Abstract
In humanized UGT1 (hUGT1) mice that express the entire UGT1 locus, the hepatic UGT1A genes are dramatically induced 12–14 days after conception. Steroid induction of the UGT1A1 gene indicates that xenobiotic sensors, such as the pregnane X receptor (PXR) and constitutive androstane receptor (CAR), may underlie the induction process. In contrast, neonatal hUGT1 mice display severe hyperbilirubinemia, with limited expression of the UGT1A genes. This study identifies PXR as both a positive and negative regulator of the UGT1A1 gene. Pregnancy hormones, in particular the glucocorticoids, target PXR as a positive regulator of human glucuronidation. Employing reverse genetics, where PXR has been genetically deleted, hUGT1/Pxr−/− mice show limited induction of the liver UGT1A genes during pregnancy, while the exact opposite occurs in the newborn mice. Neonatal hUGT1 mice show delayed expression of hepatic UGT1A1 and are severely hyperbilirubinemic. However, in hUGT1/Pxr−/− mice, hyperbilirubinemia is greatly reduced due to induction of hepatic UGT1A1. Thus, PXR serves to repress UGT1A1 gene expression during development. Transcriptional silencing of the UGT1A1 gene was relieved in neonatal hUGT1 hepatoctyes through interruption of PXR by siRNA. Conclusion. PXR is a key regulator of pregnancy induced glucuronidation capacity in addition to modulating the severity of neonatal jaundice.
Keywords: Drug metabolism, Nuclear xenobiotic receptor, Mouse genetics, Hyperbilirubinemia, Glucocorticoids
Pregnancy results in dramatic surges in hormones during gestational development, with increases in steroids such as estrogens, progestins and the glucocorticoids. These steroids have been linked to the control and regulation of xenobiotic drug metabolism, primarily through activation of the nuclear xenobiotic receptors such as the pregnane X receptor (PXR) (1–3) and the constitutive androstane receptor (CAR) (4, 5). PXR and CAR have been classified as steroid and xenobiotic sensors, so it can be anticipated that significant fluctuations in the pregnancy hormones will modulate transcriptional control of target genes such as those involved in xenobiotic or drug metabolism.
Clinical findings indicate that steroid fluctuations lead to changes in xenobiotic glucuronidation during pregnancy. For example, circulating unconjugated bilirubin is cleared from the circulation solely through UGT1A1 metabolism (6). During pregnancy, total serum bilirubin (TSB) levels are lower in woman (7), indicating that bilirubin metabolism is accelerated through induced UGT1A1. Labetalol, an antihypertensive agent, which is metabolized primarily by UGT1A1 glucuronidation, shows increased clearance in the second and third trimesters of pregnancy compared to the post-partum period (8). Lamotrigine, an antiepileptic agent metabolized by UGT1A3 and UGT1A4, has a 50% decreased elimination half-life with an increased clearance of over 200% during pregnancy, leading to a closely correlated higher incidence of epileptic seizures (9). Labetalol and lamotrigine clearance during pregnancy indicates that UGT1A1, UGT1A3 and UGT1A4 are induced and clearance is accelerated. It has been suggested that up-regulation of UGT1A4 during pregnancy may be mediated by 17β-estradiol and the ERα receptor (10). Clearly, hormonal sensors during pregnancy are leading to induction of human glucuronidation capacity.
The exact opposite is occurring during neonatal development, which is evident by the very high incidence of hyperbilirubinemia in newborn children. Since bilirubin is metabolized exclusively by UGT1A1 (6), hyperbilirubinemia develops from the inability of liver glucuronidation to match the early rise in serum bilirubin that forms from the abundance of red blood cells needed to carry oxygen. Senescence of the erythrocytes leads to an accumulation of hemoglobin that is rapidly metabolized into bilirubin and released into the circulation, where it is transported to the liver for excretion following UGT1A1 dependent glucuronidation. Jaundice is directly linked to inadequate glucuronidation of serum bilirubin stemming from reduced expression of liver UGT1A1 (11–13). It is unclear if the reduced expression of UGT1A1 in neonates is a controlled event through transcriptional silencing or simply a result of limited epigenetic factors that are eventually produced to positively regulate the UGT1A1 gene in a developmental fashion.
In this report, it will be demonstrated that PXR is linked to both pregnancy induced expression of the UGT1 locus as well as repression of the UGT1A1 gene in neonatal development. These findings were generated through the development of humanized UGT1 (hUGT1) mice (14, 15) which express the entire human UGT1 locus in a murine Ugt1-null background (16). Taking advantage of the power of reverse genetics, it will be shown that PXR plays a crucial role in pregnancy induced glucuronidation in addition to the early development of hyperbilirubinemia in neonatal hUGT1 mice.
Experimental Procedures
Animals
The generation of Tg(UGT1A1*1)Ugt1−/− (hUGT1*1) and Tg(UGT1A1*28)Ugt1−/− (hUGT1*28) mice has been reported previously (15). Pxr−/− mice were generated as previously described (17) and Car−/− mice were generously provided by Dr. Masahiko Negishi (NIEHS). All genetically modified strains have been bred for over 5 generations with C57BL/6 wild type mice before inbreeding. To generate hUGT1/Pxr−/− mice, hUGT1*1 mice were crossed with Pxr−/− mice, producing Tg(UGT1A1*1)Ugt1+/−Pxr+/− mice. These mice were backcrossed in brother/sister matings to generate Tg(UGT1A1*1)Ugt−/−Pxr−/− (hUGT1*1/Pxr−/−) mice. The same breeding strategy was used to generate hUGT1*1/Car−/− mice.
Primary hepatocyte isolation and PXR-targeted specific siRNA regulation
Hepatocytes were isolated as described previously (14). The hepatocytes were then cultured in 6-well collagen-treated plates (Discovery Labware, Bedford, MA) in 2 ml of DMEM containing penicillin/streptomycin and supplemented with 10% fetal bovine serum. Small interfering RNA (siRNA) duplexes specific for mouse PXR were provided by Bioneer (Alameda, CA) and Santa Cruz Biotechnology Inc. Four hours after primary hepatocytes were isolated from 14 day old hUGT1*1 mice, cells were transfected in the presence of 20 nM of either siRNA or control RNA with Lipofectamine 2000 (Invitrogen) in a final volume of 1 ml of OPTI-MEM. After 5 hours cells were changed with fresh medium supplemented with 10% fetal bovine serum and penicillin-streptomycin. Forty-eight hours later, cells were used for RNA extraction. Reverse transcription (RT) and real time PCR (Q-PCR) were carried out to examine gene expression levels of mouse Pxr, human UGT1A1 and mouse Cyp3a11.
Immunoblot Analysis and Real Time PCR
Mice were sacrificed and livers were perfused with ice-cold 1.15% KCL and microsomes prepared as previously outlined (14). All Western blots were performed using NuPAGE BisTris-polyacrylamide gels as previously described (15). For real time (Q)-PCR analysis, approximately 100 mg of liver tissue was homogenized into 1 ml of TRIzol and RNA prepared. Using iScript Reverse Transcriptase (BioRad), 1 μg of total RNA was used for the generation of cDNA as outlined by the manufacturer in a total volume of 20 μl. Following synthesis of cDNA, 2 μl was used in real time PCR conducted with a QuantiTect™ SYBR® Green PCR kit (Qiagen, Valencia, CA) using a MX4000 Multiplex QPCR (Stratagene, La Jolla, CA) programmed to take three fluorescence data points at the end of each annealing plateau. All PCR reactions were performed in triplicate as previously outlined (14). Ct values were normalized to mouse cyclophilin (CPH). The specific primers used to quantitate the respective gene transcripts (18) are listed in supplementary table 1.
Chromatin Immunoprecipitation
CHIP analysis was performed using the modified protocol based on the EZ-CHIP kit (Millipore). Liver tissue (100 mg) was minced and cross-linked in DMEM (Invitrogen) containing 1% formaldehyde. The procedures for cell lysis and sonication to shear DNA were followed according to the manufacturer’s protocol (EZ-CHIP kit, Millipore). One ml of cell extract was pre-cleared by incubation with 60 μl of protein A Agarose/Salmon sperm DNA overnight at 4°C. The cleared cellular extract was incubated with anti-PXR antibody (Santa Crutz, sc-25381) for 2 hr at 4°C. Following precipitation with protein A agarose the antibody-chromatin complex was then washed as outlined (19). The protein-DNA complexes were eluted in 200 μl elution buffer and DNA was then reverse cross-linked and released from the complex as indicated in the EZ-CHIP instructions. Following the DNA purification with spin columns (Qiagen), the purified DNA was further analyzed by PCR with a pair of primers (forward 5′-TTGTGGGGCAATACACTAGTA -3′, reverse 5′-GTCCGGGTTTCAGGTTATGTA -3′) for the amplification of the UGT1A1 promoter region containing the PXR binding site (3).
Results
Expression of the human UGT1A genes in TgUGT1 mice during pregnancy
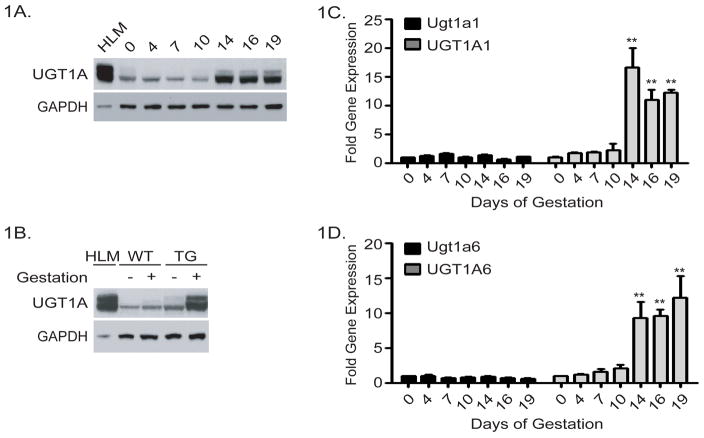
Heterozygous female TgUGT1*28 mice were mated with wild type mice and the presence of the vaginal plug in the morning was set as gestation day 1 (GD1). Starting at GD4, gravid mice were sacrificed at various times during pregnancy and liver microsomes used for Western blot analysis where the UGT1A proteins were identified with a human anti-UGT1A antibody (20). Total UGT1A protein expression was induced significantly by the end of the second trimester (GD14), and remained at a high level of expression throughout the entire third trimester (Figure 1A). This anti-UGT1A antibody also detects murine UGT1A proteins (16), but with wild-type mice as controls, no induction of murine UGT1A proteins during pregnancy was observed (Figure 1B).
Figure 1. Induction of the UGT1 locus in TgUGT1 mice.
Age matched female TgUGT1 mice were mated with wild-type mice. The following morning, female mice with the presence of a vaginal plug were removed, housed separately, and timed as gestation day 1. Pregnant mice were sacrificed at gestation days (GD) 4, 7, 10, 14, 16, and 19. Samples from at least three non-pregnant female TgUGT1 mice were used as controls. Liver microsomes and RNA from the non-pregnant control and pregnant mice were prepared. A) Immunoblot detection of UGT1A proteins. Liver microsomes prepared from pregnant TgUGT1 mice at progressive stages of pregnancy were analyzed on 10% SDS-polyacrylamide gels and the UGT1A proteins detected on immunoblots by using an anti-UGT1A antibody and anti-GAPDH antibody (Santa Cruz Biotechnology). Human liver microsomes (HLM) were used as a positive control. B) Immunoblot of UGT1A proteins from liver microsomes prepared from female wild type and TgUGT1 mice that were pregnant for 16 days. Control samples were prepared from non pregnant wild type and TgUGT1 mice. C) Total RNA was isolated from liver samples taken from the pregnant TgUGT1 mice and used in RT and Q-PCR analysis to examine murine UGT1A1 RNA (Ugt1a1) expression and human UGT1A1 RNA expression. Student t-test was used to evaluate the statistical significance (** p<0.01). D) The same RNA samples were used to quantitate murine Ugt1a6 and human UGT1A6 gene expression (** p<0.01, t-test).
Two of the UGTs that are highly conserved in function between mice and humans are UGT1A1 and UGT1A6. Expression of the murine Ugt1a1 and Ugt1a6 genes along with the human UGT1A1 and UGT1A6 genes were evaluated by RT and Q-PCR analysis using species specific primers for each of these genes. Throughout pregnancy, we observed no induction of murine Ugt1a1 or Ugt1a6 gene expression (Figure 1C, and 1D), findings which correlated with the lack of wild type UGT1A protein expression. Consistent with induction of UGT1A protein in TgUGT1*28 mice during pregnancy, UGT1A1 and UGT1A6 gene expression is induced at GD14 with the induction being sustained throughout the remainder of the gestational period. Introduction of the human UGT1 locus and the UGT1A genes in TgUGT1*28 mice is regulated throughout pregnancy in a pattern that is not replicated by the murine Ugt1 locus. Emerging principles of regulatory evolution strongly favor genetic diversity in cis-regulatory DNA and not trans-regulation of gene expression to explain interspecies differences in gene expression (21, 22). The differences in transcriptional regulation between the murine and human UGT1 locus during pregnancy may be credited to important genetic differences in the regulatory regions of these genes.
Induction of the UGT1 locus in humanized UGT1 mice
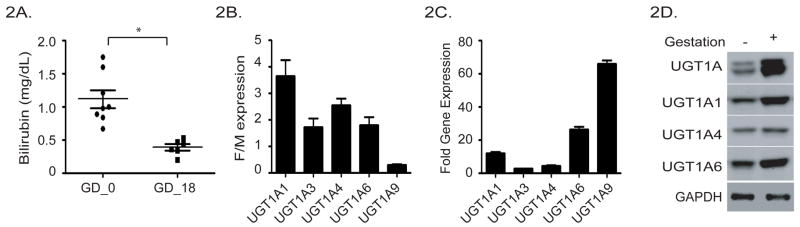
We have generated hUGT1*1 and hUGT1*28 mice, which differ predominantly in expression levels of UGT1A1 in adult liver (15). Adult hUGT1*28 mice are hyperbilirubinemic, with TSB levels that average 1 mg/dl. During pregnancy and late gestation, the TSB levels in hUGT1*28 mice averaged 0.4 mg/dl, over 50% lower than in non-pregnant mice (Figure 2A). The reduction in TSB can be accounted for by elevated levels of liver UGT1A1.
Figure 2. Regulation of the UGT1 locus during pregnancy.
A). Serum bilirubin levels in female hUGT1*28 mice were determined before pregnancy and at GD18 (* p<0.05, Student t-test). B). Quantitation of liver UGT1A gene expression by RT and Q-PCR in adult female and male mice. The relative values are expressed as a ratio value comparing female to male expression. C). Quantitation of liver UGT1A gene expression in hUGT1*1 mice pregnant for 16 days. Total liver RNA was prepared and used in RT and Q-PCR analysis with specific UGT1A gene oligonucleotides. Fold induction was calculated relative to those values obtained in non-pregnant mice. D). Immunoblot analysis of human UGT expression in non-pregnant and pregnant hUGT1*1 mice. Microsomes were prepared from non-pregnant and pregnant hUGT1*1 mice and subjected to 4–12% polyacrylamide gel electrophoresis. Following blotting, protein expression was determined using a UGT1A antibody, or isozyme specific UGT1A1, UGT1A4 and UGT1A6 antibodies.
In hUGT1*1 mice, expression of UGT1A1, -1A3, -1A4, and -1A6 are 2–4 fold greater in female liver than male liver (Figure 2B). The sole exception to the female dominance is UGT1A9, which has minimal expression in female liver. Examination of the fold increase in gene expression of each of the UGT1A genes shows that UGT1A9 expression increases to nearly 70 fold over non-pregnant values (Figure 2C). This large increase in UGT1A9 expression accounted for by fold induction during pregnancy results in part from the very low basal levels observed in non-pregnant female mice. Along with UGT1A9 gene expression, UGT1A1 and UGT1A6 gene expression are found to dominant the induction process during pregnancy. These increases are also reflected in microsomal protein abundance as determined by Western blot analysis using isoform specific antibodies (Figure 2D).
Pregnancy steroids and induction of UGT1A1
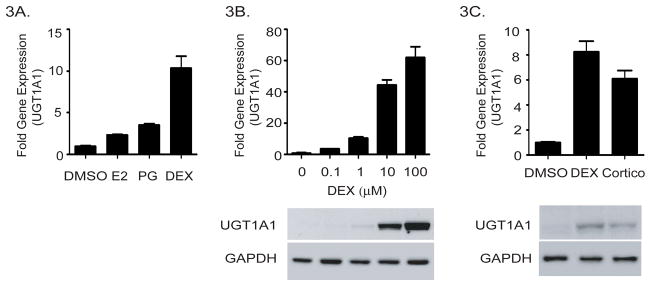
We hypothesized that hormone surges in late pregnancy play an important role in the gestational regulation of the human UGT1 locus. To examine if selective steroids are capable of regulating the UGT1A genes, primary hepatocytes from hUGT1*1 mice were isolated, placed in culture and exposed to 17β-estradiol, progesterone or the synthetic glucocorticoid, dexamethasone. We measured induction of the UGT1A1 gene, since the other UGT1A genes are refractive to expression in hepatoctyes in culture. Progesterone (50 μg/ml) and estradiol (20 μg/ml) exposure for 24 hours resulted in a minimal 2–3 fold induction of the UGT1A1 gene (Figure 3A). These concentrations were found to be optimal for UGT1A1 induction. The synthetic glucocorticoid dexamethasone (10μM) induced UGT1A1 gene expression up to 60 fold (Figure 3B). This induction of gene expression and UGT1A1 induction is dose dependent, with transcriptional induction of the UGT1A1 gene in hepatocytes being substantial at 0.1 μM. Since corticosterone is the primary form of glucocorticoids in mouse, 20 μM of corticosterone was used to treat freshly isolated hepatocytes (Figure 3C). Twenty four hours after exposure, increased UGT1A1 protein levels were detected in whole cell lysates by Western blot analysis, indicating that glucocorticoids play an important role in UGT1A gene expression.
Figure 3. Induction of UGT1A1 in primary hepatoctyes by pregnancy related hormones.
A) Primary hepatocytes were isolated from hUGT1*1 mice and treated with either estrogen (20 μg/ml), progesterone (50 μg/ml), or dexamethasone (DEX-1 μM). After 24 hours RNA was isolated and UGT1A1 gene expression was determined by RT and Q-PCR. Ct values from RT and Q-PCR were normalized to the housekeeping gene CPH, and calculated as fold induction over the expression levels of cells treated with vehicle control. B) Primary hepatocytes were exposed to different concentrations of dexamethasone. After 24 hours, RNA was isolated and UGT1A1 gene expression monitored by RT and Q-PCR. A sample of total cell extract was also prepared and used for immunodetection of UGT1A1 and GAPDH by Western blot analysis. C) Isolated hepatocytes were exposed to dexamethasone (1 μM) or corticosterone (20 μM) for 24 h, followed by RT and Q-PCR analysis and Western blot analysis to detect human UGT1A1expression, and GAPDH blotting was used as loading control.
Xenobiotic receptors and induction of the UGT1 locus during pregnancy
The progestins, corticosterone, and estradiols are low affinity substrates for PXR (23–25) and 17β-estradiol has been shown to activate CAR (4). Thus, experiments were conducted to examine precisely the role of PXR and CAR towards induction of the UGT1 locus during pregnancy.
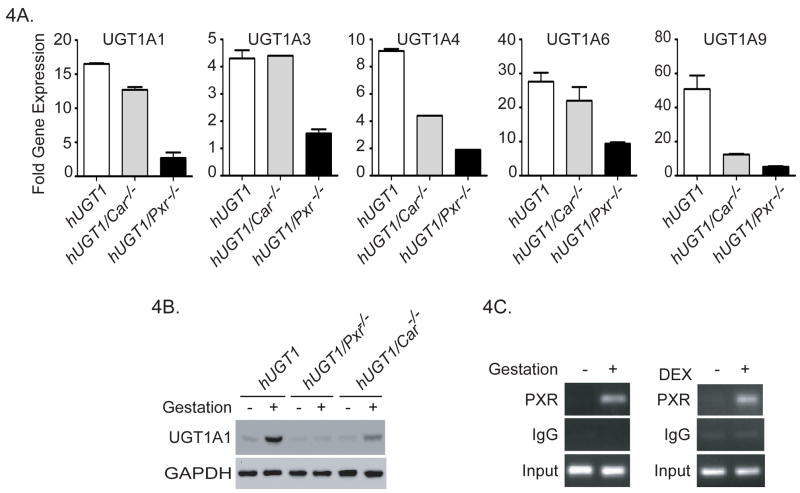
To undertake these studies, hUGT1*1 mice were crossed with Car-null mice to create hUGT1*1/Car−/− mice. On GD16, liver samples from hUGT1*1/Car−/− mice were processed for total RNA along with microsomal extracts. RT and Q-PCR analysis for liver UGT1A gene products were conducted with specific oligonucleotide primers for each gene (Figure 4A). In hUGT1*1/Car−/− mice, gestational induction of the UGT1A1, -1A3, and -1A6 genes was found to be similar to that observed in hUGT1*1 mice. Using Western blot analysis to examine UGT1A1 expression in liver microsomes, UGT1A1 was induced during pregnancy in hUGT1*1 and hUGT1*1/Car−/− mice (Figure 4B). However, CAR does play a role in the induction of UGT1A4 and UGT1A9. We observed approximately a 50% reduction in the 8-fold increase in UGT1A4 RNA accumulation observed in hUGT1*1 mice. When UGT1A9 expression was analyzed, the robust induction in hUGT1*1 pregnant mice was reduced over 75% during pregnancy in hUGT1*1/Car−/− mice, indicating an important role for CAR in the induction of the UGT1A9 gene (Figure 4A).
Figure 4. The impact of PXR and CAR deletion on gestational regulation of the human UGT1 locus in liver tissue.
Humanized UGT1/Pxr−/− or hUGT1/Car−/− mice were obtained from backcrossing hUGT1*1 mice with Pxr−/− mice or Car−/− mice. Female hUGT1*1, hUGT1/Pxr−/−, and hUGT1/Car−/− mice at 8 weeks old were used for timed pregnancy experiments. Age-matched non-pregnant female mice from each strain were used as controls. Mice were sacrificed at GD16. A) RNA was isolated from pooled liver samples followed by RT and Q-PCR analysis. Primers specific for human UGT1A1, UGT1A3, UGT1A4, UGT1A6, and UGT1A9 gene products were used (Suppl. Table 1). Q-PCR results from pregnant mice were normalized by the housekeeping gene CPH and described as fold of induction over the non-pregnant control. B) Western blot analysis using liver microsomes and anti-UGT1A1 and anti-GAPDH antibodies. C) Chromatin immunoprecipitation analysis (CHIP) of PXR associated with the human UGT1A1 gene in non-pregnant and 16 day pregnant hUGT1 mice, and DEX treated adult females.
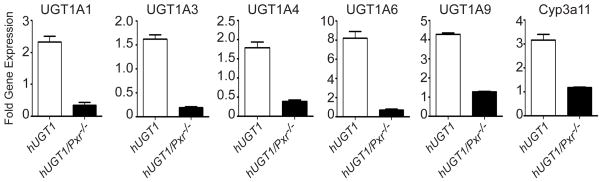
When hUGT1*1 mice are placed into a Pxr-null background, there was substantially reduced induction of each of the UGT1A genes during pregnancy when compared to expression in hUGT1*1 mice (Figure 4A). The UGT1A1 gene, robustly induced around 15-fold in hUGT1*1 and hUGT1*1/Car−/− mice during pregnancy, displays reduced expression at GD16 in hUGT1*1/Pxr−/− mice. A similar pattern of expression was observed when UGT1A1 was detected by Western blot analysis, showing little expression in hUGT1*1/Pxr−/− mice (Figure 4B). An important role for PXR binding to the UGT1A1 gene during pregnancy was reinforced when we examined PXR binding by chromatin immunoprecipitation (CHIP) analysis to the PXR binding site that flanks the UGT1A1 promoter (3). PXR is activated during pregnancy and binds to the UGT1A1 gene as demonstrated by CHIP analysis (Figure 4C), indicating that endogenous ligands are participating in regulation of this gene. Coupled with Chip analysis showing induced binding of PXR to the UGT1A1 gene following DEX treatment (Figure 4C) along with previous experiments demonstrating that PXR binding to this region of the UGT1A1 gene stimulates trans-activation of the promoter (3), these findings confirm that induction of UGT1A1 is closely linked to activation of PXR during pregnancy.
Glucocorticoids induce the UGT1 locus in a PXR dependent fashion
Since regulation of the UGT1 locus during pregnancy is linked to PXR, we examined if the genes associated with the UGT1 locus in humanized mice could be activated in a PXR dependent fashion by glucocorticoids. We treated 8 week old hUGT1*1 and hUGT1*1/Pxr−/− female mice by the i.p route with 20 mg/kg dexamethasone for 4 days and measured UGT1A gene expression in liver 48 hrs after treatment (Figure 5). Each of the five UGT1A genes expressed in liver was induced in hUGT1*1 mice. While activation of the glucocorticoid receptor by dexamethasone has been shown to activate UGT1A1 reporter gene constructs in HepG2 cells (26), dexamethasone treatment had no effect on induction of UGT1A1 in hUGT1*1/Pxr−/− mice, indicating that induction of the UGT1A genes by glucocorticoids is facilitated solely by activation of the PXR in vivo.
Figure 5. Induction of the UGT1 locus by dexamethasone in adult hUGT1*1 and hUGT1/Pxr−/− mice.
Adult hUGT1*1 and hUGT1/Pxr−/− mice were treated with desamethasone (DEX) by i.p. injection for 4 consecutive days at 20 mg/kg per dose. Non-treated mice received solvent and were used as controls. Twenty four hours after the last dose, liver RNA was prepared and RT and Q-PCR analysis of human UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A9, and murine Cyp3a11 gene expression was performed. Fold induction reflects the change in gene expression between solvent treated and DEX treated mice.
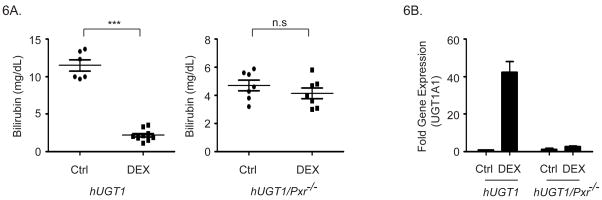
In neonatal hUGT1 mice, UGT1A1 expression controls the levels of TSB, with significant hyperbilirubinemia developing due to limited expression of hepatic UGT1A1. We examined if glucocorticoids could regulate UGT1A1 gene expression during the neonatal period in a PXR dependent fashion. In hUGT1*1 mice, TSB peaks 14 days after birth and ranges from 12–15 mg/dl (Figure 6A). Within the same litters, half of the newborn hUGT1*1 mice were treated with 8 mg/kg dexamethasone by the oral gavage while the other half received just vehicle. Serum bilirubin levels were determined 48 hours after treatment. Dexamethasone treatment led to a dramatic reduction in TSB (Figure 6A). Analysis of UGT1A1 gene expression in hepatic tissue following dexamethasone treatment confirmed a 40-fold induction of RNA in hUGT1*1 neonatal mice (Figure 6B). When we examined TSB levels in response to 8 mg/kg dexamethasone treatment in hUGT1*1/Pxr−/− mice, the serum bilirubin levels did not change when compared to vehicle treated neonatal mice (Figure 6A). There was also no induction of hepatic UGT1A1 as determined by RT and Q-PCR analysis (Figure 6B). Thus, dexamethasone induces hepatic UGT1A1 leading to bilirubin metabolism through a PXR dependent mechanism.
Figure 6. Induction of UGT1A1 by dexamethasone in neonatal hUGT1*1 and hUGT1/Pxr−/− mice.
Humanized UGT1*1 and hUGT1/Pxr−/− mice at 12 days after birth were treated by oral gavage with 8 mg/kg DEX. A). Serum bilirubin levels in untreated (Ctrl) and DEX treated mice were evaluated in 14 day old mice (*** p<0.001, n.s. no significant difference, t-test). B). After DEX treatment, fold induction of UGT1A1 gene expression in liver was determined by RT and Q-PCR analysis.
PXR represses human UGT1A1 gene expression in neonatal hUGT1 mice
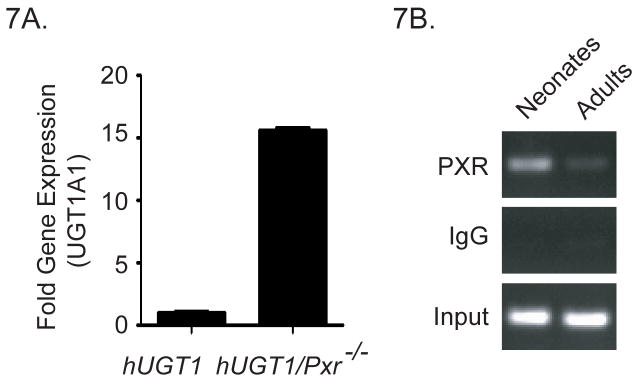
As we undertook these experiments, we also noted that TSB levels in neonatal hUGT1*1/Pxr−/− peak to only 4–6 mg/dl, almost 10 mg/dl lower than observed in hUGT1*1 mice (Figure 6A). When we examined liver UGT1A1 gene expression at 14 days after birth, there was over a 10 fold increase in gene expression in hUGT1*1/Pxr−/− mice when compared to hUGT1*1 mice (Figure 7A). This finding indicates that non-liganded PXR during development in hUGT1*1 mice is serving to repress liver UGT1A1 gene expression, since in PXR deficient mice, we observe induction of liver UGT1A1 which correlates with a reduction in serum bilirubin.
Figure 7. PXR represses UGT1A1 gene expression in neonatal hUGT1*1 mice.
7A. Comparison of the hepatic expression levels of the UGT1A1 gene in hUGT1 and hUGT1/Pxr−/− mice 14 days after birth. 7B. Chromatin immunoprecipitation analysis of PXR associated with the human UGT1A1 gene in neonatal and adult hUGT1*1 mice.
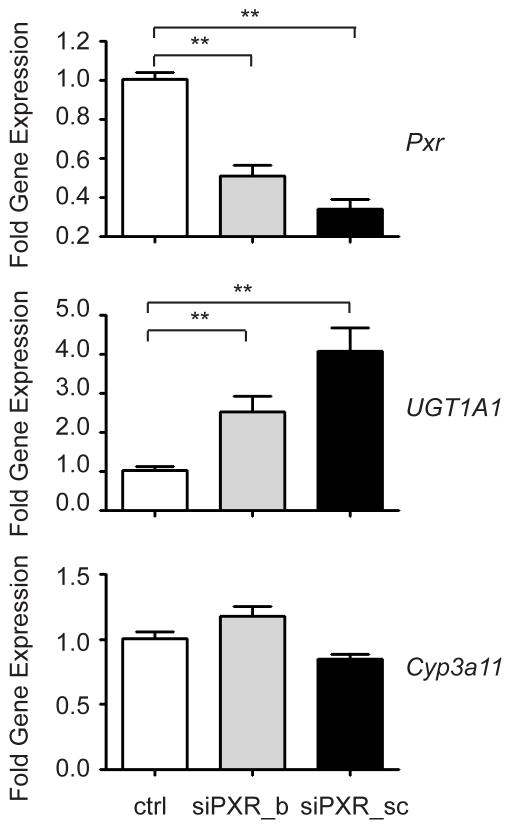
To further examine the developmental properties of PXR on UGT1A1 gene repression, we performed PXR CHIP analysis by using liver samples from both neonatal and adult hUGT1*1 mice. As shown in Figure 7B, intensified PXR signals were observed in neonatal livers in comparison to adult livers. Abundant PXR binding to the UGT1A1 gene is concordant with reduced UGT1A1 gene expression, indicating that PXR is repressing gene expression. To examine this possibility, we isolated primary hepatocytes from 14 day old hUGT1*1 mice and transfected them with PXR specific siRNAs from two sources (Figure 8). Forty-eight hours later, UGT1A1 gene expression was quantitated by using RT and Q-PCR. The fold induction was tied to the extent of the PXR mRNA knockdown. When the PXR knockdown was 50% (siPXR_b), approximately two fold induction of human UGT1A1 expression was observed. When the PXR knockdown was 70%, UGT1A1 gene induction was 4 fold. In contrast, Cyp3a11, another PXR target gene, showed no change. These findings confirm that transcriptional silencing of the UGT1A1 gene by PXR occurs during neonatal development in liver tissue.
Figure 8. Knockdown of PXR and derepression of UGT1A1 gene expression in primary hepatocytes.
Hepatocytes were isolated from 14 day-old neonatal hUGT1*1 mice. Cells were transfected with mouse siRNA oligonucleotide manufactured by Bioneer (siPXR_b) and Santa Cruz Biotechnology (siPXR_sc). Forty eight hours later, cells were harvested for RNA preparation. The relative gene expression levels of mouse Pxr, human UGT1A1, and mouse Cyp3a11 were determined by RT and Q-PCR (** p<0.01, t-test).
Discussion
Employing recently developed TgUGT1 mice with the UGT1A genes expressed in a Ugt1-null background, the function of PXR and CAR during pregnancy was investigated using reverse genetics to examine induction of the UGT1A genes in xenobiotic receptor defective mice. Previous experiments with TgUGT1 and hUGT1 mice have shown that chemical treatment with activators of either PXR or CAR leads to induction of UGT1A1, -1A3, -1A4, -1A6, and -1A9 (14). The mechanisms by which PXR and CAR control the induction of all these genes have not been determined, although PXR and CAR responsive elements have been shown to play an important role in PXR/CAR binding and induction of the UGT1A1 gene. In primary heptatocytes from hUGT1 mice, selective treatment with 17β-estradiol, progesterone and dexamethasone each led to induction of the UGT1A1 gene, with glucocorticoid treatment maximizing the induction response. In timed pregnancy experiments, each of the maternal liver specific UGT1A genes was induced in hUGT1 mice, with UGT1A1, UGT1A6 and UGT1A9 gene expression being the most prominent. During pregnancy, gestational induction of the UGT1A genes was mostly conserved in hUGT1/Car−/− mice but greatly diminished in hUGT1/Pxr−/− mice, suggesting that PXR participates in a global fashion to regulate the UGT1 locus during fetal development. The sole exception to this appears to be with UGT1A9 gene expression, which displayed reduced expression during pregnancy in hUGT1/Car−/− mice. Since both PXR/CAR appear to be necessary for induction of UGT1A9 during pregnancy, there may be cross-talk occurring between the two xenobiotic receptors to facilitate regulation of the UGT1A9 gene.
The contribution of PXR towards induction of the human UGT1 locus is not conserved with the murine Ugt1 locus, since pregnancy has no effect on regulation of the murine Ugt1a genes as determined by gene expression profiling and protein accumulation. This was surprising since it has been demonstrated previously that over expression of the human PXR in humanized PXR mice or treatment of mice with PXR ligands, such as PCN, leads to induction of the murine Ugt1a1 gene (3, 27, 28). Our results indicate that PXR is the central modulator of the human UGT1A genes during pregnancy, but additional regulatory events specifically towards control of the human UGT1 locus, and not the murine Ugt1 locus, are in place during pregnancy. Since an increase in UGT1A dependent glucuronidation occurs in humans during pregnancy, the ability to reproduce this event in transgenic mice indicates that the genetic sequence specific to the UGT1 locus is largely responsible for directing the transcriptional program of the human UGT1A genes during pregnancy. Thus, the differences observed in the induction patterns between the human UGT1A genes and the murine Ugt1a genes are not the result of interspecies differences in epigenetic machinery or the cellular environment. From this result we can infer that transcriptional factors play a secondary role in dictating the differences observed in the human UGT1 and murine Ugt1 locus during pregnancy. Such a pattern expressing conserved genes between humans and mice has been demonstrated (27–29), reinforcing the hypothesis that differences in gene expression between species are controlled by changes in cis-acting transcriptional binding sequences (21, 22, 30).
Among the UGT1A isoforms, UGT1A1 is of special physiological importance because it is the only enzyme that catalyzes the glucuronidation of bilirubin (6). Accumulation of bilirubin leads to benign levels of hyperbilirubinemia shortly after birth, but if bilirubin levels continue to rise, the more serious symptoms associated with bilirubin induced neurological dysfunction (BIND) can develop (31, 32). Phenobarbital, a CAR agonist, has been used clinically for the treatment of neonatal hyperbilirubinemia in infants at risk for severe jaundice (TSB levels more than 16 mg/dL), therefore reducing the need for exchange transfusion (33–35). However, phenobarbital treatment is not effective immediately, and it diminishes the oxidative metabolism of bilirubin increasing the risk of neurotoxic effects (36). Glucocorticoids have also been used to treat hyperbilirubinemia (37). The initial intent of glucocorticoid therapy is to help fetal lung maturation and reduce neonatal mortality in women at high risk for preterm labor before 35 gestational weeks. During these treatments, it has been observed that hyperbilirubinemia is significantly lower in the dexamethasone treated groups compared to untreated control groups. Our studies indicate that PXR serves as a major regulator following glucocorticoid treatment by inducing liver UGT1A1 expression, leading to reduction of hyperbilirubinemia. Identification of PXR as a key regulator of the UGT1A1 gene during neonatal development can be exploited as a potential therapeutic target in the treatment of hyperbilirubinemia.
Analysis of TSB levels in neonatal hUGT1 and hUGT1/Pxr−/− mice demonstrate that PXR plays a key role in controlling serum levels during development. Neonatal hUGT1 mice develop severe hyperbilirubinemia due to a reduction in liver UGT1A1 gene expression (15). In hUGT1/Pxr−/− mice, liver UGT1A1 is induced when compared to expression in hUGT1 mice. The increased levels of liver UGT1A1 in hUGT1/Pxr−/− mice lead to reduced levels of TSB. This finding indicates that in the absence of endogenous/exogenous ligands, the physiological role of PXR leads to repression of the UGT1A1 gene during early development. It may also be an underlying regulator of the UGT1A1 gene in newborns and responsible in part for neonatal hyperbilirubinemia. Hence, PXR acts as a repressor of UGT1A1 expression in the absence of ligand. It is known that the repressive function of PXR works in part through the recruitment of the co-repressor Silencing Mediator of Retinoid and Thyroid Hormone Receptors (SMRT) (38, 39). SMRT binds to nuclear receptors in the absence of ligand and alters the chromatin structure through histone modification (40, 41). Clearly, deletion of PXR releases the repression (de-repression) allowing for spontaneous induction of UGT1A1 gene expression. This finding may be useful in future studies to identify PXR modulators that might directly influence bilirubin homeostasis and accelerate bilirubin metabolism and clearance in children with abnormally high levels of TSB.
Supplementary Material
Acknowledgments
Financial Support: Funding for this work was provided by United States Public Health Service Grants GM086713 and the Superfund Research Program P42ES010337.
Abbreviations
- UGT
UDP glucuronosyltransferase
- UGT1
Human UGT1 locus
- Ugt1
murine Ugt1 locus
- PXR
pregnane X receptor
- CAR
constitutive androstane receptor
- CHIP
chromatin immunoprecipitation
- GD
gestational day
- TgUGT1
transgenic UGT1 mouse
- hUGT1
humanized UGT1 mouse
- ERα
estrogen receptor alpha
- TSB
Total serum bilirubin
Contributor Information
Shujuan Chen, Email: s18chen@ucsd.edu.
Mei-Fei Yueh, Email: mfyueh@ucsd.edu.
Ronald M Evans, Email: Evans@salk.edu.
Robert H. Tukey, Email: rtukey@ucsd.edu.
References
- 1.Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998 Oct 15;12(20):3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie W, Evans RM. Orphan nuclear receptors: the exotics of xenobiotics. J Biol Chem. 2001 Oct 12;276(41):37739–37742. doi: 10.1074/jbc.R100033200. [DOI] [PubMed] [Google Scholar]
- 3.Xie W, Yeuh MF, Radominska-Pandya A, Saini SPS, Negishi Y, Bottroff BS, et al. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci USA. 2003 Apr 1;100(7):4150–4155. doi: 10.1073/pnas.0438010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawamoto T, Kakizaki S, Yoshinari K, Negishi M. Estrogen activation of the nuclear orphan receptor CAR (constitutive active receptor) in induction of the mouse Cyp2b10 gene. Mol Endocrinol. 2000 Nov;14(11):1897–1905. doi: 10.1210/mend.14.11.0547. [DOI] [PubMed] [Google Scholar]
- 5.Timsit YE, Negishi M. CAR and PXR: the xenobiotic-sensing receptors. Steroids. 2007 Mar;72(3):231–246. doi: 10.1016/j.steroids.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosma PJ, Seppen J, Goldhoorn B, Bakker C, Oude ER, Chowdhury JR, et al. Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J Biol Chem. 1994 Jul 8;269:17960–17964. [PubMed] [Google Scholar]
- 7.Bacq Y, Zarka O, Brechot JF, Mariotte N, Vol S, Tichet J, et al. Liver function tests in normal pregnancy: a prospective study of 103 pregnant women and 103 matched controls. Hepatology. 1996 May;23(5):1030–1034. doi: 10.1002/hep.510230514. [DOI] [PubMed] [Google Scholar]
- 8.Jeong H, Choi S, Song JW, Chen H, Fischer JH. Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008 Jan;38(1):62–75. doi: 10.1080/00498250701744633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harden CL. Pregnancy Effects on Lamotrigine Levels. Epilepsy Curr. 2002 Nov;2(6):183. doi: 10.1046/j.1535-7597.2002.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Yang K, Choi S, Fischer JH, Jeong H. Up-regulation of UDP-glucuronosyltransferase (UGT) 1A4 by 17beta-estradiol: a potential mechanism of increased lamotrigine elimination in pregnancy. Drug Metab Dispos. 2009 Sep;37(9):1841–1847. doi: 10.1124/dmd.109.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boo NY, Wong FL, Wang MK, Othman A. Homozygous variant of UGT1A1 gene mutation and severe neonatal hyperbilirubinemia. Pediatr Int. 2009 Aug;51(4):488–493. doi: 10.1111/j.1442-200X.2008.02798.x. [DOI] [PubMed] [Google Scholar]
- 12.Kawade N, Onishi S. The prenatal and postnatal development of UDP-glucuronyltransferase activity towards bilirubin and the effect of premature birth on this activity in the human liver. Biochem J. 1981 Apr 15;196(1):257–260. doi: 10.1042/bj1960257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coughtrie MW, Burchell B, Leakey JE, Hume R. The inadequacy of perinatal glucuronidation: immunoblot analysis of the developmental expression of individual UDP-glucuronosyltransferase isoenzymes in rat and human liver microsomes. Mol Pharmacol. 1988;34:729–735. [PubMed] [Google Scholar]
- 14.Chen S, Beaton D, Nguyen N, Senekeo-Effenberger K, Brace-Sinnokrak E, Argikar U, et al. Tissue-specific, inducible, and hormonal control of the human UDP-glucuronosyltransferase-1 (UGT1) locus. J Biol Chem. 2005 Nov 11;280(45):37547–37557. doi: 10.1074/jbc.M506683200. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara R, Nguyen N, Chen S, Tukey RH. Developmental hyperbilirubinemia and CNS toxicity in mice humanized with the UDP glucuronosyltransferase 1 (UGT1) locus. Proc Natl Acad Sci U S A. 2010 Mar 1;107(11):5024–5029. doi: 10.1073/pnas.0913290107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen N, Bonzo JA, Chen S, Chouinard S, Kelner M, Hardiman G, et al. Disruption of the Ugt1 locus in mice resembles human Crigler-Najjar type I disease. J Biol Chem. 2008 Jan 7;283:7901–7911. doi: 10.1074/jbc.M709244200. [DOI] [PubMed] [Google Scholar]
- 17.Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000 Jul 27;406(6794):435–439. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- 18.Strassburg CP, Oldhafer K, Manns MP, Tukey RH. Differential expression of the UGT1A locus in human liver, biliary, and gastric tissue: identification of UGT1A7 and UGT1A10 transcripts in extrahepatic tissue. Mol Pharmacol. 1997 Aug;52(2):212–220. doi: 10.1124/mol.52.2.212. [DOI] [PubMed] [Google Scholar]
- 19.Okino ST, Quattrochi LC, Pookot D, Iwahashi M, Dahiya R. A dioxin-responsive enhancer 3′ of the human CYP1A2 gene. Mol Pharmacol. 2007 Dec;72(6):1457–1465. doi: 10.1124/mol.107.039826. [DOI] [PubMed] [Google Scholar]
- 20.Albert C, Vallee M, Beaudry G, Belanger A, Hum DW. The monkey and human uridine diphosphate-glucuronosyltransferase UGT1A9, expressed in steroid target tissues, are estrogen-conjugating enzymes. Endocrinology. 1999 Jul;140(7):3292–3302. doi: 10.1210/endo.140.7.6853. [DOI] [PubMed] [Google Scholar]
- 21.Wilson MD, Odom DT. Evolution of transcriptional control in mammals. Curr Opin Genet Dev. 2009 Nov 11; doi: 10.1016/j.gde.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Prud’homme B, Gompel N, Carroll SB. Emerging principles of regulatory evolution. Proc Natl Acad Sci U S A. 2007 May 15;104(Suppl 1):8605–8612. doi: 10.1073/pnas.0700488104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong H, Choi S, Song JW, Chen H, Fischer JH. Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008 Jan;38(1):62–75. doi: 10.1080/00498250701744633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blumberg B, Kang H, Bolado J, Jr, Chen H, Craig AG, Moreno TA, et al. BXR, an embryonic orphan nuclear receptor activated by a novel class of endogenous benzoate metabolites. Genes Dev. 1998 May 1;12(9):1269–1277. doi: 10.1101/gad.12.9.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore JT, Kliewer SA. Use of the nuclear receptor PXR to predict drug interactions. Toxicology. 2000 Nov 16;153(1–3):1–10. doi: 10.1016/s0300-483x(00)00300-0. [DOI] [PubMed] [Google Scholar]
- 26.Sugatani J, Nishitani S, Yamakawa K, Yoshinari K, Sueyoshi T, Negishi M, et al. Transcriptional regulation of human UGT1A1 gene expression: activated glucocorticoid receptor enhances constitutive androstane receptor/pregnane X receptor-mediated UDP-glucuronosyltransferase 1A1 regulation with glucocorticoid receptor-interacting protein 1. Mol Pharmacol. 2005 Mar;67(3):845–855. doi: 10.1124/mol.104.007161. [DOI] [PubMed] [Google Scholar]
- 27.Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005 Aug;42(2):420–430. doi: 10.1002/hep.20784. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Staudinger JL, Klaassen CD. Nuclear receptor, pregname X receptor, is required for induction of UDP-glucuronosyltranferases in mouse liver by pregnenolone-16 alpha-carbonitrile. Drug Metab Dispos. 2003 Jul;31(7):908–915. doi: 10.1124/dmd.31.7.908. [DOI] [PubMed] [Google Scholar]
- 29.Wilson MD, Barbosa-Morais NL, Schmidt D, Conboy CM, Vanes L, Tybulewicz VL, et al. Species-specific transcription in mice carrying human chromosome 21. Science. 2008 Oct 17;322(5900):434–438. doi: 10.1126/science.1160930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odom DT, Dowell RD, Jacobsen ES, Gordon W, Danford TW, MacIsaac KD, et al. Tissue-specific transcriptional regulation has diverged significantly between human and mouse. Nat Genet. 2007 Jun;39(6):730–732. doi: 10.1038/ng2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brites D, Fernandes A, Falcao AS, Gordo AC, Silva RF, Brito MA. Biological risks for neurological abnormalities associated with hyperbilirubinemia. J Perinatol. 2009 Feb;29(Suppl 1):S8–13. doi: 10.1038/jp.2008.214. [DOI] [PubMed] [Google Scholar]
- 32.Watchko JF, Lin Z. Exploring the genetic architecture of neonatal hyperbilirubinemia. Semin Fetal Neonatal Med. 2010 Jun;15(3):169–175. doi: 10.1016/j.siny.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Valaes T, Kipouros K, Petmezaki S, Solman M, Doxiadis SA. Effectiveness and safety of prenatal phenobarbital for the prevention of neonatal jaundice. Pediatr Res. 1980 Aug;14(8):947–952. doi: 10.1203/00006450-198008000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Murki S, Dutta S, Narang A, Sarkar U, Garewal G. A randomized, triple-blind, placebo-controlled trial of prophylactic oral phenobarbital to reduce the need for phototherapy in G6PD-deficient neonates. J Perinatol. 2005 May;25(5):325–330. doi: 10.1038/sj.jp.7211258. [DOI] [PubMed] [Google Scholar]
- 35.Arya VB, Agarwal R, Paul VK, Deorari AK. Efficacy of oral phenobarbitone in term “at risk” neonates in decreasing neonatal hyperbilirubinemia: a randomized double-blinded, placebo controlled trial. Indian Pediatr. 2004 Apr;41(4):327–332. [PubMed] [Google Scholar]
- 36.Allen JW, Tommarello S, Carcillo J, Hansen TW. Effects of endotoxemia and sepsis on bilirubin oxidation by rat brain mitochondrial membranes. Biol Neonate. 1998;73(5):340–345. doi: 10.1159/000013994. [DOI] [PubMed] [Google Scholar]
- 37.Madarek EO, Najati N. The effect of glucocorticoid therapy in preventing early neonatal complications in preterm delivery. J Perinat Med. 2003;31(5):441–443. doi: 10.1515/JPM.2003.069. [DOI] [PubMed] [Google Scholar]
- 38.Li CW, Dinh GK, Chen JD. Preferential physical and functional interaction of pregnane X receptor with the SMRTalpha isoform. Mol Pharmacol. 2009 Feb;75(2):363–373. doi: 10.1124/mol.108.047845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson DR, Li CW, Chen LY, Ghosh JC, Chen JD. Regulation and binding of pregnane X receptor by nuclear receptor corepressor silencing mediator of retinoid and thyroid hormone receptors (SMRT) Mol Pharmacol. 2006 Jan;69(1):99–108. doi: 10.1124/mol.105.013375. [DOI] [PubMed] [Google Scholar]
- 40.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995 Oct 5;377(6548):454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 41.Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995 Oct 5;377(6548):397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.