Abstract
Despite accumulating knowledge of porcine macrophages and dendritic cells (DCs) from in vitro studies, information regarding monocytes/macrophages and DCs in lymphoid tissues of enteric pathogen-infected neonatal animals in vivo is limited. In this study we evaluated the influence of commensal bacterial [two strains of lactic acid bacteria (LAB), Lactobacillus acidophilus and L. reuteri] colonization and rotavirus infection on distribution and frequencies of monocytes/macrophages and conventional DCs (cDCs) in ileum, spleen and blood. Gnotobiotic pigs were inoculated with LAB and virulent Wa strain human rotavirus (HRV) (LAB+HRV+), HRV only (LAB−HRV+), LAB only (LAB+HRV−) or mock (LAB−HRV−). The cDCs were characterized as SWC3+CD11R1+, whereas monocytes/macrophages were identified as SWC3+CD11R1− by flow cytometry in the gnotobiotic pigs at 10 days of age. Infection with HRV alone activated/recruited significantly more monocytes/macrophages to the intestine than LAB colonization and 56% versus 28% of these cells expressed CD14. Colonization with LAB alone also significantly increased the frequencies of monocytes/macrophages and cDCs and the CD14 expression on monocytes/macrophages in ileum and spleen compared to the controls. LAB colonization plus HRV infection significantly reduced macrophage and cDC frequencies in spleen compared to LAB colonization or HRV infection alone, suggesting that LAB colonization down-regulated HRV− infection-induced monocyte/macrophage activation/recruitment at the systemic lymphoid tissue. These results illustrated the distribution of porcine monocytes/macrophages and cDCs and the frequencies of CD14 expression on these cells in intestinal and systemic lymphoid tissues in the early stage of immune responses to intestinal colonization by LAB versus infection by an enteric pathogen HRV and will facilitate further in vivo studies on functional characterization of these immune cells in neonates.
Keywords: Dendritic cells, Monocytes/macrophages, Rotavirus, Lactic acid bacteria, Lactobacillus acidophilus, Lactobacillus reuteri, Gnotobiotic pigs
1. Introduction
Dendritic cells (DCs) and monocytes/macrophages are professional antigen-presenting cells. They express class II major histocompatibility complex (MHC II) gene products, typically process and present antigen to T cells, and stimulate T cell activation. The principal function of DCs is activation of naïve T cells and initiation of primary T cell responses (Cavanagh and Von Andrian, 2002). DCs can also activate B cells directly. Immature DCs capture microbial antigens and migrate to secondary lymphoid tissues. During this process, DCs mature to become efficient at presenting antigens and stimulating naïve T cells (Paillot et al., 2001; Randolph et al., 2005). The DCs also influence the balance between Th1 and Th2 type immunity, as well as oral tolerance (Dudda et al., 2005; Kalinski et al., 1999; Kronin et al., 2000).
Monocytes (in blood) and macrophages (in tissues) are mononuclear phagocytes, which interact on afferent and efferent aspects with adaptive immune response cells in providing initiation, regulatory and effector functions. Intestinal macrophages in lamina propria are derived from blood monocytes and they play a unique role as effector cells of the innate immune system. In the normal intestine, resident lamina propria macrophages are non-inflammatory but retain avid scavenger and host defense functions (Smith et al., 2005). In rotavirus infections, macrophages may play a role in extraintestinal infection. Rotavirus proteins were detected in macrophages in both intestinal and extraintestinal lymphoid tissues (Brown and Offit, 1998). Recent studies suggest that the rotavirus non-structural protein (NSP) 1 induces interferon regulatory factor (IRF) 7 degradation, which may allow rotavirus to move across the gut barrier by enabling the virus to replicate in specialized trafficking cells (DCs and macrophages) that constitutively express IRF7 (Barro and Patton, 2007). Rotavirus replication was confirmed in DC and potentially in macrophages and B cells of mice (Fenaux et al., 2006).
The objectives of this study were to elucidate the influence of rotavirus infection, commensal lactic acid bacteria (LAB) colonization, and the combined effect of rotavirus and LAB on the early postnatal ontogeny of innate immune responses in gnotobiotic (Gn) pigs by determining the distribution and frequencies of monocytes/macrophages and conventional dendritic cells (cDCs) and CD14 expression on these cells in the intestinal and systemic lymphoid tissues. The neonatal Gn pig model represents a unique model to study the effects of colonization by commensals and infection by rotavirus on development of the innate immune system because gnotobiotic status of the animals avoids confounding factors from extraneous enteric bacteria and viruses. Gnotobiotic pigs are devoid of maternal antibodies thus Gn pigs represent an “immunologically virgin” model (Tlaskalova-Hogenova et al., 2004). Gnotobiotic pig model of human rotavirus (HRV) infection and disease has been used extensively in studies of rotavirus pathogenesis, immunity and vaccines (Yuan and Saif, 2002). However, induction and development of innate immune responses to HRV in pigs have not been elucidated. LAB is major components of the commensal microbial flora in the gut of humans and pigs and is commonly used as probiotics (Ahrne et al., 1998). They have been shown to significantly stimulate gut epithelial cell proliferation (Ichikawa et al., 1999) and enhance non-specific and specific immunity in children or young lab animals (Fukushima et al., 1998; Herias et al., 1999; Yasui et al., 1999). Several strains of LAB, including Lactobacillus acidophilus and L. reuteri have been shown to reduce the risk, severity or duration of rotavirus diarrhea (Van Niel et al., 2002); however the mechanisms are undefined. Our hypothesis is that neonatal Gn pigs with or without LAB colonization and infected with HRV develop different monocyte/macrophage and cDC responses in intestinal and systemic lymphoid tissues. In addition, LAB colonization and/or HRV infection influences expression of CD14 on these cells differently.
In pigs, although monocytes/macrophages and DCs in various tissues have been studied, no single specific markers are known that allow definitive identification. The most frequently detected marker on porcine monocytes/macrophages and DCs is SWC3 (CD172a) (Bautista et al., 2002; Bimczok et al., 2006, 2005; Carrasco et al., 2001; Chamorro et al., 2005; Domenech et al., 2003; Haverson et al., 1994, 2000; Jamin et al., 2006; McCullough et al., 1997; Rehakova et al., 1998; Salmon et al., 2000; Summerfield et al., 2003). It is a member of the signal regulatory protein family and associates with protein–tyrosine phosphatase SHP-1 (Alvarez et al., 2000). In addition to SWC3, CD11b (CD11R1) is a marker specifically and differentially expressed on porcine DCs, but not on monocytes/macrophages (Bimczok et al., 2006, 2005; Haverson et al., 2000; Jamin et al., 2006). Several subsets of DCs have been reported in pigs (Bimczok et al., 2005; Jamin et al., 2006). In pig intestinal lymphoid tissues, four subsets were identified as SWC3+CD11b+, SWC3−CD11b+, SWC3+CD11b−, and SWC3−CD11b−, but all DCs emigrating from the intestine via lymphatic vessels in pigs are CD11b positive (SWC3+CD11b+ and SWC3− CD11b+) (Bimczok et al., 2005). Two major subsets of DCs, cDCs (SWC3+CD4−) and plasmacytoid DCs (pDCs) (SWC3+CD4+) were identified in peripheral blood mononuclear cells (MNC) of pigs (Summerfield et al., 2003). Jamin et al studied the phenotypic characteristics of cDCs and pDCs in tonsil, mesenteric lymph nodes, spleen and blood MNC of pigs and identified cDCs as SWC3+CD11R1+ and pDCs as SWC3+CD4+ (Jamin et al., 2006). In a resent study, the pDCs are more clearly defined as SWC3lowCD4+ (Gonzales et al., 2007). Besides monocytes/macrophages and DCs, the pan-myeloid marker SWC3 is also expressed on granulocytes. However, selective gating on forward scatter/side scatter (FSC/SSC) can separate most SWC3+ granulocytes from monocytes/macrophages (Summerfield et al., 2001). In this study, we used SWC3 and CD11R1 to define cDCs as SWC3+CD11R1+ and monocytes/macrophages as SWC3+CD11R1−.
The CD14 is a specific receptor that is expressed on subsets of monocytes and macrophages and to a lesser extent on neutrophils (Antal-Szalmas et al., 1997). The CD14 is also expressed on monocytes-derived DCs (Carrasco et al., 2001). Study of differentiation of porcine myeloid bone marrow haematopoietic cell populations suggests that CD14 is a maturation-dependent antigen and study of CD14 expression may be useful for assessment of cell maturity (Summerfield et al., 2001). It has been shown that CD14 plays an essential role in uptake of substrates by cells and interacts with ligands, including bacterial (i.e. LPS, peptidoglycan and phosphatidylinositol) and non-bacterial products (i.e. PolyI:C) to enhance ligand-mediated cell activation (Dunzendorfer et al., 2004; Dziarski et al., 2000; Wang and Munford, 1999). Porcine respiratory coronavirus infection induced marked increase of CD14+ monocytes/macrophages in the lung tissues of Gn pigs (Van Gucht et al., 2006, 2005). Recent findings showed that CD14 directly interacts with intracellular TLR3 and enhances dsRNA-mediated TLR3 activation by aiding uptake of dsRNA into cells. The CD14−/− mice exhibited impaired responses to PolyI:C and reduced production of inflammatory cytokines (Lee et al., 2006). These suggest that CD14 may play a role in initiation of innate immune responses to dsRNA, such as PolyI:C and dsRNAviruses, including rotavirus. Increased CD14 expression may indicate maturation and/or increased activity (e.g. TLR activation and antigen presentation) of innate immune cells. Thus we choose to study CD14 expression as a functional marker of innate immunity. Little is known about the expression of CD14 on DCs and monocytes/macrophages in neonatal pigs after rotavirus infection and how commensal/probiotic bacterial colonization influences the expression pattern of CD14.
2. Materials and methods
2.1. Experimental design
Gn pigs from two sows were derived near term by hysterectomy and maintained in sterile isolation units as described previously (Meyer et al., 1964). Gn pigs are fed (throughout the animals’ lives) a sterilized commercial infant formula (Advanced Similac, Ross Laboratories, Columbus, OH). Gn pigs (both males and females) were randomly assigned to four treatment groups with four pigs in each group as follows: HRV− infected LAB-fed (LAB+HRV+), HRV− infected non-LAB-fed (LAB − HRV+), non-infected LAB-fed (LAB+HRV−), and non-infected non-LAB-fed (LAB−HRV−). Pigs were orally dosed at 3, 5, 7 and 9 days of age with 103, 104, 105 and 106 colony forming units (CFU), respectively, of 1:1 mixture of L. acidophilus and L. reuteri in 2 ml of 0.1% of peptone water (BD Biosciences, Franklin Lakes, NJ, USA). Non-LAB-fed pigs were given an equal volume of 0.1% peptone water. At 5 days of age, pigs were orally inoculated with 105 FFU virulent Wa strain HRV (VirWaHRV) or diluent (non-infected). Pigs were euthanized at day 5 post-HRV inoculation (1 day after the last/7 days after the first LAB feeding) to isolate MNC from ileum, spleen and peripheral blood.
2.2. Virus
The VirWaHRV was passaged through Gn pigs and the pooled intestinal contents from the 23rd passage were used for inoculation at a dose of 1 × 105 fluorescent focus-forming units (FFU). The 50% infectious dose (ID50) of VirWaHRV in pigs was determined as approximately 1 FFU (Ward et al., 1996). Virus fecal shedding on post-HRV inoculation (PID) 5 in HRV− inoculated Gn pigs was detected with an antigen capture enzyme-linked immunosorbent assay (ELISA) from rectal swab fluids as previously described (Hoblet et al., 1986).
2.3. Bacterial strains
The L. reuteri strain ATCC 23272 and L. acidophilus strain NCFM™ (ATCC, Manassas, VA, USA) were used in this study. Both strains were propagated in Lactobacilli MRS broth (Weber, Hamilton, NJ, USA) overnight at 37 °C anaerobically (85% nitrogen, 10% hydrogen and 5% carbon dioxide). Cultures were subcultured once and inoculated into 10 ml MRS broth (Weber). After 24 h, serial dilutions were made in sterile 0.1% peptone water and 0.1 ml of the dilution was spread onto MRS agar plate (BD) for determining the CFU per ml. The remaining bacterial suspensions were aliquoted into 1 ml volumes, stored at −80 °C. The frozen bacterial suspension was thawed and washed with 0.1% peptone water and titrated 1 day prior to feeding pigs. For each LAB, a 0.1 ml aliquot of the bacterial suspension was diluted in 0.9 ml of 0.1% peptone water and plated onto MRS agar for enumeration (see Enumeration of LAB). The two LAB inoculums with known titers were diluted to the specified CFU/ml in 0.1% peptone water and mixed in equal amount on the day of feeding to the animals.
2.4. Enumeration of LAB
Each daily rectal swab was diluted in 4 ml of 0.1% peptone water (~1:10) and a 0.1 ml aliquot was diluted in 0.9 ml of 0.1% peptone water and plated onto MRS agar for enumeration. LAB bacteremia was also assessed by plating sera of pigs onto MRS agar plates. Plates were incubated in sealed BBL Gaspak jars (Fisher, Hanover Park, IL, USA) containing Anaerogen packs (BD) for 24 h at 37 °C. The number of CFU on plates with 20–200 colonies were subsequently counted and recorded. LAB shedding was expressed as CFU/ml.
2.5. Isolation of MNC and flow cytometry analysis of monocytes/macrophages and DCs
The ileum, spleen, and blood were collected from each pig at euthanasia and the MNC were isolated as previously described (Ward et al., 1996; Yuan et al., 1996). Following cell isolation, cell staining was performed on the same day. The freshly isolated MNC were washed with staining buffer [3% FBS and 0.09% sodium azide in 1× DPBS (0.2 mg/ml KCI, 0.2 mg/ml KH2PO4, 8 mg/ml NaCl and 2.16 mg/ml Na2HPO47H2O, pH 7.2–7.4)] and stained with primary antibodies against SWC3 conjugated with biotin, CD11R1 and CD14, followed by secondary reagent streptavidin conjugated with peridinine chlorophyll protein (PerCP) or antibodies conjugated with allophycocyanin (APC) or fluorescein isothiocyanate (FITC) (Table 1). Isotype matched antigen-irrelevant control monoclonal antibodies IgG1 (for CD11R1) (Serotec) and IgG2b (for CD14) (eBioscience, San Diego, CA, USA) were used in each staining as negative controls to set the quadrant markers for the bivariate dot plots. Control for SWC3 was omission of the primary antibody. After staining, cells were fixed in 1% paraformaldehyde. All monoclonal antibodies were titrated and used at optimal concentrations. At least 20,000 cells were acquired on a FACSCalibur flow cytometer (BD Biosciences). Flow cytometry data were analyzed using CellQuest software (BD). Results were reported as frequencies of cell populations expressing the markers of interest among gated cells.
Table 1.
| Primary antibody | Specificity | Clone | Isotype | Supplier | Secondary antibody | Isotype | Supplier |
|---|---|---|---|---|---|---|---|
| SWC3-biotin | Swine | 74–22–15 | IgG1 | Southern Biotech, Birmingham, AL, USA | Streptavidin–PerCP | BD Pharmingen, San Diego, CA, USA | |
| CD11R1 | Swine | MIL4 | IgG1 | Serotec, Raleigh, NC, USA | Rat anti-mouse IgG1–APC | IgG1 | BD Pharmingen, San Diego, CA, USA |
| CD14 | Swine | MIL2 | IgG2b | Serotec, Raleigh, NC, USA | Rat anti-mouse IgG2b–FITC | IgG2a | BD Pharmingen, San Diego, CA, USA |
SWC3+CD11R1−CD14+/−.
SWC3+CD11R1+CD14+/−.
2.6. Statistical analyses
The frequencies of monocytes/macrophages and cDCs and CD14 expression on these cells were compared among groups using the Kruskal–Wallis rank sum. When differences among these groups were detected, the same test was used in a pairwise fashion to clarify the nature of the differences. Statistical significance was assessed at P < 0.05 for all comparisons. All statistical analyses were performed using SAS program (SAS Institute, NC, USA).
3. Results
3.1. LAB colonization and HRV infection
From post-HRV inoculation day 0–5, the average fecal LAB counts in LAB+ groups ranged between 5.9 × 106 and 8.1 × 107 CFU/ml (data not shown), which were greater than the count of LAB in the inoculums (103–106 CFU/ml), indicating intestinal colonization of the LAB. The mean LAB count in the LAB+HRV+ group was significantly higher than the LAB+HRV− group on PID5 (data not shown). HRV infection was confirmed by detection of HRVantigen in the rectal swab fluids from all the pigs in the HRV+ groups (none in the HRV− groups) on PID5. There was no significant difference in mean antigen-ELISA OD values between the two HRV+ groups, suggesting that LAB colonization did not reduce rotavirus replication. The clinical signs between the LAB+HRV+ and LAB−HRV+ groups did not differ significantly (data not shown), therefore LAB colonization did not reduce rotavirus diarrhea.
3.2. Flow cytometry analysis of monocytes/macrophages and cDCs in pigs
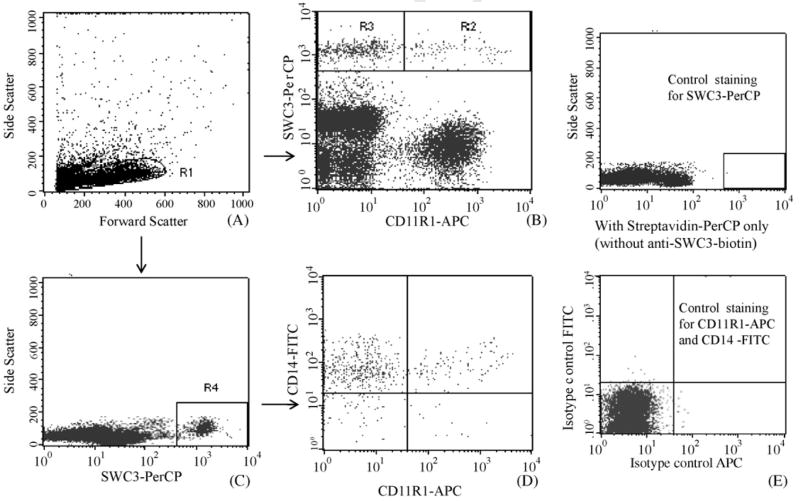
To determine the distribution of monocytes/macrophages and cDCs in different lymphoid tissues of pigs from each treatment group, flow cytometry analysis was performed on freshly isolated MNC of ileum, spleen and blood (Fig. 1). The MNC were defined by an electronic gate through FSC/SSC dot plot (R1) (Fig. 1A). The SWC3/CD11R1 dot plot was performed within R1 area to define SWC3+CD11R1+ cDCs within R2 [(cell count in R2/cell count in R1) × 100% = frequencies of cDCs] and SWC3+CD11R1− monocytes/macrophages within R3 (R3/R1 × 100% = frequencies of monocytes/macrophages) (Fig. 1B). For analysis of expression of CD14 among SWC3+ cells, SWC3/SSC dot plot was performed (within R1) to define SWC3+ cells within R4 area (Fig. 1C). Frequencies of SWC3+CD14+CD11R1− and SWC3+CD14+ CD11R1+ subsets were the percentages of cell counts in the quadrants (CD14+CD11R1− or CD14+CD11R1+) over total counts of SWC3+ cells (R4) (Fig. 1D). Besides monocytes/macrophages and cDCs, the pan-myeloid marker SWC3 is also expressed on granulocytes. However, gating on R1 (Fig. 1) avoided the detection of most SWC3+ granulocytes. Therefore, the MNC stained as SWC3+CD11R1− are mainly monocytes/macrophages and SWC3+CD11R1+ are mainly cDCs. Fig. 1E shows dot plots of the cells stained with isotype controls for CD11R1 and CD14 and without the primary antibody to SWC3.
Fig. 1.
Flow cytometry analysis of monocytes/macrophages and cDCs in Gn pigs. Forward and side scatter of MNC R1 area was used for electronic gating (A). SWC3/CD11R1 dot plot was performed within R1 area to define monocytes/macrophages and cDCs within R2 (SWC3+CD11R1−) and R3 (SWC3+CD11R1+) areas, respectively (B). Within R1, SWC3+ cells were gated on R4 (C) and CD11R1/CD14 dot plot was performed within R4 area to define CD14+ cells within cDC and monocyte/macrophage populations, respectively (D). Cells stained with isotype controls and without primary antibody to SWC3 were presented in control dot plots (E). Results are representative of spleen of four animals in LAB−HRV+ group.
3.3. Distribution of monocytes/macrophages and cDCs
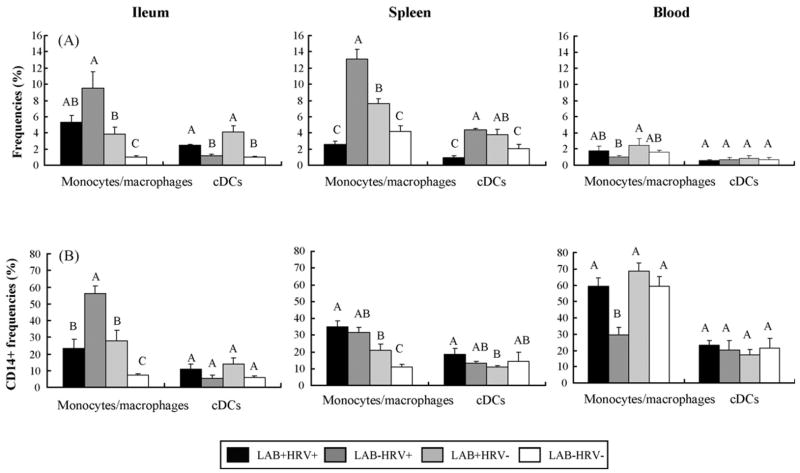
Frequencies of monocytes/macrophages and cDCs in ileum, spleen and blood from Gn pigs in the four treatment groups are depicted in Fig. 2a.
Fig. 2.
Distribution of monocytes/macrophages and cDCs in ileum, spleen and blood of Gn pigs. Gn pigs were inoculated with LAB and virulent Wa strain HRV (LAB+HRV+), HRV only (LAB−HRV+), LAB only (LAB+HRV−) or mock (LAB−HRV−). (2a) y-Axis is the frequency (%) of monocytes/macrophages or cDCs. The frequency of monocytes/macrophages is the percentage of cells in R2 over R1; the frequency of cDCs is the percentage of cells in R3 over R1 (see Fig. 1). (2b) y-Axis is the frequency (%) of CD14+ cells among monocytes/macrophages or cDCs. The frequencies of CD14+ monocytes/macrophages and CD14+ cDCs are the percentages of CD14+CD11R1− and CD14+CD11R1+ cells over R4, respectively. The data represent the mean of four pigs for each group. The error bars represent standard error of the mean. Different letters denote significant differences among groups for the same cell type from the corresponding tissue (Kruskal–Wallis rank sum test, P < 0.05).
In ileum, frequencies of monocytes/macrophages were highest in the LAB−HRV+ group (9.46%). Frequencies of monocytes/macrophages in all three treatment groups were significantly higher (3–9-fold) than those of negative controls (LAB−HRV− group), and those in the LAB−HRV+ group were significantly higher (2.5-fold) than in the LAB+HRV− group. On the other hand, the highest frequencies of cDCs in ileum were in the LAB+HRV− group (4.09%) and significantly higher in both LAB-fed pig groups (LAB+HRV+ and LAB+HRV−) than those without LAB (LAB−HRV+ and LAB−HRV−). These results suggest that HRV infection played a major role in increasing the frequencies of monocytes/macrophages, but not cDCs, whereas LAB increased both the monocytes/macrophage and cDC frequencies in intestine.
In spleen, frequencies of monocytes/macrophages were highest in the LAB−HRV+ group (13.13%), and were lowest in the LAB+HRV+ group (2.54%, not significantly lower than the controls). The LAB−HRV+ and LAB+HRV− groups had significantly higher (2–3-fold) frequencies of monocytes/macrophages than those of the LAB+HRV+ and LAB−HRV− groups. Similarly, the LAB+HRV+ group had the lowest frequencies of cDCs (0.96%), which were significantly lower than those of the LAB−HRV+ (fourfold) and LAB+HRV− (threefold) groups. The LAB−HRV+ and LAB+HRV− groups had significantly higher frequencies of cDCs than those of the control group. Though significantly higher frequencies of monocytes/macrophages were found in the LAB−HRV+ group than the LAB+HRV− group, the cDC frequencies were similar in these two groups. The most striking finding was that LAB feeding plus HRV infection significantly reduced monocytes/macrophages and cDC frequencies in spleen compared to LAB alone or HRV alone.
In blood, frequencies of the monocytes/macrophages and cDCs were similar in all groups except the frequencies of monocytes/macrophages in LAB+HRV− group were significantly higher than the LAB−HRV+ group.
3.4. Expression of CD14 on monocytes/macrophages and cDCs
Frequencies of CD14 expression on monocytes/macrophages and cDCs in ileum, spleen and blood from pigs in the four treatment groups are depicted in Fig. 2b. In ileum, frequencies of CD14+ monocytes/macrophages were significantly higher in the three treatment groups than in the controls and were significantly higher (2–8-fold) in the LAB−HRV+ group (56.12%) than in the other groups. No significant differences were found in frequencies of CD14+ cDCs in ileum among all groups.
In spleen, the three treatment groups had significantly higher (2–3-fold) frequencies of CD14+ monocytes/macrophages than the control group (10.86%). Frequencies of CD14+ monocytes/macrophages in the LAB+HRV+ group were significantly higher (twofold) than those of the LAB+HRV− group. Frequencies of CD14+ cDCs in three treatment groups were statistically similar to those of the control group (14.67%).
In blood, frequencies of CD14+ monocytes/macrophages were high and were statistically similar among LAB+HRV+, LAB+HRV− and LAB−HRV− groups, compared to significantly lower (twofold) frequencies in the LAB−HRV+ group (29.45%). Frequencies of CD14+ cDCs in blood did not differ among groups (17.4–23.32%).
4. Discussion
To better understand the influence of HRV infection, commensal colonization and their combine effect on the early postnatal ontogeny of innate immune responses, we compared the distribution of monocytes/macrophages and cDCs in intestinal (ileum) and systemic (spleen) lymphoid tissues and blood among the four treatment Gn pig groups including HRV infected, LAB colonized, HRV infected and LAB colonized, and non-infected and non-colonized controls. We also compared the expression of CD14 as a functional marker on the monocytes/macrophages and cDCs in the naïve control pigs with those infected with HRV and/or colonized with LAB.
In ileum and spleen, either HRV infection alone or LAB colonization alone induced significantly higher frequencies of monocytes/macrophages compared to the control pigs; however HRV infection alone induced significantly higher frequencies of monocytes/macrophages than LAB colonization alone. Coinciding with this observation, frequencies of monocytes/macrophages in blood of HRV only pigs were significantly lower compared to those of LAB only pigs, suggesting that HRV infection alone recruited more monocytes/macrophages from blood into lymphoid tissues than the LAB. Because tissue macrophages do not proliferate or migrate, the logical source of the monocytes/macrophages is the pool of circulating monocytes (Smith et al., 2005).
Colonization with LAB and infection with HRV induced lower frequencies of monocytes/macrophages than HRV infection alone in ileum and spleen (significantly). The mechanism for the decreased monocyte/macrophage frequencies of the LAB+HRV+ group is undefined. Considering also the significantly decreased frequencies of CD14+ monocytes/macrophages in ileum of these pigs, it is attempting to postulate that LAB down-regulated the recruitment of HRV− activated monocytes/macrophages to the systemic site (spleen) and the recruitment/maturation of these cells in ileum thereby limiting the inflammation induced by HRV infection. However this hypothesis will need to be tested in future studies. It was reported that monocyte-derived macrophages did not express CD14 in the normal mucosa of human intestines (Smith et al., 1997, 2001; Smythies et al., 2005). In our study we observed the lowest frequencies of ileal CD14+ monocytes/macrophages in the control group and lower frequencies in the pigs colonized with LAB. Several other studies have shown that a high proportion of the macrophages in the inflamed mucosa of patients with inflammatory bowel disease expressed CD14 (Smith et al., 2005). Thus CD14 expression by intestinal monocytes/macrophages is associated with inflammatory responses in the gut and the LAB (mixed L. acidophilus and L. reuteri) exhibited an anti-inflammatory effect by reducing the CD14+ monocyte/macrophage frequencies in pigs.
The patterns of CD14 expression in ileum and blood observed were similar to the patterns of monocyte/macrophage frequencies. The HRV infection alone induced the highest frequencies of CD14+ monocytes/macrophages in the ileum (56%) and the lowest in the blood (29%). Significantly reduced frequencies of CD14+ monocytes/macrophages in blood of the LAB−HRV+ pigs coincided with the significantly increased frequencies of CD14+ monocytes/macrophages in ileum and spleen of these pigs compared to the controls. The similarity between the pattern of monocyte/macrophage frequencies and the pattern of CD14 expression on these cells in ileum and blood of the four treatment groups suggests that CD14 expression is closely related to the activation/maturation of monocytes/macrophages at these sites. In naive germ-free pigs (LAB−HRV−), the frequencies of CD14+ monocytes/macrophages in ileum and spleen were significantly lower than the other three groups. However, the majority (59.38%) of blood monocytes expressed CD14 in the naïve pigs. Unlike HRV infection, the frequencies of CD14+ monocytes in blood were not significantly influenced by LAB colonization.
On the other hand, frequencies of CD14 expression on cDCs of ileum and blood did not differ significantly among the treatment groups; therefore CD14 expression may not be a sensitive indicator for activation/maturation of cDCs. In addition, frequencies of cDCs and CD14+ cDCs in ileum and blood of the LAB−HRV+ pigs did not differ from the germ-free pigs (LAB−HRV−). An explanation for this observation is that ileal and blood cDCs are not strongly involved in immune responses to rotavirus infection. This explanation concurs with the findings from an in vitro study on the interaction of rotavirus and monocyte-derived blood DCs (Narvaez et al., 2005). It was shown that rotavirus had limited effect on activation/maturation of the DCs. The rotavirus did not induce cell death of immature or mature DCs or inhibit the maturation of DCs, but also did not strongly stimulate maturation of DCs.
In spleen, the significantly increased frequencies of CD14+ monocytes/macrophages and CD14+ cDCs in the LAB+HRV+ pigs compared to LAB only pigs (Fig. 2b) contrasted with the significantly reduced frequencies of total monocytes/macrophages and cDCs (Fig. 2a). Thus, LAB colonization and HRV infection significantly reduced the total frequencies of monocytes/macrophages and cDCs in spleen of Gn pigs; but among those cells, a higher percent of them expressed CD14 compared to LAB only pigs. Therefore, there was an antagonistic effect between LAB colonization and HRV infection on the frequencies of monocytes/macrophages and cDCs and a synergistic effect on CD14 expression on these cells in the systemic lymphoid tissue. The mechanism and implication of the antagonistic/synergistic effects require further study.
Our results showed that CD14 expression on cDCs in vivo was not significantly influenced by either HRV infection or LAB colonization. These results are in contrast to in vitro studies showing that LPS up-regulated the expression of CD14 on DCs (Carrasco et al., 2001) and porcine reproductive and respiratory syndrome virus (PRRSV) reduced the expression of CD14 on DCs (Wang et al., 2007). The different experimental condition, in vivo versus in vitro, and different DC subset may partially explain the discrepancies among these studies. However the different CD14 responses on DCs most likely reflected the difference in the stimulating antigens (LPS vs. LAB and PRRSV vs. HRV). Further studies are needed to address this question.
In blood of neonatal Gn pigs, HRV infection or LAB colonization or both did not induce increases of frequencies of total cDCs or CD14+ cDCs compared to the controls, even though viremia was detected in all HRV+ pigs (data not shown). These findings are in accordance with other studies that most of porcine blood cDCs were immature (Jamin et al., 2006; Summerfield et al., 2003).
Taken together, we observed in neonatal Gn pigs that: (1) HRV infection alone activated/recruited significantly more monocytes/macrophages in the intestine and spleen than LAB colonization and a large percentage of these monocytes/macrophages expressed CD14; increased CD14 expression coincided with increased frequencies of monocytes/macrophages in these two sites but not of cDCs; (2) LAB colonization down-regulated HRV− infection-induced monocyte/macrophage activation/recruitment and CD14 expression in the intestine; (3) LAB colonization together with HRV infection significantly reduced the total frequencies of monocytes/macrophages and cDCs in spleen of Gn pigs; but among those cells, a higher percent of them expressed CD14 compared to LAB only pigs; (4) neither HRV infection nor LAB colonization induced increased frequencies of cDCs in blood.
Colonization with LAB alone also significantly increased the frequencies of monocytes/macrophages and cDCs and the CD14 expression on monocytes/macrophages in ileum and spleen compared to the control germ-free pigs at 10 days of age (7 days after the first LAB feeding). Thus LAB colonization alone had a significant impact on early postnatal development of innate immune system in both the intestinal and systemic sites. The impact of HRV infection alone was similar to that of LAB colonization alone (except for cDC frequencies in ileum) but at a greater magnitude. Therefore either intestinal commensal bacterial colonization or pathogenic enteric viral infection effectively promoted the development of innate immune system in neonatal pigs. In conclusion, this study illustrated the distribution and frequencies of porcine monocytes/macrophages and cDCs and CD14 expression on these cells in the early stage of immune responses to intestinal colonization by LAB versus infection by an enteric pathogenic virus on an “immunologically virgin” background. These results will facilitate further in vivo studies on functional characterization of these immune cells in neonatal pigs.
Acknowledgments
This work was supported by grants from the National Institutes of Health (1R21AT002524 to LY and R01AI033561 to LJS) and Ohio Agricultural Research and Development Center, The Ohio State University (OHOA1208 to LY). We thank Dr. Juliette Hanson and Mr. Rich McCormick for animal care and technical assistance. Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center (OARDC) of The Ohio State University.
References
- Ahrne S, Nobaek S, Jeppsson B, Adlerberth I, Wold AE, Molin G. The normal lactobacillus flora of healthy human rectal and oral mucosa. J Appl Microbiol. 1998;85:88–94. doi: 10.1046/j.1365-2672.1998.00480.x. [DOI] [PubMed] [Google Scholar]
- Alvarez B, Sanchez C, Bullido R, Marina A, Lunney J, Alonso F, Ezquerra A, Dominguez J. A porcine cell surface receptor identified by monoclonal antibodies to SWC3 is a member of the signal regulatory protein family and associates with protein–tyrosine phosphatase SHP-1. Tissue Antigens. 2000;55:342–351. doi: 10.1034/j.1399-0039.2000.550408.x. [DOI] [PubMed] [Google Scholar]
- Antal-Szalmas P, Strijp JA, Weersink AJ, Verhoef J, Van Kessel KP. Quantitation of surface CD14 on human monocytes and neutrophils. J Leukoc Biol. 1997;61:721–728. doi: 10.1002/jlb.61.6.721. [DOI] [PubMed] [Google Scholar]
- Barro M, Patton JT. Rotavirus NSP1 inhibits expression of type I interferon by antagonizing the function of interferon regulatory factors IRF3, IRF5, and IRF7. J Virol. 2007 doi: 10.1128/JVI.02498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista EM, Gregg D, Golde WT. Characterization and functional analysis of skin-derived dendritic cells from swine without a requirement for in vitro propagation. Vet Immunol Immunopathol. 2002;88:131–148. doi: 10.1016/s0165-2427(02)00152-6. [DOI] [PubMed] [Google Scholar]
- Bimczok D, Post A, Tschernig T, Rothkotter HJ. Phenotype and distribution of dendritic cells in the porcine small intestinal and tracheal mucosa and their spatial relationship to epithelial cells. Cell Tissue Res. 2006;325:461–468. doi: 10.1007/s00441-006-0195-3. [DOI] [PubMed] [Google Scholar]
- Bimczok D, Sowa EN, Faber-Zuschratter H, Pabst R, Rothkotter HJ. Site-specific expression of CD11b and SIRPalpha (CD172a) on dendritic cells: implications for their migration patterns in the gut immune system. Eur J Immunol. 2005;35:1418–1427. doi: 10.1002/eji.200425726. [DOI] [PubMed] [Google Scholar]
- Brown KA, Offit PA. Rotavirus-specific proteins are detected in murine macrophages in both intestinal and extraintestinal lymphoid tissues. Microb Pathog. 1998;24:327–331. doi: 10.1006/mpat.1997.0198. [DOI] [PubMed] [Google Scholar]
- Carrasco CP, Rigden RC, Schaffner R, Gerber H, Neuhaus V, Inumaru S, Takamatsu H, Bertoni G, McCullough KC, Summerfield A. Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties. Immunology. 2001;104:175–184. doi: 10.1046/j.0019-2805.2001.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh LL, Von Andrian UH. Travellers in many guises: the origins and destinations of dendritic cells. Immunol Cell Biol. 2002;80:448–462. doi: 10.1046/j.1440-1711.2002.01119.x. [DOI] [PubMed] [Google Scholar]
- Chamorro S, Revilla C, Alvarez B, Alonso F, Ezquerra A, Dominguez J. Phenotypic and functional heterogeneity of porcine blood monocytes and its relation with maturation. Immunology. 2005;114:63–71. doi: 10.1111/j.1365-2567.2004.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech N, Rodriguez-Carreno MP, Filgueira P, Alvarez B, Chamorro S, Dominguez J. Identification of porcine macrophages with monoclonal antibodies in formalin-fixed, paraffin-embedded tissues. Vet Immunol Immunopathol. 2003;94:77–81. doi: 10.1016/s0165-2427(03)00084-9. [DOI] [PubMed] [Google Scholar]
- Dudda JC, Lembo A, Bachtanian E, Huehn J, Siewert C, Hamann A, Kremmer E, Forster R, Martin SF. Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur J Immunol. 2005;35:1056–1065. doi: 10.1002/eji.200425817. [DOI] [PubMed] [Google Scholar]
- Dunzendorfer S, Lee HK, Soldau K, Tobias PS. TLR4 is the signaling but not the lipopolysaccharide uptake receptor. J Immunol. 2004;173:1166–1170. doi: 10.4049/jimmunol.173.2.1166. [DOI] [PubMed] [Google Scholar]
- Dziarski R, Ulmer AJ, Gupta D. Interactions of CD14 with components of Gram-positive bacteria. Chem Immunol. 2000;74:83–107. doi: 10.1159/000058761. [DOI] [PubMed] [Google Scholar]
- Fenaux M, Cuadras MA, Feng N, Jaimes M, Greenberg HB. Extraintestinal spread and replication of a homologous EC rotavirus strain and a heterologous rhesus rotavirus in BALB/c mice. J Virol. 2006;80:5219–5232. doi: 10.1128/JVI.02664-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y, Kawata Y, Hara H, Terada A, Mitsuoka T. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol. 1998;42:39–44. doi: 10.1016/s0168-1605(98)00056-7. [DOI] [PubMed] [Google Scholar]
- Gonzales A, Azevedo M, Yuan L, Zhang W, Saif L. Dissertation. The Ohio State University; Wooster, OH: 2007. A low human rotavirus (HRV) dose induced higher intestinal IFN-alpha producing plasmacytoid dendritic cells in-vivo and uptake or binding of rotavirus-like particles in gnotobiotic pigs compared to a higher HRV dose. [Google Scholar]
- Haverson K, Bailey M, Higgins VR, Bland PW, Stokes CR. Characterization of monoclonal antibodies specific for monocytes, macrophages and granulocytes from porcine peripheral blood and mucosal tissues. J Immunol Methods. 1994;170:233–245. doi: 10.1016/0022-1759(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Haverson K, Singha S, Stokes CR, Bailey M. Professional and non-professional antigen-presenting cells in the porcine small intestine. Immunology. 2000;101:492–500. doi: 10.1046/j.1365-2567.2000.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herias MV, Hessle C, Telemo E, Midtvedt T, Hanson LA, Wold AE. Immunomodulatory effects of Lactobacillus plantarum colonizing the intestine of gnotobiotic rats. Clin Exp Immunol. 1999;116:283–290. doi: 10.1046/j.1365-2249.1999.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoblet KH, Saif LJ, Kohler EM, Theil KW, Bech-Nielsen S, Stitzlein GA. Efficacy of an orally administered modified-live porcine-origin rotavirus vaccine against postweaning diarrhea in pigs. Am J Vet Res. 1986;47:1697–1703. [PubMed] [Google Scholar]
- Ichikawa H, Kuroiwa T, Inagaki A, Shineha R, Nishihira T, Satomi S, Sakata T. Probiotic bacteria stimulate gut epithelial cell proliferation in rat. Dig Dis Sci. 1999;44:2119–2123. doi: 10.1023/a:1026647024077. [DOI] [PubMed] [Google Scholar]
- Jamin A, Gorin S, Le Potier MF, Kuntz-Simon G. Characterization of conventional and plasmacytoid dendritic cells in swine secondary lymphoid organs and blood. Vet Immunol Immunopathol. 2006;114:224–237. doi: 10.1016/j.vetimm.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- Kronin V, Hochrein H, Shortman K, Kelso A. Regulation of T cell cytokine production by dendritic cells. Immunol Cell Biol. 2000;78:214–223. doi: 10.1046/j.1440-1711.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Lee HK, Dunzendorfer S, Soldau K, Tobias PS. Double-stranded RNA-mediated TLR3 activation is enhanced by CD14. Immunity. 2006;24:153–163. doi: 10.1016/j.immuni.2005.12.012. [DOI] [PubMed] [Google Scholar]
- McCullough KC, Schaffner R, Natale V, Kim YB, Summerfield A. Phenotype of porcine monocytic cells: modulation of surface molecule expression upon monocyte differentiation into macrophages. Vet Immunol Immunopathol. 1997;58:265–275. doi: 10.1016/s0165-2427(97)00045-7. [DOI] [PubMed] [Google Scholar]
- Meyer RC, Bohl EH, Kohler EM. Procurement and maintenance of germ-free seine for microbiological investigations. Appl Environ Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez CF, Angel J, Franco MA. Interaction of rotavirus with human myeloid dendritic cells. J Virol. 2005;79:14526–14535. doi: 10.1128/JVI.79.23.14526-14535.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillot R, Laval F, Audonnet JC, Andreoni C, Juillard V. Functional and phenotypic characterization of distinct porcine dendritic cells derived from peripheral blood monocytes. Immunology. 2001;102:396–404. doi: 10.1046/j.1365-2567.2001.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- Rehakova Z, Trebichavsky I, Sinkora J, Splichal I, Sinkora M. Early ontogeny of monocytes and macrophages in the pig. Physiol Res. 1998;47:357–363. [PubMed] [Google Scholar]
- Salmon H, Johnson I, Germana S, Haller GW, Sachs DH, Leguern C. Dendritic cells enriched from swine thymus co-express CD1, CD2 and major histocompatibility complex class II and actively stimulate alloreactive T lymphocytes. Scand J Immunol. 2000;52:164–172. doi: 10.1046/j.1365-3083.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- Smith PD, Janoff EN, Mosteller-Barnum M, Merger M, Orenstein JM, Kearney JF, Graham MF. Isolation and purification of CD14-negative mucosal macrophages from normal human small intestine. J Immunol Methods. 1997;202:1–11. doi: 10.1016/s0022-1759(96)00204-9. [DOI] [PubMed] [Google Scholar]
- Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–159. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- Smith PD, Smythies LE, Mosteller-Barnum M, Sibley DA, Russell MW, Merger M, Sellers MT, Orenstein JM, Shimada T, Graham MF, Kubagawa H. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol. 2001;167:2651–2656. doi: 10.4049/jimmunol.167.5.2651. [DOI] [PubMed] [Google Scholar]
- Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield A, Guzylack-Piriou L, Schaub A, Carrasco CP, Tache V, Charley B, McCullough KC. Porcine peripheral blood dendritic cells and natural interferon-producing cells. Immunology. 2003;110:440–449. doi: 10.1111/j.1365-2567.2003.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield A, Haverson K, Thacker E, McCullough KC. Differentiation of porcine myeloid bone marrow haematopoietic cell populations. Vet Immunol Immunopathol. 2001;80:121–129. doi: 10.1016/s0165-2427(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowska B, Lodinova-Zadnikova R, Kozakova H, Rossmann P, Bartova J, Sokol D, Funda DP, Borovska D, Rehakova Z, Sinkora J, Hofman J, Drastich P, Kokesova A. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Van Gucht S, Atanasova K, Barbe F, Cox E, Pensaert M, Van Reeth K. Effect of porcine respiratory coronavirus infection on lipopolysaccharide recognition proteins and haptoglobin levels in the lungs. Microbes Infect. 2006;8:1492–1501. doi: 10.1016/j.micinf.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gucht S, Van Reeth K, Nauwynck H, Pensaert M. Porcine reproductive and respiratory syndrome virus infection increases CD14 expression and lipopolysaccharide-binding protein in the lungs of pigs. Viral Immunol. 2005;18:116–126. doi: 10.1089/vim.2005.18.116. [DOI] [PubMed] [Google Scholar]
- Van Niel CW, Feudtner C, Garrison MM, Christakis DA. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics. 2002;109:678–684. doi: 10.1542/peds.109.4.678. [DOI] [PubMed] [Google Scholar]
- Wang PY, Munford RS. CD14-dependent internalization and metabolism of extracellular phosphatidylinositol by monocytes. J Biol Chem. 1999;274:23235–23241. doi: 10.1074/jbc.274.33.23235. [DOI] [PubMed] [Google Scholar]
- Wang X, Eaton M, Mayer M, Li H, He D, Nelson E, Christopher-Hennings J. Porcine reproductive and respiratory syndrome virus productively infects monocyte-derived dendritic cells and compromises their antigen-presenting ability. Arch Virol. 2007;152:289–303. doi: 10.1007/s00705-006-0857-1. [DOI] [PubMed] [Google Scholar]
- Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77(Pt 7):1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- Yasui H, Shida K, Matsuzaki T, Yokokura T. Immunomodulatory function of lactic acid bacteria. Antonie Van Leeuwenhoek. 1999;76:383–389. [PubMed] [Google Scholar]
- Yuan L, Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol. 2002;87:147–160. doi: 10.1016/S0165-2427(02)00046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]