Abstract
Taste loss in human patients following radiotherapy for head and neck cancer is a common and significant problem, but the cellular mechanisms underlying this loss are not understood. Taste stimuli are transduced by receptor cells within taste buds, and like epidermal cells, taste cells are regularly replaced throughout adult life. This renewal relies on a progenitor cells adjacent to taste buds, which continually supply new cells to each bud. Here we treated adult mice with a single 8 Gy dose of X-ray irradiation to the head and neck, and analyzed taste epithelium at 1–21 days post-irradiation (dpi). We found irradiation targets the taste progenitor cells, which undergo cell cycle arrest (1–3 dpi) and apoptosis (within 1 dpi). Taste progenitors resume proliferation at 5–7 dpi, with the proportion of cells in S and M phase exceeding control levels at 5–6 and 6 dpi, respectively, suggesting that proliferation is accelerated and/or synchronized following radiation damage. Using BrdU birthdating to identify newborn cells, we found that the decreased proliferation following irradiation reduces the influx of cells at 1–2 dpi, while the robust proliferation detected at 6 dpi accelerates entry of new cells into taste buds. By contrast, the number of differentiated taste cells was not significantly reduced until 7 dpi. These data suggest a model where continued natural taste cell death, paired with temporary interruption of cell replacement underlies taste loss after irradiation.
Introduction
The sense of taste is mediated by taste buds in the oral cavity. Taste buds are multicelluar receptor organs containing 60–100 cells, which are continually renewed by progenitor cells located at the basement membrane and along the lateral margins of buds (Beidler and Smallman, 1965; Miura et al., 2003; Hamamichi et al., 2006). After their terminal division, immature taste cells enter buds, and differentiate into one of 3 taste cell types. Type I cells are glial-like, and express the glutamateaspartate transporter (GLAST) and the ecto-ATPase, NTPDase 2 (Pumplin et al., 1999; Lawton et al., 2000; Bartel et al., 2006); Type I cells may also function in salt taste transduction (Vandenbeuch et al., 2008). Type II or “receptor” cells transduce sweet, bitter and umami stimuli (Bernhardt et al., 1996; Miyoshi et al., 2001; Zhang et al., 2003; Clapp et al., 2006) and express transduction elements for these tastants, including: α-gustducin, phospholipase Cβ2 (PLCß2) (Boughter et al., 1997; Clapp et al., 2004); IP3R3 (Clapp et al., 2001); and Trpm5 (Clapp et al., 2006). Type III cells detect sour (Huang et al., 2006; Kataoka et al., 2008), are the only cell type to synapse with afferent nerves (Murray, 1986; Yee et al., 2001; Yang et al., 2007), and therefore can be considered “presynaptic” cells (Chaudhari and Roper, 2010). Type III cells express NCAM (Takeda et al., 1992), SNAP-25 (Yang et al., 2000) and Car4 (Chandrashekar et al., 2009), and accumulate serotonin (Huang et al., 2005; Dvoryanchikov et al., 2007). Despite differences in function, all 3 types are thought to live for 10–14 days (Farbman, 1980), and then undergo apoptosis (Zeng and Oakley, 1999; Zeng et al., 2000; Huang and Lu, 2001; Wang et al., 2007; Ichimori et al., 2009). In this way, cells within buds are continually renewed.
Taste dysfunction after radiotherapy for head and neck cancer is a common problem for patients (Schwartz et al., 1993; Vissink et al., 2003; Sandow et al., 2006; Yamashita et al., 2009). During a 6 to 8 week course of daily radiotherapy, taste loss typically occuring by 3–4 weeks, and all taste modalities are commonly affected (Mossman and Henkin, 1978; Maes et al., 2002; Ruo Redda and Allis, 2006; Sandow et al., 2006). Distorted taste, combined with other oral dysfunctions after irradiation (xerostomia, mucositis) can dramatically and negatively affect human nutrition (Donaldson, 1977; Jensen et al., 2003).
Three models have been proposed to explain irradiation-triggered taste dysfunction: (1) Neurites that innervate sensory organs are radiosensitive, thus disruption of the contact between taste cells and nerves leads to taste cell death; (2) Irradiation directly damages differentiated taste cells; and/or (3) Irradiation targets proliferating progenitors, interrupting production of new taste cells (Nelson, 1998; Yamazaki et al., 2009). Here we show that a single, moderate dose of irradiation causes immediate cell cycle arrest in taste progenitors, followed by disruption in the supply of new cells to taste buds, which results in reduced taste cell number a week after radiation exposure. We propose that disrupted taste cell renewal is the primary mechanism responsible for functional taste loss in patients receiving radiotherapy.
Methods
Animals
Two- to 4-month-old C57Bl/6 mice of either sex were used in all experiments. Mice were maintained and sacrificed in accordance with protocols approved by the Animal Care and Use Committee at the University of Colorado, School of Medicine.
Irradiation
X-ray irradiation was delivered via an RS 2000 Biological Irradiator. Before irradiation, the mice were anesthetized with fresh Avertin i.p. (0.5 mg/gram mouse), and the body was shielded with lead, leaving only the head and neck exposed. In pilot experiments, we found that the typical 16 Gy dose used by others (Nelson, 1998; Yamazaki et al., 2009) resulted in 50% mortality by 1 dpi, and all animals were dead within 2 weeks (data not shown). We also tested lower doses of 1, 2 or 4 Gy, which were well tolerated by the mice, and produced similar, but smaller effects than the selected 8 Gy dose. Eight Gy was selected for further investigation as this dose was not lethal over the experimental period of 21 days, yet triggered a robust response in taste epithelium. Dose administration was calculated and adjusted by a dosimeter.
Tissue preparation
Mice were anesthetized as above, and perfused transcardially with 4% paraformaldehyde in 0.1M phosphate buffer (PFA). Tongues were dissected free from the lower jaw, and postfixed in 4% PFA overnight at 4°C, followed by immersion in sucrose (20% in 0.1M PB) overnight at 4°C. Cryoprotected tongues were embedded in OCT compound (Tissue Tek) and cryosectioned at 12 µm. Sections were thaw-mounted and stored at −20°C overnight before staining.
Immunofluorescence for taste cells
Sections were rehydrated in 0.1M PBS for 30 minutes, and blocked with blocking solution (0.2M PB, 0.05M NaCl, 0.1% triton X-100; 1% bovine serum albumin) with 5% normal goat serum for 2 hours at room temperature (except for goat anti-Car4 antiserum). Sections were then incubated overnight at 4°C in primary antiserum diluted in blocking solution without goat serum. Antisera include: (1) rabbit anti-Gustducin (1:1000; catalog #sc-395, Santa Cruz); (2) rabbit anti-Trpm5 (1:1000; gift from Dr. Emily Liman, USC); or (3) goat anti-Car4 (1:1000; catalog #AF2414, R&D Systems). Sections were then washed with PBS for 2 hours, and incubated in the appropriate secondary antiserum (goat anti-rabbit Alexa Fluor 546 (1:1000; Invitrogen), or donkey anti-goat Alexa Fluor 546 (1:1000; Invitrogen) in blocking solution for 2 hours at room temperature. Sections were washed in 0.1M PBS for 2 hours, counterstained with Sytox (Invitrogen), mounted in Fluoromount G and coverslipped for analysis using fluorescence and confocal microscopy.
Immunofluorescence for cell cycle markers
The method for phospho-histone3 (pH3) immunostaining has been described (Nguyen & Barlow 2010). For Ki-67 immunofluorescence, sections were washed thrice in 0.1M PBS, treated with sodium citrate buffer (pH 6.0) at 95°C for 15 minutes, and cooled to room temperature for 30 minutes. Sections were then washed in PBS, incubated in blocking solution with 5% normal goat serum for 2 hours at room temperature, followed by 15 minutes each for avidin/biotin blocking solutions (A/B blocking kit; Vector Laboratories). Sections were then incubated in rabbit anti-Ki-67 antiserum (1:200; Thermo Scientific) overnight at 4°C. After 3 washes with PBS buffer for 1 hour each, sections were incubated with biotin-conjugated anti-rabbit IgG (Vector Laboratories) diluted 1:500 in PBS with 0.1% Tween 20 and 2.5% normal goat serum for 1 hour at room temperature. Sections were washed with PBS for 1 hour, then incubated in Streptavidin 546 (1:1000; Chemicon International) in PBS buffer for 2 hours at room temperature. After final washes in PBS for 1 hour, sections were counter-stained with Sytox green (Invitrogen) and mounted in Fluoromount G (Southern Biotech).
For BrdU immunofluorescence, sections were washed in 1T buffer (0.1M Tris pH 7.5, 0.15M NaCl), then incubated in a solution of 50% formamide and 5× SSC. After 3 washes in 1T buffer, sections were blocked in 1% blocking reagent in 1T buffer for 30 minutes at room temperature. Anti-BrdU monoclonal antibody (2μg/ml; catalog #10875400, Roche) was applied to tissues together with DNaseI (10UI/ml; Roche) for 50 minutes at 37°C. Sections were washed with 1T buffer, and incubated with goat-anti-mouse Alexa 546 for 1 hour at room temperature. After washing, sections were counter-stained with Sytox green and mounted.
TUNEL assay
TUNEL (Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) was performed using the In Situ Cell Death Detection kit TMR Red (Roche Applied Science). After 3 washes in PBS, sections were treated with 3% hydrogen peroxide in methanol, bathed in sodium citrate buffer (pH 6.0) at 95°C for 15 minutes, and then cooled to room temperature for 40 minutes. Sections were washed in PBS and permeabilized for 2 minutes on ice in a solution of 0.1% triton X-100 and 0.1% sodium citrate. Sections were incubated in blocking solution (20% normal goat serum, 3% bovine serum albumin, tris-HCl 50 mM at pH 7.5) for 30 minutes. After washing in PBS for 30 minutes, sections were incubated for 1 hour at 37°C with 10% enzyme solution diluted in buffer solution (Roche kit). Sections were washed in 0.1M PBS for 1 hour, counterstained with Sytox green (Invitrogen), mounted in Fluoromount G and coverslipped for analysis using fluorescence and confocal microscopy.
Birthdating of new taste cells
Mice were injected i.p. with 5-bromo-2-deoxyuridine (BrdU; Sigma; 120 mg/kg) twice, at 10 a.m. and at 1 p.m. For the 3 different series of birthdating experiments, BrdU injections were as follows: (i) the first BrdU dose was injected 6 hours before irradiation, and mice were then euthanized at 1 or 2 dpi (Figure 4); (ii) the first BrdU dose was injected at 5 dpi, and mice euthanized at 6 or 7 dpi (Figure 6A–E); or (iii) the first BrdU dose was injected at 6 dpi, and mice euthanized at 7 or 8 dpi (Figure 6F–J). Tongue cryosections (as above) from these mice were incubated in 90% methanol containing 3% hydrogen peroxide, washed in PBS, and then treated with 0.05% trypsin solution (37°C for 5 minutes). After washing in PBS, sections were treated with 4N HCl at 50°C for 15 minutes, then blocked with M.O.M. mouse Ig blocking reagent (Vector Laboratories; 1 hour at room temperature), and incubated overnight at 4°C with mouse anti-BrdU (1:500; catalog #G3G4, Developmental Studies Hybridoma Bank). After washing with PBS for 3 hours, sections were blocked in avidin/biotin blocking solutions (A/B blocking kit; Vector Laboratories), incubated for 60 minutes with biotin-conjugated anti-mouse IgG (1:500; Vector Laboratories), washed for 1 hour in PBS, and then incubated in ABC solution (Vector Laboratories) for 90 min at room temperature. Sections were washed for 1 hour, reacted with nickel-intensified DAB (Vector Laboratories) for 6–10 minutes, dehydrated with ethanol (50%, 70%, 95% and 100%), rinsed in xylene and coverslipped with Permount (Fisher Scientific). Newborn cells within taste buds were also identified using BrdU immunofluorescence and Sytox nuclear counterstain (see above).
Analysis
Immunofluorescent images were obtained on an Olympus BX50 laser scanning confocal microscope. Images consisting of projected Z series of 0.75 μm optical sections were processed with Fluoview v5.0 software. Nomarski images were obtained with a Zeiss Axioplan 2 microscope equipped with an Axiocam cooled CCD camera and Axiovision imaging software.
The data for this study were gathered from 3 to 6 mice per time point. All quantitative measures of cells within taste buds were taken from taste bud profiles with taste pores or the middle profile of taste buds based on serial sections. For the entire study, all tallies and cell position assignments were done blind with respect to treatment and animal. Slides were identified only by number, and not the details of experimental or control treatment. The data were decoded and analyzed only after all tallies had been made.
The labeling index for each proliferation marker was calculated by dividing the number of immunopositive cells for each marker e.g. Ki67 immunoreactive (Ki67-IR), BrdU-IR, or pH3-IR, by the total number of basal epithelial cells (identified and quantified via Sytox labeling). Only basal keratinocytes along the basement membrane and within the trenches of circumvallate papillae that contain taste buds (e.g. Figure 1, basal keratinocytes lying between the arrowheads) were counted.
For BrdU birthdating studies, the border of taste buds was determined by (i) Nomarski imaging and (ii) fluorescence of Sytox green-stained nuclei. In order to include counts from individual taste bud profiles, each profile had to meet the following criteria: 1) the taste bud profile extended from the base of epithelium to its apex; and 2) if a taste bud extended across multiple sections, the middle, and therefore largest profile was selected, so that the counted profiles represent approximately the center of each taste bud. Only taste buds with clear borders were tallied in our analyses (Figures 4 and 6, taste buds with outlines). We then counted the number of BrdU-IR nuclei within each taste bud. Edge cells with elongate nuclei were considered to be outside of taste buds, whereas recently generated (24–48 hrs) BrdU+ taste bud cells were located inside the basal compartment of each bud (e.g. Figures 4A,B and 6A,C,F). BrdU+ cells outside of taste buds were also found in the basal and suprabasal compartments of non-taste epithelium within the circumvallate papilla (CVP) (examples in all panels of Figures 4 and 6).
In pilot studies comparing the average nuclear diameter of control basal epithelial cells with those from irradiated mice at 1, 3 and 5 dpi, we found that nuclear size varied significantly with radiation exposure (one-way ANOVA, n=3 mice per time point, p = 0.02). Thus to normalize the data across experimental conditions, we used Abercrombie correction (Abercrombie, 1946) as follows: Z-stack projections with a total depth of 9.75 μm were obtained from 0.75 μm optical sections of 12 μm physical sections. The top and bottom optical sections were discarded to make sure tallied optical planes were physically complete, thus minimizing any problems due to variation in section thickness. The nuclear diameter parallel to the basal membrane, and thus perpendicular to the plane of section, of 20 randomly selected basal epithelial cells (enumerated and then selected via a random number generator) was measured and averaged. This average diameter for each condition was used in the Abercrombie formula using a section thickness of 9.75 μm. As cell cycle phase correlates with nuclear size, we reasoned that each marker class could comprise a subset of similarly sized nuclei whose average diameter would be distinct from that of the total basal population. In fact, we found that the average nuclear diameter for cells at different phases of the cell cycle, and thus the Abercrombie correction factor, was distinct from that of the Sytox population as a whole. Therefore, we elected to also correct counts for all markers at each time post irradiation.
A t-test or one-way ANOVA with a Tukey post hoc multiple comparisons test was used to analyze data after ascertaining the data were normally distributed using the Anderson-Darling test. Data are presented as mean+/− SD unless otherwise noted.
Results
Irradiation targets actively cycling cells
For our studies, we focused on the circumvallate papilla (CVP), a large structure located at the posterior midline of the mouse tongue. The papilla is surrounded bilaterally by deep, epithelial trenches, and each trench contains roughly 150 taste buds. Thus, the CVP provides an anatomically focused set of taste buds to monitor for the effects of radiation exposure, including taste progenitor proliferative activity. Several immunomarkers were used to identify proliferating cells; 1) Ki-67, expressed by cells in all phases of cell cycle except early G1 and G0 (Schwarting et al., 1986); 2) BrdU, incorporated in the DNA of cells in S phase (Burns et al., 2006); and 3) phosphoHistone 3 (pH3), a marker for cells in M phase (Norbury and Nurse, 1992). We first assessed proliferation in the CVP taste epithelium of control mice to establish baseline activity. Controls (sham-irradiated) were anesthetized and placed in conical tubes, but not irradiated. We found that approximately 80% of basal epithelial cells are Ki-67 immunopositive in control CVP (Figure 1A, E), with subsets in S phase (15%), indicated by BrdU immunoreactivity assessed at 6 hours post-injection (Figures 2A,I) and M phase (12%; pH3-IR; Figure 2E, J). No dividing cells were observed inside taste buds, consistent with published reports (Beidler and Smallman, 1965; Conger and Wells, 1969; Farbman, 1980; Miura et al., 2003; Hamamichi et al., 2006; but see Sullivan et al., 2010).
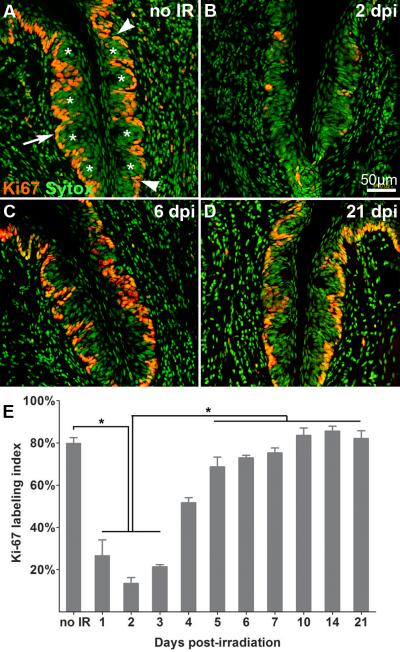
Figure 1. Irradiation targets actively cycling cells.
Proliferative cells in taste epithelium are marked by Ki67-IR (red) and nuclei are visualized with Sytox (green). Compared with controls (A), the number of basal proliferative cells is significantly reduced at 1–3 dpi (B, E), then returns to and is maintained at control levels from 5 to 21 dpi (C: 6dpi; D: 21 dpi). White asterisks indicate taste buds. White arrows indicate active cycling cells in basal epithelium. Total basal epithelial cell nuclei and Ki67-IR cells were counted between the arrowheads in each trench. E: Bar graph of the proportion of Ki67-IR basal epithelial cells at progressive time points after irradiation (Mean +/− SD; n = 3–5 mice for each time; one-way ANOVA, Tukey post hoc test, * p < 0.01.
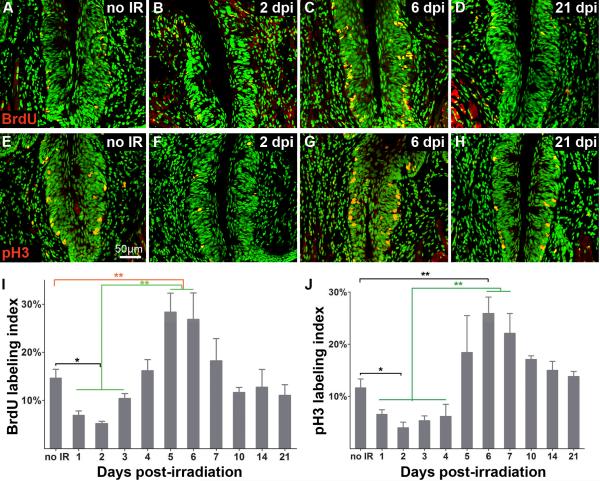
Figure 2. The proportions of cells in S and M phase drop following irradiation, then overshoot control levels, before returning to normal values.
Cells in S phase in controls and at selected times post-irradiation are identified via nuclear incorporation of BrdU (A–D; BrdU-IR; red) and those in M phase are immunoreactive for phospho Histone3 (E–H; pH3-IR, red). A, B, C, D: sham-irradiated controls, 2, 6, and 21 dpi. E, F, G, H: sham irradiated controls, 2, 6, and 21 dpi. Histograms for S (I) and M (J) phase show labeling indices of sham-irradiated epithelium versus that of epithelium harvested at progressive times post-irradiation. The proportion of basal cells in both S and M phases are significantly decreased at 2 dpi. Significantly more basal cells are in S phase at 5–6 dpi compared with controls; the proportion in M phase also overshoots controls at 6 dpi (G, J). Both indices are comparable to controls from 7 dpi onward. one-way ANOVA, Tukey post hoc test; n = 3–4 mice for each time point; * p < 0.05; ** p < 0.01.
We next quantified proliferation in taste epithelia at progressive days post-irradiation, and compared these values to those of controls. The labeling index for Ki-67 is significantly reduced compared to controls during the first 3 dpi (Figure 2B,E). However, the proportion of dividing cells returns to control levels by 5 dpi, and remains at control levels through 21 dpi (Figure 1C–E). These data demonstrate a clear and rapid effect of irradiation on proliferative activity in the taste progenitor cell population. To further evaluate the effects of irradiation on specific phases of the cell cycle, we monitored the labeling indices for BrdU at 6 hours post-injection (S phase) and pH3 (M phase). In sham irradiated taste tissue, 14.6% of basal cells have incorporated BrdU and 11.6% are in M phase. After irradiation, the labeling indices for cells in S and M phases are decreased significantly (5.2% and 4% at 2 dpi, respectively; Figure 2), which is followed by an overshoot in the proportion of cells in S and M phases at 5–6 dpi (Figure 2C, I) and 6 dpi (Figure 2G, J), respectively, compared with controls. Labeling indices for cells in S and M phases then return to control levels by 7 dpi (Figure 2D, H, I, J). This biphasic response of proliferating taste progenitor cells to irradiation suggests that proliferation of progenitor cells in the CVP is synchronized and/or accelerated following recovery from radiation.
Irradiation induces cell death in taste epithelium
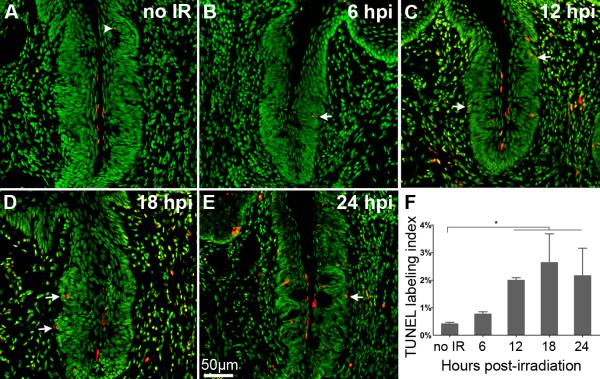
In skin and intestinal epithelium, irradiation induces double stranded breaks in nuclear DNA, which results in mitotic delay, as cells enter cell cycle arrest and attempt to repair damaged DNA (Harper and Elledge, 2007). Cells that fail to adequately repair then undergo apoptosis. In another oral tissue, the salivary gland, irradiation results in maximal cell death within 24 hours (Humphries et al., 2006). Thus, we assessed cell death in taste epithelium via TUNEL at 6, 12, 18, and 24 hours post-irradiation (hpi). In non-irradiated control tissues, only 0.4% of basal epithelial cells are TUNEL-positive, with only an occasional apoptotic nucleus detected inside a taste bud (Figure 3A, arrowhead), consistent with published reports (Wang et al., 2007). After irradiation, the TUNEL labeling index of basal epithelial cells is significantly increased at 12 hpi, and peaks at 18 hpi (Figure 3B–E, white arrows indicate examples). Although TUNEL labeled only 2.6% of basal epithelial cells at the peak of cell death, this still represents a roughly 5-fold increase over uninjured control levels. Further, considering that TUNEL only reveals the last, brief phase of apoptosis (Negoescu et al., 1998), the total number of dying cells is likely much larger. Relatively low, but nonetheless functionally significant, levels of cell death after irradiation have also been reported in epidermis, oral mucosa, and dental stem cells (Morris and Hopewell, 1988; Morris et al., 1993). Importantly, we detected few apoptotic nuclei within irradiated buds (Figure 3), further suggesting that radiation specifically and initially impacts the dividing taste progenitor cells that reside outside of taste buds. At later time points following radiation, cell death levels resembled that of controls. Specifically, examination of TUNEL-stained CVP sections at 3 and 7 days post-irradiation revealed levels of apoptosis which were no different than controls (Sham irradiated:0.42+/−0.06%; 3 dpi: 0.48+/− 0.19%; 7 dpi: 0.56+/− 0.20% TUNEL+ basal epithelial cells per CVP, one-way ANOVA, p=0.11, n=3 mice per time point).
Figure 3. Irradiation induces apoptosis in basal keratinocytes of the taste epithelium.
TUNEL (red) and Sytox (green) in sham irradiated (A), and at 6 (B), 12 (C),18 (D), and 24 (E) hours post irradiation (hpi). In sham irradiated controls, very few apoptotic cells are observed in taste epithelium; TUNEL positive cells, when present, were located within taste buds (white arrowhead). Following irradiation, the number of apoptotic cells increased, and most were located at the basement membrane (white arrows point to examples). (F) The number of TUNEL+ cells peaked at 18 hpi. one-way ANOVA, Tukey post hoc test; n=3–5 mice per time point; * p < 0.01.
Taste bud cells are indirectly affected by irradiation
Within 24 hours of their terminal division within the progenitor pool, post-mitotic taste precursor cells move into taste buds, and then, within 2–6 days following birth, differentiate into mature taste cells (Beidler and Smallman, 1965; Cho et al., 1998; Hamamichi et al., 2006; Miura et al., 2006; Oike et al., 2006; Nguyen and Barlow, 2010). As irradiation induces cell cycle arrest and apoptosis of taste progenitor cells (Figures 1–3), we hypothesized that this cessation of proliferation would lead to a decreased influx of cells into taste buds. To evaluate this possibility, we injected mice with BrdU 6 hours before irradiation, and then compared the number of newborn cells within taste buds of control and irradiated mice that had been sacrificed 1 or 2 days after irradiation (Figure 4). The number of BrdU-IR cells inside irradiated taste buds is virtually half that found in controls at both 1 dpi (mean: 0.31 vs. 0.59 cells per taste bud profile) and 2 dpi (mean: 0.34 vs. 0.69 cells per taste bud profile), indicating that the influx of cells into taste buds is significantly decreased following irradiation.
Figure 4. The influx of new cells into taste buds is reduced during the first 2 days following irradiation.
Mice were injected i.p. with BrdU 6 hours before irradiation. BrdU-IR (dark brown) cells inside taste buds were tallied at 1 dpi (A, B) and 2 dpi (C, D) in irradiated and sham-irradiated control mice. Newborn cells inside taste buds were significantly decreased at 1 dpi and 2 dpi compared with controls (E). Dashed lines indicate the borders of individual taste buds. White asterisks: newborn cells inside taste buds marked by BrdU birthdating. N=3–4 mice and 90–176 taste buds per condition and time point. T-test, * p<0.05.
Next, we tested if differentiated taste receptor cells are directly or indirectly affected by irradiation. If a single 8 Gy dose targets taste cells directly, we expect that the number of taste cells would decrease within the first few days after irradiation exposure. However, if the effect of irradiation on taste receptor cells is indirect, i.e., due to a reduction of newborn cells entering taste buds with continued natural cell death of relatively short lived taste cells, we would expect that the number of taste cells would not decrease until later time points following irradiation. To evaluate these possibilities, we compared the number of two types of differentiated cells in irradiated and control taste buds: Type II receptor cells recognized via gustducin- (Figure 5A–D) or Trpm5-IR (Figure 5E–H); and Type III presynaptic cells that are Car4-IR (Figure 5I–L). We found that the numbers of Type II and III cells did not differ from control values at the early post-irradiation time point (3dpi), but both cell types were decreased at 7 dpi (Figure 5M–O), suggesting that the effects of irradiation on taste cells are indirect. We also tried to quantify Type I taste cells via NTPDase2-IR, but because of the complex morphology of Type I cells and the localization of NTPdase2 protein at the membrane (Bartel et al., 2006), we could not reliably identify and tally individual Type I cells (data not shown). To rule out that the reduction in Type II and III cells was due to broad damage of the circumvallate taste papilla, or to total destruction of a subset of taste buds by radiation exposure, we also compared the height of the CVP, and the number of resident taste buds per circumvallate papilla section, and found no significant differences from controls at 3 dpi or 7 dpi (CVP height: sham irradiated – 459.77 +/− 40.11 μm; 3 dpi – 453.95 +/− 52.36 μm; 7 dpi – 475.00 +/− 44.23μm, one way ANOVA p= 0.31. Number of taste buds per CVP trench profile: sham irradiated – 8.50 +/− 1.69; 3 dpi – 7.95 +/− 1.15; 7 dpi – 7.60 +/− 1.30, one way ANOVA p=0.104. N=3 mice per time point).
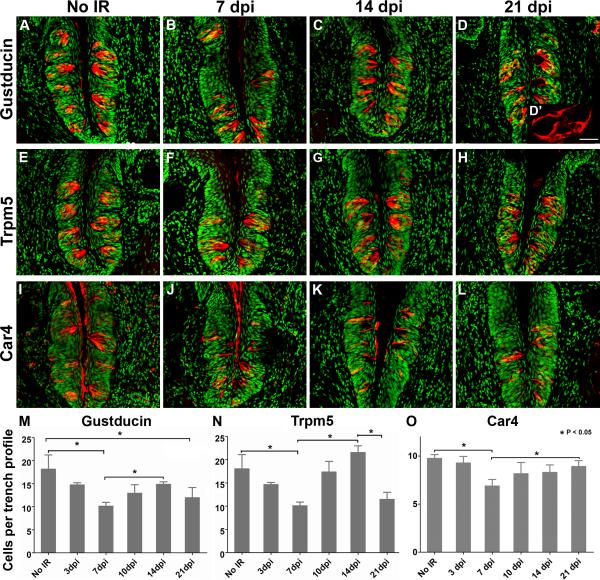
Figure 5. Loss and recovery of differentiated Type II and III taste cells is delayed compared with the immediate effect of irradiation on proliferation.
Both Type II (Gustducin-IR; Trpm5-IR - red) and Type III cells (Car4-IR - red) are significantly reduced at 7 dpi, but not 3 dpi. Type II and III cell numbers return to control levels by 14 and 10 dpi, respectively. At 21 dpi, both gustducin-IR and Trpm5-IR Type II cells are lost (M,N), while number of Type III cells remains comparable to that of controls (O). Gustducin-IR (A–D), Trpm5-IR (E–H), Car4-IR (I–L) and Sytox (all panels; green). M, N, O: Histograms of the number of Gustducin-, Trpm5- and Car4-IR cells, respectively, in sham-irradiated control versus irradiated CVPs at progressive days post irradiation. High magnification of gustducin-IR cells at 21 dpi shows their abnormal morphology (D'). One-way ANOVA, Tukey post hoc test; * p < 0.05. N=3–4 mice per time point. A total of 134–349 taste buds was counted per time point and per taste cell type marker. Scale bar (except D'): 50 μm. Scale bar for D': 20 μm.
The number of Type III cells in irradiated taste buds returned to normal by 10 dpi, and remained constant through 21 dpi (Figure 5K, L, O), consistent with the normal proliferative indices we observed at these later time points (Figures 1, 2). The number of Type II cells is also comparable to controls by 14 dpi (Figure 5C, G, M, N). These findings are broadly similar to those from (Yamazaki et al., 2009), who find reduced numbers of Type II cells between days 8–20, but no significant reduction in Type III cells during the same period following a single dose of 15Gy. However, at 21 dpi we observed an apparent second wave of Type II cell loss (Figure 5D, H, M, N), and the remaining Type II cells had somewhat abnormal morphology at this late time point (Figure 5D').
Taste cell regeneration
Following irradiation, proliferative activity resumes at 5 dpi with higher than normal proportions of cells in S and M phases (Figures 1, 2). To test if and how this accelerated and/or synchronized proliferation contributes to taste bud regeneration following radiation damage, we tallied the number of newborn taste bud cells in mice that were injected with BrdU, as proliferation resumed at 5 or 6 dpi (Figure 6). The influx of cells into taste buds was comparable to controls at 6 and 7 dpi following BrdU injection at 5 dpi (Figure 6A–D, I). Interestingly, when we injected BrdU at 6 dpi, significantly more newborn cells were detected in taste buds of irradiated mice than in controls at 24 hours post-injection (7dpi, Figure 6J; p > 0.05). These data suggest that the transient increase in cells in S and M phase following irradiation also results in a pulsed increase in the supply of new taste receptor cells to taste buds.
Figure 6. The influx of new cells into taste buds resumes and is accelerated at 6 days post-irradiation.
A–D. BrdU was injected at 5 dpi, and newborn cells inside taste buds quantified at 6 dpi (A, B) and 7 dpi (C, D) in irradiated and sham-irradiated groups. E–H. BrdU was injected at 6dpi, and newborn cells assessed at 7 dpi (E, F) and 8 dpi (G, H) irradiated and control CVP taste buds. Dashed lines indicate borders of taste buds. White asterisks mark newborn cells inside taste buds. The influx of cells into taste buds was significantly accelerated at 7 dpi, assayed at 24 hours following BrdU injection at 6 dpi (J), but not at any other time points (I, J). T-test, n=3 mice, and 60–183 taste buds tallied per time point and condition, * p < 0.05.
Discussion
Taste loss is a common problem for head and neck cancer patients following radiotherapy. Here we show that a moderate dose of radiation to the head and neck of adult mice directly affects the taste progenitor cells responsible for continual supply of new cells to taste buds. Within 24 hours of irradiation, actively cycling progenitors are dramatically reduced (Figures 1–2); concomitantly, apoptosis of progenitors is significantly increased (Figure 3). Cell cycle arrest persists for several days, as indicated by significant drops in the proliferative, S and M phase indices, while apoptosis in taste progenitors, which likely occurs in cells that fail to repair irradiation-induced DNA damage (Zhou and Sausville, 2003; Nakanishi et al., 2009), peaks within 24 hours (Figure 3). Both cell cycle arrest and death of progenitors likely contribute to the transient interruption in supply of new taste cells (Figure 4), but this dearth in cells is not apparent until several days later, when differentiated taste cells are reduced (Figure 5). In sum, irradiation targets primarily the progenitor pool, which results in a transient but delayed reduction in functional taste cells, as aged taste cells are naturally lost, but are not immediately replaced (see Figure 7).
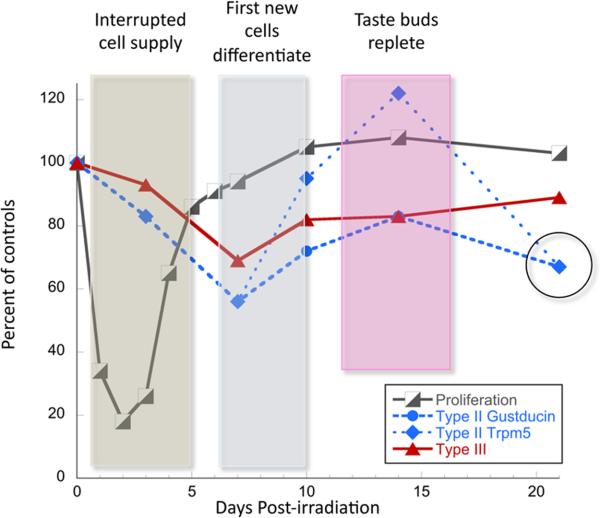
Figure 7. Summary and a model for taste epithelial cell loss and regeneration following irradiation injury.
In the first several days following irradiation, taste progenitors arrest the cell cycle (black and white boxes) and their contribution of new cells to taste buds is interrupted (blue box). During this time, the number of both Type II (blue squares and circles) and Type III (red triangles) cells trend downward, and are significantly reduced by day 7 following radiation. We propose that this loss is due to natural attrition of aged taste cells, which are not replaced by the mitotically arrested progenitor cells. As progenitors resume proliferation, new taste precursor cells enter buds, but these cells require an additional 3–5 days to differentiate (grey box), after which taste cell numbers recover to control values (pink box). Unexpectedly, a 2nd wave of loss of Type II taste cells only occurs at 21 days post-irradiation (black open circle), which may reflect synchronous, albeit naturally timed death of the cohort of Type II cells generated by the burst of proliferative activity ~14 days prior. The data from Figures 1 and 5 are shown as percent of controls for this summary diagram.
Following irradiation-induced arrest, proliferation resumes with an accelerated and/or synchronized cell cycle
Tissue-level responses to ionizing radiation depend on the rate of cell renewal (Etoh et al., 1977; Morris, 1996; Wilson, 2007). In human skin, with a 26–75 day turnover, proliferation lags for 4 weeks following radiation, whereas in mouse lip epithelium, which renews in 2–3 days, proliferative arrest lasts 1–2 days (Dorr et al., 1994; Wardley et al., 1998; Potten et al., 2002; Dorr). Taste cells renew every 10–14 days (Beidler and Smallman, 1965; Farbman, 1980), a rate congruent with our finding of an intermediate time frame for cell cycle arrest in taste epithelium, i.e., 4–5 days.
When proliferation resumes in irradiated taste epithelium, the proportion of cells in S and M phase increases to levels significantly higher than controls. Similarly, higher proportions of S and M phase cells are observed during regeneration of other tissues following irradiation (Morris, 1996; Dorr et al., 2000). If the regenerative response of taste epithelium is similar to that of other tissues, then increased S and M phase basal cells is due either to production of additional taste bud stem cells (tbSCs) via symmetric divisions, genesis of synchronized transit amplifying cells (TACs) from tbSCs, e.g. (Lehrer et al., 1998), and/or a shortened cell cycle for TACs (Kavanagh et al., 1995; Ristic-Fira et al., 2007; Dorr, 2003). Distinguishing between these mechanisms, however, is not yet possible, as to date, there are no molecular or functional markers that discriminate tbSCs from TACs (Okubo et al., 2009). Nonetheless, we conclude that proliferation of taste progenitors in general is enhanced during regeneration following damage.
Taste loss is likely due to interruption in delivery of new taste cells combined with continuing natural taste cell loss
Several models have been proposed to account for taste dysfunction following radiotherapy. Irradiation may damage nerve fibers that innervate taste buds, causing taste cell death indirectly (Conger and Wells, 1969; Nelson, 1998), as maintenance of mature taste cells requires nerve contact (Sollars et al., 2002; Miura et al., 2004; Oakley and Witt, 2004). However, at least when examined grossly at the light microscopic level, taste bud innervation appears unperturbed (Conger and Wells, 1969; Yamazaki et al., 2009). Additionally, radiation damage of salivary glands leads to severe hyposalivation, which may contribute to lessened taste acuity (de Graeff et al., 2000; Chambers et al., 2004). However, deficient salivary function is likely not a major driver of taste loss, as taste and salivary gland symptoms vary independently (see Porter et al., 2010, and references therein). In oral mucosa in general (Dorr and Weber-Frisch, 1995; Wardley et al., 1998; Potten et al., 2002), and now specifically in taste epithelium, irradiation targets proliferating cells, resulting in an insufficient supply of new cells to the epithelium and taste buds, respectively. Specifically, we find that two specific taste cell types are reduced 7 days after irradiation: Type II cells, which transduce sweet, bitter and umami (Adler et al., 2000; Nelson et al., 2001; Zhang et al., 2003), and Type III cells, which mediate sour (Huang et al., 2006) and synapses with taste afferents (Chaudhari and Roper, 2010). The timing for loss of taste cells is broadly congruent with the onset of functional taste loss in patients, which is first observed after 1 week of radiotherapy (Mossman and Henkin, 1978), with more broad taste dysfunction in patients by the 3rd – 4th weeks (Ruo Redda and Allis, 2006; Yamashita et al., 2006; Yamashita et al., 2009; Epstein and Barasch, 2010). In rodent models, changes in sucrose and sodium taste behavioral preferences are also detectable in the first and second weeks following radiation (Yamazaki et al., 2009; Nelson, 1998). However, these studies each entailed delivery of 15–17 Gy to the head, which can cause widespread tissue damage; thus in addition to targeting mitotic progenitors, post-mitotic taste cells may also have been damaged directly (Shatzman and Mossman, 1982; Urek et al., 2005; and see Figure 1 in Yamazaki et al., 2009).
Following radiation, tissues typically recover at a pace consistent with the proliferative characteristics of the tissue (Wilson, 2007). For example, regeneration of ventral tongue epithelium following radiation is complete within 15 days (Dorr and Kummermehr, 1991). Similarly, in taste epithelium, the influx of new cells into taste buds recovers to control levels 1 week after irradiation, and the numbers of Type III and II taste cells return to control levels by 10 and 14 dpi, respectively. How does this time frame compare with the timing of taste cell differentiation from the progenitor pool?
Following the last division within the progenitor domain, postmitotic precursors enter taste buds within 12–24 hours (Miura et al., 2006; Asano-Miyoshi et al., 2008; Nguyen and Barlow, 2010), and differentiation of Type II taste cells is complete between 3-6 days of birth (Cho et al., 1998; Oike et al., 2006); by contrast, little is known of the rate of renewal for cell Types I and III. Assuming, however, that each cell lives 10–14 days (Beidler and Smallman, 1965; Farbman, 1980), and taste buds in mice comprise ~60 cells (Ma et al., 2007), then 4–6 new cells must be both added and lost each day to maintain taste cell complement. Thus, when the supply of new cells is interrupted for 3–4 days by radiation (Figure 7; blue box), the first loss of differentiated cells is not detected until oldest taste cells (born before irradiation) die on their normal schedule (Zeng and Oakley, 1999; Zeng et al., 2000), while cells that should have replaced them fail to do so (Figure 7; gray box). By 14 dpi, the new cells generated during the regeneration phase (5–7 dpi) have differentiated, hence returning taste buds to their full cell complement (Figure 7; pink box). We also observed a significant but smaller effect on Type III cells. The time needed for differentiation, as well as the lifespan, of Type III cells have not been defined. Nonetheless, reduction in Type III cells at 7 dpi is consistent with a 3–6 day requirement for differentiation.
Unexpectedly, an apparent second wave of Type II, but not Type III, taste cell loss was detected at 21 dpi (Figure 7; circle around Type II data points at 21 dpi). One possibility is that if Type II cells live 10–14 days, then a synchronous pulse of new cells into taste buds at 6–8 dpi (Figure 6) would lead to synchronous death of naturally aged Type II cells at 21 dpi. Is this late loss of Type II, but not Type III cells due to different cell type-specific longevities? For example, if Type III cells renew more slowly than Type II cells, as has been suggested (Farbman 1980), but are likewise synchronously generated during regeneration following irradiation, we would predict a second wave of Type III cell loss at a later time point following initial recovery. These hypotheses, however, remain to be tested.
Fractional dose radiotherapy likely amplifies the effects of irradiation on taste cell renewal
Fractionated radiotherapies are used uniformly to treat head and neck cancer. Typically 60–70 Gy are given over 6–8 weeks, at 1.8–2 Gy per day (Sandow et al., 2006; Kamprad et al., 2008). As a result of treatment, 90% of patients experience taste loss, and while taste function typically recovers, it can take months or years (Conger, 1973; Mossman, 1986; Maes et al., 2002; Shi et al., 2004). We propose that the primary impact of low dose radiation exposure is on taste progenitors, and this damage response is compounded by daily radiotherapy. We hypothesize that repeated, daily radiation results in activation, and therefore damage, of progressively more taste progenitor cells, and this both transiently accelerates and ultimately suppresses regeneration of taste epithelium. Eventually, fractional radiotherapy will lead to the death of most or even all taste progenitor cells, providing an explanation for why recovery of taste function following radiotherapy is slow, ranging from several months to several years, or can be permanent for head and neck cancer patients.
Acknowledgements
We thank Dr. Emily Liman (USC) for the Trpm5 antiserum, Jennifer K. Scott, Greg Banninger, and especially Brendan Ross for technical assistance, and Hirohito Miura for invaluable advice, good humor and expertise during his sabbatical year in the Barlow Lab. Thanks to Drs. Tom Finger, Sue Kinnamon, Dany Gaillard and Shoba Thirumangalathu for excellent comments on earlier versions of the manuscript, and to the Rocky Mountain Taste and Smell Center for mouse, imaging and statistical support, especially advice from Dr. Rob Hallock. This work was supported by R01 DC003947 to Linda Barlow, P30 DC004657 to Diego Restrepo, and a Vietnam Education Foundation fellowship to Ha Manh Nguyen.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. see comments. [DOI] [PubMed] [Google Scholar]
- Asano-Miyoshi M, Hamamichi R, Emori Y. Cytokeratin 14 is expressed in immature cells in rat taste buds. J Mol Histol. 2008;39:193–199. doi: 10.1007/s10735-007-9151-0. [DOI] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beidler LM, Smallman RL. Renewal of cells within taste buds. J Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt SJ, Naim M, Zehavi U, Lindemann B. Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J Physiol. 1996;490(Pt 2):325–336. doi: 10.1113/jphysiol.1996.sp021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughter JD, Jr., Pumplin DW, Yu C, Christy RC, Smith DV. Differential expression of alpha-gustducin in taste bud populations of the rat and hamster. J Neurosci. 1997;17:2852–2858. doi: 10.1523/JNEUROSCI.17-08-02852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns TC, Ortiz-Gonzalez XR, Gutierrez-Perez M, Keene CD, Sharda R, Demorest ZL, Jiang Y, Nelson-Holte M, Soriano M, Nakagawa Y, Luquin MR, Garcia-Verdugo JM, Prosper F, Low WC, Verfaillie CM. Thymidine analogs are transferred from prelabeled donor to host cells in the central nervous system after transplantation: a word of caution. Stem Cells. 2006;24:1121–1127. doi: 10.1634/stemcells.2005-0463. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Garden AS, Kies MS, Martin JW. Radiation-induced xerostomia in patients with head and neck cancer: pathogenesis, impact on quality of life, and management. Head Neck. 2004;26:796–807. doi: 10.1002/hed.20045. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, Ryba NJ, Zuker CS. The taste of carbonation. Science. 2009;326:443–445. doi: 10.1126/science.1174601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YK, Farbman AI, Smith DV. The timing of alpha-gustducin expression during cell renewal in rat vallate taste buds. Chem Senses. 1998;23:735–742. doi: 10.1093/chemse/23.6.735. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2001;2:6. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger AD. Loss and recovery of taste acuity in patients irradiated to the oral cavity. Radiat Res. 1973;53:338–347. [PubMed] [Google Scholar]
- Conger AD, Wells MA. Radiation and aging effect on taste structure and function. Radiat Res. 1969;37:31–49. [PubMed] [Google Scholar]
- de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Long-term quality of life of patients with head and neck cancer. Laryngoscope. 2000;110:98–106. doi: 10.1097/00005537-200001000-00018. [DOI] [PubMed] [Google Scholar]
- Donaldson SS. Nutritional consequences of radiotherapy. Cancer Res. 1977;37:2407–2413. [PubMed] [Google Scholar]
- Dorr W. Modulation of repopulation processes in oral mucosa: experimental results. Int J Radiat Biol. 2003;79:531–537. doi: 10.1080/09553002310001600925. [DOI] [PubMed] [Google Scholar]
- Dorr W, Kummermehr J. Proliferation kinetics of mouse tongue epithelium under normal conditions and following single dose irradiation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60:287–294. doi: 10.1007/BF02899559. [DOI] [PubMed] [Google Scholar]
- Dorr W, Weber-Frisch M. Repopulation response of mouse oral mucosa during unconventional radiotherapy protocols. Radiother Oncol. 1995;37:230–236. doi: 10.1016/0167-8140(95)01666-x. [DOI] [PubMed] [Google Scholar]
- Dorr W, Brankovic K, Hartmann B. Repopulation in mouse oral mucosa: changes in the effect of dose fractionation. Int J Radiat Biol. 2000;76:383–390. doi: 10.1080/095530000138727. [DOI] [PubMed] [Google Scholar]
- Dorr W, Emmendorfer H, Haide E, Kummermehr J. Proliferation equivalent of `accelerated repopulation' in mouse oral mucosa. Int J Radiat Biol. 1994;66:157–167. doi: 10.1080/09553009414551061. [DOI] [PubMed] [Google Scholar]
- Dvoryanchikov G, Tomchik SM, Chaudhari N. Biogenic amine synthesis and uptake in rodent taste buds. J Comp Neurol. 2007;505:302–313. doi: 10.1002/cne.21494. [DOI] [PubMed] [Google Scholar]
- Epstein JB, Barasch A. Taste disorders in cancer patients: pathogenesis, and approach to assessment and management. Oral Oncol. 2010;46:77–81. doi: 10.1016/j.oraloncology.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Etoh H, Taguchi YH, Tabachnick J. Cytokinetics of regeneration in beta-irradiated guinea-pig epidermis. Radiat Res. 1977;71:109–118. [PubMed] [Google Scholar]
- Farbman AI. Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 1980;13:349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Hamamichi R, Asano-Miyoshi M, Emori Y. Taste bud contains both short-lived and long-lived cell populations. Neuroscience. 2006;141:2129–2138. doi: 10.1016/j.neuroscience.2006.05.061. [DOI] [PubMed] [Google Scholar]
- Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Lu KS. TUNEL staining and electron microscopy studies of apoptotic changes in the guinea pig vallate taste cells after unilateral glossopharyngeal denervation. Anat Embryol, (Berl) 2001;204:493–501. doi: 10.1007/s429-001-8006-1. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, Wu D, Roper SD. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ, Limesand KH, Schneider JC, Nakayama KI, Anderson SM, Reyland ME. Suppression of apoptosis in the protein kinase Cdelta null mouse in vivo. J Biol Chem. 2006;281:9728–9737. doi: 10.1074/jbc.M507851200. [DOI] [PubMed] [Google Scholar]
- Ichimori Y, Ueda K, Okada H, Honma S, Wakisaka S. Histochemical changes and apoptosis in degenerating taste buds of the rat circumvallate papilla. Arch Histol Cytol. 2009;72:91–100. doi: 10.1679/aohc.72.91. [DOI] [PubMed] [Google Scholar]
- Jensen SB, Pedersen AM, Reibel J, Nauntofte B. Xerostomia and hypofunction of the salivary glands in cancer therapy. Support Care Cancer. 2003;11:207–225. doi: 10.1007/s00520-002-0407-7. [DOI] [PubMed] [Google Scholar]
- Kamprad F, Ranft D, Weber A, Hildebrandt G. Functional changes of the gustatory organ caused by local radiation exposure during radiotherapy of the head-and-neck region. Strahlenther Onkol. 2008;184:157–162. doi: 10.1007/s00066-008-1780-z. [DOI] [PubMed] [Google Scholar]
- Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sevigny J, Kinnamon JC, Finger TE. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses. 2008;33:243–254. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh BD, Lin PS, Chen P, Schmidt-Ullrich RK. Radiation-induced enhanced proliferation of human squamous cancer cells in vitro: a release from inhibition by epidermal growth factor. Clin Cancer Res. 1995;1:1557–1562. [PubMed] [Google Scholar]
- Lawton DM, Furness DN, Lindemann B, Hackney CM. Localization of the glutamate-aspartate transporter, GLAST, in rat taste buds. Eur J Neurosci. 2000;12:3163–3171. doi: 10.1046/j.1460-9568.2000.00207.x. [DOI] [PubMed] [Google Scholar]
- Lehrer MS, Sun TT, Lavker RM. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci. 1998;111(Pt 19):2867–2875. doi: 10.1242/jcs.111.19.2867. [DOI] [PubMed] [Google Scholar]
- Ma H, Yang R, Thomas SM, Kinnamon JC. Qualitative and quantitative differences between taste buds of the rat and mouse. BMC Neurosci. 2007;8:5. doi: 10.1186/1471-2202-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes A, Huygh I, Weltens C, Vandevelde G, Delaere P, Evers G, Van den Bogaert W. De Gustibus: time scale of loss and recovery of tastes caused by radiotherapy. Radiother Oncol. 2002;63:195–201. doi: 10.1016/s0167-8140(02)00025-7. [DOI] [PubMed] [Google Scholar]
- Miura H, Kusakabe Y, Harada S. Cell lineage and differentiation in taste buds. Arch Histol Cytol. 2006;69:209–225. doi: 10.1679/aohc.69.209. [DOI] [PubMed] [Google Scholar]
- Miura H, Kusakabe Y, Kato H, Miura-Ohnuma J, Tagami M, Ninomiya Y, Hino A. Co-expression pattern of Shh with Prox1 and that of Nkx2.2 with Mash1 in mouse taste bud. Gene Expr Patterns. 2003;3:427–430. doi: 10.1016/s1567-133x(03)00081-4. [DOI] [PubMed] [Google Scholar]
- Miura H, Kato H, Kusakabe Y, Tagami M, Miura-Ohnuma J, Ninomiya Y, Hino A. A strong nerve dependence of sonic hedgehog expression in basal cells in mouse taste bud and an autonomous transcriptional control of genes in differentiated taste cells. Chem Senses. 2004;29:823–831. doi: 10.1093/chemse/bjh248. [DOI] [PubMed] [Google Scholar]
- Miyoshi MA, Abe K, Emori Y. IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses. 2001;26:259–265. doi: 10.1093/chemse/26.3.259. [DOI] [PubMed] [Google Scholar]
- Morris GM. Review article: effects of radiation on the cell proliferation kinetics of epithelial tissues--therapeutic implications. Br J Radiol. 1996;69:795–803. doi: 10.1259/0007-1285-69-825-795. [DOI] [PubMed] [Google Scholar]
- Morris GM, Hopewell JW. Changes in the cell kinetics of pig epidermis after single doses of X rays. Br J Radiol. 1988;61:205–211. doi: 10.1259/0007-1285-61-723-205. [DOI] [PubMed] [Google Scholar]
- Morris GM, Landuyt W, Whitehouse E, Vanuytsel L, Hopewell JW. Radiation response of mouse lip mucosal epithelium: a cell kinetic study. Int J Radiat Biol. 1993;63:509–517. doi: 10.1080/09553009314550671. [DOI] [PubMed] [Google Scholar]
- Mossman KL. Gustatory tissue injury in man: radiation dose response relationships and mechanisms of taste loss. Br J Cancer Suppl. 1986;7:9–11. [PMC free article] [PubMed] [Google Scholar]
- Mossman KL, Henkin RI. Radiation-induced changes in taste acuity in cancer patients. Int J Radiat Oncol Biol Phys. 1978;4:663–670. doi: 10.1016/0360-3016(78)90190-6. [DOI] [PubMed] [Google Scholar]
- Murray RG. The mammalian taste bud type III cell: a critical analysis. J Ultrastruct Mol Struct Res. 1986;95:175–188. doi: 10.1016/0889-1605(86)90039-x. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Niida H, Murakami H, Shimada M. DNA damage responses in skin biology--implications in tumor prevention and aging acceleration. J Dermatol Sci. 2009;56:76–81. doi: 10.1016/j.jdermsci.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Negoescu A, Guillermet C, Lorimier P, Brambilla E, Labat-Moleur F. Importance of DNA fragmentation in apoptosis with regard to TUNEL specificity. Biomed Pharmacother. 1998;52:252–258. doi: 10.1016/S0753-3322(98)80010-3. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Nelson GM. Biology of taste buds and the clinical problem of taste loss. Anat Rec. 1998;253:70–78. doi: 10.1002/(SICI)1097-0185(199806)253:3<70::AID-AR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Nguyen HM, Barlow LA. Differential expression of a BMP4 reporter allele in anterior fungiform versus posterior circumvallate taste buds of mice. BMC Neurosci. 2010;11:129. doi: 10.1186/1471-2202-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Oakley B, Witt M. Building sensory receptors on the tongue. J Neurocytol. 2004;33:631–646. doi: 10.1007/s11068-005-3332-0. [DOI] [PubMed] [Google Scholar]
- Oike H, Matsumoto I, Abe K. Group IIA phospholipase A(2) is coexpressed with SNAP-25 in mature taste receptor cells of rat circumvallate papillae. J Comp Neurol. 2006;494:876–886. doi: 10.1002/cne.20848. [DOI] [PubMed] [Google Scholar]
- Okubo T, Clark C, Hogan BL. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 2009;27:442–450. doi: 10.1634/stemcells.2008-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter SR, Fedele S, Habbab KM. Taste dysfunction in head and neck malignancy. Oral Oncol. 2010;46:457–459. doi: 10.1016/j.oraloncology.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Potten CS, Booth D, Cragg NJ, Tudor GL, O'Shea JA, Booth C, Meineke FA, Barthel D, Loeffler M. Cell kinetic studies in the murine ventral tongue epithelium: mucositis induced by radiation and its protection by pretreatment with keratinocyte growth factor, (KGF) Cell Prolif. 2002;35(Suppl 1):32–47. doi: 10.1046/j.1365-2184.35.s1.4.x. [DOI] [PubMed] [Google Scholar]
- Pumplin DW, Getschman E, Boughter JD, Jr., Yu C, Smith DV. Differential expression of carbohydrate blood-group antigens on rat taste-bud cells: relation to the functional marker alpha-gustducin. J Comp Neurol. 1999;415:230–239. doi: 10.1002/(sici)1096-9861(19991213)415:2<230::aid-cne7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Ristic-Fira AM, Todorovic DV, Koricanac LB, Petrovic IM, Valastro LM, Cirrone PG, Raffaele L, Cuttone G. Response of a human melanoma cell line to low and high ionizing radiation. Ann N Y Acad Sci. 2007;1095:165–174. doi: 10.1196/annals.1397.020. [DOI] [PubMed] [Google Scholar]
- Ruo Redda MG, Allis S. Radiotherapy-induced taste impairment. Cancer Treat Rev. 2006;32:541–547. doi: 10.1016/j.ctrv.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Sandow PL, Hejrat-Yazdi M, Heft MW. Taste loss and recovery following radiation therapy. J Dent Res. 2006;85:608–611. doi: 10.1177/154405910608500705. [DOI] [PubMed] [Google Scholar]
- Schwarting R, Gerdes J, Niehus J, Jaeschke L, Stein H. Determination of the growth fraction in cell suspensions by flow cytometry using the monoclonal antibody Ki-67. J Immunol Methods. 1986;90:65–70. doi: 10.1016/0022-1759(86)90384-4. [DOI] [PubMed] [Google Scholar]
- Schwartz LK, Weiffenbach JM, Valdez IH, Fox PC. Taste intensity performance in patients irradiated to the head and neck. Physiol Behav. 1993;53:671–677. doi: 10.1016/0031-9384(93)90172-c. [DOI] [PubMed] [Google Scholar]
- Shatzman AR, Mossman KL. Radiation effects on bovine taste bud membranes. Radiat Res. 1982;92:353–358. [PubMed] [Google Scholar]
- Shi HB, Masuda M, Umezaki T, Kuratomi Y, Kumamoto Y, Yamamoto T, Komiyama S. Irradiation impairment of umami taste in patients with head and neck cancer. Auris Nasus Larynx. 2004;31:401–406. doi: 10.1016/j.anl.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Smith PC, Hill DL. Time course of morphological alterations of fungiform papillae and taste buds following chorda tympani transection in neonatal rats. J Neurobiol. 2002;51:223–236. doi: 10.1002/neu.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Borecki AA, Oleskevich S. Stem and progenitor cell compartments within adult mouse taste buds. Eur J Neurosci. 2010;31:1549–1560. doi: 10.1111/j.1460-9568.2010.07184.x. [DOI] [PubMed] [Google Scholar]
- Takeda M, Suzuki Y, Obara N, Nagai Y. Neural cell adhesion molecule of taste buds. J Electron Microsc. 1992;41:375–380. [PubMed] [Google Scholar]
- Urek MM, Bralic M, Tomac J, Borcic J, Uhac I, Glazar I, Antonic R, Ferreri S. Early and late effects of X-irradiation on submandibular gland: a morphological study in mice. Arch Med Res. 2005;36:339–343. doi: 10.1016/j.arcmed.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou M, Brand J, Huang L. Inflammation activates the interferon signaling pathways in taste bud cells. J Neurosci. 2007;27:10703–10713. doi: 10.1523/JNEUROSCI.3102-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardley AM, Booth D, Roberts SA, Scarffe JH, Potten CS. A quantitative histometric murine in vivo model of radiation-induced oral mucositis. Arch Oral Biol. 1998;43:567–577. doi: 10.1016/s0003-9969(98)00031-4. [DOI] [PubMed] [Google Scholar]
- Wilson GD. Cell kinetics. Clin Oncol, (R Coll Radiol) 2007;19:370–384. doi: 10.1016/j.clon.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Nakagawa K, Hosoi Y, Kurokawa A, Fukuda Y, Matsumoto I, Misaka T, Abe K. Umami taste dysfunction in patients receiving radiotherapy for head and neck cancer. Oral Oncol. 2009;45:e19–23. doi: 10.1016/j.oraloncology.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Nakagawa K, Nakamura N, Abe K, Asakage T, Ohmoto M, Okada S, Matsumoto I, Hosoi Y, Sasano N, Yamakawa S, Ohtomo K. Relation between acute and late irradiation impairment of four basic tastes and irradiated tongue volume in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;66:1422–1429. doi: 10.1016/j.ijrobp.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Fujii S, Ochiai A. Reduction of type II taste cells correlates with taste dysfunction after X-ray irradiation in mice. J Oral Pathol Med. 2009 doi: 10.1111/j.1600-0714.2009.00823.x. [DOI] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25- like immunoreactivity. J Comp Neurol. 2000;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yang R, Ma H, Thomas SM, Kinnamon JC. Immunocytochemical analysis of syntaxin-1 in rat circumvallate taste buds. J Comp Neurol. 2007;502:883–893. doi: 10.1002/cne.21317. [DOI] [PubMed] [Google Scholar]
- Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: Immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Oakley B. p53 and Bax: putative death factors in taste cell turnover. J Comp Neurol. 1999;413:168–180. doi: 10.1002/(sici)1096-9861(19991011)413:1<168::aid-cne12>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Kwan A, Oakley B. Gustatory innervation and bax-dependent caspase-2: participants in the life and death pathways of mouse taste receptor cells. J Comp Neurol. 2000;424:640–650. doi: 10.1002/1096-9861(20000904)424:4<640::aid-cne6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Sausville EA. Drug discovery targeting Chk1 and Chk2 kinases. Prog Cell Cycle Res. 2003;5:413–421. [PubMed] [Google Scholar]