Abstract
Activated murine cytotoxic T cells express the NKG2D natural cytotoxicity receptor. This receptor recognizes MHC class I-like molecules expressed on the surface of infected cells and serves to augment T cell-mediated cytotoxicity. The role of NKG2D-mediated augmentation in the clearance of central nervous system viral infections has not been explored. Using the Theiler's murine encephalomyelitis virus model we found that NKG2D-positive CD8+ cytotoxic T cells enter the brain, that NKG2D ligands are expressed in the brain during acute infection, and that interruption of NKG2D ligand recognition via treatment with a function blocking antibody attenuates the efficacy of viral clearance from the central nervous system.
Keywords: TMEV, cytotoxic T celll, enterovirus, Rae1, H60, Mult1, neurovirulence
Members of the picornavirus family of small, non-enveloped, positive-stranded RNA viruses, which includes enterovirus 71, poliovirus, hepatitis A virus, the coxsackieviruses and the rhinoviruses, are a frequent cause of infection worldwide. Despite the fact that members of this family infect more humans than any other group of viruses (Rotbart, 2002), host factors that control picornaviral replication are poorly understood. Even less is known about host factors that control picornaviral neurovirulence and clearance of picornaviruses from the central nervous system (CNS) (Buenz and Howe, 2006). We and others have used the Theiler's murine encephalomyelitis virus (TMEV) as a mouse model of picornavirus infection of the CNS (Buenz et al, 2006; Jin et al, 2007; Rubio et al, 2006; Tsunoda et al, 2006). Previous studies have extensively characterized the role of antiviral CD8+ cytotoxic T lymphocytes (CTLs) in the clearance of TMEV from the brain during acute infection (Mendez-Fernandez et al, 2003), and it is clear in mice of the H-2b MHC class I haplotype that CTLs specific for the VP2121–130 TMEV peptide are responsible for viral clearance during the first two weeks of CNS infection (Myounget al, 2007). However, the role of additional host factors such as co-stimulatory molecules has not been thoroughly explored.
NKG2D is a natural cytotoxicity receptor expressed on natural killer cells (NKCs) and on activated CTLs in mice (Eagle and Trowsdale, 2007). This receptor recognizes stress- and infection-regulated MHC class I-like molecules such as Rae1, Mult1, and H60 expressed on the surface of murine cells (Raulet, 2003). While NKG2D is a direct mediator of cytotoxicity for NKCs, it serves as a co-stimulatory receptor for CTLs and augments cytotoxicity downstream from T cell receptor recognition of peptides presented on MHC class I (Markiewicz et al, 2005). Because the mechanisms that direct CTL-mediated clearance of infected neural cells are unclear, we asked whether NKG2D-positive immune effectors are present in the CNS during acute TMEV infection, whether NKG2D ligands are expressed in the brain following infection, and whether interruption of NKG2D-mediated co-stimulation of CTLs alters viral clearance from the CNS.
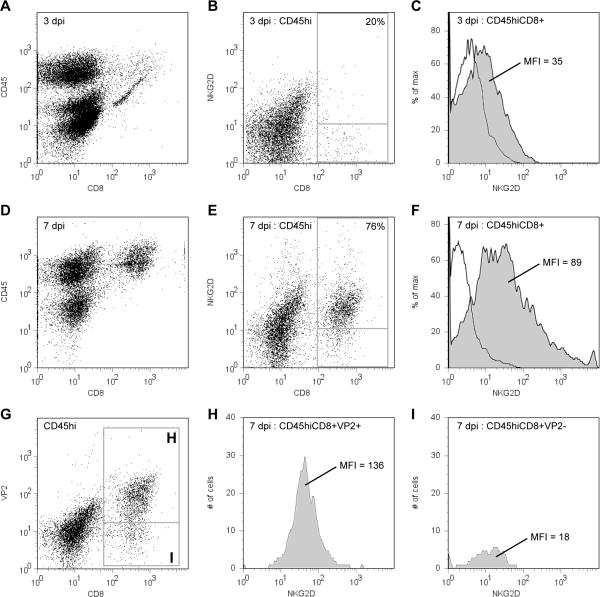
Using flow cytometric analysis of a previously characterized preparation of brain-infiltrating leukocytes (BILs) (Howe et al, 2007), we measured the number of NKG2D-positive immune cells present in the CNS at 3 and 7 days after TMEV infection (2×105 PFU i.c.; 4–6 week old C57Bl/6J mice; all experiments adhered to Mayo IACUC guidelines). We found that at 3 days postinfection (dpi) 6 ± 0.4% of CD45hi BILs were CD8-positive, of which 20 ± 0.6% were also positive for the NKG2D costimulatory receptor. By 7 dpi the BILs were comprised of 24 ± 2% CTLs, with 76 ± 2% of these cells positive for NKG2D (n=4–6 mice per timepoint; mean ± 95% CI). Thus, between 3 and 7 dpi the phenotype of immune cells infiltrating the CNS shifted toward a population that was enriched in NKG2D+CD8+CD45hi CTLs (Figure 1A–1F). The presence of NKG2D-positive CTLs within the infected hippocampus (Wada and Fujinami, 1993) was confirmed by simultaneously immunostaining cryosections from mice at 7 dpi with anti-CD8 (53-6.7, 1:100) and anti-NKG2D (CX5, 1:50) (Figure 2A–2E) (Howe et al, 2004).
Figure 1.
NKG2D-positive antiviral CD8+ T cells infiltrate the brain during acute infection with TMEV. Brain-infiltrating leukocytes (BILs) were isolated from mice at 3 (A–C) and 7 (D–F) days postinfection (dpi) and analyzed by flow cytometry. The number of CD45hiCD8+ cells increased between 3 (A) and 7 (D) dpi. Likewise, the number of CD45hi cells positive for both CD8 and NKG2D increased from 20% at 3 dpi (B) to 76% at 7 dpi (E). Moreover, the intensity of NKG2D surface labeling increased from a mean fluorescence intensity (MFI) of 35 at 3 dpi (shaded histogram in C) to 89 at 7 dpi (shaded histogram in F). The MFI for the isotype control was 4 at 3 dpi (open histogram in C) and 5 at 7 dpi (open histogram in F). BILs were further analyzed for antiviral specificity by costaining with an MHC class I tetramer loaded with the VP2121–130 H-2Db-specific viral peptide (G–I). Of the cells that were both CD8+ and tetramer-positive (box “H” in G), 81% were also NKG2D-positive, with an MFI of 136 (H). In contrast, only 12% of cells that were CD8+ but tetramer-negative (box “I” in G) were also NKG2D-positive, with an MFI of only 18 (I).
Figure 2.
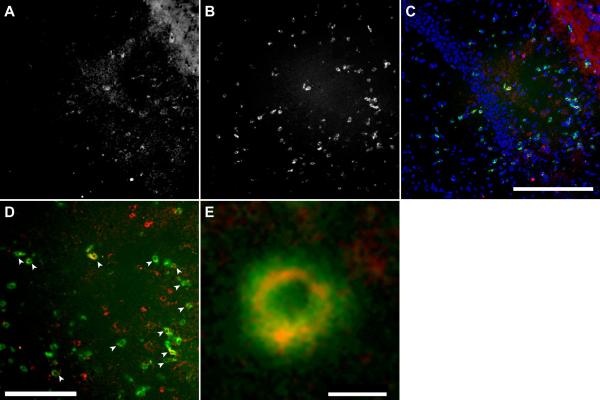
Hippocampal sections were stained with anti-NKG2D (A) and anti-CD8 (B). Colocalization (C–E) confirmed the presence of CD8+ (green) and NKG2D-positive (red) cells in the hippocampus at 7 dpi. DAPI is shown in blue in (C). Arrowheads in (D) indicate cells that are double-positive for CD8 and NKG2D. A representative double-positive cell is shown in (E). Scale bar in C is 200 microns and refers to A–C; scale bar in D is 100 microns; scale bar in E is 5 microns.
In order to determine the viral specificity of the NKG2D-positive CTLs at 7 dpi we co-stained BILs with anti-CD8, anti-NKG2D, and the TMEV VP2121–130-specific tetramer (Beckman Coulter Immunomics, San Diego, CA; 1:100) (Howe et al, 2007). Flow cytometry revealed that 74 ± 2% of CD45hiCD8+ cells were VP2-specific (Figure 1G), while none of these cells were stained by an irrelevant Db/E7 tetramer (data not shown) (Mendez-Fernandez et al, 2005). Moreover, 81 ± 2% of the VP2-specific CTLs were also NKG2D-positive (Figure 1H), while only 12 ± 2% of the VP2 tetramer-negative CTLs were positive for NKG2D (Figure 1I) (n=4 mice; mean ± 95% CI). We conclude that a robust population of NKG2D-positive VP2-specific CTLs are present within the brain at 7 days after infection with TMEV.
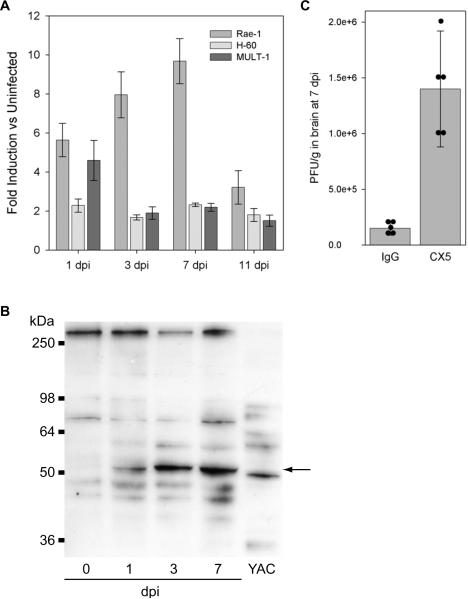
Others have reported the upregulation of NKG2D ligands following infection in a variety of peripheral tissues (Eagle and Trowsdale, 2007). However, the upregulation of these ligands by picornavirus infection has not been previously explored. We found that Rae1, Mult1, and H60 were all upregulated within the hippocampus during acute TMEV infection as determined by RTPCR analysis of RNA isolated from the excised hippocampus (see Table 1 for conditions). Of the three ligands we analyzed, Rae1 showed the most robust upregulation, with a peak induction of 10 ± 1 fold over the uninfected hippocampus at 7 dpi (F(4,9)=4.845, P=0.017 by one-way ANOVA; q(9,5)=5.841, P=0.012 with the SNK pairwise comparison between 0 and 7 dpi; mean ± 95% CI) (Figure 3A). H60 and Mult1 were also upregulated 2 ± 0.1 fold and 2 ± 0.2 fold over uninfected, respectively, at 7 dpi (n=3 mice; mean ± 95% CI) (Figure 3A).
Table 1.
RT-PCR conditions for NKG2D ligands.
| Gene | Forward Primer | Reverse Primer | UPL Probe |
|---|---|---|---|
| Rae-1γ | ATACACCAACGGGCTGGAT | CTTCGCTTCATACCAGAGAGG | cccagcag |
| H-60 | ACAGCATAGCATCTACTTTTATCCAC | TCCATGGCACTGCTGTTATC | cctggaga |
| Mult-1 | AGCTCATGTTGCACTGGAAA | TCATCAAGGTACTGAAAGATCCTG | tctggagc |
| GAPDH | AGCTTGTCATCAACGGGAAG | TTTGATGTTAGTGGGGTCTCG | catcacca |
Excised hippocampus was processed for RNA purification using the Qiagen RNeasy Lipid-tissue kit. cDNA was prepared using the Roche 1st Strand cDNA Synthesis kit and analyzed using the Roche Universal Probe Library taqman probes identified above and the Roche TaqMan Master kit. Samples were amplified for 45 cycles using: 95°C melt for 10 sec, 55°C anneal for 30 sec, and 72°C elongate for 5 sec. Primers are given as 5'-to-3'.
Figure 3.
NKG2D ligands are upregulated in the hippocampus during acute infection with TMEV and interference with NKG2D recognition of these ligands increases viral load in the CNS. qRTPCR analysis of brain mRNA revealed a 10-fold upregulation of Rae1 at 7 dpi (A) as compared to the uninfected brain. Likewise, H60 and Mult1 were also upregulated throughout the acute phase of infection (A). Western blot analysis of hippocampal protein lysates showed the translational upregulation of H60, with peak expression at 7 dpi (B). The 50 kDa band (arrow) upregulated in hippocampus was also present in YAC-1 cell lysates (B). Treatment with the CX5 anti-NKG2D function blocking antibody throughout the acute phase of infection resulted in a 10-fold increase in viral load within the brain at 7 dpi (C). Bar graph shows mean ± 95% confidence interval. Five mice were treated in each group and individual viral titers are shown as black circles in (C). All CX5-treated mice showed increased viral titer.
In order to identify the cellular locus of NKG2D ligand expression in the hippocampus we attempted, unsuccessfully, to immunostain fresh frozen or paraffin-embedded sections of hippocampus after fixation with paraformaldehyde, methanol, or ethanol:acetone. We tested specific anti-Rae1 (clone 186107; 1:25; R&D Systems) and anti-H60 (clone 205326; 1:100; R&D Systems) antibodies as well as a pan-specific NKG2D ligand antibody (goat anti-Rae1; 1:100; Santa Cruz Biotechnology) and the pan-specific NKG2D-Fc chimeric molecule (R&D Systems 139-NK; 1:10). Likewise, we attempted to confirm the expression of the NKG2D ligands at the protein level within the hippocampus by western blot analysis of hippocampal lysates. Of the numerous antibodies tested (same as immunostaining, above), only the anti-H60 antibody (clone 205326; 1:100; R&D Systems) successfully identified a prominent band that was present within the hippocampus only after infection and which was also present in lysate from the YAC-1 NK cell target line (Figure 3B). The apparent molecular weight of this prominent band (ca. 50 kDa) matches that described by Hasan and colleagues (Hasan et al, 2005). The relative difference in mobility between the putative H60 band in the hippocampal lysate and the YAC-1 cell lysate may reflect differential expression of one of the three recently identified isoforms of H60 that share high sequence homology but differ in molecular weight (Takada et al, 2008). We conclude that NKG2D ligands such as H60 are upregulated at both the transcriptional and translational level within the hippocampus following acute TMEV infection, but we are unable to identify the cells that express these ligands with the currently available immunologic tools.
Finally, we asked whether interruption of NKG2D-mediated recognition of NKG2D ligands was sufficient to alter viral load within the hippocampus. We used the CX5 function blocking antibody following a therapeutic regime adapted from Lanier and colleagues (100 ug/mouse i.p. on −1, +1, and +4 dpi; kindly provided by Dr. Lanier, UCSF) (Ogasawara et al, 2003). Control mice received an equivalent dose of polyclonal rat IgG (Kim et al, 2007). Plaque assay (Pavelko et al, 1998) of freshly excised brain homogenates at 7 dpi revealed that CX5 treatment led to 9 ± 2 times more infectious virus as compared to control IgG-treated mice (U(5,5)=15, P=0.008, Mann-Whitney rank sum test; mean ± 95% CI) (Figure 3C). Based on these observations, we propose that VP2-specific CTLs expressing NKG2D recognize NKG2D ligands such as Rae1 and H60 expressed on infected cells within the brain and thereby engage an enhanced cytotoxic response (Markiewicz et al, 2005) that contributes to the clearance of picornaviruses from the CNS.
In addition to expression on activated CTLs, NKG2D is also a primary cytotoxicity receptor for NKCs and NKC activity is certainly modulated by viral infection. We chose to specifically focus on the role of NKG2D on antiviral CTLs because the number of NKCs present within the brain during the peak of viral clearance is low compared to CTLs (ca. 10% of CD45hi cells are NK1.1-positive at 7 dpi; data not shown). However, we certainly cannot rule out a role for NKC-mediated recognition of NKG2D ligands on infected neural cells as part of an early response required for viral clearance. Likewise, NKG2D is expressed by subpopulations of CD4 T cells (Saez-Borderias et al, 2006), γδ T cells (Dandekar et al, 2005), and macrophages (Baba et al, 2006), suggesting that mutliple cells types may be inhibited by CX5 treatment. Regardless, our observations provide evidence that NKG2D-mediated recognition of virus-infected targets by CTLs participates in the clearance of picornaviruses from the CNS. These findings extend previous studies regarding the immunovirology of NKG2D ligand expression which have largely focused on viral evasion of immune surveillance (Lodoen et al, 2003; Lodoen et al, 2004; Vilarinho et al, 2007) and add to the potential repertoire of therapeutic strategies for reducing and controlling CNS picornaviral infections (Buenz and Howe, 2006; Buenz et al, 2006).
Acknowledgements
We are grateful to Reghann LaFrance-Corey for expert technical assistance and to Nikilyn Kinzel for conceptual, textual, and experimental guidance. This work was supported by a grant from the National Multiple Sclerosis Society (RG3636), by an early career development award from the Mayo Clinic (CLH), and by Donald and Frances Herdrich.
Abbreviations
- CNS
central nervous system
- TMEV
Theiler's murine encephalomyelitis virus
- CTL
cytotoxic T lymphocyte
- NKG2D
natural-killer group 2, member D
- NKC
natural killer cell
- Rae1
retinoic acid early transcript 1
- H60
minor histocompatibility protein 60
- Mult1
murine UL16-binding-protein-like transcript 1
- MHC
major histocompatibility complex
- BILs
brain-infiltrating leukocytes
- dpi
days postinfection
References
- Baba T, Ishizu A, Iwasaki S, Suzuki A, Tomaru U, Ikeda H, Yoshiki T, Kasahara M. CD4+/CD8+ macrophages infiltrating at inflammatory sites: a population of monocytes/macrophages with a cytotoxic phenotype. Blood. 2006;107:2004–12. doi: 10.1182/blood-2005-06-2345. [DOI] [PubMed] [Google Scholar]
- Buenz EJ, Howe CL. Picornaviruses and cell death. Trends Microbiol. 2006;14:28–36. doi: 10.1016/j.tim.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Buenz EJ, Rodriguez M, Howe CL. Disrupted spatial memory is a consequence of picornavirus infection. Neurobiol Dis. 2006;24:266–73. doi: 10.1016/j.nbd.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Dandekar AA, O'Malley K, Perlman S. Important roles for gamma interferon and NKG2D in gammadelta T-cell-induced demyelination in T-cell receptor beta-deficient mice infected with a coronavirus. J Virol. 2005;79:9388–96. doi: 10.1128/JVI.79.15.9388-9396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle RA, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat Rev Immunol. 2007;7:737–44. doi: 10.1038/nri2144. [DOI] [PubMed] [Google Scholar]
- Hasan M, Krmpotic A, Ruzsics Z, Bubic I, Lenac T, Halenius A, Loewendorf A, Messerle M, Hengel H, Jonjic S, Koszinowski UH. Selective down-regulation of the NKG2D ligand H60 by mouse cytomegalovirus m155 glycoprotein. J Virol. 2005;79:2920–30. doi: 10.1128/JVI.79.5.2920-2930.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CL, Bieber AJ, Warrington AE, Pease LR, Rodriguez M. Antiapoptotic signaling by a remyelination-promoting human antimyelin antibody. Neurobiol Dis. 2004;15:120–31. doi: 10.1016/j.nbd.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Howe CL, Ure D, Adelson JD, LaFrance-Corey R, Johnson A, Rodriguez M. CD8+ T cells directed against a viral peptide contribute to loss of motor function by disrupting axonal transport in a viral model of fulminant demyelination. J Neuroimmunol. 2007;188:13–21. doi: 10.1016/j.jneuroim.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Mohindru M, Kang MH, Fuller AC, Kang B, Gallo D, Kim BS. Differential virus replication, cytokine production, and antigen-presenting function by microglia from susceptible and resistant mice infected with Theiler's virus. J Virol. 2007;81:11690–702. doi: 10.1128/JVI.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Chang CK, Hayden T, Liu FC, Benjamin J, Hamerman JA, Lanier LL, Kang SM. The activating immunoreceptor NKG2D and its ligands are involved in allograft transplant rejection. J Immunol. 2007;179:6416–20. doi: 10.4049/jimmunol.179.10.6416. [DOI] [PubMed] [Google Scholar]
- Lodoen M, Ogasawara K, Hamerman JA, Arase H, Houchins JP, Mocarski ES, Lanier LL. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J Exp Med. 2003;197:1245–53. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodoen MB, Abenes G, Umamoto S, Houchins JP, Liu F, Lanier LL. The cytomegalovirus m155 gene product subverts natural killer cell antiviral protection by disruption of H60-NKG2D interactions. J Exp Med. 2004;200:1075–81. doi: 10.1084/jem.20040583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz MA, Carayannopoulos LN, Naidenko OV, Matsui K, Burack WR, Wise EL, Fremont DH, Allen PM, Yokoyama WM, Colonna M, Shaw AS. Costimulation through NKG2D enhances murine CD8+ CTL function: similarities and differences between NKG2D and CD28 costimulation. J Immunol. 2005;175:2825–33. doi: 10.4049/jimmunol.175.5.2825. [DOI] [PubMed] [Google Scholar]
- Mendez-Fernandez YV, Hansen MJ, Rodriguez M, Pease LR. Anatomical and cellular requirements for the activation and migration of virus-specific CD8+ T cells to the brain during Theiler's virus infection. J Virol. 2005;79:3063–70. doi: 10.1128/JVI.79.5.3063-3070.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Fernandez YV, Johnson AJ, Rodriguez M, Pease LR. Clearance of Theiler's virus infection depends on the ability to generate a CD8+ T cell response against a single immunodominant viral peptide. Eur J Immunol. 2003;33:2501–10. doi: 10.1002/eji.200324007. [DOI] [PubMed] [Google Scholar]
- Myoung J, Hou W, Kang B, Lyman MA, Kang JA, Kim BS. The immunodominant CD8+ T cell epitope region of Theiler's virus in resistant C57BL/6 mice is critical for anti-viral immune responses, viral persistence, and binding to the host cells. Virology. 2007;360:159–71. doi: 10.1016/j.virol.2006.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, Pertel T, Carnaud C, Bluestone JA, Lanier LL. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- Pavelko KD, van Engelen BG, Rodriguez M. Acceleration in the rate of CNS remyelination in lysolecithin-induced demyelination. J Neurosci. 1998;18:2498–505. doi: 10.1523/JNEUROSCI.18-07-02498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- Rotbart HA. Clinical significance, diagnosis, and treatment of picornavirus infections. In: Semler BL, Wimmer E, editors. Molecular Biology of Picornaviruses. ASM Press; Washington, DC: 2002. pp. 357–365. [Google Scholar]
- Rubio N, Sanz-Rodriguez F, Lipton HL. Theiler's virus induces the MIP-2 chemokine (CXCL2) in astrocytes from genetically susceptible but not from resistant mouse strains. Cell Immunol. 2006;239:31–40. doi: 10.1016/j.cellimm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Saez-Borderias A, Guma M, Angulo A, Bellosillo B, Pende D, Lopez-Botet M. Expression and function of NKG2D in CD4+ T cells specific for human cytomegalovirus. Eur J Immunol. 2006;36:3198–206. doi: 10.1002/eji.200636682. [DOI] [PubMed] [Google Scholar]
- Takada A, Yoshida S, Kajikawa M, Miyatake Y, Tomaru U, Sakai M, Chiba H, Maenaka K, Kohda D, Fugo K, Kasahara M. Two Novel NKG2D Ligands of the Mouse H60 Family with Differential Expression Patterns and Binding Affinities to NKG2D. J Immunol. 2008;180:1678–85. doi: 10.4049/jimmunol.180.3.1678. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Libbey JE, Kobayashi-Warren M, Fujinami RS. IFN-gamma production and astrocyte recognition by autoreactive T cells induced by Theiler's virus infection: role of viral strains and capsid proteins. J Neuroimmunol. 2006;172:85–93. doi: 10.1016/j.jneuroim.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0708968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y, Fujinami RS. Viral infection and dissemination through the olfactory pathway and the limbic system by Theiler's virus. Am J Pathol. 1993;143:221–9. [PMC free article] [PubMed] [Google Scholar]