Abstract
The two major classes of activity-dependent neuroplasticity predict different consequences of activity alteration on circuit response. Hebbian plasticity (positive feedback) posits that alteration of neuronal activity causes a parallel response within a circuit. In contrast, homeostatic plasticity (negative feedback) predicts that altering neuronal activity results in compensatory responses within a circuit. The relative roles of these modes of plasticity in vivo are unclear, since neuronal circuits are difficult to manipulate in the intact organism. In this study, we tested the in vivo effects of activity deprivation in the superior cervical ganglion-pineal circuit of adult rats, which can be non-invasively silenced by exposing animals to constant light. We demonstrated that total deprivation of sympathetic activity markedly decreased the presence of axonal proteins in the pineal and reduced the density and thickness of sympathetic axonal arbors. In addition, we demonstrated that sympathetic inactivity eliminated pineal function and markedly decreased pineal expression of neurotrophins. Administration of β-adrenergic agonist restored the expression of pre- and post-synaptic proteins. Furthermore, compensatory axonal growth through collateral sprouting, normally seen following unilateral denervation of the pineal, was profoundly impaired in the absence of neural activity. Thus, these data suggest that sympathetic axonal terminals are maintained by neural activity that induces neurotrophins, which may act through a retrograde mechanism to preserve the integrity of axonal arbors via a positive feedback loop. Conversely, silent yet intact circuits enter a “hibernation mode” marked by reduction of presynaptic axonal structures and dramatically reduced postsynaptic expression of neurotrophins, by utilizing Hebbian-like neuroplasticity.
INTRODUCTION
Activity dependent changes in neural circuits have attracted widespread attention, in large part due to their potential roles in learning and memory and adaptation to disease states. Two dichotomous responses to changes in neuronal activity have been observed in neural circuits: 1) Hebbian responses typified by long term potentiation (positive feedback), in which changes in network activity generate parallel changes in the response (Paulsen and Sejnowski, 2000), and 2) homeostatic neuroplasticity (negative feedback), in which a neural circuit is stabilized in response to activity changes (Nelson and Turrigiano, 2008). These principles have been extensively validated in in-vitro or ex-vivo models but major questions remain unanswered. Most significantly, it remains uncertain whether in vivo neuroplasticity is governed by Hebbian or homeostatic principles. A significant obstacle to experimental testing in vivo is that manipulation of circuit activity in animals by non-pharmacological and non-invasive methods presents significant challenges (Nelson and Turrigiano, 2008).
The superior cervical ganglion (SCG)-pineal circuit (Bowers et al., 1984) offers an attractive in vivo model (Zigmond et al., 1985) to investigate activity-dependent neuroplasticity. The pineal gland releases melatonin in response to nightly norepinephrine secretion from clock-controlled SCG neurons (Borjigin et al., 1999). Norepinephrine activates β-adrenergic receptors on pinealocytes and increases melatonin production via arylalkylamine N-acetyltransferase (AANAT) activation (Borjigin et al., 1995; Klein et al., 1997). The hypothalamus-SCG-pineal circuit offers several unique advantages for the study of activity dependent changes in neuronal networks: 1) It is a relatively simple, linear circuit; 2) The system can be rapidly and reproducibly silenced by exposure to light at night, which inhibits presynaptic norepinephrine release (Drijfhout et al., 1996) and activates AANAT degradation; 3) Melatonin production in constant light can be restored by adrenergic agonists (Huang et al., 2010). In the present study, our first objective was to test effects of prolonged inactivity on structural plasticity of axons in normal and injured pineal and to determine the ability of pharmacological activation of post-synaptic adrenergic receptors to alter structural plasticity of sympathetic axons in the absence of endogenous circuit activity.
Target derived neurotrophins including nerve growth factor (NGF) and neurotrophin 3 (NT3) promote survival and neurite extension of sympathetic neurons in vitro and in vivo (Levi-Montalcini and Angeletti, 1968; Campenot, 1977; Ruit et al., 1990; Hamburger, 1993). In adult animals, sympathetic fiber density correlates with NGF levels in target tissues (Korsching and Thoenen, 1983). Additionally, NGF promotes synaptic transmission between cultured sympathetic neurons and cardiomyocytes (Lockhart et al., 1997). It is not known, however, whether neuronal activity is continuously required for neurotrophin expression in target organs. Thus, our second objective was to test the effects of activity silencing on neurotrophin expression in normal and partially denervated pineal. Finally, we investigated whether adrenergic activation could restore neurotrophin expression under constant light.
Our data support the existence of Hebbian-like neuroplasticity within SCG-pineal circuit: activity silencing suppresses presynaptic axonal growth and postsynaptic function; and adrenergic receptor stimulation induces axonal growth of SCG neurons and postsynaptic function, both of which parallel changes in NGF expression in the pineal.
MATERIALS AND METHODS
Animals
Adult male Fischer and Sprague Dawley rats were purchased from Harlan (Indianapolis, IN, USA) and housed in a light:dark (LD) cycle of 12:12h (lights on at 6am) with food and water ad libitum at least a week prior to experiments. After acclimating to our animal housing facility, animals were kept in chambers in LD, or constant light (LL; animals do not experience any darkness in LL) conditions for the times specified, with light intensity between 250 and 450 lux at cage level. At selected time points, animals were sacrificed by decapitation in the middle of the night (zeitgeber 18:00h, or ZT18) under very dim red light for the LD animals and in the room light for the LL animals. At the time of sacrifice animals were 10–15 weeks old (210–330 g). Pineal glands were dissected and flash frozen in liquid nitrogen for subsequent analysis. All animal protocols were reviewed and approved by the University of Michigan animal care and use committee.
Surgery
Unilateral or bilateral surgical removal of the SCG was performed at 9am (ZT3), as described previously (Brammer et al., 1982). In brief, animals were anesthetized with isoflurane, a small incision of cca. 1.5 cm was made at the level of the trachea, neck muscles (cleidocephalic, sternomastoid, sternothyroid, omohyoid) were separated, gently pulled to the side and the common carotid artery was isolated. The internal and external carotid nerves (rostral) and the cervical sympathetic trunk (caudal), which connect the SCG to target tissues and spinal cord respectively, were cut and the SCG removed. Incisions were sutured, animals allowed to recover and returned to their cages where they were housed in either LD or LL conditions as described for each experiment. At set times after surgery, animals were sacrificed, pineal glands dissected and either flash-frozen in liquid nitrogen for subsequent Western blot analysis or fixed in 4% paraformaldehyde, 5% sucrose PBS for immunohistochemical analysis.
Antibodies and Chemicals
For immunoblots we used the following primary antibodies: anti-β-III tubulin (PRB-435P) (Covance, Princeton, NJ), anti-tyrosine hydroxylase (TH, T-1299) (Sigma, St. Louis, MO), anti-AANAT generated in our laboratory (Huang et al., 2005), anti-pan 14-3-3 (H-8) and anti-NGF (M-20) (Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies used were anti-rabbit IgG, HRP conjugated (AP307P) (Millipore, Billerica, MA), and IRDye800CW Conjugated anti-mouse IgG (Rockland, Gilbertsville, PA). The following antibodies were used for immunohistochemistry: anti-βIII tubulin (PRB-435P) (Covance, Princeton, NJ) and anti-TH (AB1542) (Millipore, Billerica, MA). Secondary antibodies were Alexa-Fluor 568 donkey anti-rabbit and Alexa-Fluor 488 donkey anti-mouse (Invitrogen, Carlsbad, CA). Normal Donkey Serum was purchased from Jackson Immuno-Research (West Grove, PA) and Hoechst 3332 from Molecular Probes (Eugene, OR).
Isoproterenol HCl (I-104) was purchased from Research Biochemicals International (Natick, MA). Chemicals used for the immunoblot analysis were purchased from the following companies: protease inhibitor Complete Mini from Roche Diagnostics (Mannheim, Germany), Super Signal West Pico Chemiluminescent Substrate from Thermo Scientific (Rockford, IL). Tissue-Tek OCT Compound was obtained from Electron Microscopy Sciences (Hatfield PA), RNeasy Mini Kit from Qiagen (Hilden, Germany), Superscript II First Strand Synthesis kit for RT-PCR from Invitrogen (Carlsbad, CA) and Brilliant II SYBR Green QPCR Master Mix from Stratagene (Santa Clara, CA).
Western Blot Analysis
Frozen pineals were sonicated in lysis buffer (10mM Tris HCl pH 7.5 with 1% SDS, 0.5mM EDTA, 0.1 mM Na3VO4, 0.1mM Na3P2O7, 0.1mM phenyl methyl sulfonyl fluoride and protease inhibitor), and proteins were denatured and reduced by boiling with Laemmli sample buffer with 0.1% β-mercaptoethanol and 10mM dithiothreitol. One fifth of the lysate from each pineal was resolved by SDS polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto polyvinylidene fluoride membrane and immunoblotted with primary antibodies. Specific proteins were visualized with either horseradish peroxidase conjugated secondary antibodies followed by enhanced chemiluminescence or using the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE). Immunoblots were quantified using ImageJ software, values were normalized to the expression of 14-3-3 and represented graphically as percent change relative to expression in control animals. Unpaired two-tailed t-test was calculated for statistical significance.
Immunohistochemistry
Pineals were fixed overnight in 4% paraformaldehyde, 5% sucrose PBS, cryoprotected, embedded and frozen in Tissue-Tek OCT compound. Tissues were cryosectioned at 12 microns and mounted onto glass slides. Five sections from each condition were mounted on the same slide to be processed identically. Slides were processed for immunohistochemistry as described previously (Calinescu et al., 2009). Hoechst 3332 was used as nuclear counterstain. Micrograph images were obtained with an Olympus Fluoview 500 confocal microscope using the same parameters for experimental and control samples. Adobe Photoshop CS2 (Adobe Systems, San Jose, CA) was used to construct the figures and overlays.
Quantitative Real Time PCR
Total RNA was extracted from pineals using the RNeasy Mini kit. Complementary DNA was generated with the Superscript II first strand synthesis kit using the manufacturer’s protocol with oligo(dT). Quantitative real-time PCR (QPCR) analysis was performed using the Stratagene MX3000P Cycler and Brilliant II SYBR Green QPCR master mix. Primer sequences are as follows: for ngf (XM_001067130) forward: GGACGCAGCTTTCTATCCTG and reverse: CTGTGTCAAGGGAATGCTGA, for nt3 (NM_031073) forward: CGGATGCCATGGTTACTTCT and reverse: GATATCCGCCTGGATCAACT; for aanat (NM_012818) forward: TGCCACCAGTGCGTTTGAGATTG and reverse: GCCTGTGTAGTGTCAGCGACTCCTG; for hprt (NM_012583) forward: GCAGACTTTGCTTTCCTTGG and reverse CCGCTGTCTTTTAGGCTTTG. Threshold cycles generated by the Stratagene MX3000P software were used to calculate specific mRNA expression with the ΔΔCt method, normalizing values to the expression of the housekeeping gene hprt. Results are represented graphically as percent expression relative to control. Unpaired two-tailed t-test was performed for statistical analysis. Specificity of amplification was confirmed by agarose gel electrophoresis of the amplification products, which showed single bands of the expected size.
Isoproterenol treatment
A cohort of rats received a single injection of either vehicle (PBS) or isoproterenol HCl intraperitoneally at a concentration of 1.4 mg/kg body weight at ZT6 and was sacrificed 4 hours later at ZT10. A separate cohort of animals, maintained in constant light for 12 days, received 12 consecutive daily injections of either vehicle (PBS) or isoproterenol administered between ZT11–12.
RESULTS
Silencing of sympathetic activity in the pineal results in a progressive decrease in axonal proteins
Sympathetic activity is tightly associated with the activity of NAT (or AANAT) in the pineal gland (Bowers and Zigmond, 1982). To evaluate the effects of activity-deprivation on sympathetic terminal arbors in the pineal, we exposed animals to constant light (LL) to inhibit SCG-pineal circuit activity. A light pulse at night is well known to block the release of norepinephrine (Drijfhout et al., 1996), which abolishes melatonin release (Drijfhout et al., 1996; Liu and Borjigin, 2005; Huang et al., 2008) and shuts down the production of AANAT mRNA and protein in the pineal gland (Borjigin et al., 1995; Roseboom et al., 1996; Gastel et al., 1998; Huang et al., 2008; Wongchitrat et al., 2009). Thus, the suppression of AANAT or melatonin production by light at night is indicative of the silencing of the SCG-pineal circuit activity, since the two events are coupled extremely tightly (Roseboom et al., 1996). In all analyses, the silencing of sympathetic activity by light at night was confirmed by the absence of AANAT, the key enzyme in melatonin production.
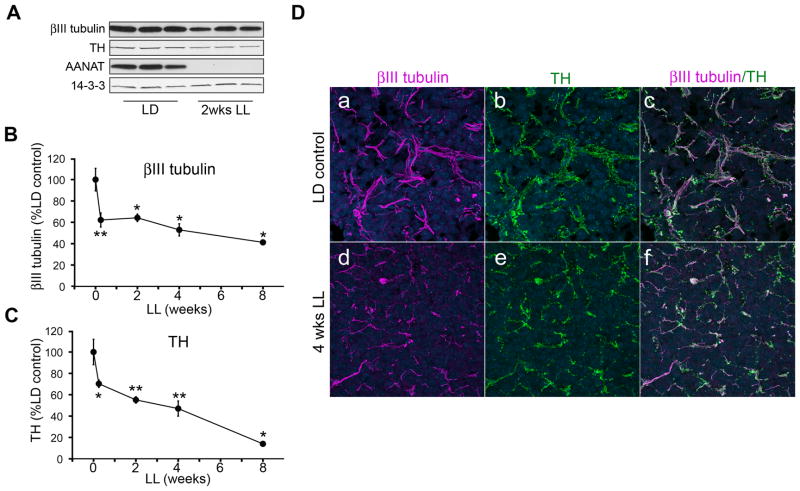
A cohort of rats was exposed to constant light for increasing periods of time (0.25, 2, 4, and 8 weeks) and the levels of axonal proteins in the pineal were analyzed by Western blot. A representative blot from the 2 weeks LL time-point is illustrated in Fig. 1A, which reveals a significant decrease in the neuronal microtubule protein βIII tubulin and tyrosine hydroxylase (TH), specific markers for sympathetic axonal fibers in the pineal. This decrease occurs most dramatically at 42h (0.25 week) of LL, and progresses further with longer exposure times (Fig. 1B and 1C). Changes in βIII tubulin and TH levels correlate throughout the time-course of light exposure (Pearson correlation coefficient = 0.9404). To investigate morphological changes of axon terminals in the pineal, we analyzed βIII tubulin and TH in the pineal gland of animals in LD or following 4 weeks of LL. Immunohistochemical analysis showed that sympathetic axon terminals co-express βIII tubulin and TH and that long-term exposure to constant light results in a reduction in the density of these fibers along with a reduction in fiber and/or fiber-bundle thickness (Fig. 1D). In constant light, the size of the fiber bundles appears to be smaller. Moreover, the distribution of the fibers appears to be more scattered than in control pineal.
Figure 1. Constant light results in a progressive decrease in expression of axonal proteins in the pineal.
(A) Representative Western blot analysis of pineal lysates from animals reared in either light:dark (LD) conditions or following 2 weeks (wks) of constant light (LL), probed with antibodies against βIII tubulin, TH, AANAT, and 14-3-3. In this and all other Western blots, each lane represents pineal lysate from a different animal and expression of 14-3-3 is monitored for quantifying protein levels. (B) βIII tubulin protein levels following light exposures of increasing durations (0, 0.25, 2, 4, and 8 wks). The values are expressed as percentage relative to LD control animals: which are: 62% at 0.25 wks (42h; n=6), 64% at 2 wks (n=3), 53% at 4 wks (n=3), and 41% at 8 wks of LL (n=3). (C) TH expression following exposure to constant light is 70% (n=6) after 0.25 wks, 55% (n=3) after 2 wks, 47% (n=3) after 4 wks, and 14% (n=3) after 8 wks. Two-tailed t-test was performed for statistical analysis, p<0.05*, p<0.01**. Error bars represent SEM. (D) Pineal glands from control animals reared in LD conditions (a–c) and after 4 wks of LL (d–f) were analyzed by immunohistochemistry for the presence of axonal markers βIII tubulin (panels a, d, c, f) and TH (panels b, e, c, f). Note co-localization of βIII tubulin and TH and marked decrease in the thickness and density of axonal terminal arbors (d–f) following exposure to constant light. In blue, Hoechst 3332 was used as nuclear counterstain. All animals were sacrificed at ZT18. The findings shown in panel D were confirmed in two rats. AANAT levels were assayed in all Western blotting experiments to ascertain the silencing of adrenergic activities when light was present at night.
Axons in the pineal represent mainly sympathetic axonal arbors originating from the two superior cervical ganglia (SCG)
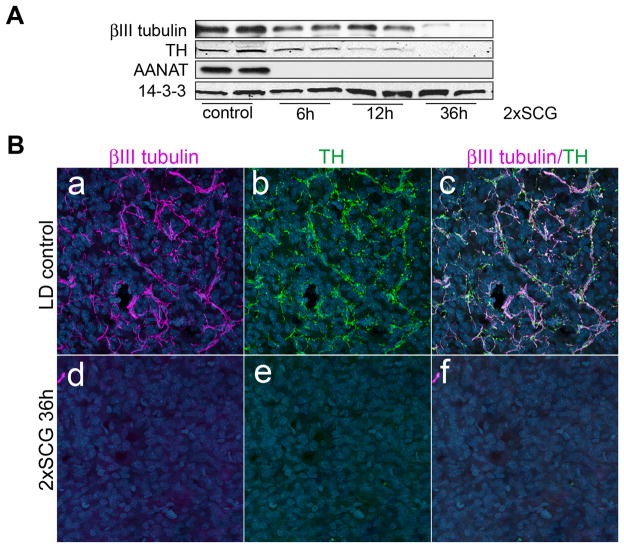
While pineal innervation originates mainly from sympathetic postganglionic neurons in the SCGs (Kappers, 1960; Bertler et al., 1964; Bowers et al., 1984; Patrickson and Smith, 1987), there have been reports of parasympathetic innervation of the pineal from the superior salivatory nucleus via the sphenopalatine ganglion (Larsen et al., 1998; Larsen, 1999). To ensure that our quantitative analysis of neuronal proteins following exposure to constant light reflects sympathetic axonal terminals originating from the SCGs, we analyzed βIII tubulin and TH by Western blot following 6, 12, and 36h after bilateral surgical removal of the SCGs (2xSCG). The levels of βIII tubulin and TH decrease rapidly, as early as 6h post surgery; 36h following 2xSCG, βIII tubulin dramatically decreased and TH is not detectable (Fig. 2A). Further, by immunohistochemistry, the neuronal proteins are undetectable in the body of the pineal 36h after 2xSCG (Fig. 2B).
Figure 2. Axonal proteins in the pineal represent mainly sympathetic axonal arborsoriginating from the two superior cervical ganglia (SCG).
(A) Western blot analysis of βIII tubulin and TH in pineals from control and ganglionectomized animals (2xSCG) following 6, 12, and 36 hours after surgical denervation. Note the progressive decrease in TH (64% of control at 6h, 17% at 12h, and 1% at 36h 2xSCG) and βIII tubulin (53% of control at 6h, 40% at 12h, and 3% at 36h post-denervation) proteins following bilateral ganglionectomy. Expression of AANAT, which is induced by norepinephrine release from the SCG axon terminals, was monitored to confirm the surgical denervation. (B) Pineal glands from control (a–c) and 36h following 2xSCG, (d–f) were analyzed by immunohistochemistry for the expression of axonal markers βIII tubulin (a,d,c,f) and TH (b,e,c,f). In blue, Hoechst 3332 was used as nuclear counterstain. Note that,βIII tubulin and TH co-localize in axonal fibers in the control sample (a–c) and that, 36h following surgical denervation, axonal proteins are undetectable. All animals were sacrificed at ZT15. These results (panels A and B) were confirmed in more than 5 independent experiments.
Expression of neurotrophins in the pineal is strongly decreased following activity-deprivation
It is generally accepted that survival of adult sympathetic neurons is less dependent on neurotrophic factors compared to neurons in the peri- and early postnatal stage (Cowen and Gavazzi, 1998). Little is known, however, about physiological factors that regulate expression of neurotrophins in targets of sympathetic neurons in adult animals. Since we observed a profound decrease in pineal innervation following activity deprivation, and since there is ample evidence documenting that neurotrophins promote sympathetic axonal growth, we asked if deprivation of sympathetic input to the pineal results in changes in expression of NGF and NT3, the most abundant neurotrophins expressed in the adult pineal (QPCR data, not shown).
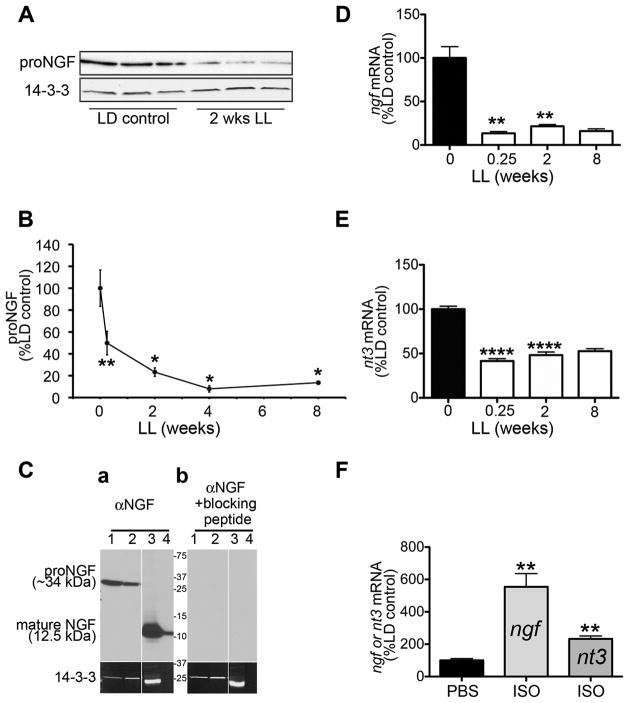
Results (Fig. 3A and 3B) show that exposure to constant light results in a profound decrease in proNGF protein (~34kDa). Western blot quantification of proNGF expression in the pineal during the time-course of light exposure shows that there is a significant decrease (50%) in proNGF expression as early as 42h in LL and this decrease is even more severe (86%) following 8 weeks of LL treatment (Fig. 3B). The decrease in proNGF expression correlates with the decrease in βIII tubulin (Pearson = 0.9109) and TH (Pearson = 0.9049). In the pineal, the most abundant form of NGF is the 34kDa protein (Bierl et al., 2005; Bierl and Isaacson, 2007; Randolph et al., 2007). The mature NGF (12.5kDa) is a minor form of NGF and is not detected by Western blot analysis. In our experimental conditions with very stringent reducing agents in the lysis buffer, the only band we observe in the pineal using the polyclonal antibody M-20 that also detects the mature NGF is the ~34kDa band (Fig. 3Ca). This band is not detected when the antibody is mixed with blocking peptide (Fig. 3Cb).
Figure 3. Neurotrophin (NGF and NT3) expression in the pineal is progressively decreased following exposure to constant light.
(A) A representative Western blot analysis of pineal lysates from animals reared in either LD conditions or following 2 weeks of LL shows decrease in expression of proNGF in animals reared in LL. (B) Time-course of changes in proNGF expression following exposure to constant light represented as percentage proNGF relative to LD control: 50% after 0.25 wks LL (n=6), 23% after 2 wks LL (n=3), 8% at 4 wks LL (n=3) and 14% after 8 wks LL (n=3). (C) Expression of proNGF in the pineal. Daytime pineal lysates from two independent rats (lanes 1 and 2) were resolved by SDS-PAGE and analyzed by immunoblot with the polyclonal anti-NGF antibody (panel a), or with the same antibody pre-incubated with blocking peptide (panel b). Two positive controls were used: lysate of male mouse submandibular gland (lane 3) and 7ng of pure NGF (Sigma, N6009) (lane 4). Note that the 34 kDa band is the only detectable NGF species in the pineal lysates. Pre-incubation of the antibody with the NGF blocking peptide results in no NGF signal (panel b), confirming specificity of the pineal 34kDa band. (D) Time-course of ngf mRNA, analyzed by quantitative real-time PCR (QPCR), in pineal glands of animals maintained in 12:12h LD conditions (n=9), or following exposure to LL, shows decrease in expression of ngf: 13% after 0.25 wks LL (n=4), 21.5% after 2 wks LL (n=3) and 16% after 8 wks LL (n=2). (E) Time-course of nt3 mRNA analyzed by QPCR in pineal glands of animals maintained in LD (n=9) or following extended periods of LL, shows decrease in expression of nt3 following exposure to constant light: 42% after 0.25 wks LL (n=4), 48 % after 2 wks LL (n=3) and 47% after 8 wks LL (n=2). All animals in panels A-D were sacrificed at ZT18. (F) β-adrenergic stimulation with isoproterenol induces expression of ngf and nt3 mRNA. Expression of ngf and nt3 in the pineal, analyzed by QPCR 4 hours after a single intraperitoneal injection of isoproterenol (ISO) administered at ZT6, increased 5.5 fold for ngf (n=4) and 2.3 fold for nt3 compared to vehicle-injected (PBS) controls (n=3). Two-tailed t-test was performed for statistical analysis, p<0.05*, p<0.01**, p<0.0001****. Error bars represent SEM. Animals were sacrificed at ZT18. Silencing of circuit activity was confirmed by the absence of AANAT protein following light exposure in panels A and B and by the suppression of aanat mRNA in panels D and E following light exposure (data not shown).
Our data agrees with other reports showing proNGF as the most abundant form of NGF protein, with little or no mature NGF detected in the brain and the pineal gland (Fahnestock et al., 2001; Bierl and Isaacson, 2007). The mature form of NGF is the biologically active form of this molecule (Edwards et al., 1988) with numerous reports showing it’s role in neural survival and neurite growth, through binding and activation of TrkA receptors and retrograde signaling (Zweifel et al., 2005; Campenot, 2009). proNGF, on the other hand, binds to the p75NTR low affinity neurotrophin receptor and induces apoptosis (Casaccia-Bonnefil et al., 1996; Frade et al., 1996; Roux and Barker, 2002). More recent studies suggest that proNGF can also possess neurotrophic activities (Fahnestock et al., 2004; Al-Shawi et al., 2008). Importantly, NGF is released in the extracellular space as proNGF where it is processed into the mature form and degraded by proteolytic enzymes (Bruno and Cuello, 2006), suggesting that changes in proNGF in vivo could reflect levels of active and mature NGF, particularly when accompanied by changes in ngf mRNA.
To test if the decrease in NGF protein is reflected at the transcriptional level, we performed real-time QPCR analysis of pineal ngf transcripts. Light exposure resulted in a profound decrease (87%) in ngf transcripts, which occurred as early as 42h in LL. Longer exposure did not produce further decrease (Fig. 3D). We observed a significant decrease in nt3 transcripts (Fig. 3E) as well (58% after 42h LL), though lower in magnitude than the decrease in ngf. These results suggest that target expression of ngf and nt3 is regulated by neural activity through the night-time release of norepinephrine and activation of β-adrenergic receptors. To test this hypothesis further, we injected animals with isoproterenol (a β-adrenergic agonist) during the day when sympathetic activity in the pineal is minimal, and analyzed expression of ngf and nt3 mRNA four hours later. Results show that expression of ngf and nt3 (Fig. 3F) is increased 5.54 fold and 2.33 fold respectively, indicating that neurotrophin expression in the pineal is regulated through sympathetic activation. Effectiveness of the isoproterenol injection was confirmed by analyzing the expression of aanat mRNA, which increased ~500 fold following isoproterenol treatment (data not shown).
Functional activation of the pineal with a β-adrenergic agonist rescues the light-induced decrease in axonal proteins and proNGF
Since we observed that functional silencing of neuronal activity results in a decrease of sympathetic innervation and this correlates with a decrease in NGF expression, and since we can induce ngf mRNA expression in the pineal with isoproterenol, we tested whether we could rescue the expression of axonal proteins in the activity-deprived pineal with β-adrenergic stimulation. We kept animals in either LD or LL conditions for 12 days, and treated them daily with either vehicle or isoproterenol. The injection was administered intraperitoneally 30 min before the normal onset of the dark period. Levels of βIII tubulin, TH, and proNGF are profoundly decreased in animals deprived of sympathetic stimulation, whereas β-adrenergic stimulation rescues the levels of axonal proteins and proNGF in the pineal despite the continued presence of light (Fig. 4). The expression of AANAT was monitored as a control to confirm efficiency of β-adrenergic activation on pinealocytes. These data strongly suggest that expression of NGF and of axonal proteins in the pineal are under the control of sympathetic activity.
Figure 4. Functional activation of the pineal with a β-adrenergic agonist rescues the light-induced decrease in axonal proteins and proNGF.
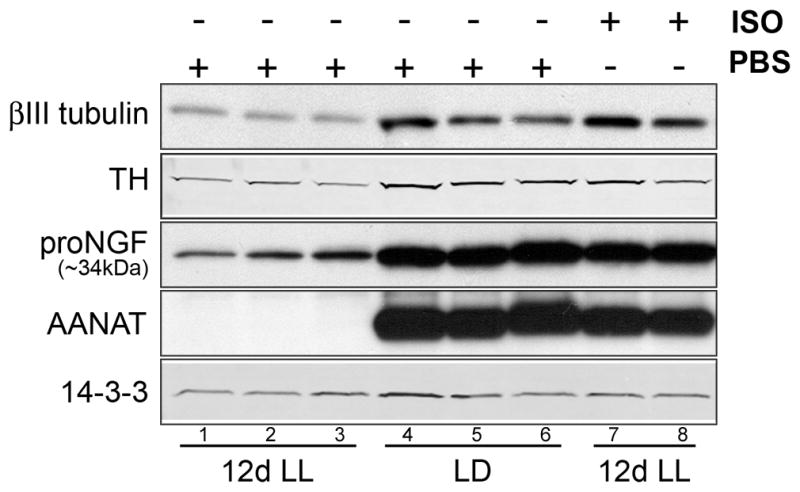
Levels of βIII tubulin, TH and proNGF were analyzed by Western blot in control pineal lysates from animals reared in LD conditions (n=3) (lanes 4–6), or maintained for 12 days in constant light (n=3) (lanes 1–3, 7 and 8) and received 12 consecutive daily injections of vehicle (lanes 1–3) or isoproterenol (ISO; lanes 7 and 8) (n=3, two lanes were shown). βIII tubulin, TH and proNGF are decreased in animals maintained in LL (lanes 1–3) compared to animals reared in LD (lanes 4–6). This decrease is rescued with 12 daily administrations of ISO (lanes 7 and 8). AANAT, which is found only during the dark phase of the light cycle and is induced by the activation of pineal β-adrenergic receptors, was monitored to confirm the effect of light exposure and efficacy of the ISO injection. All animals were sacrificed at ZT18.
Axonal growth from unlesioned sympathetic neurons is profoundly impaired in the absence of neural activity
Pineal gland displays time-dependent collateral growth of sympathetic fibers following a partial denervation (Dornay et al., 1985; Lingappa and Zigmond, 1987; Kuchel and Zigmond, 1991). Since NGF has been shown to promote collateral sprouting in other systems (Diamond et al., 1987; Doubleday and Robinson, 1994), and since we observe a decrease in NGF protein and mRNA in the pineals of animals in the absence of sympathetic activities, we tested the consequences of LL on collateral sprouting.
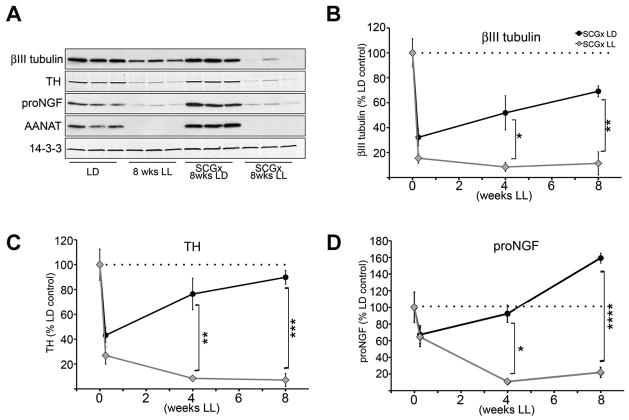
We analyzed the levels of βIII tubulin, TH, and NGF in the pineal glands of rats following unilateral sympathectomy with or without light exposures of varying durations. Results (Fig. 5) demonstrate a profound consequence of inactivity on collateral growth of sympathetic neurons spared in the partial denervation injury. Following unilateral removal of one SCG, the pineal gland displays a 50% loss of sympathetic fibers at 42h, which is gradually compensated by the growth of the contralateral SCG neurons spared by the injury following injury (black circles in Figs. 5B and 5C), if animals experience normal sympathetic activity at night in LD. Importantly, the collateral growth of the spared SCG is associated with increased NGF protein expression in the partially injured pineal gland (black circles in Fig. 5D). When the injured animals were housed in LL that blocks the sympathetic activity to the pineal gland, however, the compensatory collateral growth of SCG neurons is completely suppressed (gray diamonds in Fig. 5B and 5C). Correspondingly, NGF protein (gray diamonds in Fig. 5D) and mRNA (data not shown) expression in the injured pineal gland is also dramatically reduced.
Figure 5. Axonal growth from uninjured SCG neurons following unilateral SCG removal is profoundly impaired in the absence of neural activity.
(A) A representative Western blot of pineal lysates from control animals kept in LD or LL, and from animals which had one SCG surgically removed, and were maintained after surgery for 8 weeks in LD (SCGx 8wks LD) or in LL (SCGx 8wks LL). Membranes were probed with antibodies against βIII tubulin, TH, NGF, AANAT, and 14-3-3. (B) Time-course of βIII tubulin levels in the pineal following unilateral ganglionectomy (0, 0.25, 4, and 8 wks) from animals reared in either LD (SCGx LD) or LL (SCGx LL). Values are expressed as percentage relative to LD control animals: 32% at 0.25 wks (42h) (n=6), 52% at 4 wks (n=3) and 69% at 8 wks (n=3) in the LD samples; and 14% at 0.25 wks (n=6), 8% at 4 wks (n=3) and 11% at 8 wks (n=3) in animals maintained in LL conditions. (C) Time-course of TH protein following unilateral ganglionectomy from animals reared in either LD or LL. Values are relative to LD controls: 43% at 0.25 wks (42h) (n=6), 76% at 4 wks (n=3) and 90% at 8 wks (n=3) in the LD samples, and 27% at 0.25 wks (n=6), 8% at 4 wks (n=3) and 7% at 8 wks (n=3) in LL samples. (D) Time-course of proNGF (~34kDa) expression following unilateral ganglionectomy from animals reared in either LD or LL, expressed as percentage relative to LD control animals: 67% at 0.25 wks (42h) (n=6), 92% at 4 wks (n=3) and 159% at 8 wks (n=3) in the LD samples; and 64% at 0.25 wks (n=6), 11% at 4 wks (n=3) and 22% at 8 wks (n=3) in animals maintained in LL conditions. Unpaired, two-tailed t-test was performed for statistical analysis comparing the time-point values for each protein to the LD control values as noted in each panel above. The values between the SCGx LD and SCGx LL at each time point: p<0.05*, p<0.01**, p<0.001***, p<0.0001****. Error bars represent SEM. All animals were sacrificed at ZT18. Again, silencing of the sympathetic activity by light was confirmed by the absence of AANAT protein in the pineal gland of rats exposed to light at night. Of note, histological confirmation of sprouting following partial pineal denervation has been previously demonstrated (Lingappa and Zigmond, 1987).
DISCUSSION
Activity is critical for neural plasticity in the adult nervous system. Previous investigations of plasticity relied heavily on denervation injury to reduce neuronal activity. We took advantage of the fact that neural activity in the pineal gland can be controlled simply by light to analyze activity-dependent plasticity in the adult sympathetic nervous system. This maneuver enabled us to monitor the effect of silencing activity in living mammals without the need for invasive procedures, genetic manipulations, or in vitro analysis. We report here, that in the absence of neural activity, sympathetic terminal arbors in the pineal gland undergo profound structural changes and a corresponding decrease in structural and functional proteins. The suppression of sympathetic innervation parallels a decrease in neurotrophin expression, which can be restored by β-adrenergic stimulation. Further, axonal growth of the uninjured SCG neurons, stimulated by unilateral ganglionectomy, is severely impaired by activity deprivation. Our findings support a transsynaptic positive feedback loop between the sympathetic neurons and their target to promote activity dependent-neuroplasticity: neural activity in the SCG promotes postsynaptic function and expression of neurotrophins, which may in turn feedback onto sympathetic neurons to promote and maintain their structural integrity.
Role of neuronal activity in regulating axon structure
Numerous studies have analyzed neuroplasticity in the developing and mature sympathetic nervous system, focusing mainly on the survival and neurite extension of sympathetic neurons following injury or during aging and with or without exogenous neurotrophins (Cowen and Gavazzi, 1998). The effect of physiological neural activity on the structural plasticity of sympathetic axonal terminals is less understood. Our studies demonstrate that silencing neuronal activity dramatically reduces levels of TH, a marker of sympathetic neurons. Activity-dependent regulation of TH has been demonstrated in the peripheral nervous system (Zigmond and Chalazonitis, 1979; Zigmond et al., 1985; Rittenhouse et al., 1988; Chevalier et al., 2008). Rats continuously exposed to light for 20 days display approximately 50% decrease in TH mRNA expression in the SCG (Gallara et al., 2004). Our data complement these existing studies and demonstrate that silencing the circuit activity by light results in a marked reduction of sympathetic axon branches in the pineal and a corresponding decrease in TH protein.
One unexpected finding is the rapid regulation by activity of βIII tubulin, a component of microtubule cytoskeleton widely used as a neuronal marker (Katsetos et al., 2003). βIII tubulin expression is greatest during development when axon growth and maturation are most active; levels subsequently decrease and then stabilize in the adult nervous system (Jiang and Oblinger, 1992). Dynamic regulation of βIII tubulin in adult mammals has been reported in injured dorsal root ganglion (Jiang et al., 1994) and following stimulation of cAMP signaling pathway (Gallara et al., 2004; Liu and Brady, 2004). Our results demonstrate for the first time that silencing of neuronal activity leads to a profound decrease in βIII tubulin in a sympathetic target. Importantly, βIII tubulin levels decline by one third after only 42h of constant light. As far as we know, this is the first report to date of a rapid turnover of structural elements of adult axons. These findings suggest that structural remodeling of axon terminals is a highly dynamic process and that maintenance of integrity of sympathetic axons depends critically on sustained activity of the neural circuit. It remains to be determined if light at night exerts comparable effects on other peripheral targets controlled by the central circadian clock (Kalsbeek et al., 2006).
Previous reports have shown that sympathetic innervation to a partially denervated pineal gland is restored over time through compensatory growth of neurons in the spared SCG (Dornay et al., 1985; Lingappa and Zigmond, 1987; Kuchel and Zigmond, 1991). This experimental system is therefore suitable to investigate the anatomical correlate of the empiric observation that repeated use of the circuit enhances structural and functional recovery (a guiding principle for rehabilitation of patients with neurological injury). Using light to repress activity of the SCG-pineal circuit, we were able to unambiguously determine that neuronal activity is required for re-establishment of axonal proteins after unilateral denervation of the pineal gland in vivo. These findings provide perhaps the strongest support yet that activity of a circuit is essential for reestablishment and maintenance of anatomical connections following injury. Overall, the studies provide support of the concept that optimal rehabilitation requires activation of the dysfunctional circuit. Further work using this experimental paradigm could clarify the molecular mechanisms of activity dependent compensatory axonal growth and may prove useful in testing interventions to facilitate neural circuit rehabilitation in an in vivo system.
Role of neuronal activity in NGF expression in target tissues
Regulation of NGF expression by norepinephrine has been investigated before with mixed results. In animal models of heart disease, chronic norepinephrine treatment in vivo leads to an initial increase of NGF which decreases at later times when congestive heart failure ensues (Qin et al., 2002; Kimura et al., 2010). The early induction of NGF by norepinephrine in the heart is comparable to activation of NGF expression by a single injection of isoproterenol (Fig. 3F). The subsequent decrease of NGF expression in the heart, despite the continuous infusion of norepinephrine, may reflect negative feedback mechanisms in response to the chronic adrenergic stimulation. In contrast to the earlier report showing that decreased activity did not lead to decreased NGF in one of the sympathetic targets (Liu et al., 1996), our studies clearly demonstrate that sympathetic activity is essential for target expression of NGF.
In the pineal gland 3 weeks following bilateral SCG removal, levels of NGF (~34 kDa) increase significantly (Randolph et al., 2007). In contrast, our data show that silencing sympathetic activity by light at night decreases levels of NGF mRNA and protein; conversely, stimulation of sympathetic activity by activation of β-adrenergic signaling increases expression of NGF in the pineal. Our conflicting results can be explained by major differences in experimental models. Surgical sympathectomy causes not only circuit inactivation, but also results in Wallerian degeneration and activation of phagocytosis. Indeed, increased NGF expression is frequently seen in inflammation and following denervation of target tissues (Weskamp and Otten, 1987; Skaper, 2001). In our study, we were able to completely and non-invasively inhibit activity of the SCG without denervation. Thus, our studies, which utilize methodology that eliminates surgical stress, denervation, and inflammation, clearly demonstrate sympathetic inactivity lowers NGF in the target tissue.
Because NGF is known to promote collateral sprouting in other systems (Diamond et al., 1987; Doubleday and Robinson, 1994) and was severely suppressed by inactivity of the SCG-pineal circuit (Fig. 3), we examined the combined effects of activity silencing and partial denervation on pineal NGF expression. Our data show that NGF levels are initially decreased in the injured pineal gland but recover in parallel with the compensatory axonal growth. In the presence of constant light, however, the increase in NGF expression is completely suppressed. These studies provide compelling evidence that activity is required for neurotrophin re-expression after injury. The observation that axonal recovery and neurotrophin expression after injury both require activity is consistent with a functional connection between these two events. Furthermore, these studies provide additional support that this model system may be useful in further molecular and pharmacological investigations of activity-dependent (rehabilitative) recovery after injury.
Role of endogenous NGF in retrograde signaling in adult animals
While the role of NGF is firmly established for developing nervous systems (Zweifel et al., 2005; Sharma et al., 2010), the NGF function in adult animals is less clear. Administration or deprivation of NGF in vivo alters morphology and survival of mature SCG neurons (Ruit et al., 1990), and in vivo intracerebroventricular infusion of NGF elicits a sprouting response by sympathetic perivascular axons (Isaacson et al., 1992). These studies demonstrate that exogenous NGF influences the remodeling of mature sympathetic axons. Whether the same is true for endogenous NGF is not known.
Our data demonstrate a dramatic suppression of NGF expression when sympathetic activity is shut down by light, and an equally dramatic upregulation of endogenous NGF by adrenergic agonist, both of which are associated with parallel changes in levels of presynaptic axonal marker proteins. When the pineal gland is unilaterally denervated, the spared SCG displays marked compensatory growth, which is associated with high levels of NGF expression in the recovering pineal gland. The arrest of the axonal growth by light is once again associated with the dramatically reduced NGF levels in the pineal gland. These results strongly suggest that NGF production in adult animals is maintained by continued sympathetic activity; which in turn may retrogradely promote the structural integrity of sympathetic axons.
In circuits formed between central neurons or at neuromuscular junctions, a transsynaptic negative feedback mechanism exists to promote homeostatic neuroplasticity (Davis, 2006; Turrigiano, 2007). When central input to the pineal gland is completely shutdown in this study, however, the pineal gland responds with a transsynaptic positive feedback mechanism, likely mediated by NGF, perhaps to conserve resources of the circuit. We conclude that a Hebbian-like response is the dominant mode of neuroplasticity adopted by the sympathetic circuit in the absence of neural activity.
Acknowledgments
We thank Dr. Peter Hitchcock for critically reading this manuscript, Dr. Roman Giger for helpful discussions, and Mr. Richard Chis for help with animal care. This work was supported by NIH grant NS057583 (JB) and DoD grants FA9550-08-0149 (JB) and FA9550-09-0352 (JB).
Footnotes
Conflict of Interest: Authors declare no conflict of interest
References
- Al-Shawi R, Hafner A, Olsen J, Chun S, Raza S, Thrasivoulou C, Lovestone S, Killick R, Simons P, Cowen T. Neurotoxic and neurotrophic roles of proNGF and the receptor sortilin in the adult and ageing nervous system. Eur J Neurosci. 2008;27:2103–2114. doi: 10.1111/j.1460-9568.2008.06152.x. [DOI] [PubMed] [Google Scholar]
- Bertler A, Falck B, Owman C. Studies on 5-Hydroxytryptamine Stores in Pineal Gland of Rat. Acta Physiologica Scandinavica S. 1964;63:3. doi: 10.1111/j.1748-1716.1964.tb04118.x. [DOI] [PubMed] [Google Scholar]
- Bierl MA, Isaacson LG. Increased NGF proforms in aged sympathetic neurons and their targets. Neurobiology of Aging. 2007;28:122–134. doi: 10.1016/j.neurobiolaging.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Bierl MA, Jones EE, Crutcher KA, Isaacson LG. ‘Mature’ nerve growth factor is a minor species in most peripheral tissues. Neurosci Lett. 2005;380:133–137. doi: 10.1016/j.neulet.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Borjigin J, Wang MM, Snyder SH. Diurnal-Variation in Messenger-Rna Encoding Serotonin N-Acetyltransferase in Pineal-Gland. Nature. 1995;378:783–785. doi: 10.1038/378783a0. [DOI] [PubMed] [Google Scholar]
- Borjigin J, Li XD, Snyder SH. The pineal gland and melatonin: Molecular and pharmacologic regulation. Annual Review of Pharmacology and Toxicology. 1999;39:53–65. doi: 10.1146/annurev.pharmtox.39.1.53. [DOI] [PubMed] [Google Scholar]
- Bowers CW, Zigmond RE. The influence of the frequency and pattern of sympathetic nerve activity on serotonin N-acetyltransferase in the rat pineal gland. J Physiol. 1982;330:279–296. doi: 10.1113/jphysiol.1982.sp014341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers CW, Dahm LM, Zigmond RE. The number and distribution of sympathetic neurons that innervate the rat pineal gland. Neuroscience. 1984;13:87–96. doi: 10.1016/0306-4522(84)90261-6. [DOI] [PubMed] [Google Scholar]
- Brammer M, Binkley S, Mosher K. The rise and fall of pineal N-acetyltransferase in vitro: neural regulation in the developing rat. J Neurobiol. 1982;13:487–494. doi: 10.1002/neu.480130604. [DOI] [PubMed] [Google Scholar]
- Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Natl Acad Sci U S A. 2006;103:6735–6740. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calinescu AA, Vihtelic TS, Hyde DR, Hitchcock PF. Cellular expression of midkine-a and midkine-b during retinal development and photoreceptor regeneration in zebrafish. Journal of Comparative Neurology. 2009;514:1–10. doi: 10.1002/cne.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. NGF uptake and retrograde signaling mechanisms in sympathetic neurons in compartmented cultures. Results Probl Cell Differ. 2009;48:141–158. doi: 10.1007/400_2009_7. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- Chevalier J, Derkinderen P, Gomes P, Thinard R, Naveilhan P, Vanden Berghe P, Neunlist M. Activity-dependent regulation of tyrosine hydroxylase expression in the enteric nervous system. J Physiol. 2008;586:1963–1975. doi: 10.1113/jphysiol.2007.149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen T, Gavazzi I. Plasticity in adult and ageing sympathetic neurons. Prog Neurobiol. 1998;54:249–288. doi: 10.1016/s0301-0082(97)00071-3. [DOI] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Diamond J, Coughlin M, Macintyre L, Holmes M, Visheau B. Evidence that endogenous beta nerve growth factor is responsible for the collateral sprouting, but not the regeneration, of nociceptive axons in adult rats. Proc Natl Acad Sci U S A. 1987;84:6596–6600. doi: 10.1073/pnas.84.18.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornay M, Gilad VH, Gilad GM. Compensatory changes in contralateral sympathetic neurons of the superior cervical ganglion and in their terminals in the pineal gland following unilateral ganglionectomy. J Neurosci. 1985;5:1522–1526. doi: 10.1523/JNEUROSCI.05-06-01522.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubleday B, Robinson PP. Nerve growth factor depletion reduces collateral sprouting of cutaneous mechanoreceptive and tooth-pulp axons in ferrets. J Physiol. 1994;481 (Pt 3):709–718. doi: 10.1113/jphysiol.1994.sp020475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drijfhout WJ, van der Linde AG, de Vries JB, Grol CJ, Westerink BH. Microdialysis reveals dynamics of coupling between noradrenaline release and melatonin secretion in conscious rats. Neurosci Lett. 1996;202:185–188. doi: 10.1016/0304-3940(95)12245-1. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Selby MJ, Garcia PD, Rutter WJ. Processing of the native nerve growth factor precursor to form biologically active nerve growth factor. J Biol Chem. 1988;263:6810–6815. [PubMed] [Google Scholar]
- Fahnestock M, Yu G, Coughlin MD. ProNGF: a neurotrophic or an apoptotic molecule? Prog Brain Res. 2004;146:101–110. doi: 10.1016/S0079-6123(03)46007-X. [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Michalski B, Xu B, Coughlin MD. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol Cell Neurosci. 2001;18:210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- Frade JM, Rodriguez-Tebar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Gallara RV, Bellavia SL, Serova LL, Sabban EL. Environmental light conditions alter gene expression of rat catecholamine biosynthetic enzymes and Neuropeptide Y: differential effect in superior cervical ganglia and adrenal gland. Brain Res Mol Brain Res. 2004;124:152–158. doi: 10.1016/j.molbrainres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Gastel JA, Roseboom PH, Rinaldi PA, Weller JL, Klein DC. Melatonin production: proteasomal proteolysis in serotonin N-acetyltransferase regulation. Science. 1998;279:1358–1360. doi: 10.1126/science.279.5355.1358. [DOI] [PubMed] [Google Scholar]
- Hamburger V. The history of the discovery of the nerve growth factor. J Neurobiol. 1993;24:893–897. doi: 10.1002/neu.480240702. [DOI] [PubMed] [Google Scholar]
- Huang Z, Deng J, Borjigin J. A novel H28Y mutation in LEC rats leads to decreased NAT protein stability in vivo and in vitro. Journal of Pineal Research. 2005;39:84–90. doi: 10.1111/j.1600-079X.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- Huang Z, Liu T, Borjigin J. N-terminal residues regulate proteasomal degradation of AANAT. J Pineal Res. 2010;48:290–296. doi: 10.1111/j.1600-079X.2010.00753.x. [DOI] [PubMed] [Google Scholar]
- Huang Z, Liu T, Chattoraj A, Ahmed S, Wang MM, Deng J, Sun X, Borjigin J. Posttranslational regulation of TPH1 is responsible for the nightly surge of 5-HT output in the rat pineal gland. Journal of Pineal Research. 2008;45:506–514. doi: 10.1111/j.1600-079X.2008.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson LG, Saffran BN, Crutcher KA. Nerve growth factor-induced sprouting of mature, uninjured sympathetic axons. Journal of Comparative Neurology. 1992;326:327–336. doi: 10.1002/cne.903260302. [DOI] [PubMed] [Google Scholar]
- Jiang YQ, Oblinger MM. Differential regulation of beta III and other tubulin genes during peripheral and central neuron development. J Cell Sci. 1992;103 (Pt 3):643–651. doi: 10.1242/jcs.103.3.643. [DOI] [PubMed] [Google Scholar]
- Jiang YQ, Pickett J, Oblinger MM. Comparison of changes in beta-tubulin and NF gene expression in rat DRG neurons under regeneration-permissive and regeneration-prohibitive conditions. Brain Res. 1994;637:233–241. doi: 10.1016/0006-8993(94)91238-6. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, Ruiter M, Kreier F, Cailotto C, Buijs RM. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- Kappers JA. The Development, Topographical Relations and Innervation of the Epiphysis Cerebri in the Albino Rat. Zeitschrift Fur Zellforschung Und Mikroskopische Anatomie. 1960;52:163–215. doi: 10.1007/BF00338980. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Legido A, Perentes E, Mork SJ. Class III beta-tubulin isotype: a key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology. J Child Neurol. 2003;18:851–866. doi: 10.1177/088307380301801205. discussion 867. [DOI] [PubMed] [Google Scholar]
- Kimura K, Kanazawa H, Ieda M, Kawaguchi-Manabe H, Miyake Y, Yagi T, Arai T, Sano M, Fukuda K. Norepinephrine-induced nerve growth factor depletion causes cardiac sympathetic denervation in severe heart failure. Autonomic Neuroscience-Basic & Clinical. 2010;156:27–35. doi: 10.1016/j.autneu.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, Zatz M, Iuvone PM, Rodriguez IR, Begay V, Falcon J, Cahill GM, Cassone VM, Baler R. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307–357. discussion 357–308. [PubMed] [Google Scholar]
- Korsching S, Thoenen H. Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat: correlation with density of sympathetic innervation. Proc Natl Acad Sci U S A. 1983;80:3513–3516. doi: 10.1073/pnas.80.11.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchel GA, Zigmond RE. Functional recovery and collateral neuronal sprouting examined in young and aged rats following a partial neural lesion. Brain Res. 1991;540:195–203. doi: 10.1016/0006-8993(91)90507-r. [DOI] [PubMed] [Google Scholar]
- Larsen PJ. Tracing autonomic innervation of the rat pineal gland using viral transneuronal tracing. Microsc Res Tech. 1999;46:296–304. doi: 10.1002/(SICI)1097-0029(19990815/01)46:4/5<296::AID-JEMT6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Enquist LW, Card JP. Characterization of the multisynaptic neuronal control of the rat pineal gland using viral transneuronal tracing. Eur J Neurosci. 1998;10:128–145. doi: 10.1046/j.1460-9568.1998.00003.x. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Angeletti PU. Nerve growth factor. Physiol Rev. 1968;48:534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Lingappa JR, Zigmond RE. A histochemical study of the adrenergic innervation of the rat pineal gland: evidence for overlap of the innervation from the two superior cervical ganglia and for sprouting following unilateral denervation. Neuroscience. 1987;21:893–902. doi: 10.1016/0306-4522(87)90045-5. [DOI] [PubMed] [Google Scholar]
- Liu DT, Reid MT, Bridges DC, Rush RA. Denervation, but not decentralization, reduces nerve growth factor content of the mesenteric artery. J Neurochem. 1996;66:2295–2299. doi: 10.1046/j.1471-4159.1996.66062295.x. [DOI] [PubMed] [Google Scholar]
- Liu HH, Brady ST. cAMP, tubulin, axonal transport, and regeneration. Experimental Neurology. 2004;189:199–203. doi: 10.1016/j.expneurol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Liu T, Borjigin J. Reentrainment of the circadian pacemaker through three distinct stages. J Biol Rhythms. 2005;20:441–450. doi: 10.1177/0748730405279388. [DOI] [PubMed] [Google Scholar]
- Lockhart ST, Turrigiano GG, Birren SJ. Nerve growth factor modulates synaptic transmission between sympathetic neurons and cardiac myocytes. Journal of Neuroscience. 1997;17:9573–9582. doi: 10.1523/JNEUROSCI.17-24-09573.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Strength through diversity. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrickson JW, Smith TE. Innervation of the pineal gland in the rat: an HRP study. Experimental Neurology. 1987;95:207–215. doi: 10.1016/0014-4886(87)90018-5. [DOI] [PubMed] [Google Scholar]
- Paulsen O, Sejnowski TJ. Natural patterns of activity and long-term synaptic plasticity. Curr Opin Neurobiol. 2000;10:172–179. doi: 10.1016/s0959-4388(00)00076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Vulapalli RS, Stevens SY, Liang CS. Loss of cardiac sympathetic neurotransmitters in heart failure and NE infusion is associated with reduced NGF. Am J Physiol Heart Circ Physiol. 2002;282:H363–371. doi: 10.1152/ajpheart.00319.2001. [DOI] [PubMed] [Google Scholar]
- Randolph CL, Bierl MA, Isaacson LG. Regulation of NGF and NT-3 protein expression in peripheral targets by sympathetic input. Brain Res. 2007;1144:59–69. doi: 10.1016/j.brainres.2007.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse AR, Schwarzschild MA, Zigmond RE. Both synaptic and antidromic stimulation of neurons in the rat superior cervical ganglion acutely increase tyrosine hydroxylase activity. Neuroscience. 1988;25:207–215. doi: 10.1016/0306-4522(88)90019-x. [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Coon SL, Baler R, McCune SK, Weller JL, Klein DC. Melatonin synthesis: analysis of the more than 150-fold nocturnal increase in serotonin N-acetyltransferase messenger ribonucleic acid in the rat pineal gland. Endocrinology. 1996;137:3033–3045. doi: 10.1210/endo.137.7.8770929. [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Ruit KG, Osborne PA, Schmidt RE, Johnson EM, Jr, Snider WD. Nerve growth factor regulates sympathetic ganglion cell morphology and survival in the adult mouse. J Neurosci. 1990;10:2412–2419. doi: 10.1523/JNEUROSCI.10-07-02412.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Deppmann CD, Harrington AW, St Hillaire C, Chen ZY, Lee FS, Ginty DD. Long-distance control of synapse assembly by target-derived NGF. Neuron. 2010;67:422–434. doi: 10.1016/j.neuron.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD. Nerve growth factor: a neurokine orchestrating neuroimmune-endocrine functions. Mol Neurobiol. 2001;24:183–199. doi: 10.1385/MN:24:1-3:183. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol. 2007;17:318–324. doi: 10.1016/j.conb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Weskamp G, Otten U. An enzyme-linked immunoassay for nerve growth factor (NGF): a tool for studying regulatory mechanisms involved in NGF production in brain and in peripheral tissues. J Neurochem. 1987;48:1779–1786. doi: 10.1111/j.1471-4159.1987.tb05736.x. [DOI] [PubMed] [Google Scholar]
- Wongchitrat P, Felder-Schmittbuhl MP, Phansuwan-Pujito P, Pevet P, Simonneaux V. Endogenous rhythmicity of Bmal1 and Rev-erb alpha in the hamster pineal gland is not driven by norepinephrine. Eur J Neurosci. 2009;29:2009–2016. doi: 10.1111/j.1460-9568.2009.06742.x. [DOI] [PubMed] [Google Scholar]
- Zigmond RE, Chalazonitis A. Long-term effects of preganglionic nerve stimulation on tyrosine hydroxylase activity in the rat superior cervical ganglion. Brain Res. 1979;164:137–152. doi: 10.1016/0006-8993(79)90011-8. [DOI] [PubMed] [Google Scholar]
- Zigmond RE, Baldwin C, Bowers CW. Rapid recovery of pineal function after partial denervation: a possible role for heteroneuronal uptake of transmitter in modulating synaptic efficacy. J Neurosci. 1985;5:142–150. doi: 10.1523/JNEUROSCI.05-01-00142.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]