Abstract
Adaptation is an error-driven motor learning process that can account for predictable changes in the environment (e.g. walking on ice) or in ourselves (e.g. injury). Our ability to recall and build upon adapted motor patterns across days is essential to this learning process. We investigated how different training paradigms affect the day-to-day memory of an adapted walking pattern. Healthy human adults walked on a split-belt treadmill, and returned the following day to assess recall, re-learning rate, and performance. In the first experiment, one group adapted and de-adapted (i.e. washed-out the learning) several times on day one to practice the initial stage of learning where errors are large; another group adapted only one time and then practiced in the adapted (“learned”) state where errors were small. On day two, they performed washout trials before re-adapting. The group that repeatedly practiced the initial portion of adaptation where errors are large showed the fastest re-learning on the second day. In fact, the memory was nearly as strong as that of a third group that was left overnight in the adapted state and was not washed-out prior to re-exposure on the second day. This demonstrates that alternating exposures to early adaptation and washout can enhance re-adaptation. In the second experiment, we tested whether the opposite split-belt pattern interferes with day two re-learning. Surprisingly, it did not, and instead was similar to practicing in the adapted state. These results show that the structure of the initial phase of learning influences the ease of motor re-learning.
Keywords: motor control, gait, structure of practice, motor learning, locomotor adaptation
INTRODUCTION
The old adage “practice makes perfect” undoubtedly applies to learning movements. But what practice schedule is best for different types of motor learning? Prior work has examined the effects of practice structure on “motor skill learning”—this involves the acquisition of a new pattern of muscle activations, like a sequence of finger or arm movements (Shea and Morgan 1979; Krakauer 2009; Robertson et al. 2004). Such studies have shown that training with random practice, where a series of skills are learned and practiced in short, randomly-distributed blocks, leads to better retention days later than what results from blocked practice, where a skill is practiced exhaustively before switching (Blandin et al. 1994; Lin et al. 2008; Shea and Morgan 1979; Simon 2007).
Does practice structure also affect other types of learning, like motor adaptation? In contrast to skill learning, adaptation is an error-driven process that adjusts sensorimotor mappings of well-learned movements to account for new, predictable demands (Martin et al. 1996). This new mapping is stored and must be unlearned when normal conditions are restored (Martin et al. 1996; Reisman et al. 2005; Shadmehr and Mussa-Ivaldi 1994). Although an adapted pattern can be unlearned, it may not be forgotten. Rather, subjects who have previously adapted show some memory of the adapted state when re-exposed to the same perturbation; for example, they may be perturbed less on re-exposure or re-adapt at a faster rate (Caithness et al. 2004; Krakauer et al. 2005; Martin et al. 1996; Shadmehr and Brashers-Krug 1997). Thus, adaptation training can lead to storage of the original and the adapted pattern (Martin et al. 1996). Additionally, the memory of the adapted state may be subject to interference when it is followed by adaptation to an opposing perturbation (Krakauer et al. 2005; Shadmehr and Brashers-Krug 1997). Thus, the structure of adaptation training can influence retention.
We investigated if changing training structure in a walking adaptation task affects retention. Subjects adapted to a split-belt treadmill driving one leg faster than the other (Reisman et al. 2005). In Experiment 1, we asked if the adapted pattern could be retained from day to day, without washout, to determine if time and walking over ground would diminish the memory of the split-belt pattern. We then evaluated how different training structures affect next day retention. On day 1, subjects either switched between adapting and de-adapting repeatedly, or they practiced at the plateau of their adapted state. We hypothesized that either structure could be beneficial: switching repeatedly and the exposure to large errors may facilitate learning how to solve the problem, whereas repeating the adapted movement could reinforce the adapted pattern, possibly through a use-dependent mechanism (Diedrichsen et al. 2010; Huang et al. 2011). In Experiment 2, we asked if exposing subjects to the opposite split-belt perturbation immediately following adaptation would interfere with the day 2 memory of the adapted pattern. Interference would further support the idea that the acquisition of a specific pattern was important for the day 2 memory, whereas any benefit would suggest that subjects learn how to adapt for the general type of perturbation.
MATERIALS AND METHODS
Subjects
Fifty-two healthy volunteers (13 males, 39 females; mean age, 25.6 years) participated in this study. All subjects gave informed written consent before participating. The protocols were approved by the Johns Hopkins Institutional Review Board.
Experimental protocol
Split-belt walking adaptation was studied using a custom-built treadmill (Woodway, Waukesha, WI) that had two separate belts driven by independent motors – these belts could be driven at the same speed (“tied-belts”) or at different speeds (“split-belts”). Speed commands for each belt were sent to the treadmill through a custom MATLAB (MathWorks, Natick, MA) computer interface. Subjects were positioned in the middle of the treadmill with one leg on each belt and wore a safety harness that was suspended from the ceiling. The safety harness did not support their body weight. At the beginning of each trial, the belts were stationary and subjects were not informed of the speeds of the belts. They were also told to refrain from looking down at the belts. Subjects initially held onto a ground-referenced rail when the belts were started and were instructed to lift their hands off the rail and cross their arms within the first few strides (within 3s). Subjects remained on the treadmill (either standing or seated) between epochs for the entire session each day.
Experiment 1
The experimental paradigm is shown in Figure 1b. All subjects were naïve to the task and began the experiment with a baseline period during which the belts were tied at 0.7 m/s. Subjects were then exposed to 10 seconds of split-belts (belts split at 0.7 and 1.4 m/s; slow belt was always on the dominant side). We added this short split-belt exposure since subjects were sometimes surprised by the perturbation when they first experienced it, which exaggerated the initial asymmetry. Allowing subjects to briefly experience the split-belts before the adaptation period reduced this “surprise” reaction, resulting in a more accurate measurement of the asymmetry caused by the split-belts, and not by the perceived instability induced by this unusual walking situation. To washout any possible adaptation from the 10 second exposure, subjects walked for 1 minute on tied-belts. Following this, the subjects were adapted to split-belts.
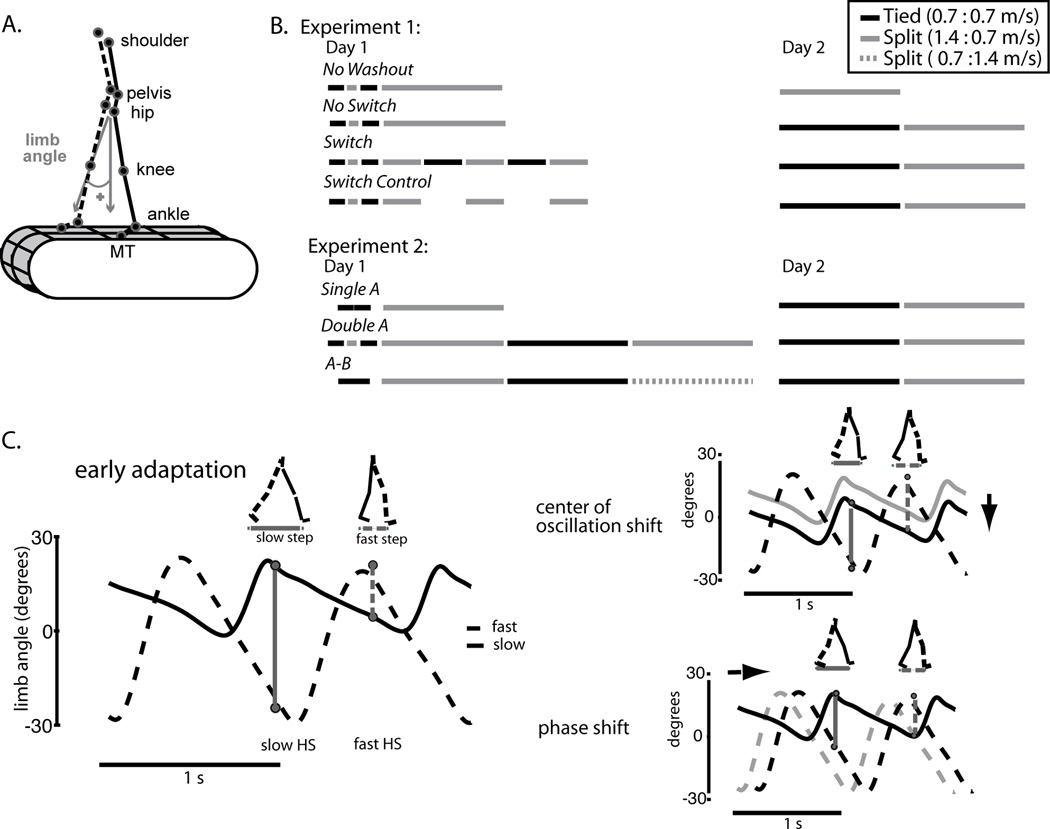
Figure 1.
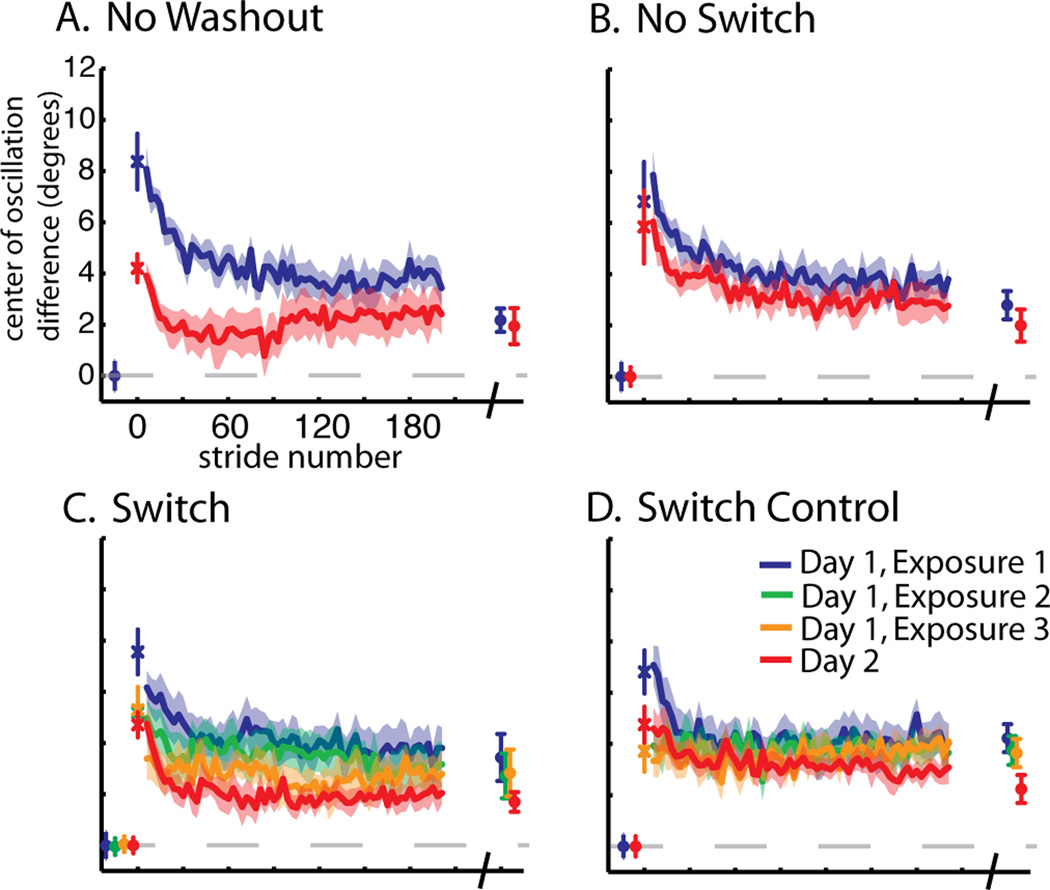
A. Diagram of marker location and limb angle convention. B. Experimental paradigm showing the periods of split-belt waking in gray lines and tied walking in black. Dotted gray lines represent split-belt walking of the opposite speed ratio. The ‘Switch Control’ group sat for five minutes between adaptation blocks. C. Schematic showing how step symmetry can be changed during adaptation. On the left are the limb angle trajectories during early adaptation where step lengths are asymmetric. Two possible ways to make stepping more symmetric are shown on the right. The center of oscillation shift (top) is a spatial change where the limbs gradually shift to oscillate about a midpoint closer to a vertical line intersecting the hip (i.e. center of oscillation = 0). Alternatively the timing of limb motion (i.e. phase shift) can be altered to equalize stepping (shown at bottom). (Figure adapted from (Malone and Bastian 2010)).
All participants were randomly assigned to one of four groups and each group received different split-belt training paradigms. Subjects in the ‘No Washout’ (N=7) and ‘No Switch’ (N=7) groups were adapted to the split-belts continuously for 15 minutes. For the ‘Switch’ (N=7) and ‘Switch Control’ (N=7) groups, we divided the adaptation period into three five-minute epochs. In the ‘Switch’ group, split-belts (S) and tied belts (T) epochs of five minutes were alternated (i.e. S-T-S-T-S), so that we could assess how switching locomotor patterns with repeated bouts of the early phase of adaptation, where errors are largest, would influence recall, re-learning, and performance. To investigate if breaks interspersed between split-belt periods were important for re-adaptation, we had the ‘Switch Control’ group sit for five minutes in between adaptation epochs, rather than walk on tied belts. All subjects ended with split-belts on the first day of training.
Twenty-four hours later subjects returned to the treadmill. The ‘No Washout’ group was immediately exposed to the split-belts for another 15 minutes to assess the previously learned split-belt pattern. To measure next day re-adaptation, the ‘No Switch’, ‘Switch’, and ‘Switch Control’ groups all completed 15 minutes of tied belts at the beginning of day 2 to wash-out any remaining split-belt adaptation from day 1 before being re-exposed to the split-belts again (15 minutes).
Experiment 2
This experiment tested whether learning the opposite split-belt pattern caused detrimental effects on recall, re-learning, or performance the second day (i.e. interference). Subjects were randomly assigned to one of three groups: ‘Single A’ (N=8), ‘Double A’ (N=8), and ‘A-B’ (N=8). All subjects experienced two minutes of baseline walking on tied belts (0.7 m/s). The ‘Double A’ subjects received the brief 10 second exposure to split-belts in between tied belts baseline periods. All groups (‘Single A’, ‘Double A’, and ‘A–B’) were adapted to split-belts, pattern ‘A,’ (belts split at 0.7 and 1.4 m/s; slow belt under dominant leg) for a continuous 15 minutes. Subjects in the ‘Double A’ and ‘A–B’ Group were then washed out with tied belts (0.7 m/s, 10–15 minutes). The ‘Double A’ group was re-exposed to the same split-belts (pattern ‘A’) again for 15 minutes, while the ‘A–B’ group were exposed to split-belts but with the opposite split pattern, pattern ‘B,’ (belts split at 1.4 and 0.7 m/s; slow belt under the non-dominant leg) for 15 minutes. All subjects then left the lab in an adapted state: ‘Single A’ and ‘Double A’ groups adapted to pattern ‘A’ and the ‘A–B’ group adapted to pattern ‘B.’ All groups returned the following day and completed a washout period of tied belts (0.7 m/s; 10–15 minutes) before being re-exposed to split-belts pattern ‘A.’
Data collection
Kinematic data were collected at 100 Hz using Optotrak (Northern Digital, Waterloo, ON). Infared-emitting markers were placed bilaterally over the toe (fifth metatarsal head), ankle (lateral malleolus), knee (lateral femoral epicondyle), hip (greater trochanter), pelvis (iliac crest), and shoulder (acromion process) (Figure 1a). Voltages reflecting treadmill belt speeds were recorded directly from treadmill motor output at 1000 Hz. Marker position and analog data (treadmill belt speeds) were synchronized and sampled simultaneously using Optotrak software. Heel strike times were approximated using the maximum angle of the limb (Figure 1a); toe-off time was approximated to be the minimum limb angle. All kinematic data was collected once the belts reached 80% of the desired speed.
Data Analysis
In this study, our primary measurement was step length symmetry which has previously been shown to adapt robustly (Choi and Bastian 2007; Choi et al. 2009; Malone and Bastian 2010; Reisman et al. 2005; Reisman et al. 2007; Reisman et al. 2009; Vasudevan and Bastian 2010). Step symmetry (SS) was defined as the normalized difference between the step lengths (SL) of the ‘fast’ and ‘slow’ leg, or
| Equation 1 |
A positive step symmetry value means that the fast step was larger than the slow step, and vice versa for negative values. A value of 0 indicates symmetry. Normalization of dividing by the sum of the step lengths was done so subjects of different height who take different sized steps could be compared. We chose this as our primary measure since our previous work suggested that it is a “global” measure of walking coordination that can be altered with modifications to either spatial or temporal coordination or a combination of both (Figure 1c) (Malone and Bastian 2010). That is, subjects can alter their step lengths during split-belt adaptation by shifting the ‘center of oscillation’ (spatial coordination – where the limbs are placed during walking) for each leg or by changing ‘phasing’ (temporal coordination – when the limbs are placed) between the legs (Malone and Bastian 2010).
Our measure of spatial coordination, ‘center of oscillation’, evaluated whether the leg was oscillating about a flexed, extended or neutral (i.e. vertical) axis. This was calculated on a stride-by-stride basis as the midpoint of the limb angle between heel strike and toe off for each leg. Limb angle was defined as the angle between a vertical line and the vector from the hip to the foot on an x–y plane (Figure 1a); it was positive when the when the foot was in front of the hip (flexion) and negative behind (extension). When the limb was oscillating symmetrically around a vertical axis drawn through the hip, the center of oscillation value was defined as zero. The center of oscillation of the ‘fast leg’ was subtracted from that of the ‘slow leg’ to give the center of oscillation difference between the two legs. When the center of oscillation difference was zero, stepping in the spatial realm was symmetric.
Our measure of temporal coordination, ‘phasing’, was determined using the time series of limb angles for each leg (Choi and Bastian 2007). It was calculated as the lag time at peak cross-correlation (Signal Processing Toolbox, MATLAB) of the limb angle trajectories over one stride cycle (Choi and Bastian 2007). Possible phasing values ranged from 0 to1 stride cycles, with symmetric walking having a value of 0.5 (i.e. outof-phase walking). The slow leg was the reference leg in this analysis.
To quantify changes in the adaptation on a single day in the three parameters (step symmetry, center of oscillation, and phasing), we measured the first stride to assess the initial perturbation, and defined the early change as the average of strides 2–30 for each individual subject, which captures the initial, large reduction in errors. The advantage of this measure of early change is that does not assume that the subjects follow a specific pattern as, for example, would a single or double exponential fit. Additionally, it allows us to compare measures across days for individual subjects. Similarly, we calculated the plateau as the last thirty strides of adaptation on day 1 and day 2. The magnitude of all first stride, early change and plateau values were used, regardless of sign convention for step symmetry, phasing and center of oscillation difference. In order to measure changes across days, we subtracted an individual subject’s values for first stride, early change and plateau on day 2 from day 1. We defined the difference in first stride across days as “recall,” the difference in early change across days as “re-learning,” and the difference in plateau across days as “performance”.
Additionally, to assess re-learning for the day 2 curves of step symmetry, we fit group curves with a single exponential with 3 floating parameters. The fit equation was of the form ae−x/t +c, where a Matlab Curve Fitting Toolbox calculated average and 95% confidence bounds on parameters a, t, and c.
Statistical analysis
One-way ANOVAs were used to compare baseline, perturbation, early adaptation and plateau values for all three parameters across groups on day 1 to confirm that all groups performed similarly. We then used repeated measures ANOVAs to compare our measures of “recall,” “re-learning,” and “performance” in these same parameters (calculation described above), which tested for effects of day and group. Post-hoc analysis was performed using Fisher’s LSD test. Statistica (StatSoft, Tulsa, OK) was used for all statistical analysis and the alpha level was set at p = 0.05.
RESULTS
Experiment 1
All subjects were able to complete the walking task without difficulty, regardless of group assignment. We first made sure there was no difference in baseline walking across groups after the brief exposure to the split-belt perturbation. Analyzing the last 30 seconds of the second tied epoch we found no difference in step symmetry (F(3,24)=0.24, p=0.85), center of oscillation difference (F(3,24)=0.57, p=0.64), or phasing (F(3,24)=2.02, p=0.14). The mean baseline values from the last 30s of this second tied epoch were subtracted from all subsequent data on day 1, thus referencing all data to each subject’s initial baseline asymmetry. The standard error of the baseline period is shown by the first points in Figures 2–4 (day 1 is blue; day 2 is red). The ‘No Washout’ group did not complete a tied period on day 2, so values from day 2 were subtracted from the day 1 baseline. For all other groups, the last thirty seconds of tied walking on day 2 were used as a second baseline period and the mean values were subtracted from subsequent values on day 2. We found no difference in the second baseline across groups in step symmetry (F(2,18)=1.02, p=0.38), center of oscillation difference (F(2,18)=0.88, p=0.43), or phasing (F(2,18)=1.67, p=0.22).
Figure 2.
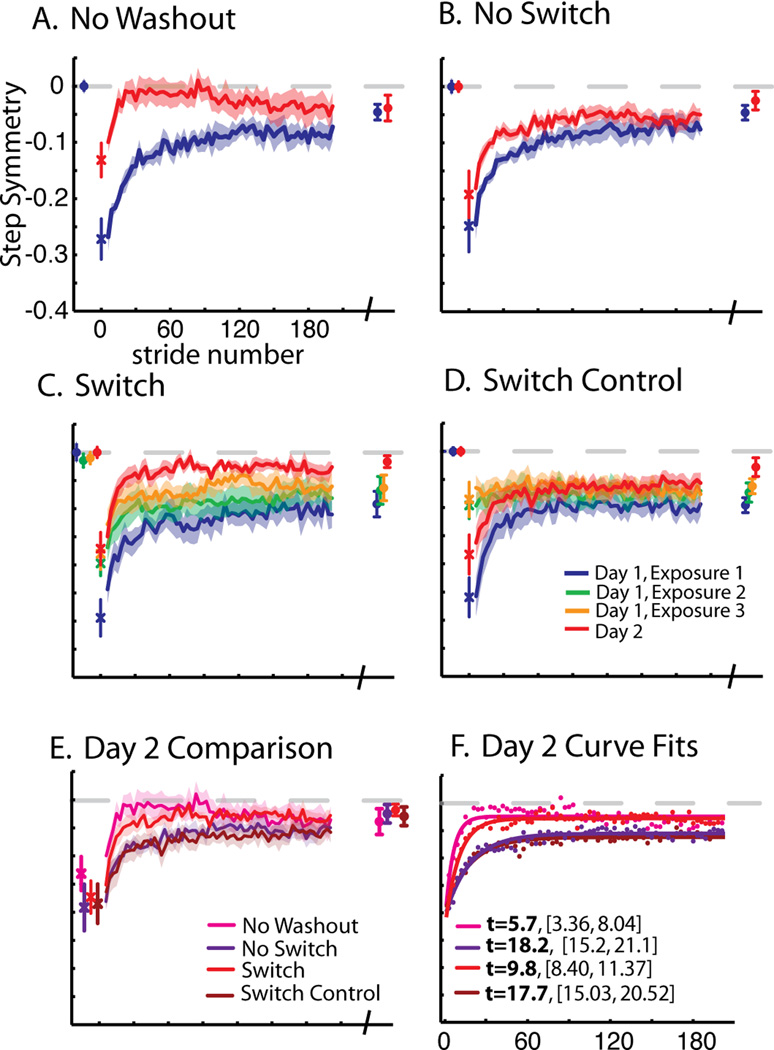
Comparison of step symmetry adaptation across groups. Baseline, first stride, adaptation curves (smoothed by 3 strides) for a portion of training epochs (2–190), and final plateau values are shown. A. No Washout. Mean step symmetry values subtracted from baseline is shown. The first stride is off-set as individual symbols, with mean and standard error bars. Average adaptation curves (± standard errors in shaded region) for strides 2–190 (approx. 5 min) of adaptation on day 1 and day 2 are shown in blue and red lines, respectively. Points plotted at the end of these curves represent means (± standard error) of the last 30 steps of adaptation on day 1 and 2, also called “plateau” values. B. No Switch. Data are shown as in (A), except that this group had a tied-belt period preceding adaptation on day 2 as well (mean of the last 30s ± standard error shown in red at beginning of plot). C. Switch. Data are shown as in (B). This group experienced three 5 min exposures to split-belts, interspersed with 5 min washout periods on day 1. Thus, the data plotted in green and yellow represent average values during the second and third exposures to split-belts, respectively. Mean baseline values for exposures 2 and 3 shown at the beginning of this plot represent mean step symmetry in the last 30s of the wash-out period preceding that exposure. D. Switch Control. Data are shown as in (C). In this group, subjects were given breaks instead of being washed-out between exposures to split-belts, thus baseline data for exposures 2 and 3 are not shown. E. Comparison of day 2 curves. Day 2 adaptation curves and plateau values for each group are shown. Note that all groups reach the same plateau, but differences are seen in the early change of adaptation, with No Washout showing the most improvement, followed by Switch, while No Switch and Switch Control show the least improvement. F. Exponential curve fits for day 2 curves. Average points are shown for the data presented in panel E. Single exponential time constants (t) with 95% confidence bounds are shown in the legend.
Figure 4.
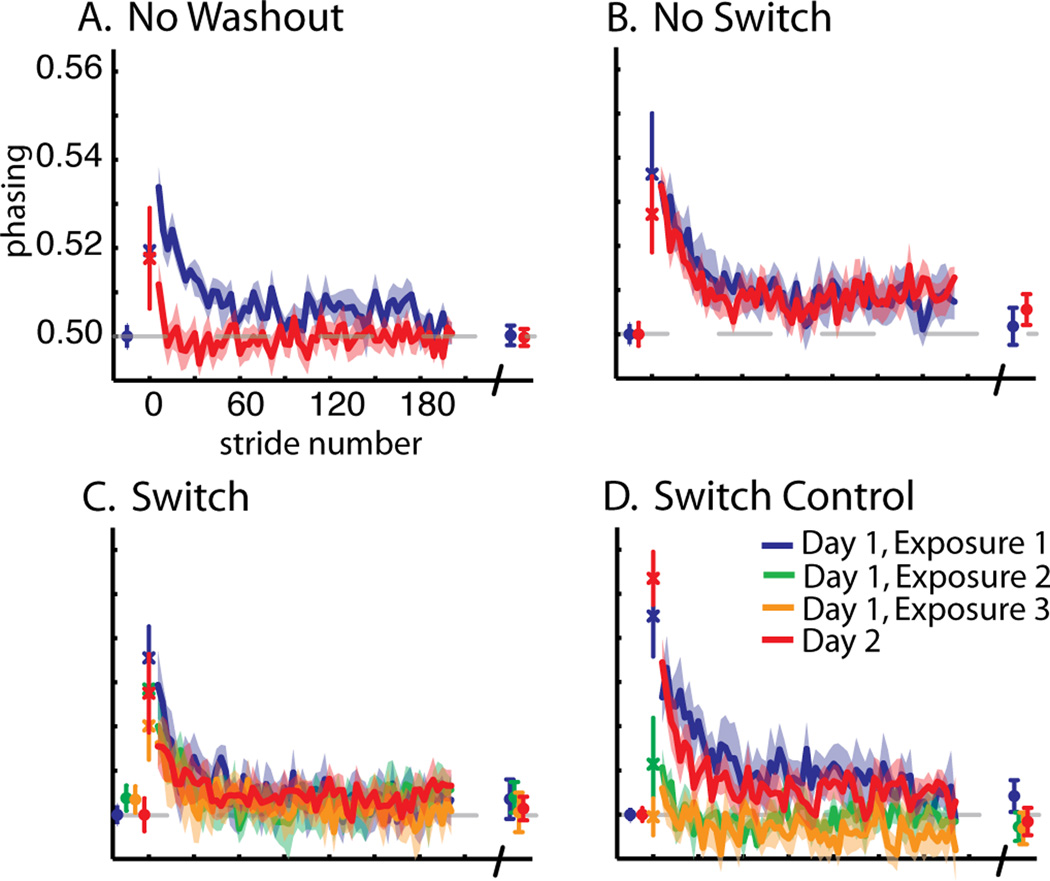
Comparison of phasing adaptation across groups. Averaged data for the No Washout (A), No Switch (B), Switch (C), and Switch Control (D) groups are shown as in Figure 2. Baseline, first stride, adaptation curves for a portion of training epochs (2–190), and final plateau values are shown.
In order to compare groups on day 2, we first assessed adaptation behavior and extent on day 1 to ensure that it was similar across groups. To quantify the initial perturbation we analyzed the first stride. No differences were found across groups: step symmetry (F(3,24)=0.28 p=0.84); center of oscillation difference (F(3,24)=0.43, p=0.74); phasing (F(3,24)=1.12, p=0.36); therefore, all groups were similarly perturbed. To assess early adaptation, we measured the early change (i.e. average of strides 2–30). No differences were found across groups: step symmetry (F(3,24)=0.27, p=0.85); center of oscillation difference (F(3,24)=0.32, p=0.81); phasing (F(3,24)=0.76, p=0.53). This indicates that subjects were perturbed and adapted similarly, regardless of group assignment. To verify that subjects adapted to similar levels by the end of training on day 1, we measured the plateau value (i.e. last thirty strides). The mean and standard errors of plateau values for each exposure are shown by the error bars at the end of the adaptation curves (only first 190 strides are shown) in Figures 2–4. For the ‘No Washout’ and ‘No Switch’ groups, where training was continuous for 15 minutes, this meant that we took the last thirty strides from the Day 1, Exposure 1 (blue symbols). For the ‘Switch’ and ‘Switch Control’ groups, we compared behavior after the complete 15 minutes of training (i.e. last 30 steps of the third exposure, yellow symbols). We found no differences in plateau in any of the parameters: step symmetry (F(3,24)=0.40, p=0.75), center of oscillation difference (F(3,24)=1.20, p=0.33), phasing (F(3,24)=0.40, p=0.76). Since all groups adapted similarly to the perturbation, and adapted the same amount on the first day, we concluded that day 1 behavior was the same across groups. In verifying that day 1 behavior was the same, we could assume that our measures assessing differences across days were due to changes in day 2 behavior.
Figure 2 shows our primary measure of step symmetry. Group means, smoothed by three strides, ± standard error are shown. We saw typical adaptation behavior on the first day, where subjects in all groups were initially perturbed by the split-belt and returned towards symmetric walking with increasing stride number. The first 190 steps (approximately five minutes) of adaptation are shown (in blue for day 1 and in red for day 2), averaging every three strides, with the final plateau shown at the end.
When subjects were brought back 24 hours later and immediately exposed to the split-belts (i.e. No Washout group, Figure 2a), the walking pattern was very well-retained (i.e. compare blue plateau with first stride of the red curve). Additionally, subjects were much less perturbed and adapted faster on the second day, seen by comparing blue and red curves in Figure 2a.
Since we found substantial retention the following day in the ‘No Washout’ group and were concerned about a ceiling effect, we included a washout prior to re-adaptation for the remaining groups so that we could assess improvements from training structure. That is, on day two we washed out the adaptation done on day one. This meant that all subjects would be in a baseline state, so that we could assess changes in re-adaptation. We used the average of the last thirty strides from this day 2 baseline period as the reference for day two behavior (i.e. red bars prior to adaptation, Figures 2–4). Following this wash-out, we then assessed re-adaptation to split-belts on the second day.
Figure 2b shows how subjects re-adapted to the perturbation in the ‘No Switch’ group. Subjects in this group showed a much smaller change across days, but still retained some memory from the previous day’s training, as evidenced by the difference between the blue and red curves. In the ‘Switch’ group, subjects were allowed to adapt and washout their walking pattern multiple times on the first day, so they would be forced to continue switching their walking pattern between the adapted and de-adapted state. Behavior for the ‘Switch’ group is shown in Figure 2c, with each exposure plotted. Note that these subjects were initially perturbed by the split-belts in each exposure (i.e. large errors in beginning of blue, green, and yellow curves in Figure 2c). Using a repeated measures ANOVA, we compared the plateau of the prior exposure to the first stride on the next exposure (e.g. blue plateau symbols and green first stride) and found a significant change (F(1,13)=48.02, p<0.001). Subjects then adapted to the perturbation with a reduction in errors as stride number increased. Interestingly, the rate at which they adapted became progressively faster with each exposure to the split-belts (i.e. compare blue, green, and yellow curves in Figure 2c). With a repeated measures ANOVA of early change values on day 1, we found an effect of exposure (Greenhouse-Geisser corrected: F(1.1,6.7)=12.97, p=0.008). When these subjects returned the second day, they adapted faster than in any of the exposures on the first day. To test if simply interspersing breaks, rather than wash-out periods, between split-belt exposures was sufficient to influence re-adaptation, we tested a ‘Switch Control’ group that sat for five minutes between adaptation epochs. We did not observe similar increases in errors from time alone between exposures as we did when split-belt periods were interspersed with tied-belt washout periods in the ‘Switch’ group (e.g., compare blue plateau to first point of green curve, Figure 2c&d). We did not find a significant change between plateau of one exposure to the first stride on the next exposure (F(1,13)=0.25, p=0.62).
Rather, subjects in the ‘Switch Control’ group returned to the adapted pattern they ended with, even after sitting for five minutes. In addition, while adaptation on day 2 was faster than in the first exposure to split-belts on day 1, it was comparable to the ‘No Switch’ group, and re-learning in ‘Switch Control’ was not as fast as in ‘Switch’.
Figure 2e shows a comparison of behavior on day 2 across the four groups. Re-learning appears to proceed faster in the ‘No Washout’ and ‘Switch’ groups, compared to ‘No Switch’ and ‘Switch Control’. We quantified the changes in step symmetry recall (i.e. difference in the first stride), re-learning (i.e. differences in early change), and performance (i.e. difference in plateau) across days in Figure 5a, b, & c.
Figure 5.
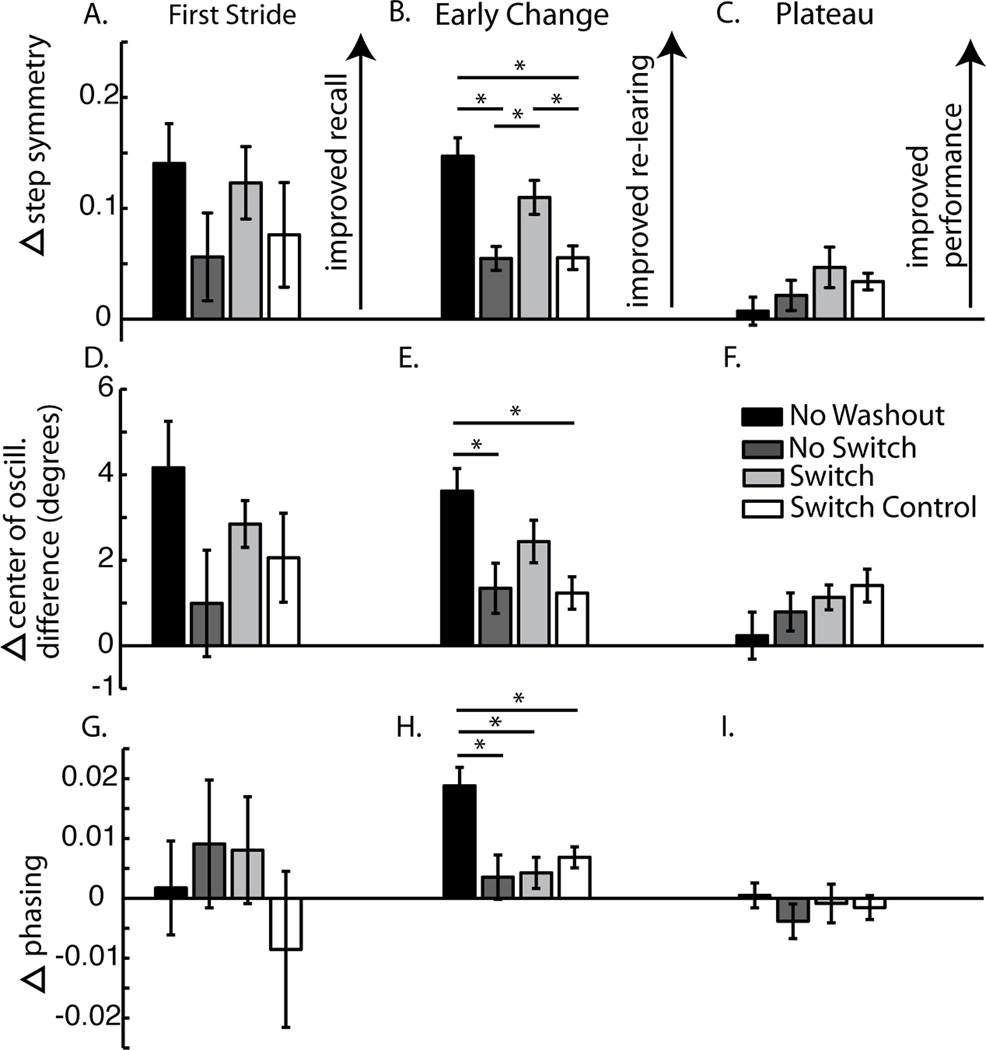
Difference between day 1 and 2 for first stride (shown in A, D, G), early change (average of strides 2–30 strides – shown in B,E, H) and plateau (average of last 30 strides – shown in C, F, I). Bars represent group means ± standard error and significant differences are denoted by asterisks. Step symmetry data are shown in A, B and C; center of oscillation in D, E and F; phasing in G, H and I. Note that there are no significant differences in the first stride (A, D, G) or plateau values for any of the measures (C,F, I); however, there are differences in early change (B, E, H). The differences between groups in early change values for center of oscillation (C) are similar to those seen in step symmetry (A), where the No Washout group shows the best recall and the No Switch and Switch Control group show the worst recall. In contrast, for phasing (E), the No Washout group still shows the most recall, but No Switch, Switch, and Switch Control groups show similar amounts of recall. Note: Higher values in Figure 5 mean larger changes across days. This normally means that subjects are closer to symmetry on day 2, but some subjects could over-adapt past symmetry, which would also result in larger delta values.
First, we found an effect of day on recall of step symmetry (F(1,24)=25.46, p<0.001). Subjects were perturbed significantly less in the first stride across days, which means that they were able to immediately recall some of the split-belt pattern. The no washout group looks to have the best recall, though we did not find an interaction of group × day on the first stride across days (F(3,24)=1.01, p=0.41). This means that although subjects on a whole were able to immediately recall the pattern, group assignment did not affect the first stride. One contributing factor to this result may be the high variability of the first stride across subjects. As such, we looked at the first 3 or first 5 strides to see if they might be less variable, and still suitable for measuring recall. Unfortunately, the different groups changed a different amount within the first 3 or 5 strides. For example, from stride 1 to 3, the no washout group changed 23% of their initial perturbation, the washout group 5%, the switch group 11% and the switch control group 6%. If we analyze the mean of the first 3 strides as reflecting “recall,” the effect is largely driven by this early change rather than what subjects recall on the first stride. This is also true for the first 5 strides. Given this information, we think that the first stride on day 2 is the best indicator of recall, despite variability.
Second, we found an effect of day on re-adaptation of step symmetry (F(1,24)=182.14, p<0.001). All groups showed a significant decrease in the early change across days (all p<0.01), signifying that all groups had improved re-learning on the second day. Third, there was a significant interaction effect found between groups for step symmetry early change across days (F(3,24)=10.95, p<0.001). As expected, we observed the fastest re-learning in the ‘No Washout’ group, since they immediately returned to split-belts on day 2. In post hoc analysis, the ‘No Washout’ group was significantly different from ‘No Switch’ (p<0.001) and ‘Switch Control’ (p<0.001), while there was a trend of a difference from ‘Switch’ (p=0.06). The ‘No Switch’ and ‘Switch Control’ groups were similar (p=0.97), thus time alone between exposures did not improve second day re-learning. The ‘Switch’ group was significantly different from both the ‘No Switch’ (p<0.01) and ‘Switch Control’ (p<0.01) groups. Finally, we found no differences in the changes in plateau values across groups (F(3,24)=1.55, p=0.23). Therefore, in sum we found that all groups recalled (i.e. first stride) the split pattern similarly. For the three groups that experienced a washout (‘No Switch’, ‘Switch’, and ‘Switch Control’), re-learning was best in the ‘Switch’ group, approaching similar early change values as what we observed when subjects were immediately re-exposed to the split-belts (i.e. ‘No Washout’ group). Despite this difference in re-learning (i.e. early change), all groups eventually converged to the same level of performance (i.e. plateau).
To ensure that our effect of re-learning wasn’t just due to a difference in the first stride, we used exponential curve fits to quantify step symmetry adaptation rates on day 2. A curve fit analysis which would assess re-learning rate without any influence from the starting point. The data with the fits overlaid are shown in Figure 2F. Time constants with 95% confidences intervals revealed that the ‘No Washout’ group re-learned the fastest (t=5.7 [3.36,8.04], r2=0.62), followed by ‘Switch’ (t=9.87 [8.40,11.37], r2=0.92), and then ‘No Switch’ (t=18.2 [15.2,21.1], r2=0.91), and ‘Switch Control’ (t=17.76 [15.03,20.52], r2=0.92).
Step symmetry can be influenced by changes in spatial (i.e. center of oscillation difference) and temporal (i.e. phasing) measures of coordination (see (Malone and Bastian 2010)). Center of oscillation difference adaptation curves are shown in Figure 3. Similar trends were noted where the ‘No Washout’ group demonstrated the fastest re-learning on day 2 (Figure 3a). In the three remaining groups, the ‘Switch’ group showed the fastest re-learning (Figure 3c) and the slowest re-learning was observed in the ‘No Switch’ and ‘Switch Control’ groups (Figures 3b&d). We found a significant effect of day on the first stride (F(1,24)=24.62, p<0.001), but not a significant interaction effect across groups (F(3,24)=1.74, p=0.19). Additionally, there was a significant effect of day for early change (F(1,24)=73.48, p<0.001). Post hoc results showed a significant decrease across days for all groups (all p<0.05). When assessing differences in early change across days (Figure 5c), we also found a significant interaction effect across groups (F(3,24)=4.89, p<0.01). Post hoc analysis revealed a significant difference between ‘No Washout’ and ‘No Switch’ (p<0.01), and ‘No Washout’ and ‘Switch Control’ (p<0.01), but not between the ‘No Washout’ and ‘Switch’ groups (p=0.11). As with step symmetry, we found that re-learning was faster with ‘Switch’ training compared with ‘No Switch’ and ‘Switch Control’ (Figure 5c), but these differences did not reach significance (p=0.14 and 0.10, respectively). There was no difference found between ‘No Switch’ and ‘Switch Control’ (p=0.88). Additionally, we found no difference in the center of oscillation difference plateau across groups (F(3,24)=1.20, p=0.33) (Figure 5d). Overall, we saw trends in the spatial elements of limb motions that were similar to the findings in step symmetry.
Figure 3.
Comparison of adaptation of center of oscillation difference across groups. Averaged data for the No Washout (A), No Switch (B), Switch (C), and Switch Control (D) groups are shown as in Figure 2. Baseline, first stride, adaptation curves for a portion of training epochs (2–190), and final plateau values are shown.
In contrast, we saw little effect of training in the temporal parameter, phasing. We did not find a significant effect of day (F(1,24)=0.25, p=0.62) or day × group (F(3,24)=0.61, p=0.61) on the first stride. However, we did find a day effect for early change (F(1,24)=33.76, p<0.001), but post hoc analysis only found a difference in the ‘No Washout’ (p<0.001) and ‘Switch Control’ (p=0.03) groups. The difference in early change values between day 1 and 2 did not reach significance for the ‘No Switch’ (p=0.23) and ‘Switch’ (p=0.15) groups. The ‘No Washout’ group re-adapts the temporal pattern much faster on the second day (Figure 4a), while the remaining three groups showed comparable re-learning (Figures 4b–d). For our early change measure, we found a significant effect across groups (F(3,24)=6.01, p<0.01), but this was due to the ‘No Washout’ group being significantly different from all other groups (all p<0.01) (Figure 5e). Similar to all the other parameters, we found no difference in the plateau for phasing (F(3,24)=0.40, p=0.76) (Figure 5f). Therefore, although subjects were able to retain the temporal pattern the second day (i.e. ‘No Washout’), the training method on the first day seemed to have little influence on phasing when subjects were washed out prior to re-adaptation.
Experiment 2
Here we tested the specificity of the learned walking pattern across days for step symmetry in three groups: ‘Single A,’ ‘Double A,’ and ‘A–B.’ We first made sure there was no difference in baseline walking across groups. Analyzing the last 30 seconds of the tied epoch we found no difference in step symmetry (F(2,21)=0.63, p=0.54). The mean baseline values from the last 30s of this tied epoch were subtracted from all subsequent data on day 1, thus referencing all data to each subject’s initial baseline asymmetry. The standard error of the baseline period is shown by the first points in Figures 6a–c (day 1 is blue; day 2 is red). The last thirty seconds of tied walking on day 2 were used as a second baseline period and the mean values were subtracted from subsequent values on day 2. We found no difference in the second baseline across groups in step symmetry (F(2,21)=1.11, p=0.35).
Figure 6.
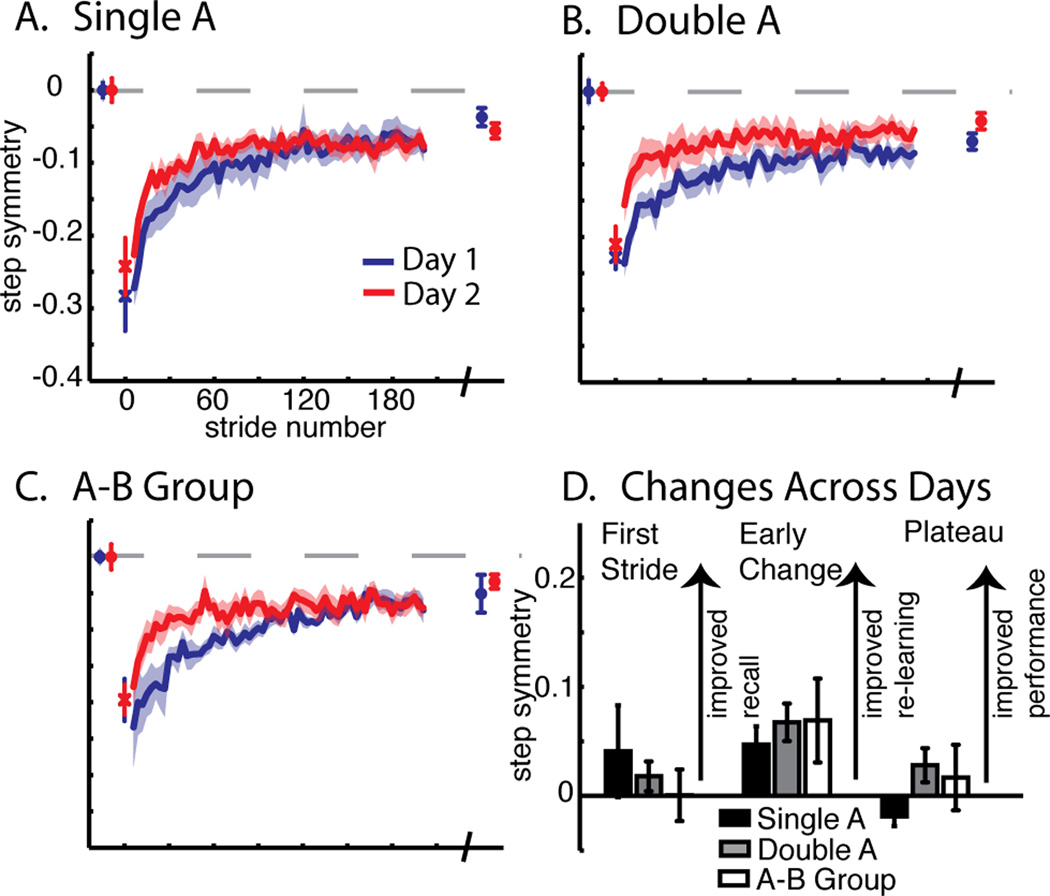
Comparison of adaptation of step symmetry across groups for Experiment 2. Averaged data for the Single A (A), Double A (B), and A–B Group (C), are shown as in Figure 2. Baseline, first stride, adaptation curves for a portion of training epochs (2–190), and final plateau values are shown. D. Difference between day 1 and 2 for first stride, early change (average of strides 2–30 strides) and plateau (average of last 30 strides). Bars represent group means ± standard error. No significant differences were found for any measure.
We assessed behavior on day 1 to ensure that it was the same across groups. Figure 6a–c shows step symmetry behavior in the same manner as Figure 2, with the first stride set apart, the first 190 steps of adaptation, and the final plateau shown by the symbols at the end. On day 1 there was no difference in the first stride (F(2,21)=1.92, p=0.17), early change (F(2,21)=0.13, p=0.88), or plateau (F(2,21)=0.79, p=0.47) for step symmetry. Therefore, day 1 adaptation perturbation, behavior, and extent were the same across groups.
Next we assessed how recall (i.e. difference in first stride), re-learning (i.e. difference in early change), and performance (i.e. difference in plateau) was influenced by a second fifteen minute exposure to the same split-belt perturbation (‘Double A’) or by the opposite split-belt perturbation (‘A–B’). We found there was not a significant group × day interaction in recall (F(2,21)=0.49, p=0.62), re-learning (F(2,21)=0.22, p=0.81), or plateau (F(2,21)=1.47, p=0.25). Therefore, as shown by the ‘Double A’ group, a second fifteen minute exposure to the same split-belt perturbation did not influence day 2 re-adaptation. Previous reaching studies of force field and visuomotor adaptation have shown that by asking subjects to learn two opposing tasks, day 2 behavior is worse for these subjects than those that learn only one adaptation (Caithness et al. 2004; Krakauer et al. 2005; Krakauer and Shadmehr 2006; Shadmehr and Brashers-Krug 1997). Perhaps surprisingly, with walking adaptation, we did not see these interference effects. When investigating the ‘A–B’ group, there was not any detrimental or enhanced effects from subjects experiencing the opposite split-belt perturbation compared to ‘Single A.’ Experiment 2 demonstrates that over-training with an additional fifteen minutes of the same or opposite split-belt perturbation does not change the re-adaptation on day 2.
DISCUSSION
We have shown that walking adaption is remembered day to day, and that the training schedule on day 1 affects re-learning on day 2. Subjects showed the fastest re-learning on day 2 when the adapted pattern was not washed-out between training days. In the groups where the adapted pattern was washed-out, re-learning was fastest when subjects adapted and de-adapted repeatedly in short intervals (i.e. switching) on day 1, and slower when subjects adapted once and continued training at the plateau. Adaptation to the opposite split-belt perturbation and ‘over-training’ (i.e. receiving two 15-minute split-belt periods) had no additional effect on day 2 re-learning—both looked similar to plateau training. Interestingly, in all cases recall (on the first stride) was present on day 2, but did not differ significantly across groups. Our interpretation is that training structure specifically affected re-learning (early change) of the pattern, and not recall (first stride).
We first assessed whether subjects retained the adapted pattern across days. While we expected some retention, we were surprised by how much subjects remembered: in ‘No Washout’, step symmetry early change was reduced from −0.18 on day 1 to −0.04 on day 2 (difference of 0.14), indicating an 80% improvement in re-learning across days. This demonstrates that the memory for this walking pattern is highly context-specific. We suspect that the calibration learned through adaptation was not used (therefore not washed out) in more natural situations, despite the fact that subjects walked as much as they liked between sessions. This is similar to reaching adaptation, which shows strong retention from day to day when no device-specific washout or alternate motor learning tasks are given (Caithness et al., 2004;Krakauer et al., 2005;Shadmehr and Brashers-Krug, 1997).
Subjects re-adapted faster to a perturbation even after the learned behavior was washed out (i.e. savings) which is similar to reaching studies (Caithness et al., 2004;Krakauer et al., 2005;Shadmehr and Brashers-Krug, 1997). Faster re-learning could imply that the nervous system retains a more permanent memory of the adapted pattern (Krakauer and Shadmehr 2006) or that it has learned about how to solve the problem more efficiently (Braun et al. 2009). Yet, our results differ from previous reaching studies in that experiencing the opposite perturbation on day 1 does not interfere with re-learning on day 2. In fact, we saw benefit from the opposite perturbation when it was performed within minutes of the initial adaptation. We speculate that subjects may have “learned to learn.” This type of phenomenon has been reported in finger sequence tasks and reaching adaptation studies whereby subjects are able to adapt more rapidly after repeated exposures to different perturbations (Miall et al. 2004; Seidler 2010). Through the exposure to multiple perturbations, subjects can learn abstract motor strategies that allow for flexibility and faster adaptation to future perturbations (Braun et al. 2010). Here subjects may have learned a bilateral rule that aids adaptation to any split-belt perturbation or that a mirror symmetric transformation could be applied to this locomotor task (i.e. the left leg does what the right was doing and vice versa). We suggest that subjects learned how to adapt to the general type of perturbation rather than being able to recall the actual solution, which we think is also consistent with the results from our switching experiment.
Switching locomotor patterns with repeated short exposures improved learning on the next day. Time between exposures alone could not account for this effect. We therefore think that the ‘Switch’ group benefitted from either repeatedly switching between adaptation and de-adaptation, more exposure to large errors, or a combination of both. It is interesting that the benefit of the switch training structure was not due to improved recall of the adapted pattern on the first stride; it was instead due to an improvement of re-learning (early change). This seems to contrast with prior work on prism adaptation training over many weeks (Martin et al. 1996). There we showed that subjects learn to improve recall of an adapted pattern from one day to the next, on the very first trial (Martin et al. 1996). However, in that study, there was a clear cue indicating the new context before the movement was made—i.e. prism goggles on versus off (Martin et al. 1996). But, despite the strong context cue, it should be noted that recall was incomplete after 1 week of training, and required 6 weeks of training to fully develop (Martin et al. 1996). So, improved recall may depend on how often the task is repeated.
One concern about the current study might be that there is no context cue to signal that the conditions are different before the treadmill belts begin to move, and therefore it may be hard to see recall on the first step. Yet, there is a proprioceptive ‘cue’ of split-belts speeds that could be processed within the first step (stance times typically take between 0.75 and 1.0 second). This is clearly sufficient to induce recall, since we see improvement on the first stride in all conditions. What we don’t see is a change in recall across training conditions. We are left to conclude that the main effect of the training condition is on re-learning, rather than recall in this study.
There are several regions of the nervous system that could contribute to day to day recall and re-learning. Two top candidate sites are the motor cortex and the cerebellum. Evidence for motor cortex involvement comes from experiments in which monkeys adapted reaching to a force field (Li et al. 2001). After washout of the adapted pattern, some neurons still remained in their adapted state, and therefore could assist in recall or more rapid re-learning. The cerebellum has been indicated via studies using reversible lesions during eye-blink conditioning (Medina et al. 2001). Those authors suggested that memories of perturbations are stored in the deep cerebellar nuclei because washout first affects cerebellar cortex, whereas the cerebellar nuclei circuits required much longer periods of extinction. They proposed that residual plasticity in the cerebellar nuclei may be activated upon re-exposure to the perturbation, resulting in faster re-learning (Medina et al. 2001).
We think that the cerebellum may be involved in the switching effects on re-learning that we observed in this study for a few reasons. First, using transcranial magnetic stimulation (TMS) we have found that the excitability of the cerebellum is specifically modulated during split-belt treadmill adaptation, where motor cortex excitability modulated with walking difficulty rather than adaptation (Jayaram et al. 2011). The cerebellum has also been shown to have greatest change in excitability during early adaptation (Galea et al. 2011), which is the period that was repeated in the switch training. We have also found that cerebellar damage impairs adaptation, particularly when perturbations are abruptly introduced and cause large errors, similar to what we see in the switch condition (Criscimagna-Hemminger et al. 2010). Finally, we know that locomotor adaptation is dependent on cerebellar integrity (Morton and Bastian 2006). Therefore, it is possible that cerebellar activity could be important for strengthening motor re-learning the following day from switch training.
It is also possible that specific cerebellar regions are involved, since switching had larger effects on the spatial versus temporal control of walking. We have previously suggested that spatial control may preferentially engage the intermediate cerebellar circuit and its connections to the cerebrum, whereas temporal control could utilize the midline cerebellar circuit and its connections to the midbrain and brainstem (Malone and Bastian 2010). It could be that cerebral-cerebellar circuits allow for more flexibility, and specifically the ability to re-learn more rapidly. We speculate that brainstem structures might be less flexible which might explain why temporal control was not influenced much by the training structure.
In sum, we have found that different adaptation training methods can influence the ease of re-learning on the next day. Switching repeatedly between adaptation and de-adaption on day 1 substantially improves re-learning on day 2. Additionally, adapting to an opposing pattern on day 1 does not interfere with re-learning on day 2—in fact, it still helps re-learning. Based on these two experiments, we suspect that the ease of re-learning reflects the fact that the subjects have somehow learned how to solve the problem more efficiently. Along these lines, we are now formally testing whether structural learning, or the ability to use general rules to improve learning of related tasks, is operational in walking (Braun et al. 2009). Other future work will be aimed at understanding how these phenomena influence long-term training, the generalization of the adapted pattern to other contexts, and most importantly motor re-learning in patient populations.
Acknowledgements
The authors would like to thank Ting Feng & Ajay Gurbani for assistance with data collection. This work was supported by NIH grant R01 HD048741.
Footnotes
Conflict of Interest: None
Reference List
- 1.Berman DE, Dudai Y. Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science. 2001;291:2417–2419. doi: 10.1126/science.1058165. [DOI] [PubMed] [Google Scholar]
- 2.Blandin Y, Proteau L, Alain C. On the cognitive processes underlying contextual interference and observational learning. J Mot Behav. 1994;26:18–26. doi: 10.1080/00222895.1994.9941657. [DOI] [PubMed] [Google Scholar]
- 3.Braun DA, Aertsen A, Wolpert DM, Mehring C. Motor task variation induces structural learning. Curr Biol. 2009;19:352–357. doi: 10.1016/j.cub.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun DA, Mehring C, Wolpert DM. Structure learning in action. Behav Brain Res. 2010;206:157–165. doi: 10.1016/j.bbr.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caithness G, Osu R, Bays P, Chase H, Klassen J, Kawato M, Wolpert DM, Flanagan JR. Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks. J Neurosci. 2004;24:8662–8671. doi: 10.1523/JNEUROSCI.2214-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- 7.Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci. 2007;10:1055–1062. doi: 10.1038/nn1930. [DOI] [PubMed] [Google Scholar]
- 8.Choi JT, Vining EP, Reisman DS, Bastian AJ. Walking flexibility after hemispherectomy: split-belt treadmill adaptation and feedback control. Brain. 2009;132:722–733. doi: 10.1093/brain/awn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010;103:2275–2284. doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Criscimagna-Hemminger SE, Shadmehr R. Consolidation patterns of human motor memory. J Neurosci. 2008;28:9610–9618. doi: 10.1523/JNEUROSCI.3071-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diedrichsen J, White O, Newman D, Lally N. Use-dependent and error-based learning of motor behaviors. J Neurosci. 2010;30:5159–5166. doi: 10.1523/JNEUROSCI.5406-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyon J, Owen AM, Petrides M, Sziklas V, Evans AC. Functional anatomy of visuomotor skill learning in human subjects examined with positron emission tomography. Eur J Neurosci. 1996;8:637–648. doi: 10.1111/j.1460-9568.1996.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 13.Galea JM, Vazquez A, Pasricha N, Orban d X, Celnik P. Dissociating the Roles of the Cerebellum and Motor Cortex during Adaptive Learning: The Motor Cortex Retains What the Cerebellum Learns. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron. 2011;70:787–801. doi: 10.1016/j.neuron.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inda MC, gado-Garcia JM, Carrion AM. Acquisition, consolidation, reconsolidation, and extinction of eyelid conditioning responses require de novo protein synthesis. J Neurosci. 2005;25:2070–2080. doi: 10.1523/JNEUROSCI.4163-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayaram G, Galea JM, Bastian AJ, Celnik P. Human Locomotor Adaptive Learning Is Proportional to Depression of Cerebellar Excitability. Cereb Cortex. 2011 doi: 10.1093/cercor/bhq263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol. 1997;77:1325–1337. doi: 10.1152/jn.1997.77.3.1325. [DOI] [PubMed] [Google Scholar]
- 18.Kandel ER. Cellular mechanisms of learning and the biological basis of individuality. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. New York: McGraw-Hill; 2000. pp. 1247–1279. [Google Scholar]
- 19.Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Science. 2001;294:1030–1048. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 20.Krakauer JW. Motor learning and consolidation: the case of visuomotor rotation. Adv Exp Med Biol. 2009;629:405–421. doi: 10.1007/978-0-387-77064-2_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krakauer JW, Ghez C, Ghilardi MF. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci. 2005;25:473–478. doi: 10.1523/JNEUROSCI.4218-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006;29:58–64. doi: 10.1016/j.tins.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li CS, Padoa-Schioppa C, Bizzi E. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron. 2001;30:593–607. doi: 10.1016/s0896-6273(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 24.Lin CH, Fisher BE, Winstein CJ, Wu AD, Gordon J. Contextual interference effect: elaborative processing or forgetting-reconstruction? A post hoc analysis of transcranial magnetic stimulation-induced effects on motor learning. J Mot Behav. 2008;40:578–586. doi: 10.3200/JMBR.40.6.578-586. [DOI] [PubMed] [Google Scholar]
- 25.Malone LA, Bastian AJ. Thinking about walking: Effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol. 2010;103:1954–1962. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain. 1996;119(Pt 4):1199–1211. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- 27.Medina JF, Garcia KS, Mauk MD. A mechanism for savings in the cerebellum. J Neurosci. 2001;21:4081–4089. doi: 10.1523/JNEUROSCI.21-11-04081.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miall RC, Jenkinson N, Kulkarni K. Adaptation to rotated visual feedback: a re-examination of motor interference. Exp Brain Res. 2004;154:201–210. doi: 10.1007/s00221-003-1630-2. [DOI] [PubMed] [Google Scholar]
- 29.Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- 30.Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94:2403–2415. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- 32.Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130:1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair. 2009;23:735–744. doi: 10.1177/1545968309332880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson EM, Pascual-Leone A, Miall RC. Current concepts in procedural consolidation. Nat Rev Neurosci. 2004;5:576–582. doi: 10.1038/nrn1426. [DOI] [PubMed] [Google Scholar]
- 35.Seidler RD. Neural correlates of motor learning, transfer of learning, and learning to learn. Exerc Sport Sci Rev. 2010;38:3–9. doi: 10.1097/JES.0b013e3181c5cce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shadmehr R, Brashers-Krug T. Functional stages in the formation of human long-term motor memory. J Neurosci. 1997;17:409–419. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shea JB, Morgan RL. Contextual interference effects on the acquisition, retention, and transfer of a motor skill. Journal of Experimental Psychology: Human Learning and Memory. 1979;5:179–187. [Google Scholar]
- 39.Simon DA. Contextual interference effects with two tasks. Percept Mot Skills. 2007;105:177–183. doi: 10.2466/pms.105.1.177-183. [DOI] [PubMed] [Google Scholar]
- 40.Squire LR, Kandel ER. Memory: From Mind to Molecules. New York: Sci. Am. Library; 1999. [Google Scholar]
- 41.Toni I, Krams M, Turner R, Passingham RE. The time course of changes during motor sequence learning: a whole-brain fMRI study. Neuroimage. 1998;8:50–61. doi: 10.1006/nimg.1998.0349. [DOI] [PubMed] [Google Scholar]
- 42.Vasudevan EV, Bastian AJ. Split-belt treadmill adaptation shows different functional networks for fast and slow human walking. J Neurophysiol. 2010;103:183–191. doi: 10.1152/jn.00501.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]