Abstract
Dementias are a varied group of disorders typically associated with memory loss, impaired judgment and/or language and by symptoms affecting other cognitive and social abilities to a degree that interferes with daily functioning. Alzheimer’s disease (AD) is the most common cause of a progressive dementia, followed by dementia with Lewy bodies (DLB), frontotemporal dementia (FTD), vascular dementia (VaD) and HIV associated neurocognitive disorders (HAND).
The pathogenesis of this group of disorders has been linked to the abnormal accumulation of proteins in the brains of affected individuals, which in turn has been related to deficits in protein clearance. Autophagy is a key cellular protein clearance pathway with proteolytic cleavage and degradation via the ubiquitin-proteasome pathway representing another important clearance mechanism. Alterations in the levels of autophagy and the proteins associated with the autophagocytic pathway have been reported in various types of dementias. This review will examine recent literature across these disorders and highlight a common theme of altered autophagy across the spectrum of the dementias.
Introduction
Neurodegenerative disorders of the aging population are clinically characterized by dementia and movement alterations; they affect over 10 million people in the US alone and represent the fifth most common cause of death for patients 65 and older (109). Dementia describes a group of symptoms affecting cognitive and social abilities severely enough to interfere with daily functioning. Dementia is usually associated with memory loss, impaired judgment and/or language (109).
Alzheimer’s disease (AD) is the most common cause of progressive dementia (33, 34), followed by dementia with Lewy bodies (DLB), frontotemporal dementia (FTD), vascular dementia (VaD) and HIV-associated neurocognitive disorder (HAND).
Most neurological disorders with dementia are characterized by progressive accumulation of misfolded proteins resulting in degeneration of selective synaptic circuitries in the neocortex, limbic system and cortico-striato nigral pathways. While the progressive accumulation of Aβ and Tau oligomers has been identified as a central pathogenic event in AD associated with synaptic dysfunction (81, 175), accumulation of α-synuclein (α-syn) and formation of oligomers has been linked to the pathogenesis of Parkinson’s Disease (PD) (43, 59, 88, 89, 93).
The pathology of AD and PD overlap in a heterogeneous group of conditions designated as Lewy body disease (LBD) (3, 16, 99, 115, 117). While in patients with DLB the clinical presentation is of dementia followed by parkinsonism, in patients with PD with dementia (PDD) the initial signs are of parkinsonism followed by dementia (71, 72, 100, 116). In PDD and the less common form of DLB, α-syn pathology predominates in both brainstem and cortical regions. In the more common form of DLB the pathological features of both AD and PD are present. Aβ promotes α-syn aggregation and toxicity in vivo (108), and Aβ and α-syn might directly interact (103) to form hybrid channel like structures (164).
Other disorders with cognitive impairment falling under the umbrella of FTD (54, 77) are usually characterized by the accumulation of Tau (45) or TDP43 (19, 42, 50, 157). Interestingly, in older individuals with chronic HIV infection, in addition to the neuroinflammatory process, proteins such as Aβ (4, 49, 123), Tau (25, 44, 130) and α-syn (78) accumulate in a distinct pattern. These patients exhibit neurodegeneration and neurocognitive disorders that in more severe instances can result in dementia.
Alterations in the rate of: 1) synthesis, 2) aggregation and 3) clearance of these proteins might be responsible for the formation of toxic Aβ, α-syn and Tau oligomers in AD, DLB, FTD and HAND (27) (Figure 1). Genetic mutations in familial cases and polymorphisms and environmental factors have been linked to alterations in the biosynthesis, aggregation and clearance. Clearance mechanisms include proteolytic cleavage, binding to chaperones and degradation via the ubiquitin-proteasome or lysosomal pathways. Of the lysosomal pathways, autophagy has become one of the most widely investigated. Autophagy is the major pathway involved in the degradation of long-lived proteins and organelles, cellular remodeling, and survival during nutrient starvation (82, 95). There are three distinct autophagic pathways (29, 86): i) macroautophagy, ii) microautophagy and iii) chaperone-mediated autophagy (CMA) (Figure 2). Autophagy has been linked to neuronal cell survival, death (23, 39) and transformation. Macroautophagy is constitutively active and highly efficient in neurons under physiological and disease conditions.
Figure 1.

Protein accumulation and subsequent neurodegeneration can result from an imbalance between the rate of synthesis, aggregation and clearance of unwanted and misfolded proteins. Dysregulation of either of these systems may be responsible for the formation of toxic Aβ, α-syn and Tau oligomers in neurodegenerative disorders.
Figure 2.

Clearance of unwanted or misfolded proteins from cells occurs via the autophagy/lysosomal pathway, the ubiquitin-proteasome or other proteolytic systems. The ubiquitin-proteasome system is the principal pathway that degrades soluble proteins, whereas the autophagy/lysosomal system is primarily responsible for clearing insoluble protein aggregates. There are three distinct autophagic pathways: Macroautophagy, microautophagy and chaperone-mediated autophagy (CMA).
In macroautophagy, organelles and macromolecular components are first surrounded by a double membrane, designated the autophagosome or autophagic vacuole (AV), which then fuses with lysosomes to form autolysosomes (Figure 3). The protein microtubule-associated protein 1, light chain 3 (LC3) is anchored to the vesicle membrane by conjugation to phosphatidylethanolamine (PE). While the un-conjugated LC3 is called LC3-I, the PE-conjugated LC3 is referred to as LC3-II and is a specific marker for autophagosomes (119). Autophagosomes then undergo several microtubule-(70) and dynein-dependent maturation events (80, 134, 141), including fusions with multivesicular bodies, early and/or late endosomes (11), before eventually fusing with lysosomes (37, 38). In microautophagy, the lysosome invaginates its own membrane, resulting in the uptake of segments of the cytoplasm (151). Finally, in CMA, individual proteins are targeted to lysosomes for degradation (102), CMA is tightly regulated, but can under extreme conditions result in cell death and carcinogenesis (47).
Figure 3.

The autophagy pathway is affected at different steps in the various types of dementias. AD is associated with a decreased expression of Beclin-1 leading to impaired autophagy initiation in addition to inhibited autophagosome degradation. PD and DLB are characterized by an increase in mTor and a decrease in Atg7 expression. These alterations are predicted to result in deficient initiation of the autophagic process, but this has not been experimentally established. Rare forms of FTD are caused by CHMP2B and VCP mutations, which lead to impaired autophagosome maturation. The HIV protein, Nef, may block autophagosome maturation by interacting with Beclin-1 leading to autophagy impairment in HIV-associated dementia. AD, Alzheimer’s disease; PD, Parkinson’s disease; DLB, Dementia with Lewy bodies; FTD, Frontotemporal dementia.
Autophagy is involved in the intracellular degradation of aggregation-prone α-syn (178) and huntingtin (136, 150) (Figure 3). Autophagic vacuoles have been identified in dystrophic neurites in AD brains and may be a site for Aβ production (184, 185). In parallel, elimination of basal neuronal autophagy is sufficient to cause neurodegeneration in the absence of other insults (55, 83). In neurodegenerative disorders such as AD, PD, Huntington’s disease (HD) (8, 128) and HAND, the autophagy pathway is deregulated and might contribute to the progressive accumulation of toxic proteins (Figure 3).
In this context, the main objective of this manuscript will be to review some of the studies in dementia patients and experimental models supporting a role for alterations in autophagy.
Autophagy alterations in Alzheimer’s Disease
Amyloidogenic processing of APP by secretases results in the release of APP C-terminal fragments (APP-CTF) and Aβ. Both contribute to the pathogenesis of AD and can display neurotoxic properties (22, 176). Alterations in autophagy might be implicated in the pathogenesis of AD by failing to clear aggregated Aβ and by playing a role in APP metabolism (Figure 4). Recent studies indicate that the autophagocytic pathology observed in AD most likely arises from impaired clearance of autophagic vacuoles (AVs) rather than strong autophagy induction alone (126–128, 184) suggesting selective alterations in molecular components of the autophagy pathway.
Figure 4.
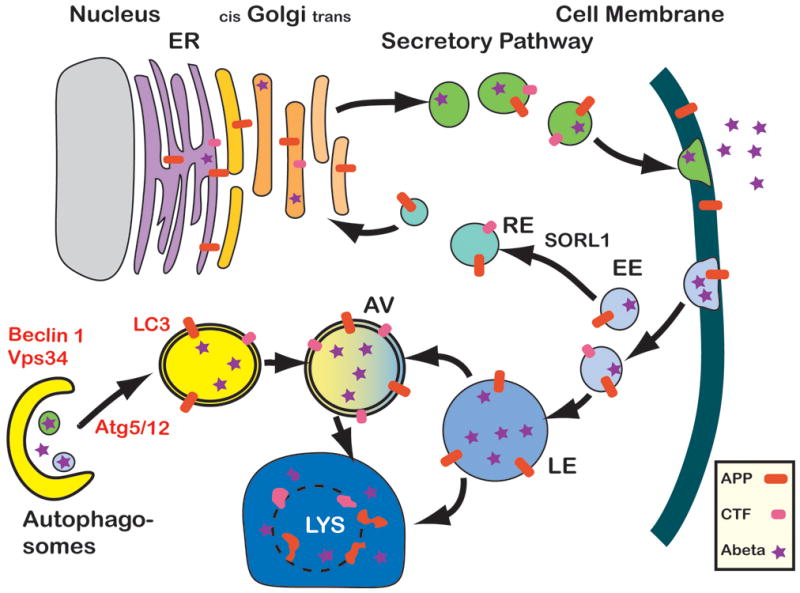
A possible role for Beclin 1 in AD. In healthy individuals, APP is transcribed in the endoplasmic reticulum (ER, grey), modified in the Golgi network and then shuttled to the cell surface through the secretory pathway. The cell can recycle APP through endocytosis. APP can then either be degraded through the autophagy - lysosome (Lys) system, or APP can be recycled via the recycling endosomes (RE) and enter the cycle again. In AD brains and Beclin 1 deficient cells, induction of autophagy (through the complex with Vps34) and autophagosomal degradation (potentially through a complex with an unknown binding partner) seem to be impaired. As a consequence, APP containing vesicles (endosomes, autophagosomes, and others) build up inside the cell. APP is increasingly cleaved by secretases and APP-CTF and Abeta are being generated and possibly released from the cell. The disruption of autophagosomal degradation includes an increasing accumulation of autophagosomes. This accumulation can serve as sites of Abeta generation, further inhibiting APP turnover and degradation. EE, early endosome; LE, late endosome, AV, Autophagic vesicle.
While it is still not completely clear how dysfunction of the autophagy pathway might contribute to neurodegeneration and AD, recent studies suggest a role for Beclin-1 in AD and mild cognitive impairment (131). Beclin-1 is the human homolog of the yeast autophagy protein Atg6 (73). Beclin-1 is necessary for autophagy (97, 132, 186, 187). It regulates the autophagy-promoting activity of Vps34 (188), and is involved in the recruitment of membranes to form autophagosomes. Beclin-1 also interacts with Bcl-2 (98) and may thus be involved in regulating cell death. Beclin-1 mRNA and protein are expressed in neurons and glia in human and mouse brains (98). Knockout mice lacking Beclin-1 (Bcn1−/−) die during embryogenesis (132, 187). In contrast, Bcn1+/− mice are viable. They have reduced autophagosome formation in skeletal muscle, bronchial epithelial cells and B lymphocytes (132), but the neuronal phenotype of these mice has not been characterized.
Recently, heterozygous deletion of Beclin-1 in mice has been reported to decrease neuronal autophagy and promote neuronal degeneration (131). Moreover, in a mouse model for AD, reduction of Beclin-1 expression results in increased accumulation of APP fragments and Aβ, increased neurodegeneration and increased inflammation (131). In contrast, gene therapy using locally injected lentivirus expressing Beclin-1 reduced amyloid pathology in APP transgenic (tg) mice. Autophagy protects neurons from Aβ induced cytotoxicity (64) and intracellular APP and APP-CTFs can be reduced by autophagy activation, indicating that the Beclin 1-PIK3C3 complex might play a role in Aβ clearance by regulating APP processing (68, 69).
Beclin-1 forms a core complex with the class III PI(3) kinase PIK3C3 (also known as Vps34) (79). Other proteins such as UVRAG, PIK3R4/Vps15, Atg14L, or Rubicon, join this complex depending on its physiological function in autophagy or endosomal trafficking (66, 96, 190). Beclin-1 and PIK3C3 mRNA and protein are expressed in human and mouse brains (67, 94). Thus, there are at least two aspects to autophagy pathology in AD (Figure 4). The first involves accumulation of abundant autophagosomes in dystrophic neurites (126, 128, 184) indicating impaired autophagosomal degradation (126). This has been confirmed in recent studies that detected increased levels of LC3-II in AD brains (28, 68). In the other end, Beclin-1 (131) and PIK3C3 are decreased in AD, suggesting impaired autophagy initiation in addition to inhibited autophagosomal degradation (Figure 3). This suggests a possible dual role of Beclin-1; one in autophagy initiation, in a complex with PIK3C3, and another in autophagosomal degradation, potentially in a complex with other proteins (68, 69, 190) (Figure 3).
Interestingly, while activation of autophagy with rapamycin has been reported to be protective in APP tg models (155), pharmacological inhibition of autophagosomal-lysosomal degradation with Bafilomycin A1 causes a comparable accumulation of APP and APP-metabolites in autophagosomes (53, 146). Although the mechanisms underlying the rapamycin findings are controversial (189), these data provide evidence that the autophagy pathway is altered in AD and modulating this pathway might represent a novel therapeutic approach for AD (Figure 4).
Alterations in autophagy in α-Synucleinopathies
Progressive accumulation of α-syn in selected regions of the CNS has been shown to play an important role in the neurodegenerative process in PD, LBD and other neurological conditions (58), for which the unifying term, synucleinopathies, has been proposed (56). In these disorders, the abnormal accumulation of α-syn is not limited to the striato-nigral system but also affects the limbic areas, the insula, frontal cortex and subcortical nuclei (14, 65, 106, 160). Fibrillar α-syn aggregates form LBs and Lewy neurites; the role of these inclusions is uncertain, however some studies suggest that they might represent a protective mechanism of isolating more toxic α-syn species (57).
Recent studies indicate that rather than the fibrils, α-syn oligomers that form protofibrils (26, 89) and probably pore-like structures (89) might be responsible for the characteristic synaptic damage and neuronal loss observed in LBD. While in familial forms of parkinsonism, mutations in the α-syn molecule (121, 173) and in other components involved in α-syn clearance (35) might promote and accelerate the formation of toxic α-syn intermediates, in sporadic forms of LBD the etiopathogenesis is less clear.
Therefore, impaired clearance of the α-syn aggregates might play an important role in the pathogenesis of PD and DLB (10, 30) (Figure 3). Among the lysosomal pathways involved, the autophagy signaling cascade has emerged as a key mechanism for the removal of α-syn aggregates. In PD recent studies have suggested that α-syn aggregates might interfere with the autophagy mechanisms and lead to neurodegeneration (30, 118, 120, 138, 140, 156). Mutant forms of α-syn found in familial PD patients (30) as well as oxidized forms of α-syn (105) found in sporadic PD and DLB have been shown to block autophagy, and α-syn contains a consensus sequence for CMA targeting (Figure 3). In neuronal cell cultures (181) and in tg mice, α-syn overexpression is associated with impaired autophagy and neurodegeneration that is reversed by Beclin-1 (154). Further supporting a role for lysosomal dysfunction in LBD, previous studies have shown that in lysosomal storage disorders such as Gaucher disease (161, 172) and Niemann-Pick disease (144), there is increased susceptibility to develop parkinsonism and α-syn accumulation.
Taken together, these lines of evidence suggest that in DLB and PD, specific molecular defects in the autophagy pathway might play a role in the pathogenesis of these disorders. In this respect, recent studies have shown that mTor levels were increased and Atg7 levels were reduced in the brains of patients with DLB and α-syn tg mice (Figure 3). Moreover, rapamycin treatment or viral-mediated delivery of Atg7 ameliorated α-syn accumulation and the related neuropathology. This is consistent with previous in vivo studies showing that rapamycin is neuroprotective in models of neurodegeneration (129, 135), AD (12) and HD (136, 145). Moreover, a recent study showed that blocking mTor by overexpression of the translation inhibitor Thor (4E-BP) could reduce the pathologic features in PD models, including degeneration of dopaminergic neurons in Drosophila (159). In addition, rapamycin activates 4E-BP in vivo and is also capable of ameliorating the pathology associated with mutations in other PD-associated genes such as Pink1 and parkin (159).
The mechanisms through which increased mTor and reduced Atg7 might participate in the neuropathology of DLB are not completely clear. Although there are mTor-independent pathways of autophagy induction, these alterations are predicted to result in deficient initiation of the autophagy process. This in turn might result in progressive accumulation of α-syn aggregates that further interfere with the fusion of lysosomes and formation of autophagosomes, as has been suggested by other studies (30, 105, 181). This may lead to the formation of enlarged and atypical AV-like structures (154). Consistent with these studies, recent evidence in cell-based models of PD-like pathology indicate that alterations in lysosomal functioning and autophagy might participate in the mechanisms of α-syn-mediated neurodegeneration (23, 30, 118, 120, 138, 140, 156).
Other animal studies have shown that deletion of Atg7 results in motor deficits and neurodegeneration (83, 84). Moreover, toxin models, such as animals exposed to the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), have also revealed autophagic dysfunction associated with alterations in signal transduction pathways (192). In addition, increased susceptibility to PD appears to be associated with polymorphisms in lysosomal genes such as those associated with Gaucher disease and Niemann-Pick disease. Moreover, recent studies have shown that reduced Cathepsin D expression results in α-syn accumulation and degeneration of the dopaminergic system in experimental models and in patients with PD (24). Cathepsin D is now considered one of the main lysosomal enzymes involved in α-syn degradation (149) and overexpression of Cathepsin D reduces the pathology associated with α-syn accumulation (31).
Multiple System Atrophy (MSA) is another neurodegenerative disorder characterized by abnormal accumulation of α-syn, however unlike the neuronal aggregation of α-syn observed in DLB and PD, in MSA α-syn accumulation is observed in oligodendrocytes in inclusions termed Glial Cell Inclusions (GCI). Despite the primarily oligodendrocytic accumulation of α-syn, MSA patients also display considerable neuronal loss in the striatum, cerebellum, brainstem and cortex, accompanied by astrogliosis, microgliosis and myelin loss (174, 183). Interestingly, a recent study has reported p62 immunoreactivity (a ubiquitin-associated autophagy substrate) in GCI’s of a MSA patient (110) highlighting the possibility that autophagocytic alterations may also play a role in this disorder.
In conclusion, these data support the notion that alterations in the autophagy pathway play a role in α-synucleinopathies such as DLB/PD and MSA and support the possibility that modulators of the autophagy pathway may also have potential therapeutic effects in these disorders (Figure 3).
Alterations in autophagy in Frontotemporal Dementia
Frontotemporal dementia is a neurodegenerative disorder characterized by progressive changes in behavior, personality, and language (13). The FTD condition is one of three syndromes caused by frontotemporal lobar degeneration (FTLD), and is the second most common early-onset dementia after Alzheimer’s Disease. Common neuropathological findings in FTD are atrophy and neuronal loss in the frontal and temporal lobes (13).
A high proportion of FTD cases have been reported to have a hereditary component and several pathogenic mutations have been identified to cause FTD. Mutations in tau (tau-positive FTD with parkinsonism, FTDP-17) or progranulin (tau-negative FTLD with ubiquitin-positive inclusions, FTLD-U) represent the most common forms of autosomal dominant inherited FTD (148). It is interesting to note that a number of mutations linked to FTD impact genes associated with later steps of autophagy including the CHMP2B (charged multivesicular body protein 2B) and VCP (valosin containing protein) genes (Figure 3).
Mutations in the CHMP2B gene are a rare cause of autosomal dominant FTD. One of the most widely studied examples of this mutation is FTD linked to chromosome 3 (FTD-3), which was discovered in a large Danish family (15, 51). This disease-causing mutation leads to C-terminal truncations of the CHMP2B protein, a component of ESCRT-III (endosomal sorting complex required for transport III) (Figure 3).
ESCRT-III is essential for formation of multivesicular bodies, which are late endosomal compartments formed through invagination and budding of vesicles into the lumen of endosomes (91). Defective ESCRT function leads to accumulation of cytoplasmic protein aggregates containing ubiquitin, p62 and TAR DNA binding protein 43 (TDP-43) (142). Recent studies using cellular and Drosophila models for HD have shown that reduced ESCRT levels inhibit the clearance of expanded polyglutamine aggregates and aggravate their neurotoxic effect (142).
Overexpression of C-terminally truncated CHMP2B in PC12 and human neuroblastoma cells produces enlarged endosomes, which disrupts normal endosomal trafficking and autophagic clearance of intracellular proteins (152, 171). Moreover, mutant CHMP2B has been shown to cause autophagosome accumulation, dendritic retraction, and subsequent neuronal cell loss in cultured cortical neurons, likely a result of insufficient fusion between autophagosomes and lysosomes (90). Furthermore, inhibition of autophagy delays neuronal cell loss caused by ESCRT-III dysfunction indicating that accumulation of autophagosomes is detrimental to the survival of neurons (92). Recently, it was shown that mutant CHMP2B impairs maturation of dendritic spines in cultured hippocampal neurons (9), a potential mechanism of neurodegeneration in FTD.
Another mutation that has been linked to FTD affects VCP/p97, one of the best characterized type II AAA (ATPases associated with diverse cellular activities) ATPases involved in vesicle fusion, proteasomal activity and autophagy (148) (Figure 3). Mutations in VCP are the cause of inclusion body myopathy associated with Paget’s disease of bone and frontotemporal dementia (IBMPFD) and a causative factor for amyotrophic lateral sclerosis (ALS). IBMPFD is a progressive, fatal genetic disorder with variable penetrance, predominately affecting muscle, bone, and brain (179). TDP-43 and ubiquitin are major components of inclusions characteristic of VCP-associated FTD, placing this disease in the group of neurodegenerative diseases termed TDP-43 proteinopathies (18).
Several studies have implicated VCP in the autophagy pathway. Expression of disease-associated VCP mutants (R155H and A232E) or overexpression of dominant-negative VCP in mouse embryonic fibroblasts cause accumulation of immature autophagic vesicles, indicating that VCP is essential for autophagosome maturation (163) (Figure 3). A Drosophila model of IBMPFD reveals that mutations of the VCP homolog, TER94, lead to redistribution of TDP-43 from the nucleus to the cytoplasm, replicating the pathological hallmark of IBMPFD and other TDP-43 proteinopathies (139). Mice expressing mutant VCP develop pathology similar to humans with IBMPFD including degeneration of muscle, bone and brain. Immunohistochemical analyses of these mice show progressive cytoplasmic accumulation of TDP-43 and ubiquitin-positive inclusion bodies in muscle and brain (7, 32). Moreover, LC3-II staining of brain sections from mice expressing VCP R155H reveals an accumulation of autophagosomes suggesting impaired autophagy in brain pathogenesis (7).
Although CHMP2B and VCP mutations are very rare among FTD patients, their involvement in the autophagy pathways may have important implications for FTD and other neurodegenerative diseases (Figure 3).
Alterations in autophagy in HIV-associated cognitive disorders
In the US, more than 1 million people are living with HIV and the aging population represents one of the fastest growing groups with HIV (1, 2, 147). The CDC estimates that by 2015, half of all Americans living with HIV will be over the age of 50 (1, 2, 153).
In the CNS, microglial cells have been identified as a primary reservoir for HIV-1 infection (41, 46, 52, 180) with productive infection also detected in astrocytes (20). With the advent of HAART, the abundance of active HIV in the brain and overt dementia has declined, however, as the number of treated subjects with chronic HIV infection increases, the prevalence of HAND is rising despite therapeutic intervention (48, 62, 63, 107, 113, 143, 170).
Neurocognitive alterations are among the most common disorders in HIV-infected patients (114), affecting 15–50% of HIV patients (62, 112), and susceptibility of the aging HIV population to cognitive alterations is becoming increasingly evident (114, 167, 168). For example, verbal memory, visual memory and psychomotor speed are more affected in HIV+ aged patients (166). The prevalence of HIV associated cognitive deficits is 7.2% in the >40 yr vs. 27.3% in the >50 yr group (2, 87). Therefore, identification of new targets that might protect the CNS from the toxic effects of HIV might be an important therapy for patients with HAND.
The mechanisms leading to neurodegeneration in HIV encephalitis (HIVE) might involve a variety of pathways including damage to the blood-brain barrier (BBB) (74, 182), excitotoxicity (61, 75), oxidative stress (122, 169), mitochondrial dysfunction (104, 165), calcium dysregulation (60, 111) and signaling alterations (36, 76, 101, 124, 133, 158, 177).
In the aging population with HIV, in addition to these factors other mechanisms might be at play. Among them, defective protein quality control might play a central role. During the aging process and in neurodegenerative disorders, defects on these clearance pathways may lead to progressive accumulation of misfolded proteins and formation of neurotoxic oligomers (28, 29, 128). In patients with HIVE, a number of studies have shown defects in proteasome function (40, 125), proteolysis (137) and autophagy (5, 6, 191) (Figure 3). We have recently shown that in the CNS of aged HIV human cases and in tg mice expressing HIV-gp120 protein (GFAP-gp120 tg) (162), abnormal functioning of neuronal autophagy (17, 21, 28, 184, 185) might result in accumulation of Aβ, α-syn and Tau. Neurodegeneration is also linked to defects in neuronal autophagy in recent studies of patients with HIVE and in the simian immunodeficiency virus encephalitis (SIVE) model (5, 6).
Likewise, recent reports have shown that autophagy in macrophages and neurons might be dysregulated in patients with HIV (5, 6, 191). Here, it is worth noting that while some studies have reported inhibition of neuronal autophagy in HIVE (5, 6, 191), we have reported an increase expression of autophagy markers (28). This apparent difference can be best explained by the possibility that in HIVE, although some markers of autophagy are increased, the overall process is actually blocked, resulting in the accumulation of molecular components of the autophagy pathway and formation of abnormal autophagosomes. Consistent with this observation, recent studies indicate that the HIV protein Nef blocks the maturation of autophagosomes (85) (Figure 3).
The mechanisms by which HIV proteins might interfere with autophagy during aging are unknown. One possibility is that HIV proteins released from infected microglia might interfere with neuronal autophagy by directly interacting with components of the autophagy pathway. Supporting this possibility, previous studies have shown that HIV1 exploits the autophagy pathway for biogenesis, that HIV1 can be eliminated via autophagy. Moreover, the HIV protein Nef acts as an anti-autophagic maturation factor (85) (Figure 3). In HIV-infected macrophages, Nef blocks autophagosome maturation, probably stabilizing an as yet unknown intracellular compartment for HIV biogenesis (85). Nef might interact with Beclin-1 in the UVRAG-Beclin-1-hVps34 complex to inhibit autophagosome maturation (85) by inhibiting lysosomal fusion and thereby affecting the membrane redistribution of hVps34 (Figure 3).
Conclusions
While there are a many different forms of dementia each characterized by a distinctive protein profile, they all share a common theme of abnormal protein aggregation linked to alterations in protein clearance mechanisms, most notably components of the autophagocytic pathway (Figure 3). Further studies into the involvement of autophagy in neurodegeneration may help elucidate common mechanisms underlying protein accumulation in these disorders and may have an impact on potential therapeutic interventions for these diseases.
Acknowledgments
This work was supported by NIH grants AG513, AG18440, AG022074, AG03197, AG010435, NS057096, MH62962, MH59745, NS44233
References
- 1.HIV/AIDS surveillance report. Centers for Disease Control; Atlanta: 2005. pp. 1–63. [Google Scholar]
- 2.HIV/AIDS surveillance report. Centers for Disease Control; Atlanta: 2007. pp. 1–54. [Google Scholar]
- 3.Aarsland D, Ballard CG, Halliday G. Are Parkinson’s disease with dementia and dementia with Lewy bodies the same entity? J Geriatr Psychiatry Neurol. 2004;17(3):137–45. doi: 10.1177/0891988704267470. [DOI] [PubMed] [Google Scholar]
- 4.Achim CL, Adame A, Dumaop W, Everall IP, Masliah E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4(2):190–9. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS ONE. 2008;3(8):e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alirezaei M, Kiosses WB, Fox HS. Decreased neuronal autophagy in HIV dementia: a mechanism of indirect neurotoxicity. Autophagy. 2008;4(7):963–6. doi: 10.4161/auto.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badadani M, Nalbandian A, Watts GD, Vesa J, Kitazawa M, Su H, Tanaja J, Dec E, Wallace DC, Mukherjee J, Caiozzo V, Warman M, Kimonis VE. VCP associated inclusion body myopathy and paget disease of bone knock-in mouse model exhibits tissue pathology typical of human disease. PLoS ONE. 2010;5(10) doi: 10.1371/journal.pone.0013183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahr BA, Bendiske J. The neuropathogenic contributions of lysosomal dysfunction. J Neurochem. 2002;83(3):481–9. doi: 10.1046/j.1471-4159.2002.01192.x. [DOI] [PubMed] [Google Scholar]
- 9.Belly A, Bodon G, Blot B, Bouron A, Sadoul R, Goldberg Y. CHMP2B mutants linked to frontotemporal dementia impair maturation of dendritic spines. Journal of cell science. 2010;123(Pt 17):2943–54. doi: 10.1242/jcs.068817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendiske J, Bahr BA. Lysosomal activation is a compensatory response against protein accumulation and associated synaptopathogenesis--an approach for slowing Alzheimer disease? J Neuropathol Exp Neurol. 2003;62(5):451–63. doi: 10.1093/jnen/62.5.451. [DOI] [PubMed] [Google Scholar]
- 11.Berg TO, Fengsrud M, Stromhaug PE, Berg T, Seglen PO. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem. 1998;273(34):21883–92. doi: 10.1074/jbc.273.34.21883. [DOI] [PubMed] [Google Scholar]
- 12.Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. 2008;28(27):6926–37. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boxer AL, Miller BL. Clinical features of frontotemporal dementia. Alzheimer Dis Assoc Disord. 2005;19(Suppl 1):S3–6. doi: 10.1097/01.wad.0000183086.99691.91. [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Braak E. Pathoanatomy of Parkinson’s disease. J neurol. 2000;247(Suppl 2):II3–10. doi: 10.1007/PL00007758. [DOI] [PubMed] [Google Scholar]
- 15.Brown J, Ashworth A, Gydesen S, Sorensen A, Rossor M, Hardy J, Collinge J. Familial non-specific dementia maps to chromosome 3. Human molecular genetics. 1995;4(9):1625–8. doi: 10.1093/hmg/4.9.1625. [DOI] [PubMed] [Google Scholar]
- 16.Burn DJ. Cortical Lewy body disease and Parkinson’s disease dementia. Curr Opin Neurol. 2006;19(6):572–9. doi: 10.1097/01.wco.0000247607.34697.a2. [DOI] [PubMed] [Google Scholar]
- 17.Butler D, Brown QB, Chin DJ, Batey L, Karim S, Mutneja MS, Karanian DA, Bahr BA. Cellular responses to protein accumulation involve autophagy and lysosomal enzyme activation. Rejuvenation Res. 2005;8(4):227–37. doi: 10.1089/rej.2005.8.227. [DOI] [PubMed] [Google Scholar]
- 18.Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, 3rd, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM, Mackenzie IR. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. The American journal of pathology. 2007;171(1):227–40. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, 3rd, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM, Mackenzie IR. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171(1):227–40. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll-Anzinger D, Al-Harthi L. Gamma interferon primes productive human immunodeficiency virus infection in astrocytes. J Virol. 2006;80(1):541–4. doi: 10.1128/JVI.80.1.541-544.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casarejos MJ, Solano RM, Gomez A, Perucho J, de Yebenes JG, Mena MA. The accumulation of neurotoxic proteins, induced by proteasome inhibition, is reverted by trehalose, an enhancer of autophagy, in human neuroblastoma cells. Neurochem Int. 2011;58(4):512–20. doi: 10.1016/j.neuint.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Chang KA, Suh YH. Pathophysiological roles of amyloidogenic carboxy-terminal fragments of the beta-amyloid precursor protein in Alzheimer’s disease. J Pharmacol Sci. 2005;97(4):461–71. doi: 10.1254/jphs.cr0050014. [DOI] [PubMed] [Google Scholar]
- 23.Chu CT. Autophagic stress in neuronal injury and disease. J Neuropathol Exp Neurol. 2006;65(5):423–32. doi: 10.1097/01.jnen.0000229233.75253.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35(3):385–98. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, Kauwe JS. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73(23):1982–7. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97(2):571–6. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crews L, Patrick C, Achim CL, Everall IP, Masliah E. Molecular Pathology of Neuro-AIDS (CNS-HIV) Int J Mol Sci. 2009;10(3):1045–63. doi: 10.3390/ijms10031045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, Hansen L, Adame A, Galasko D, Masliah E. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS ONE. 2010;5(2):e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14(2):70–7. doi: 10.1016/j.tcb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305(5688):1292–5. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 31.Cullen V, Lindfors M, Ng J, Paetau A, Swinton E, Kolodziej P, Boston H, Saftig P, Woulfe J, Feany MB, Myllykangas L, Schlossmacher MG, Tyynela J. Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo. Mol Brain. 2009;2(1):5. doi: 10.1186/1756-6606-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Custer SK, Neumann M, Lu H, Wright AC, Taylor JP. Transgenic mice expressing mutant forms VCP/p97 recapitulate the full spectrum of IBMPFD including degeneration in muscle, brain and bone. Human molecular genetics. 2010;19(9):1741–55. doi: 10.1093/hmg/ddq050. [DOI] [PubMed] [Google Scholar]
- 33.Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke RE, Hen R. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–9. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 35.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302(5646):819–22. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 36.Dewhurst S, Maggirwar SB, Schifitto G, Gendelman HE, Gelbard HA. Glycogen synthase kinase 3 beta (GSK-3 beta) as a therapeutic target in neuroAIDS. J Neuroimmune Pharmacol. 2007;2(1):93–6. doi: 10.1007/s11481-006-9051-1. [DOI] [PubMed] [Google Scholar]
- 37.Dunn WA., Jr Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J Cell Biol. 1990;110(6):1923–33. doi: 10.1083/jcb.110.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn WA., Jr Studies on the mechanisms of autophagy: maturation of the autophagic vacuole. J Cell Biol. 1990;110(6):1935–45. doi: 10.1083/jcb.110.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16(6):663–9. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Gelman BB, Nguyen TP. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: proteomic analysis of human synaptosomes. J Neuroimmune Pharmacol. 2010;5(1):92–102. doi: 10.1007/s11481-009-9168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R. The neuropathogenesis of the AIDS dementia complex. AIDS. 1997;11(Suppl A):S35–S45. [PubMed] [Google Scholar]
- 42.Geser F, Lee VM, Trojanowski JQ. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration: a spectrum of TDP-43 proteinopathies. Neuropathology. 2010;30(2):103–12. doi: 10.1111/j.1440-1789.2009.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290(5493):985–9. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 44.Gisslen M, Krut J, Andreasson U, Blennow K, Cinque P, Brew BJ, Spudich S, Hagberg L, Rosengren L, Price RW, Zetterberg H. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009;9:63. doi: 10.1186/1471-2377-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goedert M, Spillantini MG. Pathogenesis of the Tauopathies. J Mol Neurosci. 2011 doi: 10.1007/s12031-011-9593-4. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 47.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23(16):2891–906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 48.Gray F, Chretien F, Vallat-Decouvelaere AV, Scaravilli F. The changing pattern of HIV neuropathology in the HAART era. J Neuropathol Exp Neurol. 2003;62(5):429–40. doi: 10.1093/jnen/62.5.429. [DOI] [PubMed] [Google Scholar]
- 49.Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19(4):407–11. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- 50.Grossman M, Wood EM, Moore P, Neumann M, Kwong L, Forman MS, Clark CM, McCluskey LF, Miller BL, Lee VM, Trojanowski JQ. TDP-43 pathologic lesions and clinical phenotype in frontotemporal lobar degeneration with ubiquitin-positive inclusions. Arch Neurol. 2007;64(10):1449–54. doi: 10.1001/archneur.64.10.1449. [DOI] [PubMed] [Google Scholar]
- 51.Gydesen S, Hagen S, Klinken L, Abelskov J, Sorensen SA. Neuropsychiatric studies in a family with presenile dementia different from Alzheimer and Pick disease. Acta Psychiatr Scand. 1987;76(3):276–84. doi: 10.1111/j.1600-0447.1987.tb02896.x. [DOI] [PubMed] [Google Scholar]
- 52.Haas DW, Clough LA, Johnson BW, Harris VL, Spearman P, Wilkinson GR, Fletcher CV, Fiscus S, Raffanti S, Donlon R, McKinsey J, Nicotera J, Schmidt D, Shoup RE, Kates RE, Lloyd RM, Jr, Larder B. Evidence of a source of HIV type 1 within the central nervous system by ultraintensive sampling of cerebrospinal fluid and plasma. AIDS Res Hum Retroviruses. 2000;16(15):1491–502. doi: 10.1089/088922200750006010. [DOI] [PubMed] [Google Scholar]
- 53.Haass C, Capell A, Citron M, Teplow DB, Selkoe DJ. The vacuolar H(+)-ATPase inhibitor bafilomycin A1 differentially affects proteolytic processing of mutant and wild-type beta-amyloid precursor protein. J Biol Chem. 1995;270(11):6186–92. doi: 10.1074/jbc.270.11.6186. [DOI] [PubMed] [Google Scholar]
- 54.Haberland C. Frontotemporal dementia or frontotemporal lobar degeneration--overview of a group of proteinopathies. Ideggyogy Sz. 2010;63(3–4):87–93. [PubMed] [Google Scholar]
- 55.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 56.Hardy J, Gwinn-Hardy K. Genetic classification of primary neurodegenerative disease. Science. 1998;282(5391):1075–9. doi: 10.1126/science.282.5391.1075. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto M, Kawahara K, Bar-On P, Rockenstein E, Crews L, Masliah E. The Role of alpha-synuclein assembly and metabolism in the pathogenesis of Lewy body disease. J Mol Neurosci. 2004;24(3):343–52. doi: 10.1385/JMN:24:3:343. [DOI] [PubMed] [Google Scholar]
- 58.Hashimoto M, Masliah E. Alpha-synuclein in Lewy body disease and Alzheimer’s disease. Brain Pathol. 1999;9(4):707–20. doi: 10.1111/j.1750-3639.1999.tb00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hashimoto M, Rockenstein E, Masliah E. Transgenic models of alpha-synuclein pathology: past, present, and future. Ann N Y Acad Sci. 2003;991:171–88. [PubMed] [Google Scholar]
- 60.Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S55–61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- 61.Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78(3):457–67. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 62.Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hung SY, Huang WP, Liou HC, Fu WM. Autophagy protects neuron from Abeta-induced cytotoxicity. Autophagy. 2009;5(4):502–10. doi: 10.4161/auto.5.4.8096. [DOI] [PubMed] [Google Scholar]
- 65.Hurtig H, Trojanowski J, Galvin J, Ewbank D, Schmidt M, Clark C, Glosser G, Stern M, Gollomp S, Arnold S. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology. 2000;54:1916–21. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- 66.Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;19(12):5360–72. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaeger PA, Pickford F, Sun C-H, Lucin K, Masliah E, Wyss-Coray T. Regulation of amyloid precursor protein processing by the beclin 1 complex. 2009 doi: 10.1371/journal.pone.0011102. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaeger PA, Pickford F, Sun CH, Lucin KM, Masliah E, Wyss-Coray T. Regulation of amyloid precursor protein processing by the Beclin 1 complex. PLoS ONE. 2010;5(6):e11102. doi: 10.1371/journal.pone.0011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaeger PA, Wyss-Coray T. Beclin 1 complex in autophagy and Alzheimer disease. Arch Neurol. 2010;67(10):1181–4. doi: 10.1001/archneurol.2010.258. [DOI] [PubMed] [Google Scholar]
- 70.Jahreiss L, Menzies FM, Rubinsztein DC. The itinerary of autophagosomes: from peripheral formation to kiss-and-run fusion with lysosomes. Traffic. 2008;9(4):574–87. doi: 10.1111/j.1600-0854.2008.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Janvin CC, Larsen JP, Salmon DP, Galasko D, Hugdahl K, Aarsland D. Cognitive profiles of individual patients with Parkinson’s disease and dementia: comparison with dementia with lewy bodies and Alzheimer’s disease. Mov Disord. 2006;21(3):337–42. doi: 10.1002/mds.20726. [DOI] [PubMed] [Google Scholar]
- 72.Jellinger KA, Attems J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol (Berl) 2006;112(3):253–60. doi: 10.1007/s00401-006-0088-2. [DOI] [PubMed] [Google Scholar]
- 73.Kametaka S, Okano T, Ohsumi M, Ohsumi Y. Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae. J Biol Chem. 1998;273(35):22284–91. doi: 10.1074/jbc.273.35.22284. [DOI] [PubMed] [Google Scholar]
- 74.Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27(1):123–34. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 76.Kehn-Hall K, Guendel I, Carpio L, Skaltsounis L, Meijer L, Al-Harthi L, Steiner JP, Nath A, Kutsch O, Kashanchi F. Inhibition of Tat-mediated HIV-1 replication and neurotoxicity by novel GSK3-beta inhibitors. Virology. 2011;415(1):56–68. doi: 10.1016/j.virol.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kertesz A. Frontotemporal dementia, Pick’s disease. Ideggyogy Sz. 2010;63(1–2):4–12. [PubMed] [Google Scholar]
- 78.Khanlou N, Moore DJ, Chana G, Cherner M, Lazzaretto D, Dawes S, Grant I, Masliah E, Everall IP. Increased frequency of alpha-synuclein in the substantia nigra in human immunodeficiency virus infection. J Neurovirol. 2009;15(2):131–8. doi: 10.1080/13550280802578075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2(4):330–5. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kimura S, Noda T, Yoshimori T. Dynein-dependent movement of autophagosomes mediates efficient encounters with lysosomes. Cell Struct Funct. 2008;33(1):109–22. doi: 10.1247/csf.08005. [DOI] [PubMed] [Google Scholar]
- 81.Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24(4):219–24. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 82.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–21. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–4. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 84.Komatsu M, Wang QJ, Holstein GR, Friedrich VL, Jr, Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A. 2007;104(36):14489–94. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kyei GB, Dinkins C, Davis AS, Roberts E, Singh SB, Dong C, Wu L, Kominami E, Ueno T, Yamamoto A, Federico M, Panganiban A, Vergne I, Deretic V. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol. 2009;186(2):255–68. doi: 10.1083/jcb.200903070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Larsen KE, Sulzer D. Autophagy in neurons: a review. Histol Histopathol. 2002;17(3):897–908. doi: 10.14670/HH-17.897. [DOI] [PubMed] [Google Scholar]
- 87.Larussa D, Lorenzini P, Cingolani A, Bossolasco S, Grisetti S, Bongiovanni M, Moretti F, Uccella I, Zannoni P, Foresti S, Mazzarello G, Arcidiacono MI, Pedale R, Ammassari A, Tozzi V, Perno CF, Monforte AD, Cinque P, Antinori A. Highly active antiretroviral therapy reduces the age-associated risk of dementia in a cohort of older HIV-1-infected patients. AIDS Res Hum Retroviruses. 2006;22(5):386–92. doi: 10.1089/aid.2006.22.386. [DOI] [PubMed] [Google Scholar]
- 88.Lashuel HA, Hartley DM, Balakhaneh D, Aggarwal A, Teichberg S, Callaway DJ. New class of inhibitors of amyloid-beta fibril formation. Implications for the mechanism of pathogenesis in Alzheimer’s disease. J Biol Chem. 2002;277(45):42881–90. doi: 10.1074/jbc.M206593200. [DOI] [PubMed] [Google Scholar]
- 89.Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT., Jr Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322(5):1089–102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 90.Lee JA, Beigneux A, Ahmad ST, Young SG, Gao FB. ESCRT-III dysfunction causes autophagosome accumulation and neurodegeneration. Curr Biol. 2007;17(18):1561–7. doi: 10.1016/j.cub.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 91.Lee JA, Gao FB. Roles of ESCRT in autophagy-associated neurodegeneration. Autophagy. 2008;4(2):230–2. doi: 10.4161/auto.5384. [DOI] [PubMed] [Google Scholar]
- 92.Lee JA, Gao FB. Inhibition of autophagy induction delays neuronal cell loss caused by dysfunctional ESCRT-III in frontotemporal dementia. J Neurosci. 2009;29(26):8506–11. doi: 10.1523/JNEUROSCI.0924-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee M, Hyun D, Halliwell B, Jenner P. Effect of the overexpression of wild-type or mutant alpha-synuclein on cell susceptibility to insult. J Neurochem. 2001;76(4):998–1009. doi: 10.1046/j.1471-4159.2001.00149.x. [DOI] [PubMed] [Google Scholar]
- 94.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 95.Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120(2):159–62. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Liang C, Sir D, Lee S, Ou JH, Jung JU. Beyond autophagy: the role of UVRAG in membrane trafficking. Autophagy. 2008;4(6):817–20. doi: 10.4161/auto.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672–6. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 98.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72(11):8586–96. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lippa CF, Duda JE, Grossman M, Hurtig HI, Aarsland D, Boeve BF, Brooks DJ, Dickson DW, Dubois B, Emre M, Fahn S, Farmer JM, Galasko D, Galvin JE, Goetz CG, Growdon JH, Gwinn-Hardy KA, Hardy J, Heutink P, Iwatsubo T, Kosaka K, Lee VM, Leverenz JB, Masliah E, McKeith IG, Nussbaum RL, Olanow CW, Ravina BM, Singleton AB, Tanner CM, Trojanowski JQ, Wszolek ZK. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68(11):812–9. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- 100.Litvan I, MacIntyre A, Goetz CG, Wenning GK, Jellinger K, Verny M, Bartko JJ, Jankovic J, McKee A, Brandel JP, Chaudhuri KR, Lai EC, D’Olhaberriague L, Pearce RK, Agid Y. Accuracy of the clinical diagnoses of Lewy body disease, Parkinson disease, and dementia with Lewy bodies: a clinicopathologic study. Arch Neurol. 1998;55(7):969–78. doi: 10.1001/archneur.55.7.969. [DOI] [PubMed] [Google Scholar]
- 101.Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J Neurochem. 1999;73(2):578–86. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- 102.Majeski AE, Dice JF. Mechanisms of chaperone-mediated autophagy. Int J Biochem Cell Biol. 2004;36(12):2435–44. doi: 10.1016/j.biocel.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 103.Mandal PK, Pettegrew JW, Masliah E, Hamilton RL, Mandal R. Interaction between Abeta peptide and alpha synuclein: molecular mechanisms in overlapping pathology of Alzheimer’s and Parkinson’s in dementia with Lewy body disease. Neurochem Res. 2006;31(9):1153–62. doi: 10.1007/s11064-006-9140-9. [DOI] [PubMed] [Google Scholar]
- 104.Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, Nath A, Cass WA. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem. 2002;83(4):955–63. doi: 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- 105.Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, Dauer W, Przedborski S, Ischiropoulos H, Lansbury PT, Sulzer D, Cuervo AM. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118(2):777–88. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marui W, Iseki E, Nakai T, Miura S, Kato M, Ueda K, Kosaka K. Progression and staging of Lewy pathology in brains from patients with dementia with Lewy bodies. J Neurol Sci. 2002;195(2):153–9. doi: 10.1016/s0022-510x(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 107.Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART) J Neurol Neurosurg Psychiatry. 2000;69(3):376–80. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98(21):12245–50. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maslow K. 2010 Alzheimer’s disease facts and figures. Alzheimers Dement. 2010;6(2):158–94. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 110.Masui K, Nakata Y, Fujii N, Iwaki T. Extensive distribution of glial cytoplasmic inclusions in an autopsied case of multiple system atrophy with a prolonged 18-year clinical course. Neuropathology. 2011 doi: 10.1111/j.1440-1789.2011.01222.x. [DOI] [PubMed] [Google Scholar]
- 111.Mattson MP. Oxidative stress, perturbed calcium homeostasis, and immune dysfunction in Alzheimer’s disease. J Neurovirol. 2002;8(6):539–50. doi: 10.1080/13550280290100978. [DOI] [PubMed] [Google Scholar]
- 112.McArthur J, Hoover D, Bacellar H, Miller E, Cohen B, Becker J, Graham N, McArthur J, Selnes O, Jacobson L, Visscher B, Concha M, Saah A. Dementia in AIDS patients: incidence and risk factors. Neurology. 1993;43:2245–52. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 113.McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9(2):205–21. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 114.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67(6):699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 115.McKeith IG. Spectrum of Parkinson’s disease, Parkinson’s dementia, and Lewy body dementia. Neurol Clin. 2000;18(4):865–902. doi: 10.1016/s0733-8619(05)70230-9. [DOI] [PubMed] [Google Scholar]
- 116.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 117.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 118.Meredith GE, Totterdell S, Petroske E, Santa Cruz K, Callison RC, Jr, Lau YS. Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson’s disease. Brain Res. 2002;956(1):156–65. doi: 10.1016/s0006-8993(02)03514-x. [DOI] [PubMed] [Google Scholar]
- 119.Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36(12):2491–502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 120.Nakajima T, Takauchi S, Ohara K, Kokai M, Nishii R, Maeda S, Takanaga A, Tanaka T, Takeda M, Seki M, Morita Y. Alpha-synuclein-positive structures induced in leupeptin-infused rats. Brain Res. 2005;1040(1–2):73–80. doi: 10.1016/j.brainres.2005.01.099. [DOI] [PubMed] [Google Scholar]
- 121.Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M. Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. JBiolChem. 1999;274:9843–6. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 122.Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–8. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 123.Nebuloni M, Pellegrinelli A, Ferri A, Bonetto S, Boldorini R, Vago L, Grassi MP, Costanzi G. Beta amyloid precursor protein and patterns of HIV p24 immunohistochemistry in different brain areas of AIDS patients. AIDS. 2001;15(5):571–5. doi: 10.1097/00002030-200103300-00005. [DOI] [PubMed] [Google Scholar]
- 124.New DR, Maggirwar SB, Epstein LG, Dewhurst S, Gelbard HA. HIV-1 Tat induces neuronal death via tumor necrosis factor-alpha and activation of non-N-methyl-D-aspartate receptors by a NFkappaB-independent mechanism. J Biol Chem. 1998;273(28):17852–8. doi: 10.1074/jbc.273.28.17852. [DOI] [PubMed] [Google Scholar]
- 125.Nguyen TP, Soukup VM, Gelman BB. Persistent hijacking of brain proteasomes in HIV-associated dementia. Am J Pathol. 2010;176(2):893–902. doi: 10.2353/ajpath.2010.090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120(Pt 23):4081–91. doi: 10.1242/jcs.019265. [DOI] [PubMed] [Google Scholar]
- 127.Nixon RA, Cataldo AM. Lysosomal system pathways: genes to neurodegeneration in Alzheimer’s disease. J Alzheimers Dis. 2006;9(3 Suppl):277–89. doi: 10.3233/jad-2006-9s331. [DOI] [PubMed] [Google Scholar]
- 128.Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64(2):113–22. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- 129.Pan T, Kondo S, Zhu W, Xie W, Jankovic J, Le W. Neuroprotection of rapamycin in lactacystin-induced neurodegeneration via autophagy enhancement. Neurobiol Dis. 2008;32(1):16–25. doi: 10.1016/j.nbd.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 130.Patrick C, Crews L, Desplats P, Dumaop W, Rockenstein E, Achim CL, Everall IP, Masliah E. Increased CDK5 expression in HIV encephalitis contributes to neurodegeneration via tau phosphorylation and is reversed with Roscovitine. Am J Pathol. 2011;178(4):1646–61. doi: 10.1016/j.ajpath.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118(6):2190–9. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112(12):1809–20. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rappaport J, Joseph J, Croul S, Alexander G, Del Valle L, Amini S, Khalili K. Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. J Leukoc Biol. 1999;65(4):458–65. doi: 10.1002/jlb.65.4.458. [DOI] [PubMed] [Google Scholar]
- 134.Ravikumar B, Acevedo-Arozena A, Imarisio S, Berger Z, Vacher C, O’Kane CJ, Brown SD, Rubinsztein DC. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat Genet. 2005;37(7):771–6. doi: 10.1038/ng1591. [DOI] [PubMed] [Google Scholar]
- 135.Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet. 2002;11(9):1107–17. doi: 10.1093/hmg/11.9.1107. [DOI] [PubMed] [Google Scholar]
- 136.Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36(6):585–95. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 137.Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19(2):127–35. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- 138.Rideout HJ, Lang-Rollin I, Stefanis L. Involvement of macroautophagy in the dissolution of neuronal inclusions. Int J Biochem Cell Biol. 2004;36(12):2551–62. doi: 10.1016/j.biocel.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 139.Ritson GP, Custer SK, Freibaum BD, Guinto JB, Geffel D, Moore J, Tang W, Winton MJ, Neumann M, Trojanowski JQ, Lee VM, Forman MS, Taylor JP. TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30(22):7729–39. doi: 10.1523/JNEUROSCI.5894-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rockenstein E, Schwach G, Ingolic E, Adame A, Crews L, Mante M, Pfragner R, Schreiner E, Windisch M, Masliah E. Lysosomal pathology associated with alpha-synuclein accumulation in transgenic models using an eGFP fusion protein. J Neurosci Res. 2005;80(2):247–59. doi: 10.1002/jnr.20446. [DOI] [PubMed] [Google Scholar]
- 141.Rubinsztein DC, Ravikumar B, Acevedo-Arozena A, Imarisio S, O’Kane CJ, Brown SD. Dyneins, autophagy, aggregation and neurodegeneration. Autophagy. 2005;1(3):177–8. doi: 10.4161/auto.1.3.2050. [DOI] [PubMed] [Google Scholar]
- 142.Rusten TE, Simonsen A. ESCRT functions in autophagy and associated disease. Cell Cycle. 2008;7(9):1166–72. doi: 10.4161/cc.7.9.5784. [DOI] [PubMed] [Google Scholar]
- 143.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(Suppl 2):115–21. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- 144.Saito Y, Suzuki K, Hulette C, Murayama S. Aberrant phosphorylation of alpha-synuclein in human Niemann-Pick type C1 disease. J Neuropathol Exp Neurol. 2004;63(4):323–8. doi: 10.1093/jnen/63.4.323. [DOI] [PubMed] [Google Scholar]
- 145.Sarkar S, Rubinsztein DC. Small molecule enhancers of autophagy for neurodegenerative diseases. Mol Biosyst. 2008;4(9):895–901. doi: 10.1039/b804606a. [DOI] [PubMed] [Google Scholar]
- 146.Schrader-Fischer G, Paganetti PA. Effect of alkalizing agents on the processing of the beta-amyloid precursor protein. Brain Res. 1996;716(1–2):91–100. doi: 10.1016/0006-8993(96)00002-9. [DOI] [PubMed] [Google Scholar]
- 147.Scott JC, Woods SP, Carey CL, Weber E, Bondi MW, Grant I. Neurocognitive consequences of HIV infection in older adults: an evaluation of the “cortical” hypothesis. AIDS Behav. 2011;15(6):1187–96. doi: 10.1007/s10461-010-9815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Seelaar H, Rohrer JD, Pijnenburg YA, Fox NC, van Swieten JC. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. Journal of neurology, neurosurgery, and psychiatry. 2011;82(5):476–86. doi: 10.1136/jnnp.2010.212225. [DOI] [PubMed] [Google Scholar]
- 149.Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47(36):9678–87. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shibata M, Lu T, Furuya T, Degterev A, Mizushima N, Yoshimori T, MacDonald M, Yankner B, Yuan J. Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J Biol Chem. 2006;281(20):14474–85. doi: 10.1074/jbc.M600364200. [DOI] [PubMed] [Google Scholar]
- 151.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306(5698):990–5. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, Nielsen JE, Hodges JR, Spillantini MG, Thusgaard T, Brandner S, Brun A, Rossor MN, Gade A, Johannsen P, Sorensen SA, Gydesen S, Fisher EM, Collinge J. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nature genetics. 2005;37(8):806–8. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 153.Smith G. Statement of Senator Gordon H Smith. Aging hearing: HIV over fifty, exploring the new threat. Senate Committee on Aging; Washington, DC. 2005. [Google Scholar]
- 154.Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29(43):13578–88. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS ONE. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA. Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci. 2001;21(24):9549–60. doi: 10.1523/JNEUROSCI.21-24-09549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Steinacker P, Hendrich C, Sperfeld AD, Jesse S, von Arnim CA, Lehnert S, Pabst A, Uttner I, Tumani H, Lee VM, Trojanowski JQ, Kretzschmar HA, Ludolph A, Neumann M, Otto M. TDP-43 in cerebrospinal fluid of patients with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Arch Neurol. 2008;65(11):1481–7. doi: 10.1001/archneur.65.11.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sui Z, Sniderhan LF, Fan S, Kazmierczak K, Reisinger E, Kovacs AD, Potash MJ, Dewhurst S, Gelbard HA, Maggirwar SB. Human immunodeficiency virus-encoded Tat activates glycogen synthase kinase-3beta to antagonize nuclear factor-kappaB survival pathway in neurons. Eur J Neurosci. 2006;23(10):2623–34. doi: 10.1111/j.1460-9568.2006.04813.x. [DOI] [PubMed] [Google Scholar]
- 159.Tain LS, Mortiboys H, Tao RN, Ziviani E, Bandmann O, Whitworth AJ. Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat Neurosci. 2009;12(9):1129–35. doi: 10.1038/nn.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Takeda A, Hashimoto M, Mallory M, Sundsmo M, Hansen L, Sisk A, Masliah E. Abnormal distribution of the non-Ab component of Alzheimer’s disease amyloid precursor/a-synuclein in Lewy body disease as revealed by proteinase K and formic acid pretreatment. LabInvest. 1998;78:1169–77. [PubMed] [Google Scholar]
- 161.Tayebi N, Callahan M, Madike V, Stubblefield BK, Orvisky E, Krasnewich D, Fillano JJ, Sidransky E. Gaucher disease and parkinsonism: a phenotypic and genotypic characterization. Mol Genet Metab. 2001;73(4):313–21. doi: 10.1006/mgme.2001.3201. [DOI] [PubMed] [Google Scholar]
- 162.Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367(6459):188–93. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 163.Tresse E, Salomons FA, Vesa J, Bott LC, Kimonis V, Yao TP, Dantuma NP, Taylor JP. VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy. 2010;6(2):217–27. doi: 10.4161/auto.6.2.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tsigelny IF, Crews L, Desplats P, Shaked GM, Sharikov Y, Mizuno H, Spencer B, Rockenstein E, Trejo M, Platoshyn O, Yuan JX, Masliah E. Mechanisms of hybrid oligomer formation in the pathogenesis of combined Alzheimer’s and Parkinson’s diseases. PLoS ONE. 2008;3(9):e3135. doi: 10.1371/journal.pone.0003135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 165.Turchan J, Pocernich CB, Gairola C, Chauhan A, Schifitto G, Butterfield DA, Buch S, Narayan O, Sinai A, Geiger J, Berger JR, Elford H, Nath A. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology. 2003;60(2):307–14. doi: 10.1212/01.wnl.0000042048.85204.3d. [DOI] [PubMed] [Google Scholar]
- 166.Valcour V. Aging with HIV. Lessons from CROI 2009. Posit Aware. 2009;20(3):37–9. [PubMed] [Google Scholar]
- 167.Valcour V, Paul R. HIV infection and dementia in older adults. Clin Infect Dis. 2006;42(10):1449–54. doi: 10.1086/503565. [DOI] [PubMed] [Google Scholar]
- 168.Valcour V, Paul R, Neuhaus J, Shikuma C. The Effects of Age and HIV on Neuropsychological Performance. J Int Neuropsychol Soc. 2011;17(1):190–5. doi: 10.1017/S1355617710001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Valcour V, Shiramizu B. HIV-associated dementia, mitochondrial dysfunction, and oxidative stress. Mitochondrion. 2004;4(2–3):119–29. doi: 10.1016/j.mito.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 170.Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep. 2011;8(1):54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.van der Zee J, Urwin H, Engelborghs S, Bruyland M, Vandenberghe R, Dermaut B, De Pooter T, Peeters K, Santens P, De Deyn PP, Fisher EM, Collinge J, Isaacs AM, Van Broeckhoven C. CHMP2B C-truncating mutations in frontotemporal lobar degeneration are associated with an aberrant endosomal phenotype in vitro. Human molecular genetics. 2008;17(2):313–22. doi: 10.1093/hmg/ddm309. [DOI] [PubMed] [Google Scholar]
- 172.Varkonyi J, Rosenbaum H, Baumann N, MacKenzie JJ, Simon Z, Aharon-Peretz J, Walker JM, Tayebi N, Sidransky E. Gaucher disease associated with parkinsonism: four further case reports. Am J Med Genet A. 2003;116(4):348–51. doi: 10.1002/ajmg.a.10028. [DOI] [PubMed] [Google Scholar]
- 173.Volles MJ, Lansbury PT., Jr Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson’s disease. Biochemistry. 2003;42(26):7871–8. doi: 10.1021/bi030086j. [DOI] [PubMed] [Google Scholar]
- 174.Wakabayashi K, Takahashi H. Cellular pathology in multiple system atrophy. Neuropathology. 2006;26(4):338–45. doi: 10.1111/j.1440-1789.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 175.Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept Lett. 2004;11(3):213–28. doi: 10.2174/0929866043407174. [DOI] [PubMed] [Google Scholar]
- 176.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101(5):1172–84. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 177.Wang JY, Grabacka M, Marcinkiewicz C, Staniszewska I, Peruzzi F, Khalili K, Amini S, Reiss K. Involvement of alpha1beta1 integrin in insulin-like growth factor-1-mediated protection of PC12 neuronal processes from tumor necrosis factor-alpha-induced injury. J Neurosci Res. 2006;83(1):7–18. doi: 10.1002/jnr.20712. [DOI] [PubMed] [Google Scholar]
- 178.Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278(27):25009–13. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 179.Weihl CC, Pestronk A, Kimonis VE. Valosin-containing protein disease: inclusion body myopathy with Paget’s disease of the bone and fronto-temporal dementia. Neuromuscul Disord. 2009;19(5):308–15. doi: 10.1016/j.nmd.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wiley CA, Baldwin M, Achim CL. Expression of HIV regulatory and structural mRNA in the central nervous system. AIDS. 1996;10(8):843–7. doi: 10.1097/00002030-199607000-00007. [DOI] [PubMed] [Google Scholar]
- 181.Xilouri M, Vogiatzi T, Vekrellis K, Park D, Stefanis L. Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PLoS ONE. 2009;4(5):e5515. doi: 10.1371/journal.pone.0005515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang B, Akhter S, Chaudhuri A, Kanmogne GD. HIV-1 gp120 induces cytokine expression, leukocyte adhesion, and transmigration across the blood-brain barrier: modulatory effects of STAT1 signaling. Microvasc Res. 2009;77(2):212–9. doi: 10.1016/j.mvr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yoshida M. Multiple system atrophy: alpha-synuclein and neuronal degeneration. Neuropathology. 2007;27(5):484–93. doi: 10.1111/j.1440-1789.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- 184.Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Naslund J, Mathews PM, Cataldo AM, Nixon RA. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol. 2005;171(1):87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yu WH, Kumar A, Peterhoff C, Shapiro Kulnane L, Uchiyama Y, Lamb BT, Cuervo AM, Nixon RA. Autophagic vacuoles are enriched in amyloid precursor protein-secretase activities: implications for beta-amyloid peptide overproduction and localization in Alzheimer’s disease. Int J Biochem Cell Biol. 2004;36(12):2531–40. doi: 10.1016/j.biocel.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 186.Yue Z, Horton A, Bravin M, DeJager PL, Selimi F, Heintz N. A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron. 2002;35(5):921–33. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- 187.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100(25):15077–82. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119(Pt 2):259–70. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- 189.Zhang S, Salemi J, Hou H, Zhu Y, Mori T, Giunta B, Obregon D, Tan J. Rapamycin promotes beta-amyloid production via ADAM-10 inhibition. Biochem Biophys Res Commun. 2010;398(3):337–41. doi: 10.1016/j.bbrc.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhong Y, Wang QJ, Yue Z. Atg14L and Rubicon: yin and yang of Beclin 1-mediated autophagy control. Autophagy. 2009;5(6):890–1. doi: 10.4161/auto.9162. [DOI] [PubMed] [Google Scholar]
- 191.Zhou D, Masliah E, Spector SA. Autophagy is increased in postmortem brains of persons with HIV-1-associated encephalitis. J Infect Dis. 2011;203(11):1647–57. doi: 10.1093/infdis/jir163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhu JH, Horbinski C, Guo F, Watkins S, Uchiyama Y, Chu CT. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am J Pathol. 2007;170(1):75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]


