Abstract
Atherosclerosis remains the leading cause of death in the western countries and represents a complex chronic inflammatory process whose regulation is dependent on a network of cytokine and chemokine signaling between key cells such as endothelial cells, monocytes, dendritic cells, lymphocytes and smooth muscle cells. This review focuses on the biology and function of S100 proteins and their receptor RAGE with respect to the multifactorial process leading to atherosclerosis, plaque rupture, and aortic wall remodeling.
Keywords: Atherosclerosis, vascular inflammation, S100 proteins, S100A12, Receptor for advanced glycation endproducts (RAGE), mouse models of human disease
Introduction
Atherosclerosis remains the leading cause of death in the western countries and represents a chronic inflammatory process whose regulation is dependent on a complex network of cytokine and chemokine signaling between key cells such as endothelial cells, monocytes, dendritic cells, lymphocytes and smooth muscle cells. Inflammation is an adaptive response that is triggered by a variety of abnormal conditions, including infection, tissue injury and alteration of tissue homeostasis. Although infection is the best-understood trigger of inflammation, it does not play a major role in atherosclerosis. However, tissue injury leading to the release of danger signals such as S100 proteins, HMGB1, heat shock proteins, phosphatidylserine containing microparticles, and alteration of tissue homestasis with oxidation of LDL and other proteins are able to activate pattern-recognition receptors to initiate a potent inflammatory response similar to what has been shown for microbial pathogens. An increasing number of endogenous ligands are being reported as candidate stimulators of pattern recognition receptor such as toll-like receptors (TLRs), Nod-like receptors (NLR) and receptor for advanced glycation endproducts (RAGE). Clinical complications of coronary artery atherosclerosis are a consequence of chronic remodeling of the blood vessel wall possibly leading to chronic tissue ischemia and heart failure, and acute complications such as plaque rupture associated with infarction and possibly sudden death. Atherosclerosis is a multifactorial process with complex biological mechanisms at play. This review focuses on the biology and function of S100 proteins and their receptor RAGE with respect to atherosclerosis, plaque rupture, and aortic wall remodeling.
S100 proteins belong to the family of the calcium binding EF-hand proteins, and the name “S100” originates from their solubility in 100% ammonium sulfate. There are at least 21 different members of the S100 protein family and they are involved in a large number of cellular functions such as calcium homeostasis, cell growth and differentiation, dynamic regulation of the cytoskeleton related to cell invasion and metastasis, as well as regulation of inflammation and immune responses. Calcium binding to the EF-hand domain of the S100 proteins triggers structural changes that facilitate the interaction with target proteins and the modulation of their activity. S100 proteins are known by many different names, and a more uniform nomenclature according to gene name S100A1-16, S100B, S100P and S100Z has been encouraged. S100A8/9 and S100A12 share structural and functional homologies and are often named as “S100/calgranulins”. Besides their intracellular functions, the S100/calgranulins are secreted and exert cytokine-like effects in an autocrine and/or paracrine manner through the activation of cell surface receptors.
Association of S100/calgranulins with human vascular disease
S100A8 (also known as migration inhibitory factor-related protein= MRP8, or Calgranulin A), S100A9 (also known as MRP14, Calgranulin B) and S100A12 (also known as extracellular newly identified RAGE binding protein = EN-RAGE, or Calgranulin C), are endogenously expressed at various levels in cells intimately linked to vascular disease such as neutrophils, macrophages, dendritic cells and monocytes. S100/calgranulin proteins are secreted from activated myeloid cells and many clinical epidemiological studies established an association of elevated serum concentration of S100/calgranulins with disease activity in chronic inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel disease, asthma, diabetes and Kawasaki vasculitis. Hence, levels of S100/calgranulins are powerful biomarkers of generalized or localized inflammation and several clinical studies are of great interest to the cardiologist. For example, the TIMI 22 study identified the serum concentration of S100A8/9 obtained 30 days after acute coronary syndrome (ACS) as a predictor of recurrent cardiovascular events (MI or death) over the ensuing 18–36 months [1]. A similar predictive power was shown for serum S100A12 concentration in two recent studies of patients undergoing chronic hemodialysis where elevated serum S100A12 levels were found to predict cardiovascular mortality [2, 3]. In those studies, the level of S100A8/9 or S100A12 remained a predictor of adverse cardiovascular outcome even after stratification for hsCRP and IL-6 levels, suggesting that these markers have diagnostic value and are possibly directly involved in the underlying vascular pathology. Moreover, S100A8/9 [4] and S100A12 [5] -positive cells are present in human atherosclerotic plaques, and an autopsy study of victims of sudden cardiac death found enhanced expression of S100A12 and its receptor RAGE in macrophages and smooth muscle cells (SMCs) in the ruptured coronary artery plaque, with the highest levels observed in diabetic subjects [6]. These findings suggest that S100/calgranulin may predispose atherosclerotic lesions to become unstable and mediate atherothrombosis with devastating vascular complications. Indeed, an elegant study by Healy et al [7] used gene expression of the platelet transcriptome as a window in time to examine gene expression in patients with ST-elevation myocardial infarction and stable CAD. Since the small amount of mRNA in the platelets is a remnant from megakaryocytes in the bone marrow, this study reflects gene expression profiles well prior to the clinical presentation with STEMI. Interestingly, only two genes were significantly up regulated in the platelet transcriptome in STEMI compared to stable CAD: CD69 and S100A9 with an odds ratio of 6.2 and 3.3, respectively. Further studies confirm an association between S100/calgranulin and coronary artery disease. Gene expression of S100A12 in peripheral blood mononuclear cells was one of the strongest and an independent predictor of angiographically confirmed obstructive CAD in the Predict trial, a multicenter validation study involving 526 nondiabetic patients to assess a personalized risk evaluation and diagnosis of coronary tree [8]. While these clinical epidemiologic studies indicate an important role of S100/calgranulins as a biomarker for CAD, there was no proof that S100/calgranulins directly mediate vascular disease and therefore could be targeted for therapeutic interventions, until recent animal and cell culture studies shed more light on the pathological role of S100/calgranulins in mediating vascular inflammation.
S100/Calgranulins activate RAGE and TLR-4: Implication for cellular response and perturbation
Bovine S100A12 was first identified to bind to and to activate the Receptor for Advanced Glycation Endproducts (RAGE) and to mediate a pro-inflammatory response in cultured endothelial cells and macrophages, as well as in vivo in mouse models of disease. The pro-inflammatory response such as increased secretion of IL-6, IL1β and TNFα was mediated by the cell surface receptor RAGE since blocking RAGE using anti-RAGE IgG or limiting the access of S100A12 to its receptor using recombinant soluble RAGE attenuated inflammation in vitro and in vivo [9]. In addition, toll like receptor 4 (TLR-4) was identified as a binding partner for S100A8/9 to amplify S100/calgranulin mediated inflammation relevant to infection, autoimmunity and cancer [10, 11].
Both RAGE and TLR play important roles in mediating acute and chronic inflammation relevant for the initiation and progression of atherosclerosis. In support of this view, hyperlipidemic ApoE null mice lacking either RAGE or TLR signaling were found to have attenuated atherosclerosis [12, 13]. RAGE is critical for mediating acute and chronic inflammation in pathological settings, but plays a lesser role in normal physiology and development since mice lacking RAGE develop normal and have no or minimal phenotype in mice aged >15 months. These characteristics make RAGE an ideal therapeutic target in disease states characterized by enhanced ligand/RAGE axis, and would allow long-term suppression of RAGE. Longterm blockade of the ligand/RAGE axis therefore compares favorably to other strategies for example that of chronic TLR blockade since TLR signaling and lack off TLR signaling contributes to the growth of tumors in numerous organs and thus may represent a general principle of tumorigenesis [14]. RAGE was first identified in 1992 from bovine lung as a 35-kDa protein that bound Advanced Glycation Endproducts (AGEs) in a dose dependent manner [15]. A remarkable characteristic of RAGE is that it binds multiple ligands: AGEs, S100 proteins (such as S100A12, S100B, S100A4, S100A8/9, and S100P), high mobility group box 1 protein (HMGB1), amyloid beta peptide and beta sheet fibrils. The diverse repertoire of ligands binds to the extracellular domain of RAGE with different affinity. Moreover, recently it was shown that carboxylated glycans modification on the ligand binding portion of RAGE provides extra high-affinity binding sites for S100A12, and promotes increased RAGE-S100A12 signaling [16]. As shown by Srikrishna et al. glycan-enriched RAGE had a 30 fold higher binding of S100A12/mole RAGE (Bmax 44.4) compared to unmodified RAGE (Bmax 1.2), and this glycan dependent binding was much higher for S100A12 compared to S100A8/9 or S100A11 (Bmax 44.4, 0.4 and 0.04, respectively), suggesting that S100A12 may have the strongest potential to activate RAGE in conditions of increased glycosylation, such as diabetes or uremia. In general, RAGE binds S100A12 with a similar affinity (Kd ranging from 51–133 nM) to that of S100A8/9 with (Kd ranging from 7.6–34 nM) [16].
S100A12 in animal models of vascular disease
Our laboratory exploited the fact that S100A12 is not present in mice and we generated transgenic mice expressing human S100A12 in smooth muscle cells driven by the SM22a promoter. After backcrossing into the hyperlipidemic ApoE null mice, we analyzed transgenic mice with S100A12 (=S100A12tg/ApoE null) and littermate mice without the S100A12 transgene (=wild type, WT/ApoE null). We found a 1.4 fold increase in atherosclerotic plaque size and more specifically a large increase in calcified plaque area in the S100A12/ApoE null mice [5]. As shown in Figure 1, the atherosclerotic plaque in the S100A12tg/ApoE null mice showed enhanced intimal calcification, a larger necrotic core, and increased outward remodeling with breakdown of elastic fibers. These are features of human atherosclerosis that are associated with plaque rupture and atherothrombosis causing acute myocardial infarction or sudden death [17].
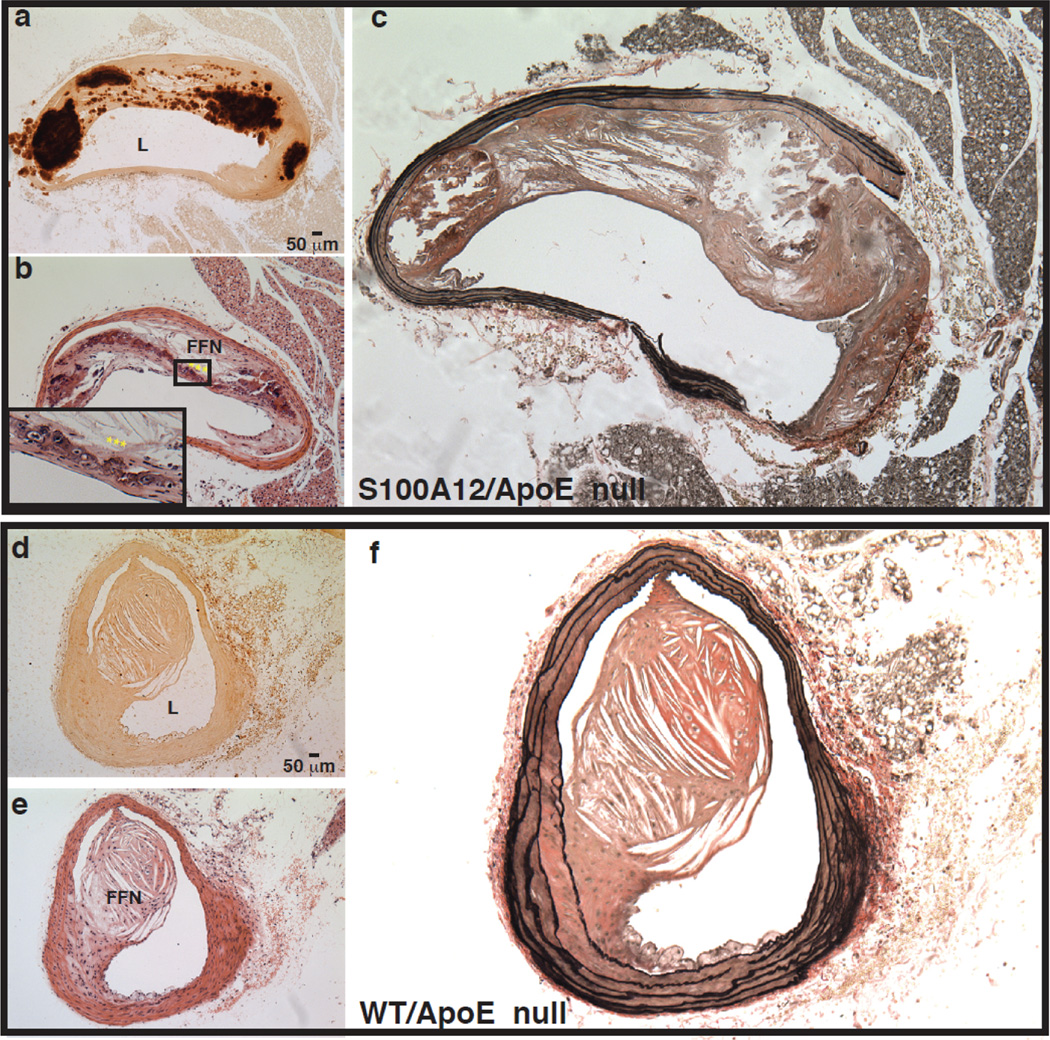
Figure 1.
Advanced remodeling with calcification, necrotic core and elastic fiber degradation of atherosclerotic plaques in ApoE null mice that express human S100A12 in the smooth muscle (a–c) compared to WT/ApoE littermate mice (d–f). Alizarin Red stain for calcium (a,d), H&E stain (b,e) and Verhoeff van Giessen stain for elastic fibers (c,f). L-lumen, FFN-fibrofatty nodule, *** osteoblast like cells. Modified and reproduced with permission from Hofmann Bowman et al [5].
The profound vascular remodeling observed in this mouse model with expression of human S100A12 are direct evidence that S100A12 is pathological for vascular disease and indeed may mediate acute myocardial infarction, sudden death and obstructive coronary disease as suggested by the clinical studies that found an association of S100A12 and these clinical outcomes. One of the key findings in the S100A12tg mice is enhanced oxidative stress. A 2–3 fold increase in urinary 8-isoprostane was observed in 4-month and 8 month old S100A12tg/ApoE null mice, and increased H2O2 production was reported in aortic smooth muscle cells harvested from S100A12tg aorta compared to WT aorta. Oxidative stress has many effects on vascular cells, including activation of Runx-2, a transcription factor regulating the expression of many osteoblastic genes [18].
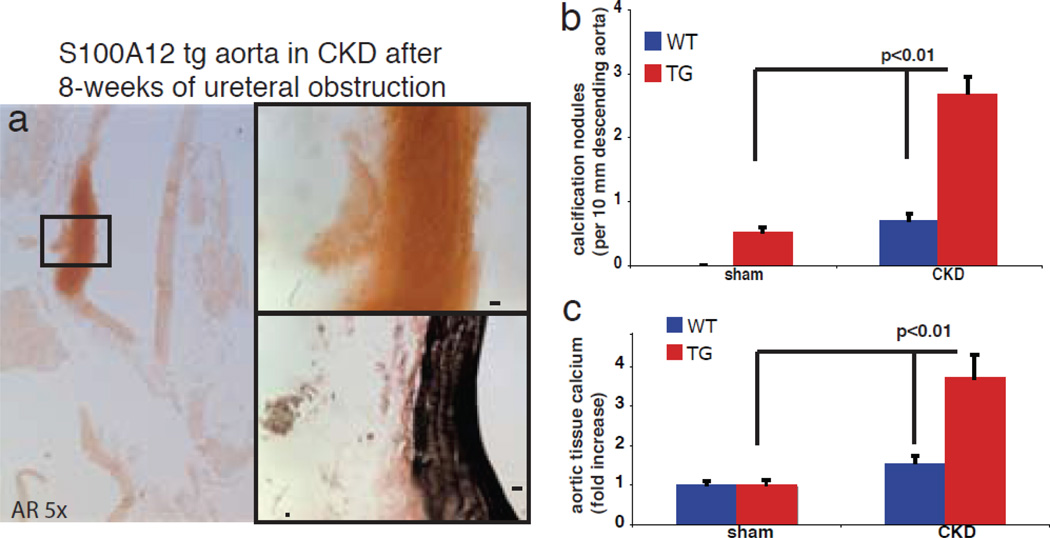
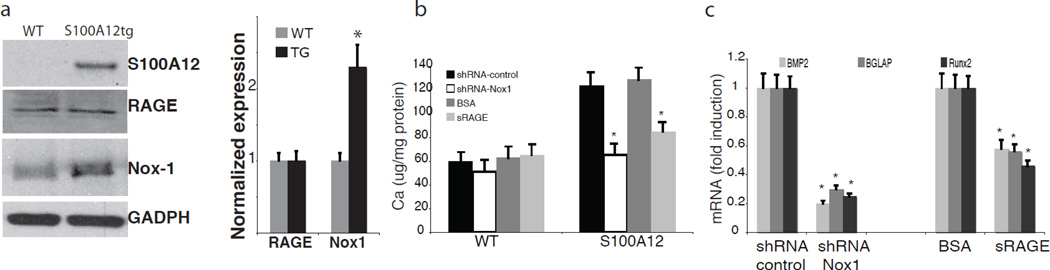
Indeed, we found increased gene expression of many bone-regulating genes in the aortic tissue of young S100A12tgApoE null mice before the onset of overt vascular calcification. Consistent with roles for oxidative stress, treatment with apocynin or diphenyliodonium (DPI), two compounds known to inhibit NADPH oxidase, attenuated calcification of cultured S100A12tg-SMCs. It is interesting to note that S100A12 expressed in SMCs alone is not sufficient to induce vascular calcification under normal metabolic conditions, since S100A12tg mice with normal lipids (C57BL6/J-ApoE +/+ mice) did not show any spontaneous vascular calcification [5]. In addition to hyperlipidemia, we found that uremia is a sufficient metabolic challenge to promote vascular calcification in the presence of S100A12. S100A12tg/C57Bl6/J mice with surgically induced chronic kidney disease, but with otherwise normal cholesterol, developed medial calcification in the aorta of S100A12tg mice but not in WT mice within 8 weeks after ureter ligation [19] (Figure 2). Medial calcification is vastly enhanced in patients with chronic and end stage kidney disease and is associated with increased adverse cardiovascular outcome. Specifically, calcification of the media usually occurs in the absence of macrophages and hyperlipidemia, and is thought to critically involve SMCs that either undergo a phenotypic switch to osteoblast like cells, or to become senescent vascular smooth muscle cells with an increased pro-calcification phenotype [20]. Our data suggest that S100A12 increases the susceptibility of SMC to undergo de-differentiation in order to gain a profile typical of osteoblast-like cells. Importantly, pretreatment of cultured aortic SMC with either soluble RAGE, the ligand-binding domain of RAGE that limits access of ligands to cell surface RAGE, or knockdown of Nox1, a critical component of NADPH oxidase in SMCs, attenuated calcification of aortic SMCs (Figure 3). We speculate that S100A12 mediated vascular calcification could be the missing link in patients with end stage renal disease to connect (i) increased S100A12 serum levels, (ii) increased vascular calcification and (iii) increased adverse cardiovascular outcome.
Figure 2.
Enhanced medial calcification in S100A12tg aorta subjected to ureteral obstruction is shown on Aizarin red (AR) stained aorta and quantified (b,c). Modified and reproduced with permission from S.Karger AG, Basel [19].
Figure 3.
In vitro calcification is attenuated in S100A12tg aortic SMCs by pretreatment with soluble RAGE and by transfection with shRNA targeted to Nox1. a) S100A12, RAGE and Nox-1 protein expression in aortic tissue from WT and S100A12tg mice, b) calcium content and c) mRNA in cultured aortic SMC form S100A12tg and WT aortas. Modified and reproduced with permission from S.Karger AG, Basel [19].
Macrovascular calcification increasingly afflicts our aging and dysmetabolic population. Once considered a passive/ degenerative process, data from many laboratories have converged to reveal that vascular calcification is in great part an actively regulated form of matrix mineral metabolism. Antecedent vasculopathy from diabetes, dyslipidemia or hypertension interacts with fluctuating hyperphosphatemic milieu of chronic kidney disease that overwhelms defenses against soft tissue mineralization and promotes low-grade widespread vascular inflammation [21]. Our data indicate that S100A12 upregulated ROS production by vascular SMCs via direct activation of RAGE, Nox1 or a heterodimeric RAGE-Nox1 signaling complex that initiates osteogenic mineralization in an appropriate cellular environment.
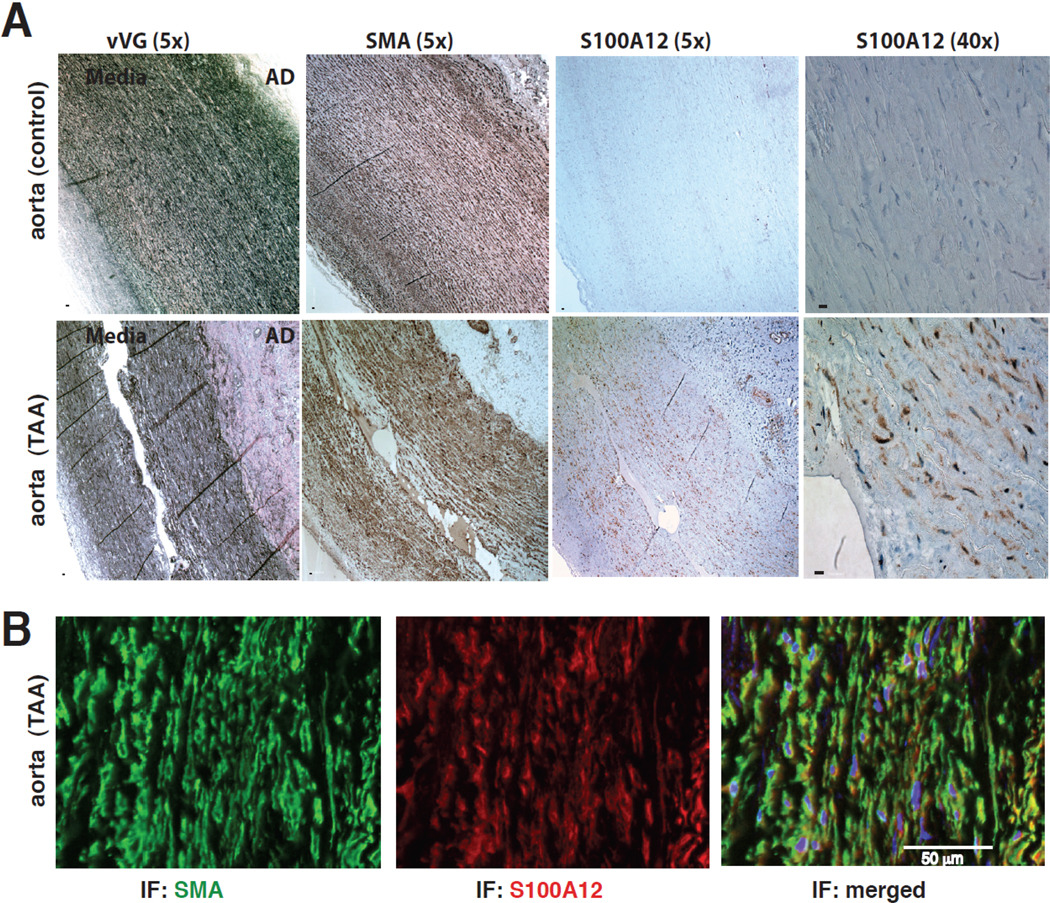
The specific phenotype of S100A12-mediated vascular disease depends on the microenvironment. In metabolically challenged mice with hyperlipidemia we found mostly enhanced intimal calcification and necrosis of atherosclerotic plaques, compared to medial calcification that was observed in uremic S100A12 tg mice. In contrast, in metabolically normal mice of the same C57BL6/J strain, we found that S100A12 modulates the phenotype of SMC from a normal contractile state to a synthetic state with increased oxidant stress and increased production of MMP2/9, IL-6, TGF-b leading to reduction of contractile fibers within the SMCs and to the formation of aortic aneurysms in S100A12tg mice [22]. In fact, the S100A12tg aorta has remarkable similarity to the aortic remodeling found in mice that carry a mutation in fibrillin-1, and serve as an animal model for Marfan syndrome [23]. Indeed, when we examined aortic tissue from patients with thoracic aortic aneurysms, we found S100A12 was upregulated in the smooth muscle rich medial layer and particularly at sites of medial dissection (Figure 4). S100A12 is not present in normal vascular SMCs but there is growing evidence that S100A12 and other S100 proteins are up-regulated in SMCs in response to injury such as lipopolysaccaride [22], mechanical stretch (unpublished data from our lab) and after endothelial wire injury[24]. S100A12 and other S100 proteins belong to the heterogenous group of damage associated molecular pattern (DAMP) molecules, and are inducible in a variety of cells linked to tissue injury, inflammation, cell death and apoptosis [25]. Studies by Tsoporis et al showed increased myocyte apoptosis after myocardial infarction that was dependent on infarct induced expression of S100B in myocytes interacting with its receptor RAGE [26]. Similarly, our studies indicate that forced expression of human S100A12 in aortic smooth muscle in bioengineered mice induces pathological ROS and inflammatory changes leading to dysfunctional SMCs with aneurismal remodeling.
Figure 4.
S100A12 is expressed in human thoracic aortic aneurysms (TAA), but not in healthy human aortic media (A), and co-localizes to SMCs (B, SMA=smooth muscle actin), Modified from Hofmann Bowman et al [22].
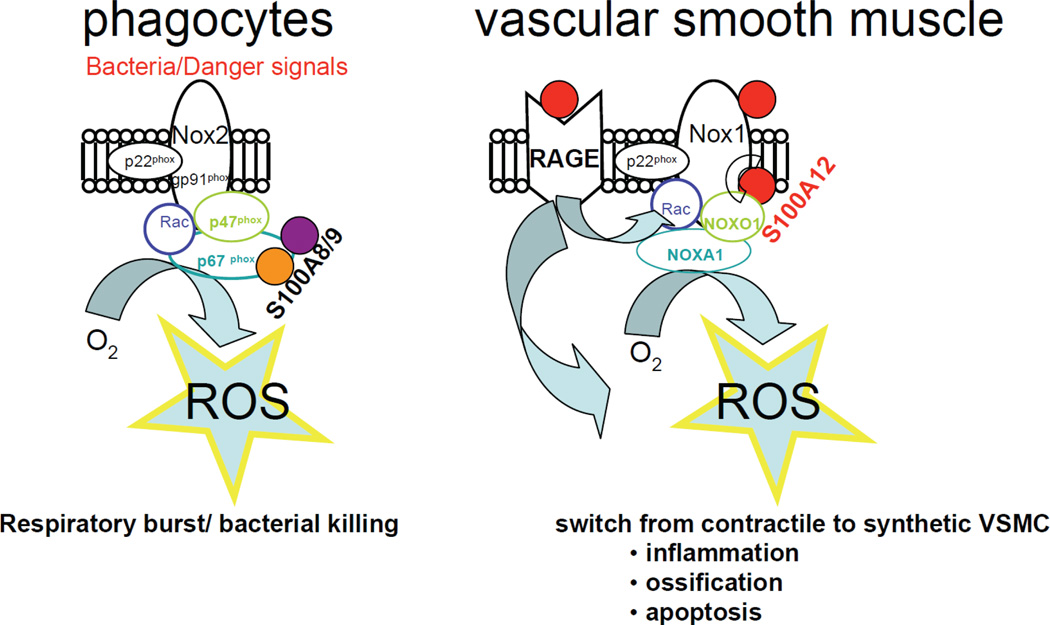
To better understand the biological mechanisms of S100/calgranulin in pathological states, it is important to understand the physiological properties of S100/calgranulins, which are endogenously expressed in myeloid cells and particularly in neutrophilic granulocytes, S100A12 constitutes 2–5% of the cytosolic protein. The normal function of S100/calgranulin in myeloid cells is only partially understood. Early studies implicated S100A12 as a protein linked to host defense with antiparasitic and antifungal ability [27]. More recent studies showed that S100A8/9 interacts with Nox2/gp91phox in phagocytes [28] resulting in ROS production, also known as respiratory burst, which is an important feature of the innate immune response to mediate microbial killing. To our knowledge, there are no known human diseases associated with insufficient or functionally abnormal S100A8/9 or S100A12 protein. However, mice lacking S100A8 display embryonic lethality [29], and mice lacking S100A9 (which is a binding partner to S100A8) have a rather mild phenotype with a modified response to LPS and reduced cytokine secretion from neutrophils and exacerbated cytokine release from dendritic cells [30]. We showed that S100A12 binds to Nox1 in SMCs, and we speculate that it may promote ROS in SMCs in a similar fashion as has been shown for S100A8/9 in phagocytes (Figure 5).
Figure 5.
Acute activation of NADPH oxidase (Nox) in phagocytes and SMC. Sensing the activation of phagocytes, S100A8/9 complexes with p67phox to rapidly move and combine components of the cytosol Nox complex to release superoxide in a respiratory burst aimed to destroy bacteria28. In SMCs S100A12 binds to RAGE and Nox1 and induces oxidative stress resulting in phenotypic changes of SMCs and possibly cell death [5].
S100/calgranulins: from friends to foes?
In summary, S100A12 is a pro-inflammatory and pro-oxidant protein that is endogenously expressed in leukocytes, and is inducible in smooth muscle and other cells under pathological conditions. In SMCs, S100A12 is sufficient to induce oxidative stress and to modulate basic cell functions ranging from increased cytokine production to the regulation of an osteogenic gene regulatory program. Our research indicates a direct role for S100A12 in mediating inflammation and apoptosis linked to vascular disease such as atherosclerosis, medial calcification, and aortic aneurysm formation. Taken together, it is intriguing to postulate that intracellular roles for S100/calgranulins such as S100A12 may be largely protective rapid response mechanisms to acute stresses. However, in chronic disorders such as those of autoimmunity where S100/calgranulins are biomarkers of long-term inflammation such as in rheumatoid arthritis and inflammatory bowel disease, sustained production of S100s and/or prolonged release of these molecules results in the gaining of new functions – chronic binding to and activation of cell surface receptors and their consequences.
In conclusion, we propose that S100A12 may serve as a therapeutic target for future drug development for chronic inflammatory disorders – from autoimmunity to atherosclerosis. There are several potential strategies to attenuate S100A12 mediated cell dysfunction: (1) blocking RAGE signaling using small molecules specific for S100A12-RAGE binding and signaling; (2) limiting access of S100A12 to its target binding proteins (RAGE, Nox1, and likely others) using a decoy protein such as soluble RAGE; (3) identification of novel S100A12 binding compounds to inhibit access to other target binding proteins; (4) effective intracellular antioxidant compounds to prevent S100A12 induced ROS production.
Our view of atherosclerosis as a chronic inflammatory disease has evolved in the last 30 years. However it is remarkable that our clinical armamentatrium to treat atherosclerosis targets mostly the treatment of hypertension, hyperlipidemia and other cardiovascular risk factors, yet no specific “anti-inflammatory” drugs are available for the treatment of atherosclerosis. We will await eagerly the results of two recently launched clinical trials, where the immunoneutralizing antibody against IL-1β Canakinumab will be given to patients with stable coronary artery disease and elevated hsCRP (=Cantos trial), and a second trial investigating a lower dose of the immune-modulatory drug methotrexate (10 mg/week) in diabetic patients with coronary artery disease (=Cardiovascular Inflammation Reduction trial, CIRT) [31]. Both trials may address the question if inflammation itself could be pharmacologically targeted to attenuate atherosclerosis. Further, these trials may be a key test of the relevance to successful therapeutic interventions in atherosclerotic mice to the human condition [32].
Acknowledgments
Dr. Hofmann Bowman is a recipient of the Doris Duke Charitable Foundation Scientist Development Award. This review is supported by the National Institute of Health grants K08-HL090917 (to Dr. Hofmann Bowman) and HL60901 (to Dr. Schmidt).
Footnotes
There are no conflicts of interest.
References
- 1.Morrow DA, Wang Y, Croce K, Sakuma M, Sabatine MS, Gao H, Pradhan AD, Healy AM, Buros J, McCabe CH, Libby P, Cannon CP, Braunwald E, Simon DI. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the Pravastatin or Atorvastatin Evaluation and Infection Therapy: Thrombolysis in Myocardial Infarction (PROVE IT-TIMI 22) trial. Am Heart J. 2008;155:49–55. doi: 10.1016/j.ahj.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakashima A, Carrero JJ, Qureshi AR, Miyamoto T, Anderstam B, Barany P, Heimburger O, Stenvinkel P, Lindholm B. Effect of Circulating Soluble Receptor for Advanced Glycation End Products (sRAGE) and the Proinflammatory RAGE Ligand (EN-RAGE, S100A12) on Mortality in Hemodialysis Patients. Clin J Am Soc Nephrol. 2010;12:2213–2219. doi: 10.2215/CJN.03360410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiotsu Y, Mori Y, Nishimura M, Sakoda C, Tokoro T, Hatta T, Maki N, Iida K, Iwamoto N, Ono T, Matsuoka E, Kishimoto N, Tamagaki K, Matsubara H, Kosaki A. Plasma S100A12 level is associated with cardiovascular disease in hemodialysis patients. Clin J Am Soc Nephrol. 6:718–723. doi: 10.2215/CJN.08310910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormick MM, Rahimi F, Bobryshev YV, Gaus K, Zreiqat H, Cai H, Lord RS, Geczy CL. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J Biol Chem. 2005;280:41521–41529. doi: 10.1074/jbc.M509442200. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann Bowman MA, Gawdzik J, Bukhari U, Husain AN, Toth PT, Kim G, Earley J, McNally EM. S100A12 in Vascular Smooth Muscle Accelerates Vascular Calcification in Apolipoprotein E-Null Mice by Activating an Osteogenic Gene Regulatory Program. Arterioscler Thromb Vasc Biol. 2011;31:337–344. doi: 10.1161/ATVBAHA.110.217745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 7.Healy AM, Pickard MD, Pradhan AD, Wang Y, Chen Z, Croce K, Sakuma M, Shi C, Zago AC, Garasic J, Damokosh AI, Dowie TL, Poisson L, Lillie J, Libby P, Ridker PM, Simon DI. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation. 2006;113:2278–2284. doi: 10.1161/CIRCULATIONAHA.105.607333. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg S, Elashoff MR, Beineke P, Daniels SE, Wingrove JA, Tingley WG, Sager PT, Sehnert AJ, Yau M, Kraus WE, Newby LK, Schwartz RS, Voros S, Ellis SG, Tahirkheli N, Waksman R, McPherson J, Lansky A, Winn ME, Schork NJ, Topol EJ. Multicenter validation of the diagnostic accuracy of a blood-based gene expression test for assessing obstructive coronary artery disease in nondiabetic patients. Ann Intern Med. 153:425–434. doi: 10.7326/0003-4819-153-7-201010050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 10.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 11.Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, Klenner L, Kuhn A, Foell D, Sorokin L, Luger TA, Roth J, Beissert S. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med. 16:713–717. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 12.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 14.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 15.Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- 16.Srikrishna G, Nayak J, Weigle B, Temme A, Foell D, Hazelwood L, Olsson A, Volkmann N, Hanein D, Freeze HH. Carboxylated N-glycans on RAGE promote S100A12 binding and signaling. J Cell Biochem. 110:645–659. doi: 10.1002/jcb.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 18.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gawdzik J, Mathew L, Kim G, Puri TS, Hofmann Bowman MA. Vascular Remodeling and Arterial Calcification Are Directly Mediated by S100A12 (EN-RAGE) in Chronic Kidney Disease. Am J Nephrol. 2011;33:250–259. doi: 10.1159/000324693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton DG, Matsubara H, Ikeda K. Pathophysiology of vascular calcification: Pivotal role of cellular senescence in vascular smooth muscle cells. Exp Gerontol. 45:819–824. doi: 10.1016/j.exger.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Towler DA. Vascular calcification: it's all the RAGE! Arterioscler Thromb Vasc Biol. 31:237–239. doi: 10.1161/ATVBAHA.110.220038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann Bowman M, Wilk J, Heydemann A, Kim G, Rehman J, Lodato JA, Raman J, McNally EM. S100A12 mediates aortic wall remodeling and aortic aneurysm. Circ Res. 2010;106:145–154. doi: 10.1161/CIRCRESAHA.109.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, Andrassy M, Marso SP, Duda S, Arnold B, Liliensiek B, Nawroth PP, Stern DM, Schmidt AM, Naka Y. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111:959–972. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 26.Tsoporis JN, Izhar S, Leong-Poi H, Desjardins JF, Huttunen HJ, Parker TG. S100B interaction with the receptor for advanced glycation end products (RAGE): a novel receptor-mediated mechanism for myocyte apoptosis postinfarction. Circ Res. 2010;106:93–101. doi: 10.1161/CIRCRESAHA.109.195834. [DOI] [PubMed] [Google Scholar]
- 27.Gottsch JD, Eisinger SW, Liu SH, Scott AL. Calgranulin C has filariacidal and filariastatic activity. Infect Immun. 1999;67:6631–6636. doi: 10.1128/iai.67.12.6631-6636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berthier S, Paclet MH, Lerouge S, Roux F, Vergnaud S, Coleman AW, Morel F. Changing the conformation state of cytochrome b558 initiates NADPH oxidase activation: MRP8/MRP14 regulation. J Biol Chem. 2003;278:25499–25508. doi: 10.1074/jbc.M209755200. [DOI] [PubMed] [Google Scholar]
- 29.Passey RJ, Williams E, Lichanska AM, Wells C, Hu S, Geczy CL, Little MH, Hume DA. A null mutation in the inflammation-associated S100 protein S100A8 causes early resorption of the mouse embryo. J Immunol. 1999;163:2209–2216. [PubMed] [Google Scholar]
- 30.Averill MM, Barnhart S, Becker L, Li X, Heinecke JW, Leboeuf RC, Hamerman JA, Sorg C, Kerkhoff C, Bornfeldt KE. S100A9 Differentially Modifies Phenotypic States of Neutrophils, Macrophages, and Dendritic Cells: Implications for Atherosclerosis and Adipose Tissue Inflammation. Circulation. 2011;123:1216–1226. doi: 10.1161/CIRCULATIONAHA.110.985523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul Ridker. Inflammation and Atherothrombosis: Where will new trials take us. ATVB scientific meeting 2011. http://my.americanheart.org/idc/groups/ahamahpublic/@wcm/@sop/@scon/documents/downloadable/ucm_426676.pdf.
- 32.Bhaskar V, Yin J, Mirza AM, Phan D, Vanegas S, Issafras H, Michelson K, Hunter JJ, Kantak SS. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in Apolipoprotein E-deficient mice. Atherosclerosis. 2011 doi: 10.1016/j.atherosclerosis.2011.02.026. [DOI] [PubMed] [Google Scholar]