Abstract
The retrotrapezoid nucleus contains Phox2b-expressing glutamatergic neurons (RTN-Phox2b neurons) that regulate breathing in a CO2-dependent manner. Here we use channelrhodopsin-based optogenetics to explore how these neurons control breathing in conscious and anesthetized adult rats. Respiratory entrainment (pacing) of breathing frequency (fR) was produced over 57% (anesthetized) and 28% (conscious) of the natural frequency range by burst activation of RTN-Phox2b neurons (3–8 × 0.5–20 ms pulses at 20 Hz). In conscious rats, pacing under normocapnic conditions increased tidal volume (VT) and each inspiration was preceded by phase 2 (active) expiration, denoting abdominal muscle contraction. During long-term pacing, VT returned to pre-stimulation levels suggesting that central chemoreceptors such as RTN-Phox2b neurons regulate VT partly independently of their effect on fR. Randomly applied light-trains reset the respiratory rhythm and shortened the expiratory phase (TE) when the stimulus coincided with late-inspiration or early-expiration. Importantly, continuous (20 Hz) photostimulation of the RTN-Phox2b neurons and a saturating CO2 concentration produced similar effects on breathing that were much larger than those elicited by phasic RTN stimulation.
In sum, consistent with their anatomical projections, RTN-Phox2b neurons regulate lung ventilation by controlling breathing frequency, inspiration and active expiration. Adult RTN-Phox2b neurons can entrain the respiratory rhythm if their discharge is artificially synchronized but continuous activation of these neurons is much more effective at increasing lung ventilation. These results suggest that RTN-Phox2b neurons are no longer rhythmogenic in adulthood and that their average discharge rate may be far more important than their discharge pattern in driving lung ventilation.
Keywords: Central chemoreceptors, breathing, respiration, retrotrapezoid nucleus, lentivirus, medulla oblongata, optogenetics
Introduction
The parafacial region of the medulla oblongata regulates breathing (Smith et al., 1989; Janczewski and Feldman, 2006; Guyenet et al., 2010; Marina et al., 2010; Kanbar et al., 2010). This heterogeneous region includes the retrotrapezoid nucleus (RTN), a group of glutamatergic, non-catecholaminergic Phox2b-expressing neurons (RTN-Phox2b neurons) that contributes to central respiratory chemoreception (Mulkey et al., 2004; Stornetta et al., 2006; Takakura et al., 2008; Gourine et al., 2010; Guyenet et al., 2010; Marina et al., 2010).
During the perinatal period, RTN-Phox2b neurons are both “inspiratory rhythmogenic” and central respiratory chemoreceptors, i.e., they are pH sensitive, have intrinsic bursting properties and their synchronized discharge defines the breathing rate by pacing the pre-Bötzinger complex (Onimaru et al., 2008; Thoby-Brisson et al., 2009; Goridis and Brunet, 2010). In adulthood, the respiratory rhythm seems generated by a more distributed network of pontomedullary neurons although the pre-Bötzinger complex remains the network’s lynchpin (Rubin et al., 2009; Nattie and Li, 2009; Abdala et al., 2009b; Feldman, 2011). While RTN-Phox2b neurons retain their central chemoreceptor properties and their ability to activate the respiratory rhythm in adulthood (Li and Nattie, 2002; Mulkey et al., 2004; Takakura et al., 2008; Gourine et al., 2010; Guyenet et al., 2010; Marina et al., 2010), the persistence of their rhythmogenic properties remains controversial (Guyenet et al., 2005; Oku et al., 2007).
Activation of RTN-Phox2b neurons increases breathing rate and inspiratory effort dramatically in conscious and anesthetized adult rats (Abbott et al., 2009b; Kanbar et al., 2010). In other preparations, inhibiting these neurons impacts active expiration primarily (Abdala et al., 2009a) with modest effects on rate and inspiratory effort (Marina et al., 2010). According to others, active expiration is generated by a parafacial expiratory oscillator that does not include the RTN-Phox2b neurons (Janczewski and Feldman, 2006; Pagliardini et al., 2011).
We postulate that, in the intact adult brain, the RTN-Phox2b neurons recruit inspiratory and expiratory muscles in an orderly sequence that depends on the degree to which they are activated by CO2 or by other mechanisms (Guyenet et al., 2010). This notion stems primarily from three observations. Anatomically verified RTN-Phox2b neurons are activated by CO2, both in slices and in vivo (Guyenet et al., 2005; Lazarenko et al., 2009). Selective activation of RTN-Phox2b neurons powerfully increases breathing rate and tidal volume in conscious rats (Kanbar et al., 2010). RTN-Phox2b neurons innervate all segments of the ponto-medullary respiratory network including where bulbospinal expiratory premotor neurons reside (Abbott et al., 2009b; Bochorishvili et al., 2011).
In the present study we used channelrhodopsin-based optogenetics to test whether stimulation of RTN-Phox2b neurons in conscious rats activates the three major components of breathing (rate, inspiration and active expiration) and whether the respiratory frequency can be set by phasically activating these neurons.
Materials and Methods
Animals
The experiments were performed in male Sprague-Dawley rats (Taconic) in accordance with NIH and Institutional Animal Care and Use Guidelines. The Animal Research Committee of the University of Virginia approved all procedures and protocols.
Lentiviral vector and injection
We used a previously described lentiviral vector that expresses enhanced humanized channelrhodopsin-2 fused with mCherry under the control of the artificial Phox2b specific promoter PRSx8 ((pLenti-PRSx8-hChR2 (H134)-mCherry-WPRE, henceforth abbreviated PRSx8-ChR2-mCherry) (Hwang et al., 2001; Abbott et al., 2009a). Phox2b is a transcription factor expressed in subsets of brainstem neurons including the RTN and catecholaminergic cells (Pattyn et al., 1997; Stornetta et al., 2006). The virus was produced by the University of North Carolina virus core and diluted to a final titer of 3.0 × 108 viral particles.ml−1 with sterile PBS before injection into brain. We verified that the above viral dilution produced specific transfection of Phox2b-expressing neurons (see Results section).
Surgery for lentiviral injection was performed in 225–250 g rats under anesthesia((ketamine (75mg/kg), xylazine (5mg/kg) and acepromazine (1mg/kg) administered i.m.)) using standard aseptic methods. A glass pipette with external tip diameter 25 μm was inserted into the brain through a dorsal craniotomy. The lentivirus was delivered through the pipette by controlled pressure injection (60 PSI, 3–8 ms pulses). The injections were targeted unilaterally to four sites separated by 200 μm (2 × 2 grid; total volume 420 nl). These sites were located 100–200 μm below the caudal end of the facial motor nucleus whose lower edge was identified in each rat by antidromic mapping as previously described (Abbott et al., 2009a). In 2 animals, anti-dopamine-β-hydroxylase antibody conjugated to saporin (antiDβH-sap; Advanced Targeting Systems) was also injected at 0.22 μg per μl bilaterally (2 sites total, 100 nl per site) into the region of the lateral horn of the second thoracic segment (1.0–1.2 mm lateral of midline, 1 mm below the lateral sulcus) in order to destroy the C1 neurons that project to the spinal cord. For experiments in conscious rats, a guide cannula (Plastics One) was implanted one week after virus injection at the same stereotaxic coordinates as the injections of virus. This cannula extended 5 mm below the surface of the skull and was secured with jeweler screws and dental cement. In all animals, the craniotomy was patched with sterile absorbable cellulose and wounds were closed with sutures, wound clips and/or cyanoacrylic glue. Following surgery, rats were treated at 0 and 24 hrs with an antibiotic (ampicillin, 100 mg/kg, i.m.) and analgesic (ketorolac, 0.6 mg/kg, i.p.). Animals were maintained for no less than 2 weeks before they were used in physiological experiments. The surgical procedures, virus injections and guide cannula placement produced no observable behavioral or respiratory effects and these rats gained weight normally.
Physiological experiments in anesthetized rats (350–425 g; N=7)
A tracheostomy was performed under general anaesthesia with 5% isoflurane in 100% oxygen. Artificial ventilation (1ml/100g, 60–80 min−1) was initiated with 3.0–3.5% isoflurane in pure oxygen and maintained throughout surgical procedures. Frequency of ventilation was adjusted as needed to maintain end-tidal CO2 at the desired level. Blood gases were not measured. Arterial PCO2 was estimated from measurements of end-tidal CO2 (Guyenet et al.,2005). This variable was monitored with a micro-capnometer (Columbus Instruments). Positive end-expiratory pressure (1.5 cm H2O) was maintained throughout to minimize atelectasis. Mean blood pressure remained above 110 mmHg and the phrenic nerve activity could be silenced by lowering end-tidal PCO2 to 3.5–4.5 %. In all animals, the femoral artery and vein were cannulated and both vagus nerves were cut distal to the carotid bifurcation as previously described (Guyenet et al., 2005b). The right phrenic nerve was dissected and recorded to monitor central respiratory drive to the diaphragm (Moreira et al., 2006; Takakura et al., 2006). The light stimulus was delivered with a 200 μm diameter fiber optic (Thorlabs) inserted vertically into the brainstem. To increase the accuracy of the fiber optic placement, the facial motor nucleus was mapped with antidromic field potentials using a glass pipette and, based on these coordinates, the optical fiber was positioned approximately 200–400 μm dorsal to the retrotrapezoid nucleus.
Upon completion of surgical procedures, urethane (1.3 g/kg, 20% w/v in sterile H2O) was slowly infused i.v. and isoflurane was withdrawn. Supplementary doses of 0.1 g/kg of urethane were administered intravenously as needed (up to an additional 0.25 g/kg during the course of an experiment) to maintain an appropriate depth of anesthesia, based on the absence of respiratory changes and blood pressure changes <10 mmHg in response to a firm paw pinch. A minimum of 30 minutes was allowed following the removal of isoflurane before commencing experiments. At that point, rats were administered the muscle relaxant pancuronium (initial dose 1 mg/kg i.v. plus additional 0.2 mg/kg doses if needed).
Integrated phrenic nerve discharge (iPND) was obtained after rectification and smoothing (τ=0.03s) of the original signal (bipolar recording, 0.2–3 kHz band). The product of the respiratory frequency (fR, in min−1) times the amplitude of iPND (PNDamp, in arbitrary units) was used to measure the total respiratory neural output of the preparation per minute (fR × amp). PNDamp and fR × amp were normalized by assigning a value of 100 to the maximum amplitude of iPND or fR × amp observed at saturation of the chemoreflex (~8% etCO2) within each experiment and a value of 0 to periods of apnea.
Whole body unrestrained plethysmography in conscious rats (300–400 g; N=6)
Tidal volume (VT, ml), breathing frequency (fR, breaths.min−1), and minute volume (MV, ml.min−1) were evaluated by whole-body plethysmography. The rats were placed in a perspex flow-through chamber (Buxco) and allowed 1–2 hrs to acclimate to their new surroundings. Afterwards, rats were briefly (<5 minutes) anesthetized with isoflurane (1.5% maintenance after 3% induction in O2) to allow insertion of a custom optic fiber assembly into the implanted guide. A fiber length of 9.5 to 10 mm produced the best results. Animals were returned to the plethysmography chamber and allowed to recover from anesthesia for over 30 minutes before commencing experimental protocols. The chamber was continuously flushed with dry room-temperature air (24 ± 0.5° Celsius) delivered by 3 computer-driven mass-flow regulators connected to pure O2, N2, and CO2 (1 L min−1). The flow signal was recorded and analyzed using EMKA IOX 2.7 (EMKA Technologies) and Spike 5.21 (CED) software. Breathing parameters were calculated from a calibrated flow signal derived from a differential pressure sensor connected to the plethysmography chamber using the equation of Drorbaugh and Fenn ( 1955). Real-time chamber conditions (temperature and humidity) and atmospheric pressure were continuously measured and the calculation of tidal volume was automatically adjusted to account for changes in these variables.
Photostimulation parameters
Photostimulation of the ventrolateral medulla was done with a diode-pumped 473 nm blue laser (CrystaLaser) controlled by a Grass model S88 stimulator (AstroMed Inc.). The light output of the laser at the tip of the optical fiber was measured before implantation into the brain and was always set at 9 mW as per our prior work (Abbott et al., 2009b). The respiratory effects elicited by continuous stimulation at 20 Hz were a saturable function of the pulse width. RTN neurons fire up to 20Hz under anesthesia (Fortuna et al., 2009). Their dynamic range in a conscious animal is unknown. A frequency of 20–23Hz was chosen based on prior evidence that using the same CHR2 variant (H134R), these neurons can follow this frequency in anesthetized rats (Abbott et al., 2009b). In order to minimize potential heat damage to the tissue, we determined the shortest pulse required to produce the maximum ventilatory effect in each rat and used this parameter throughout the experiment (range 0.5 to 10 ms). Train photostimulation was done with brief stimulus trains (anesthetized rats: 5–20 ms light pulses, 20 Hz; train duration: 328 ± 18 ms; conscious rats: 1–10 ms pulses, 20–23 Hz; train duration: 200 ± 48 ms). Each train lasted for the equivalent of 59 ± 5 (anesthesia) and 91 ± 24 degrees (conscious) of the total respiratory cycle.
Data analysis and statistics
Analysis was done using Excel 2010 and GraphPad 10 software. Results are presented as mean ± SEM. Student’s t-test, one and two-way ANOVA were used as required.
Periods of respiratory pacing were identified by the constant latency between the onset of light trains and inspiration, and a 1:1 coupling of the light train and inspiration for greater than 15 cycles. For the description of the range of entrainment and the average stimulation phase during entrainment, 15–20 cycles of entrainment at each stimulation frequency were extracted and averaged for each animal and expressed in Hz before being grouped. The stimulus trains used to test for respiratory entrainment were delivered in step increments of 5 bursts.min−1 (0.83 Hz). Train frequency ranged from resting frequency to the maximum breathing rate observed during hypercapnia, although every increment was not tested in each animal. In some cases, each stimulation bout was separated by a rest period and in others cases, the frequency was stepped up and down in a staircase fashion and maintained at the various plateaus for around one minute. The “stimulus phase” represents the interval between the beginning of inspiration and the onset of the stimulus and is expressed as a fraction of the total respiratory cycle (=360°).
The effects of artificially increasing fR on VT and MV in conscious rats was assessed by calculating the average of the first and last 10 breaths (early and late, respectively) of an entrainment period and comparing these values to a period of unperturbed breathing (rest). Data for this analysis was taken from periods when fR was held at a single elevated entrainment frequency or over several increments in entrainment frequency.
To evaluate active expiration we measured the average expiratory volume (15–20 breaths) during the first and the second half of the expiratory period and measured the ratio between the two (late: early expiration).
Phase resetting was evaluated by comparing the induced phase (time from the onset of the stimulus to inspiration in next respiratory cycle) against the stimulus phase. In this analysis, the stimulus phase reflects the period from inspiration to the stimulus in relation to the period of the preceding unperturbed respiratory cycle (i.e. expected phase or N−1). The phase of the N+1 cycle in this analysis was calculated by combining the period of the induced phase and N+1 and dividing this value by the expected phase.
To analyze the perturbations in respiratory timing and effort produced by brief activation of Phox2b neurons, we evaluated the changes in inspiratory time (TI), expiratory time (TE), total respiratory cycle time (TTot) and peak iPND (anesthetized) or VT (conscious) produced by trains of light delivered at low frequency randomly within the respiratory cycle. For TE and TTot, the perturbed respiratory cycle containing the stimulus train (N) and the following respiratory cycle (N+1) were normalized against the unperturbed cycle preceding N (i.e. N−1 or control cycle). This analysis controls for long-term changes in respiratory cycle time and effort. All data was grouped according to stimulus phase (30° bands) and significant phase perturbations of the TE and TTot were determined by comparing the values of normalized N vs. N+1 cycle using a two-way ANOVA with Bonferroni’s multiple comparisons. Analysis of TI and peakPND/VT required an evaluation of 3 breaths (N, N+1 and N+2, where N+2 is the breath following N+1), because the N and N+1 cycle were both subject to the effects of the stimulus; for example, a late stimulus phase interacts solely with the N+1 TI cycle and not the N TI cycle because the stimulus occurs after the end of the N TI cycle. The TI and PNDamp/VT of the N and N+1 (presented separately) were normalized against the N−1 cycle, grouped as per TE and TTot and statistically compared to the N+2 cycle to identify significant perturbations.
Histology
After the end of the physiological procedures the rats were deeply anesthetized with 5% isoflurane and perfused with buffered 4% paraformaldehyde. After 1–2 days of post-fixation 30 μm-thick coronal brain sections were cut with a vibrating microtome (Leica). Coronal brain sections were obtained from every rat to locate the tip of the optical fiber and to determine the distribution and phenotype of the ChR2-transfected neurons. In experiments testing the specificity of the virus, Phox2b was detected using a rabbit antibody (provided by J.-F. Brunet, ENS, France; 1:800 dilution) and visualized using appropriate fluorescent secondaries depending on the requirements of the experiments. To evaluate the proportion of ChR2-mCherry-expressing neurons that were Phox2b-positive, we identified mCherry by its natural fluorescence and Phox2b was detected by immunohistochemistry as described previously (Abbott et al., 2009b). To evaluate the proportion of catecholaminergic or cholinergic neurons that expressed ChR2-mCherry, all markers were detected by immunohistochemistry (mCherry with Living Colors rabbit anti-DsRed rabbit polyclonal antibody, Clontech, dilution 1:500; tyrosine hydroxylase with mouse anti-TH or sheep anti-TH 1:1000, both from Chemicon; Choline acetyltransferase with goat anti-ChAT, dilution 1:50, Millipore) followed by appropriate fluorescent secondaries. All histological methods and antibody specificity are detailed in (Kang et al., 2007; Stornetta et al., 2009; Abbott et al., 2009b).
All histological material was examined and photographed using a Zeiss Axioimager.Z1 (Carl Zeiss Microimaging) with a Zeiss Axiocam MRc digital camera (basic resolution 1388 × 1040 pixels). For estimation of the number and phenotype of transfected neurons, a one in six series of 30 micron coronal sections that encompassed the transfected brain area (from 7–10 sections/rat) was plotted using the Neurolucida computer assisted graphing software (MicroBrightfield) as previously described (Stornetta et al., 2002).
RESULTS
High frequency photostimulation of RTN Phox2b neurons increases breathing in anesthetized and conscious rats
Consistent with our previous observations (Abbott et al., 2009b; Kanbar et al., 2010), continuous high frequency stimulation (20–23 Hz, 30 s) produced extremely large increases in breathing rate and amplitude in anesthetized and conscious normocapnic rats (Figure 1A,B). In normocapnic (3.5–5.3% et CO2) anesthetized rats (N=5) 20 Hz stimulation increased PND frequency by xx% (from 16 ± 2 to 49 ± 4 bursts.min−1, P<0.001), PND amplitude by 57% (from 42 ± 6 to 67 ± 4 units, 100 units representing maximal amplitude at saturation of the chemoreflex, P<0.05) and the total respiratory drive (fR × amp) by 350% of baseline (from 16 ± 5 to 73 ± 9 units, 100 units being the maximum value observed at saturation of the chemoreflex, P<0.001) (Figure 1A,C). Mean arterial pressure increased modestly in normo- and hypercapnic conditions (7 ± 1 and 9 ± 2 mmHg, respectively; P=0.38). The light-induced increase in breathing frequency had fast on- and biphasic off-kinetics, the latter consisting of a virtually instantaneous but partial reduction of fR followed by a slow, approximately exponential decay to baseline (Figure 1A and Abbott et al., 2009b). The initial fast component was most conspicuous when the magnitude of the frequency response was large.
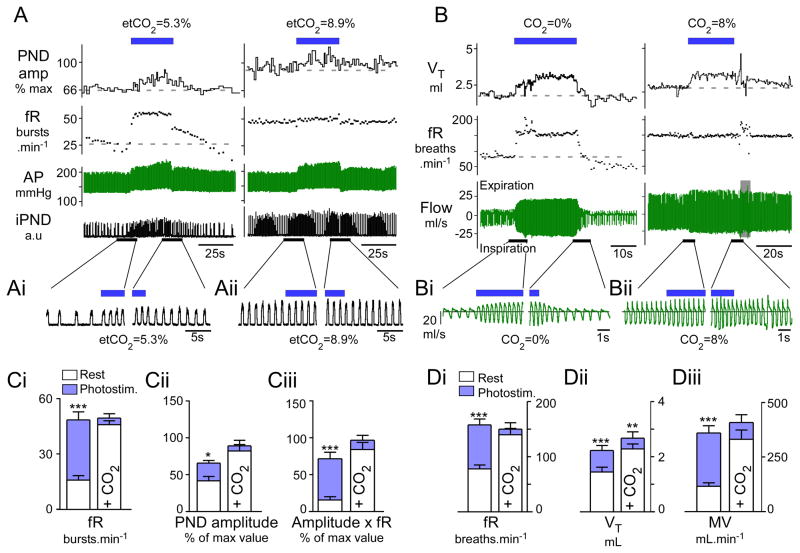
Figure 1. Breathing stimulation elicited by photostimulation of RTN Phox2b neurons is occluded by hypercapnia.
A) urethane-anesthetized, vagotomized and ventilated rat. Left panel: normocapnia (etCO2: 5.3%). Right panel: hypercapnia (etCO2: 8.9%). RTN photostimulation (20 Hz, 5 ms pulses) at blue bar. Under normocapnia, respiratory frequency (fR) increased within seconds to a steady-state level greater than 50 bursts.min−1 and decayed in two-step fashion (rapid, then slow) upon cessation of the stimulus. Phrenic nerve discharge peak amplitude (PNDamp) progressively increased during the stimulus and also had a slow decay upon cessation of the stimulus. During hyperoxic hypercapnia (right panel) the same stimulus produced no further increase of fR, a lesser increase in peak amplitude but an identical increase in arterial pressure (AP). B) Conscious rat in flow-through whole-body plethysmography chamber. Left panel: hyperoxic normocapnia (100% O2); right panel: hypercapnia (8% CO2,balance O2). Continuous RTN stimulation (23 Hz, 3ms pulses, 30s total duration, blue bar) raised breathing frequency (fR) and tidal volume (VT) with identical onset kinetics as in the anesthetized rat. Following the end of the stimulus, fR and VT dropped precipitously below baseline in the conscious rat presumably because of hyperventilation-induced hypocapnia. During hyperoxic hypercapnia (right panel), RTN photostimulation produced a small increase in VT but no increase in fR. Ai–ii, Bi–ii) Expanded traces highlighting the periods surrounding the beginning and end of the stimulus. Note that in plethysmography records, inspiration is downward. C) Group data for anesthetized rats. From left to right: Ci: fR, Cii: PND peak amplitude and, Ciii: double product (fR × PND amplitude) at normal (left columns) and elevated CO2 (right columns). D) Group data for conscious rats. From left to right: Di: fR, Dii: VT and Diii: double product MV (minute volume) at normal (left columns) and elevated CO2 (right columns; 8% CO2 in pure oxygen). *** P<0.001, **P< 0.01, * P<0.05 between pre-stimulus baseline and peak values during the stimulation period; two way ANOVA with multiple comparisons)
When the anesthetized rats were exposed to levels of CO2 that saturated the chemoreflex(etCO2 > 7%), photostimulation at 20 Hz produced no further increase in breathing parameters (fR: 47 ± 2 to 50 ± 2 bursts.min−1, NS, PNDamp: 84 ± 9 to 91 ± 8 units, NS; fR × PNDamp: 86 ± 10 to 99 ± 7% units, NS) (Figure 1B,C).
In conscious rats (N=6) breathing quietly in hyperoxic conditions (100% O2, no added CO2), photostimulation at 20–23 Hz increased breathing frequency by 202% (from 79 ± 7 to 160 ± 11 breaths.min−1, P<0.001), VT by 65% (from 1.5 ± 0.2 to 2.3 ± 0.2 ml, P<0.05), and total ventilation, MV, by 310% (from 117 ± 26 to 363 ±33 ml.min−1, P<0.01) (Figure 1B,D). The onset kinetics was similar in conscious and anesthetized rats. The increase in breathing frequency reached its maximum value almost instantaneously and remained at that level throughout the stimulus whereas the increase in amplitude had biphasic kinetics consisting of an initial rapid increase followed by a more gradual recruitment. At the end of the stimulation a large breathing undershoot (fR and VT) was observed in the conscious rats. This undershoot plausibly resulted from the reduction in arterial pCO2 caused by the preceding hyperventilation because it was not observed in the anesthetized rats in which the use of muscle relaxants and artificial ventilation should have prevented any change in blood pCO2 during activation of the respiratory network.
As in the anesthetized rats, the effects produced by high frequency photostimulation were also largely occluded when the conscious rats were exposed to high levels of CO2 (8% in pure oxygen; fR from 142 ± 12 to 152 ± 12 breaths.min−1, NS; MV from 334 ± 43 to 411 ±36 ml.min−1, NS) (Figure 1D), although VT was increased over and above the values produced by increasing inspired CO2 to 8% (from 2.3 ± 0.2 to 2.7 ± 0.2 ml, P<0.01; Figure 1Dii).
Activation of RTN Phox2b neurons paces breathing in anesthetized and conscious rats
Prior experiments revealed that, in adult rats, activation of the respiratory network by RTN neuron stimulation decays with a ten second time constant (Abbott et al., 2009b). This result suggested that RTN neurons operate via a type of synaptic transmission that is far too slow for them to have any kind of respiratory rhythmogenic role in adult rodents in which the respiratory cycle lasts from 0.5 to 1 second. We tested whether phasic activation of the RTN neurons has the capability of entraining the respiratory rhythm in the adult. A negative outcome would have ruled out a rhythmogenic function on grounds of excessively slow synaptic transmission but this is not what we observed.
We applied short trains of laser light (3–8 pulses per train, 20 Hz, 0.5–10 ms pulses) to the RTN region in order to test whether the breathing rate could be entrained to the stimuli. Entrainment was successful at train frequencies within the intrinsic operating range of breathing frequency. The elevated breathing frequency could be maintained for extended periods of time (range 0.5–15.5 min) in both anesthetized rats (Figure 2A) and conscious rats (Figure 2B).
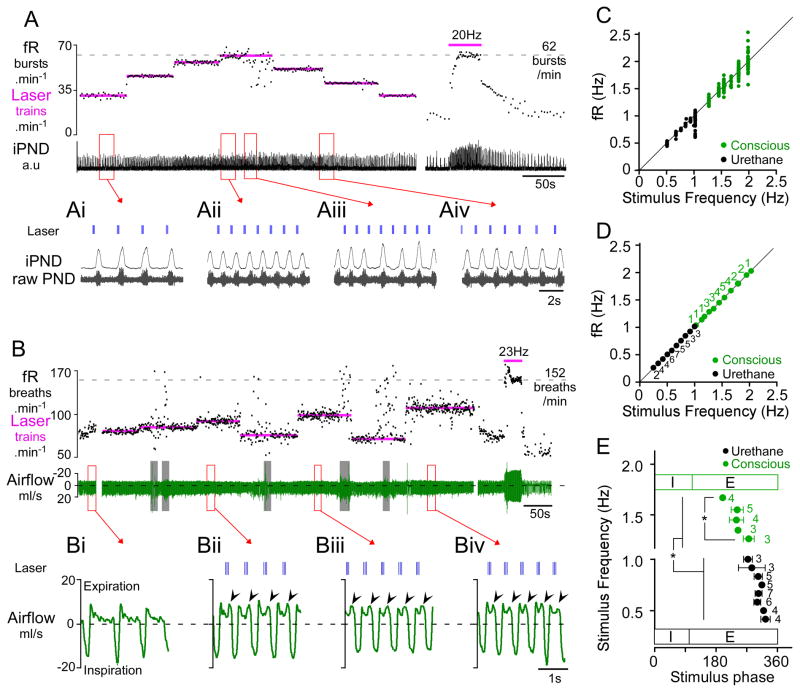
Figure 2. Respiratory pacing by intermittent photostimulation of RTN neurons in anesthetized and conscious rats.
A) Urethane-anesthetized rat. Photostimulus trains (4 × 20 ms pulses at 20 Hz) entrained PND over a range of 32 breaths.min−1 (from 25–60 bursts.min−1; see also excerpts Ai, Aii). The maximal fR observed in this rat during continuous RTN stimulation at 20 Hz was 62 bursts.min−1 (at right in panel A). At low frequencies (Ai), the laser trains settled in late expiration, whereas at higher frequencies, the stimulus train settled earlier during the expiratory phase (Aii). RTN stimulation at 60 trains.min−1 caused arrhythmia (excerpt Aiii). Aiv shows that fR follows the transition from one stimulus frequency to the next (50 to 40 trains.min−1). B) Conscious quiet rat. Light pulses (3 × 3 ms pulses at 20 Hz) entrained fR in the range of 75–120 breaths.min−1. Resting fR was close to 60 min−1 in this rat and the maximum fR seen in presence of high CO2 was 152 breaths.min−1. Continuous high frequency stimulation (23 Hz, 3 ms, at right in panel B) produced a more robust activation of breathing than short bursts. Grey bars indicate periods of behavioral artifacts (e.g sniffing, movement). Bi–Biv excerpts from panel B showing expanded plethysmography traces at various entrainment frequencies. Bi: quiet breathing in the absence of stimulation. Note monophasic decay of airflow during expiration consistent with passive lung recoil. Bii–Biv: airflow traces at three different pacing frequencies. Expiratory flow becomes biphasic with pronounced increase in flow during late expiration (arrows), indicative of active expiration. Note that the stimulation trains settle in mid-expiration during entrainment. C) Relationship between stimulus train frequency and fR for the rats shown in A (anesthetized-black) or B (conscious-green). The instantaneous frequency of each burst/breath is plotted against the frequency of the light trains. Note the increased variability of fR at higher entrainment frequencies, reflecting poor entrainment. D) Group data showing the reliability of entrainment at a range of stimulus frequencies in anesthetized (N=7) and conscious (N=6) animals. Each point represents the average fR (± SEM) when entrainment was successful. Adjacent numbers indicate the number of animals that were successfully entrained at a given stimulation frequency. Every frequency was not tested in every animal hence the varying number of determinations for each tested frequency. E) Relationship between the stimulus train frequency and the phase angle of the stimulus train during successful entrainment of the breathing cycle. Note that as stimulus (and entrainment) frequency increased, the phase angle of the stimulus train decreased. In conscious animals, the stimulus occurred at a significantly earlier phase in the respiratory cycle than in anesthetized rats. (* P<0.05).
In the urethane-anesthetized rats (N=7), PND frequency varied from 24 ± 3 bursts.min−1 just above the apneic threshold (4–5% etCO2) to 61 ± 3 bursts.min−1 at saturation of the chemoreflex (range: 36 ± 5 bursts.min−1), with a frequency of 30 ± 2 bursts.min−1 during typical resting conditions. In these rats, intermittent stimulation entrained PND over a range of 21 ± 4 bursts.min−1, equivalent to 57 ± 11% of the full operating range.
In quiet conscious rats (N=6), the breathing frequency (excluding sniffing and other behavioral artifacts) varied from 73 ± 5 breaths.min−1 under hyperoxia to 151 ± 8 breaths.min−1 during exposure to 8% CO2 (balance oxygen) corresponding to a range of 78 ± 6 breaths.min−1. Breathing frequencies as low as 25 breaths.min−1 were observed following light-induced hyperventilation but such low frequencies were never observed under any other conditions. During periods of quiet breathing in these animals, RTN burst stimulation was able to entrain fR over a range of 22 ± 5 breaths/min, equivalent to 28 ± 6 % of the total operating range. In the case shown in Figure 2B, fR was entrained between 75 and 110 breaths.min−1 over more than 15 minutes despite brief behavioral disturbances. During periods of entrainment, a biphasic expiratory flow developed in all animals (Figure 2Bii–iv).
A scatter plot of the relationship between fR and stimulus frequency during respiratory pacing is shown in Figure 2C corresponding to the cases shown in Figure 2A and 2B. At each set stimulus frequency, the instantaneous fR of 15–20 breaths were plotted (in Hz). fR was reliably entrained at frequencies slightly greater than resting frequency and tended to become more variable in relation to the stimulus frequency with increasing frequencies of entrainment. At the limit of the entrainable range (>1 Hz in anesthetized rats and >2 Hz in conscious rats), entrainment failed and breathing became arrhythmic (Figure 2Aiii).
Figure 2D shows the grouped average frequency of a 15–20 breath segment during periods of entrainment under normocapnic conditions for all animals. The number of animals entrained at a given frequency is given by the numbers adjacent to each point. Entrainment in anesthetized and conscious rats was consistently observed at frequencies above resting fR. In anesthetized rats that responded particularly strongly to a 20 Hz stimulus (Figure 1A), intermittent stimulation could entrain PND burst frequency over the entire operating range (N=2, Figure 2A & C). Entrainment at average resting frequency was possible even in animals that showed relatively weak effects during continuous high frequency stimulation. Entrainment was not possible at frequencies greater than the intrinsic maximum of fR in anesthetized rats (i.e. 61 ± 3 bursts.min−1); stimulus frequencies equal to or above the maximum fR observed at high levels of CO2 produced arrhythmic PND bursting (Figure 2Aiii). Entrainment also failed if the stimulus frequency was set below the resting fR, but entrainment was possible at frequencies below the resting fR during periods of apnea (etCO2 < 4%) in anesthetized rats (Figure 3). In rats that were hyperventilated to maintain etCO2 slightly below the apneic threshold, RTN photostimulation produced periods of quantal entrainment in 6/7 animals (2:1 or 3:1 ratio, Figure 3). Either high levels of etCO2 and/or high basal respiratory drive prevented entrainment in anesthetized animals.
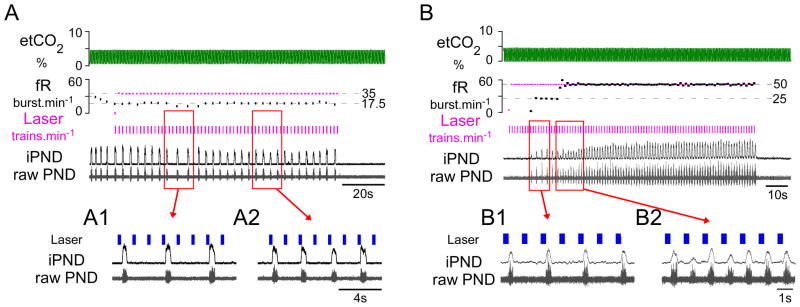
Figure 3. RTN photostimulation produces quantal breathing in urethane-anesthetized hypocapnic rats.
A) When ventilation was adjusted to maintained etCO2 close to the apneic threshold trains of laser light (7 × 5 ms pulses at 20 Hz; train frequency: 35 min−1) entrained PND in a 3:1 (Ai), or 2:1 ratio (Aii). B) Quantal entrainment in a different anesthetized rat with etCO2 maintained below apneic threshold. Laser trains (7 × 5 ms pulse at 20 Hz; train frequency: 50 min−1 ) delivered during phrenic apnea first producedquantal entrainment in a 2:1 ratio (Bi), eventually achieving 1:1 entrainment (Bii).
In conscious rats, the upper limit for entrainment was significantly lower than the maximum breathing rate (99 ± 7 vs 151 ± 10 breaths.min−1, P<0.01), but in 2 cases, entrainment was held at >120 breaths.min−1 in normocapnic conditions; and in one case we entrained fR at frequencies between 160 and 180 breaths.min−1 when inspired CO2 was elevated to 8%.
Interestingly, during entrainment, the photostimulus train always settled during mid- to late expiration in both anesthetized and conscious rats (Figure 2E). The train settled earlier during expiration as the entrainment frequency increased (P<0.0001). Finally, the phase delay between the onset of the stimulus and inspiration was longer in conscious animals than in urethane anesthetized animals (229 ± 7 vs. 244 ± 11°, P=0.0014), reflecting differences in neuronal excitability or some reorganization of the breathing network under anesthesia.
Prolonged entrainment of breathing at higher than baseline frequency causes compensatory decreases in tidal volume in conscious rats
Figure 4 shows the effect of prolonged respiratory pacing on tidal volume (VT) and total ventilation (MV) in a representative conscious rat. Light-driven hyperventilation (3 × 5 ms pulses at 20 Hz; train frequency: 87 or 95 min−1), caused a progressive reduction in VT following the initial increase caused by the stimulus. This gradual reduction of VT caused MV to decline substantially, though without ever reaching the pre-stimulation level (Figure 4A,B). On average, when fR was entrained at 18 ± 2 breaths above baseline, there was a significant reduction in VT (from 2.2 ± 0.3 to 1.8 ± 0.3 ml; P<0.01) and MV (from 207 ± 25 to 169 ± 19 ml.min−1; P<0.05) relative to the early period of the entrainment (Figure 4D). In these cases, 196± 31 s (range: 120–320 s) of entrainment was sufficient for VT to return to pre-stimulus values(1.8 ± 0.2 vs 1.8 ± 0.3 ml). MV was still elevated relative to baseline at the end of the entrainment period (169 ± 19 vs. 139 ± 16 ml.min−1; P<0.05) due to the elevated breathing frequency caused by the stimulus (P<0.01). At the end of the stimulus, fR and VT fell abruptly below resting values (Figure 4C). These after-effects were most likely caused by hypocapnia given that MV was still significantly above resting values at the end of the stimulus.
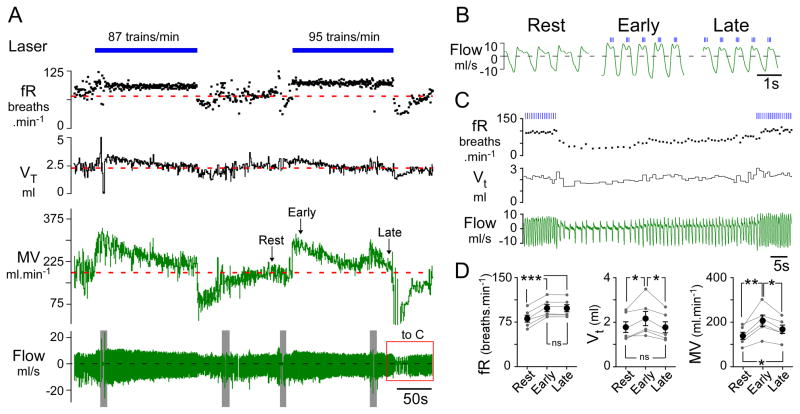
Figure 4. Respiratory pacing above resting frequency causes compensatory decrease in tidal volume in conscious rats.
A) Conscious quiet rat breathing pure oxygen. Prolonged respiratory pacing (3 × 5ms pulses at 20 Hz; train frequency: 87 and 95 trains.min−1) caused a gradual reduction of the tidal volume (VT) to the resting (prestimulation) level and a parallel decline in total ventilation (MV). After the end of the stimulus, tidal volume and frequency were transiently reduced reflecting the hypocapnia caused by an incomplete return of MV to prestimulation levels. Grey bars in flow trace indicate behavioral disturbances. B) expanded traces from A showing the compensatory changes in VT during long periods of entrainment (147 s at 95 trains.min−1). During the early part of the stimulation (middle-panel; “early”), VT was increased compared to the resting state (left panel). Two and a half minutes later (right-panel; “late”) VT had decreased to prestimulation levels. C) Expanded trace from A (red box) illustrating the post-stimulus hypoventilation that followed 147s of pacing at the rate of 95 trains min−1. fR fell to 28 breaths.min−1 before returning to resting levels. D. Group data (N=6) summarizing frequency of respiration (fR), tidal volume (VT) and total ventilation (MV) at rest vs. during the early and late part of the entrainment period (*** P<0.001, ** P< 0.01, * P<0.05; one-way ANOVA).
Active expiration is elicited during respiratory pacing in conscious normocapnic rats
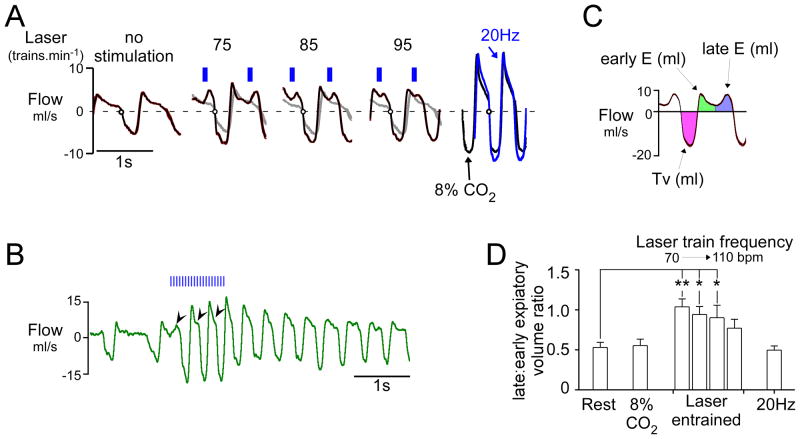
Under resting conditions, expiratory flow consists of a sharp initial peak (early expiration) followed by a slow relaxation until the next inspiration (Figure 5A). During pacing induced by RTN photostimulation at moderately elevated frequencies above resting values, expiratory flow became distinctly biphasic, the late component reflecting an observable contraction of abdominal and other thoracic expiratory muscles during late expiration. As the frequency of entrainment was increased, the separation between early and late expiratory flows became increasingly blurred and the two peaks fused into one (Figure 5A; 95 min−1). Similarly, during hypercapnia (8% CO2 added to oxygen) or continuous 20–23 Hz photostimulation of the Phox2b neurons, there was no distinguishable separation between the early and late component of expiration (compare 20 Hz and 8% CO2 in Figure 5A). The transformation from a biphasic to a monophasic expiratory pattern during continuous stimulation occurred during the first three respiratory cycles (Figure 5B), which suggests that active expiration is present during continuous activation of the RTN. To analyze this phenomenon, we divided the expiratory phase into two segments of equal duration (early expiration, late expiration) and measured the relative volume of air expelled during late expiration (late:early expiratory ratio; Figure 5C). During RTN photostimulated pacing, this ratio was much larger than at rest (P<0.01; Figure 5D). The increase in late expiratory flow was also accompanied by an increase in tidal volume from 1.8 ± 0.3 to 2.2 ± 0.3 ml (P<0.01). At high CO2 (8%) and during continuous high frequency photostimulation (23 Hz, 30 s), the late: early expiratory ratio returned to values similar to resting conditions, in part reflecting an increase in peak flow during early expiration (20.2 ± 4.1 ml/s during 23 Hz; 23.0 ± 4.1 ml/s during high CO2) compared to quiet conditions (7.0 ± 0.7; P<0.01) or photostimulated pacing at frequencies moderately above the resting rate (7.7 ±1.2 ml/s for all entrainment frequencies; P<0.01) (Figure 5C). These observations indicate that active expiration can be produced by activating the Phox2b-neurons in conscious rats and does not require hypercapnia. However active expiration can be observed by plethysmography only when the breathing rate is modestly increased from rest. Active expiration is by definition present when breathing is dramatically increased by hypercapnia but the compression of the expiratory phases combined with the large increase in early expiratory flow rate produces a monophasic expiratory flow. Presumably for the same reasons, expiratory flow loses its biphasic characteristic during continuous stimulation of the Phox2b neurons. The important result is that continuous as opposed to intermittent stimulation of the Phox2b neurons stimulates breathing in a manner that is equally coordinated and at least as powerful as hypercapnia (Figs. 1B & 5A), suggesting that intermittent activation of RTN neurons is not required for these neurons to produce an optimal breathing stimulation.
Figure 5. RTN photostimulation increases late-expiratory flow in conscious rats.
A) Trajectory of respiratory flow (waveform average, 10–15 breaths) at rest and during respiratory pacing caused by burst photostimulation of RTN at various frequencies (no stimulation, 75, 85 and 95 min−1). Traces at extreme right depict flow trajectory during continuous 20 Hz stimulus (blue trace) or during hypercapnia (8% CO2; black trace) in the same rat. At rest (left excerpt) during quiet breathing (72 breaths.min−1) expiratory flow was monotonically decremental, reflecting the passive recoil of the lungs and no active expiration. When breathing was entrained at 75, 85 and 95 breaths.min−1 respectively (middle panels), a large positive peak in flow preceded inspiration. This peak was most prominent at lower frequencies of entrainment and tended to fuse with the early expiratory peak at higher frequencies. During high frequency continuous stimulation (20 Hz, right panel-blue) under hypercapnic conditions (8% inspired, right panel-black), the expiratory flow became monophasic, although active expiration must have been present. B) original record showing the effect of a 1s stimulus train (20 Hz, 3 ms pulse). This example illustrates the progressive change in the pattern of expiratory flow from a biphasic to a monophasic pattern during continuous high frequency photostimulation. C) Tidal volume, early and late expiratory volumes are color-coded; early and late volumes were defined by dividing expiration in two periods of equal duration. D) Group data (N=6). During stimulation-induced respiratory pacing, the ratio between late expiratory and early expiratory volume was significantly increased relative to resting breathing (**P<0.01, * P<0.05 relative to rest; one-way ANOVA). The imbalance between late expiratory and early expiratory flow was most prominent at the lowest entrainment frequencies. When respiratory frequency was elevated by hypercapnia or continuous RTN stimulation at 20 Hz, the ratio between late expiratory and early expiratory volume was no different from that observed at rest.
Low frequency train stimulation produces phase-resetting of the respiratory cycle
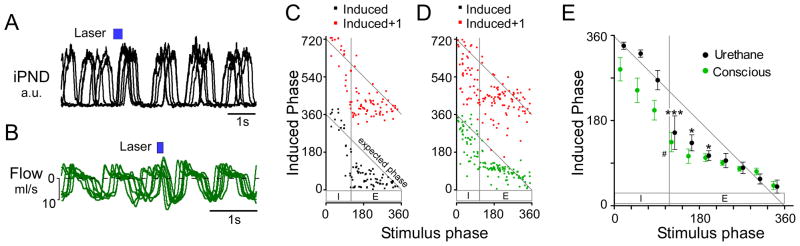
Electrical stimulation of a region co-extensive with the RTN Phox2b neurons resets the respiratory rhythm in an in vitro brainstem-spinal cord preparation of neonatal rats (Onimaru and Homma, 2003). We examined whether selective photostimulation of the RTN Phox2b neurons can reset the breathing rate in anesthetized and conscious adult rats by applying single trains at very low rates (0.05–0.1 Hz) relative to the resting breathing frequency such that each stimulus effectively occurred randomly during the breathing cycle, every 5–7 breathing cycles.
These stimuli invariably reset the respiratory rhythm in both anesthetized and conscious rats (Figure 6A,B). The largest phase advances were observed when the onset of the stimulus coincided with early expiration in both anesthetized and conscious rats with a modest or no phase advance when the stimulation occurred during early inspiration. Importantly, the stimulus did not perturb the next respiratory cycle (N+1) (Figure 6C,D). A modest lengthening of the respiratory cycle was observed in the anesthetized rats when the stimulus was delivered during inspiration but this effect was solely due to a prolongation of the phrenic nerve discharge (i.e. TI). In contrast, inspiratory-phase stimulation decreased total respiratory time in conscious rats (Figure 6D,E).
Figure 6. Low frequency train stimulation resets the respiratory cycle.
A) Overlayed recordings of integrated PND from one anesthetized rat. The waveforms are taken from periods during low frequency (<0.2 Hz) train stimulation and are aligned to the onset of the stimulus (6 traces in total). When the stimulus was delivered during expiration (in all cases), an advanced inspiratory burst occurred and the respiratory rhythm was reset, shown by the synchronization of PND activity following stimulation. B) Overlayed flow traces from one conscious rat, description as per A. C–D) phase resetting curves from a single anesthetized (C) and conscious rat (D). In both cases, a stimulus delivered during expiration dramatically reduced the induced phase (stimulus onset to following inspiration), and reset the respiratory rhythm (i.e. N+1 induced phase ≈ 360°). E) Grouped data from anesthetized (N=4) and conscious experiments (N=6) showing that stimulus induced phase resetting occurs preferentially during early expiration in anesthetized rats and conscious rats. Stimulation during late inspiration did not significantly reset the respiratory rhythm in anesthetized animals or conscious rats (*** P<0.001, * P<0.05 vs. N+1 cycle in anesthetized rats; # P<0.05 vs. N+1 cycle in conscious rats two-way ANOVA).
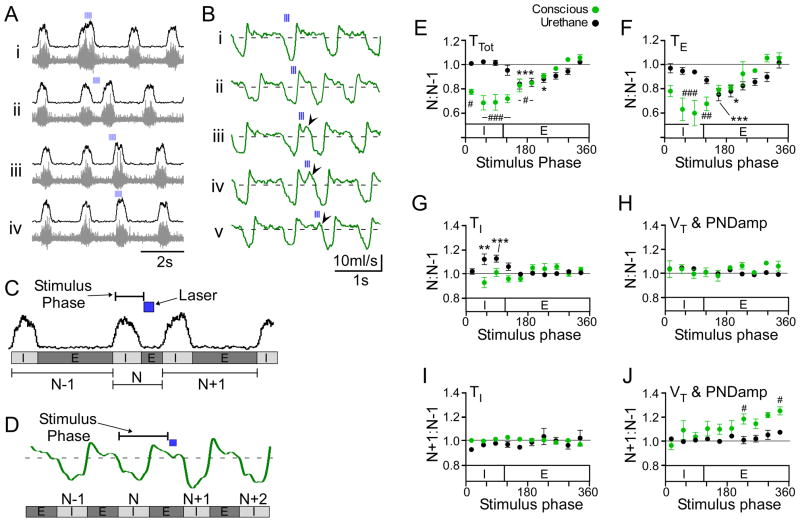
We pursued this analysis with a more detailed examination of the phase-specific perturbations in inspiratory and expiratory time, tidal volume (conscious rats) or PND amplitude (anesthesia) (Figure 7A–D). Consistent with our phase-resetting analysis, brief trains of stimuli invariably produced a phase advance (i.e. TTot reduction) relative to the natural cycle (type 1 phase response; Figure 7A,B,E). In conscious (N=5) and anesthetized rats (N=4), phase advances were entirely due to a reduction in TE (Figure 7F). Significant increases in TE were never observed. In conscious rats, stimuli applied between mid-inspiration and mid-expiration produced the largest reductions in both TTot (31 ± 7% during late inspiration; P< 0.0001, Figure 7E) and TE (41 ± 11% during late inspiration; P< 0.001, Figure 7F) whereas, stimuli applied in mid-expiration caused the greatest reductions in TTot in anesthetized rats (16 ± 4%; P< 0.001, Figure 7E) and TE (25 ± 5%; P< 0.001, Figure 7F). In anesthetized rats, TTot was unchanged when stimuli occurred during inspiration, despite modest shortening of TE (Figure 7E,F), because TI was significantly increased (12.3 ± 0.4% during late inspiration, P<0.001, Figure 7G). In contrast, stimuli delivered during inspiration produced no significant perturbation of TI in conscious rats, and we never observed a significant shortening of TI in either the anesthetized or conscious conditions irrespective of the stimulus phase (Figure 7G & I).
Figure 7. Phase-dependent perturbations of respiratory frequency and amplitude in anesthetized and conscious rats.
RTN photostimulation was applied at frequencies less than the resting breathing frequency (<0.2 Hz) so that the stimulus occurred at random within the breathing cycle. A) Original recordings of integrated (upper traces) and raw PND (lower traces) in an anesthetized rat. Train stimulation produced phase specific perturbations in total respiratory cycle (TTot), inspiratory time (TI), expiratory time (TE) and peak PND amplitude (PNDamp). In this example, stimulation during late inspiration (Ai) increased PNDamp and TI. When trains occurred during expiration (Aii–Aiii) there was a decrease in TE with a modest increase in PNDamp, but no change in TI. B) Respiratory flow in a conscious rat. As in A, brief trains (3 pulses at 20 Hz) produced phase specific perturbations in TTot, TI, TE and tidal volume (VT). Stimulation during early inspiration increased VT (Bi). Stimulation during late inspiration reduced the expiratory phase (Bii). Stimulation during early- to mid- expiration decreased TE and produced active expiration (Biii–iv). Stimulation during late expiration (Bv) shortened TE modestly and increased VT of the following inspiration. Data analysis from experiments in anesthetized (C) and conscious (D) rats. See methods for more details. E–J) Group data from anesthetized (N=4) and conscious experiments (N=5). E) Effect of stimulus on respiratory period (TTot). F) Effect of stimulus on expiratory duration (TE). G) Effect of stimulus on inspiratory duration (TI). H) Effect of stimulus on tidal volume and PNDamp. In E–H, values are expressed as ratio between respiratory cycles N and N−1 (see definitions in panels C and D). A value of 1 indicates no change from the N−1 (control) cycle. Note that in both animals, we did not observe significant increases in TE. I–J) Group data from anesthetized (N=4) and conscious experiments (N=5) showing the changes in PNDamp or VT in the N+1 cycle relative to the control cycle N−1. Brief stimulation during late expiration significantly increased VT but not PNDamp of the following inspiratory effort (N+1 cycle) (*** P<0.001, * P<0.05 vs N+1 for E–H and N+2 for I–J in anesthetized rats; ### P<0.001, ## P<0.01, # P<0.05 vs N+1 for E–H and N+2 for I–J in conscious rats).
Stimuli delivered during inspiration did not produce a statistically significant change in VT or PND amplitude of the targeted respiratory phase (Nth cycle; Figure 7H) but a clear increase was produced in 2 anesthetized and 2 conscious rats which had the most robust activation of breathing during high frequency photostimulation (for example, Figure 7A,B). In contrast, when the stimulus occurred during late expiration in conscious rats, VT of the following inspiration was consistently increased (26 ± 3%, P<0.05; Figure 7J). This augmented inspiratory effort was preceded by a marked increase in late expiratory flow similar in every detail to that observed during respiratory pacing (Figure 7Biii–iv). In anesthetized rats, there was also a tendency for PND amplitude to be greater if the stimulus fell during late expiration (Figure 8J) but the change was not statistically significant in 4 rats. These results suggest that VT is most efficiently increased by intermittent stimulation of the Phox2b neurons when the stimuli occur during mid- to late-expiration and triggers a prior forced expiration.
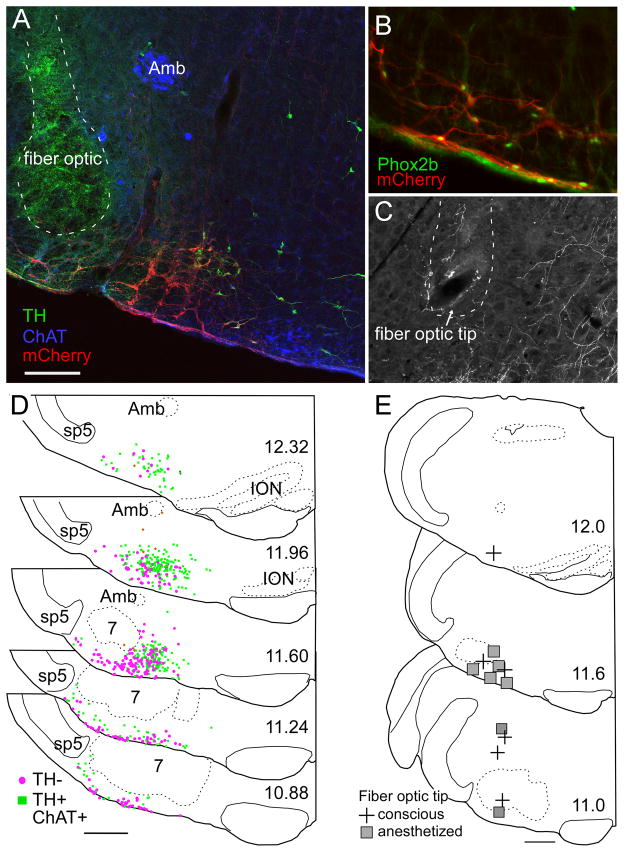
Figure 8. Anatomical distribution of ChR2-transfected cells and fiber optic locations.
A) Example of fiber optic location (dotted line, the green color within the fiber optic tract is an auto fluorescence artifact) next to the ChR2-mCherry-transfected neurons (mCherry in red). Choline acetyl-transferase-immunoreactive neurons (represented in blue) located in the ambiguus nucleus (Amb) and medial to the retrotrapezoid nucleus are not transfected. Several mCherry-positive neurons in this transverse section are catecholaminergic (tyrosine hydroxylase (TH) in green). These neurons generally occupy a medial location relative to the non-catecholaminergic, non-cholinergic neurons (the RTN-Phox2b neurons). B) Native mCherry fluorescence (red) detected along with Phox2b-immunoreactivity (green nuclei). Note the presence of Phox2b in every transfected neuron, including within the marginal layer of the retrotrapezoid nucleus. C) Example of a fiber optic tract (dotted line) found to end next to the labeled axons of ChR2-mCherry transfected neurons. D) Caudal to rostral series of transverse sections (bregma levels in mm as indicated) depicting the location of all the ChR2-mCherry-transfected neurons identified in 12 cases. Computer generated drawings with each ChR2-mCherry-transfected neuron indicated by either a green square (TH-immunoreactive (ir)), brown diamond (Choline Acetyl Transferase (ChAT)-ir) or magenta circle (not TH- or ChAT-ir) from the respective sections indicated for each bregma level from 12 animals were optimally superimposed using landmarks such as the ventral medullary surface, the edge of the trigeminal tract, the compact portion of nucleus ambiguus, the inferior olive and the facial motor nucleus. E) Location of fiber optic tips plotted on three transverse sections at the indicated bregma level (in mm). Scale bar in A= 200 μm for A and C and 100μm for B. Scale bars in D and E = 500μm.
Histology
Phox2b immunoreactivity was observed in 98.3% of the mCherry fluorescent neurons (4 transverse sections counted from each of 3 rats) demonstrating that the virus transfected Phox2b neurons with a high degree of selectivity (Figure 8B). ChR2-mCherry was detectable only in neurons and the cell distribution was confined to the region of the rostral ventrolateral medulla that harbors Phox2b-expressing neurons (Stornetta et al., 2006; Kang et al., 2007; Abbott et al., 2009b). The transfected population consisted of a mixture of catecholaminergic neurons, non-catecholaminergic neurons and a very small number of cholinergic neurons (Figure 8A).
On average (7 anesthetized + 5 conscious rats) ChR2-mCherry was detected in 383 ± 50 catecholaminergic neurons, 13.2 ± 3.5 cholinergic neurons and in 255.9 ± 35.5 non-catecholaminergic, non-cholinergic (presumptive RTN-Phox2b neurons) per rat. These figures represent the number of neurons counted in a one-in-six series of sections multiplied by six. Given our prior estimate of about 2000 RTN-Phox2b neurons per rat, between 5.4 and 26.7 % (median 13.8%) of the total population of RTN-Phox2b neurons could have been directly photoactivated.
The two rats that had received spinal injections of anti-dopamine beta-hydroxylase-conjugated saporin toxin had many fewer catecholaminergic ChR2-labeled neurons as expected (Abbott et al., 2009b) (respectively 168 and 162 per rat) but they had an average number of RTN-Phox2b neurons (respectively 234 and 474). The respiratory responses elicited by photostimulation in these animals were not different from the response in animals with an intact C1 population, confirming prior observations that, under our experimental conditions, these responses likely result from activation of the RTN-Phox2b neurons rather than the catecholaminergic neurons (Abbott et al., 2009b).
The brain distribution of the transfected neurons is represented in Figure 8D. This panel depicts the superimposed locations of all the transfected neurons identified in 12 cases (5 conscious and 7 anesthetized). The catecholaminergic neurons are all, by definition, Phox2b-containing. At the rostro-lateral edge of the facial motor nucleus these neurons are predominantly of the A5 variety (Byrum and Guyenet, 1987). Medially, they belong to the C1 adrenergic cell group. Of note, the lateral region situated between the spinal trigeminal tract and nucleus ambiguus, which may contain an expiratory oscillator (Pagliardini et al., 2011), had very few ChR2-expressing neurons and, as indicated above, the overwhelming majority of these cells in our cases were Phox2b-positive.
The location of the fiber optic tips (13 rats) is represented in Figure 8E. The fiber optic tips were generally very close to the RTN but, in several cases in which strong respiratory stimulation was elicited even with brief stimuli, the tip of the fiber optic was at a considerable distance (>1 mm) from the somata of the ChR2-expressing neurons. In such cases, the fiber optic tip was invariably close (< 250 μm) to the main axons of these cells (Figure 8C) which take an initial medio-dorsal course through the intermediate reticular nucleus ((IRt, after (Paxinos and Watson, 1986; Abbott et al., 2009b)). Therefore we presume that neuronal activation must have often resulted from stimulating the axons of the ChR2-expressing axons.
DISCUSSION
Summarizing the new results, selective activation of RTN-Phox2b neurons increases the breathing rate, inspiratory amplitude and active expiration; these effects are occluded by hypercapnia. Phasic activation of RTN-Phox2b neurons resets and entrains the breathing cycle and produces an expiration-inspiration sequence. When frequency is fixed, ventilatory homeostasis is partially restored via tidal volume reduction.
Although phasic activation of RTN-Phox2b neurons recruits both inspiratory and expiratory mechanisms, continuous activation produces far more powerful and yet effectively coordinated breathing stimulation.
Histology and experimental limitations
ChR2 was expressed by Phox2b-containing neurons consisting of catecholaminergic and non-catecholaminergic (RTN) neurons (Abbott et al., 2009b). Few ChR2-transfected neurons were within the lateral parafacial region where others located an expiratory oscillator (Pagliardini et al., 2011), except at the medullary surface under the facial motor nucleus where almost every neuron is Phox2b-positive (Takakura et al., 2008).
Destruction of most of the spinally-projecting catecholaminergic C1 neurons did not affect the breathing response to photostimulation of RTN-Phox2b neurons in anesthetized rats, consistent with our previous observations (Abbott et al., 2009b). However, our results cannot exclude a contribution of other ChR2-expressing C1 neurons, perhaps via their projections to the RTN (Rosin et al., 2006; Card et al., 2006).
We postulate that the observed breathing stimulation resulted from direct activation of the brainstem respiratory network by RTN neurons but we cannot exclude indirect effects on breathing mediated by changes in vigilance or cortical function (Boon et al., 2004; Buchanan and Richerson, 2010).
RTN induced pacing and resetting of the breathing rate
Electrical stimulation of the RTN entrains and resets the respiratory rhythm in neonates in vitro (Onimaru and Homma, 2003). As shown here, selective activation of the Phox2b neurons achieves the same result in conscious adult rats. This entrainment occurred over a limited range of breathing frequencies, possibly because only a small fraction ( ~14%) of the RTN neurons expressed ChR2. Phox2b-neuron stimulation produced quantal breathing only in anesthetized rats maintained slightly below apneic threshold consistent with the fact that this pattern is typically observed when the respiratory network is in a low excitability state (Mellen et al., 2003; Ballanyi et al., 2009). During low frequency stimulation, the greatest phase advance occurred when Phox2b neurons are stimulated during early- and mid-expiration, probably because these neurons can most productively activate pre-I/I “rhythmogenic” and early-I inhibitory neurons during this time window, thereby initiating a premature inspiration (Rubin et al., 2009). Similarly, during pacing the respiratory rhythm reset and the stimulus settled during mid- to late expiration. Respiratory entrainment by somatic afferent stimulation has the same characteristic, which has been explained by inhibition of late-expiratory neurons via the parabrachial nucleus (Potts et al., 2005). A similar mechanism could partly account for the present results because RTN neurons densely innervate the dorsal pons (Abbott et al., 2009b; Bochorishvili et al., 2011).
Photo-stimulation of unidentified parafacial neurons produced respiratory rhythm resetting and terminated inspiration in anesthetized, vagotomized, spontaneously breathing rats but such stimulation produced relatively small increases in inspiratory amplitude and frequency (Pagliardini et al., 2011). In our anesthetized ventilated preparation, brief stimulation of Phox2b neurons during inspiration actually prolonged TI, suggesting that RTN neurons can delay the I–E transition in the absence of lung stretch receptor feedback. More importantly, with or without anesthesia, continuous activation of the Phox2b cells increased tidal volume and markedly increased respiratory rate (see also (Kanbar et al., 2010)) as expected from a cell group that contributes to the effect of hypercapnia. These differences support Pagliardini et al.’s conclusion ( 2011) that the effects seen in their experiments were not primarily caused by stimulating the Phox2b neurons.
As observed previously, continuous 20 Hz activation of the RTN-Phox2b neurons under anesthesia produces a breathing activation that decays very slowly upon cessation of the stimulus ((Abbott et al., 2009b), Figure 2). Yet, we show that RTN-Phox2b neurons can also influence breathing on a breath-to-breath basis. RTN neurons may therefore utilize both fast ionotropic and slow metabotropic transmission. Indeed, the ultrastructure of RTN-Phox2b synapses resembles classical non-NMDA glutamatergic synapses (Bochorishvili et al., 2011) but the long after-discharge elicited by high frequency photostimulation and the attenuation of the central chemoreflex by certain metabotropic glutamate receptor antagonists (Takakura et al., 2011) suggest additional metabotropic mechanisms. A long after-discharge also occurs following tonic and phasic electrical activation of peripheral chemoreceptors and muscle afferents. This phenomenon is therefore not uniquely associated with RTN activation and may be a property of the rhythm and pattern generator (Eldridge et al., 1981).
Chemoreflex regulation of breathing during RTN-induced pacing
The respiratory network allows independent changes of frequency and amplitude under many conditions such as panting, sniffing, emotions, and vocalization/speech. As shown here, when breathing frequency is fixed above resting levels in conscious rats, tidal volume gradually decreases. In conscious man, tidal volume compensates for volitional breathing at elevated frequencies so that alveolar ventilation remains essentially constant (Haouzi and Bell, 2009). These data and ours indicate that the amplitude component of the central chemoreflex can be automatically adjusted somewhat independently of the breathing frequency to maintain CO2 homeostasis. RTN-Phox2b neurons innervate both the region that contains phrenic premotor neurons as well as the Pre-Bötzinger Complex (Abbott et al., 2009b; Bochorishvili et al., 2011). These projections may underlie their ability to regulate tidal volume independently of respiratory frequency.
RTN-Phox2b neurons activate phase 2 expiration in conscious rats
Phase 2 or active expiration is the recruitment of expiratory muscles for breathing which occurs predominantly during exercise, hypoxia or hypercapnia (Iscoe, 1998). Janczewski and Feldman ( 2006) first showed that the RTN region contributes to active expiration but opinions vary regarding the role of RTN-Phox2b neurons in this process (Marina et al., 2010; Pagliardini et al., 2011). Our results, obtained using conscious rats, support prior evidence that the Phox2b neurons drive active expiration (Marina et al., 2010). We add that an orderly expiration-inspiration sequence is triggered by a brief activation of these cells, even in normocapnia. The control of expiratory muscles by RTN-Phox2b neurons is consistent with their postulated function of increasing lung ventilation according to the intensity of the hypercapnic stimulus (Guyenet et al., 2010). Our experiments do not clarify whether expiration, breathing rate and inspiration are regulated by the same Phox2b neurons (Abdala et al., 2009b; Marina et al., 2010). However, in conscious rats, active expiration was produced by activating RTN-Phox2b neurons during mid- rather than late-expiration, i.e. considerably earlier than when the neurons assumed to drive abdominal muscles are active (Abdala et al., 2009a; Pagliardini et al., 2011), suggesting that the latter neurons are downstream from the Phox2b cells. These neurons may reside in the lateral medullary region identified by Pagliardini ( 2011).
Brief photostimulation of the Phox2b neurons never increased TE in conscious or anesthetized adult rats. Accordingly, the pacemaker handshake synchronization mechanism of mammalian respiratory rhythmogenesis (Wittmeier et al., 2008) does not account for the production of an expiration-inspiration sequence by mature Phox2b neurons.
Phasic activation of Phox2b neurons is not necessary to drive breathing
Under normocapnic conditions, the discharge of mature RTN-Phox2b neurons is tonic but with hypercapnia, their discharge becomes both more intense and increasingly synchronized with respiration (Guyenet et al., 2005; Moreira et al., 2007). If phasic stimulation of RTN-Phox2b neurons was required to produce strong and optimally coordinated contractions of inspiratory and expiratory muscles, then tonic stimulation should produce arrhythmic and inefficient breathing and phasic stimulation should produce the greatest increases in lung ventilation. We observed the exact opposite. Continuous high frequency activation of RTN-Phox2b neurons produced the largest increases in ventilation, reflecting the effective coordination of both inspiratory and expiratory respiratory muscles in conscious animals, whereas phasic activation tended to cause a breakdown of the breathing rhythmicity when applied at high frequency. Therefore, as previously proposed (Guyenet et al., 2005), the effectiveness of the RTN-Phox2b neurons is presumably encoded by their mean discharge rate rather than by their pattern and their respiratory synchronization during hypercapnia may simply be the manifestation of an inhibitory feedback that emanates from phasically active respiratory neurons and protects the respiratory system from excessive stimulation. The patterning of the muscle contractions elicited by CO2 via the activation of the RTN-Phox2b neurons presumably relies on the downstream respiratory pattern generator, which likely includes the parafacial region (Pagliardini et al., 2011). Expected targets of the RTN-Phox2b neurons include early-I and preI-I neurons whose coordinated excitation is presumably required to increase the breathing rate (Rubin et al., 2009), post-I neurons (Marina et al., 2010) that restrain the duration of the phrenic nerve discharge, and premotor neurons whose activation may explain why the chemoreflex can still operate when breathing frequency is fixed ((Haouzi and Bell, 2009); present data).
Conclusions
Consistent with their anatomical projections, RTN-Phox2b neurons regulate lung ventilation by recruiting inspiratory and expiratory muscles and regulating the breathing rate. Intermittent activation of adult RTN-Phox2b neurons entrains the respiratory rhythm but their tonic activation produces far greater breathing stimulation. While a rhythmogenic role in adulthood cannot be ruled out, the average discharge rats of these neurons seems to be the critical factor for their role in breathing regulation.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health to PGG: HL74011 and HL28785. SBGA is supported by a post-doctoral fellowship from the American Heart Association: 11POST7170001.
References
- Abbott SB, Stornetta RL, Socolovsky CS, West GH, Guyenet PG. Photostimulation of channelrhodopsin-2 expressing ventrolateral medullary neurons increases sympathetic nerve activity and blood pressure in rats. J Physiol. 2009a;587:5613–5631. doi: 10.1113/jphysiol.2009.177535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott SBG, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus Phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009b;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins, and implications for respiratory rhythm generation. J Physiol. 2009a;587:3539–3559. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala AP, Rybak IA, Smith JC, Zoccal DB, Machado BH, St-John WM, Paton JF. Multiple pontomedullary mechanisms of respiratory rhythmogenesis. Respir Physiol Neurobiol. 2009b;168:19–25. doi: 10.1016/j.resp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballanyi K, Ruangkittisakul A, Onimaru H. Opioids prolong and anoxia shortens delay between onset of preinspiratory (pFRG) and inspiratory (preBotC) network bursting in newborn rat brainstems. Pflugers Arch. 2009;458:571–587. doi: 10.1007/s00424-009-0645-3. [DOI] [PubMed] [Google Scholar]
- Bochorishvili G, Coates MB, Stornetta RL, Guyenet PG. Projections of the retrotrapezoid nucleus (RTN) in rats. FASEB J. 2011;25:652.1–652.1. [Google Scholar]
- Boon JA, Garnett NB, Bentley JM, Milsom WK. Respiratory chemoreflexes and effects of cortical activation state in urethane anesthetized rats 8. Respir Physiol Neurobiol. 2004;140:243–256. doi: 10.1016/j.resp.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A. 2010;107:16354–16359. doi: 10.1073/pnas.1004587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrum CE, Guyenet PG. Afferent and efferent connections of the A5 noradrenergic cell group in the rat. J Comp Neurol. 1987;261:529–542. doi: 10.1002/cne.902610406. [DOI] [PubMed] [Google Scholar]
- Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: Implications for the central control of cardiovascular regulation. J Comp Neurol. 2006;499:840–859. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- DRORBAUGH JE, Fenn WO. A barometric method for measuring ventilation in newborn infants 1. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Eldridge FL, Gill-Kumar P, Millhorn DE. Input-output relationships of central neural circuits involved in respiration in cats. J Physiol. 1981;311:81–95. doi: 10.1113/jphysiol.1981.sp013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL. Chapter 14--looking forward to breathing 4. Prog Brain Res. 2011;188:213–218. doi: 10.1016/B978-0-444-53825-3.00019-X. [DOI] [PubMed] [Google Scholar]
- Fortuna MG, Stornetta RL, West GH, Guyenet PG. Activation of the retrotrapezoid nucleus by posterior hypothalamic stimulation. J Physiol. 2009;587:5121–5138. doi: 10.1113/jphysiol.2009.176875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goridis C, Brunet JF. Central chemoreception: lessons from mouse and human genetics. Respir Physiol Neurobiol. 2010;173:312–321. doi: 10.1016/j.resp.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes Control Breathing Through pH-Dependent Release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci. 2005;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol. 2010;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haouzi P, Bell HJ. Control of breathing and volitional respiratory rhythm in humans. J Appl Physiol. 2009;106:904–910. doi: 10.1152/japplphysiol.90675.2008. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Carlezon WA, Jr, Isacson O, Kim KS. A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Hum Gene Ther. 2001;12:1731–1740. doi: 10.1089/104303401750476230. [DOI] [PubMed] [Google Scholar]
- Iscoe S. Control of abdominal muscles. Prog Neurobiol. 1998;56:433–506. doi: 10.1016/s0301-0082(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbar R, Stornetta RL, Cash DR, Lewis SJ, Guyenet PG. Photostimulation of Phox2b medullary neurons activates cardiorespiratory function in conscious rats. Am J Respir Crit Care Med. 2010;182:1184–1194. doi: 10.1164/rccm.201001-0047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, Takakura AC, Gwilt JM, Guyenet PG, Stornetta RL. Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol. 2007;503:627–641. doi: 10.1002/cne.21409. [DOI] [PubMed] [Google Scholar]
- Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie E. CO2 dialysis in one chemoreceptor site, the RTN: stimulus intensity and sensitivity in the awake rat. Respir Physiol Neurobiol. 2002;133:11–22. doi: 10.1016/s1569-9048(02)00134-9. [DOI] [PubMed] [Google Scholar]
- Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira TS, Takakura AC, Colombari E, West GH, Guyenet PG. Inhibitory input from slowly adapting lung stretch receptors to retrotrapezoid nucleus chemoreceptors. J Physiol. 2007;580:285–300. doi: 10.1113/jphysiol.2006.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol. 2009;106:1464–1466. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku Y, Masumiya H, Okada Y. Postnatal developmental changes in activation profiles of the respiratory neuronal network in the rat ventral medulla. J Physiol. 2007;585:175–186. doi: 10.1113/jphysiol.2007.138180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Kawakami K. CO2-Sensitive Preinspiratory Neurons of the Parafacial Respiratory Group Express Phox2b in the Neonatal Rat. J Neurosci. 2008;28:12845–12850. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci. 2011;31:2895–2905. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Potts JT, Rybak IA, Paton JF. Respiratory rhythm entrainment by somatic afferent stimulation. J Neurosci. 2005;25:1965–1978. doi: 10.1523/JNEUROSCI.3881-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Rubin JE, Shevtsova NA, Ermentrout GB, Smith JC, Rybak IA. Multiple rhythmic states in a model of the respiratory central pattern generator. J Neurophysiol. 2009;101:2146–2165. doi: 10.1152/jn.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Abbott SBG, Kanbar R, Guyenet PG. Photostimulation of channelrhodopsin2(ChR2)-transfected C1 neurons increases sympathetic nerve discharge (SND) and blood pressure (BP) in rats. Neuroscience Meeting Planner Program number. 2009;467.5 [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci. 2006;26:10305–10314. doi: 10.1523/JNEUROSCI.2917-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol. 2002;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Colombari E, Menani JV, Moreira TS. Ventrolateral medulla mechanisms involved in cardiorespiratory responses to central chemoreceptor activation in rats. Am J Physiol Regul Integr Comp Physiol. 2011;300:R501–R510. doi: 10.1152/ajpregu.00220.2010. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol. 2008;586:2975–2991. doi: 10.1113/jphysiol.2008.153163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat Neurosci. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Wittmeier S, Song G, Duffin J, Poon CS. Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proc Natl Acad Sci USA. 2008;105:18000–18005. doi: 10.1073/pnas.0809377105. [DOI] [PMC free article] [PubMed] [Google Scholar]