Abstract
In addition to its role in cellular development and proliferation, there are emerging in vitro data implicating the Wnt/β-catenin pathway in synaptic plasticity. Yet in vivo studies have not examined if Wnt activity is required for learning and memory. In the amygdala during fear memory formation, we found that many Wnt-signaling genes were dynamically regulated, with an immediate decrease, followed by an eventual normalization during memory consolidation. This rapid decrease in Wnt mRNA was confirmed with individual quantitative PCR and in situ hybridization. We then manipulated Wnt signaling with a specific peptide antagonist (Dkk-1) or agonist (Wnt1) injected stereotaxically into the adult amygdala during fear learning. We found that neither manipulation had an effect on locomotion, anxiety, fear acquisition or fear expression. However, both Wnt modulators prevented long-term fear memory consolidation without affecting short-term memory. Dkk-1 and Wnt infusions had destabilizing, but opposite, effects on the requisite β-catenin/cadherin dynamic interactions that occur during consolidation. These data suggest that dynamic modulation of Wnt/β-catenin signaling during consolidation is critical for the structural basis of long-term memory formation.
Introduction
Wnt/β-catenin signaling is important for a variety of cellular processes including development, cell proliferation, cell fate, and motility (Moon et al., 2004; Lee et al., 2008). Wnt proteins constitute a large family of secreted molecules that can bind to several distinct receptors, activating different signaling pathways, including the β-catenin pathway. When Wnts bind to a member of the Frizzled family of cell surface receptors, the transmembrane lipoprotein receptor related proteins 5 and 6 (LRP5/6) are recruited, and the cytoplasmic protein Dishevelled (Dvl) is activated (Bhanot et al., 1996). Dvl activation leads to the inhibition of glycogen synthase kinase-3 (GSK3), which phosphorylates β-catenin resulting in its degradation. The inhibition of β-catenin degradation increases its stability, resulting in nuclear translocation. In the nucleus, it binds to the T cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors, which regulate the expression of Wnt target genes (Logan and Nusse, 2004; Moon et al., 2004; Nelson and Nusse, 2004; Gordon and Nusse, 2006). Alternatively, β-catenin also complexes with the cytoplasmic domain of cadherin. Upon differential phosphorylation, the β-catenin/cadherin complex dissociates, leading to the transient destabilization of synapses required for new synapse formation (Bamji et al., 2006; Arikkath and Reichardt, 2008).
The role of Wnt and Wnt/β-catenin signaling in neuronal and synaptic development is increasingly well-established. The addition of Wnt7 to cultured cerebellar granule cells and hippocampal neurons increases axonal spreading and branching, as well as promoting presynaptic assembly and synaptic vesicle accumulation (Lucas and Salinas, 1997; Hall et al., 2000; Ahmad-Annuar et al., 2006). Furthermore, the addition of Wnt3 to sensory neurons in culture results in growth cone enlargement and increased axonal branching (Krylova et al., 2002). These data suggest that Wnts function in a retrograde manner to regulate presynaptic remodeling during the development of synapses. In adults, burgeoning evidence is building suggesting that dysregulation of Wnt signaling is associated with cognitive disorders, including schizophrenia and Alzheimer’s Disease (De Ferrari and Moon, 2006). Together, these data suggest that Wnts may play a role in adults beyond initial brain development.
Despite these findings, the functional role of the Wnt signaling pathway in adult neural circuits remains uncertain. Recent in vitro and slice physiology approaches have shown that Wnts may function in synaptic transmission and activity-dependent synaptic plasticity (Chen et al., 2006; Wayman et al., 2006). However, there is no evidence to date on the role of Wnt signaling in vivo underlying learning and memory formation in animals. We have previously shown that β-catenin signaling is required for fear memory formation in vivo, and transient dissociation of the β-catenin/cadherin complex is associated with the consolidation of new fear memories (Maguschak and Ressler, 2008). Given the evidence that β-catenin regulation is critical for memory formation, we hypothesized that Wnt regulation is required for β-catenin stability and regulation of synaptic plasticity in vivo. Here, using a variety of molecular, genetic and pharmacological manipulations, we demonstrate that dynamic Wnt/β-catenin signaling may be an important pathway regulating long-term memory formation in adults.
Materials and Methods
Animals
Adult male C57BL/6J mice (Jackson Labs) were used for all experiments. Mice were housed four per cage in a temperature-controlled (24 °C) animal colony, with ad libitum access to food and water, on a 12-h light-dark cycle, with all behavioral procedures done during the light cycle. All procedures used were approved by the Institutional Animal Care and Use Committee of Emory University and in compliance with National Institutes of Health (NIH) guidelines for the care and use of laboratory animals.
Stereotaxic surgery and infusion of peptides
Mice were anesthetized by intraperitoneal (i.p.) injections of a ketamine-dormitor (medetomidine) mixture and placed in a stereotaxic apparatus. Small holes were drilled into the skull and 6 mm stainless-steel guide cannulas (Plastics One) were lowered bilaterally in to the basolateral amygdala (BLA). BLA coordinates were as follows: anteriorposterior, −1.8; dorsoventral, −4.9; mediolateral, ± 3.2 relative to bregma. Dorsoventral coordinates were measured from the skull surface with the internal cannula extending 2 mm beyond the end of the guide cannula. Coordinates were based on the mouse brain atlas of Paxinos and Watston (Paxinos and Watston, 2003). The guide cannula was fixed to the skull using dental acrylic and jeweler’s screws and dummy cannulas (Plastics One) were inserted into each guide cannula to prevent clogging. All animals were allowed to recover for 7 days before testing. During this time, mice were handled daily for acclimation and inspection of cannula fixture.
Animals were infused with either Dickkopf1 (R&D Systems) (100 ng/side), Wnt1 (AbD Serotec) (100 ng/side), or PBS (vehicle). 1.0 μl infusions were made using an injection cannula (33 gauge cannula, Plastics One), which extended 2.0 mm beyond the tip of the guide cannula. Peptide was delivered manually with a 5 μl Hamilton syringe attached to the injection cannula via polyethylene tubing (PE-10). Administration of a volume of 1.0 μl/side was delivered over a period of 60 s by slowly turning the microsyringe plunger. After each infusion, the injection cannula was allowed to remain for 2 m. Visualization of injection sites was performed post-mortem to verify the location of peptide infusion.
Schematic diagrams of extent of infusions were performed on Dkk-1 infused mice. Following all behavioral studies, mice were re-injected with 1.0 μl Dkk-1 30 m prior to perfusion with 4% paraformaldehyde to observe the likely extent of infusion during the behavioral studies. After fixation, brains were equilibrated in 30% sucrose, sectioned on a cryostat, and then processed for immunohistochemistry as outlined below. Sections were examined with a 4x objective on a light microscope and extent of Dkk-1 staining was drawn on Paxinos & Franklin (2001) mouse amygdala coordinates, as illustrated in Figure 3a.
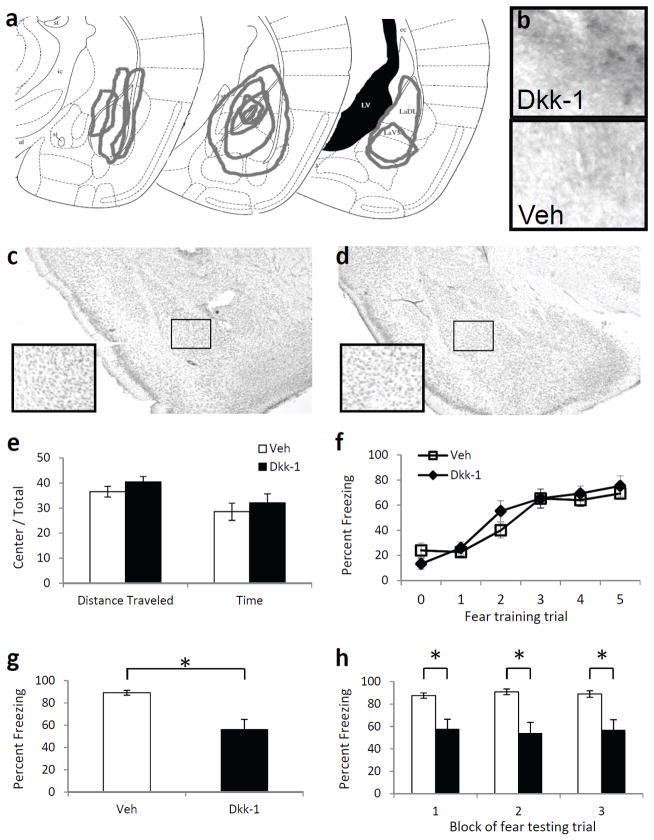
Figure 3. The effect of Dkk-1 in the amygdala on fear learning and memory.
(a) Schematic diagram, based on Paxinos & Franklin (2001), illustrating the extent of infusions across animals via stereotaxic cannulae into the amygdala based on immunohistochemistry. (b) 10× light microscopy of basolateral amygdala regions following immunohistochemical analyses of Dkk-1 in animals receiving bilateral amygdala injections of either Dkk-1 or vehicle. (c,d) Cresyl violet staining of sections showing the absence of damage in Dkk-1 (c) or vehicle (d) infected regions. (e) Activity measures of mice receiving injections of vehicle or Dkk-1 and placed in an open field apparatus for 10 m. (f) Acquisition curve showing percent time spent freezing during each tone prior to footshock presentation. (g) Percent time spent freezing when tested 48 h after fear conditioning, and presented with 15 tone presentations. (h) Freezing data from (g), presented in 3 blocks of 5 tone presentations. n = 10 per group. Mean ± s.e.m. * P < 0.01, significant differences (two-tailed t-tests).
Immunoprecipitation and Immunoblotting
After behavioral procedures, brains were blocked rapidly and kept frozen at −80 °C. Bilateral amygdala punches were obtained and homogenized. For immunoprecipitation experiments, solubilized proteins were incubated with Protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology) and centrifuged. Protein A/G PLUS-Agarose beads and antibody to β-catenin (1:200, Cell Signaling) were then added to the supernatant of each protein sample, incubated overnight at 4 °C, and washed prior to western blot analyses.
For immunoblotting, twenty five micrograms of protein per mouse were electrophoretically separated on SDS-PAGE, transferred onto nitrocellulose membranes (BioRad), blocked for 1 h in 2% nonfat dry milk, 0.1% Tween 20, 50 mM NaCl, 10 mM HEPES, pH 7.4. Membranes were incubated in primary antibody overnight at 4 °C. Antibodies to the following proteins were used: β-catenin (1:500, BD Biosciences), pan-cadherin (1:1000, Cell Signaling). Membranes were washed and incubated with a horseradish peroxidase-labeled secondary antibody (1:5000, Vector), then detected by SuperSignal West Chemiluminescence (Pierce) in an Alpha Innotech Fluorchem imaging system (Alpha Innotech).
Immunohistochemistry
Brain sections (40 μm) were blocked with 0.5 M PBS and 0.5 % Triton X-100, and incubated in a 1:100 dilution of primary Dkk-1 (Santa Cruz) or Wnt1 (Santa Cruz) antibodies overnight at 4 °C. Sections were then washed with PBS and bathed in a 1:500 dilution of secondary antibody for 2 hr. Avidin-biotin complexes were amplified using a standard Vectastain Elite ABC kit and visualized with diaminobenzidine (DAB) peroxidase staining.
Behavioral studies
Open Field Behavior
The open field consisted of a box (27.9 cm × 27.9 cm) made of Plexiglass. The mice were placed in the periphery of the arena at the start of the 10-m test period. At the end of the test, the animal was returned to its home cage. The anxiolytic-like effects were evaluated by computing percentage of time mice spent in the central zone of the open field. The central zone was defined as the central compartment of the floor centrally located 6 cm from the perimeter of the chamber walls. All testing was conducted under standard room lighting. Activity data was obtained and analyzed using the Open Field Activity Software (Med Associates Inc.)
Fear Conditioning Apparatus
Mice were fear conditioned in eight identical startle response systems (SR-LAB, San Diego Instruments). Each system consisted of a nonrestrictive Plexiglas cylinder (5.5 cm in diameter and 13 cm long) mounted on a Plexiglas platform and located in a ventilated, sound-attenuated chamber. Cylinder movements were sampled each millisecond by a piezoelectric accelerometer mounted under each platform. The footshock unconditioned stimulus (US) was generated by a programmable animal shocker (San Diego Instruments) located outside the isolation chambers and was delivered through the cage floor bars. The conditioned stimulus (CS) was a tone delivered by a speaker located about 15 cm above the chambers. Sound intensities were measured by an audiometer (Radio Shack). Stimuli presentation and data acquisition were controlled, digitized, and stored by a Dell computer using SR-LAB software.
Fear Conditioning
After five days of exposure to the conditioning chambers, mice were placed in the chamber, and after 5 m presented with five tone-shock pairings at an inter-trial interval (ITI) of 5 m. Each pairing consisted of a 30-s tone (6 kHz, 85 db, CS) that terminated with a 0.5 s footshock (1.0 mA, except where noted; US). Freezing in startle-reflex chambers during fear acquisition was assessed as described previously (Maguschak & Ressler, 2008). Forty-eight hours after training, mice were tested for freezing in a separate context: Med Associates rodent modular test chambers with an inside volume of 30.5 cm × 24.1 cm × 21.0 cm. Three minutes later, 15 conditioned stimulus tones (6 kHz, 85 db) with an ITI of 1.5 m were delivered through a high-frequency speaker attached to the side of each chamber. Percentage time spent freezing during the conditioned stimulus presentation was calculated for each mouse using FreezeFrame video monitoring software (ACT-100; Coulbourn Instruments), using settings which were previously calibrated to levels of observed freezing. For the short-term memory test, mice were trained as above, with peptide infused (1.0 μl infusions bilaterally targeting lateral/basolateral amygdala) 15 m prior to fear training. Mice were then tested for freezing in the presence of 5 tones at 1 h after training. For the long-term memory expression test, mice were trained as above with a 12 kHz tone, and 48 hours later were tested for fear expression with 30 tones. Fifteen minutes prior to fear testing, mice were infused with 1.0 μl vehicle, Dkk-1, or Wnt1.
RNA extraction and cDNA synthesis
RNA isolation was carried out using the Qiagen RNeasy Micro Kit for animal tissues (Qiagen). In short, amygdala punches were lysed and then homogenized. After centrifugation, ethanol was added to the lysates, and the samples were loaded onto the column, followed by DNase treatment. The columns were then washed to remove DNase and any other contaminants, and the remaining pure, concentrated RNA was eluted in RNase-free water. 1.0 μg of total RNA was reverse transcribed in a final reaction mix of 20 μl using RT2 First Strand Kit (SuperArray Bioscience) according to manufacturer’s instructions. cDNA was diluted by adding RNase free water.
Real-time PCR
Real-time PCR was performed using 2×SuperArray RT qPCR Master Mix (SA Biosciences) and RT2 Profiler™ PCR Array System (PAMM-043, SA Biosciences) or mouse Wnt1 PCR primer stock (SA Biosciences). The Wnt Pathway array consisted of 84 genes related to Wnt-mediated signal transduction, along with appropriate RNA quality controls, in 96-well plates. For each array, amygdala samples from each experimental group were pooled together. For the Wnt1 PCRs, amygdala samples were analyzed individually. Thermal cycling parameters were 10 m at 95 °C, followed by 40 cycles of amplifications for 15 s at 95 °C, 1 m at 60 °C. A dissociation stage, consisting of 15 s at 95 °C, 1 m at 60 °C, and 15 s at 95 °C, was added at the end. Quantification of mRNA was performed using the Applied Biosystems 7500 Real-Time PCR System. Relative levels of mRNA expression were normalized in all the samples with expression levels of glyceraldehyde-3-phosphate dehydrogenase(GAPDH). For the Wnt1 PCRs, the ΔΔCts were compared across groups. Figure 3A shows the mean +/− s.e.m. of the ΔΔCt values, and Figure 3B shows the mean fold change following previously described methods (Livak and Schmittgen, 2001).
In situ hybridization
In situ hybridization was performed and quantified as previously described (Ressler et al., 2002; Maguschak and Ressler, 2008). The Wnt1 clone was obtained by inserting the 144 bp Wnt1 RT PCR product from above into the pCR2.1-TOPO vector (Invitrogen). After sequence verification, the cloned cDNA was linearized and antisense riboprobes were generated using T7 RNA polymerase and [35S]-UTP in the reaction. After a stringent wash protocol, slides were apposed to Biomax MR autoradiography film (Eastman Kodak Co.). Hybridization density of Wnt1 mRNA in the BLA was assessed using the mean luminosity function of Adobe Photoshop.
Data analysis
Statistically significant differences were determined by Student’s t-test or ANOVA, with post hoc least-squares difference tests for multiple comparisons. The results are presented as mean ± s.e.m.
RESULTS
Genes in Wnt-mediated signaling are altered during memory formation
We first wished to examine whether Wnt-related genes showed dynamic alterations in conjunction with memory formation. Thus we performed a series of mRNA expression arrays focusing on 84 Wnt-related genes, to identify if any genes were endogenously regulated within the amygdala during the memory consolidation period following fear learning.
Following five days of habituation to the conditioning chambers, behaviorally naive mice received five tone-shock pairings. A context control group was placed in the conditioning chambers for the same amount of time, but no stimuli were presented. We collected brains from the control mice 2 h after context exposure; brains from the trained mice were collected immediately, 0.5, 2, 4, 12, or 24 h after conditioning.
Bilateral amygdala from the above mice were dissected and RNA was isolated. Equal amounts of RNA from each animal were then pooled into seven groups, one group for each time point, including context control. Total RNA was converted to cDNA, and the samples were then subjected to gene expression analysis using rtPCR arrays containing genes involved in Wnt signaling, as well as housekeeping genes.
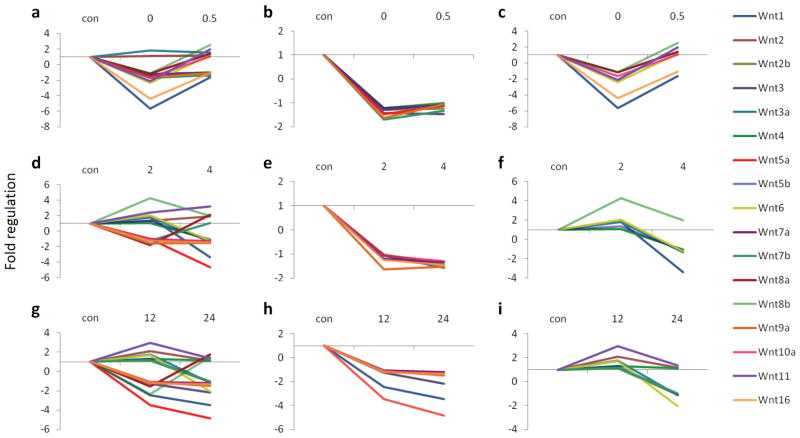
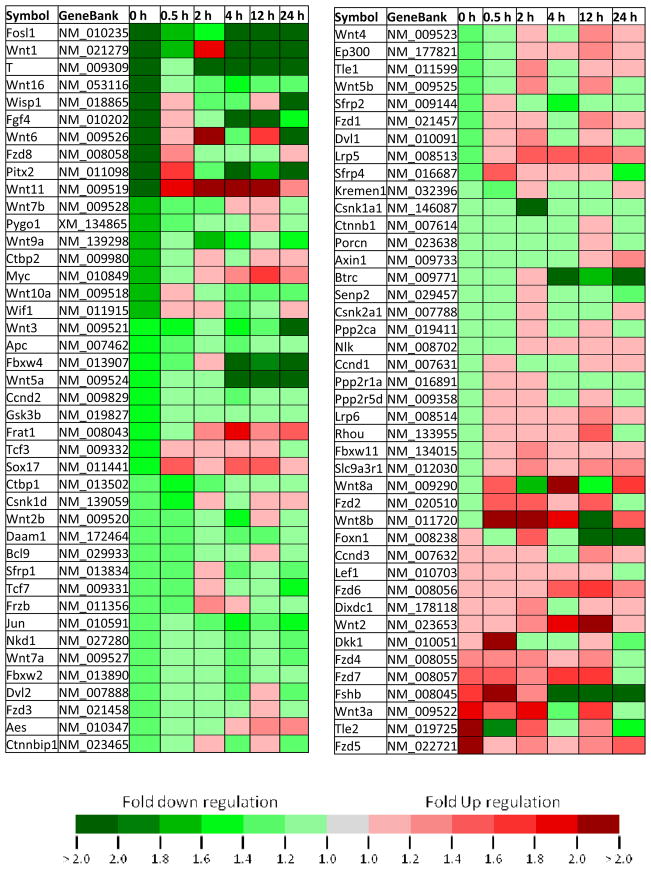
The results from the PCR arrays showed multiple patterns and amplitudes of modulation for the expression of Wnt genes following learning (Table 1). We did not find any changes in the housekeeping genes across groups. We found that 15 out of 17 Wnts (and over 50 of all Wnt-related genes examined) decrease relative to control, immediately following fear conditioning (Fig. 1A and Table 1). Furthermore, the expression patterns for most of the Wnts could be assigned to one of two categories at the 0 and 0.5 h time points, the 2 and 4 h time points, and the 12 and 24 h time points (Fig. 1). Together these data suggest that transcriptional regulation or mRNA stability of Wnt genes are highly correlated with new memory formation within the amygdala. Furthermore, the majority of Wnts show a rapid decrease in expression during the very early phase of fear memory consolidation, followed by a more variable return to normal expression levels over time.
Table 1.
Temporal changes, expressed as fold regulation, in the expression of 84 genes related to Wnt-mediated signaling following fear conditioning.
|
Figure 1. Temporal changes in Wnt gene expression after fear conditioning.
All genes are expressed as fold up or down regulation from context exposed animals. (a) The expression of 17 Wnt genes at 0 and 0.5 h after conditioning. (b) 8 of the 17 Wnt genes go down immediately after conditioning, and stay down at 0.5 h (c) 7 of the Wnt genes go down immediately after conditioning, and go back up at 0.5 h. (d) The expression of 17 Wnt genes at 2 and 4 h after conditioning. (e) 6 of the Wnts go down at 2 h after conditioning and stay down at 4 h. (f) 6 of the Wnts either stay the same or go up at 2 h and then decrease at 4 h post conditioning. (g) The expression of Wnt genes at 12 and 24 h after conditioning. (h) 7 of the Wnt genes go down at 12 h and stay down at 24 h. (i) 8 of the Wnt genes either stay the same or go up at 12 h and down at 24 h.
Wnt1 gene expression changes with learning
We first replicated the initial finding above by performing quantitative real-time PCR (qPCR) on the Wnt-related genes that were most altered with fear conditioning. Among all the genes examined in the PCR array and the follow-up qPCR study, Wnt1 showed the greatest fold change following fear conditioning. Therefore, we wanted to confirm and replicate this initial finding in an additional cohort of animals. We then performed another experiment with a new series of naive mice using qPCR specifically focused on the Wnt1 gene. After five days of habituation to the conditioning chambers, mice were either exposed to the context alone, to unpaired tone and shock presentations, or to five paired tone and shock presentations. The mice were then sacrificed as above. RNA from both amygdala of each mouse was prepared, and then analyzed by qPCR. We found that the pattern of expression from the qPCR results closely resembled the pattern observed from the PCR array (Fig. 2A,B). More specifically, we observed a significant decrease in Wnt1 gene expression immediately following fear conditioning, compared to animals exposed to the context, without any stimuli presentations (ANOVA, F7,83 = 3.18, P < 0.01. Posthoc pairwise comparisons: 0 h is significantly decreased from context, unpaired, 2, 4, 12, and 24 h). This effect does not appear to be due to the presentation of the tone or the stress of shock alone, since the unpaired group did not differ significantly from the context group (P > 0.1).
Figure 2. Wnt1 mRNA expression in the amygdala.
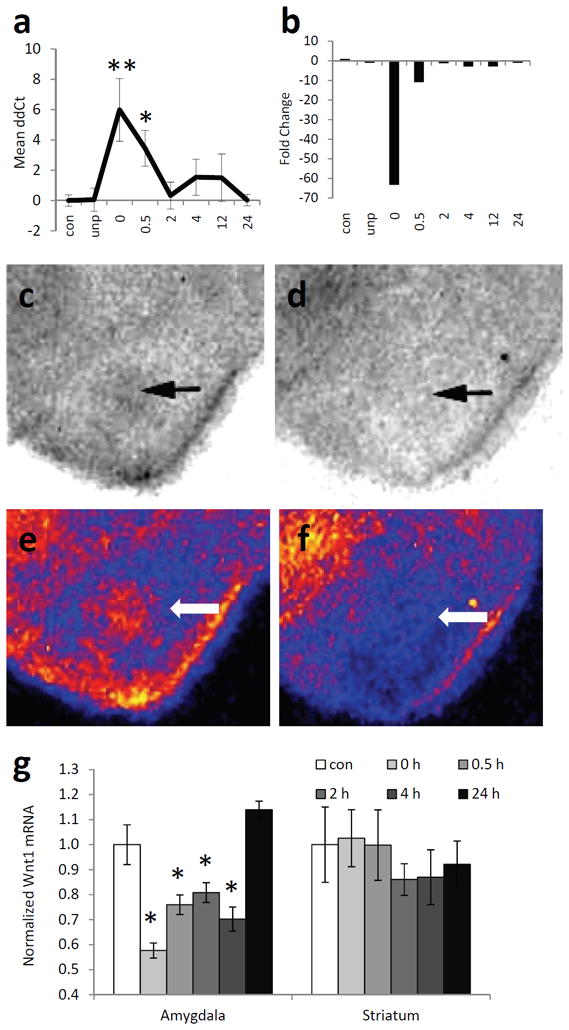
(a) Quantitative RT-PCR data showing the mean ΔΔCt values ± s.e.m. across different conditions. Note that at the 0 (immediate) timepoint post-fear conditioning, detecting the Wnt mRNA signal took on average 6 PCR cycles more than in the control and unpaired conditions. (b) The same data as in (a) presented as mean fold up or down regulation of Wnt1 mRNA compared to context-exposed mice. n = 14 for context, 7 for unpaired, 11 for 0 and 0.5 h, 12 for 2, 4, 12, and 24 h. * P < 0.05, ** P < 0.01, ANOVA with LSD posthoc analyses. (c,d) Autoradiography images of 35S-labeled Wnt1 riboprobe hybridized, in situ, to amygdala from mice sacrificed immediately after training (d) or after context exposure (c). (e,f) Pseudocolored images of (c) and (d), respectively. Yellow, highest expression; blue-black, lowest expression. Arrows point to amygdala. (g) Relative expression of Wnt1 mRNA in the amygdala and striatum, normalized to expression in context-exposed mice. n = 8 per group. Mean ± s.e.m., *P < 0.01, ANOVA with post hoc analyses.
To further validate our Wnt1 results, we fear conditioned a separate group of animals, and sacrificed the mice at the same time points as above. This time, however, we analyzed the expression of Wnt1 by in situ hybridization (Fig. 2C-F). This method allowed us to look more specifically at the regional expression pattern of Wnt1, focusing on the basolateral amygdala (BLA). Again, we found that Wnt1 mRNA in the BLA was altered with fear conditioning, similar to the pattern observed in both the array and the qPCR (F5,47 = 18.36, P < 0.01, Fig. 2G). Post hoc least-squares difference analyses indicated a significant decrease in Wnt1 mRNA expression immediately after, 0.5, 2, and 4 h after fear conditioning (P < 0.01) compared to context animals and animals sacrificed 24 h after conditioning. We did not find any significant differences in Wnt1 mRNA in the striatum (P > 0.1), a brain region not involved in cue-dependent fear conditioning. Interestingly, the pattern that was observed was remarkably similar to the β-catenin/cadherin destabilization time course that we have previously reported (Maguschak and Ressler, 2008), suggesting that Wnt1 regulation within the amygdala during fear consolidation may be highly correlated with the previous β-catenin effects we found to be required for normal memory formation.
Preventing Wnt mediated signaling impairs memory formation
Given the above data on the importance of dynamic Wnt regulation, immediate decreases followed by normalization, we sought to examine the role of the Wnt/β-catenin pathway in the amygdala of adult mice. We used Pavlovian cue-dependent fear conditioning as a model of learning and memory which is highly dependent on the amygdala. We first wished to determine if blocking the Wnt/β-catenin pathway would affect memory formation. Dickkopf-1 (Dkk-1) is a known extracellular antagonist of the Wnt pathway (Diep et al., 2004). It is expressed at low levels in the adult brain, but when present, promotes the internalization of LRP6, thereby, preventing Wnt from binding to its co-receptor.
In order to examine the effect of deregulating normal Wnt signaling in the amygdala, cannula were bilaterally implanted in the basolateral amygdala, and either Dkk-1 (100 ng/side) or saline were infused into mice. The presence and spread of the Dkk-1 peptide was verified by immunohistochemistry (Fig. 3A,B), and there did not appear to be any histological abnormalities as assessed by Nissl stain (Fig. 3C,D) due to the infusion.
After confirming that we could increase the expression of Dkk-1 within the amygdala, we first examined whether Dkk-1 affected baseline emotional behavior or locomotion. We injected Dkk-1 prior to open-field behavior, and found that there was no difference (P > 0.1) between Dkk-1 on mouse locomotion or anxiety-related behavior as measured by distance traveled and time spent in the center compared to time in the surround (Fig. 3E).
We then tested whether the presence of this peptide interfered with memory formation. After 5 days of habituation to the conditioning chambers to minimize context effects, mice received infusions of either Dkk-1 or saline bilaterally into the amygdala, and were fear conditioned 15 minutes after infusion with five tone-shock pairings. Throughout the training paradigm, we measured freezing behavior during each tone presentation (conditioned stimulus) before the presentation of footshock. We found a significant main effect of time across all mice (F 5,90 = 35.75, P < 0.01, Fig. 3F); however, there was no main effect of treatment (P > 0.1). These data demonstrate that the Dkk-1 peptide does not alter baseline locomotion or anxiety-like behavior, nor does it affect the acquisition of conditioned fear memory.
Forty-eight hours after cue fear conditioning, mice were placed in a different chamber and presented with 15 conditioned stimulus tones. The mean percent time spent freezing during these tones was recorded and used as a measure of conditioned fear. Mice that received Dkk-1 prior to fear training now showed significantly less fear than did mice that had received saline (t10.10 = 3.62, P < .01, Fig. 3G). This deficit in fear retention was present throughout the testing session (F 1,18 = 13.08, P < 0.01, Fig. 3H), as measured by percent time spent freezing in three blocks of five trials: block 1 (t10.43 = 3.33, P< 0.01), block 2 (t10.27 =3.76, P < 0.01), block 3 (t10.98 = 3.38, P < 0.01). Furthermore, the deficit did not seem to be caused by effects on locomotor behavior, as the mice did not show any significant differences across groups in activity level or freezing behavior before the first conditioned stimulus. These data suggest that a nonspecific manipulation that decreased Wnt functioning during or soon after fear conditioning leads to decreases in the expression of fear behavior 48 h later, consistent with a role for Wnt signaling within the amygdala in memory formation or consolidation.
Increasing Wnt1 signaling impairs fear memory formation
The mRNA expression data suggests that the transient decrease in Wnt1 expression immediately following fear conditioning is correlated with memory formation. Therefore, we examined if preventing this transient decrease by replacing Wnt1 in the amygdala would produce deficits in memory formation. First, we infused either a Wnt1 peptide (100 ng/side) or saline and found that infusions did not produce any structural damage to the amygdala, as visualized by Nissl staining (Fig. 4A,B). We next examined whether Wnt1 would affect baseline locomotion or anxiety-like behaviors. We injected Wnt1 prior to open-field behavior and found that there was no difference (P > 0.1) between Wnt1 and vehicle on mouse locomotion (distance traveled) or anxiety-related behavior (time spent in the center compared to time in the surround). (Fig. 4C).
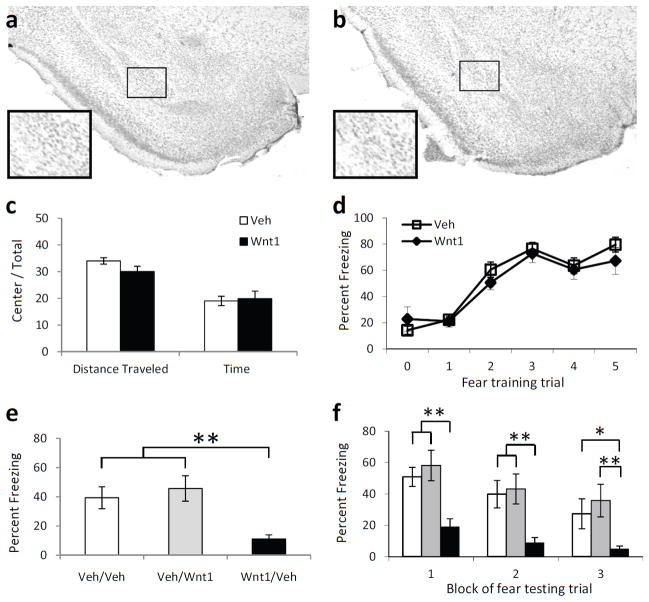
Figure 4. The effect of Wnt1 in the amygdala on fear learning and memory.
(a,b) Cresyl violet staining of parallel sections showing the absence of damage in vehicle (a) or Wnt1 (b) infected regions. (c) Activity measures of mice receiving injections of vehicle or Wnt1 and placed in an open field apparatus for 10 m. n = 13 for vehicle, n = 15 for Wnt1. (d) Acquisition curve showing percent time spent freezing during each tone prior to footshock presentation. n = 15 for vehicle, n = 23 for Wnt1. (e,f) Animals were injected with either vehicle or Wnt1 prior to training, and vehicle or Wnt1 post training (Pre-training/Post-training). (e) Percent time spent freezing when tested 48 h after fear conditioning, and presented with 15 tone presentations. (f) Freezing data from E, presented in 3 blocks of 5 tone presentations. White bars are Veh/Veh, Grey bars are Veh/Wnt1, and black bars are Wnt1/Veh animals. For both G and H, n = 8 for Veh/Veh, n = 7 for Veh/Wnt1, n = 12 for Wnt1/Veh. Mean ± s.e.m. **P < 0.01, * P < 0.05, ANOVA with post hoc analyses.
We then infused Wnt1 or saline immediately before and after fear conditioning to determine if increasing Wnt1 affects memory formation. The intensity of the unconditioned stimulus was lowered to 0.6 mA to prevent ceiling effects on fear expression. We found that all mice were able to acquire fear equally (F5,125 = 35.36, P < 0.01, Fig. 4D), irrespective of whether they had saline or Wnt1 infused into the amygdala prior to conditioning (P > 0.1). However, when tested 48 h later, there was a significant difference between animals that had received vehicle or Wnt1 prior to training (F2,26 = 10.73, P < 0.01). Post hoc analyses revealed that animals that had received Wnt1 prior to training froze significantly less compared to animals that had received either saline prior to training (P < 0.01), or Wnt1 post training (P < 0.01, Fig. 4E). This difference in fear retention across treatment was apparent throughout the testing session (F2,24 = 10.73, P < 0.01, Fig. 4F). When examined in three blocks of five tone presentations each, post hoc analyses indicated that animals receiving Wnt1 prior to training froze significantly less than animals receiving vehicle prior to- and after training during block 1 (P < 0.01), block 2 (P < 0.01) and block 3 (P < 0.05). In addition, animals receiving Wnt1 prior to training froze significantly less than animals receiving vehicle before and Wnt1 after training for all blocks (P < 0.01). These data suggest that infusing Wnt1 into the amygdala, counteracting the normal rapid decrease in Wnt1, prevents fear memory formation. If Wnt1 is given following fear conditioning, however, after this critical period of transient endogenous Wnt decrease, then normal learning occurs. Thus, the rapid decrease in endogenous Wnt1 mRNA which likely begins at the end of fear training and is observed immediately after conditioning may be critical for the formation of fear memory.
Wnt1 prevents learning-dependent disassociation between β-catenin and cadherin
We have shown that blocking Wnt signaling or preventing the Wnt1 decrease during or immediately following learning both impair memory formation. One possible explanation for this apparent discrepancy would be if the rapid decrease then normalization of Wnt signaling has equally important, but opposite effects on mechanisms of memory consolidation. To address this, we examined biochemical changes that may be occurring during consolidation at baseline and in Wnt1 and Dkk-1 treated animals.
Previous in vitro research has shown that Wnt1 increases cell-cell adhesion by promoting the binding of β-catenin to cadherin (Hinck et al., 1994). Our finding of a decrease in Wnt1 gene expression appears to be correlated with the rapid pattern of β-catenin/cadherin dissociation following learning. Since we have previously shown that the association between β-catenin and cadherin is decreased immediately following learning, we first wanted to examine if increasing Wnt1 prior to or soon after fear conditioning would prevent this observed dissociation.
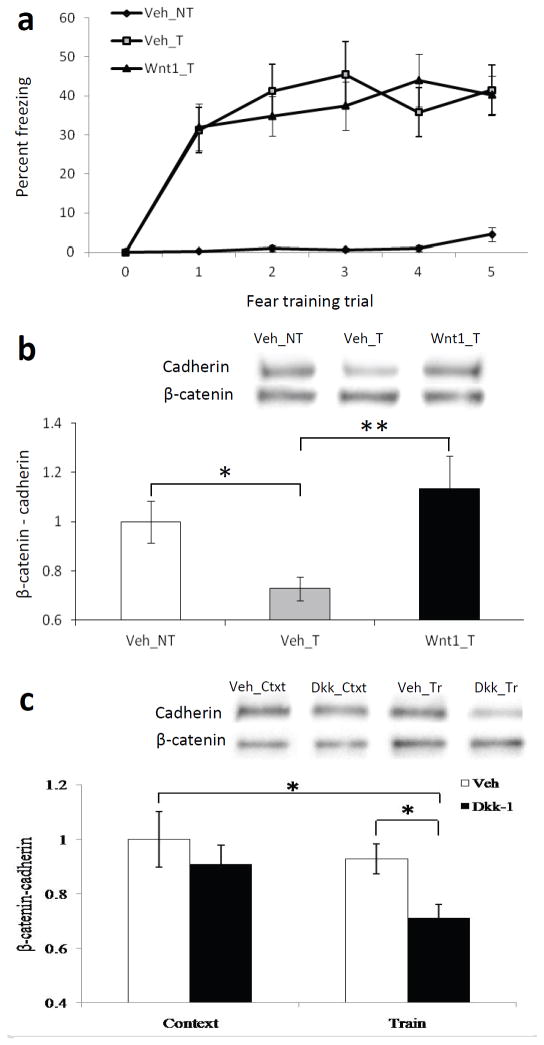
We infused either Wnt1 (Wnt1_T) or saline (Veh_T) bilaterally into the amygdala prior to fear conditioning. A third control group was included, which received saline prior to context exposure, but did not receive any stimulus presentations (Veh_NT). In agreement with our previous results, all trained animals were able to equally acquire the fear during the conditioning (F 5,145 = 26.59, P < 0.01). In addition, animals receiving training froze significantly more across time compared to the untrained control group (F1,37 = 27.57, P < 0.01, Fig. 5A). A subset of these animals was sacrificed immediately after training or exposure to the context, and brains were collected. We then immunoprecipitated β-catenin from the amygdala and probed with an antibody to pan-cadherin. We found a significant main effect for treatment (F2,25 = 3.35, P ≤ 0.05). The amount of cadherin coimmunoprecipitated with β-catenin was significantly lower in trained mice immediately after conditioning than in context control mice as we had previously demonstrated (t13 = 2.68, P < 0.5, Fig. 5B). Notably, we found that this decrease was abolished when Wnt1 was injected into the amygdala prior to training (t12.37 = −2.89, P ≤ 0.01), preventing the normal learning-dependent transient decrease in Wnt1 expression. These results suggest that exogenous Wnt1 may impair memory formation by stabilizing β-catenin/cadherin interactions during the early consolidation period, thus preventing the normal transient β-catenin/cadherin disassociation that occurs with transient Wnt1 depletion.
Figure 5. The effect of fear conditioning, Wnt1, and Dkk-1 administration on the interaction between β-catenin and cadherin.
(a) Acquisition curve showing percent time spent freezing in untrained animals (Veh_NT) compared to trained animals receiving either vehicle (Veh_T) or Wnt1 (Wnt1_T) prior to fear conditioning. n = 8 for Veh_NT, n = 12 for Veh_T, n = 19 for Wnt1_T. (b) Qualitative and quantitative western blot data showing a co-immunoprecipation of cadherin from β-catenin. n = 8 for Veh_NT, n = 7 for Veh_T, n = 11 for Wnt1_T, showing that Wnt1 prevents normal dissociation of β-catenin/cadherin interactions within 30min of fear conditioning. (c) Qualitative and quantitative western blot data showing a co-immunoprecipation of cadherin from β-catenin following vehicle or Dkk-1 treatment in context exposed (n=9 veh, n=10 Dkk-1) vs. trained animals (n=11 veh, n=9 Dkk-1), demonstrating that Dkk-1 prevents the re-stabilization of β-catenin/cadherin interactions 2 hours post fear conditioning. Mean ± s.e.m., * P < 0.05, ** P ≤ 0.01, ANOVA and two-tailed t-tests.
Previous in vitro research has shown that Dkk-1 decreases the accumulation of β-catenin and cadherin at cell membrane, resulting in a decrease in cell-cell adhesion (Kuang et al., 2009). Since we have previously shown that the association between β-catenin and cadherin is decreased immediately following learning, which then returns to baseline at 2 h, we wanted to examine if inhibiting Wnt signaling would prevent the re-association of the β-catenin/cadherin complex at the 2 h time point.
We infused either saline or Dkk-1 bilaterally into the amygdala prior to context exposure or fear conditioning, and sacrificed the animals 2 h later. We then collected the brains, immunoprecipitated β-catenin from the amygdala and probed with an antibody to pan-cadherin. We found a significant main effect for treatment (F3,35 = 2.86, P ≤ 0.05, Fig. 5C). Post hoc least-square difference analyses indicated that the amount of cadherin coimmunoprecipated with β-catenin was significantly lower in trained mice receiving injections of Dkk-1 prior to conditioning than in context control mice (P < 0.01) as well as fear conditioned mice (P < 0.05) receiving vehicle injections. These results suggest that exogenous Dkk-1 may impair memory formation by preventing the re-stabilization of the β-catenin/cadherin interaction during the early consolidation period.
Dkk-1 and Wnt1 have no effect on fear acquisition, short-term memory, or fear expression
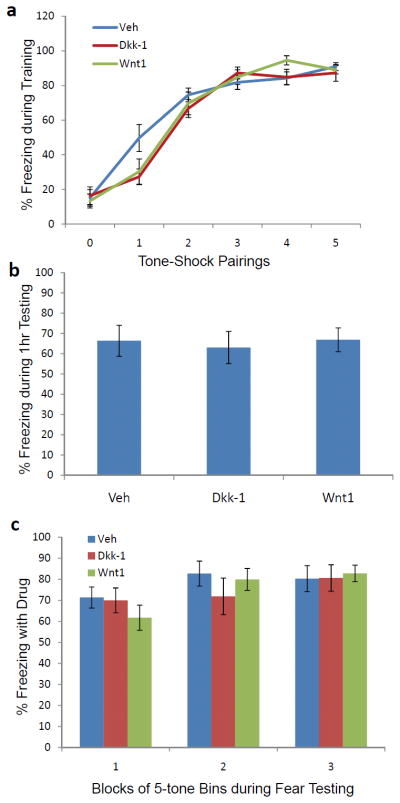
The above data suggest that Wnt1 expression is correlated with fear memory consolidation and that manipulating Wnt signaling disrupts long-term fear memories. However, it is possible that the effects are on short-term memory formation and not long-term memory consolidation. To address this question, another cohort of mice was infused with vehicle, Dkk-1, or Wnt1 as outlined above. As shown in Figure 6, we see no significant effect on the rate of acquisition of fear when these peptides are infused into bilateral amygdala sites as shown previously. Following fear acquisition, these mice were returned to their homecage. One hour after training, they received a short-term memory test, in which 5 tones were presented and freezing was measured. In contrast to the effects of Wnt1 and Dkk-1 on long-term memory consolidation, no differences were seen in this short-term memory test (Fig. 6B).
Figure 6. Dkk-1 and Wnt1 infusions are not associated with altered fear acquisition, short-term memory, or fear expression.
(a) Mice were injected via stereotaxically placed cannulae within bilateral amygdala, with vehicle, Dkk-1, or Wnt1 (n=11 per group) prior to fear conditioning with 5–6 kHz tone-footshock pairings. No differences were seen in the freezing responses during fear training. (b) At 1 h following the above training, mice were tested in a short-term memory paradigm in which 5 tones were presented and freezing was measured. In contrast to the effects of Dkk-1 and Wnt1 on long-term memory consolidation, no differences were seen in this short term memory test. (c) Forty-eight hours following training to 5 tone-shock pairings with a 12 kHz tone, mice were tested for expression of conditioned fear. In this study, mice were injected via cannulae bilaterally into the amygdala, with vehicle (n=11), Dkk-1 (n=11), or Wnt1 (n=11) prior to testing. No effect of drug was seen on expression of conditioned freezing across 3 blocks of 5-tones each.
To further confirm that Wnt1 and Dkk-1 did not have an effect on sensory processing or on long-term memory expression, we examined the role of peptide amygdala infusion on a long-term memory expression paradigm. Forty-eight hours following training to 5 tone-shock pairings with a 12 kHz tone, mice were tested for expression of conditioned fear (Fig. 6C). No effect of drug was seen on expression of conditioned freezing across 3 blocks of 5-tones each.
DISCUSSION
Our data suggest that Wnt signaling in the amygdala plays an important role in long term memory formation. We showed that during the early consolidation of memory, a number of genes in the Wnt signaling pathway are dynamically regulated with fear conditioning. The most striking difference was a rapid downregulation of many Wnt genes, with Wnt1 having the most robust effect, immediately after conditioning which was found across several replication studies using different methodology. Both preventing Wnt1 signaling and increasing Wnt1 levels within the amygdala had no effect on control behaviors, fear acquisition or fear expression, but both impaired memory consolidation. Notably, at different timepoints during consolidation, they had opposite effects on β-catenin/cadherin interactions: Wnt1 prevented the rapid dissociation of β-catenin/cadherin complexes and Dkk-1 prevented the later re-stabilization of these complexes (Fig. 7). Together these data provide evidence that dynamic regulation of endogenous Wnt/β-catenin signaling is required for memory consolidation and long-term memory formation.
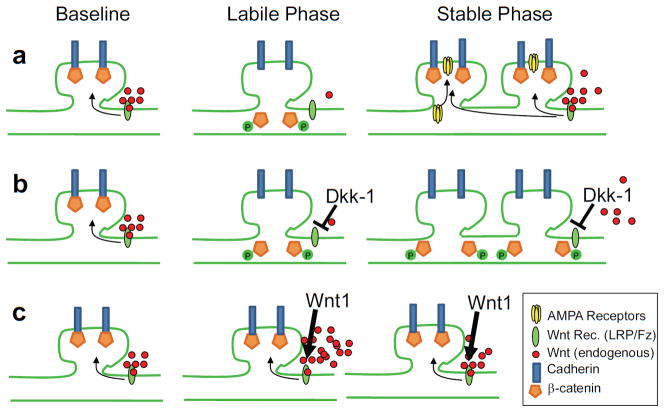
Figure 7. Schematic representation of the role of Wnt/β-catenin signaling in producing the labile and stable phases of memory formation.
(a) Baseline levels of Wnt stabilize β-catenin/Cadherin synaptic stability, transient Wnt decrease during the labile phase allowing for synapse rearrangement, followed by Wnt normalization which may mediate re-stabilization of newly formed synapses, which is also associated with increased AMPA trafficking and insertion (Rumpel et al., 2005; Yu et al., 2008; Migues et al., 2010; Nedelescu et al., 2010). (b) In the presence of Dkk-1, restabilization does not occur, preventing normal memory consolidation. (c) In the presence of Wnt1, the initial labile phase is prevented, also preventing normal memory consolidation.
The results from our PCR array allowed us to examine alterations in individual genes related to Wnt-mediated signaling. We found that over 50 Wnt signaling genes within the amygdala are dynamically regulated during the memory consolidation period following fear learning. This finding is consistent with other research showing that fear conditioning can differentially affect the expression of various genes in the amygdala (Stork et al., 2001; Ressler et al., 2002; Mei et al., 2005; Keeley et al., 2006). However, to our knowledge, this is the first evidence that rapid, dynamic regulation of Wnt signaling is involved in memory formation or in neural functioning in vivo in adult animals.
We found that many of the Wnts show rapid regulation (15 of 17 are immediately decreased) during memory consolidation. Activity-dependent changes in Wnt expression have also been shown as a result of LTP induction in a mouse hippocampal slice model (Chen et al., 2006). In the current studies, we showed that Wnt1, compared to the other Wnt genes, was found to have the most substantial changes immediately following conditioning. Therefore, we focused our remaining studies on the role of Wnt1 in memory formation.
Dkk-1 is a known negative modulator of Wnt signaling, and abnormalities in Wnt signaling have been associated with human memory disorders such as Alzheimer’s Disease (De Ferrari and Inestrosa, 2000; Caricasole et al., 2004). Previous in vitro work has shown that overexpression of Dkk-1 prevents activity-dependent dendritic branching (Yu and Malenka, 2003). Our results show that overexpression of Dkk-1 in vivo prevents memory formation. We found that infusing Dkk-1 into the amygdala before fear conditioning does not affect the acquisition or immediate expression of fear, but does produce deficits in learning when measured 48 h after training. The observed behavioral effect does not appear to be a result of neurotoxicity or alterations in baseline anxiety or locomotor activity. Notably, as found in Table 1, we find that endogenous Dkk-1 is transiently increased during fear consolidation. This is consistent with a transient decrease in Wnt signaling playing a role in plasticity and memory formation. We think that disrupting the normal temporal control of Wnt inhibition, as well as the likely more profound effect of exogenous Dkk-1 beyond endogenous levels, led to abnormal Wnt regulation. Thus, the Dkk-1 behavioral results suggest that dynamic regulation of Wnt signaling is required for the consolidation of new memory.
We chose Dkk-1 for our studies since it is a well-known, and commonly used, inhibitor of the Wnt pathway, both in vitro and in vivo (Matrisciano et al., 2011; Verani et al., 2007; Wirths et al., 2003; Shou et al., 2002; and Grotewold & Ruther, 2002). One potential limitation of this work is the lack of small molecule pharmacological antagonists targeting the Wnt pathway. Future studies utilizing a genetic silencer of Wnt signaling would be important to both target specific cell populations and more precisely target amygdala subregions.
To further examine its role in memory formation, in vivo, we infused Wnt1 into the amygdala either before or after fear conditioning. We found that infusing Wnt1 in the amygdala prior to training did not affect acquisition, but resulted in deficits in the expression of fear as measured 48 h after training. The infusion of Wnt1 after training did not have an effect on learning. Given that acquisition of fear appeared to be normal in the presence of Wnt1, these data suggest that we may be preventing the normal rapid decrease in Wnt signaling that occurs at the end of training and immediately during the early period of consolidation. These data suggest that an immediate decrease in Wnt1 with learning may be required for memory formation; thus, the infusion of Wnt1 prior to training, may allow just enough time to reverse that downregulation of Wnt1 gene expression. Conversely, infusing Wnt1 after training, may not increase expression of Wnt1 until after a critical period of early consolidation has passed. Therefore, our results suggest that inhibiting the decrease in Wnt1 expression which occurs with training produces memory deficits.
Interestingly, the observed downregulation of endogenous Wnt1 immediately following training closely resembles the alterations seen in the interaction of β-catenin with cadherin following fear conditioning (Maguschak and Ressler, 2008). There has been much discussion regarding the crosstalk between Wnt signaling and cell adhesion. Previous in vitro work in non-neuronal cells has shown that Wnt stimulation leads to an increase in cell-cell adhesion (Hinck et al., 1994; Medrek et al., 2009). Furthermore, there are many lines of convergent data suggesting that Wnts may both directly and indirectly regulate β-catenin interactions with cadherin (Nelson and Nusse, 2004). Our results showed that Wnt1 may have a similar effect in promoting β-catenin/cadherin stability in vivo within the adult brain, and conversely that Wnt inhibition or downregulation may destabilize these complexes. We found that infusing Wnt1 into the amygdala prior to training prevented the destabilization of the β-catenin/cadherin interaction that occurs immediately following fear conditioning. This lack of transient destabilization may prevent synaptic modifications from taking place which are required for new memory formation.
Of note, most of our dynamic Wnt expression appears to be localized to the basal amygdala (Fig. 2), and our Dkk-1 and Wnt1 infusions targeted basal as much as lateral subdivisions. However, the majority of work on auditory fear conditioning in rats suggests that the basal nucleus is not required for auditory fear conditioning (Amorapanth et al, 2000; Nader et al, 2001). We have several thoughts related to this: 1) as far as we are aware, most of the auditory fear conditioning pointing exclusively to the lateral amygdala studies have been performed in rats, not mice, which are used in these studies; 2) the lateral amygdala actually extends quite ventrally at the more rostral regions of the brain where these in situs were performed, making it difficult to separate ventrolateral subdivisions from basolateral; 3) we have performed other mouse genetic and pharmacological manipulations targeting the basolateral region, finding robust effects on tone fear conditioning in mice (e.g., Heldt & Ressler 2007; Maguschak & Ressler 2008); and 4) at least some work has been done suggesting that lesions of the anterior division of the basal nuclei in rats disrupts auditory fear conditioning (Goosens & Maren, 2001).
We have previously shown that β-catenin in the amygdala is required for the normal consolidation of fear memory, and that the interaction between β-catenin and cadherin is altered during this process (Maguschak and Ressler, 2008). Those data suggest that β-catenin/cadherin complexes are involved in normal synaptic stability, and that transient disruption of stable synapses may be required for new synapse formation to occur. Here, we show that Wnt signaling may contribute to β-catenin’s effect on learning and memory. As schematized in Figure 7, we propose that during the acquisition of fear memory and immediately after, normal Wnt signaling is significantly reduced, which may allow for phosphorylation of the Y654 site on β-catenin, contributing to β-catenin/cadherin destabilization (Murase et al., 2002), and subsequent synaptic weakening (Arikkath and Reichardt, 2008). Then, once the synapses have been modified during the consolidation process, Wnt signaling is normalized, the β-catenin Y654 site is dephosphorylated, and β-catenin/cadherin interaction is subsequently restored, stabilizing the new synapses (Bamji et al., 2006). These processes of Wnt-dependent β-catenin/cadherin mediated synapse stability, coupled with transient destabilization and re-stabilization during memory consolidation may provide for a structural mechanism underlying long-term memory formation and stability.
Acknowledgments
Support was provided by NIH (DA019624, P30 NS055077), the Burroughs Wellcome Fund, NSF (GRFP DGE-0234618), and Center for Behavioral Neuroscience STC Center: NSF Agmt #IBN-9876754, National Primate Research Center base grant #RR-00165, Animal Resource Program at NIH.
Footnotes
Conflict of Interest: None
References
- Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikkath J, Reichardt LF. Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci. 2008;31:487–494. doi: 10.1016/j.tins.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamji SX, Rico B, Kimes N, Reichardt LF. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions. J Cell Biol. 2006;174:289–299. doi: 10.1083/jcb.200601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, Storto M, Gaviraghi G, Terstappen GC, Nicoletti F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. J Neurosci. 2004;24:6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Inestrosa NC. Wnt signaling function in Alzheimer’s disease. Brain Res Brain Res Rev. 2000;33:1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;25:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- Diep DB, Hoen N, Backman M, Machon O, Krauss S. Characterisation of the Wnt antagonists and their response to conditionally activated Wnt signalling in the developing mouse forebrain. Brain Res Dev Brain Res. 2004;153:261–270. doi: 10.1016/j.devbrainres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem. 2001;8(3):148–55. doi: 10.1101/lm.37601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Grotewold L, Rüther U. The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. EMBO J. 2002;21(5):966–75. doi: 10.1093/emboj/21.5.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26(12):3631–44. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Nelson WJ, Papkoff J. Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing beta-catenin binding to the cell adhesion protein cadherin. J Cell Biol. 1994;124:729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley MB, Wood MA, Isiegas C, Stein J, Hellman K, Hannenhalli S, Abel T. Differential transcriptional response to nonassociative and associative components of classical fear conditioning in the amygdala and hippocampus. Learn Mem. 2006;13:135–142. doi: 10.1101/lm.86906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylova O, Herreros J, Cleverley KE, Ehler E, Henriquez JP, Hughes SM, Salinas PC. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- Kuang HB, Miao CL, Guo WX, Peng S, Cao YJ, Duan EK. Dickkopf-1 enhances migration of HEK293 cell by beta-catenin/E-cadherin degradation. Front Biosci. 2009;14:2212–2220. doi: 10.2741/3373. [DOI] [PubMed] [Google Scholar]
- Lee SH, Peng IF, Ng YG, Yanagisawa M, Bamji SX, Elia LP, Balsamo J, Lilien J, Anastasiadis PZ, Ullian EM, Reichardt LF. Synapses are regulated by the cytoplasmic tyrosine kinase Fer in a pathway mediated by p120catenin, Fer, SHP-2, and beta-catenin. J Cell Biol. 2008;183:893–908. doi: 10.1083/jcb.200807188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lucas FR, Salinas PC. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev Biol. 1997;192:31–44. doi: 10.1006/dbio.1997.8734. [DOI] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. Beta-catenin is required for memory consolidation. Nat Neurosci. 2008;11:1319–1326. doi: 10.1038/nn.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F, Busceti CL, Bucci D, Orlando R, Caruso A, Molinaro G, Cappuccio I, Riozzi B, Gradini R, Motolese M, Caraci F, Copani A, Scaccianoce S, Melchiorri D, Bruno V, Battaglia G, Nicoletti F. Induction of the Wnt antagonist Dickkopf-1 is involved in stress-induced hippocampal damage. PLoS One. 2011;6(1):e16447. doi: 10.1371/journal.pone.0016447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrek C, Landberg G, Andersson T, Leandersson K. Wnt-5a-CKI{alpha} signaling promotes {beta}-catenin/E-cadherin complex formation and intercellular adhesion in human breast epithelial cells. J Biol Chem. 2009;284:10968–10979. doi: 10.1074/jbc.M804923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei B, Li C, Dong S, Jiang CH, Wang H, Hu Y. Distinct gene expression profiles in hippocampus and amygdala after fear conditioning. Brain Res Bull. 2005;67:1–12. doi: 10.1016/j.brainresbull.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13(5):630–4. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Murase S, Mosser E, Schuman EM. Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- Nedelescu H, Kelso CM, Lázaro-Muñoz G, Purpura M, Cain CK, Ledoux JE, Aoki C. Endogenous GluR1-containing AMPA receptors translocate to asymmetric synapses in the lateral amygdala during the early phase of fear memory formation: an electron microscopic immunocytochemical study. J Comp Neurol. 2010;518(23):4723–39. doi: 10.1002/cne.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; San Diego, California: 2001. [Google Scholar]
- Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J Neurosci. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308(5718):83–8. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Shou J, Ali-Osman F, Multani AS, Pathak S, Fedi P, Srivenugopal KS. Human Dkk-1, a gene encoding a Wnt antagonist, responds to DNA damage and its overexpression sensitizes brain tumor cells to apoptosis following alkylation damage of DNA. Oncogene. 2002;21(6):878–89. doi: 10.1038/sj.onc.1205138. [DOI] [PubMed] [Google Scholar]
- Stork O, Stork S, Pape HC, Obata K. Identification of genes expressed in the amygdala during the formation of fear memory. Learn Mem. 2001;8:209–219. doi: 10.1101/lm.39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verani R, Cappuccio I, Spinsanti P, Gradini R, Caruso A, Magnotti MC, Motolese M, Nicoletti F, Melchiorri D. Expression of the Wnt inhibitor Dickkopf-1 is required for the induction of neural markers in mouse embryonic stem cells differentiating in response to retinoic acid. J Neurochem. 2007;100(1):242–50. doi: 10.1111/j.1471-4159.2006.04207.x. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Wirths O, Waha A, Weggen S, Schirmacher P, Kühne T, Goodyer CG, Albrecht S, Von Schweinitz D, Pietsch T. Overexpression of human Dickkopf-1, an antagonist of wingless/WNT signaling, in human hepatoblastomas and Wilms’ tumors. Lab Invest. 2003;83(3):429–34. doi: 10.1097/01.lab.0000059926.66359.bd. [DOI] [PubMed] [Google Scholar]
- Yu SY, Wu DC, Liu L, Ge Y, Wang YT. Role of AMPA receptor trafficking in NMDA receptor-dependent synaptic plasticity in the rat lateral amygdala. J Neurochem. 2008;106(2):889–99. doi: 10.1111/j.1471-4159.2008.05461.x. [DOI] [PubMed] [Google Scholar]
- Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]