Abstract
Background
In 1997, highly pathogenic avian influenza (HPAI) viruses caused outbreaks of disease in domestic poultry markets in Hong Kong. The virus has also been detected in infected poultry in Europe and Africa.
Objective
The objective of this study was to determine the efficacy of a heterologous vaccine administered with and without the aluminum hydroxide adjuvant in ferrets challenged with HPAI (A/Vietnam/1203/04).
Methods
Animals in four of the five groups were vaccinated twice 21 days apart, with two doses of a heterologous monovalent subvirion vaccine with or without an aluminum hydroxide adjuvant and challenged with a lethal target dose of A/Vietnam/1203/04.
Results
All animals vaccinated with the heterologous vaccine in combination with the aluminum hydroxide adjuvant survived a lethal challenge of A/Vietnam/1203/04. Four of the eight animals vaccinated with 30 µg of the vaccine without the adjuvant survived, while two of the eight animals vaccinated with 15 µg of the vaccine without the adjuvant survived. None of the unvaccinated control animals survived challenge. Additionally, changes in virus recovered from nasal washes and post-mortem tissues and serology suggest vaccine efficacy.
Conclusions
Altogether, the data suggest that the heterologous vaccine in combination with the aluminum hydroxide adjuvant offers maximum protection against challenge with A/Vietnam/1203/04 when compared to the unvaccinated control animals or animals vaccinated without any adjuvant.
Keywords: Highly Pathogenic Avian Influenza, H5N1, heterologous vaccine
Introduction
Influenza A viruses, from the family Orthomyxoviridae, are enveloped single-stranded RNA viruses that contain eight different gene segments. Influenza virus type A and B cause recurrent yearly epidemics. Additionally, the type A viruses have been associated with pandemics as observed in 1918 (Spanish Flu-claimed over 40 million lives), 1957 (Asian Flu-claimed over 4 million lives), and 1968 (Hong Kong Flu-claimed 1 million lives) 1, 2. It is thought that the 1918 pandemic arose from an adaptation of an avian influenza virus to efficient transmission in humans 3. Avian influenza viruses are contagious and widespread in birds 4–6. These viruses are categorized as low pathogenicity avian influenza or highly pathogenic avian influenza (HPAI) viruses. HPAI viruses can cause mortality of 90–100% in flocks and this virus has the ability to spread rapidly amongst flocks. In 1997, H5N1 avian influenza viruses caused disease in domestic poultry markets in Hong Kong, and this disease later spread to humans. A second outbreak of disease associated with H5N1 in humans began in 2004 and has resulted in over 560 people being infected throughout Asia, Asia Minor, Africa, and the Balkan states, with death occurring in over 60% of cases 7. H5N1 viruses isolated from humans, like those isolated from poultry, were highly pathogenic in experimentally infected chickens and genetic analyses revealed a series of multiple basic amino acids adjacent to the hemagglutinin cleavage site characteristic of HPAI viruses. Viral infections in humans resulted in various clinical outcomes, ranging from mild infections to severe respiratory illness and death. Complications in severe cases included acute respiratory distress syndrome, leukopenia, lymphopenia, hemophagocytosis, and multi-organ dysfunction. The unusual prominence of gastrointestinal symptoms, hematologic disorders, and liver and renal dysfunction suggested that the H5N1 viruses had a wider tissue tropism when compared to human influenza A H1N1 and H3N2 viruses.
The emergence of H5N1 viruses in different countries has raised concerns of a potential influenza pandemic, thus raising efforts to develop immunogenic vaccines against H5N1 viruses. However, recent clinical trials have shown that standard doses of subunit vaccines are not very immunogenic 8, 9. Thus, we aimed to test the efficacy of two doses of heterologous vaccine administered twice with and without an adjuvant in an animal model. Because ferrets (Mustela putorius furo) are naturally susceptible to infection with human influenza A and B viruses and the disease resembles that of human influenza, these animals have been widely used as a model for influenza virus pathogenesis and immunity studies. The viral disease for H5N1 is systemic and includes the central nervous system. Thus, the H5N1 A/Vietnam/1203/04 strain was utilized in this current efficacy study to determine whether administration of a heterologous vaccine administered with and without the aluminum hydroxide adjuvant provided protection in ferrets infected with a lethal dose of A/Vietnam/1203/04. We demonstrate that a heterologous monovalent subvirion vaccine administered twice in combination with the aluminum hydroxide adjuvant offers maximum protection against challenge with A/Vietnam/1203/04 when compared to the unvaccinated control animals or animals vaccinated without any adjuvant.
Materials and Methods
Test and Challenge Material
The inactivated monovalent subvirion vaccines (A/Indonesia/05/05) CLAG-1324 (30 µg) and CLAG-1323 (15 µg) and aluminum hydroxide adjuvant (CLAG-1073)were provided by DMID/NIAID/NIH. The monovalent subvirion vaccines were propagated in eggs. The animals were vaccinated intramuscularly (0.5 mL/vaccination) on Study Days 0 and 21 on the left and right hind limbs. On study day 42, HPAI A/Vietnam/1203/04 challenge stock made in embryonated eggs was diluted in PBS without divalent cations to the target concentration 1 × 106 TCID50 per 600 µL.
Intranasal inoculation
On Study Day 42, the animals were anesthetized with Telazol (20 mg/kg) and challenge material (1×106 TCID50/600 µl) was slowly introduced into the ferret nasal cavity by pipette, delivering 150 µl per nostril twice (total of 300 µl per nostril). A portion of the diluted challenge material was analyzed by TCID50 to confirm virus titer.
Animal Population
Forty-four Ferrets were purchased from Triple F Farms and were nine to 15 weeks of age upon arrival. A computer randomization program was used to divide 40 neutered and descented male ferrets (Mustela putorius furo) into five groups, according to Table 1. Forty ferrets were placed on study, with four additional ferrets intended as replacements in the event an animal was excluded from the study prior to H5N1 infection. Ear tags were used to identify each animal. Ferrets were pair housed, and cage cards were placed on each cage identifying the study number, animal number, group ID, and study director. Prior to vaccinations, all animals were bled for sera to confirm no previous exposure to influenza and all animals placed on study were confirmed to be seronegative by hemagglutination inhibition assay (HAI) for A/Vietnam/1203/04 the currently circulating influenza viruses [(A/Brisbane/10/07, A/Brisbane/59/07, B/Florida/04/06, and A/Mexico/4108/09)]. All ferrets selected for the study were in good health and free from malformations and signs of clinical disease at beginning of the study.
Table 1.
Study Design
| Vaccination | ||||||||
|---|---|---|---|---|---|---|---|---|
| Groups | No. of Animals | Vaccine | ROA1 | Days | Challenge (1 × 106 TCID50) |
Nasal Wash | Bleeds-antibody determination (MN & HAI) |
Bleeds Hematology/Clin Chem |
| 1 | 8 | CLAG-1324 (A/Indonesia/05/05, 30 µg) |
IM | 0, 21 | Days 42 | Days 45 | Days 0, 21, 42, 56, (Terminal samples if possible) |
Days 0, 42, 45, 56 (Terminal samples if possible) |
| 2 | 8 | CLAG-1323 (A/Indonesia/05/05, 15 µg) |
IM | 0, 21 | ||||
| 3 | 8 | CLAG-1324 (A/Indonesia/05/05, 30 µg) + Aluminum Hydroxide Adjuvant CLAG-1073 |
IM | 0, 21 | ||||
| 4 | 8 | CLAG-1323 (A/Indonesia/05/05, 15 µg) + Aluminum Hydroxide Adjuvant CLAG-1073 |
IM | 0, 21 | ||||
| 5 | 8 | Unvaccinated Control Group | IM | 0, 21 | ||||
ROA-route of administration
Animals from Groups 1 through 4 were vaccinated intramuscularly twice and blood for sera collection and hematology analysis was drawn according to the schedule in Table 1. All animals (including extras) underwent the appropriate pre-challenge procedures, including vaccinations, blood collections, and measurements of body weights and body temperatures. Body weights were measured on Study Days 0, 21, 41, and every second day starting on Study Day 42 (i.e., 42, 44, 46, 48, 50, 52, 54, and 56). Terminal weights were also collected. At the time of challenge, those animals assigned as extras were excluded from all study related activities and removed from study.
Temperature
Animals were implanted with programmable temperature transponder chips for monitoring body temperature prior to challenge. Temperature transponder chips (two per animal) were implanted in the shoulder and rump area (one per region). Two transponders were implanted in the event that one of the transponders was to fail during the course of the study. Temperature readings were taken from both the rump and the shoulder, unless a transponder failed, in which case readings were not reported from that site and taken from only the functional chip. Body temperatures were recorded twice daily beginning on Study Day 35. Temperatures were recorded from both the shoulder and rump transponders. The temperature values were averaged for all statistical analyses as the average temperature for each animal at each time point (AM and PM). A baseline temperature for each individual animal was calculated by averaging AM (morning) and PM (afternoon) temperatures for seven days prior to challenge.
Clinical Observations
Observations were recorded twice daily from the time the animals arrived until challenge. Following challenge and until the end of study, clinical observations were recorded twice daily (except on Study Day 56 when the animals were only observed once) and included the following scoring system based on that described by Reuman et al. 1989; see also Zitzow et al. 2002 10, 11:
0 = alert and playful
1 = alert but playful only when stimulated
2 = alert but not playful when stimulated
3 = neither alert nor playful when stimulated
Hematology and Clinical Chemistry
Blood was collected into EDTA tubes according to Table 1, and hematology analysis was conducted on the Advia 120 (Siemens). Blood was collected into SST tubes according to Table 1, and clinical chemistry analysis was conducted on the Advia 1200 (Siemens). The hematology parameters analyzed included: Red Blood Cell Count (RBC, 106 cells/µL), Hemoglobin (HGB, g/dL), Hematocrit (HCT, %), Mean Corpuscular Volume (MCV, fL), Mean Corpuscular Hemoglobin (MCH, pg), Mean Corpuscular Hemoglobin Concentration (MCHC, g/dL), Cell Hemoglobin Concentration Mean (CHCM, g/dL), Red Cell Distribution Width (RDW, %), Platelet Count (PLT, 103 cells/µL), Mean Platelet Volume (MPV, fL), White Blood Cell Count (WBC, 103 cells/µL), Neutrophils (103 cells/µL), Lymphocytes (103 cells/µL), Neutrophils/Lymphocytes Ratio (N/L Ratio), Monocytes (103 cells/µL), Eosinophils (103 cells/µL), Basophils (103 cells/µL) and Large Unstained Cells (LUC, 103 cells/µL). The clinical chemistry parameters analyzed included: Total Bilirubin (mg/dL), Aspartate Aminotransferase (AST, U/L), Alanine Aminotransferase (ALT, U/L), Sorbitol Dehydrogenase (SDH, U/L), Glucose (mg/dL), Alkaline Phosphatase (ALP, U/L), Gamma Glutamyl Transferase (GGT, U/L), Total Protein (g/dL), Albumin (g/dL), Globulin (g/dL), Albumin/Globulin Ratio (A/G Ratio), Blood Urea Nitrogen (BUN, mg/dL), Creatinine (mg/dL), BUN/Creatinine Ratio, Sodium (mEq/L), Potassium (mEq/L), Chloride (mEq/L), Calcium (mg/dL), and Phosphorus (mg/dL).
Nasal Wash Virus Recovery
Nasal washes were collected according to the schedule in Table 1, for determination of virus shedding by median tissue culture infectious dose (TCID50) analysis. Briefly, 1 mL of PBS (phosphate buffered saline) was used to flush both nostrils using a tuberculin syringe and a catheter. Once collected, nasal washes were stored at ≤−70°C until TCID50 analyses were performed.
Median Tissue Culture Infectious Dose (TCID50)
A standard TCID50 analysis was performed on collected nasal washes or tissues samples, wherein serial dilutions of nasal wash or tissue samples were inoculated onto a (Madin-Darby Canine Kidney) MDCK cell monolayer, and the presence of cytopathic effects (CPE) was scored. The dilution at which 50% of the inoculated wells demonstrated CPE (TCID50) was calculated using the Spearman Kärber method.
Microneutralization (MN) Assay
A standard microneutralization assay using MDCK cells and A/Vietnam/1203/04 was used to quantify antibody response to the vaccinations.
Hemagglutination Inhibition (HAI) Assay
A standard HAI assay using chicken red blood cells and Influenza Virus A/Brisbane/10/07, A/Brisbane/59/07, B/Florida/04/06, and A/Mexico/4108/09) was used to screen animals pre-study for possible previous exposure to the currently circulating influenza viruses. HAI assays using horse red blood cells were also used to measure titers of H5N1-specific antibody directed against A/Vietnam/1203/04.
Analytical and Statistical Plan
Statistical analysis was provided for hematology, clinical chemistry, survival, microneutralization, TCID50, weight change, temperature change, and time to death. Statistical analyses included, analysis of variance (ANOVA) models and log-rank tests. For the survival data, the SAS® MULTTEST procedure was used to adjust for multiple comparisons at the 0.05 level of significance using Bonferroni-Holm method.
Results
Vaccinating animals with the heterologous vaccine in combination with the aluminum hydroxide adjuvant significantly improved survival in HPAI-challenged animals
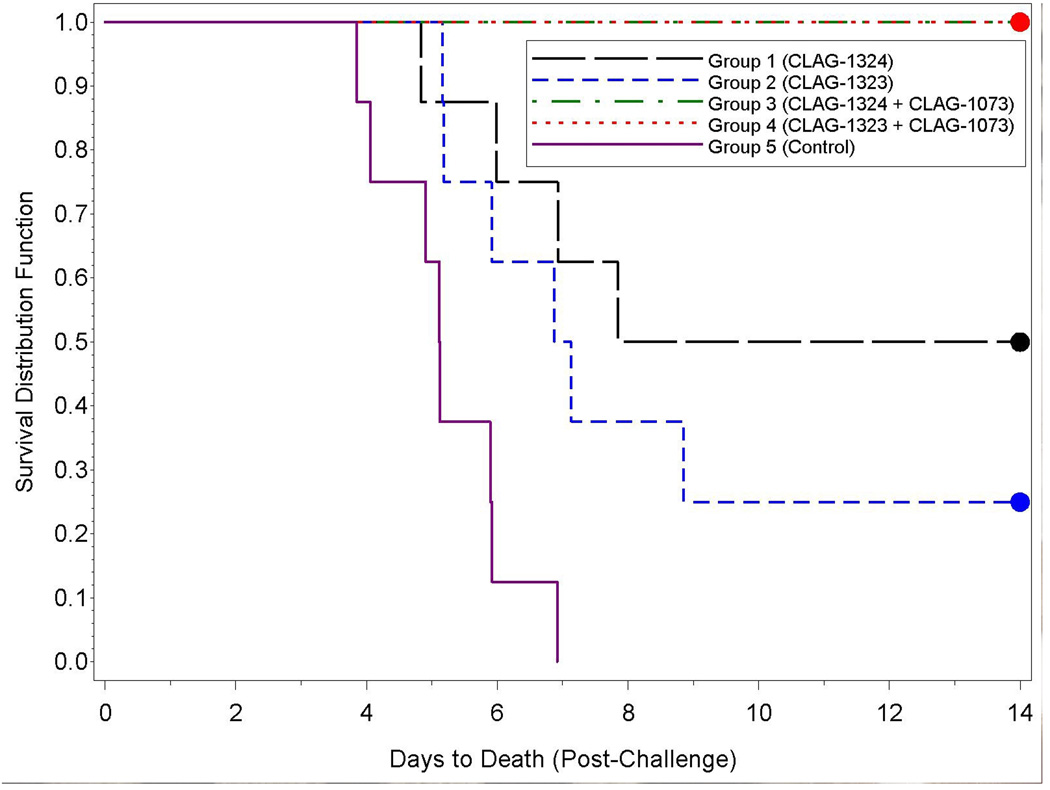
Ferrets were vaccinated with CLAG-1324 (30 µg) or CLAG-1323 (15 µg) which are monovalent (A/Indonesia/05/05) subvirion vaccines. The Aluminum Hydroxide Adjuvant (CLAG-1073) was used as an adjuvant for the Group 3 and 4 animals. Following a lethal challenge with A/Vietnam/1203/04, 4/8 animals vaccinated with CLAG-1324 (Group 1) survived; 2/8 animals vaccinated with CLAG-1323 (Group 2) survived; 8/8 animals vaccinated with CLAG-1324 and CLAG-1073 (Group 3) survived; 8/8 animals vaccinated with CLAG-1323 and CLAG-1073 (Group 4) survived; and 0/8 unvaccinated control animals (Group 5) survived (Figure 1). The highest survival rates were observed in the Group 3 and 4 animals, the animals vaccinated with the vaccine and adjuvant (CLAG-1324/CLAG-1073 and CLAG-1323/CLAG-1073, respectively). All 16 of these animals survived challenge. In comparison, all eight of the unvaccinated control animals (Group 5) died as a result of challenge. The survival of the Group 3 and 4 animals was significantly greater than the Group 2 animals (Table 2). Additionally, the distributions of time-to-death were significantly different between the Group 3 and 4 animals when compared to the Group 2 and 5 animals (Table 3).
Figure 1.
Kaplan-Meier curves representing time-to-death from challenge and survival data for each group. All animals in Groups 3 and 4 survived the length of the study; therefore, their Kaplan-Meier survival estimates overlap at 1.0 for all study times.
Table 2.
Summary of the Two-sided Pairwise Fisher’s Exact Tests Comparing Survival Among the Groups.
| Group | Unadjusted P-values | Bonferroni-Holm Adjusted P-values | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| 2 | 0.6084 | 1.0000 | ||||||
| 3 | 0.0769 | 0.0070* | 0.4615 | 0.0559 | ||||
| 4 | 0.0769 | 0.0070* | 1.0000# | 0.4615 | 0.0559 | 1.0000 | ||
| 5 | 0.0769 | 0.4667 | 0.0002* | 0.0002* | 0.4615 | 1.0000 | 0.0016* | 0.0016* |
Significance at the 0.05 level.
A p-value of 1.00 was substituted when all animals in both groups survived.
Table 3.
Summary of the Pairwise Log-rank Tests Comparing Time-to-Death and Survival Among the Groups.
| Group | Unadjusted P-values | Bonferroni-Holm Adjusted P-values | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| 2 | 0.3429 | 0.6858 | ||||||
| 3 | 0.0250* | 0.0023* | 0.0998 | 0.0158* | ||||
| 4 | 0.0250* | 0.0023* | 1.0000# | 0.0998 | 0.0158* | 1.0000 | ||
| 5 | 0.0015* | 0.0103* | <0.0001* | <0.0001* | 0.0122* | 0.0513 | 0.0004* | 0.0004* |
Significance at the 0.05 level.
A p-value of 1.00 was substituted when all animals in both groups survived.
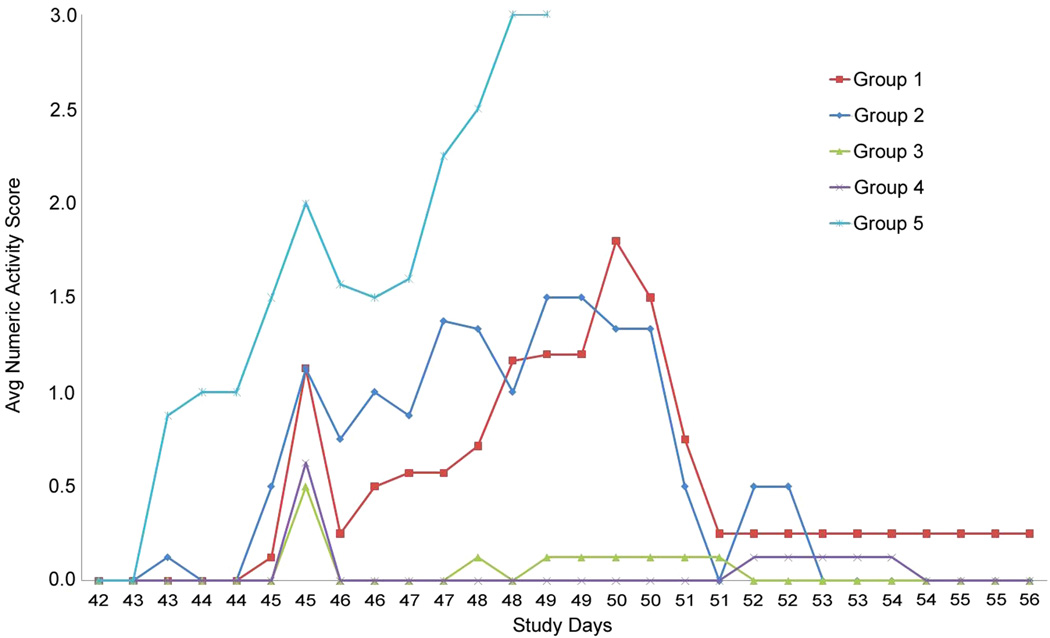
Most animals in all groups demonstrated moderate clinical signs associated with disease including inappetence, mucosal stool, and lethargy. However, animals from Group 5 demonstrated more advanced (severe) clinical signs which included diarrhea, weak limbs, hind limb paralysis, and torticollis. Activity scores were recorded twice daily along with clinical observations. Higher scores were associated with less alert and/or less playful behavior. Graphical representation of the number of animals exhibiting a maximum activity score of 0, 1, 2, or 3 illustrates a trend in which a greater number of animals with lower activity scores (i.e. more active) can be seen in vaccinated groups (Figure 2). The Group 3 and 4 animals were observed to be more playful and alert than the Group 1, 2, and 5 animals, while the Group 1 and 2 animals appeared more playful and alert when compared to the Group 5 animals. The highest activity scores (representing less alert and playful animals) were recorded among the Group 5 animals. The last of the Group 5 animals died on Study Day 49, while the Group 1 and 2 animals were the least active on Study Days 49 and 50. These animals then became more active and were relatively normal by Study Day 51. The Group 3 and 4 animals were the least active on Study Day 45, but demonstrated relatively normal activity scores by Study Day 46. The animals remained active for the remainder of the study. Altogether, these data are in agreement with the clinical observation data and demonstrate vaccine and vaccine/adjuvant efficacy against a lethal challenge of A/Vietnam/1203/04. Overall, animals vaccinated with the vaccine and adjuvant (Groups 3 and 4) were more active than the animals vaccinated with only the vaccine (Groups 1 and 2) or the unvaccinated control animals (Group 5), while the animals vaccinated with only the vaccine were more active than the unvaccinated control animals.
Figure 2.
Average activity score reported by group per day.
HPAI challenge resulted in an increase in body temperatures
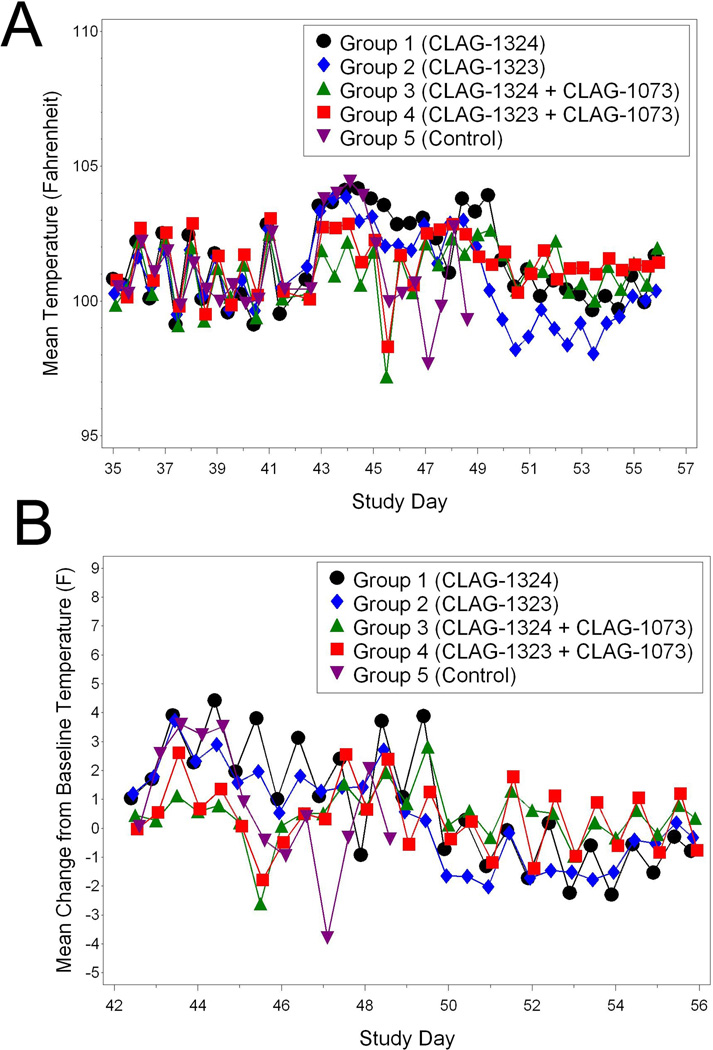
The pre-challenge animal temperatures, which were relatively constant, revealed a diurnal pattern. Therefore, the AM and PM temperatures were analyzed separately. It should be noted that no significant differences among the groups were observed for the AM or PM baseline temperatures. The Group 1 animals experienced increases in temperature from Study Days 43 through Study Day 49 when compared to baseline temperatures (Figure 3A and 3B). Increases in the Group 2 temperatures when compared to baseline were also observed from Study Days 42 through Study Day 48. The Group 3 animals experienced temperature increases from Study Days 43 through 54; while the Group 4 animals experienced increases from Study Days 43 through 55. The Group 5 animals also experienced temperature increases when compared to baseline from Study Day 43 and 44. Significant temperature differences were observed among the groups on various days from Study Days 43 through Study Day 54 (Figure 3A and 3B). The mean temperature increases from baseline observed in Groups 1, 2, and 5 were greater than the temperature observed in the Group 3 animals. In all, most animals in all Groups experienced temperature increases post-challenge when compared to baseline. However, the temperatures from the surviving vaccinated animals returned to relatively normal levels by the study end, which infers a level of recovery and protection from challenge with a lethal dose of A/Vietnam/1203/04.
Figure 3.
Temperatures associated with H5N1-challlenged animals. (A) Group mean temperatures over time (Study Day 35 through 56). (B) Group mean change from baseline temperature over time (Study Days 42 through 56).
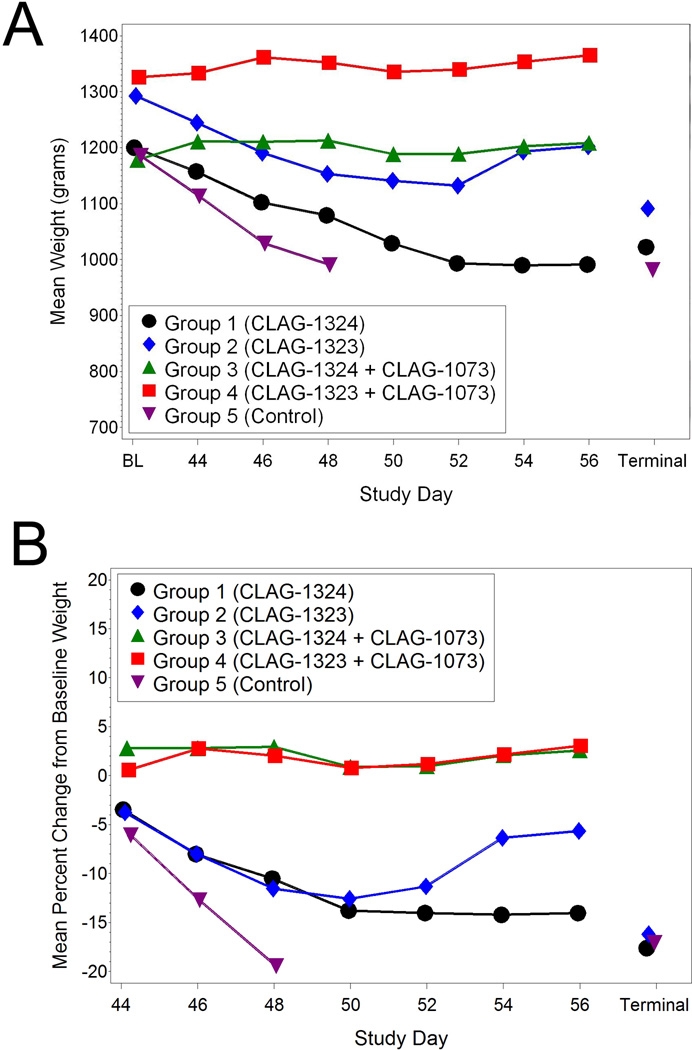
Body weights were maintained in animals vaccinated with the heterologous vaccine in combination with the aluminum hydroxide adjuvant
The Group 1 animals experienced significant decreases from baseline body weight on all post challenge study days (Figure 4A). The mean percent change in body weights from baseline eliminates bias and accounts for heavy or light animals (Figure 4B). The Group 2 animals experienced significant decreases from baseline weight on Study Days 44 through 52. However, the Group 3 and 4 animals did not experience any significant weight loss. The Group 3 animals experienced significant increases from baseline weight on Study Days 44,46, and 48 while the Group 4 animals experienced a significant increase from baseline weight on Study Day 46. The Group 5 animals experienced significant decreases from baseline weight on all post challenge study days. As a whole, surviving animals that did not receive the vaccine in combination with adjuvant (Groups 1, 2, and 5) experienced significant decreases from baseline weight, while the Group 3 and 4 animals that did receive the vaccine in combination with adjuvant never experienced mean decreases from baseline weight during the study. The mean decrease from baseline weight in the Control Group (Group 5) was significantly different than the changes from baseline weight in the groups that received the vaccine and adjuvant (Groups 3 and 4). The mean decrease from baseline weight in the Group 5 animals was also significantly greater than the Group 1 and 2 animals on study day 46. Additionally, the mean decreases from baseline weight in the Group 1 and 2 animals on study days 44 through 50 were significantly different than the mean increases from baseline weight in the Group 3 and 4 animals. In all, the Group 3 and 4 animals receiving both the vaccine and adjuvant maintained their body weights throughout the study suggesting some level of protection from administration of the vaccine and adjuvant.
Figure 4.
Weight changes associated with H5N1-challenged animals. (A) Group mean weights over time. (B) Group mean change from baseline weights over time.
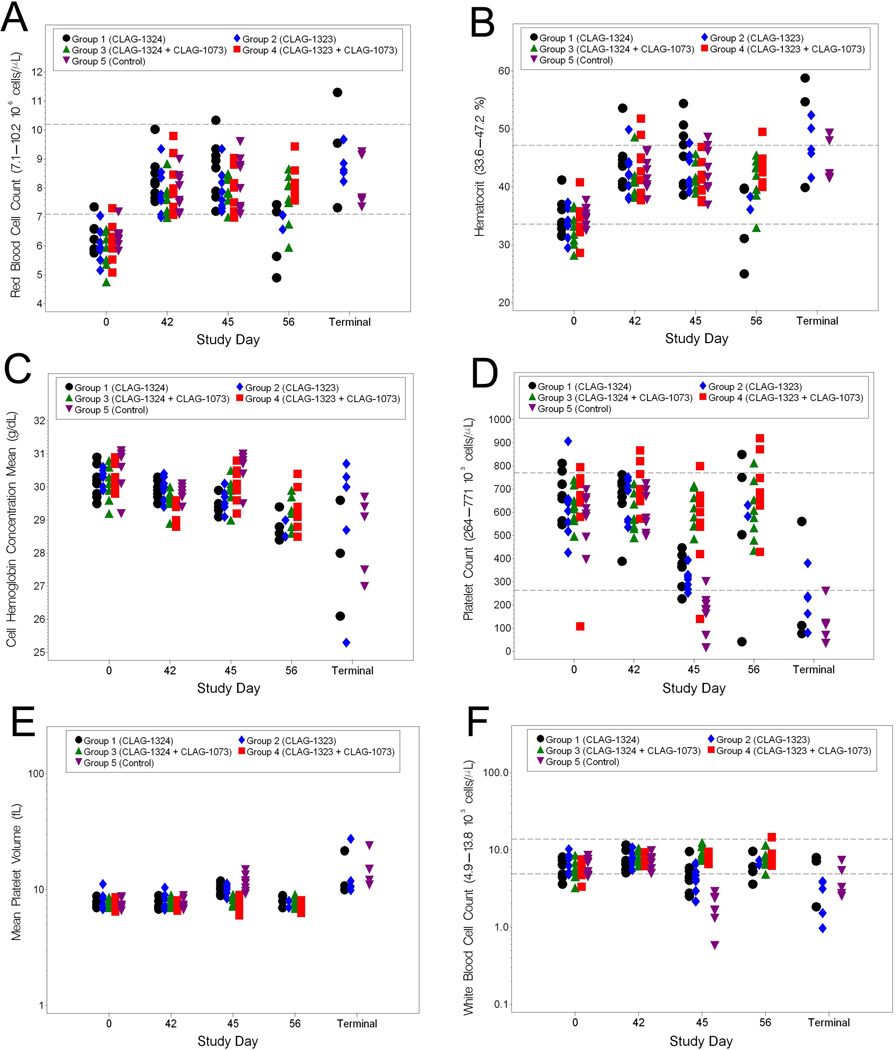
Hematology and Clinical Chemistry Changes Associated with H5N1 Challenge
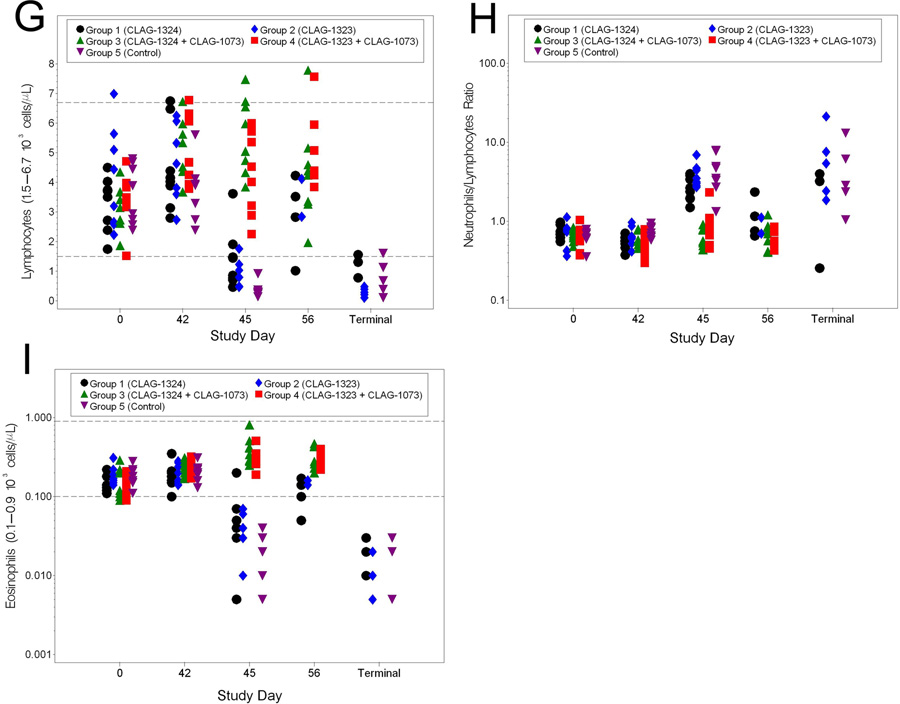
Blood samples for the hematology analyses were collected on Study Days 0 (prior to vaccination), 42 (prior to challenge), 45, 56, and at the Terminal Day, if possible. Blood collected on the day of challenge (Study Day 42), served as a baseline hematology sample for comparisons to post-challenge samples. Among the red blood cell parameters, RBC and HCT experienced similar results in infected animals (5A and 5B). There were significant increases in these three parameters from baseline in all groups on Study Day 42. However, these increases remained within the normal limits associated with ferrets. Specifically, the RBC values for all groups were similar on Study Days 42 and 45; however, as a whole, the RBC and HCT values for Groups 1 and 2 were significantly lower than those of the Groups 3 and 4 animals on Study Day 56. Increases in RBC could have resulted due to stress on the animal resulting from infection. Increases in HCT levels may have resulted from the animals suffering from slight dehydration as a result of infection. For CHCM, the mean shifts from baseline in the Group 1 and 2 animals were significantly different from those in the Group 4 and 5 animals on Study Day 45 (5C). CHCM values for the terminal samples had an extreme variation when compared to baseline. However, it should be noted that CHCM values were all within the normal range for ferrets. Thus, there is likely little biological significance related to the CHCM shifts. The mean shifts in PLT observed in the Group 1, 2, and 5 animals were significantly different from the Group 3 animals on Study Day 45 (5D and 5E). Furthermore, significantly decreased platelet counts in the Group 1, 2, and 5 animals were observed among the terminal samples. This result was expected since thrombocytopenia is a typical of H5N1 infection in ferrets. MPV in the terminal samples were elevated when compared to the baseline samples, but still within the normal range for the animals. Among the white blood cell parameters, WBC, lymphocytes, N/L ratio, and eosinophils exhibited significant changes from baseline (5F–5I). On Study Day 45, the Group 5 animals exhibited leukopenia. Additionally, leukopenia was observed in the terminal samples collected from animals that succumbed to infection. Lymphocyte numbers were also decreased in the terminal samples from the animals that succumbed to infection. The N/L ratio from the Group 1, 2, and 5 animals was increased on Study Day 45. This same pattern was observed in most of the terminal samples collected from animals that succumbed to disease. Lastly, a decrease in eosinophils was observed on Study Days 45 in the Group 1, 2, and 5 animals. This was similar to the decrease in eosinophils numbers observed in animals that succumbed to disease. In all, the data suggest a level of vaccine efficacy, especially when evaluating leukopenia and thrombocytopenia, two parameters that are hallmarks of HPAI infection in ferrets.
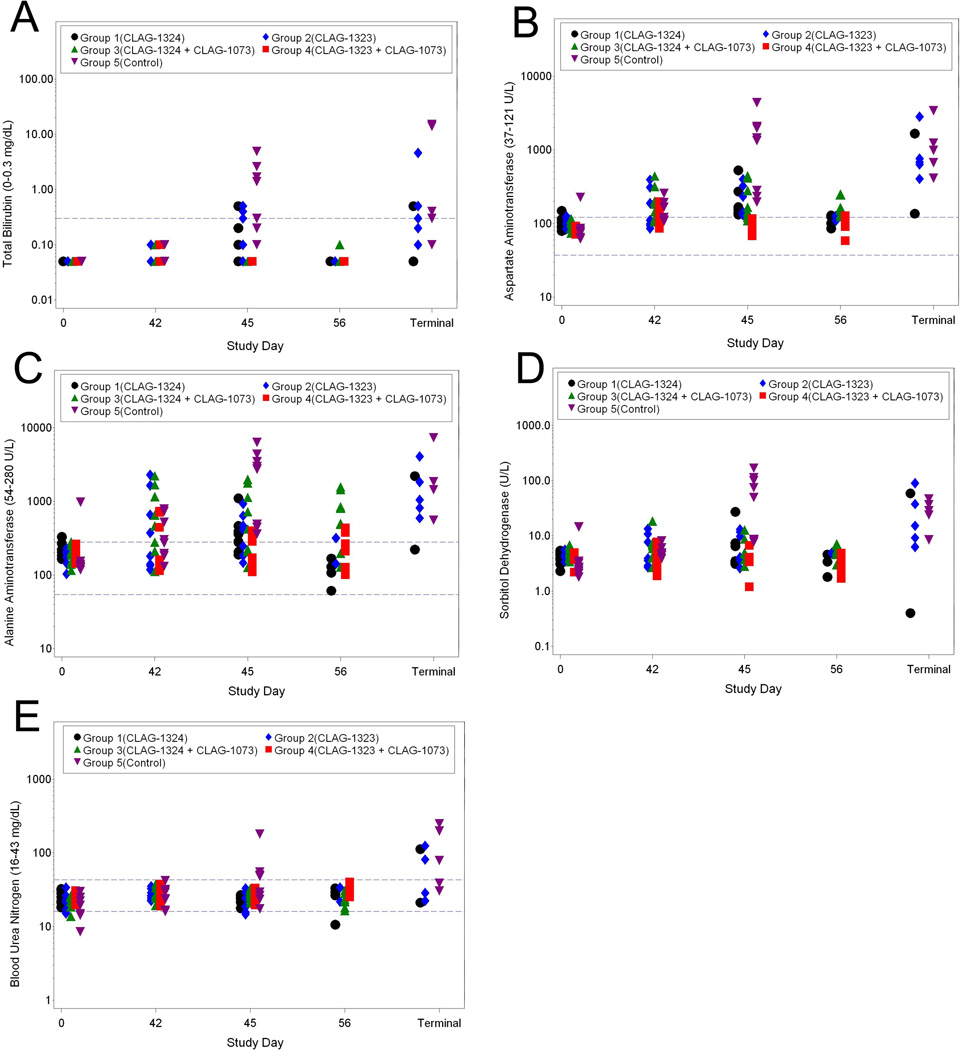
Clinical chemistry data were collected on Study Days 0 (prior to vaccination), 42 (prior to challenge), 45, 56, and at the terminal day when possible. Among the liver function parameters, there were significant increases in total bilirubin, AST, ALT, and SDH in the Control Group 5 animals on Study Day 45, which likely infers some level of liver damage (6A–6D). Additionally, increases in BUN levels on Study Day 45 were observed in the Group 5 animals when compared to the vaccinated animals, which infers some level of kidney damage in the Group 5 animals (6E). Animals that succumbed to disease also had elevated BUN. In all, these data suggest less kidney and liver damage in vaccinated animals (Groups 1–4) when compared to the unvaccinated control animals.
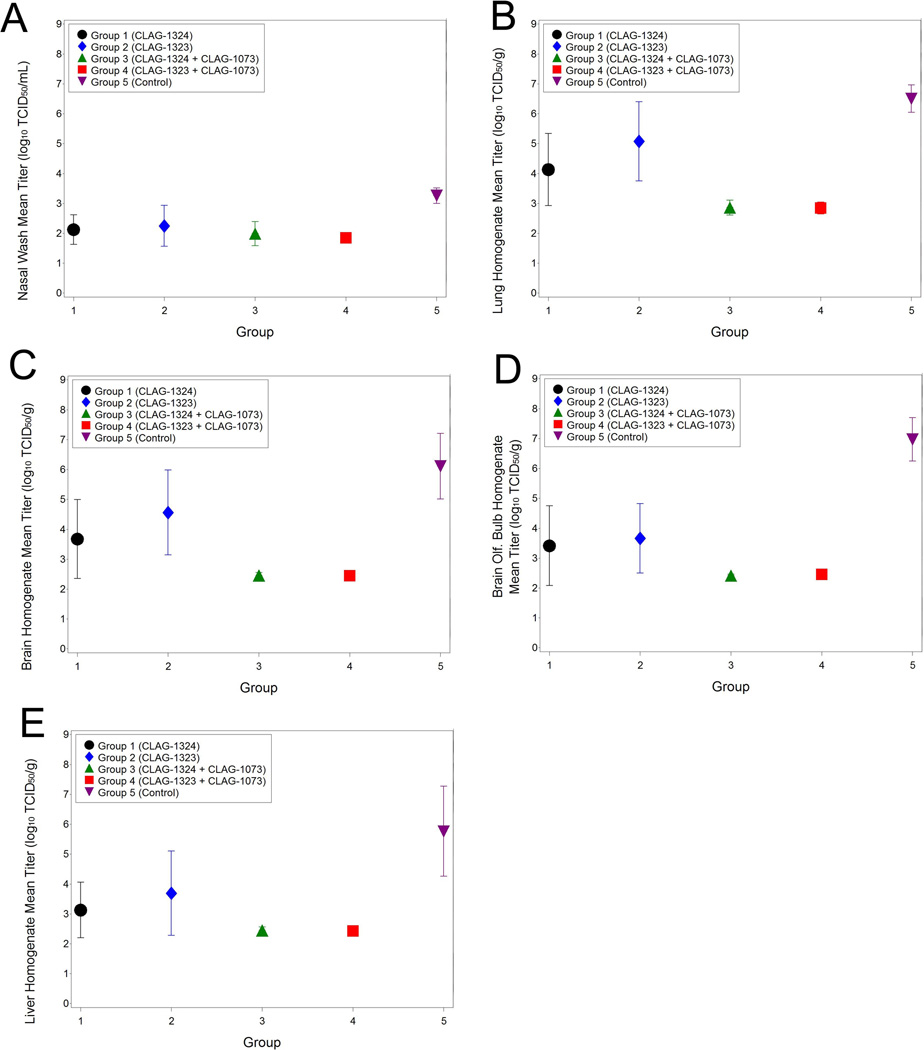
Virus recovery from nasal washes and post mortem tissues from infected animals
Virus titers were measured from nasal washes and post-mortem tissues from infected animals (Table 4). Virus was detected in the nasal wash fluid from all Group 5 ferrets (8/8) on Study Day 45. However, virus was detected in the nasal wash in only two of the Group 1, two of the Group 2 animals, one of the Group 3, and 4 of the Group 4 animals. Similar viral recovery results were observed in the tissues of the animals. Virus was recovered from the lung, brain, olfactory bulb, and liver in all of the Group 5 animals. Minimal virus was detected in the tissues collected from the Group 3 and 4 animals. However, the virus detected in the Groups 3 and 4 animals were below the limit of detection of the assay in all harvested tissues. Additionally, quantifiable virus was recovered from tissues from four of the eight Group 1 animals (the lungs [4/8 animals], brains [3/8 animals], olfactory bulb [3/8 animals], and liver [2/8 animals]) and six of the eight Group 2 animals (lungs [6/8 animals], brains [5/8 animals], olfactory bulb [4/8 animals], and liver [3/8 animals]). However, the amount of quantifiable virus harvested from all of the tissues of the Group 5 animals was significantly greater than the amount of virus harvested from the vaccinated animals (Table 4). The virus recovered from the nasal washes and tissues from the infected ferrets demonstrates vaccine efficacy since a statistically significant lower amount of quantifiable virus was collected from the vaccinated animals.
Table 4.
Virus Shedding and Virus Isolations from Specific Tissues Isolated from Individual Animals
| Animal ID | Group | Day 45 Nasal Wash log TCID50/ml |
Lung homogenate log TCID50/g |
Brain homogenate log TCID50/g |
Brain Olf. Bulb homogenate log TCID50/g |
Liver homogenate log TCID50/g |
|---|---|---|---|---|---|---|
| 4013 | 1 | <LOQ | 3.76 | <LOQ | <LOQ | <LOQ |
| 4014 | 1 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4016 | 1 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4031 | 1 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4043 | 1 | <LOQ | 5.16 | 5.44 | 2.95 | 4.63 |
| 4046 | 1 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4048 | 1 | 3.33 | 6.56 | 6.38 | 4.62 | 5.18 |
| 4030 | 1 | 2.77 | 5.61 | 4.67 | 6.94 | <LOQ |
| 4007 | 2 | <LOQ | 4.94 | <LOQ | <LOQ | 4.84 |
| 4008 | 2 | <LOQ | 4.94 | 6.28 | 4.2 | <LOQ |
| 4015 | 2 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4022 | 2 | <LOQ | 5.05 | 6.08 | 5.75 | <LOQ |
| 4023 | 2 | <LOQ | 7.43 | 5.58 | 5.42 | 5.21 |
| 4042 | 2 | 3.19 | 6.54 | 5.49 | 4.01 | 6.76 |
| 4044 | 2 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4003 | 2 | 3.89 | 5.83 | 5.42 | <LOQ | <LOQ |
| 4004 | 3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4005 | 3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4009 | 3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4012 | 3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4021 | 3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4025 | 3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4034 | 3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4037 | 3 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4020 | 4 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4024 | 4 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4027 | 4 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4029 | 4 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4033 | 4 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4035 | 4 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4036 | 4 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4047 | 4 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 4002 | 5 | 3.33 | 5.73 | 4.77 | 6.15 | 3.53 |
| 4010 | 5 | 3.33 | 6.83 | 8.2 | 7.1 | 3.67 |
| 4017 | 5 | <LOQ | 6.9 | 7.4 | 7.36 | 3.56 |
| 4018 | 5 | 3.33 | 6.29 | 6.35 | 8.06 | <LOQ |
| 4026 | 5 | 3.89 | 7.01 | 4.91 | 7.38 | 3.75 |
| 4028 | 5 | 2.91 | 5.68 | 5.31 | 5.96 | 3.79 |
| 4041 | 5 | 3.05 | 6.57 | 4.93 | 5.87 | 7.52 |
| 4045 | 5 | 3.47 | 7.03 | 7.87 | 7.87 | 3.56 |
<LOQ: Below the limit of quantitiation
Serology (Immunogenicity) following vaccinations
In order to assess immunogenicity following vaccinations, sera were collected on Study Days 0, 21, 42, and 56, along with terminal samples, if possible, and analyzed by hemagglutination inhibiting (HAI) or microneutralization (MN) assays (Figure 7A–7B). No ND50 (antibody titers neutralizing 50% of the inoculum virus) titers were measured on Study Day 0 and only two Group 3 animals demonstrated any ND50 titers on Study Day 21 as measured by the MN assay. On Study Day 42, the ND50 titers in the Groups 3 and 4 animals were significantly greater than titers in the other groups (Groups 1, 2, and 5). On Study Day 56, measurable ND50 titers were observed in all surviving vaccinated animals. When compared to the ND50 titers from vaccinated terminal samples (animals that died as result of infection), all surviving animals had greater ND50 titers, suggesting higher immunogenicity following vaccination. No hemagglutination inhibition was observed on Study Day 0, and only one Group 3 animal (the same animal with a measurable ND50 titer on Study Day 21) yielded hemagglutination inhibition on Study Day 21. By Study Day 42, HAI titers against A/Vietnam/1203/04 began to increase to measurable, albeit, low levels. However, by Study Day 56, animals from Groups 3 and 4 had significantly higher levels of HAI titers than those in Groups 1 and 2. In all, the serology data demonstrate a greater immune response in the Group 3 and 4 animals when compared to the Group 1 and 2 animals, which may have impacted the survival of the Group 3 and 4 animals when compared to the other groups.
Figure 7.
Serology results associated with H5N1 challenge: (A) Microneutralization Data by Study Day and Group (Means and 95% confidence intervals for Log10 ND50) and (B) HAI Data by Study Day and Group.
Discussion
With the emergence of H5N1 viruses in different countries around the world, economic and social well-being are being impacted. Therefore, efforts to develop immunogenic vaccines against H5N1 viruses have become a priority. At this time, the H5N1 viruses cannot be transmitted human-to-human and transmission from fowl to humans remains inefficient 12. Often, transmission from fowl to human, which is usually the result of handling sick or dead poultry during the week prior to onset of disease 13, 14, results in fatal consequences. However, specific genetic mutations may enable the virus to be transmitted from human-to-human. Because of this, and because disease continues to spread in both humans and animals, H5N1 needs to be considered a potentially serious pandemic threat 12.
Currently, antiviral agent efficacy varies among circulating strains of H5N1 15. Clade 1 viruses appear to be more sensitive to oseltamivir than clade 2 isolates from Indonesia and Turkey 15. As for vaccines, the development of an effective vaccine poses some obstacles. The H5 hemagglutinin appears to be a weak immunogen. Thus, whole virus vaccines are more immunogenic than subunit vaccines. Typically, subunit vaccines have limited cross-reactivity, so the vaccine would have to match the pandemic strain 12. Therefore, improving the immunogenicity of influenza vaccines is paramount in minimizing the impact of influenza epidemics. Thus, alum as a vaccine adjuvant has been proposed to improve the immunogenicity of Influenza vaccines 16. Subunit vaccines with adjuvants lead to a reduction in the amount of vaccine antigen required to induce an immune response. In some studies, the use of alum adjuvants does not necessarily lead to improved responses to H5 vaccines 15. However, other studies have demonstrated that the addition of alum adjuvants have led to increased antibody levels that resulted in the neutralization of homologous and heterologous Influenza strains in mice 17, 18. As reported in other mouse studies 17, 18, the use of heterologous subvirion vaccine with an aluminum hydroxide adjuvant led to increased immunogenicity in ferrets when compared to the antigen. Furthermore, all ferrets that received the vaccine and adjuvant were protected from a lethal dose of A/Vietnam/1203/04.
The primary objective of this study was to determine the efficacy of a heterologous vaccine administered with and without the aluminum hydroxide adjuvant in the A/Vietnam/1203/04 ferret challenge model. The ferret is the preferred preclinical challenge model to evaluate influenza vaccine efficacy because of the susceptibility to influenza infection and the display of clinical signs 11. Additionally, increased survival and a decrease in disease severity has been associated with a two vaccine dose regimen 19. Therefore, the four groups of ferrets received different concentrations of the antigen or the antigen in combination with the adjuvant in a two dose regimen to determine whether the adjuvant improved vaccine efficacy. The Group 5 animals served as the non-vaccinated control animals. The animals were vaccinated twice, on Study Days 0 and 21, and challenged with a lethal dose of A/Vietnam/1203/04 (Study Day 42). Vaccine efficacy was evaluated by assessing clinical signs (clinical observations, body weights, and temperatures), virus titers in nasal washes and select tissues, and survival. Vaccination of the animals led to an increase in survival and morbidity parameters when compared to the non-vaccinated control animals. Inclusion of the adjuvant to the vaccine led to increased survival when compared to the vaccinated and control (non-vaccinated) animals. No survival was observed in the untreated control animals (Groups 5). These results were similar to other published reports that observed better protection in mice vaccinated in combination with an adjuvant 17, 18. While a dose response was observed in animals vaccinated with either 30 µg or 15 µg of the vaccine, no dose response was measured when animals were vaccinated with different concentrations of the vaccine in combination with the adjuvant. The addition of the adjuvant led to total protection regardless of the concentrations tested. In addition, H5N1 influenza viruses can be isolated from the brain and lung of infected ferrets 20, thus viral titers in the various portions of the brain and lung were analyzed in the current study. Ferrets vaccinated with the vaccine and adjuvant had lower viral titers in the brain homogenate, the brain olfactory bulb homogenate, and in the lung homogenate when compared to the vaccinated and control animals. Less viral titers in the brain and lung in vaccinated animals when compared to control animals suggests a level of vaccine efficacy.
Vaccine efficacy was further supported by the lower degree of temperature increases of the vaccinated animals when compared to the untreated control animals, in addition to the absence of weight loss among the vaccinated animals. The animals that were vaccinated with both the vaccine and the adjuvant demonstrated the least amount of weight loss, which were similar. In fact, these animals, as a whole, did not lose a significant amount of weight after infection. Weight loss is one physical biomarker associated with a lethal dose of A/Vietnam/1203/04 and previous studies have used weight loss as a parameter to evaluate vaccine efficacy 19. The Group 3 and 4 animals maintained weight throughout the study. The Group 1 and 2 animals did lose weight, but the weight loss was statistically different than the weight loss associated with the unvaccinated control animals (Group 5). Thus, the lack of weight loss is another parameter that demonstrates vaccine efficacy in this current study. Additionally, severe clinical observations consistent with a lethal dose of A/Vietnam/1203/04 were consistently observed among the Group 5 unvaccinated control animals. These observations were less severe and sporadic among the surviving vaccinated animals from Groups 1, 2, 3, and 4. To further assess vaccine efficacy, clinical pathology in the infected animals was evaluated. Hematological and clinical chemistry changes among the groups also suggest a level of vaccine efficacy in treated animals when compared to the untreated animals, which is in agreement with other studies that evaluated vaccines and alum adjuvants 21. The elevated RBC count and hematocrit may be indicative of hypoxia. Thrombocytopenia and lymphopenia associated with the animals that succumbed to disease are common signs of H5N1 infection in humans 15. The elevated bilirubin, AST, ALT, SDH, and BUN observed in the animals that succumbed to disease suggest a level of liver and kidney damage. These parameters were relatively normal in the survivors at the end of the study, which may suggest a level of protection within these tissues in vaccinated animals. In all, animals vaccinated with both the vaccine and the adjuvant did not demonstrate as significant of a change from baseline in these clinical pathology parameters when compared to the unvaccinated control animals.
Altogether, the survival, clinical pathology, viral shedding, viral titers in the tissue, temperature, weight, and serology data suggest a dose response and some level of protection from a lethal challenge of A/Vietnam/1203/04 in ferrets (the preferred H5N1 animal model) vaccinated with the inactivated heterologous monovalent subvirion vaccine and more complete protection independent of dose in the animals that were vaccinated twice (separated by 21 days) with the vaccine and adjuvant. Furthermore, complete protection (100%) was observed when the alum adjuvant was administered in addition to the inactivated heterologous monovalent subvirion vaccine. Thus, inactivated monovalent subvirion vaccines in combination with an alum adjuvant require more evaluation as a vaccine platform against H5N1 influenza viruses.
Figure 5.
Hematology changes associated with H5N1-challenged animals: (A) RBC counts, (B) Hematocrit, (C) CHCM, (D) Platelet count, (E) Mean platelet volume, (F) WBC count, (G) Lymphocytes, (H) Neutrophils/Lymphocytes Ratio, and (I) Eosinophils.
Figure 6.
Clinical Chemistry changes associated with H5N1 challenge: (A) Total bilirubin, (B) AST, (C) ALT, (D) SDH, and (E) BUN.
Acknowledgements
We would like to thank Jennifer Cross, Vanessa Little, and Krisztina Janosko and the rest of the scientific staff at the Battelle for their time and effort with this project. We would also like to thank David Gilligan and Drs. Martin Crumrine and Rachelle Salomon for critically reviewing the manuscript. This study was funded with funds from the National Institute of Allergy and Infectious Diseases (contract: N01-AI-30061/TO 0011/C22).
Reference List
- 1.Crosby A. Epidemic and Peace. Westport, CT: Greenwood Press; 1976. [Google Scholar]
- 2.Webster RG, Bean W, Gorman O, Chambers T, Kawaoka Y. Evolution an decology of influenza A viurses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taubenberger J, Reid A, Lourens R, Wang R, Jin G, Fanning T. Characterization of the 1918 influenza viurs polymerase genes. Nature. 2005;437:889–893. doi: 10.1038/nature04230. [DOI] [PubMed] [Google Scholar]
- 4.Bragstad K, Jorgensen P, Handberg K, Mellergaard S, Corbet S, Fomsgaard A. New avian influenza A virus subtype combination H5N7 identified in Danish mallard ducks. Virus Research. 2005;109:181–190. doi: 10.1016/j.virusres.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Cumming G, Caron A, Abolnik C, Cattoli G, Bruinzeel L, Burger C, Cecchettin K, Chiweshe N, Mochotlhoane B, Mutumi G, Ndlovu M. The ecology of influenza A viruses in wild birds in Southern Africa. Ecohealth. 2011 doi: 10.1007/s10393-011-0684-z. in press. [DOI] [PubMed] [Google Scholar]
- 6.Williams R, Xiao X, Peterson A. Continent-wide association of H5N1 outbreaks in wild and domestic birds in Europe. Geospatial Health. 2011;5:247–253. doi: 10.4081/gh.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO. 2008
- 8.Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, Zambon MC. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. The Lancet. 2001;357:1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 9.Treanor JJ, Wilkinson BE, Masseoud F, Hu-Primmer J, Battaglia R, O'Brien D, Wolff M, Rabinovich G, Blackwelder W, Katz JM. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001;19:1732–1737. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- 10.Reuman PD, Keely S, Schiff GM. Assessment of signs of influenza illness in the ferret model. Journal of Virological Methods. 2004;24:27–34. doi: 10.1016/0166-0934(89)90004-9. [DOI] [PubMed] [Google Scholar]
- 11.Zitzow LA, Rowe T, Morken T, Shieh WJ, Zaki S, Katz JM. Pathogenesis of Avian Influenza A (H5N1) Viruses in Ferrets. J Virol. 2002;76:4420–4429. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peiris M, de Jong M, Guan Y. Avian Influenza Virus (H5N1): a Threat to Human Health. 2007:243–267. doi: 10.1128/CMR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Areechokchai D, Jiraphongsa C, Laosiritaworn Y, Hanshaoworakul W, O'Reilly M. Investigation of avian influenza (H5N1) outbreak in humans--Thailand, 2004. MMWR Morb Mortal Wkly Rep. 2006;28:3–6. [PubMed] [Google Scholar]
- 14.Dinh P, Long H, Tien N, Mai le T, Phong le H, Tuan le V, Van Tan H, Nguyen N, Van Tu P, Phuong N, et al. Risk factors for human infection with avian influenza A H5N1, Vietnam, 2004. Emerging Infectious Diseases. 2006;12:1841–1847. doi: 10.3201/eid1212.060829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Writing Committee of the Second World Health Organization Consultation of Clinical Aspects of Human Infeciton with Avian Influenza A (H5N1) Virus. Update on Avian Influenza A (H5N1) Virus Infection in Humans. N Engl J Med. 2008;358:261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 16.Hehme N, Engelmann H, Kuenzel W, Neumeier E, Saenger R. Immunogenicity of a monovalent, aluminum-adjuvanted influenza whole virus vaccine for pandemic use. Virus Research. 2004;103:163–171. doi: 10.1016/j.virusres.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Harada Y, Ninomiya-Mori A, Takahashi Y, Shirakura M, Kishida N, Kageyama T, Tada Y, Tashiro M, Odagiri T. Inactivated and adjuvanted whole-virion clade 2.3.4 H5N1 pre-pandemic influenza vaccine possesses broad protective efficacy against infection by heterologous clades of highly pathogenic H5N1 avian influenza virus in mice. Vaccine. 2011;29:8330–8337. doi: 10.1016/j.vaccine.2011.08.091. [DOI] [PubMed] [Google Scholar]
- 18.Ninomiya A, Imai M, Tashiro M, Odagiri T. Inactivated influenza H5N1 whole-virus vaccine with aluminum adjuvant induces homologous and heterologous protective immunities against lethal challenge with highly pathogenic H5N1 avian influenza viruses in a mouse model. Vaccine. 2007;25:3554–3560. doi: 10.1016/j.vaccine.2007.01.083. [DOI] [PubMed] [Google Scholar]
- 19.Gustin KM, Maines TR, Belser JA, van Hoeven N, Lu X, Dong L, Isakova-Sivak I, Chen LM, Voeten JT, Heldens JGM, van den Bosch H, Cox NJ, Tumpey TM, Klimov AI, Rudenko L, Donis RO, Katz JM. Comparative Immunogenicity and Cross-Clade Protective Efficacy of Mammalian Cell-Grown Inactivated and Live Attenuated H5N1 Reassortant Vaccines in Ferrets. Journal of Infectious Diseases. 2011;204:1491–1499. doi: 10.1093/infdis/jir596. [DOI] [PubMed] [Google Scholar]
- 20.Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, Nguyen DC, Szretter KJ, Chen LM, Thawatsupha P, Chittaganpitch M, Waicharoen S, Nguyen DT, Nguyen T, Nguyen HHT, Kim JH, Hoang LT, Kang C, Phuong LS, Lim W, Zaki S, Donis RO, Cox NJ, Katz JM, Tumpey TM. Avian Influenza (H5N1) Viruses Isolated from Humans in Asia in 2004 Exhibit Increased Virulence in Mammals. J Virol. 2005;79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Layton R, Gigliotti A, Armijo P, Myers L, Knight J, Donart N, Pyles J, Vaughan S, Plourde J, Fomukong N, Harrod K, Gao P, Koster F. Enhanced immunogenicity, mortality protection, and reduced viral brain invasion by alum adjuvant with an H5N1 split-virion vaccine in the ferret. PLoS One. 2011;6:e20641. doi: 10.1371/journal.pone.0020641. [DOI] [PMC free article] [PubMed] [Google Scholar]