Abstract
Objectives
We assessed changes in smoking prevalence and other measures associated with the July 2008 New York Office of Alcoholism and Substance Abuse Services tobacco policy, which required that all publicly funded addiction treatment programs implement smoke-free grounds, have “no evidence” of smoking among staff, and make tobacco dependence treatment available for all clients.
Methods
In a random sample of 10 programs, staff and clients were surveyed before the policy and 1 year later. Measures included tobacco-related knowledge, attitudes, and practices used by counselors and received by clients.
Results
Client smoking decreased from 69.4% to 62.8% (P = .044). However, response to the policy differed by program type. Outpatient programs showed no significant changes on any of the staff and client survey measures. In methadone programs, staff use of tobacco-related practices increased (P < .01), client attitudes toward tobacco treatment grew more positive (P < .05), and clients received more tobacco-related services (P < .05). Residential clients were more likely to report having quit smoking after policy implementation (odds ratio = 4.7; 95% confidence interval = 1.53, 14.19), but they reported less favorable attitudes toward tobacco treatment (P < .001) and received fewer tobacco-related services from their program (P < .001) or their counselor (P < .001).
Conclusions
If supported by additional research, the New York policy may offer a model that addiction treatment systems can use to address smoking in a population where it has been prevalent and intractable. Additional intervention or policy supports may be needed in residential programs, which face greater challenges to implementing tobacco-free grounds.
Persons with substance abuse and dependence smoke at higher rates1–3 and smoke more heavily4,5 than do persons in the general population. They may be more physically dependent on nicotine,6 less successful in quit attempts,7 and may die from smoking-related causes more frequently than from drug- or alcohol-related causes.8 For 30 years, research has noted the high rate of smoking among persons with other addictive disorders9–12 and several authors have argued that addiction treatment programs should address tobacco.13–16 Although this is reflected in clinical guidelines17 and policy statements,18,19 several studies have found that tobacco dependence is often not addressed in addiction treatment.20–22
Treatment of tobacco dependence in addiction settings may be accelerating.23 Veteran Affairs Medical Centers implemented smoking cessation practice guidelines for all patients, including those in addiction clinics,24 and New Jersey required tobacco-free grounds for residential drug treatment.25 Following the New Jersey initiative, all programs provided more tobacco-related treatment, half adopted smoke-free grounds, and 41% of smokers did not smoke during their residential stay.25
In 2008, the New York Office of Alcohol and Substance Abuse Services (OASAS) required all state-certified addiction treatment programs to implement tobacco-free grounds, to have no-evidence (of smoking) policies for staff, and to provide tobacco dependence intervention for clients on request.26 Tobacco-free grounds means no smoking anywhere on program grounds, including outdoor areas. No evidence of smoking means staff do not come to work smelling of tobacco smoke, or have cigarettes or other tobacco products or paraphernalia in view in the work area. Tobacco dependence intervention means smoking cessation counseling and nicotine replacement therapy (NRT). Tobacco dependence services are free to clients, with costs bundled into program contracts with the state. The policy affects 1550 programs, 20 000 staff, and 250 000 annual admissions. To support the policy, the state committed $4 million to deliver staff training and $4 million to provide NRT to treatment programs. The OASAS Web site listed volunteer mentors to help programs implement the policy, and offered online tobacco dependence training for counselors. Program licensing visits included review and grading on policy compliance. We report findings from staff and client surveys conducted in a random sample of programs before and after the policy was implemented.
METHODS
We excluded prevention, education, and short-term (< 5 days) detoxification programs. We also excluded hospital-based, criminal justice, and adolescent programs as local institutional review board review would prevent data collection before policy implementation. With these criteria, OASAS identified 610 eligible programs. Of these, 41 were randomly selected, stratified by program type (outpatient, methadone, residential). When OASAS contacted the programs, 13 expressed interest, and 10 enrolled. Four programs were in New York City boroughs, and the others were dispersed throughout the state. Four agencies offered a substitute program because the selected program was ineligible, too busy, very small, or because a different program in the same agency expressed interest. The sample included 3 outpatient, 2 methadone, and 5 residential programs.
Measures
The Smoking Knowledge, Attitudes and Practices (S-KAP)27 survey measures knowledge of the hazards of smoking (Chronbach’s α = 0.85), attitudes about treating smoking (α = 0.74), barriers to tobacco treatment (α = 0.81), counselor self-efficacy in providing such services (α = 0.72), and practices to address smoking with clients (α = 0.91).27 We calculated knowledge and attitude scales for all staff, and calculated barriers, self-efficacy, and practice scales for clinical staff only. Clinical staff are those who report a clinical job title (e.g., clinician, counselor) or report more than 5 client contact hours per week.
The Smoking Knowledge, Attitudes, and Service (S-KAS)28 survey measures knowledge (α = 0.57), attitudes (α = 0.75), and tobacco-related services received from a counselor (clinician services, α = 0.82) or from the program (program services, α = 0.82). Clinician service items asked how often in the past month the clinician had encouraged the client to reduce or quit smoking, use NRT, or arrange an appointment to discuss quitting. Program service items asked “[I]n the program where you are now, did you receive”: information, educational material, advice, referral, or medication to assist in quitting. Additional items asked whether a client attended quit-smoking groups. The distinction between the scales is that clinician service items concerned specific actions by the clinician, whereas program service items concerned whether services were available or provided “in the program where you are now.” Although the constructs are similar, the items load on separate factors,28 and are treated as separate scales in analyses. We calculated knowledge and attitude scales for all clients, and calculated clinician and program service scales for smokers only.
The New York State tobacco tax increased from $1.25 to $2.75 per pack in June 2008, and the federal excise tax increased from 39 cents to $1.00 per pack in April 2009. Tobacco tax increases represent a primary policy tool in tobacco control29 and offer potential confounding. At follow-up, clients who reported that they had quit smoking were asked 2 yes-or-no questions: (1) “[D]id you quit because of treatment program rules banning smoking?” and (2) “[D]id you quit because of the increased tobacco taxes?”
The Project Director (B. T.) interviewed a program administrator by phone following each site visit. Questions assessed beliefs concerning tobacco dependence treatment, current tobacco policies, opinion of the new state policy, and factors that facilitate or impede tobacco dependence treatment in their setting. The same questions were asked at follow-up, modified to reflect that the policy had been implemented and to assess the impact of recent tobacco tax increases.
Data Collection Procedures
The study team visited each site between June and August 2008. Seven visits were completed before the policy implementation date (July 24th) and 3 were completed within 36 days after that date. Follow-up visits occurred in June through August 2009, although 2 programs delayed follow-up until December 2009.
One staff member in each clinic served as a liaison to the study team, providing names of eligible staff and arranging a meeting for survey data collection. Survey packets labeled with a research identification number contained consent documents, the survey, and a return envelope. One team member worked with program liaisons to follow up on surveys not completed during the site visit. At baseline, 254 staff were eligible and 235 (92%) completed the survey. At follow-up, 260 staff were eligible and 237 (91%) completed the survey. Many follow-up respondents had also completed a baseline survey; however, because of staff turnover, 35% of respondents completed the survey for the first time at follow-up. Because programs change their staff complement over time, the number of eligible staff was different at baseline and follow-up. Staff samples included 173 clinicians at baseline and 166 at follow-up. Nonclinical staff included administrative, clerical, and research and training staff. Staff participants received a $25 gift card.
Client data were cross-sectional, as the same clients were unlikely to be in the program at both baseline and follow-up. In residential programs, the liaison assembled all clients in the program on the day of the site visit, and the research team explained the study and distributed survey packets. In outpatient clinics, a researcher was present after group sessions held on the site visit day. In methadone clinics, a researcher was present during morning dosing hours. The researcher explained the study, distributed survey packets, and arranged additional data collection visits until sample size was achieved. Client participants were anonymous and received a $20 gift card.
Where eligible staff numbered 25 or more, clients were recruited to a sample size of 50. Where eligible staff numbered less than 25, clients were recruited to a sample size of 25. This ensured that larger programs (those with more staff) also had more clients in the sample, while permitting a definite estimate of client sample size and associated study costs. Clients numbered 409 at baseline and 411 at follow-up. Each clinic received $1000 to $2000 to offset costs of data collection. Procedures were approved by the University of California, San Francisco, institutional review board.
Data Analysis
We used admissions data from 2008 to compare demographic characteristics for admissions to 10 programs enrolled and 31 programs not enrolled in the study. We assessed impacts of the tobacco policy by using the staff and the client survey scales. Scale scores ranged from 1 to 5, where a higher number is better—for example, more favorable beliefs toward treating tobacco dependence. For the barriers scale only, a lower score (fewer barriers) reflects a better outcome.
Because data were collected in 3 sites (2 residential, 1 outpatient) after the July 24th policy implementation date, there is potential for confounding associated with the timing of baseline data collection. To assess this, we tested the interactions of when baseline data were collected (before or after July 24th) and time in preliminary analyses. First, we tested before-or-after-by-time interactions for each outcome, for residential programs completing baseline before (3 programs) and after (2 programs) the July 24th date. Second, we tested before-or-after-by-time interactions for each outcome, for outpatient programs completing baseline before (2 programs) and after (1 program) July 24th. Significant interactions would indicate confounding, such that the pattern of change over time was different for clinics according to when baseline data were collected (before or after the implementation date). Conversely, the absence of interactions would suggest that the pattern of change over time was similar, regardless of the before-or-after distinction. Of 18 interactions tested, only 1 approached significance (outpatient client attitude scale, P = .052), suggesting little or no confounding associated with the timing of baseline data collection. To be conservative, the main analytic model included a factor for before or after, and controlled for any before-or-after-by-time interaction.
We tested linear mixed models for each scale, including effects for time (baseline, follow up), modality (outpatient, methadone, residential), and the interaction. The model controlled for whether baseline data collection occurred before or after the policy implementation date, and for the interaction of before or after by time. Models accounted for nesting of staff and clients within program. Because staff respondents may or may not be the same person at both times, the staff model allowed for correlations within site and within participant. The client model allowed for correlations within site only. We observed demographic differences by type of program at baseline. Staff and client models controlled for age, gender, Hispanic ethnicity, race, education, and smoking status. Client analyses also controlled for employment and primary substance. Staff analyses also controlled for whether the respondent was in recovery from substance abuse. We compared staff and client smoking rates from baseline to follow-up by using χ2 tests.
To assess whether change observed from baseline to follow-up may be attributed to the policy or to tax increases that occurred in the same period, we classified clients who reported having quit smoking postpolicy as to whether they quit smoking before (n = 29) or after (n = 90) entering the current treatment program. The algorithm used number of weeks the client had been in treatment, when they quit smoking (< 1 month ago, 1–3 months, 4–6 months, > 6 months), and whether they received tobacco-related services while in the program (e.g., smoking cessation referral or medication). For clients who quit smoking while in treatment, we used generalized estimating equation models to predict quitting while in treatment (yes or no) based on treatment type and with control for nesting of clients within site. We tested reasons for quitting with three 2-by-2 comparisons, 1 for each treatment type, comparing whether clients quit because of the policy (yes or no) or because of taxes (yes or no).
We recorded, transcribed, and coded administrator interviews with ATLAS.ti version 4.2 (Scientific Software Development, Berlin, Germany), a software program used in managing and analyzing qualitative data. In this study, we loaded transcribed interviews into ATLAS.ti, and developed closed codes30 to reflect current tobacco policies and services, implementation of the state policy, and how tobacco tax increases affected smoking behavior. Once data have been coded, the software enables extraction of all interview material associated with a particular code.
RESULTS
Table 1 summarizes demographic characteristics for admissions to programs enrolled (n = 2065) and not enrolled (n = 7536) in the study. Effect sizes offer an estimate of difference between groups that is independent of sample size. Cohen31 identifies 0.10 as a small, and 0.30 as a medium, effect size. All effect sizes shown are small, or are nearer to small than to medium size. Programs enrolled and not enrolled in the study were similar in terms of client characteristics, including self-reported smoking status.
TABLE 1.
Comparison of Client Demographics for Addiction Treatment Programs Enrolled (10 Programs) and Not Enrolled (31 Programs) in the Sample: New York State, 2008–2009
| Enrolled in Study (n = 2065), Mean (SD) or % | Not Enrolled in Study (n = 7536), Mean (SD) or % | t, χ2df | Effect Size | |
|---|---|---|---|---|
| Age | 36.8 (11.6) | 37.4 (12.2) | −2.349599 | 0.058 |
| Previous treatment episode | ||||
| 0 | 18.8 | 31.4 | ||
| 1 | 20.4 | 23.2 | ||
| 2 | 17.2 | 16.6 | ||
| 3 | 12.5 | 10.9 | ||
| 4 | 9.0 | 5.9 | ||
| ≥ 5 | 21.7 | 12.0 | 2325 | 0.154 |
| Education | ||||
| < high school | 34.9 | 35.4 | ||
| High school or GED | 40.7 | 35.3 | ||
| > high school | 24.4 | 29.3 | 27.22 | 0.053 |
| Employment | ||||
| Employed | 15.7 | 32.0 | ||
| Not in labor force | 68.5 | 51.0 | ||
| Unemployed | 15.8 | 17.0 | 2432 | 0.157 |
| Race/ethnicity | ||||
| African American | 28.0 | 28.8 | ||
| Hispanic | 16.7 | 24.9 | ||
| White | 53.7 | 43.3 | ||
| Other | 1.6 | 3.0 | 96.73 | 0.100 |
| Gender | ||||
| Male | 61.5 | 70.2 | ||
| Female | 38.5 | 29.8 | 56.31 | 0.076 |
| Primary substance | ||||
| Alcohol | 35.8 | 42.7 | ||
| Crack/cocaine | 21.9 | 14.3 | ||
| Marijuana/hash | 12.1 | 15.9 | ||
| Opiate | 28.4 | 24.2 | ||
| Other | 1.7 | 2.9 | 1124 | 0.108 |
| Smoked tobacco past week | ||||
| Yes | 70.5 | 69.3 | ||
| No | 29.5 | 30.7 | 1.201 | 0.011 |
Notes. GED = general equivalency diploma. All comparisons are statistically significant because of the large sample size. Based on Cohen’s w,31 0.10 is a small effect and 0.30 is a medium effect.
Participant Characteristics
Demographics for staff and clients at baseline are shown in Table 2. Residential program staff were more likely to report high-school education and less likely to report undergraduate degrees, compared with other staff. Methadone program staff, compared with others, were more often African American, and less often smokers or in recovery.
TABLE 2.
Demographic Characteristics for Staff (n = 235) and Clients (n = 409) at Baseline, by Addiction Treatment Program Type: New York State, 2008–2009
| Staff
|
Client
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Outpatient (n = 33), Mean (SD) or No. (%) | Methadone (n = 50), Mean (SD) or No. (%) | Residential (n = 152), Mean (SD) or No. (%) | P | Outpatient (n = 80), Mean (SD) or No. (%) | Methadone (n = 100), Mean (SD) or No. (%) | Residential (n = 229), Mean (SD) or No. (%) | P | |
| Age | 45.3 (10.3) | 48.1 (13.2) | 46.9 (12.2) | .59 | 39.7 (11.9) | 45.3 (9.1) | 36.7 (10.5) | <.001 |
| Gender | .19 | <.001 | ||||||
| Male | 7 (21.2) | 19 (38.0) | 42 (27.6) | 62 (77.5) | 67 (67.0) | 112 (48.9) | ||
| Female | 26 (78.8) | 30 (60.0) | 109 (71.7) | 18 (22.5) | 29 (29.0) | 116 (50.7) | ||
| Education | .014 | .37 | ||||||
| No high school diploma or GED | 0 (0.0) | 1 (2.0) | 2 (1.3) | 27 (33.8) | 37 (37.0) | 63 (27.5) | ||
| High school diploma or GED | 5 (15.2) | 12 (24.0) | 61 (40.1) | 23 (28.8) | 31 (31.0) | 76 (33.2) | ||
| Bachelor’s or associate’s degreea | 23 (69.7) | 26 (54.0) | 54 (35.5) | 29 (36.3) | 30 (30.0) | 90 (39.3) | ||
| Graduate degree | 5 (15.1) | 11 (22.0) | 33 (21.7) | |||||
| Ethnicity: Hispanic | 5 (15.1) | 6 (12.0) | 15 (9.9) | .66 | 13 (16.3) | 38 (38.0) | 33 (14.4) | <.001 |
| Race | <.001 | <.001 | ||||||
| African American | 4 (12.1) | 28 (56.0) | 33 (21.7) | 23 (28.8) | 37 (37.0) | 69 (30.1) | ||
| White | 23 (69.7) | 5 (10.0) | 91 (59.9) | 40 (50.0) | 21 (21.0) | 114 (49.8) | ||
| Otherb | 6 (18.2) | 16 (32.0) | 27 (17.8) | 16 (20.0) | 39 (39.0) | 45 (19.7) | ||
| Current smoker | 10 (30.3) | 9 (18.0) | 62 (40.8) | .015 | 60 (75.0) | 85 (85.0) | 139 (60.7) | <.001 |
| In recoveryc | 8 (24.2) | 5 (10.0) | 47 (30.9) | .013 | ||||
| Primary substance | <.001 | |||||||
| Alcohol | 25 (31.3) | 0 (0.0) | 54 (23.6) | |||||
| Crack/cocaine | 10 (12.5) | 9 (9.0) | 83 (36.2) | |||||
| Heroin | 27 (33.8) | 81 (81.0) | 61 (26.6) | |||||
| Otherd | 12 (15.0) | 2 (2.0) | 26 (11.4) | |||||
Note. GED = general equivalency diploma.
For client–graduate degree not available.
Includes Asian, Native Hawaiian/Pacific Islander, Native American/Alaskan, mixed race, and other.
Describes staff members who previously had a substance abuse problem but are now abstinent and “in recovery.”
Includes marijuana, methadone, hallucinogens, other prescription drugs, and prescription opiates.
Methadone clients tended to be older, more often of Hispanic ethnicity, more often of African American or other race, and were more often in treatment of heroin or other opiate use. Residential clients were more often female and less often smokers (Table 2). Out-patient clients were also more often employed (36.3%) than were methadone (16%) or residential (8.3%) clients (data not shown, P < .001). Smoking rates in Table 2 compare with an 18% smoking rate for New York State in 2008.32
Smoking Knowledge, Attitudes, Practices, and Services
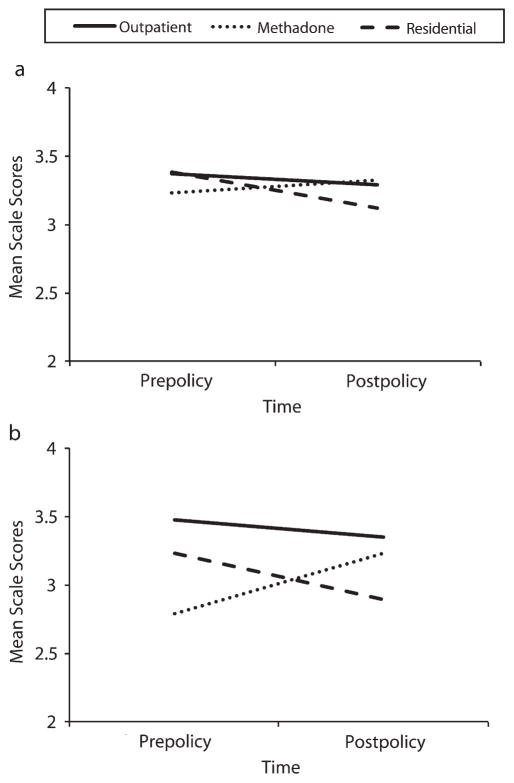
Means for staff S-KAP scales are shown in the upper half of Table 3. The last 3 columns show results of the linear mixed model analyses. For measures where there was no time-by-treatment-type interaction (knowledge, beliefs, barriers), the main effects of time were nonsignificant. There were significant interactions for counselor self-efficacy (F = 4.742,65; P = .01), and for practices used to address smoking (F = 9.042,62; P < .001. These interactions showed no change in outpatient staff, a significant increase in practice (P < .01) in methadone staff, and significant decreases for both efficacy (P < .001) and practice (P < .01) in residential staff (Figure 1).
TABLE 3.
Results of Linear Mixed Models Testing Differences for Time, Modality, and Time-by-Treatment-Type Interaction: Addiction Treatment Programs, New York State, 2008–2009
| Outpatient, Mean (SD)
|
Methadone, Mean (SD)
|
Residential, Mean (SD)
|
Time
|
Treatment Type
|
Time by Treatment Type
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Policy | After Policy | Before Policy | After Policy | Before Policy | After Policy | F | P | F | P | F | P | |
| Staffa,b | ||||||||||||
| Knowledgec | 4.11 (0.73) | 4.23 (0.52) | 4.05 (0.69) | 4.30 (0.62) | 4.07 (0.66) | 4.03 (0.74) | 1.481,100 | .23 | 1.322,100 | .27 | d | |
| Beliefs | 3.75 (0.51) | 3.75 (0.55) | 3.73 (0.65) | 3.85 (0.51) | 3.88 (0.54) | 3.75 (0.63) | 0.001,98 | .97 | 0.492,98 | .62 | d | |
| Barriers | 1.99 (0.53) | 2.01 (0.54) | 2.16 (0.61) | 2.06 (0.61) | 2.07 (0.64) | 1.97 (0.61) | 2.141,64 | .15 | 0.122,64 | .89 | d | |
| Efficacy | 3.37 (0.47) | 3.29 (0.39) | 3.23 (0.48) | 3.33 (0.56) | 3.38 (0.52) | 3.12 (0.69) | 1.861,65 | .18 | 0.642,65 | .53 | 4.742,65 | .01 |
| Practice | 3.48 (0.84) | 3.35 (0.83) | 2.79 (0.98) | 3.23 (1.08) | 3.23 (1.01) | 2.89 (0.92) | 1.101,62 | .3 | 1.352,62 | .27 | 9.042,62 | <.001 |
| Cliente | ||||||||||||
| Knowledgef | 3.72 (0.75) | 3.76 (0.81) | 3.48 (0.80) | 3.50 (0.90) | 3.82 (0.66) | 3.70 (0.74) | 0.011,710 | .92 | 0.702,710 | .5 | d | |
| Attitudes | 3.13 (0.81) | 2.97 (0.79) | 3.08 (0.71) | 3.33 (0.71) | 3.15 (0.92) | 2.80 (0.86) | 0.321,673 | .57 | 0.342,673 | .71 | 8.422,673 | <.001 |
| Program services | 2.77 (1.20) | 2.70 (1.07) | 2.51 (1.24) | 3.17 (1.18) | 3.92 (0.94) | 3.13 (1.12) | 0.831,458 | .36 | 16.692,458 | <.001 | 14.822,458 | <.001 |
| Clinician services | 2.30 (1.23) | 2.13 (1.01) | 2.08 (1.12) | 2.55 (1.15) | 2.70 (1.05) | 2.30 (1.23) | 0.621,457 | .43 | 1.552,457 | .21 | 5.062,457 | .007 |
Analysis controlled for demographics (age, gender, Hispanic ethnicity, race, education, current smoker) and for whether the baseline data collection occurred before or after the policy implementation date. Staff analyses also controlled for whether staff were in recovery. Client analyses also controlled for employment and primary substance.
Outpatient program staff before policy n = 33; after policy n = 34. Methadone program staff before policy n = 50; after policy n = 55. Residential program staff before policy n = 152; after policy n = 148.
All scales ranged from 1 to 5. Higher scores reflect more knowledge, more favorable beliefs about treating tobacco, and more tobacco practices delivered by staff or services received by clients. For the barriers scale only, a lower score is better. Scale means (SD) include all staff for knowledge and belief scales, and clinical staff only for barriers, efficacy, and practice scales.
Preliminary analyses showed no time-by-modality interaction, so this effect was dropped from the model.
Outpatient program clients before policy n = 80; after policy n = 70. Methadone program clients before policy n = 100; after policy n = 103. Residential program clients before policy n = 229; after policy n = 238.
Scale means (SD) include all clients for knowledge and attitudes scales, and smokers only for remaining scales.
FIGURE 1. Interactions of time by treatment type for (a) efficacy and (b) practice on the staff survey scales: addiction treatment programs, New York State, 2008–2009.
Note. Outpatient time effects shown in the figure were not significant for either of the measures shown. Residential time effects were significant for both efficacy (P < .001) and practice (P < .01). Methadone time effects were nonsignificant for efficacy (P = .25) and were significant for practice (P < .01). All scale scores ranged from 1 to 5.
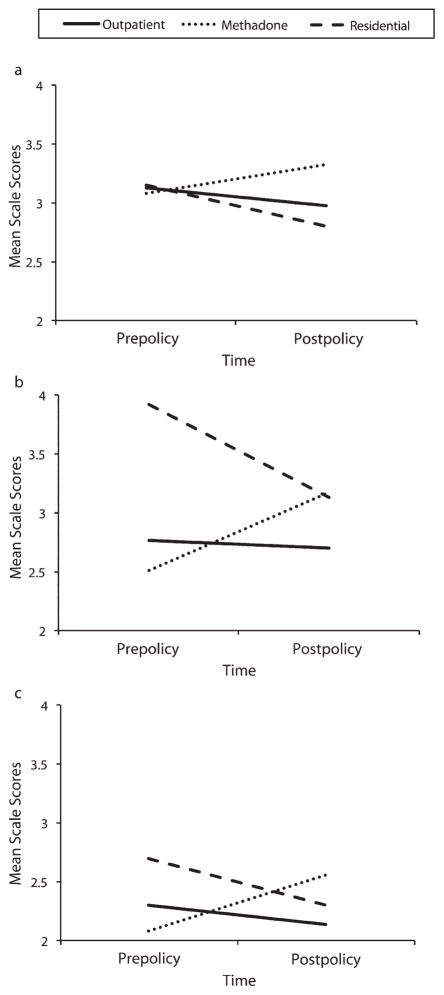
Client data are in the lower half of Table 3. There was no time-by-treatment-type interaction for the client knowledge scale, permitting direct interpretation of the main effect of time, which was nonsignificant. There were significant time-by-treatment-type interactions for the attitude (F = 8.422, 673; P < .001), program service (F = 14.822,458; P < .001), and clinician service (F = 5.062,457; P = .007) scales. These interactions (Figure 2) show no change in outpatient clients, increases (P < .05) in both attitude and program services in methadone clients, and decreases for attitudes (P < .001), program service (P < .001), and clinician service (P < .001) in residential clients.
FIGURE 2. Interactions of time by treatment type for (a) attitudes, (b) program services, and (c) clinician services on the client survey scales: addiction treatment programs, New York State, 2008–2009.
Note. Outpatient time effects shown in the figure were not significant for any of the measures shown. Residential time effects were significant for attitudes (P < .001), program services (P < .001), and clinician services (P < .001). Methadone time effects were significant for attitudes and program services at P < .05, and were not significant for clinician services (P = .21). Scale scores ranged from 1 to 5.
Smoking Behavior
Staff smoking across all programs decreased from 34.5% to 31.6% over 1 year (χ21 = 0.425; P = .51). This decrease was also non-significant when considered separately for residential (40.8% to 39.2%), outpatient (30.3% to 23.5%), and methadone (18% to 16.4%) program staff. Smoking also decreased (35.1% to 32.5%) among 154 staff who were present at both time points (χ21 = 0.274; P = .60).
Client smoking across all programs was 69.4% before policy implementation and 62.8% 1 year later (χ21 = 4.06; P = .044). Decreased client smoking was not significant when considered separately for residential (60.7% to 52.5%), outpatient (75% to 71.4%), and methadone (85% to 80.6%) program clients.
Reasons for Quitting Smoking in Treatment
Among clients who, at follow-up, quit smoking while in the treatment program, 6 were in outpatient, 14 were in methadone, and 70 were in residential treatment. Compared with those in outpatient programs, clients who smoked when entering residential treatment were almost 5 times more likely to quit smoking while in treatment (odds ratio [OR] = 4.7; 95% confidence interval [CI] = 1.53, 14.19). Comparisons for whether these participants quit because of the policy or because of the tax change were nonsignificant for outpatient quitters (P = .32). However, quitters in methadone treatment were more likely to quit because of the tax increase (P = .025), whereas those in residential treatment were more likely to quit because of the policy (P < .001). For 70 quitters in residential treatment, 60 quit because of the policy, 4 quit because of both the policy and the tax, and 6 quit for neither reason.
Administrator Interviews
For outpatient and methadone clinic administrators, the policy meant little change, as the insides of buildings were already smoke-free, and patients visit the clinic only for short periods. Residential program administrators reported efforts to adjust to the policy before implementation, such as having NRT available for clients or having already implemented tobacco-free grounds. At follow up, they noted that their clients must stop smoking to comply with the policy, and this gave way to contraband tobacco and surreptitious indoor smoking. One program discontinued nicotine lozenges because the blister packs were used to store snuff, and another program relaxed a smoking “no tolerance” policy after it led to too many discharges. Administrators thought that tax increases had little impact on smoking, noting that tax-free cigarettes were available at numerous Indian reservations, and that smokers can travel to neighboring states where taxes are lower.
DISCUSSION
During 1 year following implementation of the New York tobacco policy, client smoking prevalence in the programs studied decreased significantly (69.4% to 62.8%), and this is consistent with data for the entire New York treatment system.33 This finding is consistent with, although less dramatic than, the New Jersey findings that 41% of smokers did not smoke in residential treatment after implementation of tobacco-free grounds.25 We also found a nonsignificant decrease in staff smoking (34.5% to 31.2%). From a staff perspective, the move to tobacco-free grounds is similar to a workplace smoking ban, and such bans are shown to reduce workforce smoking.34
Some of the decrease in smoking was likely attributable to the policy, independent of taxation. During the study period, New York statewide smoking prevalence decreased by 1.2% (from 18% to 16.8%),32,35 and this amount of decrease may be associated with increased taxation or other tobacco control measures. A recent review of smoking rates among clients in addiction treatment found a 0.7% annual decrease in smoking when they reviewed published papers, and a 0.4% annual decrease in smoking when they reviewed National Survey on Drug Use and Health data.36 In the absence of the New York policy, then, staff and client smoking rates may be expected to decrease over the study period within the range of 0.4% to 1.2%. However, staff smoking in the present study decreased by 3.3% and client smoking decreased by 6.6%. Furthermore, analysis of persons who, at follow-up, reported quitting smoking while in treatment, showed that residential clients were nearly 5 times more likely to quit compared with outpatients. When asked the reasons for quitting, the majority of residential quitters said they quit because of the policy and not because of the tax increase.
Response to the New York policy differed by type of treatment. In outpatient programs, no significant pre–post policy changes were observed. That the policy had little impact in outpatient settings is supported by administrator reports of few difficulties in adapting to the policy. In methadone programs, staff use of tobacco-related practices increased, and client attitudes toward tobacco treatment grew more positive and clients reported receiving more tobacco-related services. These findings are consistent with the intended effects of the policy. Some impact of taxation is also seen, as quitters in methadone treatment were more likely to quit because of the tax increase than because of the policy. Residential clients, compared with outpatients, were almost 5 times more likely to quit smoking while in treatment, and those quitters were more likely to quit because of the policy than because of tax increases. However, residential staff reported decreased self-efficacy to address tobacco dependence over time, and decreased use of practices to address tobacco. Residential clients reported less-favorable attitudes toward treatment of tobacco dependence, and received fewer tobacco-related services.
Outpatient findings may be taken at face value, as the policy required few changes in these settings. Policy impacts may have been stronger in methadone programs because smoking rates among staff were lower than those of other programs. Methadone programs also include more medically trained staff, and previous studies of addiction treatment have shown a relationship between medical staffing and both increased availability of cessation medications for clients20 and sustained use of NRT.37 In methadone clinics, the combination of lower staff smoking, medically trained staff, and access to NRT as part of the tobacco policy may overcome frequently reported barriers to treating tobacco dependence.38
In residential settings, the demands of the policy were greater. Clients live in these programs and often for the first 1 to 2 weeks cannot leave program grounds. Those able to leave the grounds may find that opportunities to smoke are infrequent and inconvenient, and may stop smoking while in the program. This may account for the higher probability of quitting among those in residential treatment.
As to why counselor tobacco-related efficacy and practices, as well as tobacco services received by clients, would decrease in residential programs, we offer 3 possibilities. First, residential administrators reported preparing for the policy before implementation. This could elevate baseline scale scores, with later regression to the mean. This would be consistent with higher levels of program and clinician services reported by residential clients at baseline (Figure 2). Second, residential administrators reported more implementation challenges, consistent with earlier reports of implementing tobacco-free grounds in residential treatment.25 Residential programs may have initiated more tobacco-related training or services in advance of the policy, and then relaxed efforts as they confronted difficulties. Third, residential program clients were much more likely to quit smoking, and did so in response to the policy. Clients who continued to smoke in residential treatment may be more resentful of the policy and less interested in tobacco-related services. This could account for decreases in client tobacco-related attitudes and services, along with decreases in staff self-efficacy to address, and practices used to address, tobacco dependence.
Limitations
The small number of clinics and the replacement of sampled clinics with another clinic in the same agency limit generalizability. The sample was randomly selected from among those meeting eligibility criteria, all programs in the sample were invited to participate, and all of those expressing interest were enrolled until time available before policy implementation was exhausted. We allowed replacement of a selected program with another program in the same agency, as replacement programs would be subject to the same organizational approach to tobacco dependence as the selected program. Comparison of admissions data for programs included (n = 10) with those invited but not included (n = 31) enables assessment of generalizability. In terms of client demographic characteristics and self-report smoking status, the sample of programs in the study was representative of all those invited to participate. As those invited were randomly selected from 610 eligible programs, findings may reasonably generalize to those programs, representing more than one third of the New York State addiction treatment system. However, there may be other program characteristics that made sites more or less willing to participate in the study, and more or less open to the New York tobacco policy. Such program features may include, for example, attitudes of the leadership toward smoking and the tobacco initiative, and smoking prevalence among program staff.
We collected data in 3 clinics shortly after the policy implementation date. Baseline data for those clinics do not offer a “true” prepolicy baseline. This can be addressed by using the available data to represent true baseline, or by using imputation methods to estimate what the baseline data may have been approximately 1 month before the data were collected. Preliminary analyses found little evidence of confounding associated with the before-or-after timing of baseline data collection. That is, the pattern of change over time for outcome measures did not differ according to whether baseline data were collected before or after the policy implementation date. Absent evidence of before-or-after confounding, our approach was to let baseline data stand as the best estimate of a true prepolicy baseline. Our assumption is that the change 1 year after implementation is greater than that 1 month after implementation, or, stated another way, change over time may be observed over 1 year even where baseline data were collected shortly after the implementation date.
Clients were selected within programs systematically, rather than randomly. Client sampling procedures likely achieved good representation in residential programs, where all clients present on a given day were invited and incentivized to participate. Recruitment in out-patient settings occurred during specified times when clients were present and used strategies acceptable to participating clinics. It is possible that outpatient clients were not representative of all clients, particularly if smokers more often self-selected into the study. If smokers were overrepresented among outpatient clients at both time points, this would not influence estimates of change over time.
It is possible that clients underreported smoking, particularly for residential clients and if they thought that smoking status would have consequences for their treatment. Future research would be strengthened through the use of biochemical verification of client smoking status. Reported decreases in client smoking may have been short-lived, as Tesiny et al. found that clients who left programs as non-smokers often identified as smokers when they returned for a new treatment episode.33 These limitations notwithstanding, we are aware of no other data reflecting tobacco-related client-and staff-level measures, collected in the same set of clinics before and after the New York policy intervention, and there is only 1 articles to date reporting on policy efforts to address tobacco dependence in a state addiction treatment system.25
Conclusions
In 2008, 2.3 million persons received addiction treatment in specialty clinics,39 and most of those persons smoked. State addiction treatment agencies will increasingly address tobacco, and will look to New York for policy lessons. In outpatient programs, the policy had little impact, whereas in methadone programs the policy was associated with increased tobacco-related services to clients. In residential programs, the policy was associated with quitting smoking among clients, and with decreasing tobacco-related services to clients. The policy was more challenging to implement in residential settings, and states contemplating similar policies may consider additional strategies to address smoking in these settings. Organizational change protocols require the identification of an onsite “tobacco champion,” and include trained and designated tobacco counselors, change leadership teams, and external consultation to support programs in integrating tobacco dependence treatment.40,41 In 3 residential programs where such an intervention was tested, staff and client attitudes toward treating tobacco became more positive, NRT use increased, and clients received more tobacco-related services.42 Future research may assess whether some elements of an organizational change intervention, combined with a statewide policy initiative, may achieve better outcomes in residential programs. It may be helpful, for example, to prepare programs for a statewide tobacco policy by reducing smoking among staff, as policy implementation may be more difficult where many staff smoke.43 If supported by additional research, the New York policy may offer a model that other addiction treatment systems can use to address smoking in a population where it has been prevalent and intractable.
Acknowledgments
This work was supported by the National Institute on Drug Abuse (NIDA; grant R01 DA020705), the National Cancer Institute (NCI), the California–Arizona research node of the NIDA Clinical Trials Network (grant U10 DA015815), and the NIDA San Francisco Treatment Research Center (grant P50 DA009253).
The authors thank the New York Office of Alcohol and Substance Abuse Services for assistance in drawing the random sample of programs and for making initial contact with those programs on behalf of the research team.
Preliminary findings were reported at the American Society of Addiction Medicine in 2009, and the College of Problems on Drug Dependence in 2010.
Note. NIDA and NCI had no role in the project beyond financial support. R. Zavala is a researcher and L. S. Brown is the director for 2 programs—the Addiction Research and Treatment Corporation Fort Greene and the Urban Resource Institute—included in the study sample, and those programs received staff, patient, and program incentives as reported in the article.
Footnotes
Contributors
J. Guydish conceptualized and supervised the study. B. Tajima and A. Kulaga conducted all site visits and data collection. R. Zavala and L. S. Brown are affiliated with 2 of the programs in the study and provided perspectives and context concerning the New York policy. A. Bostrom planned analysis together with J. Guydish. M. Chan executed all analyses, and all participated in data interpretation. D. Ziedonis supported development of both staff and client survey measures. All authors read and commented on earlier drafts of the article.
Reprints can be ordered at http://www.ajph.org by clicking the “Reprints” link.
Human Participant Protection
This study was approved by the University of California, San Francisco, Committee on Human Research. All data collected were deidentified, and responses remain anonymous. Participants received information sheets and provided verbal consent before taking part in this study.
Contributor Information
Joseph Guydish, Philip R. Lee Institute for Health Policy Studies, University of California, San Francisco.
Barbara Tajima, Philip R. Lee Institute for Health Policy Studies, University of California, San Francisco.
Agatha Kulaga, New York University Langone Medical Center, New York, NY.
Roberto Zavala, Addiction Research and Treatment Corporation, Brooklyn, NY.
Lawrence S. Brown, Addiction Research and Treatment Corporation, Brooklyn, NY.
Alan Bostrom, Department of Epidemiology and Biostatistics, University of California, San Francisco.
Douglas Ziedonis, Department of Psychiatry, University of Massachusetts Medical School, Worcester.
Mable Chan, Philip R. Lee Institute for Health Policy Studies, University of California, San Francisco.
References
- 1.Hurt RD, Eberman KM, Slade J, Karan L. Treating nicotine addiction in patients with other addictive disorders. In: Orleans C, Slade J, editors. Nicotine Addiction: Principles and Management. New York, NY: Oxford University Press; 1993. pp. 310–326. [Google Scholar]
- 2.Kalman D. Smoking cessation treatment for substance misusers in early recovery: a review of the literature and recommendations for practice. Subst Use Misuse. 1998;33(10):2021–2047. doi: 10.3109/10826089809069815. [DOI] [PubMed] [Google Scholar]
- 3.Facts About Nicotine and Tobacco Products. Bethesda, MD: National Institute on Drug Abuse; 1998. [Google Scholar]
- 4.Hughes J. Do smokers with current or past alcoholism need different or more intensive treatment? Alcohol Clin Exp Res. 2002;26(12):1934–1935. doi: 10.1097/01.ALC.0000041282.57396.30. [DOI] [PubMed] [Google Scholar]
- 5.Sobell MB. Alcohol and tobacco: clinical and treatment issues. Alcohol Clin Exp Res. 2002;26(12):1954–1955. doi: 10.1097/01.ALC.0000041008.52475.C5. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman RS, Warheit GJ, Ulbrich PM, Auth JB. The relationship between alcohol use and attempts and success at smoking cessation. Addict Behav. 1990;15 (3):197–207. doi: 10.1016/0306-4603(90)90063-4. [DOI] [PubMed] [Google Scholar]
- 7.Drobes DJ. Cue reactivity in alcohol and tobacco dependence. Alcohol Clin Exp Res. 2002;26(12):1928–1929. doi: 10.1097/01.ALC.0000040983.23182.3A. [DOI] [PubMed] [Google Scholar]
- 8.Hurt RD, Offord KP, Croghan IT, et al. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275 (14):1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- 9.Bobo JK, Davis CM. Recovering staff and smoking in chemical dependency programs in rural Nebraska. J Subst Abuse Treat. 1993;10(2):221–227. doi: 10.1016/0740-5472(93)90047-6. [DOI] [PubMed] [Google Scholar]
- 10.Degenhardt L, Hall W. The relationship between tobacco use, substance-use disorders and mental health: results from the National Survey of Mental Health and Well-Being. Nicotine Tob Res. 2001;3(3):225–234. doi: 10.1080/14622200110050457. [DOI] [PubMed] [Google Scholar]
- 11.Friend KB, Pagano ME. Smoking initiation among nonsmokers during and following treatment for alcohol use disorders. J Subst Abuse Treat. 2004;26(3):219–224. doi: 10.1016/S0740-5472(04)00003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sussman S. Smoking cessation among persons in recovery. Subst Use Misuse. 2002;37(8–10):1275–1298. doi: 10.1081/ja-120004185. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman AL, Slade J. Following the pioneers. Addressing tobacco in chemical dependency treatment. J Subst Abuse Treat. 1993;10(2):153–160. doi: 10.1016/0740-5472(93)90040-9. [DOI] [PubMed] [Google Scholar]
- 14.Richter KP, Arnsten JH. A rationale and model for addressing tobacco dependence in substance abuse treatment. Subst Abuse Treat Prev Policy. 2006;1:23. doi: 10.1186/1747-597X-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder SA, Morris CD. Confronting a neglected epidemic: tobacco cessation for persons with mental illnesses and substance abuse problems. Annu Rev Public Health. 2010;31:297–314. doi: 10.1146/annurev.publhealth.012809.103701. 1p following 314. [DOI] [PubMed] [Google Scholar]
- 16.Stuyt EB, Order-Connors B, Ziedonis DM. Addressing tobacco through program and system change in mental health and addiction settings. Psychiatr Ann. 2003;33(7):447–456. [Google Scholar]
- 17.Fiore MC, Jaén CR, Baker TB, et al. Clinical Practice Guideline: Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 18.American Public Health Association. 2003–10 smoking cessation within substance abuse and/or mental health treatment settings. Association News Policy Statements. 2003:19–20. [Google Scholar]
- 19.Public Policy Statement on Nicotine Addiction and Tobacco (Formerly Nicotine Dependence and Tobacco) Chevy Chase, MD: American Society of Addiction Medicine; 2008. [Google Scholar]
- 20.Friedmann PD, Jiang L, Richter KP. Cigarette smoking cessation services in outpatient substance abuse treatment programs in the United States. J Subst Abuse Treat. 2008;34(2):165–172. doi: 10.1016/j.jsat.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuller BE, Guydish J, Tsoh J, et al. Attitudes toward the integration of smoking cessation treatment into drug abuse clinics. J Subst Abuse Treat. 2007;32(1):53–60. doi: 10.1016/j.jsat.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter KP, Choi WS, McCool RM, Harris KJ, Ahluwalia JS. Smoking cessation services in U.S. methadone maintenance facilities. Psychiatr Serv. 2004;55(11):1258–1264. doi: 10.1176/appi.ps.55.11.1258. [DOI] [PubMed] [Google Scholar]
- 23.Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36(2):205–219. doi: 10.1016/j.jsat.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Sherman SE. A framework for tobacco control: lessons learnt from Veterans Health Administration. BMJ. 2008;336(7651):1016–1019. doi: 10.1136/bmj.39510.805266.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams JM, Foulds J, Dwyer M, et al. The integration of tobacco dependence treatment and tobacco-free standards into residential addictions treatment in New Jersey. J Subst Abuse Treat. 2005;28(4):331–340. doi: 10.1016/j.jsat.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 26.NY Office of Alcoholism and Substance Abuse Services. [Accessed July 24, 2008];Tobacco-free services: 2008 Title 14 NYCRR Part 856. Available at: http://www.oasas.ny.gov/regs/856.cfm.
- 27.Delucchi KL, Tajima B, Guydish J. Development of the Smoking Knowledge, Attitudes, and Practices (S-KAP) instrument. J Drug Issues. 2009;39(2):347–364. doi: 10.1177/002204260903900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guydish J, Tajima B, Chan M, Delucchi KL, Ziedonis D. Measuring smoking knowledge, attitudes and services (S-KAS) among clients in addiction treatment. Drug Alcohol Depend. 2011;114(2–3):237–241. doi: 10.1016/j.drugalcdep.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh DC, Gordon NP. Legal approaches to smoking deterrence. Annu Rev Public Health. 1986;7:127–149. doi: 10.1146/annurev.pu.07.050186.001015. [DOI] [PubMed] [Google Scholar]
- 30.Boyle JS. Field research: a collaborative model for practice and research. In: Morse J, editor. Qualitative Nursing Research: A Contemporary Dialogue. Newbury Park, CA: Sage; 1991. [Google Scholar]
- 31.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates Inc; 1988. [Google Scholar]
- 32.Behavioral Risk Factor Surveillance System Survey Data. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 33.Tesiny E, Robinson J, Nottingham W. Tobacco Regulation Impact Report. Albany, NY: New York State Office of Alcoholism and Substance Abuse Services; 2010. [Google Scholar]
- 34.Evans W, Farrelly M, Montgomery E. Do workplace smoking bans reduce smoking. Am Econ Rev. 1999;89 (4):728–747. [Google Scholar]
- 35.Behavioral Risk Factor Surveillance System Survey Data. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 36.Guydish J, Passalacqua E, Tajima B, Chan M, Chun J, Bostrom A. Smoking prevalence in addiction treatment: a review. Nicotine Tob Res. 2011;13(6):401–411. doi: 10.1093/ntr/ntr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knudsen HK, Studts JL. Availability of nicotine replacement therapy in substance use disorder treatment: longitudinal patterns of adoption, sustainability, and discontinuation. Drug Alcohol Depend. 2011;118(2–3):244–250. doi: 10.1016/j.drugalcdep.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guydish J, Passalacqua E, Tajima B, Turcotte Manser S. Staff smoking and other barriers to nicotine dependence intervention in addiction treatment settings: a review. J Psychoactive Drugs. 2007;39(4):423–433. doi: 10.1080/02791072.2007.10399881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Results From the 2008 National Survey on Drug Use and Health: National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009. [Google Scholar]
- 40.Hoffman AL, Kantor B, Leech T, et al. Drug-Free Is Nicotine-Free: A Manual for Chemical Dependency Treatment Programs. New Brunswick, NJ: Tobacco Dependence Program; 1997. [Google Scholar]
- 41.Ziedonis DM, Zammarelli L, Seward G, et al. Addressing tobacco use through organizational change: a case study of an addiction treatment organization. J Psychoactive Drugs. 2007;39(4):451–459. doi: 10.1080/02791072.2007.10399884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guydish J, Ziedonis D, Tajima B, et al. Addressing Tobacco Through Organizational Change (ATTOC) in residential addiction treatment settings. Drug Alcohol Depend. 2012;121(1–2):30–37. doi: 10.1016/j.drugalcdep.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gill BS, Bennett DL. Addiction professionals’ attitudes regarding treatment of nicotine dependence. J Subst Abuse Treat. 2000;19(4):317–318. doi: 10.1016/s0740-5472(00)00106-9. [DOI] [PubMed] [Google Scholar]