Abstract
Neurofibrillary tangles (NFTs) are composed of aggregated hyperphosphorylated tau proteins and represent a common hallmark in Alzheimer’s disease (AD) and other tauopathies. Neuroinflammatory molecules derived from glial cells can trigger various cell stress response pathways, including overexpression of the secreted glycoprotein Dickopff-1 (DKK-1). DKK-1 promotes glycogen synthase kinase 3β (GSK-3β) activation, thereby disrupting the canonical Wnt pathway and causing tau hyperphosphorylation, NFT formation and neuronal degeneration. Recent studies suggest that the astrocyte-derived, Ca++ and Zn++-binding protein S100B may play a causal role in AD. However, no clear mechanistic relationship between S100B and NFT formation has been established. In the present study, we demonstrate that micromolar S100B concentrations activate c-Jun N-terminal kinase (JNK) phosphorylation through the receptors for advanced glycation (RAGE) and subsequent nuclear AP-1/cJun transcription in human neural stem cells (NSCs). AP-1/cJun activation of DKK-1 promotes GSK-3 β hyperphosphorylation and β-catenin degradation causing canonical Wnt pathway disruption and tau protein hyperphosphorylation. These findings propose a previously unrecognized link between S100B and tau hyperphosphorylation, suggesting that S100B contributes to NFT formation in AD. Moreover, S100B inhibition may serve a neuroprotective function and provide an effective strategy to delay progression of AD.
Introduction
A hallmark of AD is the NFTs seen in the pathologic brain (1). NFTs are comprised of abnormal hyperphosphorylated tau microtubule associated protein, indicating that dysregulation of tau phosphorylation may be responsible for the abnormal aggregation and impaired function of this protein in the AD brain (2,3). Several studies suggest that GSK-3 β, which phosphorylates serine/threonine and regulates the Wnt signalling pathway, likely contributes to tau hyperphosphorylation. In general, GSK-3 β phosphorylation is inhibited through the canonical Wnt pathway (4). However, aberrant processing of β–amyloid (Aβ), which is thought to contribute to the development of AD, disrupts the Wnt signalling cascade, and induces GSK-3 βactivation, subsequent tau hyperphosphorylation and neuronal cell damage (5).
Aβ not only exhibits direct cytotoxic mechanisms that impact on neuronal survival but also promotes inflammatory processes that contribute to the AD phenotype (6). While initial glial activation through Aβ beneficially contributes to the restoration of brain function, persistent reactive gliosis may result in maladaptative detrimental responses, thus substantially contributing to AD progression. For example, ongoing inflammation can trigger various cell stress response pathways, including overexpression of the secreted glycoprotein DKK-1. DKK-1 up-regulates GSK-3β activity, promotes tau hyperphosphorylation, NFT formation and neuronal degeneration. Thus, DKK-1 inhibits Wnt signalling in a manner similar to Aβ, and thereby fosters a selfsustaining feedback loop resulting in cellular injury (5,7,8).
Several lines of evidence suggest that S100B, a small soluble protein belonging to the large family of EF-related (EF helices) Ca++ and Zn++-binding proteins (for review see 9), may contribute to the AD brain phenotype. S100B overexpression has been closely associated with reactive gliosis and AD progression (10). Furthermore, at nanomolar concentrations, S100B provides a pro-survival effect on neurons and stimulates neurite outgrowth, but at higher micromolar concentrations, the protein promotes neuroinflammation and neuronal apoptosis (11). Lastly, detrimental effects of S100B are transduced by interactions with the receptor for advanced glycation ending products (RAGE) (12), which in microglial cells has been reported to lead to JNK activation (13). Perturbations in the JNK signalling cascade can regulate DKK-1 expression (14).
To date, no studies have explored whether S100B is able to disrupt Wnt signalling and promotes tau protein hyperphosphorylation through DKK-1. Here we use differentiated NSCs and demonstrate that micromolar S100B concentrations through RAGE activate JNK phosphorylation and subsequent nuclear AP-1/cJun transcription in these mixed neuronal and glial cell populations. AP- 1/cJun activation of DKK-1 promotes GSK-3 β hyperphosphorylation and β-catenin degradation. Finally, this disruption of the Wnt pathway leads to tau protein hyperphosphorylation. These findings propose a previously unrecognized link between S100B and tau hyperphosphorylation, suggesting that S100B contributes to NFT formation in AD.
Results
S100B induces JNK phosphorylation and AP-1/c-Jun activation through RAGE dependent interaction
Prior studies have shown that S100B interactions with RAGE in microglia are accompanied by increased oxidative stress signalling that is linked to the activation of different members of the Stress Activated Protein Kinases (SAPKs) family, and subsequent induction of nuclear transcription factors (13,14). Initiation of this pathway is dependent upon exposure to micromolar concentrations of exogenous S100B and interactions with the RAGE extracellular domain but is independent of RAGE transducing activity (13,15). To address whether a similar pathway might exist within a mixed human neuronal and glial cell population, we first investigated the effects of exogenous S100B challenge on phosphorylation of the stress responsive JNK. Incubation of differentiated NSCs with increasing concentrations of purified S100B protein (0.05 – 5 μM) revealed a dose dependent increase in JNK phosphorylation (pJNK) within 30 min of S100B stimulation (Table 1). We then assessed the involvement of RAGE in mediating S100B activation of pJNK. S100Btreated (maximal response obtained at 5 μM) cultures were challenged with specific RAGE blocking antibody at various dilutions (1:1000 or 1:10000 v/v). RAGE blocking antibody significantly reduced JNK phosphorylation in a dose dependent manner (Table 1). These experiments suggest that S100B interactions with RAGE leads to the activation of the stress response kinase JNK in mixed human neuronal and glial cell populations.
Table 1.
S100B induces JNK phopsphorylation through RAGE interaction in a dose-dependent fashion. Results are the mean ± S.E.M. of three independent experiments.
| Treatmen |
pJNK (OD=mm2) |
|
|---|---|---|
|
S100B (μM) |
RAGE blocking Ab (v/v dilution) |
|
| - | - | |
| 0.05 | - | ** |
| 0.5 | - | *** |
| 5 | - | *** |
| 5 | 1:10000 | ○○ |
| 5 | 1:1000 | ○○○ |
p < 0.001
p < 0.01 versus unchallenged cells
p < 0.001
p < 0.01 versus 5 μM S100B challenged cells.
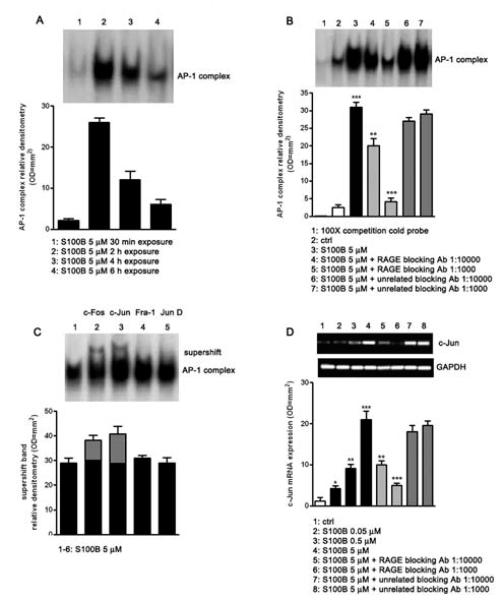
Phosphorylation of JNK as well as other SAPKs can lead to nuclear AP-1 complex up-regulation (16). We therefore tested whether S100B (5 μM) exposure resulted in AP-1 complex induction by electrophoretic mobility shift assay (EMSA). S100B induced AP-1 complex up-regulation peaked at 2 h after stimulus and progressively declined over 6 h after S100B exposure (Fig. 1A). Based on these results, we fixed the S100B exposure at 2 h for the subsequent investigations. Further EMSA analysis revealed that specific RAGE blocking antibody was able to decrease AP-1 complex up-regulation in a concentration dependent manner (1:1000 or 1:10000 v/v) within S100B induced versus untreated differentiated NSCs. Blocking using an unrelated control antibody was ineffective and the addition of 100-fold molar excess of unlabelled AP-1 oligonucleotide completely abolished AP-1 DNA complex formation, showing the specificity of action by the RAGE antibody and S100B (Fig. 1B). Supershift studies were also performed on S100B (5 μM) challenged cultures to identify activation of specific AP-1 complex components, using antibodies directed against c-Fos, c-Jun, Fra-1, and Jun-D. A supershift in the c-Fos and c-Jun complexes was appreciated at 2 h after S100B treatment, while no significant effect was detected in the other tested complexes (Fig. 1C). Finally, reverse transcriptase PCR (RT-PCR) analysis of the c-Jun/JNK pathway demonstrated that c-Jun mRNA expression was induced 6 h following cell stimulation with increasing concentrations of S100B (0.05 - 5 μM) in a concentration dependent fashion, when compared with unchallenged cells. The S100B induction of c-Jun was attenuated by RAGE blocking antibody, while unrelated blocking antibody had no effect (Fig. 1D). Collectively, these studies demonstrate that the soluble, astroglial-derived S100B interacts with RAGE, leading to JNK phosphorylation and pJNK dependent up-regulation of c-Jun, a component of the AP-1 complex.
Fig. 1.
S100B induces AP-1/DNA complex activation through RAGE interaction. A) AP-1/DNA complex activation was detected in NSCs at various time point (30 min - 6h) following the exposure to 5 μM of S100B. The time course of AP-1/DNA binding activity was evaluated by EMSA (upper panel) and densitometric analysis of corresponding bands (lower panel). B) AP-1/DNA binding activity induced by 5 μM of S100B in NSCs in the presence or absence of two different concentrations of RAGE blocking antibody or unrelated blocking antibody (1:1000 or 1:10000). Statistics show that S100B significantly enhanced the activation of AP-1/DNA complex and that the specific RAGE blocking antibody significantly blunted this effect, while the unrelated antibody failed to influence it. The AP-1/DNA binding activity was measured 2 h after S100B exposure by EMSA analysis (upper panel). The lower panel shows densitometric analysis of corresponding bands. C) AP-1 antibodies (anti Fra-1, anti c-Fos, anti c-Jun and anti Jun-D) were preincubated with nuclear extracts from NSCs exposed to 5 μM of S100B. Results of supershift analysis (upper panel) demonstrate the effect of the antibodies on the changes in the relative mobility of AP-1 species 2 h following NSCs stimulation with S100B. Densitometric analysis of corresponding bands is reported in the lower panel. D) NSCs were challenged with increasing concentrations of S100B (0.05 - 5 μM) and 6 h later lysed. c-Jun mRNA expression was evaluated by RT-PCR (upper panel) and densitometric analysis of corresponding bands (lower panel). Statistics demonstrate significant and dose-dependent effect of S100B on c-Jun mRNA expression. The figure also shows two different dilutions of RAGE blocking antibody (1:1000 or 1:10000) were able to revert the effect of the highest concentration of S100B, whereas the same dilutions of an unrelated blocking antibody were ineffective. Results are the mean ± S.E.M. of three independent experiments. *** p < 0.001, ** p < 0.01, and * p < 0.05 versus unchallenged cells (ctrl); ○○○ p < 0.001 and ○○ p < 0.01 versus 5 μM S100B challenged cells.
S100B induces DKK-1 protein expression
Among the different c-Jun molecular targets, DKK-1 is a stress stimuli-induced protein, which behaves as a potent endogenous Wnt pathway disruptor (7). In order to assess whether S100B might affect DKK-1 expression, we performed immunoblot analysis on differentiated human neuronal and glial cultures, challenged with increasing concentrations of purified S100B (0.05 - 5 μM). This treatment caused a concentration dependent rise of DKK-1 protein expression 12 h after stimulation (Table 2). Under these same experimental conditions, S100B (5 μM) challenged cells were treated with RAGE blocking antibody (1:1000 or 1:10000 v/v) in the presence or absence of the specific JNK phosphorylation inhibitor SP600125 (1 or 10 μM). S100B induction of DKK-1 up-regulation was blocked by RAGE blocking antibody in a dose-dependent fashion with almost complete inhibition of DKK-1 induction seen at the higher RAGE blocking antibody titer (1:1000 v/v) or in the presence of SP600125 (Table 2). These data thus support a pivotal role for JNK in mediating the molecular signalling mechanisms downstream of S100B stimulation, leading to DKK-1 up-regulation.
Table 2.
S100B induces DKK-1 protein expression through RAGE and JNK involvement in a dose dependent fashion. Results are the mean ± S.E.M. of three independent experiments.
| Treatment |
DKK-1 (OD=mm2) |
||
|---|---|---|---|
|
S100B (μM) |
RAGE blockingAb (v/v dilution) |
SP600125 (μM) |
|
| - | - | - | |
| 0.05 | - | - | ** |
| 0.5 | - | - | *** |
| 5 | - | - | *** |
| 5 | 1:10000 | - | ○○○ |
| 5 | 1:1000 | - | ○○ |
| 5 | 1:1000 | 1 | ### |
| 5 | 1:1000 | 10 | ### |
p < 0.001
p < 0.01 versus unchallenged cells
p < 0.001
p < 0.01 versus 5 _M S100B challenged cells
p < 0.001 versus 5 μM S100B plus RAGE block ng antibody (1:1000) challenged cells.
S100B leads to tau protein hyperphosphorylation by inducing GSK-3β phosphorylation and causing β-catenin degradation in the Wnt pathway
Activation of the canonical Wnt pathway leads to two main molecular mechanisms, represented by the inhibition of GSK-3 β and the relative accumulation/degradation of β-catenin in the cytoplasm (4,5). Different stress-related stimuli such as DKK-1 can also disrupt the Wnt pathway, inhibiting GSK-3β suppression and inducing tau protein hyperphosphorylation. Given our prior observation of DKK-1 induction by S100B, we investigated whether GSK-3 β and β –catenin expression was altered 24 h following cell stimulation with S100B (0.05 - 5 μM) by immunoblot analysis. S100B caused a concentration dependent increase in GSK-3β phosphorylation (as shown by increased immunoreactivity of p-Ser-9 bands, Fig. 2A), as well as a parallel degradation in β-catenin expression in treated versus untreated cells (Fig. 2B). Both of these effects on S100B (5 μM) challenged cells were reduced by RAGE blocking antibody (1:1000 or 1:10000 v/v), suggesting that S100B functions as a disruptor of the canonical Wnt pathway through a RAGE dependent interaction.
Fig. 2.
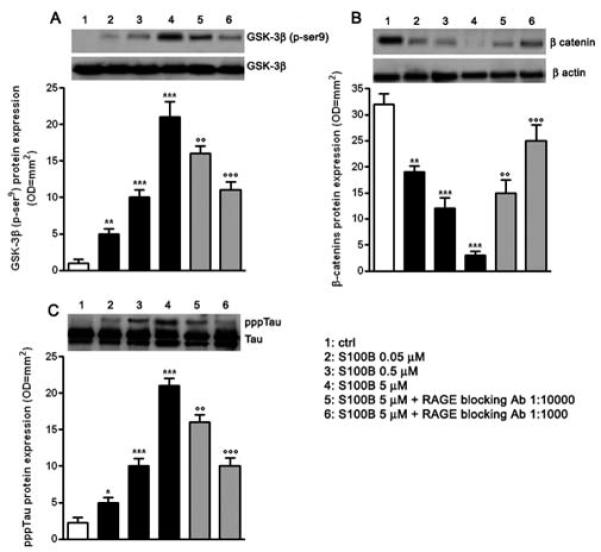
S100B induces tau protein hyperphosphorylation through Wnt pathway disruption. A) NSCs were challenged with increasing concentrations of S100B (0.05 - 5 μM). The pGSK-3β protein expression was evaluated 24 h following treatment by Western blot (upper panel) and densitometric analysis of corresponding bands (lower panel). GSK-3β served as a loading control. Statistics demonstrate significant and dose dependent effects of S100B on pGSK-3β protein expression. Two different dilutions of RAGE blocking antibody (1:1000 or 1:10000) were able to dose dependently antagonize the effect of the highest concentration of S100B. B) β-catenin protein expression was evaluated 24 h following treatment by Western blot (upper panel) and densitometric analysis of corresponding bands (lower panel). β-actin served as a loading control. Statistics show that S100B significantly and dose-dependently affected β-catenin protein expression. Two different dilutions of RAGE blocking antibody (1:1000 or 1:10000) were able to revert the effect of the highest concentration of S100B in a dose dependent manner. C) pppTau protein expression was evaluated 48 h following treatment by Western blot (upper panel) and densitometric analysis of corresponding bands (lower panel). Tau served as a loading control. Statistics indicate that S100B was able to dose dependently and significantly promote pppTau protein expression. Two different dilutions of RAGE blocking antibody (1:1000 or 1:10000) antagonized the effect of the highest concentration of S100B in a dose dependent fashion. Results are the mean ± S.E.M. of three separated experiments.*** p < 0.001, ** p < 0.01, and * p < 0.05 versus unchallenged cells (ctrl); ○○○ p < 0.001 and ○○ p < 0.01 versus 5 μM S100B challenged cells.
Tau hyperphosphorylation is an expected consequence of Wnt pathway disruption. We therefore examined whether S100B (0.05 - 5 μM) treatment altered tau protein phosphorylation levels in the mixed neuronal and glial cultures. S100B exposure induced tau protein hyperphosphorylation at 48 h following stimulation in treated versus untreated cells, as shown by the immunoreactive pppTau bands (Fig. 2C). Similar to RAGE antibody inhibition of S100B-induced GSK-3β phosphorylation and β-catenin degradation, RAGE antibody (1:1000 or 1:10000 v/v) blockade of interactions with S100B resulted in a dose-dependent reduction in tau protein hyperphosphorylation (Fig. 2C).
DKK-1 inhibition abolishes S100B induced tau protein hyperphosphorylation
The current experiments show that blocking S100B and RAGE interactions inhibits JNK phosphorylation, activation of the AP-1/cJUN complex, DKK-1 expression, components of the canonical Wnt pathway, and tau microtubule associated protein phosphorylation in mixed human neuronal and glial cultures. Moreover, inhibitors of JNK phosphorylation block DKK-1 expression, and prior studies have shown that DKK-1 overexpression promotes GSK-3β activity and tau hyperphosphorylation. To address whether S100B mediates DKK-1 protein expression and tau protein phosphorylation in neurons, we used immunoflurorescence analysis to identify neuronal staining for these proteins following S100B exposure. Cultures were exposed to S100B (5 μM) in the presence or absence of RAGE blocking antibody (1:1000 v/v), stained for the neuronal marker microtubule associated protein-2 (MAP2), DKK-1 and pppTau, and then visualized 12 or 48 h after stimulation. Consistent with results obtained by immunoblot analysis, both DKK-1 and pppTau immunopositive neurons were increased in S100B treated versus untreated cells. As expected, this increase in immunoreactivity was also significantly blocked by RAGE antibody (Fig. 3A, B).
Fig. 3.
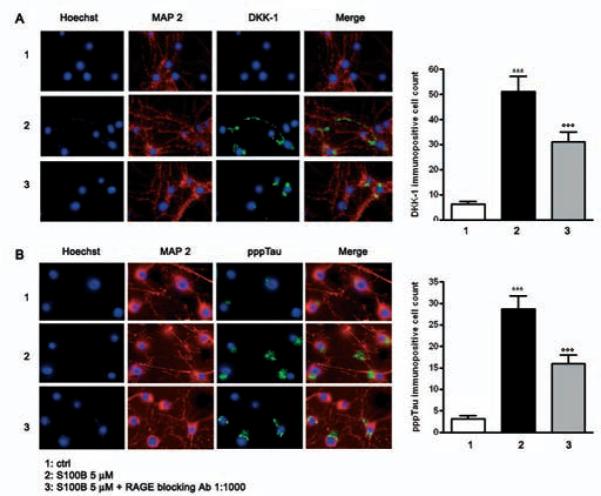
S100B induces DKK-1 expression and tau protein hyperphosphorilation through RAGE interaction. A)Left panel: representative photomicrographs of NSCs showing DKK-1 immunostaining (green). NSCs cultures were exposed to S100B 5 μM in the presence or absence of RAGE blocking antibody (1:1000), and DKK-1 protein expression was evaluated 12 h later by immunofluorescence analysis. Right panel: quantification of immunoreactivity expressed as the number of DKK-1 immunopositive neuronal cells. Statistics indicate that RAGE blocking antibody significantly reverted the effect of S100B on DKK-1 protein expression. B)Left panel: representative photomicrographs of NSCs showing pppTau immunostaining (green). NSCs cultures were exposed to S100B 5 μM in the presence or absence of RAGE blocking antibody (1:1000), and pppTau protein expression was evaluated 48 h later by immunofluorescence analysis. Right panel: quantification of immunoreactivity expressed as the number of pppTau immunopositive neuronal cells. Statistics indicate that RAGE blocking antibody significantly reverted the effect of S100B on pppTau protein expression. Anti-MAP-2 antibody was used as a neuronal marker (red), and nuclei were stained with Hoechst 33258 (blue). Scale bar = 20 μm. Data are shown as mean ± S.E.M. of five experiments. *** p < 0.001 versus unchallenged cells (ctrl); ○○○ p < 0.001 versus 5 μM S100B challenged cells.
S100B induction of both DKK-1 and pppTau expression in neurons raises the possibility that S100B promotes DKK-1 expression, and thereby disrupts the canonical Wnt pathway (increased GSK-3β phosphorylation and enhanced β-catenin degradation), leading to tau protein hyperphosphorylation. To address this possibility, we used an mRNA silencing (siRNA) approach to selectively inhibit mRNA and protein expression of DKK-1 in SHSY5Y human neuroblastoma cells. No significant changes in DKK-1 expression were seen on the transcriptional and translational level following control siRNA (Cyclophin B) or untransfected cells, 12 and 24 h after transfection, respectively (data not shown). As observed for differentiated NSCs, 5 μM S100B exposure significantly increased GSK-3β phosphorylation and β-catenin degradation by 24 h after stimulation. S100B pre-treatment also resulted in significant tau protein hyperphosphorylation at 48 h after exposure, in comparison with untreated SHSY5Y cells. Under these same experimental conditions, DKK-1 siRNA almost completely abolished GSK-3β phosphorylation, β-catenin degradation, tau protein hyperphosphorylation (Fig. 4A, B, C). Finally, these same changes in the canonical Wnt pathway were seen within SHSY5Y neuroblastoma cells transfected with DKK-1 siRNA (Fig. 5). Taken together, these findings demonstrate that S100B functions through DKK-1 in disrupting the Wnt pathway and causing tau protein hyperphosphorylation.
Fig. 4.
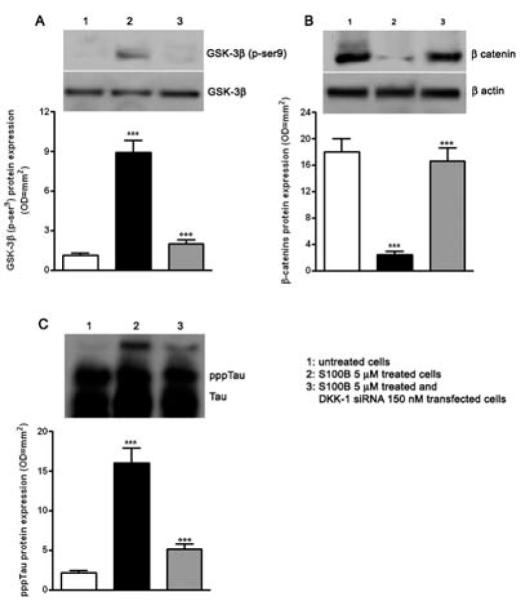
S100B requires DKK-1 activation to induce Wnt pathway disruption and tau protein hyperphosphorylation. A) SHSY5Y cells were transfected with 150 nM DKK-1 siRNA and treated with S100B 5 μM. The pGSK-3β protein expression was evaluated 24 h following treatment by Western blot (upper panel) and densitometric analysis of corresponding bands (lower panel). GSK-3β served as a loading control. B) β-catenin protein expression was evaluated 24 h following treatment by Western blot (upper panel) and densitometric analysis of corresponding bands (lower panel). β-actin served as a loading control. C) pppTau protein expression was evaluated 48 h following treatment by Western blot (upper panel) and densitometric analysis of corresponding bands (lower panel). Tau served as a loading control. Statistics show that S100B required DKK-1 activation to reduce β -catenin protein expression and to promote pGSK-3β and pppTau protein expression. Untransfected and untreated cells were used as internal controls. Results are the mean ± S.E.M. of three independent experiments.*** p < 0.001 versus untreated cells; ○○○ p < 0.001 versus untransfected cells treated with S100B 5 μM.
Fig. 5.
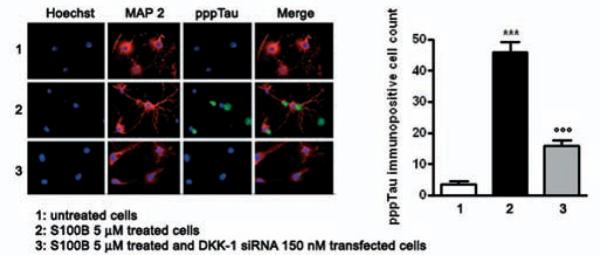
DKK-1 siRNA abolishes S100B induced tau protein hyperphosphorylation. SHSY5Y cells were transfected with 150 nM DKK-1 siRNA and treated with S100B 5 μM. pppTau protein expression was evaluated 48 h later by immunofluorescence analysis (left panel) and subsequent immunopositive cell count (right panel). Anti-MAP-2 antibody was used as a neuronal marker (red), while nuclei were stained with Hoechst 33258 (blue). Scale bar, 20 μm. Untransfected and untreated cells were used as internal controls. Results are the mean ± S.E.M. of three independent experiments. *** p < 0.001 versus untreated cells; ○○○ p < 0.001 versus untransfected cells treated with S100B 5 μM.
Discussion
The current studies extend upon known mechanisms of neuronal injury in AD. While aberrant processing of Aβ in neurons intrinsically leads to disruption of the canonical Wnt pathway (17), NFT formation and neuronal cell death, these same processes can invoke an extrinsically mediated glial inflammatory response. Here we show that elevated levels of the astrocyte-released S100B protein can similarly disrupt the Wnt signalling pathway, through interactions with RAGE and up-regulation of the DKK-1 glycoprotein. Activation of this molecular cascade leads to GSK-3β activation and tau protein hyperphosphorylation. Dysregulation of tau phosphorylation is thought to be responsible for NFT formation and neuronal death in the AD brain (1,18).
Several lines of evidence support a role for reactive gliosis in mediating AD progression. Senile plaques (SPs) and NFTs are co-localized with clusters of activated microglia and associated with a broad variety of astrocyte-derived inflammation-related proteins. In addition, both in vitro and in vivo studies have shown that Aβ peptide fragments induce a prominent neuroinflammatory response, responsible for the synthesis of different cytokines and pro-inflammatory mediators, including nitric oxide (NO), prostaglandins, interleukins and specific-glial derived molecules such as S100B protein (19,20). Finally, reactive gliosis can induce oxidative stressors and Wnt disruption, and both factors result in amyloidogenesis and tauopathy (21).
S100B expression is associated with the pathology seen in the aging AD brain (22). Mice that exhibit premature aging express higher concentrations of S100B, than can otherwise be explained by mice age alone. S100B protein is also increased both in post-mortem AD brain and in the cerebrospinal fluid of AD patients in the early stages of the disease (23). Similarly, elevated S100B levels correlate with the early onset AD seen in trisomy 21 (Down’s syndrome) (24,25), while transgenic mice that overexpress βAPP also display increased numbers of activated astrocytes and tissue concentrations of S100B several months before the appearance of Aβ deposits and SP formation (26). Finally, S100B is overexpressed in activated astrocytes surrounding SPs, where the degree of astrocyte response correlates with the degree of the neuritic pathology (22,27).
Several pathological mechanisms can be triggered from the overexpression of S100B. Elevated S100B levels exert damaging effects on neurons through pro-inflammatory cytokines and inflammatory stress-related transcription factors (28,29,30,31). In addition, S100B can directly increase βAPP expression (32), which in turn can activate astrocytes and raise S100B levels, thereby fostering a self-sustaining feedback loop that drives the progressive pathology seen in AD. Finally, the current studies demonstrate that S100B can disrupt the Wnt pathway through increases in the glycoprotein DKK-1. DKK-1 promotes activation of GSK-3 β activation, a key member of Wnt pathway implicated in tauopathy.
A central role for DKK-1 in mediating Wnt disruption and neuronal degeneration in AD brain has previously been demonstrated (5,7,8). In cortical neurons, βAPP induces the expression of the secreted DKK1, which negatively modulates the canonical Wnt signaling pathway and thus activates the tau-phosphorylating enzyme GSK-3β. DKK1 is also expressed by degenerating neurons in AD brain, and co-localizes with NFTs and distrophic neurites. Results from this work now reveal that exposure to elevated levels of S100B protein (likely from reactive astrogliosis) can similarly disrupt Wnt signalling through DKK-1. In this manner, both neuronal (βAPP) and glial (S100B) factors may promote the same cellular mechanisms that are implicated in the pathogenesis of neurodegenerative disorders.
The current studies emphasize the key role of reactive gliosis in AD progression and reinforce the need to identify factors responsible for glial-maintained neuroinflammation. Glial inflammation leads to elevated expression of astrocyte-derived S100B. While S100B can promote βAPP expression and βAPP-dependent neuronal injury, we now show that this soluble protein can also disrupt Wnt signalling through DKK-1 and promote tau hyperphosphorylation. Tau hyperphospyrlation is associated with NFT formation and AD progression. These findings suggest that reagents, which inhibit S100B function, may serve a neuroprotective function and provide an effective strategy to delay progression of AD.
Materials and methods
Cell culture
Derivation of foetal NSC has previously been described (33). NSCs were cultured in DMEM, 30% Ham’s F-12, 1% antibiotic-anti-mycotic, 2% B27 (all from Invitrogen, Milan, Italy), 20 ng/ml EGF (Sigma, St Louis, MO), 20 ng/ml FGF2 (R&D Systems Inc., Minneapolis, MN) for 1 week. NSCs were maintained at a density of 5×105 cells/ml of medium, half of the medium being replaced with fresh medium every 3 days. For differentiation, cells were resuspended in plating media (DMEM/F-12, 2% B27), treated with 10 μM retinoic acid (34) (Sigma) and allowed to differentiate in plating media for 7-10 days. During differentiation, half of the plating medium was replaced every 2 days with fresh medium. After a brief incubation for 2-4 h in Neurobasal medium (Invitrogen), cells were supplemented with 1 mM glutamine, 2% B27 and 1% foetal bovine serum (FBS) for adhesion and treatments. SHSY5Y cells were cultured in DMEM supplemented with 5% foetal calf serum (FCS), 15% horse serum (HS), 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in 5% CO2/95% air and differentiated into neurons with 10 μM retinoic acid. Depending upon the experimental procedure, cells were plated on Petri dishes or on coverslips and fixed in 4% paraformaldehyde for immunofluorescence analysis.
Protein isolation and Western blot analysis
After treatments, cells were washed with ice-cold PBS and centrifuged at 180 × g for 10 min at 4 °C. The cell pellet was resuspended in 100 μl of ice-cold hypotonic lysis buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM PMSF, 1.5 μg/ml soybean trypsin inhibitor, 7 μg/ml pepstatin A, 5 μg/ml leupeptin, 0.1 mM benzamidine, 0.5 mM DTT) and incubated on ice for 15 min. Cell lysates were centrifuged at 13,000 × g for 1 min at 4 °C. Protein concentration was determined, and equivalent amounts (100 _g) of each sample underwent SDS-PAGE electrophoresis. Proteins were then transferred onto nitrocellulose membranes according to the manufacturer’s instructions (Bio-Rad, Hercules, CA) and immunoblot was carried out with the following antibodies: anti-JNK (1:500; Santa Cruz Biotechnology, La Jolla, CA), anti-DKK-1 (1:1000, Santa Cruz Biotechnology), anti- β -actin (1:2000, Santa Cruz Biotechnology), anti-GSK-3β (1:250, Lab Vision, Fremont, CA), anti- β-catenin (1:250, Lab Vision), anti-Tau (1:1000, Neomarker, Freemont, CA). The membranes were incubated with the proper secondary antibody coupled to peroxidase (1:1000; DAKO, Golstrup, Denmark) and the immunocomplexes were visualised by the ECL chemiluminescence method (Amersham, Milan, Italy). Protein relative expression was quantified by densitometric scanning of the X-ray films with a GS 700 Imaging Densitometer (Bio-Rad) and a computer programme (Molecular Analyst, IBM).
RNA isolation and Reverse Transcriptase-PCR analysis
The mRNA level was determined using the semiquantitative RT-PCR method (Invitrogen). Total mRNA was extracted from cells by use of an ultrapure TRIzol reagent (Gibco BRL, Milan, Italy) as directed by the manufacturer. The concentration and purity of total mRNA were determined from the A260/A280 ratio using a UV spectrophotometer (DU 40, Beckman, Fullerton, CA). The primer sequences used for PCR amplification were: c-Jun sense 5′-AACCTTCTATGACGATGCCCTCA-3′ and antisense 5′-CCTGCTCATCTGTCACGTTCTT-3′; GAPDH sense 5′-GAAGGTGAAGGTCGGAGT-3′ and antisense 5′-GAAGATGGTGATGGGATTTC-3′; DKK-1 sense 5′-ACCAGACCATTGACAACTAC-3′ and antisense 5′-GTGTCTAGCACAACACAATC-3′. 1 μg of total RNA from each specimen was subjected to RT-PCR, carried out using a SuperScript TM One-Step RT-PCR with Platinum Taq Kit (Invitrogen) in a total reaction volume of 25 μl, containing 2× reaction mix, 25 μM sense primer, 25 μM antisense primer, RT-PCR platinum Taq mix, and autoclaved distilled water. Electrophoresis was performed on the amplification products using a 1% agarose gel and bands were visualized by staining with ethidium bromide. The integrated density values of the bands representing amplified products were acquired and analyzed by GS 700 Imaging Densitometer (Bio-Rad) and a computer program (Molecular Analyst, IBM).
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts, prepared from treated NSCs, were obtained according the technique described by De Stefano et al. (35). Samples were incubated with 10000 cpm of the 32P-labeled AP-1 (5′-CGCTTGATGAGTCAGCCGGAA-3′) oligonucleotide (Promega Corp., Madison WI). The binding assay was performed in the presence of poly(dI-dC) (Pharmacia Biosystem, Milton Keynes, UK) as a non specific competitor and 32P-labeled AP-1 for 30 min at room temperature. After electrophoresis, the gels were dried and autoradiographed. In supershift and competition assays, 500 μl of AP-1 antibodies (anti Fra-1, anti c-Fos, anti c-Jun and anti Jun-D, Santa Cruz Biotechnologies) were preincubated with nuclear extracts for 30 min before the addition of the labeled probe.
Immunofluorescence
NSCs were plated onto glass slide chambers coated with poly-D-lysine (BD, Milan, Italy) for several hours and were fixed with 4% paraformaldehyde in PBS. The cultures were placed in PBS blocking solution containing 10% FCS and 5% HS, and incubated with the following antibodies: anti-DKK-1 (1:100, Santa Cruz Biotechnology), anti-MAP-2 (1:300, Lab Vision), anti-Tau (1:200, Neomarker). Following PBS washing, sections were incubated in the dark for 30 min with Texas Red conjugated or fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:200, AbCam, Cambridge, UK). Nuclei were stained with Hoechst (Sigma). Laser scanning confocal microscopy was thus performed by using a Zeiss LSM 510 laser scanning system and immunopositive cells quantified by a computer program.
DKK-1 RNA interference
siRNA technique for DKK-1 was carried out on SHSY5Y cells according to protocol indicated by the manufacturer (Santa Cruz Biotechnology, see catalogue number sc-37082) and scrambled sequence for DKK-1 was used as a negative control. Specific silencing was confirmed by at least three independent immunoblot and RT-PCR experiments.
Abbreviations
- (DKK-1)
Dickopff-1
- (AD)
Alzheimer’s disease
- (GSK-3β)
Glycogen Synthase Kinase 3
- (NFTs)
Neurofibrillary tangles
- (JNK)
c-Jun N-terminal kinase
- (A_)
β -amyloid
References
- 1.Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 2.Kosik KS, Joachim CL, Selkoe DJ. Proc Natl Acad Sci USA. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Ferrari GV, Inestrosa NC. Brain Res Brain Res Rev. 2000;33:1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- 5.Caricasole A, Copani A, Caruso A, Caraci F, Iacovelli L, Sortino MA, Terstappen GC, Nicoletti F. Trends Pharmacol Sci. 2003;24:233–238. doi: 10.1016/s0165-6147(03)00100-7. [DOI] [PubMed] [Google Scholar]
- 6.McGeer PL, McGeer EG. Neurobiol Aging. 2001;22:799–809. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- 7.Scali C, Caraci F, Gianfriddo M, Diodato E, Roncarati R, Pollio G, Gaviraghi G, Copani A, Nicoletti F, Terstappen GC, Caricasole A. Neurobiol Dis. 2006;24:254–265. doi: 10.1016/j.nbd.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, Storto M, Gaviraghi G, Terstappen GC, Nicoletti F. J Neurosci. 2004;24:6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmer DB, Cornwall EH, Landar A, Song W. Brain Res Bull. 1995;37:417–429. doi: 10.1016/0361-9230(95)00040-2. [DOI] [PubMed] [Google Scholar]
- 10.Mrak RE, Griffin WS. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Van Eldik LJ, Wainwright MS. Restor Neurol Neurosci. 2003;21:97–108. [PubMed] [Google Scholar]
- 12.Adami C, Bianchi R, Pula G, Donato R. Biochim Biophys Acta. 2004;1742:169–177. doi: 10.1016/j.bbamcr.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi R, Adami C, Giambanco I, Donato R. J Leukoc Biol. 2007;81:108–118. doi: 10.1189/jlb.0306198. [DOI] [PubMed] [Google Scholar]
- 14.Grotewold L, Ruther U. EMBO J. 2002;21:966–975. doi: 10.1093/emboj/21.5.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huttunen HJ, Fages C, Rauvala H. J Biol Chem. 1999;274:19919–19924. doi: 10.1074/jbc.274.28.19919. [DOI] [PubMed] [Google Scholar]
- 16.Whitmarsh AJ, Davis RJ. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 17.De Ferrari GV, Chacon MA, Barria MI, Garrido JL, Godoy JA, Olivares G, Reyes AE, Alvarez A, Bronfman M, Inestrosa NC. Mol Psychiatry. 2003;8:195–208. doi: 10.1038/sj.mp.4001208. [DOI] [PubMed] [Google Scholar]
- 18.Dickson DW. J Clin Invest. 2004;114:23–27. doi: 10.1172/JCI22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mrak RE, Sheng JG, Griffin WS. Hum Pathol. 1995;26:816–823. doi: 10.1016/0046-8177(95)90001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mudher A, Lovestone S. Trends Neurosci. 2002;25:22–26. doi: 10.1016/s0166-2236(00)02031-2. [DOI] [PubMed] [Google Scholar]
- 22.Mrak RE, Griffin WS. Neurobiol Aging. 2001;22:915–922. doi: 10.1016/s0197-4580(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 23.Peskind ER, Griffin WS, Akama KT, Raskind MA, Van Eldik LJ. Neurochem Int. 2001;39:409–413. doi: 10.1016/s0197-0186(01)00048-1. [DOI] [PubMed] [Google Scholar]
- 24.Royston MC, McKenzie JE, Gentleman SM, Sheng JG, Mann DM, Griffin WS, Mrak RE. Neuropathol Appl Neurobiol. 1999;25:387–393. doi: 10.1046/j.1365-2990.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- 25.Griffin WS, Sheng JG, McKenzie JE, Royston MC, Gentleman SM, Brumback RA, Cork LC, Del Bigio MR, Roberts GW, Mrak RE. Neurobiol Aging. 1998;19:401–405. doi: 10.1016/s0197-4580(98)00074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheng JG, Mrak RE, Bales KR, Cordell B, Paul SM, Jones RA, Woodward S, Zhou XQ, McGinness JM, Griffin WS. J Neurochem. 2000;74:295–301. doi: 10.1046/j.1471-4159.2000.0740295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mrak RE, Sheng JG, Griffin WS. J Neuropathol Exp Neurol. 1996;55:273–279. doi: 10.1097/00005072-199603000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J, Van Eldik LJ. Biochim Biophys Acta. 1996;1313:239–245. doi: 10.1016/0167-4889(96)00095-x. [DOI] [PubMed] [Google Scholar]
- 29.Hu J, Ferreira A, Van Eldik LJ. J Neurochem. 1997;69:2294–2301. doi: 10.1046/j.1471-4159.1997.69062294.x. [DOI] [PubMed] [Google Scholar]
- 30.Lam AG, Koppal T, Akama KT, Guo L, Craft JM, Samy B, Schavocky JP, Watterson DM, Van Eldik LJ. Neurobiol Aging. 2001;22:765–772. doi: 10.1016/s0197-4580(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Li Y, Van Eldik LJ, Griffin WS, Barger SW. J Neurochem. 2005;92:546–553. doi: 10.1111/j.1471-4159.2004.02909.x. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Wang J, Sheng JG, Liu L, Barger SW, Jones RA, Van Eldik LJ, Mrak RE, Griffin WS. J Neurochem. 1998;71:1421–1428. doi: 10.1046/j.1471-4159.1998.71041421.x. [DOI] [PubMed] [Google Scholar]
- 33.Sheen VL, Ferland RJ, Harney M, Hill RS, Neal J, Banham AH, Brown P, Chenn A, Corbo J, Hecht J, Folkerth R, Walsh CA. Ann Neurol. 2006;60:137–144. doi: 10.1002/ana.20843. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs S, Lie DC, DeCicco KL, Shi Y, DeLuca LM, Gage FH, Evans RM. Proc Natl Acad Sci USA. 2006;103:3902–3907. doi: 10.1073/pnas.0511294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Stefano D, Maiuri MC, Iovine B, Ialenti A, Bevilacqua MA, Carnuccio R. J Mol Med. 2006;84:65–74. doi: 10.1007/s00109-005-0713-x. [DOI] [PubMed] [Google Scholar]