Abstract
Objectives
Reward learning is critical for survival. Animal research emphasizes the role of dopaminergic (DA) mesocorticolimbic pathways in reward learning, but few studies have evaluated extrastriatal DA functioning in humans. The purpose of this study was to examine presynaptic DA release in extrastriatal regions of the reward circuit by measuring displacement of the high affinity D2/D3 radioligand [18F]Fallypride during a reward task.
Design
Ten healthy volunteers underwent a [18F]Fallypride Positron Emission Tomography protocol while performing a reward task, allowing us to assess participants’ ability to modulate behavior as a function of reward. DA receptor ligand displacement was correlated with task performance and self-reported anhedonia.
Observations
Parametric t-maps revealed significant decrease in [18F]Fallypride binding in the medial orbitofrontal cortex (mOFC), ventromedial prefrontal cortex (vmPFC) and dorsal anterior cingulate cortex (dACC), indicating endogenous DA release in these regions. Increasing anhedonic symptoms correlated with DA release in the left vmPFC, left dACC, and right dACC emerged (all rs > 0.65, ps < 0.05). Similarly, reduced reward learning correlated with higher DA release in left vmPFC, right vmPFC, and left dACC (all rs < −0.64, ps < 0.05). Left dACC (r = 0.66, p = 0.04) and left vmPFC (r = 0.74, p = 0.01) DA release showed a significant positive correlation with impaired tendency to modulate behaviour as a function of prior positive reinforcements.
Conclusions
These findings support the hypothesis that DA release in mOFC, vmPFC and dACC regions plays an important role in reinforcement learning in the human brain.
Keywords: Positron emission tomography, PET, Reward learning, Extrastriatal Reward Circuit, Dopamine, [18F]Fallypride, Anhedonia
Introduction
Anhedonia – the loss of pleasure or lack of reactivity to pleasurable stimuli – is considered a promising endophenotype of Major Depressive Disorder (MDD) (Hasler et al. 2004; Vrieze and Claes, 2009). However, its neurobiological underpinnings in humans remain largely unknown. Various components of anhedonic behaviour have been linked to dysfunction in both striatal and extrastriatal mesocortical areas, which are part of the dopaminergic brain reward circuit (Dillon et al. 2008; Knutson and Wimmer, 2007; Kringelbach and Berridge, 2009; Lucas et al. 2004). Reward learning has been linked directly to DA neurotransmission (Hasler et al., 2009a). Striatal regions of the brain reward circuit (e.g., nucleus accumbens) have generally been implicated in anticipation of reward and reward-seeking behaviour (Ikemoto and Panksepp, 1999; Wrase et al. 2007). The extrastriatal areas appear to play an important role in reward-related decision, reinforcement learning, and reward consumption (Knutson et al. 2001; Rushworth and Behrens, 2008). Consistent with this notion, the orbitofrontal cortex (OFC) and ventromedial prefrontal cortex (vmPFC) regions have been implicated in encoding representations of expected value, whereas the anterior cingulate cortex (ACC) appears to utilize reinforcement histories to guide behavior (Cox et al. 2005; Jocham et al. 2011; Knutson et al. 2005; Roesch and Olson, 2004; Rushworth et al. 2007).
Recent developments of high affinity positron emission tomography (PET) D2/D3 radioligands, such as [18F]Fallypride, provide the unique opportunity to directly investigate regions with low D2/D3 receptor density in vivo, including the extrastriatal reward circuit (Aalto et al. 2005; Mukherjee et al. 2002; Riccardi et al. 2008), using both pharmacological challenges as well as functional stimulation tasks (Badgaiyan et al. 2009; Christian et al. 2006; Riccardi et al. 2006a). Extending this prior work, the purpose of this study was to examine presynaptic DA release in extrastriatal regions of the reward circuit by measuring D2/D3 radioligand [18F]Fallypride displacement in response to a probabilistic reward task involving monetary reward. Specifically, our goal was to correlate reward task performance and self-report measures of anhedonia with the extent of DA release in those areas showing a task-induced DA modulation. Based on prior findings (Keedwell et al. 2005; St. Onge et al., 2011; Santesso et al. 2008; Wacker et al. 2009), we expected to find task-induced DA release in the vmPFC, OFC and dorsal ACC and to uncover links between (1) the spatial extent of the estimated DA release within each specific region of interest and (2) subjective hedonic capacity as well as the ability to respond to reinforcement stimuli.
A single-scan session PET design was used. Changes in ligand binding were computed by applying the linear extension of the simplified reference region model (LSRRM), with reduced binding indicating dopamine release (Alpert et al. 2003). The reward task has been previously used to objectively measure reward responsiveness in healthy volunteers (Pizzagalli et al. 2005) and dysfunctional reinforcement learning in MDD (Pizzagalli et al. 2009). Moreover, a single dose of a DA agonist – hypothesized to activate DA autoreceptors and thus reduce DA release – blunted reward responsiveness (Pizzagalli et al. 2008) and altered reward-related dorsal ACC activation (Santesso et al. 2009) in healthy volunteers, suggesting that performance in this task is modulated by DA.
2. Material and Methods
2.1 Participants
The study included 10 healthy, non-smoking, right-handed volunteers (mean age ± SD, 33.3 ± 8.2 y). In light of gender differences in extrastriatal DA release (Riccardi et al. 2006b), only females were included. Participants with current neurological (e.g. head trauma, seizures) or somatic illnesses, current or past mood disorders, psychotic disorders, substance and/or alcohol abuse (as determined by the Structured Clinical Interview for DSM-IV-TR (SCID-I) (Spitzer et al. 1992) were excluded. None of the subjects was taking psychotropic medication. All subjects refrained from food and drinks for at least four hours before scanning. Blood and urine samples were taken prior to the assessment to exclude pregnancy, substance abuse and abnormal thyroid levels. Written informed consent was obtained from each subject. The study was approved by the local ethics committee (UZ Leuven commissie voor Medische Ethiek) and performed according to the World Medical Association Declaration of Helsinki. Participants received 150 euro for participating in the study.
2.2 Study design and procedure
Subjects meeting inclusion criteria were invited for a single day of testing. Participants started the session by completing the Snaith Hamilton Pleasure Scale (SHAPS) (Leventhal et al. 2006; Snaith et al. 1995). The SHAPS is a 14-item questionnaire probing participants’ hedonic capacity in a variety of situations. Answers are logged on a 4-point scale (strongly agree, agree, disagree or strongly disagree). A total score was computed by summing the responses to each item, with higher scores indicating lower hedonic capacity, i.e,, higher anhedonia (Franken et al. 2007). Further, participants filled out the Beck Depression Inventory (BDI) (Beck et al. 1996). The BDI is a widely used and reliable measure of depressive symptoms; higher scores reflect increasing levels of depressive symptoms.
Next, a structural T2- and volumetric T1-weighted magnetic resonance imaging (MRI) head scan was obtained on a 1.5 Tesla Vision Scanner (Siemens, Germany). For the PET assessment, a single imaging protocol was initiated using a HR+ PET (Siemens, Ehrlangen, Germany) operating in three-dimensional acquisition mode. The subjects’ head and body were fixated to minimize head movement. A brief training session of the reward task was provided before scanning. The computer task was presented on a flat screen placed in a comfortable viewing position.
The PET emission was acquired in two blocks, in accordance with the PET imaging protocol of Christian and colleagues (2006). The first PET session, representing the baseline radiotracer kinetics, consisted of 35 frames (first 6 of 60 sec/frames and 120 sec/frames thereafter) and was initiated after intravenous administration of [18F]Fallypride (mean ± SD, 179 ± 17 MBq). After a 15-min break, the second PET emission data were collected for another 70 min: the first 20 min represented an extension of the baseline scan. Then, at 100 min post-injection, the reward task was initiated, lasting on average 46 min (SD: 3 min). The scan ended 150 min post-injection, at which time all participants had completed the task. To correct for attenuation, transmission scans were acquired with a 68-germanium source before radiotracer administration and at the end of the scanning session.
2.3 Reward Task
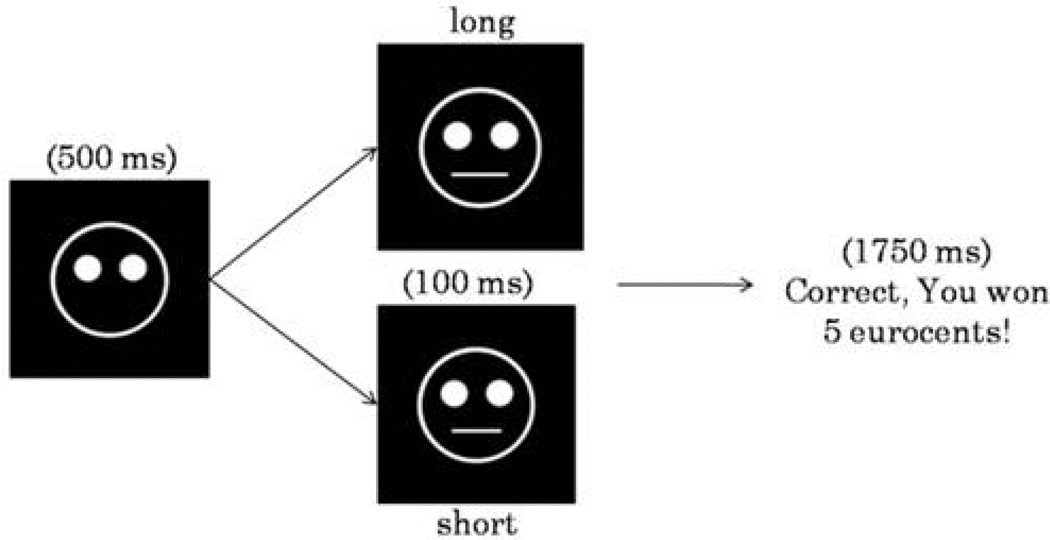
The task was a computerized probabilistic reward task in which correct identifications of two difficult-to-discriminate stimuli were differentially rewarded (Pizzagalli et al. 2005). The task consisted of 600 trials, divided in six blocks of 100 trials, separated by five short breaks (30 sec). Each trial started with a fixation point, shown for 500 msec in the middle of the screen, which was replaced by a mouthless cartoon face. After 500 msec, a short (11.5 mm) or long (13 mm) mouth appeared for 100 msec. The participants’ task was to determine, as quickly and accurately as possible, whether the short or long mouth had been presented by pressing a corresponding key. Before the PET scan, participants received verbal and written descriptions of the reward task, and were told that the goal of the task was to win as much money as possible. Moreover, they were informed that not all correct responses would result in a monetary reward. However, it was emphasized that more correct identifications would result in more reward feedback. Participants were informed that they would receive the total amount of accumulated money at the end of the experiment.
In each of the six blocks, both stimuli were shown an equal number of times. In each block, a monetary reward feedback was given to approximately 40 correct answers (Fig. 1). To induce a response bias, an asymmetrical reinforcer schedule was used, such as correct responses for one mouth (referred to as the ‘rich stimulus’) were rewarded three times more frequently (30 vs. 10) than correct responses of the other mouth (referred to as the ‘lean stimulus’). Due to this unequal frequency of reward feedback, participants with high reward responsiveness were expected to develop a response bias in favor of the rich stimulus. Subjects with low reward responsiveness, such as subjects with elevated depressive (particularly anhedonic) symptoms, were expected to develop a smaller or no bias, consistent with prior findings (Pizzagalli et al. 2005, 2009).
Figure 1.
Schematic representation of the probabilistic reward task. In each trial, the participants’ task was to decide (via key press) whether a short or long month stimulus had been presented in the mouthless face on the screen. In approximately 40% of the trials, monetary reward was presented after correct identifications.
2.4 Data reduction and statistical analysis
2.4.1 PET data
Subject images were first reconstructed using a standard three-dimensional (3D) filtered back-projection algorithm (Kinahan and Rogers, 1990) including model-based scatter as well as attenuation correction. During reconstruction, the intrinsic resolution was modeled as a 4.3-mm Gaussian (Adam et al. 1997). The image was reconstructed as a 128 × 128 × 63 voxel matrix (pixel size = 2.06 mm × 2.06 mm × 2.43 mm). Attenuation- and scatter-corrected reconstruction images were post-smoothed with a Gaussian filter with FWHM (full-width at half-maximum) equals to 4.8 mm, yielding images with a final spatial resolution of 6.5 mm.
PET frames were realigned, coregistered onto the individual MRI, and spatially normalized to the MNI template (using SPM2, Wellcome Department of Neurology).
For each individual, the mOFC (BA11), vmPFC (BA10) and dACC (BA32) were automatically defined in MNI space using sets of volume-of-interests (VOI) defined according to Brodmann areas (BA) on the basis of the Talairach Atlas (Talairach and Tournoux, 1988). VOI were constructed using the PMOD software VOI tool (PMOD Inc., Zurich, Switzerland), and then applied to each corresponding spatially normalized T1-weighted MRI image.
To measure reward-induced DA release in extrastriatal regions, we implemented a kinetic analysis of a single [18F]Fallypride scan based on a linear extension of the simplified reference region model (LSRRM) (Alpert et al. 2003) using Matlab-based in-house software (MathWorks, Natick, MA, USA). This technique includes a baseline and an activation condition. Assuming that the steady physiological state is not maintained during the single scan session, the task-induced effects are measured by time-dependent changes in ligand binding. Since binding of [18F]Fallypride to extrastriatal D2/D3 receptors is sensitive to endogenous DA levels (Mukherjee et al. 2002), a reduction in [18F]Fallypride binding potential is assumed to be caused by direct competition of the tracer with DA at the D2/D3 receptor sites. The modified LSRRM approach allows for the dissociation rate of ligand (k2a) to change over time in response to DA fluctuations, consequently to a change of binding potential. This time-dependent change of k2a is represented by the time-dependent parameter k2a + γ·h(t), where γ represents the amplitude of transient effects and the function h(t) describes a rapid change following task onset and dissipation over time. The function h(t) is an exponential decay function (Alpert et al. 2003; Christian et al. 2006) with the following form: h(t)=0 (for t < T) and h(t)=exp−τ(t-T) (for t ≥ T), where τ controls the rate at which activation effects dissipate (τ = 0.03 min−1) and T indicates the task initiation (T = 100 min). Thus, an increased k2a would be reflected in a decreased binding potential for D2/D3 receptors due to an increased DA release and would result in a positive value of γ.
For each subject, a voxel-based analysis of the data was carried out using the LSRRM, which generated individual quantitative parametric maps of the kinetic parameters. Next, in order to identify regions showing maximum radioligand displacement across subjects, these maps were combined in a t-statistic map of the γ parameter (t > 5, p < 0.000005, one-tailed). This threshold corresponded to Bonferroni-corrected p < 0.05 (0.05/average total number of voxels analyzed per subject (= 11,017) = 0.0000045). Consistent with prior studies (Christian et al. 2006), the percentage of statistically significant voxels within each region of interest was calculated to identify the spatial extent of the estimated DA release during the reward task.
2.4.2 Reward Task
We evaluated overall task performance by computing response bias (RB) and discriminability. The primary variable of interest was RB, which captures participants’ ability to modulate behaviour as a function of reward. RB was computed as:
To enable RB calculation in cases with zero in one cell of the formula, 0.5 was added to each cell in the matrix. RB is high when participants correctly classify the stimulus associated with more frequent reward (rich stimulus), and misclassify the lean stimulus. Discriminability, which captures participants’ ability to perceptually distinguish between the two stimuli and thus provides a measure of task difficulty, was computed as:
Moreover, reaction time (RT) and hit rates (% correct responses) for the rich and lean stimulus were calculated, to confirm that the task elicited the intended behavioural effects. Outlier RTs were excluded using the 2-step procedures described in prior work (Pizzagalli et al. 2005). RB and discriminability were analyzed using one-way analysis of variance (ANOVA) entering Block (1–6) as repeated measure. For RT and hit rate scores, Stimulus (rich, lean) was entered as an additional repeated measure. The Greenhouse-Geisser correction was used, when appropriate. Significant ANOVA effects were further evaluated with simple t-tests.
To directly assess overall reward learning, a difference score between RB in block 1 and 6 was computed [“Δresponse bias” = RB(Block 6) – RB(Block 1)]. In a secondary analysis, we computed the probability of rich misses as a function of which stimulus was rewarded in the immediately preceding trial. The calculated probability values allowed us to investigate the strength of the response bias as a function of the rewarded stimulus in the immediately preceding trial.
2.4.3 Correlations
Pearson correlations were computed among (1) SHAPS and BDI scores, (2) task performance, and (3) percentage of statistically significant voxels using SAS version 9.2.
3. Results
3.1 Reward task performance
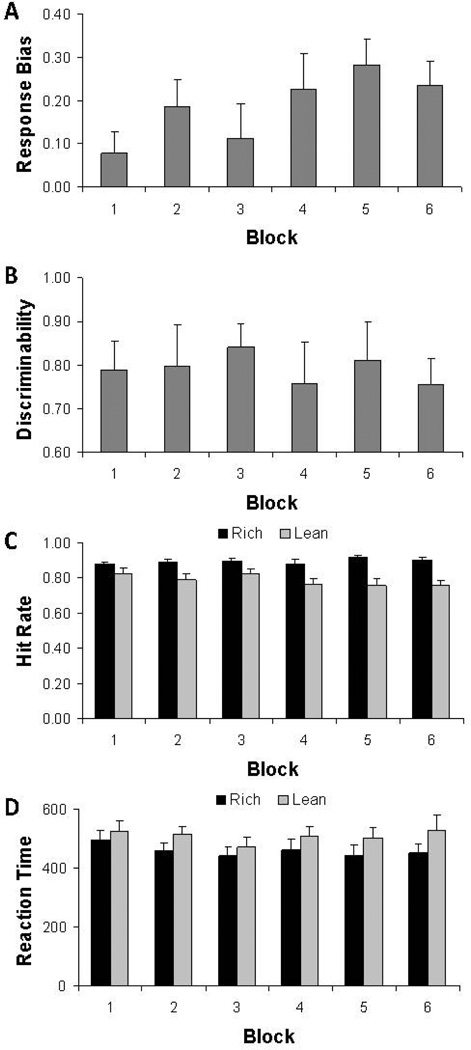
Replicating prior studies (Pizzagalli et al. 2005, 2008, 2009), the one-way ANOVA on RB scores revealed a main effect of Block (F(5,45) = 3.51, p = .041, ε = 0.48) and within-subjects analyses indicated that the linear contrast was significant (F(1,9) = 17.01, p = .003) due to a general increase in RB scores over blocks (Figure 2A). Follow-up paired t-tests indicated that RB in block 4 (t(9) = 2.24, p = .052), block 5 (t(9) = 2.79, p = .021), and block 6 (t(9) = 2.68, p = .025) was greater than in block 1. Moreover, 9 of the 10 subjects had a positive Δresponse bias (binomial p(9/10) < .01). Critically, the one-way ANOVA on discriminability scores revealed no significant effect of Block (F(5,45) = 0.48, p > .66, ε = 0.49) (Figure 2B), indicating that task difficulty was stable across blocks.
Figure 2.
Task performance during the probabilistic reward task. Response Bias (A), discriminability (B), hit rate (C) and reaction time (in ms) (D) for the whole sample (n = 10). Error bars represent standard errors. For hit rate and reaction time, the rich condition (black bars) refers to the stimulus associated with more frequent reward, whereas the lean condition (light grey bars) refers to the stimulus associated with less frequent reward.
Evidence for a behavioural preference in favour of the rich stimulus emerged also from analyses of hit rates and reaction time. For hit rates, significant Stimulus (F(1,9) = 13.86, p < .005) and Stimulus × Block (F(1,45) = 3.54, p < .009) effects emerged, due to significantly higher scores for the rich relative to the lean stimulus and the fact that differences become larger over the course of the blocks (Figure 2C). For reaction time scores, a main effect of Stimulus emerged, due to significantly faster response to the rich than lean stimulus (F(1,9) = 5.32, p < .05) (Figure 2D). Collectively, these findings indicate that participants developed a response bias in favour of the more frequently rewarded rich stimulus in the absence of fluctuations in task difficulty, confirming that the reward task produced the intended behavioural effect.
3.2 DA release during the reward task
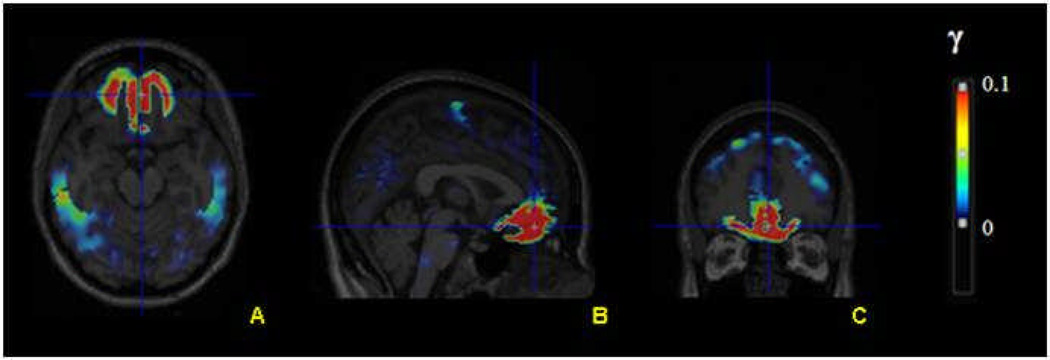
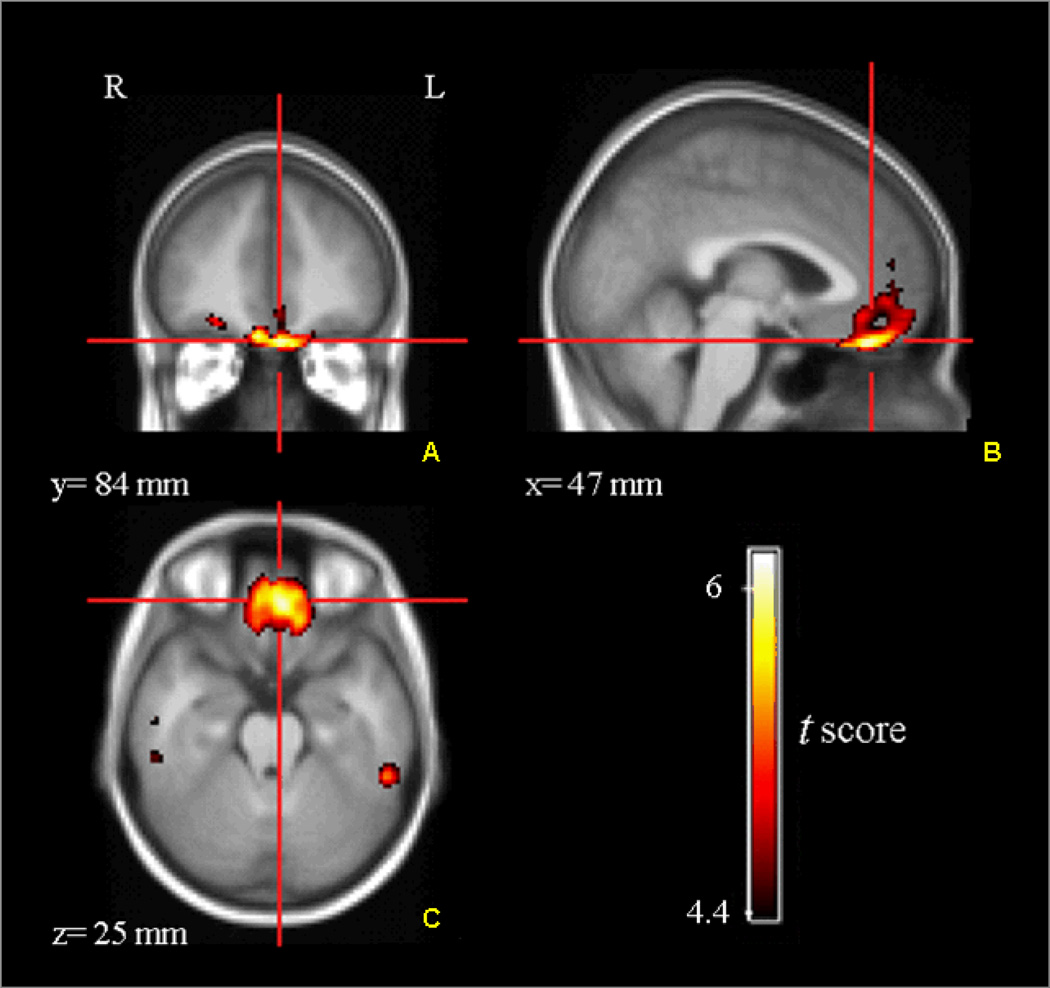
Using LSRRM, we first performed a voxel-based estimation of the kinetic parameters for each subject. Figure 3 represents an illustrative example of a γ parametric image for one subject, showing that the rate of ligand displacement increased during the reward task in the medial OFC, vmPFC, and dACC. In these regions, the mean γ obtained was between 0.008 and 0.01. All mean γ values were positive, indicating DA release. A covariance image of γ (standard deviation, sd(γ)) was calculated to generate a statistic t-map for γ [t = γ/sd(γ)], which allowed us to visualize regions with significant task-induced ligand displacement. Parametric t-map across subjects (Figure 4) confirmed significant ligand displacement in the mOFC (Brodmann area (BA) 11) and vmPFC (BA10). Significant tracer displacement was also seen in the dACC (BA32), which is not shown in these slices. Table 1 lists the percentages of significant voxels with a t score that exceeded the threshold of t > 5 within the three activated regions of interest: mOFC, vmPFC and dACC. For all three regions, there was no significant difference between the percentage of significant voxels in the left versus right hemisphere. Each subject showed a considerable ligand displacement within the mOFC, vmPFC and dACC during the task, as indicated by the average statistical parametric t map of γ, as well as the total number of significant voxels.
Figure 3.
Illustrative example of a γ parametric image (shown in transversal (A), sagittal (B) and coronal (C) views) overlaid on a structural MRI for a single subject. Increasing γ values correspond to greater ligand displacement.
Figure 4.
Statistical parametric t-map (across all subjects) showing medial OFC and vmPFC regions with significant tracer displacement during the probabilistic reward task. The color-coded t-values (t > are overlaid on a MRI template. [18F]Fallypride displacement is shown in coronal (A), sagittal (B) and transversal (C) views. L, left; R, right.
Table 1.
Spatial extent of the estimated dopamine release induced by the reward task, represented as the percentage of significant voxels exceeding the threshold of t > 5 within each activated region.
| Subject | Spatial extent of the estimated dopamine release |
Reward Task | ||||||
|---|---|---|---|---|---|---|---|---|
| BA11L | BA11R | BA10L | BA10R | BA32L | BA32R | Δresponse bias | Probability rich miss after rewarded rich trial |
|
| 1 | 46% | 56% | 18% | 10% | 11% | 8% | 0.0239 | 0.0722 |
| 2 | 29% | 21% | 16% | 9% | 6% | 9% | 0.4210 | 0.1136 |
| 3 | 8% | 7% | 30% | 14% | 20% | 35% | 0.1809 | 0.1186 |
| 4 | 8% | 4% | 7% | 16% | 4% | 21% | 0.3784 | 0 |
| 5 | 12% | 9% | 23% | 18% | 20% | 8% | 0.0814 | 0.0784 |
| 6 | 57% | 50% | 26% | 29% | 22% | 10% | 0.0080 | 0.0217 |
| 7 | 70% | 64% | 30% | 35% | 30% | 20% | 0.2938 | 0.0549 |
| 8 | 37% | 41% | 12% | 24% | 31% | 20% | 0.2220 | 0.0652 |
| 9 | 26% | 17% | 66% | 72% | 90% | 88% | −0.1945 | 0.1707 |
| 10 | 8% | 12% | 41% | 23% | 48% | 30% | 0.1628 | 0.1860 |
BA11 = medial orbitofrontal cortex (mOFC); BA10 = ventromedial prefrontal cortex (vmPFC); BA32 = dorsal anterior cingulate cortex (dACC); L = left; R = right.
Right columns represent main results from the reward task, based on Δresponse bias (computed as RB(block 6) – RB(block 1)) and the probability of a rich miss immediately after a rewarded correct identification of the rich stimulus.
3.3 Correlations between questionnaire data, task performance and ligand displacement
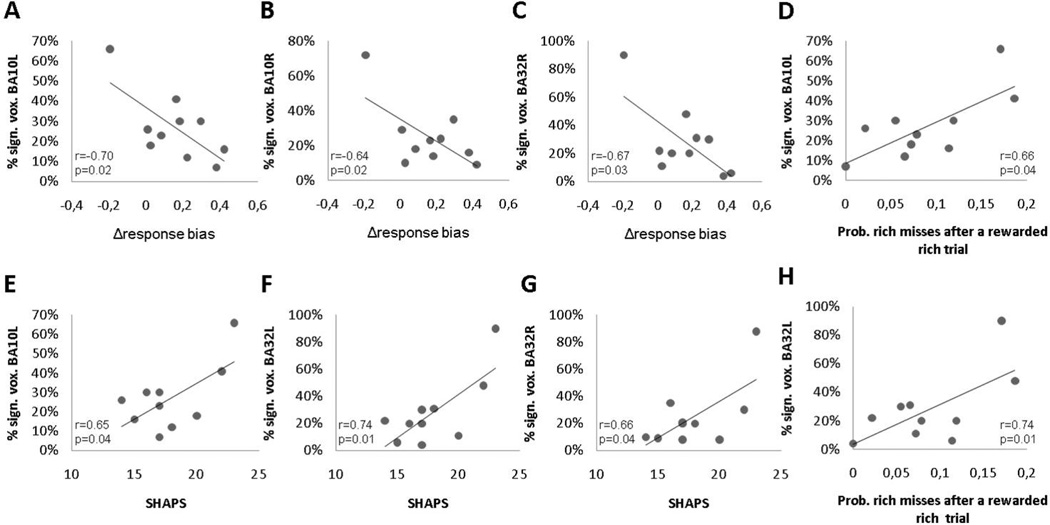
All ten subjects completed the SHAPS (mean ± SD, 17.9 ± 2.92) and BDI (mean ± SD, 1.7 ± 1.57). Kolmogorov-Smirnov tests indicated that Δresponse bias and self-rating scores across the 10 subjects were normally distributed (all ps > .15). Box and whisker plots did not detect outliers. Pearson correlations with Δresponse bias and BDI (r = −0.32, p = .37), as well as SHAPS (r = −0.52, p = .13) were not significant, likely due to the truncated range in this healthy sample and the small sample size. SHAPS scores were positively correlated with percentage of statistically significant voxels in left vmPFC (r = 0.65, p = .04), left dACC (r = 0.74, p = .01) and right dACC (r = 0.66, p = .04), indicating that lower hedonic capacity was associated with a higher number of activated voxels. Along similar lines, Δresponse bias scores were negatively correlated with the percentage of significant voxels in the left vmPFC (r = −0.70, p = .02), right vmPFC (r = −0.64, p = .04) and left dACC (r = −0.67, p = .03), suggesting that a lower ability to modulate behaviour as a function of the reinforcement schedule was associated with a higher number of activated voxels (Table 1). Highlighting the specificity of this finding, mean discriminability scores did not correlate with the percentage of significant voxels in these areas (all ps > 0.3). Finally, the probability of rich misses after an immediately preceding rewarded rich trial was significantly correlated with the percentage of significant voxels in the left dACC (r = 0.66, p = .04) and left vmPFC (r = 0.74, p = .01). These correlation data are plotted in Figure 5.
Figure 5.
Scatterplot and Pearson correlation between Δresponse bias and the percent of significant voxels in the (A) left vmPFC (BA10L), (B) right vmPFC (BA10R) and (C) left dACC (BA32L). Panel D and H show the relationship between the probability of a rich miss immediately after a rewarded rich trial and the percentage of statistically significant voxels in the left vmPFC (BA10L) and left dACC (BA32L), respectively. Panel E, F, and G show the relationship between SHAPS scores and the percentage of statistically significant voxels in the left vmPFC (BA10L), left dACC (BA32L), and right dACC (BA32R).
Box and whisker plots of the percentage of statistically significant voxels in BA10 and BA32 showed that subject 9 behaved as an outlier in our sample. She was the youngest participant (21 years old), but there were no indications of irregularities in data collection. When excluding her data, the correlations between (1) the percentage of statistically significant voxels and (2) SHAPS (0.29 < rs < 0.52) and Δresponse bias (−0.36 < rs < −0.28) were substantially weakened. The correlation with the percentage of significant voxels in the left vmPFC and the probability of rich misses in the reward task still showed a significant trend (r = 0.66, p = .052).
4. Discussion
The main goal of this study was to provide in vivo proof for the hypothesis that presynaptic DA release in specific areas of the extrastriatal reward circuit plays an important role in reward sensitivity and hedonic capacity. By measuring the high affinity D2/D3 radioligand [18F]Fallypride binding potential in response to a monetary reward learning task, we found that the tracer was successfully displaced from D2/D3 receptors in the mOFC (BA10), vmPFC (BA11), and dACC (BA32), providing indirect evidence of endogenous DA release during the task. In addition, to capture the spatial extent of extrastriatal DA release, the percentage of statistically significant voxels within the three activated regions was computed and correlated with subjective and objective measures of anhedonia. These analyses revealed that SHAPS scores (i.e., anhedonic symptoms) correlated positively with the percentage of significant voxels in the left vmPFC and left bilateral dACC. Moreover, when considering performance in the probabilistic reward task, we found a significant inverse correlation between Δresponse bias and the percentage of statistically significant voxels in the left and right vmPFC and left dACC. Accordingly, increasing anhedonia and reduced increases in response bias over the course of the experiment were associated with a spatially larger DA release in the vmPFC and dACC. These findings were further extended by the observation that the probability of rich misses after a preceding rewarded rich trial was positively correlated with the percentage of significant voxels in the left dACC and left vmPFC. Thus, a relatively lower ability to integrate reinforcement history over time was associated with a larger DA release in the dACC and vmPFC. Collectively, these findings indicate that dopamine played a direct role in reward responsiveness and more specifically in the ability to modulate behaviour as a function of reinforcement history, particularly in the presence of immediate reinforcement.
These results are in line with growing evidence implicating mOFC, vmPFC and dACC in reward processing. Using catecholamine depletion, Hasler et al. (2008, 2009b) have shown a direct relationship between DA dysfunction and reward responsiveness, as well as depressive symptoms and anhedonia, within the limbic-cortical-striatal-pallidal-thalamic circuit. In addition, the role of the OFC has specifically been reported in reward-related and goal-directed decision making (Bechara et al. 2000; Hare et al. 2008). Moreover, reward learning has been associated with the vmPFC (Rudebeck et al. 2008) and it is hypothesized that the vmPFC receives information based on the expectancy of reinforcement, which is used for adaptive decision making (Gottfried et al. 2003; Schoenbaum and Roesch, 2005). Furthermore, a growing number of studies on reinforcement learning and goal-directed decision making implicate the ACC in reward-related adaptive behaviour (Amiez et al. 2006; Kennerly et al. 2006; Mansouri et al. 2009; Rogers et al. 2004), particularly in the ability to influence a current choice by means of previous action-reinforcement history (Rudebeck et al. 2008; Williams et al. 2004).
Although a significant tracer displacement was observed in the OFC, we failed to find a reliable correlation between task performance and the percentage of significant voxels in the mOFC. This might be explained by the fact that the OFC has been primarily linked to reward consumption (O’Doherty et al. 2000, 2001) and its activity has been assumed to reflect the subjective (hedonic) experience of a decision (Peters and Büchel, 2010). The current reward task was designed to assess how behaviour is modulated by reinforcement history. Although the asymmetrical reinforcement schedule was successful in inducing a behavioral response bias, it was likely too weak and short-lived to elicit a clear-cut hedonic response. Unfortunately, we did not include an independent affective rating to assess hedonic responses during the task, which is a limitation that should be addressed in future studies.
These findings echo prior results of positive correlations between vmPFC activation in response to positive stimuli and anhedonia (Harvey et al. 2007; Keedwell et al. 2005; St. Onge et al. 2011; Wacker et al. 2009). In MDD, reward-related vmPFC responses have been interpreted as reflecting cortical compensatory mechanisms due to reduced striatal responses to positive stimuli (Dunn et al. 2002). The present findings of positive correlations between spatial extent of DA release and both objective and subjective measures of anhedonia are consistent with this speculation. Alternatively, it is possible that subjects with lower reward responsiveness might activate cortical dopaminergic mechanisms to increase attention and execute the task more accurately, leading to more correct responses for the less frequently rewarded stimulus, and thus a reduced response bias. This alternative interpretation fits accounts that depressed individuals have a more accurate view of reality and are less susceptible to positivity biases (Alloy and Abramson, 1979). Clearly, even though several studies have identified areas of the PFC as important regulators of reward-related behavior, the precise mechanism of action for processing motivationally salient information and guide adaptive behavior remains subject to much debate. Moreover, interrelations between DA levels in the PFC and striatal areas remain unclear (Karreman and Moghaddam, 1996; Murase et al. 1993; Taber and Fibiger, 1993) and PET procedures allowing for the assessment of both striatal and extrastriatal DA release will be required to evaluate such relations.
The strengths of the current study warrant mention. First, using a high-affinity D2/D3 antagonist radiotracer to explore the extrastriatal DA release was an important extension of research on the reward circuit, which until recently focused on striatal changes (Bressan and Crippa, 2005; Schultz, 2000). Second, most of the findings on the function of DA modulation in the reward circuit, such as the involvement of DA release in the vmPFC and dACC reward learning, stem from animal research, which await in vivo validation in humans. The current strategy to combine a well-established reward task with a single dynamic PET protocol is novel and afforded the opportunity to directly assess reward-related DA neuromodulation in brain regions hypothesized to be critically involved in the pathophysiology of neuropsychiatric illnesses, particularly MDD (Dunlop and Nemeroff, 2007; Nestler and Carlezon, 2005). Because we identified correlations between reward processing and anhedonic symptoms, on one hand, and a measure of DA release in the vmPFC and dACC, on the other hand, and all these variables have been suggested to be impaired in MDD, especially in patients with elevated anhedonic symptoms (Price and Drevets, 2010), we provided an important support for the function of the reward circuit as a plausible biological basis for an anhedonic endophenotype in MDD. A critical next step will be to evaluate patients with MDD. This would increase our understanding of the link between abnormal DA release and the psychopathology of symptoms and course of MDD, which may have important therapeutic implications.
The limitations of the current study should be acknowledged. First, the PET sample is relatively small and the results should be considered preliminary. Accordingly, the correlational findings emerging from the current study await independent replications in larger samples. Second, this study used a novel PET imaging technique which measured the extent of DA involvement as the percentage of significant voxels. Although we believe that the percentage of significant voxels is one of the best methods to quantify DA release in a individual areas, this quantitative approach should be tested more broadly to confirm its reliability. Third, the results do not imply causality and, as stated before, many questions about the function of each area are still unclear and should be addressed in future research. Fourth, striatal regions of the brain reward circuit, such as nucleus accumbens, were not analyzed in this experiment because the pseudo-equilibrium of the [18F]Fallypride was not ensured. Compared to lower density D2/D3 receptor regions (i.e. PFC), the relatively higher D2/D3 receptor density in the striatal regions require a longer baseline scan duration (2–3 hours) in order to reach a similar proportion of receptors to be occupied by the DA-competing ligand [18F]Fallypride, and thus achieve a stable measurement of BP (Christian et al. 2000). Accordingly, because the portion of the baseline scan, and consequently the task initiation, should have been adjusted in order to investigate different brain regions, it was not feasible to use LSRRM to measure DA release concurrently in both striatal and extrastriatal regions. To corroborate our experimental design and estimate quantitatively the ability of the LSRRM model to detect DA transmission simultaneously in both extrastriatal and striatal regions, we analyzed the kinetic characteristics of [18]Fallypride through a simulation study with variable stimulus intensity and timing (J. Ceccarini, E. Vrieze, M. Koole, T. Muylle, G. Bormans, S. Claes and K. Van Laere, unpublished observations). The suitability of the settings used in the current study was confirmed; however, in line with previous suggestions (Christian et al. 2000), a postponed task initiation at 120–150 min post-injection could even enhance the relative sensitivity of detecting DA release in striatal regions (Vernaleken et al. 2011).
Acknowledgments
The authors acknowledge the PET radiopharmacy for their skilled collaboration and declare no competing financial interests.
Financial Disclosure
This study was supported by the Fund for Scientific Research, Flanders, Belgium (FWO). Dr. Van Laere and Dr. Claes are both Senior Clinical Investigator of the FWO and Dr. Claes has received research support from Johnson and Johnson. Dr. Vrieze was supported by research grant OT 06/60 from the University of Leuven (KUL). Dr. Pizzagalli was supported by NIMH grant R01MH68376 and R21MH078979. Over the past three years, Dr. Pizzagalli has received research support from ANT North America (Advanced Neurotechnology), consulting fees from ANT and AstraZeneca, and honoraria from AstraZeneca for projects unrelated to the current study.
Footnotes
All other authors reports no biomedical financial interest.
References
- Aalto S, Brück A, Laine M, Någren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB457. J Neurosci. 2005;25(10):2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY. Judgment of contingency in depressed and nondepressed students: sadder but wiser? J Exp Psychol Gen. 1979;108(4):441–485. doi: 10.1037//0096-3445.108.4.441. [DOI] [PubMed] [Google Scholar]
- Alpert NM, Badgaiyan RD, Livni E, Fischman AJ. A novel method for noninvasive detection of neuromodulatory changes in specific neurotransmitter systems. Neuroimage. 2003;19(3):1049–1060. doi: 10.1016/s1053-8119(03)00186-1. [DOI] [PubMed] [Google Scholar]
- Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex. 2006;16(7):1040–1055. doi: 10.1093/cercor/bhj046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Dopamine release during human emotional processing. Neuroimage. 2009;47(4):2041–2045. doi: 10.1016/j.neuroimage.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotions, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd ed. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour - review of data from preclinical research. Acta Psychiatr Scand. 2005;111(427):14–21. doi: 10.1111/j.1600-0447.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- Christian BT, Lehrer DS, Shi B, Narayanan TK, Strohmeyer PS, Buchsbaum MS, Mantil JC. Measuring dopamine neuromodulation in the thalamus: using [F-18]fallypride PET to study dopamine release during a spatial attention task. Neuroimage. 2006;31(1):139–152. doi: 10.1016/j.neuroimage.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Christian BT, Narayanan TK, Shi B, Mukherjee J. Quantitation of striatal and extrastriatal D2 dopamine receptors using PET imaging of [(18)F]fallypride in nonhuman primates. Synapse. 2000;38(1):71–79. doi: 10.1002/1098-2396(200010)38:1<71::AID-SYN8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Cox SM, Andrade A, Johnsrude IS. Learning to like: a role for human orbitofrontal cortex in conditioned reward. J Neurosci. 2005;25(10):2733–2740. doi: 10.1523/JNEUROSCI.3360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Jahn AL, Bogdan R, Wald LL, Pizzagalli DA. Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology. 2008;45:36–49. doi: 10.1111/j.1469-8986.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Dunn RT, Kimbrell TA, Ketter TA, Frye MA, Willis MW, Luckenbaugh DA, Post RM. Principal components of the Beck Depression Inventory and regional cerebral metabolism in unipolar and bipolar depression. Biol Psychiatry. 2002;51(5):387–399. doi: 10.1016/s0006-3223(01)01244-6. [DOI] [PubMed] [Google Scholar]
- Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS) J Affect Disord. 2007;99(1–3):83–89. doi: 10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28(22):5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes in major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Carlson PJ, Luckenbaugh DA, Waldeck T, Geraci M, Roiser JP, Neumeister A, Meyers N, Charney DS, Drevets WC. Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch Gen Psychiatry. 2008;65(5):521–531. doi: 10.1001/archpsyc.65.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Mondillo K, Drevets WC, Blair JR. Impairments of probabilistic response reversal and passive avoidance following catecholamine depletion. Neuropsychopharmacology. 2009a;34(13):2691–2698. doi: 10.1038/npp.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Luckenbaugh DA, Snow J, Meyers N, Waldeck T, Geraci M, Roiser J, Knutson B, Charney DS, Drevets WC. Reward processing after catecholamine depletion in unmedicated, remitted subjects with major depressive disorder. Biol Psychiatry. 2009b;66(3):201–205. doi: 10.1016/j.biopsych.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry. 2007;12(8):703, 767–775. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behaviour: A unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jocham G, Tilmann A, Klein, Ullsperger M. Dopamine-Mediated Reinforcement Learning Signals in the Striatum and Ventromedial Prefrontal Cortex Underlie Value-Based Choices. J Neurosci. 2011;31:1606–1613. doi: 10.1523/JNEUROSCI.3904-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Philips ML. The neural correlates of anhedonia in major depressive disorder. Biol. Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kennerly SW, Walton ME, Behrens TEJ, Buckley MJ, Rushwoth MFS. Optimal decision making in the anterior cingulated cortex. Nature Neuroscience. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Kinahan PE, Rogers JG. analytic 3D image reconstruction using all detected events. IEEE Trans. Nucl. Sci. 1990;36(1):964–968. [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25(19):4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Wimmer GE. Splitting the difference: How does the brain code reward episodes? Ann NY Acad. Sci. 2007;1104:54–69. doi: 10.1196/annals.1390.020. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Berridge KC. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn Sci. 2009;13(11):479–487. doi: 10.1016/j.tics.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam LE, Zaers J, Ostertag H, Trojan H, Bellemann ME, Brix G. Performance evaluation of the whole-body PET scanner ECAT EXACT HR+ following the IEC standard. IEEE Trans Nucl. Sci. 1997;44(3):1172–1179. [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. J Clin Psychol. 2006;62(12):1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behaviour. Neuroscience. 2004;124(2):449–457. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci. 2009;10(2):141–152. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, Mantil J. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46(3):170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F, Kobal G, Renner B, Ahne G. Sensory-specific satiety-related olfactory activation of the human orbitofrontal cortex. Neuroreport. 2000;11(4):893–897. doi: 10.1097/00001756-200003200-00046. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C. Neural representations of subjective reward value. Behav Brain Res. 2010;213(2):135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O'Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, Culhane M. Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioural evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl) 2008;196(2):221–232. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2009b;43(1):76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi P, Baldwin R, Salomon R, Anderson S, Ansari MS, Li R, Dawant B, Bauernfeind A, Schmidt D, Kessler R. Estimation of baseline dopamine D2 receptor occupancy in striatum and extrastriatal regions in humans with positron emission tomography with [18F] fallypride. Biol Psychiatry. 2007;63(2):241–244. doi: 10.1016/j.biopsych.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B, Anderson S, Doop M, Woodward N, Schoenberg E, Schmidt D, Baldwin R, Kessler R. Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology. 2006a;31(5):1016–1026. doi: 10.1038/sj.npp.1300916. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Zald D, Li R, Park S, Ansari MS, Dawant B, Anderson S, Woodward N, Schmidt D, Baldwin R, Kessler R. Sex differences in amphetamine-induced displacement of [(18)F]fallypride in striatal and extrastriatal regions: a PET study. Am J Psychiatry. 2006b;163:1639–1641. doi: 10.1176/ajp.2006.163.9.1639. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Olson CR. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304(5668):307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, Smith SM. Distinct proportions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol. Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MF. Frontal cortex subregions play distinct roles in choices between actions and stimuli. J Neurosci. 2008;28(51):13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Curr Opin Neurobiol. 2007;17:220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11(4):389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Dillon DG, Birk JL, Holmes AJ, Goetz E, Bogdan R, Pizzagalli DA. Individual differences in reinforcement learning: behavioral, electrophysiological, and neuroimaging correlates. Neuroimage. 2008;42(2):807–816. doi: 10.1016/j.neuroimage.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Evins AE, Frank MJ, Schetter EC, Bogdan R, Pizzagalli DA. Single dose of a dopamine agonist impairs reinforcement learning in humans: evidence from event-related potentials and computational modeling of striatal-cortical function. Hum Brain Mapp. 2009;30(7):1963–1976. doi: 10.1002/hbm.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb Cortex. 2000;10(3):272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Snaith P, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- St Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal d1 and d2 receptors to risk-based decision making. J Neurosci. 8. 2011;31(23):8625–8633. doi: 10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC. Electrical stimulation of the medial prefrontal cortex increases dopamine release in the striatum. Neuropsychopharmacology. 1993;9:271–275. doi: 10.1038/npp.1993.63. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System - an Approach to Cerebral Imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Vernaleken I, Peters L, Raptis M, Lin R, Buchholz HG, Zhou Y, Winz O, Rösch F, Bartenstein P, Wong DF, Schäfer WM, Gründer G. The applicability of SRTM in [(18)F]fallypride PET investigations: impact of scan durations. J Cereb Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2011.73. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Claes S. Anhedonia and Increased Stress Sensitivity: Two promising Endophenotypes for Major Depression. Current Psychiatry Reviews. 2009;5(3):143–152. [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46(1):327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci. 2004;7(12):1370–1375. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- Wrase J, Kahnt T, Schlagenhauf F, Beck A, Cohen MX, Knutson B, Heinz A. Different neuronal systems adjust motor behaviour in response to reward and punishment. Neuroimage. 2007;36:1253–1262. doi: 10.1016/j.neuroimage.2007.04.001. [DOI] [PubMed] [Google Scholar]