Abstract
Ghrelin and peptide YY (PYY) are newly recognized gut peptides involved in appetite regulation. Plasma ghrelin concentrations are elevated in fasting and suppressed following a meal, while PYY concentrations are suppressed in fasting and elevated postprandially. We determine whether ghrelin and PYY are altered by a low-fat, high-carbohydrate (10% fat, 75% carbohydrate) or moderate-fat, moderate-carbohydrate (35% fat, 50% carbohydrate) diet and; whether these peptides are affected by intense endurance running (which is likely to temporarily suppress appetite). Twenty-one endurance-trained runners followed a controlled diet (25% fat) and training regimen for 3 days before consuming the low-fat or isoenergetic moderate-fat diet for another 3 days in random cross-over fashion. On day 7 runners underwent glycogen restoration and then completed a 90-minute pre-loaded 10-km time trial on day 8, following a control breakfast. Blood samples were obtained on days 4 and 7 (fasting), and day 8 (non-fasting) before and after exercise for analysis of ghrelin, PYY, insulin and growth hormone (GH). Insulin, GH, Ghrelin and PYY changed significantly over time (p < 0.0001) but were not influenced by diet. Ghrelin was elevated during fasting (days 4 and 7), while insulin and PYY were suppressed. Following the pre-exercise meal, ghrelin was suppressed ~17% and insulin and PYY were elevated ~157 and ~40%, respectively, relative to fasting (day 7). Following exercise, PYY, ghrelin, and GH were significantly (p < 0.0001) increased by ~11, ~16 and ~813%, respectively. The noted disruption in the typical inverse relationship between ghrelin and PYY following exercise suggests that interaction of these peptides may be at least partially responsible for post-exercise appetite suppression. These peptides do not appear to be influenced by dietary fat intake.
Keywords: Exercise-induced anorexia, gut peptides, appetite regulation, hunger, exercise
Introduction
It is well documented that intense (but not necessarily moderate or light) exercise may induce a transient suppression of hunger that has been reported in both humans (Broom et al., 2007; Burns et al., 2007; King and Blundell, 1995; King et al., 1994; Kissileff et al., 1990; Westerterp-Plantenga et al., 1997) and experimental animals (Routtenberg and Kuznesof, 1967). This short-term suppression of hunger, termed exercise-induced anorexia (King et al., 1997), however, is not reported in all studies (Imbeault et al., 1997; Pomerleau et al., 2004) and may be influenced by the mode (King et al., 1997), duration (King et al., 1994; Westerterp-Plantenga et al., 1997), and intensity (King et al., 1994; Thompson et al., 1988) of the exercise, and the body weight/adiposity of the exerciser (Kissileff et al., 1990). Several studies, for example, have documented increased hunger following 75-minutes of continuous swimming [(Verger et al., 1992)ref in (King et al., 1997)] and 2 hours of sports-specific athletic activity (Verger et al., 1994). This is in agreement with the anecdotal reports from athletes who commonly report that intense running and sports related training induces transient anorexia while swimming and cycling promotes appetite stimulation (Larson-Meyer, unpublished observations). While it is not well understood whether exercise-associated alterations in hunger (or appetite) impacts long-term energy balance (King et al., 1997), exercise-induced anorexia may potentially influence exercise recovery by delaying the onset of eating (King and Blundell, 1995; King et al., 1997) or reducing energy and nutrient intake (Kissileff et al., 1990; Westerterp-Plantenga et al., 1997) in the 30–60 minutes following exercise. Carbohydrate and protein intake in the immediate post-exercise period is needed for rapid replacement of muscle and liver glycogen and the building and repair of muscle tissue (Rodriguez et al., 2009).
Little is known about the specific mechanisms responsible for exercise-associated changes in appetite and hunger. While investigators have hypothesized that modulation of appetite may be related to elevations in body temperature (Andersson and Larsson, 1961) or blood lactate concentrations (Baile et al., 1970; Racotta and Russek, 1977), such changes alone are not likely to be mechanistically responsible for exercise-induced alterations in appetite. The recent discovery of several new gut peptides involved in appetite regulation and energy homeostasis, however, lends promising mechanisms which may help explain both training and individual (between-subject) differences in hunger, appetite and food intake following different modes, intensities and durations of acute exercise. In particular, the gut peptides ghrelin and peptide YY (PYY) are of interest because they appear to regulate hunger and food intake for up to 24-hours (Wren et al., 2001) and are not specifically controlled by body fat stores, as are the adipokines leptin and adoponectin (Cummings and Overduin, 2007). Ghrelin is a 28 amino acid gastric hormone that is released from the enteroendocrine cells of the stomach and large intestine that stimulates appetite (Cummings and Overduin, 2007; Cummings et al., 2002) and promotes gastric motility (Cummings and Overduin, 2007) whereas peptide YY 3–36 (PYY) is a 36 amino acid peptide secreted from L-cells in the intestinal mucosa that suppresses appetite and gastric emptying (Adrian et al., 1985; Cummings and Overduin, 2007; le Roux and Bloom, 2005). Ghrelin is at its highest concentration during fasting and decreases postprandially (Cummings et al., 2002), whereas PYY is lowest during fasting and increases postprandially in proportion to energy intake (Adrian et al., 1985; le Roux and Bloom, 2005). Ghrelin and PYY remain altered for 1–2 hours posstprandial and then gradually rise and fall, progressively, back toward fasting concentrations. Ghrelin has been shown to be more effectively suppressed postprandial by a high-carbohydrate (as opposed to a high-fat or high-protein) meal (Erdmann et al., 2003; Monteleone et al., 2003); whereas PYY may be more effectively elevated after a fat- compared to a carbohydrate-rich meal (Adrian et al., 1985). It is not known, however, how short- or long-term alterations in macronutrients (i.e., a long-term high-carbohydrate vs. high-fat diet) impact these gut peptides. In addition, studies have suggested that ghrelin (Broom et al., 2007; Christ et al., 2006; Ghanbari-Niaki, 2006; Kraemer et al., 2004b) and PYY (Martins et al., 2007) may be influenced by a single-bout of exercise but results are not conclusive (Burns et al., 2007; Erdmann et al., 2007; Kraemer et al., 2004a; Martins et al., 2007; Schmidt et al., 2004).
The purpose of the current study was to determine: a) whether fasting ghrelin and PYY concentrations are altered by three days of a low-fat, high-carbohydrate (10% fat, 75% carbohydrate) or moderate-fat, moderate-carbohydrate (35% fat, 50% carbohydrate) diet in endurance-trained runners and; b) whether concentrations of these peptides are affected by a bout of intense endurance exercise which is likely to promote exercise-induced anorexia. We hypothesized that: a) ghrelin and PYY would be lower during fasting and non-fasting while on the low-fat, high-carbohydrate diet compared to the moderate-fat, moderate-carbohydrate diet; 2) and that ghrelin would be lowered and PYY elevated following strenuous endurance running, thereby providing a potential mechanism for exercise associated anorexia. The results of this study will provide valuable data to enhance our understanding of the influence of diet and exercise on ghrelin and PYY concentrations and the possible causes for exercise-induced alterations of appetite.
Methods
The subjects were 21 healthy endurance trained distance runners or triathletes between the ages of 18–44 for men (n = 11) and 18–54 for women (n = 10) who were recruited for a study assessing the effect of diet on intramyocellular lipid stores (Larson-Meyer et al., 2008). To qualify, participants had to be in good general health (as determined by a study physician), be performing regular endurance running (>40 km·wk−1, have performed at least two training runs > 2 hours within the previous three months, and have a maximal aerobic fitness (VO2max) ≥50 ml·kg−1·min−1 for women and ≥55 ml·kg−1·min−1 for men. In addition, female participants had to have regularly occurring menstrual cycles (Larson-Meyer et al., 2008). Subjects were excluded if they smoked, demonstrated signs of a full or partial syndrome eating disorder, alcoholism or other substance abuse problems, were using prescription or over-the-counter medications or supplements (other than oral contraceptives) that could influence metabolism, or could not agree to consume all foods/beverages provided on the experimental diets. The study was approved by the Institutional Review Board of the Pennington Biomedical Research Center (PBRC). Volunteers were fully informed about the possible risks of all procedures before providing written informed consent.
Baseline testing
Approximately two to three weeks before initiation of the experimental protocol, VO2max was determined on a motor driven treadmill (MedTrack ST65, Quinton Industries, Inc, Bothell, WA) using a protocol specific for endurance-trained runners (Larson-Meyer et al., 2002; 2008; Russell et al., 2004), and body composition was assessed by dual-energy x-ray absorptiometry (DXA, Hologic QDR4500A). Following a 5-min warm-up, “workload” was increased by either speed (starting at the subject’s typical warm-up speed and increasing by 0.5 MPH (13.4 m/min)) or grade (starting at 0 degrees and increasing by 2.5%) every min until exhaustion. Oxygen consumption (VO2) and carbon dioxide production (VO2) were measured using a metabolic cart (V-Max 29, SensorMedics, Yorba Linda, CA), and heart rate was monitored using a portable heart rate monitor (Polar S-610, Polar Beat, Port Washington, NY). The highest VO2, respiratory exchange ratio (RER) and heart rate achieved over a 20-s period within the last 2-min of exercise were recorded as the maximum values. Subjects had to achieve two out of three of the following criteria: 1) a leveling or plateau of VO2 (defined as an increase of VO2 of < 2 ml·kg−1·min−1 with increased workload), 2) RER > 1.10, and 3) maximum heart rate within 10 beats of age-predicted maximum. Following a 30-minute rest, subjects also performed a practice endurance performance test that consisted of a 90-min submaximal run at 65%VO2max (preload) followed by a time trial. The practice performance run was performed to familiarize the subjects with the treadmill and the endurance performance test.
Experimental protocol
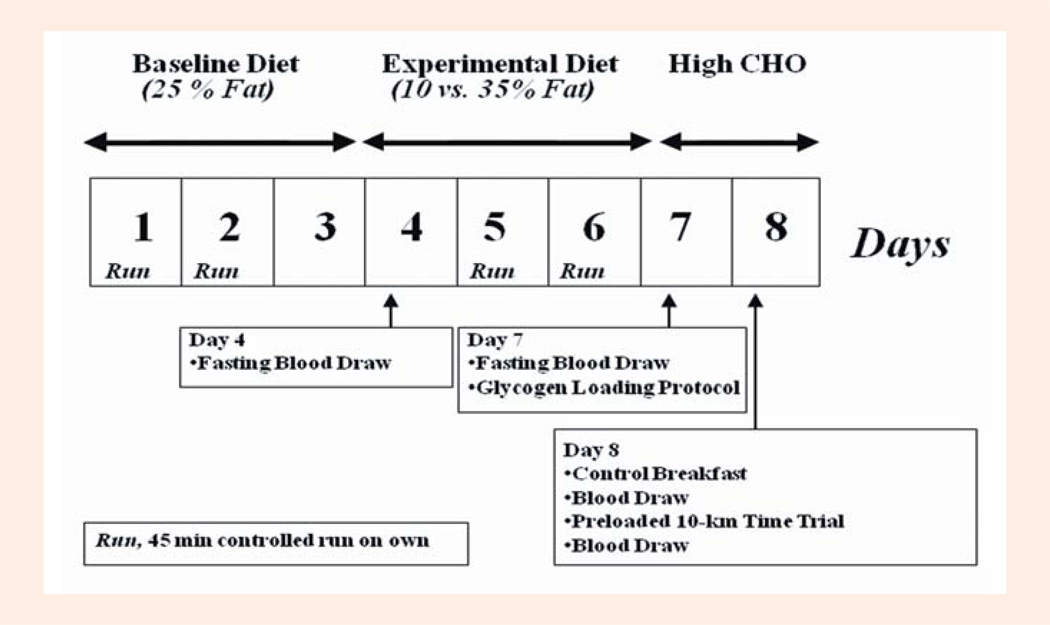
The details of this randomly assigned cross-over experiment have been published elsewhere (D. E. Larson-Meyer et al., 2008). The investigators were blinded to the diet treatment (except metabolic kitchen personnel); whereas the participants were masked (i.e., they were not told of their diet assignment but may have been able to identify certain fat-altered foods). As shown in Figure 1, participants completed two separate test weeks (eight days each) that were spaced approximately 3–4 wk apart in men and one menstrual cycle (3–5 wk) in women. The test weeks consisted of an eight day session which included a baseline weight maintenance diet for the first 3 days (15% protein, 25% fat, 60% carbohydrate), followed by four days of an isoenergetic low-fat (10% fat, 15% protein, 75% CHO; LFAT) or moderate-fat (35% fat, 15% protein, 50% CHO; MFAT) experimental diet that was provided in random order. Three days were selected because it was sufficient for altering lipid stored within skeletal muscle cells (D.E. Larson-Meyer et al., 2002) and would represent a reasonable time period an athlete might follow a specific diet regimen before competition. A glycogen normalization protocol, which consisted of a 20-min run at 70% VO2max followed by a carbohydrate loading diet (Russell et al., 2004) was completed on the last day of both experimental diets (day 7) to minimize potential differences in glycogen at the start of the performance test (morning of day 8). Diets consisted of commercially-available foods and beverages that were prepared by the PBRC Metabolic Kitchen with precise control of both macronutrient and energy content (i.e., all foods and beverages were calculated and weighed to the nearest 0.1 gram). The energy content of the diets was initially estimated using the Harris Benedict sex-specific prediction equations for basal energy expenditure multiplied by an activity factor of 1.8 for women and 2.0 for men for the baseline diet; and 2.0 and 2.2 for women and men, respectively (Larson-Meyer et al., 2002; Russell et al., 2004) for the experimental (MFAT and LFAT) diet. Energy content was adjusted (if necessary) to maintain body weight. A higher energy level was assigned for the experimental diets due to the greater energy cost of the laboratory and controlled runs during the experimental period (as explained below). Subjects ate dinner at the center and were given the following days’ breakfast, lunch and snacks to carry out. Subjects were asked to eat all food but to return anything not consumed to the research dietitian so it could be weighed and recorded.
Figure 1.
Schematic of Testing Protocol in well trained male and female runners during the randomly assigned cross over trial (see Methods for detail). Modified from Larson-Meyer et al. (2008) and reprinted with permission.
Subjects completed a number of controlled training runs during the study (Figure 1) which helped mimic free-living training conditions. Specifically these consisted of a 2-hour run in the laboratory at 63 ± 5% VO2max (62 ± 5% on LFAT and 64 ± 5% on MFAT(D. E. Larson-Meyer et al., 2008)) on the morning of day 4 and a 20 minute run at 74 ± 3% VO2max (72 ± 7 on LFAT and 75 ± 3% on MFAT (Larson-Meyer et al., 2008)) on the morning of day 7. Subjects were also asked to run on their own for 45 min at ~45 seconds/mile slower than their usual 10-km race pace, on days 1, 2, 5 and 6 and to maintain these runs on both diet conditions. Exercise was not allowed on day 3 or on days 4, 7, and 8 when exercise sessions were scheduled in the laboratory.
On the morning of day 8, three hours after consuming a controlled breakfast (603 kcal for men and 470 kcal for women, 15% protein, 15% fat, 70% carbohydrate) subjects completed the endurance running time trial that consisted of a 90-minute preload run at 62±5% of VO2max, followed by a 10-km time trial on a treadmill (LifeFitness TR 9500, Franklin Park, IL). A metabolic cart (Sensormedics Vmax 2900), was used to verify the participant’s oxygen cost (and measure the RER) during the 90-minute preload, and heart rate was measured continuously (Polar S-610, Polar Beat, Port Washington, NY). Subjects were instructed to complete the 10-km time trial as fast as possible (i.e., to “race the 10-km”) and were allowed to adjust the treadmill speed as much as desired. Gas exchange was not measured during the 10-km so as not to interfere with performance. Water consumption was encouraged ad libitum (in weighed water bottles) during the first test as well as the practice tests and was then replicated during the second session.
Blood samples and analysis
Fasting blood samples were obtained at 8:00 AM on the morning of day 4 and day 7 for analysis of insulin, glucose, GH, total ghrelin and PYY3–36 (Figure 1). On day 8, nonfasting blood samples were obtained immediately before and immediately after (within 5-minutes of exercise cessation) the preloaded time trial for analysis of these same metabolites and hormones along with lactate. Blood samples for the analysis of ghrelin and PYY3–36 were collected in EDTA-containing tubes to which 150 µl of aprotinin was added prior to centrifugation (15 min at 3000 rpm, 2–8 degrees C). The supernatant (plasma) was removed and stored in cryovials at −80 degrees C until analysis. At study completion, ghrelin and PYY were batch analyzed by Radioimmuno-assay using human-specific kits from Phoenix Pharmaceuticals Inc (Burlingame, CA 94010) and Linco Research, Inc (St. Charles, MO 63304). Insulin, glucose, GH and lactate were analyzed according to standardized procedure (Larson-Meyer et al., 2008). Briefly, insulin was analyzed via immunoassay and growth hormone analyzed via immunoassay with fluorescence using the DPC Immulite 2000 (Diagnostic Product Corporation, Los Angeles, CA). Glucose was analyzed via glucose oxidase electrode and lactate via enzymatic methods using the Beckman-Coulter Synchron CX7 (Brea, CA).
Statistical approach
The two primary response variables tested in this analysis were: a) the effect of diet on circulating fasting ghrelin and PYY concentrations; and b) the effect of exercise (or time) on circulating non-fasting ghrelin and PYY concentrations. A Split-Plot design using compound symmetry was used to test the relations between diet and exercise (time) on circulating plasma concentrations of ghrelin and PYY (as well as glucose, insulin, GH and lactate), and included elements from both randomized group designs and randomized block (repeated measures) design. Using this model, the between subjects components of the split-plot design are related to the randomized group design and the within subjects components of the split-plot design are related to randomized block or repeated measures design. A split-plot design was used rather than a repeated model because it provided better goodness of fit given that multiple factors were being measured (both diet effect and exercise/time effect). Although determining possible sex differences were not a purpose of the study, inclusion of sex as a between subject factor had little impact on the fit (as well as the results). If appropriate, post hoc pairwise comparisons were performed using Tukey-Kramer adjusted comparisons. All statistics were computed with SAS 9.1.3 on a Windows XP-Pro operating platform. Data are means±SD unless otherwise specified.
Results
The characteristics of the eleven male (age 27 ± 9 yrs, weight 69.8 ± 4.9 kg, height 1.77 ± 0.07 m, BMI 21.9 ± 1.5 kg·m−2, percent body fat 12.8±2.4%) and ten female (age 29 ± 7 yrs, weight 56.2 ± 4.9 kg, height 1.64 ± 0.04 m, BMI 21.0 ± 1.1 kg·m−2, percent body fat 20.6 ± 2.3%) endurance athletes have previously been published (D. E. Larson-Meyer et al., 2008). All were well-trained runners (V02max 63.7 ± 6.3 ml·kg−1·min−1 for men and 53.2 ± 5.4 ml·kg−1·min−1 for women) who reported training between 42 and 70 km·week−1.
Dietary control and controlled running
All participants completed the dietary and controlled running required on both treatments. Details of the energy and macronutrient intakes during the LFAT and MFAT treatments have previously been published (Larson-Meyer et al., 2008). Briefly, energy and protein intakes at baseline (days 1–3) and during the experimental diets (days 4–7) were not different between LFAT and MFAT treatments and averaged (2869 ± 504 kcal) at baseline, (2914 ± 563 kcal) for LFAT and (2913 ± 523 kcal for MFAT. Energy and macronutrients were also not different between treatments on the glycogen normalization day (day 7), averaging (2918 ± 513 kcal) and (2910 ± 523 kcal) on the LFAT and MFAT trials, respectively. Body mass remained relatively stable during the study on both diets averaging 61.7 ± 8.7 on day 1, 61.1 ± 18.7 on day 4 and 61.9 ± 8.9 on day 7 on LFAT; and 61.8 ± 9.0 on day 1, 61.4 ± 8.6 on day 4 and 62.0 ± 9.0 on day 7 on MFAT. A time effect (p = 0.02), however, was noted which was most likely due to the slight drop in body weight on day 4 of both treatments. As previously published, muscle glycogen stores were not statistically different at the start of the time trial on the LFAT and MFAT time trial and averaged 360 ± 43 and 293 ± 25mmol glucosyl units·kg−1 dry mass, respectively (Larson-Meyer et al., 2008).
Time trial
The 90-min preload was completed at an average pace of 3.12 ± 0.07 m·s−1, which elicited an average VO2 of 61 ± 4 and 63 ± 5% VO2max and heart rate of 151 ± 13 and 149 ± 13 bpm for the LFAT and MFAT trials, respectively (Larson-Meyer et al., 2008). Time to complete the 10-km time trial also did not differ by diet (Larson-Meyer et al., 2008) and was 45:32 ± 5:17 min:sec on the LFAT trial and 45:50 ± 4:35 min:sec on the MFAT trial. Participants consumed 682 ± 363 ml of water during the LFAT trial and 660 ± 414 ml during the MF trial and experienced a 0.7 ± 0.6 and 0.9 ± 0.8 kg reduction in body mass, respectively, that did not differ between trials.
Effect of diet on ghrelin PYY and other hormone and metabolites
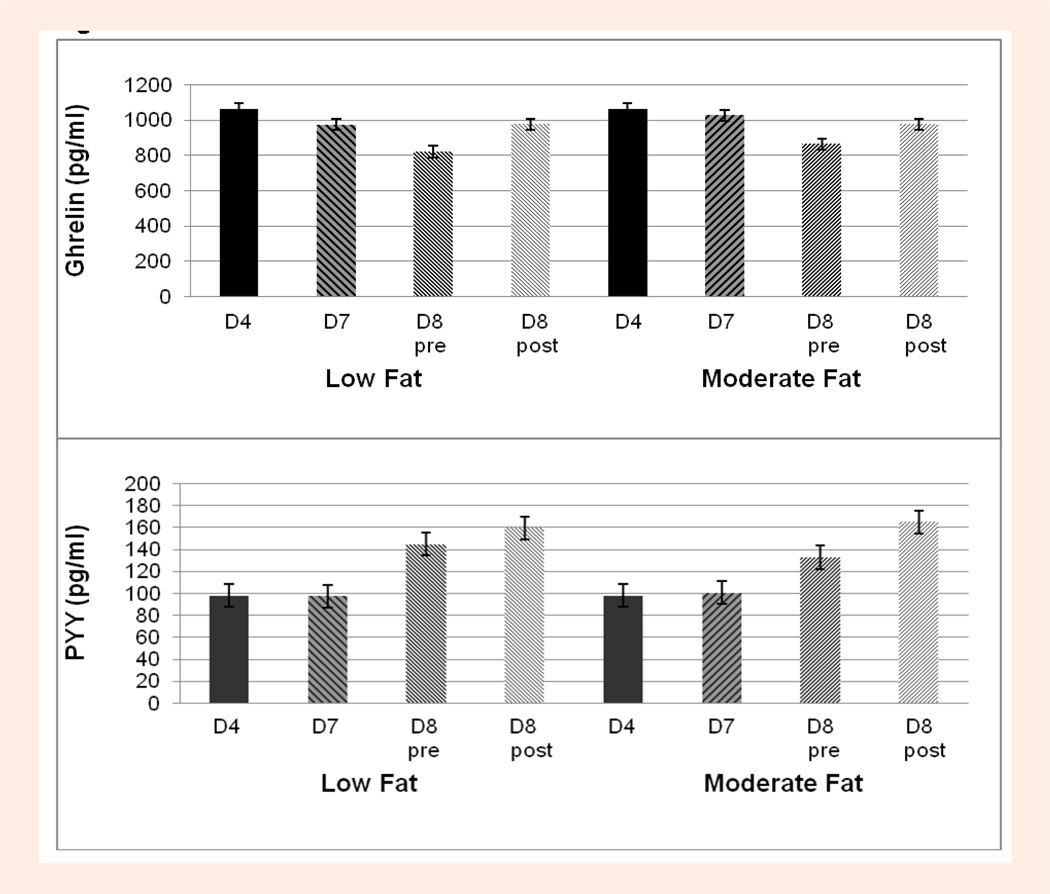
Circulating concentrations of ghrelin, PYY, glucose, insulin, growth hormone and lactate at all time points during both the LFAT and MFAT experimental trials are shown in Table 1. A main effect for diet or a diet*time interaction was not found for any of the hormones and metabolites measured including ghrelin and PYY (Figure 2). When sex was entered as a between subject factor, no significant main effects for sex or interactions with time or diet were found for ghrelin, PYY or the other hormones or metabolites.
Table 1.
Blood hormone and metabolite concentrations during fasting on Day 4 (Baseline Diet) and Day 7 (Experimental Diet), and before and after exercise on Day 8 (Experimental Diet) during the Low-fat (LFAT) and Moderate-Fat (MFAT) Trials. Data are means (±SD).
| LFAT Trial | MFAT Trial | ||||
|---|---|---|---|---|---|
| Fasting | |||||
| Day 4 | Day 7 | Day 4 | Day 7 | ||
| Ghrelin (pg·ml−1) | 1044 (95) | 987 (94) | 1022 (94) | 1029 (94) | ŧ b |
| PYY (pg·ml−1) | 104 (8) | 99 (8) | 97 (8) | 100 (8) | ŧ b |
| Glucose (mg·dl−1) | 93 (6) | 92 (8) | 91 (7) | 92 (9) | Ŧ |
| Insulin (uU·ml−1) | 7.5 (7.5) | 6.4 (3.3) | 5.8 (2.5) | 5.6 (1.7) | ŧ b |
| GH (ug·ml−1) | 3.6 (4.7) | 3.6 (5.5) | 2.4 (4.1) | 5.0 (8.2) | Ŧ |
| Exercise Following Controlled Breakfast | |||||
| Day 8 | Day 8 | Day 8 | Day 8 | ||
| Pre-Exercise | Post-Exercise | Pre-Exercise | Post-Exercise | ||
| Ghrelin | 823 (94) | 976 (94) | 853 (94) | 972 (94) | ŧ c |
| PYY | 146 (8) | 154 (8) | 131 (8) | 159 (8) | ŧ c |
| Glucose (mg·dl−1) | 83 (12) | 135 (29) | 85 (15) | 140 (31) | ŧ c |
| Insulin (uU·ml−1) | 16.5 (13.1) | 11.4 (7.6) | 14.3 (8.3) | 11.9 (8.2) | ŧ |
| GH (ug·ml−1) | 2.7 (6.0) | 23.8 (16.8) | 2.9 (4.2) | 27.3 (17.0) | ŧ c |
| Lactate (mmol·l−1) | 1.6 (.4) | 5.7 (2.0) | 1.7 (.6) | 5.6 (2.1) | ŧ |
PYY, peptide YY; GH, growth hormone.
ŧ significant effect of time (p < 0.0001);
significant difference day 7 (fasting state) vs. day 8 pre-exercise (fed state) (adjusted p < 0.05);
significant difference day 8 pre-exercise vs. post-exercise (adjusted p > 0.05).
There was no significant influence of diet (p > 0.10, diet*time and time effect) for any hormones of metabolites.
Figure 2.
Diet effect on Ghrelin (upper panel) and PYY (lower panel) concentrations on Day 4 (D4, fasting, baseline diet), Day 7 (D7, fasting, experimental diet) and before (pre) and after (post) exercise on Day 8 (control breakfast, experimental diet) on both Low Fat and Moderate Fat Trials.
Effect of time (and exercise) on ghrelin PYY and other hormone and metabolites
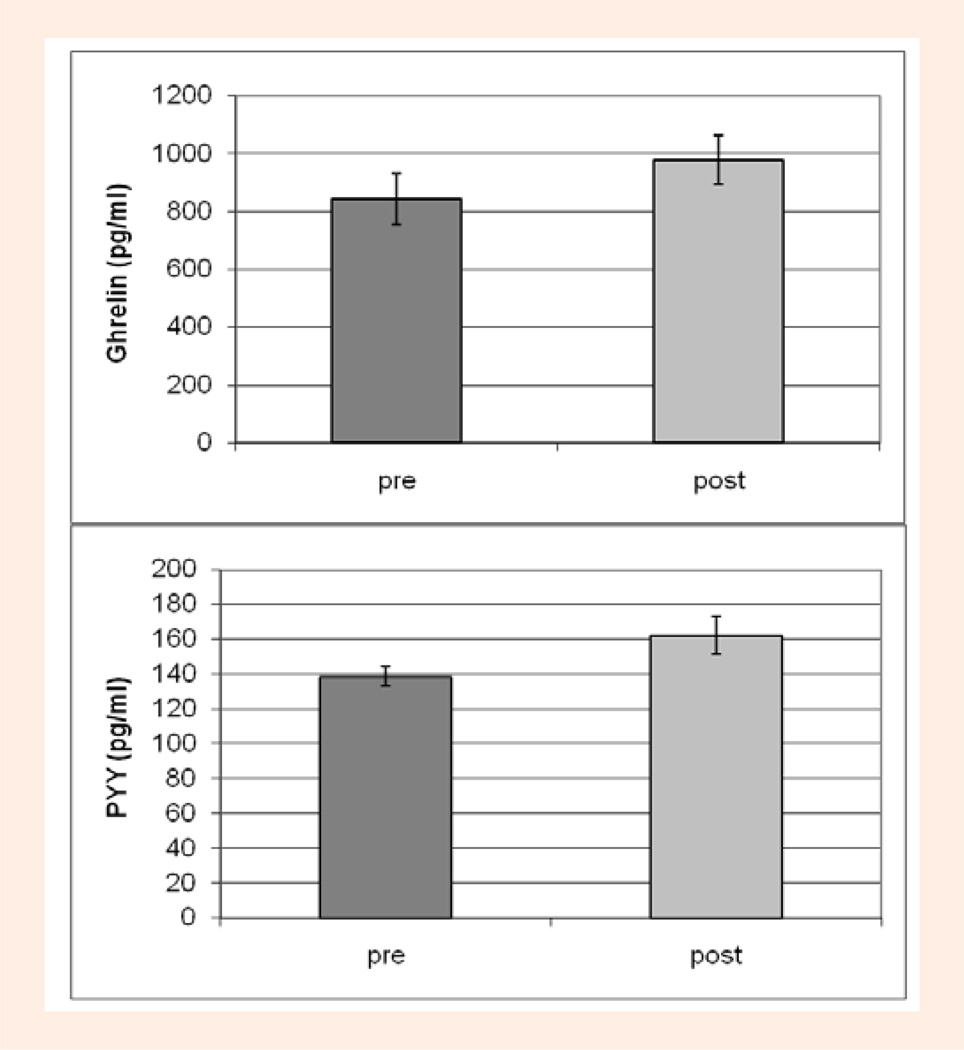
As shown in Table 1, there was a significant main effect for time for ghrelin, PYY, glucose, insulin, and growth hormone, (p < 0.0001). On both diets, ghrelin was significantly lower, by an average of 17% on day 8 pre-exercise (non-fasting, 3-hours postprandial) compared to day 7 (fasting) (p < 0.0001), and was elevated by an average of 16% after exercise (p < 0.0001) (Figure 3, upper panel) to concentrations similar to those assessed during fasting (days 4 and 7) (Figure 2, upper panel). In contrast, PYY was ~40% higher on day 8 pre-exercise (non-fasting) compared to day 7 (fasting) and also rose significantly (p = 0.03) by ~11% after exercise (Figure 3, lower panel) to concentrations higher than those measured following the control breakfast (Figure 3, lower panel). As previously reported, glucose, growth hormone and lactate were significantly elevated (p < 0.0001) after exercise by an average 64%, 813% and 242% (compared to pre-exercise) while insulin was suppressed, although not significantly (p = 0.24), by 24%. Insulin was also significantly elevated on day 8 pre-exercise (non-fasting) compared to day 7 (fasting) (p < 0.0001).
Figure 3.
Exercise/time effect on Ghrelin (upper panel) and PYY (lower panel) concentrations on Day 8 before (pre) and after (post) exercise (control breakfast) with both diet trials combined.
Correlation between ghrelin and PYY and other hormone and metabolites during exercise
The increase in ghrelin from pre-to post-exercise was significantly correlated with the change in GH on the MFAT (r = 0.61, p < 0.01) but not the LFAT trial (r = −0.01, p = 0.96), but was not correlated with the change in PYY, glucose or insulin. No significant correlations were noted between the increase in PYY and the change in GH, insulin or glucose from pre- to post-exercise.
Discussion
The present study presents novel findings which address whether the appetite-stimulating gut peptide ghrelin and the appetite-suppressing peptide PYY are influenced by the fat content of the diet and/or by a bout of intense endurance running. We found that a low-fat, high-carbohydrate pre-exercise regimen compared to a moderate-fat, moderate-carbohydrate regimen plus glycogen restoration did not influence circulating plasma ghrelin and PYY concentrations, but that both gut peptides were elevated following two to two-and-a-half hours of strenuous running. The noted disruption in the normal inverse relationship between ghrelin and PYY at rest suggests that the interaction of ghrelin and PYY (Martins et al., 2007) may be at least partially responsible for exercise-induced anorexia which is commonly reported following vigorous endurance exercise.
Exercise-induced changes in appetite are of interest over both the short and long term. Little is known, however, about whether gut peptides are altered by different exercise modes, and how such potential alterations may influence meal initiation and food intake. The gut peptides ghrelin and PYY are of particular interest because they are thought to be short-term regulators of hunger and food intake (Cummings and Overduin, 2007; Wren et al., 2001) and respond relatively rapidly to nutrient ingestion (42) and possibly also exercise (Broom et al., 2007; Christ et al., 2006; Ghanbari-Niaki, 2006; Kraemer et al., 2004b; Martins et al., 2007). The action of these gut peptides is not yet completely understood but is thought to be via central circulation where the peptides cross the blood brain barrier and directly interact with neurons located in the arcuate nucleus (ARC), which is considered to be a primary site of food intake regulation (Orr and Davy, 2005). Ghrelin, which is currently the only known appetite- (or “hunger”-) stimulating hormone, is hypothesized to initiate feeding by activating the NPY/AgRP expressing neurons within the ARC (Cummings and Overduin, 2007; Orr and Davy, 2005). Only the acylated form of ghrelin, however, is known to cross the blood brain barrier (Kojima et al., 1999) and produce its appetite stimulating effect. PYY, on the other hand, is thought to inhibit food intake primarily via reducing NPY expression in the ARC (Orr and Davy, 2005) but it has also been shown to suppress circulating ghrelin (Batterham et al., 2003) and its binding in the ARC (le Roux and Bloom, 2005). Perhaps most intriguingly, peripheral intravenous infusion of these hormones at physiological concentrations in humans has been shown to influence hunger (as assessed by visual analogue scales) and food intake (relative to a placebo infusion) at a subsequent free-choice buffet meal and cumulatively over a 24-hour period with ghrelin infusion increasing food intake by 28% (Wren et al., 2001) and PYY3–36 reducing it by 30% (Batterham et al., 2003). Although these gut peptides typically change in association with ratings of hunger (Batterham et al., 2003; Erdmann et al., 2007; Martins et al., 2007; Monteleone et al., 2003; Wren et al., 2001) and have been shown to correlate negatively with obesity (Batterham et al., 2003; Tschop et al., 2001), the significance of fasting or post-exercise ghrelin or PYY concentrations on immediate food intake or long-term body weight regulation are not yet understood.
We originally hypothesized that the pattern of ghrelin and PYY response during fasting and non-fasting (post consumption of the control breakfast) and exercise would be different after three days of a pre-exercise regimen that varied in fat and carbohydrate. Specifically we hypothesized that circulating ghrelin and PYY would be lower during fasting (day 7) on the low-fat, high-carbohydrate diet compared to the moderate-fat, moderate-carbohydrate diet and that a similar pattern would be noted in the non-fasting state before and after exercise (day 8). Several previous studies have found that carbohydrate consumption is more effective at suppressing postprandial ghrelin (Al Awar et al., 2005; Monteleone et al., 2003) and less effective at elevating PYY concentrations (Adrian et al., 1985) than is fat or protein consumption. Our lack of a diet effect, in contrast, suggests that macronutrient differences in the diet may only have an acute (single-meal) influence on these short-acting gut peptides, or that more drastic differences in the fat and carbohydrate intakes over longer periods of time (> 3days) in a larger number of subjects are needed to induce the hypothesized differences. The only other study which has investigated the influence of short-term dietary changes in the pre-exercise regimen found that the area under the curve for ghrelin during a 3-hour bout of exercise was significantly greater following a low-compared to an isocarbohydrate (but not isocaloric) high-fat diet. Pre-exercise ghrelin, however, was not significantly different following the standardized pre-exercise breakfast (Christ et al., 2006). In this study (Christ et al., 2006) as well as in the present study, however, influence of the macronutrient altered diet regimen on gut peptide concentrations in the pre-exercise fed state may have been negated by the glycogen normalization regimen and/or the control breakfast which was the same on both treatments and given to ensure that differences in glycogen stores or the carbohydrate content of the pre-exercise meal did not influence exercise.
Concerning the influence of exercise, our results were only in partial agreement with our hypothesis that ghrelin would be suppressed and PYY elevated following intense endurance running, but were in agreement with several previous studies which found elevated concentrations of ghrelin (Christ et al., 2006) and PYY (Martins et al., 2007) after a bout of aerobic exercise. Similar to our study, Christ et al. (2006) found that plasma ghrelin concentrations were significantly elevated following a three-hour bout of endurance cycling at 50% of maximal work in trained male cyclists, but PYY concentrations or hunger were not assessed. In the only previous study measuring PYY, Martins et al (Martins et al., 2007) found that PYY, glucagon-like peptide-1 (GLP-1) and pancreatic polypeptide concentrations were elevated by ~12, 60 and 100%, respectively, immediately following 60-minutes of moderate-intensity cycling in six men and six women non-athletes. Only GLP-1 and PP, however, remained elevated at 2 hours into the recovery period. Total ghrelin concentrations in this (Martins et al., 2007) and the majority of published studies, however, were not altered by exercise (Burns et al., 2007; Erdmann et al., 2007; Jurimae et al., 2007; Kraemer et al., 2004a; Martins et al., 2007; Schmidt et al., 2004) or correlated with ratings of hunger (Burns et al., 2007; Martins et al., 2007) which is in contrast to several studies that found suppressed total (Ghanbari-Niaki, 2006; Kraemer et al., 2004b) or acylated (Broom et al., 2007; Kraemer et al., 2004b) ghrelin following exercise in male and female athletes. These seemingly discrepant results concerning ghrelin’s response to exercise may be related to the intensity, duration or mode of the exercise. For example, most studies that found no exercise-associated alterations in ghrelin concentrations evaluated the response following short (< 1 hour) bouts of aerobic exercise while those that found suppressed ghrelin concentrations evaluated the response following resistant-type exercise (Ghanbari-Niaki, 2006; Kraemer et al., 2004b). The studies (including the present study) that found increased ghrelin tested its effect after exercise lasting at least 2 hours (Christ et al., 2006). Another possibility is that the exercise-associated changes in total ghrelin are not reflective of acylated (or active) ghrelin (Broom et al., 2007). Even though total ghrelin is thought to reflect the active acylated form, acylated ghrelin may respond more quickly than total ghrelin to nutrient intake (Hosoda et al., 2004) and may be better correlated with hunger ratings following exercise (Broom et al., 2007). While sex differences are also a possibility, previous studies, along with ours, have not provided evidence that ghrelin or PYY differ in woman and men (Burns et al., 2007; Martins et al., 2007; Tschop et al., 2001).
Our finding that both ghrelin and PYY are elevated following a bout of intense endurance running is intriguing and goes against previous findings in the resting state which consistently document an inverse relation between circulating ghrelin and PYY (Cummings and Overduin, 2007; Orr and Davy, 2005). This finding deserves further follow-up, given the limitations of the current study, but may suggest that exercise-induced changes in PYY can counter any increases in ghrelin and provide a potential mechanism to explain exercise-induced anorexia which is expected following a bout of intense endurance running. Although these gut peptides typically act inversely, the control and actions of these hormones in relation to each other as well as other known influencers including insulin, glucose and GH does not appear to be well understood, particularly in response to exercise when blood flow to the gut is known to be reduced. In agreement with this possibility, peripheral infusion of PYY in humans has been shown to suppress hunger and circulating ghrelin (Batterham et al., 2003) as well as its binding in the ARC (le Roux and Bloom, 2005). At rest, ghrelin has also been shown to be negatively inhibited by elevations in glucose and insulin (Flanagan et al., 2003), which may act in opposite directions during endurance exercise preceded by a pre-exercise meal. Given ghrelin’s GH secretagogue activity (Kojima et al., 1999), it may also be speculated that ghrelin could potentially stimulate the release of GH during exercise, although previous studies have not found evidence of such support (Kraemer et al., 2004a). Our finding that the increase in ghrelin from pre- to post-exercise was correlated with the elevation in GH on the MFAT but not LFAT trial, but not with glucose or insulin suggests that the mechanisms controlling ghrelin’s action and secretion may be different during exercise (as opposed to rest).
While contributing to our overall understanding of exercise-induced anorexia, our study has a number of limitations. First, we did not include a non-exercising control period as some (Broom et al., 2007; Burns et al., 2007; Kraemer et al., 2004a; Martins et al., 2007) but not all (Christ et al., 2006; Ghanbari-Niaki, 2006; Kraemer et al., 2004a; Kraemer et al., 2004b; Schmidt et al., 2004) previous studies have. Including a resting control would have allowed us to determine whether post-exercise ghrelin was elevated above pre-exercise concentrations as a function of the exercise rather than time. Since PYY would be expected to decrease (rather than increase) with increasing time following a meal, the observed change is most likely reflective of a true effect of exercise. Second, we did not assess hunger or appetite following our intense bout of exercise or obtain serum or food intake measurements into the recovery period which could have provided additional important evidence. Unfortunately, hunger ratings have been measured by only a small handful (Broom et al., 2007; Burns et al., 2007; Erdmann et al., 2007; Martins et al., 2007), but not the majority, of previous studies (Christ, 2006; Ghanbari-Niaki, 2006; Jurimae et al., 2007; Kraemer, 2004a; Schmidt, 2004). Third, we measured total rather than acylated ghrelin because total ghrelin at study initiation was thought to be reflective of its active but less stable form (Hosoda et al., 2004). Recent evidence, however, has suggested that this may not be the case following exercise (Mackelvie et al., 2007) and following glucose infusion, where acylated ghrelin responds more dramatically than total ghrelin (Hosoda et al., 2004). Finally, we did not adjust our hormone and metabolite samples for hemoconcentration which likely occurred to some degree given the minor body mass losses experienced during the run. This potentially resulted in higher post-exercise concentrations in ghrelin, PYY and the other hormones due to dehydration rather than just exercise, and may have also been a limitation of several previous studies (Christ et al., 2006; Erdmann et al., 2007; Kraemer et al., 2004b; Schmidt et al., 2004). It is important to recognize that the results of the current study should be interpreted with some caution and verified by future studies.
Conclusion
The noted disruption in the typical inverse relationship between ghrelin and PYY following two to two-and-a-half hours of strenuous running suggests that the interaction of these peptides may be at least partially responsible for exercise-induced anorexia which is common following vigorous endurance exercise. Neither ghrelin nor PYY, however, appear to be influenced by dietary fat intake. Future studies should evaluate the effect of different modes, intensities and durations of exercise on acylated ghrelin, PYY and other gut peptides and their association with post-exercise hunger, meal initiation and food intake.
Key points.
The study presents novel findings which address whether the appetite-stimulating gut peptide ghrelin and the appetite-suppressing peptide PYY are influenced by the fat content of the diet and/or by a bout of intense endurance running.
The low-fat, high-carbohydrate pre-exercise regimen compared to moderate-fat, moderate-carbohydrate regimen did not influence circulating plasma ghrelin and PYY concentrations
Most importantly, both gut peptides were elevated following two to two-and-a-half hours of strenuous running which lasted between two and two and a half hours. The noted disruption in the normal inverse relationship between ghrelin and PYY at rest suggests that the interaction of ghrelin and PYY may be at least partially responsible for exercise-induced anorexia which is commonly reported following vigorous endurance exercise.
Acknowledgments
We thank Dr James P Geaghan, Chair, Experimental Statistics, Louisiana State University for statistical assistance, and Research Dietitian Jennifer Howard, RD for assistance with the experimental diet. We also thank the volunteers. This research was supported in part by NIH K01 DK062018.
Biographies

Ryan RUSSELL
Employment
A doctoral student in exercise physiology at Louisiana State University
Degree
MSc
Research interests
The effect of exercise on appetite, and exercise training, oxidative stress and insulin sensitivity in diabetes.
E-mail: rrusse3@tigers.lsu.edu

Kentz WILLIS
Employment
Nutrition educator for the University of Wyoming Cooperative Extension Service.
Degree
MSc
Research interests
The effects of exercise and nutrition on appetite, metabolism, and immune system function.
E-mail: kwillis3@uwyo.edu

Eric RAVUSSIN
Employment
Professor in the Department of Health and Performance Enhancement at the Pennington Biomedical Research Center.
Degree
PhD
Research interests
Obesity and diabetes.
E-mail: Eric.Ravussin@pbrc.edu

Enette LARSON-MEYER
Employment
Ass. Prof. at the University of Wyoming.
Degree
MSc
Research interests
Nutrition influences the health and performance, gut peptides and vitamin D.
E-mail: enette@uwyo.edu
References
- Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide yy. Gastroenterology. 1985;89(5):1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- Al Awar R, Obeid O, Hwalla N, Azar S. Postprandial acylated ghrelin status following fat and protein manipulation of meals in healthy young women. Clinical Science (London) 2005;109(4):405–411. doi: 10.1042/CS20050072. [DOI] [PubMed] [Google Scholar]
- Andersson B, Larsson B. Influence of local temperature changes in the preoptic area and rostral hypothalamus on the regulation of food and water intake. Acta Physiologica Scandinavica. 1961;52:75–89. doi: 10.1111/j.1748-1716.1961.tb02203.x. [DOI] [PubMed] [Google Scholar]
- Baile CA, Zinn W, McLaughlin C. Exercise, blood lactate and food intake. Experientia. 1970;26(11):1227–1229. doi: 10.1007/BF01897981. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide yy3–36. New England Journal of Medicine. 2003;349(10):941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. Journal of Applied Physiology. 2007;102(6):2165–2171. doi: 10.1152/japplphysiol.00759.2006. [DOI] [PubMed] [Google Scholar]
- Burns SF, Broom DR, Miyashita M, Mundy C, Stensel DJ. A single session of treadmill running has no effect on plasma total ghrelin concentrations. Journal of Sports Sciences. 2007;25(6):635–642. doi: 10.1080/02640410600831856. [DOI] [PubMed] [Google Scholar]
- Christ ER, Zehnder M, Boesch C, Trepp R, Mullis PE, Diem P, Decombaz J. The effect of increased lipid intake on hormonal responses during aerobic exercise in endurance-trained men. European Journal of Endocrinology. 2006;154(3):397–403. doi: 10.1530/eje.1.02106. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Overduin J. Gastrointestinal regulation of food intake. Journal of Clinical Investigation. 2007;117(1):13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. New England Journal of Medicine. 2002;346(21):1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- Erdmann J, Lippl F, Schusdziarra V. Differential effect of protein and fat on plasma ghrelin levels in man. Regulatory Peptides. 2003;116(1–3):101–107. doi: 10.1016/s0167-0115(03)00195-2. [DOI] [PubMed] [Google Scholar]
- Erdmann J, Tahbaz R, Lippl F, Wagenpfeil S, Schusdziarra V. Plasma ghrelin levels during exercise - effects of intensity and duration. Regulatory Peptides. 2007;143(1–3):127–135. doi: 10.1016/j.regpep.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Flanagan DE, Evans ML, Monsod TP, Rife F, Heptulla RA, Tamborlane WV, Sherwin RS. The influence of insulin on circulating ghrelin. American Journal of Physiology Endocrinology and Metabolism. 2003;284(2):E313–E316. doi: 10.1152/ajpendo.00569.2001. [DOI] [PubMed] [Google Scholar]
- Ghanbari-Niaki A. Ghrelin and glucoregulatory hormone responses to a single circuit resistance exercise in male college students. Clinical Biochemistry. 2006;39(10):966–970. doi: 10.1016/j.clinbiochem.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Hosoda H, Doi K, Nagaya N, Okumura H, Nakagawa E, Enomoto M, Ono F, Kangawa K. Optimum collection and storage conditions for ghrelin measurements: Octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clinical Chemistry. 2004;50(6):1077–1080. doi: 10.1373/clinchem.2003.025841. [DOI] [PubMed] [Google Scholar]
- Imbeault P, Saint-Pierre S, Almeras N, Tremblay A. Acute effects of exercise on energy intake and feeding behaviour. British Journal of Nutrition. 1997;77:511–521. doi: 10.1079/bjn19970053. [DOI] [PubMed] [Google Scholar]
- Jurimae J, Hofmann P, Jurimae T, Palm R, Maestu J, Purge P, Sudi K, Rom K, von Duvillard SP. Plasma ghrelin responses to acute sculling exercises in elite male rowers. European Journal of Applied Physiology. 2007;99(5):467–474. doi: 10.1007/s00421-006-0370-y. [DOI] [PubMed] [Google Scholar]
- King NA, Blundell JE. High-fat foods overcome the energy expenditure induced by high-intensity cycling or running. European Journal of Clinical Nutrition. 1995;49(2):114–123. [PubMed] [Google Scholar]
- King NA, Burley VJ, Blundell JE. Exercise-induced suppression of appetite: Effects on food intake and implications for energy balance. European Journal of Clinical Nutrition. 1994;48(10):715–724. [PubMed] [Google Scholar]
- King NA, Tremblay A, Blundell JE. Effects of exercise on appetite control: Implications for energy balance. Medicine and Science in Sports and Exercise. 1997;29(8):1076–1089. doi: 10.1097/00005768-199708000-00014. [DOI] [PubMed] [Google Scholar]
- Kissileff HR, Pi-Sunyer FX, Segal K, Meltzer S, Foelsch PA. Acute effects of exercise on food intake in obese and nonobese women. Americal Journal of Clinical Nutrition. 1990;52(2):240–245. doi: 10.1093/ajcn/52.2.240. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kraemer RR, Durand RJ, Acevedo EO, Johnson LG, Kraemer GR, Hebert EP, Castracane VD. Rigorous running increases growth hormone and insulin-like growth factor-i without altering ghrelin. Experimental Biology of Medicine (Maywood) 2004a;229(3):240–246. doi: 10.1177/153537020422900304. [DOI] [PubMed] [Google Scholar]
- Kraemer RR, Durand RJ, Hollander DB, Tryniecki JL, Hebert EP, Castracane VD. Ghrelin and other glucoregulatory hormone responses to eccentric and concentric muscle contractions. Endocrine. 2004b;24(1):93–98. doi: 10.1385/ENDO:24:1:093. [DOI] [PubMed] [Google Scholar]
- Larson-Meyer DE, Borkhsenious ON, Gullett JC, Russell RR, Devries MC, Smith SR, Ravussin E. Effect of dietary fat on serum and intramyocellular lipids and running performance. Medicine and Science in Sports and Exercise. 2008;40(5):892–902. doi: 10.1249/MSS.0b013e318164cb33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Hunter GR, Newcomer BR. Influence of endurance running and recovery diet on intramyocellular lipid content in women: A 1h-nmr study. American Journal of Physiology Endocrinology and Metabolism. 2002;282:E95–E106. doi: 10.1152/ajpendo.2002.282.1.E95. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Bloom SR. Peptide yy, appetite and food intake. Proceedings of the Nutrition Society. 2005;64(2):213–216. doi: 10.1079/pns2005427. [DOI] [PubMed] [Google Scholar]
- Mackelvie KJ, Meneilly GS, Elahi D, Wong AC, Barr SI, Chanoine JP. Regulation of appetite in lean and obese adolescents after exercise: Role of acylated and desacyl ghrelin. Journal of Clinical Endocrinology and Metabolism. 2007;92(2):648–654. doi: 10.1210/jc.2006-1028. [DOI] [PubMed] [Google Scholar]
- Martins C, Morgan LM, Bloom SR, Robertson MD. Effects of exercise on gut peptides, energy intake and appetite. Journal of Endocrinology. 2007;193(2):251–258. doi: 10.1677/JOE-06-0030. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Bencivenga R, Longobardi N, Serritella C, Maj M. Differential responses of circulating ghrelin to high-fat or high-carbohydrate meal in healthy women. Journal of Clinical Endocrinology and Metabolism. 2003;88(11):5510–5514. doi: 10.1210/jc.2003-030797. [DOI] [PubMed] [Google Scholar]
- Orr J, Davy B. Dietary influences on peripheral hormones regulating energy intake: Potential applications for weight management. Journal of the American Dietetic Association. 2005;105(7):1115–1124. doi: 10.1016/j.jada.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Pomerleau M, Imbeault P, Parker T, Doucet E. Effects of exercise intensity on food intake and appetite in women. American Journal of Clinical Nutrition. 2004;80(5):1230–1236. doi: 10.1093/ajcn/80.5.1230. [DOI] [PubMed] [Google Scholar]
- Racotta R, Russek M. Food and water intake of rats after intraperitoneal and subcutaneous administration of glucose, glycerol and sodium lactate. Physiology & Behavior. 1977;18(2):267–273. doi: 10.1016/0031-9384(77)90132-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez NR, DiMarco NM, Langley S. Position of the american dietetic association, dietitians of canada, and the american college of sports medicine: Nutrition and athletic performance. Journal of the American Dietetic Association. 2009;109(3):509–527. doi: 10.1016/j.jada.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. Journal of Comparative Physiological Psychology. 1967;64(3):414–421. doi: 10.1037/h0025205. [DOI] [PubMed] [Google Scholar]
- Russell RD, Redmann SM, Ravussin E, Hunter GR, Larson-Meyer DE. Reproducibility of endurance performance on a treadmill using a preloaded time trial. Medicine and Science in Sports and Exercise. 2004;36(4):717–724. doi: 10.1249/01.mss.0000121954.95892.c8. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Maier C, Schaller G, Nowotny P, Bayerle-Eder M, Buranyi B, Luger A, Wolzt M. Acute exercise has no effect on ghrelin plasma concentrations. Hormone and Metabolic Research. 2004;36(3):174–177. doi: 10.1055/s-2004-814342. [DOI] [PubMed] [Google Scholar]
- Thompson DA, Wolfe LA, Eikelboom R. Acute effects of exercise intensity on appetite in young men. Medicine and Science in Sports and Exercise. 1988;20(3):222–227. doi: 10.1249/00005768-198806000-00002. [DOI] [PubMed] [Google Scholar]
- Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- Verger P, Lanteaume MT, Gournay JF, Louis-Sylvestre J. Choix alimentaire spontane apres un exercise physique de natation. Med Nutr. 1992;28:73–77. [Google Scholar]
- Verger P, Lanteaume MT, Louis-Sylvestre J. Free food choice after acute exercise in men. Appetite. 1994;22(2):159–164. doi: 10.1006/appe.1994.1015. [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga MS, Verwegen CR, Ijedema MJ, Wijckmans NE, Saris WH. Acute effects of exercise or sauna on appetite in obese and nonobese men. Physiology and Behavior. 1997;62(6):1345–1354. doi: 10.1016/s0031-9384(97)00353-3. [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. Journal of Clinical Endocrinology and Metabolism. 2001;86(12):5992–5996. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]