Abstract
Objective
To determine the extent to which hospitals vary in the use of intensive care, and the proportion of variation attributable to differences in hospital practice that is independent of known patient and hospital factors.
Data source
Hospital discharge data in the State Inpatient Database for Maryland and Washington states in 2006.
Study design
Cross sectional analysis of 90 short-term acute, care hospitals with critical care capabilities.
Data collection/methods
We quantified the proportion of variation in intensive care use attributable to hospitals using intraclass correlation coefficients derived from mixed effects logistic regression models after successive adjustment for known patient and hospital factors.
Principal findings
The proportion of hospitalized patients admitted to an ICU across hospitals ranged from 3% to 55% (median 12%; IQR:9, 17%). After adjustment for patient factors, 19.7% (95%CI: 15.1, 24.4) of total variation in ICU use across hospitals was attributable to hospitals. When observed hospital characteristics were added, the proportion of total variation in intensive care use attributable to unmeasured hospital factors decreased by 26% to 14.6% (95% CI:11, 18.3%).
Conclusions
Wide variability exists in the use of intensive care across hospitals, not attributable to known patient or hospital factors, and may be a target to improve efficiency and quality of critical care.
Keywords: intensive care, critical illness, variation, hospitals
Introduction
Critical care is the care of patients at high risk for a life-threatening deterioration, such as those with myocardial infarction, acute respiratory failure, trauma, or severe sepsis. Though provided in diverse settings, critical care is often also defined by the location of care delivery–a critical care unit, commonly an intensive care unit (ICU) or coronary care unit (CCU) – that is defined by the intensive monitoring, high nurse to patient ratio, and availability of invasive technology for organ support. Over the last twenty years there has been a dramatic rise in the use of intensive care in the United States. From 1985 to 2005, critical care beds increased by 36% (69,300 to 93,995) while non-critical care beds decreased by 35% (820,300 to 534,414).(Halpern and Pastores 2009; Halpern, Pastores, and Greenstein 2004; Halpern et al. 2007) Because care provided in the ICU is costly, critical care spending is a major contributor to the rapidly escalating healthcare costs in the US and now represents approximately 1% of the US gross domestic product.(Halpern and Pastores 2009)
The rise in ICU costs may result, in part, from specific differences in practice patterns at individual hospitals. Certain hospitals may over-use ICUs by admitting patients either with no meaningful chance of recovery, or, conversely, patients who do not require life-sustaining therapies.(Barnato et al. 2007; Rosenthal et al. 1998; Zimmerman et al. 1995) Financial incentives for both hospitals and physicians due to reimbursement for intensive care may also drive variation in practice.(Garland et al. 2006) Further, relative overuse of the ICU may result from understaffing of general medical/surgical floors in pursuit of lower costs for common non-critical illness DRGs; such an understaffed floor might be unable to care for modest acuity patients (requiring a lower threshold for transfer to the ICU) or might be late to detect incipient critical illness or fail to rescue such patients.(Ghaferi, Birkmeyer, and Dimick 2009) To address the etiology and consequences of under or over-use, researchers and policymakers must first measure the extent to which varying use of intensive care is present.
One way to indirectly measure the potential for over-use is to examine how critical care practice varies across hospitals. The decisions to admit a patient to the ICU and provide critical care is complex, and results from the interaction of specific characteristics of the patient (e.g. illness severity or co-morbidity), and specific organizational and cultural aspects of a hospital. The fraction of patients receiving care in an ICU will vary between hospitals because of variation in patients’ needs for life-sustaining therapies, the decision to admit individual patients, but also because of compositional differences between hospitals in the array of services they offer, some of which may have different requirements for ICU care (e.g., caring for advanced burns or cardiac surgery). A simple conceptual model that integrates these factors is shown in the Supplemental Digital Content (eFigure 1), drawing on the work of Andersen & Aday (Andersen et al. 1987) and Penchansky and Thomas. (Penchansky and Thomas 1981).
Our primary goal is to understand the extent to which variation between hospitals in the use of intensive care is explained by patients and hospital factors. We further wish to understand the extent to which hospital-to-hospital variation is a feature of objective differences between hospitals, particularly with regard to their case-mix and offering of specialty services, as opposed to more idiosyncratic local cultural variation in use of the ICU for observationally equivalent patients. This last category of variation—whereby comparable patients may be treated differently merely as a function of where they are hospitalized—is the area of greatest policy interest. For example, the existence of wide variation in the use of intensive care across hospitals that is not attributable to measured patient or hospital differences may suggest that some hospitals are using critical care disproportionately. By then identifying hospital outliers, researchers and policymakers can focus their efforts on select hospitals—taking advantage of this variation as natural laboratories in hospital process.
To address these questions, we used all-payor State Inpatient Data (SID) from the Agency for Healthcare Research and Quality (AHRQ) to measure variation in ICU use across hospitals without accounting for any known differences. Using multilevel regression analysis, we adjust for known patient differences to determine the proportion of total variation that remained due to hospital-level factors. We add patient-factors, including case-mix adjustment, in an a priori fashion, in order to understand the impact of more granular adjustment. We then adjust for several measured hospital characteristics to determine the residual hospital-level variation which was independent of known patient and hospital characteristics.(Merlo et al. 2005a) To further understand if hospital-level variation in intensive care is consistent across more homogeneous diagnoses or procedures, we evaluate our models in patients hospitalized with acute myocardial infarction, pneumonia, congestive heart failure, or surgery for colorectal cancer. We chose these diagnoses because they are common, have a reasonable likelihood of requiring ICU admission, and are reliably identified using administrative data.
Methods
Patient data: Healthcare Cost and Utilization Project State Inpatient Database (SID)
We used the SID for Maryland and Washington to identify all adult patients (age ≥18 years) who were hospitalized at an acute care, short term hospital in 2006. We selected these two states as they submit data to the Agency for Healthcare Research and Quality (AHRQ) as part of the healthcare utilization project (HCUP) and report revenue codes (UB-92 billing codes) for each hospitalization that allows for identification of a stay in intensive care. They are also diverse states, in distinct geographic regions, including both major urban areas as well as large rural components, and with both high and low health maintenance organization penetration. The SID includes administrative data derived from UB-92 hospital discharge forms, and provides a rich collection of variables. We used the AHRQ Clinical Classifications Software (CCS) to generate multi-level diagnosis categories for case-mix adjustment, a strategy which compares favorably to other administrative adjustment tools.(Ash et al. 2003) We also used a select group of codes for medical critical illness codes,(Ehlenbach et al. 2010) acute cardiovascular disease,(Wennberg et al. 2004) and cardiac surgery (Ghali et al. 1999) to explore more granular case-mix adjustment. A full description of patient-level adjustment variables is provided in the Supplemental Digital Content (see eMethods, eTable 1).
Hospital data: Healthcare Cost Report Information System, AHA annual survey
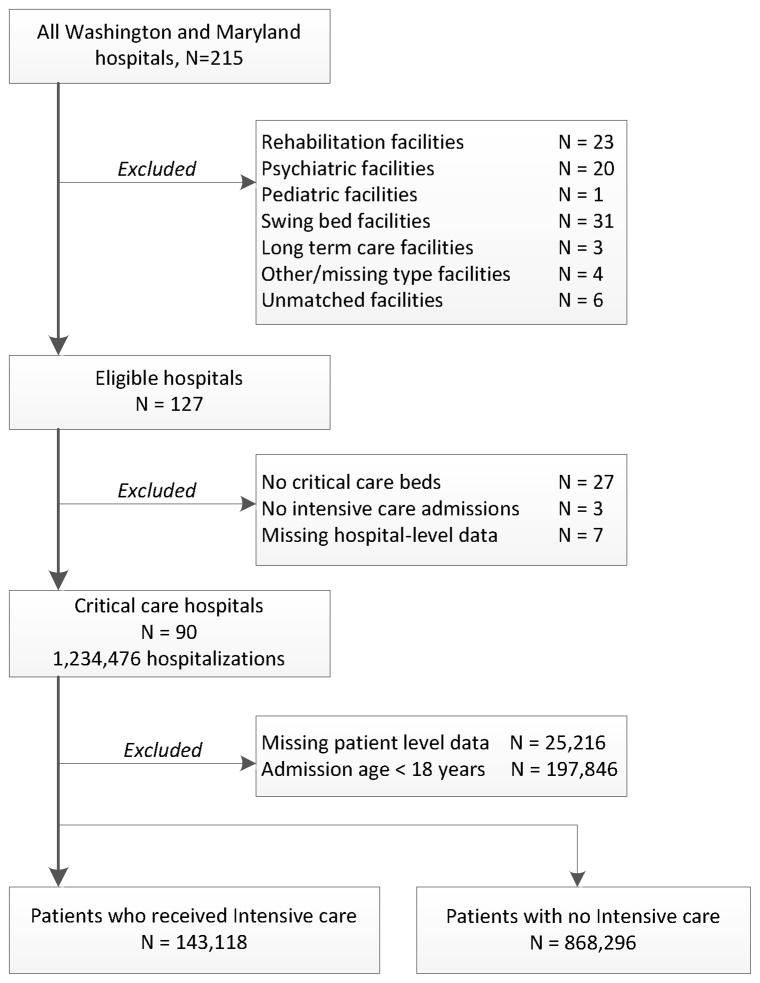
We linked eligible hospitalizations to the 2006 Healthcare Cost Report Information System (HCRIS) data from the Center for Medicare &Medicaid Services. HCRIS is a federally mandated and annually submitted Hospital Cost Report file, previously used in many studies of critical care services.(Halpern and Pastores 2009; Halpern et al. 2004; Halpern et al. 2007) We also linked hospitalizations to the 2005 American Hospital Association (AHA) annual survey, a widely used electronic dataset of self-reported data on hospital ownership, staffing, facilities, and capabilities. The AHA surveys achieve a high annual response rate and are considered highly reliable for the variables we used.(Mullner R 2002) Matching to hospital level data was successful in all but 6 facilities (Figure 1). We used commonly considered variables to capture hospital characteristics which are further detailed in the Supplemental Digital Content (see eMethods).
Figure 1.
Hospital and patient accrual
Identification of intensive care hospitalizations
We categorized each admission by the presence or absence of Medicare UB-92 billing codes for intensive care any time during hospitalization.(Halpern and Pastores 2009; Halpern et al. 2004; Iwashyna et al. 2009) We defined ICU admission as the presence of any critical care revenue code, excluding psychiatric, pediatric, or intermediate critical care. To determine intensive care use across hospitals, we determined the reliability-adjusted proportion of intensive care hospitalizations of all eligible hospitalizations at each center. Reliability adjustment reduces spurious variation in hospital-level proportions by eliminating statistical noise among hospitals with low caseloads.(Dimick, Staiger, and Birkmeyer 2010; Hayward et al. 2007) This approach is now advocated by multiple organizations for quality monitoring, including the AHRQ and the Leapfrog Group.
Statistical analyses
We present continuous descriptive data as mean ± standard deviation or median [interquartile range], depending on normality determined from graphical distributions. We present categorical variables as proportions. The range and variability in proportion of intensive care unit use across hospitals are illustrated using histograms.
Because of the hierarchical structure of the data (patients nested within hospitals), we used the latent response formulation of multilevel logistic regression analyses and constructed three models.(Rabe-Hesketh 2008) Patients are indexed i and hospitals h. Model 1 (the empty model) is the probability (pi,h) of a critical care hospitalization only as a function of the hospital:
| (1) |
βo = mean log odds of ICU admission in the sample
ζh = hospital random effect (independent across hospitals), ~N(0, ψ)
εi,h = is the residual variance (independent across hospitals and patients),~N(0,θ)
In Model 2, the probability of a critical care hospitalization is a function of the patient level factors (Xi,h):
| (2) |
β1 = vector of coefficients for patient level factors
Xi,h = vector of patient-level factors for patient i in hospital h
Using empirical Bayes methods, we determined the adjusted predicted ICU use rate and 95% confidence intervals at the hospital level for an average patient.(Morris 1983; Normand 1997) Then, we displayed the ranked order of adjusted rates across hospitals in a caterpillar plot. To illustrate the sensitivity of our result to more detailed case-mix adjustment, patient factors were added to Model 2 in a stepwise fashion. Please see the Supplemental Digital Content for more detail (see eMethods).
In Model 3, the probability of a critical care hospitalization is a function of the hospital, patient factors, and observed hospital characteristics (Zh):
| (3) |
δ = coefficient vector for the vector of hospital-specific variables (Z) in hospital h
Zh = vector of hospital-levelvariables in hospital h
To determine the proportion of variance in intensive care use attributable to hospitals, we determined the intraclass correlation (ICC) calculated, as is conventional,(Merlo et al. 2005a; Merlo et al. 2005b) as:
| (4) |
To determine the proportional change in variance (PCV) between a simple model and a model with more adjustment variables, we used an approach suggested by Merlo et al.:(Merlo et al. 2005c)
| (5) |
Sensitivity analyses
We explored if hospital level variation in ICU use was consistent for more homogeneous conditions. We restricted our models to patients with acute myocardial infarction,(Wennberg et al. 2004) pneumonia,(Fry et al. 2005) congestive heart failure,(Casper et al. 2010) and surgery for colorectal cancer,(Ho et al. 2006) respectively, using ICD-9-CM diagnosis and procedure codes. We also hypothesized that inter-facility transfer of patients may influence variation in hospitals’ use of intensive care.(Iwashyna et al. 2010) We subsequently excluded all patients with admission source coded as ‘acute care hospital’ and re-estimated our models. To ensure that the observed hospital-level variation in ICU use was true and not a result of random noise, we performed a specification test.(Huesch 2011) We chose patient marital status (married/unmarried) as an arbitrary characteristic which we did not expect to have significant variation at the hospital level. We restricted this analysis to hospitalizations in Maryland, in which marital status is reported in the SID. All models were re-estimated using this alternative outcome, and we generated corresponding ICCs for our random effects.
We used STATA/SE v11.1 (College Station, TX) for all analyses. Research involving the patient-level de-identified SID, AHA annual survey, and HCRIS was not considered human subjects research and was exempt review by the University of Washington Institutional Review Board.
Results
Patients who were cared for in an ICU were older, more often male, and were more likely to be admitted from other medical facilities, compared to patients not receiving critical care (Table 1). Among all hospitals with critical care capability (N=90), most were non-teaching facilities (n=62, 69%), half offered interventional cardiac catheterization (n=50, 56%), and few provided specialty services such as burn care or organ transplantation (<10%, respectively). Facilities had a median 17 ICU beds [IQR: 9, 34] while intermediate care ICU beds were uncommon (see Supplemental Digital Content, eTable 2).
Table 1.
Demographics and outcomes
| Variable | Critical care hospitalizations (N = 143,118) | Non-critical care hospitalizations (N = 868,291) |
|---|---|---|
| Age, yrs | 64 [51 – 77] | 55 [37 – 73] |
| Male gender, N (%) | 76,729 (53) | 322,011 (37) |
| Weighted Charlson comorbidity score | 1 [1 – 3] | 1 [0 – 2] |
| Admission source, N (%) | ||
| Routine | 129,280 (90) | 832,419 (96) |
| Acute care hospital | 8,671 (6) | 21,343 (2) |
| Long-term care facility | 5,167 (2.8) | 14,534 (2) |
| Weekend admission, N (%) | 30,661 (21) | 161,812 (19) |
| Race/Ethnicity* | ||
| White | 47,761 (67) | 309,549 (60) |
| Black | 20,038 (28) | 168,597 (33) |
| Hispanic ethnicity | 1,092 (2) | 15,506 (3) |
| Asian or Pacific islander | 959 (1) | 8,022 (2) |
| Other | 2,216 (3) | 12,257 (2) |
| Primary payer status, N (%) | ||
| Medicare | 75,535 (53) | 338,206 (39) |
| Medicaid | 14,904 (10) | 134,903 (16) |
| Private insurance | 42,023 (29) | 330,069 (38) |
| Self-pay | 6,915 (4) | 41,301 (5) |
| No charge | 643 (<1) | 4,090(<1) |
| Urbanicity | ||
| Central county, (>1 million pop.) | 34,492 (24) | 195,638 (23) |
| Fringe county, (> 1 million pop.) | 61,416 (43) | 434,803 (50) |
| Metropolitan county, (250–999,999 pop.) | 6,410 (5) | 35,308 (4) |
| Metropolitan county, (50–250,000 pop.) | 23,376 (16) | 120,749 (14) |
| Micropolitan county | 13,040 (9) | 59,504 (7) |
| Non-core county | 4,384 (3) | 22,294 (3) |
| Median household income national quartile by ZIPcode, N(%) | ||
| Quartile 1 | 24,017 (17) | 149,061 (17) |
| Quartile 2 | 28,786 (21) | 153,494 (18) |
| Quartile 3 | 45,502 (32) | 275,092 (32) |
| Quartile 4 | 44,813 (31) | 290,649 (33) |
| Died during hospitalization, N (%) | 13,005 (9) | 9,789 (1) |
| Length of stay, days^ | 5 [2 – 9] | 3 [2 – 4] |
Among Maryland hospitalizations only, missing for N=760 (0.3%)
Among hospital survivors
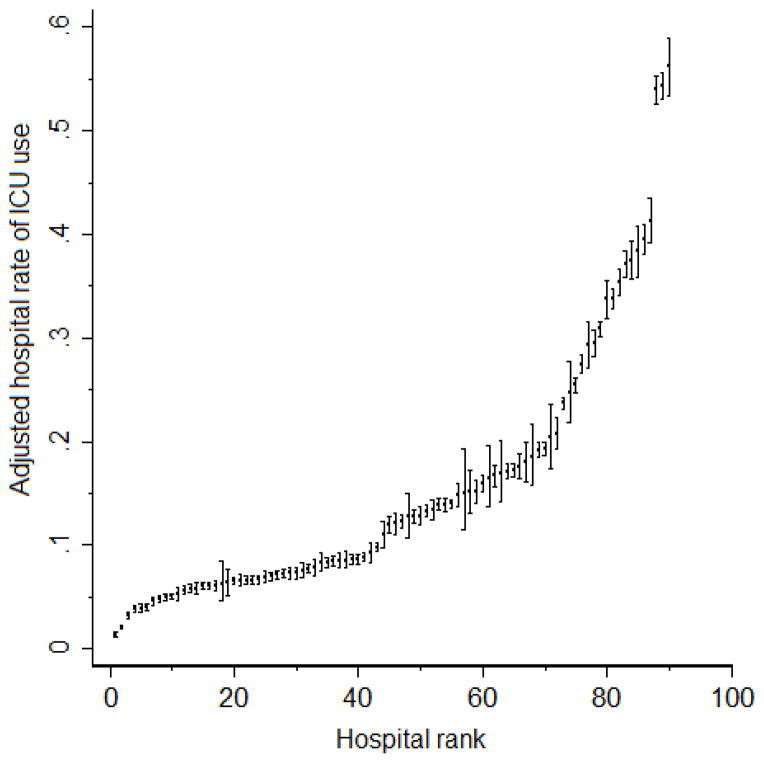
We observed broad variation in the use of intensive care (eFigure 2). The reliability adjusted proportion of total hospitalizations admitted to an ICU across hospitals ranged from 3% to 55% (median 12%; IQR: 9,17%). Table 2 reports the results from our mixed effects logistic regression models. In our unadjusted model, 10% of variation in the use of intensive care was attributed to the hospitals (ICC=10.3% (95%CI: 7.6, 13%). When patient characteristics including co-morbid status, age, gender, and case-mix were added to the model (Model 2), we observed an increase in the proportion of variance attributable to hospitals (ICC=19.7%, 95%CI: 15.1, 24.4%). This broad variation is also reflected in the caterpillar plot where each hospital was ranked according to their case-mix adjusted rate of ICU use (Figure 2). For example, hospitals in the 90th percentile were 17 times more likely to use intensive care than those in the lowest 10th percentile (54% vs. 3.3%). When patient factors were added in stepwise fashion, we observed that increasing granularity of case-mix adjustment increased variation at the hospital level (see Supplemental Digital Content, eTable 3), suggesting that any omitted patient-level comorbidity variables do not confound our hospital-level estimates..
Table 2.
Correlation coefficients for hospital-level random effects in logistic mixed regression models of intensive care use across 90 hospitals
| Empty Model Model 1 |
Model with patient factors Model 2 |
Model with patient and hospital factors Model 3 |
|
|---|---|---|---|
| Random effects variance* | |||
| Hospital-level variance (95%CI) | 0.38 (0.06, 0.28) | 0.81 (0.60, 1.1) | 0.57 (0.42, 0.76) |
| Intraclass correlations**, % | |||
| Hospital (95% CI) | 10.3 (7.6, 13) | 19.7 (15.1, 24.4) | 14.6 (11, 18.3) |
| Residual | 89.7 | 80.3 | 85.4 |
| Observations | 1,011,363 | 1,011,354 | 1,011,354 |
The residual (patient) variance in a mixed logistic regression model is fixed at π^(2/3)
ICC calculated as proportion of hospital variance divided by total variance where total variance is the sum of hospital variance and residual variance π^(2/3)
Figure 2.
Rates of ICU use ranked across hospitals (N=90) with 95% confidence intervals. Estimates are derived from Model 2, adjusted to the modal value of categorical covariates and centered mean value of continuous covariates. That is, these estimates are both risk- and reliability-adjusted.
When measured hospital characteristics were included (Model 3), we observed that hospitals still accounted for 14.6% (95%CI: 11, 18.3%) of the variation in intensive care use. Addition of known hospital characteristics only attenuated the variance attributable to hospitals by 26%. In general, we note that the sum of the contribution of estimated effects was small compared to residual, unexplained effects, which varied from 81 to 89%. Among the hospital level effects, non-teaching status, availability of intermediate care beds, presence of neurological services, and increasing hospital beds were associated with reduced hospital-level intensive care use (see Supplemental Digital Content, eTable 4).
In our sensitivity analyses, we observed no change in our estimates after we excluded all patients transferred from acute care hospitals. For specific conditions, we observed significant variation in hospital level ICU use among patients with congestive heart failure, a modest reduction for pneumonia and surgery for colorectal cancer, and even greater variation for acute myocardial infarction (Table 3). In our specification analysis, we substituted marital status (married - yes/no) for ICU use, where little unexplained variability should be captured by hospital effects (Table 3). We observed, as expected, that the contribution of hospitals to these models was minimal and substantially less than our models of ICU use. In fact, the hospital level contribution to marital status was estimated at 0.9% (95%CI: 0.5, 1.3%) after full adjustment.
Table 3.
Sensitivity analyses of hospital level variation in ICU use in specific diagnoses and procedures.#
| Pneumonia | Congestive heart failure | Surgery for colorectal cancer | Acute myocardial infarction | Excluding acute care transfers | Specification test^ | |
|---|---|---|---|---|---|---|
| Random effects variance* | ||||||
| Hospital-level variance (95%CI) | 0.27 (0.20, 0.38) | 0.64 (0.48, 0.87) | 0.29 (0.19, 0.44) | 1.24 (0.89, 1.75) | 0.57 (0.43, 0.77) | 0.03 (0.02, 0.05) |
| Intraclass correlations**, % | ||||||
| Hospital (95% CI) | 7.7 (5.4, 10.0) | 16.4 (12.2, 20.6) | 8.0 (4.9, 11.2) | 27.5 (20.7, 34.2) | 14.9 (11.1, 19.6) | 0.9 (0.5, 1.3) |
| Residual | 92.3 | 83.6 | 92 | 72.5 | 85.1 | 99.1 |
| Hospitals | 90 | 90 | 89 | 88 | 90 | 42 |
| Observations | 36,525 | 76,439 | 10,232 | 22,086 | 981,341 | 581,503 |
All analyses derive from Model 3 re-fit in after restriction to complete cases; critical illness diagnosis codes eliminated from adjustment variables for pneumonia and surgery models due to model convergence
Specification test, where outcome replaced with patient-level marital status (married – yes/no), fit only among Maryland state records
The residual (patient) variance in a mixed logistic regression model is fixed at π^(2/3)
ICC calculated as proportion of hospital variance divided by total variance where total variance is the sum of hospital variance and residual variance π^(2/3)
Discussion
Intensive care is among the most resource intensive, costly, and rapidly growing components of acute hospital care. We observed wide variation in the use of intensive care among hospitals, even after adjusting for known patient and hospital-level variables. We found that hospitals at the 90th percentile were 17 times more likely than those at the 10th percentile to admit a comparable patient to the intensive care unit. Our findings were robust to disease-specific cohorts, including acute myocardial infarction and congestive heart failure. These data suggest important, unmeasured hospital factors may contribute to the variability in intensive care unit admission for patients, and highlight the opportunity for future quality improvement and system efficiency interventions.
Despite rising critical care expenditures and critical care bed growth,(Halpern and Pastores 2009) few studies have examined the variability in intensive care across individual hospitals. Many authors describe ICU admission practice among select populations, such as those at the end-of-life, with low-acuity disease, or of advanced age, demonstrating variation across hospitals.(Angus et al. 2004; Barnato et al. 2007; Rosenthal et al. 1998; Wunsch et al. 2009) We present the first study that demonstrates the breadth of variation across hospitals in the use of intensive care for a general, acute care population. When quantified using intra-class correlations, variation in ICU use across hospitals, which we measure at 14.3%, is comparable to those reported for other areas of health services utilization. For example, hospitals may account for 10% of the variation in transfusion after coronary artery bypass graft surgery, 30% of variation in adherence to medication guidelines for secondary prevention of myocardial infarction,(Bennett-Guerrero et al. 2010; Huesch 2011; Rasmussen et al. 2008; Rogers et al. 2009) and up to 18% of the variation in the quality of care for acute stroke.(Reeves et al. 2010) These hospital level effects also exceed the proportion of variation generally attributable to providers.(Fung et al. 2010) Importantly, as in other areas of medicine, this degree of variance attribution may be responsive to targeted efforts to reduce practice variability.(Selby et al. 2010)
Although we are unable to determine whether intensive care was over- or under-utilized within hospitals, it seems very unlikely that this degree of variation can be explained without significant over-use. Our models reveal that factors other than case-mix contributed to broad variation in ICU utilization. This is an important finding as it suggests that hospitals play a significant role in determining how their ICUs are used that is not attributable to simple, clinically defined patient need. Several measured hospital characteristics helped explain some of the variability in ICU use that we observed. Specifically, smaller volume, non-teaching hospitals without intermediate care beds were more likely to use intensive care in a greater number of patients. This finding corroborates earlier data demonstrating that non-teaching and minor teaching facilities are more likely to admit lower-acuity patients to the intensive care unit (Rosenthal et al. 1998) But these measureable characteristics account for only 26% of the hospital effect – these data imply that there may be a large, seemingly discretionary aspect to the use of a very expensive component of hospital care.
There are several additional factors that likely contribute to the wide variation in ICU use across hospitals that we observed. First, hospitals that admit more patients to the ICU than average may operate under the assumption that delivering more critical care improves patient outcomes. Limited data supports this hypothesis. A single study in Pennsylvania that examined patients at high risk of death found that odds of death were lower for those who were treated in hospitals with greater end-of-life treatment intensity.(Barnato et al. 2010) Several other studies, however, suggest that greater intensive care, particularly among those who may not need it, may increase a patient’s risk for iatrogenic complications and subsequent death.(Levy et al. 2008; Thomas et al. 2009) Additional factors may include the absence of objective guidelines for whom should be admitted to the ICU,(Truog et al. 2008) or the availability of ICU beds (Joynt et al. 2001). These are only a few of potential mechanisms that may contribute to variation in ICU use across hospitals that warrant further investigation.
Our data have important implications for both policy makers and researchers seeking to optimize the efficiency of critical care delivery. Because US hospitals receive a fixed DRG-based payment for each patient regardless of whether the ICU is required, they should have strong financial incentives to minimize unnecessary ICU use for DRG-based patients. Some of the variation we observed may reflect a given hospital improving efficiency to ensure that the ICU is reserved for the most “profitable” patients (e.g. those undergoing cardiac bypass surgery). Hospitals outside the US may be less susceptible to this incentive. The dramatic variation in ICU use we observed suggests that there are opportunities for some hospitals to reduce critical care expenditures. To do so, hospital leaders could compare their hospital’s adjusted rate of ICU use to that of others, and if it deviates from the mean, examine their local practice patterns to identify the drivers of use of this expensive resource. Researchers can use a ranking of critical care capable hospitals to identify outliers and, through quantitative and qualitative study, determine the extent to which the hospital’s structure of critical care delivery, norms of care, or the professionals providing care within these hospitals explains their deviation from average hospitals, and how this deviation associates with outcome.(Barnato et al. 2009) Importantly, our data suggest, similar to quality monitoring in other areas of healthcare,(Dimick et al. 2010) that any effort to rank hospitals should first ensure that rates of ICU use are fully adjusted for patient differences and for the reliability of their estimates. Failure to do so may inappropriately identify a hospital as a high ICU user purely as a result of patient differences or infrequent admissions.
However, there may also be physician incentives to utilize the ICU that are independent of a hospital’s approach to efficiency. The majority of critically ill patients in the US are over the age of 65 years, a population of individuals largely insured by fee-for-service Medicare.(Angus et al. 2004; Milbrandt et al. 2008) During the care of fee-for-service beneficiaries, non-salaried physicians may overuse intensive care to maximize their billings. For example, by admitting patients whom may not need intensive care to the ICU, physicians have more justification to bill for greater complexity or comprehensiveness of care. The greater availability of technology in the ICU provides opportunities for physicians to perform additional diagnostic tests and invasive procedures—this may offer both financial incentives (more billing) but also non-pecuniary incentives in a world where “doing everything” is sometimes considered the hallmark of an effective and conscientious physician.(Garland et al. 2006; Song et al. 2010) These physician incentives occur in the context of an increasing supply of critical care beds in the US.(Halpern and Pastores 2010) In this respect, physicians’ opportunity to admit patients to the ICU has never been greater. Together, these issues may in part drive the variation in ICU use we observed. In the future, bundled payments which reward quality and efficiency may alleviate some of the financial incentives for physicians and hospitals which drive ICU variation. The imminent implementation of bundled payments and more broadly, accountable care organizations (ACO) - where physicians and hospitals share a fixed price for an episode of care,(Mechanic and Altman 2009) may be an effective way to eliminate ICU overuse caused by physician incentives.
We recognize several limitations to our study. First, our estimates for hospital-level variation in intensive care use are limited by the completeness of case-mix adjustment. Importantly, we used several case-mix adjustment tools (Ash et al. 2003; Ehlenbach et al. 2010; Quan et al. 2005) to account for patient differences across hospitals, yet these tools were not developed to predict intensive care utilization and may not fully capture an individual’s propensity for needing the ICU. Compared to a model without patient variables, we observed that the hospital-level ICC increased after more complete adjustment for patient-level heterogeneity. These results are reassuring, but do not remove with certainty the potential for unmeasured severity of illness to alter our estimates of variation in ICU use across hospitals. We also evaluated our models in specific conditions for which ICU admission may be more standardized (congestive heart failure, acute myocardial infarction), and found that significant hospital-level variation remained. Second, we were unable to account for physician practice variation in intensive care use within hospitals. Although debated, (Krein et al. 2002) physician-level variation may be a measureable contributor to differences in healthcare utilization across several areas of medicine, (Hershman et al. 2009; Huesch 2011) including critical care,(Escher, Perneger, and Chevrolet 2004; Garland et al. 2006) but was unmeasured in our datasets. Rather, we focused on hospitals, which have organizational characteristics and ICU structures that are inherently modifiable components of a critical care system,(Angus and Black 2004; Kim et al. 2010) and have some degree of responsibility for which physicians practice within their walls. Third, we used a dichotomous variable for the availability of intermediate care within hospitals. This variable may not capture the full breadth of structural differences that comprise individual hospitals’ critical care capabilities outside the ICU. Finally, as in much literature on variation in practice patterns,(Fung et al. 2010; Reeves et al. 2010; Selby et al. 2010) our data do not evaluate the impact of variation and patient outcomes such as mortality and morbidity. Additional measures for case mix adjustment, severity of illness, and patient-centered outcomes such as 30-day mortality and functional status, not available in our dataset, would be required for these important future analyses. As such, we can only hypothesize about the impact on patients of the observed variation in intensive care use.
In summary, there is significant variability in the use of intensive care across hospitals that is not accounted for by differences in patient characteristics or most typically measured hospital characteristics. With the goal of improving efficiency and stemming the increase in critical care costs, the present work demonstrates that there is a potential policy opportunity for a greater focus on modifiable hospital practice patterns which may contribute to the broad hospital-level variation in the use of intensive care.
Supplementary Material
Acknowledgments
Funding support: Dr. Seymour was supported in part by an National Institutes of Health (NIH) award from the National Center for Research Resources (KL2 RR025015). Dr. Wunsch is supported in part by an NIH award from the National Institutes on Aging (K08AG038477). Dr. Iwashyna is supported in part by an NIH award from the National Heart, Lung, and Blood Insitute (K08HL091249-01). Dr. Cooke was supported in part by the Robert Wood Johnson Foundation Clinical Scholars Program.
Footnotes
Address during which research was conducted: Division of Pulmonary and Critical Care Medicine, University of Washington, Harborview Medical Center, Box 359762
Christopher W. Seymour, M.D., M.Sc. (Corresponding author), Assistant Professor, Departments of Critical Care & Emergency Medicine, University of Pittsburgh School of Medicine, Core Faculty, Clinical Research, Investigation, and Systems Modeling of Acute Illness (CRISMA) Center, 639 Scaife Hall, 3550 Terrace Street, Pittsburgh, PA 15261, USA, Phone: (412)864-2993, seymourcw@upmc.edu
Theodore J. Iwashyna, M.D. PhD., University of Michigan Medical School, 3A23 300 NIB, SPC 5419, 300 North Ingalls, Ann Arbor, MI 48109-5419, Academic Office Telephone: (734) 936-5047, Academic Office Fax: (734) 936-5048, tiwashyn@umich.edu
William J. Ehlenbach, M.D., M.Sc., University of Wisconsin, Madison, School of Medicine and Public Health, 750 Highland Ave, Madison, WI 53705, Phone: 608-262-0802, Fax: 608-890-2333, wjehlen@medicine.wisc.edu
Hannah Wunsch, M.D., M.Sc., 622 West 168th Street, New York, NY 10032, Phone: (212) 305-0679, Fax: 212-305-8287, hw2125@columbia.edu
Colin R. Cooke, M.D., M.Sc., 6312 Medical Sciences Bldg. I, 1150 W. Medical Center Drive, Ann Arbor, MI 48109-5604, Office: 734-647-4844; fax: 734-647-3301, cookecr@umich.edu
References
- Agency for Healthcare Research and Quality (AHRQ) [accessed on October 1, 2010];Inpatient Quality Indicators (IQI) Composite Measure Workgroup Final Report, March 2008. 2008 Available at http://www.qualityindicators.ahrq.gov/downloads/iqi/AHRQ_IQI_Workgroup_Final.pdf.
- American Hospital Association. American Hospital Association Annual Survey Database for Fiscal Year 2001. Chicago, IL: American Hospital Association; 2001. [Google Scholar]
- Centers for Medicare and Medicaid Services. [Accessed October 1, 2010];Cost reports. Available at: http://www.cms.hhs.gov.offcampus.lib.washington.edu/CostReports/
- [accessed on June 1, 2009];Clinical Classifications Software (CCS) for ICD-9-CM. Available at: Available at http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp.
- The LeapFrog Group. [Accessed October 1, 2010];2010 website http://www.leapfroggroup.org/media/file/SurvivalPredictorWhitepaper.pdf.
- Angus DC, Barnato AE, Linde-Zwirble WT, Weissfeld LA, Watson RS, Rickert T, Rubenfeld GD. Use of intensive care at the end oflife in the United States: an epidemiologic study. Crit Care Med. 2004;32(3):638–43. doi: 10.1097/01.ccm.0000114816.62331.08. [DOI] [PubMed] [Google Scholar]
- Angus DC, Black N. Improving care of the critically ill: institutional and health-care system approaches. Lancet. 2004;363(9417):1314–20. doi: 10.1016/S0140-6736(04)16007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash AS, Posner MA, Speckman J, Franco S, Yacht AC, Bramwell L. Using claims data to examine mortality trends following hospitalization for heart attack in Medicare. Health Serv Res. 2003;38(5):1253–62. doi: 10.1111/1475-6773.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnato AE, Bost JE, Farrell MH, Lave JR, Arnold RM, Rubio DM, Angus DC. Relationship between staff perceptions of hospital norms and hospital-level end-of-life treatment intensity. J Palliat Med. 2007;10(5):1093–100. doi: 10.1089/jpm.2006.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnato AE, Chang CC, Farrell MH, Lave JR, Roberts MS, Angus DC. Is survival better at hospitals with higher “end-of-life” treatment intensity? Med Care. 2010;48(2):125–32. doi: 10.1097/MLR.0b013e3181c161e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnato AE, Farrell MH, Chang CC, Lave JR, Roberts MS, Angus DC. Development and validation of hospital “end-of-life” treatment intensity measures. Med Care. 2009;47(10):1098–105. doi: 10.1097/MLR.0b013e3181993191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett-Guerrero E, Zhao Y, O’Brien SM, Ferguson TB, Jr, Peterson ED, Gammie JS, Song HK. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304(14):1568–75. doi: 10.1001/jama.2010.1406. [DOI] [PubMed] [Google Scholar]
- Casper M, Nwaise I, Croft JB, Hong Y, Fang J, Greer S. Geographic disparities in heart failure hospitalization rates among Medicare beneficiaries. J Am Coll Cardiol. 2010;55(4):294–9. doi: 10.1016/j.jacc.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: the importance of reliability adjustment. Health Serv Res. 2010;45(6 Pt 1):1614–29. doi: 10.1111/j.1475-6773.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlenbach WJ, Hough CL, Crane PK, Haneuse SJ, Carson SS, Curtis JR, Larson EB. Association between acute care and critical illness hospitalization and cognitive function in older adults. JAMA. 2010;303(8):763–70. doi: 10.1001/jama.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher M, Perneger TV, Chevrolet JC. National questionnaire survey on what influences doctors’ decisions about admission to intensive care. BMJ. 2004;329(7463):425. doi: 10.1136/bmj.329.7463.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988–2002. JAMA. 2005;294(21):2712–9. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- Fung V, Schmittdiel JA, Fireman B, Meer A, Thomas S, Smider N, Hsu J, Selby JV. Meaningful variation in performance: a systematic literature review. Med Care. 2010;48(2):140–8. doi: 10.1097/MLR.0b013e3181bd4dc3. [DOI] [PubMed] [Google Scholar]
- Garland A, Shaman Z, Baron J, Connors AF., Jr Physician-attributable differences in intensive care unit costs: a single-center study. Am J Respir Crit Care Med. 2006;174(11):1206–10. doi: 10.1164/rccm.200511-1810OC. [DOI] [PubMed] [Google Scholar]
- Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Ann Surg. 2009;250(6):1029–34. doi: 10.1097/sla.0b013e3181bef697. [DOI] [PubMed] [Google Scholar]
- Ghali WA, Ash AS, Hall RE, Moskowitz MA. Variation in hospital rates of intraaortic balloon pump use in coronary artery bypass operations. Ann Thorac Surg. 1999;67(2):441–5. doi: 10.1016/s0003-4975(98)01138-2. [DOI] [PubMed] [Google Scholar]
- Halpern NA, Pastores SM. Critical care medicine in the United States 2000–2005: An analysis of bed numbers, occupancy rates, payer mix, and costs*. Crit Care Med. 2009 doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- Halpern NA, Pastores SM. Critical care medicine in the United States 2000–2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38(1):65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- Halpern NA, Pastores SM, Greenstein RJ. Critical care medicine in the United States 1985–2000: an analysis of bed numbers, use, and costs. Crit Care Med. 2004;32(6):1254–9. doi: 10.1097/01.ccm.0000128577.31689.4c. [DOI] [PubMed] [Google Scholar]
- Halpern NA, Pastores SM, Thaler HT, Greenstein RJ. Critical care medicine use and cost among Medicare beneficiaries 1995–2000: major discrepancies between two United States federal Medicare databases. Crit Care Med. 2007;35(3):692–9. doi: 10.1097/01.CCM.0000257255.57899.5D. [DOI] [PubMed] [Google Scholar]
- Hayward RA, Heisler M, Adams J, Dudley RA, Hofer TP. Overestimating outcome rates: statistical estimation when reliability is suboptimal. Health Serv Res. 2007;42(4):1718–38. doi: 10.1111/j.1475-6773.2006.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman DL, Buono D, McBride RB, Tsai WY, Neugut AI. Influence of private practice setting and physician characteristics on the use of breast cancer adjuvant chemotherapy for elderly women. Cancer. 2009;115(17):3848–57. doi: 10.1002/cncr.24448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho V, Heslin MJ, Yun H, Howard L. Trends in hospital and surgeon volume and operative mortality for cancer surgery. Ann Surg Oncol. 2006;13(6):851–8. doi: 10.1245/ASO.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Huesch MD. Provider-hospital “fit” and patient outcomes: evidence from Massachusetts cardiac surgeons, 2002–2004. Health Serv Res. 2011;46(1 Pt 1):1–26. doi: 10.1111/j.1475-6773.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashyna TJ, Christie JD, Moody J, Kahn JM, Asch DA. The structure of critical care transfer networks. Med Care. 2009;47(7):787–93. doi: 10.1097/MLR.0b013e318197b1f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashyna TJ, Kahn JM, Hayward RA, Nallamothu BK. Interhospital transfers among Medicare beneficiaries admitted for acute myocardial infarction at nonrevascularization hospitals. Circ Cardiovasc Qual Outcomes. 2010;3(5):468–75. doi: 10.1161/CIRCOUTCOMES.110.957993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joynt GM, Gomersall CD, Tan P, Lee A, Cheng CA, Wong EL. Prospective evaluation of patients refused admission to an intensive care unit: triage, futility and outcome. Intensive Care Med. 2001;27(9):1459–65. doi: 10.1007/s001340101041. [DOI] [PubMed] [Google Scholar]
- Kim MM, Barnato AE, Angus DC, Fleisher LA, Kahn JM. The effect of multidisciplinary care teams on intensive care unit mortality. Arch Intern Med. 2010;170(4):369–76. doi: 10.1001/archinternmed.2009.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krein SL, Hofer TP, Kerr EA, Hayward RA. Whom should we profile? Examining diabetes care practice variation among primary care providers, provider groups, and health care facilities. Health Serv Res. 2002;37(5):1159–80. doi: 10.1111/1475-6773.01102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MM, Rapoport J, Lemeshow S, Chalfin DB, Phillips G, Danis M. Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med. 2008;148(11):801–9. doi: 10.7326/0003-4819-148-11-200806030-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanic RE, Altman SH. Payment reform options: episode payment is a good place to start. Health Aff (Millwood) 2009;28(2):w262–71. doi: 10.1377/hlthaff.28.2.w262. [DOI] [PubMed] [Google Scholar]
- Merlo J, Chaix B, Yang M, Lynch J, Rastam L. A brief conceptual tutorial of multilevel analysis in social epidemiology: linking the statistical concept of clustering to the idea of contextual phenomenon. J Epidemiol Community Health. 2005a;59(6):443–9. doi: 10.1136/jech.2004.023473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo J, Gerdtham UG, Eckerlund I, Hakansson S, Otterblad-Olausson P, Pakkanen M, Lindqvist PG. Hospital level of care and neonatal mortality in low-and high-risk deliveries: reassessing the question in Sweden by multilevel analysis. Med Care. 2005b;43(11):1092–100. doi: 10.1097/01.mlr.0000182484.14608.b9. [DOI] [PubMed] [Google Scholar]
- Merlo J, Yang M, Chaix B, Lynch J, Rastam L. A brief conceptual tutorial on multilevel analysis insocial epidemiology: investigating contextual phenomena in different groups of people. J Epidemiol Community Health. 2005c;59(9):729–36. doi: 10.1136/jech.2004.023929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt EB, Kersten A, Rahim MT, Dremsizov TT, Clermont G, Cooper LM, Angus DC, Linde-Zwirble WT. Growth of intensive care unit resource use and its estimated cost in Medicare. Crit Care Med. 2008;36(9):2504–10. doi: 10.1097/CCM.0b013e318183ef84. [DOI] [PubMed] [Google Scholar]
- Morris CN. Parametric Empirical Bayes Inference: theory and applications. Journal of the American Statistical Association. 1983;78(22):47–55. [Google Scholar]
- Mullner RCK. The American Hospital Association’s Annual Survey of Hospitals: a critical appraisal. J Consum Mark. 2002;19:614–61. [Google Scholar]
- Normand ST, Glickman ME, Gatsonis CA. Statistical Methods for Profiling Providers of Medical Care: Issues and Applications. Journal of the American Statistical Association. 1997;92(439):803–14. [Google Scholar]
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using Stata. 2. College Station, Texas: StataCorp LP; 2008. [Google Scholar]
- Rasmussen S, Abildstrom SZ, Rasmussen JN, Gislason GH, Schramm TK, Folke F, Kober L, Torp-Pedersen C, Madsen M. Hospital variation in use of secondary preventive medicine after discharge for first acute myocardial infarction during 1995–2004. Med Care. 2008;46(1):70–7. doi: 10.1097/MLR.0b013e3181484952. [DOI] [PubMed] [Google Scholar]
- Reeves MJ, Gargano J, Maier KS, Broderick JP, Frankel M, LaBresh KA, Moomaw CJ, Schwamm L. Patient-level and hospital-level determinants of the quality of acute stroke care: a multilevel modeling approach. Stroke. 2010;41(12):2924–31. doi: 10.1161/STROKEAHA.110.598664. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Blumberg N, Saint S, Langa KM, Nallamothu BK. Hospital variation in transfusion and infection after cardiac surgery: a cohort study. BMC Med. 2009;7:37. doi: 10.1186/1741-7015-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal GE, Sirio CA, Shepardson LB, Harper DL, Rotondi AJ, Cooper GS. Use of intensive care units for patients with low severity of illness. Arch Intern Med. 1998;158(10):1144–51. doi: 10.1001/archinte.158.10.1144. [DOI] [PubMed] [Google Scholar]
- Selby JV, Schmittdiel JA, Lee J, Fung V, Thomas S, Smider N, Crosson FJ, Hsu J, Fireman B. Meaningful variation in performance: what does variation in quality tell us about improving quality? Med Care. 2010;48(2):133–9. doi: 10.1097/MLR.0b013e3181c15a6e. [DOI] [PubMed] [Google Scholar]
- Song Y, Skinner J, Bynum J, Sutherland J, Wennberg JE, Fisher ES. Regional variations in diagnostic practices. N Engl J Med. 2010;363(1):45–53. doi: 10.1056/NEJMsa0910881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EJ, Lucke JF, Wueste L, Weavind L, Patel B. Association of telemedicine for remote monitoring of intensive care patients with mortality, complications, andlength of stay. JAMA. 2009;302(24):2671–8. doi: 10.1001/jama.2009.1902. [DOI] [PubMed] [Google Scholar]
- Truog RD, Campbell ML, Curtis JR, Haas CE, Luce JM, Rubenfeld GD, Rushton CH, Kaufman DC. Recommendations for end-of-life care in the intensive care unit: a consensus statement by the American College [corrected] of Critical Care Medicine. Crit Care Med. 2008;36(3):953–63. doi: 10.1097/CCM.0B013E3181659096. [DOI] [PubMed] [Google Scholar]
- Wennberg DE, Lucas FL, Siewers AE, Kellett MA, Malenka DJ. Outcomes of percutaneous coronary interventions performed at centers without andwith onsite coronary artery bypass graft surgery. JAMA. 2004;292(16):1961–8. doi: 10.1001/jama.292.16.1961. [DOI] [PubMed] [Google Scholar]
- Wunsch H, Linde-Zwirble WT, Harrison DA, Barnato AE, Rowan KM, Angus DC. Use of intensive care services during terminal hospitalizations in England and the United States. Am J Respir Crit Care Med. 2009;180(9):875–80. doi: 10.1164/rccm.200902-0201OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.