Abstract
Entamoeba histolytica possesses a family of approximately 100 putative transmembrane kinases (TMKs), indicating that the parasite has an extensive means of environmental sensing. The TMKs have been divided into nine sub-groups based on the sequence composition of their intracellular kinase as well as extracellular cysteine-rich domains. EhTMKB1-9 has been recently shown to be expressed in proliferating trophozoites and induced by serum. Interference with EhTMKB1-9 by antisense RNA knockdown or expression of a truncated protein diminished proliferation, adhesion and cytotoxicity. Here we report the involvement of EhTMKB1-9 in phagocytosis and its virulence function in the formation of amebic colitis. Trophozoites induced to express higher levels of wild type EhTMKB1-9 showed increased capacity for endocytosis. In contrast, cells compromised for the EhTMKB1-9 expression through antisense inhibition showed significantly lower levels of phagocytosis and endocytosis under the experimental conditions. The role of EhTMKB1-9 as a virulence factor was studied using animal models of amebiasis. Trophozoites expressing high levels of mutant protein lacking the kinase domain showed a competitive disadvantage with regard to survival as well as invasive phenotype in the murine model of amebic colitis. The same parasites however, were not compromised in their ability to generate abscess in the gerbil model of invasive liver amebiasis. EhTMKB1-9 is the second member from the “B” group of EhTMKs which seems to be deployed by the parasite during intestinal infection. TMKs are attractive targets for drug development because of their requirement in virulence and proliferation.
Keywords: Entamoeba, Transmembrane kinase, Amebic colitis, Amebic liver abscess
1. Introduction
Entamoeba histolytica, the causative agent of amebiasis, is a protozoan parasite and responsible for severe morbidity and almost 100,000 deaths worldwide (Haque et al., 2003; Petri et al., 2009). After Plasmodium spp., E. histolytica is estimated to be the second major leading cause of deaths due to a protozoan parasite. It is therefore important to understand the virulence factors involved in the production of invasive disease in order to identify novel drug targets to treat this parasitic infection.
The parasite exhibits a biphasic life cycle consisting of non-motile, transmissible cysts and motile, replicating, invading trophozoites. Humans are the only natural host. E. histolytica infection can produce colitis characterized by ulceration and invasion of the intestinal wall. In advanced cases, trophozoites spread to distant organs including liver, lungs and brain (Samuel L, 2003). Cytolysis and phagocytosis of the target cell is a major pathological feature of the disease and therefore the factors contributing to cytolysis are important to understand amebic invasion.
Trophozoites have been demonstrated to persist in humans for many months, which suggest that they are able to evade the immune system (Haque et al., 2002). E. histolytica transmembrane kinases (EhTMKs) were initially thought to be involved in antigenic variation, as some of them showed similarity to the variant specific surface protein (VSP) of Giardia lamblia (Beck et al., 2005). Recent studies however favor the role of EhTMKs as principal signaling molecules, especially at the parasite-host interface (Buss et al., 2010). Similar to metazoan counterparts, many of the EhTMKs characterized so far (including PATMK, TMK39 and EhTMKB1-9) show cysteine-rich extracellular domains containing furin like or epidermal growth factor (EGF) -like moieties. These are signature extracellular domains in metazoan TMKs and are thought to be involved in aggregation of TMKs. E. histolytica survives in a complex gut environment competing constantly with bacteria for nutrients and space, and may need to sense changes in the environment in order to escape the host defense mechanisms, trigger transition to the persistent and environmentally resistant infective cyst life stage and express the proteins important for virulence and tissue invasion. This demands a repertoire of highly specialized networking of signaling pathways.
Transmembrane kinases represent one of the major classes of cell surface receptors and are involved in essential cellular processes in almost all the eukaryotic cells. These molecules typically sense environmental cues and transduce the signals to appropriate intracellular pathways. Completion of sequenced genomes of human-infective as well as non-infective protozoa in recent years has allowed the kinome of the parasites to be defined (Naula et al., 2005). Bioinformatic analysis predicts at least 90 TMKs in E. histolytica, 9 in Dictyostelium discoideum (Goldberg et al., 2006); 88 in Monosiga brevicollis (Manning et al., 2008), 11 in Plasmodium falciparum (Ward et al., 2004) and 10 TMKs in Trypanosoma brucei (Parsons et al., 2005). An EhTMK typically contains four domains – N-terminal signal peptide, extracellular domain rich in CXXC repeats, a single transmembrane helix and an intracellular tyrosine-kinase like domain (Beck et al., 2005). E. histolytica TMKs have been subdivided into nine sub-groups (A, B1–3, C, D1–2, E and F) based on their kinase domain as well as sequence composition of the extracellular domains.
Many signaling pathways have been shown to be active in E. histolytica and wehypothesize that these allow the pathogen toappropriately respond to various environmental stimuli. The first Entamoeba TMK to be functionally characterized TMK B1.I.1 was shown to play an active role in E. histolytica cell proliferation (Mehra et al., 2006). To date four additional EhTMKs have been studied. PATMK (phagosome associate transmembrane kinase) is also a member of EhTMK B-family that participated in erythrophagocytosis and was identified during screening for the proteins involved in the identification and ingestion of apoptotic corpses (Boettner et al., 2008). Subsequently, it was proven that a single E. histolytica trophozoite could express members of at least four sub-groups (A, B1, D1 and E) and thus utilize multiple TMKs for non-redundant functions (Bussetal., 2010). TMK-39 was shown to regulate the internalization of polyanionic macromolecules; whereas TMK-54 was shown to be a major growth factor receptor besides regulating the expression of heavy chain subunit of the Gal/GalNAc surface lectin (Bussetal., 2010). EhTMKB1-9 (TMK-95) is the most recent member of the TMK family tobe functionally characterizedin detail (Shrimal et al., 2010). EhTMKB1-9 was found to be serum responsive and one of the first examples of inducible genes in Entamoeba. The kinase domain was found to have both ser/thr and tyr kinase activities similar to dual specificity kinase splA from D. discoidium. Adhesion is one of the first steps in pathogenesis and a prerequisite for host cell killing. Cells containing reduced levels of EhTMKB1-9orhigh levels of dominant negative mutant protein resulted in decreased proliferation, target cell adherence as well as cell killing. The results clearly showed that EhTMKB1-9 was an important signaling molecule likely to be involved in parasite proliferation in vivo.
Protein phosphorylation regulates most aspect of cell life and protein kinases have been termed as one of the major drug targets of the twenty-first century. Plasmodial kinases have been considered targets for drug development and, based on structural divergence, selectivity against mammalian kinases have been viewed as a realistic possibility (Doerig, 2004). Protein kinases also are being pursued as drug targets in trypanosomes and Leishmania (Naula et al., 2005).
The objective of the present work was to validate the in vitro observations using the animal models of amebiasis and to assess EhTMKB1-9's pathogenesis properties in view of the previous studies showing its potential as an important virulence factor. Parasites over expressing the dominant negative mutant protein were tested for their competence to phagocytose and kill cells as well as cause infection at two host target tissues- gut and liver. The ability of parasite to survive and invade the gut was assessed by competition experiment using the mouse model of amebiasis. Similarly, gerbil liver abscess model was used to study parasite's ability to induce liver abscess. As in case of PATMK, EhTMKB1-9 was found to be important for intestinal infections but not for causing liver abscesses. The studies further confirm role of EhTMKB1-9 as an important virulence factor and open up possibilities of screening molecules which potentially could target this and other similar extracellular receptors.
2. Materials and methods
2.1. Strains and cell culture
E. histolytica strain HM1:IMSS trophozoites were grown under axenic conditions in TYI-S-33 medium supplemented with 15% adult bovine serum, 1× Diamond's vitamins, penicillin (100 U/ml) and streptomycin (100 μg/ml) (Diamond et al., 1978). The cloning of full length EhTMKB1-9 (TMK9-S), dominant negative mutant (TMK9-Dn) and antisense construct (TMK9-AS) into tetracycline inducible expression vector pEhHYG-tetR-O-CAT (TOC) has been described previously (Shrimal et al., 2010). Please note that the AS construct was cloned using the first 1 kb region of EhTMKB1-9 ORF. The initial 307 nucleotides are unique to EhTMKB1-9 while the rest 707 nucleotides share sequence similarity only with another member from the family viz. EhTMKB1-10. However, the expression of EhTMKB1-10 is at least 1000-fold less as compared to EhTMKB1-9 as seen by quantitative real time PCR measurements in normal HM1 proliferating cells (Shrimal et al., 2010). The transfected lines carrying different constructs were generated using established protocols (Olvera et al., 1997; Asgharpour et al., 2005). In short, amebae (2.2 × 105 ml−1) were washed in Medium 199 (Invitrogen, CA) supplemented with 5.7 mM cysteine, 1 mM ascorbic acid, 25 mM HEPES (pH 6.8) and mixed with 10 μg of DNA and 30 μl of Superfect (Qiagen). Treated amebae were incubated for 3 h at 37 °C, followed by overnight recovery in TYI-S-33 medium at 37 °C. Where mentioned, the cultures were induced using 20 μg/ml tetracycline for 48 h in TYI-33-S medium.
2.2. Murine model of amebic colitis
The murine model of amebic colitis utilized mice carrying two copies of R allele at codon 223 of the leptin receptor, as described elsewhere (Stratigopoulos et al., 2009; Duggal et al., 2011). All animal protocols were approved by the Institutional Animal Care and Use Committee. Mice were maintained under specific-pathogen-free conditions at the University of Virginia and monitored closely for general well-being. For competition experiments the respective transfectant lines were mixed in a 1:1 ratio. For all intracecal inoculations a total of 2×106 trophozoites in log phase were injected in a volume of 150 μl after laparotomy as described (Houpt et al., 2002). In mice inoculated with induced amebae, the induction was maintained by the addition of 0.2 mg/ml doxycycline and 5% sucrose in drinking water. All mice were sacrificed 4 days after infection. Amebae were isolated from two in vivo locations - the luminal gut and gut mucosal surface. DNA was extracted and samples were subjected to construct specific quantitative PCR.
2.3. Gerbil model of amebic liver abscess formation
Liver abscesses were induced as described (Chadee and Meerovitch, 1989). Transfectants carrying either the control or plasmid expressing the dominant negative EhTMKB1-9 were grown in presence or absence of 20 μg/ml tetracycline for 48 h prior to infection. 1×106 similarly treated transfectant lines were inoculated into different liver lobes of 50-60 day old Mogolian male gerbil (Meriones unguiculatus). One transfectant was injected into the right anterior lobe of the liver and the other into left lobe. This order was reversed in half of the animals. After recovery from surgery, induction was maintained where appropriate by adding 2 mg/ml doxycycline in the drinking water. Gerbils were sacrificed 7 days past infection, livers were extracted and abscess weights measured.
2.4. Quantitative PCR
Closed circular DNA was isolated from cecal contents by a modification of previously published methods (Pontes et al., 2002; Sikorski et al., 2002). Amebae were collected and washed with phosphate-buffered saline (PBS), and lysed by the addition of 700 μl Rapid One-Step Extraction buffer - 10 mM Tris (pH 8.0), 300 mM EDTA(pH 8.0), 1% sodium lauryl sulfate, 1% polyvinylpoly-pyrrolidone. The lysate was heated to 95 °C for 10 min, vortexed and returned to 95 °C for 10 min. The cell lysate was then centri-fuged at 14,000×g for 10 min at room temperature. Five hundred microliters of the supernatant was collected, mixed with 50 μl of cold 3 M sodium acetate (pH 5.3, 4 C) and 1 ml of cold ethanol (−20°C), and immediately centrifuged at 14,000×g for 15 min. DNA pellet was washed once with 500 μl of 70% ethanol (−20 °C), dried under vacuum, and resuspended in 50 mM MOPS buffer (50 mM MOPS (morpholine propane sulfonic acid), 750 mM NaCl (pH 7.5) to give a final volume of 0.75 ml. The sample was then purified using the Zymo plasmid purification kit (Zymo Inc.). The eluted DNA was passed twice over a Zymo-Spin IV-HRC filtration column, and DNA was subjected to PCR with construct specific primers. TOC plasmid was detected by using primers specific for the chloamphenicol acetyl transferase (CAT) gene. The primers used were - forward 5′-TGGAGTGAATACCACGACGA-3′ and reverse 5′-GGGCGAAGAAGTTGTCCATA-3′. The TMK9-Dn expression plasmid was detected by using the following pair of primers: forward 5′-ATTGAAGGAGGATATGCATGTGA-3′ and reverse 5′-TGATGATGATGATGATGTGATCC-3′.
2.5. Erythrophagocytosis assay
The assay was performed as described (Mora-Galindo et al., 1997). Briefly, trophozoites and red blood cells were mixed together at a ratio of 1:100, pelleted down and washed three times with distilled water to burst open non-engulfed RBCs. The pellets containing parasites and ingested RBCs were lysed in concentrated formic acid (90%), and solutions were read on a spectrophotometer at 400 nm.
2.6. RITC-dextran uptake assay
Endocytosis assay to quantitate uptake of RITC-dextran was performed as described (Sahoo et al., 2004). Briefly, trophozoites and RITC-dextran were incubated together for 30 min at 36 °C pelleted down and washed three times with PBS. The cells were lysed by resuspending in PBS containing triton-X100 and fluorescence was measured.
3. Results
3.1. EhTMKB1-9 enhances erythrophagocytosis and endocytosis
Erythrophagocytosis is one of the common assays used to assess virulence potential of laboratory strains of Entamoeba. Difference in the ability of pathogenic E. histolytica strains to ingest erythrocytes compared to the nonpathogenic species Entamoeba dispar has been used historically to diagnose Entamoeba infections from stool specimen (Trissl et al., 1978). Both the amebic Gal/GalNAc inhibitable lectin as well as transmembrane kinases have been shown to be important in mediating the attachment of ameba to erythrocytes (Petri et al., 1987; Boettner et al., 2008). EhTMKB1-9 has been previously shown to constitute about 95% of the total expressed B1 family TMKs and to be important in adhesion as well as destruction of CHO cells (Shrimal et al., 2010). We measured the ability of trophozoites over expressing or deficient in EhTMKB1-9 to phagocytose red blood cells. Parasites grown in absence or presence of tetracycline were incubated with the red blood cells and extracellular erythrocytes were lysed in distilled water. Parasites containing ingested erythrocytes were then lysed in concentrated formic acid and the amount of heme present (as measured by OD) was used as a surrogate marker for the quantification of ingested erythrocytes. The cells containing vector alone, or the sense construct for over-expression, did not show any significant change in erythrophagocytosis. Over expression of the dominant negative mutant (TMK9-Dn) or antisense RNA showed a significant reduction in phagocytosis (Fig. 1A).
Fig. 1.
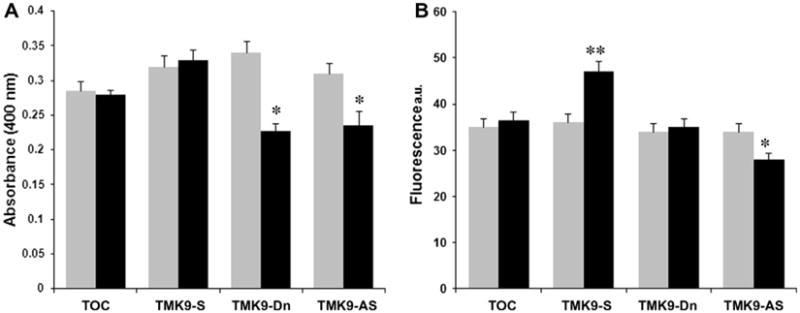
Role of EhTMKB1-9 in phagocytosis and pinocytosis. Expression of the transfectants was uninduced (grey bars) or induced (black bars) with 20 μg/ml tetracycline for 48 h. (A) Quantification of erythrophagocytosis in cells showing over-expression or suppression of EhTMKB1-9. Trophozoites were incubated with RBCs for 10 min at 37 °C followed by lysis of adherent RBCs with chilled water. The trophozoites with phagocytosed RBCs were then lysed using formic acid and amount of heme released was quantitated by measuring absorbance at 400 nm. The values are expressed as an average of three independent experiments ±SD. (B) Quantification of liquid phase pinocytosis. The different transfectants were incubated with 2 mg/ml RITC-dextran in PBS for 30 min and subjected to quantitative estimation by spectrofluorometer. The values are expressed as an average of three independent experiments ±SD. Abbreviations: TOC - tetracycline inducible vector control; TMK9 S - construct expressing full length EhTMKB1-9; TMK9 Dn - construct expressing kinase domain deletion mutant of EhTMKB1-9; TMK9 AS - construct expressing antisense RNA to N-terminal 1 kb region of EhTMKB1-9. *p value <0.05; **p value <0.001.
To determine the role of EhTMKB1-9 in a general endocytosis pathway, we measured the uptake of a 10-kDa fluid-phase marker RITC-dextran. The cells containing vector alone did not show any significant change in uptake in the presence or the absence of tet-racycline. Trophozoites induced to over express the full length EhTMKB1-9 showed significantly higher fluorescence indicating increased endocytosis activity (Fig. 1B), whereas cells induced to over express the antisense RNA showed reduced endocytosis. Surprisingly, cells induced to express the dominant negative mutant protein showed no change in the endocytic activity. The results suggested that the level of endocytosis is reduced only by anti-sense inhibition of EhTMKB1-9 gene expression. This could indicate a greater role for the extracellular domain rather than the kinase domain of EhTMKB1-9 in endocytosis.
3.2. Expression of dominant negative EhTMKB1-9 mutant confers competitive disadvantage to amebae in host gut
To test if EhTMKB1-9 acts as a virulence factor in establishing an invasive intestinal infection we used a murine model of amebic colitis (Guo et al., 2011). LepR Q223R mice were infected intrace-cally with a 1:1 mixture of amebae induced to express dominant negative EhTMKB1-9 and vector backbone. As a control, another set was used with a 1:1 mixed uninduced amebae. Amebae were induced for 48 h with 20 μg/ml tetracycline wherever indicated before animal inoculations. Eight mice were infected in each group. All mice were sacrificed 4 days post-inoculation and trophozoites were recovered from two different locations. Those isolated from the cecal contents were considered luminal whereas those removed from the luminal surface of the cecum by scraping were considered mucosal (Gilchrist et al., 2010). Closed circular DNA was extracted as described under materials and methods and used for quantitative PCR analysis. Construct-specific primer pairs allowed us to compare surviving populations of mutant or the control trophozoites quantitatively. The ratio of cultured input amebae in the induced or uninduced sets was used to correct the qPCR ratio of dominant negative to vector from each set of mice. The ratio of TMK9-Dn to TOC vector was significantly lower in the case of luminal amebae (p<0.01) as well as mucosal amebae (p<0.01) (Fig. 2). This indicated that the number of TMK9-Dn expressing amebae decreased substantially at both the gut locations. This was consistent with them having a competitive disadvantage in the gut environment. In contrast the ratio of TMK9-Dn to TOC vector were comparable when these parasites were mixed and maintained in laboratory cultures used for inoculating mice (data not shown), suggesting that the decrease in proliferation and invasion upon TMK9-Dn expression was unique to the intestinal lumen.
Fig. 2.
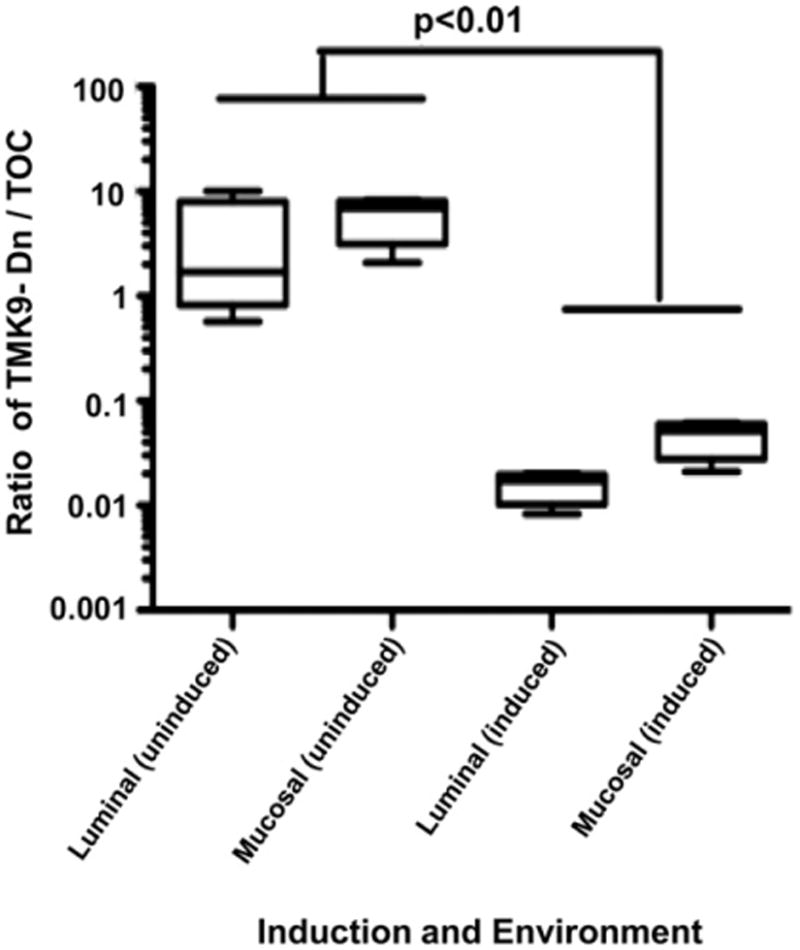
Competitive disadvantage of amebae expressing the dominant negative TMKB1-9 protein in the murine model of amebic colitis. Amebae were induced for 48 h with 20 μg/ml tetracycline where indicated. Mice carrying the susceptible Q223R Lepr allele were infected intracecally with induced TOC and TMK9-Dn amebae mixed in a 1:1 ratio. As a control, an equal number of mice were infected with the mixture of uninduced ameba. Induction was maintained by the addition of 0.2 mg/ml doxycycline and 5% sucrose in drinking water post-infectious challenge as appropriate. All mice were sacrificed 4 days after infection, and the ratios of the TMK9-Dn to the control TOC vector were determined by construct specific qPCR in amebae isolated from two different in vivo locations – the luminal gut (luminal) or the gut surface (mucosal). Values were corrected by the input data, and statistical significance was determined using t test. The difference in ratio was statistically significant in the luminal as well as mucosal samples (p < 0.01).
3.3. EhTMKB1-9 is not essential for amebic liver abscess formation
We also tested if EhTMKB1-9 which seemed to be important for intestinal amebiasis played any role in liver infection. We used the gerbil model of amebic liver abscess formation. Amebae carrying TMK9-Dn or TOC construct were grown in presence or absence of tetracycline for 48 h prior to inoculations. One million trophozoites each from similarly treated groups were used to inoculate different liver lobes of the same animal. The order in which the transfectants were inoculated was reversed in half of the animals to avoid any potential bias regarding the site of infection. Eight animals were used in each group. Gerbils were sacrificed 4 days post-infection, livers were isolated and abscess weights measured. Amebae from abscesses were cultured in TYI-S-33 broth to confirm infections (data not shown). No significant difference was noticed between the uninduced and induced control (Fig. 3A) or TMK9-Dn expressing (Fig. 3B) amebae as indicated by similar abscess weights. We concluded that expression of EhTMKB1-9 did not appear essential for liver infection.
Fig. 3.
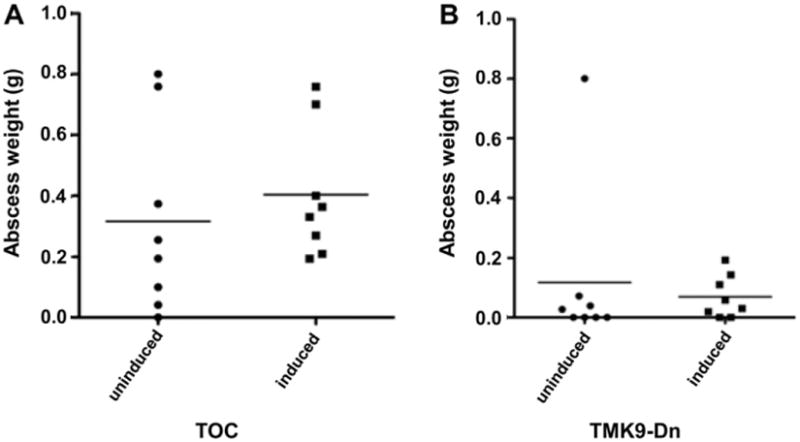
Induction of the EhTMKB1-9 dominant negative mutant did not affect liver abscess formation: Amebae transfected with the control vector or the plasmid expressing kinase domain deletion mutant of EhTMKB1-9 were grown in presence or absence of 20 μg/ml tetracycline for 48 h prior to infection. Amebae (106) were inoculated into different liver lobes of 50–60 day old Mongolian male gerbils. Induction was maintained where appropriate by adding 2 mg/ml doxycycline in the drinking water. Gerbils were sacrificed 7 days after infection, livers extracted and abscess weights measured. (A) Comparison between abscess weights formed by uninduced or induced vector transfectants (B) comparison between abscess weights formed by uninduced or induced EhTMKB9-Dn transfectants. The X-axis shows transfectant line and treatment whereas the Y-axis represents abscess weight.
TMK9-Dn transfectants in general caused smaller abscesses irrespective of the induction state. The difference in abscess weights was statistically significant between the induced TOC and TMK9-Dn transfectants (p < 0.005). This is not totally unexpected as full length TMKB1-9 has been shown to be important for cell proliferation (Shrimal et al., 2010). Surprisingly, the difference was also significant between the uninduced transfectants lines after removing the only outlier in the TMKDn group using Grubb's test. This most likely is due to TMK9-Dn background expression from the vector (Hamann et al., 1997) which in turn could be interfering with the wild type TMK function. This further emphasizes EhTMKB1-9's role in proliferation in vivo.
4. Discussion
EhTMKB1-9 is a virulence factor important for amebic colitis. Expression of the mutant EhTMKB1-9 conferred a disadvantage on the survival and invasive properties of amebae in the intestine. Competition experiment gave a direct proof for the role of EhTMKB1-9 in invasive disease as well as survival inside host gut. In contrast, EhTMKB1-9 did not seem to have any role in liver infection. Expression of EhTMK9-Dn mutant did not show any significant effect in the degree of abscess formation as seen by the comparison between abscess weights of induced EhTMK9-Dn versus control transfectants. E. histolytica seems to employ different virulence factors in different environments.
Formation of colitis and liver abscess appear to require some distinct virulence factors. Silencing of amoebapore A impaired parasite's ability to cause liver abscess but did not affect its ability to cause tissue destruction in a colonic xenograft model of amebiasis (Zhang et al., 2004). Over expression of cysteine protease-2 reduced in vitro monolayer destruction but had no effect on liver abscess formation (Hellberg et al., 2001). In our previous work we had discovered that the phagosome associated TMK (PATMK) was also important in the establishment of gut but not liver infections. We conclude that the virulence program required by E. histolytica for a successful intestinal infection differs from that required for liver infection.
It has been proposed that rapid phagocytosis of host cells might give E. histolytica an advantage for persistence in human gut by clearing dead cells and preventing release of host immunomodulatory signals which in turn could elicit infiltration of inflammatory cells (Boettner et al., 2008). PATMK participated in erythrophagocytosis (Boettner et al., 2008). Downregulation with shRNAs or expression of C terminal deletion mutant of PATMK, both decreased erythrophagocytosis by ≥50%. Expression of PATMK mutant also reduced the infection rate by 30% in mouse model of colitis. Consistent with this, EhTMKB1-9 also seemed to play a role in phagocytosis and endocytosis. In the present studies, the effect of down regulating of EhTMKB1-9 was not as dramatic compared to the downregulation of PATMK. Phagocytosis and endocytosis as says showed a ≤30% decrease. It is likely that EhTMKB1-9 may not be the primary receptor for the red blood cells and may be participating in the process as a non-receptor accessory molecule. Previous studies support this observation as no specific enrichment was seen for EhTMKB1-9 in phagocytic cups during erythrophagocytosis (Somlata et al., 2011). One of the observations made during the course of this study was that the antisense RNA against EhTMKB1-9 affected endocytosis but expression of kinase deletion mutant did not. It is possible that EhTMKB1-9 lacking the kinase domain could function similar to the epidermal growth factor receptor (ErbB) family proteins present in mammalian cells. Each ErbB member does not have an active kinase domain, but it can activate the relevant pathway through ligand induced hetero-dimerization with its partner with an active kinase domain (Linggi and Carpenter, 2006). This could explain the negative effect on endocytosis only after downregulation of the full length molecule.
Expression of TMK9-Dn showed impairment in multiple virulence functions including proliferation, adhesion, phagocytosis and target cell destruction (Shrimal et al., 2010 and this study). It is very important that the parasites maintain all these virulence functions in order to divide in gut environment, where the cells are constantly being washed away and replaced. The inability of TMK9-Dn cells to survive in the gut as suggested by the competition experiment indicates key role for EhTMKB1-9 in intestinal infection. It is possible that the parasite relies on a major virulence factor for tissue specific infection but has backup factors with overlapping functions as any interference with this may help the host to develop better immune response and hence clearance of infection. Although both EhTMKB1-9 and PATMK are important for intestinal infection, their influences on phagocytosis differ significantly. It is possible that the TMKs belonging to the same group (but different subgroups) may have retained the ability to carry redundant functions to some extent as this would ensure establishment of the parasite in its only natural host.
It would be interesting to quantitate expression of different members from the same group involved in intestinal infection in vivo. This could be done by developing antibodies to conserved regions of the closely related members and such a group of TMK proteins could also serve as an attractive target for drug design. It is remains to be discovered what ligands stimulate EhTMKB1-9 and the mechanism through which EhTMKs transduce signals intracellularly. It would also be important to see if EhTMKB1-9 modulates the amebic surface lectins since adherence is decreased.
Given the essential role EhTMKs play in the onset of infection, the idea of targeting EhTMKs as a therapeutic strategy is a promising one. Protein kinases have become the second most important group of drug targets after G-protein-coupled receptors, due to their important roles in parasite proliferation and establishment of invasive disease (Cohen, 2002). It has been proposed that protein kinases, which have less than 60% sequence identity to human counterparts, could be specifically inhibited (Vieth et al., 2004; Naula et al., 2005). Most of the protozoan protein kinases and their putative homologues in higher eukaryotes rarely show ≥60% homology (Doerig et al., 2002) and hence these could be ideal candidates for drug design. This concept was validated for trypanosomatids, where the target Leishmania CDK3 which had 54% identity human counterpart was inhibited efficiently and specifically by the designed inhibitor (Grant et al., 2004). In our work we have identified two EhTMKs belonging to the same family that are involved in non-redundant functions including cell phagocytosis and colitis. Development of inhibitor molecules that target closely related EhTMKs within a family might be advantageous. Future experiments would be directed to see the role of multiple TMKs simultaneously in the intestinal virulence program of E. histolytica.
Acknowledgments
This work was supported by NIH grant 5 R01 AI026649-20 to W.A. Petri.
Contributor Information
Mayuresh M. Abhyankar, Email: mma3a@virginia.edu.
Shiteshu Shrimal, Email: sshrimal@gmail.com.
Carol A. Gilchrist, Email: cg2p@virginia.edu.
Alok Bhattacharya, Email: alok.bhattacharya@gmail.com.
William A. Petri, Jr., Email: wap3g@virginia.edu.
References
- Asgharpour A, Gilchrist C, Baba D, Hamano S, Houpt E. Resistance to intestinal Entamoeba histolytica infection is conferred by innate immunity and Gr-1+ cells. Infect Immun. 2005;73:4522–4529. doi: 10.1128/IAI.73.8.4522-4529.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck DL, Boettner DR, Dragulev B, Ready K, Nozaki T, Petri WA., Jr Identification and gene expression analysis of a large family of transmembrane kinases related to the Gal/GalNAc lectin in Entamoeba histolytica. Eukaryotic Cell. 2005;4:722–732. doi: 10.1128/EC.4.4.722-732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner DR, Huston CD, Linford AS, Buss SN, Houpt E, Sherman NE, Petri WA., Jr Entamoeba histolytica phagocytosis of human erythrocytes involves PATMK, a member of the transmembrane kinase family. PLoS Pathog. 2008;4:e8. doi: 10.1371/journal.ppat.0040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss SN, Hamano S, Vidrich A, Evans C, Zhang Y, Crasta OR, Sobral BW, Gilchrist CA, Petri WA., Jr Members of the Entamoeba histolytica transmembrane kinase family play non-redundant roles in growth and phagocytosis. Int J Parasitol. 2010;40:833–843. doi: 10.1016/j.ijpara.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadee K, Meerovitch E. Entamoeba histolytica: diffuse liver inflammation in gerbils (Meriones unguiculatus) with experimentally induced amebic liver abscess. J Protozool. 1989;36:154–158. doi: 10.1111/j.1550-7408.1989.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Cohen P. Protein kinases – the major drug targets of the twenty-first century. Nat Rev Drug Discov. 2002;1:309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Doerig C, Meijer L, Mottram JC. Protein kinases as drug targets in parasitic protozoa. Trends Parasitol. 2002;18:366–371. doi: 10.1016/s1471-4922(02)02321-8. [DOI] [PubMed] [Google Scholar]
- Doerig C. Protein kinases as targets for anti-parasitic chemotherapy. Biochim Biophys Acta. 2004;1697:155–168. doi: 10.1016/j.bbapap.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Duggal P, Guo X, Haque R, Peterson KM, Ricklefs S, Mondal D, Alam F, Noor Z, Verkerke HP, Marie C, Leduc CA, Chua SC, Jr, Myers MG, Jr, Leibel RL, Houpt E, Gilchrist CA, Sher A, Porcella SF, Petri WA., Jr A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J Clin Invest. 2011;121:1191–1198. doi: 10.1172/JCI45294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist CA, Moore ES, Zhang Y, Bousquet CB, Lannigan JA, Mann BJ, Petri WA. Regulation of virulence of Entamoeba histolytica by the URE3-BP transcription factor. MBio. 2010;1 doi: 10.1128/mBio.00057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Manning G, Liu A, Fey P, Pilcher KE, Xu Y, Smith JL. The dictyostelium kinome-analysis of the protein kinases from a simple model organism. PLoS Genet. 2006;2:e38. doi: 10.1371/journal.pgen.0020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KM, Dunion MH, Yardley V, Skaltsounis AL, Marko D, Eisenbrand G, Croft SL, Meijer L, Mottram JC. Inhibitors of Leishmania Mexicana CRK3 cyclin-dependent kinase: chemical library screen and antileishmanialactivity. Antimicrob Agents Chemother. 2004;48:3033–3042. doi: 10.1128/AAC.48.8.3033-3042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Roberts MR, Becker SM, Podd B, Zhang Y, Chua SC, Jr, Myers MG, Jr, Duggal P, Houpt ER, Petri WA., Jr Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol. 2011;4:294–303. doi: 10.1038/mi.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann L, Buss H, Tannich E. Tetracycline-controlled gene expression in Entamoeba histolytica. Mol Biochem Parasitol. 1997;84:83–91. doi: 10.1016/s0166-6851(96)02771-5. [DOI] [PubMed] [Google Scholar]
- Haque R, Duggal P, Ali IM, Hossain MB, Mondal D, Sack RB, Farr BM, Beaty TH, Petri WA., Jr Innate and acquired resistance to amebiasis in Bangladeshi children. J Infect Dis. 2002;186:547–552. doi: 10.1086/341566. [DOI] [PubMed] [Google Scholar]
- Haque R, Huston CD, Hughes M, Houpt E, Petri WA., Jr Amebiasis. N Engl J Med. 2003;348:1565–1573. doi: 10.1056/NEJMra022710. [DOI] [PubMed] [Google Scholar]
- Hellberg A, Nickel R, Lotter H, Tannich E, Bruchhaus I. Overexpression of cysteine proteinase 2 in Entamoeba histolytica or Entamoeba dispar increases amoeba-induced monolayer destruction in vitro but does not augment amoebic liver abscess formation in gerbils. Cell Microbiol. 2001;3:13–20. doi: 10.1046/j.1462-5822.2001.00086.x. [DOI] [PubMed] [Google Scholar]
- Houpt ER, Glembocki DJ, Obrig TG, Moskaluk CA, Lockhart LA, Wright RL, Seaner RM, Keepers TR, Wilkins TD, Petri WA., Jr The mouse model of amebic colitis reveals mouse strain susceptibility to infection and exacerbation of disease by CD4+ T cells. J Immunol. 2002;169:4496–4503. doi: 10.4049/jimmunol.169.8.4496. [DOI] [PubMed] [Google Scholar]
- Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol. 2006;16:649–656. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Manning G, Young SL, Miller WT, Zhai Y. The protist, Monosiga brevicollis, has a tyrosine kinase signaling network more elaborate and diverse than found in any known metazoan. Proc Natl Acad Sci USA. 2008;105:9674–9679. doi: 10.1073/pnas.0801314105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra A, Fredrick J, Petri WA, Jr, Bhattacharya S, Bhattacharya A. Expression and function of a family of transmembrane kinases from theprotozoan parasite Entamoeba histolytica. Infect Immun. 2006;74:5341–5351. doi: 10.1128/IAI.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora-Galindo J, Gutiérrez-Lozano M, Anaya-Velázquez F. Entamoeba histolytica: kinetics of hemolytic activity, erythrophagocytosis and digestion of erythrocytes. Arch Med Res. 1997;28(Spec no):200–201. [PubMed] [Google Scholar]
- Naula C, Parsons M, Mottram JC. Protein kinases as drug targets in trypanosomes and Leishmania. Biochim Biophys Acta. 2005;1754:151–159. doi: 10.1016/j.bbapap.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvera A, Olvera F, Vines RR, Recillas-Targa F, Lizardi PM, Dhar S, Bhattacharya S, Petri W, Jr, Alagón A. Stable transfection of Entamoeba histolytica trophozoites by lipofection. Arch Med Res. 1997;28(Spec no):49–51. [PubMed] [Google Scholar]
- Parsons M, Worthey EA, Ward PN, Mottram JC. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics. 2005;6:127. doi: 10.1186/1471-2164-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri WA, Jr, Smith RD, Schlesinger PH, Murphy CF, Ravdin JI. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J Clin Invest. 1987;80:1238–1244. doi: 10.1172/JCI113198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri WA, Haque R, Mondal D, Karim A, Molla IH, Rahim A, Faruque ASG, Ahmad N, Kirkpatrick BD, Houpt E, Snider C. Prospective case-control study of the association between common enteric protozoal parasites and diarrhea in Bangladesh. Clin Infect Dis. 2009;48:1191–1197. doi: 10.1086/597580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes LA, Dias-Neto E, Rabello A. Detection by polymerase chain reaction of Schistosoma mansoni DNA in human serum and feces. Am J Trop Med Hyg. 2002;66:157–162. doi: 10.4269/ajtmh.2002.66.157. [DOI] [PubMed] [Google Scholar]
- Sahoo N, Labruyère E, Bhattacharya S, Sen P, Guillén N, Bhattacharya A. Calcium binding protein 1 of the protozoan parasite Entamoeba histolytica interacts with actin and is involved in cytoskeleton dynamics. J Cell Sci. 2004;117:3625–3634. doi: 10.1242/jcs.01198. [DOI] [PubMed] [Google Scholar]
- Samuel L SJ. Amoebiasis. Lancet. 2003;361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- Shrimal S, Bhattacharya S, Bhattacharya A. Serum-dependent selective expression of EhTMKB1-9, a member of Entamoeba histolytica B1 family of transmembrane kinases. PLoS Pathog. 2010;6:e1000929. doi: 10.1371/journal.ppat.1000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski J, Möhle M, Wackernagel W. Identification of complex composition, strong strain diversity and directional selection in local Pseudomonas stutzeri populations from marine sediment and soils. Environ Microbiol. 2002;4:465–476. doi: 10.1046/j.1462-2920.2002.00325.x. [DOI] [PubMed] [Google Scholar]
- Somlata, Bhattacharya S, Bhattacharya A. A C2 domain protein kinase initiates phagocytosis in the protozoan parasite Entamoeba histolytica. Nat Commun. 2011;2:230. doi: 10.1038/ncomms1199. [DOI] [PubMed] [Google Scholar]
- Stratigopoulos G, LeDuc CA, Matsuoka N, Gutman R, Rausch R, Robertson SA, Myers MG, Jr, Chung WK, Chua SC, Jr, Leibel RL. Functional consequences of the human leptin receptor (LEPR) Q223R transversion. Obesity (Silver Spring) 2009;17:126–135. doi: 10.1038/oby.2008.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trissl D, Martínez-Palomo A, de la Torre M, de la Hoz R, Pérez de Suárez E. Surface properties of Entamoeba: increased rates of human erythrocyte phagocytosis in pathogenic strains. J Exp Med. 1978;148:1137–1143. doi: 10.1084/jem.148.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieth M, Higgs RE, Robertson DH, Shapiro M, Gragg EA, Hemmerle H. Kinomics-structural biology and chemogenomics of kinase inhibitors and targets. Biochim Biophys Acta. 2004;1697:243–257. doi: 10.1016/j.bbapap.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Ward P, Equinet L, Packer J, Doerig C. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics. 2004;5:79. doi: 10.1186/1471-2164-5-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang Z, Alexander D, Bracha R, Mirelman D, Stanley SL., Jr Expression of amoebapores is required for full expression of Entamoeba histolytica virulence in amebic liver abscess but is not necessary for the induction of inflammation or tissue damage in amebic colitis. Infect Immun. 2004;72:678–683. doi: 10.1128/IAI.72.2.678-683.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


