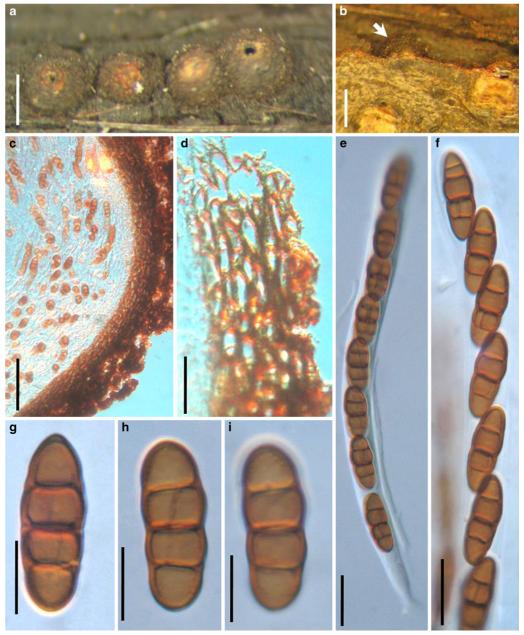
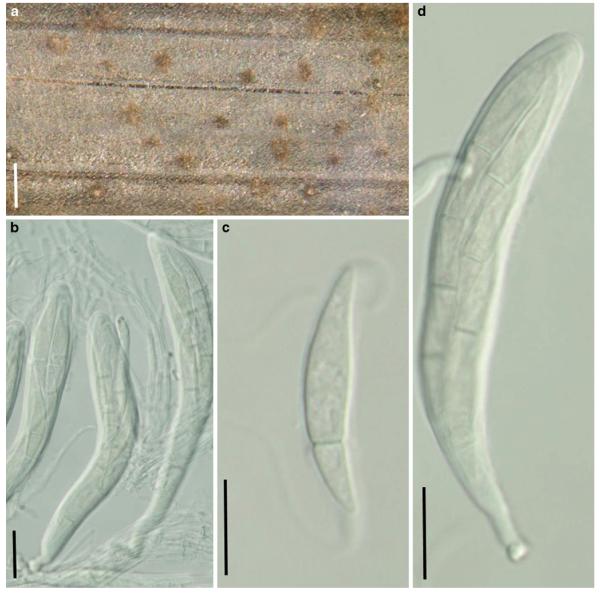
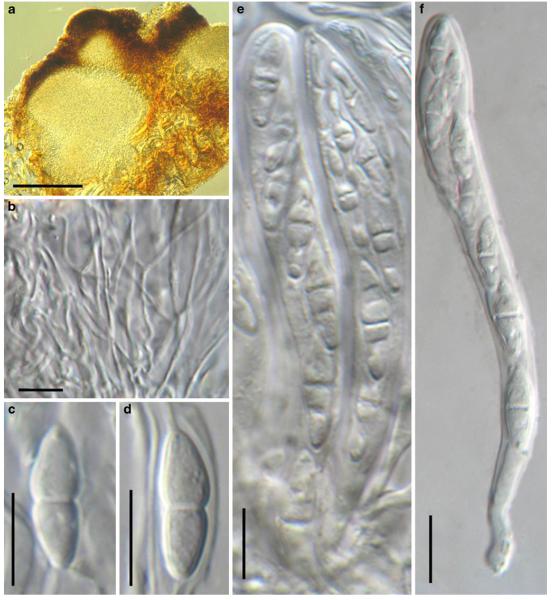
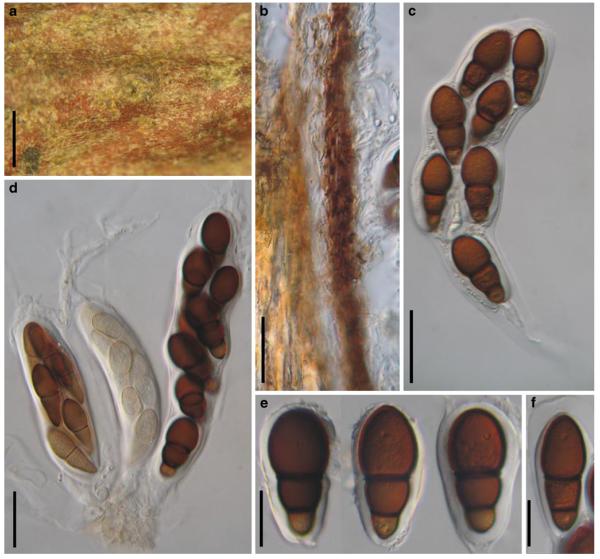
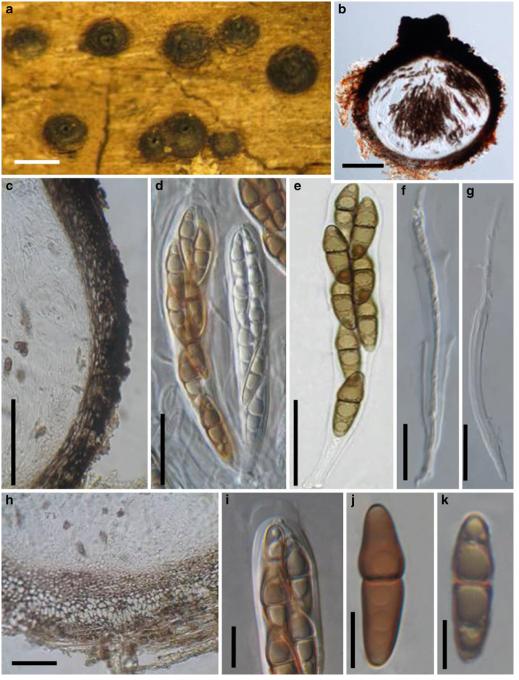
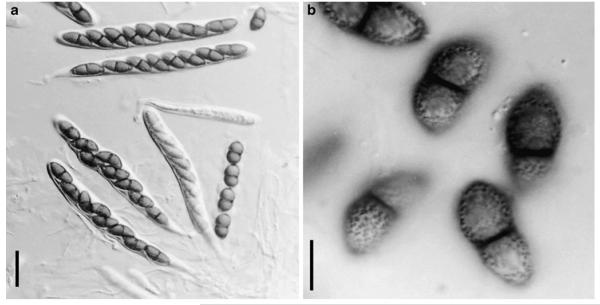
Abstract
One hundred and five generic types of Pleosporales are described and illustrated. A brief introduction and detailed history with short notes on morphology, molecular phylogeny as well as a general conclusion of each genus are provided. For those genera where the type or a representative specimen is unavailable, a brief note is given. Altogether 174 genera of Pleosporales are treated. Phaeotrichaceae as well as Krie-geriella, Zeuctomorpha and Muroia are excluded from Pleosporales. Based on the multigene phylogenetic analysis, the suborder Massarineae is emended to accommodate five families, viz. Lentitheciaceae, Massarinaceae, Montagnulaceae, Morosphaeriaceae and Trematosphaeriaceae.
Keywords: Generic type, Massarineae, Molecular phylogeny, Morphology, Pleosporales, Taxonomy
Introduction
Historic overview of Pleosporales
Pleosporales is the largest order in the Dothideomycetes, comprising a quarter of all dothideomycetous species (Kirk et al. 2008). Species in this order occur in various habitats, and can be epiphytes, endophytes or parasites of living leaves or stems, hyperparasites on fungi or insects, lichenized, or are saprobes of dead plant stems, leaves or bark (Kruys et al. 2006; Ramesh 2003).
The Pleosporaceae was introduced by Nitschke (1869), and was assigned to Sphaeriales based on immersed ascomata and presence of pseudoparaphyses (Ellis and Everhart 1892; Lindau 1897; Wehmeyer 1975; Winter 1887). Taxa in this family were then assigned to Pseudosphaeriaceae (Theissen and Sydow 1918; Wehmeyer 1975). Pseudosphaeriales, represented by Pseudosphaeriaceae, was introduced by Theissen and Sydow (1918), and was distinguished from Dothideales by its uniloculate, perithecioid ascostromata. Subsequently, the uni- or pluri-loculate ascostromata was reported to be an invalid character to separate members of Dothideomycetes into different orders (Luttrell 1955). In addition, the familial type of Pseudosphaeriales together with its type genus, Pseudosphaeria, was transferred to Dothideales, thus Pseudosphaeriales became a synonym of Dothideales. The name “Pseudosphaeriales” has been applied in different senses, thus Pleosporales (as an invalid name due to the absence of a Latin diagnosis) was proposed by Luttrell (1955) to replace the confusing name, Pseudosphaeriales, which included seven families, i.e. Botryosphaeriaceae, Didymosphaeriaceae, Herpotrichiellaceae, Lophiostomataceae, Mesnieraceae, Pleosporaceae and Venturiaceae. Muller and von Arx (1962) however, reused Pseudosphaeriales with 12 families included, viz. Capnodiaceae, Chaetothyriaceae, Dimeriaceae, Lophiostomataceae, Mesnieraceae, Micropeltaceae, Microthyriaceae, Mycosphaerellaceae, Pleosporaceae, Sporormiaceae, Trichothyriaceae and Venturiaceae.
Familial circumscriptions of the Pleosporales were based on characters of ascomata, morphology of asci and their arrangement in locules, presence and type of hamathecium, shape of papilla or ostioles, morphology of ascospores and type of habitats (Luttrell 1973) (Table 1). Based on these characters, Luttrell (1973) included eight families, i.e. Botryosphaeriaceae, Dimeriaceae, Lophiostomataceae, Mesnieraceae, Mycoporaceae, Pleosporaceae, Sporormiaceae and Venturiaceae in Pleosporales. In their review of bitunicate ascomycetes, von Arx and Muller (1975) accepted only a single order, Dothideales, with two suborders, i.e. Dothideineae (including Atichiales, Dothiorales, Hysteriales and Myriangiales) and Pseudosphaeriineae (including Capnodiales, Chaetothyriales, Hemisphaeriales, Lophiostomatales, Microthyriales, Perisporiales, Pleosporales, Pseudosphaeriales and Trichothyriales). This proposal has however, rarely been followed. Three existing families, i.e. Lophiostomataceae, Pleosporaceae and Venturiaceae plus 11 other families were accepted in Pleosporales as arranged by Barr (1979a) (largely using Luttrell’s concepts, Table 1), and she assigned these families to six suborders. The morphology of pseudo-paraphyses was given much prominence at the ordinal level in this classification (Barr 1983). In particular the Melanommatales was introduced to accommodate taxa with trabeculate pseudoparaphyses (Sporormia-type centrum development) (Barr 1983), distinguished from cellular pseudoparaphyses (Pleospora-type centrum development) possessed by members of Pleosporales sensu Barr. The order Melanommatales included Didymosphaeriaceae, Fenestellaceae, Massariaceae, Melanommataceae, Microthyriaceae, Mytilinidiaceae, Platystomaceae and Requienellaceae (Barr 1990a).
Table 1.
Major circumscription changes of Pleosporales from 1955 to 2011
Morpho-characters used in taxonomy of Pleosporales
| References | Circumscriptions distinguishing Pleosporales from other orders of Dothideomycetes |
|---|---|
| Luttrell 1955 | Pleospora-type centrum development. |
| Müller and von Arx 1962 | Ascomata perithecoid, with rounded or slit-like ostiole; asci produced within a locule, arranged regularly in a single layer or irregularly scattered, surrounded with filiform pseudoparaphyses, cylindrical, ellipsoidal or sac-like. |
| Luttrell 1973 | Ascocarps perithecioid, immersed, erumpent to superficial on various substrates, asci ovoid to mostly clavate or cylindrical, interspersed with pseudoparaphyses (sometimes form an epithecium) in mostly medium- to large-sized locules. |
| Barr 1979a | Saprobic, parasitic, lichenized or hypersaprobic. Ascomata perithecioid, rarely cleistothecioid or hysterothecioid, peridium pseudoparenchymatous, pseudoparaphyses cellular, narrow or broad, deliquescing early at times, not forming an epithecium, asci oblong, clavate or cylindrical, interspersed with pseudoparaphyses, ascospores mostly asymmetric. |
| Barr 1987b | Saprobic, biotrophic or hemibiotrophic. Ascomata globose, subglobose or conical, asci bitunicate, oblong, clavate or cylindrical, cellular pseudoparaphyses, ascospores hyaline or pigmented, asymmetric or symmetric, with or without septa. |
| Kirk et al. 2001, 2008 | Ascomata perithecioid or rarely cleistothecioid, sometimes clypeate, mostly globose, thick-walled, immersed or erumpent, black, sometimes setose, peridium composed of pseudoparenchymatous cells, pseudoparaphyses trabeculate or cellular, asci cylindrical, fissitunicate, with a well-developed ocular chamber, rarely with a poorly defined ring (J-), ascospores hyaline to brown, septate, thin or thick- walled, sometimes muriform, usually with sheath, anamorphs hyphomycetous or coelomycetous. |
| Boehm et al. 2009a, b; Mugambi and Huhndorf 2009b; Schoch et al. 2009; Shearer et al. 2009; Suetrong et al. 2009; Tanaka et al. 2009; Zhang et al. 2009a | Hemibiotrophic, saprobic, hypersaprobic, or lichenized. Habitats in freshwater, marine or terrestrial environment. Ascomata perithecioid, rarely cleistothecioid, immersed, erumpent to superficial, globose to subglobose, or lenticular to irregular, with or without conspicuous papilla or ostioles. Ostioles with or without periphyses. Peridium usually composed of a few layers of cells with various shapes and structures. Hamathecium persistent, filamentous, very rarely decomposing. Asci bitunicate, fissitunicate, cylindrical, clavate to obclavate, with or without pedicel. Ascospores hyaline or pigmented, ellipsoidal, broadly to narrowly fusoid or filiform, mostly septate. |
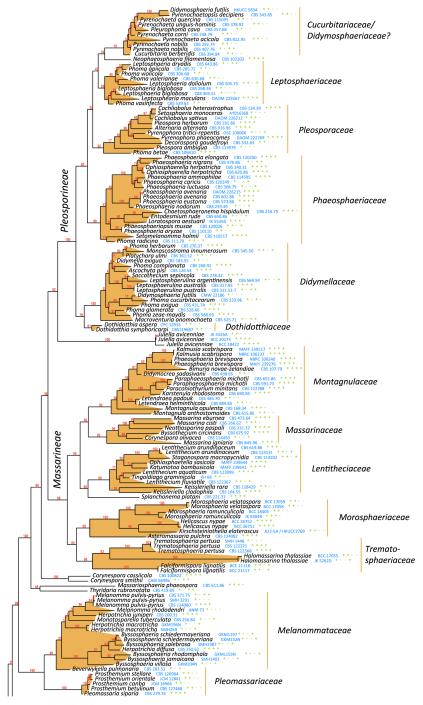
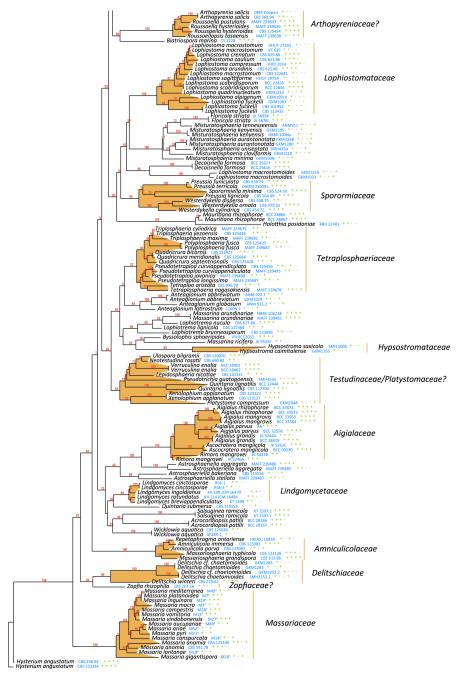
Pleosporales was formally established by Luttrell and Barr (in Barr 1987b), characterised by perithecioid ascomata, usually with a papillate apex, ostioles with or without periphyses, presence of cellular pseudoparaphyses, bitunicate asci, and ascospores of various shapes, pigmentation and septation (Table 1). Eighteen families were included, i.e. Arthopyreniaceae, Botryosphaeriaceae, Cucurbitariaceae, Dacampiaceae, Dimeriaceae, Hysteriaceae, Leptosphaeriaceae, Lophiostomataceae, Parodiellaceae, Phaeosphaeriaceae, Phaeotrichaceae, Pleomassariaceae, Pleosporaceae, Polystomellaceae, Pyrenophoraceae, Micropeltidaceae, Tubeufiaceae and Venturiaceae. Recent phylogenetic analysis based on DNA sequence comparisons, however, indicated that separation of the orders (Pleosporales and Melanommatales) based on the Pleospora or Sporormia centrum type, is not a natural grouping, and Melanommatales has therefore been combined under Pleosporales (Liew et al. 2000; Lumbsch and Lindemuth 2001; Reynolds 1991). Six more families, i.e. Cucurbitariaceae, Diademaceae, Didymosphaeriaceae, Mytilinidiaceae, Testudinaceae and Zopfiaceae, were subsequently added to Pleosporales (Lumbsch and Huhndorf 2007). After intensive sampling and multigene phylogenetic studies, 20 families were accepted in Pleosporales, namely Aigialaceae, Amniculicolaceae, Delitschiaceae, Didymellaceae, Didymosphaeriaceae, Hypsostromataceae, Lentitheciaceae, Leptosphaeriaceae, Lindgomycetaceae, Lophiostomataceae, Massarinaceae, Melanommataceae, Montagnulaceae, Morosphaeriaceae, Phaeosphaeriaceae, Pleosporaceae, Pleomassariaceae, Sporormiaceae, Tetraplosphaeriaceae and Trematosphaeriaceae (Boehm et al. 2009a, b; Mugambi and Huhndorf 2009b; Schoch et al. 2009; Shearer et al. 2009; Suetrong et al. 2009; Tanaka et al. 2009; Zhang et al. 2009a) (Table 1). In addition, another five families, i.e. Arthopyreniaceae, Cucurbitariaceae, Diademaceae, Teichosporaceae and Zopfiaceae are tentatively included (Kruys et al. 2006; Plate 1). In the most recent issue of Myconet, 28 families were included in Pleosporales (Lumbsch and Huhndorf 2010).
Plate 1.
The best scoring likelihood tree of representative Pleospor- * ales obtained with RAxML v. 7.2.7 for a concatenated set of nucleotides from LSU, SSU, RPB2 and TEF1. Family and suborder names are indicated where possible. The percentages of nodes present in 250 bootstrap pseudo replicates are shown above branches. Culture and voucher numbers are indicated after species names and the presence of the genes used in the analysis are indicated by pluses in this order: LSU, SSU, RPB2, TEF1
Species included in Pleosporales have different ecological or morphological characters. For instance, members of the Leptosphaeriaceae have saprobic or parasitic lifestyles and lightly pigmented, multi-septate ascospores. Members of the Lophiostomataceae are mostly saprobic with ascomata that usually possess a compressed apex. Members of Sporormiaceae are coprophilous, and are characterized by heavily pigmented, multi-septate ascospores with germ slits, and with or without non-periphysate ostioles. The lack of DNA sequence data for representatives of numerous families means that their inter-relationships are unclear and many genera or species are artificially placed based on morphological classification. The most recent study on Venturiaceae indicated that this group had a set of unique morphological and ecological characters, which is distinct and distantly related to other members of Pleosporales (Kruys et al. 2006; Zhang et al. unpublished). Molecular phylogenetic results indicated that members of Venturiaceae form a robust clade separate from the core members of Pleosporales, and the clade of Venturiaceae was uncertainly placed but outside of the two currently designated dothideomycetous subclasses, i.e. Pleosporomycetidae and Dothideomycetidae (Schoch et al. 2009). In addition, phylogenetic analysis of rDNA sequence data indicates that members of Zopfiaceae (as Testudinaceae) seem to lack affinity with Pleosporales (Kodsueb et al. 2006b). Thus, 26 families are temporarily accepted in Pleosporales in this study, although some such as Zopfiaceae, still require extensive DNA sequence sampling (Table 4).
Table 4.
Families currently accepted in Pleosporales (syn. Melanommatales) with included genera
| Pleosporales subordo. Pleosporineae |
| ? Cucurbitariaceae |
| Cucurbitaria Gray |
| Curreya Sacc. |
| ?Rhytidiella Zalasky |
| Syncarpella Theiss. & Syd. |
| Didymellaceae |
| ?Appendispora K.D. Hyde |
| Didymella Sacc. ex D. Sacc. |
| Didymosphaerella Cooke |
| Leptosphaerulina McAlpine |
| Macroventuria Aa |
| ?Platychora Petr. |
| Didymosphaeriaceae |
| Didymosphaeria Fuckel |
| Phaeodothis Syd. & P. Syd. |
| Dothidotthiaceae |
| Dothidotthia Höhn. |
| Leptosphaeriaceae |
| Leptosphaeria Ces. & De Not. |
| Neophaeosphaeria Câmara, M.E. Palm & A.W. Ramaley |
| Phaeosphaeriaceae |
| Barria Z.Q. Yuan |
| ?Bricookea M.E. Barr |
| ?Chaetoplea (Sacc.) Clem. |
| ?Eudarluca Speg. |
| Entodesmium Reiss |
| ?Hadrospora Boise |
| Lautitia S. Schatz |
| Loratospora Kohlm. & Volkm.-Kohlm. |
| Metameris Theiss. & Syd. |
| Mixtura O.E. Erikss. & J.Z. Yue |
| Nodulosphaeria Rabenh. |
| Ophiobolus Reiss |
| Ophiosphaerella Speg. |
| Phaeosphaeria I. Miyake |
| Phaeosphaeriopsis Câmara, M.E. Palm & A.W. |
| Ramaley |
| Pleoseptum A.W. Ramaley & M.E. Barr |
| Setomelanomma M. Morelet |
| Wilmia Dianese, Inácio & Dornelo-Silva |
| Pleosporaceae |
| Cochliobolus Drechsler |
| Crivellia Shoemaker & Inderbitzin |
| Decorospora Inderbitzin, Kohlm. & Volkm.-Kohlm. |
| Extrawettsteinina M.E. Barr |
| Lewia M.E. Barr & E.G. Simmons |
| Macrospora Fuckel |
| Platysporoides (Wehm.) Shoemaker & C.E. Babc. |
| Pleospora Rabenh. ex Ces. & De Not. |
| Pseudoyuconia Lar. N. Vasiljeva |
| Pyrenophora Fr. |
| Setosphaeria K.J. Leonard & Suggs |
| Pleosporales subordo. Massarineae |
| Lentitheciaceae |
| Lentithecium K.D. Hyde, J. Fourn. & Yin. Zhang |
| Katumotoa Kaz. Tanaka & Y. Harada |
| Keissleriella Höhn. |
| ?Wettsteinina Höhn. |
| Massarinaceae |
| Byssothecium Fuckel |
| Massarina Sacc. |
| Saccharicola D. Hawksw. & O.E. Erikss. |
| Montagnulaceae |
| Bimuria D. Hawksw., Chea & Sheridan |
| ?Didymocrea Kowalsky |
| Kalmusia Niessl |
| Karstenula Speg. |
| Letendraea Sacc. |
| Montagnula Berl. |
| Paraphaeosphaeria O.E. Erikss. |
| Tremateia Kohlm., Volkm.-Kohlm. & O.E. Erikss. |
| Morosphaeriaceae |
| ?Asteromassaria Höhn |
| Helicascus Kohlm. |
| Morosphaeria Suetrong, Sakay., E.B.G. Jones & C.L. Schoch |
| Trematosphaeriaceae |
| Falciformispora K.D. Hyde |
| Halomassarina Suetrong, Sakay., E.B.G. Jones, Kohlm., Volkm.-Koh |
| lm. & C.L Schoch |
| Trematosphaeria Fuckel |
| Other families |
| Aigialaceae |
| Aigialus Kohlm. & S. Schatz |
| Ascocratera Kohlm. |
| Rimora Kohlm., Volkm.-Kohlm., Suetrong, Sakay. & E.B.G. Jones |
| Amniculicolaceae |
| Amniculicola Y. Zhang & K.D. Hyde |
| Murispora Yin. Zhang, C.L. Schoch, J. Fourn., Crous & K.D. Hyde |
| ?Massariosphaeria (E. Müll.) Crivelli |
| Neomassariosphaeria Yin. Zhang, J. Fourn. & K.D. Hyde |
| ?Arthopyreniaceae (Massariaceae) |
| Arthopyrenia A. Massal. |
| Dothivalsaria Petr. |
| ?Dubitatio Speg. |
| Massaria De Not. |
| Navicella Fabre |
| Roussoëlla Sacc. |
| ?Roussoellopsis I. Hino & Katum. |
| Delitschiaceae |
| Delitschia Auersw. |
| Ohleriella Earle |
| Semidelitschia Cain & Luck-Allen |
| ?Diademaceae |
| Clathrospora Rabenh. |
| Comoclathris Clem. |
| Diadema Shoemaker & C.E. Babc. |
| Diademosa Shoemaker & C.E. Babc. |
| Graphyllium Clem. |
| Hypsostromataceae |
| Hypsostroma Huhndorf |
| Lindgomycetaceae |
| Lindgomyces K. Hirayama, Kaz. Tanaka & Shearer 2010 |
| Lophiostomataceae |
| Lophiostoma Ces. & De Not. |
| Melanommataceae |
| ?Astrosphaeriella Syd. & P. Syd. (Syn. Javaria) |
| ?Anomalemma Sivan. |
| ?Asymmetricospora J. Fröhl. & K.D. Hyde |
| Bertiella (Sacc.) Sacc. & P. Syd. |
| ?Bicrouania Kohlm. & Volkm.-Kohlm. |
| Byssosphaeria Cooke |
| Calyptronectria Speg. |
| ?Caryosporella Kohlm. |
| Herpotrichia Fuckel |
| ?Mamillisphaeria K.D. Hyde, S.W. Wong & E.B.G. Jones |
| Melanomma Nitschke ex Fuckel |
| Ohleria Fuckel |
| Pseudotrichia Kirschst. |
| Pleomassariaceae |
| ?Lichenopyrenis Calatayud, Sanz & Aptroot |
| ?Splanchnonema Corda |
| ?Peridiothelia D. Hawksw. |
| Pleomassaria Speg. |
| Sporormiaceae |
| Chaetopreussia Locq.-Lin. |
| Eremodothis Arx |
| Pleophragmia Fuckel |
| Preussia Fuckel |
| Sporormia De Not. |
| Westerdykella Stolk |
| ?Teichosporaceae |
| Chaetomastia (Sacc.) Berl |
| Immotthia M.E. Barr |
| Loculohypoxylon M.E. Barr |
| Sinodidymella J.Z. Yue & O.E. Erikss. |
| Teichospora Fuckel |
| Tetraplosphaeriaceae |
| Polyplosphaeria Kaz. Tanaka & K. Hirayama |
| Tetraplosphaeria Kaz. Tanaka & K. Hirayama |
| Triplosphaeria Kaz. Tanaka & K. Hirayama |
| ?Zopfiaceae (syn Testudinaceae) |
| Caryospora De Not. |
| Celtidia J.M. Janse |
| ?Coronopapilla Kohlm. & Volkm.-Kohlm. |
| Halotthia Kohlm. |
| Lepidosphaeria Parg.-Leduc |
| ?Mauritiana Poonyth, K.D. Hyde, Aptroot & Peerally |
| Pontoporeia Kohlm. |
| ?Rechingeriella Petr. |
| Richonia Boud. |
| Testudina Bizz. |
| Verruculina Kohlm. & Volkm.-Kohlm. |
| Ulospora D. Hawksw., Malloch & Sivan. |
| Zopfia Rabenh. |
| Zopfiofoveola D. Hawksw. |
| Pleosporales genera incertae sedis |
| Acrocordiopsis Borse & K.D. Hyde |
| Aglaospora De Not. |
| Anteaglonium Mugambi & Huhndorf |
| Ascorhombispora L. Cai & K.D. Hyde |
| Atradidymella Davey & Currah |
| Belizeana Kohlm. & Volkm.-Kohlm. |
| Biatriospora K.D. Hyde & Borse |
| Byssolophis Clem. |
| Carinispora K.D. Hyde |
| Cilioplea Munk |
| Decaisnella Fabre |
| Epiphegia Nitschke ex G.H. Otth |
| Isthmosporella Shearer & Crane |
| Julella Fabre |
| Lineolata Kohlm. & Volkm.-Kohlm. |
| Lophiella Sacc. |
| Lophionema Sacc. |
| Lophiotrema Sacc. |
| Moristroma A.I. Romero & Samuels |
| Neotestudina Segretain & Destombes |
| Ostropella (Sacc.) Höhn. |
| Paraliomyces Kohlm. |
| Passeriniella Berl. |
| Quintaria Kohlm. & Volkm.-Kohlm. |
| Saccothecium Fr. |
| Salsuginea K.D. Hyde |
| Shiraia P. Henn. |
| Xenolophium Syd. |
| Family excluded |
| Phaeotrichaceae |
| Echinoascotheca Matsush. |
| Phaeotrichum Cain & M.E. Barr |
| Trichodelitschia Munk |
| Genera excluded |
| Kriegeriella Höhn. |
| Muroia I. Hino & Katum. |
| Zeuctomorpha Sivan., P.M. Kirk & Govindu |
Morpho-characters used in taxonomy of Pleosporales
Sexual characters
According to the Linnean classification system, reproductive structures are the most important criteria in plant taxonomy, and this proposal is widely applied in fungal taxonomy (Gaumann 1952). In the classification of Dothideomycetes, reproductive characters such as the uni- or multilocular nature and shape of ascomata, presence and shape of ostioles/papillae, shape and apical structures of asci and shape, pigmentation and septation of ascospores play important roles at different ranks (Clements and Shear 1931; Luttrell 1951, 1955, 1973). Besides the common morphological characters possessed by Dothideomycetes (bitunicate and fissitunicate asci as well as the perithecioid-like ascostromata), most pleosporalean fungi also have pseudoparaphyses among their well-arranged asci (Zhang et al. 2009a). Currently, classification of Pleosporales at the family level focuses mostly on morphological characters of ascomata (such as size, shape of ostiole or papilla), presence or absence of periphyses, characters of centrum (such as asci, pseudoparaphyses and ascospores) as weh as on lifestyle or habitat (Barr 1990a; Shearer et al. 2009; Suetrong et al. 2009; Tanaka et al. 2009; Zhang et al. 2009a), whilst relying extensively on DNA sequence comparisons.
Ascomata
Most species of Pleosporales have uniloculate ascomata. The presence (or absence) and forms of papilla and ostiole are the pitoval character of ascomata, which serve as important characteristics in generic or higher rank classification (Clements and Shear 1931). The vertically flattened papilla has recently been shown as an effective criterion for familial level classification, e.g. in the Amniculicolaceae and the Lophiostomataceae (Zhang et al. 2009a). Papillae and ostioles are present in most species of Pleosporales, except in the Diademaceae and Sporormiaceae. Members of Diademaceae have apothecial ascomata, and some genera of Sporormiaceae have cleistothecioid ascomata. Another coprophilous pleosporalean family, Delitschiaceae, can be distinguished from Sporormiaceae by the presence of periphysate ostioles.
Pseudoparaphyses
Presence of pseudoparaphyses is a characteristic of Pleosporales (Kirk et al. 2008; Liew et al. 2000). Although pseudoparaphyses may be deliquescing in some families when the ascomata mature (e.g. in Didymellaceae), they are persistent in most of other pleosporalean members. According to the thickness, with or without branching and density of septa, pseudoparaphyses were roughly divided into two types: trabeculate and cellular, and their taxonomic significance need to be re-evaluated (Liew et al. 2000).
Asci
The asci of Pleosporales are bitunicate, usually fissitunicate, mostly cylindrical, clavate or cylindro-clavate, and rarely somewhat obclavate or sphaerical (e.g. Macroventuria anomochaeta Aa and Westerdykella dispersa). There are ocular chambers in some genera (e.g. Amniculicola and Asteromassaria), or sometimes with a large apical ring (J-) (e.g. Massaria).
Ascospores
Ascospores of Pleosporales can be hyaline or colored to varying degrees. They may be amerosporous (e.g. species of Semidelitschia), phragmosporous (e.g. Phaeosphaeria and Massariosphaeria), dictyosporous (e.g. most species of Pleospora and Bimuria), or scolecosporous (e.g. type species of Cochliobolus, Entodesmium or Lophionema). Although ascospore morphology had been regarded as a key factor in differentiating genera under some families, e.g. Arthopyreniaceae (Watson 1929) and Testudinaceae (Hawksworth 1979), it has been proven variable even within a single species. For instance, two types of ascospores are produced by Mamillisphaeria dimorphospora, i.e. one type is large and hyaline, and the other is comparatively smaller and brown. Numerous studies have shown the unreliability of ascospore characters above genus level classification (e.g. Phillips et al. 2008; Zhang et al. 2009a).
Asexual states of Pleosporales
Anamorphs of pleosporalean families
Anamorphs of Pleosporales are mostly coelomycetous, but may also be hyphomycetous. Phoma or Phoma-like anamorphic stages and its relatives are most common anamorphs of Pleosporales (Aveskamp et al. 2010; de Gruyter et al. 2009, 2010; Hyde et al. 2011). Some of the reported teleomorph and anamorph connections (including some listed below) are, however, based on the association rather than single ascospore isolation followed by induction of the other stage in culture (Hyde et al. 2011).
Pleosporales suborder Pleosporineae
Pleosporineae is a phylogenetically well supported suborder of Pleosporales, which temporarily includes seven families, namely Cucurbitariaceae, Didymellaceae, Didymosphaeriaceae, Dothidotthiaceae, Leptosphaeriaceae, Phaeosphaeriaceae and Pleosporaceae, and contains many important plant pathogens (de Gruyter et al. 2010; Zhang et al. 2009a). De Gruyter et al. (2009, 2010) systematically analyzed the phylogeny of Phoma and its closely related genera, and indicated that their representative species cluster in different subclades of Pleosporineae.
Cucurbitariaceae
Based on the molecular phylogenetic analysis, some species of Coniothyrium, Pyrenochaeta, Phoma, Phialophorophoma and Pleurophoma belong to Cucurbitariaceae (de Gruyter et al. 2010; Hyde et al. 2011). Other reported anamorphs of Cucurbitaria are Camarosporium, Diplodia- like and Pleurostromella (Hyde et al. 2011; Sivanesan 1984). The generic type of Cucurbitaria (C. berberidis Fuckel) is linked to Pyrenochaeta berberidis (Farr et al. 1989). Curreya has a Coniothyrium-like anamorphic stage (von Arx and van der Aa 1983; Marincowitz et al. 2008). The generic type of Curreya is C. conorum (Fuckel) Sacc., which is reported to be linked with Coniothyrium glomerulatum Sacc. (von Arx and van der Aa 1983). The generic type of Rhytidiella (R. moriformis, Cucurbitariaceae) can cause rough-bark of Populus balsamifera, and has a Phaeoseptoria anamorphic stage (Zalasky 1968). Rhytidiella baranyayi Funk & Zalasky, another species of Rhytidiella associated with the cork-bark disease of aspen is linked with Pseudosporella-like anamorphs (Funk and Zalasky 1975; Sivanesan 1984).
Didymellaceae, Didymosphaeriaceae and Dothidotthiaceae
As has been mentioned before, Phoma sensu lato species have been proved to be highly polyphyletic, and they cluster in six distinct familial clades within the Pleosporales (Aveskamp et al. 2010). Most Phoma species, including the generic type (P. herbarum), clustered in Didymellaceae (Aveskamp et al. 2010). The clade of Didymellaceae also comprises other sections, such as Ampelomyces, Boeremia, Chaetasbolisia, Dactuliochaeta, Epicoccum, Peyronellaea, Phoma-like, Piggotia, Pithoascus, as well as the type species of Ascochyta and Microsphaeropsis (Aveskamp et al. 2010; de Gruyter et al. 2009; Kirk et al. 2008; Sivanesan 1984). Leptosphaerulina is another genus of Didymellaceae, which has hyphomycetous anamorphs with pigmented and muriform conidia, such as Pithomyces (Roux 1986).
The other reported anamorphs of Didymosphaeria are Fusicladiella-like, Dendrophoma, Phoma-like (Hyde et al. 2011). Hyphomycetous Thyrostroma links to Dothidotthiaceae (Phillips et al. 2008).
Some important plant pathogens are included within Didymellaceae, such as Phoma medicaginis Malbr. & Roum., which is a necrotrophic pathogen on Medicago truncatula (Ellwood et al. 2006). Phoma herbarum is another plant pathogen, which has potential as a biocontrol agent of weeds (Neumann and Boland 2002). Ascochyta rabiei is a devastating disease of chickpea in most of the chickpea producing countries (Saxena and Singh 1987).
Leptosphaeriaceae
The anamorphic stages of Leptosphaeriaceae can be Coniothyrium, Phoma, Plenodomus and Pyrenochaeta. AH are coelomycetous anamorphs, and they may have phialidic or annellidic conidiogenous cells. Phoma heteromorphospora Aa & Kesteren, the type species of Phoma sect. Heterospora and Coniothyrium palmarum, the generic type of Coniothyrium, reside in Leptosphaeriaceae (de Gruyter et al. 2009).
Pleosporaceae
Various anamorphic types can occur in Pleosporaceae, which can be coelomycetous or hyphomycetous, and the ontogeny of conidiogenous cells can be phialidic, annellidic or sympodial blastic. Both Ascochyta caulina and Phoma betae belong to Pleosporaceae (de Gruyter et al. 2009).
Some species of Bipolaris and Curvularia are anamorphs of Cochliobolus. Many species of these two genera cause plant disease or even infect human beings (Khan et al. 2000). They are hyphomycetous anamorphs with sympodial proliferating conidiogenous cells, and pigmented phragmosporous poroconidia. The generic type of Lewia (L. scrophulariae) is linked with Alternaria conjuncta E.G. Simmons (Simmons 1986), and the generic type of Pleospora (P. herbarum) is linked with Stemphylium botryosum Sacc. (Sivanesan 1984). Both Alternaria and Stemphylium are hyphomycetous anamorphs characterized by pigmented, muriform conidia that develop at a very restricted site in the apex of distinctive conidiophores (Simmons 2007).
The generic type of Pleoseptum (P. yuccaesedum) is linked with Camarosporium yuccaesedum (Ramaley and Barr 1995), the generic type of Macrospora (M. scirpicola) with Nimbya scirpicola (Fuckel) E.G. Simmons (Simmons 1989), and the generic type of Setosphaeria (S. turcica) with Drechslera turcica (Pass.) Subram. & B.L. Jain (Sivanesan 1984). Pyrenophora has the anamorphic stages of Drechslera, and the anamorphic stage of Wettsteinina can be species of Stagonospora (Farr et al. 1989).
Most common anamorphs in Pleosporaceae are Alternaria, Bipolaris, Phoma-like and Stemphylium, and they can be saprobic or parasitic on various hosts. Phoma betae A.B. Frank is a notorious pathogen on sugar beet, which causes zonate leaf spot or Phomopsis of sugar beet. Alternaria porri (Ellis) Cif., Stemphylium solani G.F. Weber, S. botryosum and S. vesicarium (Wallr.) E.G. Simmons can cause leaf blight of garlic (Zheng et al. 2009). Phoma incompta Sacc. & Martelli is a pathogen on olive, and Stemphylium botryosum, the anamorph of Pleospora herbarum, causes leaf disease of olive trees (Malathrakis 1979).
Phaeosphaeriaceae
The type species of Phoma sect. Paraphoma (Phoma radicina (McAlpine) Boerema) as well as several pathogens on Gramineae, i.e. Stagonospora foliicola (Bres.) Bubak, S. neglecta var. colorata and Wojnowicia hirta Sacc. belong to Phaeosphaeriaceae (de Gruyter et al. 2009). Other anamorphs reported for Phaeosphaeriaceae are Amarenog-raphium, Ampelomyces, Chaetosphaeronema, Coniothyrium, Hendersonia, Neosetophoma, ?Parahendersonia, Paraphoma, Phaeoseptoria, Rhabdospora, Scolecosporiella, Setophoma, Sphaerellopsis and Tiarospora.
These anamorphic fungi can be saprobic, but mostly pathogenic on herbaceous plants. For instance, Stagonospora foliicola and Coniothyrium concentricum (Desm.) Sacc. can cause leaf spots on herbaceous plants (Zeiders 1975), and Ampelomyces quisqualis Ces. is a hyperparasite of powdery mildews.
Pleosporales suborder Massarineae
Massarineae species are mostly saprobic in terrestrial or aquatic environments. Five families are currently included within Massarineae, viz. Lentitheciaceae, Massarinaceae, Montagnulaceae, Morosphaeriaceae and Trematosphaeriaceae. Anamorphs of the five families are summarized as follows.
Lentitheciaceae
Stagonospora macropycnidia Cunnell nests within the clade of Lentitheciaceae (Plate 1). A relatively broad genus concept of Stagonospora is currently accepted, which comprises parasitic or saprobic taxa. Keissleriella cladophila (Niessl) Corbaz is another species nesting within Lentitheciaceae (Zhang et al. 2009a), and is linked with Dendrophoma sp., which has branching conidiogenous cells, and 1-celled, hyaline conidia (Bose 1961; Sivanesan 1984).
Massarinaceae
A relatively narrow concept tends to be accepted for Massarinaceae, which seems only to comprise limited species such as Byssothecium circinans, Massarina eburnea, M. cisti S.K. Bose, M. igniaria (C. Booth) Aptroot (anamorph: Periconia igniaria E.W. Mason & M.B. Ellis) and Neottiosporina paspali (G.F. Atk.) B. Sutton & Alcorn (Zhang et al. 2009a; Plate 1). Similarly, a relatively narrow generic concept of Massarina was accepted, containing only M. eburnea and M. cisti (Zhang et al. 2009b), and both species have been linked with species of Ceratophoma (Sivanesan 1984).
Montagnulaceae
Montagnula has an Aschersonia anamorph, and Kalmusia and Paraphaeosphaeria have Coniothyrium-like, Cytoplea, Microsphaeropsis and Paraconiothyrium anamorphs. The generic type of Paraphaeosphaeria (P. michotii) is linked with Coniothyrium scirpi Trail (Webster 1955). The Coniothyrium complex is highly polyphyletic, and was subdivided into four groups by Sutton (1980), viz. Coniothyrium, Microsphaeropsis, Cyclothyrium and Cytoplea. Paraconiothyrium was introduced to accommodate Coniothyrium minitans W.A. Campb. and C. sporulosum (W. Gams & Domsch) Aa, which are closely related to Paraphaeosphaeria based on 18S rDNA sequences phylogeny (Verkley et al. 2004).
Morosphaeriaceae
Based on the multigene phylogenetic analysis in this study, Asteromassaria is tentatively included in Morosphaeriaceae. Asteromassaria macrospora is linked with Scolicosporium macrosporium (Berk.) B. Sutton, which is hyphomycetous. No anamorphic stages have been reported for other species of Morosphaeriaceae.
Trematosphaeriaceae
Three species from three different genera were included in Trematosphaeriaceae, i.e. Falciformispora lignatilis, Halomassarina thalassiae and Trematosphaeria pertusa (Suetrong et al. data unpublished; Plate 1). Of these, only Trematosphaeria pertusa, the generic type of Trematosphaeria, produces hyphopodia-like structures on agar (Zhang et al. 2008a).
Other families of Pleosporales
Amniculicolaceae
Three anamorphic species nested within the clade of Amniculicolaceae, i.e. Anguillospora longissima (Sacc. & P. Syd.) Ingold, Repetophragma ontariense (Matsush.) W. P. Wu and Spirosphaera cupreorufescens Voglmayr (Zhang et al. 2009a). Sivanesan (1984, p. 500) described the teleomorphic stage of Anguillospora longissima as Massarina sp. II, which fits the diagnostic characters of Amniculicola well. Thus this taxon may be another species of Amniculicola.
Hypsostromataceae
A Pleurophomopsis-like anamorph is reported in the subiculum of the generic type of Hypsostroma (H. saxicola Huhndorf) (Huhndorf 1992).
Lophiostomataceae
The concept of Lophiostomataceae was also narrowed, and presently contains only Lophiostoma (Zhang et al. 2009a). Leuchtmann (1985) studied cultures of some Lophiostoma species, and noticed that L. caulium (Fr.) Ces. & De Not., L. macrostomum, L. semiliberum (Desm.) Ces. & De Not., Lophiostoma sp. and Lophiotrema nucula produced Pleurophomopsis anamorphic stages, which are similar to those now in Melanomma (Chesters 1938), but Lophiostoma and Melanomma has no proven phylogenetic relationship (Zhang et al. 2009a, b; Plate 1). Species of Aposphaeria have also been reported in Massariosphaeria (Farr et al. 1989; Leuchtmann 1984), but the polyphyletic nature of Massariosphaeria is well documented (Wang et al. 2007).
Melanommataceae
The anamorphs of the Melanommataceae are mostly coelomycetous and rarely hyphomycetous with various ontogenic structures, such as annellidic or sympodial for hyphomycetes (Exosporiella and Pseudospiropes) and coelomycetes (Aposphaeria-like and Pyrenochaeta).
Herpotrichia is reported as having a Pyrenochaeta anamorphic stage with or without seta on the surface of pycnidia (Sivanesan 1984). Aposphaeria and Phoma-like have been reported in Melanomma species (Chesters 1938; Sivanesan 1984). Similarly, the anamorphs of Karstenula are reported as coelomycetous, i.e. Microdiplodia (Constantinescu 1993). The anamorphic stage of Anomalemma is Exosporiella (Sivanesan 1983), and that of Byssosphaeria is Pyrenochaeta (Barr 1984). Ohleria brasiliensis Starback has been linked with Monodictys putredinis (Wallr.) S. Hughes (Samuels 1980). Astrosphaeriella is a contentious genus as its familial status is not determined yet. Here we temporarily assigned it under Melanommataceae, which is linked with the anamorph genus Pleurophomopsis.
Pleomassariaceae
Shearia and Prosthemium are all anamorphs of Pleomassaria, and Prosthemium betulinum is linked with the generic type of Pleomassaria (P. siparia) (Barr 1982b; Sivanesan 1984; Sutton 1980; Tanaka et al. 2010). Splanchnonema is a genus of Pleomassariaceae, the teleomorphic morphology of which is difficult to distinguish from two other genera, i.e. Asteromassaria and Pleomassaria, and the reported anamorphs of Splanchnonema are Ceuthodiplospora, Myxocyclus and Stegonsporium, which are comparable with those of Asteromassaria and Pleomassaria.
Tetraplosphaeriaceae
Tetraplosphaeriaceae was introduced to accommodate the Massarina-like bambusicolous fungi that produce Tetraploa sensu stricto anamorphs (Tanaka et al. 2009). Tetraploa aristata Berk. & Broome, the generic type of Tetraploa is widely distributed, associated with various substrates and many occur in freshwater or has been isolated from air. The polyphyletic nature of T. aristata has been well documented (Tanaka et al. 2009). Anamorphic stages can serve as a diagnostic character for this family.
Diademaceae, Massariaceae, Sporormiaceae and Teichosporaceae
The Sporormiaceae is coprophilous having Phoma or Phoma-related anamorphic states (Cannon and Kirk 2007). Comoclathris (Diademaceae) is linked with Alternaria-like anamorphs (Simmons 1952). Myxocyclus links to Massaria (Massariaceae) (Hyde et al. 2011). The anamorphic stage of Chaetomastia (Teichosporaceae) is Aposphaeria- or Conio- thyrium-like (Barr 1989c).
Generally speaking, the morphologically simple conidio-phores are usually considered phylogenetically uninformative (Seifert and Samuels 2000). Phoma-like anamorphs commonly occur in Pleosporales, while their colorless and unicellular conidia are also not phylogenetically informative (Seifert and Samuels 2000).
AH of the above mentioned anamorphic taxa of Pleosporales have phialidic, annellidic or sympodial conidiogenous cells, representing apical wall-building type (compared to ring wall-building and diffused wall-building) (Nag Raj 1993), which may indicate that the wall-building type probably has phylogenetic significance.
Molecular phylogeny of Pleosporales
Numerous genes have been applied in phylogenetic studies of Pleosporales, mostly including LSU, SSU, mtSSU and ITS as well as the protein genes, such as RPB1, RPB2, TEF1, β-tubulin (TUB1) and actin (ACT1). A single gene such as ITS or LSU, has been used to study phylogenetic relationships between Leptosphaeria and Phaeosphaeria (Camara et al. 2002) or Pleosporaceae and Tubeufiaceae (Kodsueb et al. 2006a, b) (Table 2). The use of these phylogenetic markers, although making important contributions, has not been successful in resolving numerous relationships in single gene dendrograms. One exception is the use of SSU sequences to demonstrate the phylogenetic significance of pseudopar-aphyses (Liew et al. 2000) whilst rejecting the phylogenetic utility of pseudoparaphyses morphology (cellular or trabeculate). Analyses with combined genes have had more success. For instance combined analyses with LSU and SSU sequence data could be used to define family level classification in a few cases (Dong et al. 1998; de Gruyter et al. 2009; Lumbsch and Lindemuth 2001; Pinnoi et al. 2007; Zhang et al. 2009b) (Table 2). The addition of more than two genes has been used to determine relationships between orders. For instance, genes such as LSU, SSU and mtSSU have been used to analyze ordinal relationships in Loculoascomycetes (Lindemuth et al. 2001), and to analyze phylogenetic relationships of coprophilous families in Pleosporales (Kruys et al. 2006). Phaeocryptopus gaeumannii (T. Rohde) Petr. was shown to belong in Dothideales based on LSU, SSU and ITS sequence analysis (Winton et al. 2007), while Schoch et al. (2006) used four genes, i.e. LSU, SSU, RPB2 and TEF1 to evaluate the phylogenetic relationships among different orders of the Dothideomycetes. Five genes, viz. LSU, SSU, TEF1, RPB1 and RPB2, were used to study the phylogenetic relationships of different orders within Dothideomycetes (Schoch et al. 2009) and of different families within Pleosporales (Zhang et al. 2009a) (Table 2). It is clear that even more genes will be required to address the remaining issues and the promise of genome analyses is within reach (www.jgi.doe.gov/sequencing/why/dothideomycetes.html) for Dothideomycetes.
Table 2.
List of phylogenetic studies on Pleosporales
| Year | Author(s) | Loci used | Target fungi | General conclusion |
|---|---|---|---|---|
| 1998 | Dong et al. | LSU, SSU |
Leptosphaeriaceae, Pleosporaceae and three other families |
Leptosphaeriaceae is paraphyletic and Pleosporaceae is monophyletic. |
| 2000 | Liew et al. | SSU | Pleosporales and Melanommatales |
Pleosporales and Melanommatales are not naturial groups. |
| 2001 | Lindemuth et al. | LSU, SSU, mtSSU | loculoascomycetes | Loculoascomycetes are not monophyletic. |
| 2001 | Lumbsch and Lindemuth |
LSU, SSU | Dothideomycetes | Presence of pseudoparaphyses is a major character at order level classification |
| 2002 | Câmara et al. | ITS | Leptosphaeria and Phaeosphaeria | Accepted Leptosphaeria sensu stricto. |
| 2006 | Kodsueb et al. | LSU | Pleosporaceae |
Wettsteinina should be excluded from the Pleosporaceae. |
| 2006 | Kodsueb et al. | LSU | Tubeufiaceae |
Tubeufiaceae is more closely related to the Venturiaceae. |
| 2006 | Kruys et al. | LSU, SSU, mtSSU | coprophilous familes of Pleosporales |
coprophilous familes of Pleosporales form phylogenetic monophyletic groups respectively |
| 2006 | Schoch et al. | LSU, SSU, TEF1, RPB2 |
Dothideomycetes | Proposed the subclasses Pleosporomycetidae |
| 2007 | Pinnoi et al. | LSU, SSU | Pleosporales | phylogenetic relationships of different families of Pleosporales, introduced a new fungus-- Berkleasmium crunisia |
| 2007 | Wang et al. | LSU, SSU, RPB2 | Massariosphaeria | Massariosphaeria is not monophyletic |
| 2007 | Winton et al. | LSU, SSU, ITS | Phaeocryptopus gaeumannii |
Phaeocryptopus gaeumannii nested in Dothideales. |
| 2008a | Zhang et al. | LSU, SSU | Melanomma and Trematosphaeria |
Melanomma and Trematosphaeria belong to different families |
| 2009 | de Gruyter et al. | LSU, SSU; | Phoma and related genera | They are closely related with Didymellaceae, Leptosphaeriaceae, Phaeosphaeriaceae and Pleosporaceae |
| 2009a | Zhang et al. | LSU, SSU, TEF1, RPB1, RPB2 |
Pleosporales |
Amniculicolaceae and Lentitheciaceae were introduced, and Pleosporineae recircumscribed. |
| 2009 | Mugambi and Huhndorf |
LSU, TEF1 |
Melanommataceae, Lophiostomataceae |
Recircumscribed Melanommataceae and Lophiostomataceae, and reinstated Hypsostromataceae. |
| 2009 | Nelsen et al. | LSU and mtSSU | lichenized Dothideomycetes | Pyrenocarpous lichens with bitunicate asci are not monophyletic, but belong to at least two classes (Dothideomycetes and Erotiomycetes). |
| 2009 | Suetrong et al. | LSU, SSU, TEF1, RPB1 | marine Dothideomycetes | Two new families are introduced Aigialaceae and Morosphaeriaceae. |
| 2009 | Shearer et al. | LSU, SSU | freshwater Dothideomycetes | Freshwater Dothideomycetes are related to terrestrial taxa and have adapted to freshwater habitats numerous times. |
| 2009 | Tanaka et al. | LSU, SSU, TEF1, ITS, BT | bambusicolous Pleosporales | Introduced Tetraplosphaeriaceae with Tetraploa-like anamorphs. |
| 2009 | Kruys and Wedin | ITS-nLSU, mtSSU rDNA and β-tubulin |
Sporormiaceae | Analyzed the inter-generic relationships as well as evaluated the morphological sig- nificance used in this family. |
| 2010 | Hirayama et al. | LSU, SSU | Massarina ingoldiana sensu lato |
Massarina ingoldiana sensu lato is polyphyletic, and separated into two clades within Pleosporales. |
| 2010 | Aveskamp et al. | LSU, SSU, ITS and β-tubulin |
Phoma and related genera within Didymellaceae |
Rejected current Boeremaean subdivision. |
| 2010 | de Gruyter et al. | LSU, SSU |
Phoma and related genera within Pleosporineae |
Introduced Pyrenochaetopsis, Setophoma and Neosetophoma and reinstated Cucurbitariaceae within Pleosporineae |
The importance of generic type specimens
The type specimen (collection type) is a fundamental element in the current Code of Botanical Nomenclature at familial or lower ranks (Moore 1998). A type specimen fixes the name to an exact specimen at family, genera, species and variety/subspecies rank and is ultimately based on this single specimen, i.e. a family name is based on a genus, the genus name is based on a species, and the species name is based on a specimen (Kirk et al. 2008).
The generic type is of great importance in defining generic circumscriptions in fungal taxonomy. The generic types of Pleosporales have been studied previously by many mycologists. For instance, Muller and von Arx (1962) studied the generic types of “Pyrenomycetes”, and described and illustrated them in detail. Sivanesan (1984) described and illustrated the generic representatives of Loculoascomycetes for both their teleomorphs and anamorphs, and their links were emphasized. A large number of pleosporalean genera have been studied by Barr (1990a, b). Almost all of the previous work was conducted more than 20 years ago, when no molecular phylogenetic studies could be carried out and thus had been carried out in a systematic fashion.
Aim and outline of present study
The present study had two principal objectives:
To explore genera under Pleosporales based on the generic types and provide a detailed description and illustration for the type species of selected genera, discuss the study history of those genera, and explore their ordinal, familial, and generic relationships;
To investigate the phylogeny of Pleosporales, its inter-familial relationships, and the morphological circum-scription of each family;
In order to clarify morphological characters, the generic types of the majority of teleomorphic pleosporalean genera (> 60%) were studied. Most of them are from the “core families” of Pleosporales, i.e. Delitschiaceae, Lophiostomataceae, Massariaceae, Massarinaceae, Melanommataceae, Montagnulaceae, Phaeosphaeriaceae, Phaeotrichaceae, Pleomassariaceae, Pleosporaceae, Sporormiaceae and Teichosporaceae. Notes are given for those where type specimens could not be obtained during the timeframe of this study. A detailed description and illustration of each generic type is provided. Comments, notes and problems that need to be addressed are provided for each genus. Phylogenetic investigation based on five nuclear loci, viz. LSU, SSU, RPB1, RPB2 and TEF1 was carried out using available strains from numerous genera in Pleosporales. In total, 278 pleosporalean taxa are included in the phylogenetic analysis, which form 25 familial clades on the dendrogram (Plate 1). The suborder, Massarineae, is emended to accommodate Lentitheciaceae, Massarinaceae, Montagnulaceae, Morosphaeriaceae and Trematosphaeriaceae.
Materials and methods
Molecular phylogeny
Four genes were used in this analysis, the large and small subunits of the nuclear ribosomal RNA genes (LSU, SSU) and two protein coding genes, namely the second largest subunit of RNA polymerase II (RPB2) and translation elongation factor-1 alpha (TEF1). AH sequences were downloaded from GenBank as listed in Table 3. Each of the individual ribosomal genes was aligned in SATe under default settings with at least 20 iterations. The protein coding genes were aligned in BioEdit (Hall 2004) and completed by manual adjustment. Introns were removed and all genes were concatenated in a single nucleotide alignment with 43% missing and gap characters out of a total set of 5081. The alignment had 100% representation for LSU, 75% for SSU, 48% for RPB2 and 65% for TEF1. The final data matrix had 280 taxa including outgroups (Table 3).
Table 3.
Taxa used in the phylogenetic analysis and their corresponding GenBank numbers. Culture and voucher abbreviations are indicated were available
| Species | Culture/voucher1 | LSU | SSU | RPB2 | TEF1 |
|---|---|---|---|---|---|
| Acrocordiopsis patilii | BCC 28166 | GU479772 | GU479736 | GU479811 | |
| Acrocordiopsis patilii | BCC 28167 | GU479773 | GU479737 | GU479812 | |
| Aigialus grandis | BCC 18419 | GU479774 | GU479738 | GU479813 | GU479838 |
| Aigialus grandis | JK 5244A | GU301793 | GU296131 | GU371762 | |
| Aigialus mangrovis | BCC 33563 | GU479776 | GU479741 | GU479815 | GU479840 |
| Aigialus mangrovis | BCC 33564 | GU479777 | GU479742 | GU479816 | GU479841 |
| Aigialus parvus | A6 | GU301795 | GU296133 | GU371771 | GU349064 |
| Aigialus parvus | BCC 32558 | GU479779 | GU479743 | GU479818 | GU479843 |
| Aigialus rhizophorae | BCC 33572 | GU479780 | GU479745 | GU479819 | GU479844 |
| Aigialus rhizophorae | BCC 33573 | GU479781 | GU479746 | GU479820 | GU479845 |
| Alternaria alternata | CBS 916.96 | DQ678082 | DQ678031 | DQ677980 | DQ677927 |
| Amniculicola immersa | CBS 123083 | FJ795498 | GU456295 | GU456358 | GU456273 |
| Amniculicola parva | CBS 123092 | FJ795497 | GU296134 | GU349065 | |
| Anteaglonium abbreviatum | ANM 925.1 | GQ221877 | GQ221924 | ||
| Anteaglonium abbreviatum | GKM 1029 | GQ221878 | GQ221915 | ||
| Anteaglonium globosum | ANM 925.2 | GQ221879 | GQ221925 | ||
| Anteaglonium latirostrum | L100N 2 | GQ221876 | GQ221938 | ||
| Arthopyrenia salicis | 1994 Coppins | AY607730 | |||
| Arthopyrenia salicis | CBS 368.94 | AY538339 | AY538333 | ||
| Ascochyta pisi | CBS 126.54 | DQ678070 | DQ678018 | DQ677967 | DQ677913 |
| Ascocratera manglicola | BCC 09270 | GU479782 | GU479747 | GU479821 | GU479846 |
| Ascocratera manglicola | JK 5262 C | GU301799 | GU296136 | GU371763 | |
| Asteromassaria pulchra | CBS 124082 | GU301800 | GU296137 | GU371772 | GU349066 |
| Astrosphaeriella aggregata | MAFF 239485 | AB524590 | AB524449 | ||
| Astrosphaeriella aggregata | MAFF 239486 | AB524591 | AB524450 | AB539105 | AB539092 |
| Astrosphaeriella bakeriana | CBS 115556 | GU301801 | GU349015 | ||
| Astrosphaeriella stellata | MAFF 239487 | AB524592 | AB524451 | ||
| Beverwykella pulmonaria | CBS 283.53 | GU301804 | GU371768 | ||
| Biatriospora marina | CY 1228 | GQ925848 | GQ925835 | GU479823 | GU479848 |
| Bimuria novae-zelandiae | CBS 107.79 | AY016356 | AY016338 | DQ470917 | DQ471087 |
| Byssolophis sphaerioides | IFRDCC2053 | GU301805 | GU296140 | GU456348 | GU456263 |
| Byssosphaeria jamaicana | SMH1403 | GU385152 | GU327746 | ||
| Byssosphaeria rhodomphala | GKM L153N | GU385157 | GU327747 | ||
| Byssosphaeria salebrosa | SMH2387 | GU385162 | GU327748 | ||
| Byssosphaeria schiedermayeriana | GKM1197 | GU385161 | GU327750 | ||
| Byssosphaeria schiedermayeriana | GKM152N | GU385168 | GU327749 | ||
| Byssosphaeria villosa | GKM204N | GU385151 | GU327751 | ||
| Byssothecium circinans | CBS 675.92 | AY016357 | AY016339 | DQ767646 | GU349061 |
| Chaetosphaeronema hispidulum | CBS 216.75 | EU754144 | EU754045 | GU371777 | |
| Cochliobolus heterostrophus | CBS 134.39 | AY544645 | AY544727 | DQ247790 | DQ497603 |
| Cochliobolus sativus | DAOM 226212 | DQ678045 | DQ677995 | DQ677939 | |
| Corynespora cassiicola | CBS 100822 | GU301808 | GU296144 | GU371742 | GU349052 |
| Corynespora olivacea | CBS 114450 | GU301809 | GU349014 | ||
| Corynespora smithii | CABI 5649b | GU323201 | GU371783 | GU349018 | |
| Cucurbitaria berberidis | CBS 394.84 | GQ387605 | GQ387544 | ||
| Decaisnella formosa | BCC 25616 | GQ925846 | GQ925833 | GU479825 | GU479851 |
| Decaisnella formosa | BCC 25617 | GQ925847 | GQ925834 | GU479824 | GU479850 |
| Decorospora gaudefroyi | CBS 332.63 | EF177849 | AF394542 | ||
| Delitschia cf. chaetomioides | GKM 1283 | GU385172 | |||
| Delitschia cf. chaetomioides | GKM 3253.2 | GU390656 | |||
| Delitschia chaetomioides | GKM1283 | GU385172 | GU327752 | ||
| Delitschia chaetomioides | SMH3253.2 | GU390656 | GU327753 | ||
| Delitschia winteri | CBS 225.62 | DQ678077 | DQ678026 | DQ677975 | DQ677922 |
| Didymella exigua | CBS 183.55 | EU754155 | EU754056 | ||
| Didymocrea sadasivanii | CBS 438 65 | DQ384103 | DQ384066 | ||
| Didymosphaeria futilis | CMW 22186 | EU552123 | |||
| Didymosphaeria futilis | HKUCC 5834 | GU205219 | GU205236 | ||
| Dothidotthia aspera | CPC 12933 | EU673276 | EU673228 | ||
| Dothidotthia symphoricarpi | CBS119687 | EU673273 | EU673224 | ||
| Entodesmium rude | CBS 650.86 | GU301812 | GU349012 | ||
| Falciformispora lignatilis | BCC 21117 | GU371826 | GU371834 | GU371819 | |
| Falciformispora lignatilis | BCC 21118 | GU371827 | GU371835 | GU371820 | |
| Floricola striata | JK 5603 K | GU479785 | GU479751 | ||
| Floricola striata | JK 5678I | GU301813 | GU296149 | GU371758 | |
| Halomassarina thalassiae | BCC 17055 | GQ925850 | GQ925843 | ||
| Halomassarina thalassiae | JK 5262D | GU301816 | GU349011 | ||
| Halotthia posidoniae | BBH 22481 | GU479786 | GU479752 | ||
| Helicascus nypae | BCC 36751 | GU479788 | GU479754 | GU479826 | GU479854 |
| Helicascus nypae | BCC 36752 | GU479789 | GU479755 | GU479827 | GU479855 |
| Herpotrichia diffusa | CBS 250.62 | DQ678071 | DQ678019 | DQ677968 | DQ677915 |
| Herpotrichia juniperi | CBS 200.31 | DQ678080 | DQ678029 | DQ677978 | DQ677925 |
| Herpotrichia macrotricha | GKM196N | GU385176 | GU327755 | ||
| Herpotrichia macrotricha | SMH269 | GU385177 | GU327756 | ||
| Hypsostroma caimitalense | GKM 1165 | GU385180 | |||
| Hypsostroma saxicola | SMH 5005 | GU385181 | |||
| Hysterium angustatum | CBS 123334 | FJ161207 | FJ161167 | FJ161129 | FJ161111 |
| Hysterium angustatum | CBS 236.34 | FJ161180 | GU397359 | FJ161117 | FJ161096 |
| Julella avicenniae | BCC 18422 | GU371823 | GU371831 | GU371787 | GU371816 |
| Julella avicenniae | BCC 20173 | GU371822 | GU371830 | GU371786 | GU371815 |
| Julella avicenniae | JK 5326A | GU479790 | GU479756 | ||
| Kalmusia scabrispora | MAFF 239517 | AB524593 | AB524452 | AB539093 | AB539106 |
| Kalmusia scabrispora | NBRC 106237 | AB524594 | AB524453 | AB539094 | AB539107 |
| Karstenula rhodostoma | CBS 690.94 | GU301821 | GU296154 | GU371788 | GU349067 |
| Katumotoa bambusicola | MAFF 239641 | AB524595 | AB524454 | AB539095 | AB539108 |
| Keissleriella cladophila | CBS 104.55 | GU301822 | GU296155 | GU371735 | GU349043 |
| Keissleriella rara | CBS 118429 | GU479791 | GU479757 | ||
| Kirschsteiniothelia elaterascus | A22-5A/HKUCC7769 | AY787934 | AF053727 | ||
| Lentithecium aquaticum | CBS 123099 | GU301823 | GU296156 | GU371789 | GU349068 |
| Lentithecium arundinaceum | CBS 123131 | GU456320 | GU456298 | GU456281 | |
| Lentithecium arundinaceum | CBS 619.86 | GU301824 | GU296157 | FJ795473 | |
| Lentithecium fluviatile | CBS 122367 | GU301825 | GU296158 | GU349074 | |
| Lepidosphaeria nicotiae | CBS 101341 | DQ678067 | DQ677963 | DQ677910 | |
| Leptosphaeria biglobosa | CBS 298.36 | GU237980 | GU238207 | ||
| Leptosphaeria biglobosa | CBS 303.51 | GU301826 | GU349010 | ||
| Leptosphaeria doliolum | CBS 505.75 | GU301827 | GU296159 | GU349069 | |
| Leptosphaeria dryadis | CBS 643.86 | GU301828 | GU371733 | GU349009 | |
| Leptosphaerulina argentinensis | CBS 569.94 | GU301829 | GU349008 | ||
| Leptosphaerulina australis | CBS 311.51-T | FJ795500 | GU456357 | GU456272 | |
| Leptosphaerulina australis | CBS 317.83 | GU301830 | GU296160 | GU371790 | GU349070 |
| Leptosphearia maculans | DAOM 229267 | DQ470946 | DQ470993 | DQ470894 | DQ471062 |
| Letendraea helminthicola | CBS 884.85 | AY016362 | AY016345 | ||
| Letendraea padouk | CBS 485.70 | AY849951 | GU296162 | ||
| Lindgomyces breviappendiculatus | KT 1399 | AB521749 | AB521734 | ||
| Lindgomyces cinctosporae | R56-1 | AB522431 | AB522430 | ||
| Lindgomyces cinctosporae | R56-3 | GU266245 | GU266238 | ||
| Lindgomyces ingoldianus | KH 100 JCM 16479 | AB521737 | AB521720 | ||
| Lindgomyces rotundatus | KH 114 JCM 16484 | AB521742 | AB521725 | ||
| Lophiostoma alpigenum | GKM 1091b | GU385193 | |||
| Lophiostoma arundinis | CBS 621.86 | DQ782384 | DQ782383 | DQ782386 | DQ782387 |
| Lophiostoma caulium | CBS 623.86 | GU301833 | GU296163 | GU371791 | |
| Lophiostoma compressum | IFRD 2014 | GU301834 | GU296164 | FJ795457 | |
| Lophiostoma crenatum | CBS 629.86 | DQ678069 | DQ678017 | DQ677965 | DQ677912 |
| Lophiostoma fuckelii | CBS 101952 | DQ399531 | |||
| Lophiostoma fuckelii | CBS 113432 | EU552139 | |||
| Lophiostoma fuckelii | GKM 1063 | GU385192 | |||
| Lophiostoma macrostomum | CBS 122681 | EU552141 | |||
| Lophiostoma macrostomum | HHUF 27293 | AB433274 | |||
| Lophiostoma macrostomum | KT 635 | AB433273 | AB521731 | ||
| Lophiostoma quadrinucleatum | GKM1233 | GU385184 | GU327760 | ||
| Lophiostoma sagittiforme | HHUF 29754 | AB369267 | |||
| Lophiotrema brunneosporum | CBS 123095 | GU301835 | GU296165 | GU349071 | |
| Lophiotrema lignicola | CBS 122364 | GU301836 | GU296166 | GU349072 | |
| Massarina arundinariae | MAFF 239461 | AB524596 | AB524455 | AB539096 | AB524817 |
| Massarina arundinariae | NBRC 106238 | AB524597 | AB524456 | AB539097 | AB524818 |
| Lophiotrema nucula | CBS 627.86 | GU301837 | GU296167 | GU371792 | GU349073 |
| Loratospora aestuarii | JK 5535B | GU301838 | GU296168 | GU371760 | |
| Macroventuria anomochaeta | CBS 525.71 | GU456315 | GU456346 | GU456262 | |
| Massaria anomia | CBS 123109 | GU301792 | GU296130 | GU349062 | |
| Massaria anomia | CBS 591.78 | GU301839 | GU296169 | GU371769 | |
| Massaria ariae | M52 | HQ599382 | HQ599456 | HQ599322 | |
| Massaria aucupariae | M49 | HQ599384 | HQ599455 | HQ599324 | |
| Massaria campestris | M28 | HQ599385 | HQ599449 | HQ599459 | HQ599325 |
| Massaria conspurcata | M14 | HQ599393 | HQ599441 | HQ599333 | |
| Massaria gigantispora | M26 | HQ599397 | HQ599447 | HQ599337 | |
| Massaria inquinans | M19 | HQ599402 | HQ599444 | HQ599460 | HQ599342 |
| Massaria lantanae | M18 | HQ599406 | HQ599443 | HQ599346 | |
| Massaria macra | M3 | HQ599408 | HQ599450 | HQ599348 | |
| Massaria mediterranea | M45 | HQ599417 | HQ599452 | HQ599357 | |
| Massaria platanoidea | M7 | HQ599420 | HQ599457 | HQ599462 | HQ599359 |
| Massaria pyri | M21 | HQ599424 | HQ599445 | HQ599363 | |
| Massaria vindobonensis | M27 | HQ599429 | HQ599448 | HQ599464 | HQ599368 |
| Massaria vomitoria | M13 | HQ599437 | HQ599440 | HQ599466 | HQ599375 |
| Massarina cisti | CBS 266.62 | FJ795447 | FJ795490 | FJ795464 | |
| Massarina eburnea | CBS 473.64 | GU301840 | GU296170 | GU371732 | GU349040 |
| Massarina igniaria | CBS 845.96 | GU301841 | GU296171 | GU371793 | |
| Massarina ricifera | JK 5535 F | GU479793 | GU479759 | ||
| Massariosphaeria phaeospora | CBS 611.86 | GU301843 | GU296173 | GU371794 | |
| Mauritiana rhizophorae | BCC 28866 | GU371824 | GU371832 | GU371796 | GU371817 |
| Mauritiana rhizophorae | BCC 28867 | GU371825 | GU371833 | GU371797 | GU371818 |
| Melanomma pulvis-pyrius | CBS 124080 | GU456323 | GU456302 | GU456350 | GU456265 |
| Melanomma pulvis-pyrius | CBS 371.75 | GU301845 | GU371798 | GU349019 | |
| Melanomma pulvis-pyrius | SMH 3291 | GU385197 | |||
| Melanomma rhododendri | ANM 73 | GU385198 | |||
| Misturatosphaeria aurantonotata | GKM1238 | GU385173 | GU327761 | ||
| Misturatosphaeria aurantonotata | GKM1280 | GU385174 | GU327762 | ||
| Misturatosphaeria claviformis | GKM1210 | GU385212 | GU327763 | ||
| Misturatosphaeria kenyensis | GKM1195 | GU385194 | GU327767 | ||
| Misturatosphaeria kenyensis | GKM L100Na | GU385189 | GU327766 | ||
| Misturatosphaeria minima | GKM169N | GU385165 | GU327768 | ||
| Misturatosphaeria tennesseensis | ANM911 | GU385207 | GU327769 | ||
| Misturatosphaeria uniseptata | SMH4330 | GU385167 | GU327770 | ||
| Monascostroma innumerosum | CBS 345.50 | GU301850 | GU296179 | GU349033 | |
| Monotosporella tuberculata | CBS 256.84 | GU301851 | GU349006 | ||
| Montagnula anthostomoides | CBS 615.86 | GU205223 | GU205246 | ||
| Montagnula opulenta | CBS 168.34 | DQ678086 | AF164370 | DQ677984 | |
| Morosphaeria ramunculicola | BCC 18405 | GQ925854 | GQ925839 | ||
| Morosphaeria ramunculicola | JK 5304B | GU479794 | GU479760 | GU479831 | |
| Morosphaeria velataspora | BCC 17059 | GQ925852 | GQ925841 | ||
| Morosphaeria velataspora | BCC 17058 | GQ925851 | GQ925840 | ||
| Massariosphaeria grandispora | CBS 613 86 | GU301842 | GU296172 | GU371725 | GU349036 |
| Massariosphaeria typhicola | CBS 123126 | GU301844 | GU296174 | GU371795 | |
| Neophaeosphaeria filamentosa | CBS 102202 | GQ387577 | GQ387516 | GU371773 | GU349084 |
| Neotestudina rosatii | CBS 690.82 | DQ384069 | |||
| Neottiosporina paspali | CBS 331.37 | EU754172 | EU754073 | GU371779 | GU349079 |
| Ophiosphaerella herpotricha | CBS 240.31 | DQ767656 | DQ767650 | DQ767645 | DQ767639 |
| Ophiosphaerella herpotricha | CBS 620.86 | DQ678062 | DQ678010 | DQ677958 | DQ677905 |
| Ophiosphaerella sasicola | MAFF 239644 | AB524599 | AB524458 | AB539098 | AB539111 |
| Paraconiothyrium minitans | CBS 122788 | EU754173 | EU754074 | GU371776 | GU349083 |
| Paraphaeosphaeria michotii | CBS 591.73 | GU456326 | GU456305 | GU456352 | GU456267 |
| Paraphaeosphaeria michotii | CBS 652.86 | GU456325 | GU456304 | GU456351 | GU456266 |
| Phaeosphaeria ammophilae | CBS 114595 | GU301859 | GU296185 | GU371724 | GU349035 |
| Phaeosphaeria avenaria | CBS 602.86 | AY544684 | AY544725 | DQ677941 | DQ677885 |
| Phaeosphaeria avenaria | DAOM 226215 | AY544684 | AY544725 | DQ677941 | DQ677885 |
| Phaeosphaeria brevispora | MAFF 239276 | AB524600 | AB524459 | AB539099 | AB539112 |
| Phaeosphaeria brevispora | NBRC 106240 | AB524601 | AB524460 | AB539100 | AB539113 |
| Phaeosphaeria caricis | CBS 120249 | GU301860 | GU349005 | ||
| Phaeosphaeria elongata | CBS 120250 | GU456327 | GU456306 | GU456345 | GU456261 |
| Phaeosphaeria eustoma | CBS 573.86 | DQ678063 | DQ678011 | DQ677959 | DQ677906 |
| Phaeosphaeria luctuosa | CBS 308.79 | GU301861 | GU349004 | ||
| Phaeosphaeria nigrans | CBS 576.86 | GU456331 | GU456356 | GU456271 | |
| Phaeosphaeria nodorum | CBS 259.49 | GU456332 | GU456285 | ||
| Phaeosphaeria oryzae | CBS 110110 | GQ387591 | GQ387530 | ||
| Phaeosphaeriopsis musae | CBS 120026 | GU301862 | GU296186 | GU349037 | |
| Phoma apiicola | CBS 285.72 | GU238040 | GU238211 | ||
| Phoma betae | CBS 109410 | EU754178 | EU754079 | GU371774 | GU349075 |
| Phoma complanata | CBS 268.92 | EU754180 | EU754081 | GU371778 | GU349078 |
| Phoma cucurbitacearum | CBS 133.96 | GU301863 | GU371767 | ||
| Phoma exigua | CBS 431.74 | EU754183 | EU754084 | GU371780 | GU349080 |
| Phoma glomerata | CBS 528.66 | EU754184 | EU754085 | GU371781 | GU349081 |
| Phoma herbarum | CBS 276.37 | DQ678066 | DQ678014 | DQ677962 | DQ677909 |
| Phoma radicina | CBS 111.79 | EU754191 | EU754092 | GU349076 | |
| Phoma valerianae | CBS 630.68 | GU238150 | GU238229 | ||
| Phoma vasinfecta | CBS 539.63 | GU238151 | GU238230 | ||
| Phoma violicola | CBS 306.68 | GU238156 | GU238231 | ||
| Phoma zeae-maydis | CBS 588.69 | EU754192 | EU754093 | GU371782 | GU349082 |
| Platychora ulmi | CBS 361.52 | EF114702 | EF114726 | ||
| Lophiostoma compressum | GKM1048 | GU385204 | GU327772 | ||
| Lophiostoma scabridisporum | BCC 22836 | GQ925845 | GQ925832 | GU479829 | GU479856 |
| Lophiostoma scabridisporum | BCC 22835 | GQ925844 | GQ925831 | GU479830 | GU479857 |
| Pleomassaria siparia | CBS 279.74 | DQ678078 | DQ678027 | DQ677976 | DQ677923 |
| Pleospora ambigua | CBS 113979 | AY787937 | |||
| Pleospora herbarum | CBS 191.86 | DQ247804 | DQ247812 | DQ247794 | DQ471090 |
| Polyplosphaeria fusca | CBS 125425 | AB524607 | AB524466 | AB524822 | |
| Polyplosphaeria fusca | MAFF 239687 | AB524606 | AB524465 | ||
| Preussia funiculata | CBS 659.74 | GU301864 | GU296187 | GU371799 | GU349032 |
| Preussia lignicola | CBS 264.69 | GU301872 | GU296197 | GU371765 | GU349027 |
| Preussia terricola | DAOM 230091 | AY544686 | AY544726 | DQ470895 | DQ471063 |
| Prosthemium betulinum | CBS 127468 | AB553754 | AB553644 | ||
| Prosthemium canba | JCM 16966 | AB553760 | AB553646 | ||
| Prosthemium orientale | JCM 12841 | AB553748 | AB553641 | ||
| Prosthemium stellare | CBS 126964 | AB553781 | AB553650 | ||
| Pseudotetraploa curviappendiculata | CBS 125426 | AB524610 | AB524469 | AB524825 | |
| Pseudotetraploa curviappendiculata | MAFF 239495 | AB524608 | AB524467 | ||
| Pseudotetraploa javanica | MAFF 239498 | AB524611 | AB524470 | AB524826 | |
| Pseudotetraploa longissima | MAFF 239497 | AB524612 | AB524471 | AB524827 | |
| Pseudotrichia guatopoensis | SMH4535 | GU385202 | GU327774 | ||
| Pyrenochaeta acicola | CBS 812.95 | GQ387602 | GQ387541 | ||
| Pleurophoma cava | CBS 257.68 | EU754199 | EU754100 | ||
| Pyrenochaeta corn | CBS 248.79 | GQ387608 | GQ387547 | ||
| Pyrenochaeta nobilis | CBS 292.74 | GQ387615 | GQ387554 | ||
| Pyrenochaeta nobilis | CBS 407.76 | DQ678096 | DQ677991 | DQ677936 | |
| Pyrenochaeta quercina | CBS 115095 | GQ387619 | GQ387558 | ||
| Pyrenochaeta unguis-hominis | CBS 378.92 | GQ387621 | GQ387560 | ||
| Pyrenochaetopsis decipiens | CBS 343.85 | GQ387624 | GQ387563 | ||
| Pyrenophora phaeocomes | DAOM 222769 | DQ499596 | DQ499595 | DQ497614 | DQ497607 |
| Pyrenophora tritici-repentis | OSC 100066 | AY544672 | DQ677882 | ||
| Quadricrura bicornis | CBS 125427 | AB524613 | AB524472 | AB524828 | |
| Quadricrura meridionalis | CBS 125684 | AB524614 | AB524473 | AB524829 | |
| Quadricrura septentrionalis | CBS 125428 | AB524617 | AB524476 | AB524832 | |
| Quintaria lignatilis | BCC 17444 | GU479797 | GU479764 | GU479832 | GU479859 |
| Quintaria lignatilis | CBS 117700 | GU301865 | GU296188 | GU371761 | |
| Quintaria submersa | CBS 115553 | GU301866 | GU349003 | ||
| Repetophragma ontariense | HKUCC 10830 | DQ408575 | DQ435077 | ||
| Rimora mangrovei | JK 5246A | GU301868 | GU296193 | GU371759 | |
| Rimora mangrovei | JK 5437B | GU479798 | GU479765 | ||
| Roussoella hysterioides | CBS 125434 | AB524622 | AB524481 | AB539102 | AB539115 |
| Roussoella hysterioides | MAFF 239636 | AB524621 | AB524480 | AB539101 | AB539114 |
| Roussoella pustulans | MAFF 239637 | AB524623 | AB524482 | AB539103 | AB539116 |
| Roussoellopsis tosaensis | MAFF 239638 | AB524625 | AB539104 | AB539117 | |
| Saccothecium sepincola | CBS 278.32 | GU301870 | GU296195 | GU371745 | GU349029 |
| Salsuginea ramicola | KT 2597.1 | GU479800 | GU479767 | GU479833 | GU479861 |
| Salsuginea ramicola | KT 2597.2 | GU479801 | GU479768 | GU479834 | GU479862 |
| Setomelanomma holmii | CBS 110217 | GU301871 | GU296196 | GU371800 | GU349028 |
| Setosphaeria monoceras | AY016368 | AY016368 | |||
| Massaria platani | CBS 221.37 | DQ678065 | DQ678013 | DQ677961 | DQ677908 |
| Sporormiella minima | CBS 524.50 | DQ678056 | DQ678003 | DQ677950 | DQ677897 |
| Stagonospora macropycnidia | CBS 114202 | GU301873 | GU296198 | GU349026 | |
| Tetraploa aristata | CBS 996.70 | AB524627 | AB524486 | AB524836 | |
| Tetraplosphaeria nagasakiensis | MAFF 239678 | AB524630 | AB524489 | AB524837 | |
| Lophiostoma macrostomoides | GKM1033 | GU385190 | GU327776 | ||
| Lophiostoma macrostomoides | GKM1159 | GU385185 | GU327778 | ||
| Thyridaria rubronotata | CBS 419.85 | GU301875 | GU371728 | GU349002 | |
| Tingoldiago graminicola | KH 68 | AB521743 | AB521726 | ||
| Trematosphaeria pertusa | CBS 122368 | FJ201990 | FJ201991 | FJ795476 | GU456276 |
| Trematosphaeria pertusa | CBS 122371 | GU301876 | GU348999 | GU371801 | GU349085 |
| Trematosphaeria pertusa | SMH 1448 | GU385213 | |||
| Triplosphaeria cylindrica | MAFF 239679 | AB524634 | AB524493 | ||
| Triplosphaeria maxima | MAFF 239682 | AB524637 | AB524496 | ||
| Triplosphaeria yezoensis | CBS 125436 | AB524638 | AB524497 | AB524844 | |
| Ulospora bilgramii | CBS 110020 | DQ678076 | DQ678025 | DQ677974 | DQ677921 |
| Verruculina enalia | BCC 18401 | GU479802 | GU479770 | GU479835 | GU479863 |
| Verruculina enalia | BCC 18402 | GU479803 | GU479771 | GU479836 | GU479864 |
| Westerdykella cylindrica | CBS 454.72 | AY004343 | AY016355 | DQ470925 | DQ497610 |
| Westerdykella dispersa | CBS 508.75 | DQ468050 | U42488 | ||
| Westerdykella ornata | CBS 379.55 | GU301880 | GU296208 | GU371803 | GU349021 |
| Wicklowia aquatica | AF289-1 | GU045446 | |||
| Wicklowia aquatica | CBS 125634 | GU045445 | GU266232 | ||
| Xenolophium applanatum | CBS 123123 | GU456329 | GU456312 | GU456354 | GU456269 |
| Xenolophium applanatum | CBS 123127 | GU456330 | GU456313 | GU456355 | GU456270 |
| Zopfia rhizophila | CBS 207.26 | DQ384104 | L76622 |
BCC Belgian Coordinated Collections of Microorganisms; CABI International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, U.K.; CBS Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; DAOM Plant Research Institute, Department of Agriculture (Mycology), Ottawa, Canada; DUKE Duke University Herbarium, Durham, North Carolina, U.S.A.; HHUF Herbarium of Hirosaki University, Japan; IFRDCC Culture Collection, International Fungal Research & Development Centre, Chinese Academy of Forestry, Kunming, China; MAFF Ministry of Agriculture, Forestry and Fisheries, Japan; NBRC NITE Biological Resource Centre, Japan; OSC Oregon State University Herbarium, U.S.A.; UAMH University of Alberta Microfungus Collection and Herbarium, Edmonton, Alberta, Canada; UME Herbarium of the University of Umeå, Umeå, Sweden; Culture and specimen abbreviations: ANM A.N. Miller; CPC; P.W. Crous; EB E.W.A. Boehm; EG E.B.G. Jones; GKM G.K. Mugambi; JK J. Kohlmeyer; KT K. Tanaka; SMH S.M. Huhndorf
Previous results indicated no clear conflict amongst the majority of the data used (Schoch et al. 2009). A phylogenetic analysis of the concatenated alignment was performed on CIPRES webportal (Miller et al. 2009) using RAxML v. 7.2.7 (Stamatakis 2006; Stamatakis et al. 2008) applying unique model parameters for each gene and codon (8 partitions). A general time reversible model (GTR) was applied with a discrete gamma distribution and four rate classes. Fifty thorough maximum likelihood (ML) tree searches were done in RAxML v. 7.2.7 under the same model, each one starting from a separate randomized tree and the best scoring tree selected with a final likelihood value of −95238.628839. Two isolates of Hysterium angustatum (Hysteriales, Pleosporomycetidae) were used as outgroups based on earlier work (Boehm et al. 2009a). Bootstrap pseudo-replicates were run with the GTRCAT model approximation, allowing the program to halt bootstraps automatically under the majority rule criterion (Pattengale et al. 2010). The resulting 250 replicates were plotted on to the best scoring tree obtained previously. The phylogram with bootstrap values on the branches is presented in Plate 1 by using graphical options available in TreeDyn v. 198.3 (Chevenet et al. 2006).
Morphology
Type specimens as well as some other specimens were loaned from the following herbaria: BAFC, BISH, BPI, BR, BRIP, CBS, E, ETH, FFE, FH, G, H, Herb. J. Kohlmeyer, HHUF, IFRD, ILLS, IMI, K(M), L, LPS, M, MA, NY, PAD, PC, PH, RO, S, TNS, TRTC, UB, UBC, UPS and ZT. Attempts were made to trace and borrow all the type specimens from herbaria worldwide, but only some of them could be obtained. Some of the type specimens are in such bad condition that little information could be obtained. In order to obtain the location of specimens, original publications were searched.
Ascostroma and ascomata were examined under an Olympus SZ H10 dissecting microscope. Section of the fruiting structures was carried out by cryotome or by hand-cutting. Measurements and descriptions of sections of the ascomata, hamathecium, asci and ascospores were carried out by immersing ascomata in water or in 10% lactic acid. Microphotography was taken with material mounted in water, cotton blue, Melzer’s reagent or 10-100% lactic acid.
Terminologies are as in Ulloa and Hanlin (2000). In addition, ascomata size is defined as: small-sized: < 300 μm diam., medium-sized: from 300 μm to 600 μm diam., largesized: > 600 μm diam.
Question mark (“?”) before family (or genus) name means its familial (or generic) status within Pleosporales (or some particular family) is uncertain. Other question marks after habitats, latin names or other substantives mean the correctness of their usages need verification.
Results
Molecular phylogeny
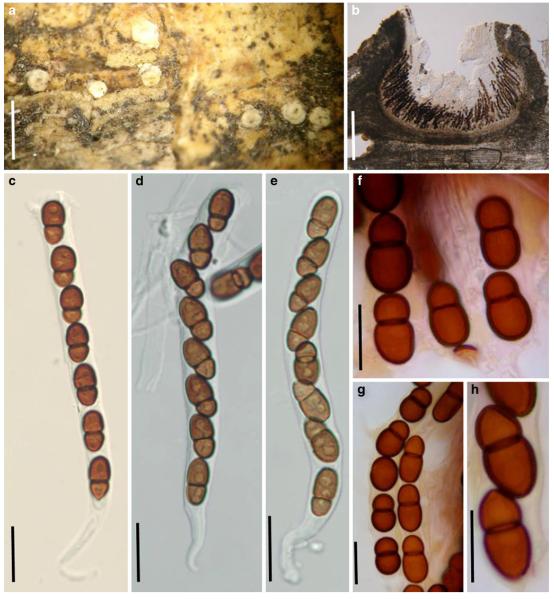
In total, 278 pleosporalean taxa are included in the phylogenetic analysis. These form 25 familial clades in the dendrogram, i.e. Aigialaceae, Amniculicolaceae, Arthopyreniaceae, Cucurbitariaceae/Didymosphaeriaceae, Delitschiaceae, Didymellaceae, Dothidotthiaceae, Hypsostromataceae, Lentitheciaceae, Leptosphaeriaceae, Lindgomycetaceae, Lophiostomataceae, Massariaceae, Massarinaceae, Melanommataceae, Montagnulaceae, Morosphaeriaceae, Phaeosphaeriaceae, Pleomassariaceae, Pleosporaceae, Sporormiaceae, Testudinaceae/Platystomaceae, Tetraplosphaeriaceae, Trematosphaeriaceae and Zopfiaceae (Plate 1). Of these, Lentitheciaceae, Massarinaceae, Montagnulaceae, Morosphaeriaceae and Trematosphaeriaceae form a robust clade in the present study and in previous studies (Schoch et al. 2009; Zhang et al. 2009a, b). We thus emended the suborder, Massarineae, to accommodate them.
Pleosporales suborder Massarineae Barr, Mycologia 71: 948. (1979a). emend.
Habitat freshwater, marine or terrestrial environment, saprobic. Ascomata solitary, scattered or gregarious, globose, subglobose, conical to lenticular, immersed, erumpent to superficial, papillate, ostiolate. Hamathecium of dense or rarely few, filliform pseudoparaphyses. Asci bitunicate, fissitunicate, cylindrical, clavate or broadly clavate, pedicellate. Ascospores hyaline, pale brown or brown, 1 to 3 or more transverse septa, rarely muriform, narrowly fusoid, fusoid, broadly fusoid, symmetrical or asymmetrical, with or without sheath.
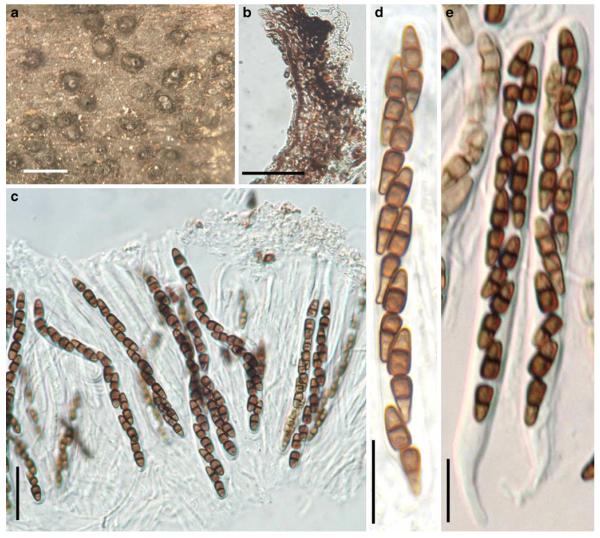
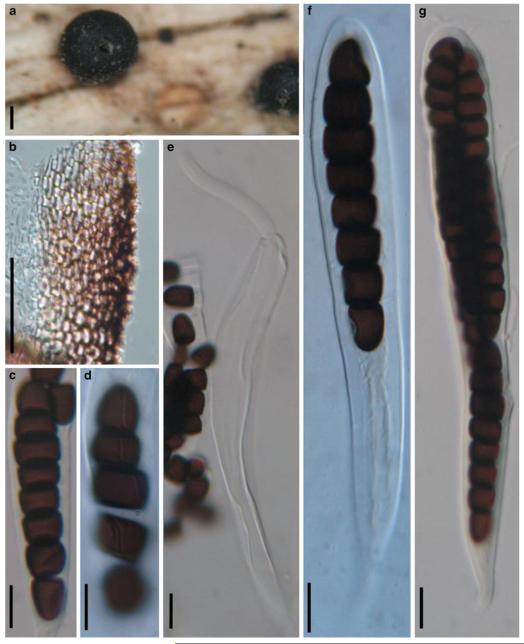
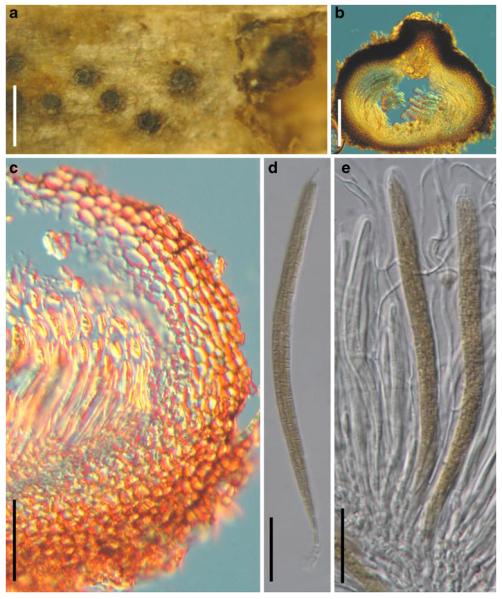
Accepted genera of Pleosporales
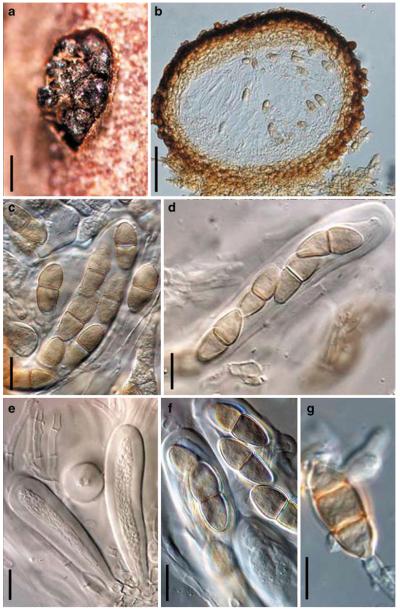
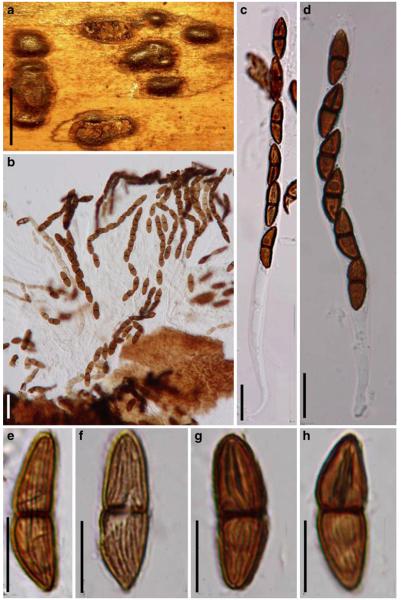
Acrocordiopsis Borse & K.D. Hyde, Mycotaxon 34: 535 (1989). (Pleosporales, genera incertae sedis)
Generic description
Habitat marine, saprobic. Ascomata seated in blackish stroma, scattered or gregarious, superficial, conical to semiglobose, ostiolate, carbonaceous. Hamathecium of dense, long trabeculate pseudoparaphyses. Asci 8-spored, cylindrical with pedicels and conspicuous ocular chambers. Ascospores hyaline, 1-septate, obovoid to broadly fusoid.
Anamorphs reported for genus: none.
Literature: Alias et al. 1999; Barr 1987a; Borse and Hyde 1989.
Type species
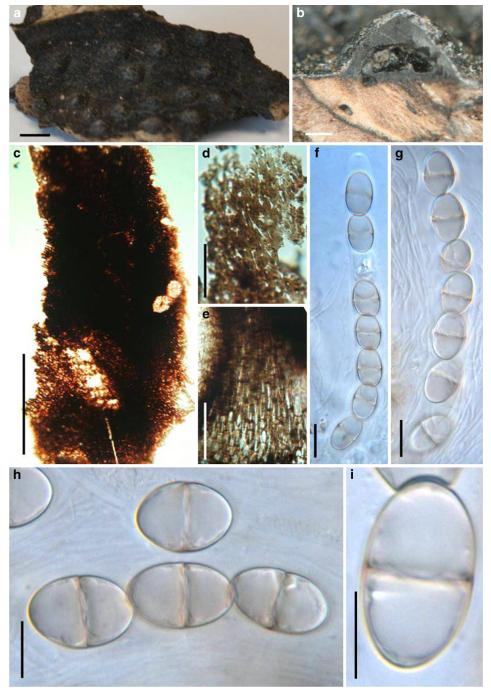
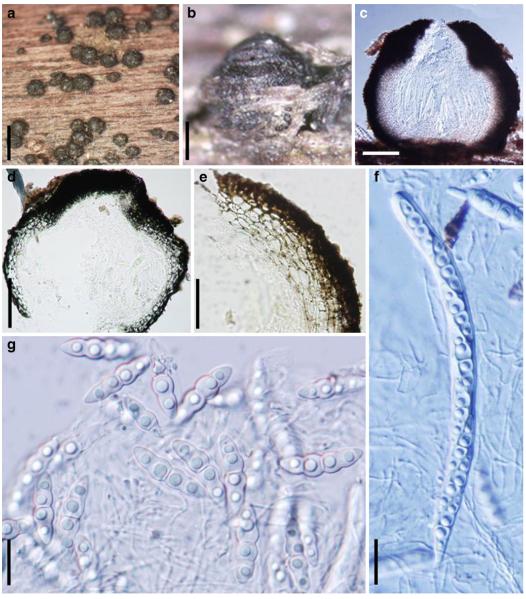
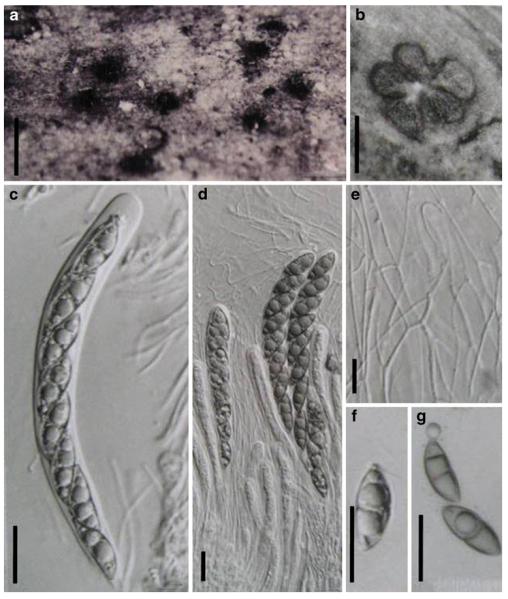
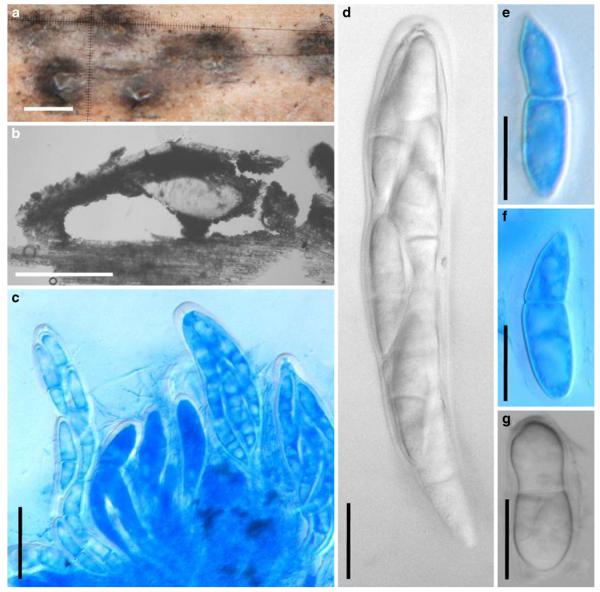
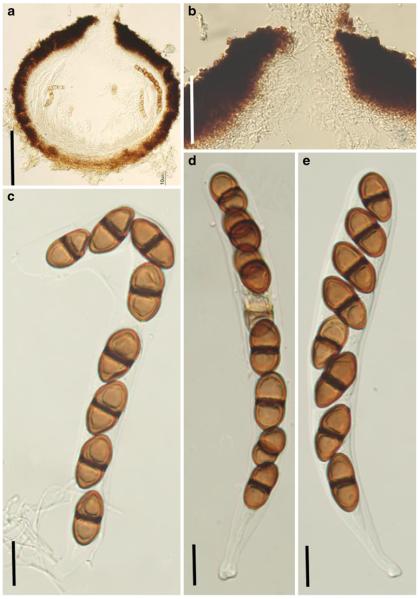
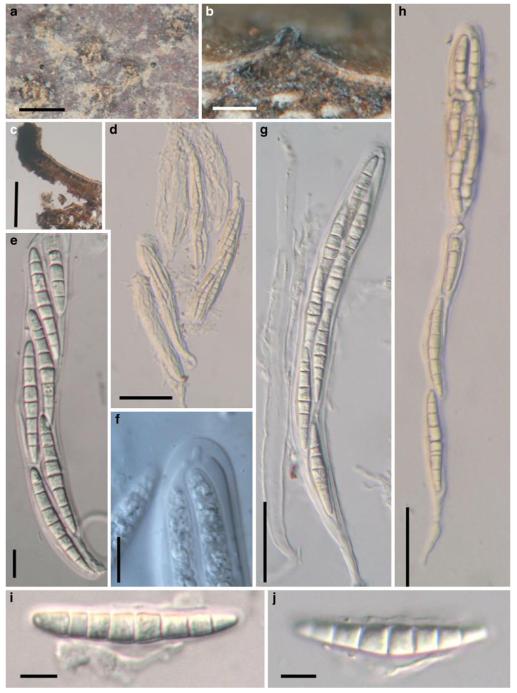
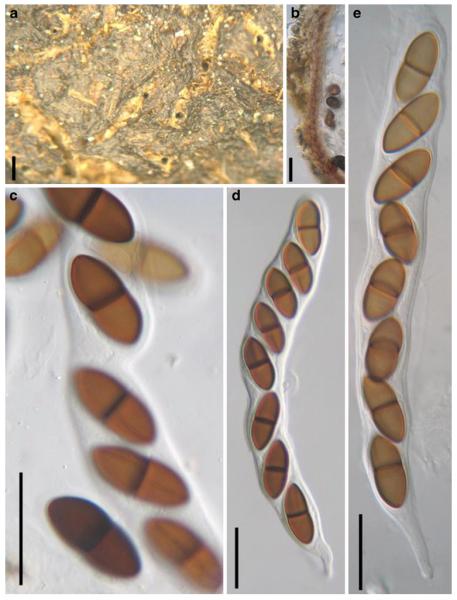
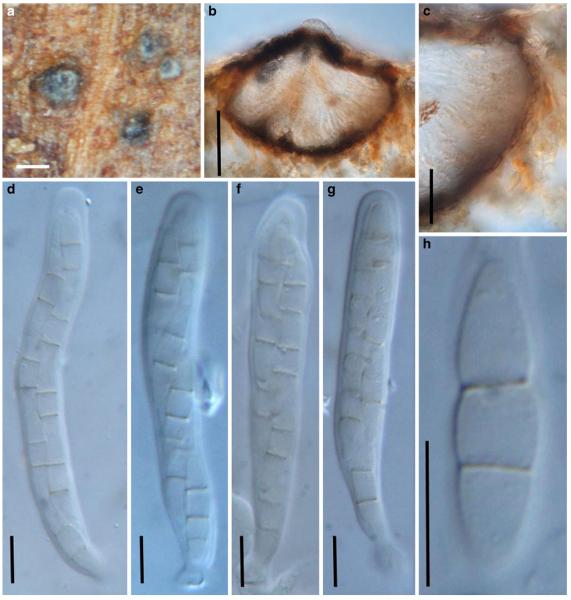
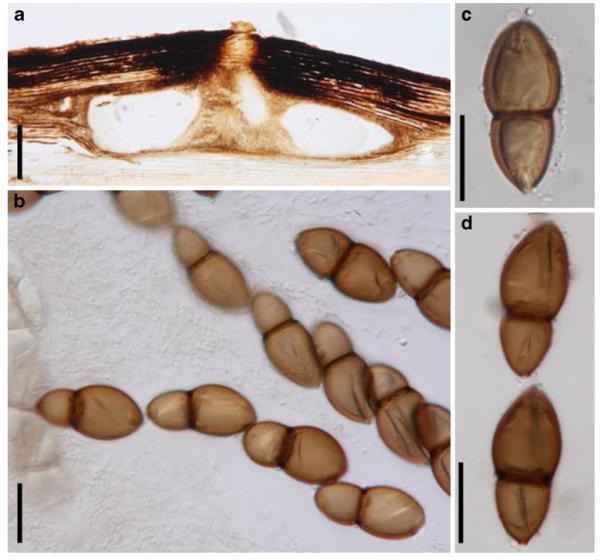
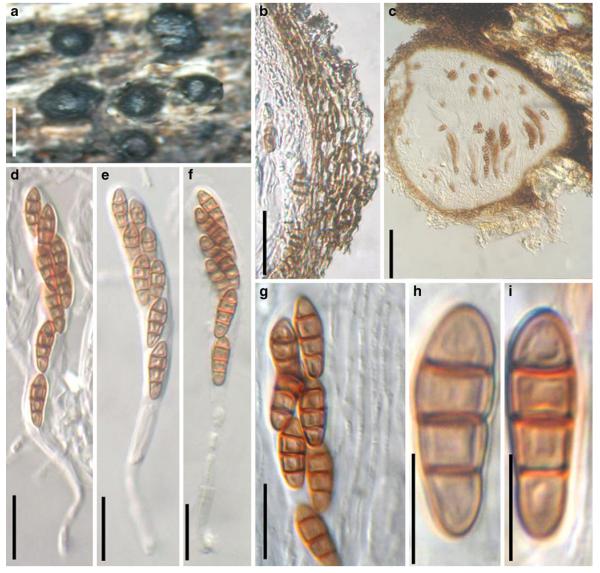
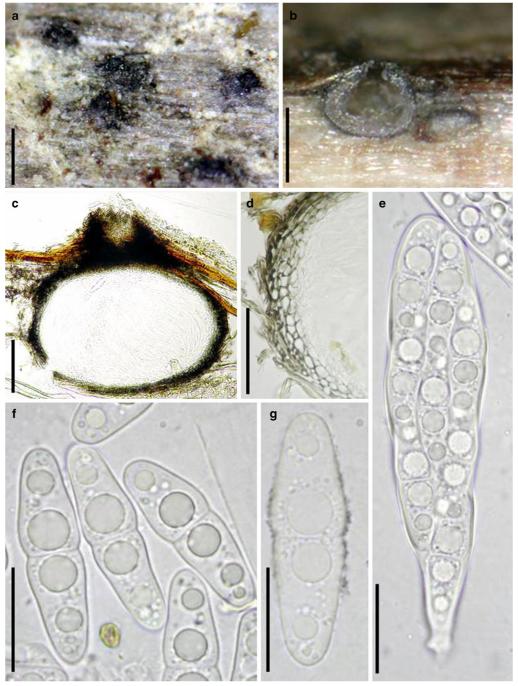
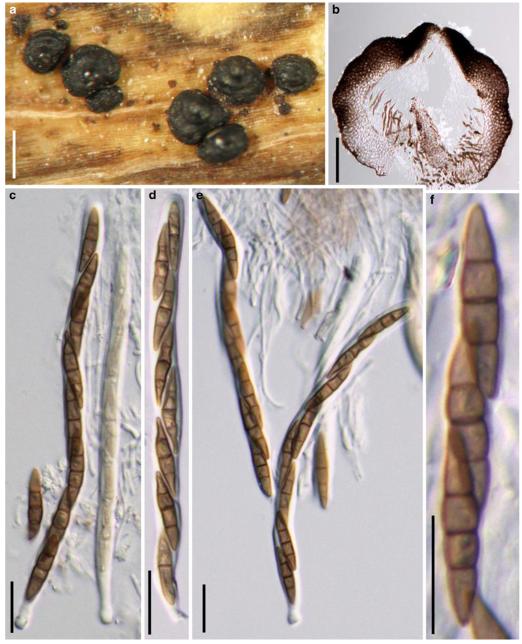
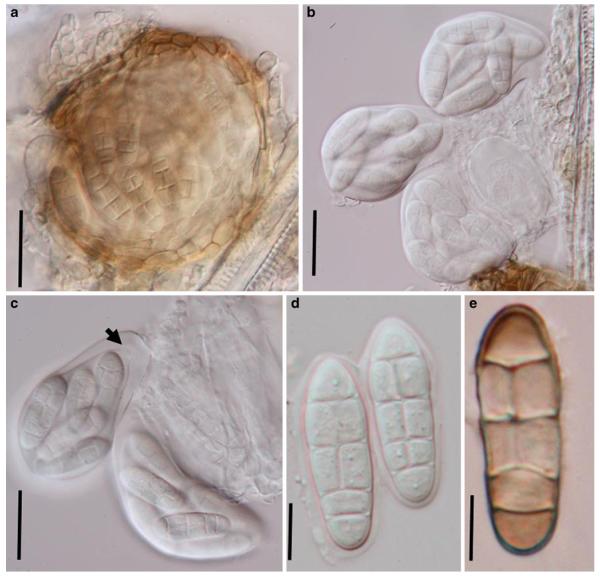
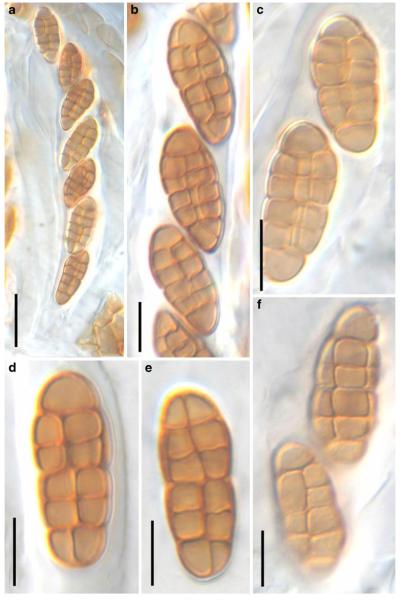
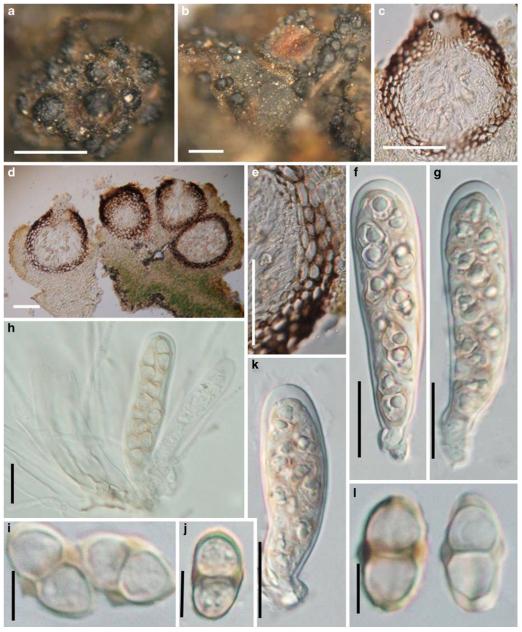
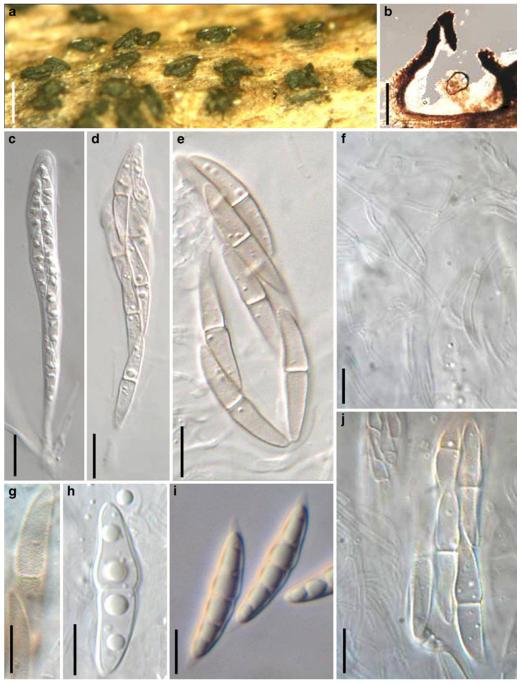
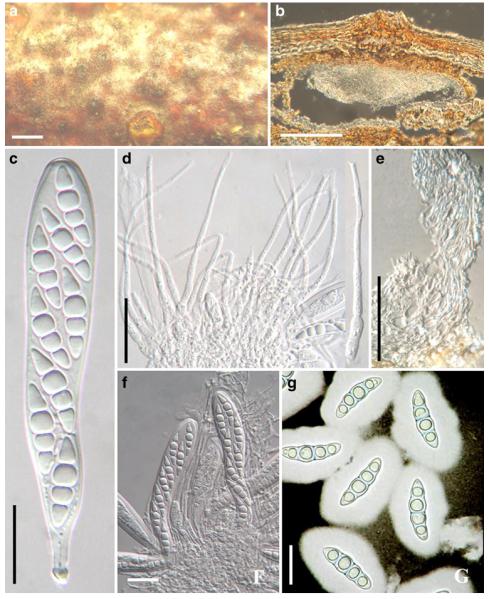
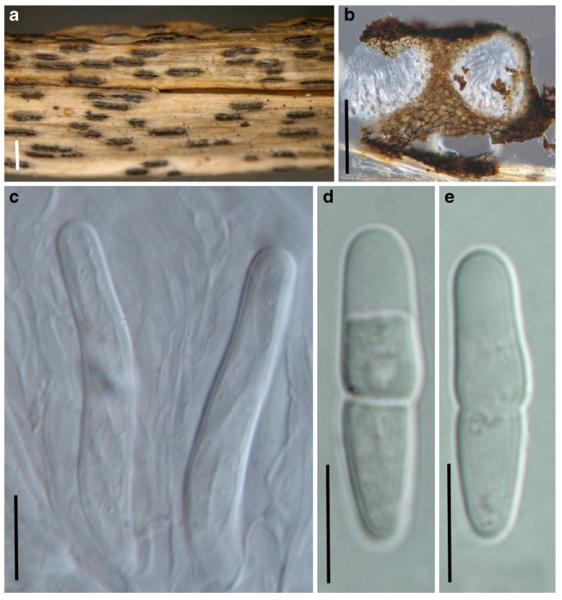
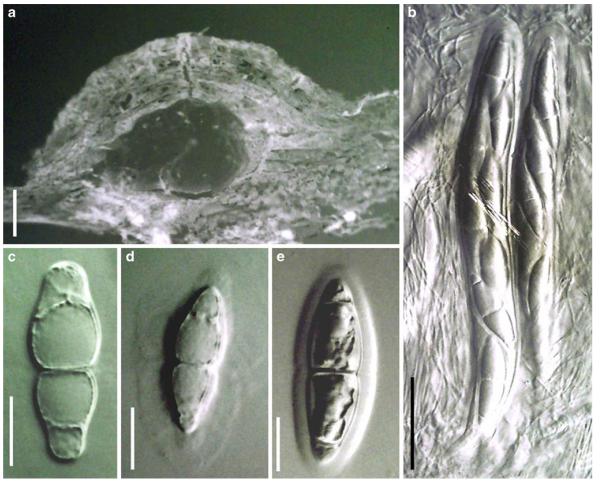
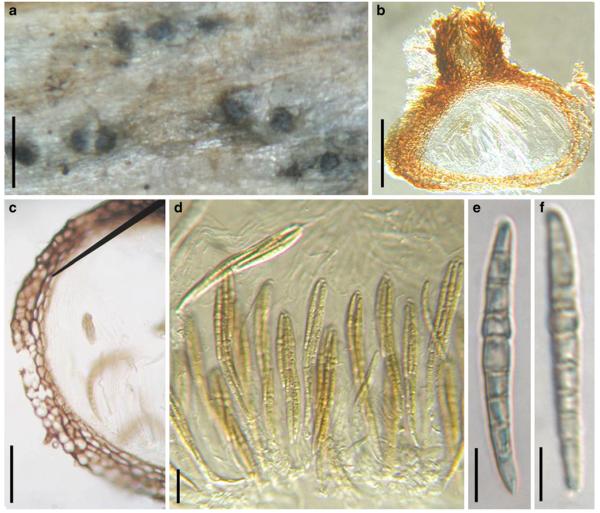
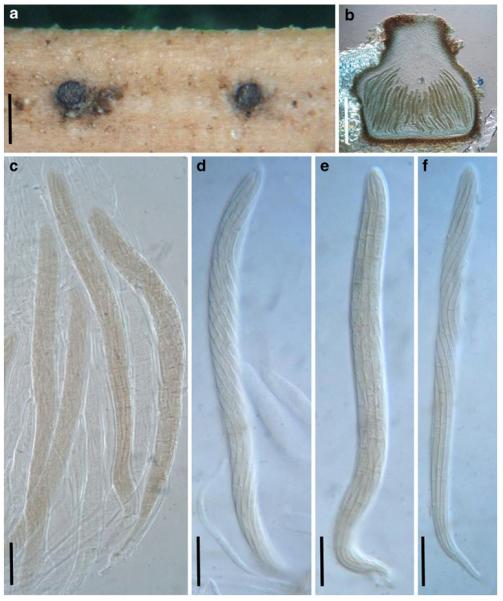
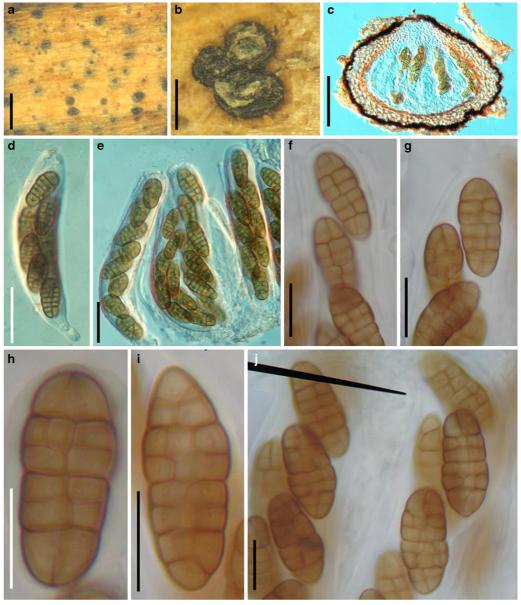
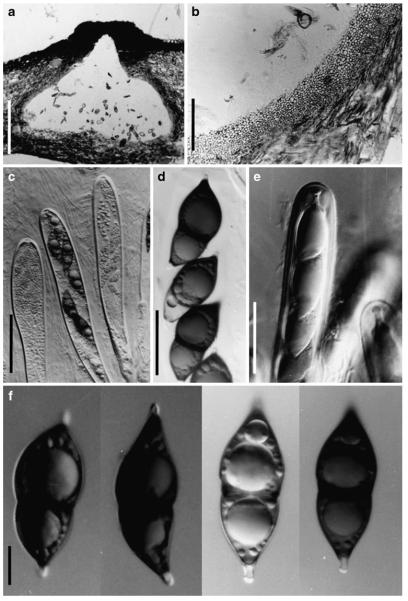
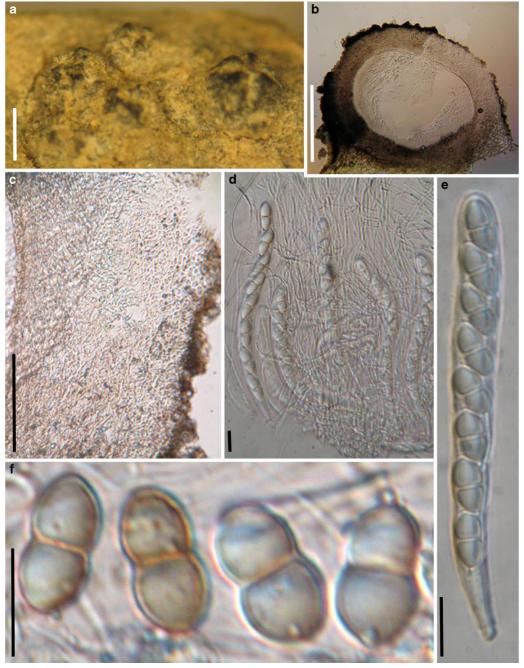
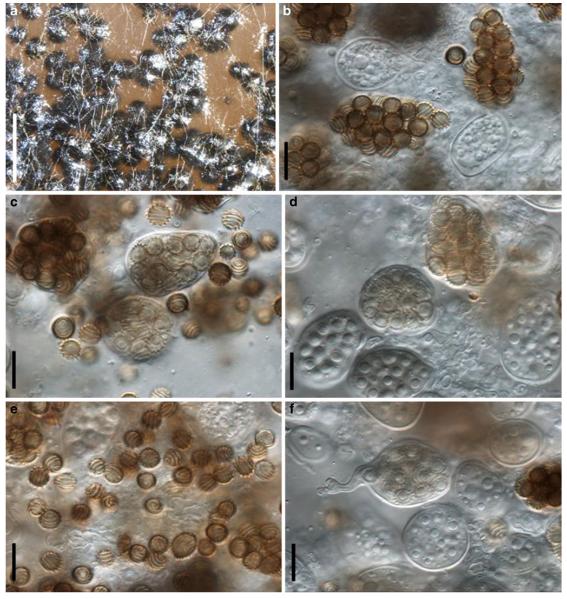
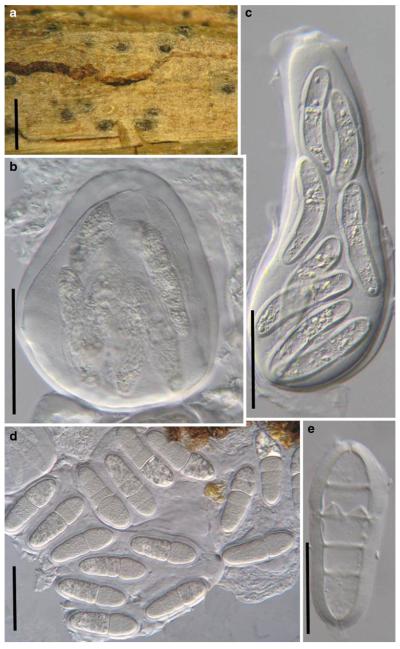
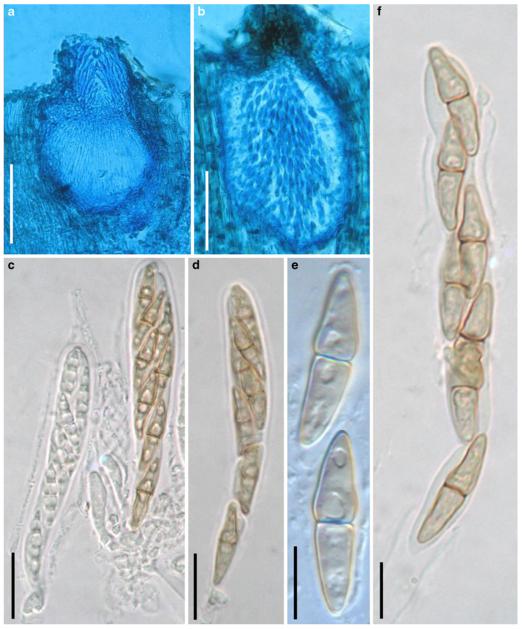
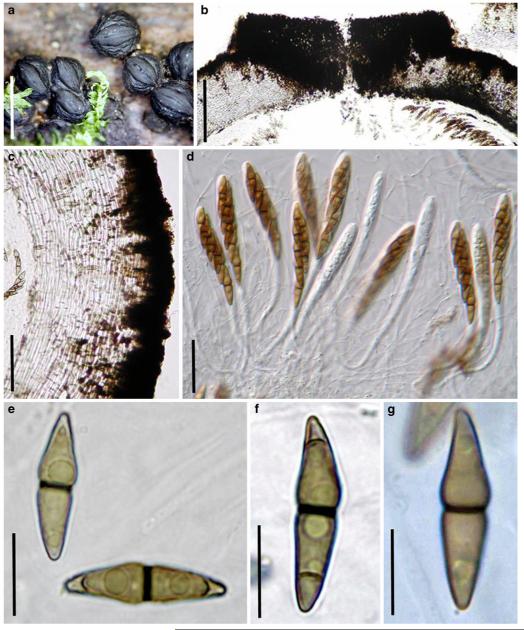
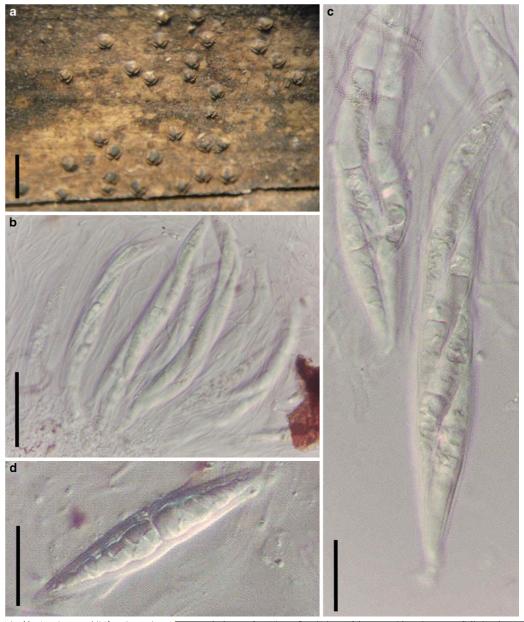
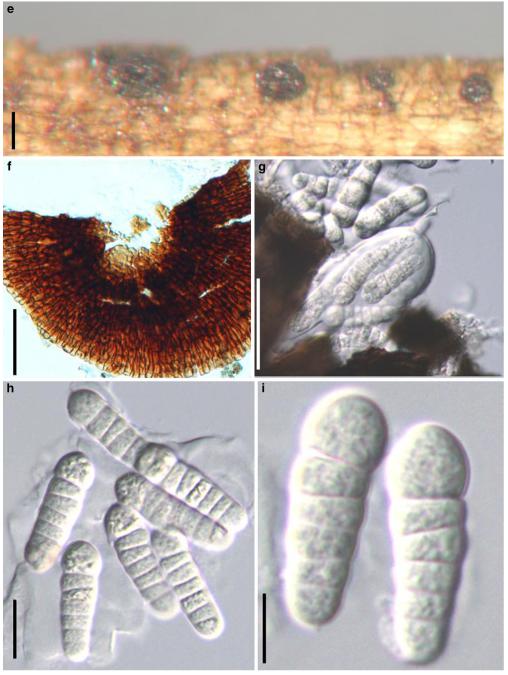
Acrocordiopsis patilii Borse & K.D. Hyde, Mycotaxon 34: 536 (1989). (Fig. 1)
Fig. 1.
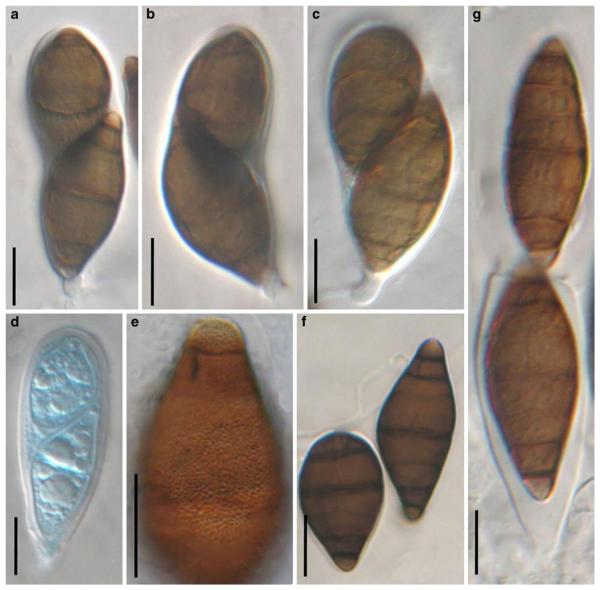
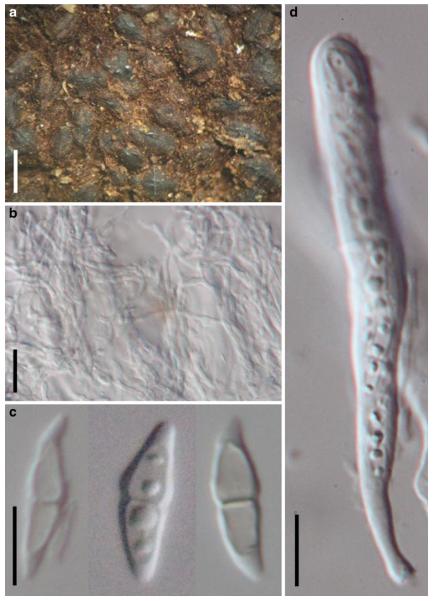
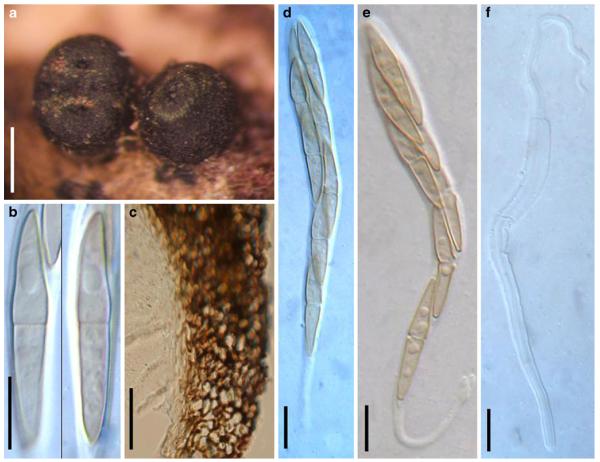
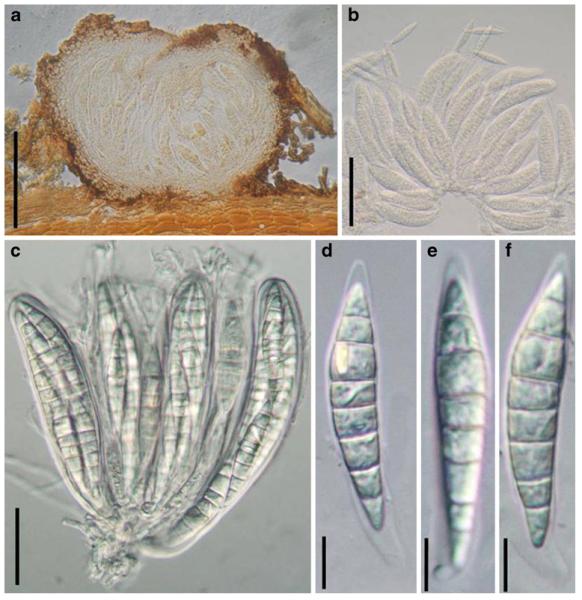
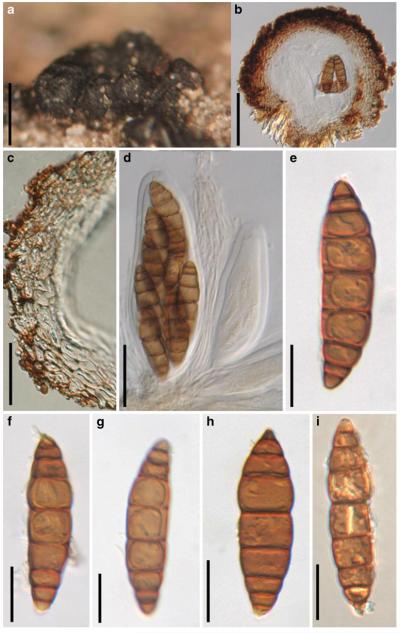
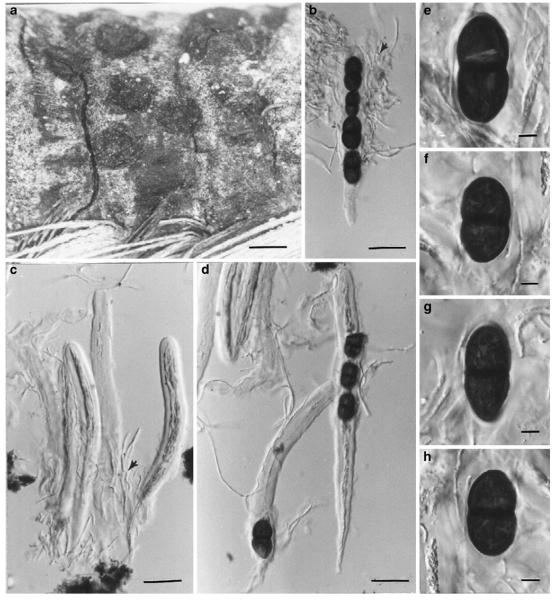
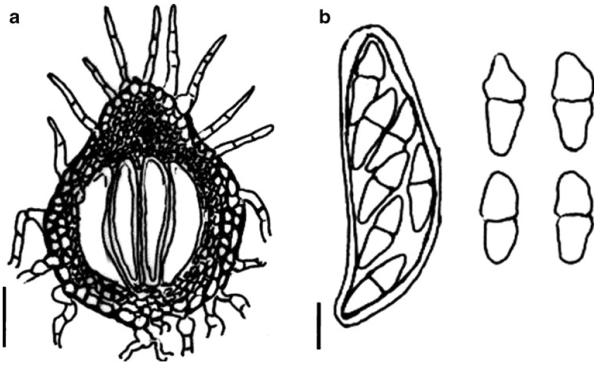
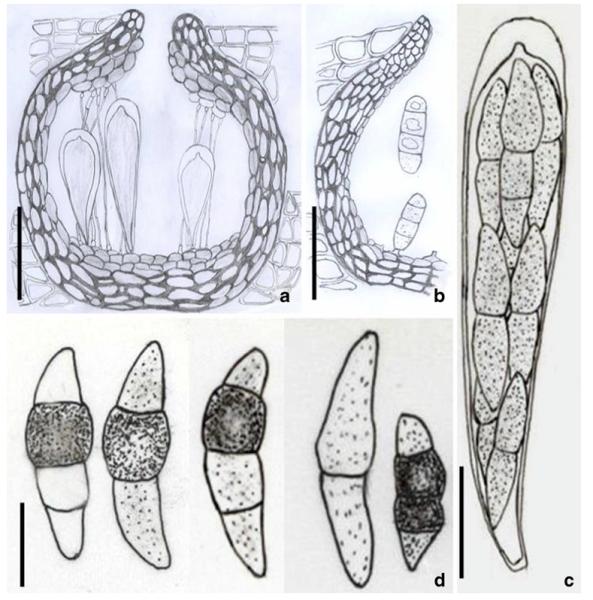
Acrocordiopsis patilii (from IMI 297769, holotype). a b Ascomata on the host surface. b Section of an ascoma. c Section of lateral peridium. d Section of the apical peridium. e Section of the basal peridium. Note the paler cells of textura prismatica. f Cylindrical ascus. g Cylindrical ascus in pseudoparaphyses. h, i One-septate ascospores. Scale bars: a=3 mm, b=0.5 mm, c=200 μm, d, e =50 μm, f-i=20 μm
Ascomata 1–2 mm highx1.8-3 mm diam., scattered or gregarious, superficial, conical or semiglobose, with a flattened base not easily removed from the substrate, ostiolate, black, very brittle and carbonaceous and extremely difficult to cut (Fig. 1a and b). Peridium 250-310 μm thick, to 600 μm thick near the apex, thinner at the base, comprising three types of cells; outer cells pseudoparenchymatous, small heavily pigmented thick-walled cells of textura epidermoidea, cells 0.6-1×6-10 μm diam., cell wall 5-9 μm thick; cells near the substrate less pigmented, composed of cells of textura prismatica, cell walls 1–3(−5) μm thick; inner cells less pigmented, comprised of hyaline to pale brown thin-walled cells, merging with pseudoparaphyses (Fig. 1c, d and e). Hamathecium of dense, long trabeculate pseudoparaphyses, ca. 1 μm broad, embedded in mucilage, hyaline, anastomosing and sparsely septate. Asci 140-220×13-17 μm (x = 165.3 × 15.6mm, n =10), 8-spored, bitunicate, fissitunicate, cylindrical, with short pedicels, 15-25(−40) μm long, with a large and conspicuous ocular chamber (Fig. 1f and g). Ascospores 17.5-25× 12.5-15(−20) μm (x = 21.5 × 13.6mm, n=10), uniseriate to partially over-lapping, ovoid or ellipsoidal, hyaline, 1-septate, not constricted at the septum, smooth-walled (Fig. 1h and i).
Anamorph: none reported.
Material examined: INDIA, Indian Ocean, Malvan (Maharashtra), on intertidal wood of Avicennia alba Bl., 30 Oct. 1981 (IMI 297769, holotype).
Notes
Morphology
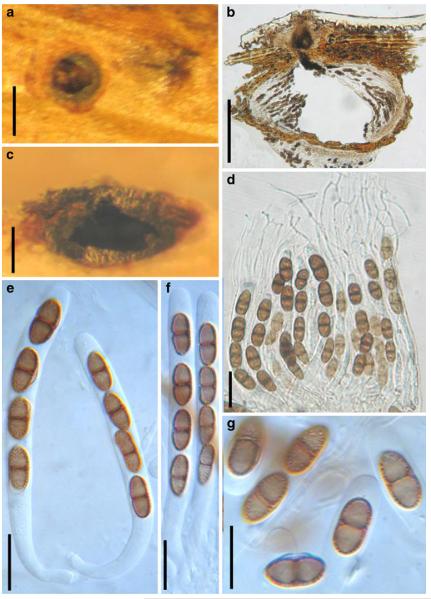
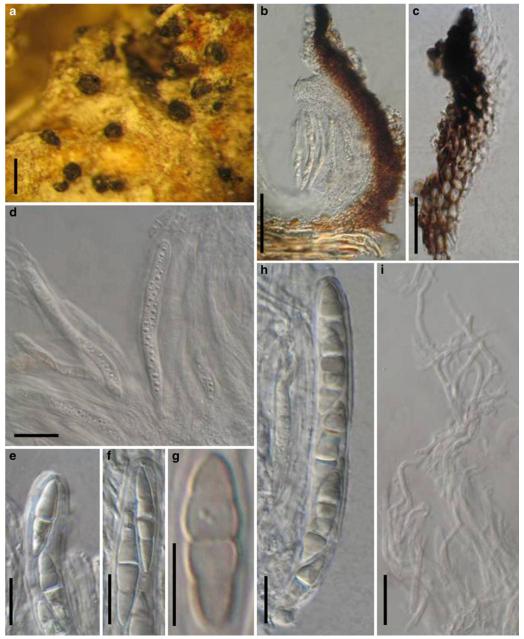
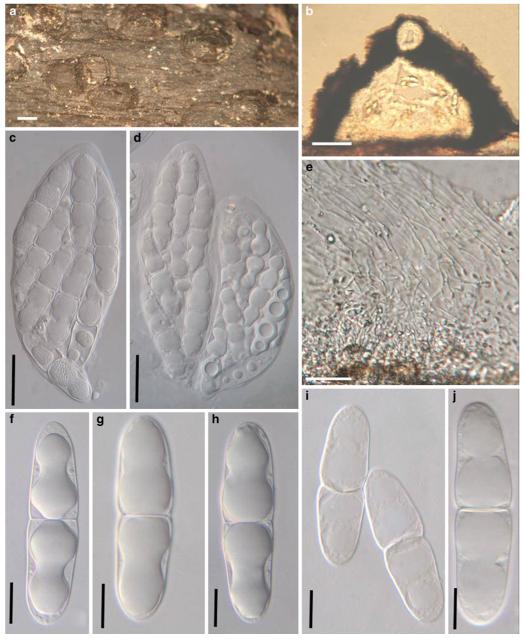
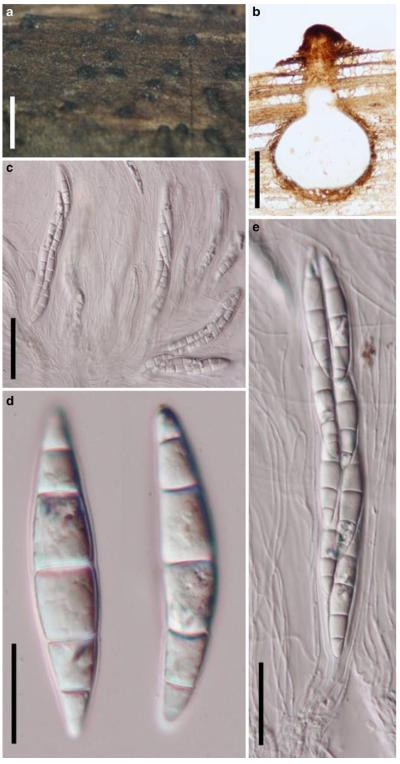
Acrocordiopsis was formally established by Borse and Hyde (1989) as a monotypic genus represented by A. patilii based on its “conical or semiglobose superficial carbonaceous ascomata, trabeculate pseudoparaphyses, cylindrical, bitunicate, 8-spored asci, and hyaline, 1-septate, obovoid or ellipsoid ascospores”. Acrocordiopsis patilii was first collected from mangrove wood (Indian Ocean) as a marine fungus, and a second marine Acrocordiopsis species was reported subsequently from Philippines (Alias et al. 1999). Acrocordiopsis is assigned to Melanommataceae (Melanommatales sensu Barr 1983) based on its ostiolate ascomata and trabeculate pseudoparaphyses (Borse and Hyde 1989). Morphologically, Acrocordiopsis is similar to Astrosphaeriella sensu stricto based on the conical ascomata and the brittle, carbonaceous peridium composed of thick-walled black cells with rows of palisade-like parallel cells at the rim area. Ascospores of Astrosphaeriella are, however, elongate-fusoid, usually brown or reddish brown and surrounded by a gelatinous sheath when young; as such they are readily distinguishable from those of Acrocordiopsis. A new family (Acrocordiaceae) was introduced by Barr (1987a) to accommodate Acrocordiopsis. This proposal, however, has been rarely followed and Jones et al. (2009) assigned Acrocordiopsis to Melanommataceae.
Phylogenetic study
Acrocordiopsis patilii nested within an unresolved clade within Pleosporales (Suetrong et al. 2009). Thus its familial placement is unresolved, but use of the Acrocordiaceae could be reconsidered with more data.
Concluding remarks
Acrocordiopsis, Astrosphaeriella sensu stricto, Mamilli-sphaeria, Caryospora and Caryosporella are morphologically similar as all have very thick-walled carbonaceous ascomata, narrow pseudoparaphyses in a gelatinous matrix (trabeculae) and bitunicate, fissitunicate asci. Despite their similarities, the shape of asci and ascospores differs (e.g. Mamillisphaeria has sac-like asci and two types of ascospores, brown or hyaline, Astrosphaeriella has cylindro-clavate asci and narrowly fusoid ascospores, both Acrocordiopsis and Caryosporella has cylindrical asci, but ascospores of Caryosporella are reddish brown). Therefore, the current familial placement of Acrocordiopsis cannot be determined. All generic types of Astrosphaeriella sensu stricto, Mamillisphaeria and Caryospora should be recollected and isolated for phylogenetic study.
Aigialus Kohlm. & S. Schatz, Trans. Br. MycoL Soc. 85: 699 (1985). (Aigialaceae)
Generic description
Habitat marine, saprobic. Ascomata mostly subglobose in front view, fusoid in sagittal section, rarely subglobose, scattered, immersed to erumpent, papillate, ostiolate, ostiole rounded or slit-like, periphysate. Peridium 2-layered. Hamathecium of trabeculate pseudoparaphyses. Asci 8-spored, cylindrical, pedicellate, with an ocular chamber and conspicuous apical ring. Ascospores ellipsoidal to fusoid, muriform, yellow brown to brown, with terminal appendages. Anamorphs reported for genus: none.
Literature: Eriksson 2006; Jones et al. 2009; Kohlmeyer and Schatz 1985; Lumbsch and Huhndorf 2007.
Type species
Aigialus grandis Kohlm. & S. Schatz, Trans. Br. MycoL Soc. 85: 699 (1985). (Fig. 2)
Fig. 2.
Aigialus grandis (from NY, J.K. 4332b, isotype). a Ascomata on the host surface. Note the longitudinal slit-like furrow which is the ostiole. b Section of the peridium. c, d Released ascospores. e Ascospores in ascus. Note the conspicuous apical ring. f Cylindrical ascus with a long pedicel. Scale bars: a=1 mm, b=200 μm, c-f= 20 μm
Ascomata 1-1.25 mm highx 1-1.3 mm diam. in front view, 250-400 μm broad in sagittal section, vertically flattened subglobose, laterally compressed, scattered, immersed to semiimmersed, papillate, with an elongated furrow at the top of the papilla, wall black, carbonaceous, ostiolate, ostiole filled with branched or forked septate periphyses (Fig. 2a). Peridium 70-100 μm thick laterally, up to 150 μm thick at the apex, thinner at the base, comprising two cell types, outer layer composed of small heavily pigmented thick-walled pseudoparenchymatous cells, cells 1–2 μm diam., cell wall 2-5 μm thick, inner layer thin, composed of small hyaline cells (Fig. 2b). Hamathecium of dense, very long trabeculate pseudoparaphyses, 0.8-1.2 μm broad, embedded in mucilage, anastomosing and branching above the asci. Asci 450-640×22–35 μm (x = 505 × 30μm, n =10), 8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a long furcate pedicel, 90-180 μm long, with a low truncate ocular chamber and a refractive apical apparatus (to 12 μm wide × 4 μm high) (Fig. 2e and f). Ascospores 75-95×15-26 μm (x = 84.3 × 17.5μm, n=10), obliquely uniseriate and partially overlapping, broadly fusoid to fusoid with narrowly rounded ends in front view, flat on one side from side view (14-20 μm thick), yellowish brown, apical cells usually hyaline, muriform, with 14-17(−18) transversal septa, 1–3 longitudinal septa in most cells, slightly constricted at the septa, with a gelatinous cap at each end (Fig. 2c and d).
Anamorph: none reported.
Material examined: BELIZE, Wee-Wee Cay, on submerged wood of roots and branches of Rhizophora mangle L., Mar. 1983, leg. J. Kohlmeyer (NY, J.K. 4332b, isotype).
Notes
Morphology
Aigialus was formally established by Kohlmeyer and Schatz (1985) based on its immersed or semi-immersed ascomata with periphysate ostiole, trabeculate pseudoparaphyses, cylindrical and fissitunicate asci, and distinctive muriform ascospores with gelatinous sheath or caps. There are five accepted species in the genus, namely A. grandis, A. mangrovei Borse, A. parvus S. Schatz & Kohlm., A. rhizophorae Borse and A. striatispora K.D. Hyde (Jones et al. 2009). Aigialus was first assigned to the Melanommatales, but its familial status was uncertain (Kohlmeyer and Schatz 1985). Barr (1990b) included Aigialus in Massariaceae based on its conspicuous apical ring in the asci and ascospore characters, and this has subsequently been widely followed (Eriksson 2006; Hawksworth et al. 1995; Kirk et al. 2001; Lumbsch and Huhndorf 2007).
Phylogenetic study
The generic type of Aigialus (A. grandis) together with other three marine species, i.e. A. mangrovei, A. parvus as well as A. rhizophorae form a robust clade on the phylogenetic tree. Thus a new family, Aigialaceae, was introduced to accommodate Aigialus together with Ascocratera and Rimora (Suetrong et al. 2009).
Concluding remarks
The pleosporalean status of Aigialus has been phylogenetically verified, and the single branch containing Aigialus, Ascocratera and Rimora represents a familial rank of Aigialaceae (Suetrong et al. 2009).
Amniculicola Yin. Zhang & K.D. Hyde, MycoL Res. 112: 1189 (2008). (Amniculicolaceae)
Generic description
Habitat freshwater, saprobic. Ascomata solitary, scattered, or in smal! groups, initially immersed, becoming erumpent, to nearly superficial, globose, subglobose to conical, wall black, roughened; apex well differentiated into two tuberculate flared lips surrounding a slit-like ostiole. Peridium thin, 2-layered, outer layer composed of small heavily pigmented thick-walled cells of textura angularis, inner layer composed of hyaline thin-walled cells of textura angularis. Hamathecium of dense, long trabeculate pseudoparaphyses, embedded in mucilage, anastomosing between and above the asci. Asci 8-spored, bitunicate, fissitunicate, cylindrical to narrowly fusoid, short pedicellate, with an ocular chamber and a small apical apparatus. Ascospores fusoid, hyaline, 1-septate, constricted at the septum, surrounded by an irregular hyaline gelatinous sheath.
Anamorphs reported for genus: Anguillospora longissima, Spirosphaera cupreorufescens and Repetophragma ontariense (Zhang et al. 2008c, 2009c).
Literature: Zhang et al. 2008c, 2009a, c.
Type species
Amniculicola lignicola Ying Zhang & K.D. Hyde, MycoL Res. 112: 1189 (2008). (Fig. 3)
Fig. 3.
Amniculicola lignicola (from PC 0092661, holotype). a Superficial ascomata gregarious on the host surface. b An erumpent ascoma with elongated papilla and slit-like ostiole. c Habitat section of a superficial ascoma. d, e Section of an ascoma and the partial peridium. f Cylindrical 8-spored ascus with a short pedicel. g Hyaline, 1-septate broadly fusoid ascospores. Scale bars: a =1 mm, b-d= 100 μm, e=50 μm, f, g=20 μm
Ascomata 350-450 μm highx300-500 μm diam., solitary, scattered, or in small groups of 2–3, initially immersed, becoming erumpent, to nearly superficial, with basal wall remaining immersed in host tissue, globose, subglobose, broadly or narrowly conical, often laterally flattened, with a flattened base not easily removed from the substrate, wall black, roughened, often bearing remnants of wood fibers; apex well differentiated into two tuberculate flared lips surrounding a slit-like ostiole, 150-250 μm long, filled with a purplish amorphous matter, oriented in the axis of the wood fibers; underlying wood stained pale purple (Fig. 3a and b). Peridium 40-55 μm thick laterally, up to 120 μm thick at the apex, thinner at the base, coriaceous, 2-layered, outer layer composed of small heavily pigmented thick-walled cells of textura angularis, cells 4-9 μm diam., cell wall 2–3 μm thick, apex cells smaller and walls thicker, inner layer composed of hyaline thin-walled cells of textura angularis, 8-16 μm diam., in places with columns of textura prismatica, oriented perpendicular to the ascomatal surface, and larger, paler cells of textura prismatica towards the interior and at the base, 10-25 μm (Fig. 3c, d and e). Hamathecium of dense, long trabeculate pseudoparaphyses <1 μm broad, embedded in mucilage (Indian ink), anastomosing between and above the asci. Asci 140-184×9-10 μm, 8-spored, bitunicate, fissitunicate, cylindrical to narrowly fusoid, with a short, narrowed, twisted, furcate pedicel which is 15-25 μm long, with a low truncate ocular chamber and a small inconspicuous apical apparatus barely seen in water (Fig. 3f). Ascospores (20.5-)28-32× (6-)8(−9) μm, obliquely uniseriate and partially overlapping, broadly fusoid to fusoid with broadly to narrowly rounded ends, hyaline, 1-septate, deeply constricted at the median septum, the upper cell often shorter and broader than the lower one, smooth, containing four refractive globules, surrounded by an irregular hyaline gelatinous sheath 4-8.5 μm thick, best seen in India ink, released senescent ascospores are greyish and 3-septate, strongly constricted at all septa (Fig. 3g).
Anamorph: none reported.
Colonies slow growing, reaching 4 cm diam. after 70 d growth on Malt Extract Agar (MEA) at 25°C, flat, with irregular to rhizoidal margin, off-white to grey, reverse reddish purple to deep reddish purple, the medium is stained pale yellow.
Material examined: FRANCE, Ariege, Prat Communal, Ruisseau de Loumet, 1000 m, on partly submerged wood of Fraxinus excelsior, 8 Aug. 2006, leg. Jacques Fournier (PC 0092661, holotype); 3 Sept. 2004 (BPI 877774; CBS: H-17932); Rimont, Ruisseau de Peyrau, 400 m, on driftwood of Alnus glutinosa (L.) Gaertn., 23 Jul. 2006 (HKU(M) 17515, isotype).
Notes
Morphology
Amniculicola is a freshwater genus which stains the woody substrate purple (Zhang et al. 2008c, 2009a, c). This genus appears only to be reported from Europe. A detailed description of the generic type was provided by Zhang et al. (2008c).
Phylogenetic study
Three species of Amniculicola cluster together with Anguillospora longissima, Spirosphaera cupreorufescens and Repetophragma ontariense as well as Pleospora rubicunda Niessl (current name Murispora rubicunda (Niessl) Y. Zhang ter, J. Fourn. & K.D. Hyde) and Massariosphaeria typhicola (P. Karst.) Leuchtm. (current name Neomassariosphaeria typhicola (P. Karst.) Yin. Zhang, J. Fourn. & K.D. Hyde). A new family, i.e. Amniculicolaceae, was introduced to accommodate these taxa (Zhang et al. 2008c, 2009a, c).
Concluding remarks
All of the five teleomorphic taxa within Amniculicolaceae are from freshwater in Europe and their ascomata stain the woody substrate purple. Purple staining makes taxa of this family easily recognized in the field.
Anomalemma Sivan., Trans. Br. MycoL Soc. 81: 328 (1983). (?Melanommataceae)
Generic description
Habitat terrestrial, fungicolous. Ascomata gregarious, superficial, papillate, ostiolate. Peridium composed cells of pseudoparenchymatous. Asci clavate, 8-spored. Hamathecium of dense, filliform pseudoparaphyses. Ascospores 1- (rarely 2- to 3-) septate, fusoid, reddish brown, constricted at the main septum.
Anamorphs reported for genus: Exosporiella (= Phanerocorynella (Sivanesan 1983).
Literature: Berkeley and Broome 1866; Keissler 1922; Massee 1887; Saccardo 1878a; Sivanesan 1983.
Type species
Anomalemma epochnii (Berk. & Broome) Sivan., Trans. Br. MycoL Soc. 81: 328 (1983). (Fig. 4)
Fig. 4.
Anomalemma epochnii (from K(M):143936, syntype). a Gregarious ascomata on the host surface. b, c Bitunicate asci. Note the wide pseudoparaphyses. d Section of the apical peridium comprising d cells of textura angularis. e-h Fusoid to broadly fusoid. Scale bars: a=0.5 mm, b-h=20 μm
≡ Sphaeria epochnii Berk. & Broome, Ann. Mag. nat. Hist., Ser. 3 18: 128 (1866).
Ascomata 340-500 μm highx170-286 μm diam., gregarious on the intertwined hyphae, superficial, papillate, wall black, coriaceous, roughened (Fig. 4a). Peridium composed of two types of cells, outer layer 17-22 μm wide, composed of heavily pigmented thick-walled cells of textura angularis, cells up to 8× 13 μm diam., cell wall 1-1.5 μm thick, inner layer 30-34 μm thick, composed of hyaline thin-walled cells (Fig. 4d). Hamathecium of dense, long cellular pseudoparaphyses, 2-4 μm broad, septate. Asci 75-108×9.5-12.5 μm (x = 92.8 × 11.1mm, n=10), 8-spored, bitunicate, fissitunicate, dehiscence not observed, cylindro-clavate to clavate, with a furcate pedicel up to 6-25 μm long, with a small ocular chamber best seen in immature asci (ca. 2 μm widex1 μm high) (Fig. 4b and c). Ascospores 20-25(−30) × 5-7.5 μm (x = 23.1 × 6.3mm, n=10), obliquely uniseriate and partially overlapping to biseriate, fusoid to narrowly fusoid with narrowly rounded ends, brown, 1-septate, rarely 2- to 3-septate, deeply constricted at the median septum, smooth (Fig. 4e, f, g and h).
Anamorph: Exosporiella fungorum (Fr.) P. Karst. (Sivanesan 1983).
≡ Epochnium fungorum Fr., Syst. mycoL 3: 449 (1832). Mycelium composed of branched, septate, pale brown hyphae. Stroma none. Conidiophores macronematous or semi-macronematous, mononematous, hyaline, smooth, branched towards the apex. Conidiogenous cells mono-blastic, cylindrical or doliform. Conidia cylindrical or ellipsoidal, dry, 3-4-septate, smooth, hyaline or pale brown.
Material examined: UK, England, Warleigh near Bath, on fungus on bark (Epochnium sp.), Mar. 1866, leg. Warbright? (K(M):143936, syntype, ex herb. C.E. Broome).
Notes
Morphology
Sphaeria epochnii was first described and illustrated by Berkeley and Broome (1866) from Britain and the anamorphic stage is the hyphomycetous Epochniella fungorum. Sphaeria epochnii has subsequently been transferred to Melanomma (as M. epochnii (Berk. & Broome) Sacc.; Saccardo 1878a), Byssosphaeria (as B. epochnii (Berk. & Broome) Cooke; Massee 1887) and Chaetosphaeria (as C. epochnii (Berk. & Broome) Keissl.; Keissler 1922). The deposition of Sphaeria epochnii in Chaetosphaeria is obviously unacceptable, as Chaetosphaeria has unitunicate asci. Melanomma has been reported having Aposphaeria or Pseudospiropes anamorphs, which differs from Exosporiella (Sivanesan 1983). In addition, the presence of well developed prosenchymatous stroma in Sphaeria epochnii can also readily distinguish it from Melanomma (Sivanesan 1983).
The gregarious ascomata and formation of prosenchymatous stroma of Anomalemma resembles those of Cucurbitaria, but the pleosporaceous dictyosporous ascospores of Cucurbitaria readily distinguish it from Anomalemma epochnii. In addition, the pseudoparenchymatous peridium, fungicolous habitat and brown 1-septate ascospores, which later becoming 3-septate differ from any other pleosporalean genus. Thus a new genus, Anomalemma, was introduced to accommodate it (Sivanesan 1983). Anomalemma is presently monotypic.
Phylogenetic study
None.
Concluding remarks
Anomalemma epochnii certainly resembles Byssosphaeria in its ascomata clustering together in groups on closely intertwined hyphae and brown ascospores, and may well be included in this genus. Its fungicolous habitat, however, distinguishes it from Byssosphaeria.
Appendispora K.D. Hyde, Sydowia 46: 29 (1994a). (?Didymellaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata small, clustered, immersed, subglobose or irregularly pyriform. Peridium thin. Hamathecium of dense, long trabeculate pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical, apical rounded with ocular chamber and faint ring, with short pedicels. Ascospores uniseriate to partially overlapping, fusoid, brown, 1-septate, slightly constricted at the septum. Anamorphs reported for genus: none.
Literature: Hyde 1994a.
Type species
Appendispora frondicola K.D. Hyde, Sydowia 46: 30 (1994a). (Fig. 5)
Fig. 5.
Appendispora frondicola (from BRIP 21354, hototype). a Immersed ascomata on host surface. b Valsoid configuration of the ascomata. c Cylindrical ascus. d Squash showing asci and numerous pseudoparaphyses. e Thin strands of anastomosing pseudoparaphyses. f, g Ascospores with one or two appendages. Scale bars: a, b=0.5 mm, c-g=30 μm
Ascomata 120-280 μm highx180-280 μm diam., clustered, immersed with minute ostioles visible through cracks or blackened dots on the host surface, subglobose or irregularly pyriform (Fig. 5a and b). Peridium 40 μm thick, comprising two types of cells; outer cells, small heavily pigmented thick-walled cells of textura angularis, inner cells compressed, hyaline. Hamathecium of dense, very long trabeculate pseudoparaphyses, ca. 1 μm broad, embedded in mucilage, hyaline, anastomosing (Fig. 5e). Asci 130-144× 11-13 μm, 8-spored, bitunicate, fissitunicate, cylindrical, with an ocular chamber and faint ring, with short pedicels (Fig. 5c and d). Ascospores 21–30×7-9 μm, uniseriate to partially over-lapping, fusoid, brown, 1-septate, slightly constricted at the septum, with an irregular ridged ornamentation and 3-5 narrow appendages at each end (Fig. 5f and g).
Anamorph: none reported.
Material examined: BRUNEL, Jalan, Muara, Simpang 835, on dead rachis ofOncosperma horridum on forest floor, Nov. 1992, K.D. Hyde 1652 (BRIP 21354, holotype).
Notes
Morphology
Appendispora was described as a saprobe of palm, and is characterized by small, immersed ascomata, bitunicate, fissitunicate asci, trabeculate pseudoparaphyses, brown, 1-septate, appendaged ascospores with irregular wall striations (Hyde 1994a). Based on its trabeculate pseudoparaphyses embedded within gel matrix and its brown ascospores, Appendispora was assigned to Didymosphaeriaceae (Barr 1987b; Hyde 1994a).
Phylogenetic study
None.
Concluding remarks
The saprobic habitat and association with monocots, cylindrical asci, trabeculate pseudoparaphyses as well as its brown, 1-septate ascospores make it difficult to determine a better phylogenetic position than Didymellaceae.
Ascorhombispora L. Cai & K.D. Hyde, Cryptog. MycoL 28: 294 (2007). (Pleosporales, genera incertae sedis)
Generic description
Habitat freshwater, saprobic. Ascomata solitary or gregarious, superficial, globose to subglobose, dark brown to black, short papillate, ostiolate, coriaceous. Peridium relatively thin, textura angularis in longitudinal section, 2-layered. Hamathecium not observed. Asci 8-spored, obpyriform, broadly clavate to saccate, pedicellate, bitunicate, apex rounded, persistent. Ascospores overlapping 2–3-seriate, broadly fusoid to rhomboid, thick-walled, surrounded by mucilaginous sheath, 3-euseptate, not constricted at septa, median septum wide, forming a darker band, central cells large, trapezoid, dark brown to black, verruculose, polar end cells small and paler.
Anamorphs reported for genus: none.
Literature: Cai and Hyde 2007.
Type species
Ascorhombispora aquatica L. Cai & K.D. Hyde, Cryptog. MycoL 28: 295 (2007). (Fig. 6)
Fig. 6.
Ascorhombispora aquatica (from HKU(M) 10859, holotype). a Section of an ascoma. b Section of a partial peridium. c Immature ascus. d-f Mature asci with ascospores. Note the deliquescent ascal wall in f. Note the wide, dark band in the medium septum of s in d and e and the mucilaginous sheath and paler end cells n e and f. Scale bars: a=20 μm, b-f=15 μm (figures referred to Cai d Hyde 2007)
Ascomata 140-170 μm highx150-185 μm diam., solitary or gregarious, superficial, globose to subglobose, dark brown to black, short papillate, ostiolate, ostioles rounded, small, coriaceous. Peridium relatively thin, 10-18 μm wide, textura angularis in longitudinal section, composed of two layers of angular cells, outer later dark brown to black, relatively thick-walled, inner layer hyaline, relatively thin-walled (Fig. 6a and b). Hamathecium not observed. Asci 100-198 × 72-102 μm (x = 186 × 88mm, n =15), 8-spored, obpyriform, broadly clavate to saccate, pedicellate, bitunicate, apex rounded, deliquescent (Fig. 6c, d and e). Ascospores 30.5-45×16-26.5 μm (x = 38.5 × 21mm, n=25), overlapping 2–3-seriate, broadly fusoid to rhomboid, thick-walled, surrounded by mucilaginous sheath, 3-euseptate, not constricted at septa, median septum wide, forming a darker band, central cells large, trapezoid, 11-18 μm long, dark brown to black, verruculose, polar end cells small, hemispherical, 3.5-4 μm long, subhyaline to pale brown, smooth (Fig. 6f).
Anamorph: none reported.
Material examined: CHINA, Yunnan, Jinghong, on submerged bamboo in a small forest stream, 26 Jan. 2003, leg. det. L. Cai, CAI-1H31 (HKU(M) 10859, holotype).
Notes
Morphology
Ascorhombispora was introduced as a monotypic genus from freshwater by Cai and Hyde (2007), and is characterized by superficial, coriaceous, non-stromatic ascomata, large, saccate asci; lack of interascal filaments and trapezoid (rhombic), 3-septate, dark brown to black ascospores with smaller end cells which are subhyaline to pale brown. Ascorhombispora is most comparable with Caryospora and Zopfia. But the globose to subglobose ascomata and thin peridium, saccate asci lacking interascal pseudoparaphyses, and the 3-septate, rhomboid ascospores with the paler end cells of Ascorhombispora differs from those of Caryospora (Cai and Hyde 2007).
Phylogenetic study
Phylogenetic analysis based on either SSU or LSU rDNA sequences indicated that Ascorhombispora aquatica belongs to Pleosporales, but its familial placement was left undetermined (Cai and Hyde 2007).
Concluding remarks
The sac-shaped asci and absence of pseudoparaphyses are uncommon in Pleosporales, especially among those from freshwater.
Asteromassaria Hohn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. I 126: 368 (1917). (?Morosphaeriaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata medium-sized, clustered, at first immersed and then breaking through the host surface and becoming superficial, globose, subglobose, coriaceous. Peridium 2-layered, thicker near the base. Hamathecium of dense, septate, cellular pseudo-paraphyses which branch and anastomosing frequently between and above asci. Asci (4-)8-spored, bitunicate, cylindro-clavate to clavate, with a short truncated pedicel and a small ocular chamber. Ascospores obliquely uniseriate and partially overlapping to biseriate, fusoid to fusoid-ellipsoidal, pale brown when mature, 1-septate, some becoming 3-septate when old, constricted at the median septum.
Anamorphs reported for genus: Scolicosporium (Sivanesan 1984).
Literature: Barr 1982a; b; 1993a; Boise 1985; Shoemaker and LeClair 1975; Sivanesan 1987; Tanaka et al. 2005.
Type species
Asteromassaria macrospora (Desm.) Hohn., F. von, Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. I 126: 368 (1917). (Fig. 7)
Fig. 7.
Asteromassaria macrospora (from L, 1004). a Ascomata b clustered in a group breaking through the host surface. b Section of an ascoma. c Section of a partial peridium. Note the cells of textura angularis. d Pseudoparaphyses. Note the branches. e Upper part of the ascus illustrating the ocular chamber. f Ascus with a short pedicel. g-j Ascospores. Note the mucilaginous sheath in G and minutely verruculose ornamentation in J. Scale bars: a=0.5 mm, b, c=100 μm, d—j =10 μm
≡ Sphaeria macrospora Desm., Ann. Sci. Nat. Bot. 10: 351 (1849).
Ascomata 400-600 μm highx450-650 μm diam., 4-20 clustered together, at first immersed and then breaking through the host surface and becoming superficial, globose, subglobose, not easily removed from the substrate, wall black, coriaceous, roughened, apex usually widely porate, with or without papilla (Fig. 7a). Peridium 70-90 μm wide, thicker near the base where it is up to 180 μm wide, comprising two cell types, outer cells composed of heavily pigmented small cells, cells 3-5 μm diam., inner layer composed of less pigmented cells of textura angularis, 10-20 μm diam. (Fig. 7b and c). Hamathecium of dense, septate, 2–3 μm broad, pseudoparaphyses which branch and anasto-mosing frequently between and above asci (Fig. 7d). Asci (180-)200-280×28-43 μm (x = 230 × 35mm, n =10), 8-spored (sometimes 4-spored), bitunicate, fissitunicate dehiscence not observed, cylindro-clavate to clavate, with a short truncated pedicel up to 30 μm, with a small ocular chamber (ca. 3 μm wide × 3 μm high) (Fig. 7e and f). Ascospores 50-58×(14-)18-21 μm (x = 55.3 × 18.2μm, n=10), obliquely uniseriate and partially overlapping to biseriate, fusoid to fusoid-ellipsoidal, with narrowly rounded ends, lightly brown when mature, 1-septate, some becoming 3-septate when old, constricted at the median septum, the upper cell often broader and longer than the lower one, minutely verrucose (Fig. 7g, h, i and j).
Anamorph: Scolicosporium macrosporium (Berk.) B. Sutton.
Acervuli immersed in bark, brown, discrete, up to 250 μm diam., opening by irregular rupture of the overlaying tissues. Peridium of thin-walled angular cells. Conidiophores cylindrical, 1–2-septate, up to 30 μm long and 3-5 μm wide. Conidiogenous cells holoblastic, 1–2-annellate, cylindrical, hyaline. Conidia 100-190×12-15 μm, fusoid, pale brown with paler or hyaline ends, 7-17 transverse septate, smooth-walled, with a tapered apex and truncate base (adapted from Sivanesan 1984).
Material examined: CZECH REPUBLIC, Mahrisch-WelBkirchen (Hranice), Wsetin (Vsetin), Berg Cap., on Fagus sylvatica L., Aug. 1938, F. Petrak (L, 1004).
Notes
Morphology
In this study we were unable to obtain the holotype, so we used a collection of Petrak’s. The main morphological characters of Asteromassaria are the medium- to large-sized, globose to depressed ascomata opening with a pore, clavate to oblong asci, narrowly cellular pseudoparaphyses, pale to dark brown, bipolar symmetric, mostly fusoid, distoseptate or euseptate ascospores (Barr 1993a). The bipolar symmetric ascospores of Asteromassaria can readily be distinguished from other genera of this family (Barr 1993a; Tanaka et al. 2005). Currently, it comprises 12 species (Tanaka et al. 2005; http://www.mycobank.org, 28-02-2009).
Phylogenetic study
Asteromassaria pulchra (Harkn.) Shoemaker & P.M. LeClair is basal to Morosphaeriaceae in the phylogenetic tree based on four genes, but its placement is influenced by taxon sampling that was different in several analyses.
Concluding remarks
Asteromassaria can be distinguished from other comparable genera, i.e. Pleomassaria and Splanchnonema by 1-septate and pale brown ascospores, thick-walled textura angularis peridium and Scolicosporium anamorphic stage (see under Pleomassaria).
Astrosphaeriella Syd. & P. Syd., Annls mycoL 11: 260 (1913). (?Melanommataceae)
Generic description
Habitat terrestrial, saprobic. Ascomata densely scattered or in small groups, erumpent through the outer layers of the host tissues to nearly superficial, reflexed pieces of the ruptured host tissue usually persisting around the base of the ascomata, often star-like, conical to semiglobose, with a central papilla. Peridium upper wall usually comprising a thick dark brittle pseudoparenchymatous layer, base usually flattened and thin-walled. Hamathecium of dense, filliform, trabeculate pseudoparaphyses, embedded in mucilage. Asci 8-spored, bitunicate, fissitunicate, cylindro-clavate to nar-rowly fusoid. Ascospores narrowly fusoid with acute ends, hyaline, pale brown or brown, 1–3-septate.
Anamorphs reported for genus: Pleurophomopsis (Hyde et al. 2011).
Literature: von Arx and Muller 1975; Barr 1990a; Chen and Hsieh 2004; Hawksworth 1981; Hawksworth and Boise 1985; Hyde and Frohlich 1998; Hyde et al. 2000; Kirk et al. 2001; Sydow and Sydow 1913; Tanaka and Harada 2005a; b; Tanaka et al. 2009.
Type species
Astrosphaeriella stellata Syd. & P. Syd., Annls mycoL 11: 260 (1913). (Fig. 8)
Fig. 8.
Astrosphaeriella fusispora (BISH 145726). a Ascomata forming a small group on host surface. Note the remains of the host forming flanges around the ascomata. b Section of the partial peridium. Note the black peridium and wedge of palisade cells between the lateral and basal walls. c Asci in trabeculate pseudoparaphyses. d-f Narrowly fusoid ascospores. Scale bars: a=1 mm, b= 100 μm, c=50 μm, d=15 μm, f =10 μm
Ascomata 360-570 μm highx 860-1150 μm diam., densely scattered or in small groups, erumpent through the outer layers of the host tissues to nearly superficial, reflexed pieces of the ruptured host tissue usually persisting around the base of the ascomata, forming star-like flanges around the ascomata from the surface view; ascomata broadly conical, with a flattened base not easily removed from the substrate, wall black; apex with a central papilla which is black and shiny at maturity, scarcely projecting (Fig. 8a). Peridium 40-70 μm thick, carbonaceous and crisp, 1-layered, composed of very small dark brown thick-walled pseudoparenchymatous cells, cells 2-5 μm diam., cell wall 2-6 μm thick, in places at the base composed of hyaline cells of textura prismatica, cells 5 × 8 μm diam. (Fig. 8b). Hamathecium of dense, very long trabeculate pseudoparaphyses, <1 μm broad, embedded in mucilage (Indian ink), anastomosing between and above the asci. Asci 130-190×11.5-15 μm (x = 161.5 × 12.8μm, n =10), 8-spored, bitunicate, fissitunicate, cylindro-clavate to narrowly fusoid, with a short, narrowed pedicel which is 10-35 μm long, with a large ocular chamber (Fig. 8c). Ascospores 35-50×5-7.5 μm (x = 43.4 × 6μm, n =10), biseriate, elongate-fusoid, gradually tapering towards the ends, hyaline, turning pale brown when mature, 1(−3)-septate, constricted at the median septum (Fig. 8d,e and f).
Anamorph: none reported.
Material examined: USA, Hawaii, Kapano Gulch, in bamboo culms, 5 Jun. 1947, leg. Kopf & Rogers, det. Miller (BISH 145726, as Astrosphaeriella fusispora Syd. & P. Syd.).
Notes
Morphology
Astrosphaeriella has been treated as a synonym of Microthelia (von Arx and Muller 1975), but the large conical ascomata, numerous trabeculate pseudoparaphyses and 1-septate and elongated ascospores of Astrosphaeriella all disagree with those of Microthelia (Hawksworth 1981). It was assigned to Platystomaceae by Barr (1990a) in Pleosporales or Melanommataceae by Kirk et al. (2001). Following a systematic study of Astrosphaeriella, only four species were accepted, i.e. A. aosimensis I. Hino & Katum., A. stellata, A. trochus (Penz. & Sacc.) D. Hawksw. and A. venezuelensis M.E. Barr & D. Hawksw. (Hawksworth 1981), and it was defined as a tropical genus, occurring exclusively on palms or bamboo. Astrosphaeriella stellata was selected as the type of Astrosphaeriella, and A. fusispora was regarded as a synonym of A. stellata (Hawksworth 1981). More taxa were subsequently added (Barr 1990a; Hawksworth and Boise 1985; Hyde and Frohlich 1998), and the generic concept extended to include three elements: 1. typical semi-immersed to superficial ascomata with flattened base, cylindro-clavate asci with fusoid ascospores and trabeculate pseudoparaphyses, i.e. Astrosphaeriella sensu stricto (e.g. A. fusispora and A. vesuvius (Berk. & Broome) D. Hawksw. & Boise); 2. Trematosphaeria-like with rounded ascomata (e. g. A. africana D. Hawksw.); and 3. Massarina-like species with immersed ascomata (e.g. A. bakeriana (Sacc.) K.D. Hyde & J. Frohl.) (Chen and Hsieh 2004; Tanaka and Harada 2005a; b). Currently, a broad generic concept of Astrosphaeriella is accepted, and 47 taxa are included in Astrosphaeriella.
Phylogenetic study
Phylogenetic analysis based on LSU and SSU nurDNA sequence data indicates that Astrosphaeriella is polyphyletic, and located in the basal region of the Pleosporales between Testudinaceae and Zopfiaceae/Delitschiaceae (Tanaka et al. 2009), or basal to Aigialaceae (Schoch et al. 2009). The genus is, however, clearly not related to Trematosphaeria as previously understood (Boise 1985).
Concluding remarks
Astrosphaeriella is currently polyphyletic and new collections of the different elements listed above are needed in order to understand the placement of various species. We suggest that some immersed bambusicolous species may belong in Tetraplospheariaceae.
Asymmetricospora J. Frohl. & K.D. Hyde, Sydowia 50: 183 (1998). (?Melanommataceae)
Generic description
Habitat terrestrial, saprobic. Ascomata solitary or in small groups, immersed, black, lenticular in section, uni- or often multi-locular, with a central ostiole without tissue differentiation. Upper peridium carbonaceous, thicker at sides and apex. Lower peridium composed of irregular-shaped, hyaline cells. Hamathecium of trabeculate pseudoparaphyses, branching and anastomosing between and above asci, embedded in mucilage. Asci 8-spored, bitunicate, fissitunicate unknown, clavate, short pedicellate. Ascospores 1-septate, hyaline, constricted at the septum, with a broad, spreading mucilaginous sheath.
Anamorphs reported for genus: none.
Literature: Frohlich and Hyde 1998.
Type species
Asymmetricospora calamicola J. Frohl. & K.D. Hyde, Sydowia 50: 184 (1998). (Fig. 9)
Fig. 9.
Asymmetricospora calamicola (from HKU(M) 7794, hototype). a Ascomata immersed in the substrate. b Section of the peridium. c Mature and immature asci in pseudoparaphyses (in cotton d Clavate ascus with a small ocular chamber. e-g Ascospores h sheath. Scale bars: a, b=0.5 mm, c=50 μm, d-g=20 μm
Ascomata 675-950 μm high×875-1500 μm diam., solitary or in small groups of 2-10, immersed and forming slightly protruding domes on the substrate surface, with near-white rim around the central ostiole; in vertical view lenticular, multi- or rarely unilocular, individual locules 175-270 μm highx320-400 μm diam., with a flattened base, ostiole a central opening without tissue differentiation (Fig. 9a). Upper peridium 32-70 μm wide, carbonaceous, composed of a few layers of black walled cells of textura angularis. Lower peridium thinner, composed of hyaline cells of textura globulosa or textura prismatica (Fig. 9b). Hamathecium of long trabeculate pseudoparaphyses, 1.2-1.6(−2) μm wide, branching and anastomosing between and above asci, embedded in mucilage. Asci 137.5-207.5×26-35 μm (x = 172.8 × 31.5mm, n=20), 8-spored, bitunicate, fissitunicate dehiscence not observed, clavate, with short pedicel (to 25 μm), with ocular chambers (ca. 3 μm wide × 4 μm high) (Fig. 9c and d). Ascospores 35-55× 10.5-15 μm (x = 44.7 × 12.4mm, n=50), biseriate, navicular to obovoid, hyaline, becoming pale brown when senescent, straight or usually curved, smooth, asymmetric, 1-septate, the upper cell larger with a rounded end, basal cell with a tapering end, constricted at the septum, with spreading mucilaginous sheath (Fig. 9e, f and g) (data from Frohlich and Hyde 1998).
Anamorph: none reported.
Material examined: AUSTRALIA, North Queensland, Palmerston, Palmerston National Park, on dead rattan of Calamus caryotoides A.Cunn. ex Mart., Mar. 1994, J. Frohlich (HKU(M) 7794, holotype).
Notes
Morphology
Asymmetricospora was introduced as a monotypic genus represented by A. calamicola based on its “absence of a subiculum, the absence of short dark setae around the papilla and its asymmetric ascospores” (Frohlich and Hyde 1998). Because of the immersed ascomata, ostiole and peridium morphology, fissitunicate asci and trabeculate pseudoparaphyses, Asymmetricospora was assigned to Melanommataceae (sensu Barr 1990a; Frohlich and Hyde 1998).
Morphologically Asymmetricospora can be distinguished from its most comparable genus, Astrosphaeriella, by its ostiole, which is a simple opening without tissue differentiation, asymmetric ascospores, and the usually multiloculate fruiting body (Frohlich and Hyde 1998).
Phylogenetic study
None.
Concluding remarks
The placement of Asymmetricospora under Melanom-mataceae remains to be confirmed.
Barria Z.Q. Yuan, Mycotaxon 51: 313 (1994). (Phaeosphaeriaceae)
Generic description
Habitat terrestrial, parasitic. Ascomata small- to medium-sized, solitary or scattered, immersed, globose, subglobose, ostiolate, coriaceous. Apex with or without papilla and with a pore-like ostiole. Peridium 2-layered. Hamathecium of dense, long cellular pseudoparaphyses, septate, embedded in mucilage. Asci bitunicate, fissitunicate, cylindrical to clavate, with a short, furcate pedicel. Ascospores ellipsoid, hyaline at first, turning brown at maturity, 1-septate, strongly constricted at the septum.
Anamorphs reported for genus: none.
Literature: Yuan 1994.
Type species
Barria piceae Z.Q. Yuan, Mycotaxon 51: 314 (1994). (Fig. 10)
Fig. 10.
Barria piceae (from NY 92003, isotype). a Ascoma on the host surface. Note the wide opening ostiole. b Section of the partial peridium with two types of cells. c, d Asci with ocular chambers and short pedicels. e, f Ellipsoid ascospores which are turning brown with thin sheath around them. Scale bars: a=0.5 mm, b=50 μm, c, d=20 μm, e, f =10 μm
Ascomata 240-370 μm highx200-320 μm diam., solitary, scattered, immersed, globose, subglobose, coriaceous, apex with or without papilla and with a pore-like ostiole (Fig. 10a). Peridium 20-35 μm thick, comprising two cell types, the outer cells comprising 3-4 layers of brown pseudoparenchymatous cells, cells 4-5 μm diam., cell wall 2–3 μm thick, inner cells comprising 3-4 layers of pale brown compressed cells, cells 2×16 μm diam., cell wall 0.5-1.5 μm thick (Fig. 10b). Hamathecium of dense, long cellular pseudoparaphyses, 2–3 μm broad, septate. Asci 135-200(−220)× 14-20 μm (x = 156 × 16.6mm, n= 10), 8-spored, bitunicate, fissitunicate, cylindrical to clavate, with a short, furcate pedicel, up to 22 μm long, with a large ocular chamber (ca. 4 μm widex3 μm high) (Fig. 10c and d). Ascospores 19-21.5×10-12 μm (x = 20.4 × 11mm, n =10), uniseriate to partially overlapping, ellipsoid, hyaline or greenish with numerous small guttules at first and olive green to smoky brown at maturity, 1-septate, strongly constricted at the septum, foveolate, surrounded with sheath (Fig. 10e and f).
Anamorph: none reported.
Material examined: CHINA, Xinjiang Province, Uygur, Urumqi, Tianshan Mountain, on needles of Picea schrenki- ana, 1 Jul. 1992, Z.Q. Yuan (NY 92003, isotype).
Notes
Morphology
Barria was established by Yuan (1994) as a monotypic genus represented by B. piceae according to its “two-celled, pigmented ascospores, pseudoparenchymatous peridium and narrowly cellular pseudoparaphyses” thus differing in its combination of characters from all of the morphologically related dothideomycetous genera, such as Didymosphaeria, Didymopleella or Stegasphaeria. The taxon was considered to belong in Phaeosphaeriaceae. Ascomata and colour or shape of ascospores, however, readily distinguish it from other 1-septate Phaeosphaeriaceae genera, i.e. Didymella, Lautitia and Metameris (Yuan 1994). Barria piceae causes blight of spruce needles.
Phylogenetic study
None.
Concluding remarks
The status of Barria with its unusual verrucose ascospores and thick gel coating is uncertain. In many ways it resembles Belizeana, with its cylindrical asci, 1-septate, ellipsoid ascospores with sheath and verruculose surface (Kohlmeyer and Volkmann-Kohlmeyer 1987). However, the latter is a marine genus while Barria causes leaf blight of terrestrial Picea (Yuan 1994). The placement in Phaeosphaeriaceae seems logical based on the parasitic life style, thin and simple peridium, wide cellular pseudoparaphyses and brown ascospores. However, molecular data are needed to confirm this.
Belizeana Kohlm. & Volkm.-Kohlm., Bot. Mar. 30: 195 (1987). (Pleosporales, genera incertae sedis)
Generic description
Habitat marine, saprobic. Ascomata solitary, scattered, or in small groups, medium-sized, immersed to semi-immersed, subglobose to broadly ampulliform, black, ostiolate, carbonaceous. Peridium thin, comprising several layers of brown thin-walled cells of textura angularis. Hamathecium of dense, filliform pseudoparaphyses, rarely branched. Asci 8-spored, bitunicate, fissitunicate, broadly cylindrical to clavate, with a short pedicel and an ocular chamber. Ascospores uniseriate, broadly ellipsoidal, hyaline, turn pale brown when senescent, 1-septate, constricted at the septum, thick-walled, 2-layered, mature spores with tuberculate ornamentation between the two layers.
Anamorphs reported for genus: Phoma-like (Kohlmeyer and Volkmann-Kohlmeyer 1987).
Literature: Kohlmeyer and Volkmann-Kohlmeyer 1987.
Type species
Belizeana tuberculata Kohlm. & Volkm.-Kohlm., Bot. Mar. 30: 196 (1987). (Fig. 11)
Fig. 11.
Belizeana tuberculata (from Herb. J. Kohlmeyer No. 4398, holotype). a Immersed to semi-immersed ascomata. b, e Vertical section of an ascoma. c Section of a partial peridium. d Squash mounts with a large number of asci. f Broadly cylindrical ascus with a large ocular chamber. g Filliform pseudoparaphyses. h Apical part of an ascus. Note the large ocular chamber. i, j One-septate ascospores. Scale bars: a=0.3 mm, b =100 μm, c=20 μm, d, e=50 μm, f-j = 10 μm
Ascomata 170-300 μm highx 160-290 μm diam., solitary, scattered, or in small groups of 2–3, immersed to semiimmersed, subglobose to broadly ampulliform, carbonaceous, black, pale brown on the sides, ostiolate, epapillate or shortly papillate, ostiolar canal filled with a tissue of hyaline cells (Fig. 11a). Peridium 25-35 μm wide, comprising several layers thin-walled cells of textura angularis, which are hyaline inwardly, near the base composed of a hyaline hyphal mass producing asci, up to 20 μm thick (Fig. 11b, c and e). Hamathecium of dense, ca. 2 μm broad, filliform pseudoparaphyses, rarely branched, embedded in mucilage (Fig. 11 g). Asci 145-170×20-30 μm (x = 163 × 25mm, n= 10), 8-spored, bitunicate, fissitunicate, broadly cylindrical to clavate with a short pedicel, thick-walled, with a small ocular chamber (Fig. 11 d, f and h). Ascospores 21–26 × 13-18 μm (x = 22 × 15mm, n = 10), uniseriate, broadly ellipsoidal, hyaline, turn pale brown when senescent, 1-septate, constricted at the septum, thick-walled, 2-layered, mature spores with tuberculate ornamentation between the two layers (Fig. 11iandj).
Anamorph: Phoma-like (Kohlmeyer and Volkmann-Kohlmeyer 1987).
Material examined: BELIZE, Twin Cays, on Laguncularia sp., 7 Apr. 1983, leg. & det. J. Kohlmeyer (Herb. J. Kohlmeyer No. 4398, holotype); AUSTRALIA, Towra Point, New South Wales, trunk of eroded tree with oysters and shipworms, intertidal zone, Botany Bay, 23 Aug. 1981 (Herb. J. Kohlmeyer No. 4209, paratype).
Notes
Morphology
Belizeana was formally established to accommodate B. tuberculata, an obligate marine fungus, which is characterized by verrucose ascospores (Kohlmeyer and Volkmann-Kohlmeyer 1987). Belizeana tuberculata can be assigned to Pleosporaceae (Pleosporales) according to Luttrell’s (1973) treatment and keys of von Arx and Muller (1975), but cannot resolve a proper family based on Barr (1979a, 1983). The unique morphology together with obligate marine habitat makes B. tuberculata readily distinguishable from all other taxa of Pleosporaceae.
Phylogenetic study
None.
Concluding remarks
The ascospores of Belizeana tuberculata are most comparable with those of Acrocordiopsis patilii, but the superficial conical ascomata of A. patilii are distinct from B. tuberculata. Thus, the familial placement of Belizeana is still undetermined.
Biatriospora K.D. Hyde & Borse, Mycotaxon 26: 263 (1986). (Pleosporales, genera incertae sedis)
Generic description
Habitat marine, saprobic. Ascomata large, solitary or gregarious, immersed, subglobose to pyriform, ostiolate, papillate, periphysate, black, branching, carbonaceous. Hamathecium of dense, long trabeculate pseudoparaphyses, embedded in mucilage. Asci 8-spored, bitunicate, fissitunicate, cylindrical, with apical apparatus. Ascospores uniseriate to partially overlapping, fusoid, hyaline when young, becoming brown to dark brown at maturity, multi-septate towards each end, with a hyaline, globose refractive chamber or appendage at each end, not constricted at the septum.
Anamorphs reported for genus: none.
Literature: Hyde and Borse 1986; Suetrong et al. 2009.
Type species
Biatriospora marina K.D. Hyde & Borse, Mycotaxon 26: 264 (1986). (Fig. 12)
Fig. 12.
1 Biatriospora marina (from IMI 297768, holotype). a, b Cylindrical asci. Note the mucilage pseudoparaphyses in (a) and the conspicuous ocular chamber in (b). c, d Ascospores with hyaline end chambers (arrowed). Scale bars: a, b= 50 μm, c, d=20 μm. 2 Line drawings of Biatriospora marina (based on holotype). a Section through ascocarp showing asci and pseudoparaphyses. b Asci and pseudoparaphyses. c Ascospores. Scale bars: a= 200 μm, b=40 μm, c=30 μm (figure with permission from Hyde and Borse 1986)
Ascomata 650-860 μm highx350-510 μm diam., solitary or gregarious, immersed, subglobose to pyriform, ostiolate, papillate, periphysate, black, carbonaceous (Fig. 12.2a). Hamathecium of dense, long trabeculate pseudoparaphyses, 1-1.5 μm broad, branching, embedded in mucilage. Asci 175-400 × 22-40 μm, 8-spored, bitunicate, fissitunicate, cylindrical, with long pedicels and apical apparatus (Fig. 12.1a, b, 2b). Ascospores 55-82×16-25 μm, uniseriate to partially overlapping, fusoid, hyaline when young, becoming brown to dark brown at maturity, 2-4-septate towards each end, and with a hyaline, globose refractive chamber or appendage at each end, 6-8×4-6 μm diam., not constricted at the septum (Fig. 12.1c, d, 2c).
Anamorph: none reported.
Material examined: SEYCHELLES, 2 Jan. 1984 (Herb. IMI 297768 holotype).
Notes
Morphology
Biatriospora was introduced to accommodate a marine fungus B. marina, which is characterized by horizontal ascomata and ascospores with polar, globose refractive chambers and polar septa (Hyde and Borse 1986). Polar refractive chambers can also occur in other marine fungi, such as Lulworthia and Aigialus. The chambers have been proposed as important for spore attachment to substrates in a liquid environment (Hyde and Borse 1986).
Phylogenetic study
Multigene phylogenetic analysis indicated that Biatriospora marina forms a separate branch, sister to other families of Pleosporales (Suetrong et al. 2009), and maybe related to species in Roussoella (Plate 1).
Concluding remarks
The familial status ofBiatriospora can not be determined.
Bicrouania Kohlm. & Volkm.-Kohlm., MycoL Res. 94: 685 (1990). (?Melanommataceae)
Generic description
Habitat marine, saprobic. Ascomata immersed gregarious, erumpent to superficial, globose to subglobose, black, periphysate, coriaceous, epapillate or papillate, ostiolate. Peridium thin, 2-layered. Hamathecium of dense, long trabeculate pseudoparaphyses, branching and anastomosing between and above the asci. Asci 8-spored, bitunicate, fissitunicate, cylindrical, with a thick, furcate pedicel lacking ocular chamber. Ascospores obliquely uniseriate and partially overlapping, ellipsoidal with broadly rounded ends, reddish brown, 1-septate, thick-walled, constricted at the septum.
Anamorphs reported for genus: none.
Literature: Jones et al. 2009; Kohlmeyer and Volkmann-Kohlmeyer 1990.
Type species
Bicrouania maritima (P. Crouan & H. Crouan) Kohlm. & Volkm.-Kohlm., MycoL Res. 94: 685 (1990). (Fig. 13)
Fig. 13.
Bicrouania maritima (from IMI 330806, isotype). a Section of an ascoma. b Section of papilla. Note the periphyses. c-e Eight-spored asci. Note the furcated pedicel. Scale bars: a, b =100 μm, c-e=20 μm
≡ Sphaeria maritima P. Crouan & H. Crouan, Florule du Finistere, Paris: 27 (1867) non Sphaeria maritima Cooke & Plowright, Grevillia 5: 120 (1877).
Ascomata 320-440 μm highx370-460 μm diam., gregarious, immersed, mostly erumpent to superficial, globose to subglobose, black, coriaceous, with a rough surface, papillate or epapillate, ostiolate, periphysate (Fig. 13a). Peridium 40-50 μm thick laterally, up to 75 μm thick at the apex, thinner at the base, 2-layered, outer layer composed of small heavily pigmented pseudoparenchymatous cells, inner layer very thin, composed of hyaline thin-walled small cells, merging into pseudoparaphyses (Fig. 13a and b). Hamathecium of dense, very long trabeculate pseudoparaphyses, 0.8-1.2 μm broad, branching and anastomosing between and above the asci. Asci 170-225× 17.5-22.5 μm (x = 199.6 × 20mm, n =10), 8-spored, bitunicate, fissitunicate, cylindrical, with a thick, furcate pedicel which is up to 70 μm long, lacking ocular chamber (Fig. 13c, d and e). Ascospores 22-26×12-15 μm (x = 24.5 × 13.3mm, n = 10), obliquely uniseriate and partially overlapping, ellipsoidal with broadly rounded ends, reddish brown, 1-septate, slightly constricted at the septum, thick-walled, with a thick darkened band around the septum, smooth (Fig. 13c, d and e).
Anamorph: none reported.
Material examined: FRANCE, Finistere, on Halimone portulacoides (IMI 330806, isotype, as Sphaeria maritima).
Notes
Morphology
When Kohlmeyer and Volkmann-Kohlmeyer (1990) studied the four marine Didymosphaeria species, the monotypic Bicrouania was established to accommodate B. maritima (as Didymosphaeria maritima (P. Crouan & H. Crouan) Sacc.), which could be distinguished from Didymosphaeria by its superficial ascomata lacking a clypeus, thick-walled asci and its association with algae (Kohlmeyer and Volkmann-Kohlmeyer 1990). Jones et al. (2009) agreed that it cannot be placed in Didymosphaeria based on its superficial ascomata, but that it does have many similarities with Didymosphaeria. Molecular data are required to determine its relationship with Didymosphaeria and to resolve its higher level placement.
Phylogenetic study
None.
Concluding remarks
Besides the morphological differences, its marine and substrate habitats also differ from Didymosphaeria.
Bimuria D. Hawksw., Chea & Sheridan, N. Z. J. Bot. 17: 268 (1979). (Montagnulaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata solitary, superficial, globose, dark brown, epapillate, ostiolate. Peridium thin, pseudoparenchymatous. Hamathecium of few, cellular pseudoparaphyses, embedded in mucilage, rarely anastomosing and branching. Asci bitunicate, fissitunicate, broadly clavate with short pedicels, 2–3-spored. Ascospores muriform, broadly ellipsoid, dark brown with subhyaline end cells, verrucose.
Anamorphs reported for genus: none.
Literature: Barr 1987b; Hawksworth et al. 1979; Lumbsch and Huhndorf 2007.
Type species
Bimuria novae-zelandiae Hawksworth, Chea & Sheridan, N. Z. J. Bot. 17: 268 (1979). (Fig. 14)
Fig. 14.
Bimuria novae-zelandiae (from CBS 107.79, isotype). a-c Asci with a short pedicel and small ocular chamber. d Immature ascus (in cotton blue). e Partial ascospore. Note the convex verrucae on the ascospore surface. f Released ascospores. Note the lighter end cells, germ pore and the longiseptum (arrowed). g Fissitunicate ascus dehiscent. Scale bars: a-g=20 μm
Ascomata (185-)200 x310(−330) μm diam., solitary, scattered, semi-immersed or superficial, globose, hyaline when young, turning dark brown to black when mature, ostiolate, the ostiole more or less sessile or raised into a very short neck. Peridium 5-8(−12) μm thick, comprising 2–3 layers of radically compressed pseudoparenchymatous cells, cells 10-15 μm diam. in surface view, cell wall 2–3 μm thick. Hamathecium consisting of few, 2.5-4 μm broad cellular pseudoparaphyses, embedded in mucilage, rarely anasto-mosing and branching, septate, 7-13 μm long between two septa. Asci (65-)80-95×20-32.5 μm (x = 75.6× 29.4mm, n = 10), (1-)2(−3)-spored, bitunicate, fissitunicate, broadly clavate, with a short and small knob-like pedicel which is up to 13 μm long, ocular chamber best seen in immature asci (Fig. 14a, b, c, d and g). Ascospores accumulating in a subglobose black shiny mass adhering together outside the ostiole, 55-68×25-28 μm (x = 59× 26mm, n =10), broadly ellipsoid but becoming narrowed towards the poles, muriform with (5-)7 transverse septa, cells with (0-)l(−2) longitudinal septa in each cell, no constriction at the septa, dark brown, the apical cells paler with no longitudinal septa, verruculose (Fig. 14e and f).
Anamorph: none reported.
Material examined: NEW ZEALAND, North Island, Wairarapa District, Nutty Farm, isolated from soil, 3 Mar. 1978, Chea Chark Yen & J.E. Sheridan (CBS 107.79, isotype).
Notes
Morphology
Bimuria novae-zelandiae was first isolated from soil of a barley field in New Zealand (Hawksworth et al. 1979). Based on B. novae-zelandiae, the genus is characterized by a very thin peridium, mostly 2-spored and fissitunicate asci as well as the muriform, dark brown, verrucose ascospores (Hawksworth et al. 1979). Because of its unique morphological characters, the familial placement of this genus has been debatable and it has been placed in Pleosporaceae (Hawksworth et al. 1979), in Phaeosphaeriaceae (Barr 1987b) and in Melanommataceae (Lumbsch and Huhndorf 2007).
Morphologically, Bimuria is most comparable with some superficially similar or allied genera, in particular Montagnula (Hawksworth et al. 1979). However, the thick carbonaceous peridium distinguishes Montagnula from that of Bimuria (Hawksworth et al. 1979). In addition, the ascospores of Montagnula are discharged forcibly through the ostiole instead of forming a mass outside of the ostiole as in Bimuria (Hawksworth et al. 1979). Ascomauritiana lignicola V.M. Rangtoo & K.D. Hyde has somewhat similar ascospores in 4-spored asci, but this taxon has unitunicate asci (Ranghoo and Hyde 1999). The morphological characters of Bimuria, such as ascospore release and large, thick-walled ascospores may be an adaptation to its soil-borne habitat (Hawksworth et al. 1979).
Phylogenetic study
Bimuria novae-zelandiae was found to be closely related to Phaeodothis winteri (Niessl) Aptroot (syn. Didymosphaerella opulenta (De Not.) Checa & M.E. Barr) and Montagnula opulenta (De Not.) Aptroot in analysis of combined sequences, i.e. SSU rDNA, LSU rDNA, RPB2 and TEF1 sequences (Schoch et al. 2006, 2009). These two species had been included by Barr (2001) in her new family Montagnulaceae.
Concluding remarks
We agree with Barr (2001) and include the genus in Montagnulaceae based on both morphological and phylogenetic characters.
Bricookea M.E. Barr, Mycotaxon 15: 346 (1982). (? Phaeosphaeriaceae)
Generic description
Habitat terrestrial, saprobic (or parasitic?). Ascomata small- to medium-sized, solitary, scattered, or in small groups, immersed, erumpent to superficial, depressed globose, papillate, ostiolate. Peridium thin. Hamathecium filliform, cellular pseudoparaphyses, embedded in mucilage, anastomosing, septate. Asci bitunicate, fissitunicate, cylindrical, cylindro-clavate or slightly obclavate, with a short knob-like pedicel, with an ocular chamber. Ascospores hyaline, ellipsoid to narrowly obovoid, 3-septate, constricted at each septum.
Anamorphs reported for genus: none.
Literature: Barr 1982a; Berlese 1896; Holm 1957; Shoemaker and Babcock 1989a.
Type species
Bricookea sepalorum (Vleugel) M.E. Barr, Mycotaxon 15: 346 (1982). (Fig. 15).
Fig. 15.
Bricookea sepalorum (from S, type). a Ascomata on host surface (arrowed). b Section of partial peridium. Note thick-walled out layer and thin-walled inner layer. c-e Cylindrical to slightly obclavate asci with short knob-like pedicels. f-j Hyaline, 3-septate smooth-walled ascospores. Scale bars: a=0.5 mm, b=50 μm, c-j = 10 μm
≡ Metasphaeria sepalorum Vleugel, Svensk bot. Tidskr. 2: 369 (1908).
Ascomata 120-250 μm high*170-440 μm diam., solitary, scattered, or in small groups, or forming locules in massive stromatic tissues, initially immersed, becoming erumpent, to nearly superficial, depressed globose, black, membraneous, roughened; apex rounded, sometimes very short and almost inconspicuous, with a somewhat slit-like or Y-shaped ostiole (Fig. 15a). Peridium 16-30 μm wide, comprising two types of cells, outer cells heavily pigmented thick-walled textura angularis, cells 4.5-8 μm diam., cell wall 1-1.5 μm thick, inner cells of subhyaline thin-walled textura angularis, cells larger than outer cells (Fig. 15b). Hamathecium of long cellular pseudoparaphyses, 1.5-2 μm broad, embedded in mucilage, anastomosing, septate. Asci 63-83*9.5-11 μm (x = 73.8× 10.8mm, n=10), 8-spored, bitunicate, fissitunicate, oblong, cylindro-clavate or slightly obclavate, with a short knob-like pedicel which is 5-13 μm long, with an ocular chamber (Fig. 15c, d and e). Ascospores (14-)15.5-19*5-7 μm (x = 16.9 × 5.9mm, n=10), obliquely uniseriate and partially overlapping to biseriate, ellipsoid to narrowly obovoid, hyaline, 3-septate, constricted at each septum, the cells above central septum often broader than the lower ones, smooth (Fig. 15f, g, h, i and j).
Anamorph: none reported.
Material examined: SWEDEN, on Juncus filliformis, Stockholm, J. Vleugel. Jul. 1907 (S, type as Metasphaeria sepalorum Vleugel).
Notes
Morphology
Bricookea was formally established by Barr (1982a) as a monotypic genus represented by B. sepalorum based on its “globose to depressed ascomata, slit-like ostiole with labial cells, bitunicate asci, cellular pseudoparaphyses, and hyaline septate ascospores”. Bricookea was morphologically assigned to Phaeosphaeriaceae. Holm (1957) checked the authentic collections from North America and type material from Europe, and observed that the ascospores of collections from North America were significantly larger than those from the type material from Sweden. Thus, Shoemaker and Babcock (1989a) considered that the collections from North America represented a new species, which they introduced as B. barrae Shoemaker & C.E. Babc. Although the short slit-like ostiole has previously been reported (Shoemaker and Babcock 1989a), it is inconspicuous in the type specimen from Sweden. Currently, only two species are accommo-dated in this genus.
Phylogenetic study
None.
Concluding remarks
The knob-shaped pedicel, slit-like ostiole, hyaline ascospores as well as the herbaceous substrate all disagree with any current pleosporalean family. Thus, we temporarily retain this genus under Phaeosphaeriaceae until DNA sequence comparisons can be carried out.
Byssolophis Clem., in Clements & Shear, Gen. fung., Edn 2 (Minneapolis): 286 (1931). (Pleosporales, genera incertae sedis)
Generic description
Habitat terrestrial, saprobic. Ascomata medium-sized, gregarious, semi-immersed to erumpent, coriaceous, ovoid, with a conspicuous elongate slit-like ostiole on the top. Peridium not observed. Hamathecium of dense, long pseudoparaphyses, anastomosing and branching between and above the asci. Asci 8-spored, bitunicate, fissitunicate, cylindrical or cylindro-clavate, with a furcate pedicel. Ascospores fusoid, hyaline, turning faintly brown when old, 1-septate, with a short terminal appendage at each end.
Anamorphs reported for genus: none.
Literature: Clements and Shear 1931; Holm 1986; Muller and von Arx 1962.
Type species
Byssolophis byssiseda (Flageolet & Chenant.) Clem., Gen. Fung. (Minneapolis): 286 (1931). (Fig. 16)
Fig. 16.
Byssolophis byssiseda (from K(M):164030, isotype). a Ascomata gregarious on the host surface. b Numerous pseudoparaphyses. c Fusoid ascospores with or without terminal appendages. d Clavate ascus with a short furcate pedicel. Scale bars: a =1 mm, b-d=10 μm
≡ Schizostoma byssisedum Flageolet & Chenant., in Chenantaise, BU11. Soc. myco1. Fr. 35: 125 (1919).
Ascomata 300-450 μm high*600-750 μm long* 350-420 μm broad, gregarious, semi-immersed to erumpent, coriaceous, ovoid with a flattened base and apex with a elongate slit-like ostiole, up to 700 μm long and 200 μm wide (Fig. 16a). Peridium not observed. Hamathecium of dense, long pseudoparaphyses, up to 1.5-2.5 μm broad, anastomosing and branching between and above the asci (Fig. 16b). Asci 80-105 *(5-)7.5-10 μm (x = 91 × 8mm; n=10), 8-spored, bitunicate, fissitunicate, cylindrical or cylindro-clavate, with a furcate pedicel and a small ocular chamber (J-) (Fig. 16d). Ascospores 18-20 (−28)*4.5-6(−7.5) μm (x = 20.8 × 5.7mm, n =10), uniseriate to biseriate, fusoid, hyaline, turning faintly brown when old, 1-septate, with 1–2 distinct oil drops in each cell and usually with a short terminal appendage at each end (Fig. 16c).
Anamorph: none reported.
Material examined: on decaying wood (K(M):164030, isotype).
Notes
Morphology
Byssolophis was introduced as a monotypic genus based on B. byssiseda, which is characterized by its semi-immersed, gregarious, ovoid ascomata, with a conspicuous central apical ostiolar slit (Holm 1986). Subsequently, two more species were introduced, viz. B. ampla (Berk. & Broome) L. Holm and B. sphaerioides (P. Karst.) E. MU11. (Holm 1986; Muller and von Arx 1962).
Phylogenetic study
The current phylogeny places Byssolophis sphaerioides in proximity of Hypsostromataceae without resolving any sister taxa (Plate 1).
Concluding remarks
The slit-like ostiole, cylindrical asci, hyaline and 1-septate ascospores as well as the form of pseudoparaphyses are similar to species in Lophiostoma. Thus, Byssolophis may be a synonym of Lophiostoma.
Byssosphaeria Cooke, Grevillea 7: 84 (1879). (Melanommataceae)
Generic description
Habitat terrestrial, saprobic. Ascomata medium-sized, scattered to gregarious, superficial, globose, subglobose to turbinate, non papillate with white, orange, red or green ostiolar region, wall black. Hamathecium of dense, long trabeculate pseudoparaphyses, embedded in mucilage, anastomosing between and above the asci. Asci bitunicate, fissitunicate, clavate to nearly cylindrical, with a furcate pedicel. Ascospores fusoid with narrow ends, straight or slightly curved, brown, 1-septate when young.
Anamorphs reported for genus: Pyrenochaeta or Chaetophoma-like (Barr 1984; Hawksworth et al. 1995; Samuels and Muller 1978).
Literature: von Arx and Muller 1975; Barr 1984; Boise 1984; Bose 1961; Chen and Hsieh 2004; Cooke and Plowright 1879; Hyde et al. 2000; Luttrell 1973; Mugambi and Huhndorf 2009b; Muller and von Arx 1962; Samuels and Muller 1978.
Type species
Byssosphaeria keitii (Berk. & Broome) Cooke [as ‘Byssos-phaeria keithii’], (1879). (Fig. 17)
Fig. 17.
Byssosphaeria schiedermayriana (from K(M):108784, holotype). a Superficial ascomata on the host surface. b Brown, 1-septate ascospores. c Section of the lateral peridium. Note the outer textura angularis and inner textura epidermoidea cells. d, e Furcate asci with a long pedicel. f Dehiscent ascus. Scale bars: a=0.5 mm, c= 50 μm, b, d-f =15 μm
≡ Sphaeria keitii Berk. & Broome [as ‘Sphaeria keithii’], Ann. Mag. Nat. Hist., IV 17: 144 (1876).
Ascomata 360-500(−600) μm highx420-640 μm diam., scattered or in small groups, superficial with basal subiculum anchoring on the substrate, globose, subglobose to turbinate, non-papillate with pore-like ostiole, ostiolar region sometimes with orange and greenish tint, wall black, roughened, coriaceous (Fig. 17a). Peridium 55-85 μm thick, peridium outside of the substrate comprising two cell types, outer layer composed of brown thick-walled cells of textura epidermoidea, cells 1–3 μm diam., inner layer composed of small hyaline cells, cells 3-5 μm diam., merging into pseudoparaphyses; peridium inside the substrate one layer, composed of large pale brown cells of textura angularis, cells 6-13 μm diam. (Fig. 17c). Hamathecium of dense, long trabeculate pseudoparaphyses, 1–2 μm broad, embedded in mucilage, anastomosing between and above the asci. Asci 90-120(−148)× 10-14 μm, 8-spored, bitunicate, fissitunicate, cylindro-clavate to clavate, biseriate above and uniseriate below, pedicel 15-20 (−53) μm long, the immature asci usually with longer and furcate pedicel (−68 μm) (Fig. 17d,e and f). Ascospores 29-34(−38)×5.5-8(−10) μm, fusoid with narrow ends, mostly straight, sometimes slightly curved, smooth, pale brown, 1-septate, becoming 3-septate after discharge, with hyaline appendages at each acute to subacute end; in some mature spores the appendage may be absent (Fig. 17b).
Anamorph: Pyrenochaeta sp. (Barr 1984; Samuels and Muller 1978).
Pycnidia 70-500 μm diam. Conidiogenous cells phialidic, lining cavity, 5-8×4-6 μm to 5-10×3-6 μm. Conidia 2.5-3.5(−4)× 1.5-2(−3) μm, hyaline, ellipsoid or subgtobose (Barr 1984).
Material examined: ERIE, Dublin, Glasnevin Botanic Garden, on old rope, Jun. 1872, W. Keit (K(M):108784, holotype, as Sphaeria keitii Berk. & Broome).
Notes
Morphology
Byssosphaeria was introduced by Cooke and Plowright (1879) based on its superficial ascomata seated on a “tomentose subiculum of interwoven threads”, which includes various species in Sphaeria and Byssisedae, and was validly typified by B. keitii (Cooke 1878). Byssosphaeria keitii was treated as a synonym of B. schiedermayeriana (Fuckel) M.E. Barr by Sivanesan (1971), and B. schiedermayeriana exclusively occurs in tropical regions or greenhouse environments in temperate regions (Barr 1984). Morphologically, B. keitii is characterized by its large ascomata with orange to reddish plain apices, and is closely related to B. rhodomphala (Berk.) Cooke (Barr 1984).
For a long time, Byssosphaeria was assigned to Herpotrichia sensu lato, and Byssosphaeria schieder-mayeriana was renamed as H. schiedermayeriana Fuckel (von Arx and Muller 1975; Bose 1961; Luttrell 1973; Muller and von Arx 1962; Sivanesan 1971). After studying Herpotrichia in North America, Barr (1984) accepted a relatively narrow generic concept, Herpotrichia sensu stricto, and revived Byssosphaeria; this proposal is supported by phylogenetic study (Mugambi and Huhndorf 2009b). Currently Byssosphaeria comprises 32 species (http://www.mycobank.org, 08-01–2009).
Phylogenetic study
The monophyletic nature of Byssosphaeria is well demonstrated, as well as its familial status in Melanommataceae (Mugambi and Huhndorf 2009b).
Concluding remarks
Orange and greenish plain apices exist in the specimen we examined, which is different from records as “orange, bright or dull reddish plain apices” by Barr (1984). This might be because different specimens have different colours, or there may be a variation of apical colour within a single species, as both orange and green can coexist on the same ascoma (see Fig. 17a). The coloured apical rim, together with the trabeculate pseudoparaphyses as well as the presence of subiculum make Byssosphaeria readily distinguishable from other morphologically comparable genera, e.g. Herpotrichia and Keissleriella (Hyde et al. 2000).
Calyptronectria Speg., Anal. Mus. nac. Hist. nat. B. Aires 19: 412 (1909). (Melanommataceae)
Generic description
Habitat terrestrial, saprobic. Ascomata small- to mediumsized, solitary, scattered, or in small groups, immersed, lenticular to subglobose, papillate, ostiolate. Hamathecium of long, filliform pseudoparaphyses, branching and anastomosing, embedded in mucilage. Asci 4- to 8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a short, furcate pedicel. Ascospores muriform, broadly fusoid to fusoid with broadly to narrowly rounded ends, hyaline.
Anamorphs reported for genus: none.
Literature: Barr 1983; Rossman et al. 1999; Spegazzini 1909.
Type species
Calyptronectria platensis Speg., Anal. Mus. nac. Hist. nat. B. Aires 19: 412 (1909). (Fig. 18)
Fig. 18.
Calyptronectria platensis (from LPS 1209, holotype). a Appearance of ascomata scattered in the substrate (after removing the out layer of the substrate). Note the protruding papilla. b Section of an ascoma. c Section of the partial peridium. Note the lightly pigmented pseudoparenchymatous cells. d Released ascospores with mucilaginous sheath. e Eight-spored asci in hamathecium and embedded in gel matrix. f Ascus with a short pedicel. Scale bars: a=0.5 mm, b= 100 μm, c=50 μm, d-f=10 μm
Ascomata 120-270 μm highx170-400 μm diam., solitary, scattered, immersed, lenticular to subglobose, papillate, ostiolate (Fig. 18a and b). Apex with a small and slightly protruding papilla. Peridium 18-30 μm wide, comprising two types of cells, outer layer composed of pseudoparenchymatous cells, cells 3-6 μm diam., cell wall 1–2 μm thick, inner layer comprising less pigmented cells, merging with pseudoparaphyses (Fig. 18b and c). Hamathecium of long, filliform pseudoparaphyses, 1–2 μm broad, branching and anastomosing, embedded in mucilage. Asci 98-140×12.5-20 μm (x = 107 × 15.4mm, n=10), 8-spored, sometimes 4-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a short, furcate pedicel, 12-20 μm long, with an ocular chamber (to 4 μm wide × 3 μm high) (Fig. 18e and f). Ascospores 1 7 – 2 2. 5 μ m × (6. 3 −) 7. 5 – 10 μ m (x = 19.8 × 7.6mm, n =10), biseriate above and uniseriate below, ellipsoid to broadly fusoid with broadly to narrowly rounded ends, hyaline, usually with (3-)5 transverse septa, with or without 1–3 longitudinal septa in the central cells, constricted at the median septum, the upper cell often broader than the lower one, smooth, surrounded by an irregular hyaline gelatinous sheath up to 3 μm thick (in dry specimen) (Fig. 18d).
Anamorph: none reported.
Material examined: ARGENTINA, La Plata, on decaying branches of Manihot carthaginensis (Jacq.) Mull., Sept. 1906, Spegazzini (LPS 1209, holotype).
Notes
Morphology
Calyptronectria is a relatively poorly studied genus, which was formally established based on C. argentinensis Speg. and C. platensis, with C. platensis being chosen as the generic type (Spegazzini 1909). Morphologically, Calyptronectria is characterized by its immersed ascomata, trabeculate pseudoparaphyses and hyaline, muriform ascospores as well as its peridium that turns reddish brown in KOH (Rossman et al. 1999) (not shown here). Subsequently, C. indica Dhaware was introduced from India, and Barr (1983) transferred Teichospora ohiensis Ellis & Everh. to Calyptronectria as C. ohiensis (Ellis & Everh.) M.E. Barr. However, this proposal is inappropriate as the type specimen of T. ohiensis is “unitunicate” (Barr 1983; Rossman et al. 1999). Subsequently, Rossman et al. (1999) transferred
Calyptronectria ohiensis to Thyridium (as T. ohiense (Ellis & Everh.) Rossman & Samuels).
Phylogenetic study
None.
Concluding remarks
The immersed ascomata, trabeculate pseudoparaphyses, bitunicate asci, hyaline and muriform ascospores as well as the reaction of peridium to KOH (turns reddish brown) make it distinguishable from all other reported genera (Rossman et al. 1999). Thus Calyptronectria is a morphologically well defined genus.
Carinispora K.D. Hyde, J. Linn. Soc., Bot. 110: 97 (1992). (Pleosporales, genera incertae sedis)
Generic description
Habitat marine, saprobic. One or two ascomata per stroma. Ascomata scattered or in small groups, developing beneath the host epidermis, erumpent, lenticular, ostiolate, lacking periphyses. Peridium pale brown, composed of thin-walled elongated cells at the sides and thick-walled cells of textura epidermoidea at the base. Hamathecium of dense, long filliform pseudoparaphyses, embedded in mucilage, anastomosing between and above the asci, rarely septate. Asci 8-spored, bitunicate, fissitunicate, clavate to cylindrical, with a short furcate pedicel, apex with an ocular chamber and apical ring. Ascospores biseriate, narrowly fusoid, yellow to pale brown, multi-septate, constricted at the septa, the two central cells being the largest, surrounded by a gelatinous sheath.
Anamorphs reported for genus: none.
Literature: Hyde 1992a, 1994b.
Type species
Carinispora nypae K.D. Hyde, J. Linn. Soc., Bot. 110: 99 (1992). (Fig. 19)
Fig. 19.
Carinispora nypae (from BRIP 17106, holotype). a b Ascomata on the host surface. b Section of an ascoma. c Section of a partial peridium. d, e, g, h Asci with ocular chambers and short pedicels. f The ocular chamber and apical ring of ascus. i-j Narrowly fusoid ascospores. Scale bars: a =1 mm, b, c =100 μm, d, g, h=50 μm, e, f, i, j =10 μm
One or two ascomata per stroma. Ascomata up to 0.8 mm diam., scattered or in sma11 groups, developing beneath the host epidermis, crust-like, as circular spots, wall brown, with a sma11 central ostiole, in section 225-285 μm highx 510-750 μm diam., lenticular, ostiolar canal lacking periphyses (Fig. 19a and b). Peridium 35-45 μm wide at sides, pale brown, at sides composed of a thin layer of thin-walled elongate cells, fusing with the stromatic tissue and host cells, at the base composed of thick-walled cells, forming a textura epidermoidea and fusing with host cells. A wedge of pale brown hyphae forming a textura porrecta is present at the rim (Fig. 19c). Hamathecium of dense, long filliform pseudoparaphyses 1–3 μm broad, embedded in mucilage, anastomosing between and above the asci, rarely septate. Asci 142-207×14.2-19.8 μm, 8-spored, bitunicate, fissitunicate, clavate to cylindrical, with a furcate pedicel, up to 40 μm long, apex with an ocular chamber and apical ring (to 2 μm wide × 3 μm high, J-), developing from ascogenous tissue at the base of the ascocarp (Fig. 19d, e, f, g and h). Ascospores 42-66×7-10.6 μm, biseriate, narrowly fusoid with broadly to narrowly rounded ends, somewhat curved, yellow to pale brown, yellow in mass, 7-8-septate, constricted at the septa, the two central cells being the largest, surrounded by a gelatinous sheath; the sheath has a central “spine” and curved polar extrusions (Fig. 19i and j).
Anamorph: none reported.
Material examined: BRUNEI DARUSSALAM, Tungit Api Api mangrove, from decaying intertidal fronds of Nypa fruticans Wurmb., 14 Apr. 1987, K.D. Hyde (BRIP 17106, holotype).
Notes
Morphology
Carinispora is distinguished from Phaeosphaeria by its saprobic life style and lenticular ascomata formed under the host epidermis, peridium structure and sheath surrounding the ascospores (Hyde 1992a, 1994b). Two species were reported, i.e. C. nypae and C. velatispora K.D. Hyde.
Phylogenetic study
Suetrong et al. (2009) could not resolve Carinispora nypae in a phylogeny based on four genes.
Concluding remarks
Both Carinispora nypae and C. velatispora are reported as marine fungi, which should be taken into consideration for their familial placement.
Caryosporella Kohlm., Proc. Indian Acad. Sci., Pl. Sci. 94: 355 (1985). (?Melanommataceae)
Generic description
Habitat marine, saprobic. Ascomata densely scattered or gregarious, superficial, subglobose, black, papillate, ostiolate, periphysate, carbonaceous. Peridium carbonaceous. Hamathecium of dense, long trabeculate pseudoparaphyses, anastomosing and branching above the asci. Asci 8-spored, bitunicate, fissitunicate, cylindrical. Ascospores ellipsoidal to broadly fusoid with narrowly hyaline rounded ends, deep reddish brown, thickwalled, 1-septate with hyaline germ pore at each end. Anamorphs reported for genus: suspected spermatia (Kohlmeyer 1985).
Literature: Eriksson 2006; Kohlmeyer 1985; Lumbsch and Huhndorf 2007.
Type species
Caryosporella rhizophorae Kohlm., Proc. Indian Acad. Sci., Pl. Sci. 94: 356 (1985). (Fig. 20)
Fig. 20.
Caryosporella rhizophoriae (from NY. Herb. J. Kohlmeyer No. 4532a, holotype). a Gregarious ascomata on host surface. b Section of an ascoma. c, d Section of partial peridium at sides (c) and base (d). Note the three layers. e Asci with long peduncles in pseudoparaphyses. f, g Ascospores. Note the “net”-like ridged ornamentation of spore surface and hyaline germ pores. Scale bars: a =1 mm, b=200 μm, c-e =100 μm, f, g =10 μm
Ascomata 0.8-1.1 mm highx0.9-1.2 mm diam., densely scattered or gregarious, superficial with a flattened base, not easily removed from the host surface, subglobose, black, short papillate, ostiolate, periphysate, carbonaceous (Fig. 20a and b). Peridium 120-150 μm thick at sides, up to 200 μm thick at the apex, thinner at the base, 3-layered, outer layer composed of golden-yellow, very thick-walled cells of textura epidermoidea, mixed with subglobose, large cells near the surface, cells 7-15 μm diam., middle layer composed of deep brown, very thick-walled cells of textura epidermoidea, inner layer composed of hyaline, thin-walled cells of textura prismatica, up to 50 × 5 μm diam., merging with pseudoparaphyses (Fig. 20b, c and d). Hamathecium of dense, long trabeculate pseudoparaphyses, 1.5-2 μm wide, anastomosing and branching above the asci. Asci 225-250 (—275)× 14-17 μm (x = 137 × 16.3mm, n =10), 8-spored, bitunicate, fissitunicate, cylindrical, with a long, narrowed, pedicel which is up to 75 μm long, apical characters not observed (Fig. 20e). Ascospores 25-28 (—30)×9-13 μm (x = 26.8 × 11mm, n =10), uniseriate to partially overlapping, ellipsoidal to broadly fusoid with narrow hyaline rounded ends, deep reddish brown, thick-walled, 1-septate with hyaline germ pore at each end, slightly constricted at the septum, verruculose, sometimes with “net”-like ridged ornamentations (Fig. 20f and g).
Anamorph: suspected spermatia (Kohlmeyer 1985). Material examined: BELIZE, Twin Cays, tip of prop root of Rhizophora mangle, 18 Mar. 1984, J. Kohlmeyer (NY. Herb. J. Kohlmeyer No. 4532a, holotype).
Notes
Morphology
Caryosporella was formally established by Kohlmeyer (1985) based on the obligate marine fungus, C. rhizophorae, which is characterized by its superficial ascomata, 3-layered peridium, filliform trabeculate pseudoparaphyses, and brown, 1-septate ascospores. Caryosporella was originally assigned to Massariaceae despite several major differences, such as the superficial ascomata, reddish brown ascospores (Kohlmeyer 1985). Subsequently, Caryosporella was assigned to Melanommataceae (Eriksson 2006; Lumbsch and Huhndorf 2007).
Phylogenetic study
Suetrong et al. (2009) showed that a single isolate of Caryosporella rhizophorae does not reside in Pleosporales, but is related to Lineolata rhizophorae (Kohlm. & E. Kohlm.) Kohlm. & Volkm.-Kohlm. and placed in Dothideomycetidae incertae sedis.
Concluding remarks
As an obligate marine fungus, the familial placement of Caryosporella rhizophorae is uncertain but it may not belong to Pleosporales.
Chaetomastia (Sacc.) Berl., Icon. fung. (Abellini) 1: 38 (1890). (Teichosporaceae)
≡ Melanomma subgen. Chaetomastia Sacc., Syll. fung. (Abellini) 2: 113 (1883).
Generic description
Habitat terrestrial, saprobic. Ascomata relatively small, scattered, or in small groups, superficial, globose or subglobose, black, papillate, ostiolate, coriaceous. Peridium relatively thin, 1-layered, composed of heavily pigmented cells of textura angularis. Hamathecium of dense, long cellular pseudoparaphyses, embedded in mucilage. Asci mostly 4-spored, bitunicate, fissitunicate, broadly cylindrical with a furcate pedicel, with a large ocular chamber, especially apparent in immature asci. Ascospores ellipsoid to broadly fusoid with broadly to narrowly rounded ends, brown, 3-septate, constricted at a11 septa.
Anamorphs reported for genus: coelomycetous where known: conidia hyaline or brown, aseptate or 1-septate (Aposphaeria- or Coniothyrium-like) (Barr 1989c).
Literature: Barr 1987b, 1989c; 1993a; b; 2002; Berlese 1890; Clements and Shear 1931; Eriksson 1999; Eriksson and Hawksworth 1987, 1998; Holm 1957; Leuchtmann 1985; Saccardo 1883.
Type species
Chaetomastia hirtula (P. Karst.) Berl., Icon. fung. (Abellini) 1: 38 (1890). (Fig. 21)
Fig. 21.
Chaetomastia hirtula (from H, FFE 825, kleptotype). a Superficial ascomata gregarious on the host surface. b Section of a partial peridium. Note the cells of textura angularis with relatively thick wall. c, d Cylindrical asci with long and furcate pedicels. e, f Brown, 3-septate ascospores. Scale bars: a=0.5 mm, b=50 μm, c-f= 15 μm
≡ Sphaeria hirtula P. Karst., Fungi Fenn. Exs. N. 825 (1869).
Ascomata 214-286 μm high×210-258 μm diam., scattered or in groups, superficial, globose, wall black; apex often opening with a broad pore within slightly raised papilla, up to 30 μm diam., coriaceous (Fig. 21a). Peridium 20-26 μm thick, 1-layered, composed of heavily pigmented cells of textura angularis, cells up to 5×15 μm diam., ce11 wall up to 3.5 μm thick (Fig. 21b). Hamathecium of dense, long cellular pseudoparaphyses, embedded in mucilage. Asci 90-130×12.5-17.5(−22.5) μm (x = 111 × 16.3mm, n = 10), mostly 4-spored, bitunicate, fissitunicate, broadly cylindrical, with a furcate pedicel, 18-48 μm long, with a large ocular chamber best seen in immature asci (to 3 μm wide × 3 μm high) (Fig. 21c and d). Ascospores 20.5-27×7-10 μm (x = 23.5× 8.2mm, n =10), uniseriate to partially over-lapping, ellipsoid to broadly fusoid with broadly to narrowly rounded ends, brown, 3-septate, verruculose, constricted at all septa, constricted at the median septum, the cell above the central septum often broader than the others (Fig. 21e and f).
Anamorph: none reported.
Material examined: FINLAND, ETELA-HAME (EH/ Ta), Tammela, Mustiala, Pa Rub. id., 8 May 1866. P.A. Karsten (H, FFE 825, kleptotype).
Notes
Morphology
Chaetomastia was introduced by Saccardo (1883) as a subgenus of Melanomma, and five species were included, i.e. M. canescens Speg., M. cucurbitarioides Speg., M. hirtulum (P. Karst.) Sacc., M. hispidulum Sacc. and M. pilosellum P. Karst. Berlese (1890) promoted it to genus rank. Subsequently, Chaetomastia hirtula (P. Karst.) Berl. was selected as the lectotype species of the genus (Clements and Shear 1931). Chaetomastia has been regarded as having unitunicate asci (Eriksson and Hawksworth 1986, 1998; Eriksson 1999). However its bitunicate status was confirmed by Holm (1957). Holm (1957) treated C. hirtula as Melanomma hirtulum (P. Karst.) Sacc., and Leuchtmann (1985) transferred this species to Montagnula sensu lato based on the ascospore morphology and the hyphae surrounding the ascomata. Barr (1987b) suggested that ascoma, peridium structure and ascospore characters pointed Montagnula sensu stricto to Phaeosphaeriaceae, while the characters of ascomata and peridium structure of Chaetomastia were thought to fit the definition of Dacampiaceae (Barr 1987b). In particular, the peridium and ascospore characters of C. hirtula are comparable with those of the generic type of Massariosphaeria (M. phaeospora). Thus, Barr (1989c) accepted Massariosphaeria sensu stricto and assigned the phragmosporous species of Massariosphaeria sensu lato to Chaetomastia.
Barr (2002) later assigned Chaetomastia to Teichosporaceae based on its saprobic or hypersaprobic lifestyle, occurring on woody stems and peridium structure, and this is widely followed (Eriksson 2006; Lumbsch and Huhndorf 2007). Currently, 11 species are accepted in this genus (http://www.indexfungorum.org/).
Phylogenetic study
None.
Concluding remarks
Familial placement of Chaetomastia is undetermined currently but has been included in the Teichosporaceae by authoritative sources (Eriksson 2006; Lumbsch and Huhndorf 2007) or the Dacampiaceae (http://www.indexfungorum.org/).
Chaetoplea (Sacc.) Clem., Gen. Fung. (Minneapolis): 275 (1931). (?Phaeosphaeriaceae)
≡ Pyrenophora subgen. Chaetoplea Sacc., Syll. fung. (Abellini) 2: 279 (1883).
Generic description
Habitat terrestrial, saprobic. Ascomata small to medium, immersed, erumpent to superficial, globose to subglobose, papillate, ostiolate. Peridium not examined. Hamathecium of dense, long, narrowly cellular pseudoparaphyses. Asci 8-spored or 4-spored, bitunicate, fissitunicate, cylindro-clavate, with a thick, furcate pedicel. Ascospores ellipsoid or fusoid, pale brown to brown, phragmosporous or muriform.
Anamorphs reported for genus: Microdiplodia-like (Barr 1990b).
Literature: Barr 1981; 1987a; b; 1990b; Clements and Shear 1931; Ramaley and Barr 1995; Yuan and Barr 1994.
Type species
Chaetoplea calvescens (Fr.) Clem., Gen. Fung. (Minneapolis): 275 (1931). (Fig. 22)
Fig. 22.
Chaetoplea calvescens (from FH-81113, isotype). a, b Fourb spored and 8-spored asci. c Released ascospores. Scale bars: a-c= 10 μm
≡ Sphaeria calvescens Fr. Scleromyc. Sueciae 401.
Ascomata not examined. Peridium not examined. Hamathecium of dense, long, narrow cellular pseudopar-aphyses, 2–3 μm broad, septate, branching and anastomosing. Asci 90-110×10-12 μm, 8-spored, rarely 4-spored, bitunicate, fissitunicate, cylindro-clavate, with a thick, furcate pedicel which is up to 30 μm long (Fig. 22a and b). Ascospores 13-18×5.5-7 μm, obliquely uniseriate and partially overlapping, broadly fusoid to oblong with broadly rounded ends, pale brown, 2–3-septate, constricted at the septa, containing four refractive globules (Fig. 22c).
Note: The specimen is only a slide, and no peridium or ascomata information could be obtained.
Anamorph: coelomycetous, conidia yellowish, 1-septate, 9-13×4-5(−8) μm (Webster and Lucas 1959); Microdiplodia henningsii Staritz=Chaetodiplodia caudina Karst. (Sutton 1980) (referred to Barr 1990b (p50)).
Material examined: SWEDEN, sub-collection: Curtis Herbarium, verified by R.A. Shoemaker, leg. E.M. Fries 401 (FH-81113, isotype, microscope slide).
Notes
Morphology
Chaetoplea was introduced based on C. calvescens, which has been regarded as similar to Pleospora or Leptosphaeria (Eriksson and Hawksworth 1987; Wehmeyer 1961; von Arx and Muller 1975). Based on the differences in ascomata, peridium structure, pseudo-paraphyses as well as its anamorphic stage, Chaetoplea was maintained as a separate genus (Barr 1990b; Yuan and Barr 1994). Chaetoplea sensu lato was accepted by Barr (1990b), which included some species of Teichospora as well as the subgenus Pleospora subg. Cylindrosporeae.
The following is from the label of specimen. “Sphaeria calvescens, Scler. Suecicae (Ed. 2) 401. No specimen of Scler. Suecicae 401 is now at Uppsala according to R. Santesson 1966. This Curtis Herbarium specimen in the Farlow Herbarium is isotype. Wehmeyer (1961) in his Pleospora monograph did not study any portion of the Scler. Suecicae exsiccatus 401, nor did Webster & Lucas in the taxonomic and life-history study (Trans. Brit. Myc. Soc. 42, 332–342. 1959) of this species.
The specimen has most of the features described by Webster & Lucas including the presence of the conidial state Microdiplodia henningsii Staritz. I did not see vertical septa in the ascospores. Webster & Lucas note that vertical septa may be occasionally be lacking. The fungus is otherwise as they describe it although some perithecia collapse and appear cupulate.”—by R.A. Shoemaker.
Phylogenetic study
None.
Concluding remarks
The substrate of Chaetoplea sensu Barr (1990b) can be herbaceous stalks, decorticated wood or periderm, or old cotton cloth and string, which may indicate its heterogeneous nature. The ascospores seem very much like Phaeosphaeria which may be an earlier name; more details concerning the ascomatal, peridial and hamathecial structures are needed to make any conclusion.
Cilioplea Munk, Dansk botanisk Arkiv 15: 113 (1953). (Pleosporales, genera incertae sedis)
Generic description
Habitat terrestrial, saprobic. Ascomata small- to medium-sized, solitary, scattered or in small groups, immersed, globose or subglobose, papilla covered with short and blackish setae, coriaceous. Peridium thin, comprising small heavily pigmented thick-walled cells of textura angularis. Hamathecium of cellular pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, broadly clavate, with a short, furcate pedicel, and small ocular chamber. Ascospores fusoid to narrowly fusoid with narrowly rounded ends, pale brown to reddish brown, multi-transverse septa, usually with one longitudinal septum in some central cells, constricted at the primary septum. Anamorphs reported for genus: none.
Literature: Barr 1990b, 1992b; Crivelli 1983; Lumbsch and Huhndorf 2007; Muller 1951; Munk 1953, 1957.
Type species
Cilioplea coronata (Niessl) Munk, Dansk botanisk Arkiv 15: 113 (1953). (Fig. 23)
Fig. 23.
Cilioplea coronata (M 175-89-290, lectotype). a Immersed ascomata in small groups on the host surface (the covering host tissue was removed). b Section of a partial ascoma. Note the thin peridium. c Clavate asci within pseudoparaphyses. d Ascus with a small ocular chamber. Scale bars: a=0.5 mm, b =100 μm, c=50 μm, d=10 μm
≡ Pleospora coronata Niessl, Notiz. Pyr.: 16 (1876).
Ascomata 170-290 μm highx200-410 μm diam., solitary, scattered, or in small groups, immersed, globose or subglobose, wall black, papilla raised, 50-80 μm high, with short and blackish setae, coriaceous (Fig. 23a). Peridium 9-15 μm thick laterally, up to 28 μm thick at the apex, thinner at the base, 1-layered, composed of small heavily pigmented thick-walled cells of textura angularis, cells up to 4×2.5 μm diam., cell wall 2–3 μm thick, apex cells smaller and walls thicker (Fig. 23b). Hamathecium of long cellular pseudoparaphyses, 2–3 μm broad. Asci (60-)80-108× 10-15 μm (x = 85.3 × 12.1mm, n=10), 8-spored, bitunicate, fissitunicate, broadly clavate, with a short, thick, furcate pedicel, 5-15 μm long, and a small ocular chamber (to 3 μm widex 2 μm high) (Fig. 23c and d). Ascospores 21–27.5 × 5.5-7.5 μm (x = 24 × 6.7mm, n=10), biseriate to uniseriate at base, fusoid to narrowly fusoid with narrowly rounded ends, pale reddish brown, 5-7 transverse septa (mostly 5), usually with one longitudinal septum in some central cells, deeply constricted at the median septum, the part above the primary septum shorter and broader, smooth-walled.
Anamorph: none reported.
Material examined: GERMANY, Hadiberg. on Reseda lutea Hadiberg, 20 Sept. 1875, Niessl (M 175-89-290, lectotype; M 175-89-291, type).
Notes
Morphology
Cilioplea was introduced by Muller (1951) as a subgenus of Pleospora, and this was followed by Munk (1957), who had earlier proposed it as a separate genus typified by C. coronata based on its hairy papilla, clavate asci as well as its “perfectly paraphysoid” (see Munk 1953). A relatively narrow concept of Pleospora was accepted by Crivelli (1983), and four species was assigned under the separate genus Cilioplea, viz. C. coronata, C. genisticola (Fautrey & Lambotte) Crivelli, C. kansensis (Ellis & Everh.) Crivelli and C. nivalis (Niessl) Crivelli. Subsequently, another six species were added (Barr 1990b, 1992b). Currently, ten species are included under Cilioplea.
Phylogenetic study
None.
Concluding remarks
The most striking character of Cilioplea is its setose papilla, which has been shown to have no phylogenetic significance in Lentitheciaceae (Zhang et al. 2009a). Cilioplea was assigned under Lophiostomataceae (Lumbsch and Huhndorf 2007), but there is little morphological similarity with the Lophiostomataceae sensu stricto (Zhang et al. 2009a). Thus its familial placement needs further study.
Crivellia Shoemaker & Inderb., in Inderbitzin, Shoemaker, O’Neill, Turgeon & Berbee, Can. J. Bot. 84: 1308 (2006). (Pleosporaceae)
Generic description
Habitat terrestrial, hemibiotrophic or parasitic. Ascomata small- to medium-sized, scattered, immersed, erumpent to nearly superficial, papillate, ostiolate. Peridium thin, composed of two cells types, outer cells of thick walled and textura angularis, inner cells thin-walled, yellow. Hamathecium of dense, long and thin pseudoparaphyses. Asci (4-) 8-spored, bitunicate, fissitunicate dehiscence not observed, broadly cylindrical to cylindrical, with a short, furcate pedicel and an ocular chamber. Ascospores fusoid to broadly fusoid, pale brown, septate, sometimes with one or two vertical septa in the middle cells, constricted at the septa.
Anamorphs reported for genus: Brachycladium (Inderbitzin et al. 2006).
Literature: Inderbitzin et al. 2006.
Type species
Crivellia papaveracea (De Not.) Shoemaker & Inderb., Can. J. Bot. 84: 1308 (2006). (Fig. 24)
Fig. 24.
Crivellia papareracea (from UBC F14995, epitype). a Gregarious ascomata immersed within the host surface. b Section of an ascoma. c Asci within pseudoparaphyses. d Cylindrical ascus with a short pedicel. Scale bars: a =1 mm, b = 100 μm, c, d=30 μm
≡ Cucurbitaria papaveracea De Not., Sfer. Ital.: 62 (1863).
Ascomata 210-260 μm highx300-380 μm diam., densely scattered, immersed, erumpent to nearly superficial, flattened globose, dark brown, papillate, ostiolate (Fig. 24a). Peridium 25-30 μm thick, thicker near the apex and thinner at the base, composed of two cell types, outer cells of thick-walled and textura angularis, cells up to 10 × 5 μm diam., cell wall 2-4 μm thick, inner cells thin-walled, yellow (Fig. 24b). Hamathecium of dense, long, 1–2 μm broad, rarely septate pseudoparaphyses. Asci 85-125×10-13 μm (x = 106× 11mm, n=10), (4-)8-spored, bitunicate, fissitunicate dehiscence not observed, broadly cylindrical to cylindrical, with a short, furcate pedicel, with a relatively large ocular chamber (Fig. 24c and d). Ascospores (16-)19-24×5-7.5 μm (x = 20.4 × 6.3mm, n =10), overlapping uniseriate to rarely biseriate, fusoid to broadly fusoid, pale brown, 3-septate, sometimes with one or two vertical septa in the middle cells, constricted at the septa, the upper cell often broader than the lower one, smooth-walled.
Anamorph: Brachycladium penicillatum (Corda) Fr. (Inderbitzin et al. 2006).
Material examined: AUSTRIA, Vienna, on decaying stems of Papaver rhoeas L., 28 Oct. 2001, W. Jaklitsch (UBC F14995, epitype).
Notes
Morphology
Crivellia was separated from Pleospora and introduced as a new genus by Inderbitzin et al. (2006) based on their differences in ascospore morphology and anamorphic stages. Crivellia is characterized by having smaU- to medium-sized ascomata, and yellow, 3-septate ascospores with one or two vertical septa in central cells. Its Brachycladium anamorphic stage with phragmosporous conidia also differs from that of Stemphylium, which is the anamorphic stage of Pleospora (Inderbitzin et al. 2006). Currently, two species are included within Crivellia, i.e. C. homothallica Inderb. & Shoemaker and C. papaveracea.
Phylogenetic study
Crivellia papaveracea was shown to be closely related to some species of Alternaria, and its pleosporaceous status was confirmed following molecular studies (Inderbitzin et al. 2006).
Concluding remarks
Crivellia seems to belong to Pleosporaceae, and may be closely related to Pleospora.
Decaisnella Fabre, Annls Sci. Nat., Bot., ser. 6 9:112 (1878). (Pleosporales, genera incertae sedis)
Generic description
Habitat terrestrial, saprobic. Ascomata medium to large, immersed to erumpent, clypeate, papillate, ostiolate. Hamathecium of dense, long, cellular pseudoparaphyses, rarely septate, embedded in mucilage. Asci mostly 4- or 8-spored, rarely 2-spored, cylindrical to cylindro-clavate, with a furcate pedicel. Ascospores muriform, dark brown, oblong with broadly rounded ends.
Anamorphs reported for genus: none.
Literature: Barr 1986; 1990a; b; Fabre 1878; Saccardo 1883.
Type species
Decaisnella spectabilis Fabre, Annls Sci. Nat., Bot., ser. 6 9: 112 (1879). (Fig. 25)
Fig. 25.
Decaisnella spectabilis (NY2082, syntype). a Appearance of ascomata on the host surface. b Section of a partial peridium (immersed in the substrate). Note the pseudoparenchymatous out layer. c, d Muriform ascospores. Note the minuitely verrucose ornamentation. e Ascus with a short pedicel. Scale bars: a=0.5 mm, b =100 μm, c-e=20 μm
Ascomata 520-680 μm highx430-600 μm diam., solitary, scattered, or in small groups of 2–3, immersed to erumpent, clypeate, globose or subglobose, black, roughened, with a blunt papilla up to 170 μm high, apex with a round ostiole, coriaceous (Fig. 25a). Peridium 70-90 μm thick at sides, thicker near the apex, comprising two types of cells; part immersed in host tissue, outer layer pseudoparenchymatous, 55-65 μm thick, pigmented, inner layer composed of lightly pigmented to hyaline thin-walled compressed cells, 15-23 μm thick, cells 3.5-7 μm diam., part above host tissue heavily pigmented covered by clypeus tissues (Fig. 25b). Hamathecium of dense, long, cellular pseudoparaphyses, 1.5-3 μm broad, rarely septate, embedded in mucilage. Asci 150–200 × 15–25 (−33) μ m (x = 181 × 20.6mm, n =10), (2−)4-spored, bitunicate, fissitunicate, broadly cylindrical, with a short, thick, furcate pedicel which is 20-40 μm long, no apical apparatus observed (Fig. 25e). Ascospores 37-45×12-17 μm (x = 43 × 15mm, n = 10), uniseriate and sometimes slightly overlapping, oblong with broadly rounded ends, dark brown, verrucose or smooth, 7-9 transverse septa and 1–3 longitudinal septa in some of the cells, no constriction at the septa (Fig. 25c and d).
Anamorph: none reported.
Material examined: GERMANY, Valsalpe in der Ramsau, Bayer, Alpen, onRhamnuspumila Turra., Jul. 1913, Karl Arnold (NY2082, syntype as Teichospora megalocarpa Rehm).
Notes
Morphology
Decaisnella was formally established by Fabre (1879), but was treated as a synonym of Teichospora by Saccardo (1883). This was followed by several mycolo gists over a long time. The main morphological differences between Decaisnella and Teichospora include the size and septation of ascospores, shape of ascomata, structure of peridium and type of pseudoparaphyses (Barr 1986). Thus Barr (1986) revived Decaisnella and assigned it to Massariaceae based on the shape of ascomata and large, distoseptate ascospores. Currently, 15 species are accepted under Decaisnella (http://www.mycobank.org/MycoTaxo.aspx). Neither the size of ascomata nor the ascospore characters have proven sufficient to place taxa at the family level in Pleosporales (Zhang et al. 2009a), and therefore familial placement of Decaisnella remains uncertain.
Phylogenetic study
Decaisnella formosa resided in the clade of Lophiostomataceae and in proximity to Lophiostoma macrostomoides De Not. (Plate 1).
Concluding remarks
The muriform ascospores, saprobic life style and 4-spored asci point Decaisnella spectabilis to Montagnulaceae, but this can only be confirmed following a molecular phylogenetic study.
Delitschia Auersw., Hedwigia 5: 49 (1866). (Delitschiaceae)
Generic description
Habitat terrestrial, saprobic (coprophilous). Ascomata medium- to large-sized, solitary or scattered, immersed to erumpent, globose or subglobose, apex with or without papilla, ostiolate. Peridium thin, composed of compressed cells. Hamathecium of dense, long pseudoparaphyses, anastomosing and branching. Asci 8-spored, cylindrical to cylindro-clavate, with short pedicel. Ascospores uni- to triseriate, pale to dark brown, ellipsoid, 1-septate, usually constricted at the septum, smooth, with a full length germ slit in each cell.
Anamorphs reported for genus: none.
Literature: Auerswald 1866; Barr 2000; Cain 1934; Dennis 1968; Eriksson 2006; Griffiths 1901; Hyde and Steinke 1996; Kirschstein 1911; Kruys et al. 2006; Luck-Allen and Cain 1975; Lumbsch and Huhndorf 2007; Moreau 1953; Munk 1957; Romero and Samuels 1991; Schoch et al. 2006; Winter 1887.
Type species
Delitschia didyma Auersw., Hedwigia 5: 49 (1866). (Fig. 26)
Fig. 26.
Delitschia didyma (from L, 1950). a Ascomata on the substrate surface. Note the ostiolar opening. b Section of peridium. Note the small cells of textura angularis. c Released and unreleased ascospores. Note the germ slit in each cell. d, e Asci with ascospores and short pedicels with rounded ends. Scale bars: a=0.5 mm, b =30 μm, c-e=70 μm
Ascomata 400-800 μm diam., solitary or scattered, immersed, globose or subglobose, black, papilla short, 70-130 μm broad, central, with a wide opening, coriaceous (Fig. 26a). Peridium ca. 15 μm thick laterally, up to 35 μm thick at the apex, up to 30 μm at the base, comprising a single layer of small lightly pigmented thin-walled cells of textura angularis, cells 4-10 μm diam., cell wall <1 μm thick, apex cells smaller and wall thicker (Fig. 26b). Hamathecium of dense, very long pseudoparaphyses, 1.5-2 μm broad, anastomosing and branching. Asci 290-380× 35-45 μm (x = 357.5 × 40.6mm, n =10), 8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with short, narrowed pedicels which are rounded at the base, 25-60 μm long, apex with a wide ocular chamber (Fig. 26d and e). Ascospores 50-58 × 20-22.5 μm (x = 54 × 21.3mm, n =10), obliquely uniseriate and partially overlapping, ellipsoid with narrowly rounded ends, reddish brown, 1-septate, slightly constricted at the septum, smooth-walled, each cell with a full length germ slit (Fig. 26c).
Anamorph: none reported.
Material examined: GERMANY, Near Konigstein, in forest, rare, Oct. 1904, W. Krieger (L, 1950).
Notes
Morphology
Delitschia was established by Auerswald (1866), and assigned to Sphaeriaceae. It was considered to be closely related to Sordariaceae and Amphisphaeriaceae. Winter (1887) assigned Delitschia under Sordariaceae, and this placement is followed in several subsequent studies (Griffiths 1901; Kirschstein 1911). Cain (1934) suggested that Delitschia might belong in Pleosporaceae, and this proposal was supported by Moreau (1953) and Dennis (1968). Finally, Munk (1957) established Sporormiaceae (Pseudosphaeriales), and Delitschia was assigned therein. Luck-Allen and Cain (1975) reviewed and redefined the genus as having bitunicate asci, pigmented and 1-septate ascospores with an elongated germ slit in each cell and surrounded by a gelatinous sheath, and in particular, the coprophilous habitat. Luck-Allen and Cain (1975) accepted 46 species. Subsequently, some wood-inhabiting species were also described (Hyde and Steinke 1996; Romero and Samuels 1991). Three genera, i.e. Delitschia, Ohleriella and Semidelitschia were separated from Sporormiaceae, and a new family, Delitschiaceae, was introduced by Barr (2000) to accommodate them. Delitschiaceae is characterized by a periphysate ostiole, wide endotunicate asci with a wide ocular chamber and ascospores having cells with germ slits. Delitschiaceae has been subsequently accepted (Eriksson 2006; Lumbsch and Huhndorf 2007).
The genus comprises 83 names (Index Fungorum) and is estimated to comprise 51 species (Kirk et al. 2008). Keys to Delitschia can be found in Luck-Allen and Cain (1975) and Hyde and Steinke (1996).
Phylogenetic study
Delitschia didyma and D. winteri (W. Phillips & Plowr.) Sacc. form a robust phylogenetic clade within Delitschiaceae, which is basal to other members of Pleosporales (Kruys et al. 2006; Schoch et al. 2006) except for Massariaceae (Voglmayr and Jaklitsch 2011). This might indicate its early derivation (Zhang et al. 2009a).
Concluding remarks
Morphologically, Delitschia is a well defined genus, and each cell of the ascospore has a full length germ slit. Currently, most species of this genus are coprophilous, although a few species are reported from wood (Hyde and Steinke 1996; Luck-Allen and Cain 1975). Whether the lignicolous habitat is an important character that might separate these taxa from the main coprophilous group, needs to be addressed, however, the morphological characters are similar.
Didymosphaeria Fuckel, Jb. nassau. Ver. Naturk. 22-23: 140 (1870). (Didymosphaeriaceae)
Generic description
Habitat terrestrial, saprobic or parasitic. Ascomata solitary, scattered, or in small groups, immersed to erumpent, globose to ovoid, papillate, ostiolate, periphysate. Ostiole with a pore-like opening. Peridium 1-layered, thin, composed of brown pseudoparenchymatous cells of textura angularis. Hamathecium of dense, trabeculate, anastomosing mostly above the asci. Asci (2-)4-spored or 8-spored, bitunicate, cylindrical, with a furcate pedicel. Ascospores uniseriate, ellipsoid, brown, 1-distoseptate.
Anamorphs reported for genus: Dendrophoma, Fusicladiella and Phoma (Aptroot 1995).
Literature: Aptroot 1995; Barr 1989a, b, 1990a, 1992a, b; 1993a; b; Fuckel 1870; Hawksworth 1985a, b; Hawksworth and Boise 1985; Hawksworth and Diederich 1988; Hyde et al. 2000; Lumbsch and Huhndorf 2007; Saccardo 1882; Scheinpflug 1958; Sivanesan 1984.
Type species
Didymosphaeria futilis (Berk. & Broome) Rehm, Hedwigia 18: 167 (1879). (Fig. 27)
Fig. 27.
Didymosphaeria futilis (from K(M): 147683, holotype). a Two immersed ascomata on the host surface (one of them is cut horizontally). b Section of an ascoma. Note the thin peridium. c Hand cut portion of ascoma showing habitat in wood. d Asci in pseudoparaphyses. Note the trabeculate pseudonparaphyses anastomosing above the asci. e, f Four-spored asci with long pedicels which are rounded at their bases. g Brown, 1-septate ascospores with spinulose ornamentation. Scale bars: a=0.3 mm, b, c= 100 μm, d-g=10 μm
≡ Sphaeria futilis Berk. & Broome, Ann. Mag. nat. Hist., Ser. 2 9: 326 (1852).
Ascomata 190-230 μm highx240-340 μm diam., scattered, or in small groups, immersed to slightly erumpent, subglobose to ovoid, membraneous, near-hyaline, under clypeus, papillate, periphysate (Fig. 27a and c). Papilla central, up to 100 μm high, black, with a pore-like ostiole (Fig. 27a and c). Peridium 30-40 μm wide upper part, 6-23 μm wide near the base, 1-layered, composed of brown pseudoparenchymatous cells of textura angularis, cell wall 2–3 μm thick (Fig. 27b). Hamathecium of dense, long trabeculate pseudoparaphyses, 0.8-1.5 μm broad, anastomosing mostly above the asci, embedded in mucilage (Fig. 27d). Asci 90-110 × 7.5-10 μm (x = 97 × 9mm, n =10), 2-4-spored, rarely 8-spored, bitunicate, fissitunicate, cylindrical, with a furcate pedicel, 17.5-27.5 μm long, with a large ocular (to 2.5 μm wide × 4 μm high) (Fig. 27d, e and f). Ascospores 14-15.5×(5.5-) 6-7.5 μm (x = 14.8× 6.9mm, n=10), uniseriate, ellipsoid with obtuse ends, brown, 1-septate, distoseptate, slightly to not constricted, capitate (Fig. 27g).
Anamorph: Dendrophoma sp., Fusicladiella sp. vel aff. (Sivanesan 1984).
Material examined: UK, England, Norfolk, King’s Cliffe; on dead stem (in ramis emortuis) Rosa sp., Mar. 1850, M.J. Berkeley (K(M): 147683, holotype).
Notes
Morphology
Didymosphaeria is a widely distributed genus with wide host range (Aptroot 1995). Didymosphaeria was formally established by Fuckel (1870) based on six ascomycetous species, and D. epidermidis (Fries) Fuckel (or D. peltigerae Fuckel) has been chosen as the lectotype species (see comments by Aptroot 1995). Hawksworth and David (1989: 494) proposed to conserve the genus with a lectotype specimen, Fungi Rhenani 1770. The genus had been considered as a depository to accommodate all types of didymosporous pyrenocarpous ascomycetes. Many workers have tried to redefine the genus and excluded some species. Saccardo (1882) restricted the genus to brown-spored species, and about 100 species have been excluded subsequently (Barr 1989a, b, 1990a, 1992a, b, 1993b; Hawksworth 1985a, b; Hawksworth and Boise 1985; Hawksworth and Diederich 1988; Scheinpflug 1958). Over 400 epithets of Didymosphaeria were included until the monograph of Aptroot (1995).
Aptroot (1995) examined more than 3000 specimens under the name Didymosphaeria. The type specimen of Didymosphaeria (Fungi Rhenani 1770) represents the widespread and common D. futilis (Aptroot 1995). In this study, we did not get the lectotype specimen, but described the type of D. futilis (Sphaeria futilis). Using a narrow concept (ignoring differences of host or country of origin), Aptroot (1995) accepted only seven species, which were closely related with the generic type of Didymosphaeria with over 100 synonyms distributed among them. Many taxa were found to belong to other groups, i.e. Aaosphaeria, Amphisphaeria, Astrosphaeriella, Dothidotthia, Flagellosphaeria, Kirschsteiniothelia, alotremis, Montagnula, Munkovalsaria, Mycomicrothelia, Parapyrenis or Phaeodothis. Didymosphaeria is mainly characterized by a peridium consisting of flattened or irregular cells or completely hyphae; a hamathecium consisting of narrow, trabeculate paraphysoids or paraphyses, richly anastomosing above the asci; and brown thinly distoseptate ascospores. Didymosphaeriaceae was maintained as a separated family within Pleosporales by Aptroot (1995) because of the distoseptate ascospores and trabeculate pseudoparaphyses mainly anastomosing above the asci. This proposal, however, has not received much support (Lumbsch and Huhndorf 2007).
Phylogenetic study
There have been few molecular investigations of Didymosphaeria when compared to the morphological studies. Didymosphaeria futilis resided in the clade of Cucurbitariaceae (or Didymosphaeriaceae) (Plate 1). The correct identification of the Didymosphaeria strain used for sequencing, however, has not been verified.
Concluding remarks
Didymosphaeria is a well established genus represented by D. futilis. Of particular significance are the narrow pseudoparaphyses which anastomose above the asci and brown 1-septate ascospores with indistinct distosepta. Familial placement of Didymosphaeria is unclear yet because of insufficient molecular data.
Dothidotthia Hohn., Ber. Deutsch. Bot. Ges. 36: 312 (1918). (Didymellaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata medium-sized, solitary, clustered or somewhat gregarious, erumpent, subglobose, apex somewhat papillate to depressed, coriaceous. Peridium composed of a few layers of dark brown cells of textura angularis, and giving rise dark brown, thick-walled hyphae in the basal region, 2-layered. Hamathecium septate pseudoparaphyses branched in upper part above asci. Asci 8-spored, bitunicate, clavate, straight to curved. Ascospores biseriate to obliquely uniseriate, ellipsoid, pale brown, 1-septate. Anamorphs reported for genus: Dothiorella and Thyrostroma (Hyde et al. 2011; Phillips et al. 2008). Literature: Barr 1989b; Phillips et al. 2008.
Type species
Dothidotthia symphoricarpi (Rehm) Hohn., Ber. Deutsch. Bot. Ges. 36: 312 (1918). (Fig. 28)
Fig. 28.
Dothidotthia symphoricarpi (from NY, holotype). a Clustered ascomata on the host stubstrate. b Longitudinal section through an ascoma. c, d Asci with pale brown, 1-septate ascospores. e Immature asci. f Pale brown, 1-septate ascospores within asci. g Conidia of Thyrostroma anamorph in association with ascomata. Scale bars: a=0.5 mm, b =100 μm, c-g =10 μm. (figure with permission from Phillips et al. 2008)
≡ Pseudotthia symphoricarpi Rehm, Ann. MycoL 11: 169 (1913).
Ascomata up to 500 μm highx550 μm diam., gregarious clustered, rarely solitary, erumpent, subglobose, apex somewhat papillate to depressed, coriaceous (Fig. 28a). Peridium 20-80 μm thick, composed of 3-6 layers of dark brown cells of textura angularis, giving rise dark brown, thick-walled hyphae in the basal region, 2-layered, outer layer wall thicker and inner layer wall thinner (Fig. 28b). Hamathecium hyaline, septate pseudoparaphyses, 2–3 μm wide, branched in upper part above asci. Asci 70-120×15-22 μm, 8-spored, bitunicate, clavate, straight to curved (Fig. 28c, d and e). Ascospores (20-)22-23 (−26)×(8-)9-10(−11) μm, biseriate to obliquely uniseriate and partially overlapping, ellipsoid tapering towards subacutely rounded ends, pale brown, 1-septate, constricted at the septum, smooth (Fig. 28f) (description referred to Phillips et al. 2008).
Anamorph: Thyrostroma negundinis (Phillips et al. 2008).
Material examined: USA, North Dakota, on branches of Symphoricarpos occidentalis Hook. (NY, holotype); Colorado, San Juan Co, c. 0.5 mile up Engineer Mountain Trail from turnoff at mile 52.5, Hwy 550, dead twigs of Symphoricarpos rotundifolius A. Gray, 24 Jun. 2004, A.W. Ramaley 0410 (BPI 871823, epitype).
Notes
Morphology
Dothidotthia was formally established to accommodate Pseudotthia symphoricarpi (Montagnellaceae, Dothideales) (von Hohnel 1918a). Many mycologists considered Dothidotthia closely related to a genus of Venturiaceae such as Dibotryon by Petrak (1927), or Gibbera by von Arx and Muller (1954) and Muller and von Arx (1962). Dothidotthia had been treated as a synonym of Gibbera (von Arx 1954; Muller and von Arx 1962), which was followed by Shoemaker (1963) and Eriksson and Hawksworth (1987). Based on the coelomycetous anamorphic stage and peridium structure, shape of asci, as well as morphology of pseudoparaphyses, Barr (1987b, 1989b) retrieved Dothidotthia, and considered it closely related to Botryosphaeria (Botryosphaeriaceae). Currently, 11 species are included within Dothidotthia (http://www.mycobank.org, 01–2011).
Phylogenetic study
Based on a multi-gene phylogenetic analysis, Dothidotthia formed a separate familial clade (Phillips et al. 2008). Thus Dothidotthiaceae was introduced to accommodate it (Phillips et al. 2008).
Concluding remarks
By comparing the morphological characters and phylogenetic dendrograms by Phillips et al. (2008) and de Gruyter et al. (2009), Dothidotthia seems closely related to Didymellaceae, but Dothidotthiaceae should still be treated as a separate family.
Dubitatio Speg., Anal. Soc. cient. argent. 12: 212 (1881). (Arthopyreniaceae (or Massariaceae))
Generic description
Habitat terrestrial, saprobic. Ascomata medium-sized, solitary, densely scattered, or in small groups of 2-4, immersed, covered with white crystaline rim, papillate, ostiolate. Hamathecium of dense pseudoparaphyses, long, 2–3 μm broad, branching and anastomosing. Asci cylindrical, pedicellate, with furcate pedicel. Ascospores 1-septate, asymmetrical, reddish to dark brown.
Anamorphs reported for genus: Aplosporella-like (Rossman et al. 1999).
Literature: Barr 1979b, 1987b; Muller and von Arx 1962; Rossman et al. 1999; Spegazzini 1881.
Type species
Dubitatio dubitationum Speg., Anal. Soc. cient. argent. 12: 212 (1881). (Fig. 29)
Fig. 29.
Dubitatio dubitationum (from NY, isotype; LPS, holotype). a Appearance of ascomata scattered on the host surface. Note the exposed white covering around the ostioles. b, c Section of an ascoma. Note the white covering (see arrow). d-f Cylindrical asci with short furcate pedicels. g, h Asymmetrical, 1-septate reddish-brown ascospores. Scale bars: a =1 mm, b = 100 μm, c-e=50 μm, f-h=20 μm
Ascomata 350-530 μm highx550-700 μm diam., solitary, densely scattered, or in small groups of 2-4, immersed, with a protruding papilla, 110-160 μm high, 160-250 μm diam., globose or subglobose, black, covered with white crystalline material which becomes hyaline and gel-like in water, ostiolate (Fig. 29a and b). Peridium 18-25 μm thick laterally (excluding the rim), up to 35 μm thick at the apex, thinner at the base, 1-layered, composed of small pale brown thin-walled cells of textura prismatica, cells 5-12×3-5 μm diam., cell wall up to 1 μm thick, apex cells smaller and walls thicker (Fig. 29b). Hamathecium of dense, long pseudoparaphyses, 2–3 μm broad, branching and anastomosing between and above the asci. Asci 150-190(−230)× 12.5-15 μm (x = 172.5 × 13.4mm, n =10), (6-)8-spored, rarely 4-spored, bitunicate, fissitunicate, cylindrical, with a furcate pedicel which is up to 40 μm long, ocular chamber not observed (Fig. 29c, d and e). Ascospores 19-22.5×10-12 μm (x = 20.2 × 11.4mm, n =10), uniseriate to obliquely uniseriate and partially overlapping, broadly ellipsoid with broadly to narrowly rounded ends, reddish brown, 1-septate, constricted at septum, asymmetric with a larger upper cell, thick-walled, possibly distoseptate (Fig. 29f, g and h).
Anamorph: Aplosporella-like (for detailed description see Rossman et al. 1999).
Conidiomata globose, ca. 300 μm diam. Conidia holoblastic, broadly fusoid, 13-15×7-10 μm, dark brown, finely spinulose (Rossman et al. 1999).
Material examined: ARGENTINA, Buenos Aires, Tuyu, on Celtis tala Gill., Jan. 1881, leg. det. C. Spegazzini (NY, isotype; LPS, holotype).
Notes
Morphology
When established Dubitatio, Spegazzini (1881) considered it as intermediate between Sphaeriaceae and Nectriaceae as has been mentioned by Rossman et al. (1999). Muller and von Arx (1962) treated Dubitatio as a synonym of Passerinula, while the differences of ascomata and ascospores could easily distinguish these two genera (Rossman et al. 1999). After checking the type specimen, Dubitatio was assigned to Dothideomycetes, and considered closely related to Dothivalsaria in the Massariaceae (Barr 1979b, 1987b). Dubitatio chondrospora was assigned to Pseudomassaria (as P. chondrospora (Ces.) Jacz.) (Barr 1964; Muller and von Arx 1962).
Phylogenetic study
None.
Concluding remarks
The black ascomata with white crystalline covering and central white ostiolar region as well as the asymmetrical reddish brown ascospores are striking characters of Dubitatio dubitationum. The genus cannot be assigned to any family with certainty based on morphological characters and fresh collections are needed for sequencing.
Entodesmium Reiss, Hedwigia 1: 28 (1854). (Phaeosphaeriaceae)
Generic description
Habitat terrestrial, saprobic (or parasitic?). Ascomata scattered or in small groups, immersed, papillate, ostiolate, periphysate. Peridium thin, comprising one cell type of pigmented pseudoparenchymatous cells. Hamathecium of dense, long pseudoparaphyses, septate, embedded in mucilage. Asci 8-spored, bitunicate, fissitunicate, cylindrical, with furcate pedicel. Ascospores ellipsoid to filliform, multiseptate, deeply constricted at the primary septum (usually near apex), breaking into partspores.
Anamorphs reported for genus: none.
Literature: von Arx and Muller 1975; Barr 1992b; Eriksson 1967a; b; Holm 1957; Liew et al. 2000; Shoemaker 1984a, b.
Type species
Entodesmium rude Reiss, Hedwigia 1: 28 (1854). (Fig. 30)
Fig. 30.
Entodesmium rude (from H, Krieger 1070). a Ascomata in groups on the host surface. Note the erumpent papilla which is cylindrical and has an inconspicuous ostiole. b Section of part of an ascoma. Note the arrangement of asci and pseudoparaphyses. c Section of the peridium comprising cells of textura angularis. d Part-spores inside the ascus. e Relatively immature ascus with filliform ascospores d low ocular chamber. f-h Mature and immature asci with pedicels. e bars: a=0.5 mm, b, c=50 μm, d-h =10 μm
Ascomata 160-250 μm highx 150-300 μm diam., in groups, immersed with long and protruding cylindrical papilla, globose to subglobose, black, coriaceous (Fig. 30a). Papilla 100-220 μm long, 70-120 μm broad, cylindrical, with periphysate ostiole. Peridium 25-33 μm wide, comprising pseudoparenchymatous cells, cells up to 10× 7.5 μm diam., cell wall up to 2 μm thick, beak cells smaller and wall thicker (Fig. 30b and c). Hamathecium of dense, long pseudoparaphyses, septate, 2–3 μm wide, embedded in mucilage. Asci 100-175 × 8-13 μm (x = 147.5 × 11.3mm, n =10), 8-spored, bitunicate, fissitunicate, cylindrical, with a furcate pedicel which is 18-50 μm long, and with a low ocular chamber (ca. 1 μm widex1 μm high) (Fig. 30e,f, g and h). Ascospores 108-138×3-3.5 μm (x = 123 × 3.2mm, n=10), filliform, brown, multi-septate, breaking into 22-28 partspores, 5-7×3-3.5 μm diam. (Fig. 30d).
Anamorph: none reported.
Material examined: GERMANY, Konigstein, on stems of Coronilla varia L., 20 May 1895, W. Krieger (H, Krieger 1070).
Notes
Morphology
Entodesmium is characterized by having immersed ascomata dark cylindrical, periphysate papillae, numerous clavate to cylindrical asci surrounded by narrowly cellular pseudoparaphyses, and ellipsoidal to filliform multi-septate ascospores (Barr 1992b; Shoemaker 1984b). Currently, five species, viz. Entodesmium eliassonii L. Holm, E. lapponicum (L. Holm) L. Holm, E. mayorii (E. Mu11.) L. Holm, E. niessleanum (Rabenh. ex Niessl) L. Holm and E. rude are accepted in this genus (Holm 1957; Shoemaker 1984b). Von Arx and Muller (1975) assigned Entodesmium to the Pleosporaceae sensu lato, and Shoemaker (1976) assigned E. rude (as Ophiobolus rudis) to Ophiobolus sensu lato based on the fragmenting filliform ascospores. According to the short, blackish beak and periphysate ostiole, Barr (1992b) assigned Entodesmium to Lophiostomataceae. The hosts of Entodesmium are restricted to stems of legumes (Barr 1992b; Shoemaker 1984b).
Phylogenetic study
Limited phylogenetic studies indicate that Entodesmium rude may have affinities to Phaeosphaeriaceae (Liew et al. 2000; Plate 1).
Concluding remarks
Species of Entodesmium share several morphological characters, such as immersed, papillate ascomata, periphysate ostioles, pale yellow to light yellowish brown, multiseptate (> 3), narrowly fusoid to filliform ascospores, and are specific to legumes. All of the above similarities indicate a close relationship among members of Entodesmium. We do not agree with Barr (1992b) who assigned Entodesmium to Lophiostomataceae because the ascomata are immersed, the papilla are not laterally compressed and the peridium comprises a single type of cells of textura angularis. These characters plus multi-septate, lightly pigmented ascospores, which break up into partspores and host specificity to legumes support inclusion in Phaeosphaeriaceae. Entodesmium multiseptatum (G. Winter) L. Holm and E. niessleanum were originally described as Leptosphaeria species (Shoemaker 1984b) indicating their similarity to Phaeosphaeria with which Leptosphaeria is commonly confused (Shoemaker 1984a; Shoemaker and Babcock 1989b). Phylogenetic study has also shown that Entodesmium rude is related to members of Phaeosphaeriaceae (Liew et al. 2000). Thus we assign Entodesmium to Phaeosphaeriaceae as a separate genus until further phylogenetic analysis is carried out on verified specimens.
Eudarluca Speg., Revta Mus. La Plata 15: 22 (1908). (?Phaeosphaeriaceae)
Generic description
Habitat terrestrial, parasitic. Ascomata small, solitary, scattered, immersed to erumpent, subglobose, ostiolate, papillate. Peridium thin, composed of a few layers cells of textura prismatica. Hamathecium of dense, cellular pseudoparaphyses, septate. Asci 8-spored, bitunicate, fissitunicate, cylindrical to fusoid, with a furcate pedicel. Ascospores broadly fusoid to fusoid, hyaline to pale yellow, rarely 1- or 3-septate, mostly 2-septate, constricted at the primary septum.
Anamorphs reported for genus: Sphaerellopsis (Sivanesan 1984).
Literature: Bayon et al. 2006; Eriksson 1966; Katumoto 1986; Ramakrishnan 1951; Spegazzini 1908.
Type species
Eudarluca australis Speg., Revta Mus. La Plata 15: 22 (1908). (Fig. 31)
Fig. 31.
Eudarluca australis (from LPS 5.415, type). a Ascomata on the host surface. b Section of an ascoma. c Section of a partial peridium. Note the thin peridium with cells of textura angularis. d-g i with short pedicels. h Ascospores. Note the 2-septate hyaline. Scale bars: a, b = 100 μm, c=50 μm, d-h =10 μm
Ascomata 160-190 μm highx180-290 μm diam., solitary, scattered, or in small groups, semi-immersed to erumpent, subglobose to broadly ellipsoid, wall black, ostiolate, apex with a short papilla, 40-70 μm broad (Fig. 31a and b). Peridium <10 μm wide laterally, up to 25 μm thick at the apex, thinner at the base, composed of lightly pigmented thin-walled cells of textura prismatica, cells up to 12×4 μm diam., cell wall <1 μm thick, apex cells heavily pigmented, smaller and walls thicker (Fig. 31b and c). Hamathecium of dense, long cellular pseudoparaphyses, 1.5-2.5 μm broad, septate. Asci 50-70×7.5-10 μm (x = 61.4 × 8.4mm, n =10), 8-spored, with a short, thick, furcate pedicel, up to 12.5 μm long, bitunicate, fissitunicate, cylindrical to fusoid, no obvious ocular chamber (Fig. 31d, e, f and g). Ascospores 16-20×4-6 μm (x = 17.3 × 5μm, n=10), obliquely uniseriate and partially overlapping to biseriate, broadly fusoid to fusoid, hyaline to pale yellow, 2-septate, sometimes 1- or 3-septate, constricted at the two main septa, the medium cell often broader than the others, smooth (Fig. 31h).
Anamorph: Sphaerellopsis filum (Biv.) B. Sutton (Sivanesan 1984).
Material examined: BRAZIL, Sao Paulo, on leaves of Canna sp., 1905, leg. Usteri, nro; det. Ove Eriksson (LPS 5.415, type).
Notes
Morphology
Eudarluca was introduced based on E. australis (Spegazzini 1908), and E. australis was subsequently treated as a synonym of E. caricis (Biv.) O.E. Erikss. (Eriksson 1966). The most striking character of E. australis is its 2-septate ascospores, which is quite rare in Pleosporales. Sphaerellopsis filum, anamorph of E. caricis, is a cosmopolitan hyperparasite associated with a large number of rust species (Plachecka 2005).
Phylogenetic study
A detailed phylogenetic study was conducted on Sphaerellopsis filum, the anamorphic stage of Eudarluca australis based on both AFLP and ITS sequences, and only limited variation between different isolates was detected (Bayon et al. 2006).
Concluding remarks
By blasting within GenBank, ITS sequences of E. caricis (= E. australis, strain MullMK, GB, access AY836374) are most comparable with species in Leptosphaeria and Phoma. Thus Eudarluca appears to be related to Leptos-phaeriaceae pending further study.
Falciformispora K.D. Hyde, MycoL Res. 96: 26 (1992). (Trematosphaeriaceae)
Generic description
Habitat freshwater, saprobic. Ascomata small, scattered to gregarious, erumpent to nearly superficial, depressed globose to ovoid, black, ostiolate, epapillate, coriaceous. Peridium thin, comprising two cells types, outer layer composed of thick-walled cells of textura angularis, inner layer composed of hyaline compressed cells. Hamathecium long and cellular pseudoparaphyses, septate, embedded in mucilage. Asci 8-spored, bitunicate, fissitunicate, broadly clavate to fusoid, with a short, thick pedicel. Ascospores fusoid to somewhat clavate, hyaline, usually slightly curved, multi-septate.
Anamorphs reported for genus: none.
Literature: Hyde 1992b; Raja and Shearer 2008.
Type species
Falciformispora lignatilis K.D. Hyde, Myco1. Res. 96: 27 (1992). (Fig. 32)
Fig. 32.
Falciformispora ligna-tilis (from BRIP 16972, holotype). a Section of a superficial ascoma. The peridium comprises two layers. b, c Squash mounts showing asci with wide pseudoparaphyses. The asci are cylindro-clavate with very short pedicels. d-f Hyaline multiseptate ascospores. Note the elongated appendage at the base (arrow head). Scale bars: a, b =100 μm, c=50 μm, d-f =10 μm
Ascomata 180-270 μm highx250-340 μm diam., scattered to gregarious, erumpent and eventually superficial, depressed globose to ovoid, black, ostiolate, epapillate, coriaceous (Fig. 32a). Peridium up to 35 μm wide, comprising two cell types, outer layer composed of thick-walled cells of textura angularis, up to 8 μm diam., cell wall up to 5 μm thick, inner layer composed of hyaline compressed cells, cells 12×3 μm diam., cell wall 1-1.5 μm thick (Fig. 32a). Hamathecium long and cellular pseudoparaphyses, 2–3 μm broad, septate, embedded in mucilage. Asci 115-130×23-31 μm, 8-spored, bitunicate, fissitunicate, broadly clavate to fusoid, with a short, thick pedicel, 8-15 μm long, with an ocular chamber (to 5 μm wide × 3 μm high) (Fig. 32b and c). Ascospores 42-50×8-10 μm, 2–3 seriate, fusoid to somewhat clavate, hyaline, usually slightly curved, 6-8-septate, mostly 7-septate, slightly constricted at all septa, smooth-walled, surrounded by a thin mucilaginous sheath which is longer at the base (up to 20-30 μm) (Fig. 32d, e and f).
Anamorph: none reported.
Material examined: MEXICO, Nova Hispania, mangrove near Boca de Pascuales, saprobic on immersed intertidal mangrove wood, Mar. 1988, K.D. Hyde (BRIP 16972, holotype).
Notes
Morphology
Falciformispora was formally established by Hyde (1992b) as a monotypic genus and was assigned to Pleosporaceae by comparing with Setosphaeria, but Setosphaeria has the anamorphic stage of Exserohilum and is exclusively parasitic on Gramineae unlike Falciformispora. The setae on the ascomata of Setosphaeria could also serve as a distinguishing character from Falciformispora. Raja and Shearer (2008) also collected this species from freshwater in Florida. They considered that the species was more closely related to Chaetomastia than Setosphaeria, but that Falciformispora differed in having hyaline ascospores.
Phylogenetic study
Phylogenetic analyses in Schoch et al. (2009) and Suetrong et al. (2009) placed Falciformispora lignatilis in Trematos-phaeriaceae in proximity to another marine species associated with mangroves, Halomassarina thalassiae.
Concluding remarks
Phylogenetic work confirmed that the saprobic habitat of Falciformispora is inconsistent with most other members of Pleosporaceae. The hyaline multi-septate ascospores with a mucilaginous sheath indicate affinities to Lophiostomataceae but this is not supported in DNA sequence comparisons. Carinispora is also similar and may be related.
Hadrospora Boise, Mem. N. Y. bot. Gdn 49: 310 (1989). (?Phaeosphaeriaceae)
Generic description
Habitat terrestrial (or freshwater?), saprobic. Ascomata small- to medium-sized, solitary, scattered, or in groups, immersed to nearly superficial, globose to subglobose, papillate. Peridium thin, comprising pseudoparenchymatous cells. Hamathecium dense, narrowly cellular, embedded in mucilage. Asci bitunicate, fissitunicate, oblong to ovoid, with a short pedicel. Ascospores ellipsoid to broadly fusoid with narrow ends, reddish brown, multi-septate, constricted at the primary septum.
Anamorphs reported for genus: Zalerion (Tanaka and Harada 2003a).
Literature: Boise 1984, 1989; Fisher and Webster 1992; Shearer and Crane 1971; Tanaka and Harada 2003a; Webster 1993.
Type species
Hadrospora fallax (Mouton) Boise, Mem. N. Y. bot. Gdn 49: 310 (1989). (Fig. 33)
Fig. 33.
Hadrospora fallax (from BR, Capsa: K 7534, holotype). a Ascomata forming a cluster on the host surface. b Section of an ascoma. Note the peridium structure. c Section of a partial peridium. Note the pseudoparenchymatous cells. d Asci in pseudoparaphyses. e-i Reddish brown multiseptate ascospores. Scale bars: a= 0.5 mm, b = 100 μm, c, d= 50 μm, e-i=20 μm
≡ Trematosphaeria fallax Mouton, BU11. Soc. R. Bot. Belg. 25: 155, (1886).
Ascomata 130-240 μm highx200-330 μm diam., solitary, scattered or in groups, initially immersed, becoming erumpent to nearly superficial, with basal wall remaining immersed in host tissue, not easily removed from the substrate, globose or subglobose, roughened, papillate, coriaceous (Fig. 33a). Peridium 30-45 μm wide, comprising cells of pseudoparenchymatous, up to 12.5×9 μm diam. (Fig. 33b and c). Hamathecium of dense, narrowly cellular pseudoparaphyses, 1–2 μm broad, embedded in mucilage. Asci 150-200×40-60 μm (x = 171.5 × 48mm, n=10), 8-spored, bitunicate, fissitunicate, oblong to ovoid, with a short pedicel, 10-24 μm long, with a ocular chamber (to 5 μm widex6 μm high) (Fig. 33d). Ascospores 55-80×16-22 μm (x = 67.1 × 18.1mm, n=10), biseriate to 4-seriate, ellipsoid to broadly fusoid with narrow ends, reddish brown with paler end cells, 8-septate, constricted at the primary septum, smooth-walled (Fig. 33e, f, g, h and i).
Anamorph: Zalerion sp. (Tanaka and Harada 2003a).
Material examined: BELGIUM, Beaufays, on cut off, still hard wood. Oct. 1922, V. Mouton (BR, Capsa: K 7534, holotype). (Note: The specimen is not in good condition, only a few ascomata left).
Notes
Morphology
Boise (1989) formally established Hadrospora to accommodate Trematosphaeria fallax and T. clarkia (Sivan.) Boise, and Hadrospora fallax (syn. T. fallax) was selected as the generic type. Hadrospora is a widely distributed species that has been reported from Belgium, China, Italy, Japan, Switzerland and the United States (Boise 1989; Fisher and Webster 1992; Shearer and Crane 1971; Tanaka and Harada 2003a; Webster 1993). Hadrospora was temporarily assigned to Phaeosphaeriaceae based on its “smaU, thin-walled ascomata and narrow cellular pseudoparaphyses” (Boise 1989), which is distinguished from other genera of Phaeosphaeriaceae by its “large, stout ascospores that form within oblong-ovoid asci” (Boise 1989). Currently, Hadrospora includes two species, i.e. H. fallax and H. clarkii (Sivan.) Boise differentiated by ascospore size.
Phylogenetic study
None.
Concluding remarks
Hadrospora seems not closely related to Phaeosphaeriaceae.
Halotthia Kohlm., Nova Hedwigia 6: 9 (1963). (’?Zopfaceae)
Generic description
Habitat marine, saprobic. Ascomata large, solitary, gregarious or confluent, broadly conical to subglobose, flattened at the base, carbonaceous, immersed to erumpent, ostiolate, epapillate. Peridium plectenchymatous. Hamathecium of dense, long, cellular pseudoparaphyses, septate, branching. Asci 8-spored, bitunicate, cylindrical, with a short pedicel. Ascospores uniseriate, ellipsoidal, subcylindrical or obtuse-fusoid, dark brown, 1-septate, constricted at the septum.
Anamorphs reported for genus: none.
Literature: Kohlmeyer 1963; Suetrong et al. 2009.
Type species
Halotthia posidoniae (Durieu & Mont.) Kohlm., Nova Hedwigia 6: 9 (1963). (Fig. 34)
Fig. 34.
Halotthia posidoniae (from S, isotype of Sphaeria posidoniae). a Ascomata gregarious on the host surface. b-d Mature or immature cylindrical asci. e-h Ellipsoidal, dark-brown, 1-septate ascospores. Scale bars: a =1 mm, b-d=50 μm, e-h=5 μm
≡ Sphaeria posidoniae Durieu & Mont. Exploratio scientifique de l’Algerie, pp. 502-503, Taf.25, Abb. 8a-i, 1849
Ascomata 0.8-1.1 mm highx1.5-2.1 mm diam., solitary, gregarious or confluent, broadly conical to subglobose, flattened at the base, carbonaceous, immersed to erumpent, ostiolate, epapillate (Fig. 34a). Peridium 165-275 μm thick at sides, thicker near the apex, plectenchymatous. Hamathecium of dense, long cellular pseudo-paraphyses, 1.5-2 μm broad, septate, branching. Asci 275-290×25-35 μm, 8-spored, bitunicate, cylindrical, with a short pedicel (Fig. 34b, c and d). Ascospores 37-60.5×16.5-26 μm, uniseriate, ellipsoidal, subcylindrical or obtuse-fusoid, dark brown, 1-septate, constricted at the septum (Fig. 34e, f, g and h) (adapted from Kohlmeyer and Kohlmeyer 1979).
Anamorph: none reported.
Material examined: ITALY, in rhizomes of Posidonia oceanica (Posidoniaceae), 1861, Caldesi (S, isotype of Sphaeria posidoniae)
Notes
Morphology
Halotthia was introduced to accommodate the marine fungus, H. posidoniae (as Sphaeria posidoniae), which is characterized by immersed to erumpent, large, carbonaceous ascomata, thick peridium, bitunicate, 8-spored, cylindrical asci, ellipsoidal, 1-septate, and dark brown ascospores (Kohlmeyer 1963). Morphologically, Halotthia is most comparable with Bicrouania maritima, but the conical ascomata with flattened base of H. posidoniae can be readily distinguished from B. maritima.
Phylogenetic study
Phylogenetically, Halotthia posidoniae, Pontoporeia biturbinata and Mauritiana rhizophorae form a robust clade, which may represent a potential family (Suetrong et al. 2009).
Concluding remarks
Currently the familial status of Halotthia is unresolved (Suetrong et al. 2009).
Helicascus Kohlm., Can. J. Bot. 47: 1471 (1969). (Morosphaeriaceae)
Generic description
Habitat marine, saprobic. Ascostromata lenticular, immersed, black, carbonaceous, enclosing several loculi, pseudoclypeus composed of host cells enclosed in black stromatic fungus material. Ascomata depressed ampulliform, horizontally arranged under a black pseudoclypeus, ostiolate, torsellioid ostioles, papillate. Peridium absent, partitions between loculi formed of brown, isodiametric or elongated cells of the stroma. Hamathecium of dense, long pseudoparaphyses. Asci 8-spored, bitunicate, subcylindrical to oblong clavate, with a short pedicel and conspicuous apical ring. Ascospores uniseriate, obovoid, brown, 1-septate, at each end with a germ pore, surrounded with dissolving sheath.
Anamorphs reported for genus: none.
Literature: Kohlmeyer 1969; Kohlmeyer and Kohlmeyer 1979; Suetrong et al. 2009.
Type species
Helicascus kanaloanus Kohlm., Can. J. Bot. 47: 1471 (1969). (Fig. 35)
Fig. 35.
Helicascus kanaloanus (from Herb. J. Kohlmeyer No. 2566, holotype). a Section of ascostroma immersed in the host tissue. Note the torsellioid ostiole. b One-septate, brown, asymmetrical ascospores within the asci. c, d Released thickwalled ascospores. Note the germ pore at the lower end of the ascospores. Scale bars: a= 0.5 mm, b-d=20 μm
Ascostromata 0.6-0.78 mm highx1.25-2.75 mm diam., lenticular, immersed, black, carbonaceous, enclosing 3-4(−5) loculi, pseudoclypeus composed of host cells enclosed in black stromatic fungus material (Fig. 35a). Ascomata 235-370 μm highx440-800 μm diam., depressed ampulliform, horizontally arranged under a black pseudoclypeus, ostioles 70-170 μm diam., torsellioid ostiole (Adams et al. 2005), papilla slightly rising over the surface of the pseudoclypeus, subconical,canal filled with thick, bright orange to yellowish periphyses, 270-435 μm high, 255-300 μm diam. Peridium absent, partitions between loculi formed of brown, isodia-metric or elongated cells of the stroma. Hamathecium of dense, very long pseudoparaphyses. Asci 250-335×25-30 μm, 8-spored, subcylindrical, finally oblong-clavate (400-480 μm long), with a short pedicel, bitunicate, thick-walled, physoclastic, apically multi-layered and annulate, ectoascus forming a third, thin permeable outer layer around the base, endoascus swelling in water and becoming coiled at maturity, finally stretching and pushing the ascus into the ostiolar canal (Fig. 35b). Ascospores 36.5-48.5 × 18-22.5 μm, uniseriate, obovoid, brown, 1-septate, at each end with a germ pore, surrounded with dissolving sheath, 2.7-5.4 μm thick, with funnel-shaped, apical indentations (Fig. 35c and d) (adapted from Kohlmeyer and Kohlmeyer 1979).
Anamorph: none reported.
Material examined: USA, Hawaii, Oahu, Kaneohe Bay, Heeia Swamp, on Rhizophora mangle, 4 Jun. 1968 (Herb. J. Kohlmeyer No. 2566, holotype; No. 2565, 2567, paratype).
Notes
Morphology
Helicascus is another marine genus, which is characterized by its thin additional sheath around the base of the asci, the coiling and stretching mechanism of the basal part of the endoascus and its conspicuous apical apparatus which is not that common in bitunicate asci (Kohlmeyer 1969). The immersed stroma comprising several loculi sharing one common ostiole is another striking character of Helicascus.
Phylogenetic study
Multigene phylogenetic analysis indicated that both Helicascus kanaloanus and H. nypae K.D. Hyde nested within Morosphaeriaceae (Suetrong et al. 2009).
Concluding remarks
Helicascus is a well defined marine genus.
Herpotrichia Fuckel, Fungi rhenani exsic.: no. 2171 (1868). (Melanommataceae)
Generic description
Habitat terrestrial, parasitic, hyperparasitic or saprobic. Ascomata medium-sized, immersed, erumpent to nearly superficial, scattered to gregarious, globose to subglobose with a broad pore. Peridium composed of pseudoparenchymatous cells. Hamathecium of dense, long pseudoparaphyses, embedded in mucilage, septate, branching. Asci cylindrical to cylindro-clavate, with a furcated pedicel. Ascospores fusoid, ellipsoid or oblong with broadly to narrowly round ends, 1-septate, constricted at the septum, uni- to biseriate.
Anamorphs reported for genus: Pyrenochaeta or Pyrenochaeta-like (Sivanesan 1984).
Literature: von Arx and Muller 1975; Barr 1984; Cannon 1982; Freyer and van der Aa 1975; Mugambi and Huhndorf 2009b; Samuels 1973; Samuels and Muller 1978; Sivanesan 1971, 1984.
Type species
Herpotrichia rubi Fuckel, Fungi rhenani exsic 2171. (1868). (Fig. 36)
Fig. 36.
Herpotrichia rubi (from g, f. rh. 2171, type). a Numerous ascomata gregariously immersed in the host tissue. b Section of an ascoma. Note the central ostiole and peridium structure and also note the arrangement of asci and pseudoparaphyses. c Section of partial lateral peridium which comprises cells of textura angularis. d Part of a mature squashed ascus. e Relatively wide, septate pseudoparaphyses. f Immature ascus. Note the furcate pedicel. g-h One-septate ascospores. Note the verruculose ornamentation which is visible at the sides. Scale bars: a=0.5 mm, b =100 μm, c=50 μm, d=20 μm, e-h =10 μm
Ascomata 220-430 μm highx240-390(−530) μm diam., scattered to gregarious, immersed to erumpent, rarely superficial, globose to subglobose, wall black, coriaceous, apex with a small sometimes inconspicuous papilla, usually with a pore, lacking periphyses (Fig. 36a and b). Peridium 32-45 μm wide at the sides, up to 60 μm wide at the apex, basal wall thinner, all walls comprising cells of textura angularis, cells 2.5-4 μm diam., ce11 wall 2-4(−7) μm thick, exterior cells more thick-walled and pigmented, inner cells thin-walled and less pigmented, comprising thin-walled cells up to 9 μm diam., apex cells smaller and walls thicker (Fig. 36b and c). Hamathecium of dense, long pseudoparaphyses, 2–3 μm broad, embedded in mucilage, septate, branching (Fig. 36e). Asci 105-150×12.5-15 μm (x = 137.5 × 13.8mm, n =10), 8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a furcate pedicel that is 20-42.5 μm long, and ocular chamber up to 2.5 μm widex2.5 μm high (Fig. 36d and f). Ascospores 17.5-25×(5.5-)6.3-9 μm (x = 20.5 × 7.3mm, n = 10), biseriate to partially overlapping uniseriate near the base, fusoid with narrowly rounded ends, hyaline when immature and becoming pale brown, 1-septate, deeply constricted at the septum, the upper cell often broader than the lower one, verruculose (Fig. 36g and h).
Anamorph: Pyrenochaeta rhenana Sacc. (Sivanesan 1984).
Material examined: AUSTRIA, on Rubus idaeus L., very rarely in the spring, in the Oestreicher meadow forest (G, F. rh. 2171, type).
Notes
Morphology
Herpotrichia was established by Fuckel (1868) comprising two species H. rhenana Fuckel and H. rubi Fuckel, but no generic type was assigned. Bose (1961) designated H. rhenana as the lectotype species with H. rubi as a synonym. This proposal was followed by Muller and von Arx (1962) and Sivanesan (1971). Herpotrichia rubi was later assigned as the generic type (Holm 1979) as it was found to be validly published 2 years earlier than H. rhenana, thus having priority (Cannon 1982). However, Cannon (1982) reported that Sphaeria herpotrichoides Fuckel (1864, cited as a synonym of H. rhenana) was the earliest name. Thus he made a new combination as H. herpotrichoides (Fuckel) P.F. Cannon and cited H. rubi as the synonym. Herpotrichia rubi is maintained as the type of the genus (Holm 1979; Cannon 1982), but the current name is H. herpotrichoides.
Herpotrichia is a morphologically we11 studied genus (Barr 1984; Bose 1961; Muller and von Arx 1962; Pirozynski 1972; Samuels and Muller 1978; Sivanesan 1971, 1984), and Herpotrichia sensu lato is characterized by having subglobose, pyriform to obpyriform ascomata and a peridium of textura angularis or comprising thick-walled polygonal cells with thin-walled hyaline cells towards the centre. Asci are clavate to cylindrical, 4-8-spored and ascospores are hyaline at first, becoming pale to dark brown, one to many septate, constricted or not at the septa and often surrounded by a mucilaginous sheath. Several morphologically distinct genera were synonymized under Herpotrichia using the above broad circumscription (Barr 1984; Muller and von Arx 1962; Sivanesan 1984). In particular, Barr kept Lojkania as a separate genus after studying its type material (Barr 1984, 1990a). Sivanesan (1984) was also of the opinion that Lojkania and Neopeckia were distinct genera as several of their characters differed. Byssosphaeria and Pseudotrichia have subsequently been assigned to Melanommataceae, Lojkania to Fenestellaceae and Neopeckia to Coccoideaceae (Barr 1984). Herpotrichia sensu stricto is represented by H. rubi and has erumpent to superficial ascomata or immersed in a subiculum, clavate to cylindrical, 4-8-spored, stalked asci with a conspicuous apical “nasse”, hyaline, 1-septate ascospores, usually becoming pale brown and several septate, constricted or not constricted at septa, usually surrounded by sheath (Sivanesan 1984). Currently, about 90 species are included in this genus (http://www.indexfungorum.org/, 12/01/2009).
Phylogenetic study
Herpotrichia diffusa (Schwein.) Ellis & Everh., H. juniperi (Duby) Petr., H. herpotrichoides and H. macro-tricha have been shown to have phylogenetic affinity with the generic types of Byssosphaeria schiedermayeriana, Melanomma pulvis-pyrius and Pleomassaria siparia, which had been assigned under Melanommataceae (Kruys et al. 2006; Mugambi and Huhndorf 2009b; Schoch et al. 2006, 2009; Zhang et al. 2009a). In this study, Pleomassaria siparia together with its closely related species of Prosthemium is kept in a separate family, viz Pleomassariaceae.
Concluding remarks
Even species under Herpotrichia sensu stricto (according to Sivanesan 1984) have diverse hosts (such as gymnosperms (H. coulteri (Peck) S.K. Bose and H. parasitica (R. Hartig) Rostr.) and angiosperms (H. diffusa and H. villosa Samuels & E. Müll.)) or substrates (like dead or living leaves, bark or decorticated wood) (Sivanesan 1984). Species of Herpotrichia sensu stricto are also reported from various locations such as Europe, Asia or America, and they have various life styles, e.g. parasitic, hyperparasitic or saprobic (Sivanesan 1984). Additional factors (like hosts or locations) may need to be considered in order to get a natural concept for Herpotrichia.
Immotthia M.E. Barr, Mycotaxon 29: 504 (1987). (Teichosporaceae)
Generic description
Habitat terrestrial, hyperparasitic. Ascomata gregarious, globose, superficial, ostiolate, periphysate. Hamathecium of cellular pseudoparaphyses. Asci 8-spored, bitunicate, cylindrical, with a short pedicel. Ascospores 1-seriate, ellipsoidal, brown to reddish brown, 1-septate, constricted at the septum, smooth.
Anamorphs reported for genus: none.
Literature: Barr 1987a, 2002; Wang et al. 2004.
Type species
Immotthia hypoxylon (Ellis & Everh.) M.E. Barr, Mycotaxon 29: 504 (1987). (Fig. 37)
Fig. 37.
Immotthia hypoxylon (from holotype of Amphisphaeria hypoxylon). a Ascomata gregarious on host surface. b-d Bitunicate asci. h Released 1-septate ascospores. Scale bars: a=0.5 mm; b-h =10 μm
≡ Amphisphaeria hypoxylon Ellis & Everh., J. Myco1. 2: 41 (1886).
Ascomata gregarious, globose, superficial, ostiolate, periphysate, papillate (Fig. 37a). Hamathecium of cellular pseudoparaphyses, 2-2.5 μm broad, septate. Asci 60-82 × 7-9 μm, 8-spored, bitunicate, cylindrical, with a short pedicel (Fig. 37b, c and d). Ascospores 10-13 x4.4-5.4 μm, 1-seriate, ellipsoidal, brown to reddish brown, 1-septate, constricted at the septum, smooth (Fig. 37f, g and h) (adapted from Wang et al. 2004).
Anamorph: none reported.
Material examined: USA, Louisiana, Pointe a la Hache, on decaying wood, a branch of Carya oliviformis (Juglandaceae) lying on the ground in grass (parasitic on some effused Hypoxylon), 30 Dec. 1885, A.B. Langlois, No. 138 (NY, holotype of Amphisphaeria hypoxylon Ellis & Everh.).
Notes
Morphology
Immotthia was introduced to accommodate a species of Amphisphaeria (A. hypoxylon), which has bitunicate asci, and is characterized by superficial, ostiolate, periphysate, papillate ascomata, cellular pseudoparaphyses, bitunicate, 8-spored, cylindrical asci, ellipsoid, smooth, brown to reddish brown, 1-septate ascospores (Barr 1987a; Wang et al. 2004).
Phylogenetic study
None.
Concluding remarks
It seems that those Amphisphaeria species with bitunicate asci should be assigned to Pleosporales. Morphologically, Immotthia is somewhat comparable with Herpotrichia.
Isthmosporella Shearer & J.L. Crane, Mycologia 91: 141 (1999). (Pleosporales, genera incertae sedis)
Generic description
Habitat freshwater, saprobic. Ascomata small- to mediumsized, scattered, immersed, erumpent to superficial, globose, papillate, ostiolate, periphysate, membranous. Peridium 2-layered, outer layer composed of brown, pseudoparenchymatic, fusoid-cylindric cells, inner layer composed of fusoid, subhyaline to pale brown, compressed cells. Hamathecium of rare, broad, septate, interascal pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, oblong to clavate, with a short pedicel, ocular chamber not observed. Ascospores 3-4 seriate, cylindrical to fusoid, isthmoid at centre, constricted at septa, isthmus 1-septate, surrounded by a gelatinous sheath. Anamorphs reported for genus: none.
Literature: Shearer and Crane 1999.
Type species
Isthmosporella pulchra Shearer & J.L. Crane, Mycologia 91: 142 (1999). (Fig. 38)
Fig. 38.
Isthmosporella pulchra (from ILLS 53086, holotype). a Section of an ascoma. b Section of a partial peridium. c-e Broadly clavate asci with short pedicels. f Pseudoparaphyses. g-j Ascospores. Note the 2celled isthmus in J and mucilaginous sheath in G and H. Scale bars: a=50 μm, b-j = 20 μm (figure with permission from http://fungi.life.illinois.edu/search/fwa_lit_refs)
Ascomata 240-330 μm diam., scattered on decorticated wood, immersed, erumpent to superficial, globose, black, papillate, papilla short, cylindrical, 60 μm long × 55 μm wide, ostiolate, periphysate, membranous (Fig. 38a). Peridium 2-layered, outer 3-4 cell layers composed of brown, pseudoparenchymatic, fusoid-cylindric cells, 2-6.5 μm long; inner layer composed of 5-7 rows of fusoid, subhyaline to pale brown compressed cells, 11–20×2–3.5 μm diam. (Fig. 38a and b). Hamathecium of rare, broad, septate, interascal pseudoparaphyses (Fig. 38f). Asci (95-) 135-160(−175)×(25-)30-45(−60) μm, 8-spored, bitunicate, fissitunicate, oblong to clavate, with a short pedicel, ocular chamber not observed (Fig. 38c, d and e). Ascospores 80-105(−110) × (7-)8-10 μm, 3-4-seriate, cylindrical to fusoid, isthmoid at centre, sometimes bent at isthmus and becoming u- or v-shaped, end cells tapering, 12-17-phragmoseptate, constricted at septa, isthmus 1-septate, 2-5.5×2-4.5 μm diam., hyaline, frequently fragmenting to form partspores; filled with lipid droplets that merge to form large guttules; surrounded by a gelatinous sheath with a dense region near the isthmus, sheath greatly enlarging in water (Fig. 38g, h, i and j).
Anamorph: none reported.
Colonies on yeast soluble starch agar containing balsa wood sticks effuse, white. Hyphae hyaline, septate.
Material examined: USA, New York, Adirondack Park. Piercefield. Tupper Lake at public boat launch from Rt. 30, UTM Zone 18, 539840 mE, 4892100mN; 44°10 59 N, 80° 31’6”W, on submerged, decorticated wood, 7 Jul. 1994, J.L. Crane & C.A. Shearer A-254-1 (ILLS 53086, holotype).
Notes
Morphology
Isthmosporella was described as a freshwater genus typified by I. pulchra, and is characterized by globose, pseudoparenchymatous ascomata, sparse, septate pseudoparaphyses, fissitunicate asci and hyaline, cylindrical to fusoid, phragmoseptate, isthmoid ascospores surrounded with a gelatinous sheath (Shearer and Crane 1999). Based on the morphological characters, i.e. small, globose ascomata, peridium with sma11 pseudoparenchymatous cells and sparse pseudoparaphyses, Isthmosporella was assigned to the Phaeosphaeriaceae (Shearer and Crane 1999). The aquatic habitat of Isthmosporella, however, disagree with the Phaeosphaeriaceae. Isthmosporella seems less likely to belong to Pleosporineae.
Phylogenetic study
None.
Concluding remarks
Molecular phylogenetic studies should be conducted to explore its familial placement within Pleosporales.
Kalmusia Niessl, Verh. nat. Ver. Brünn 10: 204 (1872). (Montagnulaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata small- to mediumsized, solitary, scattered or in small groups, immersed to erumpent, globose or subglobose, often laterally flattened, coriaceous, wall black, with or without papilla. Hamathecium of dense, filliform, delicate, septate pseudoparaphyses, branching and anastomosing between and above asci, embedded in mucilage. Asci bitunicate, fissitunicate unknown, clavate, with a long, furcate pedicel. Ascospores narrowly ovoid to clavate, pale brown, 3-septate, distoseptate.
Anamorphs reported for genus: Cytoplea (Petrak and Sydow 1926).
Literature: Barr 1987b, 1990a, 1992a; Lindau 1897; von Niessl 1872.
Type species
Kalmusia ebuli Niessl, Verh. nat. Ver. Brünn 10: 204 (1872). (Fig. 39)
Fig. 39.
Kalmusia ebuli (from BR 101525-63, holotype). a Immersed to erumpent ascomata scattered on the host surface. b Section of a partial peridium. Note the compressed peridium cells. c Section of n ascoma. d-f Eight-spored asci with long pedicels. g Partial ascus in . h, i Ascospores with 3 thick-walled septa. Scale: a=0.5 mm, b=50 μm, c=100 μm, d-g=20 μm, h, i =10 μm
Ascomata 290-360 μm highx300-520 μm diam., solitary, scattered, or in sma11 groups, immersed to erumpent, globose or subglobose, coriaceous, wall black, with or without papilla, ostiolate (Fig. 39a). Papilla sma11, up to 100 μm high, with sma11 ostioles (Fig. 39a). Peridium 15-40 μm wide, comprising one cell type of small, pigmented, thick-walled cells of textura prismatica to textura angularis, cells ca. 5×3 μm diam., ce11 wall 2–3 μm thick (Fig. 39b and c). Hamathecium of dense, delicate pseudoparaphyses, 1-1.5 μm broad, septate, branching and anastomosing between and above asci, embedded in mucilage. Asci 75-125×10-15 μm (x = 90.5 × 12μm, n = 10), 8-spored, bitunicate, fissitunicate unknown, clavate, with a long, narrowed, furcate pedicel which is up to 45 μm long, and a low ocular chamber (ca. 2 μm widex 1 μm high) (Fig. 39d, e and f). Ascospores 15-18×5.5-6.5 μm (x = 16.3 × 5.8μm, n=10), biseriate, narrowly ovoid to clavate, pale brown, 3-distoseptate, without constriction, smooth-walled (Fig. 39g, h and i).
Anamorph: none reported.
Material examined: BELGIUM, Dolembreux, onbranchlets and pieces of stumps of Sarothamnus scoparius from woodland, Oct. 1922, V. Mouton (BR 101525-63, holotype).
Notes
Morphology
Kalmusia was formally established by von Niessl (1872), and is mainly characterized as “immersed, sphaeroid ascoma with central, stout papilla, surrounded by hyphae in the substrate, stipitate asci with septate pseudoparaphyses, and brown, 3-septate, inequilateral ascospores” (Barr 1992a).
The most morphologically comparable genus to Kalmusia is Thyridaria, which had been treated as a subgenus under Kalmusia (Lindau 1897), and was subsequently transferred to Platystomaceae in Melanommatales (Barr 1987b, 1990a). Compared to Thyridaria, Kalmusia has sphaeroid ascomata, a peridium of small pseudoparenchymatous cells, basal asci and very thin pseudoparaphyses, thus it was assigned to Phaeosphaeriaceae of the Pleosporales by Barr (1990a), and the genus is utilized to accommodate both K. ebuli and K. clivensis (Berk. & Broome) M.E. Barr, as well as closely related species, i.e. K. utahensis (Ellis & Everh.) Huhndorf & M.E. Barr and K. coniothyrium (Fuckel) Huhndorf (Barr 1992a). But this proposal is questionable, as the clavate, distoseptate ascospores, as well as the clavate asci with very long pedicels are uncommon in Phaeosphaeriaceae, and most recent phylogenetic study indicated that some species of Kalmusia reside outside of Phaeosphaeriaceae (Zhang et al. 2009a).
Phylogenetic study
Both Kalmusia scabrispora Teng Kaz. Tanaka, Y. Harada & M.E. Barr and K. brevispora (Nagas. & Y. Otani) Yin. Zhang, Kaz. Tanaka & C.L. Schoch reside in the clade of Montagnulaceae (Zhang et al. 2009a). Familial placement of Kalmusia can only be verified after the DNA sequences of the generic type (K. ebuli) are obtained.
Concluding remarks
Kalmusia is distinct amongst the Pleosporales as it has pale brown ascospores with indistinct distosepta and clavate asci with long pedicels. Although both K. scabrispora and K. brevispora reside in the clade of Montagnulaceae, they both lack the distoseptate ascospores that are possessed by the generic type (K. ebuli). Thus, the familial placement of Kalmusia is stil! undetermined.
Karstenula Speg., Decades Mycologicae Italicae ad no. 94 (in sched.) (1879). (Montagnulaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata rarely small-, usually medium-sized, immersed usually under thin clypeus, scattered to gregarious, with flattened top and rounded pore-like ostiole, coriaceous. Peridium 2-layered, outer layer composed of reddish brown to dark brown small cells, inner layer of pale compressed cells. Hamathecium of dense, cellular pseudoparaphyses. Asci cylindrical to cylindro-clavate with short furcate pedicel. Ascospores muriform, ellipsoid to fusoid, reddish brown to dark brown.
Anamorphs reported for the genus: Microdiplodia (Constantinescu 1993).
Literature: Barr 1990a; Eriksson and Hawksworth 1991; Kodsueb et al. 2006a; Munk 1957; Zhang et al. 2009a.
Type species
Karstenula rhodostoma (Alb. & Schwein.) Speg., Decades Mycologicae Italicae no. 94. (1879). (Fig. 40)
Fig. 40.
Karstenula rhodostoma (from PH 01048835, type). a Line of ascomata on host surface (after remove the decaying cover). Note the wide ostiolar opening and light colored region around the ostiole. b Immersed ascoma under the decaying cover (see arrow). c, d Section of the peridium. The peridium comprises smal! thick-walled cells in the outer layer. The outside comprises defuse hyphae which is probably part of the subiculum. e Ascus with a short furcate pedicel. f Partial ascus showing arrangement of ascospores. g-i Released ascospores. Note the transverse and rarely vertical septa. Scale bars: a, b=0.5 mm, c=50 μm, d-f=20 μm, g-i =10 μm
≡ Sphaeria rhodostoma Alb. & Schwein., Consp. fung. (Leipzig): 43 (1805).
Ascomata 250-430 μm highx450-650 μm diam., scattered or gregarious, immersed in the subiculum which sometimes sloths off, globose or subglobose, black, flattened top often white or reddish and sometimes slightly protruding out of the substrate surface, usually with a wide opening ostiole after removing the cover, coriaceous (Fig. 40a and b). Peridium 30-40 μm wide, comprising two cell types, outer region 1-layered, composed of relatively small heavily pigmented thick-walled compressed cells, cells 2-4×5-10 μm diam., cell wall 2-4 μm thick, inner layer cells larger and wall thinner, comprising cells of textura angularis, merging with pseudoparaphyses (Fig. 40c and d). Hamathecium of dense, long cellular pseudoparaphyses 2–3.5 μm broad, septate, branching or anastomosing not observed. Asci 150-210× 12.5-15 μm (x = 182 × 13.1μm, n=10), 8-spored, bitunicate, fissitunicate, cylindrical, with a broad, furcate pedicel which is 12–35 μm long, and with an ocular chamber (to 4 μm widex 3 μm high) (Fig. 40e and f). Ascospores 20-26 × 7.5-10 μm (x = 22.4 × 8μm, n=10), obliquely uniseriate and partially overlapping, ellipsoid, reddish brown, with 3 transverse septa and a vertical septum in one or two central cells, constricted at the septa, verruculose (Fig. 40g, h and i).
Anamorph: Microdiplodia frangulae Allesch. (Constantinescu 1993).
Conidiomata globose to subglobose, 330-495 μm diam., in subiculum. Conidia 9-13×4-5 μm, reddish brown, 1-septate (information obtained from Barr 1990a).
Material examined: Fries, Suecia (received by herbarium in 1834) (PH 01048835, type, as Sphaeria rhodostoma Alb. & Schwein.).
Notes
Morphology
Karstenula is an ambiguous genus, which has been synonymized under Pleomassaria (Lindau 1897; Winter 1885). Some of the ascomata characters are even comparable with those of Didymosphaeria, such as ascomata seated in subiculum or beneath a clypeal thickening, the development of apex vary in a large degree, even to the occasional formation of a blackened internal clypeus, and sometimes apical cells become reddish or orange-brown (Barr 1990a). Barr (1990a) redefined the concept of Karstenula (sensu lato), which encompasses some species of Thyridium. In her concept, however, Barr (1990a) treated Karstenula as having trabeculate pseudoparaphyses and this is clearly not the case. In most cases the ascospores were brown with transverse septa and sparse longitudinal septa.
The ascomata of this species are similar to those found in Byssosphaeria and Herpotrichia, especially in the paler area around the ostiole and even in peridial structure and development under a subiculum. The numerous wide cellular pseudoparaphyses and cylindrical asci (in Herpotrichia) are also similar. The main difference of Karstenula from other two genera are the 3-septate ascospores with rare longitudinal septa (1-septate in Byssosphaeria and Herpotrichia).
Phylogenetic study
Karstenula forms a robust phylogenetic clade with Phaeodothis winteri (Niessl) Aptroot, Didymocrea sadasivanii, Bimuria novae-zelandiae, Montagnula opulenta, Curreya pityophila (J.C. Schmidt & Kunze) Arx & E. MU11. and some species of Letendraea and Paraphaeosphaeria (Kodsueb et al. 2006a; Zhang et al. 2009a). Consequently, Karstenula might be included in Montagnulaceae.
Concluding remarks
The description of the type of Karstenula here clearly excludes it from Melanommataceae as it has wide pseudoparaphyses. But its Montagnulaceae status can only be confirmed by more phylogenetic work including sequencing the generic type of Karstenula (K. rhodostoma).
Katumotoa Kaz. Tanaka & Y. Harada, Mycoscience 46: 313 (2005). (Lentitheciaceae)
Generic description
Habitat terrestrial or freshwater, saprobic. Ascomata small- to medium-sized, scattered or in small groups, immersed to erumpent, with a central protruding hairy papilla, subgtobose. Peridium thin, comprising several layers of thinwalled compressed cells. Hamathecium of dense, cellular, filliform, embedded in mucilage, branching and anastomosing. Asci 8-spored, bitunicate, fissitunicate, clavate with short furcate pedicels. Ascospores apiosporous and hyaline when young, becoming 2-septate with reddish brown echinate central cell at maturity, with long gelatinous terminal appendages.
Anamorphs reported for genus: none.
Literature: Tanaka and Harada 2005b; Tanaka et al. 2009; Zhang et al. 2009a.
Type species
Katumotoa bambusicola Kaz. Tanaka & Y. Harada, Mycoscience 46: 313 (2005). (Fig. 41)
Fig. 41.
Katumotoa bambusicola (from HHUF 28663, holotype). a Ascomata scattered on the host surface. b Asci in pseudoparaphyses. c Hyaline ascospore with long terminal appendages. d Clavate ascus with a short pedicel. Scale bars: a=0.5 mm. b-d=20 μm
Some information for the following description is from Tanaka and Harada (2005).
Ascomata 240-330 μm highx260-420 μm diam., scattered or in small groups, immersed, becoming erumpent, with a slightly protruding papilla covered with brown hyphae, subglobose (Fig. 41a). Peridium 13-30 μm thick, composed of a few layers of lightly pigmented, depressed cells. Hamathecium of dense, long cellular pseudoparaphyses, 1.5-3 μm broad, embedded in mucilage, branching and anastomosing. Asci 110-160×17.5-24 μm (x = 139 × 21μm, n=10), 8-spored, bitunicate, fissitunicate, cylindro-clavate with a short furcate pedicel which is up to 25 μm long (Fig. 41b and d). Ascospores 39-50(−57)×7-10 μm (x = 45.8 × 8.2μm, n=10), biseriate, fusoid to narrowly fusoid with acute ends, usually curved, apiosporus and hyaline when young, constricted at the primary septum, the upper cell longer and broader than the lower one, smooth, surrounded by a bipolar sheath which is up to 15 μm long, best seen in India ink, senescent ascospores yellowish brown, 2-4-septate (Fig. 41c).
Anamorph: none reported.
Material examined: JAPAN, Mt. Iwate, near Yakebashiri, Hirakasa, Nishine, Iwate, on culms of Oryza sativa L., 19 Oct. 2003, K. Tanaka (HHUF 28663, holotype).
Notes
Morphology
Katumotoa was formally established by Tanaka and Harada (2005b) to accommodate the monotypic species, K. bambusicola, which is characterized by immersed ascomata with a thin peridium comprising thin-walled compressed cells, cellular pseudoparaphyses, cylindroclavate and fissitunicate asci and fusoid ascospores with an elongated bipolar mucilaginous sheath. Based on its immersed ascomata, psuedoparenchymatous peridium cells and cellular pseudoparaphyses, Katumotoa was assigned to Phaeosphaeriaceae (Tanaka and Harada 2005b; Tanaka et al. 2009), but this classification has been shown to be incorrect in subsequent phylogenetic studies (Tanaka et al. 2009; Zhang et al. 2009a).
Phylogenetic study
Phylogenetic analysis based on five genes (LSU, SSU, RPB 1, RPB2 and EF1) indicates that Katumotoa bambusicola resides in Lentitheciaceae, and this receives high bootstrap support (Zhang et al. 2009a). In particular, K. bambusicola forms a robust clade with Ophiosphaerella sasicola (Nagas. & Y. Otani) Shoemaker & C.E. Babc., which has filliform ascospores (Shoemaker and Babcock 1989b).
Concluding remarks
The hyaline, apiosporous ascospores which become 2-4-celled with central reddish brown cells and large unraveling appendages are the most striking features of this species and readily distinguish it from other pleosporalean taxa. Both Katumotoa bambusicola and Ophiosphaerella sasicola are associated with bambusicolous hosts, which might indicate that host spectrum in this case, has greater phylogenetic significance than some morphological characters (Zhang et al. 2009a).
Keissleriella Hohn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 128: 582 (1919). (Lentitheciaceae)
Generic description
Habitat terrestrial or freshwater, saprobic. Ascomata small- to medium-sized, immersed, erumpent to nearly superficial, globose, papillate, ostiolate. Papilla covered by dark setae or small blackened cells. Peridium thick, composed of cells of pseudoparenchymatous and inner layer composed of pale cells. Hamathecium of dense, long pseudoparaphyses, rarely septate, anastomosing and branching. Asci 4- or 8-spored, bitunicate, fissitunicate, cylindro-clavate, with a furcate pedicel and a small ocular chamber. Ascospores hyaline to pale brown, ellipsoid to fusoid, 1-septate, constricted at the septum (Barr 1990a).
Anamorphs reported for genus: Dendrophoma (Bose 1961). Literature: von Arx and Muller 1975; Bose 1961; Barr 1990a; Dennis 1978; Eriksson 1967a; von Hohnel 1919; Luttrell 1973; Munk 1957; Zhang et al. 2009a.
Type species
Keissleriella aesculi (Hohn.) Hohn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 128: 582 (1919). (Fig. 42)
Fig. 42.
Keissleriella sambucina (from FH, holotype of Otthiella aesculi). a Section of an ascoma. b Pseudoparaphyses which are narrow (less than 1.5 μm) and branch and anastomosing as trabeculate. c, d Hyaline ascospores with distinct constrictions at the septa. e Asci amongst narrow pseudoparaphyses. F. Ascus with a pedicel and ocular chamber. Scale bars: a =100 μm, b-f=10 μm
≡ Pyrenochaeta aesculi Hohn., Ber. dt. bot. Ges. 35: 249 (1917).
Ascomata ca. 250 μm highx450 μm diam., gregarious, immersed to erumpent, globose or subglobose, with a small black papilla, ca. 75 μm high and 110 μm broad, with short black external setae (Fig. 42a). Peridium ca. 25-40 μm wide laterally, up to 70 μm near the apex, thinner at the base, comprising two types of cells which merge in the middle; outer cells composed of small heavily pigmented thick-walled cells, cells ca. 4 μm diam., cell wall up to 4 μm thick, and thick near the apex and thinner laterally and absent in the immersed part of the ascoma, inner cells less pigmented, comprising lightly pigmented to hyaline cells, 5-7 μm thick (Fig. 42a). Hamathecium of dense, long pseudoparaphyses, 0.8-1.2 μm broad, rarely septate, anastomosing and branching, thicker near the base, ca. 2 μm, constricted near the septum (Fig. 42b). Asci 80-120 × 6-11 μm (x = 101 × 8.5μm, n =10), 4- or 8-spored, bitunicate, fissitunicate, cylindro-clavate, with a furcate pedicel which is up to 20-40 μm long, with a small ocular chamber (Fig. 42e and f). Ascospores 13-18×4-5.5 μm (x = 14.5 × 4.8μm, n =10), obliquely uniseriate and partially overlapping, fusoid with narrowly rounded ends, hyaline, 1-septate, constricted at the septum, smooth (Fig. 42c and d).
Anamorph: none reported.
Material examined: AUSTRIA, Brentenmaistal in the Viennese forest, Aesculus hippocastanum L., 1916, Hohnel (FH, holotype of Otthiella aesculi). (Note: only two slides; setae cannot be seen from the slides but could be seen from the drawings on the cover).
Notes
Morphology
Keissleriella is characterized by ascomata with setae in and over the papilla, asci are cylindrical and ascospores are hyaline, 1-septate. Based on the morphological characters, K. aesculi was regarded as conspecific with K. sambucina; as an earlier epithet, K. sambusina typifies the genus (see comments by Barr 1990a). Munk (1957) placed Trichometasphaeria and Keissleriella in Massarinaceae, and distinguished them by their substrates (Trichometasphaeria occurs on herbaceous plants and Keissleriella on woody substrates). Bose (1961) combined Trichometasphaeria under Keissleriella, which was followed by some workers (von Arx and Muller 1975; Dennis 1978; Eriksson 1967a; Luttrell 1973). Barr (1990a), however, maintained these as distinct genera based on the differences of peridium structure and pseudoparaphyses.
Phylogenetic study
The phylogeny of Keissleriella is poorly studied. Limited phylogenetic information indicates that K. cladophila forms a robust clade with other species of Lentitheciaceae (Zhang et al. 2009a).
Concluding remarks
The presence of black setae on the surface of papilla is a striking character of Keissleriella, but phylogenetic significance of setae is undetermined yet.
Lentithecium K.D. Hyde, J. Fourn. & Yin. Zhang, Fungal Divers. 38: 234 (2009). (Lentitheciaceae)
≡ Tingoldiago K. Hirayama & Kaz. Tanaka, Mycologia 102: 740 (2010) syn. nov.
Generic description
Habitat freshwater, saprobic. Ascomata small, scattered or gregarious, immersed, slightly erumpent, depressed spherical to lenticular, ostiolate, papillate or epapillate. Peridium thin. Hamathecium of cellular pseudoparaphyses. Asci 8-ascospored, bitunicate, fissitunicate, clavate, short-stipitate. Ascospores broadly fusoid with broadly rounded ends, 1-septate, constricted, hyaline, usually with sheath.
Anamorphs reported for genus: none.
Literature: Shearer et al. 2009; Zhang et al. 2009a, b.
Type species
Lentithecium fluviatile (Aptroot & Van Ryck.) K.D. Hyde, J. Fourn. & Yin. Zhang, Fungal Divers. 38: 234 (2009). (Fig. 43)
Fig. 43.
Lentithecium fluviatile (from IFRD 2039). a Erumpent b ascomata scattering on the host surface. b Habitat section of the immersed ascomata. c, d Section of an ascoma and a partical peridium. Note the peridium cells of textura angularis. e Clavate 8-spored ascus with a short pedicel. f, g Hyaline, 1-septate broadly fusoid ascospores. Scale bars: a, b=0.5 mm, c=100 μm, d=50 μm, e-g=20 μm
≡ Massarina fluviatilis Aptroot & Van Ryck., Nova Hedwigia 73: 162 (2001).
Ascomata 230-260 μm highx280-325 μm diam., scattered or gregarious, immersed, slightly erumpent, subglobose to depressed spherical, under a small black pseudostroma originating from the apical part of the peridium, apex slightly papillate, ostiole rounded, 60-70 μm diam. (Fig. 43a and b). Peridium 15-20 μm thick at sides and at base, comprising 4-5 layers of angular cells more thick-walled outwards, 50-55 μm thick at apex, of small very thick-walled cells. Hamathecium of cellular pseudoparaphyses, 2-2.5 μm broad (Fig. 43c and d). Asci 89-100×19-21 μm, 8-spored, bitunicate, fissitunicate, clavate, bumpy, short-stipitate, apex without obvious apical chamber (Fig. 43e). Ascospores 27-35×8.5-9.4 μm,, 2–3-seriate, broadly fusoid with broadly rounded ends, straight to slightly curved, 1-septate, slightly constricted, with four large guttules, hyaline, smooth-walled, a very thin mucilaginous sheath can be occasionally observed in India ink but in most cases no sheath can be observed (Fig. 43f and g).
Anamorph: none reported.
Material examined: FRANCE, Haute Garonne: Avignonet, Lac de Rosel, artificial lake, on bark and wood of a submerged branch Populus sp., 23 Nov. 2006, leg. Michel Delpont, det. Jacques Fournier (IFRD 2039, holotype).
Notes
Morphology
Lentithecium was introduced to accommodate some freshwater fungi previous assigned under Massarina, such as M. arundinacea (Sowerby) Leuchtm. and M. fluviatilis (Zhang et al. 2009a). It is characterized by its immersed and lenticular ascomata, thin peridium which is almost equal in thickness, short pedicellate asci and fusoid or filliform, hyaline or rarely lightly pigmented, 1- to multi-septate ascospores (Zhang et al. 2009b). Lentitheciaceae was introduced to accommodate Lentithecium and some other related taxa (Zhang et al. 2009a).
Phylogenetic study
The clade of Lentitheciaceae comprises the generic type Lentithecium fluviatile, as we11 as L. arundinaceum (Sowerby) K.D. Hyde, J. Fourn. & Yin. Zhang, Stagonospora macropycnidia, Wettsteinina lacustris (Fuckel) Shoemaker & C.E. Babc., Keissleriella cladophila, and the bambusicolous species Katumotoa bambusicola and Ophiosphaerella sasicola, which receive high bootstrap support (Zhang et al. 2009a).
Concluding remarks
Tingoldiago graminicola K. Hirayama & Kaz. Tanaka form a robust clade with species of Lentithecium (Shearer et al. 2009). Tingoldiago has lenticular immersed to erumpent ascomata, numerous and septate pseudoparaphyses, cylindro-clavate asci and hyaline, 1-septate ascospores with sheath. All of these characters fit Lentithecium well. We treat Tingoldiago as a synonym of Lentithecium.
Leptosphaeria Ces. & De Not., Comm. Soc. crittog. Ital. 1: 234 (1863). (Leptosphaeriaceae)
Generic description
Habitat terrestrial, saprobic or parasitic. Ascomata small- to medium-sized, solitary, scattered or in small groups, erumpent to superficial, subglobose, broadly or narrowly conical, papillate, ostiolate. Peridium thick, comprising layers of cells of textura angularis. Hamathecium of dense cellular pseudoparaphyses, embedded in mucilage, anastomosing and branching. Asci 8-spored, bitunicate, fissitunicate unknown, cylindrical with a furcate pedicel and a large ocular chamber. Ascospores fusoid or narrowly fusoid, brown or reddish brown, 3-septate, constricted at each septum.
Anamorphs reported for genus: Coniothyrium and Phoma (Hyde et al. 2011; Sivanesan 1984).
Literature: von Arx and Muller 1975; Barr 1987a, b; Cesati and de Notaris 1863; Crane and Shearer 1991; Dong et al. 1998; Eriksson 1967a; Eriksson and Hawksworth 1986, 1991; de Greuter et al. 1988; Hedjaroude 1969; von Hohnel 1907; Holm 1957, 1975; Huhndorf et al. 1990; Luttrell 1973; Muller 1950; Munk 1957; Saccardo 1878b, 1883, 1891, 1895; Schoch et al. 2009; Shearer 1993; Shearer et al. 1990; Shoemaker 1984a; Sivanesan 1984; Zhang et al. 2009a.
Type species
Leptosphaeria doliolum Ces. & De Not., Comm. Soc. crittog. Ital. 1: 234 (1863). (Fig. 44)
Fig. 44.
Leptosphaeria doliolum (from L, lectotype). a Ascomata on the host surface. Note the shiny black surface. b Section of the partial peridium. Note the uneven thickness. c-e Asci with a short pedicel. f Three ascospores in ascus. Scale bars: a=0.5 mm, b=100 μm, c-f=20 μm
≡ Sphaeria doliolum Pers., Icon. Desc. Fung. Min. Cognit. (Leipzig) 2: 39 (1800).
Ascomata 340-450 μm highx380-500 μm diam., solitary, scattered or in small groups, superficial, subglobose, broadly or narrowly conical, with a flattened base on the host surface, black, usually with 2-4 ring-like ridges surrounding the ascomata surface, apex with a conical, usually shiny papilla (Fig. 44a). Peridium 85-110 μm wide at sides, thinner at the apex, comprising two types of cells, outer layer composed of small thick-walled cells of textura angularis, cells <2 μm diam., cell wall up to 8 μm thick, surface heavily pigmented and inner lightly pigmented, apex cells smaller, walls thicker, and cells more heavily pigmented, inner layer composed of subhyaline relatively thin-walled cells of textura angularis, 3-6 μm diam., wall up to 5 μm, cells near the base larger and wall thinner and paler (Fig. 44b). Hamathecium of dense, long cellular pseudoparaphyses, 1.5-3 μm broad, embedded in mucilage, anastomosing and branching. Asci 110-150 × 7-9(−10) μm (x = 130.6 × 8.5μm, n=10), 8-spored, bitunicate, fissitunicate unknown, cylindrical, furcate pedicel which is usually less than 25 μm long, with a large ocular chamber (Fig. 44c, d and e). Ascospores 25-31×4.5-6 μm (x = 27.7 × 5.3μm, n=10), uniseriate and somewhat partially overlapping, narrowly fusoid with sharp to narrowly rounded ends, reddish brown, 3-septate, constricted at each septum, smooth (Fig. 44f).
Anamorph: Phoma hoehnelii (Sivanesan 1984). Material examined: Herb., Persoon 910270-650 (L, lectotype).
Notes
Morphology
Leptosphaeria was first established by Cesati and de Notaris (1863) with 26 species included; L. doliolum (Pers.: Fr.) Ces. & De Not. was subsequently selected as the lectotype species (de Greuter et al. 1988; Holm 1975; Shearer et al. 1990). Leptosphaeria was originally defined based mainly on the characters of ascospores being ellipsoid or fusoid, one to many septa, hyaline to dark brown. These few common characters meant that Leptosphaeria comprised many species, and some of them should be assigned to either Euascomycetes or Loculoascomycetes (Crane and Shearer 1991). Leptosphaeria had been divided based on host and habitat (Saccardo 1878b, 1891, 1895) as we11 as the pseudothecium (glabrous, hairy, setose) and ascospore septation (see comments by Crane and Shearer 1991). von Hohnel (1907) used centrum structure in the classification of Leptosphaeria, and divided Leptosphaeria into three genera, viz. Leptosphaeria, Scleropleella and Nodulosphaeria. Muller (1950) subdivided Leptosphaeria into four sections based on pseudothecial and centrum structure as well as ascospore characters. This classification was modified by Munk (1957), who named these four sections as section I (Eu-Leptosphaeria), section II (Para-Leptosphaeria), section HI (Scleropleella) and section IV (Nodulosphaeria). Holm (1957) used a relatively narrow concept for Leptosphaeria, which included species closely related to the generic type, L. doliolum. This viewpoint was accepted by some workers (Eriksson 1967a; Hedjaroude 1969; Shoemaker 1984a). Nevertheless, it still seems a heterogeneous group of fungi (see comments by Crane and Shearer 1991). Its position among the Loculoascomycetes is also debated. It has been placed in the Pleosporaceae (von Arx and Muller 1975; Luttrell 1973; Sivanesan 1984) or Leptosphaeriaceae (Barr 1987a, b; Eriksson and Hawksworth 1991) or Phaeosphaeriaceae (Eriksson and Hawksworth 1986).
Phylogenetic study
Molecular phylogenetic analysis based on multigenes indicated that species of Leptosphaeria (including the generic type L. doliolum) and Neophaeosphaeria form a paraphyletic clade with moderate bootstrap support (Dong et al. 1998; Schoch et al. 2009; Zhang et al. 2009a), which is sister to other families of Pleosporales (Zhang et al. 2009a). Thus the familial rank of the Leptosphaeriaceae could be temporarily verified, but further molecular phylogenetic study is needed in which more related taxa should be included.
Concluding remarks
Morphologically, Leptosphaeria is mostly comparable with Amarenomyces, Bricookea, Diapleella, Entodesmium, Melanomma, Nodulosphaeria, Paraphaeosphaeria, Passeriniella, Phaeosphaeria and Trematosphaeria. While it prefers non-woody parts of dicotyledonous hosts, its cylindrical ascus with short pedicel and smooth, fusoid and multi-septate ascospores make it readily distinguishable from a11 other genera (Shoemaker 1984a).
Leptosphaerulina McAlpine, Fungus diseases of stonefruit trees in Australia and their treatment: 103 (1902). (Didymellaceae)
Generic description
Habitat terrestrial, parasitic or saprobic. Ascomata small, scattered, immersed, globose to subglobose, with a small, slightly protruding papilla, ostiolate. Peridium thin. Hamathecium of rare or decomposing cellular pseudoparaphyses. Asci bitunicate, obpyriform. Ascospores broadly clavate or cylindrical, hyaline, turning pale brown when old, asymmetrical, multi-septate, smooth-walled.
Anamorphs reported for genus: Pithoascus and Pithomyces (Hyde et al. 2011).
Literature: Barr 1972; Chlebicki 2002; Crivelli 1983; Kodsueb et al. 2006a; Zhang et al. 2009a.
Type species
Leptosphaerulina australis McAlpine, Fungus diseases of stone-fruit trees in Australia and their treatment: 103 (1902). (Fig. 45)
Fig. 45.
Leptosphaerulina australis (from NY, C.T. Rogerson 3836). a. Compressed ascoma. Note the obpyriform asci within the ascoma and the thin peridium. b, c. Eight-spored asci released from the ascomata. Note the apical apparatus (arrowed). d. Ascospores with thin sheath. e. An old pale brown ascospore. Scale bars: a-c=50 μm, d, e =10 μm
Ascomata 140-170 μm diam., scattered, immersed, globose to subglobose, with a small slightly protruding papilla, ostiolate (Fig. 45a). Peridium thin, composed of one or two layers of large cells of textura angularis, pale brown (Fig. 45a). Hamathecium of rare or decomposing cellular pseudoparaphyses, up to 5 μm broad, filling the gaps between the asci. Asci 38-53×55-75 μm (x = 67.5 × 43.3mm, n=10), 8-spored, without pedicel, bitunicate, fissitunicate dehiscence not observed, obpyriform, with a large ocular chamber and apical ring (Fig. 45b and c). Ascospores 30-40(−47)× 11-14 μm (x = 36.5 × 13μm, n= 10), broadly clavate, hyaline, turning pale brown when old, asymmetrical, upper hemisphere usually with one transverse septum and with a somewhat narrowly rounded end, lower hemisphere usually with two transverse septa and with broadly rounded ends, slighted constricted at the primary septum, mostly with one vertical septum in each central cell, smooth, with thin gelatinous sheath when young, 2–3 μm thick (Fig. 45d and e). Anamorph: none reported.
Material examined: USA, Kansas, Kansas State College, on Poa pratensis L. Grass plots, 2 Jul. 1953, leg. T. Rogerson, det. L.E. Wehmeyer (NY, C.T. Rogerson 3836).
Notes
Morphology
Leptosphaerulina, introduced by McAlpine (1902), is characterized by small immersed ascomata, obpyriform asci with a large ocular chamber and apical ring as well as muriformly septate ascospores which may be hyaline or pigmented. Species of Leptosphaerulina may occur on monocotyledons or dicotyledons. Leptosphaerulina is most comparable with Pleospora, and the only difference between them is that Leptosphaerulina has smaller ascomata and hyaline ascospores that only become pigmented after discharge, whereas the ascospores of Pleospora become brown within the asci. Currently, about 60 names are accepted in this genus, and some even reported from marine environments, e.g. L. mangrovei (Inderbitzin et al. 2000).
Phylogenetic study
Based on multigene phylogenetic analysis, two putative strains of Leptosphaerulina australis, the generic type of Leptosphaerulina, from Switzerland (CBS 311.51) and Indonesia (CBS 317.83) resided within Didymellaceae (de Gruyter et al. 2009; Zhang et al. 2009a).
Concluding remarks
Because of its morphological confusion with Pleospora and the diversity of habitats within the genus, Leptosphaerulina sensu lato is likely to be polyphyletic. Fresh collections of this species are needed from Australia to epitypify this taxon and define the genus in a strict sense. The specimen described here is a collection from USA and therefore may not represent the type.
Lewia M.E. Barr & E.G. Simmons, Mycotaxon 25: 289 (1986). (Pleosporaceae)
Generic description
Habitat terrestrial, parasitic or saprobic? Ascomata small, scattered, erumpent to nearly superficial at maturity, subglobose to globose, black, smooth, papillate, ostiolate. Papilla short, blunt. Peridium thin. Hamathecium of pseudoparaphyses. Asci (4-6-)8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a short, furcate pedicel. Ascospores muriform, ellipsoid to fusoid.
Anamorphs reported for genus: Alternaria (Simmons 1986). Literature: Kwasna and Kosiak 2003; Kwasna et al. 2006; Simmons 1986, 2007; Vieira and Barreto 2006.
Type species
Lewia scrophulariae (Desm.) M.E. Barr & E.G. Simmons, Mycotaxon 25: 294 (1986). (Fig. 46)
Fig. 46.
Lewia scrophulariae (from FH, slide from lectotype). a Cylindrical ascus with a short pedicel. b Ascospores in asci. c-f Released muriform brown ascospores. Scale bars: a=20 μm, b-f=10 μm
≡ Sphaeria scrophulariae Desm., Plantes cryptogames du Nord de la France, ed. 1 fasc. 15:no. 718 (1834).
Ascomata ca. 150-200 μm diam., scattered, erumpent to nearly superficial at maturity, subglobose to globose, black, smooth, papillate. Papilla short, blunt. Peridium thin. Hamathecium of septate pseudoparaphyses, ca. 2-2.5 μm broad, anastomosing or branching not observed. Asci 100-140×13-17 μm, (4-6-)8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a short, furcate pedicel, ocular chamber unknown (Fig. 46a). Ascospores ellipsoid, 5 (rarely 6 or 7) transversal septa and one longitudinal septum mostly through the central cells, yellowish brown to gold-brown, 20-24×8-10 μm (x = 21. 5 × 9 .1μm, n =10), constricted at median septum, smooth or verruculose (Fig. 46b, e and f).
Anamorph: Alternaria conjuncta (Simmons 1986).
Primary conidiophore simple with a single conidiogenous locus; conidia produced in chains, the first conidia in chain is larger, 30-45×10-12 μm, 7 transverse septa, 1 -2 longitudinal or oblique septa in lower cells. Secondary conidiophore with 5-7 conidiogenous loci, sometimes branched; sporulation in chains, rarely branched.
Material examined: (FH, slide from lectotype).
Note: The specimen contains only a slide, so limited structures could be observed e.g. ascospores. The information about ascomata, peridium and whole asci is referred to Simmons (1986).
Notes
Morphology
Lewia has “Pleospora-like” teleomorphs, while it has Alternaria anamorphs, which are characterized by the beakless conidia connected together with secondary conidiophore (Simmons 1986). Based on these characters, more species under this genus were subsequently reported, i.e. Lewia avenicola Kosiak & Kwasna (Kwasna and Kosiak 2003); L. chlamidosporiformans B.S. Vieira & R. W. Barreto (Vieira and Barreto 2006); L. alternarina (M. D. Whitehead & J.G. Dicks.) E.G. Simmons and L. daucicaulis E.G. Simmons (Simmons 2007). Currently Lewia comprises 15 species (http://www.mycobank.org, 24-02-2009).
Phylogenetic study
Phylogenetic analysis based either on SSU rDNA sequences or on multigenes indicated that Lewia species (Allewia eureka (E.G. Simmons) E.G. Simmons=L. eureka) form a robust clade with other members of Pleosporaceae (Schoch et al. 2006; Schoch et al. 2009; Zhang et al. 2009a).
Concluding remarks
Its position in Pleosporaceae is confirmed.
Lichenopyrenis Calat., Sanz & Aptroot, Myco1. Res. 105: 634 (2001). (?Pleomassariaceae)
Generic description
Habitat terrestrial, parasitic on lichens. Ascomata mediumsized, globose or subglobose. Hamathecium of dense, filliform, branching, septate pseudoparaphyses. Asci bitunicate, fissitunicate, clavate, with a short sometimes furcate pedicel. Ascospores ellipsoidal with broadly rounded ends, pale orange-brown, 1-distoseptate.
Anamorphs reported for genus: see below.
Literature: Calatayud et al. 2001.
Type species
Lichenopyrenis galligena Calat., Sanz & Aptroot, MycoL Res. 105: 636 (2001). (Fig. 47)
Fig. 47.
Lichenopyrenis galligena (from MA-Lichen 12715, hototype). a, b Ascomata forming in the host tissues. c, d Sections of ascomata. e Section of a partial peridium. f-h, k Broadly clavate asci. Note the short rounded pedicel. i, j, l Ascospores. Note the smal] swellings at the septa. Scale bars: a, b=0.5 mm, c, d =100 μm, e= 50 μm, f-h, k=20 μm, i, j, l=10 μm
Ascomata 140-260 μm highx 140-250 μm diam., gregarious, initially immersed in galls, later becoming erumpent, globose or subglobose, black, roughened (Fig. 47a and b).
Peridium 18-25 μm wide, composed of 2-5 layers of heavily pigmented cells of textura angularis to compressed, cells 6-11 μm diam., cell wall 1–3 μm thick (Fig. 47c, d and e). Hamathecium of dense, long filamentous pseudoparaphyses, 2.5-4 μm broad, branching, septate. Asci 65-85× 15-20 μm (x = 74 × 18μm, n = 10), 8-spored, bitunicate, fissitunicate, broadly clavate, with a short, thick, some-times furcate pedicel, up to 13 μm long, ocular chamber not observed (Fig. 47f, g, h and k). Ascospores 16-20×9-11 μm (x = 18 × 10μm, n = 10), biseriate, ellipsoidal, pale orange-brown, 1-distoseptate, with prominent swelling at the septum, containing refractive globules, smooth (Fig. 47i, j and l).
Anamorph: The following description is from Calatayud et al. (2001)).
Conidiomata pycnidial, arising in galls together with the ascomata, immersed, ca. 100-200 μm diam.; wall dark brown throughout, composed of 2-5 layers of angular to laterally compressed cells; cells relatively large, ca. 8-16 μm diam. in superficial view. Conidiophores formed by 1–3 cells, frequently branched and with the uppermost cells bearing 1-4 conidiogenous cells; cells±cylindrical, hyaline except at the base, which are sometimes pale brown, 7-15 × 3-4 μm. Conidiogenous cells tapered towards the apex, 14-18×3-4 μm. Conidia 5-7×1.5-2 μm. Vegetative hyphae hyaline.
Material examined: SPAIN, Andalucía, Province, Jaén, Andújar, lichenicolous on Leptochidium albociliatum (Desm.) M. Choisy on acid volcanic rock, 19 Apr. 2000, V. Calatayud (MA-Lichen 12715, holotype).
Notes
Morphology
Lichenopyrenis was formally established by Calatayud et al. (2001) based on its “perithecioid ascomata with peridium comprising compressed cells, fissitunicate and J-asci, wide hamathecium filaments, and 1-septate pale orange-brown ascospores with distoseptate thickenings at maturity”, and is monotypic with L. galligena. The genus was temporarily assigned to Pleomassariaceae. Lichenopyrenis galligena is a parasite of lichens, occurring in galls in the thallus of the host (Calatayud et al. 2001).
Phylogenetic study
None.
Concluding remarks
This is one of the few species that are parasitic on lichens. The most comparable species are Parapyrenis lichenicola Aptroot & Diederich and Lacrymospora parasitica Aptroot (both in Requienellaceae, Pyrenulales) as we11 as some species from Dacampiaceae. The peridium structure, cellular pseudoparaphyses, distoseptate and smooth, orange-brown ascospores as well as the anamorphic stage of Lichenopyrenis can easily distinguish from all of them (Calatayud et al. 2001).
Lineolata Kohlm. & Volkm.-Kohlm., Myco1. Res. 94: 687 (1990). (Pleosporales, genera incertae sedis)
Generic description
Habitat marine, saprobic (or perthophytic?). Ascomata medium-sized, gregarious, immersed to erumpent, obpyriform, ostiolate, papillate. Peridium thin, comprising two types of cells; outer cells thick stratum pseudostromatic, inner stratum thin, composed of a few layers of hyaline cells of textura angularis. Hamathecium of dense, long trabeculate pseudoparaphyses, embedded in mucilage, anastomosing and septate. Asci 8-spored, bitunicate, cylindrical, with short pedicels, with an ocular chamber. Ascospores uniseriate to partially overlapping, ellipsoidal, dark brown, 1-septate.
Anamorphs reported for genus: none.
Literature: Kohlmeyer and Kohlmeyer 1966; Kohlmeyer and Volkmann-Kohlmeyer 1990.
Type species
Lineolata rhizophorae (Kohlm. & E. Kohlm.) Kohlm. & Volkm.-Kohlm., Myco1. Res. 94: 688 (1990). (Fig. 48)
Fig. 48.
Lineolata rhizophorae (from Herb. J. Kolmeyer No. 2390b, isotype of Didymosphaeria rhizophorae). a Ascomata immersed in the host substrate with protruding papilla. b Ascospores within an ascus. Note the ascospore arrangement. c-f One-septate ascospores. Note the striate ornamentation in (c). Scale bars: a =100 μm, a, b=20 μm, c-f= 10 μm
≡ Didymosphaeria rhizophorae Kohlm. & E. Kohlm. Icones Fungorum Maris (Lehre) 4 & 5: tab. 62a (1967).
Ascomata 300-490 μm highx200-360 μm diam., gregarious, immersed to erumpent, obpyriform, ostiolate, papillate, subcarbonaceous to subcoriaceous, blackish brown (Fig. 48a). Peridium 37-45 μm thick, comprising two types of cells; outer cells thick stratum pseudostromatic, composed of irregular or roundish, dark brown cells, on the outside with a more or less recognizable hyphal structure, enclosing some decaying cells of the host, inner stratum thin, composed of four or five layers of hyaline, polygonal, elongate, thin-walled cells with large lumina, merging into the pseudoparaphyses. Hamathecium of dense, long trabeculate pseudoparaphyses, 1-1.5 μm broad, embedded in mucilage, anastomosing and septate. Asci 150-175×14-17.5 μm, 8-spored, bitunicate, cylindrical, with short pedicels, with an ocular chamber (Fig. 48b). Ascospores 23-32(−33)×9-12 μm, uniseriate to partially overlapping, ellipsoid, dark brown, 1-septate, not or slightly constricted at the septum, striate by delicate costae that run parallel or in a slight angle to the longitudinal axis of the ascospore (Fig. 48c, d, e and f) (adapted from Kohlmeyer and Kohlmeyer 1979).
Anamorph: none reported.
Material examined: US, Florida, Middle Torch Key, on Rhizophora mangle, 21 Nov. 1965, J. Kohlmeyer (Herb. J. Kohlmeyer No. 2390b, isotype); Pirate Grove Key, on R. mangle, 5 Jan. 1964 (Herb. J. Kohlmeyer No. 1721 paratype); Florida, Virginia Key, on R. mangle, 1 Jan. 1964, leg. E. Kohlmeyer (Herb. J. Kohlmeyer No. 1751 paratype); Florida, Torch Key, on R. mangle, 20 Nov. 1965, leg. J. Kohlmeyer (Herb. J. Kohlmeyer No. 2423 paratype).
Notes
Morphology
Lineolata was monotypified by L. rhizophorae, which was originally introduced by Kohlmeyer and Kohlmeyer (1966) as a species of Didymosphaeria (as D. rhizophorae). Based on the morphology of ascomata and asci, Barr (1990a) assigned it under Lojkania (as L. rhizophorae). Kohlmeyer and Volkmann-Kohlmeyer (1990) restudied this species and noticed that the absence of clypeus, almost superficial ascomata, coloured peridium, a hamathecium with gelatinous matrix, asci with apical ring-like structure and the ornamented ascospores are quite different from the modified concept of Didymosphaeria.
Thus they introduced Lineolata to accommodate D. rhizophorae (Kohlmeyer and Volkmann-Kohlmeyer 1990).
Phylogenetic study
Three isolates ofLineolata rhizophorae from varied geographic localities were analyzed by Suetrong et al. (2009) and shown to be related to Caryosporella rhizophorae in Dothideomycetidae and excluded from Pleosporomycetidae and Pleosporales.
Concluding remarks
Based on initial molecular work it is likely that this species does not belong to Pleosporales in spite of its dense pseudoparaphyses and other characters shared with the order.
Loculohypoxylon M.E. Barr, Mycotaxon 3: 326 (1976). (Teichosporaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata relatively small, gregarious, immersed to erumpent, globose or subglobose, forming under a clypeus, papillate, ostiolate. Peridium thin, a single layer comprising hyaline thin-walled cells of textura angularis or textura prismatica. Hamathecium of septate pseudoparaphyses. Asci (2-4-)8-spored, bitunicate, cylindrical to cylindro-clavate, with a short, furcate pedicel, and wide ocular chamber. Ascospores broadly elliptic to subglobose, often apiculate at both ends, pale to dark brown, aseptate, with a germ slit.
Anamorphs reported for genus: none.
Literature: von Arx and Muller 1975; Barr 1976.
Type species
Loculohypoxylon grandineum (Berk. & Rav.) Barr, Mycotaxon 3: 326 (1976). (Fig. 49)
Fig. 49.
Loculohypoxylon grandineum (from N.A.F. 494). a Appearance of ascomata on the host surface. b Habitat section of ascomata. c Section of an ascoma. Note the pale brown thin-walled peridium cells. d, e Uniseriate ascospores in asci. f-f Cylindro-clavate asci with ascospores. Note the ocular chamber in (g). Scale bars: a=100 μm, b=200 μm, c=50 μm, d-h=10 μm
≡ Diatrype grandinea Berk. & Rav., in Berkeley, Grevillea 4: 95 (1876).
Ascomata 85-130 μm high×75-145 μm diam., gregarious, immersed to widely erumpent, globose or subgtobose, under a reddish brown to black clypeus, papillate, ostiolate (Fig. 49a and b). Peridium 18-30 μm thick laterally, 1-layered, composed of hyaline thin-walled cells of textura angularis to prismatica, cells up to 5 × 9 μm diam., cell wall 0.5-1 μm thick, apex cells smaller and walls thicker (Fig. 49c). Hamathecium comprising 2–3 μm broad, septate pseudoparaphyses. Asci 70-90×10-12.5 μm (x = 76. 5 × 10. 9μm, n =10), (2-4-)8-spored, bitunicate, cylindrical to cylindro-clavate, with a short, furcate pedicel, up to 25 μm long, with a wide ocular chamber (Fig. 49f, g, and h). Ascospores 7.5-10×5-7 μm (x = 8. 3 × 5. 9μm, n =10), uniseriate to partially overlapping at the upper part, broadly elliptic to subglobose, often apiculate at both ends, pale to dark brown, aseptate, with a germ slit (Fig. 49d and e).
Anamorph: none reported.
Material examined: USA, New Jersey, Newfield, on bark of Quercus coccinea, Sept. 1878, as Diatrype grandinea, Ellis N.A.F. 494 (NY, MASS); on Quercus sp. wood, Nov. 1893, as Anthostoma grandinea B. & Rav., Ellis & Everhart, N.A.F. 494 (NY); Newfield, Oct. 1881, as Diatrype grandinea (NY); Newfield, Jan. 1882, on Quercus coccinea, as Diatrype grandinea B. & Rav, Ex Herb Ellis (NY); Newfield, Nov. 1893, as Anthostoma grandinea, on bark of fallen trunks of Quercus coccinea (NY).
Notes
Morphology
Loculohypoxylon grandineum is one of the rare pleosporalean species having aseptate ascospores. When emphasis is given to ascospore morphology, Semidelitschia (monotypified by S. agasmatica Cain & Luck-Allen) is the most comparable genus. The large ascomata and ascospores, the mucilaginous sheath surrounding the ascospores as well as the coprophilous habitat of S. agasmatica differ from L. grandineum greatly. Thus Loculohypoxylon was introduced as a new genus.
Phylogenetic study
None.
Concluding remarks
Aseptate ascospores are rare in Pleosporales, and the position of this fungus needs further verification. The familial status of Loculohypoxylon in Teichosporaceae is questionable, as it is simply based on the similarity ofliving habitat, ascomata and asci with Immotthia and Teichospora (Barr 2002).
Lophionema Sacc., Syll. fung. (Abellini) 2: 717 (1883). (Pleosporales, genera incertae sedis)
Generic description
Habitat terrestrial, saprobic? Ascomata solitary, scattered or in small groups, immersed to erumpent, globose to subglobose, with a flattened base, wall black, papillate, ostiolate. Peridium comprising two types of cells which merge in the middle. Hamathecium of trabeculate pseudoparaphyses, septate, rarely anastomosing and branching. Asci 8-spored, bitunicate, fissitunicate unknown, clavate to cylindro-clavate, with a short and furcate pedicel and a small inconspicuous ocular chamber. Ascospores filliform, hyaline to pale yellow, multi-septate, slightly constricted at each septum.
Anamorphs reported for genus: none.
Literature: Barr 1992b; Chesters and Bell 1970; Ellis and Everhart 1892; Hohnel 1909; Solheim 1949.
Type species
Lophionema vermisporum (Ellis) Sacc., Syll. fung. (Abellini) 2: 717 (1883). (Fig. 50)
Fig. 50.
Lophionema vermisporum (from NY-643, holotype). a Appearance of ascomata on the host surface. Note the form of the neck. b Section of the peridium. c Peridium comprising two types of cells which merge in the middle; outer cells small heavily pigmented thick-walled cells of textura angularis, inner cells less pigmented, and comprising thin-walled compressed cells. d, e Cylindro-clavate, 8-spored asci. f A 7-septate filliform ascospore. Scale bars: a=0.5 mm, b =100 μm, c=50 μm, d-f=10 μm
≡ Lophiostoma vermispora Ellis, Bull. Torrey bot. Club 9: 19 (1882).
Ascomata 320-430 μm highx280-350 μm diam., solitary, scattered or in small groups of 2–3, immersed to erumpent, globose to subglobose, black, papillate, ostiolate. Papilla 80-120 μm high, up to 150 μm broad, cylindrical to somewhat vertically flattened neck; mostly with a short slot-like ostiole, periphysate (Fig. 50a). Peridium 30-45 μm wide at the sides and slightly thicker at the apex, 2-layered, lateral walls and wall adjacent to neck comprising two types of cells which merge in the middle; outer cells small heavily pigmented thick-walled cells of textura angularis, cells 4-7 μm diam., cell wall 3.5-5 μm thick, inner cells less pigmented, comprising thin-walled compressed cells; apical wall cells smaller and walls thicker, basal wall thinner (ca. 15 μm wide), composed of lightly pigmented thin-walled compressed cells (Fig. 50b and c). Hamathecium of trabeculate pseudoparaphyses, 1–2 μm broad, septate, anastomosing and branching rarely between and mostly above the asci. Asci 105-130(−150)× 10-15 μm (x = 123 × 12μm, n =10), 8-spored, bitunicate, fissitunicate dehiscence not observed, clavate to cylindro-clavate, with a short, narrow, furcate pedicel which is 10-25 μm long, and a small inconspicuous ocular chamber (to 1.5 μm widex 1 μm high) (Fig. 50d and e). Ascospores (80-)90-115 × 3-5 μm (x = 95 × 3. 5μm, n =10), filliform, gradually tapering towards the base, hyaline to light yellow, (6-)7(−8)-septate, slightly constricted at each septum, smooth (Fig. 50f).
Anamorph: none reported.
Material examined: USA, New Jersey, Newfield, on dead stems of Oenothera biennis, Aug. 1881, Ellis (NY 643, holotype, NY 885, isotype).
Notes
Morphology
Lophionema is a relatively poorly studied genus, which was formally established by Saccardo (1883) as a monotypic genus represented by L. vermisporum based on its “globose ascomata, compressed ostiole, cylindrical to clavate ascus, and filamentous, septate, subhyaline to lightly pigmented ascospores”. Lophionema vermisporum was consequently listed as the generic type (Clements and Shear 1931). Berlese (1890) placed the genus in Lophiostomataceae but mentioned that the genus was similar to Ophiobolus according to the variable apex, and Shoemaker (1976) transferred Lophionema vermisporum to Ophiobolus sensu lato. Chesters and Bell (1970) however, had regarded Lophionema as related to Lophiostoma despite the distinct ascospore morphology. Barr (1992b) assigned Lophionema to Entodesmium based on the morphology of ascomata, papilla, peridium structure, pseudoparaphyses as well as the hyaline or slightly yellowish ascospores with a terminal appendage (not observed here). Species of Entodesmium, however, exclusively occur on legumes, but Lophionema vermisporum does not. We also note that the filliform ascospores, bitunicate asci, pseudoparaphyses and nature of the peridium may also be considered as typical of genera in the Tubeufiaceae (Barr 1980; Kodsueb et al. 2006b).
Phylogenetic study
None.
Concluding remarks
The immersed to erumpent ascomata, trabeculate pseudoparaphyses and laterally flattened papilla and periphysate ostioles indicate that this genus should be included in Lophiostomataceae. We do not accept the above proposals and, consider that Lophionema should be maintained as a separate genus with filliform ascospores in Lophiostomataceae until representative taxa can be sequenced and analyzed. Currently Lophionema comprises 10 species (http://www.mycobank.org, 08-01–2009). However, many of these are poorly studied and obscure.
Lophiostoma Ces. & De Not., Comm. Soc. crittog. Ital. 1: 219 (1863). (Lophiostomataceae)
Generic description
Habitat terrestrial, saprobic. Ascomata immersed to erumpent, usually with a distinct depressed papilla and a slot-like ostiole. Hamathecium of dense, long, septate pseudo-paraphyses, embedded in mucilage, anastomosing and branching between and above the asci. Peridium unequal in thickness, thicker near the apex and thinner at base. Asci usually clavate. Ascospores 1-septate, multi-septate or even muriform, hyaline to deep brown, usually with terminal appendages.
Anamorphs reported for genus: Pleuorphomopsis-like (Hyde et al. 2011).
Literature: Barr 1990a; Chesters and Bell 1970; Holm and Holm 1988; Hyde and Aptroot 1998; Hyde et al. 2002; Tanaka and Harada 2003b; Yuan and Zhao 1994.
Type species
Lophiostoma macrostomum (Tode) Ces. & De Not., Comm. Soc. crittog. Ital. 1: 219 (1863). (Fig. 51)
Fig. 51.
Lophiostoma macrostomum (a-h, j from UPS, leptotype; i b from IFRD 2005). a Appearance of ascomata on the host surface. Note the raised crest-like areas and full length germ slits. b Section of the peridium. c-e Cylindro-clavate asci with ascospores arranged in a 2-3-seriate manner. f Hamathecium comprising branching and septate pseudoparaphyses. g-j Released or unreleased ascospores. Note the smooth young ascospores with terminal sheath, and the verrucose senescent ascospores. Scale bars: a=0.5 mm, b=200 μm, c-j = 10 μm
≡ Sphaeria macrostoma Tode, Fung. mecklenb. sel. (Luneburg) 2: 12 (1791).
Ascomata 400-600 μm highx420-560 μm diam., densely scattered to gregarious, semi-immersed to erumpent, globose or subglobose, with a small to large flattened crest-like raised area above the ascomata which is variable in shape, up to 300 μm high and 480 μm wide, with a slit-like ostiole along the full length of the crest (Fig. 51a and b). Peridium 30-45 μm thick at the sides, thicker at the apex and thinner at the base, composed of one cell type of small lightly pigmented thin-walled cells of textura prismatica, cells ca. 6-9×3-4 μm diam., apex composed of pseudoparenchymatous cells (Fig. 51b). Hamathecium of dense, filliform, up to 3 μm near the base and less than 1.5 μm broad in the upper place, septate pseudoparaphyses, embedded in mucilage, anastomosing and branching between and above the asci (Fig. 51f). Asci 110-145×10-15 μm (x = 127.5 × 13μm, n =10), 8-spored, bitunicate, fissitunicate (ectotunica no constriction), cylindro-clavate, with a furcate pedicel and a small ocular chamber (to 1.5 μm widex 2 μm high) (J-) (Fig. 51c, d and e). Ascospores 27-38(−43)× 5-7.5 μm (x = 31.2 × 6.4μm, n = 10), biseriate, fusoid, curved, hyaline, usually 1-septate, with 3-5 septa and faintly brown when old, with (2-)3(−4) distinct oil drops in each cell and short terminal appendage at ends (Fig. 51h, i and j), and ornamented with warts when spores are senescent (Fig. 51g).
Anamorph: none reported.
Material examined: SWEDEN, Smaland, Femsjo par., Femsjo, on Prunus, 2006, Elias Fries, det. Geir Mathiassen (UPS, lectotype, as Sphaeria macrostoma Fr.). FRANCE, Ariege, Rimont, Las Muros, on dead stems of Vitis vinifera, 2 Sept. 1996 (IFRD2005).
Notes
Morphology
Lophiostoma is morphologically a well studied genus (Barr 1990a; Chesters and Bell 1970; Holm and Holm 1988; Mugambi and Huhndorf 2009b; Yuan and Zhao 1994), and currently it comprises about 30 species (Tanaka and Harada 2003b). The genus was characterized as having immersed to erumpent ascomata with a cylindrical or crest-like papilla and full length, slit-like ostiole; a peridium of unequal thickness, which was broader near the base (Lophiostoma-type); mostly clavate, bitunicate asci and 1- to several septate, hyaline to pigmented ascospores with terminal appendages or surrounded by a mucilaginous sheath (Holm and Holm 1988). This definition was followed by Barr (1990a), Yuan and Zhao (1994) and Hyde et al. (2002).
The crest-like papilla has been regarded as a prominent morphological character of Lophiostoma macrostomum (Chesters and Bell 1970; Holm and Holm 1988). In the lectotype specimen, the raised area above the ascomata is up to 300 μm high and 480 μm long, and seen as a flattened or even Y-shaped crest (Fig. 51a). In Lophiostoma curtum (Fr.) De Not. and Lophiotrema boreale Math. the raised area above the ascomata varies considerably in height or is even lacking (Holm and Holm 1988). Thus the variable “crest-like raised area in Lophiostomataceae” was explained as an evolutionarily adaptation to the hard substrate within which the ascomata develop (Holm and Holm 1988). The ascospores of L. macrostomum usually turn reddish brown when mature, and minutely verrucose ornamentation was also found on the surface of the pigmented ascospores. Hyaline ascospores that became pigmented with age are common in Lophiostoma, such as in L. appendiculatum Fuckel, L. massarioides (Sacc.) L. Holm & K. Holm, L. semiliberum, L. subcorticale Fuckel and L. winteri (Holm and Holm 1988; Tanaka and Harada 2003b). The phylogenetic significance of this character should be observed carefully in the future but at present its phylogenetic significance is unclear as this also occurs in some Lophiotrema species.
Phylogenetic study
Phylogenetic affinity with some Massarina species has been reported by Liew et al. (2002), and several Massarina species were transferred into Lophiostoma. In a systematic study of Lophiostoma- and Massarina-related fungi conducted by Zhang et al. (2009b), Lophiostoma taxa clustered into two groups; one includes the type species L. macrostomum with crest-like ostioles, L. rugulosum Yin. Zhang, J. Fourn. & K.D. Hyde with a wide, umbilicate pore surrounded by 4-6 radial ridges, and L. glabro-tunicatus with small ostiolar pores; the other cluster comprises Lophiostoma-like taxa with slot-like ostioles lacking raised crests, which includes L. arundinis (Pers.) Ces. & De Not., L. caulium, L. compressum (Pers.) Ces. & De Not., L. crenatum (Pers.) Fuckel, L. fuckelii (Sacc.) Sacc., L. macrostomoides, L. semiliberum and L. viridarium Cooke, which seems to represent a natural group at the family level. This conclusion is tentative until verified sequences of L. macrostomum are included in analyses (see comments of Zhang et al. 2009a).
Concluding remarks
We tend to accept a narrow concept of Lophiostomataceae, which only comprises species of Lophiostoma sensu stricto (Zhang et al. 2009a).
Lophiotrema Sacc., Michelia 1: 338 (1878). (Pleosporales, genera incertae sedis)
Generic description
Habitat terrestrial, saprobic. Ascomata small- to mediumsized, with or without short papilla. Hamathecium of dense, long, septate pseudoparaphyses, anastomosing and branching between and above asci. Asci cylindrical to cylindroclavate. Ascospores hyaline, 1–3-septate, usually with mucilaginous sheath.
Anamorphs reported for genus: none.
Literature: Barr 1990a; Chesters and Bell 1970; Holm and Holm 1988; Saccardo 1878a; Tanaka and Harada 2003c; Tang et al. 2003; Yuan and Zhao 1994.
Type species
Lophiotrema nucula (Fr.) Sacc., Michelia 1: 338 (1878). (Fig. 52) = Sphaeria nucula Fr., Syst. myco1. (Lundae) 2: 466 (1823). Ascomata 200-240 μm highx200-280 μm diam., scattered, erumpent to nearly superficial, with basal wall remaining immersed in host tissue, globose to subglobose, often laterally flattened, with a flattened base not easily removed from the substrate, black, roughened; with a cylindrical or slightly compressed papilla. Papilla to 120 μm long and 150 μm high, protruding, with a pore-like ostiole (Fig. 52a). Peridium 25-30 μm wide, very thin at the base, composed of heavily pigmented pseudoparenchymatous cells near the apex, cells 2-2×6 μm diam., wall 1–3(−4) μm thick, lower sides composed of pigmented cells of textura angularis, 3-5 μm diam., wall 0.8-1.5 μm thick, ostiole wall composed of heavily pigmented and thick-walled small cells (Fig. 52b and c). Hamathecium of dense, long, septate pseudoparaphyses, 1–2 μm broad, anastomosing and branching between and above asci, embedded in mucilage (Fig. 52i). Asci 90-115 × 9-11.5 μm (x = 99. 5 × 11. 5μm; n =10), 8-spored, bitunicate, fissitunicate, cylindrical, with a short, narrowed, furcate pedicel which is up to 10 μm long, with a small ocular chamber (ca. 1.5 μm widex1 μm high) (J-) (Fig. 52d, e, f and h). Ascospores 17-21(−25) × (4-)5-6.5 μm (x = 19. 5 × 5. 5μm, n =10), obliquely uniseriate and partially overlapping to biseriate, broadly fusoid to fusoid, with narrowly rounded ends, hyaline and lightly pigmented on very rare occasions when senescent, 1-septate, 3-septate when old, constricted at the median septum, the upper cell often broader than the lower one (Fig. 52g).
Fig. 52.
Lophiotrema nucula (from UPS, lectotype). a Ascomata on the host surface. b Section of a partial ascoma. c Peridium structure near the apex. d, h Cylindrical asci in the pseudoparaphyses. e, f Upper part of the asci, showing the small ocular chamber near the apex. h Mature ascospores. i Pseudoparaphyses. Scale bars: a= 0.5 mm, b = 100 μm, c, d=30 μm, e-i =10 μm
Anamorph: none reported.
Material examined: on decaying wood (UPS, lectotype as Sphaeria nucula Fr.).
Notes
Morphology
Holm and Holm (1988) provided a relatively strict definition for Lophiotrema after they examined several specimens including the type material which they lectotypified. Lophiotrema was mainly defined on the unique characters of small to medium ascomata, a “Lophiotrema-type” peridium and 1-septate ascospores. In Lophiotrema, Holm and Holm (1988) considered the ascomata to be small- to medium-sized, ca. pyriform but neck often reduced, even lacking and sometimes cylindrical. The peridium was of approximately equal thickness, 20-30 μm, composed of an outer textura angularis of uniformly pigmented cells, up to 12 μm, and an inner layer of very small hyaline cells, with somewhat thickened walls. Asci are cylindrical, spores hyaline, at first 1-septate, becoming 3-septate, with distinct guttules, often with a mucilaginous sheath. Much emphasis was given to the 1-septate ascospores by Holm and Holm (1988) when they described and distinguished the three Lophiotrema species: L. boreale, L. nucula, L. vagabundum (Sacc.) Sacc. and two other unnamed species. This concept was widely accepted by later workers (Kirk et al. 2001; Yuan and Zhao 1994). Tanaka and Harada (2003c) considered the peridium and asci to distinguish Lophiotrema from Lophiostoma, while Tang et al. (2003) introduced a new Lophiotrema species with elongated slit-like ostiole stating that the main difference between Lophiotrema and Lophiostoma were size of ascomata, structure of peridium, shape of asci and sheath of ascospores. This peridium concept however, is not supported by the lectotype specimen we examined here, which has a flattened thin-walled base. Thus the “Lophiotrema-like peridium” sensu Holm and Holm (1988) should not serve as a diagnostic character of Lophiotrema, while the ostiole, asci and ascospores might have some phylogenetic significance (Zhang et al. 2009b). No anamorph is yet known for Lophiotrema. Although the ascospores was reported by Holm and Holm (1988) to be verruculose this could not be observed in the lectotype examined under light microscope (1000 x) in the present study.
Phylogenetic study
In the phylogenetic study of Lophiostoma, Massarina and related genera (Zhang et al. 2009b), Lophiotrema nucula formed a consistent and robust clade with three other Lophiotrema species: L. lignicola Yin. Zhang, J. Fourn. & K.D. Hyde, L. brunneosporum Yin. Zhang, J. Fourn. & K.D. Hyde and L. vagabundum, separate from other members of Lophiostoma and Massarina sensu stricto. This clade might represent Lophiotrema sensu stricto, however, the correctness of strains of L. vagabundum (CBS 628.86) and L. nucula (CBS 627.86) used in the phylogenetic study are not verified and warrant further study.
Concluding remarks
Holm and Holm (1988) distinguished Lophiostoma from Lophiotrema based on the smaller ascomata, 1-septate versus multi-septate ascospores, and peridial wall structure. However, we doubt that these distinguishing characters (size of ascomata, number of septa of ascospores) can be confidently used to separate these genera and we could not establish any characters that could reliably distinguish between these two genera. The molecular data, however, does separate Lophiostoma macrostomum and Lophiotrema nucula into separate clades and provides some support that these are separate genera. Although the strain of L. nucula (CBS 627.86) was isolated by K. & L. Holm, who had examined the type specimen of L. nucula (Holm and Holm 1988), the culture of Lophiostoma macrostomum used in the analysis are unverified (see comment by Zhang et al. 2009b). For the purpose of this monograph we tentatively maintain Lophiotrema as distinct from Lophiostoma.
Macroventuria Aa, Persoonia 6: 359 (1971). (Didymellaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata small, solitary, scattered, or in groups, initially immersed, becoming erumpent, to nearly superficial, globose to subglobose, roughened with cylindrical setae erect from apex. Peridium thin, membranous. Hamathecium of cellular pseudoparaphyses, seems to easily disappear when mature. Asci bitunicate, somewhat obclavate to fusoid. Ascospores fusoid with broadly to narrowly rounded ends, hyaline, 1-septate, constricted at the septum.
Anamorphs reported for genus: none.
Literature: van der Aa 1971; von Arx and Muller 1975; Barr 1987a.
Type species
Macroventuria wentii Aa, Persoonia 6: 361 (1971). (Fig. 53)
Fig. 53.
Macroventuria wenti. a Ascomata. Note the setae. b Ascus and ascospores. Scale bars: a=50 μm, b=10 μm (figures referred to van der Aa 1971)
Ascomata 135-180 μm diam., rarely more than 200 μm diam., solitary, scattered or in groups, initially immersed, becoming erumpent, to nearly superficial, with basal wall remaining immersed in host tissue, globose to subglobose, broadly or narrowly conical, setae erect from the apical region of the ascomata, cylindrical or tapering to the rounded or pointed tip, brown, up to 90 μm long, 5-7.5 μm thick (Fig. 53a). Peridium, 25-35 μm thick, 2-layered, out layer composed of relatively thick-walled cells of textura angularis, cell wall up to 3 μm thick; inner layer cells with a thinner wall and subhyaline; near apex cells smaller (Fig. 53a). Hamathecium of cellular pseudoparaphyses, 1–2 μm thick, evanescing not sure. Asci 75-93×24-30 μm, 8-spored, without pedicel, bitunicate, somewhat obclavate to fusoid (Fig. 53b). Ascospores 22–32×8-14 μm, 1–3 seriate, fusoid with broadly to narrowly rounded ends, hyaline, 1-septate, constricted at the septum, smooth (Fig. 53b) (description adapted from van der Aa 1971).
Anamorph: none reported.
Material referred: USA, Nevada; Death Valley, plant litter, F.W. Went, 229, 1970 (CBS 526.71, holotype).
Notes
Morphology
Macroventuria was formally established by van der Aa (1971) represented by M. anomochaeta and M. wentii based on its “near-hyaline, 1-septate ascospores, setose ascomata, and saprobic life style”. Almost all of the above characters (except the saprobic life style) point this group of fungi to Venturiaceae. Thus Macroventuria was assigned to this family as a relatively primitive genus (van der Aa 1971). Subsequently, von Arx and Muller (1975) assigned Macroventuria to Pseudosphaeriaceae (Dothideales), and this proposal was followed by Barr (1987a).
Phylogenetic study
Phylogenetic analysis based on combined SSU rDNA and LSU rDNA sequences indicated that both of Macroventuria anomochaeta and M. wentii form a robust clade with Leptosphaerulina argentinensis (Speg.) J.H. Graham & Luttr., L. australis, L. trifolii (Rostr.) Petr. and Platychora ulmi, which appear to share phylogenetic affinities with the Leptosphaeriaceae and Phaeosphaeriaceae, but detached from other members of Venturiaceae and Pleosporaceae (Kodsueb et al. 2006a). In addition, culture characters also support the close relationship between Macroventuria and Leptosphaerulina (Barr 1987a). Analysis based on five genes, i.e. SSU, LSU, RPB1, RPB2 and TEF1, indicated Macroventuria anomochaeta resides in the well supported clade of Didymellaceae (Zhang et al. 2009a).
Concluding remarks
The morphological characters, such as small ascomata and hyaline, 1-septate ascospores all point at Didymellaceae, thus the familial status of Macroventuria is verified.
Mamillisphaeria K.D. Hyde, S.W. Wong & E.B.G. Jones, Nova Hedwigia 62: 514 (1996b). (?Melanommataceae)
Generic description
Habitat freshwater, saprobic. Ascomata superficial, scattered or gregarious, conical, carbonaceous, papillate. Hamathecium of dense, filliform, trabeculate pseudoparaphyses. Asci broadly clavate to clavate, with small ocular chambers and short pedicels. Ascospores of two types, (1): 2-4-seriate, ellipsoid, hyaline, slightly constricted at the main septum; with apical appendages at each end and around the ascospore; (2) 1–2-seriate, ellipsoid to fusoid, brown, with mucilaginous sheath around the ascospore (Hyde et al. 1996b).
Anamorphs reported for genus: none.
Literature: Hyde et al. 1996a, b.
Type species
Mamillisphaeria dimorphospora K.D. Hyde, S.W. Wong & E.B.G. Jones, Nova Hedwigia 62: 515 (1996b). (Fig. 54)
Fig. 54.
Mamillisphaeria dimorphospora (from HKU(M) 7425, paratype?). a Ascomata scattered on the host surface. Note the small papilla. b Section of an ascoma. c, d Asci (TYPE 1). e Trabeculate pseudoparaphyses in a gelatinous matrix. f-j Ascospores (TYPE 1). Scale bars: a=0.5 mm, b-d=100 μm, e =10 μm, f-j=20 μm
Following description is adapted from Hyde et al. 1996a, b).
Ascomata 455-650 μm high×980-1430 μm diam., scattered or in small groups, superficial, conical, carbonaceous, papillate, under pseudostroma which forms a thin layer on the host surface, up to 50 μm thick between the ascomata and 125-250 μm thick on the ascomata surface (Fig. 54a and b). Peridium 10-25 μm thick, comprising several layers of compressed, densely packed, thin-walled, hyaline cells. A wedge-shaped area of vertically orientated hyaline palisade-like cells occurs at the periphery (Fig. 54b). Hamathecium of dense, trabeculate pseudoparaphyses, ca. 1 μm broad, hyaline, branching and anastomosing, septate, embedded in mucilage (Fig. 54e). Two types of asci and ascospores exist in the same ascoma: TYPE 1: asci 185-320×40-100 μm (x = 210 × 78μm, n = 50), 8-spored, cylindro-clavate, bitunicate, fissitunicate, short-pedicellate, with an ocular chamber (to 13 μm widex5 μm high) (Fig. 54c and d). Ascospores 66-84× 20-38 μm (x = 78 × 25μm, n=50), 2-4-seriate, hyaline, ellipsoidal, constricted at the central septum, with pad-like mucilaginous appendage at each end and with some mucilage associated around the spore, and TYPE 2: asci 158-242×8-15 μm (x = 182 × 11μm, n=50), 8-spored, cylindrical, bitunicate, fissitunicate, pedicellate, with an ocular chamber and faint apical ring, ascospores 29-42× 6-9 μm (x = 35 × 7μm, n = 50), 1–2-seriate, brown, ellipsoidal-fusoid, surrounded by a thin mucilaginous sheath (Fig. 54f, g, h, i and j).
Anamorph: none reported.
Material examined: BRUNEI, on submerged wood, Aug. 1997, leg. K.D. Hyde (HKU(M) 7425).
Notes
Morphology
Mamillisphaeria was established as a monotypic genus according to its bitunicate, fissitunicate asci, trabeculate pseudoparaphyses and dimorphic ascospores, which is typified by the widely distributed freshwater fungus, M. dimorphospora (Hyde et al. 1996a, b). The most striking character of this fungus is its dimorphic ascospores, i.e. one type is large and hyaline, and the other is comparatively smaller and brown. Only a few ascomycetes have been reported having dimorphic ascospores, such as Aquasphaeria dimorphospora and Nectria heterospora Speg. (Hyde 1995b; Spegazzini 1889). Dimorphic ascospores appear to have evolutionary benefits, for example the large ascospores with mucilaginous sheaths may facilitate nutrient storage for germination and enhanced collision and attachment to substrates. The smaller brown ascospores may help resist desiccation and damage by UV light and contribute to aerial dispersal, which might explain the worldwide distribution of M. dimorphospora (Hyde et al. 1996a, b).
Phylogenetic study
None.
Concluding remarks
Although in the key by Barr (1990a), M. dimorphospora can be referred to Massariaceae, it is temporarily assigned to Melanommataceae here based on its trabeculate pseudoparaphyses embedded in mucilage (Hyde et al. 1996a, b).
Massarina Sacc., Syll. fung. (Abellini) 2: 153 (1883). emend. (Massarinaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata immersed or superficial, scattered or clustered, globose, conical globose to lenticular, papillate or epapillate, ostiolate. Hamathecium of dense, cellular pseudoparaphyses. Asci clavate to cylindrical, with short pedicels. Ascospores ellipsoid to fusoid, hyaline, 1- to 3-septate, with or without mucilaginous sheath.
Anamorphs reported for genus: Ceratophoma (Sivanesan 1984).
Literature: Aptroot 1998; Barr 1990a; Bose 1961; Eriksson and Yue 1986; Hyde 1995a; Hyde and Aptroot 1998; Liew et al. 2002; Saccardo 1883; Sivanesan 1984; Tanaka and Harada 2003d; Zhang et al. 2009a, b.
Type species
Massarina eburnea (Tul. & C. Tul.) Sacc., Syll. fung. (Abellini) 2: 153 (1883). (Fig. 55)
Fig. 55.
Massarina eburnea (from IFRD 2006). a Ascomata on the host surface. b Section of an ascoma. c Ascus with a short pedicel. d Cellular pseudoparaphyses. e Section of the peridium comprising a few layers of compressed cells. f Asci in pseudoparaphyses. g Three-septate ascospores. Scale bars: a=0.5 mm, b =100 μm, c-g=20 μm
≡ Massaria eburnea Tul. & C. Tul., Sel. Fung. Carp. 2: 239 (1863).
Ascomata to 250 μm highx500-700 μm diam., solitary or in small clusters, forming under raised dome-shaped areas, with blackened centres, with a central ostiole, immersed within the cortex of thin dead branches, ellipsoidal, rounded from above, clypeate, neck central, short and barely noticeable on host surface (Fig. 55a). Clypeus ca. 250 μm diam., 60 μm thick, brown, comprising compact brown-walled cells of textura angularis to globulosa beneath host epidermal cells (Fig. 55b). Peridium ca. 20 μm thick comprising 3-5 layers of hyaline compressed cells, fusing at the outside with the host (Fig. 55e). Hamathecium filamentous, cellular pseudoparaphyses, ca. 2 μm broad, septate, embedded in mucilage, without anastomosing (Fig. 55d). Asci 108-170×18-22 μm (x = 144.5 × 18.8μm, n =10), 8-spored, cylindro-clavate, pedunculate, bitunicate, fissitunicate, (1-)2-seriate, apically rounded, with an ocular chamber and faint ring (J-) (Fig. 55c and f). Ascospores 30-38×8-12 μm (x = 32.4 × 8.6μm, n= 10), fusoid to ellipsoid, 4-celled, constricted at the septa, hyaline, with acute rounded ends and surrounded by (5-8 μm diam.) mucilaginous sheath (Fig. 55g).
Anamorph: Ceratophoma sp. (Sivanesan 1984). Material examined: FRANCE, on twig of Fagus sp., (Desmazieres 1764. P, holotype of Sphaeria pupula var minor), (Mycotheca universalis no. 1951 lectotype). AUSTRIA, Silesia, Karlsbrunn, on dead twigs of Fagus sylvatica L., Aug. and Sept. 1890, Niessl., De Thümen, sub. Massarina eburnea, ETH. Saxonia, Konigsbrunn, on twigs of Fagus sylvatica, Apr. 1882, W. Krieger, Rabenhorst & Winter, Fungi europaei no. 2767, ETH; FRANCE, on a dead twig of Fagus sylvatica, Deux Sevres, Villiers en Bois, Foret de Chize, Rimbaud, 14 Apr. 2008, leg. det. Paul Leroy (IFRD 2006).
Notes
Morphology
Massarina was introduced by Saccardo (1883) for species of pyrenocarpous ascomycetes that had previously been placed in Massaria, but typically had hyaline ascospores (Bose 1961). The family Massarinaceae was described by Munk (1956) to accommodate Massarina. This family was not commonly used and Massarina was later placed within the Lophiostomataceae in the Pleosporales (Barr 1990a; Bose 1961; Eriksson and Yue 1986). Of the 160 epithets listed in his monograph, Aptroot accepted only 43 species (Aptroot 1998). The concept of Massarina was widely accepted as having single or aggregated, immersed to erumpent, spherical to hemispherical, pseudothecioid ascomata; cellular pseudoparaphyses; bitunicate, cylindrical to clavate or obpyriform asci; and hyaline, 1–3(−7)-septate, fusoid to long ellipsoid ascospores that mostly have a mucilaginous sheath or appendages (Aptroot 1998; Hyde and Aptroot 1998; Tanaka and Harada 2003d).
In the holotype of Sphaeria pupula var. minor (P) and lectotype of Massarina eburnea (ETH), ascospores are reported as “not constricted at the septa” (Hyde 1995a). However, in one of our recent collections, ascospores that are constricted at their septa were observed (Fig. 55g), which was consistent with the description by Fallah and Shearer (2001). This might be because this character is not clear in the old (over 100 years) and dry herbarium specimens, or it may be variable between collections.
Phylogenetic study
Recent morphological, molecular and anamorphic results indicate, however, that Massarina is polyphyletic (Hyde 1995a; Kirk et al. 2001; Liew et al. 2002). Based on the rDNA dataset, Massarina cisti and the type of Massarina (M. eburnea) forms a robust clade representing Massarina sensu stricto (Zhang et al. 2009a, b).
Concluding remarks
Massarina sensu stricto should be accepted, which seems to only include some terrestrial and saprobic species.
Massariosphaeria (E. MülL) Crivelli, Diss. Eidgenoss. Techn. Hochschule Zürich 7318: 141 (1983). (? Amniculicolaceae)
≡ Leptosphaeria subgen. Massariosphaeria E. Müll., Sydowia 4: 206 (1950).
Generic description
Habitat terrestrial or freshwater, saprobic. Ascomata medium-sized, scattered, or in small groups, immersed, erumpent to superficial, subglobose, black; apex with a wide and usually somewhat compressed papilla. Peridium thick or thin, usually thicker near the apex, composed of 2–3 layers of thick walled scleroparenchymatous cells. Hamathecium of dense, trabeculate pseudoparaphyses. Asci 8-spored, bitunicate, cylindrical to cylindro-clavate, with a short, thick, furcate pedicel. Ascospores fusoid to narrowly ellipsoid, brown or dark brown, multi-septate.
Anamorphs reported for genus: none.
Literature: Barr 1989c; Huhndorf et al. 1990; Kohlmeyer et al. 1996; Muller 1950; Tanaka and Harada 2004; Tanaka et al. 2005.
Type species
Massariosphaeria phaeospora (E. Mu11.) Crivelli, Ueber die Heterogene Ascomycetengattung Pleospora Rabh.; Vorschlag fur eine Aufteilung (Diss. Eid genossischen Tech Hochsch Zurich 7318): 141 (1983). (Fig. 56)
Fig. 56.
Massariosphaeria phaeospora (ZT, holotype). a Ascomata scattering on the host surface. Note the immersed to erumpent ascomata. b Section of a partial peridium. Note the peridium structure. c, d Released ascospores. Scale bars: a =1 mm, b-d=20 μm
≡ Leptosphaeria phaeospora E. MU11., Sydowia 4: 208 (1950).
Ascomata 400-550 μm highx300-500 μm diam., scattered, or in small groups, immersed, semi-immersed, subglobose, black, apex wide papilla, sometimes slightly compressed, 40-70(−100) μm broad (Fig. 56a). Peridium 10-20 μm wide at sides, comprising one cell type of 2–3 layers of thick walled scleroparenchymatous cells, cell wall 2-5 μm thick, peridium thicker near the apex (Fig. 56b). Hamathecium of dense, trabeculate pseudoparaphyses, 1–2 μm broad, septate, branching and anastomosing. Asci 120-173 × 18-25 μm (x = 133.2 × 20.5μm, n =10), 8-spored, bitunicate, fissitunicate dehiscence not observed, broadly cylindrical to cylindro-clavate, with a short, thick, furcate pedicel, up to 15 μ m long. Ascospores 3 2. 5 – 4 2 × 10-13 μ m (x = 36 × 11.2μm, n =10), narrowly ellipsoid, usually slightly curved, dark brown, 7-9 septa, slightly constricted at the median septum (Fig. 56c and d).
Anamorph: none reported.
Material examined: SWITZERLAND, Kt. Wallis, Findelen, Artemisiae campestris L., 10 Sept. 1895, H. Wegelin (ZT, holotype).
Notes
Morphology
Massariosphaeria was established by Müller (1950) as a section of Leptosphaeria based on its large, thick-walled ascospores with a mucilaginous sheath as well as its ascomata with a thick apex. Massariosphaeria was introduced as a separate genus by Crivelli (1983), characterized by its wide peridial apex comprising thick-walled cells, compressed to round papilla, and relatively large, thick-walled, reddish brown to brown, multi-septate to dictyosporous ascospores, usually surrounded by a sheath (Crivelli 1983; Huhndorf et al. 1990; Leuchtmann 1984). In particular, Crivelli (1983) emphasized that species of Massariosphaeria often stain the woody substrate (or culture) purple, and this was accepted by Leuchtmann (1984). Barr (1989c) had treated Massarios-phaeria as a synonym of Chaetomastia, but this viewpoint was rarely followed.
Phylogenetic study
The polyphyletic nature of Massariosphaeria is detected by analyzing SSU and LSU rDNA sequences (Wang et al. 2007). The purple staining character has shown phylogenetic significance in Amniculicolaceae, a freshwater family from France (Zhang et al. 2009a). A single isolate of M. phaeospora was shown to be unrelated to Amniculicolaceae and clustered with a single isolate of Thyridaria rubronotata (Schoch et al. 2009; Zhang et al. 2009a).
Concluding remarks
Based on phylogenetic analysis, staining the substrate purple may have more phylogenetic significance than morphological characters (Zhang et al. 2009a). Thus, the generic circumscription of Massariosphaeria should be re-evaluated by further phylogenetic study with more relevant taxa included.
Mauritiana Poonyth, K.D. Hyde, Aptroot & Peerally, Fungal Divers. 4: 102 (2000). (?Zopfiaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata medium-sized, gregarious, ovoid, immersed, ostiolate, ostiole rounded. Peridium thin, thicker near the apex. Hamathecium of dense, cellular pseudoparaphyses, branching. Asci 8-spored, bitunicate, cylindrical to cylindro-clavate, with a short pedicel and a small ocular chamber. Ascospores 2–3-seriate, fusoid with rounded ends, dark brown with paler apical cells, multi-septate, distoseptate, slightly constricted at the primary septum. Anamorphs reported for genus: none.
Literature: Hawksworth et al. 1995; Poonyth et al. 2000; Suetrong et al. 2009.
Type species
Mauritiana rhizophorae Poonyth, K.D. Hyde, Aptroot & Peerally, Fungal Divers. 4: 102 (2000). (Fig. 57)
Fig. 57.
Mauritiana rhizophorae (from HKU(M)10219, holotype). a Vertical section of an ascoma. Note the thin layer of fungal tissue (pseudostroma?) on the host surface. b Section of a partial peridium. c Pseudoparaphyses and immature ascus. d Fissitunicate asci. e Asci showing thickening of the apical wall. f-i Ascospores with transverse septa and paler polar cells. Scale bars: a=40 μm, b, d-i=10 μm, c=20 μm
Ascomata 390-410 μm highx310-325 μm diam., gregarious, ovoid, immersed, ostiolate, ostiole rounded (Fig. 57a). Peridium 40-60 μm thick laterally, thicker near the apex (Fig. 57a and b). Hamathecium of dense, long cellular pseudoparaphyses, 1.5-2 μm broad, branching. Asci 130-180×20-25 μm (x = 156 × 21.8μm, n=10), 8-spored, bitunicate, cylindrical to cylindro-clavate, with a short pedicel, with a small ocular chamber (Fig. 57c, d and e). Ascospores 29-40×9-13 μm (x = 35.4 × 11μm, n =10), 2–3-seriate, fusoid with rounded ends, dark brown with paler apical cells, 9-13-distoseptate, slightly constricted at the primary septum, smooth (Fig. 57f, g, h and i).
Anamorph: none reported.
Material examined: MAURITIUS, Grand Gaube, Melville mangrove, on dead decorticated Rhizophora mucronata Lam. wood still attached to living tree, Jan. 1995, A.D. Poonyth (HKU(M)10219, holotype).
Notes
Morphology
Mauritiana was introduced to accommodate the mangrove fungus, M. rhizophorae, which is characterized by immersed ostiolate, periphysate ascomata, thin peridium, bitunicate, 8-spored, cylindrical to cylindro-clavate asci, fusoid, smooth, hyaline to pale brown, multi-septate and distoseptate ascospores (Poonyth et al. 2000). But after carefully studying the type of M. rhizophorae, no typical distoseptate ascospores observed. The pigmented curved septum of the ascospore gives a “thickened” appearance. Based on its immersed ascomata, presence of cellular pseudoparaphyses, thick-walled, fissitunicate asci and brown, phragmosporous ascospores constricted at the primary septum, Mauritiana was assigned to the Pyrenulales sensu stricto (Melanommatales sensu lato, Dothideales sensu lato) (Hawksworth et al. 1995; Poonyth et al. 2000).
Phylogenetic study
Based on a multigene phylogenetic analysis, Mauritiana rhizophorae resided within a paraphyletic clade (Suetrong et al. 2009) sister to marine fungi Halotthia posidonia and Pontoporeia biturbinata. In this study, the dendrogram shows it to be closely related to the Sporormiaceae and Lophiostomataceae, which may indicate an uncircumscribed familial clade (Plate 1). Thus, its familial placement remains undetermined.
Concluding remarks
The “thickened” septa of ascospores of Mauritiana rhizophorae is quite unique in Pleosporales, which makes it easily distinguishable from other genera..
Melanomma Nitschke ex Fuckel, Jb. nassau. Ver. Naturk. 23-24: 159 (1870). (Melanommataceae)
Generic description
Habitat terrestrial, saprobic. Ascomata immersed, erumpent to nearly superficial, medium- to large-sized, globose to subglobose, coriaceous, gregarious, short papillate. Peridium pseudoparenchymatous cells outside with pale compressed cells inside. Asci cylindric to clavate with short pedicels. Hamathecium of dense, filamentous, branching, rarely anastomosing, septate pseudoparaphyses. Ascospores pale brown, reddish brown to olive-brown, ellipsoid to fusoid, 2 to multi-septate, constricted at the main septum.
Anamorphs reported for genus: Aposphaeria, Nigrolentilocus, Phoma-like and Pseudospiropes (Chesters 1938; Sivanesan 1984).
Literature: Barr 1990a; Chesters 1938; Fuckel 1870; Saccardo 1878; Zhang et al. 2008a.
Type species
Melanomma pulvis-pyrius (Pers.) Fuckel, Jb. nassau. Ver. Naturk. 23-24: 160 (1870). (Fig. 58)
Fig. 58.
Melanomma pulvis-pyrius (a-b, d-e, h-j from UPS, holotype; c, g, k, l from epitype). a Ascomata gregarious on the host surface. b Vertical section of an ascoma. c-f Asci with pedicels. g Dehiscent ascus. h-! Ascospores. Scale bars: a=0.5 mm, b=200 μm, C-1 =10 μm
≡ Sphaeria pulvis-pyrius Pers., Syn. meth. fung. (Gottingen) 1: 86 (1801).
Ascomata 215-471 μm highx260-440 μm diam., gregarious, substrate surface covered with a thin layer of brown psueodstroma, superficial, globose, subglobose, broadly or narrowly conical, often laterally flattened, black, roughened and irregular, often bearing remnants of wood fibers; apex short papillate, often somewhat puckered or sulcate (Fig. 58a). Peridium 70-90 μm wide, to 180 μm wide at the base, coriaceous, comprising two types of cells, outer cells small heavily pigmented thick-walled cells of textura angularis, apical cells smaller and walls thicker, individual cell walls to 6 μm thick, inner cells lightly pigmented to hyaline thin-walled cells of textura angularis, 5-8 μm diam., individual ce11 wall to 1.5-2 μm thick, in places with columns of textura prismatica, and larger, paler cells of textura prismatica towards the interior and at the base (Fig. 58b). Hamathecium of dense, filamentous, 1–2 (−2.5) μm broad, branching, rarely anastomosing, septate pseudoparaphyses. Asci 98-123 ×6.5-7.5(−9) μm (x = 109 × 7.5mm, n=10), 8-spored, bitunicate, fissitunicate, cylindrical to fusoid, with a short, furcate pedicel, to 25 μm long, with an ocular chamber (Fig. 58c, d, e, f and g). Ascospores 14-17.5(−19)×4.5-6.5 μm (x = 15.8 × 5.2mm, n =10), obliquely uniseriate and partially overlapping, broadly fusoid to fusoid with broadly rounded ends, straight or slightly curved, smooth, olive-brown, 4-celled, slightly constricted at the septa, the second cell from the top slightly wider than the others, no sheath (Fig. 58h, i, j, k and 1).
Anamorph: Aposphaeria agminalis Sacc. or Phoma agminalis Sacc. (Sivanesan 1984).
Colonies (of epitype) reaching 4 cm diam. after 20 days growth on PDA at 25°C, depressed to raised, cottony to woolly, with rhizoidal margin, grey, reverse darkened. Phoma-like anamorph has been reported by Chesters (1938) and Sivanesan (1984), but no anamorphic stage was observed in the cultures of IFRDCC 2044, CBS 109.77 and CBS 371.75 after culturing 3 months on PDA.
Material examined: ondecayingwood(UPS, Scler. suec. n. 120, holotype, as Sphaeria pulvis-pyrius Pers.); FRANCE, Ariege, Rimont, Saurine, on bark of Salix caprea, 10 Apr. 2008, Jacques Fournier (IFRD 2001, epitype).
Notes
Morphology
Melanomma, the familial type of Melanommataceae, was formally established by Fuckel (1870, p 159) based on its sma11, carbonaceous ascomata, having: “sporen meist 2–3 mal septirt, selten ohne Scheidewand, braun oder wasscrhe11.” (Chesters 1938; Fuckel 1870). Saccardo (1878, p. 344) emended this genus as “Spores ovate or oblong, multi-septate, coloured.” Subsequently, Saccardo (1883, p. 98) extended the description of Melanomma as “Perithecia gregarious, seldom scattered, somewhat superficial, sphaerical, papillate or blunt, carbonaceous, smooth or somewhat hairy. Asci elongate, for the most part accompanied by paraphyses, 8-spored. Spores oblong or somewhat spindle-shaped, two to many septate, olive or dark brown. Species of Sphaeria belong here for the most part.” Melanomma pulvis-pyrius was erected as the lectotype species (Barr 1990a; Chesters 1938). Barr (1990a) gave a detailed circumscription for Melanomma, under which Melanomma contains about 20 species (Kirk et al. 2001).
Melanomma pulvis-pyrius is characterized by its gregarious, superficial ascomata with short papillate, cylindrical asci with a short pedicel and fusoid, olive-brown, 3-septate ascospores (Chesters 1938; Zhang et al. 2008a). One of the diagnostic characters of Melanommataceae is the trabeculate pseudoparaphyses, although no typical trabeculate pseudoparaphyses could be found in the neotype (Scler. suec. n. 120, UPS) and epitype (IFRD 2001) of M. pulvis-pyrius (Zhang et al. 2008a).
Phylogenetic study
Phylogenetic analysis based on five genes (LSU, SSU, RPB 1, RPB2 and EF1) indicates that Melanomma pulvispyrius forms a robust clade with Byssosphaeria, Herpotrichia and Pleomassaria siparia (Pleomassariaceae) and likely represents a separate family (or families comprising Melanommataceae) (Zhang et al. 2008a; Mugambi and Huhndorf 2009b). A more recent phylogenetic analysis included a group of coelomycete species with stellate conidia, isolated from Fagales trees clustered in Melanommataceae (Tanaka et al. 2010; Plate 1).
Concluding remarks
The Melanomma concept based on ascospore morphology appears polyphyletic.
Metameris Theiss. & Syd., Annls myco1. 13: 342 (1915). (Phaeosphaeriaceae)
Generic description
Habitat terrestrial, saprobic or parasitic. Ascostromata erumpent through the host surface in linear rows parallel to the host fibers. Ascomata small, globose to subglobose, black, coriaceous. Peridium composed of large lightly pigmented cells of textura angularis. Hamathecium of rare, broad pseudoparaphyses, septate, constricted at the septa. Asci bitunicate, fissitunicate, broadly cylindrical to slightly obclavate, with a short, thick, knob-like pedicel. Ascospores hyaline, 1-(rarely 2-) septate. Anamorphs reported for genus: none.
Literature: von Arx and Muller 1975; Barr 1972; Clements and Shear 1931; Eriksson 2006; Lumbsch and Huhndorf 2007; Theissen and Sydow 1915.
Type species
Metameris japonica (Syd.) Syd., Annls myco1., 13(3-4): 342 (1915). (Fig. 59)
Fig. 59.
Metameris japonica (from S, F7166, type). a Ascostroma arrangement on the host surface. b Section of two ascomata from one ascostroma. c Immature asci within pseudoparaphyses. d, e Hyaline ascospores. Scale bars: a=0.5 mm. b =100 μm, c-e=20 μm
≡ Monographus japonicus Syd. Annls myco1. 10: 408 (1912).
Ascostromata erumpent through the host surface in linear rows parallel to the host fibers, 500-750 μm long and 140-200 μm wide, with three to ten ascomata arranged in a line (Fig. 59a). Ascomata 115-160 μm diam., semi-immersed in substrate to erumpent, globose, subglobose, black, coriaceous (Fig. 59b). Cells of ascostromata heavily pigmented and thick-walled, cells of peridium composed of large lightly pigmented cells of textura angularis, cells 5-15 μm diam., cell wall <1 μm thick, peridium thicker at the base, up to 50 μm (Fig. 59b). Hamathecium of rare, pseudoparaphyses 3-4 μm broad, septate, constricted at the septa, anastomosing or branching not observed. Asci (65-)80-90× 12-15 μm (x = 82.8 × 13.3mm, n=10), 8-spored, bitunicate, fissitunicate, broadly cylindrical to slightly obclavate, with a short, thick, knob-like pedicel, lacking an ocular chamber (Fig. 59c). Ascospores 25-30×5-6 μm (x = 27.4 × 5.6μm, n =10), biseriate, oblong, hyaline, 1–2-septate, the secondary septum exclusively occurring in the upper cells, slightly constricted at the primary septum which is slightly below the centre of the ascospore, the upper cells usually swollen near the main septum (Fig. 59d and e).
Anamorph: none reported.
Material examined: JAPAN, Province Mino. on Osmunda regalis L. var. japonica Milde., 10 May 1912, R. Hale (S, F7166, type, as Monographos japonicus Syd.).
Notes
Morphology
Metameris was formally established by Theissen and Sydow (1915) to accommodate Monographus japonicus Syd., which is characterized by the erumpent ascomata arranged in linear ascostromata, the presence of pseudoparaphyses and hyaline 2-septate ascospores. Clements and Shear (1931) assigned it to Dothideaceae (subfamily Dothideae), and von Arx and Muller (1975) assigned it to Pleosporaceae. Currently, it is considered as a member of Phaeosphaeriaceae (Pleosporales) (Eriksson 2006; Lumbsch and Huhndorf 2007). Scirrhodothis and Scirrhophragma are considered synonyms of Metameris (von Arx and Muller 1975), and all three closely related to Scirrhia (Barr 1972; Muller and von Arx 1962).
Phylogenetic study
None.
Concluding remarks
Its small-sized ascomata, broadly cylindrical to slightly obclavate asci with a short, thick, knob-like pedicel, as well as its monocotyledonous host preference point Metameris to the Phaeosphaeriaceae. But DNA comparisons are needed for confirmation.
Mixtura O.E. Erikss. & J.Z. Yue, Mycotaxon 38: 203 (1990). (Phaeosphaeriaceae)
Generic description
Habitat terrestrial, parasitic. Ascomata small-sized, scattered or clustered on the leaf spots, immersed, erumpent, minutely papillate, ostiolate. Papilla slightly raised. Peridium thin, comprising one cell type of lightly pigmented thin-walled cells of textura angularis. Hamathecium of dense, filliform, septate, cellular pseudoparaphyses, 4-6.3 μm broad, embedded in mucilage. Asci bitunicate, ovoid, with a very short stumpy pedicel. Ascospores fusoid to narrowly fusoid with broadly to narrowly rounded ends, curved, dark brown, multi-septate, distoseptate, with a germ pore at the lower end.
Anamorphs reported for genus: none.
Literature: Eriksson and Yue 1990.
Type species
Mixtura saginata (Syd.) O.E. Erikss. & J.Z. Yue, Mycotaxon 38: 203 (1990). (Fig. 60)
Fig. 60.
Mixtura saginata (from S reg. nr F8934, type). a, b Leaf spots in leaves of Chusquea serrulatae. Note the erumpent ascomata surrounded by white material in (b). c Section of an ascoma. Note the peridium structure which comprises cells of textura angularis. The arrangement of the asci and pseudoparaphyses can also be seen. d Immature asci in pseudoparaphyses. Note the stumpy pedicel and thickened apex with flattened ocular chamber. e, f Mature ascospores. Note the hyaline ends and distosepta. Scale bars: a=10 mm, b, c= 100 μm, d=50 μm, e-f=20 μm
≡ Leptosphaeria saginata Syd., Annls myco! 37: 376 (1939). Producing elongated yellow spots with brownish margins, leaf spots up to 45×3-5 mm, opposite side visible as a brownish spots (Fig. 60a). Ascomata 170-200 μm highx210-280 μm diam., scattered on the lower side of the leaf, immersed, erumpent, breaking through the epidermis, minutely papillate. Papilla central, slightly raised, ostiolate, ostiole surrounded by a white margin (Fig. 60b). Peridium 22–34 μm wide, thicker at the apex, thinner at the base, comprising one cell type of lightly pigmented thin-walled cells of textura angularis, cells up to 6× 8 μm diam., cell wall 0.5-1.2 μm thick, apex cells smaller and walls thicker (Fig. 60c). Hamathecium of dense, filliform, septate, cellular pseudoparaphyses, 4-6.3 μm broad, embedded in mucilage. Asci 80-128×41-53(−69) μm (x = 100.9 × 52.8μm, n =10), 8-spored, bitunicate, fissitunicate dehiscence not observed, sac-like, with a very short stumpy pedicel and a small ocular chamber (Fig. 60d). Ascospores 86-94(−106)×20.5-23.5 μm (x = 92.7 × 21.7μm, n=10), fasciculate, fusoid to narrowly fusoid, slightly curved, dark brown, 7-septate, distoseptate, with or without constriction at the primary septum, smooth-walled, with a germ pore at the lower end (Fig. 60e and f).
Anamorph: none reported.
Material examined: ECUADOR, Tungurahua, Hacienda San Antonio pr. Banos, Province, on the leaves of Chusqueae serrulatae Pilger, 9 Jan. 1938, H. Sydow. (S reg. nr F8934 type, F8935 isolectotype, as Leptosphaeria saginata).
Notes
Morphology
Mixtura was formally established by Eriksson and Yue (1990) as a monotypic genus represented by M. saginata based on its immersed and thin-walled ascomata, sparse, broad pseudoparaphyses, sac-like asci with a short pedicel and thick apex. Mixtura has a “mixture” of characters found in other pleosporalean genera. The peridium structure is comparable with Phaeosphaeria, the ascospores with Trematosphaeria and asci with Wettsteinina (Eriksson and Yue 1990). According to the structure of ascomata and hamathecium, Mixtura was provisionally assigned to Phaeosphaeriaceae (Eriksson and Yue 1990).
Phylogenetic study
None.
Concluding remarks
Morphologically, the sparse broad pseudoparaphyses and sac-like asci with a thick apical structure in Mixtura seem more comparable with the generic type of Teratosphaeria (T fibrillose Syd. & P. Syd., Teratosphaeriaceae, Capnodiales, Dothideomycetidae) than that of Phaeosphaeria (P. oryzae). The heavily pigmented, multi-septate ascospores and the persistent pseudoparaphyses of Mixtura however, differ from those of Teratosphaeria. Thus, here we assign Mixtura under Teratosphaeriaceae as a distinct genus until phylogenetic work is carried out.
Montagnula Berl., Icon. fung. (Abellini) 2: 68 (1896). (Montagnulaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata small- to mediumsized, immersed to erumpent, gregarious or grouped, globose to subglobose, black. Hamathecium of dense, narrowly cellular, septate pseudoparaphyses. Asci bitunicate, fissitunicate, usually cylindro-clavate to clavate with a long pedicel. Ascospores oblong to narrowly oblong, straight or somewhat curved, reddish brown to dark yellowish brown, muriform or phragmosporous. Anamorphs reported for genus: Aschersonia (Hyde et al. 2011).
Literature: Aptroot 1995; Barr 2001; Berlese 1896; Clements and Shear 1931; Crivelli 1983; Leuchtmann 1984; Ramaley and Barr 1995; Schoch et al. 2006; Wehmeyer 1957, 1961; Zhang et al. 2009a.
Type species
Montagnula infernalis (Niessl) Berl., Icon. fung. (Abellini). 2: 68 (1896). (Fig. 61)
Fig. 61.
Montagnula infernalis (from M 1183, holotype). a Appearance of ascomata immersed in host tissue. b Section of an immersed ascoma. Note the hyaline closely adhering cells in the ostiole region. C Section of the peridium comprising a few layers of cells. d An immature ascus with a long pedicel. e, g Mature muriform ascospores in asci. f Cellular pseudoparaphyses. Scale bars: a=0.5 mm, b, C =100 μm, d-g=20 μm
≡ Leptosphaeria infernalis Niessl, Inst. Coimbra 31: 13 (1883).
Ascomata 220-280 μm highx250-310 μm diam., immersed to erumpent, gregarious or clustered, globose to subglobose, sometimes triangular in dried material, short ostiole always filled with hyaline closely adhering cells, black (Fig. 61a and b). Peridium 40-55 μm thick at sides, up to 80 μm thick near the apex, 3-layered, outer layer composed of heavily pigmented thick-walled small cells of textura angularis, cells 3-8 μm diam., wall 1.5-3 μm thick, apex thicker with smaller cells and thicker cell wall, thinner near the base; mid layer less pigmented, cells 4-13 μm diam.; innermost layer of narrow compressed rows of cells, merging with pseudoparaphyses (Fig. 61c). Hamathecium of dense, narrow cellular pseudoparaphyses, 2-4.5 μm broad, septate (Fig. 61f). Asci 153-170(−200)× 17.5-21.5 μm (including pedicel), bitunicate, fissitunicate, cylindro-clavate to clavate, pedicel 28-60(−85) μm long, 8-spored, biseriate, with an ocular chamber best seen in immature ascus (to 3 μm widex3 μm high) (Fig. 61d and e). Ascospores 24-29×9-11 μm, oblong to narrowly oblong, straight or somewhat curved, reddish brown to dark yellowish brown, verruculose, with five transverse septa and one vertical septum in each middle cells, constricted at the primary and secondary primary septa (Fig. 61g).
Anamorph: none reported.
Material examined: PORTUGAL, Coimbra Lusitania, on leaves of Fourcroya longava pr., Feb., 1881, leg. Moller. (M 1183, holotype).
Notes
Morphology
Montagnula was introduced to accommodate two Pleospora species, i.e. P. infernalis (Niessl) Wehm. and P gigantea Mont. by Berlese (1896), based on the presence of hyphal stromatic tissues over the ascomata and asci with relatively long pedicels (Barr 2001). Montagnula infernalis was selected as the lectotype species (Clements and Shear 1931). Subsequently, Wehmeyer (1957, 1961) treated Montagnula as a subgenus of Pleospora. Crivelli (1983) accepted Montagnula as a separate genus, and divided it into two subgenera, i.e. Montagnula and Rubiginospora. Montagnula was characterized by having dark brown ascospores and exclusively occurring on Agavaceae, while Rubiginospora has reddish brown ascospores and occurs on Poaceae. This proposal was not accepted by many workers (Barr 2001). Subsequently, more species with various ascospores (such as phragmosporous species by Leuchtmann (1984) and didymosporous species by Aptroot (1995) were added in this genus), which has obviously become heterogenic. Barr (2001) assigned species of Montagnula into different genera, i.e. Kalmusia and Didymosphaerella, respectively and introduced Montagnulaceae to accommodate ah of these genera.
Phylogenetic study
Montagnula opulenta forms a robust phylogenetic clade with species of Bimuria, Curreya, Didymocrea, Letendraea, Paraphaeosphaeria, Phaeodothis and Karstenula, which might represent a familial group (Schoch et al. 2006; Zhang et al. 2009a). A more convincing conclusion can only be obtained following sequence data from more verified fungi being added to the phylogenetic tree.
Concluding remarks
One striking character of Montagnula infernalis is the very long ascal pedicel once it is released from the ascomata.
However, this character appears to have evolved more than once and can be found in Kirschsteiniothelia elaterascus Shearer which clusters with Helicascus (Shearer et al. 2009). The same ascus character is also found in Xenolophium and Ostropella in the Platystomaceae (Mugambi and Huhndorf 2009b). Montagnula opulenta is a didymosporous species, but phylogenetically closely related to those dictyosporous (Karstenula rhodostoma) and phragmosporous (Paraphaeosphaeria michotii) members of Montagnulaceae (Zhang et al. 2009a). This might indicate that compared to other morphological characters, ascospore type is not a valid character at family level classification.
Moristroma A.L Romero & Samuels, Sydowia 43: 246 (1991). (Pleosporales, genera incertae sedis)
Generic description
Habitat terrestrial, saprobic. Ascomata medium-sized, solitary, scattered, or in small groups, superficial, cushion-like, circular in outline, wall black, roughened, containing numerous locules. Peridium thin, 1-layered. Hamathecium of dense, long filliform pseudoparaphyses, 2–3 μm broad, septate, branching. Asci polysporous, with a short, laterally displaced, sometimes papillate knob-shaped pedicel, apex very thick walled, bitunicate, fissitunicate, obclavate, ocular chamber not observed. Polyspores oblong to cylindrical, hyaline, non-septate.
Anamorphs reported for genus: none.
Literature: Eriksson 2006; Romero and Samuels 1991.
Type species
Moristromapolysporum A.L Romero & Samuels, Sydowia 43: 246 (1991). (Fig. 62)
Fig. 62.
Moristroma polyspo rum (from BAFC 32036, hototype). a Two multiculate ascostroma on the host surface. b Section of an ascostroma. Note the multilocula. c Section of the peridium. Note the thick walled cells. d, e Broadly cylin drical to fusoid asci containing numerous part spores. f Released part spores. Scale bars: a=0.5 mm, b=200 μm, c= 50 μm, d-f=10 μm
Ascomata 100-210 μm highx340-600 μm diam., solitary, scattered, or in small groups of 2–3, superficial, with basal wall remaining immersed in host tissue, cushion-like, circular in outline, wall black, roughened, containing numerous locules, each locule 120-240 μm diam., ostiolate (Fig. 62a and b). Peridium 14-30 μm thick, 1-layered, composed of small heavily pigmented thick-walled cells of textura angularis, cells 2-4 μm diam., cell wall 1.5-3 μm thick, peridium between the locules hyaline (Fig. 62b and c). Hamathecium of dense, long filliform pseudoparaphyses, 2–3 μm broad, septate, branching. Asci 44-60×12-14 μm (x = 54.3 × 13μm, n=10), polysporous, with a short, papillate knob-shaped pedicel, apex very thick-walled, bitunicate, fissitunicate, obclavate, ocular chamber not observed (Fig. 62d and e). Polyspores 3-4(−5)×0.6-1.2 μm, oblong to cylindrical, hyaline, non-septate, smooth (Fig. 62f).
Anamorph: none reported.
Material examined: ARGENTINA, Buenos Aires, RamaUo, on Eucalyptus viminalis Labill., May 1982, Romero 27/4-13 (BAFC 32036, holotype); Nov. 1982, on decorticated wood, Romero 35/4-13 (BAFC 32037, paratype).
Notes
Morphology
Moristroma was formally established by Romero and Samuels (1991) based on its “cushion-shaped ascomata containing lots of locules with numerous asci inside, asci obclavate, polysporous, with a knob-shaped pedicel”. The bitunicate asci and numerous cellular pseudoparaphyses undoubtedly point it to Pleosporales, while the familial placement of Moristroma is uncertain, and it was temporarily assigned to Dacampiaceae by Romero and Samuels (1991), but no 3-layered peridium is found. Eriksson (2006) assigned it to Teichosporaceae.
Phylogenetic study
None.
Concluding remarks
The familial status of Moristroma cannot be determined yet.
Morosphaeria Suetrong, Sakay., E.B.G. Jones & C.L. Schoch, Stud. MycoL 64: 161 (2009). (Morosphaeriaceae)
Generic description
Habitat marine, saprobic. Ascomata large, solitary or gregarious, immersed to erumpent, subglobose or depressed with a flatted base, ostiolate, papillate, brown to black, coriaceous. Peridium thick. Hamathecium of dense, long cellular pseudoparaphyses, septate. Asci 8-spored, bitunicate, cylindrical, with short pedicels. Ascospores uniseriate to partially overlapping, ellipsoidal, hyaline, 1-3-septate, constricted at the septa, central cells larger, apical cells if present small and elongated, surrounded with mucilaginous sheath.
Anamorphs reported for genus: none.
Literature: Hyde and Borse 1986; Hyde 1991a, b; Suetrong et al. 2009; Zhang et al. 2009a.
Type species
Morosphaeria velataspora (K.D. Hyde & Borse) Suetrong, Sakay., E.B.G. Jones & C.L. Schoch, Stud. MycoL 64: 161 (2009). (Fig. 63)
Fig. 63.
Morosphaeria velataspora (from IMI 297770, type). a Section of an ascoma. b Cylindrical asci embedded in pseudoparaphyses. c-e Hyaline, 1-3-septate, ascospores with mucilaginous sheath. Scale bars: a =100 μm, b=50 μm, c-e=20 μm
≡ Massarina velataspora K.D. Hyde & Borse, Mycotaxon 27: 163 (1986).
Ascomata 0.7-1.2 mm diam., solitary or gregarious, immersed to erumpent, subglobose or depressed, with a flattened base not easily removed from the substrate, ostiolate, epapillate or papillate, brown to black, coriaceous (Fig. 63a). Peridium thick, the upper part of the peridium composed of brown thick-walled cells of textura angularis, cells are smaller and wall thicker near the apex, at the rim is composed of vertical, parallel, brown, elongate cells, wedge-shape in section (Fig. 63a). Hamathecium of dense, long cellular pseudoparaphyses, 1.1-1.7 μm broad, septate. Asci 220-320×23-34 μm (x = 251 × 28.2μm, n =10), 8-spored, bitunicate, cylindrical, with short pedicels (Fig. 63b). Ascospores 45-56×14-19 μm (x = 49.5 × 15.9μm, n=10), uniseriate to partially overlapping, ellipsoidal, hyaline, 1-3-septate, constricted at the septa, central cells larger, apical cells if present small and elongated, surrounded with mucilaginous sheath, 5-22 μm wide (Fig. 63c, d and e).
Anamorph: none reported.
Material examined: Jan. 1984, Herb. IMI 297770, slides 1-10 (holotype) and dried wood (isotype).
Notes
Morphology
Two Massarina sensu lato species described from the marine environment, viz. M. ramunculicola (Sacc.) O.E. Erikss. & J.Z. Yue and M. velataspora K.D. Hyde & Borse, form a robust clade, and a new genus Morosphaeria was established for them (Suetrong et al. 2009). Together with two Helicascus species, they belong to Morosphaeriaceae (another marine family) (Suetrong et al. 2009). Morphologically, Morosphaeria is characterized by solitary to gregarious, subglobose to lenticular, immersed to superficial ascomata which are ostiolate and papillate, numerous, filliform pseudoparaphyses, 8-spored, clavate to cylindrical, bitunicate, fissitunicate asci, and hyaline, 1-3-septate, fusoid to ellipsoidal ascospores which are surrounded with mucilaginous sheath.
Phylogenetic study
Species of Morosphaeria form a sister group with Helicascus and both of these genera were assigned to a new family, i.e. Morosphaeriaceae (Suetrong et al. 2009). In this study, a strain of Asteromassaria pulchra, occuring on dead twigs of Prunus spinosa, is basal to other species of Morosphaeriaceae, and gets well support. Thus here we tentatively assign Asteromassaria in Morosphaeriaceae.
Concluding remarks
The only morphological difference between M. velataspora and M. ramunculicola are their morphology of ascomata and size of ascospores (Hyde 1991b). But M. velataspora was reported staining the woody substrate (or agar in culture) purple (Hyde and Borse 1986; Hyde 1991b). Although this character could not be verified in the strain used by Suetrong et al. (2009), purple staining has been reported to have phylogenetic significance at familial rank in freshwater fungi (Zhang et al. 2009a).
Murispora Yin. Zhang, C.L. Schoch, J. Fourn., Crous & K. D. Hyde, Stud. MycoL 64: 95 (2009b). (Amniculicolaceae)
Generic description
Habitat freshwater, saprobic. Ascomata medium-sized, scattered to gregarious, immersed, lenticular, apex slightly protruding, opening through a small rounded pore, substrate stained purple. Peridium thin, composed of a few layers cells of textura angularis, thicker at the apex with pseudoparenchymatous cells. Hamathecium of narrowly cellular pseudoparaphyses, embedded in mucilage. Asci 8-spored, bitunicate, fissitunicate, biseriate, cylindroclavate with short pedicels. Ascospores curvedfusoid with narrowly rounded ends, golden yellow turning brown when senescent, multi-septate, constricted at the septa, with one, rarely two longitudinal septa in all cells except end cells, smooth or finely verruculose, surrounded by a wide mucilaginous sheath.
Anamorphs reported for genus: Phoma (Webster 1957). Literature: Zhang et al. 2009a, b.
Type species
Murispora rubicunda (Niessl) Yin. Zhang, J. Fourn. & K. D. Hyde, Stud. MycoL 64: 96 (2009a). (Fig. 64)
Fig. 64.
Murispora rubicunda (from IFRD 2017). a Habitat section of the immersed ascomata. b Section of an ascoma. Note the thin peridium and cells of textura angularis. c Mature and immature asci. d Muriform ascospores. Scale bars: a, b= 100 μm, c, d=20 μm
≡ Pleospora rubicunda Niessl, Notiz. Pyr.: 31 (1876).
Ascomata 170-200 μm highx380-410 μm diam., scattered to gregarious, immersed, lenticular, apex laterally flattened, black, slightly protruding, opening through a small rounded pore, substrate stained purple (Fig. 64a). Peridium 15-18 μm thick at sides, composed of 3-4 layers cells of textura angularis, up to 28-30 μm thick at the apex with very thick-walled cells, pseudoparenchymatous, nearly absent at the base (Fig. 64b). Hamathecium of narrowly cellular pseudoparaphyses, 1-1.7 μm broad, embedded in mucilage. Asci 124-142×19-21 μm, 8-spored, bitunicate, fissitunicate, biseriate, cylindro-clavate with a small ocular chamber, with short pedicels (Fig. 64c). Ascospores 30-38×10-12 μm, curved-fusoid with narrowly rounded ends, golden yellow turning brown when senescent, 7-9 transversally septate, constricted at the septa, with one, rarely two longitudinal septa in all cells except end cells which are often slightly paler, all cells filled with a large refractive guttule, smooth to finely verruculose, surrounded by a wide mucilaginous sheath (Fig. 64d).
Anamorph: Phoma sp. (Webster 1957).
Material examined: FRANCE, Haute Garonne, Avignonet, Lac de Rosel, 16 Jan. 2007, on submerged dead herbaceous stem (Dipsacus?), leg. Michel Delpont, det. Jacques Fournier (IFRD 2017).
Notes
Morphology
Murispora was introduced based on Pleospora rubicunda which is characterized by immersed, erumpent or nearly superficial, globose to subglobose, elongated weakly papillate ascomata which stain the woody substrate purple, trabeculate pseudoparaphyses, 8-spored, bitunicate, fissitunicate, oblong to clavate asci, fusoid, pale or reddish brown, muriform ascospores (Zhang et al. 2009a). A phylogenetic study indicated that Murispora forms a robust clade with species of Amniculicola, and Amniculicolaceae was introduced to accommodate them (Zhang et al. 2009a).
Phylogenetic study
Murispora rubicunda forms a robust clade with species of Amniculicola and Neophaeosphaeria (Zhang et al. 2009a).
Concluding remarks
As has mentioned by Eriksson (1981, P. 135), the purplestaining species of Pleospora, treated by Webster (1957), should not belong to the Pleosporaceae. Both Pleospora straminis and P. rubelloides should be closely related to Murispora.
Neomassariosphaeria Yin. Zhang, J. Fourn. & K.D. Hyde, Stud. MycoL 64: 96 (2009a). (Amniculicolaceae)
Generic description
Habitat freshwater, saprobic. Ascomata medium-sized, scattered or in small groups, immersed, with a slightly protruding elongated papilla, ostiolate, lenticular, stain the substrate purple. Peridium thin. Hamathecium of dense, long cellular pseudoparaphyses, septate. Asci 8-spored, bitunicate, fissitunicate, cylindro-clavate, with short furcate pedicels. Ascospores 2–3-seriate, narrowly fusoid, somewhat curved, reddish brown, multi-septate, slightly constricted at the primary septum.
Anamorphs reported for genus: none.
Literature: Leuchtmann 1984; Zhang et al. 2009a, b.
Type species
Neomassariosphaeria typhicola (P. Karst.) Yin. Zhang, J. Fourn. & K.D. Hyde, Stud. MycoL 64: 96 (2009a). (Fig. 65)
Fig. 65.
Neomassariosphaeria typhicola (from IFRD 2018). a Immersed ascomata gregarious in the host substrate. b-d Cylindro-clavate asci embedded in pseudoparaphyses. Note the phragmosporous ascospores. Scale bars: a=200 μm, b-d=20 μm
≡ Leptosphaeria typhicola P. Karst., Bidr. Kann. Finl. Nat. Folk 23: 100 (1873).
Ascomata 150-280 μm highx200-400 μm diam., scattered or in small groups, immersed, lenticular, with a slightly protruding elongated papilla, ostiolate, stain the substrate purple (Fig. 65a). Peridium 15-30 μm thick. Hamathecium of dense, long cellular pseudoparaphyses, 1.5-2.5 μm thick, septate. Asci 110-160×13-15 μm, 8-spored, bitunicate, fissitunicate, cylindro-clavate, with short furcate pedicels (Fig. 65b, c and d). Ascospores 30-48 × 7-11 μm, 2–3-seriate, narrowly fusoid, somewhat curved, reddish brown, 7-septate, slightly constricted at the primary septum, verruculose (Fig. 65c and d).
Anamorph: none reported.
Material examined: DENMARK, Sjaeland, Frederik-skilde, Suserup Skove, Tystrup Lake, 25 May 2007, on submerged culm of Phragmites, leg. & det. Jacques Fournier (IFRD 2018).
Notes
Morphology
Neomassariosphaeria is most comparable with Murispora, and is distinguished from Murispora by its phragmosporous ascospores. Both genera were assigned to Amniculicolaceae (Zhang et al. 2009a).
Phylogenetic study
Both Neomassariosphaeria grandispora and N. typhicola clustered with species of Murispora and Amniculicola in Amniculicolaceae (Zhang et al. 2009a,c).
Concluding remarks
Similar with those purple-staining species of Pleospora assigned to Murispora, the purple-staining species of Phaeosphaeria mentioned by Crivelli (1983) and Leuchtmann (1984) might be assigned to Neomassariosphaeria.
Neophaeosphaeria M.P.S. Camara, M.E. Palm & A.W. Ramaley, Mycol. Res. 107: 519 (2003). (Leptosphaeriaceae)
Generic description
Habitat terrestrial, parasitic or saprobic. Ascomata small, forming in leaf spots, scattered or clustered, immersed, depressed globose, under clypeus, coriaceous. Peridium thin. Hamathecium of dense, cellular pseudoparaphyses, septate, embedded in mucilage. Asci 8-spored, bitunicate, fissitunicate dehiscence not observed, broadly cylindrical to oblong, with a short furcate pedicel. Ascospores obliquely uniseriate and partially overlapping, oblong, pale brown, 1-3-septate. Anamorphs reported for genus: Coniothyrium-like (Camara et al. 2003).
Literature: Camara et al. 2001, 2003; Checa et al. 2002; Ellis and Everhart 1892.
Type species
Neophaeosphaeria filamentosa (Ellis & Everh.) M.P.S. Camara, M.E. Palm & A.W. Ramaley, MycoL Res. 107: 519 (2003). (Fig. 66)
Fig. 66.
Neophaeosphaeria filamentosa (from NY, holotype). a Ascomata as a circular cluster on the host surface. b Hamathecium of wide psuedoparaphyses. C Section of peridium comprising cells of textura angularis. d-f Cylindrical asci with thickened apex. Note the short furcate pedicel. g Pale brown, 3-septate ascospores. Note the verruculose ornamentation. Scale bars: a=200 μm, b, c = 20 μm, d-g=10 μm
≡ Leptosphaeria filamentosa Ellis & Everh., J. MycoL 4: 64 (1888).
Ascomata 115-157 μm highx 115-186 μm diam., forming in leaf spots, scattered or clustered in circular areas, immersed, depressed globose, with a small ostiolar pore slightly penetrating above the surface, under clypeus, coriaceous, papilla not conspicuous (Fig. 66a). Peridium 18-30 μm thick, composed of large pigmented thin-walled cells of textura angularis, cells up to 10 μm diam. (Fig. 66c). Hamathecium of dense, cellular pseudoparaphyses 1.5-2.5 μm broad, septate, embedded in mucilage (Fig. 66b). Asci 70-105 × 8-10 μm (x = 85.3 × 9.7μm, n=10), 8-spored, bitunicate, fissitunicate dehiscence not observed, broadly cylindrical to oblong, with a short, broad, furcate pedicel, 6-13 μm long, with a small ocular chamber, best seen in immature asci, up to 1.5 μm widex 1 μm high (Fig. 66d, e and f). Ascospores 12-15×4-5 μm (x = 13.8 × 5μm, n=10), obliquely uniseriate and partially overlapping, oblong, yellowish brown, (1–2-)3-septate, constricted at the primary septum, the upper second cell often broader than others, verruculose, containing four refractive globules (Fig. 66g).
Anamorph: Ellis and Everhart (1892) noted that the “spermogonial stage is a Coniothyrium (C. concentricum) with small (4 μm), globose, brown sporidia.”
Material examined: USA, New Jersey, Newfield, on dead parts in living leaves of Yucca filamentosa L., Jul. 1888, Ellis & Everhart (NY, holotype).
Notes
Morphology
Neophaeosphaeria was formally established by Camara et al. (2003) by segregating Paraphaeosphaeria species with 3-4-septate ascospores and anamorphs of ovoid to ellipsoid, non-septate, brown, verrucose to punctuate conidia forming from percurrently proliferating conidiogenous cells. Neophaeosphaeria filamentosa was selected as the generic type. Currently, four species are included under Neophaeosphaeria, i.e. N. barrii, N. conglomerate (M.E. Barr) M.P.S. Camara, M.E. Palm & A.W. Ramaley, N. filamentosa and N. quadriseptata (M. E. Barr) M.P.S. Camara, M.E. Palm & A.W. Ramaley (Camara et al. 2003). At present all species in Neophaeosphaeria occur on Yucca (Agavaceae).
Phylogenetic study
The four Neophaeosphaeria species form a monophyletic clade based on both ITS and SSU rDNA sequences (Camara et al. 2001; Checa et al. 2002), and they fall in the group comprising members of Phaeosphaeriaceae and Leptosphaeriaceae (Camara et al. 2003). Neophaeosphaeria filamentosa, the generic type of Neophaeosphaeria, nested in Leptosphaeriaceae with low to moderate bootstrap values (Schoch et al. 2009; Zhang et al. 2009a).
Concluding remarks
The familial status of Neophaeosphaeria under Leptosphaeriaceae is confirmed, although this family remains poorly supported in phylogenetic studies.
Nodulosphaeria Rabenh., Klotzschii Herb. Viv. MycoL, Edn 2: no. 725 (in sched.) (1858). (Phaeosphaeriaceae)
Generic description
Habitat terrestrial, saprobic or hemibiotrophic. Ascomata small, immersed to erumpent, globose or subglobose, black, papillate, ostiolate. Papilla with numerous setae in the pore-like ostiole. Peridium thin, composed of thick- or thin-walled large cells. Hamathecium of cellular pseudoparaphyses, septate and branching. Asci 8-spored, bitunicate, fissitunicate, clavate to cylindroclavate, with a very short, furcate pedicel and a small ocular chamber. Ascospores filamentous, hyaline or pale brown, multi-septate, one of the upper cells swollen.
Anamorphs reported for genus: none.
Literature: Barr 1992a; Holm 1957, 1961; Shoemaker 1984b; Shoemaker and Babcock 1987.
Type species
Nodulosphaeria hirta Rabenh., Klotzschii Herb. Viv. MycoL, Edn 2: no. 725 (in sched.) (1858). (Fig. 67)
Fig. 67.
Nodulosphaeria hirta (from BR 101945-95, holotype). a Appearance of ascomata on the host surface. b Vertical section of an ascoma. Note the setae at the apex and in the ostiole. c Section of a partial peridium. Note the outer layer cells of textura angularis and inner layer compressed cells. d Squash mount showing asci in pseudoparaphyses. e, f. The light brown filiform ascospores. Scale bars: a=0.5 mm, b =100 μm, c=50 μm, d=20 μm, e, f =10 μm
Ascomata 260-330 μm highx260-330 μm diam., scattered, or in small groups, immersed to erumpent, globose or subglobose, black, papillate, ostiolate. Papilla 50-80 μm high, numerous setae occur in the pore-like ostiole (Fig. 67a and b). Peridium 15-30 μm wide at the sides, thinner at the base, coriaceous, comprising two types of cells, outer cells of 1–2 layers of heavily pigmented cells of textura angularis, cells 6-8 μm diam., cell wall 1.5-3 μm thick, inner of compressed cells, 5×13-3×8 μm diam., wall 2–3 μm thick (Fig. 67c). Hamathecium of long cellular pseudoparaphyses 2–3 μm broad, septate and branching, mucilage not observed. Asci 100-123×12.5-15 (−17.5) μm (x = 110.8 × 14.3μm, n = 10), 8-spored, bitunicate, fissitunicate, clavate to cylindro-clavate, with a very short, furcate pedicel, with a small ocular chamber (to 2 μm widex 1 μm high) (Fig. 67d). Ascospores 48-63 x5-6.5 μm (x = 55.3 × 5.6μm, n = 10), 4-seriate, filamentous, pale brown, 8-septate, the 4th upper cell broader than the others, smooth-walled, without sheath (Fig. 67e and f).
Anamorph: none reported.
Material examined: GERMANY, Dresdae, in herbarum caulibus emortuis perrara, exeunte majo, 1858 (BR 101945-95, holotype, as Nodulosphaeria hirta).
Notes
Morphology
The name Nodulosphaeria was first used by Rabenhorst (1858) but was considered as a synonym of Leptosphaeria for many years (Clements and Shear 1931). The name was reinstated by Holm (1957) and was represented by N. hirta, which was concurrently treated as a synonym of N. derasa (Berk. & Broome) L. Holm. The most outstanding morphological characters of Nodulosphaeria were considered to be apex of ascomata often covered with setae, ascospore with three or more transverse septa with a supramedian enlarged cell or elongated to a scolecospore, mostly with terminal appendages (Barr 1992a; Holm 1961; Shoemaker 1984b). The ascomata are usually immersed and the peridium comprises a few layers of brown, relatively thin-walled cells of textura angularis and textura prismatica similar to those of Phaeosphaeria. Thus, Nodulosphaeria is likely to be a member of Phaeosphaeriaceae. However, this needs to be confirmed by molecular analysis. The boundary between Nodulosphaeria and Ophiobolus is not clear-cut, and the circumscriptions of them usually depend on the viewpoint of different mycologists. For instance, Shoemaker (1976) has assigned some Nodulosphaeria species such as N. erythrospora, N. fruticum, N. mathieui and N. megalosporus to Ophiobolus. Subsequently, more species were added to Nodulosphaeria (Barr 1992a; Shoemaker 1984b; Shoemaker and Babcock 1987). Currently, more than 60 names are included in Nodulosphaeria (http://www.mycobank.org/, 06/ 2010).
Phylogenetic study
None.
Concluding remarks
AH species included in Nodulosphaeria have an inflated ascospore cell as mentioned above. However, it is likely that this character would have evolved more than once as it is probably an adaption for ascospore ejection from the ascus (Shoemaker 1976). It occurs in Ophiobolus species and the ascomata of these species are quite dissimilar to Nodulosphaeria species and their exclusion from Nodulosphaeria seems warranted. When considering whether a species belongs in Nodulosphaeria, one must also consider the ascomata and peridium structure until DNA sequences are available.
Ohleria Fuckel, Fungi rhenani exsic.: no. 2173 (1868). (Melanommataceae)
Generic description
Habitat terrestrial, saprobic. Ascomata small to medium size, solitary, scattered, or in small groups, erumpent to nearly superficial, papillate, ostiolate. Peridium thin, thicker at the apex, 1-layered. Hamathecium of dense, long trabeculate pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical, with a short pedicel. Ascospore brown to reddish brown, broadly to narrowly fusoid, 3-septate, easily separating into two parts at the primary septum.
Anamorphs reported for genus: Monodictys (Samuels 1980). Literature: Barr 1990b; Clements and Shear 1931; Patel et al. 1997; Samuels 1980.
Type species
Ohleria modesta Fuckel, Fungi rhenani exsic. (1868) (Fig. 68)
Fig. 68.
Ohleria modesta (from g: f. rh. 2173, isotype). a Ascomata scattering on host surface. b Section of a partial peridium. C Asci embedded in pseudoparaphyses. d, e Cylindrical asci with short pedicels. Scale bars: a =1 mm, b, C=50 μm, d, e=20 μm
Ascomata 214-357 μm highx285-400 μm diam., solitary, scattered, or in small groups of 2–3, erumpent to nearly superficial, coriaceous, with basal wall remaining immersed in host tissue, broadly or narrowly conical, with a flattened base not easily removed from the substrate, black; apex with a conical protruding papilla and an often pore-like ostiole (Fig. 68a). Peridium 22-53 μm thick laterally, thicker at the apex, 1-layered, composed of heavily pigmented thick-walled cells of textura angularis, cells to 7 μm diam., cell wall 1.5-3 μm thick, apex cells smaller and walls thicker, base cells walls thinner (Fig. 68b). Hamathecium of dense, long trabeculate pseudoparaphyses 1–2 μm broad, septate, branching and anastomosing (Fig. 68c). Asci 90-130×(5.5-)7-10 μm (x = 107.3 × 8μm, n =10), 8-spored, with a short pedicel up to 20 μm long, bitunicate, fissitunicate, cylindrical, with a small ocular chamber (to 1.5 μm widex1.5 μm high) (Fig. 68c, d and e). Ascospore 15-22×4-5 μm (x = 20 × 4.4μm, n =10), biseriate near the top and uniseriate at the base, broadly fusoid to fusoid with broadly to narrowly rounded ends, brown to reddish brown, 3-septum, deeply constricted at the median septum and breaking into two conical partspores, no constriction at the secondary septum, smooth (Fig. 68d and e).
Anamorph: none reported.
Material examined: GERMANY, on decorticated, decaying roots of Fagus sylvatica, very rare, collected in autumn (G: F. rh. 2173, isotype).
Notes
Morphology
Ohleria is characterized by its subglobose to conic ascomata, produced on decorticated woody substrates, as well as its brown and phragmosporous ascospores which break into two parts at the median septum (Samuels 1980). Some species of Ohleria are widespread. For instance, O. brasiliensis is reported from New Zealand, Brazil as well as United States (Samuels 1980). Ohleria has been considered closely related to Sporormia and Preussia based on the ascosporic characters, and several species of Ohleria, such as O. aemulans Rehm, O. haloxyli Kravtzev, O. silicata Kravtzev and O. kravtzevii Schwarzman, have been transferred to these genera. Clements and Shear (1931) treated Ohleria as a synonym of Ohleriella, despite the fact that Ohleriella is a coprophilous fungus. When the ascomata and habitats are considered, Ohleria seems closely related to Melanomma and Trematosphaeria (Samuels 1980).
Phylogenetic study
None.
Concluding remarks
To some degree, habitats show phylogenetic significance (Zhang et al. 2009a). Thus, Ohleria seems less likely related to Sporormia and Preussia. But its relationship with Melanomma is uncertain, because of their differences in hamathecium and ascospores.
Ohleriella Earle, BU11 N Y Bot Gard 2: 349 (1902). (Delitschiaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata medium to large, immersed, erumpent to nearly superficial, scattered or in small groups, usually with a wide papilla, ostiolate, coriaceous. Peridium composed of small pigmented cells of textura angularis. Asci 8-spored or fewer, cylindroclavate, with a furcate pedicel. Hamathecium of trabeculate pseudoparaphyses. Ascospores brown to dark brown, cylindrical to nearly clavate with broadly to narrowly round ends, multi-septate, easily broken into partspores, smooth, with elongated germ slit in each cell.
Anamorphs reported for genus: none
Literature: Ahmed and Cain 1972; von Arx and Muller 1975; Barr 1990a; Clements and Shear 1931.
Type species
Ohleriella neomexicana Earle, Bu11 N Y Bot Gard 2: 349 (1902). (Fig. 69)
Fig. 69.
Ohleriella neomexicana (NY, holotype). a Ascoma scattering on the host surface. b Section of a partial peridium. Note the smal] cells of textura angularis. c Ascospore in ascus. d Ascospore breaking into part spores. Note the sigmoid germ slit. e Dehiscent ascus. f, g Asci with short pedicels. Scale bars: a =100 μm, b=50 μm, c-g= 10 μm
Ascomata 330-420 μm highx400-575 μm diam., solitary, scattered, or in small groups, immersed to erumpent, to nearly superficial, with basal wall remaining immersed in host tissue, coriaceous, globose or subglobose, usually a somewhat thick, short papilla, up to 100 μm high, with a pore-like ostiole (Fig. 69a). Peridium 27-35 μm thick laterally, up to 55 μm thick at the apex, 1-layered, composed of small pigmented cells of textura angularis, cells up to 5×8 μm diam., ce11 wall 1.5-2 μm thick, apex cells smaller and walls thicker (Fig. 69b). Hamathecium of dense, long trabeculate pseudoparaphyses, 1-1.5 μm broad, anastomosing and branching between and above the asci. Asci 150-208×17.5-25 μm (x = 182.5 × 22μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a narrowed, furcate, thin pedicel, 15-55 μm long, 2–3.5 μm broad, with a large truncate ocular chamber best seen in immature asci (to 4 μm widex3 μm high) (Fig. 69e, f and g). Ascospores 55-72.5× 10-12 μm (x = 63 × 10.4μm, n =10), 3-4 seriate to uniseriate near the base, cylindrical to clavate, with broadly to narrowly rounded ends, brown, 6-7 transverse septa, easily separating into partspores, with germ slits, central partspores of the ascospores shorter than broad, rectangular in vertical section, round in transverse section, 7-8×6-10 μm diam., apical cells usually longer than broad, 11-17.5×6-7 μm diam. (Fig. 69c and d).
Anamorph: none reported.
Material examined: USA, Albuquerque, Bernalillo Co., New Mexico, dry gravelly hi11, on wood, 29 Nov. 1901, T. S.A. Cockerell (NY, holotype).
Notes
Morphology
Ohleriella was formally established by Earle (1902) based on its “medium to large ascomata with a wide papilla, relatively wide peridium, cylindro-clavate asci, brown to deep brown multi-septate ascospore, with elongated germ slit on each ce11”, and was monotypified by O. neomexicana (Barr 1990a). Ohleriella subsequently has been treated as a synonym of Ohleria, Sporormiella or Preussia (Ahmed and Cain 1972; von Arx and Muller 1975; Clements and Shear 1931). Spororminula tenerifae, the generic type of Spororminula, was assigned to Ohleriella, thus Spororminula was treated as a synonym of Ohleriella (Barr 1990a). Two new species were introduced by Barr (1990a) from North America. Currently, three species are included in this genus, i.e. O. herculean (Ellis & Everh.) M.E. Barr, O. neomexicana and O. nudilignae M.E. Barr & Malloch (http://www.mdexfungorum.org; http://www.mycobank.org, 01/03/2009).
The generic type, O. neomexicana, is morphologically similar to the coprophilous genus Sporormiella, but is saprobic on grass stems.
Phylogenetic study
None.
Concluding remarks
Although we maintain Ohleriella as a separate genus here, its saprobic habitat on grasses and similarity to the coprophilous Sporormiella may indicate a close evolutionary relationship, with the grass saprobic possibly being an early relative of the coprophilous Sporormiella. Alternatively, the species/genera may simply occupy different ecological niches (i.e. dead grass vs dead grass in dung). Molecular studies are needed to resolve this issue.
Ophiobolus Reiss, Hedwigia 1:27 (1854). (Phaeosphaeriaceae)
Generic description
Habitat terrestrial, saprobic or hemibiotrophic. Ascomata medium-sized, solitary, scattered, or in groups, globose or pyriform, coriaceous, black, papillate, ostiolate, periphysate. Peridium thin, thicker near the apex, thinner at the base. Hamathecium of long cellular pseudoparaphyses, septate, anastomosing or branching not observed. Asci 8-spored, bitunicate, fissitunicate dehiscence not observed, cylindrical, with a short, furcate pedicel. Ascospores filamentous, narrower toward the lower end, pale brown, multiseptate, separating into two partspores from the middle septum, from the breaking point, the second cell of each partspore enlarged.
Anamorphs reported for genus: Coniothyrium-like, Rhabdospora, Phoma-like and Scolecosporiella (Hyde et al. 2011; Shoemaker 1976; Sivanesan 1984).
Literature: Holm 1948, 1957; Muller 1952; Reiss 1854; Shoemaker 1976; Sivanesan 1984.
Type species
Ophiobolus disseminans Reiss, Hedwigia 1:27 (1854) (Fig. 70).
Fig. 70.
Ophiobolus disseminans (from BPI-629021, type). a Immersed ascomata scattered on the host surface. Note the erumpent papilla. b Section of an ascoma. C. Section of a partial peridium. Note the thick-walled outer layer and thin-walled inner layer (orange colour due to DIC). d Ascus with a short furcate pedicel. e Squash mount showing asci in pseudoparaphyses. Scale bars: a=0.5 mm, b =100 μm, C=50 μm, d, e=20 μm
Ascomata 220-380 μm highx290-430 μm diam., solitary, scattered, or in groups often arranged in a row, immersed with a protruding papilla, globose, pyriform, coriaceous, black, periphysate. Papilla 40-90 μm high, with a pore-like ostiole (Fig. 70a and b). Peridium 40-55 μm wide at the sides, up to 70 μm thick at the apex, thinner at the base, comprising two cell types, outer layer composed of small heavily pigmented thick-walled cells of textura angularis, cells 2-5 μm diam., cell wall 2–3 μm thick, apex cells smaller and walls thicker, inner layer composed of lightly pigmented or hyaline thin-walled cells of textura angularis, 5-7 μm diam., wall 1.5-2 μm thick, merging with pseudoparaphyses (Fig. 70c). Hamathecium of long cellular pseudoparaphyses, 2–3 μm broad, septate, anastomosing or branching not observed (Fig. 70e). Asci 150-195×8-12.5 μm (x = 169.5 × 10.7μm, n =10), 8-spored, bitunicate, fissitunicate dehiscence not observed, cylindrical but narrowing towards the base, with a short, furcate pedicel which is 10-25 μm long, ocular chamber not observed (Fig. 70d and e). Ascospores 110-160×2.5-4 μm (x = 135.3 × 3μm, n = 10), filamentous, narrower toward the lower end, pale brown, 22–30-septate, separating into two partspores from the middle septum, from the breaking point the second cell of each partspore enlarged.
Anamorph: none reported.
Material examined: GERMANY, near Kassel, on dead stem of Cirsium arvense (L.) Scop., Spring 1853 (BPI-629021, type).
Notes
Morphology
Ophiobolus was established by Reiss (1854) as a monotypic genus represented by O. disseminans based on its “Perithecia discreta, ostiolis prominentibus: sporae ascis inclusae, binatae, filliformes, multiseptatae”.
A broad generic concept was adopted for the genus by Holm (1948) and Muller (1952). Shoemaker (1976) surveyed Canadian species of Ophiobolus using the broad concept of Holm (1948) and Muller (1952). A narrower generic concept was used by Holm (1957), which only included species with ascospores separating into two halves. Holm (1957) assigned species with enlarged ascospore cells to Nodulosphaeria, and those with long spirally coiled ascospores to Leptospora (Shoemaker 1976). This left only three species accepted under Ophiobolus (Holm 1957), although this concept has rarely been followed with new species recently being described (Raja and Shearer 2008).
Walker (1980) provided a detailed description from the type material and dealt with many species of scolecospored fungi that had been placed in Ophiobolus by Saccardo (1883). Thus, currently several Ophiobolus sensu lato species are separated into Acanthophiobolus, Entodesmium, Leptosphaeria and Leptospora. Ophiobolus sensu lato contains about 300 species names (Sivanesan 1984; http://www.mycobank.org/, 04/02/2009).
Phylogenetic study
Ophiobolus fulgidus (Cooke & Peck) Sacc. (as Leptos-phaeria fulgida (Cooke & Peck) M. E. Barr in Dong et al. 1998) lacks support in the clade of Leptosphaeriaceae (Dong et al. 1998). We expect it may closely related to Phaeosphaeriaceae.
Concluding remarks
We agree from morphological data that Ophiobolus should comprise species that have filamentous spores that break easily into two halves at the central septum, with the second cell on either side being swollen (Walker 1980) and that the genus presently comprises three species (i.e. O. anthrisci (L. Holm) L. Holm, O. ophioboloides (Sacc.) L. Holm and O. acuminatus). AH other Ophiobolus species need to be re-examined and should be placed in other genera such as Nodulosphaeria and Leptospora. The genus is in need of revision and molecular phylogenetic study.
Ophiosphaerella Speg., Anal. Mus. nac. Hist. nat. B. Aires 19: 401-402 (1909). (Phaeosphaeriaceae)
Generic description
Habitat terrestrial, saprobic or hemibiotrophic. Ascomata small- to medium-sized, solitary or scattered, immersed, globose or subglobose, papillate, ostiolate. Peridium thin. Hamathecium of dense, filliform, septate pseudoparaphyses. Asci bitunicate, fissitunicate dehiscence not observed, cylindrical often narrower near the base, with a short furcate pedicel. Ascospores filamentous, pale brown, multi-septate. Anamorphs reported for genus: Scolecosporiella (Farr et al. 1989).
Literature: von Arx and Muller 1975; Schoch et al. 2006, 2009; Spegazzini 1909; Walker 1980; Wetzel et al. 1999; Zhang et al. 2009a.
Type species
Ophiosphaerella graminicola Speg., Anal. Mus. nac. Hist. nat. B. Aires 19: 401 (1909). (Fig. 71)
Fig. 71.
Ophiosphaerella graminicola (from LPS 858, holotype). a Ascomata on the host surface. Note the protruding disk-like papilla. b Section of an ascoma. c Asci in pseudoparaphyses with short pedicels. d-f Cylindrical asci with short pedicels. Scale bars: a=0.5 mm, b = 100 μm, c-f =10 μm
Ascomata 280-325 μm highx250-300 μm diam., solitary or scattered, immersed with a short papilla protruding out of the substrate, globose or subglobose, often laterally flattened, dark brown to black, papillate, papilla ca. 100 μm high, 140-180 μm broad, disk-like in appearance from above, periphysate (Fig. 71a and b). Peridium 11–25 μm wide, thicker near the apex, comprising two cell types of small cells, outer wall composed 6-10 layers of lightly brown flattened cells of textura angularis, inner layer composed of paler and thin-walled cells, both layers thicker near the apex (Fig. 71b). Hamathecium of dense, long pseudoparaphyses 0.8-1.5 μm broad near the apex, septate, 2–3 μm broad between the asci. Asci 105-135×5.5-10 μm (x = 118.5 × 7μm, n =10), 8-spored, bitunicate, cylindrical and narrower near the base, with a short, furcate pedicel, up to 30 μm long, small inconspicuous ocular chamber (to 1.5 μm widex 1 μm high) (Fig. 71c, d, e and f). Ascospores 100-125 × 1.8-2.2 μm (x = 118 × 2μm, n =10), filamentous, pale brown, 12-20 septa, smooth-walled.
Anamorph: none reported.
Material examined: ARGENTINA, Tucuman, on leaf sheath of Leptochloa virgata (L.) P. Beauv., 14 Apr. 1906, C. Spegazzini (LPS 858, holotype).
Notes
Morphology
Ophiosphaerella was introduced by Spegazzini (1909) who described and illustrated a single new species, O. graminicola, and thus the genus was validly published (Walker 1980, p. 70). After checking the type specimen, Petrak and Sydow (1936) transferred the generic type to Ophiobolus graminicolus (Speg.) Petrak & Syd, and assigned Ophiosphaerella as a synonym of Ophiobolus. This was followed by von Arx and Muller (1975). Ophiosphaerella differs from Phaeosphaeria by its scole-cospores without swollen cells or appendages, and from Ophiobolus by its ascospores without swollen cells or separating into partspores, thus was kept as a separating genus (Eriksson 1967a; Walker 1980).
Phylogenetic study
Ophiosphaerella forms a monophyletic group as a sister group of Phaeosphaeria located in Phaeosphaeriaceae (Schoch et al. 2006, 2009; Wetzel et al. 1999; Zhang et al. 2009a).
Concluding remarks
Numerous Ophiobolus species are likely to belong in Ophiosphaerella. The two genera are distinguished as Ophiobolus sensu Shoemaker (1976) has swollen central cells or breaking into partspores or with long spirally coiled ascospores, and Ophiosphaerella (sensu Walker 1980) has scolecospores without swollen central cells or breaking into partspores.The recent introduction of Ophiobolus shoemakeri Raja & Shearer (Raja and Shearer 2008) is probably incorrect since the ascospores do not split up into partspores and there is no swelling above septum either. In particular, its freshwater habitat also distinguishes it from other species of Ophiobolus. Like Ophiobolus, Ophiosphaerella is in need of phylogenetic analysis but appears to be closely related to Phaeosphaeriaceae (Schoch et al. 2006).
Ostropella (Sacc.) Hohn., Annls myco1 16: 144 (1918). (Pleosporales, genera incertae sedis)
≡ Ostropa subgen. Ostropella Sacc., Syll. fung. (Abellini) 2: 805 (1883).
Generic description
Habitat terrestrial, saprobic. Ascomata large, erumpent to superficial, solitary or gregarious, globose to subglobose, with broad and compressed papilla and slit-like ostiole. Peridium carbonaceous. Hamathecium of dense, long trabeculate pseudoparaphyses, anastomosing and branching, rarely septate, embedded in mucilage. Asci clavate with very long and thin and furcate pedicels. Ascospores pale brown, ellipsoid to fusoid, 1-septate, constricted.
Anamorphs reported for genus: none.
Literature: Barr 1990a; Chesters and Bell 1970; Holm and Yue 1987; Huhndorf 1993; Muller and von Arx 1962; Muller and Dennis 1965; Saccardo 1883.
Type species
Ostropella albocincta (Berk. & M.A. Curtis) Hohn., Annls myco1. 16: 144 (1918). (Fig. 72)
Fig. 72.
Ostropella albocincta (K(M): 143941, syntype). a Ascomata^. gregarious on host surface. b Section of the partial peridium. Note the peridium comprising two cell types and the whitening tissue (arrowed). c Pseudoparaphyses. d, e Asci with long pedicel. f-h Ascospores, which are strongly constricted at the central septum. Scale bars: a=1 mm, b =100 μm, d, e, h=20 μm, c, g, f=10 μm
≡ Ostropa albocincta Berk & M.A. Curtis, in Berkeley, J. Linn. Soc., Bot. 10: 372 (1868).
Ascomata 1000-1730 μm highx 1050-1450 μm diam., scattered to gregarious, erumpent to superficial, globose to subglobose, roughened, often covered with white crustose covering, with subiculum, with a broad compressed papilla and long and slit-like ostiole (Fig. 72a). Peridium 100-250 μm thick, not of uniform thickness throughout entire wall area, composed of two cell types, one is of lightly pigmented thin-walled cells of textura prismatica, cells up to 17 × 3 μm diam., ce11 wall <1 μm thick, intermingled with small heavily pigmented thick-walled cells of textura globosa, cells up to 5 μm diam., cell wall 2–3 μm thick (Fig. 72b). Hamathecium of dense, long trabeculate pseudoparaphyses, 1.2-1.8 μm broad, anastomosing and branching, rarely septate, embedded in mucilage (Fig. 72c). Asci 90-150(−180) × 8-13(−17) μm (x = 120.5 × 11.5μm, n=10), 8-spored, bitunicate, fissitunicate dehiscence not observed, cylindro-clavate, with a long, narrowed, furcate pedicel which is up to 75 μm long, and with a small ocular chamber best seen in immature asci (up to 2 μm widex 1 μm high) (Fig. 72d and e). Ascospores 18-26× 5-6 μm (x = 22.4 × 5.6μm, n=10) biseriate in upper part and uniseriate in lower part, fusoid, pale brown, 1-septate, deeply constricted at the septum, smooth or rarely verrucose (Fig. 72f, g and h).
Anamorph: none reported.
Material examined: Wright s.n., Herb. G.E. Massee, (NY 921990, possible isotype); CUBA, as Ostropa albocincta, C. Wright 345, 1879 (K(M): 143941, syntype).
Notes
Morphology
Ostropella was established by Saccardo (1883) as a subgenus of Ostropa and was monotypic being represented by O. albocincta. The genus was formally established (as Ostropella) and redescribed by von Hohnel (1918b) and later the description was modified by several workers (Barr 1990a; Huhndorf 1993; Muller and von Arx 1962; Muller and Dennis 1965). Ostropella is characterized by having large ascomata, a conspicuous ridged compressed papilla with an elongated slit-like ostiole, and 1-septate lightly pigmented ascospores.
The affinity of Ostropella to Schizostoma sensu Sacc. was first recognized by von Hohnel (1918b) and this was accepted by Muller and von Arx (1962) and they transferred Schizostoma pachythele (Berk. & Broome) Sacc. and Ostreionella fusispora Seaver to Ostropella. Holm and Yue (1987), however, disagreed with this transfer because of the differences in ascomatal vestiture and the rather thick wall comprising two cell types of Ostropella albocincta differ from those of Schizostoma pachythele. Chesters and Bell (1970) suggested that S. pachythele, Xenolophium leve and X. verrucosum Syd. are three varieties under Lophiostoma pachythele (Berk. & Broome) Chesters & A.E. Bell. The conspecific status of the three taxa was supported by Holm and Yue (1987). Although no combination was made, Holm and Yue (1987) assigned these taxa to Xenolophium instead of Lophiostoma. Barr (1990a) suggested that either Ostropella or Xenolophium could accommodate these closely related taxa, i.e. O. fusispora (Seaver) E. Mu11., S. pachythele, X. leve, and X. verrucosum. Huhndorf (1993) formally transferred S. applanata Petch and S. pachythele to Xenolophium.
Phylogenetic study
Phylogenetic analysis based on LSU sequences indicated that Ostropella albocincta clusters together with Xenolophium applanatum as we11 as species of Platystomum, but they receive poor support (Mugambi and Huhndorf 2009b). They a11 were temporarily assigned under Platystomaceae (Mugambi and Huhndorf 2009b).
Concluding remarks
Although the placement of Ostropella albocincta under Platystomaceae lacks support, Ostropella should be excluded from Melanommataceae despite its trabeculate pseudoparaphyses.
Paraliomyces Kohlm., Nova Hedwigia 1: 81 (1959). (Pleosporales, genera incertae sedis)
Generic description
Habitat marine, saprobic. Ascostromata immersed, penetrating into the substrate with dark brown hyphae. Ascomata medium-sized, solitary, immersed or erumpent, subglobose to pyriform, subiculate or nonsubiculate, papillate or epapillate, ostiolate, periphysate, carbonaceous. Peridium thick. Hamathecium of long trabeculate pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical, with a short furcate pedicel, without apical apparatus, uniseriate. Ascospores ellipsoid to broadly fusoid with broadly rounded ends, 1-septate, constricted at the septum, hyaline, smoothwalled, surrounded by a gelatinous sheath.
Anamorphs reported for genus: none.
Literature: Kohlmeyer 1959; Tam et al. 2003.
Type species
Paraliomyces lentifer Kohlm. [as ‘lentiferus’], Nova Hedwigia 1: 81 (1959). (Fig. 73)
Fig. 73.
Paraliomyces lentifer (from Herb. J. Kohlmeyer No. 1720). a Section of an immersed ascoma. b Eight-spored cylindrical asci embedded in pseudoparaphyses. c, d Cylindrical asci with short pedicels. e-h One-septate hyaline ascospores. Scale bars: a =100 μm, b-d=20 μm, e-h =10 μm
Ascostromata black, immersed, penetrating into the substrate with dark brown hyphae. Ascomata up to 680 μm highx540 μm diam., solitary, immersed or erumpent, subglobose to pyriform, subiculate or nonsubiculate, papillate or epapillate, ostiolate, periphysate, carbonaceous (Fig. 73a). Peridium thick. Hamathecium of long trabeculate pseudoparaphyses, 1-1.5 μm broad. Asci 90-130×12-17 μm (x = 116 × 15μm, n =10), bitunicate, fissitunicate, cylindrical, 8-spored, uniseriate, with a short furcate pedicel, without apical apparatus (Fig. 73b, c and d). Ascospores 17.5-25×10-12.5 μm (x = 21 × 11μm, n= 10), ellipsoid to broadly fusoid with broadly rounded ends, 1-septate, constricted at the septum, hyaline, smooth-walled, surrounded by a gelatinous sheath that contracts to form a lateral, lentiform, viscous appendage over the septum, 7.5-12.5 μm diam., 1-3 μm thick (Fig. 73e, f, g and h). Anamorph: none reported.
Material examined: USA, Florida, Charlotte Harbor in Punta Garda, 10 Jan. 1964, leg., det. J. J. Kohlmeyer (Herb. J. Kohlmeyer No. 1720).
Notes
Morphology
Paraliomyces was introduced to accommodate the marine fungus P. lentifer, which is characterized by immersed ascomata produced within the ascostroma, trabeculate pseudoparaphyses, cylindrical, 8-spored asci, ellipsoidal, hyaline, 1-septate ascospores surrounded by a gelatinous sheath, which forms a lentiform, viscous appendage over the septum (Kohlmeyer 1959).
Phylogenetic study
Based on analysis of SSU sequences, Paraliomyces lentifer nested within Pleosporales, but its familial status was left undetermined (Tam et al. 2003).
Concluding remarks
None.
Phaeosphaeria I. Miyake, Bot. Mag., Tokyo 23: 93 (1909). (Phaeosphaeriaceae)
Generic description
Habitat terrestrial, saprobic or hemibiotrophic. Ascomata small, solitary, scattered, or in small groups, immersed, globose, subglobose, wall black. Apex with a pore-like ostiole. Peridium thin. Hamathecium of dense, filliform, septate pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, broadly cylindrical to narrowly fusoid, with a short pedicel. Ascospores fusoid to narrowly fusoid, pale brown to brown, 3-septate.
Anamorphs reported for genus: Amarenographium, Hendersonia-like, Phaeoseptoria, Scolecosporiella and Stagonospora (Hyde et al. 2011; Leuchtmann 1984; Shoemaker and Babcock 1989b).
Literature: von Arx and Muller 1975; Camara et al. 2002; Eriksson 1967a, 1981; Holm 1957; Khashnobish and Shearer 1996; Leuchtmann 1984; Miyake 1909; Shoemaker and Babcock 1989b.
Type species
Phaeosphaeria oryzae I. Miyake, Bot. Mag., Tokyo 23: 136 (1909). (Fig. 74)
Fig. 74.
Phaeosphaeria oryzae (from S nr F9572, F9573, lectotype). a Appearance of ascomata on the host surface. b Section of an ascoma. c Squash mount showing asci in pseudoparaphyses. Note that asci with short pedicels. d, e Asci with short pedicels. F, G. Light brown 3-septate ascospores. Scale bars: a=100 μm, b-g= 10 μm
Ascomata 120-140 μm highx100-140 μm diam., solitary, scattered, or in small groups, immersed, globose, subglobose, wall black, forming black spots on the leaves of hosts (Fig. 74a). Apex with a pore-like ostiole. Peridium 4-8 μm wide at the sides, composed of heavily pigmented thin-walled cells of textura angularis, cells 2-2.5×3-5 μm diam., cell wall less than 1 μm thick (Fig. 74b). Hamathecium of dense, long cellular pseudoparaphyses 2-2.5 μm broad, embedded in mucilage, rarely branched, septate. Asci 53-80(−90)×7-10 μm (x = 65.3 × 8.3μm, n =10), 8-spored, bitunicate, fissitunicate, broadly cylindrical to narrowly fusoid, with a short pedicel which is ca. 8 μm long, with a small ocular chamber and an inconspicuous apical apparatus (to 2 μm widex 1 μm high) (Fig. 74c, d and e). Ascospores 1 7 – 2 2 (− 2 8) × 4 – 5 μ m (x = 20.5 × 4.6μm, n=10), obliquely uniseriate, partially overlapping or biseriate, narrowly fusoid with rounded ends, pale brown, 3-septate, slightly constricted at primary septum, granulate (Fig. 74f and g).
Anamorph: none reported.
Material examined: JAPAN, Suruya, Shizuoka, on the leaves of Oryza sativa, Sept. 1907 (S nr F9572, F9573, lectotype).
Notes
Morphology
Phaeosphaeria was introduced by Miyake (1909), but was regarded as a synonym of Leptosphaeria for a long time. Holm (1957), however, reinstated Phaeosphaeria, assigning some Leptosphaeria sensu lato species with relatively small ascomata and which occurred on monocotyledons to Phaeosphaeria. Although this division based on host range is considered unnatural by some workers (Dennis 1978; Sivanesan 1984), it has been widely accepted (von Arx and Muller 1975; Eriksson 1967a; Hedjaroude 1969; Shoemaker and Babcock 1989b). Eriksson (1981) further revised the generic concept of Phaeosphaeria by including dictyosporous taxa as well as some perisporium taxa. Phaeosphaeria was further divided into six subgenera, i.e. Ovispora, Fusispora, Phaeosphaeria, Spathispora, Vagispora and Sicispora, based on differences in ascospore shape and the number of septa (Shoemaker and Babcock 1989b). Phaeosphaeria species are usually associated or parasitic on annual monocots, such as Cyperaceae, Juncaceae or Poaceae but have also been recorded as saprobes and on dicotyledons (e.g. P. viridella and P. vagans).
Phylogenetic study
The separation of Phaeosphaeria from Leptosphaeria sensu stricto was supported by phylogenetic studies based on ITS sequences. The peridium structure, pseudoparenchymatous cells in Phaeosphaeria versus scleroplectenchymatous cells in Leptosphaeria had phylogenetic significance in the distinction between these two genera, while the subgenus division was not supported by the phylogenetic results (Camara et al. 2002; Morales et al. 1995). The familial status of both Phaeosphaeriaceae and Leptosphaeriaceae was verified by multigene phylogenetic analysis (Schoch et al. 2009; Zhang et al. 2009a).
Concluding remarks
Phaeosphaeria was originally thought to be a synonym of Leptosphaeria (Muller 1950; Munk 1957), however, molecular analysis has shown these two genera differ with Phaeosphaeria having pseudoparenchymatous peridium, Stagonospora-like anamorph and mostly monocotyledonous hosts and Leptosphaeria having scleroplectenchymatous peridium, Phoma-like anamorph and mostly dicotyledonous hosts (Camara et al. 2002; Schoch et al. 2009; Shoemaker and Babcock 1989b; Zhang et al. 2009a). It is now recognized that Phaeosphaeria is the type genus of Phaeosphaeriaceae and related genera include Entodesmium and Setomelanomma and probably Ophiosphaerella (Schoch et al. 2009; Zhang et al. 2009a). Paraphaeosphaeria was introduced as an off-shoot of Phaeosphaeria and differs in ascospore shape and septation as well as anamorphic stages (Eriksson 1967a, b). Similarly, Nodulosphaeria was recently reinstated and differs from Phaeosphaeria because of setae over the apex as well as its ascospores with swelling supramedian cells and terminal appendages (Holm 1957, 1961). While the newly reinstated Phaeosphaeria was confined to monocotyledons and particularly grasses, there are now many species that have been described from dicotyledons (Farr et al. 1989). Whether these taxa form a monophyletic group needs to be investigated with fresh collections and molecular data.
Phaeosphaeriopsis M.P.S. Camara, M.E. Palm & A.W. Ramaley, Myco1. Res. 107: 519 (2003). (Phaeosphaeriaceae)
Generic description
Habitat terrestrial, saprobic or hemibiotrophic? Ascomata small, scattered or in small groups, immersed, globose, subglobose. Peridium thin, comprising one cell type of textura angularis. Hamathecium of dense, wide cellular pseudoparaphyses. Asci 8-spored, bitunicate, cylindrical to broadly fusoid, with a short pedicel and a small ocular chamber. Ascospores obliquely uniseriate and partially overlapping to biseriate even triseriate, cylindrical, pale brown, multi-septate, primary septum submedian, with or without constriction, verrucose or baculate.
Anamorphs reported for genus: Coniothyrium-like, Phaeostagonospora (Camara et al. 2003).
Literature: Camara et al. 2003.
Type species
Phaeosphaeriopsis glaucopunctata (Grev.) M.P.S. Camara, M.E. Palm & A.W. Ramaley, Myco1. Res. 107: 519 (2003). (Fig. 75)
Fig. 75.
Phaeosphaeriopsis glauco-punctata (from M.C. Cooke 166). a Ascomata immersed in the substrate. b Eightspored cylindrical asci. c-f. Pale brown baculate ascospores which are released from asci. Scale bars: a=200 μm, b=20 μm, c, d-f=10 μm
≡ Cryptosphaeria glaucopunctata Grev. Fl. Edin.: 362 (1824).
Ascomata 120-150 μm highx140-200 μm diam., scattered, or in small groups, immersed, globose, subglobose (Fig. 75a). Peridium 10-25 μm wide, comprising one type of cells, composed of thick-walled cells of textura angularis, cells 4-9 μm diam., ce11 wall 2–3 μm thick, almost equal in thickness. Hamathecium of dense, wide cellular pseudoparaphyses, 3-5 μm broad. Asci (50-)60-110×10-15 μm (x = 82.3 × 12μm, n =10), 8-spored, bitunicate, fissitunicate dehiscence not observe, cylindrical to broadly fusoid, with a short pedicel, with a small ocular chamber (to 0.8 μm widex1 μm high) (Fig. 75b). Ascospores 18-28×5-7.5 μm (x = 23.5 × 6.2μm, n = 10), obliquely uniseriate and partially overlapping to biseriate even triseriate, cylindrical, pale brown, 4(−5)-septate, without constriction or slightly constricted at the basal septum, the forth cell from the apex usually slightly inflated, the basal cell often longer, baculate (Fig. 75c, d, e and f).
Anamorph: none reported.
Material examined: UK, Epping, Sept. 1863 (E, M.C. Cooke 166, barcode: E00074286).
Notes
Morphology
Phaeosphaeriopsis was introduced to accommodate some species of Paraphaeosphaeria based on both morphological characters and results of SSU rDNA sequence analyses (Camara et al. 2003). Most of the Phaeosphaeriopsis species occur on the Agavaceae, although P. glaucopunctata occurs on Liliaceae (Ruscus). Phaeosphaeriopsis is characterized by having uni- or multioculate stromata and 4- or 5-septate ascospores. Although the morphological characters of Phaeosphaeriopsis species is more diverse than those of Paraphaeosphaeria sensu stricto or Neophaeosphaeria, the ITS sequences are more similar to each other than those of the other two genera (Camara et al. 2003). Currently, Phaeosphaeriopsis comprises seven species, namely P. agavensis (A.W. Ramaley, M.E. Palm & M.E. Barr) M.P.S. Camara, M.E. Palm & A. W. Ramaley, P. amblyospora A.W. Ramaley, P. glaucopunctata, P. musae Arzanlou & Crous, P. nolinae (A.W. Ramaley) M.P.S. Camara, M.E. Palm & A.W. Ramaley, P obtusispora (Speg.) M.P.S. Camara, M.E. Palm & A.W. Ramaley and P. phacidiomorpha (Ces.) D.F. Farr & M.E. Palm (http://www.mycobank.org/, 06/2010).
Phylogenetic study
The generic type of Phaeosphaeriopsis, P. glaucopunctata, located in Phaeosphaeriaceae based on SSU rDNA sequences (Camara et al. 2003). Phaeosphaeriopsis musae is also shown to belong to Phaeosphaeriaceae in recent phylogenetic studies (Schoch et al. 2009; Plate 1).
Concluding remarks
None.
Platysporoides (Wehm.) Shoemaker & C.E. Babc., Can. J. Bot. 70: 1648 (1992). (Pleosporaceae)
≡ Pleospora subgenus Platysporoides Wehmeyer, A World Monograph of the genus Pleospora and its Segregates, p. 236. 1961.
Generic description
Habitat terrestrial, saprobic? Ascomata small, scattered, immersed, semi-immersed to nearly superficial, globose, subglobose, black, smooth; apex with a protruding papilla and pore-like ostiole, without periphyses. Perid-ium thin, composed of a few layers of textura angularis. Hamathecium of numerous, cellular pseudoparaphyses, anastomosing, septate. Asci bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a short, furcate pedicel. Ascospores broadly ellipsoid, reddish brown, muriform.
Anamorphs reported for genus: none.
Literature: Shoemaker and Babcock 1992; Wehmeyer 1961.
Type species
Platysporoides chartarum (Fuckel) Shoemaker & C.E. Babc., Can. J. Bot. 70: 1650 (1992) (Fig. 76)
Fig. 76.
Platysporoides chartarum (from G NASSAU: 210558, type). a, b Ascomata scattered among fibers. Note the central ostioles. c Asci in numerous cellular pseudoparaphyses. d, e Cylindro-clavate asci with short pedicels. f-h. Muriform ascospores. Scale bars: a, b=200 μm, c-e=20 μm, f-h =10 μm
≡ Pleospora chartarum Fuckel, Jb. nassau. Ver. Naturk. 23-24: 133-134 (1870).
Ascomata 150-230 μm highx180-260 μm diam., scattered, immersed, semi-immersed to rarely superficial, globose, subglobose, black, smooth; apex with a protruding papilla, 50-85 μm long, 60-85 μm broad, ostiolate (Fig. 76a and b). Peridium 8-22 μm wide, composed of 2-4 layers of brown cells of textura angularis, cells 5-9 μm diam., cell wall 1–2.5 μm thick, without periphyses. Hamathecium of dense, long cellular pseudoparaphyses, 2–3 μm broad, anastomosing, septate (Fig. 76c). Asci 110-140×12.5-16.5 μm (x = 121.5 × 14.7μm, n =10), (6-)8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a short, furcate pedicel, 8-17 μm long, ocular chamber not observed (Fig. 76c, d and e). Ascospores 20-26×8-11 μm (x = 23.7 × 9μm, n = 10), obliquely uniseriate and partially overlapping, flattened, broadly ellipsoid in front view, reddish brown, 3 transverse septa, 1 longitudinal septum in each central cell, 1 oblique septum in each end cell, constricted at all septa, granulate, with a sheath 2–3 μm wide (as reported in Shoemaker and Babcock 1992) (Fig. 76f, g and h).
Anamorph: none reported.
Material examined: GERMANY, Budenheim, Leopold Fuckel, Nassau’s Flora, on old paper (G NASSAU: 210558 (a), as Sphaeria chartarum Wallr., type).
Notes
Morphology
Platysporoides was introduced as a subgenus of Pleospora by Wehmeyer (1961) and was typified by Pleospora chartarum. Shoemaker and Babcock (1992) raised Platysporoides to generic rank and placed it in the Pleosporaceae based on its “applanodictyospore” and “terete pored beak of the ascomata”. Currently, eleven species are included in this genus (Shoemaker and Babcock 1992). Another comparable pleosporalean family is Diademaceae, which is distinguished from Platysporoides by its ascoma opening as “an intraepidermal discoid lid” (Shoemaker and Babcock 1992).
Phylogenetic study
None.
Concluding remarks
Aigialus grandis is another pleosporalean fungus with flattened and muriform ascospores as well as papilla and ostioles, which belongs to Aigialaceae, a phylogenetically well supported marine family (Suetrong et al. 2009). Thus, it is highly likely that flattened and muriform ascospores are of little phylogenetic significance.
Pleomassaria Speg., Anal. Soc. cient. argent. 9: 192 (1880). (Pleomassariaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata medium to large, solitary, scattered, or in small groups, immersed, erumpent by a minute slit or a small conical swelling in the bark, flattened, papillate, ostiolate. Hamathecium of dense, cellular pseudoparaphyses, embedded in mucilage. Asci bitunicate, fissitunicate, broadly cylindrical to broadly cylindro-clavate, with a short, thick pedicel. Ascospores muriform, brown, constricted at the septa.
Anamorphs reported for genus: Prosthemium and Shearia (Barr 1982b; Sivanesan 1984).
Literature: Barr 1982b, 1990b, 1993a; Clements and Shear 1931; Eriksson 2006; Lumbsch and Huhndorf 2007; Shoemaker and LeClair 1975; Sivanesan 1984; Tanaka et al. 2005.
Type species
Pleomassaria siparia (Berk. & Broome) Sacc., Syll. fung. 2: 239 (1883) (Fig. 77)
Fig. 77.
1 Pleomassaria siparia (from BR, type). a Ascomata on the host surface (after removing the cortices). b Section of a partial peridium. C, d Asci with short pedicels. e-g Ascospores with thin sheath. Scale bars: a=0.5 mm, b-d=50 μm, e-g= 20 μm. 2 Prosthemium betulinum (from BR, type). h – j Conidia with arms. Scale bars: h-j=20 μm
≡ Sphaeria siparia Berk. & Broome, Ann. Mag. nat. Hist., Ser. 2 9: 321 (1852).
Ascomata 150-410 μm highx440-740 μm diam., solitary, scattered, or in small groups, immersed, erumpent by a minute slit or a small conical swelling in the bark, depressed globose, papillalte, ostiolate (Fig. 77a). Peridium 45-60 μm wide, thicker at the apex, thinner at the base, 1-layered, composed of small pigmented thick-walled compressed cells, cells ca. 15×3 μm diam., ce11 wall 2–3.5 μm thick, apex cells larger, base composed of small pigmented thick-walled cells of textura angularis, ca. 5 μm diam. (Fig. 77b). Hamathecium of dense, cellular pseudoparaphyses, 1–2 μm broad, embedded in mucilage, anastomosing or branching not observed. Asci 180-250× 28-42 μm (x=206.3 × 36.8μm, n = 10), 8-spored, bitunicate, fissitunicate, broadly cylindrical to broadly cylindroclavate, with a short, thick pedicel, 15-45 μm long, with inconspicuous ocular chamber (Fig. 77c and d). Ascospores 45-58×12.5-17.5 μm (x = 50.5 × 14.8μm, n = 10), biseriate, narrowly oblong with broadly to narrowly rounded ends, brown, muriform with 5-8 transverse septa and 1–2 vertical septa in some cells, smooth to verrucose, constricted at the septa, surrounded by a mucilaginous sheath (Fig. 77e, f and g).
Anamorph: Prosthemium betulinum Kunze (Sivanesan 1984).
Conidia to 120 μm diam., with 3-5 arms, each arm 3-5-septate, 40-55×13-16 μm, connected to a central ce11 (Fig. 77h, i and j).
Material examined: UK, Wiltshire, Spye Park, on branch of Betulina with Hendersonia polycystis Berk., et Br. leg. C.E. Broome, 1850? (BR, type).
Notes
Morphology
Pleomassaria as characterized by Barr (1982b) has medium- to large-sized, immersed ascomata, cellular pseudoparaphyses, clavate to oblong asci and large, muriform ascospores (Barr 1982b; Sivanesan 1984). The muriform and somewhat asymmetrical ascospores with a submedian primary septum distinguish Pleomassaria from Asteromassaria in the family Pleomassariaceae, while in Splanchnonema ascospores have distinct bipolar asymmetry. Barr (1982b) included five North American species in the genus, while Kirk et al. (2008) listed four species.
Barr (1993a) treated Pleomassaria as a synonym of Splanchnonema based on a morphological cladistic analysis, but this proposal was not followed by later workers (Eriksson 2006; Lumbsch and Huhndorf 2007; Tanaka et al. 2005).
Phylogenetic study
Pleomassaria siparia forms a robust phylogenetic clade with Melanomma pulvis-pyrius (generic type) (Schoch et al. 2009; Zhang et al. 2009a), which might represent a phylogenetic family (or suborder?).
Concluding remarks
The genera Asteromassaria, Pleomassaria and Splanchnonema of Pleomassariaceae are considered to be closely related and difficult to separate (Barr 1982b; Crivelli 1983). They all have ascomata which are immersed in bark and are visible as slightly raised pustules with small ostioles, but may eventually become erumpent (e.g. Asteromassaria macrospora). Pseudoparaphyses are cellular, asci are bitunicate, while ascospores vary from 1-septate and pale brown (e.g. Asteromassaria macrospora) to muriform (e.g. Pleomassaria siparia) and may be symmetrical (e.g. Asteromassaria macrospora) or highly asymmetrical (e.g. Splanchnonema pustulatum). The peridium ranges from thick-walled textura angularis (e.g. Asteromassaria macrospora) to thin-walled compressed cells (e.g. Splanchnonema pustulatum) and medium textura prismatica (e.g. Pleomassaria siparia). Anamorphs also vary distinctly, Prosthemium in Pleomassaria siparia, Scolicosporium in Asteromassaria macrospora but no anamorphic stage reported for Splanchnonema pustulatum. Furthermore, Asteromassaria pulchra clusters in Morosphaeriaceae in this study, thus here we tentatively assign Asteromassaria in Morosphaeriaceae (Plate 1). There seems to be considerable confusion in this family, especially when Pleomassaria siparia forms a robust phylogenetic clade with Melanomma pulvis-pyrius (Melannomataceae). Thus in this study, Pleomassariaceae is restated as a separate family from Melannomataceae. Therefore, fresh collections of the types of these genera are needed for molecular analysis and to establish which characters are important for classification.
Pleophragmia Fuckel, Jb. nassau. Ver. Naturk. 23-24: 243 (1870). (Sporormiaceae)
Generic description
Habitat terrestrial, saprobic (coprophilous). Ascomata small- to medium-sized, gregarious, immersed to erumpent, globose to subglobose, black, coriaceous; apex with a short papilla, or sometimes forming an ostiolar pore. Peridium thin, composed of several layers of thin-walled cells of textura angularis. Hamathecium of dense, delicate pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, clavate to cylindro-clavate, with a relatively long pedicel and an ocular chamber. Ascospores muriform, narrow oblong to cylindrical with rounded ends, dark brown, constricted at each septum.
Anamorphs reported for genus: none.
Literature: von Arx and Muller 1975; Cain 1934.
Type species
Pleophragmia leporum Fuckel, Jb. nassau. Ver. Naturk. 23-24 (1870) [1869-70]. (Fig. 78)
Fig. 78.
Pleophragmia leporum (from G. Fungi rhenani n2272, type). a Appearance of ascomata on the substrate surface. Note the ostiolar pore. b Section of a partial peridium. c, h Apical part of an ascus. Note the apical apparatus in (c). d Released ascospores. e, f Clavate Asci with pedicels. g Part of a broken ascospore. Note the crossing septa. Scale bars: a=0.5 mm, B=50 μm, c-f=20 μm, g, h =10 μm
Ascomata 330-480 μm highx320-430 μm diam., gregarious, immersed to slightly erumpent, globose to subglobose, black; apex with a short papilla, sometimes forming a ostiolar pore (Fig. 78a). Peridium 25-35 μm thick at the sides, composed of one cell type of lightly pigmented thin-walled cells of textura angularis, cells 6-10 μm diam., cell wall 1.5-2 μm thick (Fig. 78b). Hamathecium of numerous, long pseudoparaphyses, 1 – 2 μm broad, anastomosing not observed. Asci 160-250× 22.5-27.5 μm (x = 203.6 × 25μm, n =10), 8-spored, bitunicate, fissitunicate, clavate to cylindro-clavate, with a 20-50 μm long pedicel and an ocular chamber (to 5 μm wide × 2 μm high) (Fig. 78e and f). Ascospores 42-50×8-10 μm (x = 46 × 10μm, n =10), biseriate to uniseriate and partially overlapping, narrowly oblong to cylindrical with rounded ends, dark brown, often slightly curved, with 9 transverse septa with two crossing longitudinal septa in the centre, constricted at each septum, smooth-walled (Fig. 78c, d, g and h).
Anamorph: none reported.
Material examined: GERMANY, between Konigstein and Glashutten, on the same dung with Delitschia minuta. s.d. (G, Fungi rhenani n2272, type).
Notes
Morphology
Pleophragmia was formally established by Fuckel (1870) and monotypified by Pleophragmia leporum. The most comparable genus to Pleophragmia is Sporormia, as ascospores of both have no germ slits and the inner layer of wall is considerably thinner than the outer layer (Barr 1990a, b). But the muriform ascospores of Pleophragmia can be readily distinguished from the phragmosporous ascospores of Sporormia. Currently, only four species are accommodated under this genus (http://www.mycobank.org, 28-02-2009).
Phylogenetic study
None.
Concluding remarks
The presence of both transverse and crossing longitudinal septa is the most striking character of Pleophragmia, although the phylogenetic significance of this character is unclear.
Pleoseptum A.W. Ramaley & M.E. Barr, Mycotaxon 54: 76 (1995). (Phaeosphaeriaceae)
Generic description
Habitat terrestrial, saprobic? Ascomata medium-sized, scattered, or in small groups, immersed, globose to conoid, black, papillate, ostiolate. Peridium 1-layered. Hamathecium of dense, long cellular pseudoparaphyses, septate, branching. Asci 8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with furcate pedicel. Ascospores obliquely uniseriate and partially overlapping, muriform, ellipsoid, ovoid to fusoid, yellowish to dark brown.
Anamorphs reported for genus: Camarosporium (Ramaley and Barr 1995).
Literature: Ramaley and Barr 1995.
Type species
Pleoseptum yuccaesedum A.W. Ramaley & M.E. Barr, Mycotaxon 54: 76 (1995). (Fig. 79)
Fig. 79.
Pleoseptum yuccaesedum (from BPI 802381, holotype). a Appearance of ascomata scattered on the host surface. Only the upper region is visible. b Squash mount of asci in pseudoparaphyses. c Section of an ascoma. Note the peridium comprising cells of textura angularis. d, e Asci with short furcate pedicels. f, g Muriform dark-brown ascospores. Scale bars: a=0.5 mm, b = 40 μm, c =100 μm, d, e=20 μm, f, g =10 μm
Ascomata 300-500 μm diam., scattered, or in small groups of 2–3, immersed with a flattened top, globose to conoid, black, papillate, ostiolate (Fig. 79a). Papilla small, slightly protruding from the host surface. Peridium 30-50 μm thick at sides, up to 100 μm thick at the apex, I-layered, composed of 5-8 layers of heavily pigmented purplish-brown cells of textura angularis, cells 5-12 μm diam., cell wall 1–2 μm thick, apex cells smaller and walls thicker (Fig. 79c). Hamathecium of dense, long cellular pseudoparaphyses 1–2 μm broad, septate, branching (Fig. 79b). Asci 125-170(−195)× 15-22 μm (x = 153.8 × 19.3μm, n =10), 8-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a short, narrowed, furcate pedicel which is 10-20 μm long, with an ocular chamber best seen in immature asci (to 5 μm broadx3 μm high) (Fig. 79d and e). Ascospores 22–30×II-14 μm (x = 27.1 × 12.6μm, n =10) obliquely uniseriate and partially overlapping, ellipsoid, ovoid to fusoid, yellowish to yellowish brown, becoming reddish brown to dark brown, muriform, with 3-(4) transverse septa, constricted at the primary septum, part above central septum wider, vertical septa exist in each cell, ornamentation of foveolae in linear rows (Fig. 79f and g).
Anamorph: Camarosporium yuccaesedum Fairm. (Ramaley and Barr 1995).
Conidiomata 200-450 μm diam., pycnidial, immersed, scattered, subglobose to conoid, ostiolate. Macroconidiogenous cells determinate or indeterminate, enteroblastic, hyaline, smooth. Macroconidia holoblastic, 20-36×10-15 μm diam., ellipsoid to narrowly ovoid, muriform, yellowish brown, 3-7 transverse septa, constricted at the septa. Microconidiogenous cells produced near or in the ostiole, hyaline, smooth. Microconidia 5-10×5-7 μm diam., globose to ovoid, aseptate, hyaline, smooth.
Material examined: USA, Colorado, Montezuma County, hillside near entrance to Mesa Verde National Park, on dead leaves of Yucca baccata, 11 Oct. 1992, Ramaley Annette (9237A) (BPI 802381, holotype).
Notes
Morphology
Pleoseptum is a monotypic genus established by Ramaley and Barr (1995) and represented by P. yuccaesedum based on its “immersed ascomata, thick peridium, muriform ascospores, anamorphic stage and the linoeate ornamentation of the ascospores and conidia”. The shape of ascomata of Pleoseptum is comparable with that of Chaetoplea, but the peridium structure easily distinguishes them. Some species of Curreya, Leptosphaeria and Heptameria are comparable with Pleoseptum, but their anamorphic stages differ.
Pleoseptum yuccaesedum and its Camarosporium yuccaesedum anamorph both formed in the leaves of Yucca baccata and the ascomata and conidiomata were indistinguishable. Camarosporium is the anamorph of diverse teleomorph genera included in Botryosphaeriales and Cucurbitariaceae (Kirk et al. 2008). The genus is in need of revision (Sutton 1980) and is no doubt polyphyletic.
Phylogenetic study
None.
Concluding remarks
The placement of Pleoseptum under Phaeosphaeriaceae is still tentative.
Pleospora Rabenh. ex Ces. & De Not., Comm. Soc. crittog. Ital. 1: 217 (1863). (Pleosporaceae)
Generic description
Habitat terrestrial, saprobic or parasitic. Ascomata small- to medium-sized, immersed, erumpent to superficial, papillate, ostiolate. Peridium thin. Hamathecium of dense, cellular pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical to clavate, with furcate pedicel and small inconspicuous ocular chamber. Ascospores muriform, brown or pale brown, with or without sheath.
Anamorphs reported for genus: Stemphylium (Simmons 1985).
Literature: Barr 1981; Frisullo and Braun 1996; Kodsueb et al. 2006a; Luttrell 1951; Wehmeyer 1946, 1961, 1975; Zhang et al. 2009a.
Type species
Pleospora herbarum (Pers.) Rabenh., Klotzschii Herb. Viv. Myco1. 2: no. 547 (1854). (Fig. 80)
Fig. 80.
Pleospora herbarium (from E, Krieger 683). a Immersed ascomata scattering on host surface. b Ascomata in small groups. Note: the surface layer of the host is removed. c Section of an ascoma. Note the peridium cells of textura angularis. d, e. Asci with short pedicels f-j Ascospores. Scale bars: a, b=0.5 mm, c=100 μm, d, e=30 μm, f-j = 20 μm
≡ Sphaeria herbarum Pers., Syn. meth. fung. (Gottingen) 1: 78 (1801).
Ascomata 130-220 μm highx250-420 μm diam., scattered, or in small groups of 2–3, immersed, semi-immersed to erumpent, broadly to narrowly oblong and flattened, with flattened base not easily removed from the substrate, wall black, papillate, ostiolate (Fig. 80a and b). Peridium 30-50 μm thick on sides, thinner at the base, coriaceous, 2-layered, outer layer composed of one or two layers of heavily pigmented thick-walled cells of textura angularis, cells 5-10 μm diam., ce11 wall 2-4 μm thick, apex cells smaller and walls thicker, inner layer composed of hyaline thin-walled cells of textura angularis, 8-12 μm diam., wall hyaline, 0.5-1.5 μm thick (Fig. 80c). Hamathecium of dense, cellular pseudoparaphyses, 2–3 μm broad, filling the gaps between the asci. Asci 100-210 × 27.5-30 μm (x = 142.2 × 28.3μm, n =10), 8-spored, bitunicate, fissitunicate, broadly cylindrical to clavate, with a short, thick, furcate pedicel, 8-12(−20) μm long, with small inconspicuous ocular chamber (ca. 3 μm widex 1 μm high) (Fig. 80d and e). Ascospores 28-38× 12.5-15 μm (x = 33 × 14.5μm, n =10), ellipsoidal, straight or sometimes curved, with broadly rounded ends and upper hemispore slightly shorter and broader; spores usually divided by 3 A-transsepta, all 4 segments by longisepta and then by one stratum of B-transsepta (mature spores as a rule with 7 transsepta, 3A+4B), yellowish brown, smooth; each hemispore with thick gelatinous sheath, the lower one with umbilicus (sheaths fused in mature spores) (Fig. 80f, g, h, i, j and k).
Anamorph: Stemphyllium herbarum E. Simmons (Simmons 1985).
Material examined: GERMANY, on stalks of MelUotusalla? at the bank of the Elbe in Konigstein, 1882 (E, Krieger 683); as Sphaeria herbarum Persoon Syn. fung. p. 78 (E, 81); as Sphaeria herbarum Fries, Scleromyceti Sueciae 38 (E, lectotype).
Notes
Morphology
Pleospora was originally assigned within Sphaeriales. Subsequently, it was assigned within Pseudosphaeriales and Pleosporales (Wehmeyer 1961). Pleospora is a large group, which is widely distributed and associated with a wide range of species of monocotyledons as well as dicotyledons (Wehmeyer 1975). A11 species of Pleospora have muriform ascospores (Wehmeyer 1961, 1975). Pleospora has downward growing pseudoparaphyses within the ascomata of “Pleospora-type” development (Luttrell Univ. Mo. Stud. 1951), which subsequently served as a diagnostic character. However, only a limited number of species had detailed studies on this character (Wehmeyer 1961). The heterogeneous nature of Pleospora has been noted, and several subgenera have been erected, such as Scleroplea to include all “sclerotioid” species of Pleospora, Teichosporoides to accommodate species of Pleospora with immersed ascomata, Pleosphaeria for those having superficial and setose ascomata (Wehmeyer 1961). Similarly, Cucurbitaria, Fenestella and Montagnula are also separated as a section from Pleospora. Most of these subgenera are currently at genus rank.
Phylogenetic study
The polyphyletic nature of Pleospora is clear (Kodsueb et al. 2006a), and those that stain the woody substrate purple should be assigned to Amniculicolaceae (Zhang et al. 2009a).
Concluding remarks
As some Pleospora species have a wide range of host spectrum, especially on both monocotyledons and dicotyledons, it is highly possible they are cryptic species.
Preussia Fuckel, Hedwigia 6: 175 (1867) [1869-70]. (Sporormiaceae)
Generic description
Habitat terrestrial, saprobic (on decaying fibers or coprophilous). Ascomata small- to medium-sized, cleistothecial or perithecial, solitary or scattered on substrate surface, globose, membraneous, black. Peridium thin, composed of thick-walled, poly-angular cells from the surface view. Pseudoparaphyses not observed. Asci (4-) 8-spored, bitunicate, clavate to broadly clavate, with a long and thin and furcate pedicel. Ascospores 3-6 seriate to uniseriate near the base, cylindrical with rounded ends, brown, septate, easily breaking into partspores, with germ slits in each cell. Anamorphs reported for genus: Phoma (von Arx 1973; Cain 1961; Malloch and Cain 1972).
Literature: Ahmed and Cain 1972; Arena1 et al. 2005; von Arx 1973; von Arx and van der Aa 1987; Auerswald 1866; Barr 1987b, 1990a; Boylan 1970; Cain 1961; Eriksson 1992; Fuckel 1866; Guarro et al. 1981, 1997a, b; Khan and Cain 1979a, b; Kruys and Wedin 2009; Lodha 1971; Lorenzo 1994; Luck-Allen and Cain 1975; Maciejowska and Williams 1963; Malloch and Cain 1972; Munk 1957; Narendra and Rao 1976; Rai and Tewari 1963; Sultana and Malik 1980.
Type species
Preussia funiculata (Preuss) Fuckel, Jb. nassau. Ver. Naturk. 23-24: 91 (1870) [1869-70]. (Fig. 81)
Fig. 81.
Preussia funiculata (from TRTC 46985). a Superficial cleistothecoid ascomata. b Part of peridium from front view. c Squash mounts showing a large number of asci. d A clavate ascus with a long and thin pedicel. Scale bars: a= 0.5 mm, b=20 μm, c, d=50 μm
≡ Perisporium funiculatum Preuss, Fung. Hoyersw.: no. 145 (1851).
Ascomata 240-500 μm diam., cleistothecial, solitary, scattered on substrate, superficial, globose, membraneous, black (Fig. 81a). Peridium thin, composed of thick-walled, poly-angular cells in front view (Fig. 81b). Pseudoparaphyses not observed. Asci 42-65×20-25 μm (x = 55.8 × 21.8μm, n= 10), (4-)8-spored, bitunicate, broadly clavate, with a long and thin and furcate pedicel, up to 115 μm long, ocular chamber not observed (Fig. 81c and d). Ascospores 30-40 × 6.3-7.5 μm (x = 35.6 × 6.9μm, n =10), 3-6 seriate to uniseriate near the base, cylindrical with rounded ends, brown, with 3 transverse septa, easily breaking into partspores, central cells round in transverse section but rectangular in vertical section, with a germ slit in each ce11, 6.5-8.5× 4-7.5 μm broad, apical cells 8.8-10×5-7 μm broad, sheath not observed.
Anamorph: none reported.
Material examined: USA, Ontario, York Co., Nashville, on old jute sack on ground, 1 Jul. 1960, leg. & det. R.F. Cain (in part Preussia typharum) (TRTC 46985).
Notes
Morphology
Preussia was introduced by Fuckel (1866) to accommodate species having cleistothecioid ascomata, bitunicate asci, multi-septate ascospores with a germ slit in each cell and with a gelatinous sheath, and occurring in soil or plant debris. Preussia, Sporormia and Sporormiella are regarded as closely related genera, which share numerous morphological characters. Sporormia can be distinguished from Preussia by its perithecioid ascomata and cylindrical asci. The only distinguishing morphological character for Preussia from Sporormiella are the cleistothecioid ascomata in Preussia (Barr 2000; Cain 1961), but this has been shown to have little phylogenetic significance (von Arx 1973; Zhang et al. 2009a). Substrate preference has been used to distinguish species of Sporormiella and Preussia, with Sporormiella being restricted to a coprophilous habitat, while Preussia grows in plant debris, wood or soil (von Arx and van der Aa 1987). This proposal was rejected, as P. intermedia (Clum) Cain can be isolated from either soil or dung (Guarro et al. 1997b). In a review of Preussia, Cain (1961) accepted 12 species, and some of them are coprophilous. Subsequently, numerous additional new species have been published (Arena1 et al. 2005; Barr 1987b, 1990a; Boylan 1970; Eriksson 1992; Guarro et al. 1981, 1997a, b; Khan and Cain 1979a; Lodha 1971; Lorenzo 1994; Luck-Allen and Cain 1975; Maciejowska and Williams 1963; Malloch and Cain 1972; Narendra and Rao 1976; Rai and Tewari 1963; Sultana and Malik 1980). Currently, 84 species are listed under Preussia (http://www.mycobank.org/mycotaxo.aspx, 10/2010) and Kirk et al. (2008) estimates there are 51 species.
Phylogenetic study
In phylogenetic analysis based on ITS, nLSU, mtSSU and β-tubulin gene fragments, Preussia, Sporormiella and Spororminula clustered together. Thus, Sporormiella to- gether with Spororminula are treated as synonyms of Preussia (Kruys and Wedin 2009).
Concluding remarks
Preussia sensu lato (including Sporormiella and Spororminula) based on both morphology and molecular data should be accepted pending further research.
Quintaria Kohlm. & Volkm.-Kohlm., Bot. Mar. 34: 34 (1991). (Pleosporales, genera incertae sedis)
Habitat marine, saprobic. Ascomata medium-sized, scattered or loosely gregarious, immersed, mostly subglobose, rarely globose, with a protruding papilla, ostiolate. Peridium thin, 2-layered, coriaceous, thicker near the apex. Hamathecium of dense, filamentous, trabeculate pseudoparaphyses, branching and anastomosing between and above asci. Asci 8-spored, bitunicate, fissitunicate, cylindro-clavate, with a short furcate pedicel. Ascospores biseriate, broadly fusoid to fusoid, hyaline, mostly 5-septate, rarely up to 7-septate.
Anamorphs reported for genus: none.
Literature: Hyde and Goh 1999; Kohlmeyer and Volkmann-Kohlmeyer 1991; Suetrong et al. 2009; Zhang et al. 2008b.
Type species
Quintaria lignatilis (Kohlm.) Kohlm. & Volkm.-Kohlm., Bot. Mar. 34: 35 (1991). (Fig. 82)
Fig. 82.
Quintaria lignitalis (from J. Kohlmeyer No. 4365a, holotype). a Ascomata immersed in substrate. b Section of an ascoma. Note the thin peridium and elongated papilla. c, e Asci embedded in pseudoparaphyses. d Five septate fusoid hyaline ascospores. Scale bars: a=0.5 mm, b=200 μm, c, e= 50 μm, d =20 μm
≡ Trematosphaeria lignatilis Kohlm., Marine Ecology, [Pubblicazioni della Stazione Zoologica Napoli I] 5(4): 365 (1984).
Ascomata 240-500 μm diam., scattered or loosely gregarious, immersed, globose to subglobose, coriaceous, ostiolate, ostiole is encrusted with thick-walled black cells, papilla up to 400 μm long (Fig. 82a). Peridium thin, 20-30 μm wide, thinner at the base, thicker near the apex, up to 300 μm, 2-layered, outer layer composed of hyphoid cells, inner layer composed of compressed cells of textura angularis (Fig. 82b). Hamathecium of dense, filamentous, trabeculate pseudoparaphyses, 0.8-1.5 μm broad, branching and anastomosing between and above asci (Fig. 82e). Asci 175-250×25-35 μm (x = 220 × 28mm, n =10), 8-spored, bitunicate, fissitunicate, cylindro-clavate, with a short, furcate pedicel, to 20 μm long (Fig. 82c and e). Ascospores 55-73×12.5-15 μm (x = 63.3 × 13.1mm, n= 10), biseriate, broadly fusoid to fusoid, usually slightly curved, smooth, hyaline, mostly 5-septate, rarely up to 7-septate, smooth-walled, lacking a sheath.
Anamorph: none reported.
Material examined: BELIZE, Twin Cays, on attached dead tip of prop root of Rhizophora mangle, with ship-worms, 3 Apr. 1983, leg. & det. J.K. Kohlmeyer (J. Kohlmeyer No. 4365a, holotype).
Notes
Morphology
Quintaria was introduced to accommodate the marine fungus, Trematosphaeria lignatilis, based on its immersed ascomata with rounded bases, black incrustations surrounding the sides of the ostiolar canal as well as its hyaline ascospores (Kohlmeyer and Volkmann-Kohlmeyer 1991). Subsequently, three more species were introduced to this genus, viz. Q. aquatica K.D. Hyde & Goh, Q. microsporum Yin. Zhang, K.D. Hyde & J. Fourn. and Q. submerse K.D. Hyde & Goh, which are all from freshwater (Hyde and Goh 1999; Zhang et al. 2008b).
Phylogenetic study
Multigene phylogenetic study indicated that Quintaria lignatilis forms a separate sister clade to other families of Pleosporales, which may represent a new familial linage (Suetrong et al. 2009). This was supported by phylogenetic studies which place the freshwater Q. submersa separate from Q. lignatilis (Schoch et al. 2009; Suetrong et al. 2009; Plate 1).
Concluding remarks
The freshwater members of Quintaria should likely be excluded from this genus, and only the generic type, Q. lignatilis retained, but this needs confirmation.
Roussoella Sacc., in Saccardo & Paoletti, Atti Inst. Veneto Sci. lett., ed Arti, Ser. 3 6: 410 (1888). (Arthopyreniaceae (or Massariaceae))
Generic description
Habitat terrestrial, saprobic. Ascomata medium-sized, clustered, immersed in host tissue, forming under darkened, slightly raised, somewhat liner or dome-shaped stroma on the host, with a flush intra-epidermal papilla; immersed under clypeus, papillate, ostiolate. Peridium thin, comprising several layers of compressed cells. Hamathecium of dense, long trabeculate pseudoparaphyses, embedded in mucilage, hyaline, anastomosing and septate. Asci 8-spored, bitunicate, cylindrical, with furcate pedicel, and a conspicuous ocular chamber. Ascospores uniseriate to partially overlapping, fusoid or ellipsoidal, brown, 1-septate, constricted at the septum. Anamorphs reported for genus: Cytoplea (Hyde et al. 1996a).
Literature: Hyde et al. 1996a; Hyde 1997; Ju et al. 1996; Tanaka et al. 2009.
Type species
Roussoella nitidula Sacc. & Pao1., Atti Ist. Veneto Sci., Ser. 6, 6:410. (1888). (Fig. 83)
Fig. 83.
Roussoella nitidula (from PAD Pacl. 2484, hototype). a Appearance of the stroma on host surface. b Asci and pseudoparaphyses. c, d Long cylindrical furcate asci. e-h. Ascospores. Note the striate ornamentation. Scale bars: a=0.5 mm, b-d=20 μm, e-h =10 μm
Ascomata 160-200 μm highx400-500 μm diam., clustered, immersed in host tissue, forming under darkened, slightly raised, somewhat liner or dome-shaped stroma on the host, with a flush intra-epidermal papilla; in vertical section subglobose with a flattened base, immersed under clypeus, subglobose with a flattened base, papillate, ostiolate (Fig. 83a). Peridium up to 20 μm thick, comprising several layers of compressed cells. Hamathecium of dense, long trabeculate pseudoparaphyses, 1-1.5 μm broad, embedded in mucilage, anastomosing and septate. Asci 123-220×7-11 μm, 8-spored, bitunicate, cylindrical, with furcate pedicels, and a conspicuous ocular chamber (Fig. 83b, c and d). Ascospores 17.5-22×5.5-8 μm, uniseriate to partially overlapping, fusoid or ellipsoidal, brown, 1-septate, constricted at the septum, ornamented with longitudinal wall striations and surrounded by a wide mucilaginous sheath (Fig. 83e, f, g and h).
Anamorph: Cytoplea hysterioides K.D. Hyde (Hyde et al. 1996a).
Material examined: MALAYSIA, Malacca, on culms of Bambusa Bar & Grill, 1885, B. Scortechini 15 (PAD, Roussoella nitidula Sacc. Pao1. 2484, holotype, on a loose label Roussoella nitidula S. & P. Est Phyllachora phaeo-didym./15 prob. original material from Malacca Peninsula).
Notes
Morphology
Roussoella was introduced by Saccardo for the single species R. nitidula Sacc. & Paol. (Saccardo and Paoletti 1888). It was redescribed by Hyde et al. (1996a) and the anamorph of Roussoella hysterioides (Ces.) Hohn., Cytoplea hysterioides K.D. Hyde was determined and described. Roussoella was then reviewed by Hyde (1997) and a modified key for Roussoella species was provided based on the one proposed by Ju et al. (1996). Roussoella is characterized as having immersed ascomata containing long cylindrical asci and brown 1-septate ornamented ascospores. In this study, we have checked the type species and it matches Hyde et al. (1996a). The asci are bitunicate, but we could not observe the fissitunicate dehiscence.
Phylogenetic study
Species of Roussoella, Roussoellopsis as well as Arthopyrenia salicis form a robust phylogenetic clade, which form a sister group with pleosporalean families, but the generic type of Roussoella (R. nitidula) was not included in the phylogenetic study (Tanaka et al. 2009).
Concluding remarks
The bambusicolous habitat of Roussoella is a striking character at generic rank classification but its relationship to the lichenized Arthopyrenia is unexpected and will require more analysis.
Saccharicola D. Hawksw. & O.E. Erikss., in Eriksson & Hawksworth, Mycologia 95: 431 (2003). (Massarinaceae)
Generic description
Habitat terrestrial, parasitic. Ascomata medium-sized, solitary, scattered, immersed, globose to subglobose, carbonaceous, papillate, ostiolate. Peridium relatively thin, composed of one cell type of pale brown to hyaline pseudoparenchymatous cells. Hamathecium of trabeculate pseudoparaphyses. Asci bitunicate, 8-spored, cylindro-clavate to clavate. Ascospores biseriate and sometimes laterally uniseriate, fusoid with narrowly rounded ends, septate, constricted at the septa, the upper second cell becoming pigmented when mature, smooth or verruculose. Anamorphs reported for genus: Stagonospora (Eriksson and Hawksworth 2003; Kaiser et al. 1979; Leuchtmann 1984).
Literature: Eriksson and Hawksworth 2003.
Type species
Saccharicola bicolor (D. Hawksw., W.J. Kaiser & Ndimande) D. Hawksw. & O.E. Erikss., Mycologia 95: 431 (2003). (Fig. 84)
Fig. 84.
Saccharicola bicolor (from IMI 215888, holotype). a Section of an ascomata immersed in the host tissue. b Section of a partial pycnidia. Note the phragmosporous conidia. c Clavate ascus with ocular chamber and short pedicel. d Ascospores. Note the pigmented central cell(s). Scale bars: a, b = 50 μm, c=20 μm, d =10 μm
≡ Leptosphaeria bicolor D. Hawksw., W.J. Kaiser & Ndimande, Mycologia 71: 483 (1979).
Ascomata 125-175 μm highx175-220 μm diam., solitary, scattered, immersed, globose to subglobose, wall black, carbonaceous, with a protruding papilla, with a central ostiole (Fig. 84a). Peridium 15-20 μm thick composed of one cell type of pale brown to hyaline pseudoparenchymatous cells, becoming thicker near the apex (Fig. 84a). Hamathecium of 1–2 μm broad, filliform, hyaline, septate pseudoparaphyses, branching and anastomosing in mucilage. Asci (90-)125-150×(20-)25-30 μm, 8-spored, with a short pedicel, bitunicate, cylindro-clavate to clavate, with a small ocular chamber at the apex (Fig. 84c). Ascospores 29-42×8-11 μm, biseriate and sometimes laterally uniseriate, fusoid with narrowly rounded ends, (2-)3-septate, deeply constricted at the septa, the upper second cell subhyaline to pale brown when young and becoming dark brown to almost black at maturity, smooth or verruculose (Fig. 84d). (data from the original description by Kaiser et al. (1979) because of the bad condition of the type material).
Anamorph: Pycnidia typical of Stagonospora (Sphaeropsidales), “scattered, arising singly both on the host and in pure culture, in culture generally surrounded by an envelope of mycelial hyphae, numerous, immersed on the host, but nearly superficial in culture, subglobose to slightly applanate, black, 150-250 μm diam., with a central slightly papillate ostiole, lacking a distinct neck; walls mainly 15-20 μm thick, composed of three to six layers of pseudoparenchymatous cells, the outermost layers dark brown and inner pale brown to hyaline cells somewhat compressed radially, very variable in size, cells of the outer layers mainly 7-12 μm long × 4-6 μm wide in vertically section and 10-12 μm diam. in surface view, wall not or only slightly thicked near the ostiole. Conidiogenous cells lining the inner surface of the pycnidial cavity, holoblastic, minute and difficult to distinguish from the pseudoparenchymatous cells with which they are mixed, mammiform with a flattened apex, hyaline, smooth walled, about 4-6 μm tall and 4-6 μm wide. Conidia copiously produced, ellipsoid, with somewhat truncated ends, hyaline, smooth walled, (2-)3 septate, not or slightly constricted at the septa, often guttulate, rather thin walled, (21-)24-28(−34) μmx7-8.5(−11.5) μm” (from Kaiser et al. 1979).
Material examined: KENYA, near Nairobi, on leaves of Saccharum officinarum L.; 24 Aug. 1977; leg. W.J. Kaiser (IMI 215888, holotype).
Notes
Morphology
Saccharicola was separated from Leptosphaeria as a new genus based on its Stagonospora anamorph and its biotrophic habitat in leaves of sugar cane, and two species were included, i.e. Saccharicola bicolor and S. taiwanensis (J.M. Yen & C.C. Chi) O.E. Erikss. & D. Hawksw. (Eriksson and Hawksworth 2003). Saccharicola is characterized by its parasitic habitat on monocots, small ascomata, bitunicate asci, presence of pseudoparaphyses as we11 as its 3-septate ascospores (Eriksson and Hawksworth 2003).
Phylogenetic study
Based on the limited phylogenetic analysis of SSU sequences, Saccharicola is considered to be closely related to Massarina eburnea, the generic type of Massarina (Eriksson and Hawksworth 2003). Thus, Saccharicola was assigned to Massarinaceae, which includes Keissleriella, Massarina and Saccharicola (Eriksson and Hawksworth 2003).
Concluding remarks
Based on the parasitic habitat on monocots and its small ascomata and Stagonospora (or Cercospora? for S. taiwanensis, see Eriksson and Hawksworth 2003; Shoemaker and Babcock 1989b) anamorph, Saccharicola seems more similar to Pleosporineae. Further molecular study is needed for confirmation.
Salsuginea K.D. Hyde, Bot. Mar. 34: 315 (1991). (Pleosporales, genera incertae sedis)
Generic description
Habitat marine, saprobic. Ascomata large, solitary, fusoid, conical or subglobose, with or without a flattened base, immersed under a darkened clypeus, papillate, ostiolate. Peridium thin, composed of round cells (in cross section) at sides, fusing at the top with the clypeus, thin at the base. Hamathecium of dense, long trabeculate pseudoparaphyses, anastomosing, embedded in mucilage. Asci 8-spored, bitunicate, fissitunicate, clavate to cylindro-clavate, pedunculate, with a large ocular chamber and conspicuous apical ring. Ascospores uniseriate, obovoid, brown to black, with hyaline apical germ pores, 1-septate, constricted at the septum, dark brown with paler apical cells, lacking sheath, smooth. Anamorphs reported for genus: none.
Literature: Hyde 1991a; Suetrong et al. 2009.
Type species
Salsuginea ramicola K.D. Hyde, Bot. Mar. 34: 316 (1991). (Fig. 85)
Fig. 85.
Salsuginea ramicola (from BRIP 17102, holotype). a Habitat section of an ascoma. b Section of the partial peridium. C Clavate mature and immature asci. d Ascospores within ascus. e Apical part of immature asci. f Ascospores with an apical chamber at each end. Scale bars: a=0.5 mm, b-e=50 μm, f= 10 μm
Ascomata 1040-2600 μm highx455-1430 μm diam., solitary, fusoid, conical or subglobose, with or without a flattened base, immersed under a darkened clypeus, papillate, ostiolate, ostiole rounded (Fig. 85a). Peridium up to 39 μm thick, composed of round cells (in cross section) at sides, fusing at the top with the clypeus, thin at the base (Fig. 85b). Hamathecium of dense, long trabeculate pseudoparaphyses, 1–2 μm broad, anastomosing, embedded in mucilage. Asci 440-512×29-34 μm, 8-spored, bitunicate, fissitunicate, clavate to cylindro-clavate, pedunculate, with a large ocular chamber and conspicuous apical ring (Fig. 85c and e). Ascospores 59-72×24-30 μm, uniseriate, obovoid, brown to black, with hyaline apical germ pores, 1-septate, constricted at the septum, dark brown with paler apical cells, lacking sheath, smooth (Fig. 85d and f).
Anamorph: none reported.
Material examined: THAILAND, Ranong mangrove, Aegiceras corniculatum (L.) Blanco., Oct. 1988, leg. & det. K.D. Hyde (BRIP 17102, holotype).
Notes
Morphology
Salsuginea was introduced to accommodate the mangrove fungus, S. ramicola, which is characterized by large, immersed, ostiolate and papillate ascomata under a clypeus, dense, trabeculate pseudoparaphyses embedded in gel matrix, fissitunicate, 8-spored, cylindrical asci with short pedicel and conspicuous apical apparatus, 1-septate, dark brown ascospores with paler apical cells (Hyde 1991a). Salsuginea is considered closely related to Helicascus and Caryospora, and they are all proposed to Melanommataceae (Hyde 1991a).
Phylogenetic study
Based on a multigene phylogenetic analysis, Salsuginea ramicola nested in a paraphyletic clade within Pleosporales; its familial status is undetermined (Suetrong et al. 2009).
Concluding remarks
It has been shown that trabeculate pseudoparaphyses has no phylogenetic significance at familial rank, so a well resolved phylogeny based on DNA comparisons will be necessary to categorize this genus.
Semidelitschia Cain & Luck-Allen, Mycologia 61: 581 (1969). (Delitschiaceae)
Generic description
Habitat terrestrial, saprobic (coprophilous). Ascomata immersed to slightly erumpent, scattered, coriaceous, papillate, ostiolate. Hamathecium of non-typical trabeculate pseudoparaphyses, thin, septate, rarely branching. Asci cylindrical, pedicellate, each with a conspicuous large apical ring. Ascospores non-septate, dark brown to nearly black, each with an elongated germ slit.
Anamorphs reported for genus: none.
Literature: Barr 2000; Cain and Luck-Allen 1969.
Type species
Semidelitschia agasmatica Cain & Luck-Allen, Mycologia 61: 581 (1969). (Fig. 86)
Fig. 86.
Semidelitschia agasmatica (from TRTC 40697, holotype). a Immersed ascomata scattered on the surface of the substrate. b Squash of ascoma. Note the numerous released asci. C Apical ring of cylindrical asci. d One-celled ascospores. Note the germ slits (see arrow). e Cylindrical ascus. Note the tapering pedicel. Scale bars: a=0.5 mm, b-e =100 μm
Ascomata 550-900 μm diam., solitary, immersed to erumpent, globose to subglobose, black, semicoriaceous, smooth-walled, with a protruding papilla and a conspicuous ostiole (Fig. 86a). Peridium thin, comprising multi-angular cells from front view. Hamathecium of non-typical trabeculate pseudoparaphyses, 1–2 μm broad, septate, rarely branching, anastomosing not observed. Asci 410-505 × (38-) 43-50 μm (x = 470.6 × 46.4μm, n =10), 8-spored, bitunicate, fissitunicate dehiscence not observed, cylindrical, with a thick pedicel which is up to 90 μm long, and with a large and conspicuous dome-shaped ocular chamber surrounded by apical ring (to 18 μm widex4 μm high) (Fig. 86b and e). Ascospores 53-65×30-38 μm (x = 61.3 × 34.1μm, n=10), uniseriate to obliquely uniseriate and partially over-lapping, broad fusoid to subglobose, hyaline when young, then becoming yellowish brown, reddish brown and nearly black and opaque when mature, non-septate, smooth-walled, with a full length germ slit, surrounded by a broad gelatinous sheath (Fig. 86c and d).
Anamorph: none reported.
Material examined: CANADA, Alberta, North of Beaver Mines, on sheep dung, 28 Jul. 1962, E.R. Luck-Allen, (TRTC 41607, paratype); USA, Montana: Gallatin County, 60 min S of Bozeman, on sheep dung, 2 Sept. 1957, Cain (TRTC 42032, paratype); Stillwater County Columbus, on cow dung, 3 Sept. 1957, Cain (TRTC 42031, paratype); South Dakota, Meade Co.: South of Wall, on cow dung, 3 Sept. 1962, Cain (TRTC 40697, holotype).
Notes
Morphology
Semidelitschia was formally established by Cain and Luck-Allen (1969) and was assigned to Sporormiaceae. Although it is similar to Delitschia, it differs as the ascospores are 1-celled, as opposed to 2-celled. Subsequently, Semidelitschia was transferred to Delitschiaceae together with Delitschia (Barr 2000). Currently, three species are listed under this genus, i.e. S. agasmatica Cain & Luck-Allen, S. nanostellata A.E. Bell & Mahoney and S. tetraspora J.H. Mirza & S.M. Khan (Index Fungorum) although the number of species in the genus are given as only two in Kirk et al. (2008).
Phylogenetic study
None.
Concluding remarks
This is a clearly defined genus that differs from Delitschia in having 1-celled ascospores. Cultures of S. agasmatica are needed for sequencing and for establishing the placement and uniqueness of the genus.
Setomelanomma M. Morelet, BU11. Soc. Sci. nat. Arch. Toulon et du Var 227:15 (1980). (Phaeosphaeriaceae)
Generic description
Habitat terrestrial, hemibiotrophic or biotrophic. Ascomata small, solitary, scattered, immersed, erumpent to superficial, globose to subglobose, black; with or without a small papilla, apex covered with setae and a periphysate ostiole. Peridium thin, 1-layered, composed of several layers of cells of textura angularis. Hamathecium of dense, 1–2 μm broad pseudoparaphyses, septate, anastomosing. Asci 8-spored, bitunicate, broadly cylindrical. Ascospores fusoid to broadly clavate, pale brown to brown, 3-septate. Anamorphs reported for genus: none.
Literature: Leonard and Suggs 1974; Morelet 1980; Rossman et al. 2002; Schoch et al. 2009; Zhang et al. 2009a.
Type species
Setomelanomma holmii M. Morelet, Bulletin de la Societe des Sciences naturelles et d’Archeologie de Toulon et du Var 36 (no. 227): 15 (1980). (Fig. 87)
Fig. 87.
Setomelanomma holmii (from UPS F-117969 (slide), isotype). b a, b Asci with short pedicels in pseudoparaphyses. C Partial view of ascus. d Branching and septate pseudoparaphyses. a Three-septate lightly pigmented ascospores in ascus. Scale bars: a-e =10 μm
(Some information in the following description is from Rossman et al. (2002))
Ascomata 80-250 μm diam., solitary, scattered, immersed, erumpent to superficial, globose to subglobose, black, with setae; with or without a small papilla, apex covered with setae and a periphysate ostiole. Peridium 15-25 μm thick, 1-layered, composed of several layers of cells of textura angularis, ce11 wa11 thinner and more lightly pigmented towards centrum, cell wall thicker near the apex. Hamathecium of dense, 1–2 μm broad pseudoparaphyses, thicker near the base, septate, anastomosing (Fig. 87a and d). Asci 70-100×11-14 μm, 8-spored, bitunicate, broadly cylindrical with a short, thick, furcate pedicel, with a small ocular chamber (Fig. 87a, b and c). Ascospores 16-21×5-6.5 μm, obliquely uniseriate and partially overlapping to biseriate, fusoid to broadly clavate with broadly to narrowly rounded ends, pale brown to brown, 3-septate, slightly constricted at the median septum, smooth (Fig. 87e).
Anamorph: none reported.
Material examined: FRANCE, Leuglay, on dying twigs of Picea pungens. 8 May 1987, leg. M. Morelet (UPS F-117969 (slide), isotype).
Notes
Morphology
Setomelanomma was formally established by Morelet (1980) as a monotypic genus represented by S. holmii, which was collected in France. The description, however, is not detailed and lacks illustrations. Rossman et al. (2002) collected this species in North America and detailed studies were conducted including both morphology and phylogeny. The bitunicate, broadly cylindrical asci, cellular pseudopar-aphyses as we11 as the pale brown, septate ascospores with a median primary septum point Setomelanomma to Phaeosphaeriaceae as defined by Barr (1992a) and Eriksson et al. (2002) (Rossman et al. 2002). However, its setose ascomata, brown and 3-septate ascospores together with its residence in conifers distinguish it from all other genera under Phaeosphaeriaceae (Rossman et al. 2002). Setomelanomma is mostly comparable with Kalmusia and Phaeosphaeria. Setomelanomma can be distinguished from Kalmusia by its erumpent to superficial ascomata with almost no papilla, and Phaeosphaeria differs from Setomelanomma by its host spectrum and reported anamorphic stages (Rossman et al. 2002). Currently, five species are included in Setomelanomma, namely S. holmii, S. monoceras, S. prolata K.J. Leonard & Suggs, S. rostrata (K.J. Leonard) K. J. Leonard & Suggs and S. turcica (Luttr.) K.J. Leonard & Suggs (http://www.mycobank.org/, 06/2010).
Phylogenetic study
Setomelanomma forms a well supported phylogenetic clade with other members of Phaeosphaeriaceae (Schoch et al. 2009; Zhang et al. 2009a).
Concluding remarks
None.
Shiraia Henn., Bot. Jb. 28: 274 (1900). (Pleosporales, genera incertae sedis)
Generic description
Habitat terrestrial, parasitic. Ascostroma warty-like or tuber-like. Ascomata medium to large, subglobose, gregarious on the surface layer of ascostroma, immersed, ostiolate, with a small black opening seen on the surface of the ascostroma, ostiole rounded. Hamathecium of dense, long trabeculate pseudoparaphyses, anastomosing and branching between the asci. Asci bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a short furcate pedicel, with a big and truncate ocular chamber. Ascospores obliquely uniseriate and partially overlapping, narrowly fusoid to fusoid or broadly fusoid with tapering or narrowly rounded ends, hyaline to pale brown or brown, muriform.
Anamorphs reported for genus: coelomycetous with muriform conidia (see Liu 2009).
Literature: Cheng et al. 2004; Hino 1961; Kishi et al. 1991; Liu 2009; Morakotkarn et al. 2008.
Type species
Shiraia bambusicola Henn., Bot. Jb. 28: 274 (1900). (Fig. 88)
Fig. 88.
Shiraia bambusium (from IFRD 2040). a Ascostroma form a nubby structures on the twigs of host. b Vertical section of an ascostroma. Note the reddish staining of the inner tissue. c, d Cylindrical asci with a short pedicel. e-g Muriform fusoid hyaline ascospores. Scale bars: a=1 cm, b =1 mm, c, d= 50 μm, e-g=20 μm
Ascostroma 1-1.5 cm highx1–2.5 cm diam., subgtobose, oblong to irregular, slightly pink with cracking surface. Ascomata 350-800 μm highx300-700 μm diam., subglobose, gregarious on the surface layer of ascostroma, immersed, ostiolate, with a small black opening seen on the surface of the ascostroma, ostiole rounded, the inner tissue of ascostroma carnation red (Fig. 88a and b). Hamathecium of dense, long trabeculate pseudoparaphyses, 0.8-1.5 μm broad, anastomosing and branching between the asci. Asci 3 0 0 – 4 2 5 × 2 0 – 3 5 μm (x = 360.5 × 28,um, n = 10), 6-spored, bitunicate, fissitunicate, cylindrical to cylindro-clavate, with a short furcate pedicel, up to 50 μm long, with a big and truncate ocular chamber (Fig. 88c and d). Ascospores 62.5-80×17.5-22.5 μm (x = 72.3 × 19.3mm, n =10), obliquely uniseriate and partially overlapping, narrowly fusoid to fusoid with tapering or narrowly rounded ends, hyaline turning pale brown when mature, muriform, with 9-13 transversal septa, 1-3 longitudinal septa in central cells, slightly constricted at the septa, usually with a gelatinous cap at each end (Fig. 88e, f and g).
Anamorph: coelomycetous with muriform conidia (see Liu 2009).
Material examined: CHINA, Zhejiang, Hangzhou, Panan, on bamboom, 15 Jun. 2009, leg. Liu Yongxiang (IFRD 2040).
Notes
Morphology
Shiraia is reported as a parasite on branches of several genera of bamboo distributed mainly in southern regions of China and Japan (Hino 1961; Kishi et al. 1991; Liu 2009). Shiraia is characterized by its bambusicolous habitat, large ascostroma and muriform ascospores. Asci comprise 6 ascospores in this study and some previous studies (Hino 1961; Liu 2009). Shiraia bambusicola is we11 studied because of its medical effect in anticancer treatment (Kishi et al. 1991).
Phylogenetic study
Based on the SSU and ITS rDNA sequences analysis, its pleosporalean status was verified, and Shiraia was suggested to be closely related to Leptosphaeriaceae and/or Phaeosphaeriaceae (Pleosporineae) (Cheng et al. 2004). Based on the molecular phylogenetic analysis, another Shiraia-like fungus was reported which produced distinctive prawn-shaped conidioma-like structures (Morakotkarn et al. 2008), and differed from conidiomata in the anamorph of S. bambusicola described by Liu (2009).
Concluding remarks
A relatively broad species concept of Shiraia bambusicola is currently used, which could comprise several species.
Sinodidymella J.Z. Yue & O.E. Erikss., Mycotaxon 24: 295 (1985). (Teichosporaceae)
Generic description
Habitat terrestrial, saprobic? Ascomata medium to large, scattered, or in small groups, immersed, erumpent, to superficial, globose, subglobose, coriaceous, apex flattened, with radial ridges arranged around the central region. Peridium thick, 2-layered. Hamathecium of dense, broadly trabeculate pseudoparaphyses, anastomosing and branching between the asci. Asci 8-spored, with a short, furcate pedicel, bitunicate, cylindrical. Ascospores broadly ellipsoid, hyaline, becoming pale brown when mature, 1-septate, constricted at the median septum. Anamorphs reported for genus: none.
Literature: Yue and Eriksson 1985.
Type species
Sinodidymella verrucosa (Petr.) J.Z. Yue & O.E. Erikss., Mycotaxon 24: 295 (1985). (Fig. 89)
Fig. 89.
Sinodidymella verrucosa (from W 16366, type). a Ascomata b on the host surface. Note the radial ridges around the pseudostiolar region. b Section of an ascoma. c Section of peridium. Note the hyaline small cells and interwoven hyphae. d Cylindrical asci in pseudoparaphyses. e Eight-spored ascus with short pedicel. f Hyaline, 1-septate ascospores which turn pale brown when mature. Scale bars: a =1 mm, b = 100 μm, c=50 μm, d-f=20 μm
≡ Amphididymella verrucosa Petr., Meddn Goteb. Bot. 17: 129 (1947).
Ascomata 620-930 μm highx800-1250 μm diam., scattered, or in small groups, immersed, becoming erumpent, to nearly superficial, globose, subglobose, coriaceous, apex flattened, with 3-6 radial ridges arranged around the central region, with a flattened base not easily removed from the substrate, wa11 black, roughened (Fig. 89a and b). Peridium 100-150 μm thick, thinner at the base, 2-layered, outer layer thin, up to 40 μm thick, composed of small heavily pigmented thick-walled cells of textura globulosa, cells up to 5 μm diam., ce11 wa11 3-6 μm thick, inner layer thick, composed of hyaline small cells of textura epidermoidea, 2-4 μm diam., cell wall 1-3 μm thick, interspersed with interwoven mycelium in places (Fig. 89b and c). Hamathecium of dense, broadly trabeculate pseudoparaphyses 1–2 μm broad, anastomosing between and above the asci (Fig. 89d). Asci 140-190 (—205)× 12.5-15(−17.5) μm (x = 164 × 14.3μm, n=10), 8-spored, bitunicate, cylindrical, with a short, furcate pedicel, 20-45 μm long, and an inconspicuous ocular chamber (to 2 μm wide × 1 μm high) (Fig. 89d and e). Ascospores 20-25 × 10-12 μm (X = 22.1 × 10.3μm, n=10), obliquely uniseriate and partially overlapping, broadly ellipsoid with rounded ends, hyaline, becoming pale brown when mature, 1-septate, constricted at the median septum, smooth (Fig. 89f).
Anamorph: none reported.
Material examined: CHINA, Kansu Prov., between Scharakuto and Kweite, on rotten stems of Salsola gemmascens Pall., 25 Jul. 1935, G. Fenzel 2400 (W 16366, type).
Notes
Morphology
Sinodidymella was formally established by Yue and Eriksson (1985) as they noticed that Amphididymella verrucosa Petr. was not congeneric with the generic type, A. adeana Petr., which is a pyrenolichen. Thus a new monotypic genus, Sinodidymella was introduced to accommodate it. The most outstanding morphological character of Sinodidymella is its radial ridges, which are somewhat comparable with that of Lophiostoma rugulosum Yin. Zhang, J. Fourn. & K.D. Hyde, although their pseudoparaphyses are dissimilar. Lophiostoma rugulosum has “tightly aggregated cellular pseudoparaphyses” and “apically ending into bunches of clavate cells” (Zhang et al. 2009b).
Phylogenetic study
None.
Concluding remarks
The radial ridges have little phylogenetic significance in genus level classification (Zhang et al. 2009b), but the broadly trabeculate pseudoparaphyses of Sinodidymella may fit Melanommataceae.
Splanchnonema Corda, in Sturm, Deutschl. Fl., 3 Abt. (Pilze Deutschl.) 2(9), Tome 3: 115 (1829). (?Pleomassariaceae)
Generic description
Habitat terrestrial, saprobic. Ascomata medium to large, solitary or scattered, immersed in cortex with a pseudostromal covering, with a small ostiole appearing on the host surface, flattened subglobose. Peridium thin. Hamathecium of dense, cellular pseudoparaphyses, embedded in mucilage, anastomosing and branching. Asci bitunicate, fissitunicate, clavate to broadly cylindrical, with a short, narrowed, furcate pedicel. Ascospores clavate with a rounded apex and acute base, reddish brown, constricted at the septa.
Anamorphs reported for genus: Myxocyclus, Steganosporium (Barr 1982b).
Literature: Barr 1982b, 1993a; Boise 1985; Corda 1829; Eriksson 1981; Ramaley and Barr 1995; Shoemaker and LeClair 1975; Sivanesan 1984; Tanaka et al. 2005.
Type species
Splanchnonemapustulatum Corda, in Sturm, Deutschl. Fl., 3 Abt. (Pilze Deutschl.) 2(9), Tome 3: 115 (1829). (Fig. 90) Ascomata 400-600 μm highx550-1000 μm diam., solitary or scattered, immersed in cortex with a pseudostromal covering, with a small ostiole appearing on the host surface, flattened subglobose (Fig. 90a). Peridium 15-25 μm thick, composed of small lightly pigmented thin-walled compressed cells (Fig. 90b). Hamathecium of dense, long cellular pseudoparaphyses 2–3 μm broad, embedded in mucilage, anastomosing and branching. Asci 200-250×30-45 μm (x = 219.6 × 38.2μm, n = 10), 8-spored, bitunicate, fissitunicate, clavate to broadly cylindrical, with a short, narrowed, furcate pedicel up to 35 μm long, without conspicuous ocular chamber (Fig. 90c and d). Ascospores 45-53 x20-24 μm (X = 48.5 × 22.3μm, n =10), obliquely uniseriate and partially overlapping to biseriate, clavate with a rounded apex and acute base, reddish brown, 2-septate, apical cell largest, broader than the lower cells, basal cell smallest, constricted at the septa, smooth-walled, surrounded by a regular hyaline gelatinous sheath, 3-6 μm thick (Fig. 90e and f).
Fig. 90.
Splanchnonema pustulatum (from L, No. 910.251-352, No. 910.251-371). a Appearnce of ascomata on the host surface beneath a slightly raised area with minute ostiolar opening. b Section of the partial peridium. Note the compressed cells. c Dehiscent ascus. d Cluster of three asci joined in hymenium and pseudoparaphyses. e, f Asymmetric ascospores. Note the conspicuous sheath. Scale bars: a= 1 mm, b-d=50 μm, e, f=20 μm
Anamorph: none reported.
Material examined: UK, Avon, nr Bath, Batheaston, on branch of Ulmus, C.E. Broome (L, No. 910.251-352, No. 910.251-371).
Notes
Morphology
A confusing outline of the history of Splanchnonema was provided by Shoemaker and LeClair (1975), which at the time was a valid, but little used name. Eriksson (1981) and Sivanesan (1984) stated (without comment) that the lectotype of Splanchnonema is S. pupula (Fr.) O. Kuntze. However, S. pustulatum is listed as the generic type in the online databases MycoBank and Index Fungorum. We assume Eriksson (1981) gained his data from Shoemaker and LeClair (1973), who considered S. pustulatum to be a synonym of S. pupula. Since we were unable to locate material of Corda or Fries we used a later collection of C.E. Broome.
Splanchnonema can be distinguished from the morphologically comparable genera, i.e. Pleomassaria or Splanchospora by its depressed ascomata, and obovoid and asymmetrical ascospores (Barr 1982b). Currently, about 40 species are included in this genus. Barr (1993a) provided a key to 27 North American species, however, the inclusion of species with a range of ascospore types and immersed to superficial ascomata suggests the genus to be polyphyletic. Tanaka et al. (2005) suspected that the genus might include species of Pleomassaria, thus this genus needs further study.
Phylogenetic study
Splanchnonema platani (= Massaria platani) is poorly supported to be related to Lentitheciaceae (Schoch et al. 2009).
Concluding remarks
Splanchnonema pustulatum has unique ascospores formed in immersed ascomata with thin walls, indicating that Splanchnonema sensu stricto should be confined to a few similar species. The type needs recollecting, sequencing and epitypifying in order to establish the phylogenetic relationships of this genus and to study what may be important defining characters. Also see entry under Pleomassaria.
Sporormia De Not., Micromyc. Ital. Novi 5: 10 (1845). (Sporormiaceae)
Generic description
Habitat terrestrial, saprobic (coprophilous). Ascomata small, solitary, scattered, immersed to erumpent, globose, subglobose, wa11 black; apex without obvious papilla, ostiolate. Peridium thin. Hamathecium of rare, broad, septate pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate dehiscence not observed, short cylindrical, with a short, narrowed, furcate pedicel. Ascospores fasciculate, broadly filliform, reddish brown, multi-septate, easily separating into partspores, without visible germ-slits or pores.
Anamorphs reported for genus: none.
Literature: Ahmed and Asad 1968; Ahmed and Cain 1972; Kirschstein 1944; de Notaris 1849.
Type species
Sporormia fimetaria De Not., Micromyc. Ital. Novi 5: 10 (1845). (Fig. 91)
Fig. 91.
Sporormia fimetaria (from RO, type). a Appearance of b ascomata on the host surface. Note the scattered distribution. b-d Broad cylindrical asci with a short and thick pedicel. e Released filiform ascospores which may break up into part spores. Scale bars: a=0.5 mm, b-d=20 μm, e =10 μm
Ascomata 100-150 μm diam., solitary, scattered, immersed to erumpent, globose, subglobose, wa11 black; apex without obvious papilla, ostiolate (Fig. 91a). Peridium thin (other characters unknown). Hamathecium of rare, 2–3 μm wide, septate pseudoparaphyses. Asci 70-100×13-18 μm (x = 86.4 × 14.9mm, n = 10), 8-spored, bitunicate, fissitunicate dehiscence not observed, shortly cylindrical, with a short, narrowed, furcate pedicel up to 20 μm long, no apical apparatus could be observed (Fig. 91b, c and d). Ascospores 50-58×4-5 μm (x = 54.7 × 4.8mm, n = 10), fasciculate, broadly filliform, reddish brown, with 16 cells, easily separating into partspores, central cells of the ascospores shorter than broad, rectangular in vertical section, round in transverse section, 4-5×2.5-3.5 μm, without visible germ-slits or pores, apical cells usually longer than broad, 5-6.5 μm long, also without apertures (sheath is reported (Ahmed and Cain 1972), but not observed in this study) (Fig. 91e).
Anamorph: none reported.
Material examined: 1832, (RO, type, as Hormospora fimetaria De Not.).
Notes
Morphology
Sporormia was formally established by de Notaris (1849), and only one species was described, i.e. S. fimetaria, which subsequently was selected as the generic type. Sporormia sensu stricto was accepted by several workers, and only includes members with a fasciculate ascospore arrangement, parallel to the ascus, and the part cells of the ascospores lacking germ-slits (Ahmed and Asad 1968; Ahmed and Cain 1972; Kirschstein 1944). Species whose ascospores are not fasciculate and have partspores with germ-slits were assigned to Sporormiopsis by Kirschstein (1944) and to Sporormiella by Ahmed and Cain (1972).
Phylogenetic study
The generic status of Sporormia in Pleosporales was verified based on a phylogenetic analysis of ITS-nLSU rDNA, mtSSU rDNA and β-tubulin sequences (Kruys and Wedin 2009). Sporormia clustered together with species of Westerdykella (including Eremodothis and Pycnidiophora), but lacks clear statistical support. Thus, the relationship of Sporormia with other genera of Sporormiaceae is unclear and not resolved yet.
Concluding remarks
Several coprophilous taxa (e.g. Chaetopreussia and Pleophragmia as we11 as Sporormiella nigropurpurea) in the Pleosporales were not included in the study by Kruys and Wedin (2009). Strains of these genera need to be collected and analyzed and their relationship with Sporormia established.
Trematosphaeria Fuckel, Jb. nassau. Ver. Naturk. 23-24: 161 (1870). (Trematosphaeriaceae)
Generic description
Habitat terrestrial or freshwater, saprobic. Ascomata subglobose, unilocular, erumpent to superficial, with papillate ostiole. Peridium thin, comprising several cell types. Hamathecium of dense, delicate, filliform, septate pseudoparaphyses. Asci bitunicate, fissitunicate, cylindro-clavate, normally 8-spored. Ascospores ellipsoid-fusoid to biconic, septate, smooth to finely verruculose, brown.
Anamorphs reported for genus: hyphopodia-like (Zhang et al. 2008a).
Literature: von Arx and Muller 1975; Barr 1979a; Boise 1985; Clements and Shear 1931; Zhang et al. 2008a.
Type species
Trematosphaeria pertusa (Pers.) Fuckel, Jb. nassau. Ver. Naturk. 23-24: 161 (1870). (Fig. 92)
Fig. 92.
Trematosphaeria pertusa (a, d, f-i from epitype, b, c, e, j b from neotype). a Ascomata on the host surface. b Section of an ascoma. c, h Section of the peridium. c shows the peridium structure at sides, and h indicates the basal peridium structure. Note the hyaline and thinwalled cells in (h). d Asci amongst pseudoparaphyses. e Ascus with pedicle. f, g Dehiscent ascus. i Upper part of the ascus, showing the ocular chamber and the mucilage covering the apex. j, k Ascospores. Scale bars: a=0.5 mm, b, c =100 μm, d-h=20 μm, i-k=10 μm
≡ Sphaeria pertusa Pers., Syn. meth. fung. (Gottingen) 1: 83 (1801).
Ascomata 350-550 μm highx320-480 μm diam., solitary, scattered, or in groups, initially immersed, becoming erumpent, to semi-immersed, subglobose, black; apex with a short ostiole usually slightly conical and widely porate, to 100 μm high (Fig. 92a and b). Peridium 48-55 μm wide laterally, to 80 μm at the apex, thinner at the base, 30-40 μm thick, coriaceous, 3-layered, comprising several cell types, one is of small heavily pigmented thick-walled cells of textura angularis, cells 4-8 μm diam., ce11 wa11 1.5-3 μm thick in places with columns of textura prismatica orientated perpendicular to the ascomatal surface, apex cells smaller and walls thicker, forming thick-walled cells of textura pseudoparenchymata, and larger, paler cells of mixture of textura epidermoidea and textura angularis at the base (Fig. 92b, c and h). Hamathecium of dense, filamentous, 1.5-2.5 μm broad, septate pseudoparaphyses, embedded in mucilage, branching and anastomos-ing between and above the asci (Fig. 92d, e and f). Asci 100-145×15-17 μm (x = 118 × 15.5mm, n =10), 8-spored, bitunicate, fissitunicate, cylindro-clavate, with a short, thick, furcate pedicel which is 12–30 μm long, with a truncate ocular chamber (Fig. 92d, e, f, g and i). Ascospores 27.5-32.5×7.5-8.5 μm (x = 29.5 × 8mm, n =10), biseriate to uniseriate near the base, fusoid with broadly to narrowly rounded ends, dark brown, 1-3-septate, secondary septum forming late or often absent, constricted at the median septum, the upper cell often shorter and broader than the lower one, smooth to finely verruculose, containing refractive globules (Fig. 92j and k).
Anamorph: Onlyhyphopodia-like structures (orconidia?) observed (Zhang et al. 2008a).
Colonies (of epitype) reaching 5 cm diam. after 20 days growth on MEA at 25°C, raised, woolly, deep grey, with irregular to rhizoidal margin, reverse darkened. Hyphopodia-like structures (or conidia?) produced after 6 months, hyaline to pale brown, lobed, 4-4.5(−5) μm long and 3-3.5 μm diam.
Material examined: EUROPE, Upsala, on decaying wood, designated by Boise (1985), (L-Pers 910269-172, as Sphaeria pertusa Pers., neotype); FRANCE, Deux Sevres, Sansais, Le Vanneau, Les Grandes Mottines, swamp, on bark of a dead stump of Fraxinus excelsior, 25 Apr. 2004, J. Fournier (IFRD 2002, epitype); Haute Garonne, Avignonet, Canal du Midi, on submerged wood of Platanus in a canal, 23 Nov. 2006, Michel Delpont, det. J. Fournier (IFRD2003).
Notes
Morphology
Trematosphaeria was formally established in ‘Rhenish fungi’ by Fuckel (1870) based on the broadly pertuse ascomata, and Fries (1823) assigned it under Ascomycetes, Pyrenomycetes, Lophiostomataceae. Subsequently, Winter (1885) placed Trematosphaeria in Amphisphaeriaceae. Berlese (1890), however, treated Trematosphaeria as a synonym of Melanomma (Melanommataceae). After establishment of Loculoascomycetes (Luttrell 1955), Trematosphaeria was assigned to Pleosporaceae (Loculoascomycetes, Pleosporales) (Holm 1957), and this was followed by von Arx and Muller (1975). Trematosphaeria was assigned to Melanommataceae by Barr (1979a), and this has been widely followed (Eriksson 2006; Kirk et al. 2001; Lumbsch and Huhndorf 2007).
Trematosphaeria pertusa, the lectotype species of Trematosphaeria (Clements and Shear 1931), is characterized by having semi-immersed to erumpent ascomata, filamentous pseudoparaphyses, cylindro-clavate asci, fusoid, 1-septate reddish brown to dark brown ascospores (Zhang et al. 2008a). All of these characters are quite different from those of Melanomma, the familial type of Melanommataceae.
Phylogenetic study
Trematosphaeria pertusa forms a robust phylogenetic clade with Falciformispora lignatilis and Halomassarina thalassiae, and they are all assigned to Trematosphaeriaceae (Suetrong et al. 2009; Zhang et al. 2009a; Plate 1).
Concluding remarks
Trematosphaeria pertusa is a terrestrial species which can also survive in a freshwater environment. However, both Falciformispora lignatilis and Halomassarina thalassiae are marine fungi. Their habitat difference may indicate their distant relationship, at least above genus level.
Verruculina Kohlm. & Volkm.-Kohlm., Myco1. Res. 94: 689 (1990). (Testudinaceae)
Generic description
Habitat marine, saprobic. Ascomata medium-sized, solitary under clypeate, immersed to semi-immersed, subglobose to depressed ellipsoidal, papillate, ostiolate, periphysate, black, carbonaceous. Peridium thin, comprising a few layers of cells of textura angularis. Hamathecium of long cellular pseudoparaphyses, embedded in mucilage, hyaline, septate and sparsely branching. Asci 8-spored, bitunicate, fissitunicate, cylindrical, with short pedicels, ocular chamber not observed. Ascospores biseriate, ovoid or ellipsoidal, dark brown, 1-septate, constricted at the septum, verrucose or verruculose, with or without germ pore.
Anamorphs reported for genus: none.
Literature: Kohlmeyer and Volkmann-Kohlmeyer 1990; Suetrong et al. 2009.
Type species
Verruculina enalia (Kohlm.) Kohlm. & Volkm.-Kohlm., Myco1. Res. 94: 689 (1990). (Fig. 93)
Fig. 93.
Verruculina enalia (from KDH 2137, slide). a Cylindrical asci with short pedicels. b One-septate verruculose ascospores. Scale bars: a=20 μm, b =10 μm
≡ Didymosphaeria enalia Kohlm., Ber. dt. bot. Ges. 79: 28 (1966).
Ascomata 295-480 μm highx140-520 μm diam., solitary under clypeate, immersed to semi-immersed, subglobose to depressed ellipsoidal, ostiolate, papillate, periphysate, black, carbonaceous. Peridium thin, comprising a few layers of cells of textura angularis. Hamathecium of long cellular pseudoparaphyses, 1.5-2 μm broad, embedded in mucilage, hyaline, septate and sparsely branching. Asci 177-135× 12.5-15.5 μm, 8-spored, bitunicate, fissitunicate, cylindrical, with short furcate pedicels, ocular chamber not observed (Fig. 93a).
Ascospores 16.5-23 x7.5-10 μm, biseriate, ovoid or ellipsoidal, dark brown, 1-septate, constricted at the septum, verrucose or verruculose, with or without germ pore (Fig. 93b).
Anamorph: none reported.
Material examined: SEYCHELLES, Victoria, on submerged branch of Rhizophora mangle L., Mar. 2004, K.D. Hyde (KDH 2137, slide).
Notes
Morphology
Verruculina was introduced to accommodate an obligate marine species, i.e. Verruculina enalia (Kohlmeyer and Volkmann-Kohlmeyer 1990). Verruculina is characterized by immersed, clypeate, carbonaceous, ostiolate and papillate ascomata. The peridium is composed of cells of textura angularis. Pseudoparaphyses are trabeculate and embedded in mucilage. Asci are 8-spored, cylindrical with short pedicels and ocular chamber, and ascospores are ellipsoidal, 1-septate, dark brown, verrucose or verruculose. The partly or completely immersed clypeate ascomata of V. enalia is comparable with those of Didymosphaeria futilis, but it differs from the later by the dark peridium, gelatinous matrix around the pseudoparaphyses, stipitate asci with an ocular chamber, and the verruculose ascospores (Kohlmeyer and Volkmann-Kohlmeyer 1990).
Phylogenetic study
Based on multigene phylogenetic analysis, Verruculina enalia nested within Testudinaceae (Suetrong et al. 2009). Thus, its familial placement seems clarified.
Concluding remarks
None.
Westerdykella Stolk, Trans. Br. Myco1. Soc. 38: 422 (1955). (Sporormiaceae)
Generic description
Habitat terrestrial, saprobic (coprophilous). Ascomata small, scattered on the upper layer of the culture medium, wall black. Peridium thin, composed of one layer of cells of polygonal, dark brown, thick-walled cells. Hamathecium not observed. Asci 32-spored, bitunicate nature undetermined, fissitunicate dehiscence not observed, subglobose to ellipsoid, arranged in the centre of the ascomata, with or without a short pedicel. Ascospores globose, brown, 1-celled, without germ pore.
Anamorphs reported for genus: Phoma-like (von Arx 1974). Literature: von Arx 1973, 1981; Kruys et al. 2006; Kruys and Wedin 2009; Stolk 1955a.
Type species
Westerdykella ornata Stolk, Trans. Br. Myco1. Soc. 38: 422 (1955). (Fig. 94)
Fig. 94.
Westerdykella ornata (from CBS 379.55, holotype). a Appearance of the ascomata on culture substrate surface. b-f Mature and immature asci as wel! as the released ascospores. Note the spiral bands around the ascospores. Scale bars: a=1 mm, b-f=10 μm
Ascomata 100-300 μm diam., cleistothecoid, scattered on the upper layer of the culture medium, wall black (Fig. 94a). Peridium composed of one layer of cells of polygonal in front view, dark brown, thick-walled cells, ca. 5 μm diam. Hamathecium not observed. Asci 25-32× 16-22 μm, 32-spored, bitunicate nature undetermined, fissitunicate dehiscence not observed, subglobose to ellipsoid, arranged in the centre of the ascomata, with a short furcate pedicel best seen in immature asci (Fig. 94b, c, d and f). Ascospores 6.2-7×6-6.8 μm, globose, brown, 1-celled, ornamented with irregular spiral bands, which occur in four to five coils, without germ pore (Fig. 94e).
Anamorph: none reported.
On MEA colonies spreading, but somewhat erumpent, with moderate aerial mycelium and even, lobate margins; surface dirty white with luteous to orange patches; reverse orange to sienna. On PDA similar but with sparse aerial mycelium; surface with patches of orange to luteous and dirty white; reverse luteous with cream margins. On OA flat, spreading with sparse aerial mycelium; surface with luteous and dirty white patches and transparent margins; sporulating on OA, visible as black masses of aggregated ascomata; colonies reaching 4 cm diam. on all media (based on CBS 379.55).
Material examined: MOZAMBIQUE, Inhaca, leg. H.J. Swart, mangrove mud (CBS 379.55, holotype).
Notes
Morphology
Westerdykella was introduced to accommodate a coprophilous fungus, which is characterized by cleistothecioid and membraneous ascomata (Stolk 1955a). Asci are subglobose to ellipsoid, stalked, many-spored and evanescent. Ascospores are globose to subglobose, brown, ornamented with spiral bands, without germ pores (Stolk 1955a). Westerdykella was assigned under Phaeosporeae of the Eurotiaceae (Stolk 1955a), and was assigned to Sporormiaceae by von Arx and Muller (1975). Based on the spore ornamentation, von Arx and van der Aa (1987) and Barr (2000) accepted Westerdykella as a separate genus, but this is not supported by molecular phylogenetic analysis (Kruys and Wedin 2009).
Phylogenetic study
Phylogenetic reconstructions indicated that both Pycnidiophora and Eremodothis should be treated as synonyms of Westerdykella (Kruys and Wedin 2009).
Concluding remarks
Westerdykella is another example where ascospore ornamentation can be phylogenetically uninformative. Westerdykella is proved a good genus of Sporormiaceae (Kruys and Wedin 2009).
Wettsteinina Hohn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. I 116: 126 (1907). (?Lentitheciaceae)
Generic description
Habitat terrestrial or freshwater? hemibiotrophic or saprobic. Ascomata generally small, scattered, immersed with a protruding broad papilla. Peridium very thin, composed of few layers of thin-walled large polygonal cells in surface view. Hamathecium deliquescing at maturity. Asci bitunicate, fissitunicate, subglobose to obpyriform, without a pedicel, with small truncate ocular chamber. Ascospores hyaline and turning pale brown when mature, septate, upper second cell enlarged, slightly constricted at the second septum, smooth, surrounded by a hyaline gelatinous sheath.
Anamorph reported for genus: Stagonospora (Farr et al. 1989).
Literature: Barr 1972; Muller 1950; Shoemaker and Babcock 1987, 1989b.
Type species
Wettsteinina gigaspora Hohn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl., Abt. 1 116: 126 (1907). (Fig. 95)
Fig. 95.
Wettsteinina gigantospora (from S, holotype of Massarina gigantospora). a Ascomata with protruding papilla scattered on the host surface. b Obpyriform thick-walled ascus with small apical apparatus. c Fissitunicate ascus. d Released hyaline ascospores. Note the distinct primary septum and less distinct secondary septa. e Ascospore with sheath. Scale bars: a=0.5 mm, b-d= 100 μm, e=50 μm
Ascomata 150-250 μm diam., scattered, immersed with protruding broad papillae, 50-90 μm diam. Peridium thin, composed of few layers of thin-walled large polygonal cells in surface view, 6-15 μm diam. (Fig. 95a). Hamathecium deliquescing at maturity. Asci 140-200×75-120 μm, 8-spored, bitunicate, fissitunicate, subglobose to obpyriform, lacking a pedicel, with a small truncate ocular chamber (to 8 μm wide × 5 μm high) (Fig. 95b and c). Ascospores 90-110 × 25-30 μm, 2-4-seriate, hyaline and turning pale brown when mature, broadly clavate, 4-septate, primary septum distinct and constricted forming 1/3r from the apex of the ascospore, complete, secondary septa less distinct and slightly constricted, incomplete, with one forming above and two forming below the primary septum, largest cell the second cell from apex, smooth, surrounded by a hyaline gelatinous sheath 5-8 μm thick (Fig. 95d and e).
Anamorph: none reported.
Material examined: SLOVENIA, Postojna, on Genista sagittalis leg. Stapf. det. H. Rehm. (S, holotype of Massarina gigantospora).
Notes
Morphology
Confusion exists in the generic type of Wettsteinina. Hohnel (1907) described W. gigaspora when introducing Wettsteinina, and listed it as the first species of Wettsteinina. Clements and Shear (1931) accepted W. gigaspora as the generic type of Wettsteinina, which is followed by Shoemaker and Babcock (1987). But according to http://www.indexfungorum.org (June 2011), W. gigantospora is the generic type of Wettsteinina. Both W. gigantospora and W. gigaspora were treated as the synonyms of W. mirabilis (Niessl) Hohn. http://www.indexfungorum.org (June, 2011, Synonymy Contributor: CBS (2010)). We tentatively described the generic type of W. gigantospora as a representing of the type of W. gigaspora here.
New family names, i.e. Pseudosphaeriaceae and Wettsteininaceae (as Wettsteiniaceae) and a new order, Pseudosphaeriales had been introduced to accommodate Wettsteinina and its synonym Pseudosphaeria (Hohnel 1907; Locquin 1972). After a systematic study, Wettsteinina was included in Pleosporaceae based on its “Pleospora-type” centrum, and Pseudosphaeriaceae and Wettsteininaceae are treated as synonyms of Pleosporaceae (Shoemaker and Babcock 1987).
Phylogenetic study
Wettsteinina macrotheca (Rostr.) E. MU11., W. pachyasca (Niessl) Petr. and W. dryadis (Rostr.) Petr. were reported to be closely related to Pleomassaria siparia (Melanommataceae) (Kodsueb et al. 2006a), and W. lacustris (Fuckel) Shoemaker & C.E. Babc. nested within Lentitheciaceae (Schoch et al. 2009). The generic type has not been sequenced.
Concluding remarks
The most striking character for Wettsteinina is its asymmetrical ascospores, thick-walled obpyriform asci and lack of pseudoparaphyses at maturity. These characters are comparable with genera in the Capnodiales and Venturiales. The phylogenetic significance of these characters are not fully understood, while the hemibiotrophic or saprobic life style may indicate its polyphyletic nature (Shoemaker and Babcock 1987). Strains from the genus, in particular the generic type require DNA sequence data so that the phylogenetic placement can be investigated.
Wilmia Dianese, Inacio & Dorn.-Silva, Mycologia 93: 1014 (2001). (Phaeosphaeriaceae)
Generic description
Habitat terrestrial, hemibiotrophic or biotrophic. Ascomata small, scattered, immersed, globose to subglobose, papillate. Peridium thin, composed of a few layers of brown, thick-walled cells of textura angularis to prismatica. Hamathecium comprising filliform, septate, rarely branching, evanescent, cellular pseudoparaphyses embedded in mucilage. Asci bitunicate, fissitunicate, cylindrical to clavate, with a short, furcate pedicel and ocular chamber. Ascospores fusoid, pale brown, 1-septate.
Anamorphs reported for genus: see below.
Literature: Dianese et al. 2001.
Type species
Wilmia brasiliensis Dianese, Inacio & Dorn.-Silva, Mycologia 93: 1014 (2001). (Fig. 96)
Fig. 96.
Wilmia brasiliensis (from UB CO1. MicROl 8438, holotype). a Section of an ascoma. Note the setae in the ostiole. b Conidioma of the coelomycetous anamorphic stage. c, d Clavate asci with short e pedicels. e, f Released 1-septate pale brown ascospores. Scale: a, b =100 μm, c, d=20 μm, e, f =10 μm
Ascomata 175-240 μm highx95-145 μm diam., scattered, immersed, globose to subglobose; apex with a short papilla, 40-80 μm long, ostiolate, periphysate, periphyses up to 90 μm long (Fig. 96a and b). Peridium 6-15 μm wide, 1-layered, composed of 3-7 layers of brown, thick-walled cells of textura angularis to prismatica, cells 4-9 μm diam., cell wall 2-4 μm thick (Fig. 96a and b). Hamathecium of long cellular pseudoparaphyses 2–3 μm broad, septate, rarely branching, embedded in mucilage, evanescent. Asci 65-95×9.5-14 μm (x = 78.5× 11.5mm, n =10), 8-spored, bitunicate, fissitunicate, cylindrical to clavate, with a short, furcate pedicel and a small ocular chamber (Fig. 96c, d and f). Ascospores 22.5-28×5-8.5 μm (x = 26.5 × 6.8mm, n =10), biseriate, fusoid with narrowly rounded ends, pale brown, 1-septate, constricted at the septum, the upper cell often shorter and broader than the lower one, smooth, with or without sheath (Fig. 96d and e).
Anamorph: Conidiomata 170-200 μm highx85-130 μm diam., eustromatic, immersed, subglobose to irregular, ostiolate, brown. Peridium thin, 1–2 wall layers, 6-8 μm thick, thicker near the apex. Ostiole 50-63 μm highx30-35 broad. Conidiogenous cells ampulliform or lageniform, phialidic, aseptate. Conidia 13-20×4-7 μm, ellipsoid, oblong, ovoid, hyaline (Dianese et al. 2001).
Material examined: BRAZIL, Distrito Federal, Vargem Bonita, Fazenda Agua Limpa, on leaves of Memora pedunculata (Vell.) Miers, 18 May 1995, Carlos A. Inacio (UB Co1. Micro1 8438 holotype).
Notes
Morphology
Wilmia was formally established by Dianese et al. (2001) as a monotypic genus represented by W. brasiliensis, which causes leaf spots on Memora pedunculata. The peridium of W. brasiliensis comprises a few layers of brown, thick-walled textura angularis to prismatica cells, and it also has cellular pseudoparaphyses, clavate asci, 1-septate pale brown ascospores (Dianese et al. 2001).
Phylogenetic study
None.
Concluding remarks
The dicotyledonous host habit of Wilmia brasiliensis seems in agreement with Leptosphaeriaceae rather than Phaeosphaeriaceae. But a verified conclusion can only be reached by further molecular phylogenetic study.
Xenolophium Syd., Bulletin of the Bernice P. Bishop Museum, Honolulu, Hawaii 19: 96 (1925). (Pleosporales, genera incertae sedis)
Generic description
Habitat terrestrial, saprobic on wood. Ascomata nearly superficial, scattered to gregarious, globose, large, with a conspicuous compressed papilla and large slit-like ostiole. Peridium carbonaceous. Hamathecium of dense, long trabeculate pseudoparaphyses, branching and anastomosing between and among asci. Asci 8-spored, clavate, with very long furcate pedicels. Ascospores fusoid to narrowly fusoid, light to dark brown, 1-septate, constricted at the septum. Anamorphs reported for genus: none.
Literature: Chesters and Bell 1970; Huhndorf 1993; Mugambi and Huhndorf 2009b; Muller and von Arx 1962; Stevens 1925.
Type species
Xenolophium leve Syd., Bulletin of the Bernice P. Bishop Museum, Honolulu, Hawaii 19: 97 (1925) (Fig. 97)
Fig. 97.
Xenolophium applanatum (from IFRD 2038). a Gregarious ascomata on the host surface. Note protruding papilla and slit-like ostiole. b Vertical section of the papilla and ostiole. c Section of the partial peridium. Note the two layers of the peridium. d Eight-spored i in trabeculate pseudoparaphyses. Note the long pedicels. e-g Pale n ascospores. Scale bars: a=2 mm, b=200 μm, c=50 μm, d= 0 μm, e-g =10 μm
Current name: Xenolophium applanatum (Petch) Huhndorf, Mycologia 85: 493 (1993).
≡ Schizostoma applanatum Petch, Ann. Roy. Bot. Gard. (Peradeniya) 6: 231 (1916).
Ascomata 1-1.5 mm diam., scattered to clustered, erumpent to superficial, globose with base immersed in host tissue, wall black, carbonaceous, roughened with ridges, papillate. Apex with a conspicuous hysteriform papilla extending on the sides, 1-1.4 mm long, 0.4-0.5 mm wide, 0.2-0.3 mm high, smooth, ostiole slit-like, nearly as long as papilla length (Fig. 97a). Peridium 140-160 μm thick, pseudoparenchymatous, composed of two distinct layers: outer crust 16-45 μm thick, blackish, of heavily melanized, nearly opaque thick-walled angular cells, of uneven thickness forming irregular strands extending into the inner layer; inner layer subhyaline, composed of thick-walled prismatic to angular cells, with columns or patches of darker thick-walled cells extending inwardly from the outer layer; papilla wall 200-220 μm thick, of heavily melanized angular thick-walled cells (Fig. 97b and c). Hamathecium of dense, very long trabeculate pseudoparaphyses 0.8-1.5 μm broad, embedded in mucilage, anastomosing and branching between and above the asci. Asci 104-152* 9-12 μm (excluding pedicel) (x = 149 × 10.2mm, n=10), 8-spored, bitunicate, fissitunicate dehiscence not observed, clavate, with a long, narrowed, furcate pedicel which is 50-75 μm long (Fig. 97d). Ascospores 17-26*4-5.5 μm (x = 22.5 × 4.8mm, n =10), upper biseriate and lower uniseriate, fusoid, straight to slightly curved, equally 1-septate, constricted at the septum, the upper cell slightly wider, with one or rarely two additional septa appearing on a small number of senescent ascospores, pale brown, median septum darker, constricted, smooth, without sheath or appendages (Fig. 97e, f and g).
Anamorph: none reported.
Material examined: MARTINIQUE, Morne Rouge, on rotten wood, leg C. Lecuru, det Jacques Fournier, 29 Aug. 2007, IFRD 2038.
Notes
Morphology
Xenolophium was formally established by Sydow (in Stevens 1925) to accommodate two species, i.e. X. leve and
X. verrucosum, of which X. leve is selected as the generic type (Huhndorf 1993). Because of its morphological similarity with some genera, such as Ostropella and Schizostoma, Xeno-lophium has been treated as a synonym of Ostropella (Muller and von Arx 1962) or even of Lophiostoma (Chesters and Bell 1970). Huhndorf (1993) clarified the circumscription of Xenolophium and treated X. leve as a synonym of Schizostoma applanata. Xenolophium mainly differs from Ostropella in lack of “organized cell composition and triangular pattern of melanization” in the peridium (Huhndorf 1993).
Phylogenetic study
The polyphyletic nature of Xenolophium has been demonstrated (Mugambi and Huhndorf 2009b). The generic type of Xenolophium (X. leve, current name X. applanatum) clustered together with Ostropella albocincta (generic type of Ostropella), and both locate in Platystomaceae (Mugambi and Huhndorf 2009b).
Concluding remarks
The large ascomata with slit-like ostioles, hamathecium of numerous and trabeculate pseudoparaphyses, clavate asci with long pedicels, and the pale brown, 1-septate ascospores of Xenolophium leve are all comparable with those of Ostropella albocincta. However, the phylogenetic results do not support them being congeneric (Mugambi and Huhndorf 2009b).
Synonyms
Javaria Boise, J.R., Acta Amazonica 14(Supl.): 50 (1984). (Melanommataceae)
Current name: Astrosphaeriella Syd. & P. Syd., Annls mycoL 11: 260 (1913).
Generic description
Habitat terrestrial, saprobic. Ascomata medium-sized, scattered, erumpent to nearly superficial, reflexed pieces of the ruptured host tissue usually persisting around the surface of the ascomata; ascomata broadly conical, with a flattened base not easily removed from the substrate, wall black, papillate. Peridium carbonaceous. Hamathecium of trabeculate pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindro-clavate to narrowly fusoid, with a short, narrowed, furcate pedicel. Ascospores elongate-fusoid, hyaline, 1-septate, constricted at the septum.
Anamorphs reported for genus: none.
Literature: Barr 1990a; Boise 1984.
Type species
Javaria samuelsii Boise, J.R., Acta Amazonica 14(Supl.): 50 (1984) (Fig. 98)
Fig. 98.
Javaria samuelsii (from isotype). a Ascoma on the host surface. Note reflexed pieces of the ruptured host tissue. b, c Cylindro-clavate asci within narrow pseudoparaphyses in gelatinous matrix. d Released ascospore with sheath. Scale bars: a =1 mm, b=50 μm, c, d=20 μm
Current name: Astrosphaeriella samuelsii Boise, Acta Amazon., Supl. 14(1–2, Suppl.): 50 (1986) [1984].
Ascomata 300-380 μm diam., scattered, erumpent through the outer layers of the host tissues, to nearly superficial, reflexed pieces of the ruptured host tissue usually persisting around the surface of the ascomata; ascomata broadly conical, with a flattened base not easily removed from the substrate, wall black, papillate (Fig. 98a). Peridium 50-80 μm thick, carbonaceous and crisp, 1-layered. Hamathecium of dense, long trabeculate pseudoparaphyses, 0.8-1.5 μm broad, embedded in mucilage, anastomosing between and above the asci. Asci 140-185× 17.5-20 μm (x = 158 × 19.4mm, n =10), 8-spored, bitunicate, fissitunicate, cylindro-clavate to narrowly fusoid, with a short, narrowed, furcate pedicel up to 20 μm long (Fig. 98b and c). Ascospores 48-55(−60)×6-7.5(−10) μm (x = 52.2 × 7.7mm, n = 10), biseriate, elongatefusoid, gradually tapering towards the ends, hyaline, surrounded with sheath, 2-5 μm thick, 1-septate, constricted at the septum (Fig. 98d).
Anamorph: none reported.
Material examined: Serra Araca, 60 m, terra firme, open forest, deep litter. Dry. 10-13 Mar. 1984, det. Jean R. Boise, G.J. Samuels (isotype).
Notes
Morphology
Javaria was introduced by Boise (1984) based on seven Amazonian collections on decaying palm petioles; it is comparable with Astrosphaeriella in numerous characters. But Javaria differs from Astrosphaeriella by its hyaline ascospores with sheath, and its apical ring can be stained with Congo Red, as well as its small ascomata. Barr (1990a) introduced a second species J. shimekii which occurs on woody substrate. Some mycologists treat Javaria as a synonym of Astrosphaeriella (Hyde and Frohlich 1998).
Phylogenetic study
None.
Concluding remarks
The size of ascomata and pigmentation of ascospores has little significance at generic level classification (Zhang et al. 2009a). Likewise, the staining of endotunica with Congo Red has not been shown to have great significance. Thus, we accept Javaria as a synonym of Astrosphaeriella.
Pycnidiophora Clum, Mycologia 47: 899 (1955). (Sporormiaceae)
Current name: Westerdykella Stolk, Trans. Br. Mycol. Soc. 38(4): 422 (1955).
Generic description
Habitat terrestrial, saprobic (coprophilous). Ascomata small, cleistothecial, scattered on surface of agar media, semi-immersed, globose to subglobose, black. Peridium thin, composed of thin-walled, polyangular cells from front view. Hamathecium not apparent. Asci numerous, irregularly arranged, bitunicate nature undetermined, fissitunicate nature undetermined, globose, without pedicel. Ascospores gathering in the globose asci, smooth.
Anamorphs reported for genus: Phoma-like. Literature: Cain 1961; Clum 1955; Stolk 1955b; Thompson and Backus 1966.
Type species
Pycnidiophora dispersa Clum, Mycologia 47: 900 (1955) [1955]. (Fig. 99)
Fig. 99.
Pycnidiophora dispersa (a from CBS 297.56; b-d from MSC 133.118, type). a Ascomata scattering on the surface of the substrate. b Crashed ascoma. Note the numerous released asci. c Globose asci and released ascospores. d One-celled ascospores. Scale bars: a= 200 μm, b-d=20 μm
Current name: Westerdykella dispersa (Clum) Cejp & Milko.
Ascomata 200-290 μm diam., cleistothecial, scattered on surface of agar media, semi-immersed, globose to subglobose, black (Fig. 99a). Peridium thin, composed of thin-walled, poly-angular cells from front view (Fig. 99b). Hamathecium not apparent. Asci numerous, 11-14 μm diam. (x = 12.3mm, n =10), irregularly arranged, 32-spored when mature, bitunicate nature undetermined, fissitunicate nature undetermined, globose, without pedicel (Fig. 99b and c). Ascospores 4-5.5×2.5-3 μm (x = 4.7 × 2.8mm, n= 10), in the globose asci, olivaceous, oblong, 1-celled, smooth (Fig. 99d).
Anamorph: Phoma-like coelomycetes.
On MEA colonies spreading, flat with sparse aerial mycelium, covering the dish after 1 month; surface smokegrey with dirty white margins; reverse olivaceous-grey with luteous patches. On PDA spreading without aerial mycelium, colonies transparent, sporulating profusely with black, globose ascomata and pycnidia of a Phoma-like anamorph. On OA similar, lacking aerial mycelium, sporulating profusely with black, globose ascomata (based on CBS 297.56).
Material examined: USA, Michigan, East Lansing, Science Greenhouse, isolated from damped off Phlox seedling, Dec. 1952, F.M. Clum (No. 27) (MSC 133.118, type).
Notes
Morphology
Pycnidiophora was formally established by Clum (1955) based on its “imperfect stage of pycnidium”, which was subsequently confirmed as the sexual stage (Cain 1961; Thompson and Backus 1966). Clum (1955) has described and tentatively assigned P. dispersa (Clum) Cain to Aspergillaceae (= Eurotiaceae), and Stolk (1955b) has proposed to assign the morphologically comparable species. multispora Saito & Minoura ex Cain to Eurotiaceae as. Cain (1961), however, suspected that the 32 asco-spores are actually the disarticulated segments of eight 4-celled ascospores, thus assigned it under Preussia (Sporormiaceae). After detailed study, Thompson and Backus (1966) confirmed that the so-called “eight 4-celled ascospores” do not exist in the development of the asci in both P. dispersa and P. multisporum. Thus, Pycnidiophora was assigned to Eurotiaceae (Eurotiales) (Thompson and Backus 1966).
Phylogenetic study
Phylogenetic study based on the ITS-nLSU rDNA sequences indicated that Pycnidiophora dispersa nested within clade of Westerdykella (including the generic type, W ornata) (Kruys and Wedin 2009). Morphologically, both genera have cleistothecioid ascomata, asci with short or without pedicels and ascospores 1-celled and no germ slits. Thus, Pycnidiophora is treated as a synonym of Westerdykella (Kruys and Wedin 2009).
Concluding remarks
Although the pleosporalean status of Pycnidiophora is verified, morphological characters such as the cleistothecioid ascomata and irregularly arranged asci, which do not show typical bitunicate or fissitunicate characters, absence of pseudoparaphyses as well as the ascospores separating into partspores very early all challenge the traditional concept of Pleosporales (Zhang et al. 2009a). Obviously, most of these morphological characters overlap with those of the Eurotiales.
Sporormiella Ellis & Everh., N. Amer. Pyren.: 136 (1892). (Sporormiaceae)
Current name: Preussia Fuckel, Hedwigia 6: 175 (1867) [1869-70].
Generic description
Habitat terrestrial, saprobic (coprophilous). Ascomata medium-sized, solitary, scattered, or in small groups, semi-immersed to nearly superficial, globose, subglobose, black, coriaceous, ostiolate, periphysate. Peridium thin, composed of small heavily pigmented cells of textura angularis, apex cells smaller and walls thicker. Hamathecium of dense, septate, cellular pseudoparaphyses, embedded in mucilage. Asci 8-spored, bitunicate, fissitunicate, cylindro-clavate, with a narrowed, furcate pedicel. Ascospores cylindrical with rounded ends, brown, 3-septate, deeply constricted at each septa, with sigmoid germ slit in each cell.
Anamorphs reported for genus: none.
Literature: Ahmed and Cain 1972; Ellis and Everhart 1892; Khan and Cain 1979a, b; Luck-Allen and Cain 1975.
Type species
Sporormiella nigropurpurea Ellis & Everh., N. Amer. Pyren.: 136 (1892). (Fig. 100)
Fig. 100.
Sporormiella nigropurpurea (from NY, holotype). a Section of an ascoma. b Section of the papilla. Note the dense pseudoparaphyses. c Section of a partial peridium. d, e Eight-spored cylindro-clavate asci with furcate pedicels. f, g Four-celled, brown ascospores. Note the sigmoid germ slit in each cell. Scale bars: a= 200 μm, b, c=50 μm, d, e=20 μm, f, g =10 μm
Current name: Preussia nigropurpurea (Ellis & Everh.) Kruys, Syst. Biod. 7: 476.
Ascomata 314-528 μm high*(250-)357-500 μm diam., solitary, scattered, or in small groups, immersed, semi-immersed to nearly superficial, globose, subglobose, wall black, coriaceous, smooth, papillate, papilla 43-115 μm long, 72-157 μm broad, ostiolate, ostiole filled with periphyses (Fig. 100a and b). Peridium 20-28 μm thick laterally, up to 40 μm thick at the apex, composed of small heavily pigmented cells of textura angularis, cells 5-8 μm diam., cell wall 1-3 μm thick, apex cells smaller and walls thicker (Fig. 100c). Hamathecium of dense, long, septate, cellular pseudoparaphyses, 1.5-2 μm broad, embedded in mucilage. Asci (70-)110-158*9-12.5(−15) μm (x = 114.3 × 11.1mm, n= 10), 8-spored, bitunicate, fissitunicate, cylindrical to cylindroclavate, with a narrowed, furcate pedicel, 13-38 μm long, ocular chamber apparent (Fig. 100d and e). Ascospores 15-20*4-5.5 μm (x = 17.3 × 4.9mm, n=10), obliquely uniseriate and partially overlapping to biseriate, shortly cylindrical with rounded ends, brown, 3-septate, deeply constricted at each septum, with sigmoid germ slit in each cell, smooth-walled (Fig. 100f and g).
Anamorph: none reported.
Material examined: USA, New field, New Jersey: Gloucester Co., on cow dung, Mar. 1891 (NY, holotype).
Notes
Morphology
Sporormiella was formally established by Ellis and Everhart (1892) based on the single species, Sporormiella nigropurpurea, which is characterized by its “immersed to semi-immersed, papillate ascomata, cylindrical to cylindroclavate asci with a pedicel, three to multi-septate ascospores with elongated germ slits through the whole cell” (Ahmed and Cain 1972; Khan and Cain 1979a, b). Barr (1990a) has indicated that Sporormiella might be a synonym of Ohleriella, while Sporormiella is assigned to Sporormiaceae as a separate genus (Eriksson 2006; Lumbsch and Huhndorf 2007). Currently, about 90 species are included in this genus (http://www.mycobank.org).
Phylogenetic study
The phylogenetic analysis based on ITS-nLSU rDNA, mtSSU rDNA and β-tubulin sequences indicated that Sporormiella nested in Preussia, and a Sporormiella-Preussia complex is formed (Kruys and Wedin 2009). Thus, Sporormiella was assigned under Preussia (Kruys and Wedin 2009).
Concluding remarks
It is clear that the presence or absence of an ostiole cannot distinguish Sporormiella from Preussia according to the findings of Guarro et al. (1997a, b) and Kruys and Wedin (2009). Thus, Sporormiella should be treated as a synonym of Preussia (Kruys and Wedin 2009).
Spororminula Arx & Aa, Trans. Br. Myco1. Soc. 89: 117 (1987). (Sporormiaceae)
Current name: Preussia Fuckel, Hedwigia 6: 175 (1867) [1869-70].
Generic description
Habitat terrestrial, saprobic (coprophilous). Ascomata small to medium, solitary, scattered, immersed to erumpent, globose, subglobose, to ovate, black, membraneous, papillate, ostiolate. Peridium thin, membraneous, composed of several layers of heavily pigmented, elongate cells of textura angularis. Hamathecium of dense trabeculate, aseptate, decomposing pseudoparaphyses. Asci bitunicate, broadly cylindro-clavate with a narrow furcated pedicel. Ascospores cylindrical to cylindro-clavate, with round ends, brown, multi-septate, easily breaking into partspores.
Anamorphs reported for genus: none.
Literature: von Arx and van der Aa 1987.
Type species
Spororminula tenerifae Arx & Aa, Trans. Br. Myco1. Soc. 89: 117 (1987).(Fig. 101)
Fig. 101.
Spororminula tenerifae (from HCBS 9812, holotype). a b Appearance of ascomata on the host surface. b, c Sections of the partial peridium. Note the elongate cells of textura angularis. d, e Asci with thin pedicels. f, g Ascospores, which may break into part spores. Scale bars: a=0.5 mm, b =100 μm, c=50 μm, d-g=20 μm
Current name: Preussia tenerifae (Arx & Aa) Kruys, Syst. Biod. 7: 476.
Ascomata 290-400 μm diam., solitary, scattered, initially immersed, becoming erumpent when mature, globose, subglobose to ovate, black, membraneous, with a cylindrical or somewhat conical beak, 90-150(−230) μm broad and 110-190 μm high (Fig. 101a). Peridium 20-33 μm thick, 1-layered, composed of several layers of heavily pigmented, elongate cells of textura angularis, cells up to 6.3 x5 μm diam., cell wall 1-1.5 μm thick (Fig. 101b and c). Hamathecium of dense, long trabeculate pseudoparaphyses 1–2 μm broad, hyaline, aseptate, decomposing when mature. Asci 165-220×33-42.5 μm, 8-spored, bitunicate, broadly clavate, with a small, thin and furcate pedicel, 35-50 μm long, 3-5 μm broad, ocular chamber not observed (Fig. 101d and e). Ascospores 68-93×12.5-16 μm, 3-4 seriate to uniseriate near the base, cylindrical to cylindro-clavate with rounded ends, brown, (6-)7 transverse septa, easily breaking into partspores, central cells triangular in transverse section but rectangular in vertical section, 14-16.5×8-10 μm long, apical cells 12.5-15×11.5-17.5 μm long (Fig. 101f and g).
Anamorph: none reported.
Material examined: SPAIN, Canary Islands, Tenerifa Las Canadas, on rabbit? droppings, Mar. 1986, J.A. von Arx (HCBS 9812, holotype).
Notes
Morphology
Spororminula was formally established by von Arx and van der Aa (1987) according to its “ostiolate ascomata, elongated ascospore separated into part cells by transverse septa and without germ slits”, and was monotypified by S. tenerifae. Currently, only one species was included in this genus.
Phylogenetic study
Based on a phylogenetic analysis of ITS-nLSU rDNA, mtSSU rDNA and β-tubulin sequences, Spororminula tenerifae nested in the clade of Preussia, thus Spororminula was treated as a synonym of Preussia (Kruys and Wedin 2009).
Concluding remarks
To clarify its relationship with other genera of Sporormiaceae, further phylogenetic study is needed, which should include additional related taxa.
Excluded and doubtful genera
Kriegeriella Hohn., Annls myco1. 16: 39 (1918). (Dothideomycetes, families incertae sedis, Microthyriaceae)
Generic description
Habitat terrestrial, saprobic? Ascomata small, solitary, scattered, superficial, subglobose, black, roughened, apex no obvious opening. Peridium thin, composed of a single type of lightly pigmented thin-walled cells. Hamathecium long cellular pseudoparaphyses, septate. Asci 8-spored, bitunicate, obpyriform. Ascospores hyaline, turning brown when mature, multi-septate, constricted at each septum. Anamorphs reported for genus: none.
Literature: von Arx and Muller 1975; Barr 1975, 1987b; Eriksson 2006; Lumbsch and Huhndorf 2007.
Type species
Kriegeriella mirabilis Hohn., Annls myco1. 16: 39 (1918) (Fig. 102)
Fig. 102.
Kriegeriella mirabilis (from S reg. nr F12638, isolectotype). a Section of a superficial ascoma. b Anamorphic stage. c Obpyriform ascus. Note the pigmented ascospores and hyaline ascospores coexisted in a single ascus. d Ascospores. Scale bars: a= 50 μm, b-d =10 μm. e Ascomata on the host surface. f, g Crashed ascoma. Note the peridium structure. h, i Hyaline asymmetric ascospores. Scale bars: e, f =100 μm, g=50 μm, h, i=10 μm
Ascomata 100-120 μm highx 150-220 μm diam., solitary, scattered, superficial, with basal wall flattened on the surface of the substrate, subglobose, black, roughened, apex no obvious opening (Fig. 102a and e). Peridium thin, composed of a single type of lightly pigmented thin-walled cells, cells up to 12× 5 μm diam. in front view, cell wall less than 1 μm thick, apex cells smaller and walls thicker (Fig. 102a and f). Hamathecium long cellular pseudoparaphyses, 1.5-2 μm wide, septate. Asci 65-85×31-36 μm (x = 63.1 × 33mm, n= 10), 8-spored, bitunicate, fissitunicate undetermined, obpyriform, no pedicel, no ocular chamber was seen (Fig. 102c and g). Ascospores 28-37.5×8-11 μm (x = 32.6 × 10mm, n= 10), 8-seriate, fusoid with broadly rounded ends, hyaline, turning brown when mature, 5-6-septate, constricted at each septum, the upper first-cell longer and broader than the lower ones with semi-round shape, smooth (Fig. 102d, h, i).
Anamorph: see Fig. b.
Material examined: On the leaves of Faulenden nadeln von Pinus silvestris, bei Ronigstein, Sept. 1896, W. Rueges. (S reg. nr F12638, isolectotype).
Notes
Morphology
Kriegeriella was formally established by von Hohnel (1918b) and was represented by two species, i.e. K. mirabilis and K. transiens; it was typified by K. mirabilis and assigned to Microthyriaceae. Subsequently, Kriegeriella was assigned to the subfamily of Aulographiodeae (Microthyriaceae) (Batista et al. 1959), Asterinaceae (Hemisphaeriales) (Luttrell 1973) and Pseudosphaeriaceae (Dothideales) (Barr 1975).
After checking the original description and the type specimens of K. mirabilis and K. transiens, no significant difference could be observed, and both are described from rotting needles of conifers (Barr 1975; Batista et al. 1959; Hohnel 1918b). Morphologically, Extrawettsteinina is comparable with Kriegeriella. In particularly, E. pinastri could not be distinguished from K. transiens or K. mirabilis. Thus, K. transiens including Extrawettsteinina pinastri was treated as synonyms of K. mirabilis, and was included in the section of Kriegeriella under the genus Kriegeriella (von Arx and Muller 1975; Barr 1975). The other section of Kriegeriella, Extrawettsteinina, includes two previous Extrawettsteinina species, i.e. K. minuta and K. mediterranea. Barr (1987b) introduced a family, i.e. Kriegeriellaceae (Dothideales) to accommodate Kriegeriella and Extrawettsteinina. This proposal is rarely followed, and Kriegeriella is usually assigned to Pleosporaceae (Pleosporales) (Eriksson 2006; Lumbsch and Huhndorf 2007).
Phylogenetic study
None.
Concluding remarks
Kriegeriella might belong to Microthyriaceae, although it would be unusual in this family in having 5-6-septate ascospores. Micropeltidaceae better accommodates the ascospores, however, the parallel arrangement of cells of the upper peridium are not typical. Asterinaceae may be most suitable as Luttrell (1973) suggested.
Phaeotrichum Cain & M.E. Barr, Can. J. Bot. 34: 676 (1956). (Dothideomycetes, family incertae sedis, Phaeotrichaceae)
Generic description
Habitat terrestrial, saprobic (coprophilous). Ascomata small, cleistothecial, solitary, or in small groups, superficial, with long straight or slightly flexed, thin, black appendages evenly scattered on the surface of the ascomata, globose, black. Peridium thin, carbonaceous-membraneous, 1-layered, composed of dark brown thick-walled cells of textura angularis. Hamathecium not observed. Asci bitunicate form not clear, fissitunicate dehiscence not observed, broadly clavate, with a relatively thick pedicel. Ascospores oblong to ellipsoid, hyaline when young, turning reddish brown at maturity, 1-septate, deeply constricted at the septum, each end with a subhyaline and broadly rounded germ pore, readily forming partspores at the septum at maturity. Anamorphs reported for genus: none.
Literature: Cain 1956; Malloch and Cain 1972.
Type species
Phaeotrichum hystricinum Cain & M.E. Barr, Can. J. Bot. 34: 677 (1956). (Fig. 103)
Fig. 103.
Phaeotrichum hystricinum (from TRTC 4361, hototype). a Superficial ascomata on host surface. Note the long and black appendages. b Part of peridium. Note the large cells in surface view. c-f Released reddish brown ascospores with hyaline end cells. Note the strongly constricted middle septum. Scale bars: a=0.5 mm, b-f =20 μm
(Some information for the following description is from Cain 1956)
Ascomata 170-280 μm diam., cleistothecial, solitary, or in small groups, superficial, with 15-20 long straight or slightly flexed, thin, black appendages evenly scattered on the surface of the ascomata, 0.5-1 mm long, 15-25 μm wide at base, tapering to less than 5 μm at the blunt apex, with few or without septa, globose, black, smooth (Fig. 103a). Peridium thin, carbonaceous-membraneous, 1-layered, composed of dark brown thick-walled cells of textura angularis, cells 8-16 μm diam., cell wall 0.5-1.5 μm thick (data obtained from Cain 1956) (Fig. 103b). Hamathecium not observed. Asci 42-48 × 14-17 μm, 8-spored, bitunicate form not typical, lacking fissitunicate dehiscence, broadly clavate, with a relatively thick pedicel which is about 18 μm (data obtained from Cain 1956). Ascospores 14-16×4-5 μm, 4-seriate, oblong to ellipsoid, hyaline when young, turning reddish brown at maturity, 1-septate, deeply constricted at the septum, each end with a subhyaline and broadly rounded germ pore, smooth, readily separating into partspores at the septum at maturity (Fig. 103c, d, e and f).
Anamorph: none reported.
Material examined: CANADA, Ontario, Muskoka, Stoneleigh, on porcupine dung, 18 Aug. 1932, Cain (TRTC 4361, holotype).
Note: the ascomata of the specimen are fragile and no asci could be obtained.
Notes
Morphology
Phaeotrichum was formally established by Cain (1956) to accommodate two new coprophilous fungi, i.e. P. hystricinum and P. circinatum Cain, and P. hystricinum was selected as the generic type. Phaeotrichum is mainly characterized by its coprophilous habitat, superficial cleistothecial ascocarps covered by long hairy appendages, reddish brown 1-septate ascospore with a broadly rounded germ pore at each end, readily breaking into partspores (Cain 1956). According to Cain (1956), Phaeotrichum possesses untypical bitunicate ascus, and the ascospore releasing is described as “simply break down and allow the contents to become free in the cavity of the ascocarp”. This ascospore releasing mechanism is considered as evolutionarily developed compared to those that “discharge the ascospores through an apical pore” (Cain 1956). Although lacking a typical bitunicate ascus, Phaeotrichum is still assigned to Pleosporales, because the lack of bitunicate ascus does not “taken by itself, exclude a fungus from close relationship” (Cain 1956).
Phylogenetic study
A single unverified isolate of Phaeotrichum benjaminii is placed well outside of Pleosporales in a broad phylogenetic study (Schoch et al. 2009).
Concluding remarks
The superficial cleistothecial ascocarps covered by long hairy appendages, the absence of hamathecium as well as the nontypical bitunicate ascus are all distinct from members of Pleosporales, but definite conclusions could only be obtained by further molecular phylogenetic analysis. In this study, we assign it to Dothideomycetes incertae cedis.
Zeuctomorpha Sivan., P.M. Kirk & Govindu, Bitunicate Ascomycetes and their Anamorphs: 572 (1984). (Venturiaceae)
Generic description
Habitat terrestrial, hemibiotrophic. Ascomata small, gregarious, superficial, globose to slightly flattened, ostiolate, covered with setae. Peridium thin, composed of heavily pigmented pseudoparenchymatous cells of textura angularis. Hamathecium of rare, septate, branching and anastomosing pseudoparaphyses. Asci 8-spored, with a short thick pedicel, bitunicate, fissitunicate, broadly clavate to obclavate. Ascospores ellipsoid, dark brown, 1-septate, asymmetrical, deeply constricted at the septum.
Anamorphs reported for genus: Acroconidiellina (Sivanesan 1984).
Literature: Sivanesan 1984.
Type species Zeuctomorpha arecae Sivan., P.M. Kirk & Govindu, in Sivanesan, Bitunicate Ascomycetes and their Anamorphs: 572 (1984). (Fig. 104)
Fig. 104.
Zeuctomorpha arecae (from IMI 246067, holotype). a Gregarious ascomata on host surface. Note the numerous setae on the surface of ascomata. b Asci with ocular chamber and short peduncles. c, d Ascus with ocular chamber and knob-like pedicel. e-i One septate ascospores which are slightly asymmetrical. Scale bars: a=0.5 mm, b-i=20 μm
Ascomata 175-300 μm diam., gregarious, superficial, globose to slightly flattened, collapsed at the apex when dry, ostiolate, covered with numerous long setae (Fig. 104a). Peridium up to 25 μm wide, composed of heavily pigmented pseudoparenchymatous cells of textura angularis, to 7 μm diam. Hamathecium of rare, 2-5 μm broad, septate, branching and anastomosing pseudoparaphyses. Asci 83-185×29-40(−50) μm (x = 134 × 35.3mm, n= 10), 8-spored, bitunicate, fissitunicate, broadly clavate to obclavate, with a short thick pedicel, up to 40 μm long, apically rounded, with a small ocular chamber (to 4 μm widex 7 μm high) (Fig. 104b, c and d). Ascospores 35-43× 12.5-18 μm (x = 36.5 × 15.4mm, n=10), 2-4 seriate, ellipsoid, dark brown, 1-septate, deeply constricted at the septum, usually slightly asymmetric, smooth (Fig. 104e, f, g, h and i).
Anamorph: Acroconidiellina arecae (Sivanesan 1984). Material examined: INDIA, Shimogee, on Areca catechu L. leaf, 1 Nov. 1979, H.C. Govindu (IMI 246067, holotype).
Notes
Morphology
Zeuctomorpha was formally established by Sivanesan (1984) based on its superficial setose ascomata, clavate asci, ellipsoid and 1-septate ascospores, and presence of pseudoparaphyses, and was monotypified by Z. arecae. Zeuctomorpha arecae is widely distributed in tropical regions of East South Asia exclusively on the leaves of Areca catechu (Sivanesan 1984).
Phylogenetic study
None.
Concluding remarks
This taxon is unusual amongst the Pleosporaceae as it has hairy superficial ascomata, few pseudoparaphyses, broadly clavate to obclavate asci and 1-septate pigmented ascospores. All of these morphological characters are most comparable with species of Acantharia, which might be closely related to Venturiaceae (Zhang et al. data unpublished).
Muroia I. Hino & Katum., J. Jap. Bot. 33: 79 (1958). (Ascomycota)
Generic description
Habitat terrestrial, saprobic or parasitic. Ascostromata erumpent through the host surface in linear rows parallel to the host fibers. Ascomata small- to medium-sized, semi-immersed to erumpent, subglobose to rectangular, black, coriaceous, cells of ascostromata pseudoparenchymatous, cells of peridium composed of pigmented cells of textura angularis. Hamathecium of rare, pseudoparaphyses. Asci bitunicate, clavate to cylindro-clavate. Ascospores oblong to elongated oblong, hyaline, 1-celled, usually slightly curved.
Anamorphs reported for genus: none.
Literature: Hino and Katumoto 1958.
Type species
Muroia nipponica I. Hino & Katum., J. Jap. Bot. 33: 79 (1958). (Fig. 105)
Fig. 105.
Muroia nipponica (TNS-F-230252, isotype). a Linear ascostroma parallel to the host fibers. b Crashed ascus with ascospores released. c-e Released hyaline ascospores. Scale bars: a=5 mm, b-e=30 μm
Ascostroma 1-6 mm long, 360-470 μm broad, linear parallel to the host fibers with several linearly arranged ascomata (Fig. 105a). Ascomata 250-400 μm diam., semi-immersed in substrate to erumpent, subglobose to rectangular with a furrow-shaped ostiole, black, coriaceous, cells of ascostromata pseudoparenchymatous. Peridium composed of pigmented cells of textura angularis. Hamathecium of rare, 3-4.5 μm broad pseudoparaphyses. Asci (120-)150-190×30-45 μm, 8-spored, bitunicate, fissitunicate dehiscence not observed, clavate to cylindro-clavate, with a short, thin, knob-like pedicel, lacking an ocular chamber (Fig. 105b). Ascospores 43-50×13-18 μm (x = 46.6 × 15.2mm, n=10), biseriate, ob-long to elongated oblong, hyaline, 1-celled, usually slightly curved (Fig. 105c,d and e).
Anamorph: none reported.
Material examined: JAPAN, Province Ugo. on moribund culm of Sasa kurilensis, 4 Aug. 1957, coll. H. Muroi, Det. I. Hino & K. Katumoto (TNS-F-230252, isotype).
Notes
Morphology
Muroia was introduced based on M. nipponica, which is a parasite on the lower part of Sasa kurilensis (Hino and Katumoto 1958). Muroia is characterized by its 1-celled ascospores. Considering the perithecial structure and linear ostiole, it was assigned to the Lophiostomataceae, and was regarded as closely related to the amerosporous Lophiella (Hino and Katumoto 1958).
Phylogenetic study
None.
Concluding remarks
The linear ascostroma and 1-celled, hyaline ascospores make it less likely to fit the concept of Lophiostomataceae. Because of the condition of the specimen, its bitunicate nature could not be confirmed.
Genera not studied
Aglaospora De Not., G. bot. ital. 2: 43 (1844).
Type species: Aglaospora profusa (Fr.) De Not., G. bot. ital. 2: 43 (1844).
Aglaospora, which was introduced by de Notaris (1844), has 35 species epithets (http://www.mycobank.org/mycotaxo.aspx) and was considered to be a synonym of Massaria (Voglmayr and Jaklitsch 2011) or separate (Barr 1990a). In a recent phylogenetic study, Voglmayr and Jaklitsch (2011) confirmed that Aglaospora is a synonym of Massaria and is treated as such here. The immersed ascomata with short beaks, together with ascostroma under pseudostromatic tissues, cylindrical asci with a large and refractive apical ring, trabeculate pseudoparaphyses within a gel matrix, and distoseptate ascospores, are all similar to species of Massaria. The large and conspicuous apical ring of the ascus of Aglaospora has the appearance of being unitunicate, and thus Shoemaker and Kokko (1977) treated it as a unitunicate taxon. Currently, its bitunicate status is widely accepted.
Allewia E.G. Simmons, Mycotaxon 38: 260 (1990).
Type species: Allewia proteae E.G. Simmons, Mycotaxon 38: 262 (1990).
Allewia was introduced by Simmons (1990) to accommodate Lewia-like species but with Embellisia anamorphs. Embellisia differs from other similar genera by a combination of characters including the percentage of dictyoconidia, shape of conidia, thickness of septa, umbilicate sites of conidiophore geniculation, proliferating chlamydospores and hyphal coils in culture (Simmons 1971). Based on multigene phylogenetic analysis, A. eureka, which is closely related to A. proteae, clustered together with species of Alternaria. Thus, Allewia should be treated as a synonym of Lewia.
Anteaglonium Mugambi & Huhndorf, System. Biodivers. 7: 460 (2009).
Type species: Anteaglonium abbreviatum (Schwein.) Mugambi & Huhndorf, System. Biodivers. 7: 460 (2009).
≡ Hysterium abbreviatum Schwein., Trans. Am. phil. Soc., New Series 4: no. 2094 (1832).
Anteaglonium was introduced to accommodate a monophyletic hysterothecial clade within Pleosporales, and four species (A. abbreviatum, A. globosum Mugambi & Huhndorf, A. parvulum (W.R. Gerard) Mugambi & Huhndorf and A. latirostrum Mugambi & Huhndorf) are included (Mugambi and Huhndorf 2009a). Anteaglonium is characterized by erumpent to superficial, globose to subglobose or elongate, fusoid to oblong ascomata, which are brown to shiny black, opening by a pronounced or indistinct longitudinal slit running entire length of fruit body or apex raised and laterally compressed; asci cylindrical with short pedicel, 8-spored, uniseriate or biseriate; ascospores fusoid to oblong, septate, constricted at the primary septum, hyaline or pigmented. A phylogenetic analysis based on DNA comparisons indicated that Anteaglonium resides as a separate clade but related to Tetraplosphaeria, Lophiotrema and other species without clear resolution. Therefore, the familial placement of Anteaglonium remains unclear (Mugambi and Huhndorf 2009a).
Arthopyrenia A. Massal., Ric. auton. lich. crost. (Verona): 165 (1852).
Type species: Arthopyrenia rhyponta (Ach.) A. Massal., Ric. auton. lich. crost. (Verona): 166, fig. 329 (1852).
≡ Verrucaria rhyponta Ach., K. Vetensk-Acad. Nya Hand1.: 150 (1809).
Arthopyrenia is a lichen genus with a Trentepohlia photobiont and is characterized by dimidiate perithecoid ascomata, which are scattered to irregularly confluent, and have an upper thick clypeate wall composed of periderm cells intermixed with dark hyphae. The pseudoparaphyses are branched and asci are obpyriform, obclavate to subcylindrical and 8-spored. Ascospores are oblong, ovoid, slipper-shaped, 1-3-septate, hyaline and smooth-walled (Coppins 1988; Upreti and Pant 1993). Multigene phylogenetic studies indicated that Arthopyrenia salicis, a typical species of Arthopyrenia, is located within Pleosporales in close proximity to bambusicolous species in the genus Roussoella, with its familial status remaining undetermined (Del Prado et al. 2006; Schoch et al. 2009; Zhang et al. 2009a).
Ascocratera Kohlm., Can. J. Bot. 64: 3036 (1986).
Type species: Ascocratera manglicola Kohlm., Can. J. Bot. 64(12): 3036 (1986).
Ascocratera is a monotypic obligate marine fungus and is characterized by conical, crater-like, erumpent to superficial and carbonaceous ascomata, a depressed ostiole, a thick peridium, trabeculate pseudoparaphyses, bitunicate, fissitunicate and cylindrical asci, and ellipsoidal, hyaline, 1-septate (3-septate when senescent) ascospores surrounded by a sheath (Kohlmeyer 1986). Ascocratera was reported to be one of the most common marine fungi of the upper intertidal zone of dead mangrove roots, trunks and branches (Kohlmeyer 1986). Based on a multigene phylogenetic analysis, Ascocratera nested within the clade of Aigialaceae (Schoch et al. 2009; Suetrong et al. 2009).
Atradidymella M.L. Davey & Currah, Am. J. Bot. 96: 1283 (2009).
Type species: Atradidymella muscivora M.L. Davey & Currah, Am. J. Bot. 96: 1283 (2009).
Atradidymella was introduced as a pleosporalean genus parasitic on boreal bryophytes, and is characterized by minute, unilocular, setose pseudothecia with 2–3 wall layers; brown, fusoid, 1-septate ascospores, and an anamorphic stage (Phoma muscivora M.L. Davey & Currah) (Davey and Currah 2009). Based on an ITS rDNA sequences analysis, Atradidymella nested within Didymellaceae (Davey and Currah 2009).
Bertiella (Sacc.) Sacc. & P. Syd., in Saccardo, SyU. fung. (Abellini) 14: 19 (1899).
≡ Bertia subgen. Bertiella Sacc., SyU. fung. (Abellini) 1: 584 (1882).
Type species: Bertiella macrospora (Sacc.) Sacc. & Traverso, SyU. fung. (Abellini) 19: 147 (1910).
≡ Bertia macrospora Sacc., Michelia 1(no. 8): 452 (1882). Bertia subg. Bertiella was raised to generic rank by Saccardo (1899), and is typified by B. macrospora. After studying the type specimen of B. macrospora, Eriksson and Yue (1986) assigned it to Massarina (as M. macrospora (Sacc.) O.E. Erikss & J.Z. Yue). Concurrently, Bertiella is treated as a synonym of Massarina. Hyde et al. (2002) assigned Bertia macrospora to Lophiostoma as (L. bertiellum Aptroot & K.D. Hyde).
The superficial ascomata, cylindro-clavate asci and hyaline 1-septate ascospores which may become 3-septate and pale brown when senescent and, in particular, the woody habitat indicate that B. macrospora may be related to Lophiostoma sensu Holm and Holm (1988). A single isolate of Bertiella macrospora clusters with Byssosphaeria in the Melanommataceae in a recent DNA based phylogeny (Mugambi and Huhndorf 2009b). The relationship between Bertiella and Byssosphaeria needs further study.
Byssothecium Fuckel, Bot. Ztg. 19: 251 (1861).
Type species: Byssothecium circinans Fuckel, Bot. Ztg. 19: 251 (1861).
The isotype of Byssothecium circinans is in FH as exiccatae (Fungi rhenani 730c); it was described by Boise (1983) and could not be loaned. Byssothecium circinans is regarded as a saprobe or weak parasite of Medicago sativa (Semeniuk 1983), and a Pleospora-type centrum was observed (Boise 1983). A Chaetophoma-like anamorph was produced in culture, however, no culture or herbarium specimen is listed (Boise 1983). Boise (1983) regarded Byssothecium circinans as closely related to Teichospora, however, confirmation is required. An isolate of Byssothecium circinans was sequenced and a multigene phylogeny placed it in close proximity to members of Massarinaceae (Schoch et al. 2009; Zhang et al. 2009a; Plate 1).
Caryospora De Not., Micr. Ital. Novi 9: 7 (1855).
Type species: Caryospora putaminum (Schwein.) De Not., Micr. Ital., Dec. 9: 7 (1855).
After studying the Caryospora species in North America, Barr (1979b) indicated that species of Caryospora may closely relate to Trematosphaeria. Boise (1985) distinguished Caryospora from Trematosphaeria based on the structure of ascospores. Currently, 17 taxa, from freshwater, marine, or terrestrial habitats (Raja and Shearer 2008), are included within Caryospora and might be polyphyletic.
Celtidia J.D. Janse, Ann. Jard. Bot. Buitenzorg 14: 202 (1897). Type species: Celtidia duplicispora J.D. Janse, Ann. Jard. Bot. Buitenzorg 14: 202 (1897).
Celtidia is a monotypic genus, which is characterized by its echinulate ascospores (Hawksworth 1979). It is only known from an illustration accompanying the original description from root nodules of Celtis in Java. A new collection is needed for further study of this genus.
Chaetopreussia Locq.-Lin., Revue MycoL, Paris 41: 185 (1977). . Type species: Chaetopreussia chadefaudii Locq.-Lin., Revue MycoL, Paris 41: 187 (1977).
Chaetopreussia is a monotypic genus characterized by cleistothecioid ascomata with seta, and 3-septate ascospores without germ slits. Recent molecular analysis has shown that cleistothecioid ascomata and the presence of germ slits lack significance at the generic rank (Kruys and Wedin 2009). Chaetopreussia is possibly another synonym of Preussia.
Clathrospora Rabenh., Hedwigia 1(18): 116 (1857).
Type species: Clathrospora elynae Rabenh., Hedwigia 1: 116 (1857).
The most striking character of Clathrospora is its ascomata opening with an intraepidermal discoid lid and muriform applanate ascospores with more than one row of longitudinal septa (Shoemaker and Babcock 1992). The form of opening and applanate ascospores, however, might have limited significance at generic rank and thus, Clathrospora may be closely related to Pleosporaceae. Phylogenetic analysis based on nLSU, nSSU and mtSSU indicate that C. diplospora (Ellis & Everh.) Sacc. & Traverso nests in Pleosporaceae (Kruys et al. 2006). Clathrospora elynae is saprobic on monocots (Shoemaker and Babcock 1992).
Cochliobolus Drechsler, Phytopathology 24: 973 (1934). Type species: Cochliobolus heterostrophus (Drechsler) Drechsler, Phytopathology 24: 973 (1934).
Cochliobolus and its asexual relatives are well studied taxa in Pleosporales because of their economic importance. Cochliobolus includes both saprobic and pathogenic species that are significant monocot pathogens worldwide, which attack corn, rice, barley, sugarcane, wheat, and oats, all major cereal crops. Cochliobolus is characterized by globose or subglobose ascomata with a well defined long ostiolar papilla or cylindrical neck, a peridium composed of pseudoparenchymatous cells, filliform, septate and branched pseudoparaphyses, and thin-walled cylindrical or broadly clavate asci. Ascospores are distinctively hyaline or pale brown, filliform, and strongly helicoid to loosely coiled in the asci (Sivanesan 1984). The anamorphs of Cochliobolus belong to Bipolaris and Curvularia (Sivanesan 1984). Bipolaris and Curvularia can be distinguished by characters of conidial morphology, conidial germination, hilum structure, conidial septum and wall structure, conidial septum ontogeny (Sivanesan 1987). Multigene phylogenetic analysis indicated that Cochliobolus heterostrophus and C. sativus (S. Ito & Kurib.) Drechsler ex Dastur nested within the clade of Pleosporaceae (Zhang et al. 2009a; Plate 1). Thus, its familial placement is confirmed.
Comoclathris Clem., Gen. fung. (Minneapolis): 37, 173 (1909).
Type species: Comoclathris lanata Clem. [as ‘Comochlatris’], Gen. fung. (Minneapolis) (1909).
Comoclathris is temporarily placed in Diademaceae, and its pivotal characters are the circular lid-like opening and applanate reddish-brown to dark reddish-brown muriform ascospores with single longitudinal septa (versus two or more rows of longitudinal septa of Clathrospora) (Shoemaker and Babcock 1992). Barr (1990b) treated it as a synonym of Graphyllium. Comoclathris has been linked with an Alternaria-like anamorphs (Simmons 1952), which may suggest its close relationship with Pleosporaceae.
Coronopapilla Kohlm. & Volkm.-Kohlm., Myco1. Res. 94: 686 (1990).
Type species: Coronopapilla avellina Kohlm. & Volkm.-Kohlm., Mycol. Res. 94: 687 (1990).
Coronopapilla is characterized by immersed ascomata with a conical papilla, thin peridium, 8-spored and thick-walled, cylindrical and fissitunicate asci. Ascospores are ellipsoidal, 1-3-septate, brown and distoseptate. Coronopapilla avellina is an obligate marine species, and was originally assigned to Didymosphaeriaceae (Kohlmeyer and Volkmann-Kohlmeyer 1990). The marine habitat of Coronopapilla makes it readily distinguishable from Didymosphaeria futilis (the generic type of Didymosphaeria). Thus, the familial placement of Coronopapilla is yet to be determined.
Cucurbitaria Gray, Nat. Arr. Brit. Pl. (London) 1: 508, 519 (1821).
Type species: Cucurbitaria berberidis (Pers.) Gray, Nat. Arr. Brit. Pl. (London) 1: 508, 519 (1821).
≡ Sphaeria berberidis Pers., Neues Mag. Bot. 1: 83 (1794).
A narrow generic concept of Cucurbitaria was accepted by Welch (1926), who restricted Cucurbitaria to five closely related species, which have turbinate ascomata that develop cespitosely in a massive subiculum or over compressed stromatic tissues and have a thick and obconoid base. A broader generic concept was accepted by Mirza (1968), who also included species with globose or ovoid to pyriform ascomata that are gregarious on the substrate with only sparse subiculum and lack an obconoid region in the base of the locule. Barr (1990b) accepted an intermediate concept, and described 11 related species from North America. Currently, 450 species are accepted in Cucurbitaria (http://www.mycobank.org/mycotaxo.aspx), and the genus was assigned to Cucurbitariaceae. In this study, an isolate of C. berberidis clustered with some species of Pyrenochaeta and Didymosphaeria futilis, and they get moderate bootstrap support (Plate 1). Cucurbitariaceae may be another family within Pleosporineae.
Curreya Sacc., Syll. fung. (Abellini) 2: 651 (1883).
Type species: Curreya conorum (Fuckel) Sacc., Syll. fung. (Abellini) 2: 651 (1883).
Curreya is a contentious genus which had been assigned to Pleospora (Barr 1981). von Arx and van der Aa (1983), however, maintained it as distinct, because of its Coniothyrium anamorph, and considered Curreya should be closely related to Didymosphaeria, Melanomma, Paraphaeosphaeria or Massarina. Because of the small sclerotial cells of its peridium, the narrower, thinner-walled asci and its Coniothyrium-like anamorph, Barr (1990b) assigned it to the Leptosphaeriaceae. Previous phylogenetic studies indicated that a strain of Curreya pityophila (J.C. Schmidt & Kunze) Petr. nested within Massarineae (Kruys et al. 2006).
Decorospora Inderb., Kohlm. & Volkm.-Kohlm., Mycologia 94: 657 (2002).
Type species: Decorospora gaudefroyi (Pat.) Inderb., Kohlm. & Volkm.-Kohlm., Mycologia 94: 657 (2002).
≡ Pleospora gaudefroyi Pat., Tabl. analyt. Fung. France (Paris) 10: 40 (no. 602) (1886).
Decorospora gaudefroyi (as Pleospora gaudefroyi) had been considered a synonym of Pleospora herbarum, despite its striking sheath of ascospores (Wehmeyer 1961). Molecular phylogenetic analysis based on partial SSU and ITS rDNA sequences indicated that Decorospora gaudefroyi was a sister taxon in the Pleosporaceae represented by Alternaria alternata (Fr.) Keissl., Cochliobolus sativus, Pleospora herbarum, Pyrenophora triticirepentis (Died.) Drechsler and Setosphaeria rostrata K.J. Leonard (Inderbitzin et al. 2002). Decorospora was introduced as a monotypic genus represented by Decorospora gaudefroyi, which is characterized by black ascomata becoming superficial on the substrate at maturity, septate and branched pseudoparaphyses, fissitunicate, clavate asci, as well as yellowish brown ascospores with seven transverse septa and one to three longitudinal septa in each segment, enclosed in a sheath with 4-5 apical extensions (Inderbitzin et al. 2002). Decorospora gaudefroyi is an obligate marine fungus, growing at or above the high water mark (Inderbitzin et al. 2002).
Diadema Shoemaker & C.E. Babc., Can. J. Bot. 67: 1349 (1989).
Type species: Diadema tetramerum Shoemaker & C.E. Babc. [as ‘tetramera’], Can. J. Bot. 67: 1354 (1989).
During their study of Leptosphaeria and Phaeosphaeria, Shoemaker and Babcock (1989c) found some alpine fungi with typical pleosporalean characters (such as perithecoid ascomata, bitunicate asci and presence of pseudoparaphyses) having relatively large, very dark brown ascospores, mostly with a peculiar disc-like opening (as reported in some species of Wettsteinina, Shoemaker and Babcock 1987). Thus, they introduced a new genus Diadema (typified by D. tetramerum) to accommodate them (Shoemaker and Babcock 1989c). Currently, Diadema is assigned to Diademaceae, and differs from other genera in the family in having ascospores which lack longitudinal septa (Shoemaker and Babcock 1992). The large, dark brown ascospores and the disc-like opening, however, may be an adaptation to environmental factors.
Diademosa Shoemaker & C.E. Babc., Can. J. Bot. 70: 1641 (1992).
Type species: Diademosa californiana (M.E. Barr) Shoemaker & C.E. Babc. [as ‘californianum’], Can. J. Bot. 70: 1641 (1992).
≡ Graphyllium californianum M.E. Barr, Mem. N. Y. bot. Gdn 62: 40 (1990).
Diademosa is the only genus in Diademaceae that has terete (cylindrical, circular in cross section) ascospores (Shoemaker and Babcock 1992).
Didymella Sacc., Michelia 2(no. 6): 57 (1880).
Type species: Didymella exigua (Niessl) Sacc., SyU. fung. (Abellini) 1: 553 (1882).
≡ Didymosphaeria exigua Niessl, Ost. bot. Z.: 165 (1875).
The type specimen of Didymella (D. exigua) is lost and a neotype specimen was selected by de Gruyter et al. (2009). Didymella was characterized by the immersed or erumpent, globose or flattened and ostiolate ascomata with dense, rare (or lack?) of pseudoparaphyses. Asci are cylindrical, clavate or saccate, and 8-spored. Ascospores are hyaline, 1-septate (symmetrical or asymmetrical) and constricted at the septum. Didymella has been assigned under Mycosphaerellaceae, Pleosporales (Sivanesan 1984), Phaeosphaeriaceae (Barr 1979a; Silva-Hanlin and Hanlin 1999), Venturiaceae (Reddy et al. 1998) or Pleosporales genera incertae sedis (Lumbsch and Huhndorf 2007). Based on a multigene phylogenetic analysis, the Didymella clade forms a familial rank within Pleosporineae, thus the Didymellaceae was introduced (Aveskamp et al. 2010; de Gruyter et al. 2009; Zhang et al. 2009a; Plate 1). Anamorphs of Didymellaceae include Ascochyta, Ampelomyces, Boeremia, Chaetasbolisia, Dactuliochaeta, Epicoccum, Microsphaeropsis, Peyronellaea, Phoma, Piggotia, Pithoascus, Pithomyces and Stagonosporopsis (Aveskamp et al. 2010; de Gruyter et al. 2009; Hyde et al. 2011).
Didymocrea Kowalski, Mycologia 57: 405 (1965).
Type species: Didymocrea sadasivanii (T.K.R. Reddy) Kowalski, Mycologia 57: 405 (1965).
≡ Didymosphaeria sadasivanii T.K.R. Reddy, Mycologia 53: 471 (1962).
Didymocrea is a monotypic genus, and was separated from Didymosphaeria based on its “unitunicate asci”, presence of pseudoparaphyses and absence of spermatia, and assigned under Hypocreales (Kowalski 1965). Following Kowalski (1965), Luttrell (1975) also studied the centrum development of Didymocrea, and concluded that it should be a true pleosporalean fungus with functionally unitunicate asci, and retained it in Didymosphaeria. After studying the type specimen of Didymocrea sadasivanii, Aptroot (1995) concluded that it should be closely related to the loculoascomycetous genus Zopfia. Rossman et al. (1999) also kept it as a unique genus in Pleosporales. Based on a multigene phylogenetic analysis, D. sadasivanii nests within Montagnulaceae (Kruys et al. 2006; Schoch et al. 2009).
Dothivalsaria Petr., Sydowia 19: 283 (1966) [1965].
Type species: Dothivalsaria megalospora (Auersw.) Petr., Sydowia 19: 283 (1966) [1965].
≡ Valsaria megalospora Auersw., Leipzig. Bot. Tauschver. 5. (1866).
Dothivalsaria is monotypic and is represented by D. megalospora (Petrak 1965). The taxon is characterized by immersed, medium- to large-sized ascomata which usually aggregate under blackened stromatic tissues and have trabeculate pseudoparaphyses. Asci are cylindrical, while ascospores are brown, ellipsoid, and 1-septate and uniseriate in the asci (Barr 1990a). The ascostroma of D. megalospora is comparable with those of Aglaospora profusa as has been mentioned by Barr (1990a), but their relationships are unclear.
Epiphegia G.H. Otth, Mitt. naturf. Ges. Bern: 104 (1870). Type species: Epiphegia alni G.H. Otth, Mitt. naturf. Ges. Bern: 104 (1870).
Epiphegia was reinstated to accommodate a species which has Phragmoporthe-like ascocarps and Massarina- like asci, pseudoparaphyses and ascospores (Aptroot 1998). Ascomata are grouped within stromatic tissues, pseudoparaphyses are cellular, asci are bitunicate and ascospores are hyaline and trans-septate (Aptroot 1998).
Eremodothis Arx, Kavaka 3: 34 (1976) [1975] (IMI 90223 = CBS 610.74 type).
Type species: Eremodothis angulata (A.C. Das) Arx, Kavaka 3: 34 (1976) [1975].
≡ Thielavia angulata A.C. Das, Trans. Br. Myco1. Soc. 45: 545 (1962).
The type species of Eremodothis (E. angulata) was originally isolated from rice field soil in Fulta, India and it was assigned to Sporormiaceae because of the orange pigmentation of the colony (von Arx 1976). The cleistothecoid ascomata, sphaerical asci and 1-celled ascospores of E. angulata are comparable with those of Pycnidiophora. Based on a multigene phylogenetic study, both Eremodothis and Pycnidiophora were treated as synonyms of Westerdykella (Kruys and Wedin 2009).
Extrawettsteinina M.E. Barr, Contr. Univ. Mich. Herb. 9 (8): 538 (1972).
Type species: Extrawettsteinina minuta M.E. Barr, Contr. Univ. Mich. Herb. 9(8): 538 (1972).
Extrawettsteinina was introduced to accommodate three species, i.e. E. minuta, E. pinastri M.E. Barr and E. mediterranea (E. MU11.) M.E. Barr, which are saprobic on the dead leaves of gymnosperms and angiosperms, in North America and Europe (Barr 1972). Subsequently, a fourth species was introduced, viz. E. andromedae (Auersw.) M.E. Barr (Barr 1987a). Extrawettsteinina is characterized by superficial, conical ascomata, containing a few saccate bitunicate asci, ellipsoidal, obovate-clavate, septate, smooth and hyaline ascospores which turn dull brown at maturity (Barr 1972). The diagnostic character of Extrawettsteinina is its conic ascocarps which are superficial on the substrate, and radiating arrangement of wall cells, which makes it distinguishable from comparable genera such as Stomatogene and Wettsteinina.
Graphyllium Clem., Botanical Survey of Nebraska 5: 6 (1901). Type species: Graphyllium chloes Clem., Botanical Survey of Nebraska 5: 6 (1901).
Graphyllium was first described as a hysteriaceous fungus with elongate ascomata, but von Hohnel (1918b, 1919) recognized its similarity to Clathrospora. Petrak (1952) transferred Graphyllium to Pleospora, and noted that the elongate ascomata and closely grouped rows of small ascomata are not sufficient to recognize the genus. Barr (1987b, 1990b) supported this proposal and considered Graphyllium differs from Clathrospora by shape, septation and pigmentation of ascospores. A narrow generic concept of Graphyllium was adapted by Shoemaker and Babcock (1992), which is characterized by hysterothecia, applanate ascospores that are at least 3-septate in side view and have some longitudinal septa in front view, and it was assigned under Hysteriaceae (order Pleosporales, Shoemaker and Babcock 1992). But subsequent classification systems tend to assign it to Diademaceae (e.g. Lumbsch and Huhndorf 2007, 2010). This seems unlikely as pointed out by Zhang et al. (2011) and the genus could be placed in one of five families containing hysteriotheciod ascomata. Recollection and molecular studies are needed.
Halomassarina Suetrong, Sakay., E.B.G. Jones, Kohlm., Volkm.-Kohlm. & C.L. Schoch, Stud. Myco1. 64: 161 (2009). Type species: Halomassarina thalassiae (Kohlm. & Volkm.-Kohlm.) Suetrong, Sakay., E.B.G. Jones, Kohlm., Volkm.-Kohlm. & C.L. Schoch, Stud. Myco1. 64: 161 (2009).
≡ Massarina thalassiae Kohlm. & Volkm.-Kohlm., Can. J. Bot. 65: 575 (1987).
Halomassarina is another marine genus which morphologically fits Massarina sensu lato, and is typified by H. thalassiae, which is characterized by subglobose to pyriform, immersed or erumpent, ostiolate, periphysate, papillate or epapillate, coriaceous ascomata, simple, rarely anastomosing pseudoparaphyses, 8-spored, cylindrical to clavate, pedunculate, thick-walled, fissitunicate asci, and ellipsoidal, (1-)3-septate, hyaline ascospores. Based on a multigene phylogenetic analysis, Halomassarina thalassiae clustered together with Trematosphaeria pertusa and another marine fungus Falciformispora lignatilis, and they are all assigned to Trematosphaeriaceae (Suetrong et al. data unpublished).
Hypsostroma Huhndorf, Mycologia 84: 750 (1992).
Type species: Hypsostroma saxicola Huhndorf, Mycologia 84: 750 (1992).
Hypsostroma was introduced as a tropical wood-inhabiting genus by Huhndorf (1992). Hypsostroma has several striking characters including the large superficial ascomata which form on a subiculum, pseudoparenchymatous peridial cells, trabeculate pseudoparaphyses, clavate asci with long pedicels and a conspicuous apical apparatus, and ascospores that separate into partspores with a germ slit in each partspore (Huhndorf 1992). Phylogenetic study indicated that Hypsostroma should be a new genus and the Hypsostromataceae was reinstated to accommodate Hypsostroma (Mugambi and Huhndorf 2009b; Plate 1).
Julella Fabre, Annls Sci. Nat., Bot., ser. 6 9:113 (1879) [1878]. Type species: Julella buxi Fabre, Annls Sci. Nat., Bot., ser. 6 9: 113 (1879) [1878].
Julella has been assigned to Thelenellaceae, a family of Ostropomycetidae (Lumbsch and Huhndorf 2007), and Arthopyreniaceae (= Xanthopyreniaceae sensu O. Eriksson, Pleosporales) (Barr 1985). Julella is characterized by its immersed, medium-sized ascomata with pseudoparenchymatous peridial cells, cellular pseudoparaphyses, and hyaline and muriform ascospores (Barr 1985). With the exception of hyaline ascospores, these characters are typical of Montagnulaceae. The taxonomic affinity of the generic type of Julella needs confirmation following recollection. Julella avicenniae (Borse) K.D. Hyde is a marine fungus. A DNA based phylogeny containing most currently accepted families placed two isolates of J. avicenniae as sister to the families in the Pleosporineae with good support, which might suggest a novel family within Pleosporales (Suetrong et al. 2009). However, J. avicenniae is not the generic type and therefore this conclusion must be treated with caution as only J. avicenniae can be considered pleosporalean.
Lautitia S. Schatz, Can. J. Bot. 62: 31 (1984).
Type species: Lautitia danica (Berl.) S. Schatz, Can. J. Bot. 62: 31 (1984).
≡ Leptosphaeria danica Berl., Icon. fung. (Abellini) 1: 87 (1892).
Lautitia is monotypified by L. danica, which is characterized by subglobose, immersed, ostiolate ascomata with a pseudoclypeus, a thin peridium, broad, cellular pseudoparaphyses, and 8-spored, bitunicate, cylindrical to clavate asci. Ascospores are hyaline, 1-septate, and obovate and the fungus is parasitic on algae (Schatz 1984). Marine or maritime fungi have been reported in Phaeosphaeria, such as P. spartinae (Ellis & Everh.) Shoemaker & C.E. Babc. and P. ammophilae (Lasch) Kohlm. & E. Kohlm. (Zhang et al. 2009a). In addition, the prosenchymatous peridium of L. danica agrees with that of Phaeosphaeriaceae (Schatz 1984).
Lepidosphaeria Parg.-Leduc, C. r. hebd. Seanc. Acad. Sci., Paris, Ser. D 270: 2786 (1970).
Type species: Lepidosphaeria nicotiae Parg.-Leduc, Pubbl. Staz. ZooL Napoli, 1 270: 2786 (1970).
Lepidosphaeria is a genus likely in Testudinaceae, which is distinguished from other genera of this family by its smaller ascospores, which lack furrows, and have minute granulate ornamentation (Hawksworth 1979). In DNA sequence-based phylogenies, L. nicotiae clustered with species of Ulospora and Verruculina (Schoch et al. 2009; Zhang et al. 2009a), but more recent work including species of Platystomaceae lacks support (Plate 1).
Letendraea Sacc., Michelia 2: 73 (1880).
Type species: Letendraea eurotioides Sacc., Michelia 2: 73 (1880).
Letendraea was introduced for L. eurotioides, which is characterized by superficial, globose to conical ascomata, filliform pseudoparaphyses, obclavate to cylindrical, 8-spored asci, and fusoid to oblong, 1-septate ascospores (Saccardo 1880). Because L. helminthicola (Berk. & Broome) Weese clustered with Karstenula rhodostoma, Letendraea was assigned to Melanommataceae (Kodsueb et al. 2006b). But subsequent multigene phylogenetic analysis indicated that both L. helminthicola and L. padouk Nicot & Parg.-Leduc nested within Montagnulaceae
(Schoch et al. 2009; Zhang et al. 2009a; Plate 1), and its familial status seems confirmed.
Lindgomyces K. Hirayama, Kaz. Tanaka & Shearer, Mycologia 102: 133 (2010).
Type species: Lindgomyces ingoldianus (Shearer & K.D. Hyde) K. Hirayama, Kaz. Tanaka & Shearer, Mycologia 102: 733 (2010).
≡ Massarina ingoldiana Shearer & K.D. Hyde, Mycologia 89: 114 (1997).
Lindgomyces was introduced to accommodate a freshwater lineage, which belongs to Massarina ingoldiana sensu lato, and is characterized by scattered, subglobose to globose, erumpent, papillate, ostiolate ascomata, cellular pseudoparaphyses, and 8-spored, fissitunicate, cylindrical to clavate asci. Ascospores are fusoid to narrowly fusoid, hyaline and 1-septate but become 3-5-septate when senescent (Hirayama et al. 2010). A new family, Lindgomycetaceae, was introduced to accommodate Lindgomyces (Hirayama et al. 2010).
Lophiella Sacc., Michelia 1: 337 (1878).
Type species: Lophiella cristata (Pers.) Sacc., Michelia 1: 337 (1878).
≡ Sphaeria cristata Pers., Syn. meth. fung. (Gottingen) 1: 54 (1801).
The generic type of Lophiella, L. cristata, was treated as a synonym of Lophiostoma angustilabrum var crenatum (Pers.) Chesters & A.E. Bell (see http://www.indexfungorum.org/names/Names.asp).
Loratospora Kohlm. & Volkm.-Kohlm., Syst. Ascom. 12: 10 (1993).
Type species: Loratospora aestuarii Kohlm. & Volkm.-Kohlm., Syst. Ascom. 12: 10 (1993).
Loratospora was introduced as a marine genus and is monotypified by L. aestuarii (Kohlmeyer and Volkmann-Kohlmeyer 1993). The generic type is characterized by ellipsoid, immersed to erumpent, carbonaceous ascomata, which are ostiolate, and with or without a papilla. Pseudoparaphyses comprise small subglobose cells forming irregular chains and finally breaking apart, and asci are 8-spored, clavate to ellipsoidal, and fissitunicate. Ascospores are hyaline, cylindrical, 3-septate and surrounded by a mucilaginous sheath (Kohlmeyer and Volkmann-Kohlmeyer 1993). The distinctive pseudoparaphyses of Loratospora aestuarii makes it readily distinguishable from other taxa. Based on a multigene phylogenetic analysis, Loratospora aestuarii nested within the clade of Phaeosphaeriaceae (Schoch et al. 2009; Suetrong et al. 2009; Plate 1), and ascospores of L. aestuarii are in agreement with those of Phaeosphaeria as has been mentioned by Kohlmeyer and Volkmann-Kohlmeyer (1993).
Macrospora Fuckel, Jb. nassau. Ver. Naturk. 23-24: 139 (1870) [1869-70].
Type species: Macrospora scirpicola (DC.) Fuckel, Jb. nassau. Ver. Naturk. 23-24: 139 (1870) [1869-70].
≡ Sphaeria scirpicola DC., in Lamarck & de Candolle, Fl. franç., Edn 3 (Paris) 2: 300 (1805).
Macrospora had been assigned to Diademaceae based on its applanate and muriform ascospores with 1-row of longitudinal septa, with a sheath, 2–3 μm wide and constricted at first septum and ascospores are paler and larger than those of Comoclathris (Shoemaker and Babcock 1992). Macrospora was however, considered as a synonym of Pyrenophora by Eriksson and Hawksworth (1991) which was assigned in Pleosporaceae, and this proposal was widely followed (Eriksson 2006; Lumbsch and Huhndorf 2010). Nimbya anamorphs were reported for Macrospora (Johnson et al. 2002).
Massaria De Not., G. bot. ital. 1: 333 (1844).
Type species: Massaria inquinans (Tode) De Not., G. bot. ital. 1: 333 (1844).
≡ Sphaeria inquinans Tode, Fung. mecklenb. sel. (Luneburg) 1: Fig. 85 (1790).
Colonies on MEA erumpent, not spreading; surface irregular, folded; margins even, feathery; surface olivaceous grey, with thin, umber margin; reverse olivaceous-grey. On PDA similar; surface olivaceous grey, margin dirty white; reverse smoke-grey to olivaceous grey; colonies reaching 1 cm diam. On OA similar, surface olivaceous grey in centre, margins wide, dirty white; colonies reaching 12 mm diam. on all media tested; colonies sterile (based on CBS 125591).
Massaria was formally established by de Notaris (1844), and is typified by M. inquinans. Numerous fungi with brown septate ascospores surrounded by gelatinous sheath were included in the genus (Barr 1979b; Shoemaker and LeClair 1975). Shoemaker and LeClair (1975) accepted a narrow concept for Massaria, with only a few species characterized by large, symmetric, 4-celled ascospores surrounded by a massive gelatinous sheath. Barr (1979b, 1990a) had considered Aglaospora a separate genus, but this subsequently proved congeneric with Massaria (Voglmayr and Jaklitsch 2011). Based on intensive sample collection and multi-gene phylogenetic analysis, Voglmayr and Jaklitsch (2011) accepted Massaria as the sole genus within Massariaceae, which is characterized by a set of we11 defined morphological and ecological characters; Europe is regarded as the centre of diversity.
Misturatosphaeria Mugambi & Huhndorf, Stud. Myco1. 64: 108 (2009).
Type species: Misturatosphaeria aurantonotata Mugambi & Huhndorf, Stud. Myco1. 64: 108 (2009).
Misturatosphaeria was introduced to accommodate a group of fungi which are phylogenetically closely related to
Amniculicolaceae, Lophiostomataceae sensu stricto and Sporormiaceae (Mugambi and Huhndorf 2009b; Zhang et al. 2009a). Species of Misturatosphaeria are characterized by erumpent to superficial ascomata which are scattered or in groups, with or without papilla; asci cylindrical or clavate, 8-spored; pseudoparaphyses numerous, septate, ascospores brown or hyaline, phragmosporous or dictyosporous, with or without sheath. The terrestrial saprobic habitat on wood, as well as its distinct morphological characters may indicate that this genus belongs to an undescribed family. A close relationship with the marine anamorphic species Floricola striata is unexpected and may suggest that some of the species in this genus could have marine affinities (Plate 1).
Navicella Fabre, Annls Sci. Nat., Bot., ser. 6 9: 96 (1879) [1878].
Type species: Navicella julii Fabre, Annls Sci. Nat., Bot., ser. 6 9: 96 (1879) [1878].
Navicella is characterized by medium- to large-sized, immersed to erumpent, globose ascomata, apex elongated or rarely rounded, asci clavate or cylindrical, pseudoparaphyses trabeculate, ascospores reddish to dark brown, ellipsoid to fusoid, multi-septate, the primary septum is euseptate, and others distoseptate, obliquely uniseriate or biseriate (Barr 1990a). Navicella is saprobic on bark, and was considered closely related to the Lophiostomataceae (Holm and Holm 1988). Based on the wide endotunica, thin apical ring and distoseptate ascospores, Barr (1990a) transferred it to the Massariaceae. The morphological characters of Navicella do not match the Massariaceae sensu stricto (Voglmayr and Jaklitsch 2011).
Neotestudina Segretain & Destombes, C. r. hebd. Seanc. Acad. Sci., Paris 253: 2579 (1961).
Type species: Neotestudina rosatii Segretain &Destombes, C. r. hebd. Seanc. Acad. Sci., Paris 253: 2579 (1961).
Neotestudina is characterized by medium- to large-sized, superficial, gregarious, cleistothecioid and globose ascomata which split on opening. Asci are 4- or 8-spored, and cylindrical or oblong, pseudoparaphyses are sparse and trabeculate, and ascospores are dark brown, ellipsoid, 1-septate, with a small germ pore at each end, and uniseriate or crowded in the asci (Barr 1990a). Based on the cleistothecioid ascomata, Neo-testudina was assigned under Zopfiaceae (von Arx and Muller 1975) or Testudinaceae (Hawksworth 1979). Barr (1990a) assigned it to Didymosphaeriaceae based on its ascospore morphology. A DNA based phylogeny showed that sequence obtained from Neotestudina rosatii resides as sister to Ulospora bilgramii (D. Hawksw., C. Booth & Morgan-Jones) D. Hawksw., Malloch & Sivan. and other species that may represent Testudinaceae or Platystomaceae (Kruys et al. 2006; Plate 1).
Paraphaeosphaeria O.E. Erikss., Ark. Bot., Ser. 2 6: 405 (1967).
Type species: Paraphaeosphaeria michotii (Westend.) O.E. Erikss., Cryptogams of the Himalayas 6: 405 (1967).
≡ Sphaeria michotii Westend., BU11. Acad. R. Sci. Belg., Cl. Sci., ser. 2 7: 87 (1859).
Paraphaeosphaeria was separated from Leptosphaeria (Eriksson 1967a), and it is also quite comparable with Phaeosphaeria. Paraphaeosphaeria can be distinguished from Phaeosphaeria by its ascospores. Ascospores of Paraphaeosphaeria michotii have two septa, and they are biseriate, straight, subcylindrical with broadly rounded ends, rather dark brown and punctate. The primary septum is laid down closer to the distal end than to the proximal, and the larger, proximal hemispore is divided by one transversal septum. There are more septa in the proximal hemispore of other species such as Par. castagnei (Durieu & Mont.) O.E. Erikss., Par. obtusispora (Speg.) O.E. Erikss. and Par. vectis (Berk. & Broome) Hedjar. Anamorphic characters can also distinguish Paraphaeosphaeria and Phaeosphaeria. Paraphaeosphaeria has Paraconiothyrium or Coniothyrium-related anamorphs, but Phaeosphaeria has Hendersonia-Phaeoseptoria anamorphs (Eriksson 1967a). Shoemaker and Babcock (1985) redescribed some Canadian and extralimital species, and excluded Par. longispora (Wegelin) Crivelli and Par. oblongata (Niessl) Crivelli from Paraphaeosphaeria based on their longitudinal septa as well as beak-like papilla and wall structures. Molecular phylogenetic results based on multigenes indicated that Paraphaeosphaeria should belong to Montagnulaceae (Zhang et al. 2009a; Plate 1).
Passeriniella Berl., Icon. fung. (Abellini) 1: 51 (1890). Type species: Passeriniella dichroa (Pass.) Berl., Icon. fung. (Abellini) 1: 51 (1890).
≡ Leptosphaeria dichroa Pass.
Passeriniella was introduced by Berlese in 1890 based on the black, ostiolate and papillate ascomata, 8-spored asci, as well as transverse septate ascospores, with pigmented central cells and hyaline terminal cells. Two species were included, i. e. P. dichroa and P. incarcerata (Berk. & M.A. Curtis) Berl. (Berlese 1890). Subsequently, more species were introduced including some marine taxa such as P. mangrovei G.L. Maria & K.R. Sridhar, P. obiones (P. Crouan & H. Crouan) K.D. Hyde & Mouzouras and P. savoryellopsis K.D. Hyde & Mouzouras (Hyde and Mouzouras 1988; Maria and Sridhar 2002). Currently, eight species are included (http://www.mycobank.org, Jan. 2011). Both P. dichroa and P. incarcerata were considered as synonyms of Leptosphaeria obiones (P. Crouan & H. Crouan) Sacc. (Kohlmeyer and Kohlmeyer 1979). The familial placement of the marine species P. savoryellopsis could not be resolved in a DNA based phylogeny but it did suggest a close relationship to Acrocordiopsis patilii (Suetrong et al. 2009) in Pleosporales.
Peridiothelia D. Hawksw., Bull. Br. Mus. nat. Hist., Bot. 14: 120 (1985).
Type species: Peridiothelia fuliguncta (Norman) D. Hawksw., Bull. Br. Mus. nat. Hist., Bot. 14: 121 (1985).
≡ Microthelia fuliguncta Norman, Ofvers. kongl. Svensk. Vetensk.-Akad. Forhandl., Stockholm 41(no. 8): 36 (1884).
When dealing with the names under Microthelia, Peridiothelia was introduced to accommodate species having non-clypeate peridium which composed cells of textura globulosa but sometimes angularis, “dark reddish brown except below the generative locule where the wall is poorly developed or almost absent at maturity, colour not changed significantly in potassium hydroxide, centrum turning blue in iodine” (Hawksworth 1985a). Three species were included, i. e. P. grandiuscula (Anzi) D. Hawksw., P. fuliguncta and P oleae (Korb.) D. Hawksw., and Peridiothelia was referred to Phaeosphaeriaceae (Hawksworth 1985a, b). However, its familial placement is not confirmed yet.
Phaeodothis Syd. & P. Syd., Annls myco1. 2: 166 (1904). Type species: Phaeodothis tricuspidis Syd. & P. Syd., Annls myco1. 2: 166 (1904).
Phaeodothis is characterized by its 1-septate euseptate ascospores with a sparse hamathecium consisting of thin pseudoparaphyses and immersed to superficial ascomata (Aptroot 1995). The genus had been previously assigned to Didymosphaeria, but Aptroot (1995) considered it to be closely related to Phaeosphaeriaceae. A strain named Phaeodothis winteri (a synonym of P tricuspidis Syd. & P. Syd.) nested within the clade of Montagnulaceae (Schoch et al. 2009).
Platychora Petr., Annls myco1. 23: 102 (1925).
Type species: Platychora ulmi (Schleich.) Petr., Annls myco1. 23(1/2): 103 (1925).
Platychora is characterized by immersed to erumpent crust-like ascostroma with globose locules scattered inside (Barr 1968). Asci are oblong to saccate or nearly cylindrical and bitunicate, and ascospores are hyaline 1-septate apiosporous and turn olivaceous when old. Platychora had been previously assigned to Venturiaceae by Barr (1968), but molecular phylogenetic analysis indicated that a strain named Platychora ulmi (the generic type of Platychora) belongs to Didymellaceae (Winton et al. 2007; Plate 1). The generic type needs recollecting and epitypifying to stabilize the generic name.
Platystomum Trevis., Bull. Soc. R. Bot. Belg. 16: 16 (1877). Type species: Platystomum compressum (Pers.) Trevis., Bull. Soc. R. Bot. Belg. 16: 16 (1877).
≡ Sphaeria compressa Pers., Syn. meth. fung. (Gottingen) 1: 56 (1801).
Platystomum was introduced by Trevisan in 1877, and has been considered a synonym of Lophidium, as the ascospores of Platystomum have both transverse and vertical septa (Barr 1990a, b; Chesters and Bell 1970). However, the boundary between Lophiostoma and Platystomum is not clear (Chesters and Bell 1970). Holm and Holm (1988) treated Platystomum as a synonym of Lophiostoma, and concurrently, the Platystomaceae should be treated as a synonym of Lophiostomataceae. Based on a phylogenetic analysis, however, the generic type of Platystomum (P. compressum) separated from other species of Lophiostoma, and nested with the clade of Platystomaceae (Mugambi and Huhndorf 2009b) which may be closely related to species in the Testiduniaceae (Plate 1).
Polyplosphaeria Kaz. Tanaka & K. Hirayama, Stud. Myco1. 64: 192 (2009).
Type species: Polyplosphaeria fusca Kaz. Tanaka & K. Hirayama, Stud. Myco1. 64: 193 (2009).
Polyplosphaeria is characterized by globose ascomata surrounded by numerous brown hyphae and a reddish pigment on the host surface around the ascomata (Tanaka et al. 2009). Asci are cylindro-clavate with fissitunicate dehiscence and ascospores are narrowly fusoid surrounded by a sheath. The anamorph is Piricauda-like (Tanaka et al. 2009). The cylindro-clavate asci, narrowly fusoid ascospores as well as its thin and numerous pseudoparaphyses are comparable with those of Massarina sensu lato, especially Lentithecium (Zhang et al. 2009a). The terrestrial and bambusicolous habitat of Polyplosphaeria and Piricauda anamorph readily distinguishes the genus from Lentithecium.
Pontoporeia Kohlm., Nova Hedwigia 6: 5 (1963).
Type species: Pontoporeia biturbinata (Durieu & Mont.) Kohlm., Nova Hedwigia 6: 5 (1963)
≡ Sphaeria biturbinata Durieu & Mont., Flora Algericae 1: 497 (1849).
Pontoporeia was introduced by Kohlmeyer in 1963, and is monotypified by P. biturbinata. Pontoporeia was treated as a synonym of Zopfia (Malloch and Cain 1972), which is followed by Hawksworth and Booth (1974). Based on its asci originating at the periphery of the subglobose locus, filaments occupying the center of the ascocarps, the irregular peridial structure, the ascospores having 2-layered walls with a germ pore at each end and its marine habitat, Pontoporeia was kept as a separate genus within Pleosporaceae (Kohlmeyer and Kohlmeyer 1979). A DNA based phylogeny placed an isolate on a long branch in relationship with other marine species, Halotthia posidoniae and Mauritiana rhizophorae, but a familial placement awaits further resolution (Suetrong et al. 2009).
Pseudotrichia Kirschst., Annls myco1. 37: 125 (1939).
Type species: Pseudotrichia stromatophila Kirschst., Annls myco1. 37: 125 (1939).
Pseudotrichia can be distinguished from Byssosphaeria, Herpotrichia and Lojkania by its lacking of subiculum, larger ascomata usually with compressed apices, the peripheral arrangement of asci and trabeculate pseudoparaphyses (Barr 1984). Phylogenetic study of strains Pseudotrichia mutabilis and some Herpotrichia species indicated that these species are closely related, and both nested within Melanommataceae (Mugambi and Huhndorf 2009b). But in this study, Pseudotrichia guatopoensis nested in the Testudinaceae (or Platystomaceae) (Plate 1). The types of both Herpotrichia and Pseudotrichia need recollecting, redescribing and epitypifying in order to stabiles the use of these generic names and clarify their familial status.
Pseudoyuconia Lar.N. Vassiljeva, Nov. sist. Niz. Rast. 20: 71 (1983).
Type species: Pseudoyuconia thalictri (G. Winter) Lar. N. Vassiljeva [as ‘thalicti’], Nov. sist. Niz. Rast. 20: 71 (1983).
≡ Leptosphaeria thalictri G. Winter, Hedwigia 10: 40 (1872).
Pseudoyuconia was introduced by Vassiljeva (1983), and was monotypified by P. thalictri. Currently, Pseudoyuconia is included in Pleosporaceae (Lumbsch and Huhndorf 2010).
Pyrenophora Fr., Summa veg. Scand., Section Post. (Stockholm): 397 (1849).
Type species: Pyrenophora phaeocomes (Rebent.) Fr., Summa veg. Scand., Section Post. (Stockholm): 397 (1849).
≡ Sphaeria phaeocomes Rebent., Prodr. fl. neomarch. (Berolini): 338 (1804).
Pyrenophora is characterized by immersed, erumpent to nearly superficial ascomata, indefinite pseudoparaphyses, clavate to saccate asci usually with a large apical ring, and muriform terete ascospores. Morphologically, the terete ascospores of Pyrenophora can be readily distinguished from Clathrospora and Platyspora. The indefinite pseudoparaphyses and smaller ascospores of Pyrenophora can be readily distinguished from those of Pleospora (Sivanesan 1984). Based on both morphology and molecular phylogeny, Pyrenophora is closely related to Pleosporaceae (Zhang et al. 2009a).
Rechingeriella Petr., in Rechinger et al. Annln naturh. Mus. Wien 50: 465 (1940).
Type species: Rechingeriella insignis Petr., Annln naturh. Mus. Wien, Ser. B, Bot. ZooL 50: 465 (1940).
Rechingeriella is characterized by its erumpent to superficial, cleistothecioid ascomata and thin, branching pseudoparaphyses (Hawksworth and Booth 1974). Asci are obovate, thick-walled, bitunicate and evanescent, and ascospores are globose, simple, dark brown to black (based on the type specimen of R. insignis) (Hawksworth and Booth 1974). Based on these characters, R. insignis was treated as a species of Zopfia (as Z. insignis (Petr.) D. Hawksw. & C. Booth). Rechingeriella has been assigned to Botryosphaeriaceae by von Arx and Muller (1975). Further study should be conducted on the type specimen of R. insignis in order to clarify its taxonomic status and fresh collections are needed for epitypification.
Rhytidiella Zalasky, Can. J. Bot. 46: 1383 (1968).
Type species: Rhytidiella moriformis Zalasky, Can. J. Bot. 46: 1383 (1968).
Rhytidiella was introduced based on R. moriformis, which causes perennial rough-bark of Populus balsamifera (Zalasky 1968), and produces macroconidia belonging to Phaeoseptoria. Subsequently, three more species were introduced, viz. R. baranyayi A. Funk & Zalasky, R. hebes P.R. Johnst. and R. beloniza (Stirt.) M.B. Aguirre (Aguirre-Hudson 1991; Funk and Zalasky 1975; Johnston 2007), Both R. baranyayi and R. hebes seem closely related to R. moriformis on both biology and morphology (Funk and Zalasky 1975; Johnston 2007), but R. beloniza is saprobic on Cordyline australis bark (Aguirre-Hudson 1991). Rhytidiella was temporarily assigned to Cucurbitariaceae (Barr 1987b).
Richonia Boud., Revue myco1., Toulouse 7: 224 (1885). Type species: Richonia variospora Boud., Revue myco1., Toulouse 7: 265 (1885).
Richonia is characterized by its 1-septate, relatively large ascospores which are broadly rounded at both ends, and have a thick ornamented undulating sheath giving an irregularly ridged appearance to mature spores (Hawksworth 1979). Richonia variospora has been isolated from several localities in France, but it is rare (Hawksworth 1979). Richonia was assigned under Zopfiaceae (von Arx and Muller 1975; Hawksworth 1979), and there are presently no better suggestions for its familial placement. The taxon needs recollecting and epitypifying.
Rimora Kohlm., Volkm.-Kohlm., Suetrong, Sakay. & E.B. G. Jones, Stud. Myco1. 64: 166 (2009).
Type species: Rimora mangrovei (Kohlm. & Vittal) Kohlm., Volkm.-Kohlm., Suetrong, Sakay. & E.B.G. Jones, Stud. Myco1. 64: 166 (2009).
≡ Lophiostoma mangrovei Kohlm. & Vittal [as ‘man-grovis’], Mycologia 78: 487 (1986).
Rimora was introduced based on a marine fungus R. mangrovei (syn. Lophiostoma mangrovei), and is characterized by its erumpent ascomata with elongated flat tops, cellular pseudoparaphyses and cylindrical asci (Suetrong et al. 2009). Ascospores are fusoid, hyaline, 3-septate and surrounded with an evanescent sheath (Kohlmeyer and Vittal 1986; Suetrong et al. 2009). Rimora forms a robust clade with other marine fungi, such as species of Aigialus and Ascocratera, and a new family, Aigialaceae was introduced to accommodate them (Suetrong et al. 2009).
Roussoellopsis I. Hino & Katum., J. Jap. Bot. 40: 86 (1965). Type species: Roussoellopsis japonica (I. Hino & Katum.) I. Hino & Katum., J. Jap. Bot. 40: 86 (1965).
≡ Didymosphaeria japonica I. Hino & Katum., Bulletin of the Faculty of Agriculture, Yamaguchi University 5: 229 (1954).
Roussoellopsis was introduced by Hino and Katumoto (1965) based on three bambusicolous fungal species, i.e. R. japonica, R. macrospora (I. Hino & Katum.) I. Hino & Katum. and R. tosaensis (I. Hino & Katum.) I. Hino & Katum. These three species have immersed and gregarious ascomata, clavate to cylindro-clavate asci, numerous and filliform pseudoparaphyses, and 1-septate, asymmetrical ascospores (Hino and Katumoto 1965). All these characters point Roussoellopsis to Pleosporales, but its familial placement cannot be determined.
Saccothecium Fr., Fl. Scan.: 349 (1836).
Type species: Saccothecium sepincola (Fr.) Fr. [as ‘saepincola’], Summa veg. Scand., Section Post. (Stockholm): 398 (1849).
≡ Sphaeria sepincola Fr. [as ‘saepincola’], Observ. myco1. (Havniae) 1: 181 (1815).
Saccothecium is characterized by its subglobose, immersed to erumpent ascomata, absence of pseudoparaphyses and hyaline, muriform to phragmosporous ascospores. It has been assigned to the Dothioraceae (Barr 1987b; Muller and von Arx 1950). Molecular phylogenetic analysis indicated that a strain named S. sepincola nested within Didymellaceae (Schoch et al. 2009; Plate 1). The generic type needs recollecting, redescribing and epitypifying.
Setosphaeria K.J. Leonard & Suggs, Mycologia 66: 294 (1974).
Type species: Setosphaeria turcica (Luttr.) K.J. Leonard & Suggs, Mycologia 66: 295 (1974).
≡ Trichometasphaeria turcica Luttr., Phytopathology 48: 282 (1958).
Setosphaeria was segregated from Keissleriella on the basis of lacking a clypeus, lysigenous development of the ostiole, occurrence of setae on the perithecial wall, the absence of periphyses in the ostiole, and the hyphomycetous conidial states, and four species were included, i.e. S. prolata, S. holmii, S. pedicellata (R.R. Nelson) K.J. Leonard & Suggs and S. turcica (Leonard and Suggs 1974). Currently, nine species are included in Setosphaeria (http://www.mycobank.org, Jan/2011). Setosphaeria monoceras Alcorn nested within Pleosporaceae based on multigene phylogenetic analysis (Schoch et al. 2009; Plate 1).
Syncarpella Theiss. & Syd., Annls myco1. 13: 631 (1915). Type species: Syncarpella tumefaciens (Ellis & Harkn.) Theiss. & Syd., Annls myco1. 13(5/6): 633 (1915).
≡ Sphaeria tumefaciens Ellis & Harkn., J. Myco1. 2: 41 (1886).
Syncarpella was introduced by Theissen and Sydow (1915) as a genus of Montagnellaceae within Dothideales. A detailed description of S. tumefaciens can be seen in Barr and Boise (1989). Syncarpella was considered closely related to Leptosphaeria, and was treated as a synonym (Clements and Shear 1931). Syncarpella is characterized by its abundant globose, ovoid to turbinate ascomata with minute papillae which are seated on a common basal stroma and which are erumpent through fissures in the host tissues (Barr and Boise 1989). The peridium is thicker at the base, pseudoparaphyses are cellular, and asci are bitunicate, clavate to oblong with a furcate pedicel. Ascospores are pale brown to brown, oblong to narrowly obovoid, ends obtuse, transversely septate, smooth-walled. All these characters fit Cucurbitariaceae, where Barr and Boise (1989) transferred Syncarpella.
Teichospora Fuckel, Jb. nassau. Ver. Naturk. 23-24: 160 (1870) [1869-70].
Type species: Teichospora trabicola Fuckel, Jb. nassau. Ver. Naturk. 23-24: 161 (1870) [1869-70].
Teichospora was introduced by Fuckel (1870), and was typified by T. trabicola, with four more species included, i.e. T. brevirostris Fuckel, T. dura Fuckel, T. morthieri Fuckel and T. obducens (Schumach.) Fuckel. Only T. brevirostris and T. trabicola were kept in Teichospora (Barr 1987b). After studying the type specimens, Barr (1987b) indicated that Teichospora was different from Strickeria with Teichospora belonging to Pleosporales, and Strickeria closely related to Melanomma (Melanommatales). Currently, more than 250 names are included within Teichospora (http://www.mycobank.org, Jan/2011), but almost no molecular phylogenetic study has been conducted on this genus.
Testudina Bizz., Atti Inst. Veneto Sci. lett., ed Arti, Ser. 6 3: 303 (1885).
Type species: Testudina terrestris Bizz., Fungi venet. nov. vel. Crit. 3: 303 (1885).
Testudina terrestris is characterized by its reticulately ridged ascospores, which readily distinguish it from other genera of Zopfiaceae (Hawksworth 1979). The species is usually associated with other fungi, or on the wood of Abies? and Pinus or on the fallen leaves of Taxus in Europe (Hawksworth and Booth 1974; Hawksworth 1979).
Tetraplosphaeria Kaz. Tanaka & K. Hirayama, Stud. Myco1. 64: 177 (2009).
Type species: Tetraplosphaeria sasicola Kaz. Tanaka & K. Hirayama, Stud. Myco1. 64: 180 (2009).
Tetraplosphaeria was introduced by Tanaka et al. (2009) to accommodate bambusicolous fungi with immersed to erumpent, globose to subglobose and smaller (mostly< 300 μm) ascomata. The peridium is thin, and is composed of thin-walled cells of textura angularis. The pseudoparaphyses are cellular, and asci are fissitunicate, 8-spored, cylindrical to clavate with short pedicels. Ascospores are narrowly fusoid, hyaline and surrounded with a sheath. Species of Tetraplosphaeria have Tetraploa sensu stricto anamorphic stage, which is quite unique in Tetraplosphaeriaceae (Tanaka et al. 2009).
Tingoldiago K. Hirayama & Kaz. Tanaka, Mycologia 102: 740 (2010).
Type species: Tingoldiago graminicola K. Hirayama & Kaz. Tanaka, Mycologia 102(3): 740 (2010).
Tingoldiago is a genus of freshwater ascomycetes characterized by flattened, globose, immersed to erumpent ascomata, and numerous cellular pseudoparaphyses (Hirayama et al. 2010). Asci are fissitunicate and cylindrical, and ascospores are 1-septate, which usually turn 3-septate and pale brown when old, usually with a sheath (Hirayama et al. 2010). Based on both morphology and multigene phylogenetic analysis, Tingoldiago should be treated as a synonym of Lentithecium (Shearer et al. 2009a; Zhang et al. 2009a).
Tremateia Kohlm., Volkm.-Kohlm. & O.E. Erikss., Bot. Mar. 38: 165 (1995).
Type species: Tremateia halophila Kohlm., Volkm.-Kohlm. & O.E. Erikss., Bot. Mar. 38: 166 (1995).
Tremateia was introduced as a facultative marine genus which is characterized by depressed globose, immersed ascomata, numerous and cellular pseudoparaphyses, fissitunicate and clavate asci, ellipsoid muriform ascospores, and a Phoma-like anamorph (Kohlmeyer et al. 1995). These characters point Tremateia to Pleosporaceae (Kohlmeyer et al. 1995). DNA sequence based phylogenies placed T. halophila as sister to Bimuria novae-zelandiae in Montagnulaceae (Schoch et al. 2009; Suetrong et al. 2009).
Triplosphaeria Kaz. Tanaka & K. Hirayama, Stud. Mycol. 64: 186 (2009).
Type species: Triplosphaeria maxima Kaz. Tanaka & K. Hirayama, Stud. Myco1. 64: 188 (2009).
Triplosphaeria was introduced as a bambusicolous genus characterized by immersed ascomata, numerous cellular pseudoparaphyses, bitunicate, cylindrical to clavate asci with a short pedicel, fusoid, hyaline, 1-septate ascospores surrounded with a sheath, and with a Tetraploa-like anamorph (Tanaka et al. 2009). Together with Tetraplosphaeria, Pseudotetraploa, Quadricrura and Polyplosphaeria, Triphsphaeria was assigned to the Tetraplosphaeriaceae (Tanaka et al. 2009).
Ulospora D. Hawksw., Malloch & Sivan., in Hawksworth, Can. J. Bot. 57: 96 (1979).
Type species: Ulospora bilgramii (D. Hawksw., C. Booth & Morgan-Jones) D. Hawksw., Malloch & Sivan., Can. J. Bot. 57: 96 (1979).
Ulospora was introduced as a monotypic genus to accommodate taxa of Testudinaceae whose ascospore has 3-6 fissures (Hawksworth 1979). Genera of Testudinaceae are distinguished based on the morphology of ascospores, although the validity of this classification needs to be confirmed by molecular study. DNA sequence based phylogenies placed sequences from an unverified culture of U. bilgramii in a clade together with Verruculina enalia, and Lepidosphaeria nicotiae and it may have a close relationship to species in Platystomaceae (Mugambi and Huhndorf 2009b; Schoch et al. 2009; Plate 1).
Zopfia Rabenh., Fungi europ. exsicc.: no. 1734 (1874). Type species: Zopfia rhizophila Rabenh., Fungi europ. exsicc.: no. 1734 (1874).
Zopfia was introduced by Rabenhorst (1874) as a monotypic genus (typified by Z. rhizophila), and it was assigned to the Perisporiaceae by Saccardo (1882) and Winter (1884). Arnaud (1913) described the Zopfiaceae to accommodate Zopfia, and considered that it should be excluded from the Perisporiaceae. A relatively broad generic concept was accepted by Hawksworth and Booth (1974), in which they take the ascospore size and ornamentation variation as criteria under generic rank classification, and they treat Celtidia, Lepidosphaeria, Marchaliella, Neotestudina, Pontoporeia, Pseudophaeotrichum, Rechingeriella, Richonia and Testudina as synonyms of Zopfia. A narrow generic concept was adopted by Hawksworth (1979), and Zopfia is characterized by 1-septate ascospores, which are apiculate at both ends, smooth-walled by light microscope, with minute irregular pitting by SEM, and larger than other species of Zopfia sensu Hawksworth and Booth (1974). Three species were accepted, viz. Z. albiziae Farr, Z. biturbinata (Dur. & Mont.) Malloch & Cain and Z. rhizophila, and they all occur on roots of plants (Hawksworth 1979). DNA sequences from an unverified culture of Zopfia rhizophila placed it in close proximity to species in Delitschiaceae without strong statistical support (Kruys et al. 2006; Schoch et al. 2009; Plate 1).
Zopfiofoveola D. Hawksw., Can. J. Bot. 57: 98 (1979). Type species: Zopfiofoveola punctata (D. Hawksw. & C. Booth) D. Hawksw., Can. J. Bot. 57: 98 (1979).
≡ Zopfia punctata D. Hawksw. & C. Booth, Myco1. Pap. 153: 23 (1974).
Zopfiofoveola was hesitantly separated from Zopfia as a monotypic new genus based on its evenly distributed ornamentation with pale minute pits readily visible under the light microscope, and the more elongate shape and less pronounced apical papilla than those of Zopfia (Hawksworth 1979). The type specimen of this species however, cannot be redescribed, because “the type species is only known from a microscopic preparation obtained from earthworm excrements in Sweden” as has been mentioned by Hawksworth (1979).
General discussion
Molecular phylogenetic studies based on four to five genes indicate that 20 families should be included in Pleosporales (Schoch et al. 2009; Shearer et al. 2009; Suetrong et al. 2009; Tanaka et al. 2009; Zhang et al. 2009a). Together with five unverified families (marked with “?”), 26 families are currently assigned under Pleosporales (Table 4). The Phaeotrichaceae lacks pseudoparaphyses, has cleistothecial ascomata with long setae, and conspicuous ascospores with germ pores at each end. These characters do not agree with the current concept of Pleosporales (Zhang et al. 2009a), and therefore Phaeotrichaceae is excluded from Pleosporales (Table 4).
Families in Pleosporales
Based on LSU and SSU rDNA, RPB1, RPB2 and TEF1 sequence analysis, Pleosporineae is emended, and in this study, seven families are tentatively included, i.e. Cucurbitariaceae, Didymellaceae, Didymosphaeriaceae, Dothidotthiaceae, Leptosphaeriaceae, Phaeosphaeriaceae and Pleosporaceae (Zhang et al. 2009a; Plate 1). In this study, Massarineae was emended to accommodate another five families, viz. Lentitheciaceae, Massarinaceae, Montagnulaceae, Morosphaeriaceae, Trematosphaeriaceae. The subordinal affinity of other families remained undetermined. Most of the families accepted within Pleosporales received high bootstrap support (Plate 1). The characters used to define a family, however, do not appear to have clear cut boundaries, as the ascomatal and hamathecial characters also seem to be poorly defined in some families. For example, both trabeculate and cellular pseudoparaphyses coexist in the Amniculicolaceae. Pycnidiophora, a genus of Sporormiaceae, has cleistothecial ascomata with spherical asci irregularly arranged in it. Brown phragmosporous ascospores are reported in Amniculicolaceae, Leptosphaeriaceae, Lophiostomataceae, Melanommataceae, Montagnulaceae, Phaeosphaeriaceae and Pleosporaceae. Similarly muriform ascospores occur in Aigialaceae, Amniculicolaceae, Didymellaceae, Lophiostomataceae, Montagnulaceae, Pleosporaceae and Sporormiaceae. Anamorphs of Pleosporales are also variable to a large degree at the family level. Both hyphomycetous and coelomycetous anamorphs co-exist in Didymellaceae, Melanommataceae or Pleosporaceae. Phoma and Phoma-like anamorphs exist in Didymellaceae, Leptosphaeriaceae, Phaeosphaeriaceae, Pleosporaceae and Melanommataceae (de Gruyter et al. 2009; Zhang et al. 2009a). It is clear that some characters, e.g. cleistothecial or perithecial ascomata, shape, colour and septation of ascospores, shape or arrangement (regular or irregular) of asci, or even presence or absence of pseudoparaphyses have evolved on numerous occasions which make the use of morphological characters in segregating families complicated. It is therefore unclear with our present state of knowledge which characters are taxonomically important at the family level or whether a suit of characters are necessary to define a family. DNA sequence comparisons are essential in delineating these taxa in combination with other characters. It is hoped that additional characters, i.e. biochemical, genomic and subcellular will be used to further distinguish these groups into natural taxa. Below we discuss each of the families, their genera and their considered important characteristics.
Aigialaceae Suetrong, Sakay., E.B.G. Jones, Kohlm., Volkm.-Kohlm. & C.L. Schoch 2010
The Aigialaceae was introduced by Suetrong et al. (2009) based on its carbonaceous ascomata without papilla, cylindrical asci with apical apparatus, trabeculate pseudoparaphyses and ascospores with a sheath. The type genus (Aigialus) of the Aigialaceae was previously incorporated within the Massariaceae (Lumbsch and Huhndorf 2007). Currently, three genera are assigned under Aigialaceae, viz. Ascocratera, Aigialus and Rimora (Suetrong et al. 2009). The genera included in Aigialaceae have a wide range of morphological variation, with very few shared features as mentioned above, but all are found in mangrove habitats (Suetrong et al. 2009). The ascospores, however, vary widely from having 1 to 3 transverse septa and being hyaline to muriformly septate and brown (Suetrong et al. 2009). It is still unclear which characters unify the family and therefore placement of unsequenced genera is difficult. Further molecular work is needed to better understand this family.
Amniculicolaceae Yin. Zhang, C.L. Schoch, J. Fourn., Crous & K.D. Hyde 2009
Members of Amniculicolaceae form a well supported clade, and all are freshwater fungi which usually stain the woody substrate purple (Zhang et al. 2009a, c). Genera of Amniculicolaceae have ascomata with compressed papilla and cylindrical to cylindro-clavate asci. Neomassariosphaeria typhicola was traditionally assigned to Massariosphaeria (as M. typhicola), and Massariosphaeria is characterized by staining the woody substrate purple (Crivelli 1983; Leuchtmann 1984). Eriksson (1981 p. 135) had pointed out that “Purple-staining species of Pleospora, treated by Webster (1957), are not congeneric with P. herbarum (Eriksson 1967b: 13), and certainly do not even belong to the Pleosporaceae’. This is mirrored in Murispora rubicunda, a previous Pleospora species (as P. rubicunda) staining the woody substrate purple, closely related to the Amniculicolaceae in a subsequent phylogenetic study (Zhang et al. 2009a). The anamorphs of this family are possibly Anguillospora longissima, Spirosphaera cupreorufescens and Repetophragma ontariense (Zhang et al. 2009a).
?Arthopyreniaceae (or Massariaceae) W. Watson 1929
The Arthopyreniaceae was introduced as a lichenized family of Pyrenocarpales, which comprises Acrocordia, Arthopyrenia, Athrismidium, Bottaria, Celothelium, Laurera, Leptorhaphis, Microthelia, Microtheliopsis, Polyblastiopsis, Pseudosagedia, Raciborskiella and Tomasellia (Watson 1929). Subsequently, Arthopyreniaceae was assigned under Dothideales (suborder Pseudosphaeriineae) (von Arx and Muller 1975). The generic type of Massaria (M. inquinans) and Torula herbarum and Arthopyrenia salicis together with members of Roussoella as well as Roussoellopsis form a robust clade, which makes their familial placement uncertain (Massariaceae or Arthopyreniaceae) (Schoch et al. 2009; Zhang et al. 2009a).
?Cucurbitariaceae G. Winter 1885
The Cucurbitariaceae is characterized by its aggregated ascomata which form from a basal stromatic structure, ostiolate, fissitunicate and cylindrical asci, and pigmented, phragmosporous or muriform ascospores (Cannon and Kirk 2007). Currently, no molecular study has been able to resolve its ordinal status, but some characters are similar to Leptosphaeriaceae or Phaeosphaeriaceae (Cannon and Kirk 2007). Cucurbitaria elongata clustered within Pleosporales (Schoch et al. 2006).
Delitschiaceae M.E. Barr 2000
The Delitschiaceae was established to accommodate some species of the Sporormiaceae, which is characterized by its ascomata with periphysate ostioles, ocular chamber surrounded by a dome and usually in having four refractive rods, ascospores with or without a septum, having a germ slit in each cell and being surrounded by a mucilaginous sheath (Barr 2000). Species of the Delitschiaceae are hypersaprotrophic on old dung or exposed wood (Barr 2000). Based on a molecular phylogenetic studies, Delitschia didyma and D. winteri form a robust clade basal to other pleosporalean fungi (Schoch et al. 2009; Zhang et al. 2009a). The familial status of two other genera, Ohleriella and Semidelitschia, remains undetermined.
?Diademaceae Shoemaker & C.E. Babc. 1992
The Diademaceae was introduced by Shoemaker and Babcock (1992) based on its ascomata opening as a flat circular lid and bitunicate asci, ascospores are fusiform, brown, mostly applanate, and having three or more transverse septate and with or lacking longitudinal septa and usually having a sheath. Five genera had been included viz. Clathrospora, Comoclathris, Diadema, Diademosa and Macrospora (Shoemaker and Babcock 1992).
Didymellaceae Gruyter, Aveskamp & Verkley 2009
The generic type of Didymella (D. exigua) together with some Phoma or Phoma-related species form a robust familial clade on the phylogenetic tree, thus the Didymellaceae was introduced to accommodate them (de Gruyter et al. 2009). Subsequently, Didymellaceae was assigned to Pleosporineae (suborder of Pleosporales) (Zhang et al. 2009a). A detailed study was conducted on the Didymellaceae based on LSU, SSU rDNA, ITS as we11 as β-tubulin, which indicated that many Phoma or Phoma-related species/fungi reside in this clade of the Didymellaceae (Aveskamp et al. 2010).
Didymosphaeriaceae Munk 1953
The Didymosphaeriaceae was introduced by Munk (1953), and was revived by Aptroot (1995) based on its distoseptate ascospores and trabeculate pseudoparaphyses, mainly anastomosing above the asci. The familial status of the Didymosphaeriaceae is debatable, and Lumbsch and Huhndorf (2007) assigned it to the Montagnulaceae, while von Arx and Muller (1975) treated it as a synonym of the Pleosporaceae. In this study, Didymosphaeria futilis (the generic type of Didymosphaeria) is closely related to the Cucurbitariaceae (Plate 1). Herein, we accept it as a separate family containing three genera, namely Appendispora, Didymosphaeria and Phaeodothis. More information could only be obtained by further molecular work based on correctly identified strains.
Dothidotthiaceae Crous & A.J.L. Phillips 2008
Dothidotthiaceae was introduced to accommodate the single genus Dothidotthia, which is characterized by gregarious, erumpent, globose ascomata, hyaline, septate pseudoparaphyses, 8-spored, bitunicate, clavate asci, ellipsoid, 1-septate ascospores, and has anamorphic Thyrostroma (Phillips et al. 2008). In this study, Dothidotthiaceae is closely related to Didymellaceae, but it is still treated as a separate family (Plate 1).
Hypsostromataceae Huhndorf 1994
Hypsostromataceae was introduced based on two tropical genera (i.e. Hypsostroma and Manglicola), which have superficial, large, elongate ascomata with a soft-textured, pseudoparenchymatic wall, trabeculate pseudoparaphyses and stipitate asci attached in a basal arrangement in the centrum; asci with an apical chamber and fluorescing ring; and fusiform, septate ascospores (Huhndorf 1994). Hypsostromataceae was assigned to Melanommatales sensu Barr (Huhndorf 1994). In a subsequent phylogenetic study, Hypsostromataceae was recovered as a strongly supported monophyletic group nested within Pleosporales (Mugambi and Huhndorf 2009b).
Lentitheciaceae Yin. Zhang, C.L. Schoch, J. Fourn., Crous & K.D. Hyde 2009
Phylogenetic analysis based on multi-genes indicate that freshwater taxa, e.g. Lentithecium fluviatile, L. arundinaceum, Stagonospora macropycnidia, Wettsteinina lacustris, Keissleriella cladophila, Katumotoa bambusicola and Ophiosphaerella sasicola form a well supported clade, which most likely represent a familial rank (Zhang et al. 2009a).
Their morphology, however, varies widely, e.g. ascomata small- to medium-sized, ascospores fusoid to filliform, hyaline to pale yellow, 1- to multi-septate (Zhang et al. 2009a). In particular, they are saprobic on monocotyledons or dicotyledons. Currently, no conspicuous, unique morphological character has been noted in Lentitheciaceae, which makes it difficult to recognize based on morphology.
Leptosphaeriaceae M.E. Barr 1987a
The Leptosphaeriaceae was introduced by Barr (1987a) based on Leptosphaeria. The familial status of the Leptosphaeriaceae is subsequently supported by molecular phylogenetic studies, in which members of the Leptosphaeriaceae form a paraphyletic clade with moderate bootstrap support (Dong et al. 1998; de Gruyter et al. 2009; Schoch et al. 2009; Zhang et al. 2009a). Coniothyrium palmarum, the generic type of Coniothyrium nested within this family (de Gruyter et al. 2009). Further molecular phylogenetic study is needed, in which more related taxa are included.
Lindgomycetaceae K. Hirayama, Kaz. Tanaka & Shearer 2010
Lindgomycetaceae was introduced as a monotypic family represented by Lindgomyces (Hirayama et al. 2010). Lindgomycetaceae is another freshwater family in Pleosporales, which is characterized by its subglobose to globose, ostiolate and papillate ascomata, numerous, septate, branching and anastomosing pseudoparaphyses, fissitunicate, cylindrical to clavate, 8-spored asci, fusiform to cylindrical, uni- to multiseptate, hyaline to brown ascospores usually covered with an entire sheath and/or bipolar mucilaginous appendages (Hirayama et al. 2010).
Lophiostomataceae Sacc. 1883
The Lophiostomataceae had been characterized by its slot-like ostiole on the top of a flattened neck (Holm and Holm 1988). Based on this, 11 genera were assigned under the Lophiostomataceae, viz. Byssolophis, Cilioplea, Entodesmium, Herpotrichia, Lophiella, Lophionema, Lophiostoma, Lophiotrema, Massariosphaeria, Muroia and Quintaria (Holm and Holm 1988). The Lophiostomataceae was thought to be heterogeneous, as the ”papilla form is an unstable and highly adaptive character“ (Holm and Holm 1988). Most recent phylogenetic analysis support the monophyletic status of the Lophiostomataceae sensu stricto (which tends to comprise a single genus of Lophiostoma) (Zhang et al. 2009a, b). The familial placement of other genera, however, remains unresolved.
Massarinaceae Munk 1956
The Massarinaceae was established based on Keissleriella, Massarina, Metasphaeria, Pseudotrichia and Trichometasphaeria (Munk 1956). Subsequently, the Massarinaceae is sometimes treated as a synonym of Lophiostomataceae (Barr 1987b). Based on a multigene phylogenetic study, the generic type of Massarina (M. eburnea) together with M. cisti, Neottiosporina paspali and Byssothecium circinans form a we11 supported clade (Zhang et al. 2009a, b). It seems that a relatively narrow familial concept should be accepted.
Melanommataceae G. Winter 1885
The traditional circumscription of the Melanommataceae was based on its globose or depressed perithecial ascomata, bitunicate and fissitunicate asci, pigmented phragmosporous ascospores as well as the trabeculate pseudoparaphyses (Barr 1990a; Sivanesan 1984). However, the family has recently proved polyphyletic (Liew et al. 2000; Kodsueb et al. 2006a; Kruys et al. 2006; Wang et al. 2007). Bimuria, Ostropella, Trematosphaeria and Xenolophium occur outside Melanommataceae (Mugambi and Huhndorf 2009b; Zhang et al. 2009a). Species of Byssosphaeria, Bertiella, Herpotrichia, Pseudotrichia, Pleomassaria as well as Melanomma resided in the clade of Melanommataceae (Mugambi and Huhndorf 2009b; Schoch et al. 2009; Zhang et al. 2009a). The familial status of many genera previously listed under this family remains to be sorted out (Lumbsch and Huhndorf 2007).
Montagnulaceae M.E. Barr 2001
The Montagnulaceae was introduced to accommodate some pleosporalean genera with ascomata immersed under a clypeus, a pseudoparenchymatous peridium with small cells, cylindric or oblong asci with pedicels and brown ascospores (Barr 2001). Three genera were included, i.e. phragmosporous Kalmusia, dictyosporous Montagnula and didymosporous Didymosphaerella (Barr 2001). Our molecular phylogenetic analysis based on multi-genes indicated that species from Kalmusia, Phaeosphaeria, Bimuria, Didymocrea, Paraphaeosphaeria, Karstenula, Letendraea as we11 as Montagnula resided in the monophylogenetic clade of the Montagnulaceae (Schoch et al. 2009; Zhang et al. 2009a).
Morosphaeriaceae Suetrong, Sakay., E.B.G. Jones & C.L. Schoch 2009
Four marine species, viz. Massarina ramunculicola (as Morosphaeria ramunculicola), Massarina velataspora (Morosphaeria velataspora), Helicascus kanaloanus and H. nypae together with the freshwater species Kirschsteiniothelia elaterascus form a we11 supported clade, which most likely represent a familial rank (Suetrong et al. 2009). Thus, Morosphaeriaceae was introduced to accommodate these taxa (Suetrong et al. 2009). In this study, Asteromassaria pulchra is basal to other species of Morosphaeriaceae, and gets well support (Plate 1). Thus we tentatively assign Asteromassaria under Morosphaeriaceae.
Phaeosphaeriaceae M.E. Barr 1979a
The Phaeosphaeriaceae was introduced to accommodate some pleosporalean genera which have saprobic, parasitic or hyperparasitic lifestyles and have small- to medium-sized, subglobose or conical ascomata, bitunicate asci and hyaline or pigmented ascospores with or without septation (Barr 1979a). Fourteen genera were included, viz. Comoclathris, Didymella, Eudarluca, Heptameria, Leptosphaeria, Loculohypoxylon, Metameris, Microthelia, Nodulosphaeria, Ophiobolus, Paraphaeosphaeria, Rhopographus, Scirrhodothis and Teichospora (Barr 1979a), which were subsequently assigned to various families, such as Loculohypoxylon and Teichospora to the Teichosporaceae, Paraphaeosphaeria to the Montagnulaceae, Leptosphaeria to the Leptosphaeriaceae, Comoclathris to the Diademaceae, Didymella to the Didymellaceae and Heptameria and Rhopographus to genera incertae sedis of Dothideomycetes (Aveskamp et al. 2010; de Gruyter et al. 2009; Lumbsch and Huhndorf 2007; Zhang et al. 2009a). Based on multi-gene phylogenetic analysis, a relatively narrow familial concept is accepted, which is mostly associated with monocotyledons, with perithecoid, small- to medium-sized ascomata, and septate ascospores which are fusiform to filliform (Zhang et al. 2009a). Four genera were accepted, Ophiosphaerella, Phaeosphaeria, Entodesmium and Setomelanomma (Zhang et al. 2009a). Together with Cucurbitariaceae, Didymellaceae, Didymosphaeriaceae, Dothidotthiaceae, Leptosphaeriaceae and Pleosporaceae, the Phaeosphaeriaceae is assigned under Pleosporineae (Zhang et al. 2009a).
Pleomassariaceae M.E. Barr 1979a
Both Asteromassaria and Splanchnonema were designated as representative genera of Pleomassariaceae (Barr 1979a). Currently, four genera are included in Pleomassariaceae, viz.?Lichenopyrenis,?Splanchnonema,?Peridiothelia and Pleomassaria (Table 4). The generic type of Pleomassaria (P. siparia) clustered with species of Melanommataceae in previous and present studies (Schoch et al. 2009; Zhang et al. 2009a; Plate 1). Zhang et al. (2009a) has attempted to assign Pleomassariaceae to Melanommataceae (Zhang et al. 2009a). Based on the distinct morphology and anamorphic stage of Pleomassaria siparia as well as the divergence of dendrogram, we hesitantely reinstate Pleomassariaceae as a separate family in this study.
Pleosporaceae Nitschke 1869
The Pleosporaceae is one of the earliest introduced families in Dothideomycetes. The Pleosporaceae was originally assigned under Sphaeriales, which accommodated species with paraphyses and immersed perithecia (Ellis and Everhart 1892; Lindau 1897; Winter 1887). Subsequently, many of the Pleosporaceae species were transferred to the Pseudosphaeriaceae, which was subsequently elevated to ordinal rank as Pseudosphaeriales (Theissen and Sydow 1918). Luttrell (1955) introduced the Pleosporales (lacking a Latin description), which is characterized by its Pleospora-type of centrum development. Based on this, the Pleosporaceae and the Lophiostomataceae as well as other five families were placed in Pleosporales (Luttrell 1955). Pleosporaceae is the largest and most typical family in Pleosporales. Wehmeyer (1975) stated that the Pleospora- type centrum development is verified in a small number of genera, and centrum development in the majority of genera is unknown; thus the placement of families or genera is quite arbitrary. In addition, the circumscription of Pleosporaceae is not clear-cut, and “ ascostromata of many different types, which are previously placed in various other families (Trichosphaeriaceae, Melanommataceae, Cucurbitariaceae, Amphisphaeriaceae etc.) are to be found here” (Wehmeyer 1975). Thus, the heterogeneous nature of Pleosporales is obvious (Eriksson 1981), and had been confirmed by subsequent molecular phylogenetic studies (e.g. Kodsueb et al. 2006a). Based on the multi-gene phylogenetic analysis, some species from Lewia, Cochliobolus, Pleospora, Pyrenophora and Setosphaeria resided in the Pleosporaceae (Zhang et al. 2009a).
Sporormiaceae Munk 1957
The Sporormiaceae is the largest coprophilous family in Pleosporales, which bears great morphological variation. Ascomata vary from cleistothecoid to perithecoid, asci are regularly or irregularly arranged, clavate or spherical, ascospores with or without germ slits or ornamentations. Based on phylogenetic analysis, Sporormiaceae is most likely monophyletic as currently circumscribed (Kruys et al. 2006; Kruys and Wedin 2009).
?Teichosporaceae M.E. Barr 2002
The Teichosporaceae was introduced by segregating some non-lichenized members of the Dacampiaceae which are apostrophic on woody stems and periderm or hypersaprotrophic on other ascomycetous fungi (Barr 2002). The Dacampiaceae together with its synonym, Pyrenidiaceae was only maintained to accommodate its lichenicolous genera (Barr 2002). This proposal does not have any molecular phylogenetic support.
Tetraplosphaeriaceae Kaz. Tanaka & K. Hirayama 2009
The Tetraplosphaeriaceae was introduced to accommodate five genera, i.e. Tetraplosphaeria, Triplosphaeria, Polyplosphaeria and the anamorphic genera Pseudotetraploa and Quadricrura (Tanaka et al. 2009). The Tetraplosphaeriaceae is characterized by its Massarina-like teleomorphs and its Tetraploa-like anamorphs with setae-like appendages, and its monophylogenetic status has been recently confirmed based on DNA phylogenetic studies (Tanaka et al. 2009).
Trematosphaeriaceae
Three species, viz. Falciformispora lignatilis, Halomassarina thalassiae and Trematosphaeria pertusa form a robust clade, which forms a sister group with other pleosporalean families (Schoch et al. 2009; Suetrong et al. 2009). Trematosphaeriaceae is waiting to be formally proposed (Suetrong et al. data unpublished).
?Zopfiaceae G. Arnaud ex D. Hawksw. 1992
The Zopfiaceae was introduced by Arnaud (1913), but was invalid due to the lack of a Latin diagnosis (see comments by Eriksson and Hawksworth 1992). The Zopfiaceae was formally introduced by Eriksson and Hawksworth (1992), and is characterized by its cleistothecial ascomata, thick-walled peridium, globose or saccate asci and oneseptate, dark brown ascospores (Cannon and Kirk 2007). Currently, eleven genera are included, but the family is likely polyphyletic (Kruys et al. 2006).
Excluded family
Phaeotrichaceae Cain 1956
The cleistothecioid ascomata, ascospores with germ pore at each end and the absence of pseudoparaphyses indicate that the Phaeotrichaceae may not be closely related to Pleosporales. This was confirmed by DNA based phylogenies (Schoch et al. 2009). Thus, we exclude it from Pleosporales.
Final remarks
Problems and concerns
Recently, many new pleosporalean lineages from freshwater (Shearer et al. 2009; Zhang et al. 2009a), marine (Suetrong et al. 2009) or from bambusicolous hosts (Tanaka et al. 2009) have been reported. In particular, large-scale phylogenetic analysis indicate that numerous unresolved clades still exist, which may also indicate that a large number of fungal lineages are not resolved. As has been estimated, 95% of all fungi are unreported (Hawksworth 1991), and a large portion of them might exist only as hyphae (or DNA-only fungi, Taylor 1993). Under the influence of human activities, environmental situations are changing quickly, which may result in numerous fungal taxa losing their habitats and/or become endangered. More field work is urgently needed.
A future polyphasic approach to study Pleosporales
The use of DNA sequence comparisons have proved invaluable in modern concepts of fungal taxonomy. It is now clear many fungi do not produce reproductive structures or only do so under very rare circumstances and many fungi cannot be cultured (Begerow et al. 2010). More and more morpho-species have proven to be cryptic taxa, and already a large percentage of fungal diversity is documented only by DNA sequences. DNA sequence analysis is an essential way to resolve these problems. But are they enough for fully informed fungal taxonomy? Each single morphological character may be the outcome of the expression of one to numerous genes, which might be composed of thousands of base pairs. DNA barcoding methods are “a breakthrough for identification, but they will not supplant the need to formulate and rigorously test species hypothesis” (Wheeler et al. 2004). Thus, integration of classical morphological approaches and DNA and protein based sequence comparisons are critical to produce a modern taxonomy that reflects evolutionary similarities and differences (DeSalle et al. 2005; Godfray 2002). In particular, the advent of comparative genomics and advances in our understanding of secondary metabolites and host or habitat spectra allow the possibility to tie phylogenetic hypotheses derived from DNA and protein sequence to the biology of the organisms. (Bitzer et al. 2008; Stajich et al. 2009; Zhang et al. 2009a, b).
Acknowledgement
We are grateful to the Directors and Curators of the following herbaria for loan of specimens in their keeping: BAFC, BISH, BPI, BR, BRIP, CBS, E, ETH, FFE, FH, G, H, Herb. J. Kohlmeyer, HHUF, IFRD, ILLS, IMI, K(M), L, LPS, M, MA, NY, PAD, PC, PH, RO, S, TNS, TRTC, UB, UBC, UPS and ZT; to Dr. L. Cai, Dr. A.J.L. Phillips, Dr. C. Shearer and some other mycologists for their permission to use or refer to their published figures, to J.K. Liu, H. Zhang, Y.L. Yang and J. Fournier for helping me loan or collect specimens, to H. Leung for technical help. The third coauthor acknowledges the Intramural Research Program of the NIH, National Library of Medicine. The Global Research Network and King Saud University are also thanked for support.
Footnotes
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Ying Zhang, Division of Microbiology, School of Biological Sciences, The University of Hong Kong, Pokfulam Road, Hong Kong, SAR, People’s Republic of China.
Pedro W. Crous, CBS-KNAW Fungal Biodiversity Centre, RO. Box 85167, 3508 AD Utrecht, The Netherlands
Conrad L. Schoch, National Center for Biotechnology Information, National Library of Medicine, National Institutes of Health, 45 Center Drive, MSC 6510, Bethesda, MD 20892-6510, USA
Kevin D. Hyde, School of Science, Mae Fah Luang University, Tasud, Muang, Chiang Rai 57100, Thailand; Botany and Microbiology Department, College of Science, King Saud University, Riyadh 11442, Saudi Arabia
References
- Adams GC, Wingfield MJ, Common R, Roux J. Phylogenetic relationships and morphology of Cytospora species from Eucalyptus. Stud Mycol. 2005;52:1–146. [Google Scholar]
- Aguirre-Hudson B. A taxonomic study of the species referred to the ascomycete genus Leptorhaphis. Bull Br Mus Nat Hist (Bot) 1991;21:85–192. [Google Scholar]
- Ahmed SI, Asad F. Sporormia fimicola sp. nov. and Sporormiella inaequalis sp. nov. from West Pakistan. Sydowia. 1968;21:290–294. [Google Scholar]
- Ahmed SI, Cain RF. Revision of the genera Sporormia and Sporormiella. Can J Bot. 1972;50:419–478. [Google Scholar]
- Alias SA, Jones EBG, Torres J. Intertidal fungi from the Philippines, with a description of Acrocordiopsis sphaerica sp. nov. (Ascomycota) Fungal Divers. 1999;2:35–41. [Google Scholar]
- Aptroot A. A monograph of Didymosphaeria. Stud Mycol. 1995;37:1–160. [Google Scholar]
- Aptroot A. A world revision of Massarina (Ascomycota) Nova Hedw. 1998;66:89–162. [Google Scholar]
- Arena1 F, Platas G, Pelaez F. Two new Preussia species defined based on morphological and molecular evidence. Fungal Divers. 2005;20:1–15. [Google Scholar]
- Arnaud G. Sur les genres Zopfia, Richonia et Caryospora. Bull Soc Mycol Fr. 1913;29:253–260. [Google Scholar]
- Auerswald B. Delitschia nov. gen. e grege Sphaeriacearum simplicium. Hedwigia. 1866;5:49–64. [Google Scholar]
- Aveskamp MM, de Gruyter J, Woudenberg JHC, Verkley GJM, Crous PW. Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Stud Mycol. 2010;65:1–60. doi: 10.3114/sim.2010.65.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr ME. The genus Pseudomassaria in North America. Mycologia. 1964;56:841–862. [Google Scholar]
- Barr ME. The Venturiaceae of North America. Can J Bot. 1968;46:799–864. [Google Scholar]
- Barr ME. Preliminary studies on the Dothideales in temperate North America. Contrib Univ Mich Herb. 1972;9:523–638. [Google Scholar]
- Barr ME. A note on Extrawettsteinina. Mycotaxon. 1975;2:104–106. [Google Scholar]
- Barr ME. Hypoxylon grandineum: a Loculoascomycete. Mycotaxon. 1976;3:325–329. [Google Scholar]
- Barr ME. A classification of Loculoascomycetes. Mycologia. 1979a;71:935–957. [Google Scholar]
- Barr ME. On the Massariaceae in North America. Mycotaxon. 1979b;9:17–37. [Google Scholar]
- Barr ME. On the family Tubeufiaceae (Pleosporales) Mycotaxon. 1980;12:137–167. [Google Scholar]
- Barr ME. The genus Curreya: an example of taxonomic confusion in the Ascomycetes. Mycologia. 1981;73:599–609. [Google Scholar]
- Barr ME. Leptosphaeria sepalorum. Mycotaxon. 1982a;15:345–348. [Google Scholar]
- Barr ME. On the Pleomassariaceae (Pleosporales) in North America. Mycotaxon. 1982b;15:349–383. [Google Scholar]
- Barr ME. Muriform ascospores in class Ascomycetes. Mycotaxon. 1983;18:149–157. [Google Scholar]
- Barr ME. Herpotrichia and its segregates. Mycotaxon. 1984;20:1–38. [Google Scholar]
- Barr ME. On Julella, Delacourea, and Decaisnella, three dictyosporous genera described by J.H. Fabre. Sydowia. 1985;38:11–19. publ. 1986. [Google Scholar]
- Barr ME. New taxa and combinations in the Loculoascomycetes. Mycotaxon. 1987a;29:501–505. [Google Scholar]
- Barr ME. Amherst. University of Massachusetts; Massachusetts; 1987b. Prodromus to Class Loculoascomycetes. [Google Scholar]
- Barr ME. Some unitunicate taxa excluded from Didymosphaeria. Stud Mycol. 1989a;31:23–27. [Google Scholar]
- Barr ME. The genus Dothidotthia (Botryosphaeriaceae) in North America. Mycotaxon. 1989b;34:517–526. [Google Scholar]
- Barr ME. The genus Chaetomastia (Dacampiaceae) in North America. Mycotaxon. 1989c;34:507–515. [Google Scholar]
- Barr ME. Melanommatales (Loculoascomycetes) N Amer Fl. 1990a;13(H):1–129. [Google Scholar]
- Barr ME. Some dictyosporous genera and species of Pleosporales in North America. Mem N Y Bot Gard. 1990b;62:1–92. [Google Scholar]
- Barr ME. Additions to and notes on the Phaeosphaeriaceae (Pleosporales, Loculoascomycetes) Mycotaxon. 1992a;43:371–400. [Google Scholar]
- Barr ME. Notes on the Lophiostomataceae (Pleosporales) Mycotaxon. 1992b;45:191–221. [Google Scholar]
- Barr ME. Notes on the Pleomassariaceae. Mycotaxon. 1993a;49:129–142. [Google Scholar]
- Barr ME. Redisposition of some taxa described by J.B. Ellis. Mycotaxon. 1993b;46:45–76. [Google Scholar]
- Barr ME. Notes on coprophilous bitunicate Ascomycetes. Mycotaxon. 2000;76:105–112. [Google Scholar]
- Barr ME. Montagnulaceae, a new family in the Pleosporales, and lectotypification of Didymosphaerella. Mycotaxon. 2001;77:193–200. [Google Scholar]
- Barr ME. Teichosporaceae, another family in the Pleosporales. Mycotaxon. 2002;82:373–389. [Google Scholar]
- Barr ME, Boise JR. Syncarpella (Pleosporales, Cucurbitariaceae) Mem N Y Bot Gard. 1989;49:298–304. [Google Scholar]
- Bayon C, Yuan Z-W, Ruiz C, Liesebach M, Pei MH. Genetic diversity in the mycoparasite Sphaerellopsis filum inferred from AFLP analysis and ITS-5.8S sequences. Mycol Res. 2006;110:1200–1206. doi: 10.1016/j.mycres.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Batista AC, Costa CA, Peres GEP, Leal FB. Novos e antigos fungos Microthyriaceae. Anais Soc Biol Pernambuco. 1959;16:129–140. [Google Scholar]
- Begerow D, Nilsson H, Unterseher M, Maier W. Current state and perspectives of fungal DNA barcoding and rapid identification procedures. Appl MicroBiol Biotechno1. 2010;87:99–108. doi: 10.1007/s00253-010-2585-4. [DOI] [PubMed] [Google Scholar]
- Berkeley MJ, Broome CE. Notices of British fungi. Ann Mag Nat Hist. 1866;18(3):128. [Google Scholar]
- Berlese AN. Icones Fungorum. I. fasc. 1890:1–66. [Google Scholar]
- Berlese AN. Icones fungorum. II. fasc. 1896:29–68. [Google Scholar]
- Bitzer J, L^ssoe T, Fournier J, Kummer V, Decock C, Tichy H-V, Piepenbring M, Persoh D, Stadler M. Affinities of Phylacia and the daldinoid Xylariaceae, inferred from chemotypes of cultures and ribosomal DNA sequences. Mycol Res. 2008;112:251–270. doi: 10.1016/j.mycres.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Boehm EWA, Mugambi GK, Miller AN, Huhndorf SM, Marincowitz S, Spatafora JW, Schoch CL. A molecular phylogenetic reappraisal of the Hysteriaceae, Mytilinidiaceae and Gloniaceae (Pleosporomycetidae, Dothideomycetes) with keys to world species. Stud Mycol. 2009a;64:49–83. doi: 10.3114/sim.2009.64.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm EWA, Schoch CL, Spatafora JW. On the evolution of the Hysteriaceae and Mytilinidiaceae (Pleosporomycetidae, Dothideomycetes, Ascomycota) using four nuclear genes. Mycol Res. 2009b;113:461–479. doi: 10.1016/j.mycres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Boise JR. On Trematosphaeria circinans and reinstatement of the genus Byssothecium. Mycologia. 1983;75:666–669. [Google Scholar]
- Boise JR. New and interesting fungi (Loculoascomycetes) from the Amazon. Acta Amazonica. 1984;14:49–53. [Google Scholar]
- Boise JR. An amended description of Trematosphaeria. Mycologia. 1985;77:230–237. [Google Scholar]
- Boise JR. On Hadrospora, a new genus in the Phaeosphaeriaceae and Byssothecium alpestris. Mem N Y Bot Gard. 1989;49:308–310. [Google Scholar]
- Borse BD, Hyde KD. Marine fungi from India. III. Acrocordiopsis patilii gen. et sp. nov. from mangrove wood. Mycotaxon. 1989;34:535–540. [Google Scholar]
- Bose SK. Studies on Massarina Sacc. and related genera. Phytopath Z. 1961;41:151–213. [Google Scholar]
- Boylan BV. The cytology and development of Preussia anaganii sp. nov. Can J Bot. 1970;48:163–166. [Google Scholar]
- Cai L, Hyde KD. Ascorhombispora aquatica gen. et sp. nov. from a freshwater habitat in China, and its phylogenetic placement based on molecular data. Crypt Mycol. 2007;28:291–300. [Google Scholar]
- Cain RF. Studies of coprophilous Sphaeriales in Ontario. Univ Toronto Stud Biol Ser. 1934;38:1–126. [Google Scholar]
- Cain RF. Studies on coprophilous ascomycetes. II. Phaeotrichum, a new cleistocarpous genus in a new family, and its relationships. Can J Bot. 1956;34:675–684. [Google Scholar]
- Cain RF. Studies of coprophilous ascomycetes. VII. Preussia. Can J Bot. 1961;39:1633–1666. [Google Scholar]
- Cain RF, Luck-Allen ER. Semidelitschia, a new genus of the Sporormiaceae. Mycologia. 1969;61:580–585. [Google Scholar]
- Calatayud V, Sanz MJ, Aptroot A. Lichenopyrenis galligena (Pleomassariaceae), a new genus of gall-forming lichenicolous fungi on Leptochidium. Mycol Res. 2001;105:634–637. [Google Scholar]
- Camara MPS, Palm ME, van Berkum P, Stewart EL. Systematics of Paraphaeosphaeria: a molecular and morphological approach. Mycol Res. 2001;105:41–56. [Google Scholar]
- Camara MPS, Palm ME, van Berkum P, O’Neill NR. Molecular phylogeny of Leptosphaeria and Phaeosphaeria. Mycologia. 2002;94:630–640. doi: 10.1080/15572536.2003.11833191. [DOI] [PubMed] [Google Scholar]
- Camara MP, Ramaley AW, Castlebury LA, Palm ME. Neophaeosphaeria and Phaeosphaeriopsis, segregates of Paraphaeosphaeria. Mycol Res. 2003;107:516–522. doi: 10.1017/s0953756203007731. [DOI] [PubMed] [Google Scholar]
- Cannon PF. A note on the nomenclature of Herpotrichia. Trans Br Mycol Soc. 1982;79:338–339. [Google Scholar]
- Cannon PF, Kirk PM. Fungal families of the world. CABI; Wallingford: 2007. [Google Scholar]
- Cesati V, De Notaris G. Schema di classificazione degle sferiacei italici aschigeri piu’ o meno appartenenti al genere Sphaeria nell’antico significato attribuitoglide Persono. Comm Soc crittog Ital. 1863;1:177–420. [Google Scholar]
- Checa J, Ramaley AW, Palm-Hernandez ME, Camara MPS. Paraphaeosphaeria barrii, a new species on Yucca schidigera from Mexico. Mycol Res. 2002;106:375–379. [Google Scholar]
- Chen CY, Hsieh WH. Astrosphaeriella from Taiwan, including two new species. Bot Bull Acad Sin. 2004;45:171–178. [Google Scholar]
- Cheng TF, Jia XM, Ma XH, Lin HP, Zhao YH. Phylogenetic study on Shiraia bambusicola by rDNA sequence analyses. J Basic MicroBiol. 2004;44:339–350. doi: 10.1002/jobm.200410434. [DOI] [PubMed] [Google Scholar]
- Chesters CGC. Studies on British pyrenomycetes II. A comparative study of Melanomma pulvis-pyrius (Pers.) Fuckel, Melanomma fuscidulum Sacc. and Thyridaria rubro-notata (B. & Br.) Sacc. Trans Br Mycol Soc. 1938;22:116–150. [Google Scholar]
- Chesters CGC, Bell A. Studies in the Lophiostomataceae Sacc. Mycol Pap. 1970;120:1–55. [Google Scholar]
- Chevenet F, Brun C, Banuls AL, Jacq B, Christen R. TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinforma. 2006;7:439. doi: 10.1186/1471-2105-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebicki A. Biogeographic relationships between fungi and selected glacial relict plants Use of host-fungus data as aid to plant geography on the basis of material from Europe, Greenland and northern Asia. Monogr Bot. 2002;90:1–90. [Google Scholar]
- Clements FE, Shear CL. Genera of fungi. 2nd edn. H.W. Wilson Company; New York: 1931. [Google Scholar]
- Clum FM. A new genus in the Aspergillaceae. Mycologia. 1955;47:899–901. [Google Scholar]
- Constantinescu O. Teleomorph anamorph connection in ascomycetes: Microdiplodia anamorph of Karstenula rhodostoma. Mycol Res. 1993;97:377–380. [Google Scholar]
- Cooke MC, Plowright CB. British Sphaeriacei. Grevillea. 1879;7:77–89. [Google Scholar]
- Coppins BJ. Notes on the genus Arthopyrenia in British Isles. Lichenologist. 1988;20:305–325. [Google Scholar]
- Corda ACJ. Deutschlands Flora, Abt. HI. Die Pilze Deutschlands. 1829;2-9:105–136. [Google Scholar]
- Crane JL, Shearer CA. A nomenclator of Leptosphaeria V. Cesati & G. de Notaris (Mycota – Ascomycotina – Loculoascomycetes) Illinois Nat Hist Surv Bull. 1991;34:1–355. [Google Scholar]
- Crivelli PG. Dissertation ETH Nr. 7318. Zurich, Germany: 1983. Über die heterogene Ascomycetengattung Pleospora Rabh.: vorschlag fur eine Aufteilung. [Google Scholar]
- Davey ML, Currah RS. Atradidymella muscivora gen. et sp. nov. (Pleosporales) and ITS anamorph Phoma muscivora sp. nov.: a new pleomorphic pathogen of boreal bryophytes. Am J Bot. 2009;96:1281–1288. doi: 10.3732/ajb.0900010. [DOI] [PubMed] [Google Scholar]
- de Gruyter JD, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW. Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycol Res. 2009;113:508–519. doi: 10.1016/j.mycres.2009.01.002. [DOI] [PubMed] [Google Scholar]
- de Gruyter JD, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW. Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia. 2010;102:1066–1081. doi: 10.3852/09-240. [DOI] [PubMed] [Google Scholar]
- de Notaris G. Micromicetes italici novi vel minus cogniti, decas 5. Mem Reale Accad Sci Torino. 1849;10:333–350. [Google Scholar]
- del Prado R, Schmitt I, Kautz S, Palice Z, Lucking R, Lumbsch HT. Molecular data place Trypetheliaceae in Dothideomycetes. Mycol Res. 2006;110:511–520. doi: 10.1016/j.mycres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Dennis RWG. British Ascomycetes. 2nd edn. J. Cramer; Vaduz: 1968. [Google Scholar]
- Dennis RWG. British Ascomycetes. 3rd edn. J. Cramer; Vaduz: 1978. [Google Scholar]
- DeSalle R, Egan MG, Siddall M. The unholy trinity: taxonomy, species delimitation and DNA barcoding. Philos T R Soc B. 2005;360:1905–1916. doi: 10.1098/rstb.2005.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianese JC, Inacio CA, Dornelo-Silva D. Wilmia, a new genus of phaeosphaeriaceous ascomycetes on Memora pedunculata in central Brazil. Mycologia. 2001;93:1014–1018. [Google Scholar]
- Dong JW, Chen WD, Crane JL. Phylogenetic studies of the Leptosphaeriaceae, Pleosporaceae and some other Loculoascomycetes based on nuclear ribosomal DNA sequences. Mycol Res. 1998;102:151–156. [Google Scholar]
- Earle FS. Mycological studies. Bull N Y Bot Gard. 1902;2:331–350. [Google Scholar]
- Ellis JB, Everhart BM. The North American Pyrenomycetes. Published by authors; Newfield, New Jersey: 1892. [Google Scholar]
- Ellwood SR, Kamphuis LG, Oliver RP. Identification of sources of resistance to Phoma medicaginis isolates in Medicago truncatula SARDI core collection accessions, and multigene differentiation of isolates. Phytopathology. 2006;96:1330–1336. doi: 10.1094/PHYTO-96-1330. [DOI] [PubMed] [Google Scholar]
- Eriksson OE. On Eudarluca caricis (Fr.) O. Erikss., comb. nov., a cosmopolitan uredinicolous pyrenomycete. Bot Not. 1966;119:33–69. [Google Scholar]
- Eriksson OE. On graminicolous pyrenomycetes from Fennoscandia. I, II, HI. Ark Bot Ser. 1967a;26:339–466. [Google Scholar]
- Eriksson OE. Studies on graminicolous pyrenomycetes from Fennoscandia. Acta Univ Upsal. 1967b;88:1–16. [Google Scholar]
- Eriksson OE. The families of bitunicate ascomycetes. Opera Bot. 1981;60:1–220. [Google Scholar]
- Eriksson OE. Eriksson; Lund: 1992. Non-lichenized Pyrenomycetes of Sweden. [Google Scholar]
- Eriksson OE. Outline of Ascomycota – 1999. Myconet. 1999;3:1–88. [Google Scholar]
- Eriksson OE. Outline of Ascomycota – 2006. Myconet. 2006;12:1–82. [Google Scholar]
- Eriksson OE, Hawksworth DL. An alphabetical list of the generic names of ascomycetes – 1986. Syst Ascomyc. 1986;5:3–111. [Google Scholar]
- Eriksson OE, Hawksworth DL. Outline of the Ascomycetes- 1987. Syst Ascomyc. 1987;6:259–337. [Google Scholar]
- Eriksson OE, Hawksworth DL. Outline of the Ascomycetes – 1990. Syst Ascomyc. 1991;9:39–271. Reprint of Volumes 1-4 (1982-1985) [Google Scholar]
- Eriksson OE, Hawksworth DL. Notes on ascomycete systematics – nos 1294-1417. Syst Ascomyc. 1992;11:49–82. [Google Scholar]
- Eriksson OE, Hawksworth DL. Outline of the Ascomycetes – 1998. Syst Ascomyc. 1998;16:83–296. [Google Scholar]
- Eriksson ove E, Hawksworth DL. Saccharicola, a new genus for two Leptosphaeria species on sugar cane. Mycologia. 2003;95:426–433. [PubMed] [Google Scholar]
- Eriksson OE, Yue JZ. Bertiella (Sacc.) Sacc. & Sydow, a synonym of Massarina Sacc. Mycotaxon. 1986;27:247–253. [Google Scholar]
- Eriksson OE, Yue JZ. Notes on bambusicolous pyrenomycetes. Mycotaxon. 1990;38:201–220. [Google Scholar]
- Eriksson OE, Baral HO, Currah RS, Hansen K, Kurtzman CP, Rambold G, Laessoe T. Outline of Ascomycota – 2002. Myconet. 2002;8:1–54. [Google Scholar]
- Fabre JH. Essai sur les Spheriacees du departement de Vaucluse. Ann Sci Nat Bot Ser. 1878;6:66–118. [Google Scholar]
- Fallah PM, Shearer CA. Freshwater ascomycetes: new or noteworthy species from north temperate lakes in Wisconsin. Mycologia. 2001;93:566–602. [Google Scholar]
- Farr DF, Bills GF, Chamuris GP, Rossman AY. Fungi on plants and plant products in the United States. APS Press; St. Paul: 1989. [Google Scholar]
- Fisher PJ, Webster J. A Trematosphaeria endophyte from rice roots and its Zalerion anamorph. Nova Hedw. 1992;54:77–81. [Google Scholar]
- Freyer K, HA van der Aa. Über Pyrenochaeta parasitica sp. nov., die Nebenfruchtform von Herpotrichia parasitica (Hartig) E. Rostrup (=Trichosphaeria parasitica Hartig) Eur J For Path. 1975;5:177–182. [Google Scholar]
- Fries EM. Systema Mycologicum. 1823;2:275–621. [Google Scholar]
- Frisullo S, Braun U. Etiology of some leaf spot diseases on Dichondra repens. Phytopath Mediterr. 1996;35:137–143. [Google Scholar]
- Frohlich J, Hyde KD. Fungi from palms. XXXIX. Asymmetricospora gen. et sp. nov. (Melanommataceae) Sydowia. 1998;50:182–186. [Google Scholar]
- Fuckel L. Fungi rhenani. Suppl Fasc. 1866;3:1750. [Google Scholar]
- Fuckel L. Fungi rhenani exsic. 1868;7:2171. [Google Scholar]
- Fuckel L. Symbolae Mycologicae. Jahrb Nassau Ver Naturk. 1870;23(24):1–459. [Google Scholar]
- Funk A, Zalasky H. Rhytidiella baranyayi n. sp., associated with cork-bark of aspen. Can J Bot. 1975;53:752–755. [Google Scholar]
- Gaumann EA. The fungi. Hafner Publishing Company; New York: 1952. A description of their morphological features and evolutionary development. Translated from the German by Frederic Lyle Wynd. [Google Scholar]
- Godfray HCJ. Challenges for taxonomy. Nature. 2002;417:17–19. doi: 10.1038/417017a. [DOI] [PubMed] [Google Scholar]
- Greuter W, Burdet HM, Chaloner WG, Demoulin V, Grolle R, Hawksworth DL, Nicolson DH, Silva PC, Stafleu FA, Voss EG, McNeill J, editors. International Code of Botanical Nomenclature. Adopted by the Fourteenth International Botanical Congress, Berlin, July-August 1987. Regnum Veg. 1988;118:1–328. [Google Scholar]
- Griffiths D. The North American sordariaceae. Mem Torrey Club. 1901;11:1–134. [Google Scholar]
- Guarro J, Calvo MA, Ramirez C. Soil ascomycetes from Catalunya (Spain) II. Nova Hedw. 1981;34:285–299. [Google Scholar]
- Guarro J, Abdullah SK, Gene J, Alsaadoon AH. A new species of Preussia from submerged plant debris. Mycol Res. 1997a;101:305–308. [Google Scholar]
- Guarro J, Al-Saadon AH, Abdullah SK. Two new coprophilous species of Preussia (Ascomycota) from Iraq. Nova Hedw. 1997b;64:177–183. [Google Scholar]
- Hall T. Bioedit v7.0.1. Isis Pharmaceuticals; 2004. [Google Scholar]
- Hawksworth DL. Ascospore sculpturing and generic concepts in the Testudinaceae (syn. Zopfiaceae) Can J Bot. 1979;57:91–99. [Google Scholar]
- Hawksworth DL. Astrosphaeriella Sydow, a misunderstood genus of melanommataceous pyrenomycetes. Bot J Linn Soc. 1981;82:35–59. [Google Scholar]
- Hawksworth DL. A redisposition of the species referred to the ascomycete genus Microthelia. Bull Br Mus (nat Hist J), Bot. 1985a;14:43–181. [Google Scholar]
- Hawksworth DL. Kirschsteiniothelia, a new genus for the Microthelia incrustans-group (Dothideales) Bot J Linn Soc. 1985b;91:181–202. [Google Scholar]
- Hawksworth DL. The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycol Res. 1991;95:641–655. [Google Scholar]
- Hawksworth DL, Boise JR. Some addditional species of Astrosphaeriella, with a key to the members of the genus. Sydowia. 1985;38:114–124. [Google Scholar]
- Hawksworth DL, Booth C. A revision of the genus Zopfia Rabenh. Mycol Pap. 1974;135:1–38. [Google Scholar]
- Hawksworth DL, Diederich P. A synopsis of the genus Polycoccum (Dothideales), with a key to accepted species. Trans Br Mycol Soc. 1988;90:293–312. [Google Scholar]
- Hawksworth DL, Chea CY, Sheridan JE. Bimuria novaezelandiae gen. et sp. nov., a remarkable ascomycete isolated from a New Zealand barley field. N Z J Bot. 1979;17:267–273. [Google Scholar]
- Hawksworth DL, David JC. Proposals for nomina conservanda and rejicienda for ascomycete names (lichenized and nonlichenized) Taxon. 1989;38:493–499. [Google Scholar]
- Hawksworth DL, Kirk PM, Sutton BC, Pegler DN. Ainsworth & bisby’s dictionary of the fungi. 8th edn. CABI; Wallingford: 1995. [Google Scholar]
- Hedjaroude A. Etudes taxonomiques sur les Phaeosphaeria Miyake et leurs formes voisines (ascomycetes) Sydowia. 1969;22:57–107. [Google Scholar]
- Hino I. Fuji Bamboo Garden; Gotenba, Japan: 1961. Icones fungorum bambusicolorum japonicorum. [Google Scholar]
- Hino I, Katumoto K. On Murioa, a new genus of the Lophiostomataceae. J Jpn Bot. 1958;33:77–80. [Google Scholar]
- Hino I, Katumoto K. Notes on bambusicolous fungi. 1. J Jpn Bot. 1965;40:81–89. [Google Scholar]
- Hirayama K, Tanaka K, Raja HA, Miller AN, Shearer CA. A molecular phylogenetic assessment of Massarina ingoldiana sensu lato. Mycologia. 2010;102:729–746. doi: 10.3852/09-230. [DOI] [PubMed] [Google Scholar]
- Holm L. Taxonomical notes on Ascomycetes. 1. The Swedish species of the genus Ophiobolus Riess sensu Sacc. Sven Bot Tidskr. 1948;42:337–347. [Google Scholar]
- Holm L. Etudes taxonomiques sur les pleosporacees. Symb Bot Upsaliens. 1957;14:1–188. [Google Scholar]
- Holm L. Taxonomical notes on Ascomycetes. IV. Notes of Nodulosphaeria Rbh. Sven Bot Tidskr. 1961;55:63–80. [Google Scholar]
- Holm LM. Nomenclatural notes on pyrenomycetes. Taxon. 1975;24:475–488. [Google Scholar]
- Holm L. In: Index Nominum Genericorum (Plantarum) Farr ER, Leussink JA, Stafleu FA, Junk W, editors. The Hague; 1979. p. 1896. [Google Scholar]
- Holm L. A note on Byssolophis ampla. Windahlia. 1986;16:49–52. [Google Scholar]
- Holm L, Holm K. Nordic equiseticolous Pyrenomycetes. Nord J Bot. 1981;1:109–119. [Google Scholar]
- Holm L, Holm K. Studies in the Lophiostmataceae with emphasis on the Swedish species. Symb Bot Upsaliens. 1988;28:1–50. [Google Scholar]
- Holm L, Yue JZ. Notes on some fungi referred to Schizostoma Ces. & de Not. ex Sacc. Acta Mycol Sinica Suppl. 1987;1:82–89. [Google Scholar]
- Hohnel F., von Fragmente zur Mykologie: VI. Mitteilung (Nr. 182 bis 288) Sber Akad Wiss Wien, Math-naturw Kl, Abt I. 1909;118:275–452. [Google Scholar]
- Huhndorf SM. Neotropical ascomycetes 2. Hypsostroma, a new genus from the Dominican Republic and Venezuela. Mycologia. 1992;84:750–758. [Google Scholar]
- Huhndorf SM. Neotropical ascomycetes 3. Reinstatement of the genus Xenolophium and two new species from French Guiana. Mycologia. 1993;85:490–502. [Google Scholar]
- Huhndorf SM. Neotropical ascomycetes 5. Hypostromataceae, a new family of Loculoascomycetes and Manglicola samuelsii, a new species from Guyana. Mycologia. 1994;86:266–269. [Google Scholar]
- Huhndorf SM, Crane JL, Shearer CA. Studies in Leptosphaeria. Transfer of L. massarioides to Massariosphaeria. Mycotaxon. 1990;37:203–210. [Google Scholar]
- Hyde KD. Helicascus kanaloanus, H. nypae sp. nov. and Salsuginea ramicola gen. et sp. nov. from intertidal mangrove wood. Bot Mar. 1991a;34:311–318. [Google Scholar]
- Hyde KD. Massarina velatospora and a new mangrove-inhabiting species, M. ramunculicola sp. nov. Mycologia. 1991b;83:839–845. [Google Scholar]
- Hyde KD. Fungi from decaying inter-tidal fronds of Nypa fruticans, including three new genera and four new species. J Linn Soci, Bot. 1992a;110:95–110. [Google Scholar]
- Hyde KD. Intertidal mangrove fungi from the west coast of Mexico, including one new genus and two new species. Mycol Res. 1992b;96:25–30. [Google Scholar]
- Hyde KD. Fungi from palms. XI. Appendispora frondicola gen. et sp. nov. from Oncosperma horridum in Brunei. Sydowia. 1994a;46:29–34. [Google Scholar]
- Hyde KD. Fungi from palms. XII. Three new intertidal ascomycetes from submerged palm fronds. Sydowia. 1994b;46:257–264. [Google Scholar]
- Hyde KD. The genus Massarina, with a description of M. eburnea and an annotated list of Massarina names. Mycol Res. 1995a;99:291–296. [Google Scholar]
- Hyde KD. Tropical Australasian fungi. VII. New genera and species of ascomycetes. Nova Hedw. 1995b;61:119–140. [Google Scholar]
- Hyde KD. The genus Roussoella, including two new species from palms in Cuyabeno, Ecuador. Mycol Res. 1997;101:609–616. [Google Scholar]
- Hyde KD, Aptroot A. Tropical freshwater species of the genera Massarina and Lophiostoma (Ascomycetes) Nova Hedw. 1998;66:489–502. [Google Scholar]
- Hyde KD, Borse BD. Marine fungi from Seychelles V. Biatriospora marina gen. et sp.nov. from mangrove wood. Mycotaxon. 1986;26:263–270. [Google Scholar]
- Hyde KD, Frohlich J. Fungi from palms XXXVU. The genus Astrosphaeriella, including ten new species. Sydowia. 1998;50:81–132. [Google Scholar]
- Hyde KD, Goh TK. Some new melannommataceous fungi from woody substrata and a key to genera of lignicolous Loculoascomycetes in freshwater. Nova Hedw. 1999;68:251–272. [Google Scholar]
- Hyde KD, Mouzouras R. Passeriniella savoryellopsis sp. nov. a new ascomycete from intertidal mangrove wood. Trans Br Mycol Soc. 1988;91:179–185. [Google Scholar]
- Hyde KD, Steinke TS. Two new species of Delitschia from submerged wood. Mycoscience. 1996;37:99–102. [Google Scholar]
- Hyde KD, Eriksson OE, Yue JZ. Roussoella, an ascomycete genus of uncertain relationships with a Cytoplea anamorph. Mycol Res. 1996a;100:1522–1528. [Google Scholar]
- Hyde KD, Wong SW, Jones EBG. Tropical Australian fresh water fungi. 11. Mamillisphaeria dimorphospora gen. et sp. nov. and notes on fresh water ascomycetes with dimorphic ascospores. Nova Hedw. 1996b;62:513–520. [Google Scholar]
- Hyde KD, Taylor JE, Frohlich J. Fungal Diversity research Series. Vol. 2. Fungal Diversity Press; Hong Kong: 2000. Genera of Ascomycetes from Palms. [Google Scholar]
- Hyde KD, Wong WS, Aptroot A, Hyde KD. Marine and estuarine species of Lophiostoma and Massarina. Fungi in Marine Environments, Fungal Diversity Research Series. 2002;7:93–109. [Google Scholar]
- Hyde KD, McKenzie EHC, KoKo TW. Towards incorporating anamorphic fungi in a natural classification – checklist and notes for 2010. Mycosphere. 2011;2:1–88. [Google Scholar]
- Inderbitzin P, Jones EBG, Vrijmoed LLP. A new species of Leptosphaerulina from decaying mangrove wood from Hong Kong. Mycoscience. 2000;41:233–237. [Google Scholar]
- Inderbitzin P, Kohlmeyer J, Volkmann-Kohlmeyer B, Berbee ML. Decorospora, a new genus for the marine ascomycete Pleospora gaudefroyi. Mycologia. 2002;94:651–659. doi: 10.1080/15572536.2003.11833193. [DOI] [PubMed] [Google Scholar]
- Inderbitzin P, Shoemaker RA, O’Neill NR, Turgeon BG, Berbee ML. Systematics and mating systems of two fungal pathogens of opium poppy: the heterothallic Crivellia papaveracea with a Brachycladium penicillatum asexual state and a homothallic species with a Brachycladium papaveris asexual state. Can J Bot. 2006;84:1304–1326. [Google Scholar]
- Johnson DA, Simmons EG, Miller JS, Stewart EL. Taxonomy and pathology of Macrospora/Nimbya on some north American bulrushes (Scirpus spp.) Mycotaxon. 2002;84:413–428. [Google Scholar]
- Johnston PR. Rhytidiella hebes sp. nov. from the subantarctic Auckland Islands. N Z J Bot. 2007;45:151–153. [Google Scholar]
- Jones EBG, Sakayaroj J, Suetrong S, Somrithipo1 S, Pang KL. Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Divers. 2009;35:1–187. [Google Scholar]
- Ju Y-M, Rogers JD, Huhndorf SM. Valsaria and notes on Endoxylina, Pseudothyridaria, Pseudovalsaria, and Roussoella. Mycotaxon. 1996;58:419–481. [Google Scholar]
- Kaiser WJ, Ndimande BN, Hawksworth DL. Leaf-scorch disease of sugar cane in Kenya caused by a new species of Leptosphaeria. Mycologia. 1979;71:479–492. [Google Scholar]
- Katumoto K. Two new species of Eudarluca hyperparasitic to Botryosphaeria. Trans Mycol Soc J. 1986;27:11–16. [Google Scholar]
- Keissler K. Mykologische Mitteilungen. Ann Naturhist Mus Wien. 1922;35:1–35. [Google Scholar]
- Khan RS, Cain RF. The genera Sporormiella and Sporormia in East Africa. Can J Bot. 1979a;57:1174–1186. [Google Scholar]
- Khan RS, Cain RF. The genera Sporormiella and Sporormia in Africa. Can J Bot. 1979b;57:1827–1887. [Google Scholar]
- Khan JA, Hussain ST, Hasan S, McEvoy P, Sarwari A. Disseminated bipolaris infection in an immunocompetent host: an atypical presentation. J Pak Med Assoc. 2000;50:68–71. [PubMed] [Google Scholar]
- Khashnobish A, Shearer CA. Phylogenetic relationships in some Leptosphaeria and Phaeosphaeria species. Mycol Res. 1996;100:1355–1363. [Google Scholar]
- Kirk PM, Cannon PF, David JC, Stalpers JA. Dictionary of the Fungi. 9th edn. CABI; Wallingford: 2001. [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Staplers JA. Dictionary of the Fungi. 10th edn. CABI Bioscience; UK: 2008. [Google Scholar]
- Kirschstein W. Sphaeriales. Kryptogamen-Flora der Mark Brandenburg. 1911;7:164–304. [Google Scholar]
- Kirschstein W. Über neue und kritische Kleinpilz. Hedwigia. 1944;81:204–205. [Google Scholar]
- Kishi T, Tahara S, Taniguchi N, Tsuda M, Tanaka C, Takahashi S. New perylenequinones from Shiraia bambusicola. Planta Med. 1991;57:376–379. doi: 10.1055/s-2006-960121. [DOI] [PubMed] [Google Scholar]
- Kodsueb R, Dhanasekaran V, Aptroot A, Lumyong S, McKenzie EHC, Hyde KD, Jeewon R. The family Pleosporaceae: intergeneric relationships and phylogenetic perspectives based on sequence analyses of partial 28S rDNA. Mycologia. 2006a;98:571–583. doi: 10.3852/mycologia.98.4.571. [DOI] [PubMed] [Google Scholar]
- Kodsueb R, Jeewon R, Vijaykrishna D, McKenzie EHC, Lumyong P, Lumyong S, Hyde KD. Systematic revision of Tubeufiaceae based on morphological and molecular data. Fungal Divers. 2006b;21:105–130. [Google Scholar]
- Kohlmeyer J. Neufunde holzbesiedelnder Meerespilze. Nova Hedw. 1959;1:77–99. [Google Scholar]
- Kohlmeyer J. Zwei neu Ascomyceten-Gattungen aufPosidonia-Rhizomen. Nova Hedw. 1963;6:5–13. [Google Scholar]
- Kohlmeyer J. Marine fungi of Hawaii including the new genus Heliascus. Can J Bot. 1969;49:1469–1487. [Google Scholar]
- Kohlmeyer J. Caryosporella rhizophorae gen. et sp. nov. (Massariaceae), a marine ascomycete from Rhizophora mangle. Proc Indian Acad Sci (Plant Sci) 1985;94:355–361. [Google Scholar]
- Kohlmeyer J. Ascocratera manglicola gen. et sp. nov. and key to the marine Loculoascomycetes on mangroves. Can J Bot. 1986;64:3036–3042. [Google Scholar]
- Kohlmeyer JJ, Kohlmeyer E. Icones Fungorum Maris. 1966:4–5. 62a. [Google Scholar]
- Kohlmeyer J, Kohlmeyer E. Marine mycology: the higher fungi. Academic; New York: 1979. [Google Scholar]
- Kohlmeyer J, Schatz S. Aigialus gen. nov. (Ascomycetes) with two new marine species from mangroves. Trans Br Mycol Soc. 1985;85:699–707. [Google Scholar]
- Kohlmeyer J, Vittal BPR. Lophiostoma mangrovis, a new. marine ascomycete from the tropics. Mycologia. 1986;78:489–492. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B. Marine fungi from Belize with a description of two new genera of ascomycetes. Bot Mar. 1987;30:195–204. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B. Revision ofmarine species of Didymosphaeria (Ascomycotina) Mycol Res. 1990;94:685–690. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B. Illustrated key to the filamentous higher marine fungi. Bot Mar. 1991;34:1–61. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B. Atrotorquata and Loratospora: new ascomycete genera on Juncus roemerianus. Syst Ascomyc. 1993;12:7–22. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B, Eriksson OE. Fungi on Juncus roemerianus 2. New dictyosporous ascomycetes. Bot Mar. 1995;38:165–174. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B, Eriksson OE. Fungi on Juncus roemerianus. 8. New bitunicate ascomycetes. Can J Bot. 1996;74:1830–1840. [Google Scholar]
- Kowalski DT. The development and cytology of Didymocrea sadasavanii. Mycologia. 1965;57:404–416. [Google Scholar]
- Kruys A, Wedin M. Phylogenetic relationships and an assessment of traditionally used taxonomic characters in the Sporormiaceae (Pleosporales, Dothideomycetes, Ascomycota), utilising multi-gene phylogenies. System Biodivers. 2009;7:465–478. [Google Scholar]
- Kruys A, Eriksson OE, Wedin M. Phylogenetic relationships of coprophilous Pleosporales (Dothideomycetes, Ascomycota), and the classification of some bitunicate taxa of unknown position. Mycol Res. 2006;110:527–536. doi: 10.1016/j.mycres.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Kwasna H, Kosiak B. Lewia avenicola sp. nov. and its Alternaria anamorph from oat grain, with a key to the species of Lewia. Mycol Res. 2003;107:371–376. doi: 10.1017/s0953756203007378. [DOI] [PubMed] [Google Scholar]
- Kwasna H, Ward E, Kosiak B. Lewia hordeicola sp. nov. from barley grain. Mycologia. 2006;98:662–668. [PubMed] [Google Scholar]
- Leonard KJ, Suggs EG. Setosphaeria prolata, the ascigerous state of Exserohilum prolatum. Mycologia. 1974;66:281–297. [Google Scholar]
- Leuchtmann A. Über Phaeosphaeria Miyake und andere bitunicate Ascomyceten mit mehrfach querseptierten Ascosporen. Sydowia. 1984;37:75–194. [Google Scholar]
- Leuchtmann A. Kulturversuche mit einigen Arten der Gattung Lophiostoma Ces. & de Not. Sydowia. 1985;38:158–170. [Google Scholar]
- Liew ECY, Aptroot A, Hyde KD. Phylogenetic significance of the pseudoparaphyses in Loculoascomycete taxonomy. Mol Phylogenet Evo1. 2000;16:392–402. doi: 10.1006/mpev.2000.0801. [DOI] [PubMed] [Google Scholar]
- Liew ECY, Aptroot A, Hyde KD. An evaluation of the monophyly of Massarina based on ribosomal DNA sequences. Mycologia. 2002;94:803–813. [PubMed] [Google Scholar]
- Lindau G. Pyrenomycetineae, Laboulbeniineae. In: Engler A, Prantl K, editors. Die NatÜrlichen Pflanzenfamilien 1. Verlag von Wilhelm Engelmann; Leipzig: 1897. pp. 321–505. [Google Scholar]
- Lindemuth R, Wirtz N, Lumbsch HT. Phylogenetic analysis of nuclear and mitochondrial rDNA sequences supports the view that loculoascomycetes (Ascomycota) are not monophyletic. Mycol Res. 2001;105:1176–1181. [Google Scholar]
- Liu YX, Suranaree University of Technology . Dissertation. 2009. Biological characteristics of a bamboo fungus, Shiraia bambusicola, and screening for hypocrellin high-yielding iso-lates. [Google Scholar]
- Locquin MV. Synopsis generalis fungorum, excerpts ex libro ‘De Taxia Fungorum’. Rev Mycol P. 1972;(Suppl) [Google Scholar]
- Lodha BC. Studies on coprophilous fungi. IV. Some cleistothecial ascomycetes. J Ind Bot Soc. 1971;50:196–208. [Google Scholar]
- Lorenzo LE. A new hairy species of Sporormiella. Mycol Res. 1994;98:10–12. [Google Scholar]
- Luck-Allen ER, Cain RF. Additions to the genus Delitschia. Can J Bot. 1975;53:1827–1887. [Google Scholar]
- Lumbsch HT, Huhndorf SM, editors. Outline of Ascomycota – 2007. Myconet. 2007;13:1–58. [Google Scholar]
- Lumbsch HT, Huhndorf SM. Outline of Ascomycota – 2009. Fieldiana Life and Earth Sciences. 2010;1:1–60. [Google Scholar]
- Lumbsch HT, Lindemuth R. Major lineages of Dothideomycetes (Ascomycota) inferred from SSU and LSU rDNA sequences. Mycol Res. 2001;105:901–908. [Google Scholar]
- Luttrell ES. Taxonomy of the Pyrenomycetes. Univ Mo Stud. 1951;24:1–120. [Google Scholar]
- Luttrell ES. The ascostromatci Ascomycetes. Mycologia. 1955;47:511–532. [Google Scholar]
- Luttrell ES. Loculoascomycetes. In: Ainsworth GC, Sparrow FK, Sussman AS, editors. The fungi, an advanced treatise, a taxonomic review with keys: ascomycetes and fungi imperfecti. Academic; New York: 1973. [Google Scholar]
- Luttrell ES. Centrum development in Didymosphaeria sadasivanii (Pleosporales) Am J Bot. 1975;62:186–190. [Google Scholar]
- Maciejowska Z, Williams EB. Studies on a multiloculate species of Preussia. Mycologia. 1963;53:300–308. [Google Scholar]
- Malathrakis NE, Agricultural College of Athens . Dissertation. 1979. A study of an olive tree disease caused by the fungus Phoma incompta Sacc. et Mart. [Google Scholar]
- Malloch D, Cain RF. New species and combinations of cleistothecial ascomycetes. Can J Bot. 1972;50:61–72. [Google Scholar]
- Maria GL, Sridhar KR. A new ascomycete, Passeriniella mangrovei sp. nov. from the mangrove forest of India. Indn J For. 2002;25:319–322. [Google Scholar]
- Marincowitz S, Crous PW, Groenewald JZ, Wingfield MJ. Microfungi occurring on Proteaceae in the fynbos. CBS Biodiversity Series. 2008;7:1–166. [Google Scholar]
- Massee G. British pyrenomycetes. Grevillea. 1887;16:34–39. [Google Scholar]
- McAlpine D. Fungus diseases of stone-fruit trees in Australia and their treatment. Melbourne; R.S. Brain, government printer: 1902. [Google Scholar]
- Miller MA, Holder MT, Vos R, Midford PE, Liebowitz T, Chan L, Hoover P, Warnow T. The CIPRES Portals. 2009 http://www.phylo.org/sub_sections/portal.
- Mirza F. Taxonomic investigations on the ascomycetous genus Cucurbituria S.F. Gray. Nova Hedw. 1968;16:161–213. [Google Scholar]
- Miyake I. Studies on the parasitic fungi of rice in Japan. Bot Mag (Tokyo) 1909;23:85–97. [Google Scholar]
- Moore G. A comparison of traditional and phylogenetic nomenclature. Taxon. 1998;47:561–579. [Google Scholar]
- Morakotkarn D, Kawasaki H, Tanaka K, Okane I, Seki T. Taxonomic characterization of Shiraia-like fungi isolated from bamboos in Japan. Mycoscience. 2008;49:258–265. [Google Scholar]
- Morales VM, Jasalavich CA, Pelcher LE, Petrie GA, Taylor JL. Phylogenetic relationships among several Leptosphaeria species based on their ribosomal DNA sequences. Mycol Res. 1995;99:593–603. [Google Scholar]
- Moreau C. Les genres Sordaria et Pleurage. Encycl mycol. 1953;25:1–130. [Google Scholar]
- Morelet M. Sur quatre Dothideales. Bull Soc Sci Nat Archeo1 Toulon. 1980;227:14–15. [Google Scholar]
- Mugambi GK, Huhndorf SM. Parallel evolution of hysterothecial ascomata in ascolocularous fungi (Ascomycota, Fungi) System Biodivers. 2009a;7:453–464. [Google Scholar]
- Mugambi GK, Huhndorf SM. Molecular phylogenetics of Pleosporales: Melanommataceae and Lophiostomataceae recircumscribed (Pleosporomycetidae, Dothideomycetes, Ascomycota) Stud Mycol. 2009b;64:103–121. doi: 10.3114/sim.2009.64.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜller E. Die schweizerischen Arten der Gattung Leptosphaeria und ihrer Verwandten. Sydowia. 1950;4:185–319. [Google Scholar]
- MÜller E. Die Schweizerischen Arten der Gattungen Clathrospora, Pleospora, Pseudoplea und Pyrenophora. Sydowia. 1951;5:248–310. [Google Scholar]
- MÜller E. Die schweizerischen Arten der Gattung Ophiobolus Riess. Ber Schweiz Bot Ges. 1952;62:307–339. [Google Scholar]
- MÜller E, Arx JA., von Einige aspekte zur Systematik pseudosphaerialer Ascomyceten. Ber Schweiz Bot Gesell. 1950;60:329–397. [Google Scholar]
- MÜller E, Dennis RWG. Fungi Venezuelani. VIII. Plectascales, Sphaeriales, Loculoascomycetes. Kew Bull. 1965;19:357–386. [Google Scholar]
- MÜller E, von Arx JA. Die Gattungen der didymosporen Pyrenomyceten. Beitr Krypt Fl Schweiz. 1962;11:1–922. [Google Scholar]
- Munk A. The system of the pyrenomycetes. A contribution to a natural classification of the group Sphaeriales sensu Lindau. Dansk Bot Ark. 1953;15:1–163. [Google Scholar]
- Munk A. On Metasphaeria coccodes (Karst.) Sacc. and other fungi probably related to Massarina Sacc. Massarinaceae n. fam. Friesia. 1956;5:303–308. [Google Scholar]
- Munk A. Danish pyrenomycetes. A preliminary flora. Dansk Bot Ark. 1957;17:1–491. [Google Scholar]
- Nag Raj TR. Coelomycetous anamorphs with appendage-bearing conidia. Mycologue Publications; WaterloÜ: 1993. [Google Scholar]
- Narendra DV, Rao VG. Studies on coprophilous fungi of Maharashtra (India) V. Nova Hedw. 1976;27:631–645. [Google Scholar]
- Neumann S, Boland GJ. Influence of host and pathogen variables on the efficacy of Phoma herbarum, a potential biological control agent of Taraxacum officinale. Can J Bot. 2002;80:425–429. [Google Scholar]
- Nitschke TRJ. Grundlage eines Systems der Pyrenomyceten. Verh Naturhist Vereines Preuss Rheinl. 1869;26:70–77. [Google Scholar]
- Patel US, Pandey AK, Rajak RC. Two new species of Fungi. Indian Phytopath. 1997;50:194–199. [Google Scholar]
- Pattengale ND, Alipour M, Bininda-Emonds OR, Moret BM, Stamatakis A. How many bootstrap replicates are necessary? J Comput Biol. 2010;17:337–354. doi: 10.1089/cmb.2009.0179. [DOI] [PubMed] [Google Scholar]
- Petrak F. Mykologische Notizen. IX. Annls Mycol. 1927;25:193–343. [Google Scholar]
- Petrak F. Ergebnisse einer Revision der Grundtypen verschiedener Gattungen der Askomyzeten und Fungi Imperfecti. Sydowia. 1952;6:336–343. [Google Scholar]
- Petrak F. Über Valsaria megalospora Auersw. und die Gattung Massariovalsa Sacc. Sydowia. 1965;19:279–283. [Google Scholar]
- Petrak F, Sydow H. Die Gattungen der Pyrenomyzeten, Sphaeropsideen und Melanconieen. 1. Teil. Die Phaeosporen, Sphaeropsideen und dei Gattung Macrophoma (Repertorium spec.) Novarum Regni Veg Beihefte Nr. 1926;1:1–160. [Google Scholar]
- Petrak F, Sydow H. Kritisch-systematische Originaluntersuchungen Über Pyrenomyzeen, Spaeropsideen und Melanconieen. Annls Mycol. 1936;34:11–52. [Google Scholar]
- Phillips AJL, Alves A, Pennycook SR, Johnston PR, Ramaley A, Akulov A, Crous PW. Resolving the phylogenetic and taxonomic status of dark-spored teleomorph genera in the Botryosphaeriaceae. Persoonia. 2008;21:29–55. doi: 10.3767/003158508X340742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnoi A, Jeewon R, Sakayaroj J, Hyde KD, Jones EBG. Berkleasmium crunisia sp. nov. and its phylogenetic affinities to the Pleosporales based on 18S and 28S rDNA sequence analyses. Mycologia. 2007;99:378–384. doi: 10.3852/mycologia.99.3.378. [DOI] [PubMed] [Google Scholar]
- Pirozynski KA. Microfungi of Tanzania. I. Miscellaneous fungi on oil palm. II. New Hyphomycetes. Mycol Pap. 1972;129:1–64. [Google Scholar]
- Plachecka A. Microscopical observations of Sphaerellopsis filum, a parasite of Puccinia recondita. Acta Agrobot. 2005;58:67–71. [Google Scholar]
- Poonyth AD, Hyde KD, Aptroot A, Peerally A. Mauritiana rhizophorae gen. et sp. nov. (Ascomycetes, Requienellaceae), with a list of terrestrial saprobic mangrove fungi. Fungal Divers. 2000;4:101–116. [Google Scholar]
- Rabenhorst . Herb myc. ed. 2. 1858. p. 725. in sched. [Google Scholar]
- Rabenhorst . Fungi europaei exsiccatino. 1874. p. 1734. [Google Scholar]
- Rai JN, Tewari JP. On some isolates of the genus Preussia Fuckel from Indian soils. Proc Indian Acad Sci B. 1963;57:45–55. [Google Scholar]
- Raja HA, Shearer CA. Freshwater Ascomycetes: new and noteworthy species from aquatic habitats in Florida. Mycologia. 2008;100:467–489. doi: 10.3852/07-167r. [DOI] [PubMed] [Google Scholar]
- Ramaley AW, Barr ME. New dictyosporous species from leaves of Agavaceae. Mycotaxon. 1995;54:75–90. [Google Scholar]
- Ramakrishnan TS. Additions to fungi of Madras – XI. Proc Indian Acad Sci B. 1951;34:157–164. [Google Scholar]
- Ramesh Ch. Loculoascomycetes from India. In: Rao GP, Manoharachari C, Bhat DJ, editors. Frontiers of fungal diversity in india. International Book Distributing Company; Lucknow: 2003. pp. 457–479. [Google Scholar]
- Ranghoo VM, Hyde KD. Ascomauritiana lignicola gen. et. sp. nov., an ascomycete from submerged wood in Mauritius. Mycol Res. 1999;103:938–942. [Google Scholar]
- Reddy PV, Patel R, White JF., Jr. Phylogenetic and developmental evidence supporting reclassification of cruciferous pathogens Phoma lingam and Phoma wasabiae in Plenodomus. Can J Bot. 1998;76:1916–1922. [Google Scholar]
- Reiss MLC. Neue Kernpilze. Hedwigia. 1854;1:23–28. [Google Scholar]
- Reynolds DR. A phylogeny offissitunicate ascostromatic fungi. Mycotaxon. 1991;42:99–123. [Google Scholar]
- Romero AI, Samuels GJ. Studies on xylophilous fungi from Argentina. VI. Ascomycotina on Eucalyptus viminalis (Myrtaceae) Sydowia. 1991;43:228–248. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, Lowen R. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes) Stud Mycol. 1999;41:1–248. [Google Scholar]
- Rossman AY, Farr DF, Castlebury LA, Shoemaker R, Mengistu A. Setomelanomma holmii (Pleosporales, Phaeosphaeriaceae) on living spruce twigs in Europe and North America. Can J Bot. 2002;80:1209–1215. [Google Scholar]
- Roux C. Leptosphaerulina chartarum sp. nov., the teleomorph of Pithomyces chartarum. Trans Br Mycol Soc. 1986;86:319–323. [Google Scholar]
- Saccardo PA. Fungi Italici autographice delineati a Prof. P.A. Saccardo. Patavii 1878. Michelia. 1878a;1:326–350. [Google Scholar]
- Saccardo PA. Fungi Veneti novi vel critici vel mycologiae Venetae addendi. Series IX. Michelia. 1878b;1:361–445. [Google Scholar]
- Saccardo PA. Fungi Gallici lecti a cl. viris P. Brunaud, Abb. Letendre, A. Malbranche, J. Therry vel editi in Mycotheca Gallica C. Roumegueri. Series II. Michelia. 1880;2:39–135. [Google Scholar]
- Saccardo PA. Sylloge fungorum 1. Padova; 1882. p. 766. [Google Scholar]
- Saccardo PA. Sylloge Fungorum 2. Pavia; Italy: 1883. p. 815. [Google Scholar]
- Saccardo PA. Sylloge Fungorum 9. Pavia; Italy: 1891. p. 1141. [Google Scholar]
- Saccardo PA. Sylloge Fungorum 11. Pavia; Italy: 1895. p. 753. [Google Scholar]
- Samuels GJ. Ascomycetes of New Zealand. 1. Ohleria brasiliensis and its Monodictys anamorph, with notes on taxonomy and systematics of Ohleria and Monodictys. N Z J Bot. 1980;18:515–523. [Google Scholar]
- Samuels GJ. The genus Macbridiella with notes on Calostilbe, Herpotrichia, Phaeonectria, and Letendrea. Can J Bot. 1973;51:1275–1283. [Google Scholar]
- Samuels GJ, MÜller E. Life-history studies of Brazilian Ascomycetes 4. Three species of Herpotricia and their Pyrenochaeta-like anamorphs. Sydowia. 1978;31:157–168. [Google Scholar]
- Saxena MC, Singh KB. The chickpea. In: Saxena MC, Varma S, editors. Faba beans, kabuli chickpeas and lentils in the 1980s. CABI Wallingford; UK: 1987. pp. l39–151. [Google Scholar]
- Schatz S. The life history, developmental morphology, and taxonomy of Lautitia danica gen. nov., comb. nov. Can J Bot. 1984;62:28–32. [Google Scholar]
- Scheinpflug H. Untersuchungen Über die Gattung Didymosphaeria Fuck. und einige verwandte Gattungen. Ber Schweiz Bot Ges. 1958;68:325–385. [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW. A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia. 2006;98:1041–1052. doi: 10.3852/mycologia.98.6.1041. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, et al. A class-wide phylogenetic assessment of Dothideomycetes. Stud Mycol. 2009;64:1–15. doi: 10.3114/sim.2009.64.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert RA, Samuels GJ. How should we look at anamorphs? Stud Mycol. 2000;45:5–18. [Google Scholar]
- Semeniuk G. Association of Trematosphaeria circinans with crown and root rot of Alfafa in South Dakota. Mycologia. 1983;75:744–747. [Google Scholar]
- Shearer CA. Reexamination of eight taxa originally described in Leptosphaeria on members of the Asteraceae. Mycologia. 1993;85:825–834. [Google Scholar]
- Shearer CA, Crane JL. Fungi of the Chesapeake Bay and its tributaries. I. Patuxent River. Mycologia. 1971;63:237–260. [Google Scholar]
- Shearer CA, Crane JL. Freshwater Ascomycetes: Isthmosporella pulchra gen. and sp. nov. Mycologia. 1999;91:141–144. [Google Scholar]
- Shearer CA, Crane JL, Chandra Reddy KR. Studies in Leptosphaeria. Lectotypification of Sphaeria doliolum. Mycologia. 1990;82:496–500. [Google Scholar]
- Shearer CA, Raja HA, Miller AN, Nelson P, Tanaka K, Hirayama K, Marvanova L, Hyde KD, Zhang Y. The molecular phylogeny of freshwater Dothideomycetes. Stud Mycol. 2009;64:145–153. doi: 10.3114/sim.2009.64.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker RA. Generic correlations and concepts: Griphosphaerioma and Labridella. Can J Bot. 1963;41:1419–1423. [Google Scholar]
- Shoemaker RA. Canadian and some extralimital Ophiobolus species. Can J Bot. 1976;54:2365–2404. [Google Scholar]
- Shoemaker RA. Canadian and some extralimital Leptosphaeria species. Can J Bot. 1984a;62:2688–2729. [Google Scholar]
- Shoemaker RA. Canadian and some extralimital Nodulos-phaeria and Entodesmium species. Can J Bot. 1984b;62:2730–2753. [Google Scholar]
- Shoemaker RA, Babcock CE. Canadian and some extralimital Paraphaeosphaeria species. Can J Bot. 1985;63:1284–1291. [Google Scholar]
- Shoemaker RA, Babcock CE. Wettsteinina. Can J Bot. 1987;65:373–405. [Google Scholar]
- Shoemaker RA, Babcock CE. Bricookea barrae n. sp. compared with Bricookea sepalorum. Stud Mycol. 1989a;31:165–169. [Google Scholar]
- Shoemaker RA, Babcock CE. Phaeosphaeria. Can J Bot. 1989b;67:1500–1599. [Google Scholar]
- Shoemaker RA, Babcock CE. Diadema. Can J Bot. 1989c;67:1349–1355. [Google Scholar]
- Shoemaker RA, Babcock CE. Applanodictyosporous Pleosporales: Clathrospora, Comoclathris, Graphyllium, Macrospora, and Platysporoides. Can J Bot. 1992;70:1617–1658. [Google Scholar]
- Shoemaker RA, Kokko EG. 1977 [Google Scholar]
- Shoemaker RA, LeClair PM. Type studies of Massaria from the Wehmeyer collection. Can J Bot. 1975;53:1568–1598. [Google Scholar]
- Silva-Hanlin DMW, Hanlin RT. Small subunit ribosomal RNA gene phylogeny of several loculoascomycetes and its taxonomic implications. Mycol Res. 1999;103:153–160. [Google Scholar]
- Simmons EG. Culture studies in the genera Pleospora, Clathrospora, and Leptosphaeria. Mycologia. 1952;44:330–365. [Google Scholar]
- Simmons EG. Helminthosporium allii as type of a new genus. Mycologia. 1971;63:380–386. [Google Scholar]
- Simmons EG. Perfect states of Stemphylium II. Sydowia. 1985;38:284–293. publ. 1986. [Google Scholar]
- Simmons EG. Alternaria themes and variations (22-26) Mycotaxon. 1986;25:287–308. [Google Scholar]
- Simmons EG. Perfect states of Stemphylium HI. Mem New York Bot Gdn. 1989;49:305–307. [Google Scholar]
- Simmons EG. Embellisia and related teleomorphs. Mycotaxon. 1990;38:251–265. [Google Scholar]
- Simmons EG. Alternaria: an identification manual. CBS Fungal Biodiversity Center; Utrercht: 2007. [Google Scholar]
- Sivanesan A. The genus Herpotrichia Fuckel. Mycol Pap. 1971;127:1–37. [Google Scholar]
- Sivanesan A. Studies on ascomycetes. Trans Brit Mycol Soc. 1983;81:313–332. [Google Scholar]
- Sivanesan A. The bitunicate ascomycetes and their anamorphs. J. Cramer; Vaduz: 1984. [Google Scholar]
- Sivanesan A. Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycol Pap. 1987;158:1–261. [Google Scholar]
- Solheim WG. Studies on Rocky Mountain Fungi – I. Mycologia. 1949;41:623–631. [Google Scholar]
- Spegazzini C. Fungi argentini additis nonnullis brasiliensibus montevideensibusque. Pugillus quartus (Continuacion) Anales Soc Cienc Argentina. 1881;12:193–227. [Google Scholar]
- Spegazzini C. Fungi Puiggariani. Pugillus 1. Boletín, Academia nacional de Ciencias. Córdoba. 1889;11:381–622. [Google Scholar]
- Spegazzini C. Fungi aliquot paulistani. Revista del Museo de La Plata. 1908;15:7–48. [Google Scholar]
- Spegazzini C. Mycetes Argentinenses. Series IV. Anales del Museo nacional de Historia natural. Buenos Aires. 1909;19:257–458. [Google Scholar]
- Stajich JE, Berbee ML, Blackwell M, Hibbett DS, James TY, Spatafora JW, Taylor JW. The fungi. Curr Biol. 2009;19:R840–R845. doi: 10.1016/j.cub.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Stevens FL. Hawaiian fungi. Bernice P. Bishop Mus Bull. 1925;19:1–189. [Google Scholar]
- Stolk AC. Emericellopsis minima sp. nov. and Westerdykella ornata gen. nov., sp. nov. Trans Br Mycol Soc. 1955a;38:419–424. [Google Scholar]
- Stolk AC. The genera Anixiopsis Hansen and Pseudeurotium van Beyma. Leeuwenhoek Ned Tidjschr. 1955b;21:65–79. doi: 10.1007/BF02543800. [DOI] [PubMed] [Google Scholar]
- Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann-Kohlmeyer B, Sakayaroj J, Phongpaichit S, Tanaka K, Hirayama K, Jones EBG. Molecular systematics of the marine Dothideomycetes. Stud Mycol. 2009;64:155–173. doi: 10.3114/sim.2009.64.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana K, Malik KA. A new coprophilous ascomycete from Pakistan. Bull Mycol. 1980;1:33–35. [Google Scholar]
- Sutton BC. The Coelomycetes: fungi Imperfecti with pycnidia, acervuli and stromata. Commonwealth Mycological Institute; Kew, Surrey, England: 1980. [Google Scholar]
- Sydow H, Sydow P. Novae fungorum species – X. Ann Mycol. 1913;11:254–271. [Google Scholar]
- Tam WY, Pang KL, Jones EBG. Ordinal placement of selected marine Dothideomycetes inferred from small subunit ribosomal DNA sequence analysis. Bot Mar. 2003;46:487–494. [Google Scholar]
- Tanaka E, Harada Y. Hadrospora fallax (Pleosporales) found in Japan. Mycoscience. 2003a;44:245–248. [Google Scholar]
- Tanaka K, Harada Y. Pleosporales in Japan (1): the genus Lophiostoma. Mycoscience. 2003b;44:85–96. [Google Scholar]
- Tanaka K, Harada Y. Pleosporales in Japan (2): the genus Lophiotrema. Mycoscience. 2003c;44:115–121. [Google Scholar]
- Tanaka K, Harada Y. Pleosporales in Japan (3): the genus Massarina. Mycoscience. 2003d;44:173–185. [Google Scholar]
- Tanaka K, Harada Y. Pleosporales in Japan (4). The genus Massariosphaeria. Mycoscience. 2004;45:96–105. [Google Scholar]
- Tanaka K, Harada Y. Bambusicolous fungi in Japan (4): a new combination, Astrosphaeriella aggregata. Mycoscience. 2005a;46:114–118. [Google Scholar]
- Tanaka K, Harada Y. Bambusicolous fungi in Japan (6): Katumotoa, a new genus of phaeosphaeriaceous ascomycetes. Mycoscience. 2005b;46:313–318. [Google Scholar]
- Tanaka K, Ooki Y, Hatakeyama S, Harada Y, Barr ME. Pleosporales in Japan (5): Pleomassaria, Asteromassaria, and Splanchnonema. Mycoscience. 2005;46:248–260. [Google Scholar]
- Tanaka K, Hirayama K, Yonezawa H, Hatakeyama S, Harada Y, Sano T, Shirouzu T, Hosoya T. Molecular taxonomy of bambusicolous fungi: Tetraplosphaeriaceae, a new pleosporalean family with Tetraploa-like anamorphs, and notes on the phylogeny of selected species from bamboo. Stud Mycol. 2009;64:175–209. doi: 10.3114/sim.2009.64.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Mel’nik VA, Kamiyama M, Hirayama K, Shirouzu T. Molecular phylogeny of two coelomycetous fungal genera with stellate conidia, Prosthemium and Asterosporium, on Fagales trees. Botany. 2010;88:1057–1071. [Google Scholar]
- Tang AMC, Hyde KD, Tsui CKM, Corlett RT. A new species of Lophiotrema from wild fruit in Hong Kong. Persoonia. 2003;18:265–269. [Google Scholar]
- Taylor JW. A contemporary view of the holomorph: nucleic acid sequences and computer databases are changing fungal classification. In: Reynolds DR, Taylor JW, editors. The fungal holomorph: mitotic. Meiotic and pleomorphic speciation in fungal systematics. CABI; Wallingford: 1993. pp. 3–15. [Google Scholar]
- Theissen F, Sydow H. Die Dothideales. Kritisch-systematisch Originaluntersuchungen. Ann Mycol. 1915;13:147–746. [Google Scholar]
- Theissen F, Sydow H. VorentwÜrfe zu den Pseudosphaeriales. Ann Mycol. 1918;16:1–34. [Google Scholar]
- Thompson TW, Backus MP. Further notes on Pycnidiophora dispersa and Pseudeurotium multisporum. Mycologia. 1966;58:650–654. [Google Scholar]
- Ulloa M, Hanlin RT. Illustrated dictionary of mycology. APS Press, The American Phytopathological Society; St Paul: 2000. [Google Scholar]
- Upreti DK, Pant G. Notes on Arthopyrenia species from India. Bryologist. 1993;96:226–232. [Google Scholar]
- van der Aa HA. Macroventuria, a new genus of the Venturiaceae. Persoonia. 1971;6:359–363. [Google Scholar]
- Vassiljeva LN. De Buergenerula thalictri (Wint.) E. MÜller. Nov Sist Nizsh Rast. 1983;20:70–72. [Google Scholar]
- Verkley GJM, da Silva M, Wicklow DT, Crous PW. Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Stud Mycol. 2004;50:323–335. [Google Scholar]
- Vieira BS, Barreto RW. Lewia chlamidosporiformans sp. nov. from Euphorbia heterophylla. Mycotaxon. 2006;94:245–248. [Google Scholar]
- Voglmayr H, Jaklitsch WM. Molecular data reveal high host specificity in the phylogenetically isolated genus Massaria (Ascomycota, Massariaceae) Fungal Divers. 2011;46:133–170. [Google Scholar]
- von Arx JA. Ostiolate and nonostiolate Pyrenomycetes. Proc K Ned Akad Wet Ser C. 1973;76:289–296. [Google Scholar]
- von Arx JA. The genera of fungi sporulating in pure culture. 2nd edn. J Cramer; Vaduz: 1974. [Google Scholar]
- von Arx JA. On Thielavia angulata and some recently described Thielavia species. Kavaka. 1976;3:33–36. [Google Scholar]
- von Arx JA. The genera of fungi sporulating in pure culture. 3 rd edn J Cramer; Vaduz: 1981. [Google Scholar]
- von Arx JA. Revision einiger Gattungen der Ascomyceten. Acta Bot Neerl. 1954;3:83–93. [Google Scholar]
- von Arx JA, MÜller E. Die Gattungen der amerosporen Pyrenomyceten. Beitr Kryptogamenflora Schweiz. 1954;11:1–434. [Google Scholar]
- von Arx JA, MÜller E. A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Stud Mycol. 1975;9:1–159. [Google Scholar]
- von Arx JA, van der Aa HA. Notes on Curreya (Ascomycetes, Dothideales) Sydowia. 1983;36:1–5. [Google Scholar]
- von Arx JA, van der Aa HA. Spororminula tenerifae gen. et sp. nov. Trans Br Mycol Soc. 1987;89:117–120. [Google Scholar]
- von Hohnel F. Fragmente zur Mykologie. Sber Akad Wiss Wien, Math-nat Kl, Abt I. 1907;116:83–635. [Google Scholar]
- von Hohnel F. Dritte vorlaufige Mitteilung mycologischer Ergebnisse (nr. 201-304) Ber Deutsch Bot Ges. 1918a;36:309–317. [Google Scholar]
- von Hohnel F. Mykologische fragmente. Ann Mycol. 1918b;16:35–174. [Google Scholar]
- von Hohnel FXR. Fragmente zur Mykologie. 1175. Uber die Gattung Graphyllium Clements. Sber Akad Wiss Wien, Math-nat Kl, Abt I. 1919;128:589–590. [Google Scholar]
- von Niessl G. Beitrage zur Kenntniss der Pilze. Beschreibung neuer und wenig bekannter Pilze. Verhandl d naturf Ver in BrÜnn. 1872;10:153–217. [Google Scholar]
- Walker JM. Gaeumannomyces, Linocarpon, Ophiobolus and several other genera of scolecospored ascomycetes and Phialophora conidial states, with a note on hyphopodia. Mycotaxon. 1980;11:1–129. [Google Scholar]
- Wang YZh, Aptroot A, Hyde KD. Revision of the Ascomycete genus Amphisphaeria. Fungal Diversity Press; Hong Kong: 2004. [Google Scholar]
- Wang HK, Aptroot A, Crous PW, Jeewon R, Hyde KD. The polyphyletic nature of Pleosporales: an example from Massariosphaeria based on rDNA and RBP2 gene phylogenies. Mycol Res. 2007;111:1268–1276. doi: 10.1016/j.mycres.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Watson W. The classification of lichens Part II. New Phytol. 1929;28:1–36. [Google Scholar]
- Webster J. Graminicolous pyrenomycetes. V. Conidial states of Leptosphaeria michotii, L. microscopica, Pleospora vagans and the perfect state of Dinemasporium graminum. Trans Br Mycol Soc. 1955;38:347–365. [Google Scholar]
- Webster J. Pleospora straminis, P. rubelloides and P. rubicunda, three fungi causing purple-staining of decaying tissues. Trans Br Mycol Soc. 1957;40:177–186. [Google Scholar]
- Webster J. A rice root endophyte identified as Hadrospora fallax. Nova Hedw. 1993;57:141–142. [Google Scholar]
- Webster J, Lucas MT. Observations on British species of Pleospora. Trans Brit Mycol Soc. 1959;42:332–342. [Google Scholar]
- Wehmeyer LE. Studies on some fungi from north-western Wyoming. II. Fungi Imperfecti. Mycologia. 1946;38:306–330. [PubMed] [Google Scholar]
- Wehmeyer LE. The genus Montagnula Berl. Sydowia Beiheft. 1957;1:257–263. [Google Scholar]
- Wehmeyer LE. A world monograph of the genus pleospora and its segregates. University of Michigan Press; Michigan: 1961. [Google Scholar]
- Wehmeyer LE. The New York Botanical Garden. J. Cramer; Germany: 1975. The pyrenomycetous fungi. Mycologia Memoir No. 6. [Google Scholar]
- Welch DC. A monographic study of the genus Cucurbitaria. Mycologia. 1926;18:51–86. [Google Scholar]
- Wetzel HC, Hulbert SH, Tisserat NA. Molecular evidence for the presence of Ophiosphaerella narmari n. comb., a cause of spring dead spot of bermuda grass, in North America. Mycol Res. 1999;103:981–989. [Google Scholar]
- Wheeler QD, Raven PH, Wilson EO. Taxonomy: impediment or expedient? Science. 2004;303:285. doi: 10.1126/science.303.5656.285. [DOI] [PubMed] [Google Scholar]
- Winter G. Pilze – Ascomyceten. In GL Rabenhorst’s Kryptogamen-Flora von Deutschland. Oesterreich und der Schweiz. 1885;1:65–528. [Google Scholar]
- Winter G. Rabenhorst’s Die’ Pilze Deutschlands. Oesterreichs und der Schweiz; Bd I, Abt II: 1887. Ascomyceten. [Google Scholar]
- Winton LM, Stone JK, Hansen EM, Shoemaker RA. The systematic position of Phaeocryptopus gaeumannii. Mycologia. 2007;99:240–252. doi: 10.3852/mycologia.99.2.240. [DOI] [PubMed] [Google Scholar]
- Yuan ZQ. Barria, a new ascomycetous genus in the Phaeosphaeriaceae. Mycotaxon. 1994;51:313–316. [Google Scholar]
- Yuan ZQ, Barr ME. Species of Chaetoplea on desert plants in China. Mycotaxon. 1994;52:495–499. [Google Scholar]
- Yuan ZQ, Mohammed C. Seiridium papillatum, a new species (mitosporic fungus) described on stems of Eucalypts in Australia. Aust Syst Bot. 1997;10:69–75. [Google Scholar]
- Yuan ZQ, Zhao ZY. Studies on lophiostomataceous fungi from Xinjiang, China. Sydowia. 1994;46:162–184. [Google Scholar]
- Yue JZ, Eriksson O. Studies on Chinese ascomycetes. 2. Sinodidymella verrucosa. Mycotaxon. 1985;24:293–300. [Google Scholar]
- Zalasky H. Rhytidiella moriformis n. gen., n. sp. causing rough-bark of Populus balsamifera. Can J Bot. 1968;46:1383–1387. [Google Scholar]
- Zeiders KE. Stagonospora foliicola a pathogen of reed canarygrass spray-irrigated with municipal sewage effluent. Plant Dis Reptr. 1975;59:779–783. [Google Scholar]
- Zhang Y, Fournier J, Pointing SB, Hyde KD. Are Melanomma pulvis-pyrius and Trematosphaeria pertusa congeneric? Fungal Divers. 2008a;33:47–60. [Google Scholar]
- Zhang Y, Fournier J, Jeewon R, Hyde KD. Quintaria microsporum sp. nov., from a stream in France. Crypt Mycol. 2008b;29:179–182. [Google Scholar]
- Zhang Y, Jeewon R, Fournier J, Hyde KD. Multi-gene phylogeny and morphotaxonomy of Amniculicola lignicola: a novel freshwater fungus from France and its relationships to the Pleosporales. Mycol Res. 2008c;112:1186–94. doi: 10.1016/j.mycres.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Fournier J, Crous PW, Pointing SB, Hyde KD. Phylogenetic and morphological assessment of two new species of Amniculicola and their allies (Pleosporales) Persoonia. 2009a;23:48–54. doi: 10.3767/003158509X472187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schoch CL, Fournier J, Crous PW, De Gruyter J, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Hyde KD. Multi-locus phylogeny of the Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Stud Mycol. 2009b;64:85–102. doi: 10.3114/sim.2009.64.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang HK, Fournier J, Crous PW, Jeewon R, Pointing SB, Hyde KD. Towards a phylogenetic clarification of Lophiostoma/Massarina and morphologically similar genera in the Pleosporales. Fungal Divers. 2009c;38:225–251. [Google Scholar]
- Zhang YM, Koko TW, Hyde KD. Towards a monograph of Dothideomycetes: Studies on Diademaceae. Crypt Mycol. 2011 accepted. [Google Scholar]
- Zheng L, Lv R, Hsiang T, Huang J. Host range and phytotoxicity of Stemphylium solani, causing leaf blight of garlic (Allium sativum) in China. Eur J Plant Pathol. 2009;124:21–30. [Google Scholar]