census bureau estimate in July 2009 was used to provide the age stratified population: 21.3 million (under 5 years), 20.6 million (5–9 years), 20.0 million (10–14 years), and 21.5 million (15–19 years).[10]
Data Inputs
Table 1 lists the probabilities, costs, durations, and utilities used in the model along with their corresponding distributions and sources. Costs of annual vaccination are based on the average whole sale price and administration cost.[11] Mortality values are from the CDC National Vital Statistics Reports of Number of Deaths and Death Rates, by Age, Race, and Sex: United States 2007.[12] A 3% discount rate converted costs and QALYs from other years into 2010 values.[13] Death resulted in a QALY loss based on the QALY-adjusted life expectancy of the person's age.[14] Each influenza episode resulted in age-adjusted QALY decrements for the duration of the condition.[8]
Table 1.
Data inputs
| Description (units) | Distribution | Mean | Standard Deviation or Range | Source |
|---|---|---|---|---|
| COSTS ($US) | ||||
|
| ||||
| Annual vaccine | Point Estimate | 20 | - | [11,35] |
| Influenza Treatment: | ||||
| Outpatient Visit | ||||
| Pediatric Outpatient Visit | Point Estimate | 74.90 | [36] | |
| Adult Outpatient Visit | Triangular | 104.77 | 69.14 – 140.39 | [37] |
| Elderly Outpatient visit | Triangular | 155.92 | 118.39 – 193.44 | [37] |
| Hospitalization | ||||
| Age 1 to 4 | Gamma | 5992 | 515 | [38] |
| Age 5 to 9 | Gamma | 5761 | 561 | [38] |
| Age 10 to 14 | Gamma | 8735 | 1231 | [38] |
| Age 15 to 17 | Gamma | 6559 | 816 | [38] |
| Age 18 to 44 | Gamma | 6506 | 461 | [38] |
| Age 45 to 64 | Gamma | 7580 | 759 | [38] |
| Age 65 to 84 | Gamma | 7568 | 234 | [38] |
| Age 85 and Over | Gamma | 7698 | 240 | [38] |
| General death | Triangular | 6921 | 5191 – 9025 | [39] |
| Treatment of vaccine side effects | Triangular | 0.79 | 0.70 – 3.93 | [11] |
| Median hourly wage | Point Estimate | 15.57 | - | [35] |
|
| ||||
| DURATIONS | ||||
|
| ||||
| Work hours per day | - | 8 | - | Assumption |
| Absenteeism from influenza (days) | Uniform | 3.2 | 1.5 – 4.9 | [40] |
| Time being sick from the flu | Uniform | 6 | 5 – 7 | [41–42] |
| Time after having vaccine side effects | Uniform | 0.75 | 0.5 – 1 | [43] |
|
| ||||
| UTILITIES (QALYs) | ||||
|
| ||||
| One year of life | ||||
| Age 0 to 17 | Point Estimate | 1 | - | [8] |
| Age 18 to 64 | Point Estimate | 0.92 | - | [8] |
| Age 65 and Over | Point Estimate | 0.84 | - | [8] |
| Influenza with no hospitalization | Triangular | 0.65 | 0.49 – 0.81 | [44–45] |
| Influenza with hospitalization | Triangular | 0.50 | 0.38 – 0.63 | [44,46] |
| Vaccine side effects | Triangular | 0.95 | 0.71 – 1.00 | [46] |
|
| ||||
| PROBABILITIES | ||||
|
| ||||
| Clinical Outcomes without Vaccination | ||||
| Influenza throughout the year | Triangular | 0.125 | 0.05 – 0.2 | [7] |
| Outpatient Visit given influenza | ||||
| Age 0 to 4 | Beta | 0.455 | 0.098 | [47] |
| Age 5 to 17 | Beta | 0.318 | 0.061 | [47] |
| Age 18 to 64 | Beta | 0.313 | 0.014 | [47] |
| Age 65 and Over | Beta | 0.620 | 0.027 | [47] |
| Age 0 to 4 (High-risk) | Beta | 0.910 | 0.250 | [47] |
| Age 5 to 17 (High-risk) | Beta | 0.635 | 0.167 | [47] |
| Age 18 to 64 (High-risk) | Beta | 0.625 | 0.118 | [47] |
| Age 65 and Over(High-risk) | Beta | 0.850 | 0.093 | [47] |
| Hospitalization given influenza | ||||
| Age 0 to 4 | Beta | 0.0141 | 0.0047 | [47] |
| Age 5 to 17 | Beta | 0.0006 | 0.0002 | [47] |
| Age 18 to 49 | Beta | 0.0042 | 0.0014 | [47] |
| Age 50 to 64 | Beta | 0.0193 | 0.0064 | [47] |
| Age 65 and Over | Beta | 0.0421 | 0.0140 | [47] |
| Mortality given influenza | ||||
| Age 0 to 4 | Beta | 0.00004 | 0.00001 | [47] |
| Age 5 to 17 | Point Estimate | 0.00001 | [47] | |
| Age 18 to 49 | Beta | 0.00009 | 0.00003 | [47] |
| Age 50 to 64 | Beta | 0.00134 | 0.00045 | [47] |
| Age 65 and Over | Beta | 0.01170 | 0.00390 | [47] |
| Vaccine efficacy | Triangular | 0.45 | 0.56 – 0.68 | [7] |
| Vaccine side effects | Point Estimate | 0.03 | - | [48] |
Sensitivity Analyses
Sensitivity analyses systematically varied the cost of the universal vaccine ($100, $200), universal vaccine efficacy (range: 50%–75%), probability of influenza infection being symptomatic (50% or 67%), initial age of the individual (range: 2–18 years), compliance with annual vaccine (range: 25%, 50%, 75%, and 100%), and the duration of universal vaccine protection (5 or 10 years).[15–16] Probabilistic sensitivity analyses simultaneously varied the values of each parameter across the ranges listed in Table 1.
Results
Cost Effectiveness Analysis when Universal Protection Duration is 5 years
Table 2 shows how the ICER of universal vaccination compares to annual vaccination varying with differing universal vaccine efficacy, cost, and annual vaccine compliance when the duration of universal vaccine protection is 5 years. Universal vaccine is the dominant strategy (i.e., saves costs and provides health benefits) when vaccine cost is ≤$100/dose and vaccine efficacy is ≥75% for all scenarios tested. The annual vaccine dominates the $100 universal vaccine, only when the universal is 50% efficacious and annual compliance is 100%. When increasing the cost to $200/dose, universal vaccine is cost-effective only when annual compliance is ≤25% and universal vaccine efficacy ≥75% for both symptomatic rates. A $200 universal vaccine with an efficacy ≤50% was not cost-effective for any annual compliance rate. For high-risk children, a $100 universal vaccine dominated the annual vaccine or had ICER values ≥$185,060/QALY for all probabilities of annual compliance.
Table 2.
Cost, Effectiveness, and Incremental Cost-Effectiveness Ratio (ICER; Cost per QALY) of Switching from Annual to Universal Vaccine when Universal Vaccine Provides 5 years of Protection (50% Symptomatic Influenza Rate)
| Annual Vaccine Compliance | Vaccination Strategy | Cost | Effectiveness | ICER | ||
|---|---|---|---|---|---|---|
| Vaccine Cost $100 | Vaccine Efficacy 75% | 100% | Universal | 1,580 – 2,120 | 25.52 – 28.29 | Universal Dominates |
| Annual | 1,684 – 2,385 | 25.52 – 28.29 | ||||
| 75% | Universal | 1,579 – 2,118 | 25.53 – 28.29 | Universal Dominates | ||
| Annual | 1,649 – 2,560 | 25.52 – 28.29 | ||||
| 50% | Universal | 1,579 – 2,120 | 25.52 – 28.29 | Universal Dominates | ||
| Annual | 1,616 – 2,320 | 25.52 – 28.28 | ||||
| 25% | Universal | 1,577 – 2,118 | 25.53 – 28.29 | Universal Dominates | ||
| Annual | 1,578 – 2,286 | 25.52 – 28.28 | ||||
|
| ||||||
| Vaccine Efficacy 50% | 100% | Universal | 1,775 – 2,473 | 25.52 – 28.29 | Annual Dominates | |
| Annual | 1,685 – 2,387 | 25.52 – 28.30 | ||||
| 75% | Universal | 1,777 – 2,474 | 25.53 – 28.29 | 39,482 – 52,197 | ||
| Annual | 1,650 – 2,351 | 25.53 – 28.29 | ||||
| 50% | Universal | 1,775 – 2,475 | 25.53 – 28.29 | 31,544 – 74,353 | ||
| Annual | 1,612 – 2,320 | 25.53 – 28.29 | ||||
| 25% | Universal | 1,775 – 2,474 | 25.53 – 28.29 | 33,987 – 49,354 | ||
| Annual | 1,579 – 2,282 | 25.52 – 28.29 | ||||
|
| ||||||
| Vaccine Cost $200 | Vaccine Efficacy 75% | 100% | Universal | 2,019 – 2,718 | 25.52 – 28.30 | 77,108 – 124,575 |
| Annual | 1,684 – 2,384 | 25.53 – 28.29 | ||||
| 75% | Universal | 2,214 – 2,893 | 25.52 – 28.29 | 171,099 – 319,601 | ||
| Annual | 1,648 – 2,353 | 25.52 – 28.28 | ||||
| 50% | Universal | 2,020 – 2,717 | 25.53 – 28.29 | 79,422 – 81,349 | ||
| Annual | 1,614 – 2,317 | 25.52 – 28.29 | ||||
| 25% | Universal | 2,018 – 2,718 | 25.53 – 28.30 | 33,562 – 47,763 | ||
| Annual | 1,579 – 2,288 | 25.52 – 28.28 | ||||
|
| ||||||
| Vaccine Efficacy 50% | 100% | Universal | 2,411 – 3,072 | 25.53 – 28.29 | Annual Dominates | |
| Annual | 1,682 – 2,387 | 25.53 – 28.29 | ||||
| 75% | Universal | 2,411 – 3,071 | 25.53 – 28.29 | Annual Dominates – 495,957 | ||
| Annual | 1,650 – 2,353 | 25.52 – 28.29 | ||||
| 50% | Universal | 2,413 – 3,073 | 25.52 – 28.29 | 257,930 – 806,958 | ||
| Annual | 1,614 – 2,560 | 25.52 – 28.29 | ||||
| 25% | Universal | 2,412 – 2,284 | 25.53 – 28.29 | 144,542 – 172,231 | ||
| Annual | 1,580 – 3,073 | 25.52 – 28.29 | ||||
Note: Bold ICER values are cost-effective.
Budget Impact Analysis when Universal Protection Duration is 5 years
Switching from the annual vaccine to the universal vaccine can yield cost savings from the societal perspective. A $100/dose universal vaccine with a vaccine efficacy ≥75% will provide cost savings per pediatric patient vaccinated: $1–$104 (ages below 5 years), $5–$102 (5–9 years), $6–$96 (10–14 years), and $168–$266 (15–18 years). Therefore, switching the entire pediatric population to universal vaccination could generate cost savings of $15 million - $2.2 billion for those below 5 years, $101 million - $2.1 billion for 5–9 years, $121 million - $1.9 billion for 10–14 years, and $3.6 billion - $5.7 billion for 15–18 years over their lifetimes. Increasing the proportion of developing symptomatic influenza from 50% to 67% will provide more cost savings.
Cost Effectiveness Analysis when Universal Protection Duration is 10 years
Table 3 demonstrates the incremental cost-effectiveness ratio when duration of protection by the universal vaccine increases from 5 to 10 years. Universal vaccine is optimal (i.e., economically dominant) compared to annual vaccine when its efficacy ≥50% and cost ≤$100/dose for all annual compliance and symptomatic rates explored.
Table 3.
Cost, Effectiveness, and Incremental cost-effectiveness ratio (ICER) of Switching from Annual to Universal Vaccine when Universal Vaccine Provides 10 years of Protection (50% Symptomatic Influenza Rate)
| Annual Vaccine Compliance | Vaccination Strategy | Cost | Effectiveness | ICER | ||
|---|---|---|---|---|---|---|
| Vaccine Cost $100 | Vaccine Efficacy 75% | 100% | Universal | 1,287 – 2,021 | 25.53 – 28.29 | Universal Dominates |
| Annual | 1,685 – 2,385 | 25.52 – 28.29 | ||||
| 75% | Universal | 1,286 – 2,021 | 25.52 – 28.29 | Universal Dominates | ||
| Annual | 1,648 – 2,353 | 25.52 – 28.29 | ||||
| 50% | Universal | 1,286 – 2,019 | 25.53 – 28.29 | Universal Dominates | ||
| Annual | 1,613 – 2,319 | 25.52 – 28.29 | ||||
| 25% | Universal | 1,286 – 2,022 | 25.53 – 28.29 | Universal Dominates | ||
| Annual | 1,581 – 2,283 | 25.52 – 28.29 | ||||
|
| ||||||
| Vaccine Efficacy 50% | 100% | Universal | 1,483 – 2,197 | 25.53 – 28.29 | Universal Dominates | |
| Annual | 1,685 – 2,386 | 25.52 – 28.29 | ||||
| 75% | Universal | 1,485 – 2,201 | 25.53 – 28.29 | Universal Dominates | ||
| Annual | 1,649 – 2,351 | 25.53 – 28.29 | ||||
| 50% | Universal | 1,482 – 2,200 | 25.52 – 28.29 | Universal Dominates | ||
| Annual | 1,615 – 2,316 | 25.52 – 28.29 | ||||
| 25% | Universal | 1,484 – 2,200 | 25.52 – 28.29 | Universal Dominates | ||
| Annual | 1,579 – 2,282 | 25.52 – 28.29 | ||||
|
| ||||||
| Vaccine Cost $200 | Vaccine Efficacy 75% | 100% | Universal | 1,630 – 2,347 | 25.53 – 28.29 | 9,180 – 45,456 |
| Annual | 1,683 – 2,386 | 25.53 – 28.29 | ||||
| 75% | Universal | 1,649 – 2,348 | 25.52 – 28.29 | 1,285 – 31,956 | ||
| Annual | 1,629 – 2,354 | 25.53 – 28.29 | ||||
| 50% | Universal | 1,615 – 2,348 | 25.52 – 28.29 | 4,222 – 4,755 | ||
| Annual | 1,616 – 2,378 | 25.52 – 28.29 | ||||
| 25% | Universal | 1,629 – 2,346 | 25.53 – 28.30 | 5,194 – 5,970 | ||
| Annual | 1,580 – 2,285 | 25.52 – 28.29 | ||||
|
| ||||||
| Vaccine Efficacy 50% | 100% | Universal | 1,829 – 2,526 | 25.53 – 28.29 | Annual Dominates | |
| Annual | 1,684 – 2,384 | 25.53 – 28.29 | ||||
| 75% | Universal | 1,827 – 2,530 | 25.52 – 28.29 | Annual Dominates | ||
| Annual | 1,651 – 2,350 | 25.52 – 28.29 | ||||
| 50% | Universal | 1,827 – 2,527 | 25.52 – 28.29 | 69,797 – 380,364 | ||
| Annual | 1,613 – 2,322 | 25.52 – 28.29 | ||||
| 25% | Universal | 1,827 – 2,527 | 25.53 – 28.29 | 59,443 – 74,421 | ||
| Annual | 1,580 – 2,286 | 25.52 – 28.28 | ||||
Note: Bold ICER values are cost-effective.
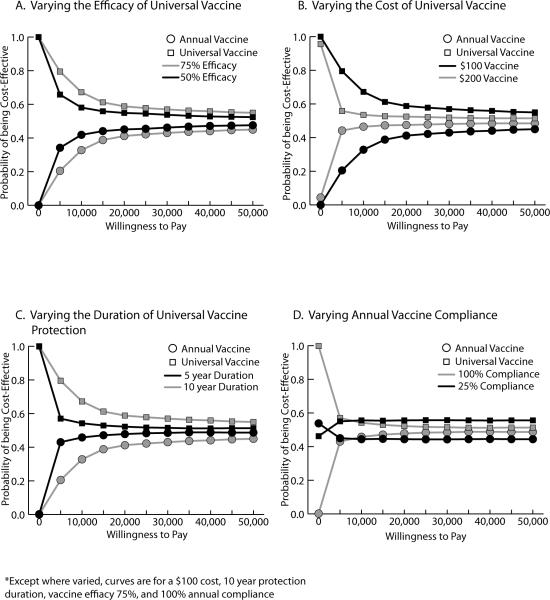
Figure 2 shows acceptability curves for the universal and annual vaccine when the universal protects for 10 years and costs $100. The universal vaccine consistently has a higher probability of being cost-effective, even with an increasing willingness to pay. A $200/dose universal vaccine is cost-effective only when its efficacy is ≥75%. At an efficacy of 50%, a $200 universal vaccine is not cost-effective compared to the annual vaccine. Figure 2b shows the curves for this change in cost.
Figure 2.
Acceptability Curves a) varying the efficacy of universal vaccine, b) varying the cost of universal vaccine, c) varying the duration of universal vaccine protection, d) varying annual vaccine compliance
Budget Impact Analysis when Universal Protection Duration is 10 years
Increasing the duration of universal protection to 10 years further augments the potential cost savings to society. A $100/dose universal vaccine with ≥75% efficacy can provide cost savings of $295 – $398 per pediatric patients (ages below 5 years), $284 – $388 (5–9 years), $274 – $377 (10–14 years), and $261–$364 (15–18 years) vaccinated. Therefore, switching the entire pediatric population to universal vaccination could generate cost savings of $6.2 billion - $8.5 billion for those below 5 years, $5.9 billion - $8.0 billion for 5–9 years, $5.5 billion - $7.5billion for 10–14 years, and $5.6 billion - $7.8 billion for 15–18 years over their lifetimes. As before, increasing the probability of being symptomatic will provide even more cost savings.
Discussion
Our results suggest that a universal vaccine could provide substantial economic value by overcoming the annual vaccine's current drawbacks. This favors investment in universal vaccine development, helps establish efficacy and duration of protection targets for developers, and prepares policy makers for reimbursement questions. Addressing these issues early in a vaccine's development when changes are easier to make could help avoid considerable problems in the future.[5]
In many ways, our study underestimates the potential value of a universal vaccine. Not only is compliance with the annual vaccine far less than 100%, many children do not get vaccinated until later into the influenza season, i.e., after October or even November. As previous studies have demonstrated the value of annual influenza vaccine drops the later in the season the vaccine is administered, because the longer the patient remains unvaccinated, the more susceptible they are to being infected.[17–18] Moreover, our model did not account for how the universal vaccine may prevent the vaccinated individual from transmitting the influenza virus to others. Unvaccinated individuals are not only more susceptible to infection but may shed more virus when infected compared to vaccinated individuals. Our model focuses on the individual and does not consider influenza transmission and herd immunity. If the universal vaccine were to results in a greater proportion of the populations protected, then it could more substantially reduce transmission than the standard annual vaccine and therefore would be more cost effective. Finally, in our model, individuals are healthy children without co-morbidities that may worsen influenza outcomes.
The 2009 influenza pandemic identifies another possible benefit of the universal vaccine. A universal vaccine that provides protection against novel strains may circumvent the need to develop a specific vaccine against an emerging pandemic strain. As computer simulation studies have suggested, timely and effective vaccination of the population may be the most important mitigation intervention.[17–20]
Bringing a universal vaccine to market requires surmounting numerous hurdles. First, the vaccine must contain an appropriate antigen common to all possible circulating influenza viruses. Second, the antigen should be stable and not prone to mutation. Third, the antigen must not occur in other common human tissues. Fourth, the antigen needs to generate an adequate immune response. Fifth, the vaccine must remain effective and not wane for the duration of vaccine coverage.
Du and colleagues describe the possible approaches in developing a universal influenza vaccine which focus on the conserved sequences of M2e, HA (HA1, HA2), NP, and epitopes from different influenza viral proteins.[21] These sequences occur across many known subtypes of influenza virus making them ideal universal vaccine targets. Some candidates use a combination of these conserved epitopes from different viral proteins, potentially offering further cross-protection across varying subtypes.[21] Other candidates focus on the sequences of major structural proteins of the virus surface, ectodomain of matrix protein 2.[22–23] Scientists have also targeted human antibodies that could cross-react with and neutralize several different hemagglutinin viral subtypes.[24–27] Several candidate “universal” influenza vaccines are currently at different stages of development based on these targets. Five companies, Acambis Inc. (Cambridge, UK), Cytos Biotechnology (Schlieren, Switzerland), Merck & Co Inc. (NJ,USA), and VaxInnate Corp. (NJ,USA) have reported promising preliminary Phase 1 clinical study results.[3, 28] BiondVax's (Ness Ziona, Israel) Mulitmeric-001 Universal Flu Vaccine successfully navigated through phase I/II trials and will enter Phase II trials in 2010.[29–30] BiondVax is currently recruiting patients 55 to 75 years old for its next study.[31]
A recently published article reports significant human B cell responses towards the 2009 pandemic H1N1 influenza.[32] Most of the neutralizing antibodies induced by the virus are able to cross-react against epitopes in the hemagglutinin head and stalk of various influenza strains. Tested antibodies show broad protection against H1N1 and H5N1 influenza strain with abundantly stalk-reactive antibodies in H1N1 patients. Such universal vaccine may have a stronger cross-protection to divergent virus subtypes, reduced production time and cost. This advantage may serve as an important direction in the development of a universal influenza vaccine.
Another study provides evidence that a universal vaccine which covers all influenza strains is achievable. This novel influenza vaccine is able to reactivate and induce T-cell responses (CD8+ and CD4+) towards NP and M1 proteins of the virus that is common in all influenza type A strains.[33] It proves to be safe and well tolerated with less local side effects. Extensive protection against seasonal and pandemic influenza is promising. According to researchers, introduction of such a vaccine would provide protection for at least 5–10 years.[34]
Limitations
In addition to the limitations identified earlier, all models are simplifications of real life. A model cannot represent all possible influenza outcomes and the heterogeneity that exist among the patient population. Rather than make decisions, a model provides information for decision makers such as public health officials, scientists, insurance companies, investors, manufacturers, and clinicians. Models are designed to elucidate relationships, raise questions, and approximate orders of magnitude instead of providing exact answers. Although our model does not explicitly represent natural immunity from infection, which may persist for several years, especially when occurring in children, the various outcome probabilities (e.g., risk of influenza) did draw from studies where natural immunity was present.
Conclusion
Limitations of the current annual influenza vaccine have led to ongoing efforts to develop a “universal“ influenza vaccine, i.e., one that targets a conserved portion of the influenza virus so that the coverage of a single vaccination can persist for multiple years. Our results suggest that a universal vaccine could provide substantial economic value by overcoming the annual vaccine's current drawbacks. This favors investment in universal vaccine development, helps establish efficacy and duration of protection targets for developers, and prepares policy makers for reimbursement questions. Addressing these issues early in a vaccine's development when changes are easier to make could help avoid considerable problems in the future. Although development of a universal vaccine requires surmounting scientific hurdles, our results delineated the circumstances under which such a vaccine would be a cost-effective alternative to the annual influenza vaccine.
Acknowledgements
This work was supported by the National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) [1U54GM088491-0109], the National Library of Medicine [5R01LM009132-02], and the Centers for Disease Control and Prevention (CDC) University of Pittsburgh Center for Advanced Study of Informatics [1P01HK000086-01]. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. B.Y.L. has served as an advisor for Novartis and Glaxosmithkline. R.K.Z. has received grants for clinical research from and served as an advisor for MedImmune and Merck & Co. This manuscript has been presented in the following conferences: 1) CDC's 2010 Conference “Modeling for Public Health Action: From Epidemiology to Operations,“ in the Behavioral and Economic Modeling track, December 8–9, 2010, Atlanta, GA, 2) 45th National Immunization Conference, March 28–31, 2011, Washington, D.C., Abstract no. 25588.
References
- 1.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009 Jul 31;58(RR-8):1–52. [PubMed] [Google Scholar]
- 2.Influenza vaccination coverage among children and adults - United States, 2008–09 influenza season. MMWR Morb Mortal Wkly Rep. 2009 Oct 9;58(39):1091–5. [PubMed] [Google Scholar]
- 3.Schotsaert M, De Filette M, Fiers W, Saelens X. Universal M2 ectodomain-based influenza A vaccines: preclinical and clinical developments. Expert Rev Vaccines. 2009 Apr;8(4):499–508. doi: 10.1586/erv.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DJ, Lapedes AS, de Jong JC, Bestebroer TM, Rimmelzwaan GF, Osterhaus AD, et al. Mapping the antigenic and genetic evolution of influenza virus. Science. 2004 Jul 16;305(5682):371–6. doi: 10.1126/science.1097211. [DOI] [PubMed] [Google Scholar]
- 5.Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine. 2010 Apr 1;28(16):2806–9. doi: 10.1016/j.vaccine.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . Seasonal Influenza (Flu): Key facts about seasonal flu vaccine. Atlanta, GA: [Google Scholar]
- 7.Rivetti D, Jefferson T, Thomas R, Rudin M, Rivetti A, Di Pietrantonj C, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2006;3:CD004876. doi: 10.1002/14651858.CD004876.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998 Jun;36(6):778–92. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Neumann PJ, Sandberg EA, Bell CM, Stone PW, Chapman RH. Are pharmaceuticals cost-effective? A review of the evidence. Health Aff (Millwood) 2000 Mar–Apr;19(2):92–109. doi: 10.1377/hlthaff.19.2.92. [DOI] [PubMed] [Google Scholar]
- 10.Bureau USC, editor. Annual Estimates of the Resident Population by Sex and Five-Year Age Groups for the United States: April 1, 2000 to July 1, 2009. [Google Scholar]
- 11.PDR Red Book: Pharmacy's Fundamental Reference. Thompson Healthcare, INC; Montvale, NJ: 2010. [Google Scholar]
- 12.Xu J, Kochanek DK, Tejada-Vera B, B.S. SL. Deaths: preliminary data for 2007. Natl Vital Stat Rep. 2009;58(19) [PubMed] [Google Scholar]
- 13.Shepard DS. In: Cost-effectiveness in Health and Medicine. Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Oxford University Press; New York: 1996. J Ment Health Policy Econ. 1999 Jun 1;2(2):91-2. [Google Scholar]
- 14.Wilmoth J. V. S. Human Mortality Database. 2008. [Google Scholar]
- 15.Ling LM, Chow AL, Lye DC, Tan AS, Krishnan P, Cui L, et al. Effects of early oseltamivir therapy on viral shedding in 2009 pandemic influenza A (H1N1) virus infection. Clin Infect Dis. 2010 Apr 1;50(7):963–9. doi: 10.1086/651083. [DOI] [PubMed] [Google Scholar]
- 16.Carrat F, Vergu E, Ferguson NM, Lemaitre M, Cauchemez S, Leach S, et al. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am J Epidemiol. 2008 Apr 1;167(7):775–85. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 17.Lee BY, Tai JH, Bailey RR, Smith KJ. The timing of influenza vaccination for older adults (65 years and older) Vaccine. 2009 Nov 23;27(50):7110–5. doi: 10.1016/j.vaccine.2009.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BY, Tai JH, Bailey RR, Smith KJ, Nowalk AJ. Economics of influenza vaccine administration timing for children. Am J Manag Care. 2010 Mar;16(3):e75–e85. [PMC free article] [PubMed] [Google Scholar]
- 19.Lee BY, Brown ST, Cooley PC, Zimmerman RK, Wheaton WD, Zimmer SM, et al. A computer simulation of employee vaccination to mitigate an influenza epidemic. Am J Prev Med. 2010 Mar;38(3):247–57. doi: 10.1016/j.amepre.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee BY, Brown ST, Korch GW, Cooley PC, Zimmerman RK, Wheaton WD, et al. A computer simulation of vaccine prioritization, allocation, and rationing during the 2009 H1N1 influenza pandemic. Vaccine. 2010 May 16; doi: 10.1016/j.vaccine.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du L, Zhou Y, Jiang S. Research and development of universal influenza vaccines. Microbes Infect. 2010 Apr;12(4):280–6. doi: 10.1016/j.micinf.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Fiers W, De Filette M, El Bakkouri K, Schepens B, Roose K, Schotsaert M, et al. M2e-based universal influenza A vaccine. Vaccine. 2009 Oct 23;27(45):6280–3. doi: 10.1016/j.vaccine.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009 Apr 10;324(5924):246–51. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen GL, Subbarao K. Attacking the flu: neutralizing antibodies may lead to 'universal' vaccine. Nat Med. 2009 Nov;15(11):1251–2. doi: 10.1038/nm1109-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nabel GJ, Fauci AS. Induction of unnatural immunity: prospects for a broadly protective universal influenza vaccine. Nat Med. 2010 Dec;16(12):1389–91. doi: 10.1038/nm1210-1389. [DOI] [PubMed] [Google Scholar]
- 26.Donis RO, Cox NJ. Prospecting the influenza hemagglutinin to develop universal vaccines. Clin Infect Dis. 2011 Apr 15;52(8):1010–2. doi: 10.1093/cid/cir129. [DOI] [PubMed] [Google Scholar]
- 27.Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, et al. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):18979–84. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO . Tables on the clinical trials of pandemic influenza prototype vaccines. [Google Scholar]
- 29.Clinical Trials Gov . In: A double-dose safety study of an influenza vaccine (Multimeric-001) Health USNIo, editor. 2009. [Google Scholar]
- 30.Ben-Yedidia T. Multimeric-001: BiondVax's universal flu vaccine. Expert Rev Vaccines. 2010 Mar;9(3):241–2. doi: 10.1586/erv.10.2. Interview by Duc Le. [DOI] [PubMed] [Google Scholar]
- 31.Clinical Trials Gov . In: A double-dose safety study of an influenza vaccine (Multimeric-001) injected to elderly volunteers. Health USNIo, editor. 2009. [Google Scholar]
- 32.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011 Jan 17;208(1):181–93. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berthoud TK, Hamill M, Lillie PJ, Hwenda L, Collins KA, Ewer KJ, et al. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin Infect Dis. 2011 Jan;52(1):1–7. doi: 10.1093/cid/ciq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkinson E. Universal Flu Vaccine Tests Start. BBC News; 2008. [Google Scholar]
- 35.Labor USDo, editor. May 2008 National Occupation Employment and Wage Estimates, United States. 2008. [Google Scholar]
- 36.American Medical Association . In: Socioeconomics of Medical Practice, 1997. Gonzales M, editor. American Medical Assoication; Chicago, IL: 1997. p. 69.p. 89.p. 107. [Google Scholar]
- 37.Services USDoHH . Centers for Medicare & Services. 2009. [PubMed] [Google Scholar]
- 38.HCUP facts and figures . In: Statistics on hospital-based care in the United States in 2007. Services USDoHH, editor. 2008. [PubMed] [Google Scholar]
- 39.Gould MK, Dembitzer AD, Sanders GD, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A cost-effectiveness analysis. Ann Intern Med. 1999 May 18;130(10):789–99. doi: 10.7326/0003-4819-130-10-199905180-00002. [DOI] [PubMed] [Google Scholar]
- 40.Keech M, Beardsworth P. The impact of influenza on working days lost: a review of the literature. Pharmacoeconomics. 2008;26(11):911–24. doi: 10.2165/00019053-200826110-00004. [DOI] [PubMed] [Google Scholar]
- 41.Smith KJ, Roberts MS. Cost-effectiveness of newer treatment strategies for influenza. Am J Med. 2002 Sep;113(4):300–7. doi: 10.1016/s0002-9343(02)01222-6. [DOI] [PubMed] [Google Scholar]
- 42.Hawkes M, Schuh S, Ipp M, Bitnun A, Richardson SE, Parkin PC, et al. Natural history of pandemic H1N1 2009 influenza infection in healthy pediatric outpatients. Acad Pediatr. 2011 Jan–Feb;11(1):66–74. doi: 10.1016/j.acap.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention . Possible Side-effects from Vaccines. Atlanta, GA: [cited 2009 August 11]; Available from: http://www.cdc.gov/vaccines/vac-gen/side-effects.htm#flu. [Google Scholar]
- 44.Sackett DL, Torrance GW. The utility of different health states as perceived by the general public. J Chronic Dis. 1978;31(11):697–704. doi: 10.1016/0021-9681(78)90072-3. [DOI] [PubMed] [Google Scholar]
- 45.Allsup S, Haycox A, Regan M, Gosney M. Is influenza vaccination cost effective for healthy people between ages 65 and 74 years? A randomised controlled trial. Vaccine. 2004 Dec 16;23(5):639–45. doi: 10.1016/j.vaccine.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000 Jun;38(6):583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007 Jun 28;25(27):5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 48.Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007 Feb 15;356(7):685–96. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]