Abstract
Many viruses are capable of infecting the human respiratory tract to cause disease. These viruses display various transmission patterns among humans; however, they all share the ability to transmit from person to person, and their human transmissibility is influenced by the environment in which pathogen and host meet. This review aims to summarize recent and significant observations regarding the impact of environmental factors such as weather and climate, humidity, temperature, and airflow on the transmission of human respiratory viruses. Where possible, knowledge gaps that require further scientific study will be identified.
INTRODUCTION
Viral respiratory tract infections are both ubiquitous and burdensome, accounting for many millions of lost school- and workdays and millions more physician visits each year [1]. Although there are similarities in the clinical syndromes caused by the many viruses capable of infecting and causing disease in the human respiratory tract, they possess varying transmission patterns among humans. The mode or modes by which a virus transmits from person to person are critical to understanding how the environment in which they transmit impacts person-to-person spread. The Centers for Disease Control and Prevention (CDC) consensus definition for the modes of transmission of influenza virus is broadly applicable to other respiratory (i.e., non-vector-borne) viruses as well (Box 1). In this review, for consistency, we will adhere to the CDC terminology; thus, “contact transmission” encompasses both direct and indirect transmission, while “airborne transmission,” comprising both droplet spray and aerosol modes, describes the direct inoculation of virus particles from the air into the respiratory tract without an intermediate.
Box 1. Modes of person-to-person transmission of respiratory viruses.
| Contact transmission | In both modes of contract transmission (direct and indirect), contaminated hands play an important role in carrying virus to mucous membranes. |
| Direct transmission | Virus is transferred by contact from an infected person to another person without a contaminated intermediate object (fomite). |
| Indirect transmission | Virus is transferred by contact with a contaminated intermediate object (fomite). |
| Droplet spray transmission | Virus transmits through the air by droplet sprays (such as those produced by coughing or sneezing); a key feature is deposition of droplets by impaction on exposed mucous membranes. |
| Aerosol transmission | Virus transmits through the air by aerosols in the inspirable size range or smaller; aerosol particles are small enough to be inhaled into the oronasopharynx and distally into the trachea and lung. |
Adapted from Centers for Disease Control and Prevention (CDC); URL: http://www.cdc.gov/influenzatransmissionworkshop2010/
Respiratory viruses display a great deal of variety not only in their virion structure and genome composition but also in their modes of transmission among humans (Table 1). For instance, evidence supports a primary role for direct and indirect contact in respiratory syncytial virus (RSV) and adenovirus transmission, while airborne routes (droplet spray and aerosol) seemed to be more important in SARS coronavirus spread. With other viruses, evidence is either contradictory or incomplete, and mode(s) of transmission are yet to be fully resolved.
Table 1.
Modes of transmission of several human respiratory tract viruses.
| Virus | Family | Primary mode(s) of respiratory transmission |
|---|---|---|
| Adenoviruses | Adenoviridae | Contact, possibly droplet spray and/or aerosol (limited data) [2–4] |
| Influenza viruses | Orthomyxoviridae | Contact, droplet spray and/or aerosol (conflicting data) [5–9] |
| Human parainfluenza viruses (HPIV) | Paramyxoviridae | Uncertain (limited data) [10–12] |
| Metapneumovirus | Paramyxoviridae | Uncertain (limited data) [2] |
| Respiratory syncytial virus (RSV) | Paramyxoviridae | Direct and indirect contact [7,13], possibly droplet spray [14] |
| Rhinoviruses | Picornaviridae | Contact, droplet spray and/or aerosol (conflicting data) [7,15] |
| SARS coronavirus | Coronaviridae | Droplet spray and aerosol [2,4,16], possibly contact [17] |
Here we will review the effects of certain environmental factors on respiratory virus transmission, with an emphasis on influenza and respiratory syncytial viruses. We have referenced several large-scale surveillance studies, as well as experimentally generated data in small animal models. We will also discuss remaining uncertainties as to the relative importance of these factors, as well the possible contributions of non-environmental factors on the infectiousness and transmissibility of respiratory viruses.
ENVIRONMENTAL FACTORS AFFECTING RESPIRATORY VIRUS TRANSMISSION
Temperature and Humidity
Multiple hypotheses have been advanced to explain the specific effect of humidity and temperature on the pronounced seasonality of influenza and, to a lesser extent, disease caused by RSV and other respiratory viruses [18–22]. These include changes in host behavior (for instance, more time spent indoors, in closed environments, during cold or rainy weather), changes in host defense mechanisms (such as impairment of mucociliary clearance with inhalation of cold, dry air) and changes in the virus infectivity and stability in different climactic conditions (Figure 1).
Figure 1.
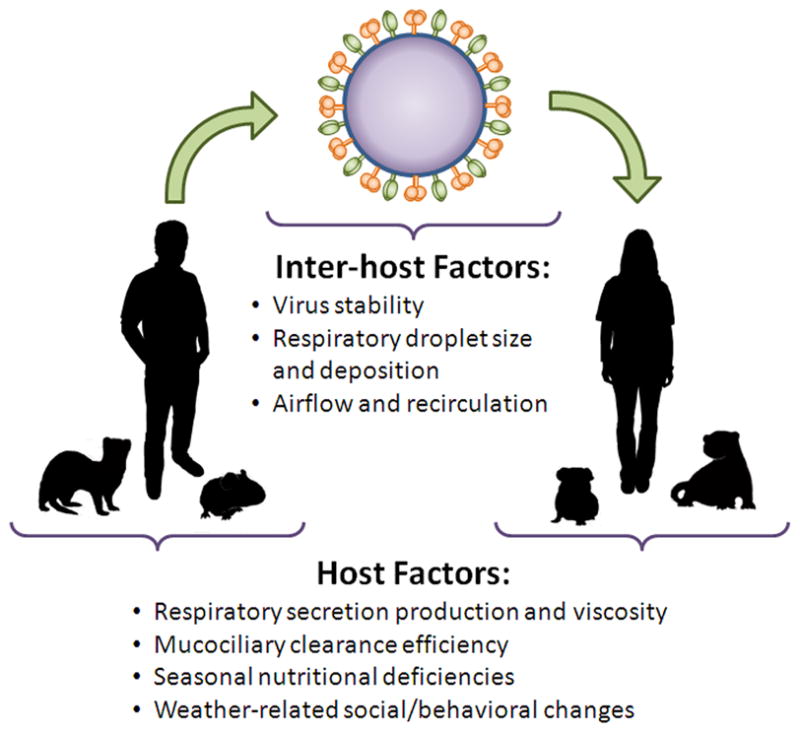
Animal models have elucidated potential mechanisms by which humidity and temperature influence human influenza virus transmission. In the early 1960s, Schulman and Kilbourne developed an influenza virus transmission model in mice. Although mouse-to-mouse transmission is relatively inefficient, they still observed a significant decrease in transmission efficiency with increasing relative humidity (RH) [23] and during summer months, even when laboratory temperature and RH were controlled during experiments [24]. Lowen et al. expanded upon these experiments in the guinea pig transmission model. At 20°C, transmission efficiency of an influenza A/H3N2 isolate displayed a bimodal dependence on RH, with airborne (i.e., droplet or aerosol) transmission being maximal at 20–35% RH, poor at 50% RH, moderate at 65% RH, and absent at 80% RH. At 5°C, transmission was overall more efficient than at 20°C, and the relationship between RH and transmission efficiency was montonic, with efficiency decreasing with increasing RH [25]. Transmission was abolished at high temperature (30°C), regardless of RH [26].
Transmission of viruses via airborne routes may be affected by ambient humidity, which affects not only the virus’ stability but also respiratory droplet size, as water content evaporates. In turn, droplet size influences whether the particle will quickly settle to the ground or remain airborne long enough to be inhaled into the respiratory tract of a susceptible host. For influenza virus, mathematical modeling suggests that RH is an important variable in airborne transmission of influenza virus; high RH favors removal of infectious particles both by increasing the settling of large, water-laden droplets and by hastening virus inactivation [27]. In aerosol viability experiments, adenovirus [28,29] and rhinovirus [30] were more stable at high RH; in contrast, a bovine parainfluenza virus was more stable at low RH [31], while RSV demonstrated bimodal peak stability at 20% or 40–60% RH with relative instability at 30% [32]. Influenza viruses are also generally more stable at lower RH; some studies have observed a bimodal stability similar to that seen by Lowen et al., while others have not [20]. However, these data should not be over-interpreted, as aerosol generation protocols and thus particle size and composition were not necessarily uniform across experiments.
RH is a ratio that describes the actual water vapor pressure of air, relative to its vapor pressure at saturation. Because saturation vapor pressure is exponentially related to temperature, RH varies both with the temperature and with the water vapor content of air. Absolute humidity (AH), on the other hand, describes the actual water vapor content of air, without respect to temperature. Thus, at equivalent RH, warm air contains more water vapor (i.e., has higher AH) than cold air [33]. A reanalysis of the data of Lowen et al. showed that, although RH and temperature were both weakly correlated with influenza virus transmission efficiency, AH was strongly associated with transmission efficiency, with efficiency decreasing as vapor content of air increases [33]. Subsequent analyses of epidemiological and meteorological data in temperate areas in the United States [34] and Japan [35] suggest that low AH correlates strongly with the onset of influenza epidemic activity, more so than RH.
However, as Tamerius and colleagues observe [20], hypotheses correlating AH and influenza epidemics are best suited to explain the seasonality of influenza virus transmission in temperate climates. While influenza virus transmission has been thought not to display marked seasonality in the tropics, accumulating data suggest that equatorial regions can experience not only year-round transmission (such as Colombia; 4°N) [36] but also distinct annual epidemics that are unimodal (Fortaleza, Brazil; 3°S) or bimodal (Singapore; 1°N) [20]. In other tropical areas, influenza epidemics correlate with the rainy season, when AH is highest (such as Dakar, Senegal; 14°N [37], or Belem, Brazil; 1°S [38]). The question remains to what extent the seasonality (or lack thereof) of influenza epidemics is attributable to seasonal factors like humidity and temperature, what other environmental or seasonal variables matter, and which variables are causative and which are merely correlated or even confounding [20].
Precipitation
Another environmental factor that may influence viral transmissibility is precipitation. Several large-scale studies have been conducted in tropical and equatorial countries in order to determine the relationship between rainfall and respiratory disease, particularly that associated with RSV and, to a lesser extent, influenza virus. A 3-year study of RSV infections in Lombok, Indonesia (8°S) found an association between rainfall and RSV hospitalizations; interestingly, total monthly precipitation was less important than the number of days on which it rained [39]. In a study from 1982 to 1997 in Malaysia (4°N) involving over 5,000 children, Chan et al. also documented a significant correlation between number of rainy days and RSV infection [40]. An association between rain and RSV infection has also been seen in several other studies [41–44]. In contrast, a large, 3378-children study in Northern Taiwan (23°N) did not find any association between rainfall and RSV infection [45], nor did a 2002 study in Santiago, Chile (33°S). Of note, however, the Chilean study focused on cases in just one public pediatric hospital [46]; it is possible that a limited sampling of cases in only one hospital would hinder the ability to draw statistically significant conclusions. However, studies in other locations have found the relationship between RSV disease and rainfall to be inversely related. In a 24-month study of over 1000 symptomatic children in India (22°N), RSV infection rates were negatively correlated with millimeters of rainfall; these findings were statistically significant [47].
Inconsistencies in the role that rain plays in infection rates is not altogether surprising; discrepancies can even be reported within a given country. Following a 43-month study in Fortaleza (3°S), in the northeast of Brazil, an association between rainfall and RSV infection was documented [48]; Nasciemento-Carvalho et al. reported a similar correlation in Salvadore (12°N) [49]. However, RSV infection did not correlate with the rainy season in Sao Paolo (23°N) [50]. While these studies employed varying techniques and collected samples over different time periods, it is possible that rain plays a unique role in RSV infection depending on a variety of factors, including geographic location. In addition, weather-dependent behavioral factors such as crowding following rainfall, could also influence RSV infection rates [51], as RSV is thought to transmit through direct or indirect contact [7]. Indeed, family structure, living conditions and person-to-person contact are risk factors for RSV infection [51,52].
Epidemiological data appear generally to support a relationship between influenza virus infection and rainfall. In a two-year study, a significant association was found between rainfall and influenza A virus infection in India (22°N), with little to no reported infections during the dry season [47]. These findings are in agreement with earlier studies by Rao and Banerjee, who reported a statistically significant association between rainfall and influenza virus infection, though the relative contribution to influenza A or B virus infection was not determined [53]. While it has been noted that peak influenza virus activity coincided with the first rainfall in Chennai, India (13°N) [54], the study lacked sufficient statistical rigor to determine if a relationship in fact exists. In a fairly limited study of pediatric influenza virus infections in an urban slum in Bangladesh (23°N) in 2007, 77% of the influenza B cases occurred during the monsoon season (July to September); conversely, 70% of the influenza A cases occurred during the pre-monsoon period (April to June) [55]. This study also did not determine statistical significance, and has several limitations in the study design, including the retrospective collection of samples from children who displayed clinical symptoms.
Like RSV, influenza virus transmission may be more affected by rainfall in one geographic location than another. When comparing incident influenza and weather trends in Singapore (1°N), Hong Kong (22°N), Ulaanbaatar (47°N), Vancouver (49°N), Brisbane (27°S), Melbourne (37°S), and Sydney (33°S) from 2000 to 2007, rainfall was not significantly correlated with infection [56]. Murray et al. reported that influenza A/H5N1 infections in Egypt (26°N) were negatively correlated with precipitation between 2006 and 2008, as the peak incidence of human infections coincided with an average of 0.2 mm of rainfall [57]. These results were not statistically significant, likely due to the small number of human cases during the study period. In the same study, Murray et al. did not find any association between H5N1 infection and rainfall in Indonesia, though the authors attributed this result to variation in climate across the country, societal differences that may affect how poultry is handled, or differences in susceptibility of the inhabitants and/or poultry due to previous exposures [57].
Much less work has been done to assess the relationship between precipitation and infection with other respiratory viruses. In a study that investigated the seasonal patterns of viral and bacterial infections among hospitalized children with radiologically diagnosed pneumonia in Salvadore, Brazil (12°N), adenovirus infection was significantly correlated with total precipitation. However, parainfluenza virus infection was inversely correlated in the same study [49]. Indeed, there are substantial knowledge gaps as to how rainfall affects transmission of respiratory viruses. The effects of rain would be difficult to examine experimentally, such as in a small animal model, and it is possible that weather- or climate-related factors other than precipitation affect seasonal infection rates.
Airflow and Ventilation
Though relatively few data exist, airflow (the speed of air currents flowing through indoor spaces) and ventilation (the degree of mixing between indoor and outdoor air) seem play a role in respiratory virus infectivity and transmission. Schulman and Kilbourne again made prescient early observations of the effect of airflow on the transmissibility of influenza viruses in the mouse model, demonstrating that the rate of transmission decreased with increasing ventilation of a closed chamber in which mice were housed [23]. A similar phenomenon was observed with rhinovirus; the probability of detecting airborne picornavirus RNA in office buildings was directly correlated with the carbon dioxide (CO2) content of the air, which is in turn inversely related to ventilation with fresh outside air [58]; however, there were too few positive nasal samples to correlate CO2 content with actual human infection.
Although inadequate ventilation has been implicated in the airborne transmission of respiratory viruses [59,60], the Severe Acute Respiratory Syndrome (SARS) coronavirus outbreak in 2002–2003 provides an interesting case study for the outdoor airborne transmission of a viral respiratory pathogen. More than 300 residents of the Amoy Gardens high-rise apartment complex in Hong Kong were infected with the virus, in a dispersion pattern consistent with a single index patient, a visitor to the complex. The index patient was found to have extremely high viral loads in fecal and urine samples; computational fluid-dynamics modeling of the dispersion plume was most consistent with transit of virus aerosols through improperly sealed plumbing U-traps, up an airshaft in the index building, and then along prevailing winds into neighboring buildings up to 60 meters away [61,62]. In the largest nosocomial SARS outbreak in Hong Kong, 17 of the 30 infected patients were housed in different multi-bed wards from the index patient. A recent reanalysis of this outbreak found that, even though the patient wards were designed to be at positive pressure relative to the hospital corridor (thus preventing bioaerosols from entering the wards), small differences in temperature – as little as 0.5°C – between corridor and wards was sufficient to allow two-way airflow at ward entrances, thus permitting SARS coronavirus entry into patient wards [63].
CONCLUSIONS
Viral infections of the respiratory tract are common acute illnesses among humans, and virus transmission, by either direct or indirect routes, occurs in disparate regions around the globe. A more detailed understanding of how these viruses transmit can have broad public health implications. Indeed, a variety of meteorological factors have at times been associated with rates of virus infection as well as transmission among individuals. As presented in this review, precipitation, humidity, temperature, and airflow can be determinants of virus infection and transmission; however, despite robust investigation of the effects of these environmental factors, inconsistencies and uncertainties in the data remain. It is possible that meteorological determinants play greater roles in some geographic regions than others, or simply that differences in experimental design affect outcomes and data interpretation. Non-environmental effects, including but not limited to seasonal changes in behavior, family and social structures, and pre-existing immunity, could also be playing a role in respiratory virus transmissibility and rates of infection. Discrepancies in collected data suggest that more vigilant surveillance over large geographic regions and further controlled experiments in animal models and perhaps in humans will likely be necessary to determine with increased certainty the role that environmental factors play on the transmission of viral pathogens.
HIGHLIGHTS.
Respiratory viruses are spread from person to person via various modes of transmission, including direct and indirect contact, droplet spray, and aerosol.
Virus transmission is affected by a number of factors, including environmental determinants, host behavior, host defense mechanisms, and virus infectivity.
Uncertainties remain with respect to the relative importance of these factors and the roles that they play. A more complete understanding of environmental factors impacting respiratory virus transmission will require enhanced epidemiological and surveillance data, as well as controlled experiments in small animal models.
Acknowledgments
This work was funded by the National Institutes of Health (NIH) RC1 AI086061-01 and the NIH Center of Excellence for Influenza Research and Surveillance (CEIRS) HHSN266200700010C (NP). NMB is funded by an NIAID Career Development Grant (K08 AI089940).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Turner RB. Chapter 53 -- The Common Cold. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7. Churchill Livingstone Elsevier; 2009. pp. 809–813. [Google Scholar]
- 2.Sandrock C, Stollenwerk N. Acute febrile respiratory illness in the ICU: reducing disease transmission. Chest. 2008;133:1221–1231. doi: 10.1378/chest.07-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musher DM. How contagious are common respiratory tract infections? N Engl J Med. 2003;348:1256–1266. doi: 10.1056/NEJMra021771. [DOI] [PubMed] [Google Scholar]
- 4.Forgie S, Marrie TJ. Healthcare-associated atypical pneumonia. Semin Respir Crit Care Med. 2009;30:67–85. doi: 10.1055/s-0028-1119811. [DOI] [PubMed] [Google Scholar]
- 5*.Belser JA, Maines TR, Tumpey TM, Katz JM. Influenza A virus transmission: contributing factors and clinical implications. Expert Rev Mol Med. 2010;12:e39. doi: 10.1017/S1462399410001705. This comprehensive review presents recent progress made in understanding the viral and environmental factors that impact upon efficient person-to-person transmission of influenza virus, as well as the clinical implications of these findings. [DOI] [PubMed] [Google Scholar]
- 6.Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7:257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 7*.Goldmann DA. Transmission of viral respiratory infections in the home. Pediatr Infect Dis J. 2000;19:S97–102. doi: 10.1097/00006454-200010001-00002. This review, although less recent, provides one of the few comprehensive summaries of prior published work regarding the modes of transmission of RSV, rhinovirus, and influenza virus. [DOI] [PubMed] [Google Scholar]
- 8.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interface. 2009;6(Suppl 6):S783–790. doi: 10.1098/rsif.2009.0302.focus. This paper provides an updated review of the literature, particularly with reference to new evidence supporting the role of influenza virus transmission through the aerosol/droplet routes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meissner HC, Murray SA, Kiernan MA, Snydman DR, McIntosh K. A simultaneous outbreak of respiratory syncytial virus and parainfluenza virus type 3 in a newborn nursery. J Pediatr. 1984;104:680–684. doi: 10.1016/s0022-3476(84)80943-9. [DOI] [PubMed] [Google Scholar]
- 11.Brady MT, Evans J, Cuartas J. Survival and disinfection of parainfluenza viruses on environmental surfaces. Am J Infect Control. 1990;18:18–23. doi: 10.1016/0196-6553(90)90206-8. [DOI] [PubMed] [Google Scholar]
- 12.Ansari SA, Springthorpe VS, Sattar SA, Rivard S, Rahman M. Potential role of hands in the spread of respiratory viral infections: studies with human parainfluenza virus 3 and rhinovirus 14. J Clin Microbiol. 1991;29:2115–2119. doi: 10.1128/jcm.29.10.2115-2119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol. 2011;46:324–347. doi: 10.1002/ppul.21377. [DOI] [PubMed] [Google Scholar]
- 14.Hall CB, Douglas RG., Jr Modes of transmission of respiratory syncytial virus. J Pediatr. 1981;99:100–103. doi: 10.1016/s0022-3476(81)80969-9. [DOI] [PubMed] [Google Scholar]
- 15.Winther B. Rhinovirus infections in the upper airway. Proc Am Thorac Soc. 2011;8:79–89. doi: 10.1513/pats.201006-039RN. [DOI] [PubMed] [Google Scholar]
- 16.Hui DS, Chan PK. Severe acute respiratory syndrome and coronavirus. Infect Dis Clin North Am. 2010;24:619–638. doi: 10.1016/j.idc.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peiris JS, Yuen KY, Osterhaus AD, Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349:2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 18.Casanova LM, Jeon S, Rutala WA, Weber DJ, Sobsey MD. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl Environ Microbiol. 2010;76:2712–2717. doi: 10.1128/AEM.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sloan C, Moore ML, Hartert T. Impact of pollution, climate, and sociodemographic factors on spatiotemporal dynamics of seasonal respiratory viruses. Clin Transl Sci. 2011;4:48–54. doi: 10.1111/j.1752-8062.2010.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Tamerius J, Nelson MI, Zhou SZ, Viboud C, Miller MA, Alonso WJ. Global influenza seasonality: reconciling patterns across temperate and tropical regions. Environ Health Perspect. 2011;119:439–445. doi: 10.1289/ehp.1002383. This comprehensive and recent review summarizes the scientific evidence for mechanisms that potentially explain influenza disease seasonality around the world, highlighting further research that is needed to resolve central questions in influenza seasonality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welliver RC., Sr Temperature, humidity, and ultraviolet B radiation predict community respiratory syncytial virus activity. Pediatr Infect Dis J. 2007;26:S29–35. doi: 10.1097/INF.0b013e318157da59. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf S, Piedimonte G, Auais A, Demmler G, Krishnan S, Van Caeseele P, Singleton R, Broor S, Parveen S, Avendano L, et al. The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiol Infect. 2007;135:1077–1090. doi: 10.1017/S095026880600776X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulman JL, Kilbourne ED. Airborne transmission of influenza virus infection in mice. Nature. 1962;195:1129–1130. doi: 10.1038/1951129a0. [DOI] [PubMed] [Google Scholar]
- 24.Schulman JL, Kilbourne ED. Experimental Transmission of Influenza Virus Infection in Mice. Ii. Some Factors Affecting the Incidence of Transmitted Infection. J Exp Med. 1963;118:267–275. doi: 10.1084/jem.118.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Lowen AC, Mubareka S, Steel J, Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:1470–1476. doi: 10.1371/journal.ppat.0030151. This influential study provided direct experimental evidence that airborne transmission of influenza virus in the guinea pig model is affected by ambient temperature and humidity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowen AC, Steel J, Mubareka S, Palese P. High temperature (30 degrees C) blocks aerosol but not contact transmission of influenza virus. J Virol. 2008;82:5650–5652. doi: 10.1128/JVI.00325-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang W, Marr LC. Dynamics of airborne influenza a viruses indoors and dependence on humidity. PLoS One. 2011;6:e21481. doi: 10.1371/journal.pone.0021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elazhary MA, Derbyshire JB. Aerosol stability of bovine adenovirus type 3. Can J Comp Med. 1979;43:305–312. [PMC free article] [PubMed] [Google Scholar]
- 29.Miller WS, Artenstein MS. Aerosol stability of three acute respiratory disease viruses. Proc Soc Exp Biol Med. 1967;125:222–227. doi: 10.3181/00379727-125-32054. [DOI] [PubMed] [Google Scholar]
- 30.Karim YG, Ijaz MK, Sattar SA, Johnson-Lussenburg CM. Effect of relative humidity on the airborne survival of rhinovirus-14. Can J Microbiol. 1985;31:1058–1061. doi: 10.1139/m85-199. [DOI] [PubMed] [Google Scholar]
- 31.Elazhary MA, Derbyshire JB. Aerosol stability of bovine parainfluenza type 3 virus. Can J Comp Med. 1979;43:295–304. [PMC free article] [PubMed] [Google Scholar]
- 32.Rechsteiner J, Winkler KC. Inactivation of respiratory syncytial virus in aerosol. J Gen Virol. 1969;5:405–410. [Google Scholar]
- 33.Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci U S A. 2009;106:3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaman J, Pitzer VE, Viboud C, Grenfell BT, Lipsitch M. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 2010;8:e1000316. doi: 10.1371/journal.pbio.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoji M, Katayama K, Sano K. Absolute humidity as a deterministic factor affecting seasonal influenza epidemics in Japan. Tohoku J Exp Med. 2011;224:251–256. doi: 10.1620/tjem.224.251. [DOI] [PubMed] [Google Scholar]
- 36.Viboud C, Alonso WJ, Simonsen L. Influenza in tropical regions. PLoS Med. 2006;3:e89. doi: 10.1371/journal.pmed.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dosseh A, Ndiaye K, Spiegel A, Sagna M, Mathiot C. Epidemiological and virological influenza survey in Dakar, Senegal: 1996–1998. Am J Trop Med Hyg. 2000;62:639–643. doi: 10.4269/ajtmh.2000.62.639. [DOI] [PubMed] [Google Scholar]
- 38.de Mello WA, de Paiva TM, Ishida MA, Benega MA, Dos Santos MC, Viboud C, Miller MA, Alonso WJ. The dilemma of influenza vaccine recommendations when applied to the tropics: the Brazilian case examined under alternative scenarios. PLoS One. 2009;4:e5095. doi: 10.1371/journal.pone.0005095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Omer SB, Sutanto A, Sarwo H, Linehan M, Djelantik IG, Mercer D, Moniaga V, Moulton LH, Widjaya A, Muljati P, et al. Climatic, temporal, and geographic characteristics of respiratory syncytial virus disease in a tropical island population. Epidemiology and infection. 2008;136:1319–1327. doi: 10.1017/S0950268807000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan PW, Chew FT, Tan TN, Chua KB, Hooi PS. Seasonal variation in respiratory syncytial virus chest infection in the tropics. Pediatric pulmonology. 2002;34:47–51. doi: 10.1002/ppul.10095. [DOI] [PubMed] [Google Scholar]
- 41.Robertson SE, Roca A, Alonso P, Simoes EA, Kartasasmita CB, Olaleye DO, Odaibo GN, Collinson M, Venter M, Zhu Y, et al. Respiratory syncytial virus infection: denominator-based studies in Indonesia, Mozambique, Nigeria and South Africa. Bulletin of the World Health Organization. 2004;82:914–922. [PMC free article] [PubMed] [Google Scholar]
- 42.Reese PE, Marchette NJ. Respiratory syncytial virus infection and prevalence of subgroups A and B in Hawaii. Journal of clinical microbiology. 1991;29:2614–2615. doi: 10.1128/jcm.29.11.2614-2615.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Tropical medicine & international health : TM & IH. 1998;3:268–280. doi: 10.1046/j.1365-3156.1998.00213.x. [DOI] [PubMed] [Google Scholar]
- 44.Loscertales MP, Roca A, Ventura PJ, Abacassamo F, Dos Santos F, Sitaube M, Men ndez C, Greenwood BM, Saiz JC, Alonso PL. Epidemiology and clinical presentation of respiratory syncytial virus infection in a rural area of southern Mozambique. The Pediatric infectious disease journal. 2002;21:148–155. doi: 10.1097/00006454-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 45**.Shek LP, Lee BW. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatric respiratory reviews. 2003;4:105–111. doi: 10.1016/s1526-0542(03)00024-1. This review summarizes the seasonal trends of respiratory viral infections in the tropics, with a special emphasis on RSV. [DOI] [PubMed] [Google Scholar]
- 46.Avendano LF, Parra J, Padilla C, Palomino MA. The influence of winter 2002 in pediatric health: dissociation between environmental factors and respiratory syncytial viruses, in Santiago. Revista medica de Chile. 2003;131:902–908. [PubMed] [Google Scholar]
- 47.Agrawal AS, Sarkar M, Chakrabarti S, Rajendran K, Kaur H, Mishra AC, Chatterjee MK, Naik TN, Chadha MS, Chawla-Sarkar M. Comparative evaluation of real-time PCR and conventional RT-PCR during a 2 year surveillance for influenza and respiratory syncytial virus among children with acute respiratory infections in Kolkata, India, reveals a distinct seasonality of infection. Journal of medical microbiology. 2009;58:1616–1622. doi: 10.1099/jmm.0.011304-0. [DOI] [PubMed] [Google Scholar]
- 48.Moura FE, Nunes IF, Silva GB, Jr, Siqueira MM. Respiratory syncytial virus infections in northeastern Brazil: seasonal trends and general aspects. The American journal of tropical medicine and hygiene. 2006;74:165–167. [PubMed] [Google Scholar]
- 49.Nascimento-Carvalho CM, Cardoso MR, Barral A, Araujo-Neto CA, Oliveira JR, Sobral LS, Saukkoriipi A, Paldanius M, Vainionpaa R, Leinonen M, et al. Seasonal patterns of viral and bacterial infections among children hospitalized with community-acquired pneumonia in a tropical region. Scandinavian journal of infectious diseases. 2010;42:839–844. doi: 10.3109/00365548.2010.498020. [DOI] [PubMed] [Google Scholar]
- 50.Vieira SE, Stewien KE, Queiroz DA, Durigon EL, Torok TJ, Anderson LJ, Miyao CR, Hein N, Botosso VF, Pahl MM, et al. Clinical patterns and seasonal trends in respiratory syncytial virus hospitalizations in Sao Paulo, Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo. 2001;43:125–131. doi: 10.1590/s0036-46652001000300002. [DOI] [PubMed] [Google Scholar]
- 51.Stensballe LG, Devasundaram JK, Simoes EA. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. The Pediatric infectious disease journal. 2003;22:S21–32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- 52.Bulkow LR, Singleton RJ, Karron RA, Harrison LH. Risk factors for severe respiratory syncytial virus infection among Alaska native children. Pediatrics. 2002;109:210–216. doi: 10.1542/peds.109.2.210. [DOI] [PubMed] [Google Scholar]
- 53.Rao BL, Banerjee K. Influenza surveillance in Pune, India, 1978–90. Bulletin of the World Health Organization. 1993;71:177–181. [PMC free article] [PubMed] [Google Scholar]
- 54.Ramamurty N, Pillai LC, Gunasekaran P, Elango V, Priya P, Sheriff AK, Mohana Influenza activity among the paediatric age group in Chennai. The Indian journal of medical research. 2005;121:776–779. [PubMed] [Google Scholar]
- 55.Abdullah Brooks W, Terebuh P, Bridges C, Klimov A, Goswami D, Sharmeen AT, Azim T, Erdman D, Hall H, Luby S, et al. Influenza A and B infection in children in urban slum, Bangladesh. Emerging infectious diseases. 2007;13:1507–1508. doi: 10.3201/eid1310.070368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang JW, Lai FY, Nymadawa P, Deng YM, Ratnamohan M, Petric M, Loh TP, Tee NW, Dwyer DE, Barr IG, et al. Comparison of the incidence of influenza in relation to climate factors during 2000–2007 in five countries. Journal of medical virology. 2010;82:1958–1965. doi: 10.1002/jmv.21892. [DOI] [PubMed] [Google Scholar]
- 57.Murray EJ, Morse SS. Seasonal Oscillation of Human Infection with Influenza A/H5N1 in Egypt and Indonesia. PloS one. 2011;6:e24042. doi: 10.1371/journal.pone.0024042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Myatt TA, Johnston SL, Zuo Z, Wand M, Kebadze T, Rudnick S, Milton DK. Detection of airborne rhinovirus and its relation to outdoor air supply in office environments. Am J Respir Crit Care Med. 2004;169:1187–1190. doi: 10.1164/rccm.200306-760OC. [DOI] [PubMed] [Google Scholar]
- 59.Rudnick SN, Milton DK. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air. 2003;13:237–245. doi: 10.1034/j.1600-0668.2003.00189.x. [DOI] [PubMed] [Google Scholar]
- 60.Moser MR, Bender TR, Margolis HS, Noble GR, Kendal AP, Ritter DG. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110:1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- 61.Yu IT, Li Y, Wong TW, Tam W, Chan AT, Lee JH, Leung DY, Ho T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 62.McKinney KR, Gong YY, Lewis TG. Environmental transmission of SARS at Amoy Gardens. J Environ Health. 2006;68:26–30. quiz 51–22. [PubMed] [Google Scholar]
- 63.Chen C, Zhao B, Yang X, Li Y. Role of two-way airflow owing to temperature difference in severe acute respiratory syndrome transmission: revisiting the largest nosocomial severe acute respiratory syndrome outbreak in Hong Kong. J R Soc Interface. 2011;8:699–710. doi: 10.1098/rsif.2010.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]


