Abstract
Multiple myeloma (MM) still remains incurable in most of the patients. Despite of treatments with high-dose chemotherapy, stem cell transplantation and other novel therapies, most patients will become refractory to the therapies and relapse. Thus, it is urgent to develop new approaches for MM treatment. Currently, antibody-targeted therapy has been extensively utilized in hematological malignancies, including MM. Several novel monoclonal antibodies (mAbs) against MM have been generated and developed over the past several years. These mAbs aim to target not only tumor cells alone but also tumor microenvironment, including interaction of tumor-bone marrow stromal cells and the components of bone marrow milieu, such as cytokines or chemokines that support myeloma cell growth and survival. These include mAbs specific for CD38, CS1, CD40, CD74, CD70, HM1.24, interleukin-6 and β2-microglobulin (β2M). We have shown that anti-β2M mAbs may be a potential antitumor agent for MM therapy due to their remarkable efficacy to induce myeloma cell apoptosis in tumor cell lines and primary myeloma cells from patients in vitro and in established myeloma mouse models. In this article, we will review advances in the development and mechanisms of MM-targeted mAbs and especially, anti-β2M mAbs. We will also discuss the potential application of the mAbs as therapeutic agents to treat MM.
Keywords: Multiple myeloma, monoclonal antibodies, anti-β2M mAbs, therapy
INTRODUCTION
Multiple myeloma (MM) is a plasma cell neoplasm, characterized as malignant plasma cell infiltrating and growing in the bone marrow (BM) and development of a progressive osteolytic bone disease [1]. This disease is one of the most common hematological malignancies among people older than 65 years in the United States and is more prevalent than lymphocytic leukemia, myelocytic leukemia or Hodgkin disease [2]. Estimated by the American Cancer Society, approximately 20,580 new cases were diagnosed and about 10,580 patients died from this disease in 2009 [3]. Although advances in the treatment of MM by new therapeutic agents, such as thalidomide, lenalidomide, and the pro-teasome inhibitor bortezomib, has been reported to prolong patient survival to 5-7 years over the past decades [4], this disease still remains a largely incurable and fetal, and patients are prone to quickly relapse after high-dose chemotherapy, stem cell transplantation and other novel therapies [4]. Therefore, development of a novel therapeutic approach to eradicate tumor cells is necessary, and will be helpful to improve overcomes of patients with MM.
Application of monoclonal antibodies (mAbs) is one of the successful approaches and has been utilized in current cancer therapy. Although the mechanism of mAb action to initiate and induce tumor cell death is not entirely known so far, it has been proposed that mAbs are able to bind to and cross-link target molecules and subsequently, elicit antibody-dependent cell-mediated cytotoxicity (ADCC) and activate complement-dependent cytotoxicity (CDC), and/or directly induce tumor cell apoptosis [5]. For induction of mAb-mediated ADCC, binding of the Fc portion of mAbs to Fcγ receptors on immune cells is necessary. The immune cells including monocytes, natural killer cells, and granulocytes can destruct mAb-bound tumor cells either by phagocytosis or by release of cytotoxic granules contained in immune effector cells. To induce antibody-mediated CDC, cross-linking of mAbs activates complement cascades, which trigger assembly of membrane attack complex and subsequently, osmotic cell lysis. Moreover, a few of mAbs can directly induce tumor cell apoptosis through transduction of an apoptotic signal to cells, which triggers intracellular apoptotic signaling pathways and cleaves caspase and poly (ADP-ri-bose) polymerase (PARP), leading to tumor cell apoptosis [5].
Thus far, several mAbs have been successfully used in solid tumors, such as trastuzumab for breast cancer [6]; bevacizumab for renal cell carcinoma and colorectal cancer [7, 8] and cetuximab for squamous-cell carcinoma of the head and neck [9, 10]. Because therapeutic efficacy of mAbs can be achieved at low doses and response can be achieved rapidly, mAbs also have been extensively used in hematological malignances. One successful example is rituximab, a chimeric human-mouse mAb specific for CD20, a cell surface glycoprotein expressed on the majority of B cells. This mAb so far has been used as a frontline therapy for diffuse large B-cell lymphoma and other B-cell tumors [11-13] [14], even though its therapeutic efficacy may vary in individual patients. Derived from rituximab, several novel anti-CD20 mAbs have been developed, such as ofatumumab, ocrelizumab, veltuzumab, GA101, AME-133v and PRO131921 [5, 15]. The potential of their therapeutic efficacy is currently under investigation in preclinical and early clinical studies. Unfortunately, the majority of myeloma patients are not sensitive to anti-CD20 mAb treatment, because only 20% of malignant plasma cells from patients with MM express CD20 [15]. To develop specific and potential therapeutic mAbs for MM, several novel mAbs have been generated recently. In this review, we will focus on mAbs that have been developed in the past years and may become potential therapeutic agents in MM in the near future.
MABS CURRENTLY UNDER INVESTIGATION FOR THEIR THERAPEUTIC ACTIVITY IN MM
Several novel mAbs with strong anti-myeloma activity have been developed over the past years, including but not limited to mAbs specific for CD38 [16, 17], CS1 [18], CD40 [19], CD74 [20, 21], CD70 [22, 23], HM1.24 [24], interleukin (IL)-6 [25, 26],[27], and β2M [28]. The targets of these potential therapeutic mAbs include not only tumor cells alone but also tumor microenvironment, such as the interaction of tumor-BM stromal cells (BMSCs), and the components of BM milieu, including cytokines or chemokines that support myeloma cell growth and survival.
1. MAbs Targeting Tumor Cells
Anti-CD38 mAbs
CD38 is a 46-KDa type II transmembrane glycoprotein with a short 20-amino acid N-terminal cytoplasmic tail and a long 256-amino acid extracellular domain. The biological function of CD38 includes receptor-mediated adhesion [29] and signaling transduction and regulation of intracellular calcium mobilization [30]. In MM, malignant plasma cells express relatively high levels of CD38 [31]. Currently, Daratumumab, a human mAb specific for CD38, is in a phase I/II safety and dose finding study for MM therapy [16]. In vitro studies show that Daratumumab is able to effectively kill tumor cells isolated from patients with MM and myeloma-derived cell lines by ADCC and/or CDC [16]. The effects of Daratumumab-mediated ADCC and/or CDC are not affected by the presence of BMSCs. Moreover, Daratumumab has in vivo therapeutic effects in a myeloma xenograft SCID mouse model at low doses [32]. These results indicate that Daratumumab may be a therapeutic mAb with potential for the treatment of CD38-positive MM.
Anti-CD70 mAbs
CD70 is a member of tumor necrosis factor (TNF) family. Interaction of CD70 and its ligand CD27 regulates the expansion and differentiation of effector and memory T-cell populations [33] and promotes B-cell expansion, germinal center formation and plasma cell differentiation [34]. CD70 is only transiently expressed on activated B cells, T cells, mature dendritic cells and thymic medulla stromal cells, but it is not expressed in other normal, non-hematopoietic tissues [33]. However, aberrant CD70 expression in tumor cells has been reported in diffuse large B-cell lymphoma, follicular lymphomas, B-cell lymphocytic leukemias, Burkitt and mantle cell lymphomas, Waldenstrom macroglobulinemia, Hodgkin disease Teed-Sternberg cells, and MM [22]. SGN-70, a humanized mAb specific for CD70, has been developed recently [23]. SGN-70 exhibits potent anti-myeloma activity in vitro and significantly prolonged the survival of tumor-bearing mice in vivo. This mAb induces Fc -mediated effector functions, such as ADCC, complement fixation and CDC [22].
Anti-CD74 mAbs
CD74 is a HLA-DR (MHC class II) invariant chain, associated with the α and β chains of HLA-DR and plays a role in antigen presentation [35]. It may interact with macrophage migration inhibitory factors that are critical mediators of the host defense and is involved in both acute and chronic response [36]. Recent studies have shown that CD74 is frequently expressed in MM [37]. Malignant plasma cells from 80% of MM patients and from majority of myeloma cell lines express CD74 mRNA and protein [37]. Anti-CD74 mAb, Milatuzumab (hLL1), has been developed and is currently in clinical evaluation for MM therapy [20]. Pre-clinical studies showed that Milatuzumab has in vitro growth inhibitory effects on myeloma cell lines and in vivo therapeutic effects on established myeloma in SCID mouse models [20]. Combination of Milatuzumab with other drugs such as bortezomib, doxorubicin or dexamethasone, causes more tumor growth inhibition and extends longer survival time of tumor-bearing mice in vivo than application of the drug alone via inducing more apoptosis and cleaved caspase-3 [20].
2. MAbs Targeting Tumor-BMSC Interaction
Anti-CS1 mAbs
CS1 (CD2 subset 1, CRACC, SLAMF7, CD319 or 19A24) is a cell surface glycoprotein belonging to the immunoglobulin gene superfamily[38]. CS1 mRNA and protein are highly expressed in tumor cells from majority of myeloma cell lines and from more than 97% of patients with MM [39]. Moreover, low levels of circulating CS1 are present in myeloma patient sera [39]. Except activated B, natural killer (NK), CD8+ T cells, and mature dendritic cells with low expression levels, it is not expressed in normal tissues or stem cells [39]. Recent studies have shown that CS1 mediates tumor cell adhesion and supports tumor growth and proliferation via c-maf-mediated interaction of tumor cells with BMSCs in MM [39, 40]. Moreover, it regulates NK cell cytolytic activity via recruiting EWS-activated transcript-2 and activating the PI3K/PLCg signaling pathways [41, 42]. Currently, a humanized anti-CS1 mAb, Elotuzumab (HuLuc63), has been developed [18]. The studies showed that Elotuzumab significantly inhibits myeloma cell binding to BMSCs and induces ADCC in vitro in a dose-dependent fashion. Administration of Elotuzumab markedly induces tumor regression in myeloma xenograft mouse models [18, 43]. Furthermore, combination of Elotuzumab with bortezomib significantly enhances the in vivo therapeutic efficacy to eradiate patient-derived myeloma cells in a SCID-hu mouse model [44]. This mAb is currently in a phase I clinical trial in relapsed/refractory myeloma.
Anti-CD40 mAbs
CD40 is a transmembrane protein belonging to TNF superfamily [45]. Recent studies have shown that CD40 is essential to mediate a broad variety of immune and inflammatory responses, such as T cell-dependent immunoglobulin class switching, memory B cell development and germinal center formation [46]. CD40 is expressed in many resting cell types but is highly expressed in B and dendritic cells [46]. In MM, CD40 is highly expressed on malignant plasma cells [47]. It binds to its ligand CD154 and induces myeloma cell proliferation and migration via PI3K/Akt/NF-κB signaling pathways [48]. CD40 is also expressed on BMSCs [49]. The interaction of CD40–CD154 increases myeloma growth factor secretion from BMSCs, such as IL-6 and VEGF [50]. Blockade of CD40-CD154 interaction by anti-CD40 mAbs inhibits intracellular signaling pathways in both tumor cells and BMSCs and displays remarkable anti-myeloma activity. Pre-clinical studies observed that Dacetuzumab (SGN-40), a recently generated humanized anti-CD40 mAb, induces strong tumor cell death in MM via breaking CD40-CD40L interaction and activating NK cell-mediated ADCC [19, 51, 52]. This mAb is currently in a phase I study in patients with advanced MM [51].
Anti-HM1.24 mAbs
HM1.24 (BST2/CD317) was originally identified as a plasma cell-specific antigen [53, 54]. Currently it has been proposed that HM1.24 serves as a target molecule for myeloma therapy [54]. A humanized mAb specific to HM1.24 antigen has been developed. Injection of the mAb significantly reduces M-protein levels in sera and tumor cell numbers in BM, and prolongs survival of myeloma-bearing mice in myeloma xenograft mouse models [55]. However, its anti-myeloma activity is diminished when the mice are pre-treated with anti-Fcγ receptor III/II antibodies, indicating that anti-HM1.24 mAb kills myeloma cells via ADCC and/or CDC [56]. In vitro studies validated the mAb action mechanism via NK- and monocyte/macrophage-mediated ADCC [56, 57]. These results suggest the potential application of anti-HM1.24 mAb in MM therapy.
3. MAbs Targeting Components of Bone Marrow Milieu
Anti-IL-6 mAbs
IL-6, mainly secreted from BMSCs, is a major cytokine for myeloma growth, survival, and drug resistance [58]. IL-6 activates several signaling pathways, such as JAK/STAT3, MEK/ERK and PI3K/Akt signaling pathways [59]. In MM, elevated levels of IL-6 and soluble IL-6 receptor are frequently present in the BM plasma and sera of MM patients [60], and are considered as an indicator of poor prognosis [61]. Thus far, several anti-IL-6 mAbs have been generated for MM therapy. Siltuximab (CNTO 328) [25], a chimeric human-mouse neutralizing mAb against IL-6, has demonstrated promising antimyeloma activity in combination with bortezomib [26], dexamethasone [62] and melphalan [25] in preclinical studies. Combination of Siltuximab with chemotherapy drugs enhances the levels of tumor cell apoptosis [25],[26],[62]. Siltuximab inhibits IL-6-mediated phosphorylation of ERK1/2, STAT1 and STAT3 in myeloma cells [25]. In addition, Siltuximab also inhibits bortezomib-induced increase of myeloid cell leukaemia-1 and heat shock protein-70 [26], both of which may be involved in inducible drug resistance. Other anti-IL-6 mAbs, such as single-chain fragment NRI [63] and humanized mAb 1339 [27], have also demonstrated therapeutic effects on myeloma.
ANTI-B2M MABS AS A NOVEL THERAPEUTIC AGENT IN MM
1. Human β2M and MM
Human β2M, an 11.6-kDa nonglycosylated polypeptide, interacts with and stabilizes the tertiary structure of the MHC class I α-chain [64]. Because it is non-covalently associated with the α-chain and has no direct attachment to the cell membrane, β2M on the cell surface can be exchanged with free β2M present in body fluids under physiologic conditions and in serum-containing medium [65, 66]. β2M is almost exclusively catabolized in the kidney, and 95% to 100% of circulating β2M is eliminated via glomerular filtration, reabsorbed in the tubes, and degraded by proteolytic enzymes. In healthy individuals, the serum concentration of β2M is usually < 2 mg/L and urinary excretion < 400 μg/24 h [67]. Increased synthesis and release of β2M, as indicated by an elevation of serum β2M concentration, occurs in inflammatory, autoimmune and infectious diseases [67]. The stimulation of T- and B-cell antigen or cytokines, such as TNF-α, IL-2, interferon (IFN)-α and IFN-γ, increases cellular β2M release [68].
There is a close association of free β2M with many hematological malignancies, such as MM [69, 70], leukemias [71, 72] and lymphomas [73]. Elevated levels of β2M in serum are present and correlate with a poor patient outcome in MM [70, 74, 75]. In fact, the serum levels of β2M are one of the most important prognostic factors for patients with MM, giving a reliable survival prediction [70, 76]. Moreover, in long-term survivors of MM, an elevated level of β2M is still an unfavorable factor [77, 78]. In vitro studies have shown that primary myeloma cells and myeloma cell lines produce β2M [79]. Myeloma cells are presumed to contribute to the high levels of serum β2M, which is considered a surrogate marker for tumor burdens in myeloma patients. In addition, β2M is also involved in negative regulation of immune system [80] and in activation of bone resorption [81]. These results point to an important yet unidentified role of β2M in the pathogenesis of MM.
2. Mechanism of Anti-β2M mAb Action in Myeloma Cells
Anti-β2M mAbs have been developed recently [28]. The studies showed that anti-β2M mAbs may be a novel agent for MM therapy, because the mAbs have: (1) remarkably strong tumoricidal activities to kill all examined myeloma, including tumor cell lines and primary CD138+ malignant plasma cells isolated from patients with MM, and β2M+/HLA-ABC+ hematological malignant cells; (2) direct induction of tumor cell death without the need for exogenous immunological effector cells and/or molecules; (3) ability to kill chemotherapy-refractory myeloma; (4) therapeutic efficacy in vivo in xenograft mouse models of myeloma; and (5) effectively kill myeloma cells in the presence of high concentrations of soluble β2M or myeloma cell-survival cytokines, such as IL-6 and insulin-like growth factor (IGF)-I, or BMSCs. Although the mechanisms of its action in myeloma cell death need to be further investigated, evidence has shown that anti-β2M mAbs directly induce tumor cell apoptosis without immunological effector mechanisms [28].
Anti-β2M mAb-induced apoptotic signaling pathways in myeloma cells
β2M/MHC class I complex has been shown to serve as an important signal-transducing molecule, which is involved in responses ranging from anergy and apoptosis to cell proliferation and IL-2 production [82, 83]. Earlier studies showed that cross-linking of β2M/MHC class I leads to a rise of intracellular free calcium concentration and activation of STAT3 and/or JNK through phosphorylation of a signaling motif at tyrosine 320 residue in the cytoplasmic domain of MHC class I α-chain [84, 85]. However, previous studies also demonstrate that deletion of all but the four proximal amino acids from the cytoplasmic tail does not alter their signal transduction capabilities [84]. Nevertheless, our studies demonstrate that binding of anti-β2M mAbs to myeloma cells results in internalization and down-modulation of surface β2M/MHC class I molecules, and induction of myeloma cell apoptosis [28]. Knockdown of surface β2M by specific small interference RNAs (siRNAs) significantly abrogates the mAb-induced tumor cell apoptosis. As a result, cross-linking of β2M/MHC class I molecules by the mAbs activates JNK and inhibits PI3K/Akt and ERK, leading to compromised mitochondrial integrity, cytochrome-c release, and activation of the caspase-9 cascade [28]. However, the mechanisms underlying the mAb-mediated binding to and cross-linking of surface β2M/MHC class I molecules, and transduction of apoptotic signals to cells needs further investigation.
Lipid rafts as a linker mediate anti-β2M mAb-induced tumor apoptosis
Lipid rafts, cholesterol- and glycosphingolipid-enriched dynamic patches in the plasma membrane, function to organize plasma membrane into functional units [86] and act as platforms for conducting different signals into cells [87]. Integral proteins in the cellular membrane, such as caveolins and flotillins, can modify lipid rafts structurally and functionally, and may therefore affect subsequent cellular functions [88, 89]. Our studies showed that lipid rafts play an important role in anti-β2M mAb-induced myeloma cell apoptosis (Figure 1). Anti-β2M mAbs bind to surface β2M/MHC class I molecules and recruit them to lipid rafts, leading to MHC class I binding to caveolin-1 and consequently activating the downstream kinases of JNK [28, 90]. On the other hand, stimulation of myeloma cells by IL-6 or IGF-I leads to relocation of their receptors gp130, or IGF-IRβ, respectively, and its substrate IRS-1, to lipid rafts and an increased affinity of receptor binding to caveolin-1, which regulates the structure and function of lipid rafts. Treatment of anti-β2M mAbs excludes gp130, IGF-IRβ and IRS-1 out of lipid rafts in the presence of IL-6 and/or IGF-I and abrogates IL-6 - or IGF-I-mediated JAK/STAT3, PI3K/Akt, and Ras/Raf/ERK pathway signaling [90]. These findings indicate that modification of lipid rafts may enhance the ability of mAbs to induce tumor cell apoptosis.
Figure 1.
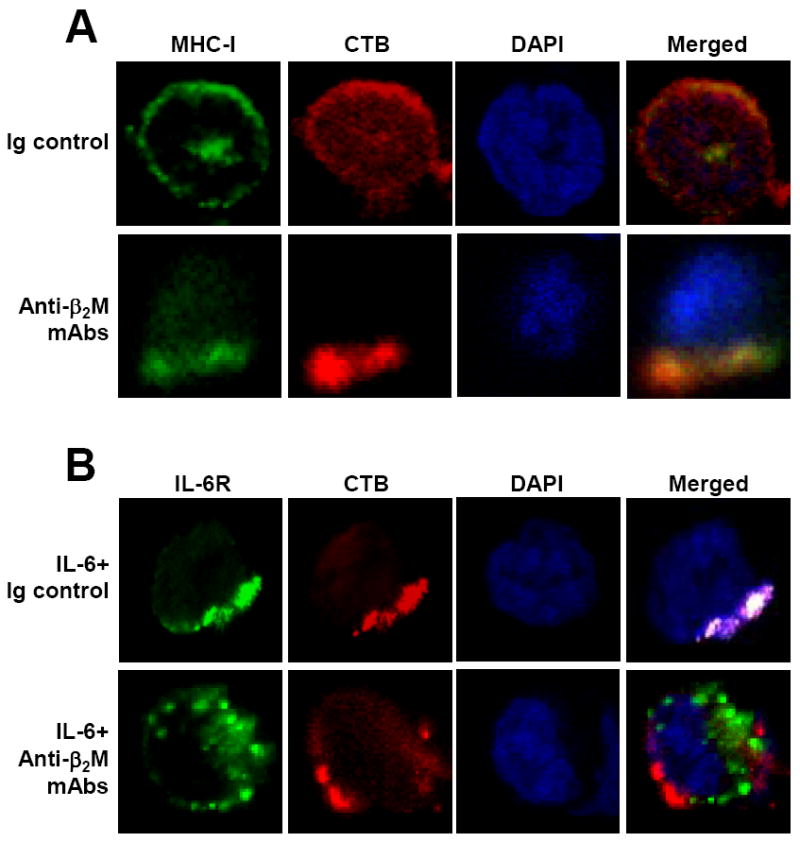
Anti-β2M mAbs recruit MHC class I into and exclude IL-6R from lipid rafts. Visualization by confocal microscopy shows the distribution and localization of: (A) MHC class I (MHC-I) and (B) IL-6 receptor (IL-6R) in relationship with lipid rafts on myeloma cells ARP-1 treated with 50 µg/mL of anti-β2M mAbs in presence or absence of IL-6 (10 ng/mL) for 1 hour. Cells treated with mouse Ig control served as controls. After the treatment, cells were fixed with 4% paraformaldehyde and stained with anti-MHC class I mAb W6/32 (A), or with anti-gp130 antibody for IL-6R (B), followed by incubation with Alexa Fluor 488-conjugated secondary antibody (green color). Cells were then stained with anti-CTB antibody (red color) for detection of lipid rafts and DAPI staining (blue color) to visualize nuclei. Representative results of three independent experiments are shown.
3. Therapeutic Efficacy and Potential Toxicity of Anti-β2M mAbs
For future clinical application of the mAbs, a major concern is whether anti-β2M mAbs will be therapeutic in patients, in whom every tissue expresses low densities of β2M/MHC class I molecules and elevated levels of soluble β2M are present in the circulation. In vitro studies showed that anti-β2M mAb treatment kill myeloma cells with the same efficiency even though they are surrounded and outnumbered by β2M/MHC class I-expressing normal peripheral blood mononuclear cells (PBMCs) in the cocultures. In addition, cocultures with BMSCs, such as osteoclasts, has no effects to protect myeloma cells from mAb-induced apoptosis [28]. Furthermore, addition of higher molar concentrations of soluble β2M (50–100 μg/mL), which is 3- to 10-fold higher than those in patients with myeloma, in the culture of myeloma cells does not abrogate the mAb effects on tumor apoptosis [28]. To examine the in vivo anti-myeloma effects of the mAbs, a human-like mouse model, i.e., myeloma-HLA-A2-transgenic NOD/SCID mice, has been generated [91]. The mice are transgenic for HLA-A2 α-chain but not human β2M. However, with established myeloma, myeloma-derived human β2M forms mature MHC class I molecules with the HLA-A2 α-chain on murine cells, and high levels of circulating human β2M were detected. Moreover, the human MHC class I molecules on murine cells were functional. Although there is a concern that the surrounding, β2M/MHC class I-expressing BMSCs would prevent anti-β2M mAb-induced tumor cell apoptosis [91], anti-β2M mAbs still effectively suppressed myeloma growth, activated caspase-9 and -3 and induced myeloma cell apoptosis in this mouse model.
On the other hand, as β2M is expressed in almost all cells, another concern is whether anti-β2M mAb treatment will be safe to treat patients. In vitro examination of the mAb-mediated potential toxicity on normal hematopoietic cells showed that normal PBMCs, resting and activated CD3+ T cells and CD19+ B cells, CD16+ NK cells, and BM CD34+ stem cells are resistant to mAb-induced apoptosis [28]. In myeloma-HLA -A2-transgenic NOD/SCID mice, although the mAbs can be detected on different organs including the heart, lung, spleen, liver, and kidney, no normal tissue damage or normal cell apoptosis or associated caspase activation is observed [91]. Treatment of anti-β2M mAbs does not change the body weight of the mice or impair the implanted human BM tissues in SCID-hu mice [28]. Moreover, no tissue damage are observed in vivo in human BM tissue implanted in the SCID-hu mice, in which anti-β2M mAbs and subsequently purified human NK cells were injected directly into the implanted human bones [28]. These findings provide evidence that application of the mAbs would have limited direct or indirect (via CDC or ADCC) toxicity on normal tissues and organs, and their therapeutic efficacy would be high if this approach was translated into a therapeutic strategy, despite the ubiquitous expression of β2M and class I MHC on the majority of tissues.
4. Future Perspectives of Anti-β2M mAbs in MM
Our recent studies showed that IgM anti-β2M mAbs are more potent than IgG mAbs to induce myeloma cell apoptosis, due to IgM mAb pentameric structure of 10 antigenic binding sites with stronger cross-linking capacity (unpublished data). Disruption of IgM pentamers by beta-mercaptoethanol [92, 93] impaired the ability of IgM anti-β2M mAbs to induce tumor apoptosis. This observation indicates that enhancing the cross-linking ability of anti-β2M IgG mAbs or using IgM anti-β2M mAbs may improve the mAb-induced anti-myeloma activity and their therapeutic potential. In addition, combination therapy has demonstrated more efficacy to eradicate tumor cells than monotherapy in MM. Combination of the mAbs with chemotherapy drugs may improve antimyeloma activity of the mAbs. Furthermore, although anti-β2M mAbs induce strong tumor cell apoptosis, other mechanisms, such as ADCC and CDC, may be utilized in vivo by the mAbs. Therefore, it is necessary to examine whether application of ADCC and/or CDC may improve the antitumor activity of the mAbs in MM in future studies.
Nevertheless, the mechanisms why anti-β2M mAbs selectively kill tumor cells but not normal cells need further investigation. One of the potential mechanisms may be partially due to overexpression of the antigens in tumor cells. Our and other studies have shown that myeloma cells express significantly higher levels of surface β2M than normal cells, even though the expression levels of β2M vary in myeloma cell lines or patient samples [28]. Additionally, it is a possibility that the components of MHC class I or class I-like complexes are different in myeloma and normal cells, which warrants future studies to find out which MHC class I or class I-like molecules the mAbs bind to in tumor cells but not in normal cells. Another potential mechanism may involve mAb-mediated signaling transduction. Our studies have shown that the mAbs do not recruit MHC class I molecules to lipid raft and activate the downstream apoptotic signaling pathways in normal B cells [28]. Therefore, resistance to mAb-induced apoptosis in normal cells may be partially due to the inability of the mAbs to cross-link β2M/MHC class I molecules and activate apoptotic signaling pathways in normal cells. Finally, our studies also showed that the mAbs exclude growth receptors out of lipid rafts and inhibit survival signaling pathways [90]. It is possible that growth receptors may be physically associated with β2M/MHC class I molecules and higher levels of these receptors are expressed in tumor cells than in normal cells. Cross-linking of β2M/MHC class I molecules with the mAbs may exclude more growth receptors out of lipid rafts and induce stronger inhibition of growth factor-mediated anti-apoptotic signaling.
CONCLUSION
Advances in molecular biology and tumor biology over the past years have enhanced our understanding of the mechanisms of MM pathogenesis. Therefore, many potential tumor-associated antigens or targets have been identified, which include those expressed in tumor cells, involving in tumor-BMSC interaction, and in BM microenvironment. Based on these findings, several mAbs, including anti-β2M mAbs, have been developed and are being examined in preclinical and early clinical studies. Most of them showed remarkable anti-myeloma activity. Although these mAbs have the potential to be therapeutic agents for MM therapy in the future, enhancement of the therapeutic efficacy of the mAbs is still necessary. Moreover, to overcome drug resistance and to improve patient outcome, development of more potent and specific mAbs for myeloma cells and myeloma-associated BM microenvironment are still needed.
Acknowledgments
National Cancer Institute R01 CA96569, R01 CA103978, and R01 CA138402, the Leukemia and Lymphoma Society Translational Research Grants, Multiple Myeloma Research Foundation, Commonwealth Foundation for Cancer Research, and funds from the University Cancer Foundation and the Center for Targeted Therapy of The University of Texas M. D. Anderson Cancer Center (Q. Yi); and National Cancer Institute K99/R00 CA137158, Lymphoma Research Foundation, and American Society of Hematology (J. Yang).
References
- 1.Anderson KC, Shaughnessy JD, Jr, Barlogie B, Harousseau JL, Roodman GD. Multiple myeloma. Hematology Am Soc Hematol Educ Program. 2002:214–240. doi: 10.1182/asheducation-2002.1.214. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Laubach JP, Mitsiades CS, Mahindra A, Schlossman RL, Hideshima T, Chauhan D, Carreau NA, Ghobrial IM, Raje N, Munshi NC, Anderson KC, Richardson PG. Novel therapies in the treatment of multiple myeloma. J Natl Compr Canc Netw. 2009;7:947–960. doi: 10.6004/jnccn.2009.0062. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KC. Clinical update: novel targets in multiple myeloma. Semin Oncol. 2004;31:27–32. doi: 10.1053/j.seminoncol.2004.10.016. discussion 33. [DOI] [PubMed] [Google Scholar]
- 5.Bello C, Sotomayor EM. Monoclonal antibodies for B-cell lymphomas: rituximab and beyond. Hematology Am Soc Hematol Educ Program. 2007:233–242. doi: 10.1182/asheducation-2007.1.233. [DOI] [PubMed] [Google Scholar]
- 6.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 7.Sonpavde G. Bevacizumab in renal-cell cancer. N Engl J Med. 2003;349:1674. doi: 10.1056/NEJM200310233491719. [DOI] [PubMed] [Google Scholar]
- 8.Hurwitz H, Fehrenbacher L, Novotny W, Cart-wright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 10.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radio-therapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 11.Overman MJ, Feng L, Pro B, McLaughlin P, Hess M, Samaniego F, Younes A, Romaguera JE, Hagemeister FB, Kwak L, Cabanillas F, Rodriguez MA, Fayad LE. The addition of rituximab to CHOP chemotherapy improves overall and failure-free survival for follicular grade 3 lymphoma. Ann Oncol. 2008;19:553–559. doi: 10.1093/annonc/mdm511. [DOI] [PubMed] [Google Scholar]
- 12.Liu YY, Leboeuf C, Shi JY, Li JM, Wang L, Shen Y, Garcia JF, Shen ZX, Chen Z, Janin A, Chen SJ, Zhao WL. Rituximab plus CHOP (R-CHOP) overcomes PRDM1-associated resistance to chemotherapy in patients with diffuse large B-cell lymphoma. Blood. 2007 doi: 10.1182/blood-2006-09-049189. [DOI] [PubMed] [Google Scholar]
- 13.Spina M, Simonelli C, Tirelli U. Phase II trial of CHOP plus rituximab in patients with HIV-associated non-Hodgkin’s lymphoma. J Clin Oncol. 2007;25:e7. doi: 10.1200/JCO.2006.09.0407. [DOI] [PubMed] [Google Scholar]
- 14.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 15.Kapoor P, Greipp PT, Morice WG, Rajkumar SV, Witzig TE, Greipp PR. Anti-CD20 monoclonal antibody therapy in multiple myeloma. Br J Haematol. 2008;141:135–148. doi: 10.1111/j.1365-2141.2008.07024.x. [DOI] [PubMed] [Google Scholar]
- 16.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, Peipp M, Valerius T, Slootstra JW, Mutis T, Bleeker WK, Anderson KC, Lokhorst HM, van de Winkel JG, Parren PW. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 186:1840–1848. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 17.Ellis JH, Barber KA, Tutt A, Hale C, Lewis AP, Glennie MJ, Stevenson GT, Crowe JS. Engineered anti-CD38 monoclonal antibodies for immunotherapy of multiple myeloma. J Immunol. 1995;155:925–937. [PubMed] [Google Scholar]
- 18.Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, Lee AI, Podar K, Hideshima T, Rice AG, van Abbema A, Jesaitis L, Caras I, Law D, Weller E, Xie W, Richardson P, Munshi NC, Mathiot C, Avet-Loiseau H, Afar DE, Anderson KC. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112:1329–1337. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein R, Mattes MJ, Cardillo TM, Hansen HJ, Chang CH, Burton J, Govindan S, Goldenberg DM. CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clin Cancer Res. 2007;13:5556s–5563s. doi: 10.1158/1078-0432.CCR-07-1167. [DOI] [PubMed] [Google Scholar]
- 20.Stein R, Smith MR, Chen S, Zalath M, Goldenberg DM. Combining milatuzumab with bortezomib, doxorubicin, or dexamethasone improves responses in multiple myeloma cell lines. Clin Cancer Res. 2009;15:2808–2817. doi: 10.1158/1078-0432.CCR-08-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alinari L, Yu B, Christian BA, Yan F, Shin J, Lapalombella R, Hertlein E, Lustberg ME, Quinion C, Zhang X, Lozanski G, Muthusamy N, Praetorius-Ibba M, O’Connor OA, Goldenberg DM, Byrd JC, Blum KA, Baiocchi RA. Combination anti-CD74 (milatuzumab) and anti-CD20 (rituximab) monoclonal antibody therapy has in vitro and in vivo activity in mantle cell lymphoma. Blood. doi: 10.1182/blood-2010-08-303354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEarchern JA, Oflazoglu E, Francisco L, McDonagh CF, Gordon KA, Stone I, Klussman K, Turcott E, van Rooijen N, Carter P, Grewal IS, Wahl AF, Law CL. Engineered anti-CD70 antibody with multiple effector functions exhibits in vitro and in vivo antitumor activities. Blood. 2007;109:1185–1192. doi: 10.1182/blood-2006-07-034017. [DOI] [PubMed] [Google Scholar]
- 23.McEarchern JA, Smith LM, McDonagh CF, Klussman K, Gordon KA, Morris-Tilden CA, Duniho S, Ryan M, Boursalian TE, Carter PJ, Grewal IS, Law CL. Preclinical characterization of SGN-70, a humanized antibody directed against CD70. Clin Cancer Res. 2008;14:7763–7772. doi: 10.1158/1078-0432.CCR-08-0493. [DOI] [PubMed] [Google Scholar]
- 24.Amano J, Masuyama N, Hirota Y, Tanaka Y, Igawa Y, Shiokawa R, Okutani T, Miyayama T, Nanami M, Ishigai M. Antigen-dependent internalization is related to rapid elimination from plasma of humanized anti-HM1.24 monoclonal antibody. Drug Metab Dispos. 38:2339–2346. doi: 10.1124/dmd.110.035709. [DOI] [PubMed] [Google Scholar]
- 25.Hunsucker SA, Magarotto V, Kuhn DJ, Kornblau SM, Wang M, Weber DM, Thomas SK, Shah JJ, Voorhees PM, Xie H, Cornfeld M, Nemeth JA, Orlowski RZ. Blockade of interleukin-6 signalling with siltuximab enhances melphalan cytotoxicity in preclinical models of multiple myeloma. Br J Haematol. 152:579–592. doi: 10.1111/j.1365-2141.2010.08533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voorhees PM, Chen Q, Kuhn DJ, Small GW, Hunsucker SA, Strader JS, Corringham RE, Zaki MH, Nemeth JA, Orlowski RZ. Inhibition of interleukin-6 signaling with CNTO 328 enhances the activity of bortezomib in preclinical models of multiple myeloma. Clin Cancer Res. 2007;13:6469–6478. doi: 10.1158/1078-0432.CCR-07-1293. [DOI] [PubMed] [Google Scholar]
- 27.Fulciniti M, Hideshima T, Vermot-Desroches C, Pozzi S, Nanjappa P, Shen Z, Patel N, Smith ES, Wang W, Prabhala R, Tai YT, Tassone P, Anderson KC, Munshi NC. A high-affinity fully human anti-IL-6 mAb, 1339, for the treatment of multiple myeloma. Clin Cancer Res. 2009;15:7144–7152. doi: 10.1158/1078-0432.CCR-09-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Qian J, Wezeman M, Wang S, Lin P, Wang M, Yaccoby S, Kwak LW, Barlogie B, Yi Q. Targeting beta2-microglobulin for induction of tumor apoptosis in human hematological malignancies. Cancer Cell. 2006;10:295–307. doi: 10.1016/j.ccr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 29.Gallay N, Anani L, Lopez A, Colombat P, Binet C, Domenech J, Weksler BB, Malavasi F, Herault O. The role of platelet/endothelial cell adhesion molecule 1 (CD31) and CD38 antigens in marrow microenvironmental retention of acute myelogenous leukemia cells. Cancer Res. 2007;67:8624–8632. doi: 10.1158/0008-5472.CAN-07-0402. [DOI] [PubMed] [Google Scholar]
- 30.Partida-Sanchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, Kusser K, Goodrich S, Howard M, Harmsen A, Randall TD, Lund FE. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- 31.Santonocito AM, Consoli U, Bagnato S, Milone G, Palumbo GA, Di Raimondo F, Stagno F, Guglielmo P, Giustolisi R. Flow cytometric detection of aneuploid CD38(++) plasmacells and CD19(+) B-lymphocytes in bone marrow, peripheral blood and PBSC harvest in multiple myeloma patients. Leuk Res. 2004;28:469–477. doi: 10.1016/j.leukres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 32.van der Veer MS, de Weers M, van Kessel B, Bakker JM, Wittebol S, Parren PW, Lokhorst HM, Mutis T. Towards effective immunotherapy of myeloma: enhanced elimination of myeloma cells by combination of lenalidomide with the human CD38 monoclonal antibody daratumumab. Haematologica. 96:284–290. doi: 10.3324/haematol.2010.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Gisbergen KP, van Olffen RW, van Beek J, van der Sluijs KF, Arens R, Nolte MA, van Lier RA. Protective CD8 T cell memory is impaired during chronic CD70-driven costimulation. J Immunol. 2009;182:5352–5362. doi: 10.4049/jimmunol.0802809. [DOI] [PubMed] [Google Scholar]
- 34.Arens R, Tesselaar K, Baars PA, van Schijndel GM, Hendriks J, Pals ST, Krimpenfort P, Borst J, van Oers MH, van Lier RA. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity. 2001;15:801–812. doi: 10.1016/s1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- 35.Faure-Andre G, Vargas P, Yuseff MI, Heuze M, Diaz J, Lankar D, Steri V, Manry J, Hugues S, Vascotto F, Boulanger J, Raposo G, Bono MR, Rosemblatt M, Piel M, Lennon-Dumenil AM. Regulation of dendritic cell migration by CD74, the MHC class II-associated invariant chain. Science. 2008;322:1705–1710. doi: 10.1126/science.1159894. [DOI] [PubMed] [Google Scholar]
- 36.Veillat V, Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A. Macrophage migration inhibitory factor elicits an angiogenic phenotype in human ectopic endometrial cells and triggers the production of major angiogenic factors via CD44, CD74, and MAPK signaling pathways. J Clin Endocrinol Metab. 95:E403–412. doi: 10.1210/jc.2010-0417. [DOI] [PubMed] [Google Scholar]
- 37.Burton JD, Ely S, Reddy PK, Stein R, Gold DV, Cardillo TM, Goldenberg DM. CD74 is expressed by multiple myeloma and is a promising target for therapy. Clin Cancer Res. 2004;10:6606–6611. doi: 10.1158/1078-0432.CCR-04-0182. [DOI] [PubMed] [Google Scholar]
- 38.Boles KS, Stepp SE, Bennett M, Kumar V, Mathew PA. 2B4 (CD244) and CS1: novel members of the CD2 subset of the immunoglobulin superfamily molecules expressed on natural killer cells and other leukocytes. Immunol Rev. 2001;181:234–249. doi: 10.1034/j.1600-065x.2001.1810120.x. [DOI] [PubMed] [Google Scholar]
- 39.Tai YT, Soydan E, Song W, Fulciniti M, Kim K, Hong F, Li XF, Burger P, Rumizen MJ, Nahar S, Podar K, Hideshima T, Munshi NC, Tonon G, Carrasco RD, Afar DE, Anderson KC. CS1 promotes multiple myeloma cell adhesion, clonogenic growth, and tumorigenicity via c-maf-mediated interactions with bone marrow stromal cells. Blood. 2009;113:4309–4318. doi: 10.1182/blood-2008-10-183772. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Retraction. CS1 promotes multiple myeloma cell adhesion, clonogenic growth, and tumorigenicity via c-maf-mediated interactions with bone marrow stromal cells. Blood. 115:2983. doi: 10.1182/blood-2010-03-273136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tassi I, Colonna M. The cytotoxicity receptor CRACC (CS-1) recruits EAT-2 and activates the PI3K and phospholipase Cgamma signaling pathways in human NK cells. J Immunol. 2005;175:7996–8002. doi: 10.4049/jimmunol.175.12.7996. [DOI] [PubMed] [Google Scholar]
- 42.Tassi I, Presti R, Kim S, Yokoyama WM, Gilfillan S, Colonna M. Phospholipase C-gamma 2 is a critical signaling mediator for murine NK cell activating receptors. J Immunol. 2005;175:749–754. doi: 10.4049/jimmunol.175.2.749. [DOI] [PubMed] [Google Scholar]
- 43.Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, Huseni M, Powers D, Nanisetti A, Zhang Y, Rice AG, van Abbema A, Wong M, Liu G, Zhan F, Dillon M, Chen S, Rhodes S, Fuh F, Tsurushita N, Kumar S, Vexler V, Shaughnessy JD, Jr, Barlogie B, van Rhee F, Hussein M, Afar DE, Williams MB. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14:2775–2784. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rhee F, Szmania SM, Dillon M, van Abbema AM, Li X, Stone MK, Garg TK, Shi J, Moreno-Bost AM, Yun R, Balasa B, Ganguly B, Chao D, Rice AG, Zhan F, Shaughnessy JD, Jr, Barlogie B, Yaccoby S, Afar DE. Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma. Mol Cancer Ther. 2009;8:2616–2624. doi: 10.1158/1535-7163.MCT-09-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodrigues-Lima F, Josephs M, Katan M, Cassinat B. Sequence analysis identifies TTRAP, a protein that associates with CD40 and TNF receptor-associated factors, as a member of a superfamily of divalent cation-dependent phosphodiesterases. Biochem Biophys Res Commun. 2001;285:1274–1279. doi: 10.1006/bbrc.2001.5328. [DOI] [PubMed] [Google Scholar]
- 46.Morel Y, Truneh A, Sweet RW, Olive D, Costello RT. The TNF superfamily members LIGHT and CD154 (CD40 ligand) costimulate induction of dendritic cell maturation and elicit specific CTL activity. J Immunol. 2001;167:2479–2486. doi: 10.4049/jimmunol.167.5.2479. [DOI] [PubMed] [Google Scholar]
- 47.Pellat-Deceunynck C, Bataille R, Robillard N, Harousseau JL, Rapp MJ, Juge-Morineau N, Wijdenes J, Amiot M. Expression of CD28 and CD40 in human myeloma cells: a comparative study with normal plasma cells. Blood. 1994;84:2597–2603. [PubMed] [Google Scholar]
- 48.Mizuno T, Rothstein TL. B cell receptor (BCR) cross-talk: CD40 engagement creates an alternate pathway for BCR signaling that activates I kappa B kinase/I kappa B alpha/NF-kappa B without the need for PI3K and phospholipase C gamma. J Immunol. 2005;174:6062–6070. doi: 10.4049/jimmunol.174.10.6062. [DOI] [PubMed] [Google Scholar]
- 49.Solanilla A, Dechanet J, El Andaloussi A, Dupouy M, Godard F, Chabrol J, Charbord P, Reiffers J, Nurden AT, Weksler B, Moreau JF, Ripoche J. CD40-ligand stimulates myelopoiesis by regulating flt3-ligand and thrombopoietin production in bone marrow stromal cells. Blood. 2000;95:3758–3764. [PubMed] [Google Scholar]
- 50.Futagami S, Hiratsuka T, Shindo T, Hamamoto T, Tatsuguchi A, Nobue U, Shinji Y, Suzuki K, Kusunoki M, Tanaka S, Wada K, Miyake K, Gudis K, Tsukui T, Sakamoto C. COX-2 and CCR2 induced by CD40 ligand and MCP-1 are linked to VEGF production in endothelial cells. Prostaglandins Leukot Essent Fatty Acids. 2008;78:137–146. doi: 10.1016/j.plefa.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 51.Hussein M, Berenson JR, Niesvizky R, Munshi N, Matous J, Sobecks R, Harrop K, Drachman JG, Whiting N. A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica. 95:845–848. doi: 10.3324/haematol.2009.008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tai YT, Catley LP, Mitsiades CS, Burger R, Podar K, Shringpaure R, Hideshima T, Chauhan D, Hamasaki M, Ishitsuka K, Richardson P, Treon SP, Munshi NC, Anderson KC. Mechanisms by which SGN-40, a humanized anti-CD40 antibody, induces cytotoxicity in human multiple myeloma cells: clinical implications. Cancer Res. 2004;64:2846–2852. doi: 10.1158/0008-5472.can-03-3630. [DOI] [PubMed] [Google Scholar]
- 53.Ozaki S, Kosaka M, Wakatsuki S, Abe M, Koishihara Y, Matsumoto T. Immunotherapy of multiple myeloma with a monoclonal antibody directed against a plasma cell-specific antigen, HM1.24. Blood. 1997;90:3179–3186. [PubMed] [Google Scholar]
- 54.Wang W, Nishioka Y, Ozaki S, Jalili A, Abe S, Kakiuchi S, Kishuku M, Minakuchi K, Matsumoto T, Sone S. HM1.24 (CD317) is a novel target against lung cancer for immunotherapy using anti-HM1.24 antibody. Cancer Immunol Immunother. 2009;58:967–976. doi: 10.1007/s00262-008-0612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawai S, Yoshimura Y, Iida S, Kinoshita Y, Koishihara Y, Ozaki S, Matsumoto T, Kosaka M, Yamada-Okabe H. Antitumor activity of humanized monoclonal antibody against HM1.24 antigen in human myeloma xenograft models. Oncol Rep. 2006;15:361–367. [PubMed] [Google Scholar]
- 56.Ono K, Ohtomo T, Yoshida K, Yoshimura Y, Kawai S, Koishihara Y, Ozaki S, Kosaka M, Tsuchiya M. The humanized anti-HM1.24 antibody effectively kills multiple myeloma cells by human effector cell-mediated cytotoxicity. Mol Immunol. 1999;36:387–395. doi: 10.1016/s0161-5890(99)00029-2. [DOI] [PubMed] [Google Scholar]
- 57.Ozaki S, Kosaka M, Wakahara Y, Ozaki Y, Tsuchiya M, Koishihara Y, Goto T, Matsumoto T. Humanized anti-HM1.24 antibody mediates myeloma cell cytotoxicity that is enhanced by cytokine stimulation of effector cells. Blood. 1999;93:3922–3930. [PubMed] [Google Scholar]
- 58.Hideshima T, Chauhan D, Hayashi T, Akiyama M, Mitsiades N, Mitsiades C, Podar K, Munshi NC, Richardson PG, Anderson KC. Protea-some inhibitor PS-341 abrogates IL-6 triggered signaling cascades via caspase-dependent downregulation of gp130 in multiple myeloma. Oncogene. 2003;22:8386–8393. doi: 10.1038/sj.onc.1207170. [DOI] [PubMed] [Google Scholar]
- 59.Nakashima K, Taga T. gp130 and the IL-6 family of cytokines: signaling mechanisms and thrombopoietic activities. Semin Hematol. 1998;35:210–221. [PubMed] [Google Scholar]
- 60.Nilsson K, Jernberg H, Pettersson M. IL-6 as a growth factor for human multiple myeloma cells--a short overview. Curr Top Microbiol Immunol. 1990;166:3–12. doi: 10.1007/978-3-642-75889-8_1. [DOI] [PubMed] [Google Scholar]
- 61.Suematsu S, Hibi M, Sugita T, Saito M, Murakami M, Matsusaka T, Matsuda T, Hirano T, Taga T, Kishimoto T. Interleukin 6 (IL-6) and its receptor (IL-6R) in myeloma/plasmacytoma. Curr Top Microbiol Immunol. 1990;166:13–22. doi: 10.1007/978-3-642-75889-8_2. [DOI] [PubMed] [Google Scholar]
- 62.Voorhees PM, Chen Q, Small GW, Kuhn DJ, Hunsucker SA, Nemeth JA, Orlowski RZ. Targeted inhibition of interleukin-6 with CNTO 328 sensitizes pre-clinical models of multiple myeloma to dexamethasone-mediated cell death. Br J Haematol. 2009;145:481–490. doi: 10.1111/j.1365-2141.2009.07647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshio-Hoshino N, Adachi Y, Aoki C, Pereboev A, Curiel DT, Nishimoto N. Establishment of a new interleukin-6 (IL-6) receptor inhibitor applicable to the gene therapy for IL-6-dependent tumor. Cancer Res. 2007;67:871–875. doi: 10.1158/0008-5472.CAN-06-3641. [DOI] [PubMed] [Google Scholar]
- 64.Bjorkman PJ, Burmeister WP. Structures of two classes of MHC molecules elucidated: crucial differences and similarities. Curr Opin Struct Biol. 1994;4:852–856. doi: 10.1016/0959-440x(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 65.Malik P, Klimovitsky P, Deng LW, Boyson JE, Strominger JL. Uniquely conformed peptide-containing beta 2-microglobulin-free heavy chains of HLA-B2705 on the cell surface. J Immunol. 2002;169:4379–4387. doi: 10.4049/jimmunol.169.8.4379. [DOI] [PubMed] [Google Scholar]
- 66.Fellous M, Bono R, Hyafil F, Gresser I. Interferon enhances the amount of membrane-bound beta2-microglobulin and its release from human Burkitt cells. Eur J Immunol. 1981;11:524–526. doi: 10.1002/eji.1830110616. [DOI] [PubMed] [Google Scholar]
- 67.Revillard JP, Vincent C, Clot J, Sany J. beta 2 -Microglobulin and beta 2-microglobulin-binding proteins in inflammatory diseases. Eur J Rheumatol Inflamm. 1982;5:398–405. [PubMed] [Google Scholar]
- 68.Moe SM, Hack BK, Cummings SA, Sprague SM. Role of IL-1 beta and prostaglandins in beta 2-microglobulin-induced bone mineral dissolution. Kidney Int. 1995;47:587–591. doi: 10.1038/ki.1995.74. [DOI] [PubMed] [Google Scholar]
- 69.Barlogie B, Jagannath S, Desikan KR, Mattox S, Vesole D, Siegel D, Tricot G, Munshi N, Fassas A, Singhal S, Mehta J, Anaissie E, Dhodapkar D, Naucke S, Cromer J, Sawyer J, Epstein J, Spoon D, Ayers D, Cheson B, Crowley J. Total therapy with tandem transplants for newly diagnosed multiple myeloma. Blood. 1999;93:55–65. [PubMed] [Google Scholar]
- 70.Bataille R, Durie BG, Grenier J. Serum beta2 microglobulin and survival duration in multiple myeloma: a simple reliable marker for staging. Br J Haematol. 1983;55:439–447. doi: 10.1111/j.1365-2141.1983.tb02158.x. [DOI] [PubMed] [Google Scholar]
- 71.Molica S, Levato D, Cascavilla N, Levato L, Musto P. Clinico-prognostic implications of simultaneous increased serum levels of soluble CD23 and beta2-microglobulin in B-cell chronic lymphocytic leukemia. Eur J Haematol. 1999;62:117–122. doi: 10.1111/j.1600-0609.1999.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 72.Shvidel L, Hofstein R, Berrebi A. Serum beta-2 microglobulin as a marker of B-cell activation in chronic lymphoid malignancies. Am J Hematol. 1996;53:148–149. doi: 10.1002/(SICI)1096-8652(199610)53:2<148::AID-AJH21>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 73.Cooper EH, Plesner T. Beta-2-microglobulin review: its relevance in clinical oncology. Med Pediatr Oncol. 1980;8:323–334. doi: 10.1002/mpo.2950080403. [DOI] [PubMed] [Google Scholar]
- 74.Alexanian R, Barlogie B, Fritsche H. Beta 2 microglobulin in multiple myeloma. Am J Hematol. 1985;20:345–351. doi: 10.1002/ajh.2830200405. [DOI] [PubMed] [Google Scholar]
- 75.Alexanian R, Barlogie B, Fritsche H. Beta 2 microglobulin in multiple myeloma. American Journal of Hematology. 1985;20:345–351. doi: 10.1002/ajh.2830200405. [DOI] [PubMed] [Google Scholar]
- 76.Child JA, Spati B, Illingworth S, Barnard D, Corbett S, Simmons AV, Stone J, Worthy TS, Cooper EH. Serum beta 2 microglobulin and C-reactive protein in the monitoring of lymphomas: findings in a multicenter study and experience in selected patients. Cancer. 1980;45:318–326. doi: 10.1002/1097-0142(19800115)45:2<318::aid-cncr2820450220>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 77.Cuzick J, De Stavola BL, Cooper EH, Chapman C, MacLennan IC. Long-term prognostic value of serum beta 2 microglobulin in myelomatosis. Br J Haematol. 1990;75:506–510. doi: 10.1111/j.1365-2141.1990.tb07790.x. [DOI] [PubMed] [Google Scholar]
- 78.Cuzick J, Cooper EH, MacLennan IC. The prognostic value of serum beta 2 microglobulin compared with other presentation features in myelomatosis. Br J Cancer. 1985;52:1–6. doi: 10.1038/bjc.1985.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rossi JF, Durie BG, Duperray C, Braich T, Marion SL, Pike JW, Haussler MR, Janbon C, Bataille R. Phenotypic and functional analysis of 1,25-dihydroxyvitamin D3 receptor mediated modulation of the human myeloma cell line RPMI 8226. Cancer Res. 1988;48:1213–1216. [PubMed] [Google Scholar]
- 80.Xie J, Wang Y, Freeman ME, 3rd, Barlogie B, Yi Q. Beta 2-microglobulin as a negative regulator of the immune system: high concentrations of the protein inhibit in vitro generation of functional dendritic cells. Blood. 2003;101:4005–4012. doi: 10.1182/blood-2002-11-3368. [DOI] [PubMed] [Google Scholar]
- 81.Canalis E, McCarthy T, Centrella M. A bone-derived growth factor isolated from rat calvariae is beta 2 microglobulin. Endocrinology. 1987;121:1198–1200. doi: 10.1210/endo-121-3-1198. [DOI] [PubMed] [Google Scholar]
- 82.Skov S, Nielsen M, Bregenholt S, Odum N, Claesson MH. Activation of Stat-3 is involved in the induction of apoptosis after ligation of major histocompatibility complex class I molecules on human Jurkat T cells. Blood. 1998;91:3566–3573. [PubMed] [Google Scholar]
- 83.Pedersen AE, Skov S, Bregenholt S, Ruhwald M, Claesson MH. Signal transduction by the major histocompatibility complex class I molecule. APMIS. 1999;107:887–895. doi: 10.1111/j.1699-0463.1999.tb01488.x. [DOI] [PubMed] [Google Scholar]
- 84.Gur H, Geppert TD, Lipsky PE. Structural analysis of class I MHC molecules: the cytoplasmic domain is not required for cytoskeletal association, aggregation and internalization. Mol Immunol. 1997;34:125–132. doi: 10.1016/s0161-5890(97)00007-2. [DOI] [PubMed] [Google Scholar]
- 85.Gur H, Geppert TD, Wacholtz MC, Lipsky PE. The cytoplasmic and the transmembrane domains are not sufficient for class I MHC signal transduction. Cell Immunol. 1999;191:105–116. doi: 10.1006/cimm.1998.1417. [DOI] [PubMed] [Google Scholar]
- 86.Pierce SK. Lipid rafts and B-cell activation. Nat Rev Immunol. 2002;2:96–105. doi: 10.1038/nri726. [DOI] [PubMed] [Google Scholar]
- 87.Rao R, Logan B, Forrest K, Roszman TL, Goebel J. Lipid rafts in cytokine signaling. Cytokine Growth Factor Rev. 2004;15:103–110. doi: 10.1016/j.cytogfr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 88.Podar K, Tai YT, Cole CE, Hideshima T, Sattler M, Hamblin A, Mitsiades N, Schlossman RL, Davies FE, Morgan GJ, Munshi NC, Chauhan D, Anderson KC. Essential role of caveolae in interleukin-6- and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cells. J Biol Chem. 2003;278:5794–5801. doi: 10.1074/jbc.M208636200. [DOI] [PubMed] [Google Scholar]
- 89.Maggi D, Biedi C, Segat D, Barbero D, Panetta D, Cordera R. IGF-I induces caveolin 1 tyrosine phosphorylation and translocation in the lipid rafts. Biochem Biophys Res Commun. 2002;295:1085–1089. doi: 10.1016/s0006-291x(02)00809-4. [DOI] [PubMed] [Google Scholar]
- 90.Yang J, Zhang X, Wang J, Qian J, Zhang L, Wang M, Kwak LW, Yi Q. Anti beta2-microglobulin monoclonal antibodies induce apoptosis in myeloma cells by recruiting MHC class I to and excluding growth and survival cytokine receptors from lipid rafts. Blood. 2007;110:3028–3035. doi: 10.1182/blood-2007-06-094417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang J, Cao Y, Hong S, Li H, Qian J, Kwak LW, Yi Q. Human-like mouse models for testing the efficacy and safety of anti-beta2-microglobulin monoclonal antibodies to treat myeloma. Clin Cancer Res. 2009;15:951–959. doi: 10.1158/1078-0432.CCR-08-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shachar I, Rabinovich E, Kerem A, Bar-Nun S. Thiol-reducing agents and calcium perturbants alter intracellular sorting of immunoglobulin M. J Biol Chem. 1994;269:27344–27350. [PubMed] [Google Scholar]
- 93.Lankester AC, van Schijndel GM, Fromme J, Cordell JL, van Lier RA, van Noesel CJ. Evidence for a direct physical interaction of membrane IgM, IgD, and IgG with the B29 gene product. J Immunol. 1994;152:2157–2162. [PubMed] [Google Scholar]


